Abstract
Objective:
Ovarian steroid cell tumor (SCT) is a rare tumor with steroid-producing ability. We report a 22-year-old woman with secondary amenorrhea and hirsutism caused by an ovarian SCT-not otherwise specified (NOS), who underwent successfully laparoscopic resection of the tumor.
Case report:
A 22-year-old null gravida woman presented to a hospital, having amenorrhea for 18 months and increasing facial hair. Physical examination revealed obesity (body mass index, 37.3 kg/m2) with evident facial and trunk hair. Total and free serum testosterone, and dehydroepiandrosterone sulfate levels were found to be elevated. Levels of serum adrenocorticotropic hormone, gonadotropins, cortisol, aldosterone, and ovarian steroids were observed to be within reference intervals. Although polycystic ovaries were not found, a hyperechogenic solid tumor (3 cm) was detected on transvaginal ultrasonography. Laparoscopic resection of the tumor was performed. One month post-surgery, total and free testosterone levels were observed to have decreased, and menstruation resumed two months thereafter. The patient was histologically diagnosed with ovarian SCT-NOS. Expression of ovarian steroidogenic enzymes, which are related to hyperandrogenism, was observed. No disease recurrence has been reported for more than 5 years post-surgery.
Keywords: amenorrhea, ovarian tumor, steroid cell tumor not other specified, hyperandrogenism, laparoscopic surgery
Introduction
Ovarian steroid cell tumor (SCT) is a very rare entity, comprising 0.1% of all ovarian tumors1). Ovarian SCTs are of three subtypes based on the source of cell origin: stromal luteoma, Leydig cell tumor, and not otherwise specified (NOS)2). SCT-NOS, which accounts for over 50% of the ovarian SCTs, derives from an indeterminate lineage of ovarian tissue3). All subtypes of SCT have the potential of producing steroid hormones such as androgen, estrogen, and cortisol3). Androgen producing SCT-NOS causes various virilizing symptoms including hirsutism, baldness, and amenorrhea1). Here, we report on a 22-year-old female with secondary amenorrhea and hirsutism caused by androgen-producing SCT-NOS. She was treated successfully with laparoscopic resection of the tumor. Additionally, we examined the immune expression of steroid synthetic enzymes which are involved in androgen synthesis. We obtained informed consent from the patient to publish this case report.
Case presentation
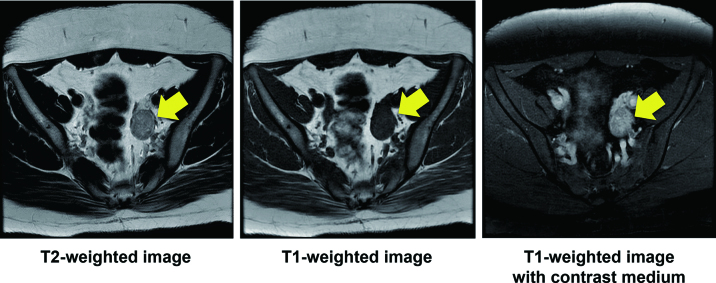
A 22-year-old null gravida woman presented with amenorrhea for 18 months and increasing facial hair (Fig. 1). She was referred to our hospital as a possible case of endocrine abnormality, presumably polycystic ovary syndrome (PCOS). Since menarche at 11 years old, she never had regular menstruation. She was 148 cm tall and weighed 78 kg; her body mass index (BMI) was 37.3 kg/m2. Waist and hip circumferences were recorded as 101 and 110 cm, respectively, with a waist to hip ratio of 0.918. Systolic and diastolic blood pressure and heart rate were observed to be 141/102 mmHg and 86/min, respectively. She presented with hirsutism of the face and trunk; however, no remarkable signs of hoarseness, frontotemporal baldness, or clitoromegaly were observed. Pelvic examination, performed manually for reasons of body habitus, was reported as unremarkable. Biochemical and hormonal data are shown in Table 1. Biochemical data shows the levels of serum uric acid and triglyceride were as high as 6.8 mg/dl and 419 mg/dl, respectively. For glucose tolerance and insulin resistance, the levels of fasting-plasma glucose and HbA1c were within reference intervals. Furthermore, fasting immunoreactive insulin and homeostatic model assessment index were reported to be as high as 32.8 µIU/mL and 6.8, respectively. Serum adrenocorticotropic hormone, gonadotropins, cortisol, aldosterone, and ovarian steroids were found within reference intervals. Total and free serum testosterone, and dehydroepiandrosterone sulfate levels were found to be as high as 1.3 ng/mL (reference interval, 0.15-0.44 ng/mL), 4.5 pg/mL (reference interval, < 2.7 pg/mL), and 332 µg/dL (reference interval, 73-322 µg/dL), respectively. Transvaginal ultrasound identified a 3.0 × 2.3-cm left ovarian solid mass without sign of polycystic ovaries (Fig. 2). Magnetic resonance imaging with contrast medium confirmed the presence of a well-enhanced left ovarian tumor (Fig. 3). We diagnosed an androgen-producing ovarian tumor and metabolic syndrome. Expression levels of tumor markers for alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), cancer antigen 19-9 (CA19-9), CA125, and squamous cell carcinoma antigen (SCC) were within reference intervals. On diagnosis of androgen-producing ovarian tumor, we performed laparoscopic resection. In the peritoneal cavity, no ascites was observed, and peritoneal washing was collected for cytological examination. Laparoscopic findings revealed that the left ovary was slightly enlarged with no sign of rupture or metastasis in the abdominal cavity. The left ovary was excised and a well-circumscribed soft hemorrhagic mass was carefully removed using scissors and forceps (Fig. 4). Cytological examination of peritoneal washing was negative. In this case, we did not perform rapid intraoperative pathological examination; subsequent treatment was decided based on permanent-section pathology. The excised surface of the specimen appeared yellow with a solid hemorrhagic mass. Microscopic findings revealed tumor cells arranged in a diffuse pattern or columns, or arranged in nets, and the cell morphology was observed to be polygonal with abundant cytoplasm (Fig. 5). As crystals of Reinke were not observed, the pathological diagnosis was SCT-NOS. Average mitotic activity was found to be 1.8 over 10 high power fields. No signs of necrosis, hemorrhage, and nuclear atypia were observed. We analyzed the expression of steroidogenic enzymes of the tumor by immunohistochemistry. Different expressions of the steroidogenic enzymes in the specimen are illustrated in Table 2. Immunoexpression of ovarian steroidogenic enzymes such as 3-β-hydroxysteroid dehydrogenase (3-β-HSD), P450c17, 5-α-reductase type 1/2, 17-β-HSD 5, and 17-β-HSD 1 were observed to be high, whereas the expression of P450 side-chain cleavage (P450scc) enzyme, aromatase, and steroid sulfatase were undetected. On the other hand, adrenal steroidogenic enzymes such as P450c21, dehydroepiandrosterone sulfotransferase (DHEA-ST), steroid 11-β-hydroxylase (CYP11B1), and aldosterone synthase (CYP11B2) were not detected. As ovarian SCT-NOS is classified as a borderline malignancy, we offered an additional operation of ipsilateral oophorectomy. However, she did not agree to additional surgery and wanted diligent follow-up. After the operation, total and free testosterone levels were found to have rapidly decreased while menstruation was reported to have resumed spontaneously 2 months thereafter (Fig. 6). No recurrence of disease has been observed for more than 5 years post-surgery.
Fig. 1.
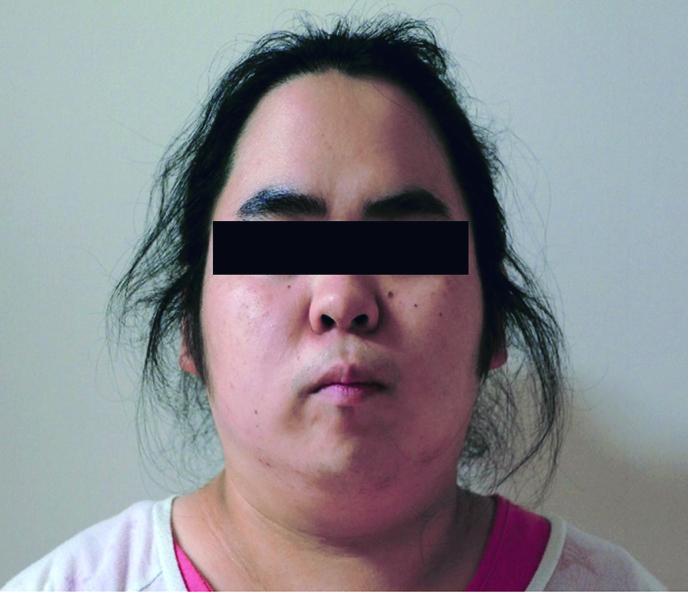
Picture shows facial hirsutism.
Table 1.
Biochemical and hormonal data
| Total protein | 7.5 g/dl (6.7-8.3 g/dl) |
| Alb | 4.2 g/dl (3.8-5.2 g/dl) |
| AST | 12 U/l (10-40 U/l) |
| ALT | 17 U/l (5-40 U/l) |
| γGTP | 24 U/l (< 30 U/l) |
| BUN | 9 mg/dl (8-22 mg/dl) |
| Creatinine | 0.42 mg/dl (0.47-0.79 mg/dl) |
| UA | 6.8 mg/dl (2.5-7.0 mg/dl) |
| Na | 142 mmol/l (136-147 mmol/l) |
| K | 4.1 mmol/l (3.6-5.0 mmol/l) |
| Cl | 106 mmol/l (93-109 mmol/l) |
| Ca | 9.3 mg/dl (8.5-10.2 mg/dl) |
| Triglyceride | 419 mg/dl (50-149 mg/dl) |
| Total cholesterol | 199 mg/dl (150-219 mg/dl) |
| LDL-cholesterol | 137 mg/dl (70-139 mg/dl) |
| HDL-cholesterol | 36 mg/dl (40-96) |
| Fasting glucose | 84 mg/dl (70-109) |
| HbA1c | 5.3% (4.6-6.2) |
| Fasting insulin | 32.8 μIU/ml (1.84-12.2 μIU/ml) |
| HOMA-IR | 6.8 (< 1.6) |
| ACTH | 8.5 pg/ml (7.2-63.3 pg/ml) |
| Cortisol | 1.7 μg/dl (6.24-18.0 μg/dl) |
| LH | 2.3 mIU/ml (1.76-10.24 mIU/ml) |
| FSH | 4.9 mIU/ml (3.01-14.72 mIU/ml) |
| PRL | 25.7 ng/ml (4.91-29.3 ng/ml) |
| Estradiol | 58 pg/ml (28.8-196.8 pg/ml) |
| Progesterone | 0.82 ng/ml (< 0.28 ng/ml) |
| Total testosterone | 1.3 ng/ml (0.15-0.44 ng/ml) |
| Free testosterone | 4.5 pg/ml (< 2.7 pg/ml) |
| DHEA-S | 332 μg/dl (73-322 μg/dl) |
HOMA-IR: homeostasis model assessment for insulin resistance, DHEA-S:dehydroepiandrosterone sulfate. Reference intervals are shown in parentheses.
Fig. 2.
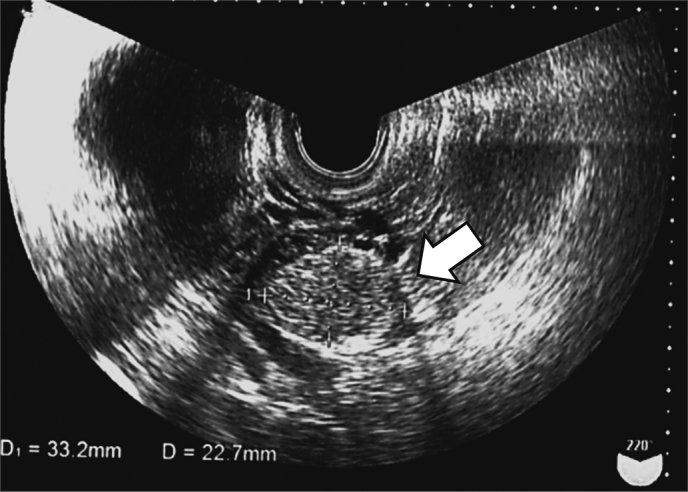
Transvaginal ultrasonography findings: Arrow indicates the ovarian tumor.
Fig. 3.
MRI findings: Arrows indicate the ovarian tumor.
Fig. 4.
Laparoscopic operative procedure: Images show laparoscopic resection of the left ovarian tumor (a, b, c, d, e, and f).
Fig. 5.
Pathological findings: Macroscopic appearance (A). Microscopic findings (B: low power field, C: high power field).
Table 2.
Immunoexpression of steroidogenic enzymes
| Positive expression | Negative expression |
| 3 β-HSD | P450scc |
| P450c17 | aromatase |
| 5 α-reductase1/2 | steroid sulfatase |
| 17 β-HSD5 | P450c21* |
| 17 β-HSD1 | DHEA-ST* |
| CYP11B1/2* |
HSD: hydroxysteroid dehydrogenase, scc: side chain cleavage, DHEA-ST: dehydroepiandrosterone sulfotransferase, CYP11B1: steroid 11 β-hydroxylase, CYP11B2: aldosterone synthase. *, adrenal steroidogenic enzymes.
Fig. 6.
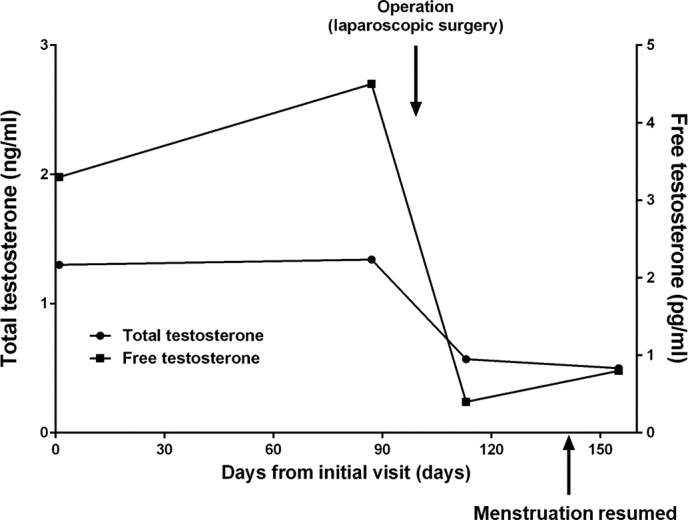
Chart of clinical course
Discussion
We encountered a case of a 22-year-old woman who presented with amenorrhea and virilization due to an androgen-producing ovarian tumor. Laparoscopic partial resection of the ovarian tumor was performed, and histopathological examination revealed ovarian SCT-NOS.
It is crucial to differentiate SCT-NOS from other types of steroid cell tumors, such as stromal luteoma or Leydig cell tumors. Leydig cell tumors are commonly present in a hilar location and have cytoplasmic Reinke crystals. Stromal luteoma is commonly located in the ovarian stroma and is associated with stromal hyperthecosis. In the present case, as pathological findings such as cytoplasmic Reinke crystals and stromal luteoma were absent, this case was diagnosed as SCT-NOS. Moreover, while immunohistochemical staining with inhibin and calretinin is useful to differentiate among other non-sex cord tumors4), staining for such tumors was not performed.
Although the majority of ovarian sex cord-stromal tumors, including ovarian SCT, are benign, ovarian SCT-NOS exhibits a malignant potential that has been reported in approximately one-third of all cases1,2). Further, ovarian SCT-NOS is classified as a borderline malignancy. Standard treatment for women with no pregnancy intention is hysterectomy and bilateral salpingo-oophorectomy with omentectomy. On the other hand, those women who desire future fertility can be treated with ipsilateral adnexectomy and omentectomy by laparotomy. In the present case, as she wanted to have a child, partial resection of the tumor was performed by laparoscopic surgery.
Laparoscopic surgery is a common procedure for benign ovarian lesions. Recently, there have been reports about laparoscopic surgery for ovarian tumors of borderline malignancy including SCT-NOS. As per our literature survey, there have been only 11 cases of SCT-NOS who underwent laparoscopic surgery5-16). Many patients undergoing laparoscopic surgery have opted for oophorectomy, and only three cases have been operated by partial resection5,7,10). Patients who were treated by laparoscopic partial resection were 30 years, 16 years, and 23 years old; all were considered to be in early stage of their disease. Although the follow-up period after surgery was within three years for those patients, there was no recurrence of the disease.
Since ovarian SCT-NOS is rare and standard treatments have not been established, treatment strategies need to be determined with reference to pathological risk factors. Hayes and Scully reported that poor prognostic factors for SCT-NOS are: tumor size > 7 cm, presence of 2 or more mitoses, presence of necrosis, hemorrhage, and grade 2 or 3 nuclear atypia1). In this case, according to the Hayes’s criteria, none of the risk factors were observed. Thus, the tumor was not considered to be aggressive. Furthermore, the patient had careful follow-up with no sign of recurrence even 5 years after surgery.
We evaluated the origin of increased serum testosterone levels by immunostaining steroidogenic enzymes. Ovarian steroidogenic enzymes, such as 3 β-HSD, P450c17, 5 α-reductase 1/2, 17 β HSD 5, and 17 β HSD 1, were significantly expressed in the tumor cells. Our result was consistent with a testosterone-synthesizing tumor. However, since the expression of P450scc was scarcely observed, it was unlikely that steroidogenesis was active in the tumor itself. Furthermore, as aromatase and steroid sulfatase were not expressed in the tumor, estrogen synthesis ability was considered to be low. These results suggested ovarian-derived hyperandrogenism with normal ovarian steroidogenesis. On the other hand, although cases showing Cushing’s syndrome had been reported in ovarian SCT-NOS1,17), adrenal-specific steroidogenic enzymes such as P450c21, DHEA-ST, and CYP11B1/2 were not found to be expressed in this case. This indicates that adrenocortical hormone was not being synthesized.
Differential diagnosis of female virilization is essential18). There is female virilization by androgen excess originating from the adrenal gland or ovary19). Adrenal hyperandrogenemia is caused by primary adrenal tumors, adrenocorticotropic hormone-producing tumors such as Cushing disease, or congenital adrenal hyperplasia. Conversely, ovarian hyperandrogenism is caused by PCOS or androgen-producing tumors of the ovary. In the present case, there were no findings of adrenal hyperandrogenism and no sign of polycystic ovary. Subsequently, we did find a solid ovarian tumor, which may produce androgens.
Clinical symptoms induced by steroid hormones that are derived from ovarian SCT-NOS are crucial for diagnosis. It has been reported that ovarian SCT-NOS synthesizes diverse steroid hormones such as androgen, estrogen, and cortisol1). The frequency of hyperandrogenism induced by the tumor is reported to be 56% to 77%1,20). Thus, most patients show clinical symptoms of virilization such as hirsutism, hoarseness, and frontal baldness1). Hyperandrogenism also affects ovarian follicle development and causes anovulation followed by amenorrhea1). There has been a report on primary amenorrhea with ovarian SCT-NOS in a 16-year old female patient7).
In this case, Cushing syndrome was potential candidate for differential diagnosis. The frequency of Cushing syndrome caused by cortisol production from ovarian SCT-NOS is reported to be around 10%1,20). Our present patient suffered from obesity, hypertension, and dyslipidemia, which met the criteria for metabolic syndrome and amenorrhea. Although these symptoms were consistent with Cushing syndrome, serum cortisol was found to be within the reference interval. Moreover, in the present study, we confirmed that none of the steroidogenic enzymes related to cortisol synthesis were expressed in the tumor. This result aligns with the average level of serum cortisol in the patient.
In summary, the ovarian SCT-NOS is a rare tumor that produces steroid hormones and causes virilization and menstrual irregularity. When patients are of reproductive age, fertility preservation should be considered, depending on the degree of malignancy. If fertility-sparing surgery is performed, patients must be followed-up carefully.
Disclosures
Conflicts of interest: The authors declare no conflict of interest.
Acknowledgements: We acknowledge Hironobu Sasano, a professor at Tohoku University Graduate School of Medicine, Department of Anatomic Pathology, for assisting with the immunobiological expression analysis of steroidogenic enzymes.
References
- 1.Hayes MC, Scully RE. Ovarian steroid cell tumors (not otherwise specified). A clinicopathological analysis of 63 cases. The American journal of surgical pathology, 11: 835-845, 1987. [DOI] [PubMed] [Google Scholar]
- 2.Kurman R, Carcangiu M, Herrington C, et al. WHO classification of tumours of female reproductive organs (World Health Organization classification of tumours). Lyon, France: International Agency for Research on Cancer, 2014. [Google Scholar]
- 3.Young RH. Sex cord-stromal, steroid cell, and other ovarian tumors with endocrine, paraendocrine, and paraneoplastic manifestations. Blaustein’s Pathology of the female Genital Tract: 785-846, 2011. [Google Scholar]
- 4.Zhao C, Vinh TN, McManus K, et al. Identification of the most sensitive and robust immunohistochemical markers in different categories of ovarian sex cord-stromal tumors. The American journal of surgical pathology, 33: 354-366, 2009. [DOI] [PubMed] [Google Scholar]
- 5.Alves P, Sa I, Brito M, et al. An Early Diagnosis of an Ovarian Steroid Cell Tumor Not Otherwise Specified in a Woman. Case reports in obstetrics and gynecology, 2019: 2537480, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sood N, Desai K, Chindris AM, et al. Symptomatic Ovarian Steroid Cell Tumor not Otherwise Specified in a Post-Menopausal Woman. Rare tumors, 8: 6200, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yokozawa T, Asano R, Nakamura T, et al. Steroid cell tumour, not otherwise specified: Rare case with primary amenorrhoea in a 16-year-old. Journal of obstetrics and gynaecology: the journal of the Institute of Obstetrics and Gynaecology, 35: 867-868, 2015. [DOI] [PubMed] [Google Scholar]
- 8.Chung DH, Lee SH, Lee KB. A case of ovarian steroid cell tumor, not otherwise specified, treated with surgery and gonadotropin releasing hormone agonist. Journal of menopausal medicine, 20: 39-42, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas TT, Ruscher KR, Mandavilli S, et al. Ovarian steroid cell tumor, not otherwise specified, associated with congenital adrenal hyperplasia: rare tumors of an endocrine disease. Journal of pediatric surgery, 48: E23-27, 2013. [DOI] [PubMed] [Google Scholar]
- 10.Jiang W, Tao X, Fang F, et al. Benign and malignant ovarian steroid cell tumors, not otherwise specified: case studies, comparison, and review of the literature. Journal of ovarian research, 6: 53, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chun YJ, Choi HJ, Lee HN, et al. An asymptomatic ovarian steroid cell tumor with complete cystic morphology: A case report. Obstetrics & gynecology science, 56: 50-55, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai HJ, Chen SC, Wei HY, et al. Hypothyroidism and hyperlipidemia with a virilizing ovarian steroid cell tumor, not otherwise specified. Gynecological endocrinology: the official journal of the International Society of Gynecological Endocrinology, 23: 69-71, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Liu AX, Sun J, Shao WQ, et al. Steroid cell tumors, not otherwise specified (NOS), in an accessory ovary: a case report and literature review. Gynecologic oncology, 97: 260-262, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Ashraf A, Abdul-Latif H, Hardin W, et al. Vaginal bleeding and galactorrhea in a child with ovarian steroid cell tumor. Endocrine practice: official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists, 11: 346-349, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Faraj G, Di Gregorio S, Misiunas A, et al. Virilizing ovarian tumor of cell tumor type not otherwise specified: a case report. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology, 12: 347-352, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Bettegowda A, Rangaiah N, Prasad N, et al. Virilizing Ovarian Steroid Cell Tumor: A Rare Case. Journal of clinical and diagnostic research: JCDR, 9: QD05-06, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zang L, Ye M, Yang G, et al. Accessory ovarian steroid cell tumor producing testosterone and cortisol: A case report. Medicine, 96: e7998, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azziz R. Differential diagnosis and evaluation of hyperandrogenism In: Handbook of diagnostic endocrinology. Springer; 2003: 323-330. [Google Scholar]
- 19.Rachon D. Differential diagnosis of hyperandrogenism in women with polycystic ovary syndrome. Exp Clin Endocrinol Diabetes, 120: 205-209, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Taylor HB, Norris HJ. Lipid cell tumors of the ovary. Cancer, 20: 1953-1962, 1967. [DOI] [PubMed] [Google Scholar]