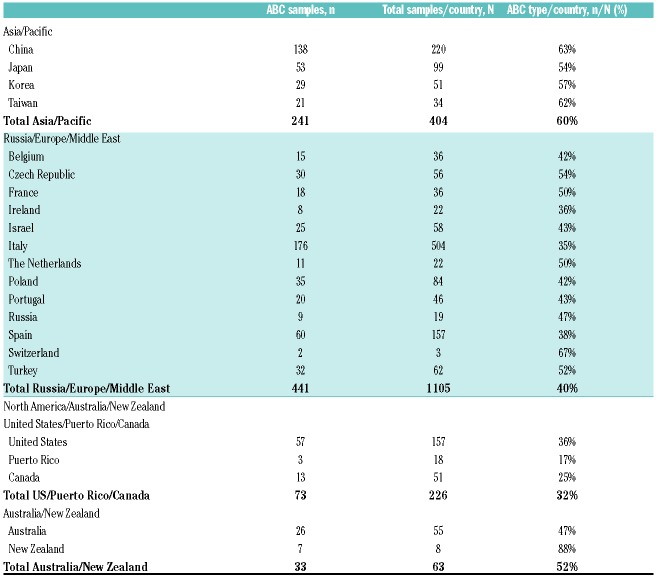
Although molecular heterogenicity of diffuse large B-cell lymphoma (DLBCL) with regards to cell-of-origin (COO) was first described nearly 20 years ago, limitations of technology used to assess COO precluded prospective, real time implementation in clinical trials until recently. Advances in COO methodology have enabled incorporation of COO into ongoing clinical studies, such as the international phase III ROBUST study (clinicaltrials.gov identifier: 02285062), which is designed to evaluate the efficacy and safety of lenalidomide plus R-CHOP (R2-CHOP) versus placebo/R-CHOP in patients with activated B-cell-like (ABC) DLBCL.1 Based on our experience with the ROBUST study, we feel it is important to report our observations from the COO analysis, as they have the potential to influence the design and size estimation of studies that are currently being planned and will be planned in the future. Here, we report notable differences in the proportion of ABC-type DLBCL based on global regions: 60% in samples from China/Japan/South Korea/Taiwan; 40% in samples from Russia/Europe/Middle East; and 37% in samples from North America/Australia/New Zealand.
The importance of identifying COO is now well established as a key component for classifying patients with DLBCL.2 Two distinct DLBCL subtypes were discovered by gene expression profiling (GEP). These were then translated into an immunohistochemistry (IHC) algorithm which recognized two broad subgroups defined as germinal center B-cell-like (GCB) and non-GCB subtypes.3–5 The use of GEP allows more accurate assignment of COO and specifically differentiates GCB from the ABC subtype, with a small group of tumors that cannot be assigned with high confidence labeled as unclassified.5,6 Patients with ABC-DLBCL who received R-CHOP have historically shown inferior outcomes, as demonstrated by 5-year overall survival (OS) rates of approximately 80% versus approximately 50% in DLBCL subtypes GCB versus ABC, respectively (P<0.001).6 However, newer combination treatment is challenging that notion. Two phase II studies (MC078E and REAL07) demonstrated that the addition of the IMiD® immunomodulatory agent lenalidomide to standard R-CHOP (i.e. R2-CHOP) resulted in similar survival outcomes for newly diagnosed DLBCL patients with either GCB or non-GCB COO subtype (per IHC3), and may actually improve survival outcomes for those with the non-GCB subtype (as compared with historic controls receiving R-CHOP with no lenalidomide).7,8 These studies laid the foundation for the ongoing phase III ROBUST study, which integrates COO identification by GEP in the screening process to prospectively target patients with ABC-type DLBCL.1 Table 1. ROBUST study: proportion of ABC-type diffuse large B-cell lymphoma by region.
Table 1.
ROBUST study: proportion of ABC-type diffuse large B-cell lymphoma by region.
The ROBUST study, which includes 257 active study sites in 21 countries worldwide, identifies ABC-type DLBCL patients and randomizes them 1:1 to R2-CHOP (15 mg/day oral lenalidomide, days 1-14, plus standard R-CHOP21) or placebo/R-CHOP (primary end point is progression-free survival by central review). Further details of the ROBUST study design have been previously reported.1 For these analyses, tumor biopsy samples provided in a formalin-fixed, paraffin-embedded (FFPE) block or unstained slides were submitted to central pathology for diagnosis, COO subtyping, and evaluation of CD20+ status, as required by study inclusion criteria. To minimize the potential for pre-screening bias, study investigators were discouraged from pre-screening patients with IHC and thoroughly educated on the process for submitting tissue samples for GEP analysis to central pathology for COO typing. COO subtype was determined by the DLBCL Lymphoma Subtyping Test (LST) assay, which was based on the Lymph2Cx assay analyzing FFPE samples using the commercial NanoString® nCounter® Analysis System (NanoString Technologies Inc., Seattle, WA, USA).4 This system was successfully validated against the original COO model defined by Wright et al.9 with a very low subtype misassignment rate of 2%, comparing favorably to IHC (6-17%) and with ≥95% concordance of COO assignment by two independent laboratories.4 The turnaround time for sample identification was 2.4 days and was defined as the number of calendar days between central pathology sample receipt and results being provided to the study site. Central pathology centers were located in Beijing (China), Edinburgh/Heston (UK), and Valencia (CA, USA).
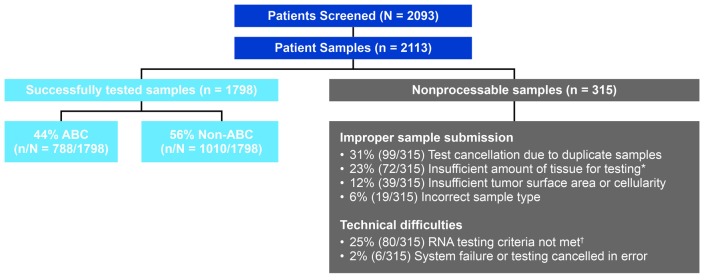
ROBUST study enrollment was completed on August 3rd 2017; 2,113 samples from 2,093 patients were screened. Of these 2,113 samples, 1,798 (85%) were successfully tested for COO subtype, whereas 315 (15%) were non-processable for various reasons, including improper sample submission and technical difficulties with the assay (Figure 1). Of successfully tested samples in the ROBUST study, 788 of 1,798 (44%) samples were identified as ABC-DLBCL [56% non-ABC (GCB and unclassified)]; of these 788 samples, 570 patients fulfilled the remaining inclusion/exclusion criteria and were enrolled. The overall proportion of enrolled patients who had ABC-type DLBCL was 27% (570 of 2,093 screened patients). Subtype incidence using the same assay was consistent with two separate studies of patients receiving R-CHOP at the British Columbia Cancer (BCC) agency10 and in patients randomized to R-CHOP or obinutuzumab plus CHOP (i.e. G-CHOP) from the GOYA study.11 In the BCC study, 108 of 335 (32%) patients with evaluable samples were identified as ABC-DLBCL.10 For the global phase III GOYA study, 933 of 1,418 patients enrolled were assessed for COO, 243 of 933 (26%) of whom had ABC-type DLBCL.11
Figure 1.
ROBUST study: enrollment and sample processing. *Tissue/block from site was small core or tissue biopsy, block from site was nearly exhausted, insufficient slide numbers, or no tissue or tumor on slides. †RNA concentration and/or purity did not meet criteria or low RNA signal at hybridization step.
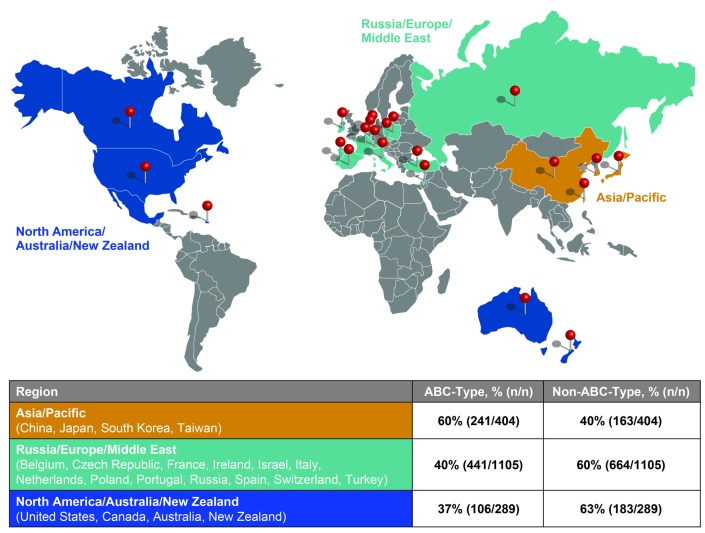
Notably, there were global variations in COO for ROBUST based on geographical region of origin. The proportion of ABC-DLBCL from all 1,798 successfully tested samples was 60% (241 of 404 samples) from China/Japan/South Korea/Taiwan, 40% (441 of 1,105 samples) from Russia/Europe/Middle East, and 37% (106 of 289 samples) from North America/Australia/New Zealand (Figure 2). A small number of patients were found in the Australia and New Zealand combined region [33 of 63 samples (52% ABC)], which is why they were combined with North American samples [73 of 226 samples (32% ABC)] (Table 1). The noticeably higher ABC-subtype in Asian patients aligns with non-GCB subtype rates identified by IHC,12 and from a Korean study classifying COO as ABC, and utilizing the same GEP methodology used here.13 In 82 Korean patients, the COO distribution was numerically similar (59% ABC, 35% GCB, and 6% unclassified) to the ROBUST percentages reported for the Asian cohort [60% ABC and 40% non-ABC (GCB/unclassified combined)].13
Figure 2.
ROBUST study: results of COO testing by region. The proportion of COO subtypes from 1,798 successfully tested diffuse large B-cell lymphoma patient samples were evaluated from three regions: North America/Australia/New Zealand, Russia/Europe/Middle East, and China/Japan/South Korea/Taiwan.
The two main COO subtypes harbor unique genetic profiles, which may in part explain differences in responses to therapy.5 The ABC subtype is associated with genetic aberrations leading to activation of B-cell receptor and Toll-like receptor signaling, along with downstream NF-κB activation.5 Similarly, Epstein-Barr virus (EBV)-associated proteins have been known to play an important role in immune cell evasion, preventing cell apoptosis, and regulating tumor cell proliferation.14 One possible relationship with the increased proportion of ABC-type DLBCL is a higher incidence of EBV positivity in Asian countries over those seen in Western countries. In a meta-analysis of 4,111 patients from 13 studies (10 Asian countries, 2 Caucasian countries, and 1 from Peru), EBV positivity (measured by EBV-encoded small RNA) was correlated with unfavorable OS (P<0.001).14 High EBV positivity was also associated with known poor prognosis factors [older age >60 years, high International Prognostic Index (IPI), >1 extranodal involvement, presence of B symptoms, elevated lactate dehydrogenase] and the non-GCB subtype.
Rapid identification of patient COO subtype was important for enabling patients to receive treatment quickly after diagnosis and screening. For 1,798 of 2,113 successfully tested patient samples, mean turnaround time for COO determination was an average of 2.4 calendar days, allowing patients to be randomized to a treatment arm at the study outset. These results provide support for the feasibility of real-time COO assessment by central laboratory analysis for multiple regions globally, with a short turnaround, minimizing the delay for patients in receiving treatment. The REMoDL-B study reported a median of 12 working days to identify COO results by GEP, allowing patients to be randomized to treatment arms beginning with the second cycle based on subtype assignment.15
This is the first report assessing the geographical distribution of ABC subtype as measured by GEP analysis in multiple global regions. It has the potential to guide future studies of newly diagnosed DLBCL patients based on COO. Overall, these results provide evidence of rapid COO testing to enable a significant advance in precision medicine in DLBCL, with the goal of enhancing accuracy of COO subtype assignment and ensuring appropriate patient selection for trials of novel therapies for ABC-type DLBCL. The potential impact on patient prognosis, study designs, and geographical-based treatment needs for ABC-DLBCL patients warrants further study.
Supplementary Material
Acknowledgments
We thank all of the co-investigators on the ROBUST clinical study; patients, families, and caregivers who are participating in the study; and support from Celgene Corporation for sponsoring the study. This work was supported by Celgene Corporation, Summit, NJ, USA and is a joint scientific collaboration among Fondazione Italiana Linfomi, Mayo Clinic, and Celgene. Editorial support was provided by Julie Kern, PhD, CMPP of Bio Connections LLC and funded by Celgene Corporation. The authors directed development of the manuscript and are fully responsible for all content and editorial decisions.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Nowakowski GS, Chiappella A, Witzig TE, et al. ROBUST: Lenalidomide-R-CHOP versus placebo-R-CHOP in previously untreated ABC-type diffuse large B-cell lymphoma. Future Oncol. 2016;12(13):1553–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282. [DOI] [PubMed] [Google Scholar]
- 4.Scott DW, Wright GW, Williams PM, et al. Determining cell-of-origin subtypes of diffuse large B-cell lymphoma using gene expression in formalin-fixed paraffin-embedded tissue. Blood. 2014;123(8):1214–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503–511. [DOI] [PubMed] [Google Scholar]
- 6.Lenz G, Wright G, Dave SS, et al. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med. 2008;359(22):2313–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vitolo U, Chiappella A, Franceschetti S, et al. Lenalidomide plus R-CHOP21 in elderly patients with untreated diffuse large B-cell lymphoma: results of the REAL07 open-label, multicentre, phase 2 trial. Lancet Oncol. 2014;15(7):730–737. [DOI] [PubMed] [Google Scholar]
- 8.Nowakowski GS, LaPlant B, Macon WR, et al. Lenalidomide combined with R-CHOP overcomes negative prognostic impact of non-germinal center B-cell phenotype in newly diagnosed diffuse large B-Cell lymphoma: a phase II study. J Clin Oncol. 2015;33(3):251–257. [DOI] [PubMed] [Google Scholar]
- 9.Wright G, Tan B, Rosenwald A, Hurt EH, Wiestner A, Staudt LM. A gene expression-based method to diagnose clinically distinct subgroups of diffuse large B cell lymphoma. Proc Natl Acad Sci U S A. 2003;100(17):9991–9996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott DW, Mottok A, Ennishi D, et al. Prognostic significance of diffuse large B-cell lymphoma cell of origin determined by digital gene expression in formalin-fixed paraffin-embedded tissue biopsies. J Clin Oncol. 2015;33(26):2848–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vitolo U, Trneny M, Belada D, et al. Obinutuzumab or rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in previously untreated diffuse large B-cell lymphoma. J Clin Oncol. 2017;35(31):3529–3537. [DOI] [PubMed] [Google Scholar]
- 12.Shiozawa E, Yamochi-Onizuka T, Takimoto M, Ota H. The GCB subtype of diffuse large B-cell lymphoma is less frequent in Asian countries. Leuk Res. 2007;31(11):1579–1583. [DOI] [PubMed] [Google Scholar]
- 13.Yoon N, Ahn S, Yong Yoo H, Jin Kim S, Seog Kim W, Hyeh Ko Y. Cell-of-origin of diffuse large B-cell lymphomas determined by the Lymph2Cx assay: better prognostic indicator than Hans algorithm. Oncotarget. 2017;8(13):22014–22022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao X, Li J, Wang Y, Liu S, Yue B. Clinical characteristics and prognostic significance of EBER positivity in diffuse large B-cell lymphoma: a meta-analysis. PLoS One. 2018;13(6):e0199398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies AJ, Caddy J, Maishman T, et al. A prospective randomised trial of targeted therapy for diffuse large B-cell lymphoma (DLBCL) based upon real-time gene expression profiling: the Remodl-B study of the UK NCRI and SAKK lymphoma groups. Blood. 2015; 126(23):812. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.