Abstract
Since salicylic acid (SA) was discovered as an elicitor of tobacco plants inducing the resistance against Tobacco mosaic virus (TMV) in 1979, increasing reports suggest that SA indeed is a key plant hormone regulating plant immunity. In addition, recent studies indicate that SA can regulate many different responses, such as tolerance to abiotic stress, plant growth and development, and soil microbiome. In this review, we focused on the recent findings on SA’s effects on resistance to biotic stresses in different plant-pathogen systems, tolerance to different abiotic stresses in different plants, plant growth and development, and soil microbiome. This allows us to discuss about the safe and practical use of SA as a plant defense activator and growth regulator. Crosstalk of SA with different plant hormones, such as abscisic acid, ethylene, jasmonic acid, and auxin in different stress and developmental conditions were also discussed.
Keywords: abiotic stress tolerance, resistance, salicylic acid, susceptibility
As a sessile organism, plant growth and response to environmental cues are largely governed by plant hormones, also called phytohormones. Phytohormones act spatially and temporally as endogenous signals at a very low dose to regulate various physiological functions. Unlike the animal systems producing hormones in specialized organs and transferring them to another parts via blood stream, each living plant cell can produce hormones on their own (Went and Thimann, 1937). To successfully survive under the biotic and abiotic stress conditions, plants evolved highly sophisticated crosstalk between different phytohormones. Fine-tuning of complex phytohormone network enables the balanced response of plants to developmental and environmental cues, thus minimizing defense-associated fitness costs. Harmony and/or disharmony of phytohormones, such as jasmonic acid (JA), ethylene (ET), abscisic acid (ABA), auxin (IAA), and salicylic acid (SA), result in specific response to specific stimuli.
Phytohormones JA and ET play a pivotal role in the regulation of plant immune response against necrotrophic pathogens and herbivory insect pathogens (Wasternack and Song, 2017). JA is biosynthesized from α-linolenic acid via octadecanoid pathway. During the activation of JA signaling, JA stimulates interaction of coronatine-insensitive 1 (COI1), which is a component of SCFCOI1 E3 ubiquitin ligase complex, with jasmonate-zim-domain (JAZ) proteins, a suppressor of JA-responsive transcription factors (TFs; e.g., MYC2). This JA-induced interaction between COI1 and JAZs facilitate degradation of JAZs through the 26S proteasome, thereby releasing downstream TFs to regulate gene expression and activate JA responses. ET, a gaseous plant hormone, is biosynthesized from S-adenosylmethionine via the action of two key enzymes ACC synthase and ACC oxidase (Wang et al., 2002). It regulates many different aspects of the plant physiology, including germination, senescence, abscission, fruit ripening, and response to biotic and abiotic stresses. Five ethylene receptor genes (ETHYLENE RESPONSE1 [ETR1], ETHYLENE RESPONSE SENSOR1 [ERS1], ETR2, ETHYLENE INSENSITIVE4 [EIN4], and ERS2) are identified from the Arabidopsis plants (Ju and Chang, 2015). Mutant plant lacks JA/ET signaling showed highly susceptible phenotype against infection by necrotrophic pathogens and infestation by herbivory insect pathogens.
ABA, a sesquiterpenoid hormone, is biosynthesized from C40 epoxycarotenoid precursors through an oxidative cleavage reaction in plastids (Xiong and Zhu, 2003). The C15 intermediate xanthoxin is converted to ABA by a two-step reaction via ABA-aldehyde oxidase in cytosol. ABA plays an important role in many cellular processes including seed development, dormancy, germination, and water stress responses (Ng et al., 2014). In the absence of ABA signaling, type 2C protein phosphatases (PP2Cs) constitutively dephosphorylate Snf1-related protein kinases 2 (SnRK2). In response to ABA signal, regulatory component of ABA receptor (RCAR)/pyrabactin resistance (PYR)/PYR1-like (PYLs) proteins, collectively termed PYLs, bind and inhibit PP2Cs. Inhibition PP2Cs in turn allows SnRK2 activation through autophosphorylation. Active SnRK2 mediate the downstream ABA response through the phosphorylation of target proteins (e.g., slow-type anion channel [SLAC1] and inward-rectifying potassium channel [KAT1]).
Both IAA and SA are synthesized from shikimate pathway (Pérez-Llorca et al., 2019). In the first step, shikimate is converted into chorismate by chorismate synthase. For IAA and SA biosynthesis, chorismate is further converted into either tryptophan (Trp) or isochorismate, respectively. Then, Trp is converted into IAA through several reaction steps, including the conversion of Trp to indole-3-pyruvic acid by the tryptophan aminotransferase. IAA plays an essential role in almost every aspect of plant growth and development processes, including the cell division and differentiation, as well as in biotic and abiotic stress responses (Korver et al., 2018; Pérez-Llorca et al., 2019). Perception of IAA via its cognate receptor TRANSPORT INHIBITOR RESPONSE1 (TIR1), the F-box subunit of the ubiquitin ligase complex SCFTIR1, stabilizes the interaction between TIR1 and Aux/IAA proteins. This interaction results in Aux/IAA ubiquitination and subsequent degradation. Degradation of Aux/IAA led to release and activation of AUXIN RESPONSE FACTOR proteins for activation of downstream IAA signaling.
SA is biosynthesized from the chorismate via two independent pathways, isochorismate synthase- and phenylalanine ammonia-lyase–dependent pathways (Dempsey and Klessig, 2017). Role of SA in plant defense response has only become evident during the past 30 years. Before SA is recognized as an important plant hormone, it was assumed to be relatively unimportant products together with many other phenolic secondary metabolites. However, the findings regarding (1) enhanced resistance phenotype in SA-treated tobacco plants against Tobacco mosaic virus (TMV) infection, and (2) over 20-fold increase in endogenous SA level in TMV-infected resistant tobacco plants boost the researches on SA as a plant hormone regulating disease resistance (Klessig et al., 2018). Increasing reports suggest that SA plays important roles not only in regulating plant disease resistance, but also in thermogenesis, abiotic stress tolerance, DNA damage/repair, fruit yield, seed germination, and etc. (Dempsey and Klessig, 2017). In this review, we focused on the diverse effect of SA on different aspect of plant phenotypes in relation with other plant hormones. For SA-mediated defense signaling pathway at the molecular level, we recommend to read reviews by Lu (2009), Yan and Dong (2014), and Zhang and Li (2019).
SA and JA/ET in Plant Resistance to Biotic Stresses
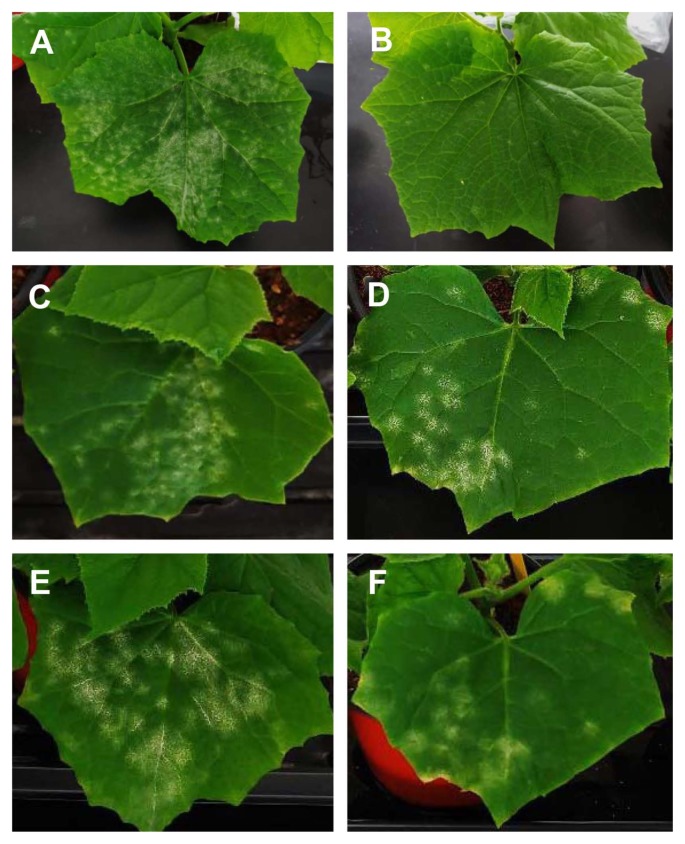
SA is a defense-related plant hormone that plays a key role in resistance to different microbial pathogens, such as virus, bacteria, fungi, and oomycetes (Kunkel and Brooks, 2002; Vlot et al., 2009). In plants, the positive correlation between endogenous levels of SA and resistance responses against biotrophic and hemibiotrophic pathogens are well established (Glazebrook, 2005). In addition, the exogenous SA application induces local and systemic acquired resistance in different plant species against various types of pathogens, including Fusarium oxysporum, Alternaria alternata, Magnaporthe grisea, Colletotrichum gloeosporides, Xanthomonas spp., different kinds of viruses and etc. (Table 1) (Daw et al., 2008; Esmailzadeh et al., 2008; Jendoubi et al., 2017; Kundu et al., 2011; Le Thanh et al., 2017; Mohan Babu et al., 2003; Radwan et al., 2007; Saikia et al., 2003; Wang and Liu, 2012; Wang et al., 2006). Notably, exogenous application of 1 mM SA almost completely suppressed powdery mildew disease development in cucumber plants (Fig. 1). However, SA’s roles in plant defense against necrotrophic pathogens are not fully understood yet, due to its complexity. JA and ET are known to be essential for plant resistant against necrotrophic pathogens (Erb et al., 2012; Wang et al., 2015a). Among different plants-necrotrophic pathogens interactions, a few cases of exogenous SA treatment-induced enhanced susceptibility was reported (Table 2). In broad bean, SA treatment compromised red light-induced resistance against the necrotrophic pathogen Botrytis cinerea; however, it does not further enhanced dark light-induced susceptibility (Khanam et al., 2005). Application of SA-induced enhanced susceptibility in tomato against B. cinerea in a dose-dependent manner. Controversially, the SA-induced enhanced resistance of tomato and Arabidopsis plants against B. cinerea is also reported (Ferrari et al., 2003; Li and Zou, 2017). Generally, SA-dependent defense singling is known to be antagonistic against JA-/ET-dependent defense signaling (Glazebrook, 2005). However, the hormone signaling pathways between SA and ET/JA are not exclusively antagonistic (Robert-Seilaniantz et al., 2011), thus it needs to be carefully analyzed in different plant-pathogen systems and field conditions.
Table 1.
Enhanced disease resistance upon exogenous SA application in different plants
| Host plant | Pathogen (infection style) | SA conc. and treatment method | Effect | References |
|---|---|---|---|---|
| Tomato (Lycopersicon esculentum) | Fusarium oxysporum (hemibiotrophic) | 0.2 mM | ~55% reduction in disease incidence | Jendoubi et al. (2017) |
| Botrytis cinerea (necrotrophic) | 2 mM | ~62% reduction in disease severity | Li and Zou (2017) | |
| Alternaria alternata (necrotrophic) | 0.4 mM | ~57% reduction in disease severity | Esmailzadeh et al. (2008) | |
| Potato purple top (PPT) phytoplasma (biotrophic) | 100 ml of 0.1 mM SA is sprayed and 100 ml of 0.1 mM soil-drenched | ~47% reduction in disease incidence | Wu et al. (2012) | |
| Pepper (Capsicum annuum) | Ralstonia solanacearum (hemibiotrophic) | 0.5 mM | R. solanacearum-induced seedling growth inhibition is recovered. Notably, 0.5 mM SA itself enhanced seedling growth by ~150% | Chandrasekhar et al. (2017) |
| Fusarium oxysporum (hemibiotrophic) | 0.5 mg/l | ~50% reduction in disease incidence | Yousif (2018) | |
| Rice (Oryza sativa) | Magnaporthe grisea (hemibiotrophic) | 8 mM | ~70% reduction in disease severity | Daw et al. (2008) |
| Xanthomonas oryzae (hemibiotrophic) | 1 mM | Leaf blight lesion length is reduced | Mohan Babu et al. (2003) | |
| 1 mM | ~30% reduction in disease severity | Le Thanh et al. (2017) | ||
| Oebalus pugnax (piercing and sucking insect) | 16 mM | ~35% reduction in number of bugs found in plots; retarded nymph development to adult insect | Stella de Freitas et al. (2019) | |
| Orange (Citrus sinensis) | Xanthomonas axonopodis (biotrophic) | 0.25 mM | ~45% reduction in disease incidence | Wang and Liu (2012) |
| Banana (Musa acuminata) | Fusarium oxysporum (hemibiotrophic) | Roots were dipped in 0.1 mM SA for 2 days | Disease symptom (corm browning) is not observed 3 weeks after inoculation with the pathogen Note, 0.2 mM SA-induced necrosis on roots |
Wang et al. (2015b) |
| Chickpea (Cicer arietinum) | Fusarium oxysporum (hemibiotrophic) | 10 μl of ~14.5 mM SA is injected at the base of stem | ~20% reduction in disease severity (also increased ~6% in both shoot and root growth length) | Saikia et al. (2003) |
| 10 ml of ~0.58 mM SA is soil-drenched | ~20% reduction in disease severity (also increased ~10 and 4.5% in shoot and root growth length, respectively) | |||
| Black gram or urdbean (Vigna mungo) | Mungbean yellow mosaic Indian virus (MYMIV) (biotrophic) | 0.1 mM | ~71% reduction in disease severity | Kundu et al. (2011) |
| Pumpkin (Cucurbita pepo) | Zucchini yellow mosaic virus (ZYMV) (biotrophic) | 0.1 mM | ~66% reduction in disease severity | Radwan et al. (2007) |
| Peanut (Arachis hypogaea) | Peanut mottle virus (PeMoV) (biotrophic) | 0.2 mM | ~42% reduction in disease severity | Kobeasy et al. (2011) |
| Tea flower (Camelia oleifera) | Colletotrichum gloeosporioides (hemibiotrophic) | ~1 mM | >40% reduction in disease severity | Wang et al. (2006) |
| Rubber tree (Hevea brasiliensis) | Phytophthora palmivora (hemibiotrophic) | 5 mM | ~41% reduction in disease severity (>10 mM SA-induced leaf shrinkage) | Deenamo et al. (2018) |
| Arabidopsis (Arabidopsis thaliana) | Botrytis cinerea (necrotrophic) | 5 mM | ~62% reduction in lesion size | Ferrari et al. (2003) |
SA, salicylic acid.
Fig. 1.
Enhanced resistance of cucumber plants against powdery mildew disease by exogenous salicylic acid (SA) treatment. (A, B) Powdery mildew disease symptom developed 7 days after inoculation. Before the pathogen inoculation, cucumber plants were sprayed with steriled tap water (A) or 1 mM SA (B). (C–F) Disease control effect of SA. (C, D) Cucumber leaves developing powdery mildew disease symptoms before the SA treatment. (E, F) Disease progression was observed 7 days after spray with steriled tap water (E) or 1 mM SA (F). SA effectively suppressed new infection (B) and disease progression (F) in cucumber plants.
Table 2.
Enhanced disease susceptibility upon exogenous SA application in different plants
| Host plant | Pathogen (infection style) | SA conc. and treatment method | Effect | References |
|---|---|---|---|---|
| Tomato (Lycopersicon esculentum) | Botrytis cinerea (necrotrophic) | 0.05, 0.5, and 2.5 mM | Lesion size are increased by ~325, 416, and 425%, respectively | El Oirdi et al. (2011) |
| Broad bean (Vicia faba) | Botrytis cinerea (necrotrophic) | 0.05–1 mM (under red light = resistance inducing condition) | ~746% increase in disease severity by 1 mM SA | Khanam et al. (2005) |
| 0.05–1 mM (under dark = disease inducing condition) | No significant difference in disease severity upto 1 mM SA |
SA, salicylic acid.
SA and ABA in Plant Tolerance to Abiotic Stresses
Continuous cropping and climate change threaten plant production via multiple abiotic stresses induced by heavy metals, salinity, ozone, ultraviolet, temperature and drought (Connor, 2002). Intriguingly, SA is not only regulating the resistance to biotic stresses, but also the tolerance to various abiotic stresses (Horváth et al., 2007; Khan et al., 2015) (Table 3). The underlying mechanisms of SA-induced abiotic stress tolerance include that SA-mediated (1) accumulation of osmolytes, such as glycinebetaine, proline, soluble sugars and amines, which can help maintain osmotic homeostasis, (2) regulation of mineral nutrition uptake, (3) enhanced reactive oxygen species scavenging activity, (4) enhanced secondary metabolite production, such as terpenes, phenolics, and compounds with nitrogen (alkaloids, cyanogenic glucosides, non-protein amino acids) and sulfur (glutathione, glucosinolates, phytoalexins, thionins, defensins, and allinin), and (5) regulation of other hormone pathways (Horváth et al., 2007; Khan et al., 2015).
Table 3.
Enhanced abiotic stress upon exogenous SA application in different plants
| Host plant | Stress | SA conc. and treatment method | Effect | References |
|---|---|---|---|---|
| Tomato (Lycopersicon esculentum) and Bean (Phaseolus vulgaris) | Heat/chilling/drought | Seed imbibed or soil drenched with 0.05, 0.1, 0.5, and 1 mM SA | Upon heat, chilling, and drought stress treatments, untreated control or 0.05 and 1.0 mM SA-treated plants died 100%, while 0.1 and 0.5 mM SA-treated plants survived 100% | Senaratna et al. (2000) |
| Tomato (Lycopersicon esculentum) | Salt | 0.1 mM SA is added in hydroponic cluture solution (3 weeks) | Photosynthetic activity is partially recovered. Endogenous ABA content is increased | Horváth et al. (2015) |
| Corn (Zea mays) | Cadmium (Cd) | SI with 0.5 mM SA for 6 h | Shoot FW and root DW are increased by ~262% and ~121%, respectively. However, SA treatment itself reduced shoot FW by 27% | Krantev et al. (2008) |
| Barley (Hordeum vulgare) | Cadmium (Cd) | SI with 0.5 mM SA for 6 h | Shoot and root FW are increased by ~133% and ~127%, respectively. However, SA treatment itself reduced shoot and root FW by 22% and 12%, respectively | Metwally et al. (2003) |
| Osmotic stress | The root systems of 20-day-old seed lings were immersed in aerated solutions of 30, 60, and 120 nM SA for 24 h | Osmotic stress-induced membrane injury (cell death) reduced by ~50%. Endogenous ABA content is increased | Bandurska and Stroiński (2005) | |
| Wheat (Triticum aestivum) | Freezing | 0.01, 0.1, and 1 mM SA sprayed on wheat leaves at the four-leaf stage three times, with an interval of 12 h | 0.01 and 0.1 mM SA significantly inhibited freezing stress-induced PS II quantum yield reduction and cell death. SA enhanced production of ABA and H2O2 | Wang et al. (2018) |
SA, salicylic acid; ABA, abscisic acid; FW, fresh weight; DW, dry weight; PS II, photosystem II.
Exogenous SA treatment induces the expression of a set of pathogenesis-related (PR) genes, including PR1, PR2, and PR5 (Ali et al., 2018). Interestingly, transgenic overexpression of some PR genes not only enhanced the resistance to different pathogens, but also enhanced the tolerance to different abiotic stresses (Hong and Hwang, 2005; Sarowar et al., 2005; Wu et al., 2016). Transgenic tobacco overexpressing pepper PR-1 showed enhanced heavy metal tolerance (Sarowar et al., 2005). Overexpression of pepper PR-1 enhanced drought and salt stress tolerance in Arabidopsis plants (Hong and Hwang, 2005). However, the underlying molecular mechanisms on how these PR proteins enhancing the abiotic stress tolerance need further investigation.
ABA is known as a key plant hormone conferring abiotic stress tolerance, and it antagonistically regulates SA-mediated defense signaling (Robert-Seilaniantz et al., 2011). ABA-induced suppression of SA-dependent signaling pathway often led to enhanced disease susceptibility in different plant-pathogen interactions (Audenaert et al., 2002; Jiang et al., 2010; Ulferts et al., 2015). However, under the certain abiotic stress conditions, such as freezing and salt stresses, ABA and SA together seems to be able to positively regulate stress tolerance response (Horváth et al., 2015; Szalai et al., 2011; Wang et al., 2018). Exogenous SA pretreatment significantly induced freezing tolerance of wheat via enhancing biosynthesis of ABA (Wang et al., 2018). In tomato plants, exogenous SA treatment-induced ABA biosynthesis in a dose-dependent manner, and partially recovered lowered photosynthetic activity under salt stress condition (Horváth et al., 2015). Pretreatment of barley plants with SA increased the ABA content and reduced the damage induced by water deficit condition (Bandurska and Stroiński, 2005). Notably, an important cluster of genes that are similarly regulated by ABA and SA (28% of ABA-induced genes were also induced by SA, while 40% of ABA-repressed genes were also repressed by SA) is identified in Arabidopsis plants, suggesting plants equipped the common transcriptomic responses regulated by ABA and SA (Kalachova et al., 2016). Taken together, although ABA is antagonistically regulating the SA-mediated disease resistance, ABA and SA seem to be able to cooperate for developing abiotic stress tolerance.
SA and IAA in Plant Growth and Development
SA has controversial roles in plant growth and development depending on its concentration and plant growth conditions and developmental stages (Rivas-San Vicente and Plasencia, 2011). Generally, high levels of SA (It depends on the plant species, however, >1 mM SA considered as high concentration.) negatively regulate plant development and growth. Nevertheless, the application of optimal concentrations of SA showed beneficial effects on it. Depending on the experimental conditions, SA distinctly stimulated growth under both normal and different abiotic stress conditions in different plant species (Gunes et al., 2007; Gutiérrez-Coronado et al., 1998; Kováčik et al., 2009; Manzoor et al., 2015; Sakhabutdinova et al., 2003; Yildirim et al., 2008, 2015) (see also Table 3).
Exogenous SA application also showed different effects on plant development, including seed germination, budding, flowering, and fruit setting and ripening. In Finger Millet plants, SA stimulated flowering (Appu and Muthukrishnan, 2014). SA-induced enhanced fruit setting and weight were observed in strawberry (Kazemi, 2013), apple (Shaaban et al., 2011) and mango (Ngullie et al., 2014). Germination of barley and maize seeds imbibed in >3 mM SA were completely blocked (Guan and Scandalios, 1995; Xie et al., 2007). On the other hands, imbibing maize seeds in ~0.3 mM to ~0.9 mM SA showed higher germination speed, percentage and shoot length (Sallam and Ibrahim, 2015). Notably, ~0.43 mM SA exhibited the best germination stimulating effect, but its effect was decreased at the higher concentrations. Taken together, different concentrations of SA in different plants have either stimulating or blocking effects on plant development.
IAA influences plant growth and development, including tropic growth responses, vascular development, leaf and flower initiation, root growth, and lateral root formation. Recently, Pasternak et al. (2019) reported that SA regulates IAA biosynthesis and transport thereby changing the Arabidopsis root meristem patterning. Low-concentration SA (below 50 μM) promoted adventitious roots and altered architecture of the root apical meristem, whereas high-concentration SA (greater than 50 μM) inhibited overall growth processes in the root. Importantly, both SA and IAA are known to be biosynthesized from the shikimate pathway (Pérez-Llorca et al., 2019). This may suggest that higher biosynthetic activity of SA during the defense response may limit the resource required for the production of plant growth regulating hormone IAA, and vice versa, thereby fine-tuning the cost for the growth or defense depending on external and internal conditions.
SA and Plant Microbiome
Recently, researches on plant microbiome in relation with plant’s health are getting more attention from plant science community (Bulgarelli et al., 2012; Lundberg et al., 2012). In model plant Arabidopsis thaliana, the effect of SA on microbiome was analyzed after exogenous SA application or by using the mutants with altered endogenous SA levels (Lebeis et al., 2015). It revealed that SA application distinctly enriched [Flavobacterium sp. 40 (Bacteroidetes) and Terracoccus sp. 273 (Actinobacteria)] and depleted [Mitsuaria sp. 370 (β-Proteobacteria)] specific bacterial isolates from synthetic community (SynCom) experiment. In addition, population density of nine Actinobacteria and 12 Proteobacteria families were reduced and increased, respectively, in cpr5 mutants that constitutively produces SA. This suggests that soil (and/or rhizosphere) microbiome can be distinctly altered by SA. So far, SA’s effects on plants after soil drench application are majorly focused on induction of systemic immune response; however little is known about its effect on plant root or endophytic microbiomes. Thus further studies on SA’s effects on diverse soil or endophytic microbiome will shed the lights on how SA influences different aspect of plant physiology, including immunity, growth, development and etc.
Concluding Remarks and Perspectives
Increasing numbers of reports suggesting that use of SA and its derivatives, collectively termed salicylates, reduces the risk of multiple chronic diseases in humans, including heart attack, stroke, arthritis, diabetes, certain type of cancers and Alzheimer’s diseases (Castro-Torres et al., 2015; Chang et al., 2016; Rothwell et al., 2010; Steinberg et al., 2013; Tschanz et al., 2013). Given beneficial effects of SA on both plant and human health, SA and salicylates can be used as an efficient plant protector with very minor side effect on environments and humans. To use SA as an effective and environmentally friendly plant protector and/or plant growth regulator, following questions need to be addressed. First, the effective concentrations of SA for specific plants and/or purpose need to be determined. As mentioned above, high dose of SA (>2 mM) not only induces the enhanced disease resistance, but also has adverse effects on plant growth and productivity, which caused by undesirable balancing between cost and benefit of limited energy that plant can use. In humans, >2.5 mM SA in the plasma lead to acute toxicity (Choi et al., 2015a, 2015b, 2019). Thus, SA concentrations ranging from micromolar (~100 μM) to low milimolar (<2 mM) concentrations can be tested to screening the safe and effective concentrations for specific purpose. In addition, the use of high concentration of SA (~2 mM) as a plant growth regulator may also be considered, especially in case of growers need to reduce the growth rate of specific plants with disease control effect. Finally, natural SA derivatives, the amorfrutins, from the medicinal legume licorice Glycyrrhiza foetida, showed ~1,000 times stronger binding and inhibitory activities on selected SA-binding proteins (Choi et al., 2015b, 2019). Thus, the screening of salicylates with higher efficiency and specificity may warrant a novel plant protection method. Further studies on practical use of SA in different crop plants will contribute to developing the cost-effective and environmental friendly crop management system.
Acknowledgments
This work was supported by a Research Grant of Andong National University.
References
- Ali S, Ganai BA, Kamili AN, Bhat AA, Mir ZA, Bhat JA, Tyagi A, Islam ST, Mushtaq M, Yadav P, Rawat S, Grover A. Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiol Res. 2018;212–213:29–37. doi: 10.1016/j.micres.2018.04.008. [DOI] [PubMed] [Google Scholar]
- Appu M, Muthukrishnan S. Foliar application of salicylic acid stimulates flowering and induce defense related proteins in finger millet plants. Univers J Plant Sci. 2014;2:14–18. [Google Scholar]
- Audenaert K, De Meyer GB, Höfte MM. Abscisic acid determines basal susceptibility of tomato to Botrytis cinerea and suppresses salicylic acid-dependent signaling mechanisms. Plant Physiol. 2002;128:491–501. doi: 10.1104/pp.010605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandurska H, Stroiński A. The effect of salicylic acid on barley response to water deficit. Acta Physiol Plant. 2005;27:379–386. doi: 10.1007/s11738-005-0015-5. [DOI] [Google Scholar]
- Bulgarelli D, Rott M, Schlaeppi K, Ver Loren van Themaat E, Ahmadinejad N, Assenza F, Rauf P, Huettel B, Reinhardt R, Schmelzer E, Peplies J, Gloeckner FO, Amann R, Eickhorst T, Schulze-Lefert P. Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature. 2012;488:91–95. doi: 10.1038/nature11336. [DOI] [PubMed] [Google Scholar]
- Castro-Torres Y, Katholi RE, Yar Khan N. Aspirin for primary prevention of cardiovascular diseases: current concepts, unanswered questions and future directions. Hellenic J Cardiol. 2015;56:461–474. [PubMed] [Google Scholar]
- Chandrasekhar B, Umesha S, Naveen Kumar HN. Proteomic analysis of salicylic acid enhanced disease resistance in bacterial wilt affected chilli (Capsicum annuum) crop. Physiol Mol Plant Pathol. 2017;98:85–96. doi: 10.1016/j.pmpp.2017.04.002. [DOI] [Google Scholar]
- Chang C-W, Horng J-T, Hsu C-C, Chen J-M. Mean daily dosage of aspirin and the risk of incident alzheimer’s dementia in patients with type 2 diabetes mellitus: a nationwide retrospective cohort study in Taiwan. J Diabetes Res. 2016;2016 doi: 10.1155/2016/9027484. 9027484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HW, Tian M, Manohar M, Harraz MM, Park S-W, Schroeder FC, Snyder SH, Klessig DF. Human GAPDH is a target of aspirin’s primary metabolite salicylic acid and its derivatives. PLoS ONE. 2015a;10:e0143447. doi: 10.1371/journal.pone.0143447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HW, Tian M, Song F, Venereau E, Preti A, Park S-W, Hamilton K, Swapna GVT, Manohar M, Moreau M, Agresti A, Gorzanelli A, De Marchis F, Wang H, Antonyak M, Micikas RJ, Gentile DR, Cerione RA, Schroeder FC, Montelione GT, Bianchi ME, Klessig DF. Aspirin’s active metabolite salicylic acid targets high mobility group box 1 to modulate inflammatory responses. Mol Med. 2015b;21:526–535. doi: 10.2119/molmed.2015.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HW, Wang L, Powell AF, Strickler SR, Wang D, Dempsey DA, Schroeder FC, Klessig DF. A genome-wide screen for human salicylic acid (SA)-binding proteins reveals targets through which SA may influence development of various diseases. Sci Rep. 2019;9:13084. doi: 10.1038/s41598-019-49234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor D. Climate change and global crop productivity. Crop Sci. 2002;42:978. [Google Scholar]
- Daw BD, Zhang LH, Wang ZZ. Salicylic acid enhances antifungal resistance to Magnaporthe grisea in rice plants. Australas Plant Pathol. 2008;37:637–644. doi: 10.1071/AP08054. [DOI] [Google Scholar]
- Deenamo N, Kuyyogsuy A, Khompatara K, Chanwun T, Ekchaweng K, Churngchow N. Salicylic acid induces resistance in rubber tree against Phytophthora palmivora. Int J Mol Sci. 2018;1883;19 doi: 10.3390/ijms19071883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey DA, Klessig DF. How does the multifaceted plant hormone salicylic acid combat disease in plants and are similar mechanisms utilized in humans? BMC Biol. 2017;15:23. doi: 10.1186/s12915-017-0364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Oirdi M, El Rahman TA, Rigano L, El Hadrami A, Rodriguez MC, Daayf F, Vojnov A, Bouarab K. Botrytis cinerea manipulates the antagonistic effects between immune pathways to promote disease development in tomato. Plant Cell. 2011;23:2405–2421. doi: 10.1105/tpc.111.083394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb M, Meldau S, Howe GA. Role of phytohormones in insect-specific plant reactions. Trends Plant Sci. 2012;17:250–259. doi: 10.1016/j.tplants.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmailzadeh M, Soleimani MJ, Rouhani H. Exogenous applications of salicylic acid for inducing systemic acquired resistance against tomato stem canker disease. J Biol Sci. 2008;8:1039–1044. doi: 10.3923/jbs.2008.1039.1044. [DOI] [Google Scholar]
- Ferrari S, Plotnikova JM, De Lorenzo G, Ausubel FM. Arabidopsis local resistance to Botrytis cinerea involves salicylic acid and camalexin and requires EDS4 and PAD2, but not SID2, EDS5 or PAD4. Plant J. 2003;35:193–205. doi: 10.1046/j.1365-313X.2003.01794.x. [DOI] [PubMed] [Google Scholar]
- Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol. 2005;43:205–227. doi: 10.1146/annurev.phyto.43.040204.135923. [DOI] [PubMed] [Google Scholar]
- Guan L, Scandalios JG. Developmentally related responses of maize catalase genes to salicylic acid. Proc Natl Acad Sci U S A. 1995;92:5930–5934. doi: 10.1073/pnas.92.13.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunes A, Inal A, Alpaslan M, Eraslan F, Bagci EG, Cicek N. Salicylic acid induced changes on some physiological parameters symptomatic for oxidative stress and mineral nutrition in maize (Zea mays L.) grown under salinity. J Plant Physiol. 2007;164:728–736. doi: 10.1016/j.jplph.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Coronado MA, Trejo-López C, Larqué-Saavedra A. Effects of salicylic acid on the growth of roots and shoots in soybean. Plant Physiol Biochem. 1998;36:563–565. doi: 10.1016/S0981-9428(98)80003-X. [DOI] [Google Scholar]
- Hong JK, Hwang BK. Induction of enhanced disease resistance and oxidative stress tolerance by overexpression of pepper basic PR-1 gene in Arabidopsis. Physiol Plant. 2005;124:267–277. doi: 10.1111/j.1399-3054.2005.00515.x. [DOI] [Google Scholar]
- Horváth E, Csiszár J, Gallé Á, Poór P, Szepesi Á, Tari I. Hardening with salicylic acid induces concentration-dependent changes in abscisic acid biosynthesis of tomato under salt stress. J Plant Physiol. 2015;183:54–63. doi: 10.1016/j.jplph.2015.05.010. [DOI] [PubMed] [Google Scholar]
- Horváth E, Szalai G, Janda T. Induction of abiotic stress tolerance by salicylic acid signaling. J Plant Growth Regul. 2007;26:290–300. doi: 10.1007/s00344-007-9017-4. [DOI] [Google Scholar]
- Jendoubi W, Harbaoui K, Hamada W. Salicylic acid-induced resistance against Fusarium oxysporumf.s.pradicis lycopercisi in hydroponic grown tomato plants. J New Sci Agric Biotechnol. 2017;21:985–995. [Google Scholar]
- Jiang C-J, Shimono M, Sugano S, Kojima M, Yazawa K, Yoshida R, Inoue H, Hayashi N, Sakakibara H, Takatsuji H. Abscisic acid interacts antagonistically with salicylic acid signaling pathway in rice-Magnaporthe grisea interaction. Mol Plant-Microbe Interact. 2010;23:791–798. doi: 10.1094/MPMI-23-6-0791. [DOI] [PubMed] [Google Scholar]
- Ju C, Chang C. Mechanistic insights in ethylene perception and signal transduction. Plant Physiol. 2015;169:85–95. doi: 10.1104/pp.15.00845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalachova T, Puga-Freitas R, Kravets V, Soubigou-Taconnat L, Repellin A, Balzergue S, Zachowski A, Ruelland E. The inhibition of basal phosphoinositide-dependent phospholipase C activity in Arabidopsis suspension cells by abscisic or salicylic acid acts as a signalling hub accounting for an important overlap in transcriptome remodelling induced by these hormones. Environ Exp Bot. 2016;123:37–49. doi: 10.1016/j.envexpbot.2015.11.003. [DOI] [Google Scholar]
- Kazemi M. Foliar application of salicylic acid and calcium on yield, yield component and chemical properties of strawberry. Bull Environ Pharmacol Life Sci. 2013;2:19–23. [Google Scholar]
- Khan MIR, Fatma M, Per TS, Anjum NA, Khan NA. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front Plant Sci. 2015;6:462. doi: 10.3389/fpls.2015.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanam NN, Ueno M, Kihara J, Honda Y, Arase S. Suppression of red light-induced resistance in broad beans to Botrytis cinerea by salicylic acid. Physiol Mol Plant Pathol. 2005;66:20–29. doi: 10.1016/j.pmpp.2005.03.006. [DOI] [Google Scholar]
- Klessig DF, Choi HW, Dempsey DA. Systemic acquired resistance and salicylic acid: past, present, and future. Mol Plant-Microbe Interact. 2018;31:871–888. doi: 10.1094/MPMI-03-18-0067-CR. [DOI] [PubMed] [Google Scholar]
- Kobeasy MI, El-Beltagi HS, El-Shazly MA, Khattab EAH. Induction of resistance in Arachis hypogaea L against Peanut mottle virus by nitric oxide and salicylic acid. Physiol Mol Plant Pathol. 2011;76:112–118. doi: 10.1016/j.pmpp.2011.07.005. [DOI] [Google Scholar]
- Korver RA, Koevoets IT, Testerink C. Out of shape during stress: a key role for auxin. Trends Plant Sci. 2018;23:783–793. doi: 10.1016/j.tplants.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kováčik J, Klejdus B, Hedbavny J, Bačkor M. Salicylic acid alleviates NaCl-induced changes in the metabolism of Matricaria chamomilla plants. Ecotoxicology. 2009;18:544–554. doi: 10.1007/s10646-009-0312-7. [DOI] [PubMed] [Google Scholar]
- Krantev A, Yordanova R, Janda T, Szalai G, Popova L. Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J Plant Physiol. 2008;165:920–931. doi: 10.1016/j.jplph.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Kundu S, Chakraborty D, Pal A. Proteomic analysis of salicylic acid induced resistance to Mungbean Yellow Mosaic India Virus in Vigna mungo. J Proteomics. 2011;74:337–349. doi: 10.1016/j.jprot.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Kunkel BN, Brooks DM. Cross talk between signaling pathways in pathogen defense. Curr Opin Plant Biol. 2002;5:325–331. doi: 10.1016/S1369-5266(02)00275-3. [DOI] [PubMed] [Google Scholar]
- Le Thanh T, Thumanu K, Wongkaew S, Boonkerd N, Teaumroong N, Phansak P, Buensanteai N. Salicylic acid-induced accumulation of biochemical components associated with resistance against Xanthomonas oryzae pv. oryzae in rice. J Plant Interact. 2017;12:108–120. doi: 10.1080/17429145.2017.1291859. [DOI] [Google Scholar]
- Lebeis SL, Paredes SH, Lundberg DS, Breakfield N, Gehring J, McDonald M, Malfatti S, Glavina del Rio T, Jones CD, Tringe SG, Dangl JL. Salicylic acid modulates colonization of the root microbiome by specific bacterial taxa. Science. 2015;349:860–864. doi: 10.1126/science.aaa8764. [DOI] [PubMed] [Google Scholar]
- Li L, Zou Y. Induction of disease resistance by salicylic acid and calcium ion against Botrytis cinerea in tomato (Lycopersicon esculentum) Emir J Food Agric. 2017;29:78–82. doi: 10.9755/ejfa.2016-10-1515. [DOI] [Google Scholar]
- Lu H. Dissection of salicylic acid-mediated defense signaling networks. Plant Signal Behav. 2009;4:713–717. doi: 10.4161/psb.4.8.9173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg DS, Lebeis SL, Paredes SH, Yourstone S, Gehring J, Malfatti S, Tremblay J, Engelbrektson A, Kunin V, Del Rio TG, Edgar RC, Eickhorst T, Ley RE, Hugenholtz P, Tringe SG, Dangl JL. Defining the core Arabidopsis thaliana root microbiome. Nature. 2012;488:86–90. doi: 10.1038/nature11237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoor K, Ilyas N, Batool N, Ahmad B, Arshad M. Effect of salicylic acid on the growth and physiological characteristics of maize under stress conditions. J Chem Soc Pakistan. 2015;37:588–593. [Google Scholar]
- Metwally A, Finkemeier I, Georgi M, Dietz K-J. Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol. 2003;132:272–281. doi: 10.1104/pp.102.018457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan Babu R, Sajeena A, Vijaya Samundeeswari A, Sreedhar A, Vidhyasekaran P, Seetharaman K, Reddy MS. Induction of systemic resistance to Xanthomonas oryzae pv. oryzae by salicylic acid in Oryza sativa (L.) J Plant Dis Prot. 2003;110:419–431. doi: 10.1007/BF03356119. [DOI] [Google Scholar]
- Ng LM, Melcher K, Teh BT, Xu HE. Abscisic acid perception and signaling: structural mechanisms and applications. Acta Pharmacol Sin. 2014;35:567–584. doi: 10.1038/aps.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngullie CR, Tank RV, Bhanderi DR. Effect of salicylic acid and humic acid on flowering, fruiting, yield and quality of mango (Mangifera indica L.) cv. KESAR. Adv Res J Crop Improv. 2014;5:136–139. doi: 10.15740/HAS/ARJCI/5.2/136-139. [DOI] [Google Scholar]
- Pasternak T, Groot EP, Kazantsev FV, Teale W, Omelyanchuk N, Kovrizhnykh V, Palme K, Mironova VV. Salicylic acid affects root meristem patterning via auxin distribution in a concentration-dependent manner. Plant Physiol. 2019;180:1725–1739. doi: 10.1104/pp.19.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Llorca M, Muñoz P, Müller M, Munné-Bosch S. Biosynthesis, metabolism and function of auxin, salicylic acid and melatonin in climacteric and non-climacteric fruits. Front Plant Sci. 2019;10:136. doi: 10.3389/fpls.2019.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwan DEM, Fayez KA, Mahmoud SY, Hamad A, Lu G. Physiological and metabolic changes of Cucurbita pepo leaves in response to zucchini yellow mosaic virus (ZYMV) infection and salicylic acid treatments. Plant Physiol Biochem. 2007;45:480–489. doi: 10.1016/j.plaphy.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Rivas-San Vicente M, Plasencia J. Salicylic acid beyond defence: its role in plant growth and development. J Exp Bot. 2011;62:3321–3338. doi: 10.1093/jxb/err031. [DOI] [PubMed] [Google Scholar]
- Robert-Seilaniantz A, Grant M, Jones JDG. Hormone crosstalk in plant disease and defense: more than just jasmonate-salicylate antagonism. Annu Rev Phytopathol. 2011;49:317–343. doi: 10.1146/annurev-phyto-073009-114447. [DOI] [PubMed] [Google Scholar]
- Rothwell PM, Wilson M, Elwin C-E, Norrving B, Algra A, Warlow CP, Meade TW. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376:1741–1750. doi: 10.1016/S0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- Saikia R, Singh T, Kumar R, Srivastava J, Srivastava AK, Singh K, Arora DK. Role of salicylic acid in systemic resistance induced by Pseudomonas fluorescens against Fusarium oxysporum f. sp. ciceri in chickpea. Microbiol Res. 2003;158:203–213. doi: 10.1078/0944-5013-00202. [DOI] [PubMed] [Google Scholar]
- Sakhabutdinova AR, Fatkhutdinova DR, Bezrukova MV, Shakirova FM. Salicylic acid prevents the damaging action of stress factors on wheat plants. Bulg J Plant Physiol. 2003;29(3–4 Spec issue):314–319. [Google Scholar]
- Sallam AM, Ibrahim HIM. Effect of grain priming with salicylic acid on germination speed, seedling characters, anti-oxidant enzyme activity and forage yield of teosinte. Am Eurasian J Agric Environ Sci. 2015;15:744–753. [Google Scholar]
- Sarowar S, Kim YJ, Kim EN, Kim KD, Hwang BK, Islam R, Shin JS. Overexpression of a pepper basic pathogenesis-related protein 1 gene in tobacco plants enhances resistance to heavy metal and pathogen stresses. Plant Cell Rep. 2005;24:216–224. doi: 10.1007/s00299-005-0928-x. [DOI] [PubMed] [Google Scholar]
- Senaratna T, Touchell D, Bunn E, Dixon K. Acetyl salicylic acid (Aspirin) and salicylic acid induce multiple stress tolerance in bean and tomato plants. Plant Growth Regul. 2000;30:157–161. doi: 10.1023/A:1006386800974. [DOI] [Google Scholar]
- Shaaban MM, Abd El-Aal AMK, Ahmed FF. Insight into the effect of salicylic acid on apple trees growing under sandy saline soil. Res J Agric Biol Sci. 2011;7:150–156. [Google Scholar]
- Steinberg GR, Dandapani M, Hardie DG. AMPK: mediating the metabolic effects of salicylate-based drugs? Trends Endocrinol Metab. 2013;24:481–487. doi: 10.1016/j.tem.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella de Freitas TF, Stout MJ, Sant’Ana J. Effects of exogenous methyl jasmonate and salicylic acid on rice resistance to Oebalus pugnax. Pest Manag Sci. 2019;75:744–752. doi: 10.1002/ps.5174. [DOI] [PubMed] [Google Scholar]
- Szalai G, Pál M, Janda T. Abscisic acid may alter the salicylic acid-related abiotic stress response in maize. Acta Biol Szeged. 2011;55:155–157. [Google Scholar]
- Tschanz JT, Norton MC, Zandi PP, Lyketsos CG. The Cache County Study on Memory in Aging: factors affecting risk of Alzheimer’s disease and its progression after onset. Int Rev Psychiatry. 2013;25:673–685. doi: 10.3109/09540261.2013.849663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulferts S, Delventhal R, Splivallo R, Karlovsky P, Schaffrath U. Abscisic acid negatively interferes with basal defence of barley against Magnaporthe oryzae. BMC Plant Biol. 2015;15:7. doi: 10.1186/s12870-014-0409-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlot AC, Dempsey DA, Klessig DF. Salicylic Acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol. 2009;47:177–206. doi: 10.1146/annurev.phyto.050908.135202. [DOI] [PubMed] [Google Scholar]
- Wang C, Liu Y, Li SS, Han GZ. Insights into the origin and evolution of the plant hormone signaling machinery. Plant Physiol. 2015a;167:872–886. doi: 10.1104/pp.114.247403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Chen S-H, Huang Y-F, Sun S. Induced resistance to anthracnose of Camelia oleifera by salicylic acid. For Res. 2006;19:629–632. [Google Scholar]
- Wang KL-C, Li H, Ecker JR. Ethylene biosynthesis and signaling networks. Plant Cell. 2002;(14 Suppl):S131–S151. doi: 10.1105/tpc.001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Wang X, Huang M, Cai J, Zhou Q, Dai T, Cao W, Jiang D. Hydrogen peroxide and abscisic acid mediate salicylic acid-induced freezing tolerance in wheat. Front Plant Sci. 2018;9:1137. doi: 10.3389/fpls.2018.01137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liu J-H. Exogenous treatment with salicylic acid attenuates occurrence of citrus canker in susceptible navel orange (Citrus sinensis Osbeck) J Plant Physiol. 2012;169:1143–1149. doi: 10.1016/j.jplph.2012.03.018. [DOI] [PubMed] [Google Scholar]
- Wang Z, Jia C, Li J, Huang S, Xu B, Jin Z. Activation of salicylic acid metabolism and signal transduction can enhance resistance to Fusarium wilt in banana (Musa acuminata L. AAA group, cv. Cavendish) Funct Integr Genomics. 2015b;15:47–62. doi: 10.1007/s10142-014-0402-3. [DOI] [PubMed] [Google Scholar]
- Wasternack C, Song S. Jasmonates: biosynthesis, metabolism, and signaling by proteins activating and repressing transcription. J Exp Bot. 2017;68:1303–1321. doi: 10.1093/jxb/erw443. [DOI] [PubMed] [Google Scholar]
- Went FW, Thimann KV. Phytohormones. Macmillan; New York, NY, USA: 1937. p. 294. [Google Scholar]
- Wu J, Kim SG, Kang KY, Kim J-G, Park S-R, Gupta R, Kim YH, Wang Y, Kim ST. Overexpression of a pathogenesis-related protein 10 enhances biotic and abiotic stress tolerance in rice. Plant Pathol J. 2016;32:552–562. doi: 10.5423/PPJ.OA.06.2016.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Ding Y, Wei W, Davis RE, Lee I-M, Hammond RW, Zhao Y. Salicylic acid-mediated elicitation of tomato defence against infection by potato purple top phytoplasma. Ann Appl Biol. 2012;161:36–45. doi: 10.1111/j.1744-7348.2012.00550.x. [DOI] [Google Scholar]
- Xie Z, Zhang Z-L, Hanzlik S, Cook E, Shen QJ. Salicylic acid inhibits gibberellin-induced alpha-amylase expression and seed germination via a pathway involving an abscisic-acid-inducible WRKY gene. Plant Mol Biol. 2007;64:293–303. doi: 10.1007/s11103-007-9152-0. [DOI] [PubMed] [Google Scholar]
- Xiong L, Zhu J-K. Regulation of abscisic acid biosynthesis. Plant Physiol. 2003;133:29–36. doi: 10.1104/pp.103.025395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S, Dong X. Perception of the plant immune signal salicylic acid. Curr Opin Plant Biol. 2014;20:64–68. doi: 10.1016/j.pbi.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim E, Ekinci M, Turan M, Dursun A, Kul R, Parlakova F. Roles of glycine betaine in mitigating deleterious effect of salt stress on lettuce (Lactuca sativa L.) Arch Agron Soil Sci. 2015;61:1673–1689. doi: 10.1080/03650340.2015.1030611. [DOI] [Google Scholar]
- Yildirim E, Turan M, Guvenc I. Effect of foliar salicylic acid applications on growth, chlorophyll, and mineral content of cucumber grown under salt stress. J Plant Nutr. 2008;31:593–612. doi: 10.1080/01904160801895118. [DOI] [Google Scholar]
- Yousif DYM. Effects sprayed solution of salicylic acid to prevent of wilt disease caused by Fussarium oxysporium. J Phys Conf Ser. 2018;1003:012001. doi: 10.1088/1742-6596/1003/1/012001. [DOI] [Google Scholar]
- Zhang Y, Li X. Salicylic acid: biosynthesis, perception, and contributions to plant immunity. Curr Opin Plant Biol. 2019;50:29–36. doi: 10.1016/j.pbi.2019.02.004. [DOI] [PubMed] [Google Scholar]