Abstract
A series of DNA primers containing nucleotides with various sugar pucker conformations at the 3′-terminus were chemically synthesized by solid-phase synthesis. The ability of wild-type (WT) HIV-1 reverse transcriptase (RT) and AZT-resistant (AZTr) RT to excise the 3′-terminal nucleotide was assessed. Nucleosides with a preference for the North conformation were more refractory to excision by both WT-RT and AZTr-RT. We found that DNA primers that contain North puckered-nucleotides at the 3′-terminus can also affect the translocation status of the RT/template/primer complex, which provides an underlying mechanism to avoid being excised. Together, these results point to a correlation between the sugar conformation of the 3′-terminal nucleotide, the precise position of HIV-1 RT on its nucleic acid substrate, and, in turn, its catalytic function. Nucleotide sugar conformation is therefore an important parameter in defining the susceptibility to RT-catalyzed phosphorolytic excision.
Graphical Abstract
Nucleoside (or nucleotide) reverse transcriptase inhibitors (NRTIs) are widely used clinically as first-line treatment for HIV infection as important components of Highly Active Anti-Retroviral Therapy (HAART).1–5 Zidovudine (AZT), the first FDA approved NRTI to treat HIV, has been shown to inhibit HIV replication by competing with thymidine triphosphate for incorporation into the growing DNA strand and causing chain termination. However, with prolonged treatment, HIV can mutate the gene encoding reverse transcriptase (RT) to acquire AZT resistance. These mutations are known as thymidine analogue mutations (TAMs) and result in a number of amino acid changes in RT including D67N/K70R/T215F/K219Q.6–8 TAMs confer high-level resistance to AZT by facilitating the removal of incorporated 3′-AZT by a process termed nucleotide phosphorolytic excision.7,8 While in this report we refer to RT with the indicated TAMs as AZT-resistant RT (AZTr-RT), these TAMs also provide some degree of resistance to most other NRTIs. Removal of the chain-terminating 3′-terminal NRTI enables restoration (or rescue) of RT-catalyzed DNA synthesis. Nucleotide excision is thus a major phenotypic mechanism of resistance to the NRTI class of antiretroviral agents.9
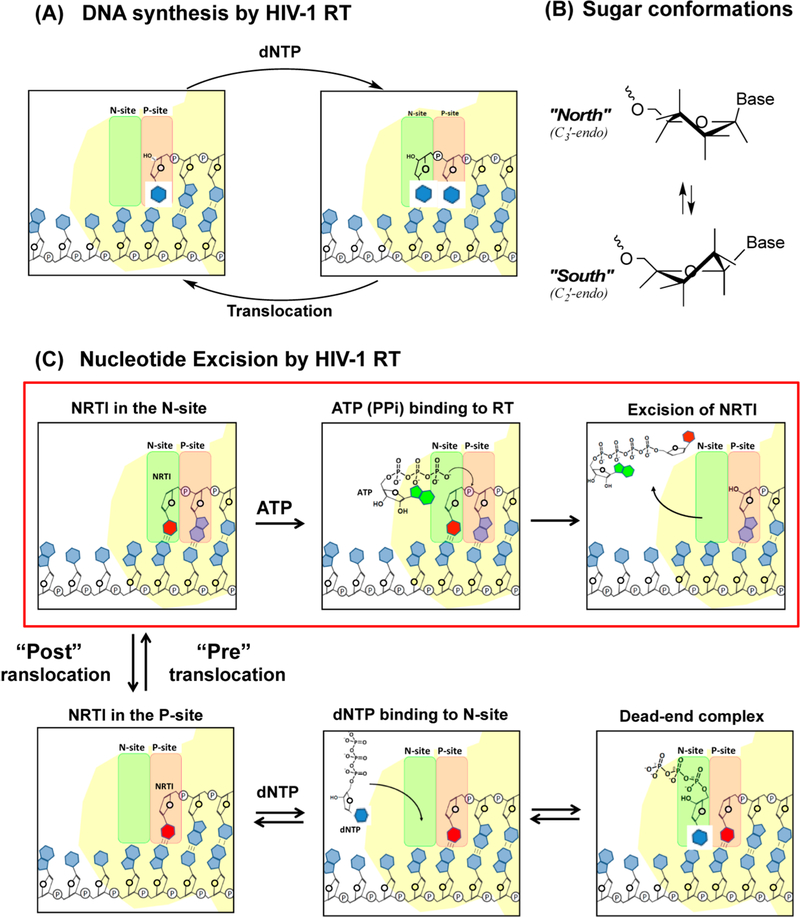
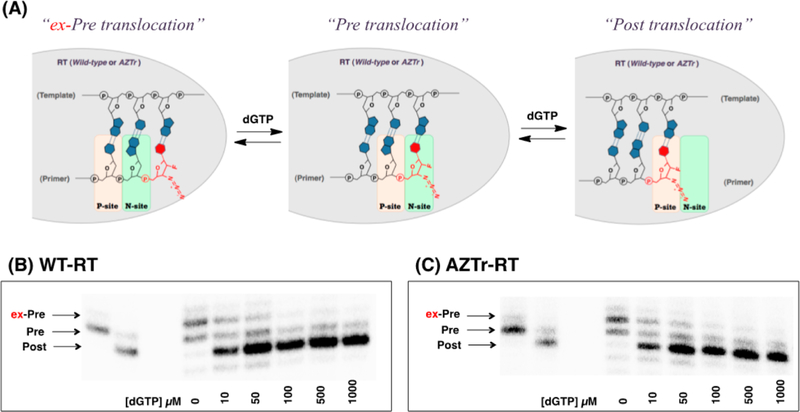
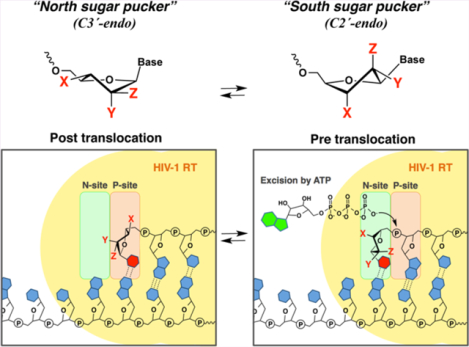
The nucleotide excision process involves the attack of pyrophosphate or a pyrophosphate donor, such as ATP, on the 5′-phosphate moiety of the 3′-terminal NRTI to form an NRTI-triphosphate or a 5′,5′-linked dinucleoside tetraphosphate (ATP-pNRTI), respectively8 (Figure 1C). For excision to occur, the 3′-terminal nucleotide must occupy the nucleotide binding site (N-site), also called the pretranslocation state. In contrast, nucleotide binding requires RT translocation relative to its nucleic acid substrate, which moves the 3’-terminus of the primer to the primer site (P-site).10,11 The enzyme shuttles between pre- and post-translocational states, which constitutes a dynamic equilibrium (Figure 1C).
Figure 1.
Schematic illustration of (A) polymerization cycles of HIV-1 RT, (B) sugar conformation of nucleosides, and (C) the excision of a chain terminator (NRTI). Once the NRTI is incorporated into the N-site, further chain extension is stopped due to the lack of a 3’-hydroxyl group in NRTI. HIV-1 RT locates this 3’-terminal NRTI at the N-site. Cellular ATPs bind to the complex and nucleophilically attack the 5’-phosphodiester linkage of the NRTI, excising the NRTI by forming a 5’,5’-linked dinucleoside tetraphosphate (ATP-pNRTI).
The presence of TAMs in RT substantially enhances the rate of ATP-mediated NRTI excision compared to the WT drug-sensitive enzyme. Other factors that influence nucleotide excision include the concentrations of cellular ATP, the concentration of the next complementary nucleotide, which can trap the primer 3′-terminus at the P-site by virtue of nucleotide binding to the N-site, and other less well-characterized factors such as the sequence of the primer-template.12 Structural features of the primer 3′-terminal nucleotide have an impact on the efficiency of excision. For example, it has been proposed that the bulky azido group of AZT may cause steric conflicts in the post-translocational state, shifting the translocational equilibrium toward pre-trans-location.13,14 AZT, thus, remains in the N-site, enabling potential excision. Furthermore, the nature of the nucleobase in 3′-azido-nucleotides affects the rate of excision, indicating that the 3′-azido group is not the only determinant for excision.15
The present study describes the impact of primer 3′-terminal nucleotide sugar pucker on the ability of these nucleotides to undergo phosphorolytic excision. Pucker is the twist of the pentose ring to relieve ring and torsional strain, and this results in some of the nucleoside sugar atoms protruding above and below the plane of the ring (Figure 1B). The majority of nucleosides show sugar puckers that equilibrate between C3′-endo (North, or A-form RNA) and C2′-endo (South, or B-form DNA) conformations. Preference for a North or South sugar pucker has implications on nucleic acid structure and biological function. For example, HIV-1 RT prefers nucleotides with the North pucker as substrates for DNA polymerization, as determined from X-ray crystallography and studies evaluating incorporation of conformationally biased nucleotides.16–18 However, less was known about the impact of nucleotide conformation on RT-catalyzed phosphorolytic excision of 3’-terminal nucleotides. To evaluate this, we synthesized a library of DNA primers containing 3’-terminal nucleotides with different sugar conformational preferences and compared their rates of excision by WT-RT and AZTr-RT, as well as the 3′-terminal nucleotide translocational equilibrium (pretranslocation N-site/post-translocation P-site). Interestingly, we found that North-puckered nucleotides tended to occupy the P-site and were poorly excised, whereas South-puckered nucleotides favored the N-site and were rapidly excised.
RESULTS AND DISCUSSION
Synthesis of DNA Primer Library.
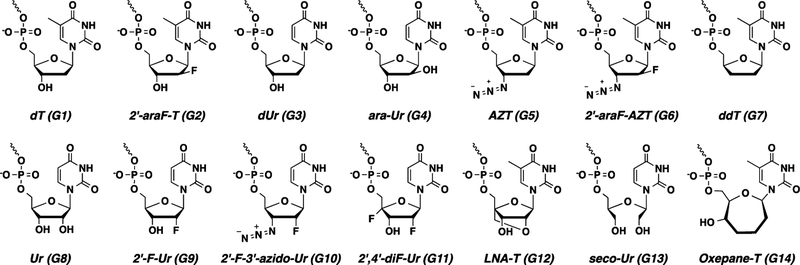
Previous studies have uncovered sugar preferences for the first phosphorylation step and incorporation, but they have never addressed the conformational requirement(s), if any, for excision. Hence, a key goal of this study was to evaluate the impact of nucleotide sugar conformation on the phosphorolytic excision reaction catalyzed by HIV-1 RT. We required a variety of primers with diverse terminal nucleotide chemistries which we obtained via solid-phase oligonucleotide synthesis. This is in contrast to other reported excision studies which employed HIV-1 RT to enzymatically incorporate the terminal nucleotides immediately prior to assessing excision.19,20 Using solid-phase synthesis, we were able to access virtually any desired nucleotide chemistry for our study without being limited by the incorporation efficiency by HIV-1 RT. In addition, this allowed us to decouple polymerization from excision and investigate the latter independently as a discrete reaction. We confined our analysis to T- and U-modified nucleosides only to avoid complications due to nucleobase-mediated effects. The 3′-terminal nucleotides with South sugar pucker included those based on thymidine (dT), 2′-deoxy-2′-fluoro-arabinothymidine (2′-araF-T), 2′-deoxyuridine (dUr), arabinouridine (ara-Ur), and 2′-(β)-fluoro-3′-azido-2′,3′-dideoxythymidine (2′-araF-AZT). Those favoring a North sugar pucker included 2′,3′-dideoxythymidine (ddT), uridine (Ur), 2′-fluoro-2′-deoxyur-idine (2′-F-Ur), 2′,4′-difluoro-2′-deoxyuridine (2′,4′-diF-Ur), 2′-(α)-fluoro-3′-azido-2′,3′-dideoxyuridine (2′-F-3′-azido-Ur), and LNA-thymidine (LNA-T). In order to examine the effect of size and flexibility of the sugar moiety of excised nucleotides on excision by RTs, we also prepared primers terminated with size-expanded oxepane-thymidine (oxepane-T) and conformationally unlocked flexible 2′,3′-seco-uridine (seco-Ur; Figure 2).
Figure 2.
Library of DNA primers that contain unmodified or chemically modified nucleotides at the 3’-terminus.
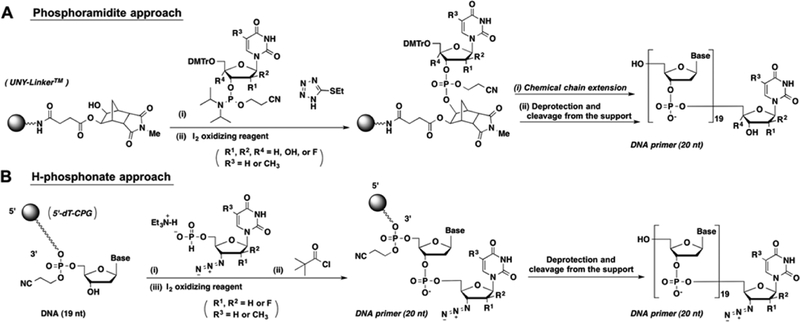
The synthesis of primers containing chain terminators was carried out from the 5′ to 3′ direction, as the 3′-terminal nucleotides have no 3′-hydroxyl group. This was achieved in excellent coupling yields using reversed 3′-O-DMT-5′-phos-phoramidite building blocks. The incorporation of the chain terminator was done in the final step. For primer G7, the 5′-phosphoramidite of ddT was coupled using a DNA synthesizer. However, this strategy is not compatible with chain terminators containing azido functional groups (primers G5, G6, and G10), as azido groups react with trivalent phosphorus.21 Coupling of nucleosides containing terminal azido groups was carried out from their respective 5′-H-phosphonates (Scheme 1), which were activated in situ by trimethylacetyl chloride in pyridine.22
Scheme 1. Synthetic Strategy for the Synthesis of Chemically Modified DNA Primers.
(A) Phosphoramidite approach for the chemical incorporation of nucleosides without azido-substituents. (B) H-phosphonate approach for the chemical incorporation of nucleosides containing azido-substituent.
RT-catalyzed Phosphorolytic Excision of 3′-Termmal Nucleotides.
The susceptibility of the various 3′-terminal nucleotides to phosphorolytic excision catalyzed by WT and AZTr-RT is summarized in Table 1 and Table S2. Both enzymes provided similar results, although the AZTr-RT provided a greater extent of excision, as anticipated.
Table 1.
Correlation between Sugar Conformation, Excision, and Translocation
| primers | nucleosidea | Northern Pb | Southern Pb | % Northc | excision by WT-RTd | translocation % pre (WT-RT)e |
|---|---|---|---|---|---|---|
| G12 | LNA-T23 | 17 | n.a. | 100 | − | 41 |
| G11 | 2′,4′-diF-Ur24 | 18 | n.a. | 100 | − | 27 |
| G9 | 2′-F-Ur26 | 21 | 159 | 87 | − | 26 |
| G10 | 2′-F-3′-N3–Ur25 | 17 | 155 | 85 | − | 32 |
| G7 | ddT27 | 11 | 154 | 75 | + | 66 |
| G8 | Ur26 | 18 | 162 | 58 | − | 19 |
| G5 | AZT30 | 22 | 160 | 50 | ++ | 80 |
| G4 | ara-Ur28 | 22 | 151 | 46 | − | 37 |
| G6 | 2′-araF-AZT27 | 11 | 120 | 40 | + | 76 |
| G3 | dUr26 | 18 | 162 | 39 | +++ | 66 |
| G1 | dT31 | n.a. | n.a. | 37 | +++ | 73 |
| G2 | 2′-araF-T29 | −6 | 126 | 31 | + | 70 |
| G13 | seco-Ur | n.a. | n.a. | n.a. | − | 24 |
| G14 | oxepane-T | n.a. | n.a. | n.a. | − | 28 |
Nucleoside sugar conformational preferences were obtained from indicated references.
Northern (Southern) P is indicative of the pseudorotational angle of the nucleoside in the most stable form of the North (South) conformation.
%N = 100 − %S; for ribonucleosides, 2′-(a)-fluoro-2′-deoxyribonucleosides, and ddT: %S29 =10 × J1′2′; for 2′-deoxyribonucleosides, 2′-(β)-fluoro-2′-deoxyribonucleosides, and ara-Ur: % S32 = 100 [J1′2′ (cis) + J1′2′ (trans) − 7.1]/9.
Legend: - no excision, + moderate excision, ++ fast excision, +++ very fast excision.
For South conformers and AZT: % Pre = Pre/[Pre + Post] × 100. For North conformers, seco-Ur, and oxepane-T: %Pre = Pre/[ex-Pre + Pre + Post] × 100.
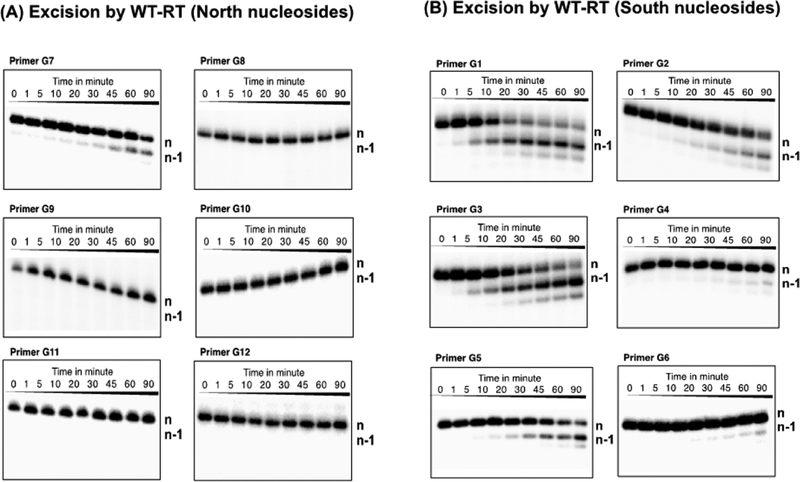
Primers with North-pucker 3′-terminal nucleotides (G7–G12) were refractory to phosphorolytic excision, with the exception of G7 (3′-terminal ddT; Figure 3A). In contrast, substantial excision was observed with DNA primers containing South- or South/East-pucker 3′-terminal nucleotides at the 3′-terminus (G1–G6, Figure 3B). The relative order of excision susceptibility with WT and AZTr-RT RT is G1 > G3 > G5 = G2 ≫ G4 = G6 (Figures S1 and S2). Excision of South pucker 3′-terminal nucleotides was affected by the nature of the substituents on the pentose sugar ring. Nucleotides with an arabinofuranose sugar (2′-β-OH or 2′-β-F; G2, G4, G6) showed diminished excision compared to the corresponding 2′-deoxyribose analogues (G1, G3, G5).
Figure 3.
Nucleotide excision by WT-RT of primers containing 3′-end North nucleosides (A) and South nucleosides (B). Aliquots were taken at each time point (1, 5, 10, 20, 30, 45, 60, and 90 min, respectively). For the control experiment, aliquots were taken before mixing “ATP-mix” solutions, which were used as aliquots at 0 min.
Primers G5, G6, and G10 have a bulky 3′-azido group that should promote pretranslocation, the site favoring excision. While primer G5 (3′-azido, 2′-deoxyribose) showed substantial excision activity, primer G10, with a North sugar pucker bias, was refractory to excision. Interestingly, primer G6 (2-araF-AZT) was also a poor substrate for excision. 2′-Ara-F-modified nucleosides are categorized as South/East-pucker conformers (Table 1), an intermediate conformer in the equilibrium between South and North sugar puckering. Our data thus suggest that 3′-terminal nucleotides must adopt a South conformation to allow excision and that this conformation seems to supersede the positive impact on excision traditionally attributed to the 3′-azido functionality. Primers containing seco-Ur (G13) and oxepane-T (G14), which consist of flexible or size-expanded sugars, were also highly refractory to RT-catalyzed excision (Figure S5). We therefore suggest that nucleotide sugar conformation is an important parameter in defining the susceptibility to RT-catalyzed phosphorolytic excision.
Rescue Assay Analysis of Excision of Primer 3′-Chain Terminating Nucleotides.
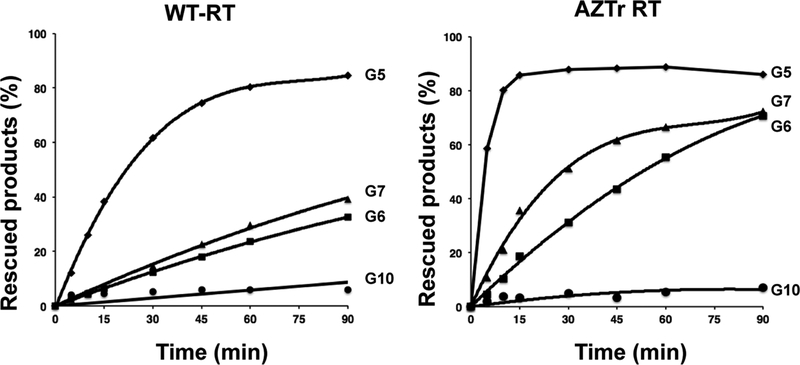
We complemented the direct excision assays by carrying out “rescue” assays in which DNA synthesis from a chain-terminated primer is evaluated in the presence of dNTPs and the pyrophosphate donor ATP. Primers G5, G6, G7, and G10 were used as they have 3′-terminal nucleotides lacking a 3′-OH and thus, cannot be extended without first removing the terminating nucleotide. As seen in Figure 4 and Figure S3, the rescue assays effectively recapitulated the direct excision assay data.
Figure 4.
Rescue of DNA synthesis of primers G5, G6, G7, and G10 by WT or AZTr-RT. Reactions were monitored in a time course. For the control experiment, aliquots were taken before initiation of the reaction (indicated as 0 min).
KOONO-dependent OH-Radial Footprinting.
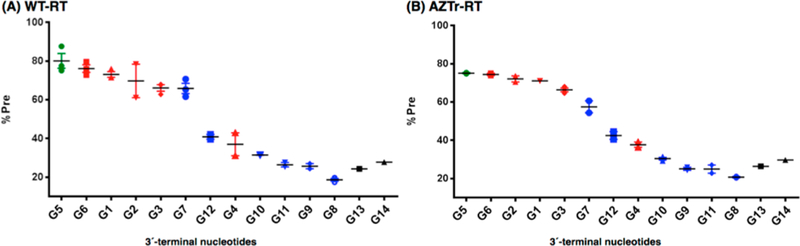
Nucleotide excision can only occur if the primer terminus occupies the N-site. Therefore, the influence of the sugar pucker on the translocation state also needs to be assessed when considering factors that affect excision. We carried out peroxynitrite footprinting analyses in which the DNA template of the RT binary complex is cleaved in a site-specific manner, thereby indicating the ratio of N-site and P-site occupancy by the primer 3′-terminal nucleotide.11 We observed that primers with greater excision efficiencies prefer the N-site (pretranslocation) compared to the P-site (post-translocation; Figure 5). Most of the DNA primers with South or South/East puckered-nucleotides at the 3′-terminus occupied the N-site greater than 60% of the time and were efficiently excised. In contrast, most DNA primers with North puckered-nucleotides at the 3′-terminus resided 40% or less in the N-site and were not excised. For example, G9 and G11 which contain terminal nucleotides that strongly prefer North sugar puckers reside predominantly in the post-translocation state, which explains their high resistance to excision. Primers also appear to escape excision by residing further away from the N-site. This was observed with primers G8, G10, G11, and G13, as the footprinting assays indicated a shift of RT (WT and AZTr) by one or two bases toward the 5′-end of the primer (i.e., ex-pre-translocation; Figures S4, S6). We also conducted Fe-mediated footprinting for G13 and G14 to avoid the possibility of method-dependent translocation, but no significant differences were observed (Figure S6).
Figure 5.
KOONO-mediated site-specific footprinting assays. Highlighted markers in green, red, and blue indicate 50:50 North/South conformation, North conformation preferred, and South conformation preferred respectively. The highlighted marker in black indicates nucleosides containing flexible or size-expanded sugars. (A) Translocation of the complex of DNA primers/template/RT (WT). (B) Translocation of the complex of DNA primers/template/RT (AZTr). For South conformers and AZT: % Pre = Pre/[Pre + Post] × 100 for North conformers, seco-Ur and oxepane-T: % Pre = Pre/[ex-Pre + Pre + Post] × 100.
Notable exceptions to the above trend included primer G7 containing a terminal ddT (75% North), which shows preference for the N-site but was moderately excised. We hypothesize this is because ddT is devoid of 2′ and 3′ substituents and thus, has relatively less energy barriers to overcome when equilibrating between North and South puckers. When it does equilibrate to South, it rapidly gets excised. Another exception was primer G4, which contains ara-Ur (54% South pucker) but did not show preference for the N-site and showed very little excision. As ara-Ur does not show a very strong bias for the South, it may easily flip to the North conformation in HIV-1 RT’s active site, thereby escaping excision. The debate over flexibility vs rigidity was further tested with G12 (LNA, rigid and locked North) and G13 (seco-Ur, flexible and unlocked), both of which did not exhibit any excision. The latter result further demonstrates the merits of using acyclic sugars as nucleotide analogues for antivirals, which may contribute to the success of drugs like tenofovir. Primer G12 with LNA-T occupied the N-site to some degree but showed high-level resistance to excision, presumably since LNA is locked in the North conformation and cannot adopt the South conformation required for excision. In summary, nucleoside flexibility can at times play in favor of excision (G7) or disfavor it (G13, G14).
The Effect of Next-Incoming dGTP on the Translocation of G5, G6, and G7.
Taking into account the results of the excision and rescue assays where we observed different excision efficiencies for AZT and 2′-araF-AZT, we proceeded to determine in detail the translocational status of primers having AZT (G5), 2′-araF-AZT (G6), and ddT (G7), in the presence of the next-complemental dGTP (Figure S7). We found that no significant difference was observed between G5 and G6 even in the presence of dGTP, whereas ddT easily shifted toward the P-site in the presence of dGTP.
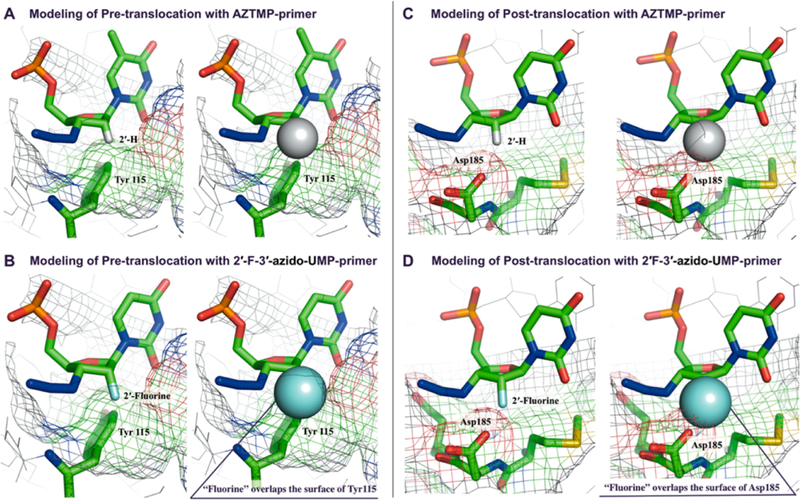
The origin for the sugar conformational preferences for excision and translocation is not entirely clear, but our understanding of incorporation may provide some insight. During the incorporation of dNTPs, RT discriminates between dNTP and rNTP substrates through the “steric gate” effect mediated by residue Y115 in the dNTP binding pocket.14,15 Residue Y115 clashes with 2′ substituents in rNTPs, and also directs dNTPs to adopt a North conformation to reduce steric hindrance by the 3′-hydroxyl group in the N-site. We suspected the steric gate also plays a role in translocation, therefore we employed computational models, biochemical assays, and translocation assays with RT mutants to uncover which steric interactions may direct excision. Based on modeling of the AZTMP-terminated primer/template/WT RT complex in the pretranslocated state (PDB: 1N6Q), a 2′-substituent (fluorine in the case of G9, G10, and G11, or OH in the case of G8) in the sugar ring of AZTMP could induce a steric clash with the Y115 amino acid side chain of RT (Figure 6A,B), if the terminal nucleoside is North. This interaction may prevent the nucleotide from occupying the N-site, resulting in translocation to the P-site where it is released from steric clashes, suggesting that Asp185 is relatively flexible (Figure 6C,D).
Figure 6.
(A, left) Modeling of complexes of AZT-terminated DNA primer/DNA template/RT (WT) in the crystal structure of the pretranslocation complex (PDB: 1N6Q) indicating structural fitting to Tyr115, (A, right) Van-der-Waals radii of 2′-(α)-hydrogen in (A) was shown. (B, left) Modeling of complexes of 2′-(α)-F-3′-azido-Ur-terminated DNA primer/DNA template/RT (WT) in the structure of the pretranslocation complex indicates steric clashes to Tyr115, (B, right) Van-der-Waals radii of 2′-(α)-fluorine was shown. (C, left) Modeling of complexes of AZT-terminated DNA primer/DNA template/RT (WT) in the crystal structure of the post-translocation complex indicates structural fitting to Asp185. (C, right) Van-der-Waals radii of 2′-(α)-hydrogen was shown. (D, left) Modeling of complexes of 2′-(α)-F-3′-azido-Ur-terminated DNA primer/DNA template/RT (WT) in the structure of the post-translocation complex indicates steric interaction with Aps185, (D, right) Van-der-Waals radii of 2′-(α)-fluorine was shown. 2′ C–F bond length in C and D was adjusted to 1.399 Å.
However, this hypothesis does not account for the instances when the terminal nucleotide was located neither in the N-site nor the P-site, but at positions one nucleotide (G8 and G10) or two nucleotides (G13 and G14) preceding the N-site. It is clear, however, that the preference for this site is not very strong as it was easily subverted. With the addition of very small amounts of dGTP, the primer terminus of G10 was brought to the P-site, which is in line with the trend observed with other North puckered terminal nucleotides (Figure 7). This result also proves that the 3′-azido group in North puckers, such as G10, are not obstructing dGTP from binding the N-site, despite the impact of proximal amino acid residues on the sugar conformation. Finally, we carried out translocation experiments with a mutant reverse transcriptase that contained the Y115V mutation to determine if indeed the Y115 side chain pushes the terminal nucleotide out of the N-site. All three primers (G8–10) tested with the Y115V mutant showed a preference for the P-site (Figures S4 and S8), and no aberrant translocation was observed. However, when another mutant (Y115F) was used, the aberrant translocation was observed (2′F-3′ azido-Ur; Figure S9). This supports our conjecture that the phenolic side chain ofY115 affects the translocation of G8 and G10.
Figure 7.
(A) Illustration of the sliding of the RT from ex-Pre to Pre, and to Post translocation. (B, C) Fe-mediated footprintings of the complex containing 2′-F-3′-azido-Ur performed in a different range of concentration of next-incoming dGTP.
Conclusion.
This work suggests that nucleotide excision by HIV-1 RT has a distinct sugar conformational preference for the South sugar conformation, and that the 3′-terminal nucleotide of primers in the polymerase active site of RT is relatively flexible. Remarkably, this subtle change in sugar structure can dictate the translocation state of HIV-1 RT and thus control the extent of phosphorolytic excision of the primer 3′-terminal nucleotide. For the purpose of developing better drugs, both incorporation and excision efficiency should be considered. While incorporation is invariably the route the NRTI must take to generate a chain-terminated complex, the rates of incorporation vs excision should give a better measure on a potential drug’s success. Knowledge of the sugar pucker preferences during nucleoside metabolism into active inhibitors and during their incorporation into HIV-RT has guided researchers to develop more efficient antiviral drugs. As such, we believe our studies provide groundwork for development of future nucleosides refractory to excision.
MATERIALS AND METHODS
3′-Azido-2′,3′-dideoxy-2′-fluoro-5-methyl-D-arabinouridine 5′-H-Phosphonate triethylammonium Salt (2).
3′-Azido-2′,3′-dideoxy-2′-fluoro-5-methyl-D-arabinouridine (1)33,34 (143 mg, 0.5 mmol) was rendered anhydrous by repeated coevaporation with anhydrous pyridine and finally dissolved in anhydrous THF-pyridine (6:1, v/v, 3.0 mL). 2-Chloro-4-H-1,3,2-benzodioxaphosphorin-4-one (152 mg, 0.75 mmol) was added to the solution, and the resulting mixture was stirred at RT for 2 h. The reaction was quenched by adding triethylamine-H2O (1:1, v/v, 3.0 mL) and stirred for 2 h. The mixture was evaporated under reduced pressure, and the crude product was purified by C-60 silica gel column chromatography with CH2Cl2-MeOH containing 1% triethylamine to yield 2 (166 mg, 74%). 1H NMR (300 MHz, CDCl3): δ 1.20–1.25 (9H, t, CH3 on triethylammonium salt, Jhh = 7.3 Hz), 1.82 (3H, s), 2.95–3.03 (6H, m, CH2 on triethylammonium salt), 3.88–3.93 (1H, m), 4.07–4.11 (2H, m), 4.44 (1H, ddd, Jhf = 20.5 Hz, JHH = 3.4 Hz, JHH = 5.5 Hz), 5.01 (1H, dt, JHH = 3.7, JHF = 52.2 Hz), 6.85 (1H, d, JPH = 629.7 Hz), 6.09 (1H, dd, Jhh = 4.2 Hz, Jhf = 15.3 Hz), 7.9 (1H, s, 6H), 10.5 (1H, s, NH). 13C NMR (CDCl3, 75.4 MHz): δ 8.4, 12.4, 45.5, 61.5 (d, JCP = 3.4 Hz), 63.7 (d, Jcf = 25.3 Hz), 79.9 (dd, Jcf= 3.4 Hz, Jcf = 7.0 Hz), 83.0 (d, JCF = 14.0 Hz), 93.6 (d, JCF = 196.8 Hz), 110.4, 136.1, 155.5, 164.2. 19F NMR (CDCl3, 282.23 MHz): δ −195.9 (ddd, F-2′, JHF = 15.3 Hz, JHF = 20.5 Hz, JHF = 52.2 Hz). 31P NMR (CDCl3, 161 MHz): δ 4.25. HRMS (ESI−) m/z calcd for C10H12FN5O6P− [M − H]: 348.0515. Found: 348.0502.
3′-Azido-2′,3′-dideoxy-2′-fluoro-uridine 5′-H-phosphonate Triethylammonium Salt (4).
3′-Azido-2′,3′-dideoxy-2′-fluoro-uri-dine (3)35 (68.5 mg, 0.3 mmol) was rendered anhydrous by repeated coevaporation with anhydrous pyridine and finally dissolved in anhydrous pyridine (0.6 mL). Diphenyl phosphite (402.1 μL, 2.1 mmol) in anhydrous pyridine (0.6 mL) was added dropwise to the solution of compound 3 and stirred at RT for 30 min. The reaction was quenched by adding triethylamine-H2O (1:1, v/v, 3.0 mL) and stirred for 2 h. The crude reaction mixture was evaporated to dryness under reduced pressure and then purified by C-60 silica gel column chromatography with CH2Cl2-MeOH containing 1% triethylamine to yield 4 (117.3 mg, 89%). 1H NMR (400 MHz, CDCl3): δ 1.22 (9H, t, CH3 on triethylammonium salt, JHH = 7.2 Hz), 2.98–3.03 (6H, m), 4.02–4.19 (3H, m), 4.29 (1H, dt, Jhh = 5.7 Hz, Jhf = 17.4 Hz), 5.18 (1H, d, Jhf = 52.4 Hz), 5.59 (1H, d, Jhh = 8.0 Hz), 6.01 (1H, d, Jhf = 16.9 Hz), 6.82 (1H, d, Jph = 627.7 Hz), 7.76 (1H, d, Jhh = 8.0 Hz), 11.07 (1H, br), 11.72 (1H, br). 13C NMR (CDCl3, 75.4 MHz): δ 8.5, 45.5, 58.6 (d, Jcp = 15.6 Hz), 61.5 (d, Jcf = 4.0 Hz), 88.3 (d, J = 6.7 Hz), 93.7 (d, JCF = 34.5 Hz), 93.7 (d, JCF = 191.3 Hz), 102.7, 140.3, 150.4), 163.72. 19F NMR (CDCl3, 282.23 MHz): δ −199.4 (dt, F-2′, Jhf = 16.9 Hz, Jhf = 52.4 Hz). 31P NMR (CDCl3 161 MHz): δ 4.11. HRMS (ESI−) m/z calcd for C9H10FN5O6P− [M − H]: 334.0358. Found: 334.0350.
Oligonucleotide Synthesis.
The sequence of the DNA primer used in the study (5′-TTAAAAGAAAAGGGGGGACX-3′, where X represents the varying terminal nucleotides) is derived from the HIV-1 primer binding sequence (PBS). Commercially available DNA phosphoramidite building blocks of 6N-Bz-dA, 4N-Bz-dC, and 2N-iBu-dG were used. 5′-O-DMTr-3′-phosphoramidites of 2′-F-ara-T and 2′ F-Ur were purchased from Glen Research, and 5′-O-DMTr-3′-phosphoramidites of dT, dUr, ara-Ur, and Ur were purchased from ChemGenes. The synthesis of primers containing a 3′-OH (G1, G2, G3, G4, G5, G8, and G9) was achieved by coupling the 5′-O-DMTr-3′-phosphoramidites of the 3′-terminal nucleotide to the Unylinker solid support (ChemGenes) and followed by standard solid phase oligonucleotide synthesis in the 3′ to 5′ direction on a DNA synthesizer (Scheme 1). For the synthesis of the DNA primer containing 2′,4′-difluoro-uridine (2′,4′-diF-Ur) at the 3′-terminus (G11), the 5′-O-DMTr-3′-phosphoramidite of 2′,4′-diF-Ur was prepared according to a previously published method,24 and DNA synthesis was carried out in the same manner as described above (Scheme 1). The DNA primer modified with LNA-T at the 3′-teminus (Primer G12) was purchased from Exiqon. For the synthesis of primers containing 3′-terminal modifications with seco-Ur (G13) and oxepane-T (G14), each nucleoside was synthesized using previously published methods, loaded onto succinylated solid supports36–38 and synthesized from the 3′ to 5′ direction using the standard phosphoramidite method. For the synthesis of DNA primers having nucleotides that lack 3′-hydroxyl groups (G5, G6, G7, and G10), 3′-O-DMTr-dT-loaded CPG (ChemGenes) was used as a starting material, and the oligonucleotide synthesis was carried out in the direction of 5 to 3 using 3′-O-DMTr-nucleoside-5′-O-phosphoramidites. Compounds 1 and 3 were synthesized using previously published methods33–35 and converted to their respective 5′-H-phosphonates. For the synthesis of primers containing nucleosides having a 3′-azido group (G5, G6, and G10), the 5′-H-phosphonates of AZT, compound 2, and compound 4 were coupled to the 3′-end of the respective oligonucleotide according to previously described methods (Scheme 1).39 The 5′-phosphoramidite of ddT was synthesized and coupled at the 3′-terminus of an oligonucleotide grown from the 5′ to 3′ direction on the DNA synthesizer to obtain G7. The deprotection and cleavage from solid support was carried out in 28% NH4OH at room temperature for 36 h and evaporated under reduced pressure. For the primers having 2′-O-TBDMS-protected uridine, further deprotection of the silyl group was carried out in TREAT-HF at 65 °C for 3 h. The deprotected oligonucleotide was precipitated in 3 M sodium acetate (pH 5.2) and excess ice-cold n-butanol. Purification of crude materials were carried out on 12% polyacrylamide gels (7 M urea) containing 50 mM Tris borate, pH 8.0, and 1 mM EDTA. Purified DNA primers were eluted from gel slices by using 0.5 M sodium acetate and 0.1% SDS. The DNA template used in this study (3′-AAGAATCGGTGA-AAAATTTTCTTTTCCCCCCTGACCTTCCCGACTAAG-TGACTGTT GC-5′) was purchased from IDT.
ATP-dependent Phosphorolysis.
Recombinant WT and AZTr-RT was expressed and purified as previously described.40,41 5′-End labeling of DNA primers and DNA templates was conducted with [γ−32P] ATP and T4 polynucleotide kinase (Fermentas) using the manufacturer’s recommended procedure. The template and 32P-labeled DNA primers (3:1 ratio) were dissolved in a buffer containing 50 mM Tris-HCl and 50 mM KCl (pH 8.0) and were heated at 95 °C for 5 min and cooled to RT in 60 min. A total of 100 nM of the hybrid was preincubated with 750 nM of WT or AZTr-RT at 37 °C in 50 mM Tris-HCl buffer (pH 8.0) containing 50 mM KCl. The reaction was initiated by adding 6 mM MgCl2 and 3 mM pyrophosphatase-treated ATP and then incubated at 37 °C. Aliquots were taken at each time point 1, 5, 10, 20, 30, 45, 60, and 90 min, and the reaction was stopped by adding formamide and subsequent heating at 95 °C for 5 min. For the zero time point, the sample containing the primer/template hybrid and RT mixture without MgCl2 or ATP was used. The remaining 32P-labeled full-length primers and excised products at each time point were analyzed on 12% polyacrylamide gels (7 M urea) containing 50 mM Tris borate, pH 8.0, and 1 mM EDTA and were quantitated after exposure to a phosphorus imager screen to evaluate the excision efficiency.
Rescue DNA Synthesis from 3′-end Terminated DNA Primers.
The 32P-labeled DNA primer-template hybrid was prepared as described above. The hybrid (250 nM) was incubated with 750 nM of WT or AZTr-RT at 37 °C in a buffer containing 50 mM Tris-HCl (pH 8.0) and 50 mM NaCl. To the preincubated solution, 6 mM MgCl2 and 3 mM pyrophosphatase-treated ATP, 100 μM dTTP, and 100 μM ddGTP were added to initiate the reaction, and aliquots at 5, 10, 15, 30, 45, 60, and 90 min were collected and analyzed as described.42
Site-specific Footprinting.
Hybrids of the 0.5 μM of 32P-labeled DNA template and 1.5 μM of the DNA primer annealed in a buffer containing 160 mM sodium cacodylate (pH 7) and 100 mM NaCl. To the 10 μL of the hybrid was added 3.3 μL of 5 mM DTT, 2.0 μL of 1 M MgCl2, 4.4 μL of 1 M sodium cacodylate (pH 7.0), and 81.3 μL of ddH2O. A total of 20 μL of this mixture was preincubated with 750 nM 2RT at 37 °C for 10 min. The treatment with KOONO was performed essentially according to the method previously described.43–45 The treatment with Fe-dependent OH radical was conducted according to the method previously described.45 As an additional control experiment, the radical-mediated site-specific strand cleavage in the presence of PFA (foscarnet), which is known to induce pretranslocation, was conducted.46 Another control experiment was carried out in the presence of 5-methyl-1-(4-nitro-phenyl)-2-oxo-2,5-dihydro-1H-pyrido[3,2-b]indole-3-carbonitrile (INDOPY-1), which is known to trap the post-translocational state.47
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by an NSERC Discovery Grant (M.J.D.), a McGill University CIHR Drug Development Training Program (S.M.-M. and M.H.), a grant from the Canadian Institutes of Health Research CIHR (M.G.). J.A.B. is the recipient of a Doctoral Training Scholarship from the FRSQ and a Chemical Biology Training Program Scholarship from the CIHR, a Strategic Young Researcher Overseas Visits Program for Accelerating Brain Circulation. K.Y. thanks a Japan Society for the Promotion of Science (JSPS) fellowship. M.A.P. thanks the national Institutes of Health for grants AI076119 and AI079801.
Footnotes
Supporting Information
1H, 13C, 31P, and 19F NMR spectra for compounds 2 and 5. Oligonucleotide MS characterization. Additional figures showing KOONO-mediated site-specific footprinting and excision assays. The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acschembio.5b00263.
The authors declare no competing financial interest.
REFERENCES
- (1).Hammer SM, Katzenstein DA, Hughes MD, Gundacker H, Schooley RT, Haubrich RH, Henry WK, Lederman MM, Phair JP, Niu M, Hirsch MS, and Merigan TC (1996) A trial comparing nucleoside monotherapy with combination therapy in HIV-infected adults with CD4 cell counts from 200 to 500 per cubic millimeter. N. Engl. J. Med 335, 1081–1090. [DOI] [PubMed] [Google Scholar]
- (2).Yeni P (2006) Update on HAART in HIV. J. Hepatol 44, S100–S103. [DOI] [PubMed] [Google Scholar]
- (3).Asboe D, Aitken C, Boffito M, Booth C, Cane P, Fakoya A, Geretti AM, Kelleher P, Mackie N, Muir D, Murphy G, Orkin C, Post F, Rooney G, Sabin C, Sherr L, Smit E, Tong W, Ustianowski A, Valappil M, Walsh J, Williams M, and Yirrell D (2012) British HIV Association guidelines for the routine investigation and monitoring of adult HIV-1-infected individuals 2011. HIV Med. 13, 1–44. [DOI] [PubMed] [Google Scholar]
- (4).Patel N, and Miller CD (2012) New option for management of HIV-1 infection in treatment-naive patients: once-daily, fixed-dose combination of rilpivirine-emtricitabine-tenofovir. HIV/AIDS 4, 61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Montaner JS, Lima VD, Harrigan PR, Lourenco L, Yip B, Nosyk B, Wood E, Kerr T, Shannon K, Moore D, Hogg RS, Barrios R, Gilbert M, Krajden M, Gustafson R, Daly P, and Kendall P (2014) Expansion of HAART coverage is associated with sustained decreases in HIV/AIDS morbidity, mortality and HIV transmission: the ″HIV Treatment as Prevention″ experience in a Canadian setting. PLoS One 9, e87872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Meyer PR, Matsuura SE, Mian AM, So AG, and Scott WA (1999) A mechanism of AZT resistance: an Increase in nucleotide-dependent primer unblocking by mutant HIV-1 reverse transcriptase. Mol. Cell 4, 35–43. [DOI] [PubMed] [Google Scholar]
- (7).Arion D, Kaushik N, McCormick S, Borkow G, and Parniak MA (1998) Phenotypic mechanism of HIV-1 resistance to 3′-azido-3′-deoxythymidine (AZT): increased polymerization processivity and enhanced sensitivity to pyrophosphate of the mutant viral reverse transcriptase. Biochemistry 37, 15908–15917. [DOI] [PubMed] [Google Scholar]
- (8).Meyer PR, Matsuura SE, So AG, and Scott WA (1998) Unblocking of chain-terminated primer by HIV-1 reverse transcriptase through a nucleotide-dependent mechanism. Proc. Natl. Acad. Sci. U. S. A 95, 13471–13476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Menéndez-Arias L (2013) Molecular basis of human immunodeficiency virus type 1 drug resistance: overview and recent developments. Antiviral Res. 98, 93–120. [DOI] [PubMed] [Google Scholar]
- (10).Sarafianos SG, Clark AD Jr., Das K, Tuske S, Birktoft JJ, Ilankumaran P, Ramesha AR, Sayer JM, Jerina DM, Boyer PL, Hughes SH, and Arnold E (2002) Structures of HIV-1 reverse transcriptase with pre- and post-translocation AZTMP-terminated DNA. EMBO J. 21, 6614–6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Marchand B, and Götte M (2003) Site-specific footprinting reveals differences in the translocation status of HIV-1 reverse transcriptase. Implications for polymerase translocation and drug resistance. J. Biol. Chem 278, 35362–35372. [DOI] [PubMed] [Google Scholar]
- (12).Marchand B, and Götte M (2004) Impact of the translocational equilibrium of HIV-1 reverse transcriptase on the efficiency of mismatch extensions and the excision of mispaired nucleotides. Int. J. Biochem. Cell Biol 36, 1823–1835. [DOI] [PubMed] [Google Scholar]
- (13).Tong W, Lu CD, Sharma SK, Matsuura S, So AG, and Scott WA (1997) Nucleotide-induced stable complex formation by HIV-1 reverse transcriptase. Biochemistry 36, 5749–5757. [DOI] [PubMed] [Google Scholar]
- (14).Boyer PL, Sarafianos SG, Arnold E, and Hughes SH (2001) Selective excision of AZTMP by drug-resistant human immunodeficiency virus reverse transcriptase. J. Virol 75, 4832–4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Sluis-Cremer N, Arion D, Parikh U, Koontz D, Schinazi RF, Mellors JW, and Parniak MA (2005) The 3′-azido group is not the primary determinant of 3′-azido-3′-deoxythymidine (AZT) responsible for the excision phenotype of AZT-resistant HIV-1. J. Biol. Chem 280, 29047–29052. [DOI] [PubMed] [Google Scholar]
- (16).Mu L, Sarafianos SG, Nicklaus MC, Russ P, Siddiqui MA, Ford H Jr., Mitsuya H, Le R, Kodama E, Meier C, Knispel T, Anderson L, Barchi JJ Jr., and Marquez VE (2000) Interactions of conformationally biased north and south 2′-fluoro- 2′,3′-dideoxynucleoside 5′-triphosphates with the active site of HIV-1 reverse transcriptase. Biochemistry 39, 11205–11215. [DOI] [PubMed] [Google Scholar]
- (17).Boyer PL, Julias JG, Marquez VE, and Hughes SH (2005) Fixed conformation nucleoside analogs effectively inhibit excision-proficient HIV-1 reverse transcriptases. J. Mol. Biol 345, 441–450. [DOI] [PubMed] [Google Scholar]
- (18).Peng CG, and Damha MJ (2008) Probing DNA polymerase activity with stereoisomeric 2′ F-fluoro-β-D-arabinose (2′ F-araNTPs) and 2′-fluoro-β-D-ribose (2′ F-rNTPs) nucleoside 5′-triphosphates. Can. J. Chem 86, 881–891. [Google Scholar]
- (19).Sluis-Cremer N, Sheen CW, Zelina S, Torres PS, Parikh UM, and Mellors JW (2007) Molecularmechanism by which the K70E mutation in human immunedeficiency virus type 1 reverse transcriptase confers resistance to nucleoside reverse transcriptase inhibitors. Antimicrob. Agents Chemother 51, 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Michailidis E, Marchand B, Kodama EN, Singh K, Matsuoka M, Kirby KA, Ryan EM, Sawani AM, Nagy E, Ashida N, Mitsuya H, Parniak MA, and Sarafianos SG (2009) Mechanism of inhibition of HIV-1 reverse transcriptase by 4′-Ethynyl-2-fluoro-2′-deoxyadenosine triphosphate, a translocation-defective reverse transcriptase inhibitor. J. Biol. Chem 18, 35681–35691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Leffler JE, and Temple RD (1967) Staudinger reaction between triarylphosphines and azides. A study of the mechanism. J. Am. Chem. Soc 89, 5235–5246. [Google Scholar]
- (22).Wada T, Mochizuki A, Higashiya S, Tsuruoka H, Kawahara S, Ishikawa M, and Sekine M (2001) Synthesis and properties of 2-azidodeoxyadenosine and its incorporation into oligodeoxynucleotides. Tetrahedron Lett 42, 9215–9219. [Google Scholar]
- (23).Morita K, Takagi M, Hasegawa C, Kaneko M, Tsutsumi S, Sone J, Ishikawa T, Imanishi T, and Koizumi M (2003) Synthesis and properties of 2′-O,4′-C-ethylene-bridged nucleic acids (ENA) as effective antisense oligonucleotides. Bioorg. Med. Chem 11, 2211–2226. [DOI] [PubMed] [Google Scholar]
- (24).Martínez-Montero S, Deleavey GF, Kulkarni A, Martín-Pintado N, Lindovska P, Thomson M, González C, Götte M, and Damha MJ (2014) Rigid 2′,4′-difluororibonucleosides: synthesis, conformational analysis, and incorporation into nascent RNA by HCV polymerase. J. Org. Chem 79, 5627–5635. [DOI] [PubMed] [Google Scholar]
- (25).The minimization of the South and North conformations to calculate the corresponding P values for 2′-araF and 3′-azido-Ur were carried out with the program Gaussian 09 at the HF/6–31+G(d,p) level.
- (26).Guschlbauer W, and Jankowski K (1980) Nucleoside conformation is determined by the electronegativity of the sugar substituent. Nucleic Acids Res 8, 1421–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Jagannadh B, Reddy DV, and Kunwar AC (1991) 1H-NMR study of the sugar pucker of 2′,3′-dideoxynucleosides with antihuman immunedeficiency virus (HIV) activity. Biochem. Biophys. Res. Commun 179, 386–391. [DOI] [PubMed] [Google Scholar]
- (28).Houseknecht JB, Altona C, Hadad CM, and Lowary TL (2002) Conformational analysis of furanose rings with PSEUROT: parametrization for rings possessing the arabino, lyxo, ribo, and xylo stereochemistry and application to arabinofuranosides. J. Org. Chem 67, 4647–4651. [DOI] [PubMed] [Google Scholar]
- (29).Watts JK, Sadalapure K, Choubdar N, Pinto BM, and Damha MJ (2006) Synthesis and conformational analysis of 2′-fluoro-5-methyl-4′-thioarabinouridine (4′ S-FMAU). J. Org. Chem 71, 921–925. [DOI] [PubMed] [Google Scholar]
- (30).Plavec J, Koole LH, Sandström A, and Chattopadhyaya J (1991) Structural studies of anti-HIV 3′-(α-fluorothymidine and 3′-(α-azidothymidine by 500 MHz 1H-NMR spectroscopy & molecular mechanics (MM2) calculation. Tetrahedron 47, 7363–7376. [Google Scholar]
- (31).Thibaudeau C, Plavec J, and Chattopadhyaya J (1996) Quantitation of the pD Dependent Thermodynamics of the NS pseudorotational equilibrium of the pentofuranose moiety in nucleosides gives a direct measurement of the strength of the tunable anomeric effect and the pKa of the nucleobase. J. Org. Chem 61, 266–286. [Google Scholar]
- (32).Altona C, and Sundaralingam M (1973) Conformational analysis of the sugar ring in nucleosides and nucleotides. Improved method for the interpretation of proton magnetic resonance coupling constants. J. Am. Chem. Soc 95, 2333–2344. [DOI] [PubMed] [Google Scholar]
- (33).Watanabe KA, Harada K, Zeidler J, Matulic-Adamic J, Takahashi K, Ren WY, Cheng LC, Fox JJ, Chou TC, Zhu QY, Polsky B, Gold JWM, and Armstrong D (1990) Synthesis and anti-HIV-1 activity of 2′-”up”-fluoro analogues of active anti-AIDS nucleosides 3′-azido-3′-deoxythymidine (AZT) and 2′,3′-dideoxycyti-dine (DDC). J. Med. Chem 33, 2145–2150. [DOI] [PubMed] [Google Scholar]
- (34).Sterzycki RZ, Ghazzouli I, Brankovan V, Martin JC, and Mansuri MM (1990) Synthesis and anti-HIV activity of several 2-fluoro-containing pyrimidine nucleosides. J. Med. Chem 33, 2150–2157. [DOI] [PubMed] [Google Scholar]
- (35).van Aerschot A, and Herdewijn P (1989) 2,3′-Difluoro- and 3′-azido-2′-fluoro substituted dideoxypyrimidines as potential anti-HIV agents. Bull. Soc. Chim. Belg 98, 937–941. [Google Scholar]
- (36).Mangos MM, Min KL, Viazovkina E, Galarneau A, Elzagheid MI, Parniak MA, and Damha MJ (2003) Efficient RNase H-directed cleavage of RNA promoted by antisense DNA or 2′ F-ANA constructs containing acyclic nucleotide inserts. J. Am. Chem. Soc 125, 654–661. [DOI] [PubMed] [Google Scholar]
- (37).Sabatino D, and Damha MJ (2007) Oxepane nucleic acids: synthesis, characterization, and properties of oligonucleotides bearing a seven-membered carbohydrate ring. J. Am. Chem. Soc 129, 8259–8270. [DOI] [PubMed] [Google Scholar]
- (38).Damha MJ, Giannaris PA, and Zabarylo SV (1990) An improved procedure for derivatization of controlled-pore glass beads for solid-phase oligonucleotide synthesis. Nucleic Acids Res 18, 3813–3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Stawinski J, and Stromberg R (2005) Di- and oligonucleotide synthesis using H-Phosphonate chemistry. Methods Mol. Biol 288, 81–100. [PubMed] [Google Scholar]
- (40).Sluis-Cremer N, Arion D, Parikh U, Koontz D, Schinazi RF, Mellors JW, and Parniak MA (2005) The 3′-azido group is not the primary determinant of 3′-azido-3′-deoxythymidine (AZT) responsible for the excision phenotype of AZT-resistant HIV-1. J. Biol. Chem 280, 29047–29052. [DOI] [PubMed] [Google Scholar]
- (41).Le Grice SF, and Grüninger-Leitch F (1990) Rapid purification of homodimer and heterodimer HIV-1 reverse transcriptase by metal chelate affinity chromatography. Eur. J. Biochem 187, 307–314. [DOI] [PubMed] [Google Scholar]
- (42).Götte M, Arion D, Parniak MA, and Wainberg MA (2000) The M184V mutation in the reverse transcriptase of human immunodeficiency virus type 1 impairs rescue of chain-terminated DNA synthesis. J. Virol 74, 3579–3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Götte M, Marquet R, Isel C, Anderson VE, Keith G, Gross HJ, Ehresmann C, Ehresmann B, and Heumann H (1996) Probing the higher order structure of RNA with peroxonitrous acid. FEBS Lett. 390, 226–228. [DOI] [PubMed] [Google Scholar]
- (44).Götte M, Maier G, Gross HJ, and Heumann H (1998) Localization of the active site of HIV-1 reverse transcriptase-associated RNase H domain on a DNA template using site-specific generated hydroxyl radicals. J. Biol. Chem 273, 10139–10146. [DOI] [PubMed] [Google Scholar]
- (45).Marchand B, and Gotte M (2003) Site-specific footprinting reveals differences in the translocation status of HIV-1 reverse transcriptase. Implications for polymerase translocation and drug resistance. J. Biol. Chem 278, 35362–35372. [DOI] [PubMed] [Google Scholar]
- (46).Marchand B, Tchesnokov EP, and Gotte M (2007) The pyrophosphate analogue foscarnet traps the pre-translocational state of HIV-1 reverse transcriptase in a brownian ratchet model of polymerase translocation. J. Biol. Chem 282, 3337–3346. [DOI] [PubMed] [Google Scholar]
- (47).Jochmans D, Deval J, Kesteleyn B, Van MH, Bettens E, De Baere I, Dehertogh P, Ivens T, Van GM, Van SB, Ehteshami M, Wigerinck P, Gotte M, and Hertogs K (2006) Indolopyridones inhibit human immunodeficiency virus reverse transcriptase with a novel mechanism of action. J. Virol 80, 12283–12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.