Abstract
Objective
To investigate the effect of recombinant adenovirus-mediated HIF-1 alpha (HIF-1α) on the expression of vascular endothelial growth factor (VEGFA) and HIF-1α in hypoxic brain microvascular endothelial cells (BMEC) in rats.
Methods
Primary cultured rat BMEC in vitro were treated without or with either recombinant adenovirus-mediated hypoxia-inducible factor-1 alpha (AdHIF-1α) or recombinant adenovirus empty vector (Ad) in the presence of CoCl2 (simulating hypoxia conditions), or were grown under normoxia conditions. The expression of VEGFA and HIF-1α was analyzed at 12h, 24h, 48h and 72h incubation time, respectively. We also accessed a GEO dataset of stroke to analyze in vivo the alteration of HIF-1α and VEGFA expression, and the correlations between HIF-1α, VEGFA and CD31 mRNA levels in vascular vessels after stroke.
Results
VEGFA and HIF-1α expression were significantly higher in at each time point in the AdHIF-1α than other groups (p<0.05), whereas the Ad group and hypoxia group, showed no statistically significant difference (p>0.05). Moreover, VEGFA and HIF-1α levels were significantly higher in BMEC under hypoxia conditions than normoxia conditions (p <0.05). Both HIF-1α and VEGFA expression significantly increased after stroke in vivo with 1.30 and 1.57 fold-change in log2, respectively. There were significantly positive associations between HIF-1α, VEGFA and CD31 mRNA levels in vivo after stroke.
Conclusion
Hypoxia-induced HIF-1α and VEGFA expression in vascular vessels, and recombinant AdHIF-1α could up-regulate VEGFA, and enhance HIF-1ααlevels in BMEC in vitro, which may play an important role in the recovery of stroke.
Keywords: AdHIF-1α, brain microvascular endothelial cells, hypoxia, VEGFA, stroke
Introduction
Stroke ranks the second most common cause of death and a leading risk of disability in the world.1 Stroke is characteristic of insufficient blood supply to brain, and leads to the damage to brain tissue in the affected area. Local blood flow restoration and improvement are a key therapeutic target in the clinical management of stroke, and the induction of angiogenesis and the repair of cerebral vasculature play a critical role in long-term recovery of stroke.
Vascular endothelial growth factor (VEGF) is a member of growth factors. By binding to its receptor, VEGF exerts its mitogenic, angiogenic and neurogenic functions.2 It has been reported that VEGF expression increases in human brain tissue and circulating system after ischemic stroke in response to hypoxia condition.3,4 Over-expressed VEGF, in turn, stimulates the formation of new vessels to bypass the blocked vessels, which has been observed in both stroke patients and experimental animals.5,6 Delayed inhibition of VEGF signaling after stroke could improve functional recovery in animal models.7 However, endogenous VEGF seems insufficient to completely protect the brain from stroke. Several previous studies have shown promising angiogenic changes and functional outcome of stroke with exogenous administration of VEGF.8–10 A double-blind, placebo-controlled clinical trial showed that chronic myocardial ischemia patients who were treated with high-dose VEGF had a significant improvement in their angina class.11 Another Phase II clinical trial of chronic myocardial ischemia demonstrated a significant increase in myocardial perfusion in the Adenoviral-VEGF-treated patients.12
Hypoxia-inducible factor-1 (HIF-1) is a heterodimeric transcriptional factor, consisting of an inducible HIF-1α subunit and a constitutive HIF-1β subunit.13 HIF-1 is a master regulator in response to hypoxia, which regulates the expression of genes involved in a variety of physiological and biological processes such as angiogenesis, erythropoiesis, cell proliferation, apoptosis and energy production, facilitating cells’ adaptation to low oxygen microenvironments. In the animal models, it has been shown that the exposure of mice to low oxygen for 30 to 60 min leads to the upregulation of HIF-1 level in the brain.14 HIF-1 activation was demonstrated in mouse brains after systemic hypoxia for 1 hr.15 Stroka and colleagues16 reported that the expression of HIF-1α reached maximum after 5 hrs of continuous hypoxia, and then declined to basal levels after 12 hrs. Inactivation of HIF-1α accelerates brain injury in a mouse model of transient focal cerebral ischemia.17 In our previous animal model study, we have found that recombinant Adenovirus-mediated HIF-1α could significantly reduce ischemia-induced brain damage.18 However, the underlying mechanisms are yet to be characterized. Given that VEGF is one of direct transcriptional targets of HIF-1α,19 the administration of exogenous HIF-1α may be able to protect brain from hypoxia-induced injury by inducing VEGF expression. Thus in this study, we aimed to investigate the effect of recombinant adenovirus-HIF-1ααon VEGFA and HIF-1α expression using brain microvascular endothelial cells (BMEC) in vitro under hypoxia conditions, as well as the correlations between the mRNA levels of HIF-1α, VEGFA and CD31 (an angiogenesis marker) in vascular vessels in vivo after stroke using a publicly accessed dataset.
Materials and Methods
Animals and Primary Cultured Brain Microvascular Endothelial Cells (BMEC)
Newly-born male and female Sprague-Dawley (SD) rats (aging 24 h) were purchased from the Animal Research Centre of Guizhou Medical University (License number:SCXK (Qian) 2010–0003). The protocol for animal care and experiments was approved by the Institutional Animal Care and Use Committee (IACUC) of Guizhou Medical University according to the National Guidelines of China for the care and use of laboratory animals. After sacrificing the rats with anesthesia, the primary BMEC from rats were cultured in DMEM complete medium (GE Hyclone Laboratories Inc. USA) according to the methods described elsewhere with the minor modification.20,21 The cultures were incubated in a humidified incubator with 5% CO2 at 37ºC in vitro. The third generation cultured BMEC cells were characterized using the staining method with rabbit anti-mouse factor VIII antibody (Santa Cruz Biotechnology Inc, Dallas, TX, USA). All cell culture media were replaced every other day if not specially noted.
Hypoxia Model of BMEC
Cobalt chloride (CoCl2) (Sigma-Aldrich, USA) is a chemical agent widely used in in vitro cell lines to mimic hypoxia, since Co2+ can substitute Fe2+ in a heme protein, and has a low affinity to oxygen.22 The third generation BMEC were cultured to the eleventh day, and the medium were replaced by CoCl2-containing medium (100 µmol/L) for experiments.23
AdHIF-1α/Ad Construction
The recombinant adenoviral HIF-1α (AdHIF-1α) plasmid containing GFP cassette (obtained from Professor Tang Hong at the Chinese Academy of Science) was constructed as previously described elsewhere.18 Human embryonic kidney cells HEK-293 cells (ATCC® CRL-1573TM) (purchased from ATCC, USA) were used as host cells for adenovirus infection to package the recombinant AdHIF-1α. The AdHIF-1α virus titer was calculated based on the formula as follows: AdHIF-1α virus titer (pfu/mL) = GFP positive cell counts (pfu) × supernatant dilution factor/0.2 mL. AdHIF-1ααviruses were harvested as previously described elsewhere.18
Transfection of Hypoxia BMEC with AdHIF-1α/Ad
The third generation of BMEC (1 x 106/mL) in DMEM complete medium were seeded into each well of 6-well plates, and incubated for 11 days in a humidified incubator with 5% CO2 at 37ºC. Then, we treated the cell cultures under four different conditions.1 Normoxia control group: the cells were maintained in DMEM complete medium containing 2% fetal bovine serum;2 Hypoxia group: the cells were treated with CoCl2 (100 µmol/L);3 AdHIF-1α group: after 24 h-treatment of the cells with CoCl2 (100 µmol/L), the AdHIF-1α/Ad was added to the cells based on MOI 35;4 Ad group (empty group): the adenovirus (without AdHIF-1α) only was added to the 24 h CoCl2 (100 µmol/L)-treated cells. All cell cultures were incubated in a humidified incubator with 5% CO2 at 37ºC.
VEGFA and HIF-1α Expression
The cultured BMEC cells under each condition were harvested at 12-, 24-, 48- and 72-h post-transfection to prepare slides for the determination of VEGFA and HIF-1α expression using immunohistochemistry (IHC) analysis or to extract proteins for VEGFA expression measurement using Western Blot. VEGFA and HIF-1α antibodies (1:100) were purchased from Santa Cruz Biotechnology Inc (Dallas, TX, USA). In each assay, the negative control of PBS in replacement of the antibodies was used.
The color development of IHC slides was performed using freshly prepared 3, 3ʹ-diaminobenzidine (DAB). Images were taken using biomedical image analysis system, and 10 high-resolution visual fields each IHC slide were randomly selected for the optical density measurement.
In the Western blot assay, a total amount of approximately 10 μg protein from each sample, which was determined by using the BCA method (Thermo Fisher Scientific Inc., CA, USA) following the manufacturer’s instruction, was loaded into each well of SDS-PAGE gel. After the electrophoresis, the proteins were transferred to NC membrane, which was then incubated in 5% skimmed milk blocking solution containing VEGFA antibodies (1:100). Horseradish peroxidase-conjugated goat anti-rabbit IgG (1:500) was used. The color intensity was determined using image-pro-plus 6.0 software. β-actin was used as an internal control.
RT-PCR Analysis
Total RNA was extracted from each group, and cDNA was prepared using reverse transcriptase kit (ThermoFisher Scientific Inc.). The primer sequences are as follows: β-actin (as a reference), forward 5ʹ-ATC CGT AAA GAC CTC TAT GCC AAC A; reverse 5ʹ- GCT AGG AGC CAG GGC AGT AAT C, and the PCR product size is 105 bp. VEGFA: forward 5ʹ- GGA CTT GAG TTG GGA GGA GGA T; reverse 5ʹ- CAG GGA TGG GTT TGT CGT GTT, and the PCR product size is 188 bp. The PCR reaction conditions include: denature at 94 ºC for 7 min, and 28 cycles (for VEGFA) or 30 cycles (for β-actin) of denature at 94ºC for 45 s, annealing at 57.8ºC for 45 s, and extension at 72ºC for 2 min, and final extension at 72ºC for 7 min. After the completion of PCR reaction, the PCR products were analyzed by running 2% agarose gel electrophoresis. Images were taken with the gel image processing system, and the integral light density value was recorded. The relative VEGFA expression (%) = (optical density of amplified VEGFA product/optical density of β-actin product) x 100%
Alteration of HIF-1α and VEGFA Expression After Stroke
We retrieved the publicly accessed data GSE13353 from GEO datasets (https://www.ncbi.nlm.nih.gov) to examine the alteration of HIF-1α, VEGFA and CD31 expression in vivo after stroke. This dataset was generated from a whole-genome expression array analysis on 11 ruptured saccular intracranial aneurysm (a devastating form of stroke) wall samples, which were resected at a median of 15 hrs after rupture, and 8 unruptured ones. The data were analyzed using GEO2R for differential expression between ruptured vs unruptured wall samples.
Statistical Analysis
An ANOVA model was used to compare the differences in the means between multiple groups with Bonferroni post hoc pair comparisons for groups at each time point using SPSS13.0. The values presented in the tables are expressed as mean (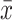 ) ± standard deviation (s). Differential expression of whole-genome expression array data was analyzed using GEO2R of R package. All p values <0.05 at two-tailed were considered statistically significant.
) ± standard deviation (s). Differential expression of whole-genome expression array data was analyzed using GEO2R of R package. All p values <0.05 at two-tailed were considered statistically significant.
Results
Characterization of Primary BMEC
Figure 1 is representative images of primary BMEC. The morphology of fresh primary BMEC displayed a single branch, or branched. After one-day culture, most of the microvascular segments and individual cells have attached the plates, and the edges of the microvascular segments grow into a single endothelial cell with the typical triangular or long spindle-shaped appearance (Figure 1A). The cells were arranged randomly with pale nucleus and clear cell membrane. The scattered cell colonies were formed after 3-4d culture and proliferation. Until 10–13 days, they formed a monolayer of tightly packaged, non-overlapping longitudinally aligned cells (Figure 1B). A high purity of over 95% of the cells reached as demonstrated by vascular endothelial cell marker factor VIII (Figure 1C).
Figure 1.
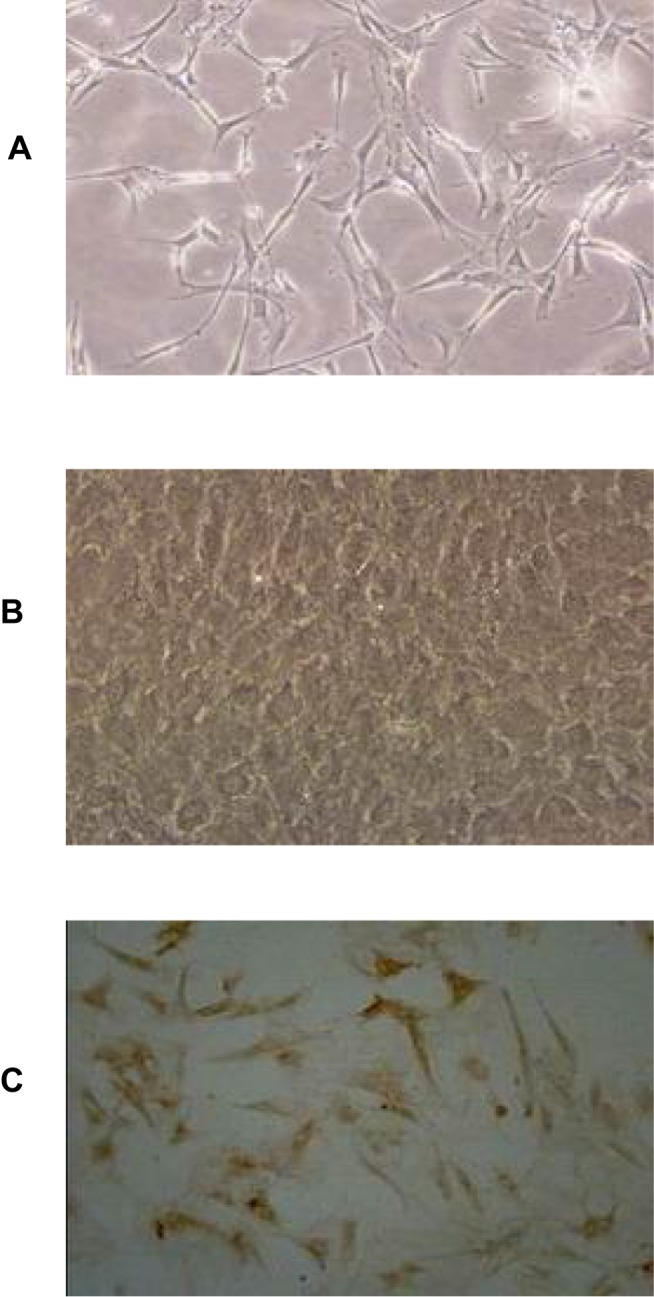
Morphology of primary BMEC. (A) Representative image of 1-day primary cultured BMEC under light microscope (×100), (B) Representative image of 10–13d primary cultured BMEC under light microscope (×100), (C) Immunohistochemistry staining for vascular endothelial cell marker factor VIII under light microscope (×100).
AdHIF-1α Increases VEGFA and HIF-1α Expression in BMEC Under Hypoxia Conditions
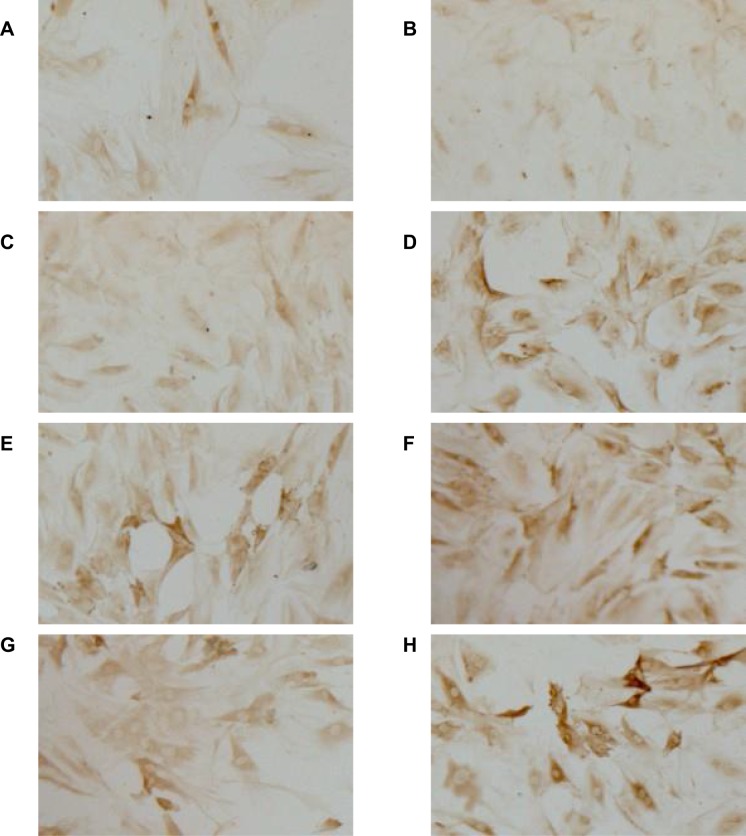
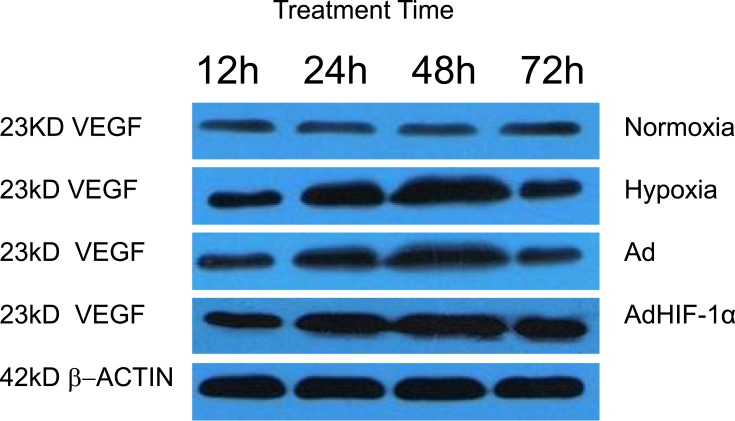
To examine whether recombinant adHIF-1α can increase VEGFA expression in BMEC under hypoxia conditions, we treated the BMEC with AdHIF-1α in the presence of CoCl2 in the medium. Figure 2 shows the representative images of IHC staining, and Table 1 is the level of VEGFA expression in each group at each time point. The results showed that hypoxia could significantly induce VEGFA expression in BMEC compared to the normoxia. In the period of 3-day observation, the level of VEGFA expression remained stable in the normoxia group, whereas VEGFA expression kept increasing until 48 h, at which time point, the VEGFA level reached peak under hypoxia conditions. At each time point, the AdHIF-1α group showed a significantly higher VEGFA expression compared to the other three (p<0.05). However, there was no significant difference in VEGFA expression between the hypoxia group and the Ad group (p >0.05). Western blot analysis (Figure 4 and Table 5) shows a similar change pattern and the differences of VEGFA expression in BMEC in the time course across the groups as observed in the IHC results.
Figure 2.
Immunohistochemistry staining for VEGFA protein in BMEC. Representative light microscopy images (×100) of the effect of AdHIF-1α on VEGFA protein level in BMEC at 12 h (A) and 48 (B) in the normoxia group, at 12 h (C) and 48 h (D) in the hypoxia group, at 12 h (E) and 48 h (F) in the AdHIF-1α group, and at 12 h (G) and 48 h (H) in the Ad group, respectively.
Table 1.
Effect of Hypoxia and AdHIF-1α on VEGFA Protein in BMEC in Four Groups (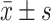 )
)
| Group | n | VEGFA Protein | |||
|---|---|---|---|---|---|
| 12h | 24h | 48h | 72h | ||
| Normoxia | 6 | 0.21±0.02a* | 0.23±0.04a | 0.22±0.03a | 0.21±0.03a |
| Hypoxia | 6 | 0.36±0.03b | 0.82±0.06b | 1.17±0.16b | 0.41±0.03b |
| Ad | 6 | 0.38±0.02b | 0.81±0.07b | 1.19±0.18b | 0.39±0.04b |
| AdHIF-1α | 6 | 0.53±0.06c | 0.97±0.11c | 1.46±0.23c | 0.66±0.04c |
Note: *At the same time point, the different letters indicate statistically significant (p<0.05), whereas the same letters indicate no significant difference in VEGF expression between groups (p>0.05).
Figure 4.
Representative Western Blot images of VEGFA expression in BMEC.
Table 5.
Western Blot Results of VEGFA Expression in BMEC Among Four Groups (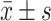 )
)
| Group | n | VEGFA Expression | |||
|---|---|---|---|---|---|
| 12h | 24h | 48h | 72h | ||
| Normoxia | 6 | 0.20±0.03a* | 0.20±0.02a | 0.21±0.02a | 0.23±0.02a |
| Hypoxia | 6 | 0.35±0.03b | 0.54±0.03b | 0.82±0.04b | 0.36±0.02b |
| Ad | 6 | 0.33±0.04b | 0.57±0.03b | 0.79±0.05b | 0.39±0.03b |
| AdHIF-1α | 6 | 0.63±0.03d | 0.74±0.03d | 1.19±0.06d | 0.69±0.03d |
Note: *At the same time point, the different letters indicate statistically significant (p<0.05), whereas the same letters indicate no significant difference in VEGF expression between groups (p>0.05).
Again, at the mRNA level, RT-PCR results showed the similar results of the differences in VEGFA expression between the groups (Table 2).
Table 2.
Effect of Hypoxia and AdHIF-1α on VEGFA mRNA Level in BMEC ( )
)
| Group | n | VEGFA mRNA Level (%) | |||
|---|---|---|---|---|---|
| 12h | 24h | 48h | 72h | ||
| Normoxia | 6 | 20.87±2.04a* | 20.89±1.39a | 20.02±1.43a | 20.04±1.82a |
| Hypoxia | 6 | 24.37±2.35b | 28.09±1.62b | 34.02±2.37b | 26.12±1.03b |
| Ad | 6 | 24.32±2.32b | 29.41±1.93b | 36.78±2.71b | 27.23±1.53b |
| AdHIF-1α | 6 | 28.15±2.67ce | 36.46±2.48df | 48.32±2.58df | 33.36±1.89df |
Note: *At the same time point, the different letters indicate statistically significant (p<0.05), whereas the same letters indicate no significant difference in VEGFA expression between groups (p>0.05).
Table 3 shows the levels of HIF-1α expression in each group at each time point. The results showed that hypoxia could significantly induce HIF-1α expression in BMEC compared to the normoxia. In the period of 3-day observation, the level of HIF-1α expression remained stable in the normoxia group, whereas HIF-1α expression kept increasing and reached peak until 48 h under hypoxia conditions. At each time point, the AdHIF-1α group showed a significantly higher HIF-1α expression compared to the other three (p<0.05). However, there was no significant difference in HIF-1α expression between the hypoxia group and the Ad group (p >0.05).
Table 3.
Effect of Hypoxia and AdHIF-1α on HIF-1α Protein in BMEC in Four Groups (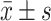 )
)
| Group | N | HIF-1α (OD Ratio) | |||
|---|---|---|---|---|---|
| 12h | 24h | 48h | 72h | ||
| Normoxia | 6 | 0.23±0.02a* | 0.25±0.01a | 0.23±0.03a | 0.24±0.04a |
| Hypoxia | 6 | 0.27±0.03b | 0.33±0.03b | 0.37±0.02b | 0.30±0.03b |
| Ad | 6 | 0.27±0.02b | 0.32±0.02b | 0.36±0.04b | 0.29±0.02b |
| AdHIF-1α | 6 | 0.30±0.04c | 0.37±0.02c | 0.45±0.03c | 0.35±0.03c |
Note: *At the same time point, the different letters indicate statistically significant (p<0.05), whereas the same letters indicate no significant difference in VEGFA expression between groups (p>0.05).
Up-Regulated HIF-1α and VEGFA Expression After Stroke
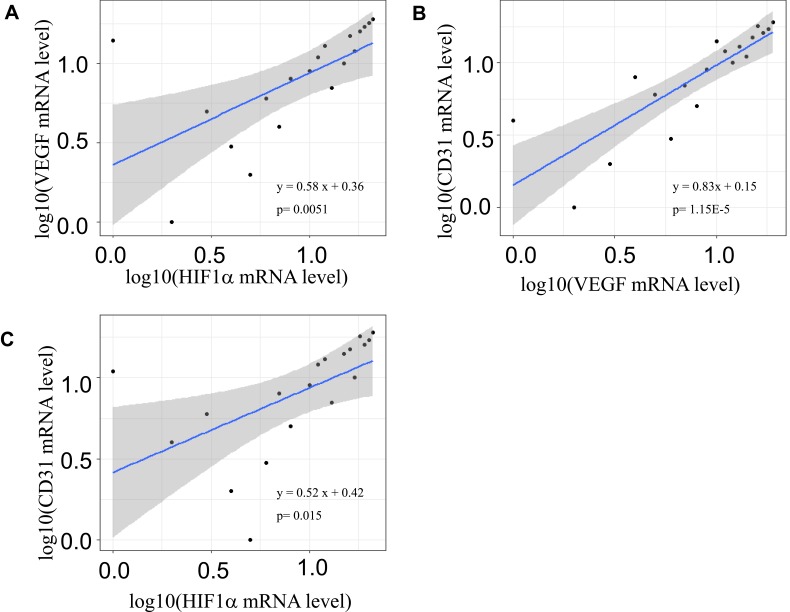
Table 4 shows the differential expression of HIF-1ααand VEGF in ruptured vascular walls vs unruptured ones. Both HIF-1α and VEGFA expression significantly increased in vascular walls at a median of 15 hrs after stroke. The fold-changes in log were 1.30 for HIF-1α and 1.57 for VEGFA, respectively at approximately 15 hrs after stroke, and p values were 5.50E-7, and 0.0018, respectively. Again, Figure 3 shows the correlations between the mRNA levels in log10 of HIF-1α, VEGFA and CD31 (a marker of angiogenesis). There were significantly positive associations between three markers in vascular cells after stroke. The parameters (β) in log10 were 0.58 for HIF-1α and VEGFA (p = 0.0051), 0.83 for VEGFA and CD31 (p = 1.15E-5), and 0.52 for HIF-1α and CD31 (p = 0.015), respectively.
Table 4.
Differential Expression of HIF-1α and VEGFA in Ruptured vs Unruptured Vessels
| Gene | Ruptured vs Unruptured Vascular Walls | |||
|---|---|---|---|---|
| Log (FC) | p- value | FDR | Probe-ID | |
| HIF-1a | 1.30 | 5.50E-07 | 0.00602 | 200989_at |
| VEGFA | 1.57 | 0.0018 | 0.05435 | 210513_s_at |
Abbreviations: log(FC), fold-change in log2; FDR, false discovery rate.
Figure 3.
Scatter-plots of HIF-1α, VEGFA and CD31 mRNA levels in vascular walls after stroke. A significantly positive association between HIF1α and VEGFA mRNA levels in log10 (p=0.0051) (A), and between CD31 and VEGFA mRNA levels in log10 (p = 1.15E-5) (B), and between CD31 and HIF1α mRNA levels in log10 (p = 0.015) (C). The regression line is in blue and 95% confidence intervals are in grey shadow.
Discussion
In this study, we demonstrated the effect of AdHIF-1α on VEGFA and HIF-1α expression in primary BMEC in vitro under hypoxia conditions. As expected, when the cells were exposed to hypoxia conditions, VEGFA and HIF-1α expression significantly increase with the time, reaching the peak at 48 h after the hypoxia. The addition of recombinant AdHIF-1α significantly augmented the production of VEGFA and enhanced the levels of endogenous HIF-1α in BMEC. These findings are consistent with the proof-of-concept coined in the previous studies,3,4 indicating that the recombinant AdHIF-1α has the similar activities as endogenous HIF-1α in stimulating VEGF expression, an angiogenesis factor in vessel remodeling after stroke.
VEGF is a glycoprotein, containing eight conserved cysteine residues sequence,24 and is a member of the super-family growth factors.25,26 In the healthy adult mammalian brain, VEGF expression maintains at a low level to stabilize mature vessels.27 However, VEGF and its receptors VEGFR-1 and VEGFR-2, which are expressed on neurons, glial and vascular endothelial cells, are upregulated under hypoxia conditions to protect brain from insufficient oxygen supply-induced injury.3,28 The response of VEGF expression in response to stroke could start as early as 2 to 4 h after the onset, and last for almost a month.29 Biphasic increase of VEGF expression after stroke was observed in a previous study,30 which showed the first peak of VEGF increase at 6 h and the second one at 7 days after reperfusion. This response is not limited to the stroke and penumbra area only, where VEGF expression is predominately increased though, and can also spread to the function- and behavior-related cortical areas.31 In addition, VEGF-induced angiogenesis may alter the dynamic of blood flow, which is reduced in the ischemic areas while increased in the areas outside of the lesion,32 thereby protecting neurons from ischemic cell death directly or indirectly via the formation of neo-vessels.
VEGF is pro-angiogenic and neuroprotective. It has been shown that VEGF can increase the permeability of venules and small veins, and promote the angiogenesis by stimulating the growth of vascular endothelial cells.33,34 In the early stage of cerebral ischemia, the increase of VEGF expression and neo-vessels was observed.35,36 Via binding to VEGFR-1/2, VEGF significantly increases the number of CD31+ vessels in an in vivo angiogenesis model.37 Similarly, after the vessel rupture-caused stroke, the findings in this study that both the HIF-1α and VEGFA mRNA levels increased and are positively associated with CD31 mRNA levels suggest that hypoxia leads to HIF-1a, VEGFA expression and consequently angiogenesis initiation. The transplantation of VEGF-over-expressing neural stem cells leads to angiogenesis and functional recovery in mouse stroke model.38 Dzietko et al reported that VEGF could promote angiogenesis to execute its neuroprotective effect.39 Bozayan et al showed that the astrocytes were able to utilize the VEGF signaling pathway to control the formation of vascular network.40 In turn, VEGF could induce the transdifferentiation of astrocytes into neurons, consequently stimulating neurogenesis,41 and this may explain the clinic phenomenon that neuron system injury gradually improves in stroke patients. Other studies showed that VEGF might have different effects on brain in the different stages of stroke.42,43 In animal stroke models, they found that the elevated level of VEGF at the early stage increased the permeability of blood-brain barrier, whereas at the late stage, VEGF stimulated angiogenesis and promoted the nervous system repair by activating the PI3K signaling pathway, which is critical in the angiogenic process. Moreover, in vitro cell line experiments showed neuronal cells survived hypoxia in the presence of VEGF,44 maintaining its functions once neo-vessels are remodeled. In in vivo animal stroke models, local application of VEGF significantly decreased the hypoxia-induced brain damage, including infarct volume and edema formation.45 In contrast, the intraventricular application of anti-VEGF antibody increased the ischemia-induced brain lesion.46 VEGF-overexpressing transgenic mice showed an increase of both neurogenesis and the migration of newly-formed neurons to the peri-infarcted cortex.47
In stroke, HIF-1 is a critical transcription factors in the hypoxic brain, triggering the downstream genes involved in angiogenesis and neurogenesis, as well as differentiation of stem cells.48 Under normoxia conditions, the turnover of HIF-1 is quick, which is rapidly degraded by ubiquitinylation. In contrast, the stabilization of HIF-1 is enhanced under hypoxia conditions, resulting in an increase of HIF-1 in hypoxic brain.49 VEGF is one of the well-documented downstream genes of HIF-1. The response of HIF-1 to hypoxia and consequently the increase of VEGF facilitates the cells’ adaptation to hypoxia,50 and to counteract ischemia-induced brain damage.28,44 A previous report demonstrated that the proline hydroxylase inhibitor-4 (PHI-4) could reduce ischemia-induced neuron apoptosis by increasing HIF-1α and its target gene VEGF in animal models.51 In line with these findings, we found that VEGFA and HIF-1α significantly increased both in vivo after stroke and in in vitro BMEC under hypoxia conditions, and that the addition of recombinant AdHIF-1α could increase VEGFA and HIF-1α expression in BMEC in vitro in this study. However, since VEGF is a vascular permeability factor, causing capillary permeability that occurs first and precedes the formation of neo-vessel in vessel remodeling, it is a trade-off between vessel permeability and neo-vessel formation. Particularly neo-vessel formation can efficiently ameliorate permeability-caused edema and improve the supply of oxygen and nutrients. In future studies, it will be warranted to in vivo investigate the optimal dose of recombinant AdHIF-1α as a therapy of stroke.
In summary, we used the primary BMEC cells to first demonstrate the effect of recombinant AdHIF-1α on VEGFA and HIF-1α expression in vitro, and as expected, we found that hypoxia conditions led to the increase of HIF-1ααand VEGFA, and that recombinant AdHIF-1α could significantly increase VEGFA in endogenous HIF-1α-manner and HIF-1α expression in BMEC under hypoxia conditions. Hemorrhage-caused stroke led to significantly increased HIF-1α and VEGFA in vascular vessels. A significantly positive association exists between HIF-1α, VEGFA and CD31 mRNA levels. These findings suggest that the delivery of recombinant AdHIF-1ααcan promotes VEGFA expression via enhancing the levels of HIF-1ααunder hypoxia conditions, consequently may help stroke-induced damage recovery.
Acknowledgments
We thank Professor Tang Hong from the Institute Pasteur of Shanghai, Chinese Academy of Sciences for his gift of recombinant adenovirus plasmids.
Funding Statement
Guizhou Province Outstanding Scholars and Talents Fund Project 2006-60, Guizhou Science and Technology Fund Project 2007-2096, Guizhou Province Department of Health Science and Technology Fund Project 2014-41.
Disclosure
The authors report grants from Guizhou Province Outstanding Scholars and Talents Fund Project 2006-60, grants from Guizhou Science and Technology Fund Project 2007-2096, grants from Guizhou Province Department of Health Science and Technology Fund Project 2014-41, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
- 1.Thrift AG, Thayabaranathan T, Howard G, et al. Global stroke statistics. Int J Stroke. 2017;12:13–32. doi: 10.1177/1747493016676285 [DOI] [PubMed] [Google Scholar]
- 2.Greenberg DA, Jin K. From angiogenesis to neuropathology. Nature. 2005;438:954–959. doi: 10.1038/nature04481 [DOI] [PubMed] [Google Scholar]
- 3.Margaritescu O, Pirici D, Margaritescu C. VEGF expression in human brain tissue after acute ischemic stroke. Rom J Morphol Embryol. 2011;52:1283–1292. [PubMed] [Google Scholar]
- 4.Matsuo R, Ago T, Kamouchi M, et al. Clinical significance of plasma VEGF value in ischemic stroke - research for biomarkers in ischemic stroke (REBIOS) study. BMC Neurol. 2013;13:32. doi: 10.1186/1471-2377-13-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liman TG, Endres M. New vessels after stroke: postischemic neovascularization and regeneration. Cerebrovasc Dis. 2012;33:492–499. doi: 10.1159/000337155 [DOI] [PubMed] [Google Scholar]
- 6.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215 [DOI] [PubMed] [Google Scholar]
- 7.Reeson P, Tennant KA, Gerrow K, et al. Delayed inhibition of VEGF signaling after stroke attenuates blood-brain barrier breakdown and improves functional recovery in a comorbidity-dependent manner. J Neurosci. 2015;35:5128–5143. doi: 10.1523/JNEUROSCI.2810-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang JP, Liu HJ, Liu XF. VEGF promotes angiogenesis and functional recovery in stroke rats. J Invest Surg. 2010;23:149–155. doi: 10.3109/08941930903469482 [DOI] [PubMed] [Google Scholar]
- 9.Sun Y, Jin K, Xie L, et al. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–1851. doi: 10.1172/JCI200317977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen F, Fan Y, Su H, et al. Adeno-associated viral vector-mediated hypoxia-regulated VEGF gene transfer promotes angiogenesis following focal cerebral ischemia in mice. Gene Ther. 2008;15:30–39. doi: 10.1038/sj.gt.3303048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henry TD, Annex BH, McKendall GR, et al. The VIVA trial: vascular endothelial growth factor in Ischemia for vascular angiogenesis. Circulation. 2003;107:1359–1365. doi: 10.1161/01.CIR.0000061911.47710.8A [DOI] [PubMed] [Google Scholar]
- 12.Hedman M, Hartikainen J, Syvanne M, et al. Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia: phase II results of the Kuopio Angiogenesis Trial (KAT). Circulation. 2003;107:2677–2683. doi: 10.1161/01.CIR.0000070540.80780.92 [DOI] [PubMed] [Google Scholar]
- 13.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiener CM, Booth G, Semenza GL. In vivo expression of mRNAs encoding hypoxia-inducible factor 1. Biochem Biophys Res Commun. 1996;225:485–488. doi: 10.1006/bbrc.1996.1199 [DOI] [PubMed] [Google Scholar]
- 15.Bernaudin M, Nedelec AS, Divoux D, MacKenzie ET, Petit E, Schumann-Bard P. Normobaric hypoxia induces tolerance to focal permanent cerebral ischemia in association with an increased expression of hypoxia-inducible factor-1 and its target genes, erythropoietin and VEGF, in the adult mouse brain. J Cereb Blood Flow Metab. 2002;22:393–403. doi: 10.1097/00004647-200204000-00003 [DOI] [PubMed] [Google Scholar]
- 16.Stroka DM, Burkhardt T, Desbaillets I, et al. HIF-1 is expressed in normoxic tissue and displays an organ-specific regulation under systemic hypoxia. FASEB J. 2001;15:2445–2453. doi: 10.1096/fj.01-0125com [DOI] [PubMed] [Google Scholar]
- 17.Baranova O, Miranda LF, Pichiule P, Dragatsis I, Johnson RS, Chavez JC. Neuron-specific inactivation of the hypoxia inducible factor 1 alpha increases brain injury in a mouse model of transient focal cerebral ischemia. J Neurosci. 2007;27:6320–6332. doi: 10.1523/JNEUROSCI.0449-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang ML, Tao T, Xu J, Liu Z, Xu D. Antiapoptotic effect of gene therapy with recombinant adenovirus vector containing hypoxia-inducible factor-1alpha after cerebral ischemia and reperfusion in rats. Chin Med J (Engl). 2017;130:1700–1706. doi: 10.4103/0366-6999.209909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Komatsu DE, Hadjiargyrou M. Activation of the transcription factor HIF-1 and its target genes, VEGF, HO-1, iNOS, during fracture repair. Bone. 2004;34:680–688. doi: 10.1016/j.bone.2003.12.024 [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Hu G, Zhang T. Isolation, culture and identification of rat cerebral cortex microvascular endothelial cells. Animal Husbandry Veterinary Med. 2008;70–73. [Google Scholar]
- 21.Frye CA, Patrick CW Jr. Isolation and culture of rat microvascular endothelial cells. In Vitro Cell Dev Biol Anim. 2002;38:208–212. doi: [DOI] [PubMed] [Google Scholar]
- 22.Wu D, Yotnda P. Induction and testing of hypoxia in cell culture. J Vis Exp. 2011;12(54):2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ciavarella C, Fittipaldi S, Pasquinelli G. CoCl2 administration to vascular MSC cultures as an in vitro hypoxic system to study stem cell survival and angiogenesis. Methods Mol Biol. 2016;1516:309–317. [DOI] [PubMed] [Google Scholar]
- 24.Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989;425:540–547. doi: 10.1016/j.bbrc.2012.08.021 [DOI] [PubMed] [Google Scholar]
- 25.Ciulla TA, Danis RP, Criswell M, Pratt LM. Changing therapeutic paradigms for exudative age-related macular degeneration: antiangiogenic agents and photodynamic therapy. Expert Opin Investig Drugs. 1999;8:2173–2182. doi: 10.1517/13543784.8.12.2173 [DOI] [PubMed] [Google Scholar]
- 26.Muller YA, Christinger HW, Keyt BA, de Vos AM. The crystal structure of vascular endothelial growth factor (VEGF) refined to 1.93 A resolution: multiple copy flexibility and receptor binding. Structure. 1997;5:1325–1338. doi: 10.1016/S0969-2126(97)00284-0 [DOI] [PubMed] [Google Scholar]
- 27.Maharaj AS, Saint-Geniez M, Maldonado AE, D’Amore PA. Vascular endothelial growth factor localization in the adult. Am J Pathol. 2006;168:639–648. doi: 10.2353/ajpath.2006.050834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marti HJ, Bernaudin M, Bellail A, et al. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am J Pathol. 2000;156:965–976. doi: 10.1016/S0002-9440(10)64964-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang ZG, Zhang L, Tsang W, et al. Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. J Cereb Blood Flow Metab. 2002;22:379–392. doi: 10.1097/00004647-200204000-00002 [DOI] [PubMed] [Google Scholar]
- 30.Zan L, Zhang X, Xi Y, et al. Src regulates angiogenic factors and vascular permeability after focal cerebral ischemia-reperfusion. Neuroscience. 2014;262:118–128. doi: 10.1016/j.neuroscience.2013.12.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stowe AM, Plautz EJ, Eisner-Janowicz I, et al. VEGF protein associates to neurons in remote regions following cortical infarct. J Cereb Blood Flow Metab. 2007;27:76–85. doi: 10.1038/sj.jcbfm.9600320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Kilic E, Kilic U, et al. VEGF overexpression induces post-ischaemic neuroprotection, but facilitates haemodynamic steal phenomena. Brain. 2005;128:52–63. doi: 10.1093/brain/awh325 [DOI] [PubMed] [Google Scholar]
- 33.Yu Y, Zhao Y, Chang X. Synthesis and antitumor activity of novel aryl urea derivatives as vascular endothelial growth factor receptor inhibitors. Pharm J Chin PLA. 2016;32:198–201. [Google Scholar]
- 34.Luo M, Wang Z, An Z. Research progress on human vascular endothelial growth factor. Chin J Gerontol. 2012;32:3835–3837. [Google Scholar]
- 35.SanGiovanni JP, Chen J, Sapieha P, et al. DNA sequence variants in PPARGC1A, a gene encoding a coactivator of the omega-3 LCPUFA sensing PPAR-RXR transcription complex, are associated with NV AMD and AMD-associated loci in genes of complement and VEGF signaling pathways. PLoS One. 2013;8:e53155. doi: 10.1371/journal.pone.0053155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chao HM, Chuang MJ, Liu JH, et al. Baicalein protects against retinal ischemia by antioxidation, antiapoptosis, downregulation of HIF-1alpha, VEGF, and MMP-9 and upregulation of HO-1. J Ocul Pharmacol Ther. 2013;29:539–549. doi: 10.1089/jop.2012.0179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park K, Amano H, Ito Y, et al. Vascular endothelial growth factor receptor-1 (VEGFR-1) signaling enhances angiogenesis in a surgical sponge model. Biomed Pharmacother. 2016;78:140–149. doi: 10.1016/j.biopha.2016.01.005 [DOI] [PubMed] [Google Scholar]
- 38.Lee SW, Moskowitz MA, Sims JR. Sonic hedgehog inversely regulates the expression of angiopoietin-1 and angiopoietin-2 in fibroblasts. Int J Mol Med. 2007;19:445–451. [PubMed] [Google Scholar]
- 39.Dzietko M, Derugin N, Wendland MF, Vexler ZS, Ferriero DM. Delayed VEGF treatment enhances angiogenesis and recovery after neonatal focal rodent stroke. Transl Stroke Res. 2013;4:189–200. doi: 10.1007/s12975-012-0221-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bozoyan L, Khlghatyan J, Saghatelyan A. Astrocytes control the development of the migration-promoting vasculature scaffold in the postnatal brain via VEGF signaling. J Neurosci. 2012;32:1687–1704. doi: 10.1523/JNEUROSCI.5531-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen SW, Duan CL, Chen XH, et al. Neurogenic effect of VEGF is related to increase of astrocytes transdifferentiation into new mature neurons in rat brains after stroke. Neuropharmacology. 2016;108:451–461. doi: 10.1016/j.neuropharm.2015.11.012 [DOI] [PubMed] [Google Scholar]
- 42.Moretti R, Pansiot J, Bettati D, et al. Blood-brain barrier dysfunction in disorders of the developing brain. Front Neurosci. 2015;9:40. doi: 10.3389/fnins.2015.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu KW, Yang P, Li SS, Liu CW, Sun FY. VEGF attenuated increase of outward delayed-rectifier potassium currents in hippocampal neurons induced by focal ischemia via PI3-K pathway. Neuroscience. 2015;298:94–101. doi: 10.1016/j.neuroscience.2015.04.015 [DOI] [PubMed] [Google Scholar]
- 44.Jin KL, Mao XO, Greenberg DA. Vascular endothelial growth factor: direct neuroprotective effect in in vitro ischemia. Proc Natl Acad Sci U S A. 2000;97:10242–10247. doi: 10.1073/pnas.97.18.10242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hayashi T, Abe K, Itoyama Y. Reduction of ischemic damage by application of vascular endothelial growth factor in rat brain after transient ischemia. J Cereb Blood Flow Metab. 1998;18:887–895. doi: 10.1097/00004647-199808000-00009 [DOI] [PubMed] [Google Scholar]
- 46.Bao WL, Lu SD, Wang H, Sun FY. Intraventricular vascular endothelial growth factor antibody increases infarct volume following transient cerebral ischemia. Zhongguo Yao Li Xue Bao. 1999;20:313–318. [PubMed] [Google Scholar]
- 47.Wang Y, Jin K, Mao XO, et al. VEGF-overexpressing transgenic mice show enhanced post-ischemic neurogenesis and neuromigration. J Neurosci Res. 2007;85:740–747. doi: 10.1002/(ISSN)1097-4547 [DOI] [PubMed] [Google Scholar]
- 48.Ferriero DM. Protecting neurons. Epilepsia. 2005;46(Suppl 7):45–51. doi: 10.1111/j.1528-1167.2005.00302.x [DOI] [PubMed] [Google Scholar]
- 49.Dery MA, Michaud MD, Richard DE. Hypoxia-inducible factor 1: regulation by hypoxic and non-hypoxic activators. Int J Biochem Cell Biol. 2005;37:535–540. doi: 10.1016/j.biocel.2004.08.012 [DOI] [PubMed] [Google Scholar]
- 50.Giaccia A, Siim BG, Johnson RS. HIF-1 as a target for drug development. Nat Rev Drug Discov. 2003;2:803–811. doi: 10.1038/nrd1199 [DOI] [PubMed] [Google Scholar]
- 51.Trollmann R, Richter M, Jung S, Walkinshaw G, Brackmann F. Pharmacologic stabilization of hypoxia-inducible transcription factors protects developing mouse brain from hypoxia-induced apoptotic cell death. Neuroscience. 2014;278:327–342. doi: 10.1016/j.neuroscience.2014.08.019 [DOI] [PubMed] [Google Scholar]