Abstract
Background
Indomethacin is used as standard therapy to close a patent ductus arteriosus (PDA) but is associated with reduced blood flow to several organs. Ibuprofen, another cyclo‐oxygenase inhibitor, may be as effective as indomethacin with fewer adverse effects.
Objectives
To determine the effectiveness and safety of ibuprofen compared with indomethacin, other cyclo‐oxygenase inhibitor(s), placebo, or no intervention for closing a patent ductus arteriosus in preterm, low‐birth‐weight, or preterm and low‐birth‐weight infants.
Search methods
We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL 2017, Issue 10), MEDLINE via PubMed (1966 to 30 November 2017), Embase (1980 to 30 November 2017), and CINAHL (1982 to 30 November 2017). We searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles for randomised controlled trials and quasi‐randomised trials.
Selection criteria
Randomised or quasi‐randomised controlled trials of ibuprofen for the treatment of a PDA in preterm, low birth weight, or both preterm and low‐birth‐weight newborn infants.
Data collection and analysis
Data collection and analysis conformed to the methods of the Cochrane Neonatal Review Group. We used the GRADE approach to assess the quality of evidence.
Main results
We included 39 studies enrolling 2843 infants.
Ibuprofen (IV) versus placebo: IV Ibuprofen (3 doses) reduced the failure to close a PDA compared with placebo (typical relative risk (RR); 0.62 (95% CI 0.44 to 0.86); typical risk difference (RD); ‐0.18 (95% CI ‐0.30 to ‐0.06); NNTB 6 (95% CI 3 to 17); I2 = 65% for RR and I2 = 0% for RD; 2 studies, 206 infants; moderate‐quality the evidence). One study reported decreased failure to close a PDA after single or three doses of oral ibuprofen compared with placebo (64 infants; RR 0.26, 95% CI 0.11 to 0.62; RD ‐0.44, 95% CI ‐0.65 to ‐0.23; NNTB 2, 95% CI 2 to 4; I2 test not applicable).
Ibuprofen (IV or oral) compared with indomethacin (IV or oral): Twenty‐four studies (1590 infants) comparing ibuprofen (IV or oral) with indomethacin (IV or oral) found no significant differences in failure rates for PDA closure (typical RR 1.07, 95% CI 0.92 to 1.24; typical RD 0.02, 95% CI ‐0.02 to 0.06; I2 = 0% for both RR and RD; moderate‐quality evidence). A reduction in NEC (necrotising enterocolitis) was noted in the ibuprofen (IV or oral) group (18 studies, 1292 infants; typical RR 0.68, 95% CI 0.49 to 0.94; typical RD ‐0.04, 95% CI ‐0.07 to ‐0.01; NNTB 25, 95% CI 14 to 100; I2 = 0% for both RR and RD; moderate‐quality evidence). There was a statistically significant reduction in the proportion of infants with oliguria in the ibuprofen group (6 studies, 576 infants; typical RR 0.28, 95% CI 0.14 to 0.54; typical RD ‐0.09, 95% CI ‐0.14 to ‐0.05; NNTB 11, 95% CI 7 to 20; I2 = 24% for RR and I2 = 69% for RD; moderate‐quality evidence). The serum/plasma creatinine levels 72 hours after initiation of treatment were statistically significantly lower in the ibuprofen group (11 studies, 918 infants; MD ‐8.12 µmol/L, 95% CI ‐10.81 to ‐5.43). For this comparison, there was high between‐study heterogeneity (I2 = 83%) and low‐quality evidence.
Ibuprofen (oral) compared with indomethacin (IV or oral): Eight studies (272 infants) reported on failure rates for PDA closure in a subgroup of the above studies comparing oral ibuprofen with indomethacin (IV or oral). There was no significant difference between the groups (typical RR 0.96, 95% CI 0.73 to 1.27; typical RD ‐0.01, 95% CI ‐0.12 to 0.09; I2 = 0% for both RR and RD). The risk of NEC was reduced with oral ibuprofen compared with indomethacin (IV or oral) (7 studies, 249 infants; typical RR 0.41, 95% CI 0.23 to 0.73; typical RD ‐0.13, 95% CI ‐0.22 to ‐0.05; NNTB 8, 95% CI 5 to 20; I2 = 0% for both RR and RD). There was low‐quality evidence for these two outcomes. There was a decreased risk of failure to close a PDA with oral ibuprofen compared with IV ibuprofen (5 studies, 406 infants; typical RR 0.38, 95% CI 0.26 to 0.56; typical RD ‐0.22, 95% CI ‐0.31 to ‐0.14; NNTB 5, 95% CI 3 to 7; moderate‐quality evidence). There was a decreased risk of failure to close a PDA with high‐dose versus standard‐dose of IV ibuprofen (3 studies 190 infants; typical RR 0.37, 95% CI 0.22 to 0.61; typical RD ‐ 0.26, 95% CI ‐0.38 to ‐0.15; NNTB 4, 95% CI 3 to 7); I2 = 4% for RR and 0% for RD); moderate‐quality evidence).
Early versus expectant administration of IV ibuprofen, echocardiographically‐guided IV ibuprofen treatment versus standard IV ibuprofen treatment, continuous infusion of ibuprofen versus intermittent boluses of ibuprofen, and rectal ibuprofen versus oral ibuprofen were studied in too few trials to allow for precise estimates of any clinical outcomes.
Authors' conclusions
Ibuprofen is as effective as indomethacin in closing a PDA. Ibuprofen reduces the risk of NEC and transient renal insufficiency. Therefore, of these two drugs, ibuprofen appears to be the drug of choice. The effectiveness of ibuprofen versus paracetamol is assessed in a separate review. Oro‐gastric administration of ibuprofen appears as effective as IV administration. To make further recommendations, studies are needed to assess the effectiveness of high‐dose versus standard‐dose ibuprofen, early versus expectant administration of ibuprofen, echocardiographically‐guided versus standard IV ibuprofen, and continuous infusion versus intermittent boluses of ibuprofen. Studies are lacking evaluating the effect of ibuprofen on longer‐term outcomes in infants with PDA.
Plain language summary
Ibuprofen for the treatment of patent ductus arteriosus in preterm or low‐birth‐weight (or both) infants
Review question
Is the use of ibuprofen compared with indomethacin, other cyclo‐oxygenase inhibitors, placebo, or no intervention for closing a patent ductus arteriosus (PDA) safe and effective for improving the rate of ductal closure and other important clinical outcomes in preterm or low‐birth‐weight (or both) infants?
Background
A common complication for very preterm (premature) or very small babies is PDA. PDA is an open vascular channel between the lungs and the heart. It should close after birth, but sometimes remains open because of the baby's immature stage of development. PDA can lead to life‐threatening complications. The usual treatment for PDA has been indomethacin, a medicine that will successfully close the PDA in the majority of babies, but can cause serious side effects such as reduced blood flow to several organs. Another option is the drug ibuprofen.
Study characteristics
We searched scientific databases for randomised controlled trials (clinical studies where people are randomly put into one of two or more treatment groups) in preterm infants (born at less than 37 weeks into pregnancy), low‐birth‐weight (weighing less than 2500 g) infants, or preterm and low‐birth‐weight infants with a PDA. The treatments were ibuprofen, indomethacin, another cyclo‐oxygenase inhibitor, placebo or no treatment. The evidence is current to 30 November 2017.
Key Results
This review of 39 trials (2843 infants) found that ibuprofen was as effective as indomethacin in closing a PDA, caused fewer transient side effects on the kidneys, and reduced the risk of necrotising enterocolitis, a serious condition that affects the gut. Whether ibuprofen confers any important long‐term advantages or disadvantages on development is not known. Additional long‐term follow‐up studies to 18 months of age and to the age of school entry are needed to decide whether ibuprofen or indomethacin is the drug of choice for closing a PDA.
Quality of Evidence: According to GRADE (a method to score the quality of the trials supporting each outcome), the quality of the evidence varied from very low to moderate but was moderate for the important outcomes of failure to close a PDA, need for surgical closure of the PDA, duration of ventilator support, necrotizing enterocolitis, oliguria and serum/plasma creatinine levels when we compared intravenous or oral ibuprofen with intravenous or oral indomethacin.
Summary of findings
for the main comparison.
| Intravenous ibuprofen compared with placebo for patent ductus arteriosus | ||||||
|
Patient or population: preterm infants with patent ductus arteriosus Settings: NICU Intervention: intravenous ibuprofen Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | IV ibuprofen | |||||
| Failure to close a patent ductus arteriosus (after 3 doses) | High risk population | RR 0.62 (0.44 to 0.86) | 206 (2) | ⊕⊕⊕⊝ moderate | Bias: there was unclear bias for random sequence generation and allocation concealment in the two included studies. The blinding of personnel was unclear in both studies and the blinding of outcome assessments was low risk in one study and unclear in the other study. We did not downgrade the evidence. Heterogeneity/consistency: we noted moderate heterogeneity (65%) for RR but no heterogeneity (0%) for RD. We downgraded the evidence by one step. Directness of evidence: studies were conducted in the target population. Precision: the confidence intervals around the point estimates for RR and RD were quite narrow Presence of publication bias: this category was not applicable as only two studies were included in the analysis. |
|
| 471 per 1000 | 294 per 1000 29 to 432 | |||||
| Necrotising enterocolitis | High risk population | RR 1.84 (0.87 to 3.90) | 264 (2) | ⊕⊕⊕⊝ moderate | Bias: the information about random sequence generation, allocation concealment, blinding of personnel and blinding of outcome assessments was unclear in one of the two studies. In the second study there were no concerns about these items. We did not downgrade the evidence. Heterogeneity/consistency: we noted high heterogeneity (77%) for RR and moderate heterogeneity (67%) for RD. We downgraded the evidence by one step. Directness of evidence: studies were conducted in the target population. Precision: the confidence intervals around the point estimates for RR and RD were quite narrow. Presence of publication bias: this category was not applicable as only two studies were included in the analysis. |
|
| 68 per 1000 | 129 per 1000 (119 to 139) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; IV: intravenous; NICU: Neonatal intensive care unit: RD: risk difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
2.
| Intravenous or oral ibuprofen compared with intravenous or oral indomethacin for patent ductus arteriosus | ||||||
|
Patient or population: preterm infants with patent ductus arteriosus Settings: NICU Intervention: intravenous or oral ibuprofen Comparison: intravenous or oral indomethacin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Indomethacin (IV or oral) | Ibuprofen (IV or oral) | |||||
| Failure to close a patent ductus arteriosus (PDA) (after single or 3 doses) | High risk population | RR 1.07 (0.92 to 1.24) | 1590 (24) | ⊕⊕⊕⊝ moderate | Bias: there was low risk of bias for random sequence generation in 7 of the studies and there was unclear risk in the remaining 17 studies.There was low risk of bias for allocation concealment in 13 studies, high risk of bias in one study and unclear risk in the remaining 10 studies. The blinding of personnel was adequate in three studies, unclear in six studies and there was high risk of bias in 15 studies. Blinding of outcome assessments was at low risk of bias in 11 studies, unclear in six studies and there was high risk of bias in seven studies. We downgraded the evidence by one step. Heterogeneity/consistency: there was no heterogeneity (0%) for either RR or for RD. Directness of evidence: studies were conducted in the target population. Precision: the confidence intervals around the point estimates for RR and RD were narrow. Presence of publication bias: the funnel plot was symmetric based on 24 studies. |
|
| 280 per 1000 | 305 per 1000 (0 to 708) | |||||
| Need for surgical closure of the PDA | High risk population | RR 1.06 (0.81 to 1.39) | 1275 (16) | ⊕⊕⊕⊝ moderate | Bias: there was low risk of bias for random sequence generation in seven of the studies and there was unclear risk in the remaining 9 studies.There was low risk of bias for allocation concealment in 10 studies, high risk of bias in one study and unclear risk in the remaining 5 studies. The blinding of personnel was adequate in three studies, unclear in two studies and there was high risk of bias in 11 studies. Blinding of outcome assessments was at low risk of bias in 9 studies, unclear in three studies and there was high risk of bias in four studies. We downgraded the evidence by one step. Heterogeneity/consistency: there was no heterogeneity (0%) for either RR or for RD. Directness of evidence: studies were conducted in the target population. Precision: the confidence intervals around the point estimates for RR and RD were narrow. Presence of publication bias: the funnel plot was symmetric based on 16 studies. |
|
| 135 per 1000 | 144 per 1000 (0 to 250) | |||||
| Duration of ventilator support (days) | The mean duration of ventilator support (days) ranged across control groups from 8 to 26 days | The mean duration of ventilator support (days) in the intervention groups was 2.35 days lower (3.71 to 0.99 days lower) | MD ‐2.35 (‐3.71 to ‐0.99) | 471 (6) | ⊕⊕⊕⊝ moderate | Bias: there was low risk of bias for random sequence generation in two of the studies and there was unclear risk in the remaining four studies.There was low risk of bias for allocation concealment in five studies, and unclear risk in one study. The blinding of personnel was adequate in two studies, and there was high risk of bias in four studies. Blinding of outcome assessments was at low risk of bias in four studies, and unclear in two studies. We downgraded the evidence by one step. Heterogeneity/consistency: there was no heterogeneity (19%) for MD. Directness of evidence: studies were conducted in the target population. Precision: the confidence intervals around the point estimates for RR and RD were narrow. Presence of publication bias: only 6 studies were included in the analysis so a funnel plot was not constructed. |
| Necrotising enterocolitis (any stage) | High risk population | RR 0.68 (0.49 to 0.94) | 1292 (18) | ⊕⊕⊕⊝ moderate | Bias: there was low risk of bias for random sequence generation in seven of the studies and there was unclear risk in the remaining 11 studies.There was low risk of bias for allocation concealment in eleven studies, high risk in one study and unclear risk in six studies. The blinding of personnel was adequate in two studies, and there was high risk of bias in 13 studies and an unclear risk of bias in three studies. Blinding of outcome assessments was at low risk of bias in ten studies, high risk of bias in five studies and unclear in three studies. We downgraded the evidence by one step. Heterogeneity/consistency: there was no heterogeneity (0%) for RR and RD. Directness of evidence: studies were conducted in the target population. Precision: the confidence intervals around the point estimates for RR and RD were narrow. Presence of publication bias: the funnel plot was symmetric based on 18 studies. |
|
| 111 per 1000 | 73 per 1000 (0 to 400) | |||||
| Oliguria (urine output < 1 mL/kg/hour) | High risk population | RR 0.28 (0.14 to 0.54) | 576 (6) | ⊕⊕⊕⊝ moderate | Bias: there was low risk of bias for random sequence generation in two of the studies and there was unclear risk in the remaining four studies.There was low risk of bias for allocation concealment in four studies, and unclear risk in two studies. The blinding of personnel was adequate in two studies, unclear in one study and there was high risk of bias in three studies. Blinding of outcome assessments was at low risk of bias in all six studies. We did not downgrade the evidence. Heterogeneity/consistency: there was no heterogeneity (24%) for RR and moderate for RD (69%). We downgraded the evidence by one step. Directness of evidence: studies were conducted in the target population. Precision: the confidence intervals around the point estimates for RR and RD were narrow. Presence of publication bias: only 6 studies were included in the analysis so a funnel plot was not constructed. |
|
| 124 per 1000 | 34 per 1000 (0 to 68) | |||||
|
Serum/plasma creatinine levels (μmol/L) 72 hours after treatment Normal values for male and female newborns 17.7 to 88.4 µmol/L |
The mean serum/plasma creatinine level ranged across control groups from 45.97 to 147.63 μmol/L | The mean serum/plasma creatinine level in the intervention groups was 8.12 μmol/L lower (‐10.81 to ‐ 5.43 lower) | MD ‐8.12 μmol/L (‐10.81 to ‐ 5.43) | 918 (11) | ⊕⊕⊝⊝ low | Bias: there was low risk of bias for random sequence generation in four of the studies and there was unclear risk in the remaining seven studies.There was low risk of bias for allocation concealment in seven studies and unclear risk in four studies. The blinding of personnel was adequate in two studies, there was high risk of bias in seven studies, and the risk of bias was unclear in two studies. Blinding of outcome assessments was at low risk of bias in six studies but there was high risk of bias in 5 studies. We downgraded the evidence by one step. Heterogeneity/consistency: there was high heterogeneity (83%) for MD. We downgraded the evidence by one step. Directness of evidence: studies were conducted in the target population. Precision: the confidence intervals around the point estimates for MD were narrow. Presence of publication bias: the funnel plot was symmetric based on 11 studies. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; IV: intravenous; MD: mean difference; NICU: Neonatal intensive care unit; RD: risk difference; RR: Risk Ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
3.
| Oral ibuprofen compared with intravenous or oral indomethacin for patent ductus arteriosus | ||||||
|
Patient or population: preterm infants with patent ductus arteriosus Settings: NICU Intervention: oral ibuprofen Comparison: intravenous or oral indomethacin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous or oral indomethacin | Oral ibuprofen | |||||
| Failure to close a patent ductus arteriosus (PDA) (after 3 doses) | High risk population | RR 0.96 (0.73 to 1.27) | 272 (8) | ⊕⊕⊝⊝ low |
Bias: there was low risk of bias for random sequence generation in two of the studies and there was unclear risk in the remaining six studies.There was low risk of bias for allocation concealment in 3 studies, high risk of bias in one study and unclear risk in four studies. The blinding of personnel was inadequate in seven studies and unclear in one study. Blinding of outcome assessments was good in two of the studies but with high risk of bias in six studies. We downgraded the evidence by two steps. Heterogeneity/consistency: we noted no heterogeneity (0%) for RR and RD. Directness of evidence: studies were conducted in the target population. Precision: the confidence interval around the point estimates for MD was quite narrow Presence of publication bias: only 8 studies were included in the analysis so a funnel plot was not constructed. |
|
| 386 per 1000 | 393 per 1000 (0 to 708) | |||||
| Need for surgical closure of the PDA | High risk population | RR 0.93 (0.50 to 1.74) | 174 (4) | ⊕⊕⊝⊝ low | Bias: there was low risk of bias for random sequence generation in two of the studies and there was unclear risk in two studies.There was low risk of bias for allocation concealment in one study, high risk of bias in one study and unclear risk in two studies. The blinding of personnel was inadequate in all four studies as was blinding of outcome assessments. We downgraded the evidence by two steps. Heterogeneity/consistency: we noted no heterogeneity (0%) for RR and RD. Directness of evidence: studies were conducted in the target population. Precision: the confidence interval around the point estimate for RR and RD was quite narrow. Presence of publication bias: only 4 studies were included in the analysis so a funnel plot was not constructed. |
|
| 188 per 1000 | 181 per 1000 (0 to250) | |||||
| Necrotising enterocolitis (any stage) | High risk population | RR 0.41 (0.23 to 0.73) | 249 (7) | ⊕⊕⊝⊝ low | Bias: there was low risk of bias for random sequence generation in two of the studies and there was unclear risk in the remaining five studies.There was low risk of bias for allocation concealment in 3 studies, high risk of bias in one study and unclear risk in three studies. The blinding of personnel was inadequate in six studies and unclear in one study. Blinding of outcome assessments was good in two of the studies but with high risk of bias in five studies. We downgraded the evidence by two steps. Heterogeneity/consistency: we noted no heterogeneity (0%) for RR and RD. Directness of evidence: studies were conducted in the target population. Precision: the confidence interval around the point estimates for MD was quite narrow. Presence of publication bias: only 7 studies were included in the analysis so a funnel plot was not constructed. |
|
| 224 per 1000 | 83 per 1000 (0 to 400) | |||||
|
Serum/plasma creatinine levels (µmol/L) 72 hours after treatment Normal values for male and female newborns 17.7 to 88.4 µmol/L |
The mean serum/plasma creatinine levels ranged across control groups from 45.97 to 106.08 µmol/L | The mean serum/plasma creatinine level in the intervention groups was 0.51 µmol/L (‐6.04 to 5.01) | MD ‐0.51 (‐6.04 to 5.01) | 190 (5) | ⊕⊝⊝⊝ very low | Bias: there was low risk of bias for random sequence generation in one of the studies and there was unclear risk in the remaining 4 studies.There was low risk of bias for allocation concealment in 3 studies, and unclear risk in two studies The blinding of personnel was inadequate in four studies and unclear in one study. Blinding of outcome assessments was good in one of the studies but with high risk of bias in four studies. We downgraded the evidence by two steps. Heterogeneity/consistency: we noted moderate heterogeneity (72%) for MD. We downgraded the evidence by one step. Directness of evidence: studies were conducted in the target population. Precision: the confidence interval around the point estimates for MD was quite narrow. Presence of publication bias: only 5 studies were included in the analysis so a funnel plot was not constructed. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: Mean difference; RR: Risk Ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
4.
| Oral ibuprofen compared with intravenous ibuprofen for patent ductus arteriosus | ||||||
|
Patient or population: preterm infants with patent ductus arteriosus Settings: NICU Intervention: oral ibuprofen Comparison: intravenous ibuprofen | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| intravenous ibuprofen | oral ibuprofen | |||||
| Failure to close a patent ductus arteriosus (after single or 3 doses) | High risk population | RR 0.38 (0.26 to 0.56) | 406 (5) | ⊕⊕⊕⊝ moderate | Bias: there was unclear risk of bias for random sequence generation in all 5 studies. There was low risk of bias for allocation concealment in 4 studies, and unclear risk in one study. The blinding of personnel was inadequate in all five studies and blinding of outcome assessments was good in three studies but inadequate in two studies. We downgraded the evidence by one step. Heterogeneity/consistency: we noted no heterogeneity for RR and for RD (0%). Directness of evidence: studies were conducted in the target population. Precision: the confidence intervals around the point estimates for RR and RD were quite narrow. Presence of publication bias: only 5 studies were included in the analysis so a funnel plot was not constructed. |
|
| 363 per 1000 | 139 per 1000 (115 to 156) | |||||
| Need for surgical closure of the ductus | High risk population | RR 0.41 (0.41 to 1.21) | 406 (5) | ⊕⊕⊕⊝ moderate | Bias: there was unclear risk of bias for random sequence generation in all 5 studies. There was low risk of bias for allocation concealment in 4 studies, and unclear risk in one study. The blinding of personnel was inadequate in all five studies and blinding of outcome assessments was good in three studies but inadequate in two studies We downgraded the evidence by one step. Heterogeneity/consistency: we noted no heterogeneity for RR and for RD (0%). Directness of evidence: studies were conducted in the target population. Precision: the confidence intervals around the point estimates for RR and RD were quite narrow. Presence of publication bias: only 5 studies were included in the analysis so a funnel plot was not constructed. |
|
| 51 per 1000 | 19 per 1000 (0 to 31) | |||||
| Duration of ventilatory support | High risk population | MD 0.54 (days) (‐0.01 to 1.10) | 134 (2) |
⊕⊕⊝⊝ low | Bias: there was unclear risk of bias for random sequence generation in both studies. There was low risk of bias for allocation concealment in one study, and unclear risk in one study. The blinding of personnel was inadequate in both studies and blinding of outcome assessments was good in one study but inadequate in two one study. We downgraded the evidence by one step. Heterogeneity/consistency: we noted no heterogeneity for MD (10%). Directness of evidence: studies were conducted in the target population. Precision: the confidence intervals around the point estimates for RR and RD were quite wide. We downgraded the evidence by one step. Presence of publication bias: only two studies were included in the analysis so a funnel plot was not constructed. |
|
| The mean duration of ventilatory support (days) ranges across control groups from 3 to 5.1 days | The mean duration of ventilatory support was 0.54 (days) higher in the in the oral ibuprofen group (‐0.01 to 1.10) | |||||
|
Serum/plasma creatinine levels (μmol/L) after treatment Normal values for male and female newborns 17.7 to 88.4 µmol/L |
The mean serum/plasma creatinine levels ranged across control groups from 69.84 to 76.02 (μmol/L) | The mean serum/plasma creatinine level in the intervention groups was 22.47 (μmol/L) lower (‐32.40 to ‐12.53) | MD 22.47 (μmol/L) (‐32.40 to ‐12.53) | 170 (2) | ⊕⊕⊝⊝ low | Bias: there was unclear risk of bias for random sequence generation in both studies. There was low risk of bias for allocation concealment in one study, and unclear risk in one study. The blinding of personnel was inadequate in both studies and blinding of outcome assessments was good in one study but inadequate in the other study. We downgraded the evidence by one step. Heterogeneity/consistency: there was high heterogeneity for MD (81%). We downgraded the evidence by one step. Directness of evidence: studies were conducted in the target population. Precision: the confidence intervals around the point estimates for MD were quite narrow. Presence of publication bias: only 2 studies were included in the analysis so a funnel plot was not constructed. |
| Oliguria (Urine output < 1 mL/kg/hour) | High risk population | RR 0.14 (0.01 to 2.66) | 304 (4) | ⊕⊕⊝⊝ low | Bias: there was unclear risk of bias for random sequence generation in all 4 studies. There was low risk of bias for allocation concealment in three studies, and unclear risk in one study. The blinding of personnel was inadequate in all four studies and blinding of outcome assessments was good in three studies but inadequate in one study. We downgraded the evidence by one step. Heterogeneity/consistency: tests for heterogeneity were not applicable for RR as there were only outcomes in one group in one trial. We noted no heterogeneity for RD (19%). Directness of evidence: studies were conducted in the target population. Precision: the confidence interval around the point estimate for RR was quite wide. We downgraded the evidence by one step. Presence of publication bias: only 4 studies were included in the analysis so a funnel plot was not constructed. |
|
| 2 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; IV: intravenous; MD: Mean difference; NICU: Neonatal intensive care unit; RR: Risk Ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
5.
| High‐dose oral or intravenous ibuprofen compared with standard‐dose oral or intravenous ibuprofen for patent ductus arteriosus | ||||||
|
Patient or population: preterm infants with patent ductus arteriosus Settings: NICU Intervention: high‐dose oral or intravenous ibuprofen Comparison: standard‐dose oral or intravenous ibuprofen | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard‐dose ibuprofen | High‐dose ibuprofen | |||||
| Failure to close a patent ductus arteriosus after 3 doses of ibuprofen | High risk population | RR 0.37 (0.22 to 0.61) | 190 (3) | ⊕⊕⊕⊝ moderate | Bias: there was unclear risk of bias for random sequence generation in all three studies. The allocation was concealed in two of the studies and unclear in one study. The blinding of personnel was unclear in all three studies and blinding of outcome assessments was unclear in one of the three studies, with low risk of bias in the other two studies. We downgraded the evidence by one step. Heterogeneity/consistency: we noted no heterogeneity for RR (4%) or for RD (0%). Directness of evidence: studies were conducted in the target population. Precision: the confidence intervals around the point estimates for RR and RD were quite narrow. Presence of publication bias: only 3 studies were included in the analysis so a funnel plot was not constructed. |
|
| 411 per 1000 | 147 per 1000 (0 to 300) | |||||
| Necrotising enterocolitis | High risk population | RR 1.00 (0.40 to 2.50) | 130 (2) | ⊕⊕⊝⊝ low | Bias: there was unclear risk of bias for random sequence generation in both studies. The allocation was concealed in both studies. The blinding of personnel was unclear in both studies but there was low risk of bias for blinding of outcome assessments. We downgraded the evidence by one step. Heterogeneity/consistency: we noted no heterogeneity for RR or for RD (0% for both). Directness of evidence: studies were conducted in the target population. Precision: the confidence intervals around the point estimates for RR and RD were quite wide as the sample size was small. We downgraded the evidence by one step. Presence of publication bias: only 2 studies were included in the analysis so a funnel plot was not constructed. |
|
| 123 per 1000 | 123 per 1000 (114 to 133) | |||||
| Oliguria | High risk population | RR 1.57 (0.44 to 5.63) | 120 (2) |
⊕⊕⊝⊝ low | Bias: there was unclear risk of bias for random sequence generation in both studies. The allocation was concealed in one study. The blinding of personnel was unclear in both studies but there was low risk of bias for blinding of outcome assessments (by cardiologist) in one study. We downgraded the evidence by one step. Heterogeneity/consistency: we noted no heterogeneity for RR or for RD (0% for both). Directness of evidence: studies were conducted in the target population. Precision: the confidence intervals around the point estimates for RR and RD were quite wide as the sample size was small. We downgraded the evidence by one step. Presence of publication bias: only 2 studies were included in the analysis so a funnel plot was not constructed. |
|
| 50 per 1000 | 83 per 1000 (33 to 133) |
|||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; NICU: Neonatal intensive care unit; RR: Risk Ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
Description of the condition
Normal fetal circulation is dependent on the placenta and the patency of the ductus arteriosus (PDA) (Mathew 1998). Following birth and with the separation of the placenta and initiation of breathing, the circulation changes and closure of the ductus starts immediately (Mathew 1998). However, in about a third of low‐birth‐weight (LBW; weighing less than 2500 g) infants, the PDA remains open, especially during the early days of life (Ellison 1983). In preterm neonates, the PDA often fails to close. The haemodynamic instability caused by the left to right shunt and associated run off causes renal or gastrointestinal effects including spontaneous perforation and necrotising enterocolitis (NEC), chronic lung disease (CLD) and, if not managed, may lead to mortality (Cotton 1978a). The presence of a PDA is associated with reduced middle cerebral artery blood flow velocity (Weir 1999).
The surgical closure of the symptomatic PDA reduces duration of mechanical ventilation, improves haemodynamics, and improves lung compliance (Cotton 1978b; Naulty 1978). However, medical treatment is still considered the treatment of choice in the majority of cases because of the risks related to the surgery. In a large Canadian cohort of 3779 very low‐birth‐weight (VLBW, weighing less than 1500 g) infants, 28% required treatment for a PDA; 75% were treated with indomethacin alone, 8% with surgical ligation alone, and 17% required both indomethacin and surgical ligation (Lee 2000). Infants with lower birth weight (BW) were more likely to be treated surgically (Lee 2000).
Description of the intervention
Prostaglandins play a significant role in keeping the ductus arteriosus patent (Mathew 1998). PDA‐related morbidity and mortality reduce with the use of indomethacin, which acts as an inhibitor of prostaglandin‐forming cyclo‐oxygenase enzymes (Mahony 1982; Stefano 1991). However, indomethacin use has been associated with transient or permanent derangement of renal function, NEC, gastrointestinal haemorrhage or perforation, alteration of platelet function and impairment of cerebral blood flow/cerebral blood flow velocity (Edwards 1990; Ohlsson 1993; Seyberth 1983; Wolf 1989). These negative effects of indomethacin are possibly related to mechanisms other than inhibition of prostaglandin synthesis.
In one large trial of 1202 extremely low‐birth‐weight infants, indomethacin prophylaxis did not significantly improve the rate of survival without neurosensory impairment at 18 months, despite the fact that it reduced the frequency of PDA and severe periventricular and intraventricular haemorrhage (IVH) (Schmidt 2001). One Cochrane review confirmed that prophylactic treatment with indomethacin has a number of short‐term benefits, in particular a reduction in symptomatic PDA, the need for ductal ligation, and severe IVH (Fowlie 2010). The same review found no evidence of either benefit or harm concerning longer‐term outcomes, including neurodevelopment (Fowlie 2010).
How the intervention might work
The complications associated with the use of indomethacin have encouraged the search for an alternate drug to treat a PDA. Ibuprofen, a propionic acid derivative and non‐selective cyclo‐oxygenase inhibitor, has been reported to close a PDA, but without gastrointestinal haemodynamic disturbance and potentially harmful cerebral adverse effects (Chemtob 1991; Coceani 1979; Varvarigou 1996). Ibuprofen has some neuro‐protective effects in animal models (Chemtob 1990; Pellicer 1999). Ibuprofen enhances cerebral autoregulation without affecting cerebral blood flow, cerebral metabolism, or intestinal or renal haemodynamics (Grosfeld 1983; Hardy 1996; Kaplan 1994).
Another non‐steroidal anti‐inflammatory drug, mefenamic acid, has been reported to close a PDA (Ito 1994; Niopas 1994; Sakhalkar 1992). Mefenamic acid is currently being used in Japan to close a PDA (Uchiyama 2011), but, as of July 2014, we have not been able to identify any randomised studies.
Why it is important to do this review
One previous meta‐analysis of three trials of small sample size (Patel 2000; Van Overmeire 1997; Van Overmeire 1998) suggested that ibuprofen may be as effective as indomethacin in closing a PDA (Ohlsson 2000). The meta‐analysis included 176 neonates who were randomised to either ibuprofen (10 mg/kg followed at 24 and 48 hours later by a dose of 5 mg/kg) or indomethacin (0.2 mg/kg at 12‐hour interval for three doses). The typical risk ratio (RR) for failure of PDA closure using ibuprofen versus indomethacin was 1.0 (95% confidence interval (CI) 0.85 to 1.17) (Ohlsson 2000). This meta‐analysis was included in a commentary on a publication of a randomised controlled trial (Patel 2000), and the publication type did not allow for detailed description of the methodology used or the inclusion of outcomes other than ductal closure (Ohlsson 2000). Additional trials have been published since the year 2000. Therefore, systematic reviews according to Cochrane methodology were justified (Ohlsson 2003), as were the current and previous updates (Ohlsson 2005; Ohlsson 2008; Ohlsson 2010; Ohlsson 2013; Ohlsson 2015).
Objectives
Primary objective
To determine the effectiveness and safety of ibuprofen compared with indomethacin, other cyclo‐oxygenase inhibitor(s), placebo, or no intervention for closing a patent ductus arteriosus in preterm, low‐birth‐weight, or preterm and low‐birth‐weight infants.
Secondary objectives
To determine the effectiveness and safety of ibuprofen to close a PDA in relation to gestational age, birth weight, method used to diagnose a PDA, and dosing regimen for ibuprofen.
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised controlled trials.
Types of participants
Preterm infants less than 37 weeks' gestational age or LBW infants (weighing less than 2500 grams) with a PDA, diagnosed either clinically or by echocardiographically‐guided criteria in the neonatal period (less than 28 days).
Types of interventions
The following is a list of the ten interventions/comparisons that were included in this update:
Intravenous ibuprofen verus place
Oral ibuprofen versus placebo
Intravenous or oral ibuprofen versus intravenous or oral indomethacin
Oral ibuprofen versus intravenous or oral indomethacin
Oral ibuprofen versus intravenous ibuprofen
High‐dose (oral or intravenous) versus standard‐dose ibuprofen (oral or intravenous)
Early versus expectant administration of intravenous ibuprofen
Echocardiographically (ECHO) ‐ guided intravenous ibuprofen treatment versus standard intravenous ibuprofen treatment
Continuous infusion of ibuprofen versus intermittent boluses of ibuprofen
Rectal ibuprofen versus oral ibuprofen
For previous versions of this review and for this update, we did not compare ibuprofen to paracetamol, as that is the topic of a separate Cochrane review (Ohlsson 2018).
Types of outcome measures
Primary outcomes
Failure of permanent PDA closure within one week of administration of the first dose of ibuprofen (PDA diagnosed either clinically or by ECHO criteria).
Secondary outcomes
All‐cause mortality during initial hospital stay.
Neonatal mortality (mortality during the first 28 days of life).
Infant mortality (mortality during the first year of life).
Reopening of the ductus arteriosus.
Need for surgical closure of the PDA.
Need for treatment with indomethacin to close the PDA*.
Duration of ventilator support (days).
Duration of need for supplementary oxygen (days).
Pneumothorax.
Pulmonary haemorrhage*.
Pulmonary hypertension*.
Chronic lung disease (CLD) (defined as oxygen requirement at 28 days' postnatal age in addition to compatible clinical and roentgenographic findings).
CLD (defined as oxygen requirement at 36 weeks' postmenstrual age (PMA) in addition to compatible clinical and roentgenographic findings).
CLD (age at diagnosis not stated)*.
Intraventricular haemorrhage (IVH) (grades I to IV).
Severe IVH (grades III and IV).
Periventricular leukomalacia (PVL).
Necrotising enterocolitis (NEC) (any stage).
Intestinal perforation*.
Gastrointestinal bleed.
Time to full enteral feeds (postnatal age at time of achieving full enteral feeds).
Time to regain birth weight* (days).
Retinopathy of prematurity (ROP) (according to the international classification of ROP).
Definite sepsis (clinical symptoms and signs of sepsis and a positive bacterial culture in a specimen obtained from normally sterile fluids or tissue obtained at postmortem).
Oliguria (defined as less than 1 mL/kg/hour).
Serum/plasma levels of creatinine (µmol/L) after treatment*.
Increase in serum/plasma levels of creatinine (μmol/L) after treatment*.
Cystatin‐C plasma levels (mg/dL) after treatment***.
Duration of hospitalisation (total length of hospitalisation from birth to discharge home or mortality) (days).
Neurodevelopmental outcome (assessed by a standardised and validated assessment tool, a child developmental specialist or both) at any age reported (outcome data grouped at 12, 18 and 24 months, if available).
Bilirubin albumin binding*.
Proportion of infants who required rescue treatment for PDA (indomethacin or surgery), died, or dropped out to study day 14**.
Other adverse effects reported by the authors.
Outcomes marked with an asterisk (*) were not included in the original protocol but were included in the update of this review in August 2007. These outcomes were included in updates of the review as they were closely related to previous outcomes already included and were considered to be of importance to establish the effectiveness and safety of ibuprofen versus indomethacin. The outcome 'Proportion of infants that required rescue treatment for PDA (indomethacin or surgery), died, or dropped out through study day 14** ' was the primary outcome of the only study (until this update) that compared IV ibuprofen with placebo (Aranda 2009), and was, therefore, included from the 2007 update. 'Cystatin‐C plasma levels (mg/dL) after treatment*** ' were included in the 2012 update.
Search methods for identification of studies
We used the criteria and standard methods of Cochrane and Cochrane Neonatal (see the Cochrane Neonatal search strategy for specialised register).
Electronic searches
We conducted a comprehensive search including: Cochrane Central Register of Controlled Trials (CENTRAL 2017, Issue 10) in The Cochrane Library; MEDLINE via PubMed (1966 to 30 November 2017); Embase (1980 to 30 November 2017); and CINAHL (1982 to 30 November 2017) using the following search terms: ((Ibuprofen[MeSH]) OR (Mefenamic Acid[MeSH]) OR ibuprofen OR mefenamic acid) AND ((Ductus Arteriosus, Patent[MeSH]) OR patent ductus arteriosus or PDA), plus database‐specific limiters for RCTs and neonates (see Appendix 1 for the full search strategies for each database). We did not apply language restrictions. See Appendix 2 for information on past searches.
We searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov; the World Health Organization’s International Trials Registry and Platform, and the ISRCTN Registry).
Searching other resources
We searched the reference lists of any articles selected for inclusion in this review in order to identify additional relevant articles.
Data collection and analysis
We used the standard review methods of the Cochrane Neonatal Review Group (CNRG) in data collection and analysis. One review author (AO) performed the updates conducted in 2005, 2007, and 2010. All three review authors (AO, RW, SS) conducted the 2012, 2014, and 2017 updates.
Selection of studies
In the original review, two review authors (AO, SS) assessed all abstracts and published full reports identified as potentially relevant by the literature search for inclusion. For the 2012 and 2014 updates, all three review authors (AO, RW, SS) assessed the articles for possible inclusion. For this update in 2017, two review authors (AO, SS) selected the studies for inclusion.
Data extraction and management
Each review author independently extracted data using pre designed data abstraction forms. The review authors compared results and resolved differences. One review author (AO) entered data into Review Manager 5 (Review Manager 2014), and the other review authors (RW and SS) cross checked the printout against their own data abstraction forms and corrected errors by consensus.
For the studies identified as abstracts, we contacted some primary authors to ascertain whether a full publication was available if the full paper was not identified in an electronic database.
We obtained information from the primary author if the published article provided inadequate information for the review. We independently assessed retrieved articles and abstracted the data.
Assessment of risk of bias in included studies
Two review authors (AO, SS) independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane ‘Risk of bias’ tool (Higgins 2017) for the following domains:
Sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Any other bias
Any disagreements were resolved by discussion or by a third assessor. See Appendix 3 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
The statistical analyses followed the recommendations of the CNRG. The estimates of treatment effects included RR, risk difference (RD), number needed to treat for an additional beneficial outcome (NNTB) or additional harmful outcome (NNTH) for dichotomous outcomes, and mean difference (MD) for continuous outcomes. All estimates of treatment effects were reported with 95% CI. We considered a P‐value < 0.05 as statistically significant.
Unit of analysis issues
The unit of randomisation was the individual infant. We did not include cross‐over or cluster‐randomised trials as those trial designs are unlikely for the intervention studied in this review. No cross‐over or cluster‐randomised trials were identified. An infant was only considered once even if the infant may have been randomised twice by investigators. We planned to contact the authors in order to provide data resulting from the first randomisation. If we could not separate data from the first randomisation, it was planned that the study would be excluded.
Dealing with missing data
We requested additional data from the authors of each included trial when data on important outcomes were missing or needed clarification. We received clarifying information from one of the authors or coauthors of the following studies: Bagnoli 2013; Bravo 2014; Dani 2012; Fesharaki 2012; Patel 1995; ElHassan 2014.
Assessment of heterogeneity
We performed heterogeneity tests including the I2 test to assess the appropriateness of pooling the data using the following categories for heterogeneity: less than 25% no heterogeneity; 25% to 49% low heterogeneity; 50% to 74% moderate heterogeneity and 75% or greater high heterogeneity (Higgins 2003).
Assessment of reporting biases
To ascertain the possibility of publication bias, we produced a funnel plot for the primary outcome of 'failure to close a PDA (after single or three doses)' (Analysis 3.1) and for the outcome of NEC (Analysis 3.17). Both funnel plots were quite symmetric indicating that there was no obvious indication of publication bias (Figure 1; Figure 2).
3.1. Analysis.
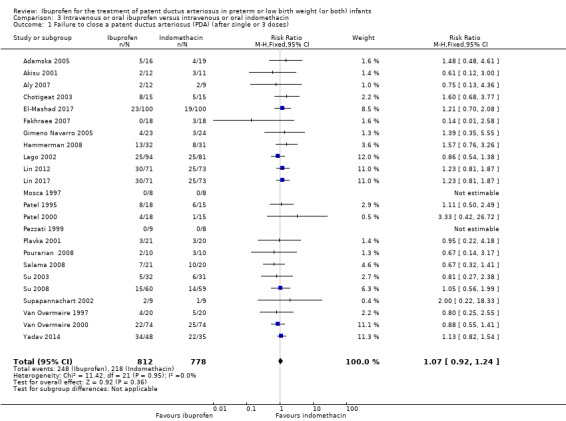
Comparison 3 Intravenous or oral ibuprofen versus intravenous or oral indomethacin, Outcome 1 Failure to close a patent ductus arteriosus (PDA) (after single or 3 doses).
3.17. Analysis.
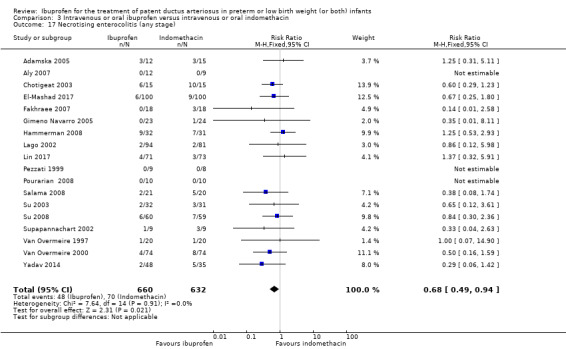
Comparison 3 Intravenous or oral ibuprofen versus intravenous or oral indomethacin, Outcome 17 Necrotising enterocolitis (any stage).
1.
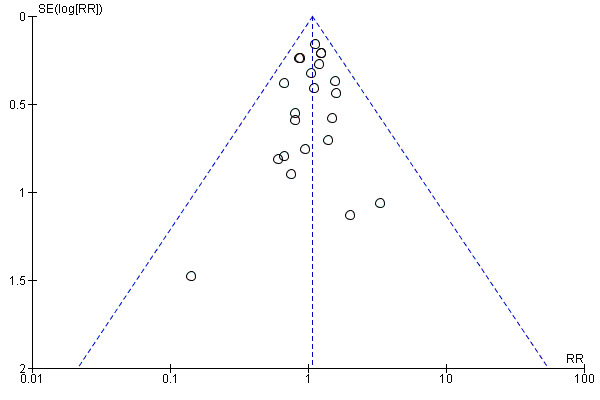
Funnel plot of comparison: 3 Intravenous or oral ibuprofen versus intravenous or oral indomethacin, outcome: 3.1 Failure to close a patent ductus arteriosus (PDA) (after single or 3 doses).
2.
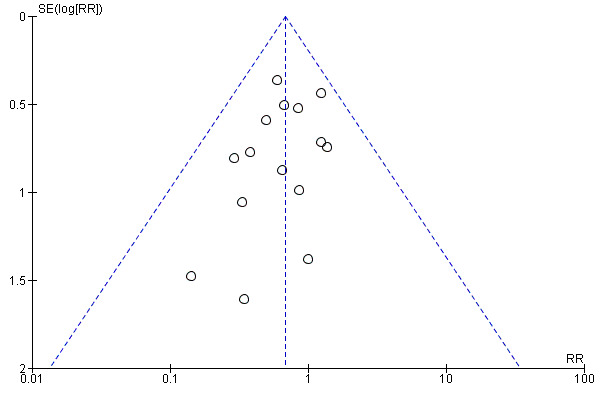
Funnel plot of comparison: 3 Intravenous or oral ibuprofen versus intravenous or oral indomethacin, outcome: 3.17 Necrotising enterocolitis (any stage).
Data synthesis
We performed meta‐analyses using Review Manager 5 (Review Manager 2014). For estimates of typical RR and RD, we used the Mantel‐Haenszel method. We calculated mean difference (MD) for continuous outcomes. For measured quantities, we used the inverse variance method. We used a fixed‐effect model for all meta‐analyses. We used the formulas proposed by Hozo and coworkers to estimate means and standard deviations (SD) from medians and ranges presented by the authors of some of the included studies (Hozo 2005).
Quality of evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes:
1. Failure to close a PDA after three doses;
2. Need for surgical closure of the PDA;
3. Necrotising enterocolitis;
4. Duration of ventilatory support;
5. Oliguria (urine output < 1mL/kg/hr);
6. Serum/plasma creatinine levels (µmol/L) 72 hours after treatment.
We included only clinically important outcomes that were reported by at least two trials.
Two authors (AO, SS) independently assessed the quality of the evidence for each of the outcomes above. We considered evidence from randomised controlled trials as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias. We used the GRADEpro GDT Guideline Development Tool to create a ‘Summary of findings’ table to report the quality of the evidence. We developed ‘Summary of findings’ tables for comparisons that included at least two trials.
The GRADE approach results in an assessment of the quality of a body of evidence in one of four grades:
High: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses:
gestational age (less than 28 weeks, 28 to 32 weeks, 33 to 36 weeks);
BW (less than 1000 grams, 1000 to 1500 grams, 1501 to 2500 grams);
method used to diagnose a PDA (by ECHO criteria or only by clinical criteria);
a dosing regimen of ibuprofen 10 mg/kg followed by ibuprofen 5 mg/kg 24 and 48 hours later, or indomethacin 0.2 mg/kg at 12‐hour intervals for three doses;
oral ibuprofen versus indomethacin (this was added in 2008 as a new comparison as studies now have used oral ibuprofen) (Ohlsson 2008);
oral ibuprofen versus IV ibuprofen (this was included as a new comparison in 2013 as studies now have been published assessing this comparison) (Ohlsson 2013);
timing of ibuprofen administration as early versus expectant management (this was included as a new comparison in 2013 as one trial studied this intervention) (Ohlsson 2013);
higher dosing regimen of ibuprofen 20 mg/kg/day followed by ibuprofen 10 mg/kg/day for two doses compared with the standard‐dose of ibuprofen 10 mg/kg/day followed by ibuprofen 5 mg/kg/day for two doses (this was included in 2013 as a new comparison as one trial studied this intervention) (Ohlsson 2013);
ECHO‐guided ibuprofen treatment versus standard ibuprofen treatment (this was included in 2014 as a new comparison as one trial studied this intervention) (Ohlsson 2015);
continuous infusion of ibuprofen versus standard boluses of ibuprofen (this was included in 2014 as a new comparison as one trial studied this intervention) (Ohlsson 2015).
rectal ibuprofen versus oral ibuprofen (this was included in the 2017 update as a new comparison as one trial studied this intervention).
Sensitivity analysis
The prespecified subgroup analyses excluding studies that used only one dose of medication and studies that were published as abstracts only were abandoned for the updates in 2007, 2010, 2012, 2014 and this 2017 update of the review. Only one study used a single dose and we identified only one abstract. We incorporated the results of these studies with the other studies. All studies used ECHO criteria to diagnose a PDA, so this prespecified subgroup analysis was also abandoned.
Results
Description of studies
We identified one study comparing oral ibuprofen with placebo for the update in 2013 (Lin 2012). For the same update, we added a comparison of oral ibuprofen versus IV ibuprofen as three trials studied this comparison (Cherif 2008; Erdeve 2012; Gokmen 2011). We included one study that compared IV high‐dose ibuprofen versus a standard‐dose regimen of ibuprofen (Dani 2012). We included one additional comparison for early versus expectant administration of IV ibuprofen (Sosenko 2012). Thus, we included six additional studies for the update in 2013. For the update in 2014, we identified and included six additional trials (Bagnoli 2013; Bravo 2014; Fesharaki 2012; Lago 2014; Pistulli 2014; Yadav 2014), and one study reported on long‐term follow‐up for Gokmen 2011. For this update in 2017, we included the following ongoing trials (ACTRN12616000195459; ChiCTR‐TRC‐14004719; EUCTR2016‐002974‐11‐ES; IRCT201205029611N1; IRCT2015111024977N1; NCT02128191; NCT02884219; NCT01149564; NCT01630278; NCT01758913; NCT02128191). The following full‐text reports were included in this 2017 update; one study compared IV ibuprofen to placebo (Ding 2014); two studies compared IV ibuprofen to IV indomethacin (Lin 2017; El‐Mashad 2017); one study compared high versus standard‐dose of ibuprofen (Pourarian 2015); one study compared rectal versus oral ibuprofen (Demir 2017); and one study compared IV ibuprofen to oral ibuprofen (Akar 2017).
Results of the search
The results of the search conducted in November, 2017 are shown in Figure 3.
3.
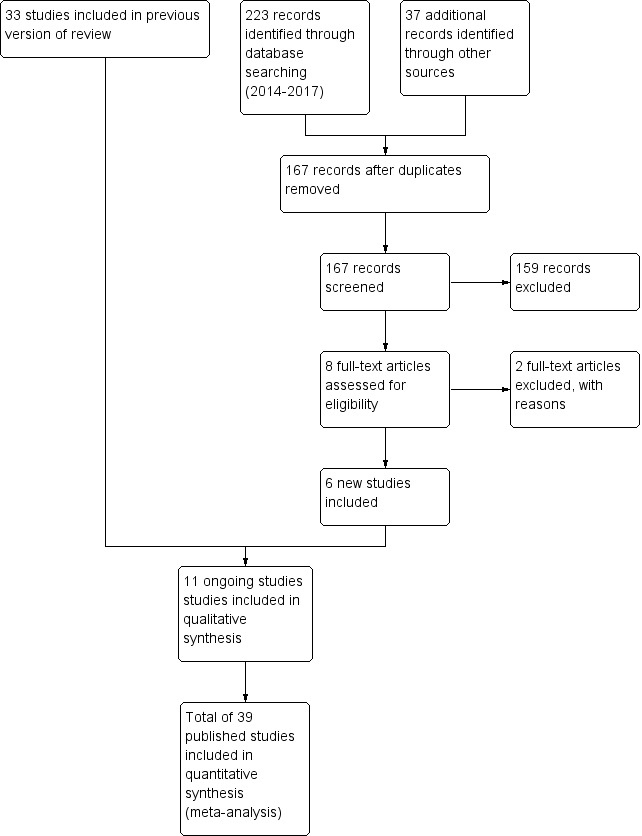
Study flow diagram: review update
Included studies
Intravenous ibuprofen versus placebo or no intervention (Comparison 1)
The study by Aranda and coworkers was a multi centre study conducted at 11 sites in the US and published as an abstract in 2005 (full study published in 2009) (Aranda 2009).
Objective: to compare the efficacy and safety of IV ibuprofen (L‐lysine) with placebo for the early closure of a nonsymptomatic PDA within 72 hours of birth in extremely low‐birth‐weight preterm infants with evidence of ductal shunting by ECHO.
Population: 136 preterm infants (PMA < 30 weeks, BW 500 to 1000 grams) with evidence of ductal shunting by ECHO within 72 hours after birth.
Intervention: infants were allocated to either a three‐day treatment course of IV ibuprofen of 10 mg/kg, 5 mg/kg and 5 mg/kg (68 infants) or placebo (saline) (68 infants).
Outcomes: primary outcome measure was the proportion of infants that required rescue treatment for PDA (indomethacin or surgery), died, or dropped out prior to study day 14. Secondary outcomes included mortality, need for PDA ligation, IVH, PVL, NEC, ROP, pulmonary haemorrhage, pulmonary hypertension, ROP, BPD (supplemental oxygen at 28 days), BPD (supplemental oxygen at 36 weeks' PMA).
The study by Bagnoli and coworkers was a single centre study conducted in Siena, Italy (Bagnoli 2013).
Objective: to evaluate the renal adverse effects of IV ibuprofen.
Population: 134 preterm newborns with ECHO‐confirmed PDA (PMA less than 32 weeks, BW less than 1500 grams, postnatal age greater than 72 hours.
Intervention: infants were allocated to a three‐day treatment course of IV ibuprofen of 10 mg/kg, 5 mg/kg and 5 mg/kg given IV over 10 minutes (67 infants) or placebo (0.9% NaCl given IV) (67 infants).
Outcomes: failure to close a PDA, need for surgical ligation of the PDA, oliguria, NEC, creatinine, and blood urea nitrogen (BUN) before and after treatment, mortality at 28 days of life.
The study by Ding and coworkers was a single‐centre study conducted in a provincial hospital affiliated to Shandong University, Jinan, China (Ding 2014).
Objective: To clarify the role of N‐terminal pro brain natriuretic peptide, (NT‐proBNP) in ibuprofen on preterm infants with patent ductus arteriosus PDA.
Population: 72 preterm infants with mean PMA of 30.24 +/‐ 1.49 weeks and an ECHO‐confirmed PDA.
Intervention: Ibuprofen group received oral ibuprofen 10 mg/kg, followed by 5 mg/kg after 24 and 48 hours, and the placebo group received the same volume of 5% glucose.
Outcomes: PDA and NT‐proBNP were detected at 24 hours, 3 and 7 days of age. We included only rate of failure of PDA closure at 7 days.
Oral ibuprofen versus placebo or no intervention (Comparison 2)
The study by Lin and coworkers was a single centre study conducted in Xiamen City, Xiamen, Fujian, China (Lin 2012). The study was published in Chinese and only the information in the abstract published in English was understood by the review authors. We have written to the authors to obtain further details, but we have not received a response as of January 22nd, 2018.
Objective: to study the therapeutic effect and safety of early administration of oral ibuprofen in VLBW infants with a PDA.
Population: 64 symptomatic VLBW infants with ECHO‐confirmed PDA were enrolled within 24 hours after birth.
Intervention: in the ibuprofen group, 32 infants received oral ibuprofen 10 mg/kg as an initial dose within 24 hours after birth, followed by a second and a third dose of ibuprofen 5 mg/kg 24 and 48 hours after the initial dose. In the placebo group, 32 infants received normal saline 1 mL/kg followed by saline 0.5 mL 24 and 48 hours later.
Outcomes: primary outcome was PDA closure rate following the initial course of treatment (three doses).
Intravenous or oral ibuprofen versus intravenous or oral indomethacin (Comparison 3)
The study by Adamska and coworkers was a single centre study conducted in Poland (Adamska 2005).
Objective: to assess the efficacy and safety of early treatment with IV ibuprofen or IV indomethacin in preterm infants.
Population: 35 preterm (less than 33 weeks' PMA and BW less than 1500 grams) infants with a PDA diagnosed by Doppler ECHO.
Intervention: infants were randomised to receive three doses of indomethacin (0.2 mg/kg IV given at 24‐hour intervals, 19 infants) or three doses of ibuprofen (10, 5 and 5 mg/kg IV given at 24‐hour intervals, 16 infants).
Outcomes: primary outcome was ductal closure. Other outcomes included: need for surgical ligation, IVH, PVL, NEC, intestinal perforation, oliguria, time to full oral feeds, CLD (at 28 days of age), pulmonary haemorrhage, pulmonary hypertension, duration of mechanical ventilation, and days on supplemental oxygen.
The study by Akisu and coworkers was a single centre study conducted in Turkey (Akisu 2001).
Objective: to investigate the efficacy and safety of enteral ibuprofen for the treatment of PDA and to compare it with enteral indomethacin.
Population: 23 preterm infants (less than 35 weeks' PMA) with a PDA diagnosed by Doppler ECHO.
Intervention: infants were randomised to receive either enteral ibuprofen 10 mg/kg as the initial dose followed by 5 mg/kg 24 and 48 hours later (12 infants) or three doses of enteral indomethacin (0.2 mg/kg) every 12 hours (11 infants).
Outcomes: primary outcome was ductal closure. Other outcomes included need to re‐treat a PDA with indomethacin or ibuprofen, urine output, serum creatinine after treatment, thrombocyte counts, gastrointestinal haemorrhage, IVH, sepsis, and mortality.
The study by Aly and coworkers was a single centre study conducted in Egypt (Aly 2007).
Objective: to evaluate the feasibility of the use of oral ibuprofen suspension versus IV indomethacin in the treatment of PDA in preterm infants.
Population: 21 preterm infants (less than 35 weeks' gestation) aged two to seven days with respiratory distress and PDA diagnosed by Doppler ECHO.
Intervention: infants were randomised to receive three doses of IV indomethacin 0.2 mg/kg at 12‐hour intervals (nine infants) or an initial oral dose of ibuprofen 10 mg/kg, followed by two doses of 5 mg/kg after 24 and 48 hours (12 infants).
Outcomes: primary outcome was ductal closure. Secondary outcomes included pulmonary haemorrhage, gastrointestinal haemorrhage, NEC, gastrointestinal perforation, and change in serum creatinine following treatment.
The study by Chotigeat and coworkers was a single centre study conducted in Thailand (Chotigeat 2003).
Objective: to compare efficacy and adverse effects of oral ibuprofen versus IV indomethacin treatment for symptomatic PDA in preterm infants.
Population: preterm infants with a symptomatic PDA confirmed by ECHO.
Intervention: 30 infants were randomised to receive either three oral doses of ibuprofen (dose not stated) given at 24‐hourly intervals (15 infants) or three doses of IV indomethacin (dose not stated) given at 12‐hourly intervals (15 infants) starting within 10 days of life.
Outcomes: primary outcome measure was ductal closure. Secondary outcomes included the need for surgical closure of a PDA, the need for re‐treatment with ibuprofen or indomethacin, mortality by 28 days, CLD (at 28 days), sepsis, ROP, and serum creatinine levels after treatment.
The study by El‐Mashad and coworkers was a single centre study conducted in Egypt (El‐Mashad 2017).
Objective: the effectiveness of indomethacin, ibuprofen, and paracetamol in PDA closure in preterm neonates.
Population: preterm neonates with haemodynamically significant PDA.
Intervention: group I (paracetamol group) received 15 mg/kg/6 H IV paracetamol infusion for 3 days; group II (ibuprofen group) received 10 mg/kg IV ibuprofen infusion followed by 5 mg/kg/day for 2 days; group III (indomethacin group) received 0.2 mg/kg/12 H indomethacin IV infusion for three doses. Each study group included 100 infants. Total sample 300.
Outcomes: primary outcome: failure to close the PDA. Secondary outcomes included: surgical ligation, ROP, GI bleeding, NEC, pulmonary haemorrhage, IVH, sepsis, daily urine output, serum creatinine, serum bilirubin, platelet count.
The study by Fakhraee and coworkers was conducted in a single centre in Iran (Fakhraee 2007).
Objective: to compare the efficacy and safety of oral ibuprofen and oral indomethacin for the treatment of PDA in preterm infants.
Population: 36 preterm infants (less than 34 weeks' PMA).
Intervention: 18 infants were randomised to receive three oral doses of indomethacin 0.2 mg/kg at 24‐hour intervals and 18 infants to three doses of oral ibuprofen (first dose of 10 mg/kg, followed by 5 mg/kg/dose at 24‐hour intervals).
Outcomes: primary outcome was ductal closure. Secondary outcomes included maximum serum BUN and creatinine levels after treatment, NEC, mortality at one month of age, and IVH (grades III and IV).
The study by Gimeno Navarro and coworkers was a single centre study conducted in Spain (Gimeno Navarro 2005).
Objective: to compare the safety and efficacy of IV ibuprofen and IV indomethacin in the treatment of PDA in preterm infants.
Population: preterm infants (less than 34 weeks' PMA) with a haemodynamically significant PDA, confirmed by ECHO in the first week of life and who required respiratory support.
Intervention: during the first week of life (mean of two days of life), 47 ventilated infants were randomised to receive either indomethacin 0.2 mg/kg/dose IV every 12 hours for three doses (24 infants) or an initial dose of IV ibuprofen 10 mg/kg, followed by two doses of ibuprofen IV every 24 hours (23 infants).
Outcomes: primary outcome was ductal closure. Other outcomes included mortality, ductal reopening, need for surgical ligation, NEC, isolated bowel perforation, intestinal haemorrhage, pulmonary haemorrhage, CLD (need for supplemental oxygen at 28 days of age), IVH (grades III and IV), days on assisted ventilation, days on supplemental oxygen, and days in neonatal intensive care unit (NICU).
The study by Hammerman was conducted in a single centre in Israel (Hammerman 2008).
Objective: to show that treating a PDA with continuous IV indomethacin was similar to IV ibuprofen in its effect on urine output, renal function and blood flow velocities.
Population: 64 preterm infants (PMA 33 weeks or less, BW 1750 grams or less) with PDA.
Intervention: 31 infants received continuous IV infusion of indomethacin for 36 hours at a rate of 17 μg/kg/hour and 32 infants received ibuprofen 10 mg/kg IV followed by two doses of 5 mg/kg at 24‐hour intervals. One boy assigned to the ibuprofen group was withdrawn by his parents before he started therapy and he was not included in the analysis.
Outcomes: primary outcome was ductal closure. Other outcomes included need for surgical ligation, need for re‐treatment with either indomethacin or ibuprofen, need for surgical treatment, bronchopulmonary dysplasia (BPD), IVH (grades III and IV), ROP, NEC.
The study by Lago and coworkers was conducted in two centres in Italy (Lago 2002).
Objective: to compare IV indomethacin and IV ibuprofen with regard to efficacy and safety for the early treatment of PDA.
Population: preterm infants (PMA 34 weeks or less, postnatal age 48 to 72 hours) with respiratory distress syndrome (RDS) treated with mechanical ventilation and ECHO‐confirmed PDA.
Intervention: 175 infants were randomised to either IV ibuprofen (94 infants) at an initial dose of 10 mg/kg followed by two doses of 5 mg/kg each after 24 and 48 hours or three doses of IV indomethacin 0.2 mg/kg at 12‐hour intervals (81 infants). When the ductus arteriosus was still patent after the randomly assigned treatment in infants in either group receiving mechanical ventilation, another three doses of the same medication were given as a non‐randomised rescue treatment. If this therapy did not induce ductal closure, the infant continued to receive mechanical ventilation and, if the ductus was judged to be haemodynamically significant or if further pharmacological treatment was contraindicated, surgical ligation of the ductus was performed.
Outcomes: primary outcome was ductal closure. Other outcomes included mortality, oliguria, IVH, PVL, surgical ligation of PDA, serum creatinine, CLD at 36 weeks, NEC, sepsis, mortality, duration of ventilator support, days on supplemental oxygen, duration of hospital stay, and time to full feeds.
The study by Mosca and coworkers was conducted in a single centre in Italy (Mosca 1997).
Objective: to compare the effects of IV indomethacin and IV ibuprofen on cerebral perfusion and oxygenation in preterm infants with PDA.
Population: preterm infants (less than 31 weeks' PMA) with PDA and receiving mechanical ventilation.
Intervention: 16 infants received either IV ibuprofen 10 mg/kg dissolved in saline 1 mL and infused over one minute or IV indomethacin 0.2 mg/kg (eight infants). A second and third dose of ibuprofen 5 mg/kg at 24‐hour intervals or indomethacin 0.1 mg/kg (eight infants) was administered, provided no significant adverse effect was observed.
Outcomes: near‐infrared spectroscopy was used to measure changes in cerebral blood volume and in oxidised cytochrome oxidase concentration. Cerebral blood flow velocity in the pericallosal artery was measured using Doppler ultrasonography. Ductal closure, reopening of a PDA and the need for re‐treatment with indomethacin or ibuprofen were reported.
The study by Lin and coworkers was conducted in one NICU in Chicago, USA and one NICU in Taiwan (Lin 2017).
Objective: to compare renal function and ductal response between indomethacin and ibuprofen.
Population: preterm neonates < 1000 grams with ECHO‐proven and clinically significant PDA.
Intervention: group I (indomethacin group) received IV indomethacin in an initial dose of 0.2 mg/kg (1.0 ml/kg) followed by 2 doses of 0.1 mg/kg (0.5 ml/kg) at 24‐hour intervals. Group II (ibuprofen group) received 10 mg/kg IV ibuprofen infusion followed by 2 doses of 5 mg/kg/day at 24 hour intervals. Total number of infants enrolled was 144.
Outcomes: renal function, ductal closure, surgical ligation, mortality, ROP stage 3‐4, BPD, NEC (stage ≥ 2), IVH (grade ≥ 2).
The study by Patel and coworkers was a single centre pilot study conducted in England (Patel 1995).
Objective: to compare the cerebral effects of IV ibuprofen with IV indomethacin in preterm infants.
Population: 33 infants with a median PMA of 26 weeks (range 23 to 28) and an ECHO‐confirmed PDA.
Intervention: infants were randomised to receive either ibuprofen 5 mg/kg (12 infants), ibuprofen 10 mg/kg (six infants) or indomethacin 0.1 mg/kg (15 infants). The drugs were infused IV over 15 minutes.
Outcomes: near‐infrared spectroscopy was used to observe the effect of treatment on cerebral perfusion, indicated by changes in cerebral blood volume and cerebral mitochondrial oxygenation, determined by the change in concentration of oxidised cytochrome aa3. Ductal closure was reported.
The second study by Patel and coworkers was conducted in four centres in England (Patel 2000).
Objective: to compare the effects of IV ibuprofen to IV indomethacin on cerebral haemodynamics measured using near‐infrared spectroscopy in preterm infants during treatment for PDA.
Population: 33 preterm infants (less than 35 weeks' PMA).
Intervention: infants were randomly assigned to three IV doses of either ibuprofen 5 to 10 mg/kg every 24 hours (18 infants) or indomethacin 0.20 to 0.25 mg/kg every 12 hours (15 infants) and also received a dose of saline.
Outcomes: primary outcomes were the effects of the first dose on cerebral blood flow and cerebral blood volume. The PDA closure rates, need for surgical ligation of PDA, and need for re‐treatment with indomethacin or ibuprofen were reported.
The study by Pezzati and coworkers was conducted in a single centre in Italy (Pezzati 1999).
Objective: to evaluate the effect of IV ibuprofen and IV indomethacin for treatment of PDA on mesenteric and renal blood flow velocity in preterm infants.
Population: preterm mechanically ventilated infants (less than 33 weeks' PMA) with a PDA diagnosed by Doppler ECHO.
Intervention: 17 infants were randomised to receive either IV indomethacin 0.2 mg/kg (eight infants) or IV ibuprofen 10 mg/kg (nine infants) as a continuous infusion over 15 minutes. Regardless of ductal closure after the first dose, all infants received a second and third dose of indomethacin 0.1 mg/kg or ibuprofen 5 mg/kg at 24‐hour intervals.
Outcomes: primary outcomes were mesenteric and renal blood flow velocity. Secondary outcomes included ductal closure, ductal reopening, and NEC.
The study by Plavka and coworkers was conducted in three centres in the Czech Republic (Plavka 2001).
Objective: to compare adverse effects and efficacy of IV ibuprofen with IV indomethacin for treatment of PDA in very preterm infants.
Population: 41 preterm infants with clinical and ECHO signs of PDA.
Intervention: infants received either IV ibuprofen 8 mg/kg every 24 hours for three doses (21 infants) or IV indomethacin 0.2 mg/kg every 24 hours for three doses (20 infants). If PDA persisted, treatment was repeated at half dose every 24 hours for six doses. Resistant PDA was ligated.
Outcomes: primary outcome was PDA closure. Secondary outcomes included reopening of the duct, need for surgical ligation rates, and cerebral blood flow velocities.
The study by Pourarian and coworkers was conducted in a single centre in Iran (Pourarian 2008).
Objective: to evaluate the therapeutic effects of oral administration of indomethacin or ibuprofen suspension on closure of PDA in preterm infants.
Population: 20 preterm infants with ECHO‐confirmed PDA
Intervention: for the indomethacin group, the powder content of a 25 mg indomethacin capsule was freshly prepared by dissolving in 25 mL distilled water. This was given orally as 0.2 mg/kg for three doses at 24‐hour intervals (10 infants). For the ibuprofen group, an ibuprofen oral suspension containing 100 mg/5 mL was given as an initial dose of 10 mg/kg, followed by two further doses of 5 mg/kg at 24‐hour intervals (10 infants). Administration of the second or third doses of each drug was dependent on achievement of ductal closure after the initial doses.
Outcomes: primary outcome was ductal closure. Secondary outcomes included need for surgical closure, NEC, change in mean serum creatinine levels before and after treatment, increase in BUN level greater than 14 µmol/L, and thrombocytopenia less than 50,000/mm3.
The study by Salama and coworkers was conducted in a single centre in Qatar (Salama 2008).
Objective: to compare the efficacy of oral ibuprofen with IV indomethacin for closure of a significant PDA in preterm infants.
Population: 41 preterm infants (PMA less than 34 weeks, BW less than 2500 grams) diagnosed with haemodynamically significant PDA.
Intervention: 20 infants received IV indomethacin (three doses of 0.2 mg/kg/dose every 24 hours) and 21 received oral ibuprofen (10 mg/kg on the first day followed by 5 mg/kg for two more days). Ibuprofen was mixed with 0.5 mL of milk before its administration via an oro‐gastric tube.
Outcomes: primary outcome was complete closure of the PDA. Secondary outcomes included need for surgical ligation, bowel perforation, and mortality.
The study by Su and coworkers was conducted in a single centre in Taiwan (Su 2003).
Objective: to compare IV ibuprofen and IV indomethacin with regard to efficacy and safety for the early treatment of PDA in preterm infants.
Population: 63 preterm infants (PMA 32 weeks or less, BW 1500 grams or less) with ECHO evidence of a significant PDA.
Intervention: 32 infants received IV ibuprofen 10 mg/kg initially followed by 5 mg/kg after 24 and 48 hours and 31 received IV indomethacin 0.2 mg/kg every 12 hours for three doses.
Outcomes: primary outcome was PDA closure. Secondary outcomes included need for surgical ligation, mortality, NEC, CLD at 36 weeks' PMA, IVH, PVL, ROP, hospital stay, duration of mechanical ventilation, days to full enteral feeds, and gastric haemorrhage.
The study by Su and coworkers was conducted in a single centre in Taiwan (Su 2008).
Objective: to ascertain whether ibuprofen was effective and safe in inducing PDA closure in extremely preterm infants.
Population: 119 infants (PMA 28 weeks or less) with RDS and PDA confirmed by ECHO.
Intervention: 59 infants received IV indomethacin 0.2 mg/kg (1 mL) as the initial dose and then 0.1 mg/kg in infants less than 48 hours old, and 0.2 mg/kg in infants over 48 hours old at 24‐hour intervals as indicated by PDA flow patterns. Sixty infants received IV ibuprofen 10 mg/kg (1 mL) and then 5 mg/kg at 24‐hour intervals, as indicated by PDA flow patterns.
Outcomes: PDA closure rate, need for ductal ligation, mortality, NEC, bowel perforation, gastrointestinal haemorrhage, BPD, sepsis, IVH, PVL, days to full enteral feeds, days to regain BW, days on ventilation, days of supplemental oxygen, post‐treatment serum creatinine levels, and oliguria (less than 1 mL/kg/hour).
The study by Supapannachart and coworkers was conducted in a single centre in Thailand (Supapannachart 2002).
Objective: to assess whether oral ibuprofen daily for three days was as effective as indomethacin to treat symptomatic PDA in preterm infants and to compare the adverse effects of oral ibuprofen to oral or IV indomethacin.
Population: 18 preterm (less than 34 weeks' PMA) infants with a symptomatic PDA.
Intervention: nine infants received oral ibuprofen 10 mg/kg/dose for three doses given at 24‐hourly intervals and nine infants received three doses of oral or IV indomethacin 0.2 mg/kg/dose given at 12‐hourly intervals.
Outcomes: primary outcome was PDA closure. Secondary outcomes included mortality, CLD (age not stated), IVH (grade not stated), and NEC.
The study by Van Overmeire and coworkers was conducted in a single centre in Belgium (Van Overmeire 1997).
Objective: to evaluate the efficacy and adverse effects of IV ibuprofen for the early treatment of PDA and compare it with IV indomethacin.
Population: preterm infants (PMA less than 33 weeks) with PDA diagnosed by ECHO.
Intervention: 40 infants were randomly assigned at two to three days of life to receive either IV ibuprofen (20 infants) with an initial dose of 10 mg/kg followed by 5 mg/kg 24 and 48 hours later or IV indomethacin (20 infants) 0.2 mg/kg every 12 hours for three doses. Presence of a PDA was verified by Doppler ECHO prior to enrolment, after the last dose of the randomised treatment, and at the age of seven days. When a PDA was present after the randomised treatment and the infant required mechanical ventilation, the infant was treated with indomethacin 0.2 mg/kg every 12 hours for three doses.
Outcomes: primary outcome was ductal closure. Secondary outcomes included need for surgical ligation of a PDA, need for re‐treatment with indomethacin, mortality, CLD (at 28 days of age), duration of assisted ventilation, duration of supplemental oxygen, sepsis, NEC, age to regain BW, and ROP.
The second study by Van Overmeire and coworkers was conducted in five centres in Belgium (Van Overmeire 2000).
Objective: to compare IV ibuprofen and IV indomethacin with regard to efficacy and safety for the early treatment of PDA in preterm infants.
Population: 148 infants (PMA 24 to 32 weeks) with RDS and a PDA confirmed by ECHO.
Intervention: 74 infants received IV ibuprofen 10 mg/kg as an initial dose followed by two doses of 5 mg/kg at 24 and 48 hours and 74 infants received IV indomethacin 0.2 mg/kg every 12 hours for three doses. When the ductus arteriosus was still patent after the randomly assigned treatment in an infant in either group who was still receiving mechanical ventilation, indomethacin (three doses of 0.2 mg/kg at 12‐hour intervals) was given as non‐randomised rescue treatment. If this therapy did not promote ductal closure and the infant continued to receive mechanical ventilation, or if there was a contraindication to the second pharmacological treatment, surgical ligation of the ductus was performed.
Outcomes: primary outcome was ductal closure. Other outcomes included mortality by one month, NEC, localised bowel perforation, extension of IVH during treatment, PVL, CLD (need for supplemental oxygen for more than 28 days), duration of supplemental oxygen, duration of mechanical ventilation, time to regain BW, time to full enteral feeding, urine output, and serum creatinine.
The study by Yadav and coworkers was conducted in two tertiary care institutes in New Delhi, India (Yadav 2014).
Objective: to assess the efficacy and safety of oral ibuprofen for PDA closure in preterm neonates and compare it with oral indomethacin.
Population: 83 preterm infants with a haemodynamically significant PDA (PMA less than 37 weeks, BW less than 2500 grams) confirmed by ECHO.
Intervention: 48 infants received oral ibuprofen 10 mg/kg on the first day followed by 5 mg/kg every 24 hours for two doses and 35 infants received indomethacin as three doses of 0.20 to 0.25 mg/kg every 24 hours depending on the gestational age (initial dose was 0.2 mg/kg, subsequent doses at two to seven days of age were 0.2 mg/kg/dose every 24 hours for two doses, and at seven days of age were 0.25 mg/kg/dose every 24 hours for two doses).
Outcomes: primary outcome was PDA closure. Secondary outcomes included need for a repeat course of medications (after 48 hours of the third dose of treatment), reopening of the duct, need for surgical ligation (after two courses of treatment), oliguria, gastrointestinal bleed, NEC, IVH, derangement of renal functions, and pulmonary hypertension.
Oral ibuprofen versus intravenous or oral indomethacin (Comparison 4)
The study by Akisu and coworkers was conducted in a single centre in Turkey (Akisu 2001).
Objective: to investigate the efficacy and safety of enteral ibuprofen for the treatment of PDA and to compare it with enteral indomethacin.
Population: 23 preterm infants (less than 35 weeks' PMA) with a PDA diagnosed by Doppler ECHO.
Intervention: 12 infants received enteral ibuprofen 10 mg/kg as the initial dose followed by 5 mg/kg 24 and 48 hours later and 11 infants received three doses of enteral indomethacin 0.2 mg/kg every 12 hours.
Outcomes: primary outcome was ductal closure. Other outcomes included need to re‐treat a PDA with indomethacin or ibuprofen, urine output, serum creatinine after treatment, thrombocyte counts, gastrointestinal haemorrhage, IVH, sepsis, and mortality.
The study by Aly and coworkers was a single centre study conducted in Egypt (Aly 2007).
Objective: to evaluate the feasibility of the use of oral ibuprofen suspension versus IV indomethacin in the treatment of PDA in preterm infants.
Population: 21 preterm infants (less than 35 weeks' PMA) aged two to seven days with respiratory distress and PDA diagnosed by Doppler ECHO.
Intervention: nine infants received three doses of IV indomethacin 0.2 mg/kg at 12‐hour intervals and 12 infants received an initial oral dose of ibuprofen 10 mg/kg, followed by two doses of 5 mg/kg after 24 and 48 hours.
Outcomes: primary outcome was ductal closure. Secondary outcomes included biochemical tests (serum creatinine), pulmonary haemorrhage, gastrointestinal bleed, NEC, gastrointestinal perforation, and increase in serum creatinine following treatment.
The study by Chotigeat and coworkers was a single centre study in Thailand (Chotigeat 2003).
Objective: to compare efficacy and adverse effects of ibuprofen versus indomethacin treatment for symptomatic PDA in preterm infants.
Population: preterm infants with a symptomatic PDA confirmed by ECHO.
Intervention: 30 infants were randomised to receive either three oral doses of ibuprofen (dose not stated) given at 24‐hourly intervals or three doses of IV indomethacin (dose not stated) given at 12‐hourly intervals starting within 10 days of life.
Outcomes: primary outcome measure was ductal closure. Secondary outcomes included the need for surgical closure of a PDA, the need for re‐treatment with ibuprofen or indomethacin, mortality by 28 days, CLD (at 28 days), sepsis, ROP, and serum creatinine levels after treatment.
The study by Fakhraee and coworkers was conducted in a single centre in Iran (Fakhraee 2007).
Objective: to compare the efficacy and safety of oral ibuprofen and oral indomethacin for the treatment of PDA in preterm infants.
Population: 36 preterm infants (less than 34 weeks' PMA).
Intervention: 18 infants were randomised to receive three oral doses of indomethacin 0.2 mg/kg at 24‐hour intervals and 18 infants received three doses of oral ibuprofen (first dose of 10 mg/kg, followed by 5 mg/kg/dose at 24‐hour intervals).
Outcomes: primary outcome was ductal closure. Secondary outcomes included maximum serum BUN and creatinine levels after treatment, NEC, mortality at 1 month of age, and IVH (grades III and IV).
The study by Pourarian and coworkers was conducted in a single centre in Iran (Pourarian 2008).
Objective: to evaluate the therapeutic effects of oral administration of indomethacin or ibuprofen suspension on closure of PDA in preterm infants.
Population: 20 preterm infants with ECHO‐confirmed PDA.
Intervention: for the indomethacin group, the powder content of a 25 mg indomethacin capsule was freshly prepared by dissolving in 25 mL distilled water. This was given orally as 0.2 mg/kg for three doses at 24‐hour intervals. For the ibuprofen group, an ibuprofen oral suspension containing 100 mg/5 mL was given as an initial dose of 10 mg/kg, followed by two further doses of 5 mg/kg at 24‐hour intervals. Administration of the second or third doses of each drug was dependent on achievement of ductal closure after the initial doses.
Outcomes: primary outcome was ductal closure. Secondary outcomes included need for surgical closure, NEC, change in mean serum creatinine levels before and after treatment, increase in BUN level more than 14 µmol/L, and thrombocytopenia less than 50,000 mm3.
The study by Salama and coworkers was conducted in a single centre in Qatar (Salama 2008).
Objective: to compare the efficacy of oral ibuprofen with IV indomethacin for closure of a significant PDA in preterm infants.
Population: 41 preterm infants (PMA less than 34 weeks, BW less than 2500 grams) diagnosed with haemodynamically significant PDA.
Intervention: 20 infants received IV indomethacin 0.2 mg/kg/dose every 24 hours for three doses and 21 infants received oral ibuprofen 10 mg/kg on the first day followed by 5 mg/kg for two more days. Ibuprofen was mixed with 0.5 mL of milk before its administration via an oro‐gastric tube.
Outcomes: primary outcome was complete closure of the PDA. Secondary outcomes included need for surgical ligation, bowel perforation, and mortality.
The study by Supapannachart and coworkers was conducted in a single centre in Thailand (Supapannachart 2002).
Objective: to assess whether oral ibuprofen was as effective as indomethacin to treat symptomatic PDA in preterm infants and to compare the adverse effects of oral ibuprofen to indomethacin.
Population: 18 preterm (less than 34 weeks' PMA) infants with a symptomatic PDA.
Intervention: infants were randomly assigned to receive either oral ibuprofen 10 mg/kg/dose for three doses given at 24‐hourly intervals or three doses of oral or IV indomethacin 0.2 mg/kg/dose given at 12‐hourly intervals.
Outcomes: primary outcome was PDA closure. Secondary outcomes included mortality, CLD (age not stated), IVH (grades not stated), and NEC.
The study by Yadav and coworkers was conducted in two tertiary care institutes in New Delhi, India (Yadav 2014).
Objective: to assess the efficacy and safety of oral ibuprofen therapy for PDA closure in preterm neonates and compare it with oral indomethacin.
Population: 83 preterm infants with a haemodynamically significant PDA (PMA less than 37 weeks, BW less than 2500 grams) confirmed by ECHO.
Intervention: 48 infants received oral ibuprofen 10 mg/kg on the first day followed by 5 mg/kg every 24 hours for two doses and 35 infants received indomethacin as three doses of 0.20 to 0.25 mg/kg every 24 hours depending on the gestational age (initial dose was 0.2 mg/kg, subsequent doses at two to seven days of age were 0.2 mg/kg/dose every 24 hours for two doses, and at seven days of age 0.25 mg/kg/dose every 24 hour for two doses).
Outcomes: primary outcome was PDA closure. Secondary outcomes included need for a repeat course of medications (after 48 hours of the third dose of treatment), reopening of the duct, need for surgical ligation (after two courses of treatment), oliguria, gastrointestinal bleed, NEC, IVH, derangement of renal functions, and pulmonary hypertension.
Oral ibuprofen versus intravenous ibuprofen (Comparison 5)
The study by Akar and coworkers was conducted in a single centre in Ankara, Turkey Akar 2017.
Objective: To evaluate the interaction between oxidative status and the medical treatment of PDA with different forms of ibuprofen.
Population: 102 preterm infants of < 32 weeks' PMA, birth weight < 1500 grams, and postnatal age 48 to 96 hours with PDA.
Intervention: IV Ibuprofen 10 mg/kg initial dose followed by 5 mg/kg after 24 and 48 hours; PO ibuprofen 10 mg/kg initial dose followed by 5 mg/kg after 24 and 48 hours.
Outcomes: The primary outcome of the study was the effect of different forms of ibuprofen treatment on the antioxidant and oxidant status of the patients. Secondary outcomes were the relationship between pretreatment total antioxidant capacity and total oxidant status levels and the success rate of PDA closure and need for surgical ligation. We included PDA closure rates and need for surgical ligation in the analyses.
The study by Cherif and coworkers was conducted in a single centre in Tunis, Tunisia (Cherif 2008).
Objective: to compare efficacy and tolerance between oral and IV ibuprofen in early closure of PDA in VLBW infants.
Population: 64 VLBW infants with ECHO‐confirmed PDA, PMA less than 32 weeks, BW less than 1500 grams, postnatal age 48 to 96 hours, respiratory distress requiring more than 25% oxygen supplementation.
Intervention: 32 infants received oral ibuprofen 10 mg/kg as the initial dose and 32 infants received IV ibuprofen 10 mg/kg as the initial dose. After the first dose of treatment in both groups, ECHO evaluation was performed to determine the need for a second or a third dose. In each group, in case the ductus was still open after the third dose, IV ibuprofen (an initial dose of 10 mg/kg followed by two doses of 5 mg/kg each, after 24 and 48 hours) as a non‐randomised rescue treatment was given. If this therapy did not promote ductal closure and the infant continued to receive mechanical ventilation, surgical ligation of the ductus was performed.
Outcomes: PDA closure rate, need for surgical ligation, rate of reopening of the ductus, oliguria, increase in serum creatinine level greater than 16 mg/dL, change in creatinine concentrations, IVH grades I or II and grades III or IV, PVL, NEC, bowel perforation, sepsis, duration of intubation, survival at one month, and duration of hospital stay.
The study by Erdeve and coworkers was conducted in a single centre in Ankara, Turkey (Erdeve 2012).
Objective: to compare the efficacy and safety of oral versus IV ibuprofen for the pharmacological closure of PDA in less mature preterm infants.
Population: 80 infants with PMA 28 weeks or less, BW less than 1000 grams, postnatal age 48 to 96 hours and with ECHO‐confirmed significant PDA.
Intervention: 36 infants received oral ibuprofen and 34 infants received IV ibuprofen at a dose of 10 mg/kg followed by 5 mg/kg at 24 and 48 hours.
Outcomes: primary outcome was PDA closure rate. Secondary outcomes included mortality, need for re‐treatment or surgical treatment of the PDA, duration of ventilation, duration of hospital stay, increase in serum bilirubin level after treatment, plasma creatinine after the first course of treatment, rate of ductal reopening, pneumothorax, pulmonary haemorrhage, pulmonary hypertension, BPD (supplemental oxygen at 36 weeks' PMA), IVH (grades I to IV), NEC, ROP, and ROP requiring laser treatment.
The study by Gokmen and coworkers was conducted in a single centre in Ankara, Turkey (Gokmen 2011).
Objective: to compare oral ibuprofen versus IV ibuprofen for closure of PDA in VLBW infants.
Population: 108 VLBW infants with PDA, verified by ECHO (PMA 32 weeks or less, BW 1500 grams or less, postnatal age 48 to 96 hours).
Intervention: 54 infants received either IV ibuprofen and 54 infants received oral ibuprofen at an initial dose of 10 mg/kg, followed by 5 mg/kg at 24 and 48 hours. Six infants (four in the IV group and two in the oral group) died before they completed the treatment and were excluded from the analyses except for the outcome of mortality during hospital stay.
Outcomes: renal tolerance, mean plasma creatinine after treatment, urine output after treatment, cystatin‐C levels, failure to close a PDA, need for second course of ibuprofen, need for surgical ligation, oliguria, hospital stay, NEC, gastrointestinal bleed, sepsis, pneumothorax, BPD (supplemental oxygen at 36 weeks' PMA or at discharge, which ever came first), ROP requiring laser treatment, and mortality during hospital stay.
Notes: in 2013, 57 children (56%) of the original 102 infants enrolled in this study were followed to an age of 18 to 24 months' corrected age; 30 infants in the oral ibuprofen group and 27 infants in the IV ibuprofen group were assessed for long‐term outcomes. The following outcomes were reported; Mental (MDI) and Psychomotor (PDI) Developmental Index on Bayley Scales of Infant Development II, moderate/severe cerebral palsy with functional deficits that required rehabilitation services, bilateral hearing loss (requiring amplification), blindness in either eye, MDI less than 70, and PDI less than 70.
The study by Pistulli and coworkers was conducted in a single centre in Tirana, Albania (Pistulli 2014).
Objective: to compare the efficacy and safety of oral ibuprofen versus IV ibuprofen in LBW preterm infants.
Population: 80 preterm LBW infants with PMA 28 to 32 weeks, BW 2000 grams or less, postnatal age 48 to 96 hours, RDS treated with mechanical ventilation with oxygen requirement greater than 30%, and PDA verified by ECHO. Twelve infants were excluded from the analysis.
Intervention: 44 infants received ibuprofen at an initial dose of 10 mg/kg via an oro‐gastric tube and 36 infants received 10 mg/kg of ibuprofen infused IV over a 15‐minute period with a syringe pump followed by 5 mg/kg for two consecutive days. When the PDA was still haemodynamically significant 24 hours after the third dose, as demonstrated by ECHO, and there was no evidence of deterioration in brain ultrasonography, a second course of ibuprofen with three other doses was administered. Infants with persistent PDA even after the second course were treated surgically. In the oral ibuprofen group, seven infants were excluded because of mortality before the complete treatment course and one infant was excluded because of pulmonary haemorrhage (total, eight infants). Outcomes were reported for 36 infants in the oral ibuprofen group. In the IV ibuprofen group, three infants were excluded because of a gastrointestinal bleed and one infant was excluded because only two doses of ibuprofen were administered (total, four infants). Outcomes were reported for 32 infants.
Outcomes: failure to close a PDA, need for a second course of ibuprofen, need for surgical ligation, plasma creatinine following treatment, and oliguria.
High‐dose ibuprofen (IV or PO) versus standard‐dose regimen of ibuprofen (IV or PO)(Comparison 6)
The study by Dani and coworkers was conducted as a multi centre study in four NICUs in Italy (Dani 2012).
Objective: to assess whether a high‐dose of IV ibuprofen versus a standard‐dose IV ibuprofen was more effective in closing a PDA, without increasing adverse effects.
Population: 95 infants underwent randomisation; 48 were allocated to standard ibuprofen and 47 to high‐dose ibuprofen. There were 70 infants with PMA less than 29 weeks, ECHO evidence of significant PDA, age 12 to 24 hours and RDS necessitating respiratory support.
Intervention: 35 infants received a high‐dose of IV ibuprofen (20‐10‐10 mg/kg/day) and 35 infants received a standard‐dose of IV ibuprofen (10‐5‐5 mg/kg/day). Thirty‐five infants (mean (SD) PMA 25.6 (1.8) weeks; BW 781 (225) grams) were randomised to a high‐dose ibuprofen and 35 infants (mean (SD) PMA 26.0 (1.7) weeks; BW 835 (215) grams) were randomised to standard‐dose ibuprofen.
Outcomes: ductal closure, serum creatinine on day three of treatment, oliguria (1 mL/kg/hour or less during a 24‐hour collection period), peak total serum bilirubin during the first week of life, IVH (all grades and grades III and IV), PVL, ROP (all stages, stage greater than 2), NEC, BPD (oxygen requirement at 36 weeks' PMA), sepsis, mortality, and hospital stay (days).
The study by Fesharaki and coworkers was conducted in a single centre in Tehran, Iran (Fesharaki 2012).
Objective: to compare the effects of high‐dose ibuprofen versus standard ibuprofen.
Population: 60 preterm infants with a haemodynamically significant PDA confirmed by ECHO (PMA 29 weeks 6/7 to 35 weeks 6/7; BW 1000 to 2500 grams; postnatal age 72 to 120 hours).
Intervention: 30 infants received ibuprofen 15 mg/kg on the first day followed by two doses of 7.5 mg/kg on next two days and 30 infants received 10 mg/kg ibuprofen on the first day followed by 5 mg/kg on next two days.
Outcomes: failure to close a PDA, urine output less than 0.5 mL/kg after the onset of treatment, and gastrointestinal bleed.
The study by Pourarian and coworkers was conducted in two NICUs in Shiraz, Iran (Pourarian 2015).
Objective: to compare the efficacy and possible adverse effects of oral high‐dose ibuprofen to that of standard regimen in closing a PDA
Population: 60 preterm infants < 37 weeks' PMA and postnatal age of 3 to 7 days with ECHO diagnosis of haemodynamically significant PDA
Intervention: high‐dose ibuprofen group received 20 mg/kg of ibuprofen orally followed by 10 mg/kg/dose after 24 and 48 hours. The normal‐dose ibuprofen group received 10mg/kg of ibuprofen followed by 5 mg/kg/dose after 24 and 48 hours.
Outcomes: primary outcome: failure to close the PDA after the first course of ibuprofen. Secondary outcomes included bleeding disorders, GI bleeding, NEC, pulmonary haemorrhage, mortality, oliguria (≤ 1 ml/kg/H), serum creatinine, urine output (ml/kg/hour), and platelet count after treatment.
Early versus expectant administration of intravenous ibuprofen (Comparison 7)
The study by Sosenko and coworkers was conducted in a single NICU in Miami, Florida, USA (Sosenko 2012).
Objective: to determine whether early ibuprofen treatment at the onset of subtle PDA symptoms would improve respiratory outcome in preterm infants compared with expectant management, with ibuprofen treatment only when the PDA became haemodynamically significant.
Population: infants born with BW 500 to 1250 grams and PMA 23 to 32 weeks, who were more than 24 hours but 14 days or less old and who had ECHO for subtle PDA symptoms (metabolic acidosis, murmur, bounding pulses).
Intervention: infants were randomised to 'early' treatment (54 infants received blinded ibuprofen) or 'expectant' management (51 infants received blinded placebo). If the PDA became haemodynamically significant (pulmonary haemorrhage, hypotension, respiratory deterioration), infants received open‐label ibuprofen. Infants with haemodynamically significant PDA at enrolment were excluded from the study.
The dosing schedule for ibuprofen was an initial dose of 10 mg/kg, followed by two doses of 5 mg/kg each, every 24 hours, by slow IV infusion; dosing of placebo involved equivalent volumes of dextrose by slow IV infusion on the same schedule.
Outcomes: days on supplemental oxygen during the first 28 days of life, mortality during hospital stay, supplemental oxygen at 36 weeks' PMA, intestinal perforation, NEC requiring surgery, IVH (grades III and IV), PVL, sepsis, and ROP (stage 3 or greater).
Echocardiographically guided intravenous ibuprofen versus standard intravenous ibuprofen (Comparison 8)
The study by Bravo and coworkers was conducted in a single NICU in Madrid, Spain (Bravo 2014).
Objective: to explore the efficacy of ECHO‐guided pharmacological closure of the ductus arteriosus in reducing the number of required ibuprofen doses without increasing the reopening rate.
Population: 49 preterm infants with an ECHO‐confirmed PDA measuring 1.5 mm or greater (PMA 24 to 34 weeks).
Intervention: infants received the first dose of ibuprofen 10 mg/kg and were then randomised to receive either standard treatment (21 infants) or ECHO‐guided treatment (28 infants). Infants in the standard group received two additional doses of ibuprofen 5 mg/kg at 24‐hour intervals after the initial dose of 10 mg/kg, independently of ductal size, as long as additional doses were not contraindicated. Infants in the ECHO‐guided group received additional doses of ibuprofen 5 mg/kg at 24‐hour intervals only if the PDA was still 1.5 mm or greater at the time of the corresponding ibuprofen dose. Decision on whether to treat the PDA when the diameter was less than 1.5 mm in the ECHO‐guided group was at the discretion of the treating consultant. Additional ibuprofen doses were administered only when the PDA was greater than 1.5 mm 24 hours after a complete ibuprofen course (therapeutic failure) or when a reopening was documented.
Outcomes: primary outcome was reopening of PDA; secondary outcomes included failure to close a PDA, number of ibuprofen doses used, need for surgical ligation, mortality, BPD (need for supplemental oxygen at 36 weeks' PMA), IVH (grade II or III), PVL, oliguria, creatinine after treatment, and laser therapy for ROP.
Notes: Dr. Bravo clarified that ibuprofen was given IV. She communicated that the random sequence was computer‐generated and that the allocation to one of the two groups was by sequential numbered, opaque and sealed envelopes.
Continuous intravenous infusion of ibuprofen versus standard intravenous ibuprofen (boluses) (Comparison 9)
The study by Lago and coworkers was conducted in a single centre in Padua, Italy (Lago 2014).
Objective: to establish whether continuous infusion of ibuprofen was more effective in VLBW infants with no additional adverse effects and reduce the need for surgical ligation compared with infants treated with conventional 15‐minute intermittent boluses.
Population: 112 VLBW infants (mean (SD) PMA 27.2 (2) weeks; weight 1019 (330) grams).
Intervention: 56 infants were given IV ibuprofen in conventional 15‐minute intermittent boluses, while the other 56 were administered IV ibuprofen as a 24‐hour continuous infusion, both at standard‐doses (10/5/5 mg/kg). One infant in the continuous infusion group was excluded because informed consent was withdrawn, leaving 55 infants in that group.
Outcomes: primary outcome: PDA closure rate after two standard‐dose ibuprofen courses. Secondary outcomes included mortality, PDA closure after one ibuprofen course, rate of PDA reopening, need for surgical ligation, oliguria (urine output 1 mL/kg/hour or less), creatinine after treatment, gastrointestinal haemorrhage, isolated intestinal perforation, NEC (according to modified Bell's criteria (all stages), BPD (supplemental oxygen at 36 weeks' PMA), IVH (any grade and grade III or IV), cystic PVL, duration of hospital stay, and survival without morbidity. Brain ultrasound was performed on admission and before starting each ibuprofen course, then twice a month or when clinically indicated. Any IVH was graded according to Papille's classification, and PVL was defined as periventricular white matter cysts. Any other gastrointestinal and pulmonary bleeding disorders due to interference with local prostaglandin metabolism were also recorded. BPD was defined as the need for oxygen supplementation and typical chest X‐ray features at a postconceptional age of 36 weeks. ROP was diagnosed according to international criteria.
Notes: Dr Lago clarified that ibuprofen in the continuous infusion group was given continuously IV over 24 hours.
Rectal ibuprofen versus oral ibuprofen (Comparison 10)
The study by Demir and coworkers was conducted in a single centre in Van, Turkey (Demir 2017).
Objective: to compare rectal ibuprofen with oral ibuprofen for the closure of haemodynamically significant patent ductus arteriosus (hsPDA).
Population: 72 VLBW preterm infants.
Intervention: A total of three ibuprofen doses were administered; the initial dose was 10 mg/kg and the following two doses at 24 and 48 hours were 5 mg/kg. Both rectal and oral ibuprofen were given via an oro‐gastric tube, which was flushed with 1‐2 ml of sterile water to ensure the delivery of the drug.
Outcomes: Failure to close the PDA, need for a 2nd course, need for surgical ligation, plasma bilirubin and plasma creatinine after treatment, urine output after treatment.
Excluded studies
Five studies were excluded (Alipour 2016; Amoozgar 2010; Cherif 2008; Desfrere 2005; Kalani 2016). The studies by Alipour 2016 and Amoozgar 2010 were conducted in term infants. Cherif 2008 did not include a control group. The study by Desfrere 2005 was a dose‐finding study and Kalani 2016 compared early ibuprofen with indomethacin administration to prevent intraventricular haemorrhage among preterm infants.
Risk of bias in included studies
For details, see the 'Risk of bias' summary (Figure 4) and 'Risk of bias' graph (Figure 5). These were all randomised controlled trials, but whether the randomisation was concealed or not was not always clear. In several studies, the timing of the doses of ibuprofen and indomethacin did not coincide, and therefore, the caregivers would be aware of group assignment (for details see 'Risk of bias' tables).
4.
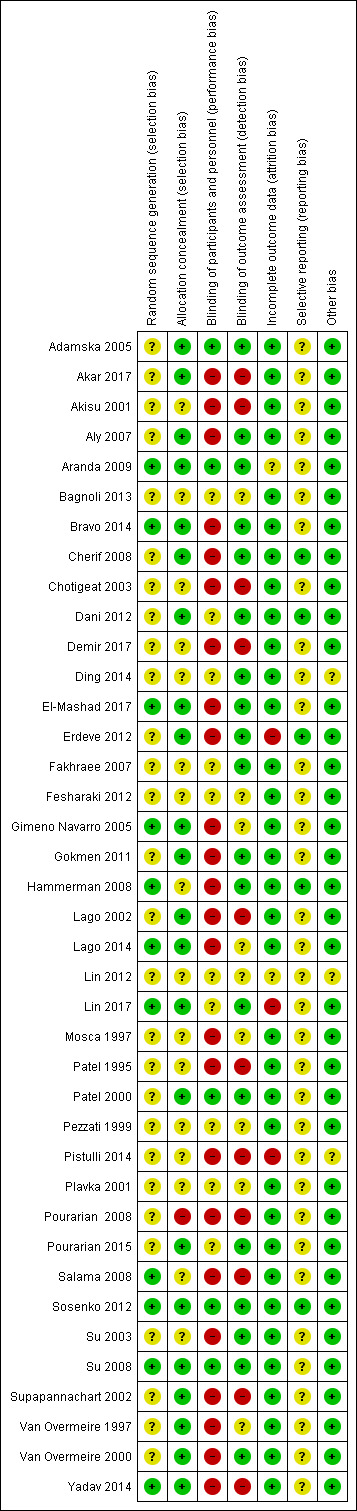
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
5.
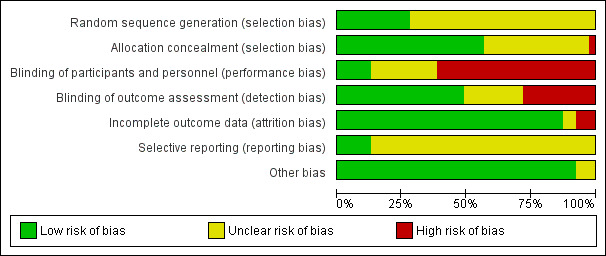
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
The random sequence was properly generated (low risk of bias) in 28% of the trials and was unclear in the remaining 72% of the trials. With regards to allocation concealment, there was low risk of bias in 56% of the trials, unclear risk in 41% and high risk of bias in 3% of the trials.
Blinding
There was adequate blinding of personnel in 13% of the trials (low risk of bias). The blinding of personnel was unclear in 26% of the trials and there was high risk of bias in 62% of the trials.
There was blinding of outcome assessments in 51% of the trials (low risk of bias). The risk of bias was unclear in 23% of the trials and the risk was high in 26% of the trials.
Incomplete outcome data
There was low risk of bias in 87% of the trials, unclear risk in 5% of the trials and the risk of bias was high in 8% of the trials.
Selective reporting
For most of the included studies, the protocol for the study was not available to us and for several studies for which the protocol was registered in a trials registry, the registration had occurred after the trials had been completed (retrospective registration). There was low risk of bias in 13% of the trials, unclear risk in 87%, and no trials had high risk of bias.
Other potential sources of bias
We did not detect any other sources of potential bias in 90% of the studies (low risk of bias). There was unclear risk in 10% of the studies and no trials had high risk of other potential sources of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
See: Summary of findings tables for the main comparisons: Table 1; Table 2; Table 3; Table 4; Table 5.
When only one study is included in an analysis the test for heterogeneity is not applicable.
Intravenous ibuprofen versus placebo or no intervention (Comparison 1)
Primary outcomes
Failure to close a patent ductus arteriosus after three doses (Analysis 1.1)
There was a statistically significantly reduced typical RR for failure to close a PDA after three doses of ibuprofen versus placebo (two studies, 206 infants); typical relative risk (RR); 0.62 (95% CI 0.44 to 0.86); typical risk difference (RD); ‐0.18 (95% CI ‐0.30 to ‐0.06); NNTB 6 (95% CI 3 to 17); I2 = 65% (moderate heterogeneity) for RR and I2 = 0% (no heterogeneity) for RD (Analysis 1.1). The quality of the evidence was moderate according to GRADE.
1.1. Analysis.
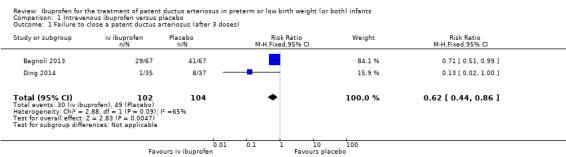
Comparison 1 Intravenous ibuprofen versus placebo, Outcome 1 Failure to close a patent ductus arteriosus (after 3 doses).
Secondary outcomes
Necrotising enterocolitis (Analysis 1.13)
There was no statistically significant difference in the incidence of NEC (two studies, 264 infants; typical RR 1.84, 95% CI 0.87 to 3.90; typical RD 0.06, 95% CI ‐0.01 to 0.13; Analysis 1.13). There was high heterogeneity for the RR (I2 = 77%) and moderate heterogeneity for the RD (I2 = 67%) (Analysis 1.13). The quality of the evidence was moderate according to GRADE.
1.13. Analysis.
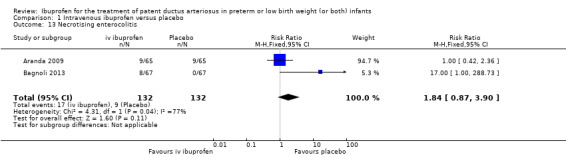
Comparison 1 Intravenous ibuprofen versus placebo, Outcome 13 Necrotising enterocolitis.
For the following outcomes, there was only one study included in each analysis and there was no significant difference between the IV ibuprofen group versus the placebo or no intervention group (for details for each outcome, see the specific analysis; tests for heterogeneity were not applicable); need for surgical ligation (Analysis 1.2); intraventricular haemorrhage (any grade) (Analysis 1.3); intraventricular haemorrhage (grades III and IV) (Analysis 1.4); periventricular leukomalacia (Analysis 1.5); pulmonary haemorrhage (Analysis 1.6); pulmonary hypertension (Analysis 1.7); retinopathy of prematurity (any stage) (Analysis 1.8); retinopathy of prematurity (stage 3 or 4) (Analysis 1.9); retinopathy of prematurity (plus disease) (Analysis 1.10); chronic lung disease (supplemental oxygen at 28 days of age) (Analysis 1.11); chronic lung disease (supplemental oxygen at 36 weeks' PMA) (Analysis 1.12); mortality by 28 days (Analysis 1.14); mortality during hospital stay (Analysis 1.18).
1.2. Analysis.
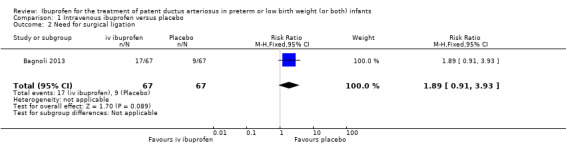
Comparison 1 Intravenous ibuprofen versus placebo, Outcome 2 Need for surgical ligation.
1.3. Analysis.
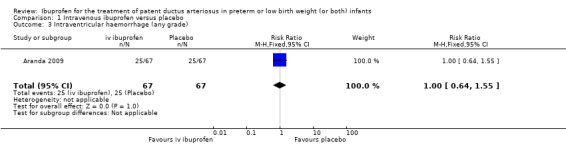
Comparison 1 Intravenous ibuprofen versus placebo, Outcome 3 Intraventricular haemorrhage (any grade).
1.4. Analysis.
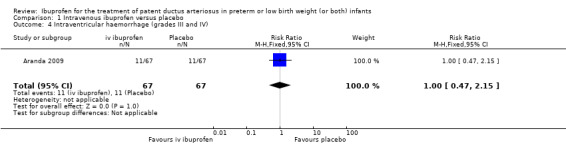
Comparison 1 Intravenous ibuprofen versus placebo, Outcome 4 Intraventricular haemorrhage (grades III and IV).
1.5. Analysis.
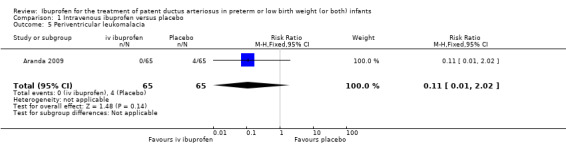
Comparison 1 Intravenous ibuprofen versus placebo, Outcome 5 Periventricular leukomalacia.
1.6. Analysis.
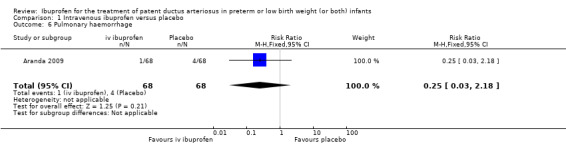
Comparison 1 Intravenous ibuprofen versus placebo, Outcome 6 Pulmonary haemorrhage.
1.7. Analysis.
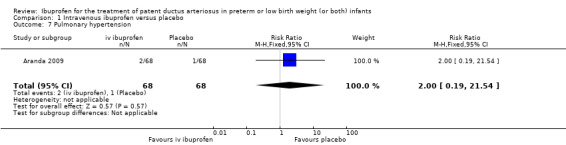
Comparison 1 Intravenous ibuprofen versus placebo, Outcome 7 Pulmonary hypertension.
1.8. Analysis.
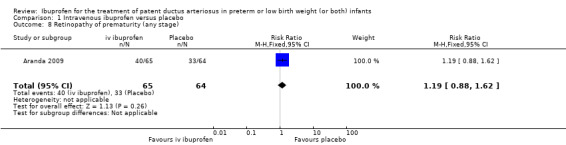
Comparison 1 Intravenous ibuprofen versus placebo, Outcome 8 Retinopathy of prematurity (any stage).
1.9. Analysis.
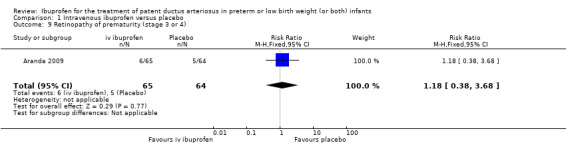
Comparison 1 Intravenous ibuprofen versus placebo, Outcome 9 Retinopathy of prematurity (stage 3 or 4).
1.10. Analysis.
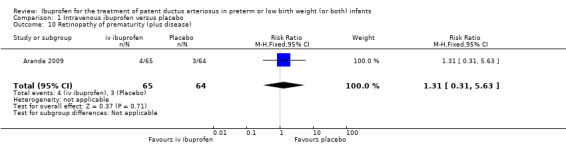
Comparison 1 Intravenous ibuprofen versus placebo, Outcome 10 Retinopathy of prematurity (plus disease).
1.11. Analysis.
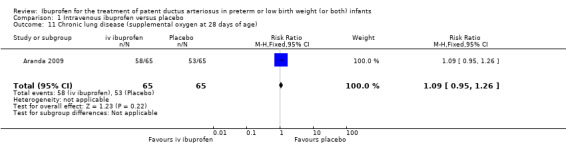
Comparison 1 Intravenous ibuprofen versus placebo, Outcome 11 Chronic lung disease (supplemental oxygen at 28 days of age).
1.12. Analysis.
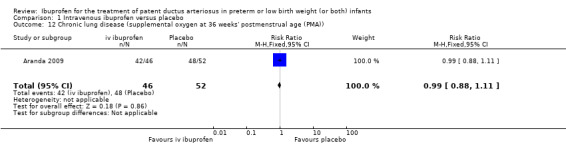
Comparison 1 Intravenous ibuprofen versus placebo, Outcome 12 Chronic lung disease (supplemental oxygen at 36 weeks' postmenstrual age (PMA)).
1.14. Analysis.
Comparison 1 Intravenous ibuprofen versus placebo, Outcome 14 Mortality by 28 days of life.
1.18. Analysis.
Comparison 1 Intravenous ibuprofen versus placebo, Outcome 18 Mortality.
For the following outcomes, there was only one study included in each analysis, but there were significant differences between the IV ibuprofen group versus the placebo or no intervention group:
Oliguria (urine output less than 1 mL/kg/hour) (Analysis 1.15)
There was a statistically significant difference in the incidence of oliguria with a higher incidence in the ibuprofen group (one study, 134 infants; RR 39, 95% CI 2.40 to 633.01; RD 0.28, 95% CI 0.17 to 0.39; NNTH 4, 95% CI 3 to 6; Analysis 1.15).
1.15. Analysis.
Comparison 1 Intravenous ibuprofen versus placebo, Outcome 15 Oliguria (urine output < 1 mL/kg/hour).
Creatinine after treatment (Analysis 1.16)
There was a statistically significantly higher creatinine level in the ibuprofen group (one study, 134 infants; MD 29.17 µmol/L, 95% CI 12.60 to 45.74; Analysis 1.16).
1.16. Analysis.
Comparison 1 Intravenous ibuprofen versus placebo, Outcome 16 Creatinine (µmol/L) after treatment.
Blood urea nitrogen after treatment (Analysis 1.17)
There was a statistically significantly higher BUN level in the ibuprofen group (one study, 134 infants; MD 18.45 µmol/L, 95% CI 12.76 to 24.14; Analysis 1.17).
1.17. Analysis.
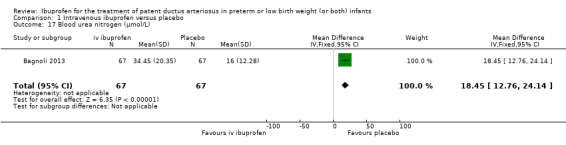
Comparison 1 Intravenous ibuprofen versus placebo, Outcome 17 Blood urea nitrogen (µmol/L).
Since this review was first published, oral ibuprofen has been introduced to close a PDA. Therefore, we included additional comparisons that were not planned a priori.
Oral ibuprofen versus placebo or no intervention (Comparison 2)
Failure to close a patent ductus arteriosus after single or three doses of ibuprofen (Analysis 2.1)
One study reported on failure to close a PDA after single or three doses of ibuprofen (Lin 2012). There was a significant reduction in the failure rate to close a PDA (64 infants; RR 0.26, 95% CI 0.11 to 0.62; RD ‐0.44, 95% CI ‐0.65 to ‐0.23; NNTB 2, 95% CI 2 to 4. (Analysis 2.1).
2.1. Analysis.
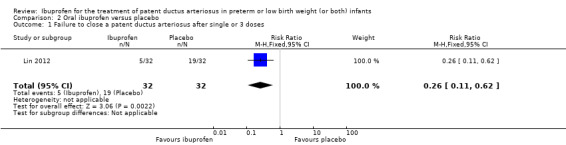
Comparison 2 Oral ibuprofen versus placebo, Outcome 1 Failure to close a patent ductus arteriosus after single or 3 doses.
The authors reported that the incidence of PVL and BPD were significantly lower in the ibuprofen group than in the placebo group (P value < 0.05). The duration of mechanical ventilation and hospitalisation were significantly shorter in the ibuprofen group than in the placebo group (P value < 0.05). There were no significant differences in the incidence of IVH, early pulmonary haemorrhage and NEC between the two groups (P value > 0.05). Only the abstract was available to us and numbers for these outcomes were not reported in the abstract. We have written to the authors to try to obtain more information, but we have not received any feedback.
Intravenous or oral ibuprofen versus intravenous or oral indomethacin (Comparison 3)
We included 24 studies for one or more of the outcomes listed under this comparison.
Primary outcome
Failure to close a patent ductus arteriosus after single or three doses (Analysis 3.1)
Twenty‐four studies reported failure rates for PDA closure after one or three doses of ibuprofen compared with indomethacin included in this comparison and none found a statistically significant difference between the groups. In the meta‐analysis, there was no statistically significant difference between the groups (1590 infants; typical RR 1.07, 95% CI 0.92 to 1.24; typical RD 0.02, 95% CI ‐0.02 to 0.06) (Analysis 3.1; Figure 6; Figure 1). There was no between‐study heterogeneity (I2 = 0% for both RR and RD). The quality of the evidence was moderate according to GRADE.
6.
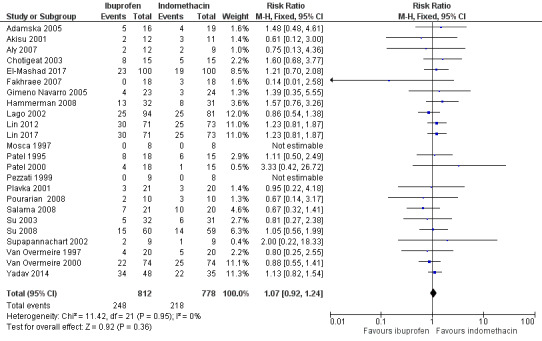
Forest plot of comparison: 3 Intravenous or oral ibuprofen versus intravenous or oral indomethacin, outcome: 3.1 Failure to close a patent ductus arteriosus (after single or three doses).
Secondary outcomes
All‐cause mortality (Analysis 3.2)
Ten studies reported on mortality that occurred at an unspecified time while in hospital and none found a statistically significant difference between the groups. The meta‐analysis showed no statistically significant difference between the groups (697 infants; typical RR 0.79, 95% CI 0.54 to 1.17; typical RD ‐0.03, 95% CI ‐0.08 to 0.02) (Analysis 3.2). There was no between‐study heterogeneity (I2 = 0% for both RR and RD).
3.2. Analysis.
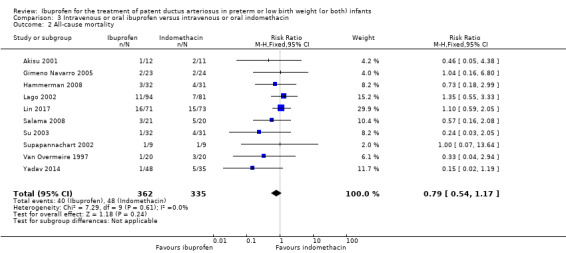
Comparison 3 Intravenous or oral ibuprofen versus intravenous or oral indomethacin, Outcome 2 All‐cause mortality.
Neonatal mortality (death during first 28/30 days of life) (Analysis 3.3)
Four studies reported on mortality by 28 or 30 days of age. There was no statistically significant difference between the groups in the individual studies or in the meta‐analysis (333 infants; typical RR 1.12, 95% CI 0.59 to 2.11; typical RD 0.01, 95% CI ‐0.05 to 0.08) (Analysis 3.3). There was no between‐study heterogeneity (I2 = 0% for both RR and RD).
3.3. Analysis.
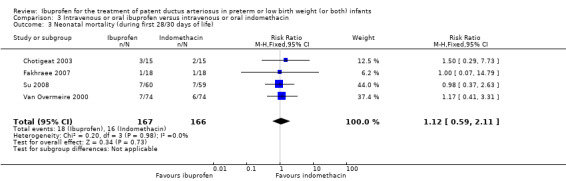
Comparison 3 Intravenous or oral ibuprofen versus intravenous or oral indomethacin, Outcome 3 Neonatal mortality (during first 28/30 days of life).
Infant mortality (mortality during the first year of life)
None of the studies reported on infant mortality.
Reopening of the ductus arteriosus (Analysis 3.4)
Seven studies reported on reopening of the PDA and none of the individual studies found a statistically significant difference between the groups. In the meta‐analysis, there was no statistically significant difference between the groups (305 infants; typical RR 1.57, 95% CI 0.83 to 2.99; typical RD 0.05, 95% CI ‐0.02 to 0.12) (Analysis 3.4). There was no between‐study heterogeneity (I2 = 0% for both RR and RD).
3.4. Analysis.
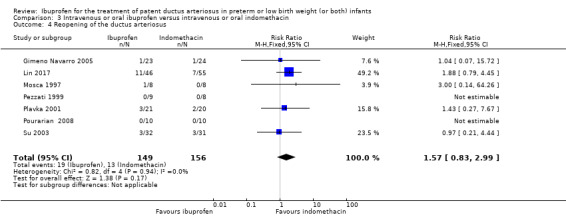
Comparison 3 Intravenous or oral ibuprofen versus intravenous or oral indomethacin, Outcome 4 Reopening of the ductus arteriosus.
Need for surgical closure of the patent ductus arteriosus (Analysis 3.5)
Sixteen studies reported on need for surgical closure of the PDA and none found a statistically significant difference between the groups. In the meta‐analysis, there was no statistically significant difference between the groups (1275 infants; typical RR 1.06, 95% CI 0.81 to 1.39; typical RD 0.01, 95% CI ‐0.03 to 0.05) (Analysis 3.5). There was no between‐study heterogeneity (I2 = 0% for both RR and RD). The quality of the evidence was moderate according to GRADE.
3.5. Analysis.
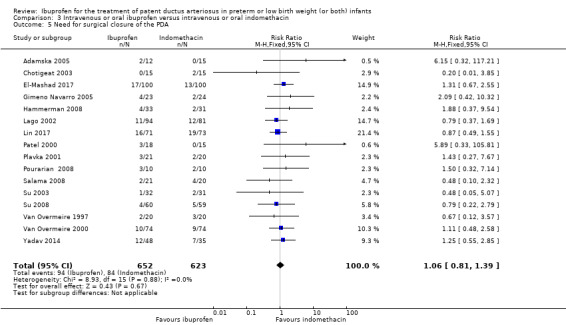
Comparison 3 Intravenous or oral ibuprofen versus intravenous or oral indomethacin, Outcome 5 Need for surgical closure of the PDA.
Need for re‐treatment with indomethacin or ibuprofen to close the patent ductus arteriosus (Analysis 3.6)
Seven studies reported on need for re‐treatment with indomethacin or ibuprofen to close the PDA and none of the studies found a statistically significant difference between the groups. In the meta‐analysis, there was no statistically significant difference between the groups (241 infants; typical RR 1.20, 95% CI 0.76 to 1.90; typical RD 0.04, 95% CI ‐0.06 to 0.14) (Analysis 3.6). There was no between‐study heterogeneity (I2 = 0% for both RR and RD).
3.6. Analysis.
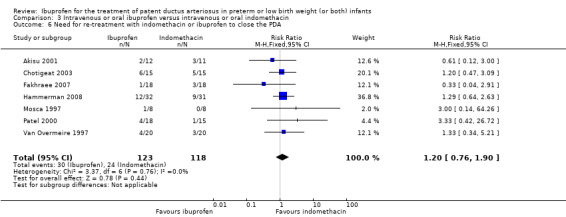
Comparison 3 Intravenous or oral ibuprofen versus intravenous or oral indomethacin, Outcome 6 Need for re‐treatment with indomethacin or ibuprofen to close the PDA.
Duration of ventilator support (Analysis 3.7)
Six studies reported on duration of ventilator support. There was a statistically significant difference between the groups favouring ibuprofen (six studies, 471 infants; MD ‐2.35 days, 95% CI ‐3.71 to ‐0.99) (Analysis 3.7). There was no heterogeneity between the studies (I2 = 19%). The quality of the evidence was moderate according to GRADE.
3.7. Analysis.
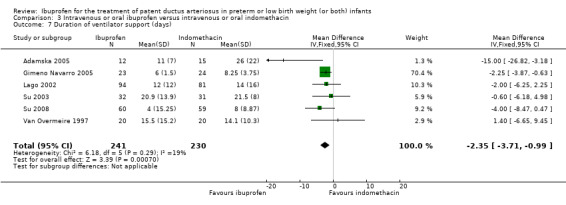
Comparison 3 Intravenous or oral ibuprofen versus intravenous or oral indomethacin, Outcome 7 Duration of ventilator support (days).
Duration of supplementary oxygen (Analysis 3.8)
Six studies reported on duration of supplementary oxygen. There was no statistically significant difference between the groups (556 infants; MD ‐0.33 days, 95% CI ‐1.66 to 0.99) (Analysis 3.8). There was low between‐study heterogeneity for this outcome (I2 = 46%).
3.8. Analysis.
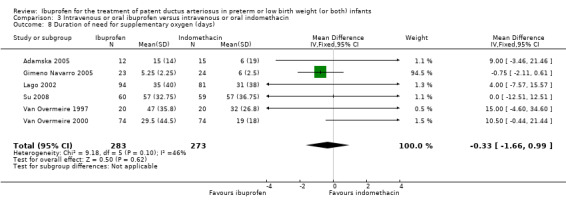
Comparison 3 Intravenous or oral ibuprofen versus intravenous or oral indomethacin, Outcome 8 Duration of need for supplementary oxygen (days).
Pneumothorax
No study reported on pneumothorax.
For the following outcomes, there were no statistically significant differences between the groups (for details see the corresponding analyses); pulmonary haemorrhage (Analysis 3.9); pulmonary hypertension (Analysis 3.10); chronic lung disease (at 28 days) (Analysis 3.11); chronic lung disease at 36 weeks' PMA (Analysis 3.12); chronic lung disease (age not stated) (Analysis 3.13); intraventricular haemorrhage (grades I to IV) (Analysis 3.14); intraventricular haemorrhage (grades III and IV) (Analysis 3.15); periventricular leukomalacia (Analysis 3.16); intestinal perforation (Analysis 3.18); gastrointestinal bleed (Analysis 3.19); time to full enteral feeds (Analysis 3.20); time to regain birth weight (Analysis 3.21); retinopathy of prematurity (according to the international classification of retinopathy of prematurity) (Analysis 3.22); sepsis (Analysis 3.23); duration of hospitalisation (Analysis 3.27).
3.9. Analysis.
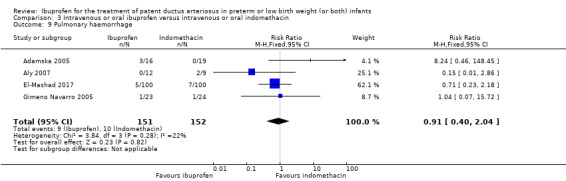
Comparison 3 Intravenous or oral ibuprofen versus intravenous or oral indomethacin, Outcome 9 Pulmonary haemorrhage.
3.10. Analysis.
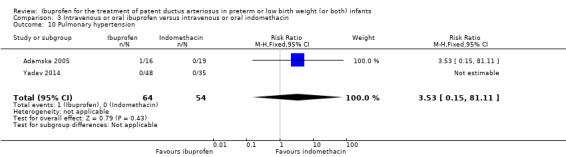
Comparison 3 Intravenous or oral ibuprofen versus intravenous or oral indomethacin, Outcome 10 Pulmonary hypertension.
3.11. Analysis.
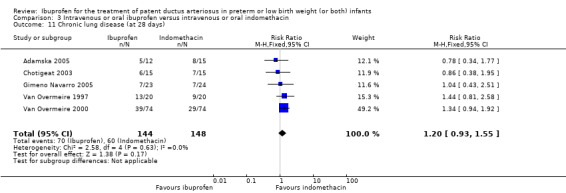
Comparison 3 Intravenous or oral ibuprofen versus intravenous or oral indomethacin, Outcome 11 Chronic lung disease (at 28 days).
3.12. Analysis.
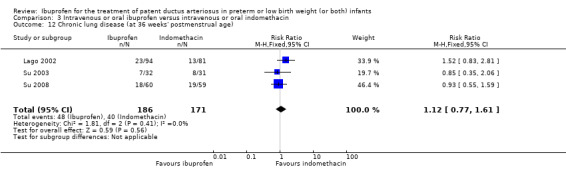
Comparison 3 Intravenous or oral ibuprofen versus intravenous or oral indomethacin, Outcome 12 Chronic lung disease (at 36 weeks' postmenstrual age).
3.13. Analysis.
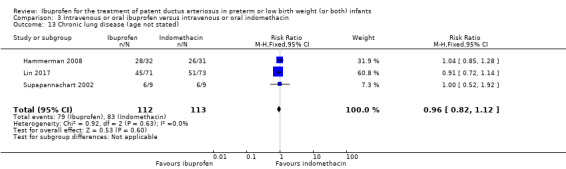
Comparison 3 Intravenous or oral ibuprofen versus intravenous or oral indomethacin, Outcome 13 Chronic lung disease (age not stated).
3.14. Analysis.
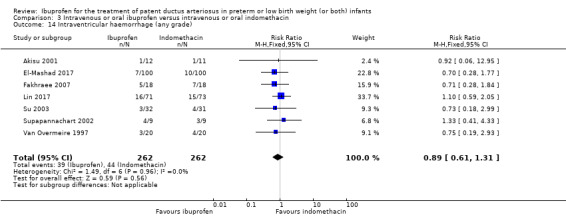
Comparison 3 Intravenous or oral ibuprofen versus intravenous or oral indomethacin, Outcome 14 Intraventricular haemorrhage (any grade).
3.15. Analysis.
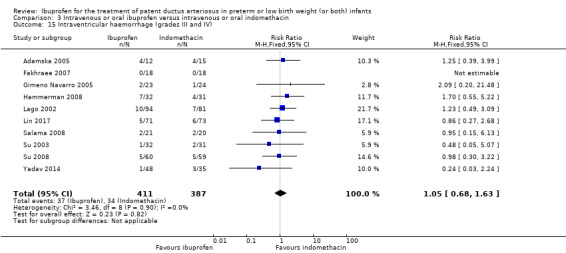
Comparison 3 Intravenous or oral ibuprofen versus intravenous or oral indomethacin, Outcome 15 Intraventricular haemorrhage (grades III and IV).
3.16. Analysis.
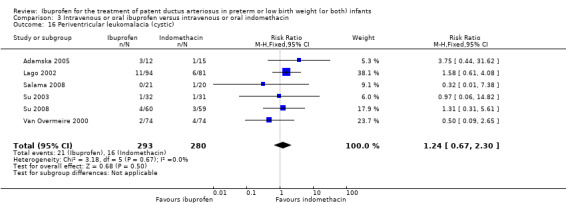
Comparison 3 Intravenous or oral ibuprofen versus intravenous or oral indomethacin, Outcome 16 Periventricular leukomalacia (cystic).
3.18. Analysis.
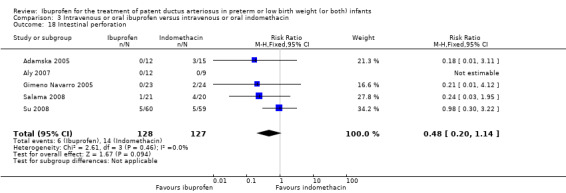
Comparison 3 Intravenous or oral ibuprofen versus intravenous or oral indomethacin, Outcome 18 Intestinal perforation.
3.19. Analysis.
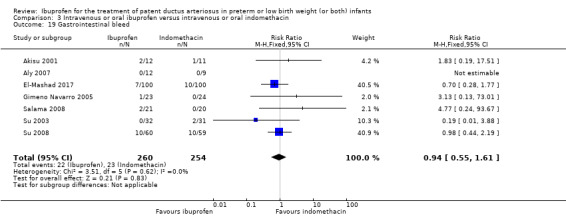
Comparison 3 Intravenous or oral ibuprofen versus intravenous or oral indomethacin, Outcome 19 Gastrointestinal bleed.
3.20. Analysis.
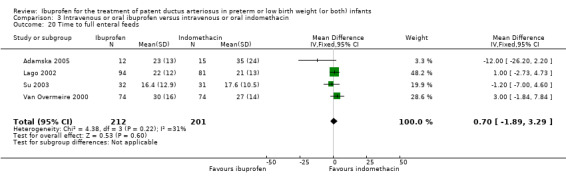
Comparison 3 Intravenous or oral ibuprofen versus intravenous or oral indomethacin, Outcome 20 Time to full enteral feeds.
3.21. Analysis.
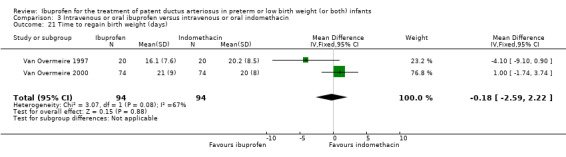
Comparison 3 Intravenous or oral ibuprofen versus intravenous or oral indomethacin, Outcome 21 Time to regain birth weight (days).
3.22. Analysis.
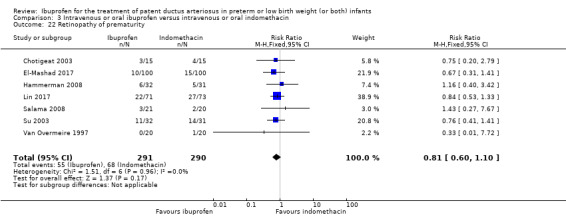
Comparison 3 Intravenous or oral ibuprofen versus intravenous or oral indomethacin, Outcome 22 Retinopathy of prematurity.
3.23. Analysis.
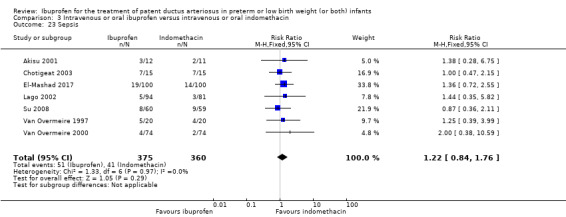
Comparison 3 Intravenous or oral ibuprofen versus intravenous or oral indomethacin, Outcome 23 Sepsis.
3.27. Analysis.
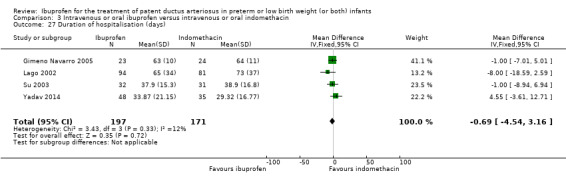
Comparison 3 Intravenous or oral ibuprofen versus intravenous or oral indomethacin, Outcome 27 Duration of hospitalisation (days).
Necrotising enterocolitis (any stage) (Analysis 3.17)
Eighteen studies reported on NEC (any stage) and none of the studies found a statistically significant difference between the groups. In one study, the rates of NEC were exceptionally high in both groups (Chotigeat 2003). In the meta‐analysis, there was a statistically significant difference between the groups favouring the ibuprofen group (1292 infants; typical RR 0.68, 95% CI 0.49 to 0.94; typical RD ‐0.04, 95% CI ‐0.07 to ‐0.01; NNTB 25, 95% CI 14 to 100) (Analysis 3.17; Figure 7; Figure 2). There was no between‐study heterogeneity (I2 = 0% for both RR and RD). The quality of the evidence was moderate according to GRADE.
7.
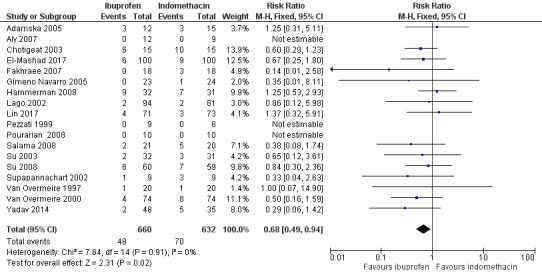
Forest plot of comparison: 3 Intravenous or oral ibuprofen versus intravenous or oral indomethacin, outcome: 3.17 Necrotising enterocolitis (any stage).
Oliguria (urine output less than 1 mL/kg/hr) (Analysis 3.24)
Six studies reported on oliguria. Two trials found a statistically significant decrease in the proportion of infants with oliguria in the ibuprofen group (Lago 2002; Van Overmeire 2000). In the meta‐analysis, there was a statistically significant reduction in the proportion of infants with oliguria in the ibuprofen group (576 infants; typical RR 0.28, 95% CI 0.14 to 0.54; typical RD ‐0.09, 95% CI ‐0.14 to ‐0.05; NNTB 11, 95% CI 7 to 20; Analysis 3.24). There was no between‐study heterogeneity for RR (I2 = 21%) and moderate for RD (I2 = 69%). Hammerman and colleagues reported no statistically significant differences in urine output between the ibuprofen and indomethacin groups at pretreatment and at 24 and 48 hours after treatment (Hammerman 2008). The quality of the evidence was moderate according to GRADE.
3.24. Analysis.
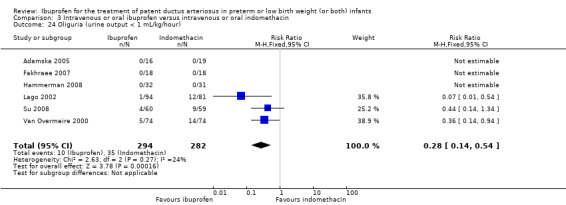
Comparison 3 Intravenous or oral ibuprofen versus intravenous or oral indomethacin, Outcome 24 Oliguria (urine output < 1 mL/kg/hour).
Serum/plasma creatinine levels 72 hours after treatment (Analysis 3.25)
Eleven studies (918 infants) reported on serum/plasma creatinine levels 72 hours after treatment in such a format that the data could be used to summarise the information. Four individual studies found statistically significant lower serum/plasma creatinine levels 72 hours after initiation of treatment in the ibuprofen group compared with the indomethacin group (Chotigeat 2003; El‐Mashad 2017; Lago 2002; Supapannachart 2002). In the meta‐analysis, the serum/plasma creatinine levels 72 hours after initiation of treatment were statistically significantly lower in the ibuprofen group (eleven studies, 918 infants; MD ‐8.12 µmol/L, 95% CI ‐10.81 to ‐5.43; Analysis 3.24). There was high between‐study heterogeneity (I2 = 83%). The quality of the evidence was low according to GRADE.
Pezzati and coworkers noted significantly lower serum creatinine levels on day three in the ibuprofen group compared with the indomethacin group (P value < 0.05; data provided in graph form only) (Pezzati 1999). Plavka and coworkers reported lower serum creatinine levels in the ibuprofen group compared with the indomethacin group in the first 96 hours of treatment (P value < 0.01; data for the two groups not provided) (Plavka 2001). Van Overmeire and coworkers noted that the maximal difference in serum creatinine levels between the ibuprofen and the indomethacin groups occurred on day three (P value = 0.07; data provided in graph form only) (Van Overmeire 1997). The lower levels were observed in the ibuprofen group. In their second trial, Van Overmeire and coworkers noted significantly lower serum creatinine levels in the ibuprofen group compared with the indomethacin group (P value = 0.04 overall; data provided in graph form only) (Van Overmeire 2000). Pourarian and coworkers reported that the MDs in serum creatinine before and after treatment were 0.35 mg/dL in the ibuprofen group and 0.45 mg/dL in the indomethacin group (SDs were not provided) (Pourarian 2008).
Increase in serum/plasma creatinine levels 72 hours after treatment (Analysis 3.26)
One study reported on the increase in serum/plasma creatinine levels 72 hours after treatment (Aly 2007). The increase in serum creatinine levels was significantly lower in the ibuprofen group compared with the indomethacin group (21 infants; MD ‐15.91 µmol/L, 95% CI ‐31.78 to ‐0.04) (Analysis 3.26).
3.26. Analysis.
Comparison 3 Intravenous or oral ibuprofen versus intravenous or oral indomethacin, Outcome 26 Increase in serum/plasma creatinine levels (mg/dL) following treatment.
Significant decrease in urine output (> 20% decrease in urine output after starting therapy) (Analysis 3.28)
One study reported a significant decrease in urine output after starting therapy. There was a lower risk of this outcome in the ibuprofen group compared with the indomethacin group (144 infants; RR 0.51, 95% CI 0.30 to 0.87; RD ‐0.20, 95% CI ‐0.35 to ‐0.05; NNTB 5, 95% CI 3 to 20) (Analysis 3.28).
3.28. Analysis.
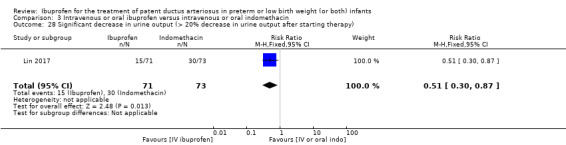
Comparison 3 Intravenous or oral ibuprofen versus intravenous or oral indomethacin, Outcome 28 Significant decrease in urine output (> 20% decrease in urine output after starting therapy).
Daily urine output (mL/kg/hr) after treatment (Analysis 3.29)
One study reported significantly higher urine output in the ibuprofen group compared with indomethacin group (200 infants; MD 0.59 mL/kg/hr, 95% CI 0.45 to 0.73) (Analysis 3.29).
3.29. Analysis.
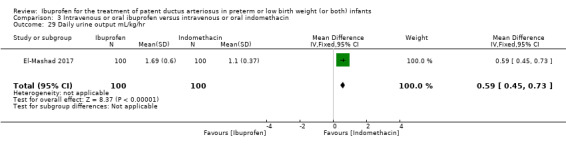
Comparison 3 Intravenous or oral ibuprofen versus intravenous or oral indomethacin, Outcome 29 Daily urine output mL/kg/hr.
Serum bilirubin (µmol/L) after treatment (Analysis 3.30)
One study reported on significantly higher serum bilirubin in the ibuprofen group compared with indomethacin group (200 infants; MD 12.65 µmol/L, 95% CI 9.96 to 15.34) (Analysis 3.30).
3.30. Analysis.
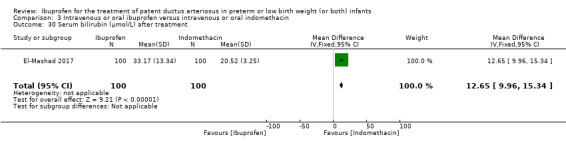
Comparison 3 Intravenous or oral ibuprofen versus intravenous or oral indomethacin, Outcome 30 Serum bilirubin (µmol/L) after treatment.
Platelet count (x109/L) after treatment (Analysis 3.31)
One study reported a significantly higher platelet count in the ibuprofen group compared with indomethacin group (200 infants; MD 72.00 x109/L, 95% CI 58.07 to 85.93) (Analysis 3.31).
3.31. Analysis.
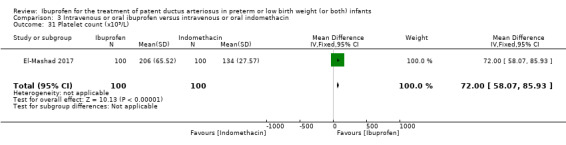
Comparison 3 Intravenous or oral ibuprofen versus intravenous or oral indomethacin, Outcome 31 Platelet count (x109/L).
Neurodevelopmental outcome (neurodevelopmental outcome assessed by a standardised and validated assessment tool or a child developmental specialist, or both) at any age reported (no analysis)
No long‐term outcome data were reported on neurodevelopmental outcome.
The effects on cerebral blood flow velocity or cerebral blood flow were not included as predetermined outcomes in this review. However, several authors reported on these outcomes. All results favoured the ibuprofen group with less reduction in cerebral blood flow velocity or cerebral blood flow.
Oral ibuprofen versus intravenous or oral indomethacin (Comparison 4)
Failure to close a patent ductus arteriosus (after three doses) (Analysis 4.1)
Eight trials reported on failure to close a PDA. There was no statistically significant difference for oral ibuprofen versus IV or oral indomethacin (272 infants; typical RR 0.96, 95% CI 0.73 to 1.27; typical RD ‐0.01, 95% CI ‐0.12 to 0.09) (Analysis 4.1). There was no heterogeneity for this outcome (I2 = 0% for both RR and RD). The quality of the evidence was low according to GRADE.
4.1. Analysis.
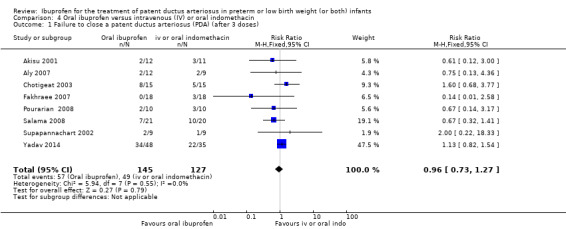
Comparison 4 Oral ibuprofen versus intravenous (IV) or oral indomethacin, Outcome 1 Failure to close a patent ductus arteriosus (PDA) (after 3 doses).
All‐cause mortality (during hospital stay) (Analysis 4.2)
Four studies reported on all‐cause mortality. There was no statistically significant difference in any of the trials comparing oral ibuprofen with indomethacin and the meta‐analysis showed a difference of borderline statistical significance (165 infants; typical RR 0.41, 95% CI 0.17 to 1.00; typical RD ‐0.10, 95% CI ‐0.20 to ‐0.00; P value = 0.05 for both RR and RD) (Analysis 4.2). There was no heterogeneity for this outcome (I2 = 0% for both RR and RD).
4.2. Analysis.
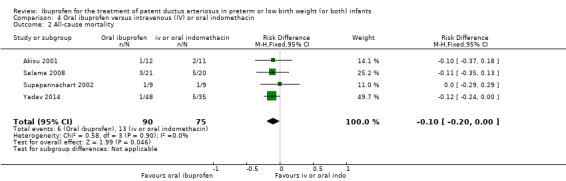
Comparison 4 Oral ibuprofen versus intravenous (IV) or oral indomethacin, Outcome 2 All‐cause mortality.
Neonatal mortality (during first 28/30 days of life) (Analysis 4.3)
Two studies reported on neonatal mortality. There was no statistically significant difference in either of the trials comparing oral ibuprofen with indomethacin and the meta‐analysis showed no statistically significant difference (66 infants; typical RR 1.33, 95% CI 0.33 to 5.39; typical RD 0.03, 95% CI ‐0.12 to 0.18) (Analysis 4.3). There was no heterogeneity for this outcome (I2 = 0% for both RR and RD).
4.3. Analysis.
Comparison 4 Oral ibuprofen versus intravenous (IV) or oral indomethacin, Outcome 3 Neonatal mortality (during first 28/30 days of life).
Reopening of the ductus arteriosus (Analysis 4.4)
One study reported on reopening of the ductus arteriosus. There was no case of reopening of the ductus in either of the groups (20 infants; RR not estimable; RD 0.00, 95% CI ‐0.17 to 0.17) (Analysis 4.4).
4.4. Analysis.
Comparison 4 Oral ibuprofen versus intravenous (IV) or oral indomethacin, Outcome 4 Reopening of the ductus arteriosus.
Need for surgical closure of the patent ductus arteriosus (Analysis 4.5)
Four studies reported on need for surgical closure of the PDA. There was no statistically significant difference in any of the trials comparing oral ibuprofen with indomethacin and the meta‐analysis showed no statistically significant difference (174 infants; typical RR 0.93, 95% CI 0.50 to 1.74; typical RD ‐0.01, 95% CI ‐0.13 to 0.10) (Analysis 4.5). There was no heterogeneity for this outcome (I2 = 0% for both RR and RD). The quality of the evidence was low according to GRADE.
4.5. Analysis.
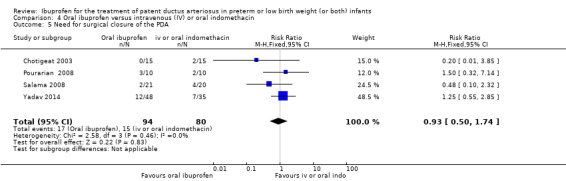
Comparison 4 Oral ibuprofen versus intravenous (IV) or oral indomethacin, Outcome 5 Need for surgical closure of the PDA.
Necrotising enterocolitis (any stage) (Analysis 4.13)
Seven trials reported on NEC. There was no statistically significant difference in any of the trials comparing oral ibuprofen with indomethacin but the meta‐analysis showed a statistically significant difference in favour of oral ibuprofen (249 infants; typical RR 0.41, 95% CI 0.23 to 0.73; typical RD ‐0.13, 95% CI ‐0.22 to ‐0.05; NNTB 8, 95% CI 5 to 20) (Analysis 4.13). There was no heterogeneity for this outcome (I2 = 0% for both RR and RD). The quality of the evidence was low according to GRADE.
4.13. Analysis.
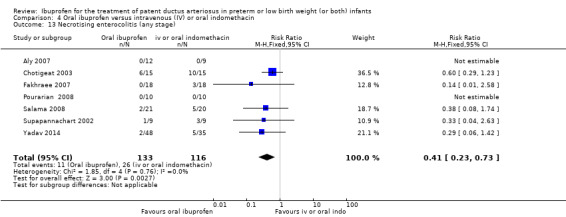
Comparison 4 Oral ibuprofen versus intravenous (IV) or oral indomethacin, Outcome 13 Necrotising enterocolitis (any stage).
For the following outcomes, there were no statistically significant differences between the oral ibuprofen versus IV or oral indomethacin (for details see the corresponding analyses); pulmonary haemorrhage (Analysis 4.6); pulmonary hypertension (Analysis 4.7); chronic lung disease (at 28 days) (Analysis 4.8); chronic lung disease (age not stated) (Analysis 4.9); intraventricular haemorrhage (grades I to IV) (Analysis 4.10); intraventricular haemorrhage (grades III and IV) ( Analysis 4.11); periventricular leukomalacia (Analysis 4.12); intestinal perforation (Analysis 4.14); gastrointestinal bleed (Analysis 4.15); retinopathy of prematurity ( Analysis 4.16); sepsis (Analysis 4.17); oliguria (less than 1 mL/kg/hour) (Analysis 4.18); serum/plasma creatinine levels 72 hours after treatment (the quality of the evidence was very low according to GRADE) (Analysis 4.19); duration of hospital stay (Analysis 4.20).
4.6. Analysis.
Comparison 4 Oral ibuprofen versus intravenous (IV) or oral indomethacin, Outcome 6 Pulmonary haemorrhage.
4.7. Analysis.
Comparison 4 Oral ibuprofen versus intravenous (IV) or oral indomethacin, Outcome 7 Pulmonary hypertension.
4.8. Analysis.
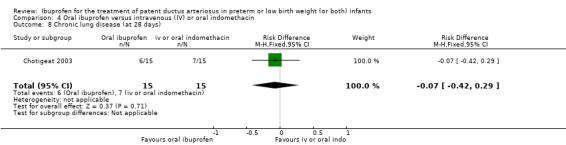
Comparison 4 Oral ibuprofen versus intravenous (IV) or oral indomethacin, Outcome 8 Chronic lung disease (at 28 days).
4.9. Analysis.
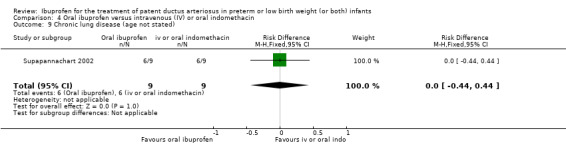
Comparison 4 Oral ibuprofen versus intravenous (IV) or oral indomethacin, Outcome 9 Chronic lung disease (age not stated).
4.10. Analysis.
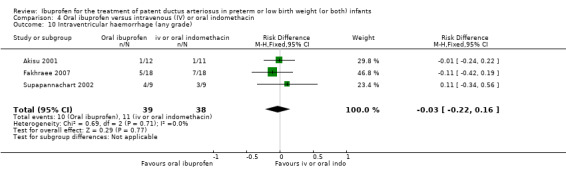
Comparison 4 Oral ibuprofen versus intravenous (IV) or oral indomethacin, Outcome 10 Intraventricular haemorrhage (any grade).
4.11. Analysis.
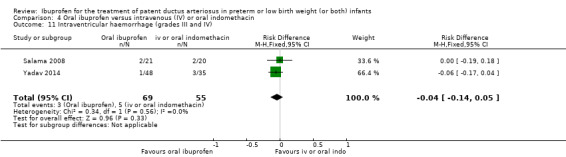
Comparison 4 Oral ibuprofen versus intravenous (IV) or oral indomethacin, Outcome 11 Intraventricular haemorrhage (grades III and IV).
4.12. Analysis.
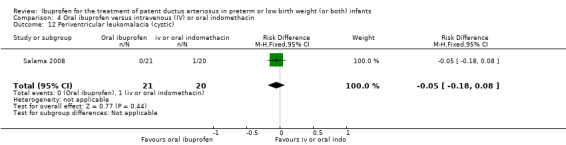
Comparison 4 Oral ibuprofen versus intravenous (IV) or oral indomethacin, Outcome 12 Periventricular leukomalacia (cystic).
4.14. Analysis.
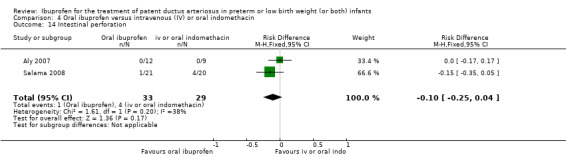
Comparison 4 Oral ibuprofen versus intravenous (IV) or oral indomethacin, Outcome 14 Intestinal perforation.
4.15. Analysis.
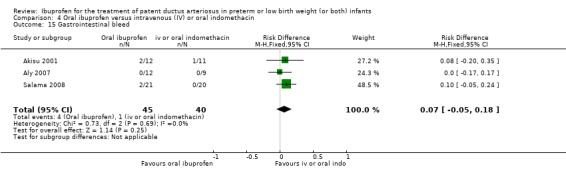
Comparison 4 Oral ibuprofen versus intravenous (IV) or oral indomethacin, Outcome 15 Gastrointestinal bleed.
4.16. Analysis.
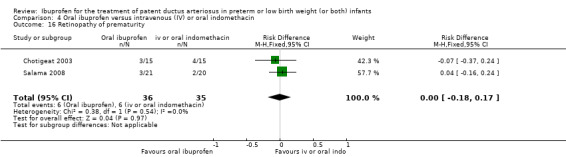
Comparison 4 Oral ibuprofen versus intravenous (IV) or oral indomethacin, Outcome 16 Retinopathy of prematurity.
4.17. Analysis.
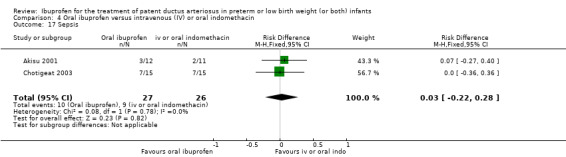
Comparison 4 Oral ibuprofen versus intravenous (IV) or oral indomethacin, Outcome 17 Sepsis.
4.18. Analysis.
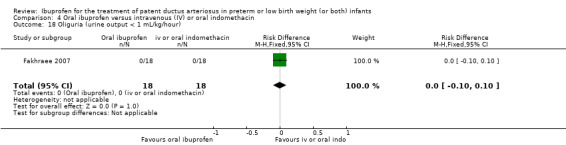
Comparison 4 Oral ibuprofen versus intravenous (IV) or oral indomethacin, Outcome 18 Oliguria (urine output < 1 mL/kg/hour).
4.19. Analysis.
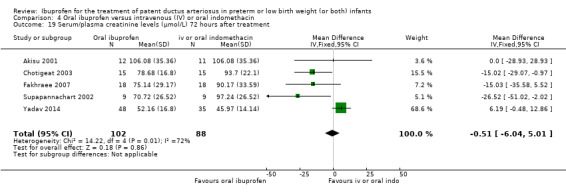
Comparison 4 Oral ibuprofen versus intravenous (IV) or oral indomethacin, Outcome 19 Serum/plasma creatinine levels (µmol/L) 72 hours after treatment.
4.20. Analysis.
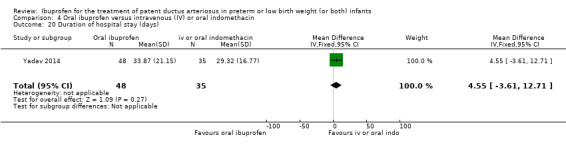
Comparison 4 Oral ibuprofen versus intravenous (IV) or oral indomethacin, Outcome 20 Duration of hospital stay (days).
Chronic lung disease (36 weeks' postmenstrual age)
No trial reported on CLD at 36 weeks' PMA.
Oral ibuprofen versus intravenous ibuprofen (Comparison 5)
Primary outcome
Failure to close a patent ductus arteriosus (after three doses) (Analysis 5.1)
Five studies reported on failure to close a PDA. The meta‐analysis showed a statistically significant difference favouring the oral ibuprofen group (406 infants; typical RR 0.38, 95% CI 0.26 to 0.56; typical RD ‐0.22, 95% CI ‐0.31 to ‐0.14; NNTB 5, 95% CI 3 to 7) (Analysis 5.1). There was no heterogeneity for this outcome (I2 = 0% for both RR and RD). The quality of the evidence was moderate according to GRADE.
5.1. Analysis.
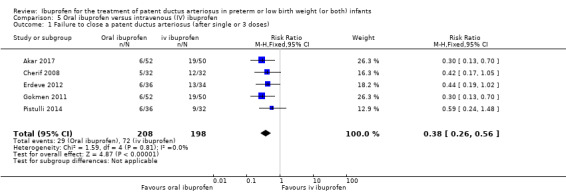
Comparison 5 Oral ibuprofen versus intravenous (IV) ibuprofen, Outcome 1 Failure to close a patent ductus arteriosus (after single or 3 doses).
Secondary outcomes
Mortality (during first 28/30 days of life) (Analysis 5.2)
One study reported on mortality during the first 28/30 days of life. There was no significant difference comparing oral ibuprofen with IV ibuprofen (64 infants; RR 1.13, 95% CI 0.50 to 2.55; RD 0.03, 95% CI ‐0.19 to 0.25) (Analysis 5.2).
5.2. Analysis.
Comparison 5 Oral ibuprofen versus intravenous (IV) ibuprofen, Outcome 2 Mortality (during first 28/30 days of life).
Mortality (during hospital stay) (Analysis 5.3)
Two studies reported on mortality during hospital stay. There was no significant difference comparing oral ibuprofen with IV ibuprofen in either of the two studies and the meta‐analysis showed no statistically significant difference (188 infants; typical RR 0.83, 95% CI 0.38 to 1.82; typical RD ‐0.02, 95% CI ‐0.11 to 0.07) (Analysis 5.3). There was no heterogeneity for this outcome (I2 = 0% for both RR and RD).
5.3. Analysis.
Comparison 5 Oral ibuprofen versus intravenous (IV) ibuprofen, Outcome 3 Mortality (during hospital stay).
Plasma cystatin‐C after treatment (Analysis 5.4)
One study reported on plasma cystatin‐C after treatment. There was a statistically significant difference comparing oral ibuprofen with IV ibuprofen (102 infants; MD ‐0.25 mg/dL, 95% CI ‐0.37 to ‐0.13) (Analysis 5.4).
5.4. Analysis.
Comparison 5 Oral ibuprofen versus intravenous (IV) ibuprofen, Outcome 4 Mean plasma cystatin‐C (mg/L) after treatment.
Need for surgical closure of the ductus (Analysis 5.5)
Five studies reported on need for surgical closure of the ductus. The meta‐analysis showed no statistically significant difference between the two groups (406 infants; typical RR 0.41, 95% CI 0.14 to 1.21; typical RD ‐0.03, 95% CI ‐0.07 to 0.01) (Analysis 5.5). There was no heterogeneity for this outcome (RR: I2 = 0%; RD: I2 = 22%). The quality of the evidence was moderate according to GRADE.
5.5. Analysis.
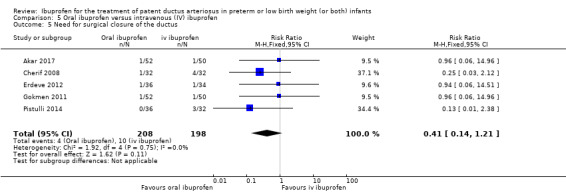
Comparison 5 Oral ibuprofen versus intravenous (IV) ibuprofen, Outcome 5 Need for surgical closure of the ductus.
Serum/plasma creatinine levels 72 hours after treatment (Analysis 5.19)
Two studies reported on serum/plasma creatinine levels 72 hours after treatment. There was a statistically significant reduction with oral ibuprofen compared with IV ibuprofen (170 infants; MD ‐22.47 μmol/L, 95% CI ‐32.40 to ‐12.53; Analysis 5.19). There was high heterogeneity for this outcome (I2 = 81%). The quality of the evidence was low according to GRADE.
5.19. Analysis.
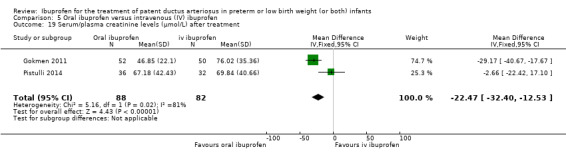
Comparison 5 Oral ibuprofen versus intravenous (IV) ibuprofen, Outcome 19 Serum/plasma creatinine levels (μmol/L) after treatment.
For the following outcomes, there were no statistically significant differences between the two groups (for details see the corresponding analyses); duration of ventilatory support (Analysis 5.6) (the quality of the evidence was low according to GRADE); duration of hospitalisation (Analysis 5.7); pneumothorax (Analysis 5.8); pulmonary haemorrhage (Analysis 5.9); pulmonary hypertension (Analysis 5.10); chronic lung disease (at 36 weeks' postmenstrual age or at discharge) (Analysis 5.11); intraventricular haemorrhage (grades I to IV) (Analysis 5.12); periventricular leukomalacia (Analysis 5.13); necrotising enterocolitis (Analysis 5.14); intestinal perforation (Analysis 5.15); gastrointestinal bleed (Analysis 5.16); sepsis (Analysis 5.17); retinopathy of prematurity that required laser treatment (Analysis 5.18); and oliguria (Analysis 5.20) (the quality of the evidence was low according to GRADE)
5.6. Analysis.
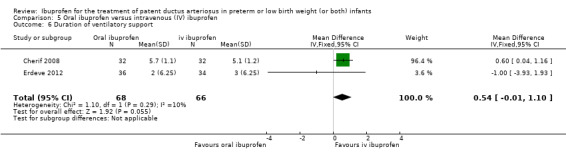
Comparison 5 Oral ibuprofen versus intravenous (IV) ibuprofen, Outcome 6 Duration of ventilatory support.
5.7. Analysis.
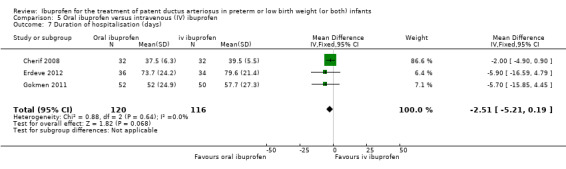
Comparison 5 Oral ibuprofen versus intravenous (IV) ibuprofen, Outcome 7 Duration of hospitalisation (days).
5.8. Analysis.
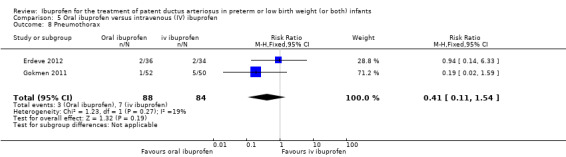
Comparison 5 Oral ibuprofen versus intravenous (IV) ibuprofen, Outcome 8 Pneumothorax.
5.9. Analysis.
Comparison 5 Oral ibuprofen versus intravenous (IV) ibuprofen, Outcome 9 Pulmonary haemorrhage.
5.10. Analysis.
Comparison 5 Oral ibuprofen versus intravenous (IV) ibuprofen, Outcome 10 Pulmonary hypertension.
5.11. Analysis.
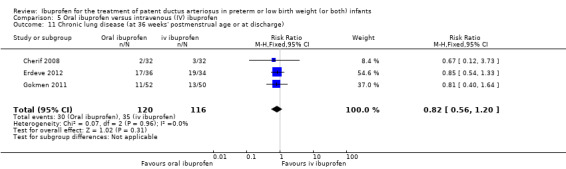
Comparison 5 Oral ibuprofen versus intravenous (IV) ibuprofen, Outcome 11 Chronic lung disease (at 36 weeks' postmenstrual age or at discharge).
5.12. Analysis.
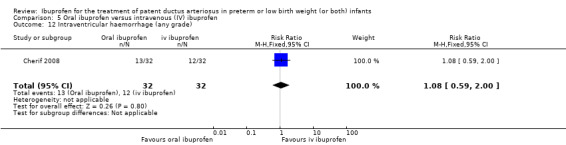
Comparison 5 Oral ibuprofen versus intravenous (IV) ibuprofen, Outcome 12 Intraventricular haemorrhage (any grade).
5.13. Analysis.
Comparison 5 Oral ibuprofen versus intravenous (IV) ibuprofen, Outcome 13 Periventricular leukomalacia.
5.14. Analysis.
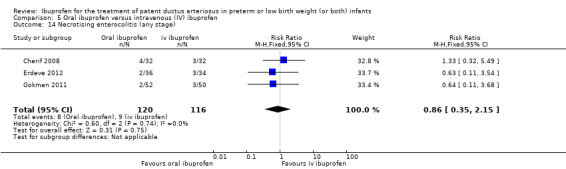
Comparison 5 Oral ibuprofen versus intravenous (IV) ibuprofen, Outcome 14 Necrotising enterocolitis (any stage).
5.15. Analysis.
Comparison 5 Oral ibuprofen versus intravenous (IV) ibuprofen, Outcome 15 Intestinal perforation.
5.16. Analysis.
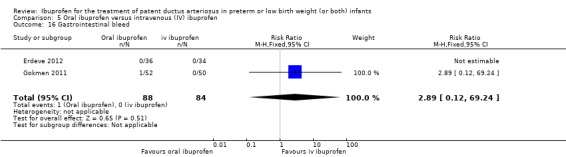
Comparison 5 Oral ibuprofen versus intravenous (IV) ibuprofen, Outcome 16 Gastrointestinal bleed.
5.17. Analysis.
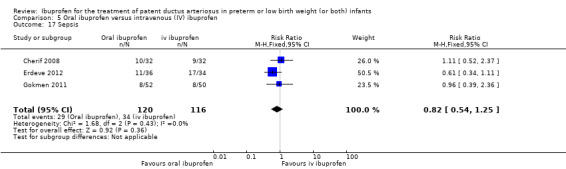
Comparison 5 Oral ibuprofen versus intravenous (IV) ibuprofen, Outcome 17 Sepsis.
5.18. Analysis.
Comparison 5 Oral ibuprofen versus intravenous (IV) ibuprofen, Outcome 18 Retinopathy of prematurity that required laser treatment.
5.20. Analysis.
Comparison 5 Oral ibuprofen versus intravenous (IV) ibuprofen, Outcome 20 Oliguria (Urine output < 1 mL/kg/hour).
No study reported on the following outcomes; chronic lung disease (at 28 days); chronic lung disease (age not stated); intraventricular haemorrhage (grades III and IV).
Mental Developmental Index (Bayley II) at 18 to 24 months (Analysis 5.21)
One study reported on the Mental Developmental Index (Bayley II) at 18 to 24 months. There was no statistically significant difference between oral ibuprofen and IV ibuprofen (57 infants; MD ‐9.00, 95% CI ‐23.89 to 5.89) (Analysis 5.21).
5.21. Analysis.
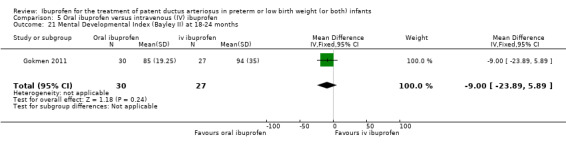
Comparison 5 Oral ibuprofen versus intravenous (IV) ibuprofen, Outcome 21 Mental Developmental Index (Bayley II) at 18‐24 months.
Psychomotor Developmental Index (Bayley II) at 18 to 24 months (Analysis 5.22)
One study reported on the Psychomotor Developmental Index (Bayley II) at 18 to 24 months. There was no statistically significant difference between oral ibuprofen and IV ibuprofen (57 infants; MD 5.00, 95% CI ‐7.67 to 17.67) (Analysis 5.22).
5.22. Analysis.
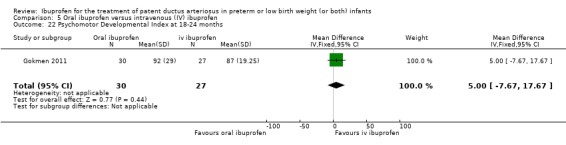
Comparison 5 Oral ibuprofen versus intravenous (IV) ibuprofen, Outcome 22 Psychomotor Developmental Index at 18‐24 months.
Moderate/severe cerebral palsy at 18 to 24 months (Analysis 5.23)
One study reported on moderate/severe cerebral palsy at 18 to 24 months. There was no statistically significant difference between oral ibuprofen and IV ibuprofen (57 infants; RR 1.35, 95% CI 0.24 to 7.48; RD 0.03, 95% CI ‐0.12 to 0.17) (Analysis 5.23).
5.23. Analysis.
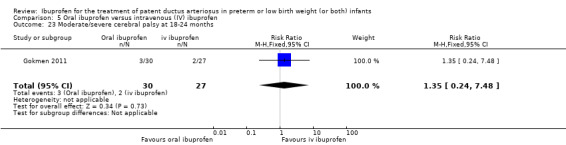
Comparison 5 Oral ibuprofen versus intravenous (IV) ibuprofen, Outcome 23 Moderate/severe cerebral palsy at 18‐24 months.
Blindness at 18 to 24 months (Analysis 5.24)
One study reported on blindness at 18 to 24 months. There was no case of blindness either in the oral ibuprofen group or the IV ibuprofen group (57 infants; RR not estimable; RD 0.00, 95% CI ‐0.07 to 0.07) (Analysis 5.24).
5.24. Analysis.
Comparison 5 Oral ibuprofen versus intravenous (IV) ibuprofen, Outcome 24 Blindness at 18‐24 months.
Deafness at 18 to 24 months (Analysis 5.25)
One study reported on deafness at 18 to 24 months. There was no case of deafness either in the oral ibuprofen group or the IV ibuprofen group (57 infants; RR not estimable; RD 0.00, 95% CI ‐0.07 to 0.07; Analysis 5.25).
5.25. Analysis.
Comparison 5 Oral ibuprofen versus intravenous (IV) ibuprofen, Outcome 25 Deafness at 18‐24 months.
High‐dose ibuprofen (oral or IV) versus standard‐dose regimen of ibuprofen (oral or IV) (Comparison 6)
Three studies compared a high‐dose of ibuprofen (20‐10‐10 mg/kg/day) (Dani 2012; Pourarian 2015) or (15‐7.5‐7.5 mg/kg/day) (Fesharaki 2012) versus standard‐dose of ibuprofen (10‐5‐5 mg/kg/day).
The study by Dani 2012 randomised 95 infants. We reported on the outcomes included by the authors and these included 70 infants for all reported outcomes, except for mortality during hospital stay, which included 95 infants (all infants randomised) (a total of 25 infants were excluded because of 20 deaths and five infants with incomplete data). The study by Fesharaki 2012 randomised 60 infants. The only outcome that was reported by the three studies was failure to close a PDA. The study by Pourarian 2015 randomised 60 infants and outcomes were reported for all randomised infants.
Failure to close a patent ductus arteriosus (after three doses) (Analysis 6.1)
Three studies reported on failure to close a PDA. There was a significant reduction in favour of high‐dose ibuprofen versus standard‐dose ibuprofen (190 infants; typical RR 0.37, 95% CI 0.22 to 0.61; typical RD ‐0.26, 95% CI ‐0.38 to ‐0.15; NNTB 4, 95% CI 3 to 7) (Analysis 6.1). There was no heterogeneity for RR (I2 = 4%) nor for RD (I2 = 0%). The quality of the evidence was moderate according to GRADE.
6.1. Analysis.
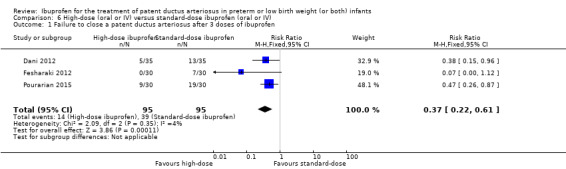
Comparison 6 High‐dose (oral or IV) versus standard‐dose ibuprofen (oral or IV), Outcome 1 Failure to close a patent ductus arteriosus after 3 doses of ibuprofen.
Reopening after a second course of ibuprofen (Analysis 6.2)
One study reported on reopening after a second course of ibuprofen. There was no significant difference between high‐dose ibuprofen and standard‐dose ibuprofen (70 infants; RR 2.00, 95% CI 0.39 to 10.22; RD 0.06, 95% CI ‐0.07 to 0.19) (Analysis 6.2).
6.2. Analysis.
Comparison 6 High‐dose (oral or IV) versus standard‐dose ibuprofen (oral or IV), Outcome 2 Reopening after second course of ibuprofen.
Need for surgical closure (Analysis 6.3)
One study reported on need for surgical closure. There was no significant difference between high‐dose ibuprofen and standard‐dose ibuprofen (70 infants; RR 1.00, 95% CI 0.15 to 6.71; RD 0.00, 95% CI ‐0.11 to 0.11) (Analysis 6.3).
6.3. Analysis.
Comparison 6 High‐dose (oral or IV) versus standard‐dose ibuprofen (oral or IV), Outcome 3 Need for surgical closure.
Mortality during hospital stay (Analysis 6.4)
Two studies reported on mortality during hospital stay. There was no significant difference between high‐dose ibuprofen and standard‐dose ibuprofen (95 infants; RR 1.02, 95% CI 0.58 to 1.79; RD 0.00, 95% CI ‐0.12 to 0.13) (Analysis 6.4). There was no heterogeneity for this outcome (I2 = 0 % for both RR and RD).
6.4. Analysis.
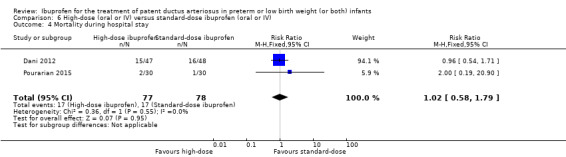
Comparison 6 High‐dose (oral or IV) versus standard‐dose ibuprofen (oral or IV), Outcome 4 Mortality during hospital stay.
Urine output on day three of treatment (Analysis 6.5)
Two studies reported on urine output on day three. There was no significant difference between high‐dose ibuprofen and standard‐dose ibuprofen (130 infants; MD 0.21, 95% CI ‐0.43 to 0.85; Analysis 6.5). There was no heterogeneity for this outcome (I2 = 0 % for both RR and RD).
6.5. Analysis.
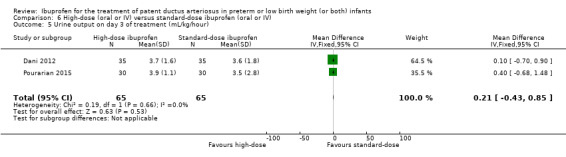
Comparison 6 High‐dose (oral or IV) versus standard‐dose ibuprofen (oral or IV), Outcome 5 Urine output on day 3 of treatment (mL/kg/hour).
Oliguria (less than 1 mL/kg/hour during 24 hours) after onset of treatment (Analysis 6.6)
One study reported on oliguria (less than 1 mL/kg/hour). There was no significant difference in the incidence of oliguria between high‐dose ibuprofen and standard‐dose ibuprofen (70 infants; RR 1.50, 95% CI 0.27 to 8.43; RD 0.03, 95% CI ‐0.09 to 0.15) (Analysis 6.6).
6.6. Analysis.
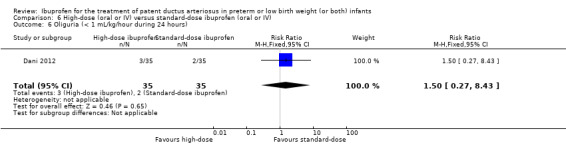
Comparison 6 High‐dose (oral or IV) versus standard‐dose ibuprofen (oral or IV), Outcome 6 Oliguria (< 1 mL/kg/hour during 24 hours).
For the following outcomes, there were no statistically significant differences between high‐dose ibuprofen and standard‐dose ibuprofen (for details see the corresponding analyses); intraventricular haemorrhage (all grades) (Analysis 6.7); intraventricular haemorrhage (grades III and IV) (Analysis 6.8); periventricular leukomalacia (Analysis 6.9); retinopathy of prematurity (all stages) (Analysis 6.10); retinopathy of prematurity (stage 3 or 4) (Analysis 6.11); necrotising enterocolitis (the quality of the evidence was low according to GRADE) (Analysis 6.12); chronic lung disease (at 36 weeks' PMA) (Analysis 6.13); sepsis (Analysis 6.14); hospital stay (Analysis 6.15); oliguria (< 0.5 mL/kg/hr) (Analysis 6.16) (The quality of the evidence was low according to GRADE); gastrointestinal bleed (Analysis 6.17); platelet count (x109/L) Analysis 6.18; serum creatinine after treatment (Analysis 6.19).
6.7. Analysis.
Comparison 6 High‐dose (oral or IV) versus standard‐dose ibuprofen (oral or IV), Outcome 7 Intraventricular haemorrhage (any grade).
6.8. Analysis.
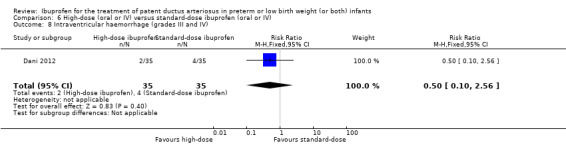
Comparison 6 High‐dose (oral or IV) versus standard‐dose ibuprofen (oral or IV), Outcome 8 Intraventricular haemorrhage (grades III and IV).
6.9. Analysis.
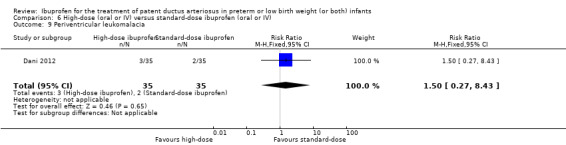
Comparison 6 High‐dose (oral or IV) versus standard‐dose ibuprofen (oral or IV), Outcome 9 Periventricular leukomalacia.
6.10. Analysis.
Comparison 6 High‐dose (oral or IV) versus standard‐dose ibuprofen (oral or IV), Outcome 10 Retinopathy of prematurity (any stage).
6.11. Analysis.
Comparison 6 High‐dose (oral or IV) versus standard‐dose ibuprofen (oral or IV), Outcome 11 Retinopathy of prematurity (stage 3 or 4).
6.12. Analysis.
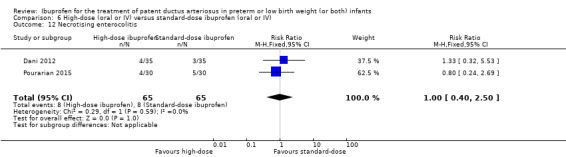
Comparison 6 High‐dose (oral or IV) versus standard‐dose ibuprofen (oral or IV), Outcome 12 Necrotising enterocolitis.
6.13. Analysis.
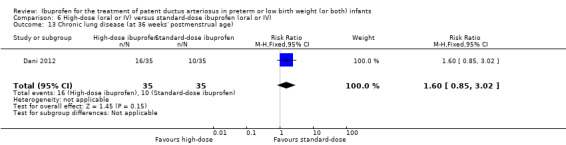
Comparison 6 High‐dose (oral or IV) versus standard‐dose ibuprofen (oral or IV), Outcome 13 Chronic lung disease (at 36 weeks' postmenstrual age).
6.14. Analysis.
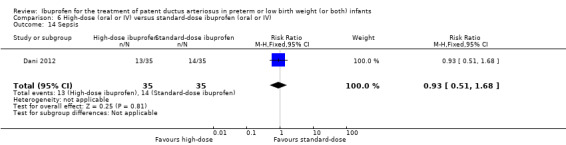
Comparison 6 High‐dose (oral or IV) versus standard‐dose ibuprofen (oral or IV), Outcome 14 Sepsis.
6.15. Analysis.
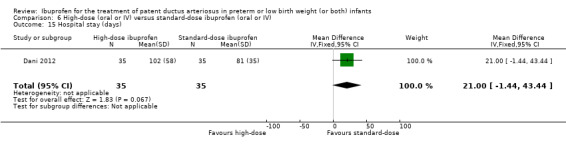
Comparison 6 High‐dose (oral or IV) versus standard‐dose ibuprofen (oral or IV), Outcome 15 Hospital stay (days).
6.16. Analysis.
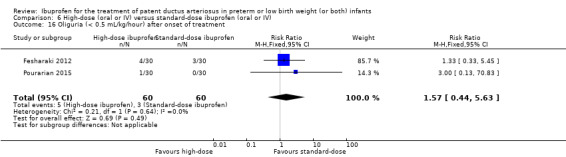
Comparison 6 High‐dose (oral or IV) versus standard‐dose ibuprofen (oral or IV), Outcome 16 Oliguria (< 0.5 mL/kg/hour) after onset of treatment.
6.17. Analysis.
Comparison 6 High‐dose (oral or IV) versus standard‐dose ibuprofen (oral or IV), Outcome 17 Gastrointestinal bleed.
6.18. Analysis.
Comparison 6 High‐dose (oral or IV) versus standard‐dose ibuprofen (oral or IV), Outcome 18 Platelet count (x 109/L) after treatment.
6.19. Analysis.
Comparison 6 High‐dose (oral or IV) versus standard‐dose ibuprofen (oral or IV), Outcome 19 Serum creatinine (µmol/L) after treatment.
Early versus expectant administration of intravenous ibuprofen (Comparison 7)
Only one study compared early versus expectant administration of ibuprofen (Sosenko 2012). The study enrolled 105 infants. We reported on the outcomes included by the authors and these included 105 infants for all reported outcomes. As only one study was included for each of the outcomes, tests for heterogeneity were not applicable.
Primary outcome
Days on supplemental oxygen during the first 28 days (Analysis 7.1)
There was a statistically significant difference between early and expectant administration favouring expectant administration of ibuprofen (105 infants; MD 2.00 days, 95% CI 0.04 to 3.96; P value = 0.05) (Analysis 7.1).
7.1. Analysis.
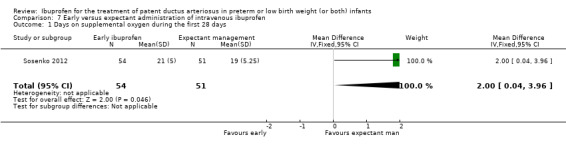
Comparison 7 Early versus expectant administration of intravenous ibuprofen, Outcome 1 Days on supplemental oxygen during the first 28 days.
Secondary outcomes
For the following outcomes, there were no statistically significant differences between early and expectant administration of IV ibuprofen (for details see the corresponding analyses); days on supplemental oxygen (Analysis 7.2); days on mechanical ventilation first 28 days (Analysis 7.3); days on mechanical ventilation (Analysis 7.4); chronic lung disease (at 36 weeks' PMA) (Analysis 7.5); mortality or chronic lung disease (at 36 weeks' postmenstrual age) (Analysis 7.6); mortality during hospital stay (Analysis 7.7); pneumothorax (Analysis 7.8); intraventricular haemorrhage (grades III and IV) (Analysis 7.9); periventricular leukomalacia (Analysis 7.10); necrotising enterocolitis (requiring surgery) (Analysis 7.11); intestinal perforation (Analysis 7.12); sepsis (Analysis 7.13); retinopathy of prematurity (Analysis 7.14).
7.2. Analysis.
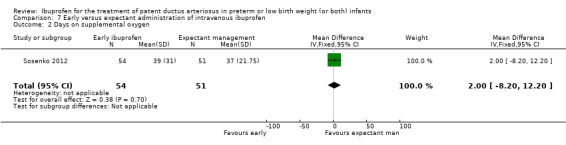
Comparison 7 Early versus expectant administration of intravenous ibuprofen, Outcome 2 Days on supplemental oxygen.
7.3. Analysis.
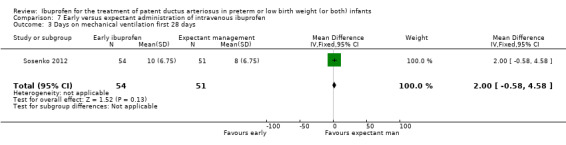
Comparison 7 Early versus expectant administration of intravenous ibuprofen, Outcome 3 Days on mechanical ventilation first 28 days.
7.4. Analysis.
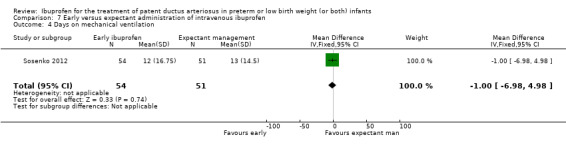
Comparison 7 Early versus expectant administration of intravenous ibuprofen, Outcome 4 Days on mechanical ventilation.
7.5. Analysis.
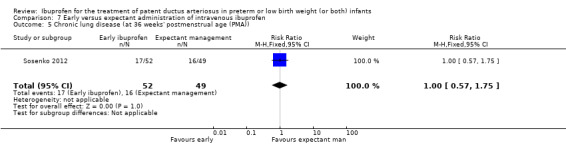
Comparison 7 Early versus expectant administration of intravenous ibuprofen, Outcome 5 Chronic lung disease (at 36 weeks' postmenstrual age (PMA)).
7.6. Analysis.
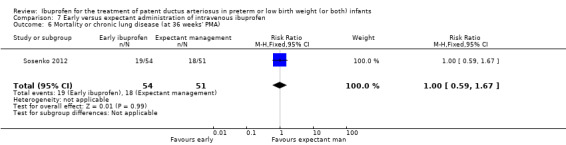
Comparison 7 Early versus expectant administration of intravenous ibuprofen, Outcome 6 Mortality or chronic lung disease (at 36 weeks' PMA).
7.7. Analysis.
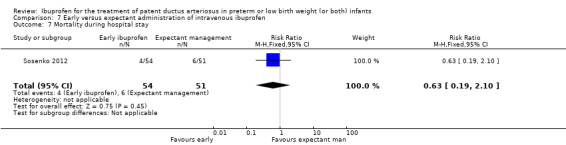
Comparison 7 Early versus expectant administration of intravenous ibuprofen, Outcome 7 Mortality during hospital stay.
7.8. Analysis.
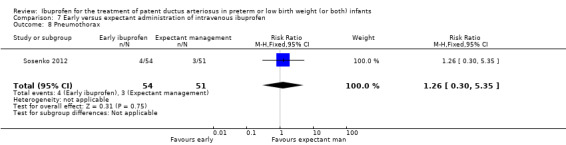
Comparison 7 Early versus expectant administration of intravenous ibuprofen, Outcome 8 Pneumothorax.
7.9. Analysis.
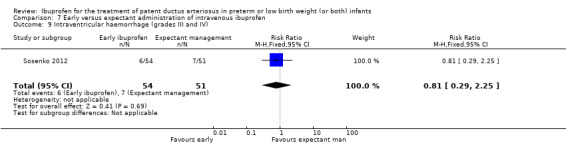
Comparison 7 Early versus expectant administration of intravenous ibuprofen, Outcome 9 Intraventricular haemorrhage (grades III and IV).
7.10. Analysis.
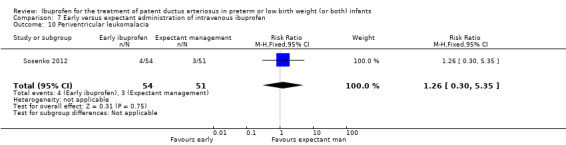
Comparison 7 Early versus expectant administration of intravenous ibuprofen, Outcome 10 Periventricular leukomalacia.
7.11. Analysis.
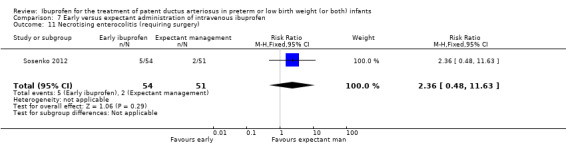
Comparison 7 Early versus expectant administration of intravenous ibuprofen, Outcome 11 Necrotising enterocolitis (requiring surgery).
7.12. Analysis.
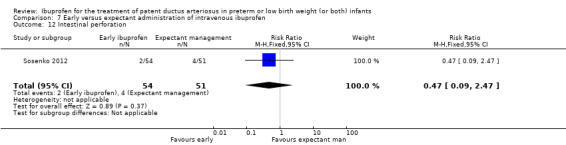
Comparison 7 Early versus expectant administration of intravenous ibuprofen, Outcome 12 Intestinal perforation.
7.13. Analysis.
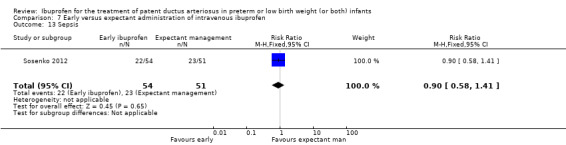
Comparison 7 Early versus expectant administration of intravenous ibuprofen, Outcome 13 Sepsis.
7.14. Analysis.
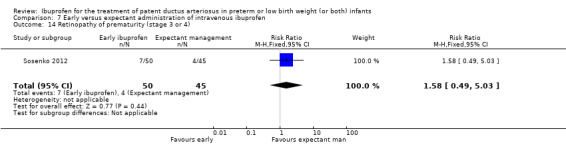
Comparison 7 Early versus expectant administration of intravenous ibuprofen, Outcome 14 Retinopathy of prematurity (stage 3 or 4).
Echocardiographically guided intravenous ibuprofen versus standard intravenous ibuprofen (Comparison 8)
Only one study reported on this comparison (Bravo 2014). As only one study was included for any of the outcome analyses listed below, tests for heterogeneity were not applicable.
Primary outcome
Failure to close a patent ductus arteriosus (Analysis 8.1)
There was no statistically significant difference between ECHO‐guided IV ibuprofen and standard IV ibuprofen for failure to close a PDA (49 infants; RR 1.31, 95% CI 0.44 to 3.91; RD 0.06, 95% CI ‐0.17 to 0.29) (Analysis 8.1).
8.1. Analysis.
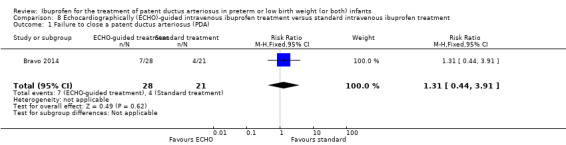
Comparison 8 Echocardiographically (ECHO)‐guided intravenous ibuprofen treatment versus standard intravenous ibuprofen treatment, Outcome 1 Failure to close a patent ductus arteriosus (PDA).
Secondary outcomes
Reopening of patent ductus arteriosus (Analysis 8.2)
There was no statistically significant difference between ECHO‐guided IV ibuprofen and standard IV ibuprofen for reopening of PDA (49 infants; RR 2.25, 95% CI 0.25 to 20.13; RD 0.06, 95% CI ‐0.09 to 0.21) (Analysis 8.2).
8.2. Analysis.
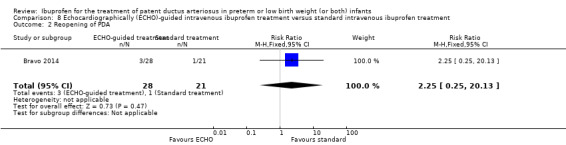
Comparison 8 Echocardiographically (ECHO)‐guided intravenous ibuprofen treatment versus standard intravenous ibuprofen treatment, Outcome 2 Reopening of PDA.
Number of ibuprofen doses (Analysis 8.3)
There was a statistically significant difference between ECHO‐guided IV ibuprofen and standard IV ibuprofen for number of ibuprofen doses favouring ECHO‐guided IV ibuprofen (49 infants; MD ‐1.25 doses, 95% CI ‐1.70 to ‐0.80) (Analysis 8.3).
8.3. Analysis.
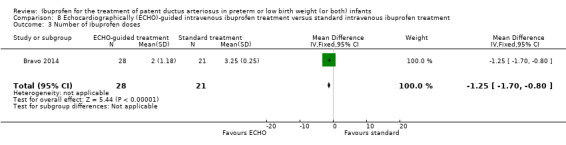
Comparison 8 Echocardiographically (ECHO)‐guided intravenous ibuprofen treatment versus standard intravenous ibuprofen treatment, Outcome 3 Number of ibuprofen doses.
Need for surgical ligation
The study did not report on need for surgical ligation.
Mortality during hospital stay (Analysis 8.4)
There was no statistically significant difference between ECHO‐guided IV ibuprofen and standard IV ibuprofen for mortality during hospital stay (49 infants; RR 0.56, 95% CI 0.14 to 2.25; RD ‐0.08, 95% CI ‐0.29 to 0.12; Analysis 8.4).
8.4. Analysis.
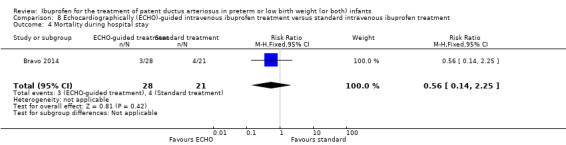
Comparison 8 Echocardiographically (ECHO)‐guided intravenous ibuprofen treatment versus standard intravenous ibuprofen treatment, Outcome 4 Mortality during hospital stay.
For the following outcomes, there were no statistically significant differences between ECHO‐guided IV ibuprofen and standard IV ibuprofen (for details see the corresponding analyses); bronchopulmonary dysplasia (supplemental oxygen at 36 weeks' PMA) (Analysis 8.5); necrotising enterocolitis (Analysis 8.6); intraventricular haemorrhage (grade II and III) (Analysis 8.7); white matter damage (Analysis 8.8); oliguria (urine output less than 1 mL/kg/hour) (Analysis 8.9); serum/plasma creatinine after treatment (Analysis 8.10); laser therapy for retinopathy of prematurity (Analysis 8.11).
8.5. Analysis.
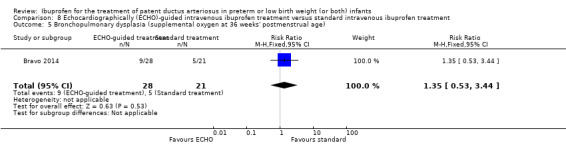
Comparison 8 Echocardiographically (ECHO)‐guided intravenous ibuprofen treatment versus standard intravenous ibuprofen treatment, Outcome 5 Bronchopulmonary dysplasia (supplemental oxygen at 36 weeks' postmenstrual age).
8.6. Analysis.
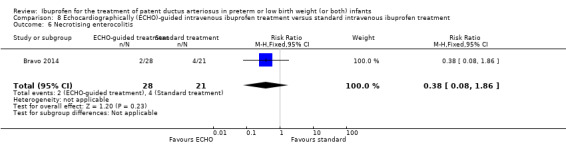
Comparison 8 Echocardiographically (ECHO)‐guided intravenous ibuprofen treatment versus standard intravenous ibuprofen treatment, Outcome 6 Necrotising enterocolitis.
8.7. Analysis.
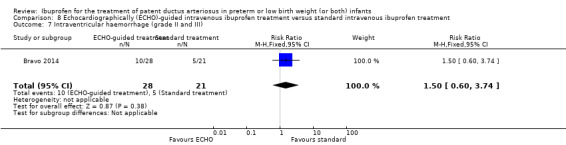
Comparison 8 Echocardiographically (ECHO)‐guided intravenous ibuprofen treatment versus standard intravenous ibuprofen treatment, Outcome 7 Intraventricular haemorrhage (grade II and III).
8.8. Analysis.
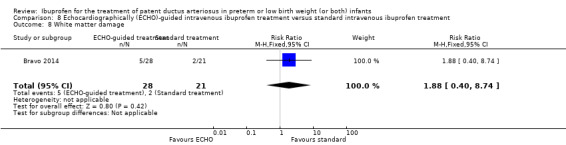
Comparison 8 Echocardiographically (ECHO)‐guided intravenous ibuprofen treatment versus standard intravenous ibuprofen treatment, Outcome 8 White matter damage.
8.9. Analysis.
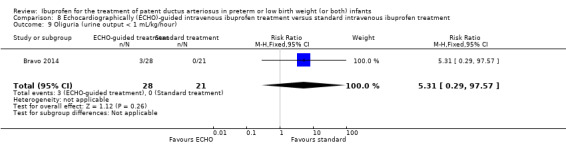
Comparison 8 Echocardiographically (ECHO)‐guided intravenous ibuprofen treatment versus standard intravenous ibuprofen treatment, Outcome 9 Oliguria (urine output < 1 mL/kg/hour).
8.10. Analysis.
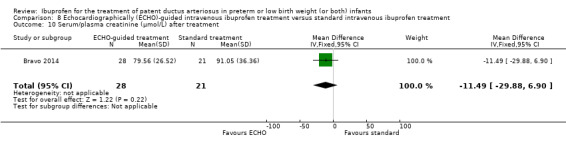
Comparison 8 Echocardiographically (ECHO)‐guided intravenous ibuprofen treatment versus standard intravenous ibuprofen treatment, Outcome 10 Serum/plasma creatinine (µmol/L) after treatment.
8.11. Analysis.
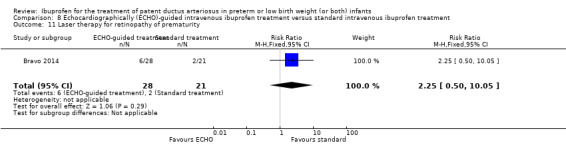
Comparison 8 Echocardiographically (ECHO)‐guided intravenous ibuprofen treatment versus standard intravenous ibuprofen treatment, Outcome 11 Laser therapy for retinopathy of prematurity.
Continuous intravenous infusion of ibuprofen versus standard intravenous ibuprofen (boluses) (Comparison 9)
Only one study reported on this comparison (Lago 2014). As only one study was included for any of the outcome analyses listed below, tests for heterogeneity were not applicable.
Primary outcome
Failure to close a patent ductus arteriosus after one course of ibuprofen (Analysis 9.1)
There was no statistically significant difference between continuous infusion of ibuprofen and intermittent boluses of ibuprofen for failure to close a PDA after one course of ibuprofen (111 infants; RR 1.18, 95% CI 0.88 to 1.58; RD 0.10, 95% CI ‐0.08 to 0.28) (Analysis 9.1).
9.1. Analysis.
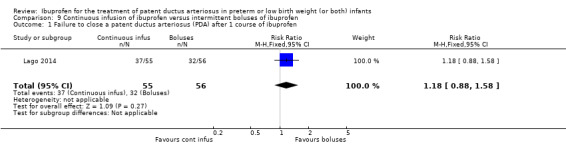
Comparison 9 Continuous infusion of ibuprofen versus intermittent boluses of ibuprofen, Outcome 1 Failure to close a patent ductus arteriosus (PDA) after 1 course of ibuprofen.
Secondary outcomes
Reopening of patent ductus arteriosus (Analysis 9.2)
There was no statistically significant difference between continuous infusion of ibuprofen and intermittent boluses of ibuprofen for reopening of PDA (111 infants; RR 3.05, 95% CI 0.33 to 28.47; RD 0.04, 95% CI ‐0.03 to 0.11) (Analysis 9.2).
9.2. Analysis.
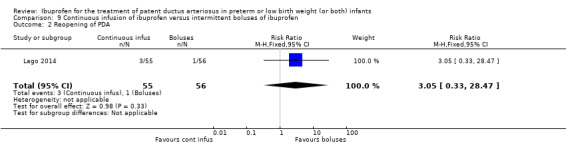
Comparison 9 Continuous infusion of ibuprofen versus intermittent boluses of ibuprofen, Outcome 2 Reopening of PDA.
Need for surgical ligation (Analysis 9.3)
There was a statistically significant difference between continuous infusion of ibuprofen and intermittent boluses of ibuprofen for need for surgical ligation favouring the continuous infusion of ibuprofen group (111 infants; RR 0.28, 95% CI 0.08 to 0.94; RD ‐0.14, 95% CI ‐0.26 to ‐0.02; NNTB 7, 95% CI 4 to 50) (Analysis 9.3).
9.3. Analysis.
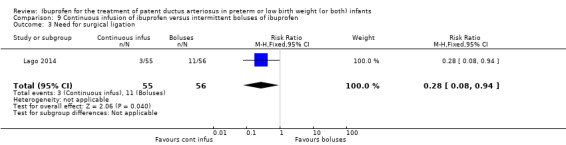
Comparison 9 Continuous infusion of ibuprofen versus intermittent boluses of ibuprofen, Outcome 3 Need for surgical ligation.
Mortality (in hospital) (Analysis 9.4)
There was no statistically significant difference between the continuous infusion of ibuprofen and the intermittent boluses of ibuprofen for mortality (in hospital) (111 infants; RR 1.02, 95% CI 0.07 to 15.87; RD 0.00, 95% CI ‐0.05 to 0.05) (Analysis 9.4).
9.4. Analysis.
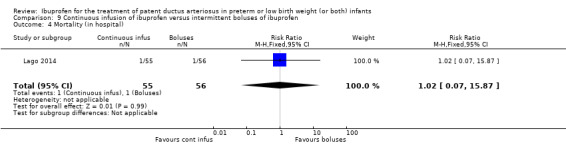
Comparison 9 Continuous infusion of ibuprofen versus intermittent boluses of ibuprofen, Outcome 4 Mortality (in hospital).
For the following outcomes, there were no statistically significant differences between continuous IV infusion of ibuprofen and standard IV ibuprofen (for details see the corresponding analyses); Chronic lung disease (at 36 weeks' postmenstrual age) (Analysis 9.5); Retinopathy of prematurity (any stage) (Analysis 9.6); Retinopathy of prematurity (stage 3 or 4) (Analysis 9.7); Intraventricular haemorrhage (any grade) (Analysis 9.8); Intraventricular haemorrhage (grade III and IV) (Analysis 9.9); Cystic periventricular leukomalacia (Analysis 9.10); Necrotising enterocolitis (Analysis 9.11); Isolated intestinal perforation (Analysis 9.12); Oliguria (1 mL/kg/hour or less) (Analysis 9.13); Serum/plasma creatinine after treatment (Analysis 9.14); Gastrointestinal haemorrhage (Analysis 9.15).
9.5. Analysis.
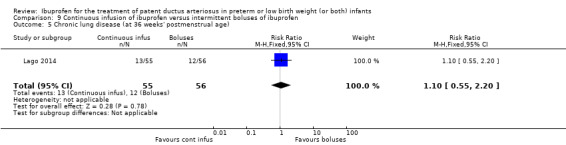
Comparison 9 Continuous infusion of ibuprofen versus intermittent boluses of ibuprofen, Outcome 5 Chronic lung disease (at 36 weeks' postmenstrual age).
9.6. Analysis.
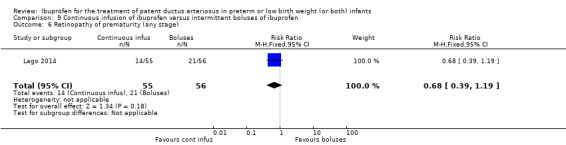
Comparison 9 Continuous infusion of ibuprofen versus intermittent boluses of ibuprofen, Outcome 6 Retinopathy of prematurity (any stage).
9.7. Analysis.
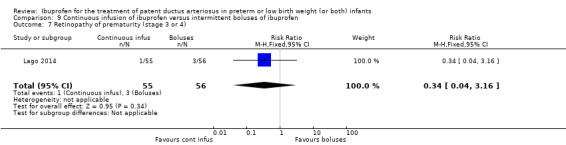
Comparison 9 Continuous infusion of ibuprofen versus intermittent boluses of ibuprofen, Outcome 7 Retinopathy of prematurity (stage 3 or 4).
9.8. Analysis.
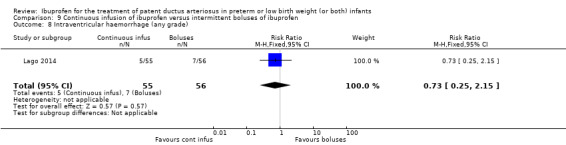
Comparison 9 Continuous infusion of ibuprofen versus intermittent boluses of ibuprofen, Outcome 8 Intraventricular haemorrhage (any grade).
9.9. Analysis.
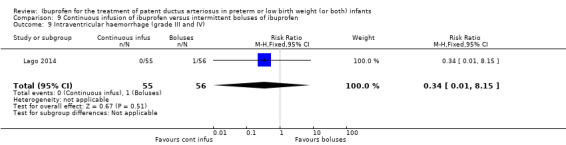
Comparison 9 Continuous infusion of ibuprofen versus intermittent boluses of ibuprofen, Outcome 9 Intraventricular haemorrhage (grade III and IV).
9.10. Analysis.
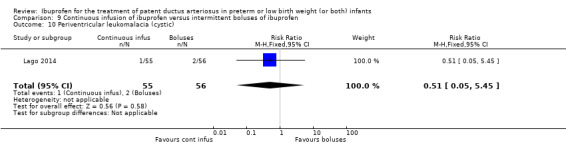
Comparison 9 Continuous infusion of ibuprofen versus intermittent boluses of ibuprofen, Outcome 10 Periventricular leukomalacia (cystic).
9.11. Analysis.
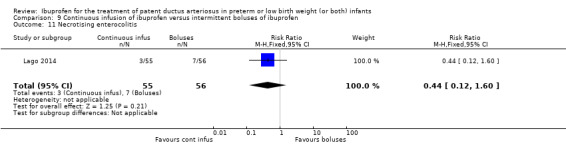
Comparison 9 Continuous infusion of ibuprofen versus intermittent boluses of ibuprofen, Outcome 11 Necrotising enterocolitis.
9.12. Analysis.
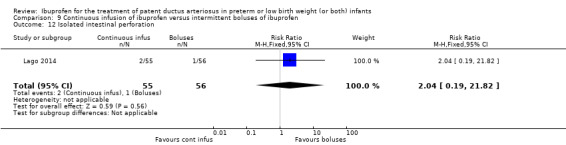
Comparison 9 Continuous infusion of ibuprofen versus intermittent boluses of ibuprofen, Outcome 12 Isolated intestinal perforation.
9.13. Analysis.
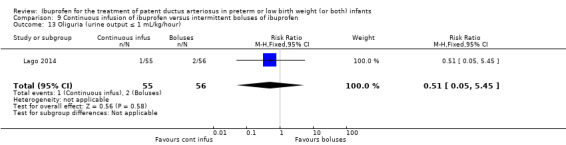
Comparison 9 Continuous infusion of ibuprofen versus intermittent boluses of ibuprofen, Outcome 13 Oliguria (urine output ≤ 1 mL/kg/hour).
9.14. Analysis.
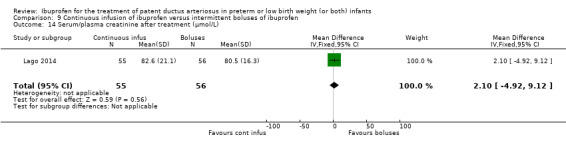
Comparison 9 Continuous infusion of ibuprofen versus intermittent boluses of ibuprofen, Outcome 14 Serum/plasma creatinine after treatment (µmol/L).
9.15. Analysis.
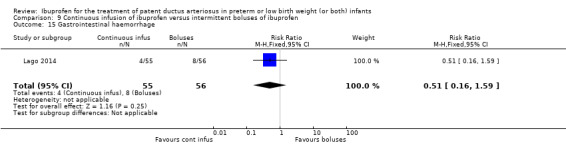
Comparison 9 Continuous infusion of ibuprofen versus intermittent boluses of ibuprofen, Outcome 15 Gastrointestinal haemorrhage.
Rectal ibuprofen versus oral ibuprofen (Comparison 10)
One study (Demir 2017) randomised 75 infants, but three infants (one in the rectal ibuprofen group and two in the oral ibuprofen group) died before they had completed treatment. The following outcomes were reported in 72 infants. As only one study was included in each analysis, tests for heterogeneity were not applicable.
Failure to close a patent ductus arteriosus (Analysis 10.1)
There was no statistically difference between rectal and oral ibuprofen for failure to close a PDA (72 infants; RR 0.83, 95% CI 0.28 to 2.49); RD ‐0.03, 95% CI ‐0.19 to 0.14) (Analysis 10.1).
10.1. Analysis.
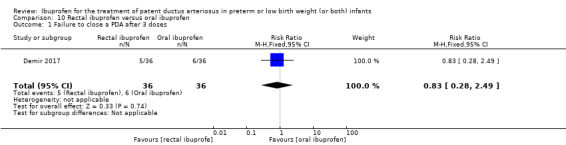
Comparison 10 Rectal ibuprofen versus oral ibuprofen, Outcome 1 Failure to close a PDA after 3 doses.
Need for surgical ligation (Analysis 10.2)
There was no statistically significant difference in the need for surgical ligation between rectal and oral ibuprofen (72 infants; RR 1.00, 95% CI 0.15 to 6.72; RD 0.00, 95% CI ‐0.11 to 0.11) (Analysis 10.2).
10.2. Analysis.
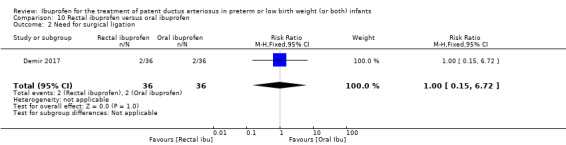
Comparison 10 Rectal ibuprofen versus oral ibuprofen, Outcome 2 Need for surgical ligation.
Plasma creatinine (µmol/L) after treatment (Analysis 10.3)
The plasma creatinine was statistically significantly lower in the rectal versus the oral ibuprofen group (72 infants: MD ‐6.18 µmol/L, 95% CI ‐7.22 to ‐ 5.14) (Analysis 10.3).
10.3. Analysis.
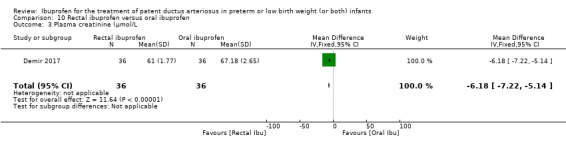
Comparison 10 Rectal ibuprofen versus oral ibuprofen, Outcome 3 Plasma creatinine (µmol/L.
Plasma bilirubin (µmol/L)) after treatment (Analysis 10.4)
There was no statistically significant difference between the plasma bilirubin levels after treatment in the rectal versus the oral ibuprofen group (72 infants: MD 7.01 µmol/L, 95% CI ‐11.23 to 25.25) (Analysis 10.4).
10.4. Analysis.
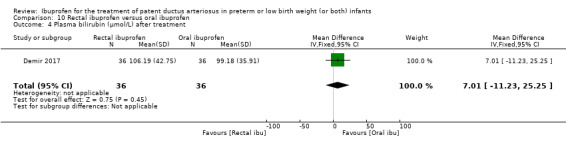
Comparison 10 Rectal ibuprofen versus oral ibuprofen, Outcome 4 Plasma bilirubin (µmol/L) after treatment.
Urine output (mL/kg/hr) after treatment (Analysis 10.5)
There was no statistically significant difference between the urine output after treatment in the rectal versus the oral ibuprofen group (72 infants: MD ‐0.22 mL/kg/hr, 95% CI ‐0.45 to 0.01) (Analysis 10.5).
10.5. Analysis.
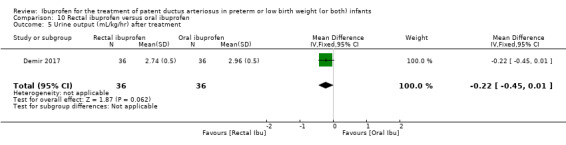
Comparison 10 Rectal ibuprofen versus oral ibuprofen, Outcome 5 Urine output (mL/kg/hr) after treatment.
Subgroup analyses
We abandoned the prespecified subgroup analyses (excluding studies that used only one dose of medication and studies that were published as abstracts only for this and previous updates of the review). Only one study used a single dose and we identified only one abstract. The results of these studies were incorporated with the other studies.
We found no randomised controlled trials on the use of mefenamic acid for the treatment or prevention of a PDA.
Funnel plots
To ascertain the possibility of publication bias, we conducted two funnel plots for the comparison 'intravenous or oral ibuprofen versus IV or oral indomethacin' for the primary outcome of 'failure to close a PDA (after single or three doses)' (Figure 1), and for the same comparison for the secondary outcome of NEC (Figure 2). Both funnel plots were quite symmetric indicating that there was no obvious indication of publication bias.
Discussion
Summary of main results
The 39 studies completed to date have reported on 2843 infants. We used the data from these studies and compared IV ibuprofen to placebo or no intervention (three studies); oral ibuprofen to placebo or no intervention (one study); IV or oral ibuprofen to IV or oral indomethacin (24 studies); oral ibuprofen to IV or oral indomethacin (8 studies); oral ibuprofen to IV ibuprofen (5 studies); high‐dose (oral or IV) ibuprofen to standard‐dose ibuprofen (oral or IV) (3 studies); early administration of ibuprofen to expectant administration of ibuprofen (one study); ECHO‐guided IV ibuprofen to standard IV ibuprofen (one study); continuous infusion of ibuprofen to intermittent boluses of ibuprofen (one study); and rectal ibuprofen to oral ibuprofen (one study). Some studies were included in more than one comparison.
In this review, we have reported on the following comparisons. To avoid repetition, we included the GRADE score for the 'quality of evidence' for comparisons and outcomes that we have included in the 'Summary of findings' tables.
Intravenous ibuprofen was significantly more effective in reducing the outcome 'failure to close a PDA after three doses than placebo or no intervention (moderate‐quality evidence) without any significant effect on NEC (moderate‐quality evidence). However, with IV ibuprofen there was an increased risk of oliguria, and increase in serum/plasma creatinine and blood urea nitrogen compared with placebo. Oral ibuprofen reduced the risk of 'failure to close a PDA after three doses' compared to placebo.
There was no significant difference for intravenous or oral ibuprofen versus intravenous or oral indomethacin for the primary outcome of 'failure to close a PDA after three doses' (moderate‐quality evidence) or for the outcome of need for surgical closure of the PDA (moderate‐quality evidence). Duration of ventilator support was significantly reduced (moderate‐quality evidence) as were the outcomes of NEC (moderate‐quality evidence), and oliguria (urine output < 1 ml/kg/hour) (moderate‐quality evidence) and serum/plasma creatinine (low‐quality evidence) was significantly lower in the ibuprofen versus the indomethacin group.
For the comparison oral ibuprofen versus IV or oral indomethacin, there was no significant difference for the outcomes 'failure to close a PDA after three doses' (low‐quality evidence), need for surgical closure of the PDA (low‐quality evidence), but there was a significantly lower risk of NEC in the oral ibuprofen group (low‐quality evidence). There was no significant difference in the serum/plasma creatinine levels between the groups (very low‐quality evidence).
There was a significantly lower risk of 'failure to close a PDA after three doses' of oral ibuprofen compared with IV ibuprofen (moderate‐quality evidence), but no significant difference for the outcome of need for surgical closure of the PDA (moderate‐quality evidence). The serum/plasma creatinine levels were significantly lower in the oral ibuprofen group compared with the IV ibuprofen group (low‐quality evidence), but there was no difference in the risk of oliguria (urine output < 1 ml/kg/hour) (low‐quality evidence) between the two groups.
For the comparison high‐dose oral or IV ibuprofen compared with standard‐dose oral or IV ibuprofen, there was a significantly decreased risk of 'failure to close a PDA after three doses' in the high‐dose group (moderate‐quality evidence) but there was no difference in the risk of NEC between the groups (low‐quality evidence).
For the following comparisons, only one study was available for each of the included analyses and the number of included infants varied from 49 to 111 in the individual analyses. We did not perform 'summary of findings' tables for these comparisons.
There were no significant differences in any of the outcomes for the comparison 'early versus expectant administration of IV ibuprofen'.
For the comparison ECHO‐guided IV ibuprofen treatment versus standard IV ibuprofen treatment, the number of ibuprofen doses administered was significantly reduced in the ECHO‐guided IV ibuprofen treatment group.
In the comparison continuous infusion of ibuprofen versus intermittent boluses of ibuprofen, the need for surgical ligation was significantly reduced in the continuous infusion group.
In the comparison rectal ibuprofen versus oral ibuprofen, the plasma creatinine level was significantly reduced in the rectal ibuprofen group.
Overall completeness and applicability of evidence
Since first published in 2003 (Ohlsson 2003), the review has been regularly updated (Ohlsson 2005; Ohlsson 2008; Ohlsson 2010; Ohlsson 2013; Ohlsson 2015), and this update, initiated in 2017, includes data from 39 studies with results reported on 2843 infants. There are currently 11 ongoing studies.
Researchers have reported on additional comparisons that we did not include in the original review in 2003 (Ohlsson 2003), and we have added these comparisons.
Ibuprofen is more effective than placebo in closure of a PDA. In the two trials that compared IV ibuprofen with placebo, the closure rates were 71% for ibuprofen versus 53% for placebo.
There was moderate GRADE quality evidence that ibuprofen (IV or orally) is as effective as indomethacin (IV or orally) to close a PDA (24 studies; 1590 infants). None found a statistically significant difference in failure to close a PDA. In the meta‐analysis, there was no statistically significant difference between the groups (typical RR 1.07, 95% CI 0.92 to 1.24; typical RD 0.02, 95% CI ‐0.02 to 0.06) (Figure 6). There was no between‐study heterogeneity (I2 = 0% for both RR and RD). The CIs around the point estimates were very narrow for the primary outcome (Figure 6). Likewise, there was moderate GRADE quality evidence that ibuprofen (IV or orally) compared with indomethacin (IV or orally) reduces the risk of NEC (18 studies, 1292 infants) (typical RR 0.68, 95% CI 0.49 to 0.94; typical RD ‐0.04, 95% CI ‐0.07 to ‐0.01; NNTB 24, 95% CI 14 to 100) (Figure 7). There was no significant between‐study heterogeneity (I2 = 0% for both RR and RD). The funnel plots for these two outcomes were symmetrical, suggesting that there was no publication bias (Figure 1; Figure 2).
Data on long‐term follow‐up are still largely missing, which is a serious concern. Long‐term follow‐up of 18 to 24 months has been reported in only one study (Gokmen 2011) of oral versus IV ibuprofen. Only 57 of the original cohort of 102 infants were assessed at follow‐up. To date, no long‐term follow‐up studies have been published for the other comparisons included in this review. As mentioned in the Background, prophylactic use of indomethacin does reduce the risk of severe IVH and surgical duct ligation but does not confer any significant advantages at 18 months' corrected age with regards to intact survival (Fowlie 2010; Schmidt 2001). There were no significant differences in the outcomes of IVH (any grade and grade II‐IV) in any of the comparisons.
One study of the prophylactic use of ibuprofen was stopped after 135 infants had been enrolled (Gournay 2002). Three infants developed severe hypoxaemia in the ibuprofen group. Hypoxaemia was thought to be due to pulmonary hypertension, as ECHO showed severely decreased pulmonary blood flow. Hypoxaemia resolved quickly on inhaled nitric oxide (Gournay 2002). The authors postulated that this could be due to early administration of ibuprofen (less than six hours) preventing the normal fall in pulmonary vascular resistance, acidification of their ibuprofen solution (buffered with tromethamine) causing precipitation and micro embolism in the lungs, or due to a specific effect of ibuprofen. This adverse effect has not been reported in other trials using ibuprofen for prophylaxis of PDA (Ohlsson 2011). In the 2007 update of the review (Ohlsson 2008), one randomised controlled trial reported one case of pulmonary hypertension in the ibuprofen group (Adamska 2005). In the 2010 update, there were three cases of pulmonary hypertension reported in the study by Aranda and coworkers (Aranda 2009); two in the ibuprofen group and one in the placebo group.
In an extensive search of the literature, including study designs other than randomised controlled trials, one additional case report following L‐lysine ibuprofen therapy in a preterm infant with a PDA (Bellini 2006) was identified in the 2008 update of the review (Ohlsson 2008). A repeat literature search in 2010 did not identify any new case of pulmonary hypertension associated with the treatment of a PDA in neonates (Ohlsson 2010). For the 2013 update (Ohlsson 2013), the literature was searched in July 2012 and three additional case reports of pulmonary hypertension in preterm infants treated with ibuprofen were identified (Amendolia 2012; Sehgal 2013). A repeat PubMed search in July 2014 did not identify any additional cases of pulmonary hypertension following ibuprofen treatment. For this update, we identified additional studies that reported on 35 cases of pulmonary hypertension associated with ibuprofen treatment for PDA (Bravo 2014a; ElHassan 2014; Malikiwi 2015; Rodriguez‐Castano 2016; Kim 2016). In the study by ElHassan 2014, data were extracted from the Pediatric Health Information System; extremely low birth weight infants (ELBW) infants born between January 1, 2007 and December 31, 2010 and admitted on day of life 0 were eligible for inclusion. Seven hundred thirty‐two infants had a PDA diagnosis and met inclusion criteria. Persistent pulmonary hypertension of the newborn (PPHN) occurred in 19 of 306 infants in the ibuprofen group (6%) and in 32 of 426 infants in the indomethacin group (8%) (ElHassan 2014).
In the 2008 update of this review (Ohlsson 2008), we stated, "In view of the lack of long‐term outcome data and potential side effects for both drugs, one drug cannot be recommended over the other as the therapy of choice for a PDA". In the 2010 update of the review, we found a significant reduction in the incidence of NEC in the ibuprofen versus indomethacin group (Ohlsson 2010). As the closure rates for PDA by ibuprofen and indomethacin are similar, the reduced rate of NEC is an important finding and favours the use of ibuprofen over indomethacin for the treatment of a PDA. Kidney function is less affected by ibuprofen. In the update in 2014, the closure rates for ibuprofen versus indomethacin were identical with no heterogeneity and the risk of NEC remained reduced as did the risk of adverse effects on the kidneys. This update confirms these findings in larger samples. Some results favoured oral ibuprofen over IV ibuprofen. Oral ibuprofen is more readily available in some countries. For the comparisons 'high‐dose versus standard‐dose ibuprofen'; 'early versus expectant administration of ibuprofen' , 'ECHO‐guided IV ibuprofen treatment versus standard IV treatment', 'continuous infusion of ibuprofen versus intermittent boluses of ibuprofen' and for 'rectal ibuprofen versus oral ibuprofen', evidence is lacking on which treatment is preferable.
Quality of the evidence
Study quality was variable and the results of this review were based on small to moderately large trials. As can be seen in Figure 4, 'Risk of bias summary: review authors' judgements about each risk of bias item for each included study' and in Figure 5, 'Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies', we identified concerns about bias in most individual studies and therefore for the group of studies included as well. The main concerns were the lack of blinding and unclear information about concealed allocation to the treatment groups. As stated in above under 'Summary of main results' most outcomes in the 'summary of findings' tables were rated as of moderate quality according to GRADE. The sample sizes varied from 16 (Mosca 1997) to 200 (El‐Mashad 2017) infants enrolled. For many of the outcomes, the sample size lacked power to detect a significant difference and the estimates were imprecise. The studies were conducted in 18 different countries (Albania, Belgium, China, Czech Republic, Egypt, India, Iran, Israel, Italy, Poland, Qatar, Spain, Taiwan, Thailand, Tunisia, Turkey, the UK, the US), which increases the applicability of the results internationally. There was no heterogeneity for the primary outcome of 'failure to close a PDA' in any of the comparisons or for NEC in the comparisons of ibuprofen versus indomethacin. These findings increased the validity of these results. In addition, the funnel plot for the primary outcome 'failure to close a PDA' was symmetrical, with no obvious absence of smaller studies having a protective effect of ibuprofen versus indomethacin (Figure 1). For the important secondary outcome of 'NEC', the funnel plot was also symmetrical (Figure 2).
Potential biases in the review process
We are not aware of any potential biases in the review process.
Agreements and disagreements with other studies or reviews
Indomethacin decreases cerebral blood flow in a preterm infant with a PDA (Ohlsson 1993), while ibuprofen has some neuro protective effects in animal models (Chemtob 1990; Pellicer 1999). Future studies comparing the two drugs should include long‐term follow‐up (intact survival) to at least 18 months of age. Sample size calculations could be based on this review and two related Cochrane reviews (Fowlie 2010; Ohlsson 2011).
Coceani and coworkers suggested that a membrane‐bound prostaglandin E synthase inhibitor, once developed for therapeutic use, could become the agent of choice for PDA treatment, particularly when preterm birth is complicated by infectious or inflammatory conditions (Coceani 2005).
One systematic review that used meta‐analytic techniques, but included fewer trials, has come to similar conclusions as us (Neumann 2012). Likewise, one narrative review by Oncel 2016 reported similar findings to ours. Mitra 2016 and coworkers have published a protocol for a systematic review and network meta‐analysis of the effectiveness and safety of treatments used for the management of PDA in preterm infants.
Authors' conclusions
Implications for practice.
Ibuprofen is more effective in closing a patent ductus arteriosus (PDA) compared with placebo. We found no statistically significant difference in the effectiveness of ibuprofen compared with indomethacin in closing a PDA. Ibuprofen reduced the risk of necrotising enterocolitis (NEC), time on assisted ventilation, and had fewer negative effects on renal function. Pulmonary hypertension was observed in three infants after the prophylactic use of ibuprofen, in one case in this review and in additional case reports for the treatment of a PDA. Either ibuprofen or indomethacin can be used to close a PDA. Based on currently available information, ibuprofen does appear to confer net benefits over indomethacin for the treatment of a PDA, but the clinician needs to be aware that both drugs are associated with adverse effects.
Implications for research.
Future research would benefit from long‐term follow‐up (intact survival) to at least 18 months' corrected age, and preferably to the age of school entry.
What's new
| Date | Event | Description |
|---|---|---|
| 3 February 2020 | New citation required but conclusions have not changed | Contact author changed, and contact details updated. |
| 3 February 2020 | Amended | Arne Ohlsson deceased. |
History
Protocol first published: Issue 1, 2002 Review first published: Issue 2, 2003
| Date | Event | Description |
|---|---|---|
| 22 March 2018 | New citation required and conclusions have changed | New citations, conclusions changed. For this update, we identified 6 new published studies. One study compared IV ibuprofen to placebo (Ding 2014); one study compared IV ibuprofen to IV indomethacin (Lin 2017); one study compared high versus standard dose of ibuprofen (Pourarian 2015); one study compared rectal versus oral ibuprofen (Demir 2017); one study compared IV ibuprofen to oral ibuprofen (Akar 2017) and one study compared IV ibuprofen to IV indomethacin( El‐Mashad 2017) |
| 11 February 2018 | New search has been performed | Thirty‐nine studies reporting on 2843 infants are included in this review. Currently there are at least 11 ongoing studies relevant to this review. |
| 20 May 2015 | Amended | Risk Difference fixed to Risk Ratio in data tables. |
| 19 August 2014 | New search has been performed | This updates the review "Ibuprofen for the treatment of patent ductus arteriosus in preterm and/or low birth weight infants" (Ohlsson 2013). |
| 19 August 2014 | New citation required but conclusions have not changed | For this update we identified 6 new studies and one follow‐up study from a previously reported trial. One study compared ibuprofen to placebo (Bagnoli 2013); one study compared continuous infusion of ibuprofen vs. bolus administration (Lago 2014); one study compared oral vs. iv administration of ibuprofen (Pistulli 2014); one study compared a high dose of ibuprofen vs. a standard dose of ibuprofen (Fesharaki 2012); one study compared standard vs. echocardiographically guided ibuprofen treatment (Bravo 2014); and one study compared oral ibuprofen with oral indomethacin for patent ductus arteriosus closure in preterm infants (Yadav 2014). One study reported on long‐term follow‐up in a limited cohort of an earlier published study (Gokmen 2011); Thirty‐three studies enrolling 2190 infants are included in this review. Currently there are at least four ongoing trials (Gournay 2012; Su 2010; Sung 2014; Yeh 2012). |
| 16 November 2012 | New citation required and conclusions have changed | This updates the review "Ibuprofen for the treatment of patent ductus arteriosus in preterm and/or low birth weight infants" (Ohlsson 2010). For this update six additional studies were included; one study compared oral ibuprofen with placebo (Lin 2012); three studies compared oral ibuprofen with iv ibuprofen (Cherif 2008; Erdeve 2012; Gokmen 2011, one study compared iv high dose of ibuprofen versus standard dose of ibuprofen (Dani 2012) and one study compared early versus expectant administration of iv ibuprofen (Sosenko 2012). Two studies are awaiting classification. The results, as before, show that ibuprofen is as effective as indomethacin in closing a patent ductus arteriosus (PDA). There is no statistically significant increase in the risk of chronic lung disease with ibuprofen. The incidence of necrotising enterocolitis is lowered by ibuprofen compared to indomethacin. Kidney function is less affected by ibuprofen than indomethacin and less by oral compared to intravenous (iv) ibuprofen. Oral ibuprofen may be more effective in closing a PDA than iv ibuprofen and reduces the risk of necrotising enterocolitis. Ibuprofen is now recommended over indomethacin to close a PDA. Additional studies are warranted to assess the effectiveness of high‐dose ibuprofen versus a standard dose regimen and early versus expectant administration of ibuprofen. Long‐term follow‐up studies are still warranted. |
| 28 February 2010 | New search has been performed | This updates the review "Ibuprofen for the treatment of patent ductus arteriosus in preterm and/or low birth weight infants" (Ohlsson 2008). This review was updated in February, 2010. One study comparing ibuprofen to placebo was identified and 5 new trials comparing ibuprofen to indomethacin were identified. The results, as before, show that ibuprofen is as effective as indomethacin in closing a PDA. There is now clearly no statistically significant increase in the risk of chronic lung disease with ibuprofen. A new important finding is that ibuprofen reduces the risk of necrotizing enterocolitis. Ibuprofen is now recommended over indomethacin to close a PDA. Long‐term follow‐up studies are still warranted. |
| 26 June 2008 | Amended | Converted to new review format. |
| 19 September 2007 | New search has been performed | This review updates the existing review "Ibuprofen for the treatment of patent ductus arteriosus in preterm and/or low birth weight infants", published in Issue 4, 2005 of The Cochrane Library (Ohlsson 2005). This update of the review conducted in August 2007 identified four previously not included trials (Adamska 2005, Aly 2007, Gimeno Navarro 2005, Pezzati 1999). In addition, two trials previously included as abstracts have now been published as full articles (Chotigeat 2003, Supapannachart 2002). The current review includes a total of 16 trials enrolling 876 infants. The increase in sample size made the point estimates more precise and changed the results of one important outcome. In the previous review there was a statistically significant increase in chronic lung disease in the ibuprofen group. Although a trend towards an increase in chronic lung disease remained in this review, the summary estimates did not reach statistical significance. In this review, the outcome of serum/plasma levels of creatinine following treatment was included and the results showed significantly lower levels in the ibuprofen group. As in previous reviews, the risk of decreased urine output was lower in the ibuprofen group. There is not enough data available regarding the effectiveness of oral ibuprofen to close a patent ductus arteriosus. One case of pulmonary hypertension associated with ibuprofen treatment was reported in one trial. Long‐term neurodevelopmental data are still lacking. Based on the available evidence clinicians may prefer one of the two drugs currently available for closure of a patent ductus arterious over the other: a) Either drug is effective in closing a patent ductus arterious b) Ibuprofen may be preferred because of its less negative impact on the kidney function c) Indomethacin may be preferred because of the trend towards increase in chronic lung disease in the ibuprofen group and the potential risk of pulmonary hypertension associated with the use of ibuprofen This review has previously been updated in 2005 (Ohlsson 2005). An updated search in July 2005 identified one trial of ibuprofen versus placebo, but the results were not reported unblinded to group. However, the search identified three trials that compared ibuprofen to indomethacin for the treatment of a PDA. The addition of the results from these three trials confirmed our previous findings that ibuprofen is no more effective than indomethacin and may cause more adverse effects. There were no important changes to the conclusions of that review. An updated search in October 2004 found no new eligible trials for inclusion in this review. There was no trial identified using mefenamic acid in the original review or in any of the updates. |
| 19 September 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We are thankful to Ms. Elizabeth Uleryk for her assistance in the search of the literature and to Ms. Tara Pourdowlat, RN, for help in translating the study by Akisu and colleagues from Turkish to English (Akisu 2001).
Dr. David Edwards provided additional information on the study by Patel and colleagues (Patel 1995).
We appreciate the help of Ms. Ana Marie Nagy, RN, for helping with the interpretation of the study by Gimeno Navarro (Gimeno Navarro 2005).
We thank Dr. Z Badiee for providing an electronic copy of the paper by Fakhraee and coworkers (Fakhraee 2007), and to Dr. J Aranda for providing a copy of his paper (Aranda 2009).
Dr. C. Dani provided clarifying information regarding his paper (Dani 2012).
In 2013, Dr F. Nayeri provided an English translation of the study originally published in Persian (Fesharaki 2012).
In 2014, Dr. Annalisa Rossetti provided additional outcome data for the study by Bagnoli and coworkers (Bagnoli 2013).
Dr. María Carmen Bravo provided clarifying information and additional outcome data in 2014 for the study by herself and coworkers (Bravo 2014).
Dr. Nahed El‐hassan provided us with additional information on the study (ElHassan 2014).
Ms. Jennifer Spano, Information Specialist at Cochrane Neonatal, conducted the literature searches in November 2017, and provided us with some of the reprints.
The methods section of this review is based on a standard template used by Cochrane Neonatal.
Appendices
Appendix 1. Cochrane Neonatal standard search strategy
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]))
Embase: ((exp infant) OR (infan* OR newborn or neonat* OR premature or very low birth weight or low birth weight or VLBW or LBW).mp AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial).mp
CINAHL: (infan* OR newborn OR neonat* OR premature OR low birth weight OR VLBW OR LBW) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
Cochrane Library: (infan* or newborn or neonat* or premature or preterm or very low birth weight or low birth weight or VLBW or LBW)
Appendix 2. Previous Search Strategy
This review is the fifth update of the original review. We searched the Cochrane Library, MEDLINE, Embase, Clincialtrials.gov, Controlled‐trials.com, www.abstracts2view.com/pas, the reference lists of identified studies, meta‐analyses and personal files in May 2014. We subscribed to weekly updates from Ovid AutoAlert on the topic (Ovid AutoAlert (autorun@ovid.com)).
For this update, as with previous updates, the search started by review of personal files and the Cochrane Central Register of Controlled Trials (CENTRAL, the Cochrane Library); we searched MEDLINE (1966 to May 2014) using MeSH terms: ibuprofen (or mefenamic acid), newborn, infant, premature (or preterm) or low birth weight infant, patent ductus arteriosus or PDA. Other data bases searched included: Embase (1980 to May 2014), CINAHL (1982 to May 2014) and the reference list of identified trials and abstracts published in Pediatric Research (1991 to April Issue, 2005, and electronically on the Pediatric Academic Societies (PAS) website from 2006 to 2014) (www.abstracts2view.com/pas) from conference proceedings of PAS and the European Society of Pediatric Research. We identified no new trials since the first publication of this review in the searches undertaken in October 2004. The searches in July 2005 identified four new trials of which one was published in abstract form. A search by first review author (AO) and coauthors of any abstracts identified in Pediatric Research was done in July 2005 in MEDLINE and EMBASE to try to identify any corresponding full manuscripts published. The searches in August 2007 identified four additional studies. In the 2012 update of the review, we identified six additional trials. In this 2014 update of the review, we identified seven relevant publications, six were reports of previously unpublished trials and one was a follow‐up study of a previously published trial. We reviewed reference lists of published narrative and systematic reviews. We sought unpublished data. We contacted authors of some published trials to clarify or provide additional information. We searched the literature for any reports (regardless of publication type) of pulmonary hypertension associated with the treatment with ibuprofen or indomethacin. We did not apply any language restrictions.
Appendix 3. Risk of bias tool
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorised the method used to generate the allocation sequence as:
low risk (any truly random process e.g. random number table; computer random number generator);
high risk (any nonrandom process e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorised the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or nonopaque envelopes, alternation; date of birth); or
unclear risk
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorised the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk, high risk or unclear risk for participants; and
low risk, high risk or unclear risk for personnel.
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorised the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or class of outcomes. We categorised the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorised the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk.
6. Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we compared prespecified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we contacted study authors to gain access to the study protocol. We assessed the methods as:
low risk (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
7. Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk;
unclear risk.
If needed, we explored the impact of the level of bias through undertaking sensitivity analyses.
Data and analyses
Comparison 1. Intravenous ibuprofen versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to close a patent ductus arteriosus (after 3 doses) | 2 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.44, 0.86] |
| 2 Need for surgical ligation | 1 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [0.91, 3.93] |
| 3 Intraventricular haemorrhage (any grade) | 1 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.64, 1.55] |
| 4 Intraventricular haemorrhage (grades III and IV) | 1 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.47, 2.15] |
| 5 Periventricular leukomalacia | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.02] |
| 6 Pulmonary haemorrhage | 1 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.18] |
| 7 Pulmonary hypertension | 1 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.54] |
| 8 Retinopathy of prematurity (any stage) | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.88, 1.62] |
| 9 Retinopathy of prematurity (stage 3 or 4) | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.38, 3.68] |
| 10 Retinopathy of prematurity (plus disease) | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.31, 5.63] |
| 11 Chronic lung disease (supplemental oxygen at 28 days of age) | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.95, 1.26] |
| 12 Chronic lung disease (supplemental oxygen at 36 weeks' postmenstrual age (PMA)) | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.88, 1.11] |
| 13 Necrotising enterocolitis | 2 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [0.87, 3.90] |
| 14 Mortality by 28 days of life | 1 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Oliguria (urine output < 1 mL/kg/hour) | 1 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 39.0 [2.40, 633.01] |
| 16 Creatinine (µmol/L) after treatment | 1 | 134 | Mean Difference (IV, Fixed, 95% CI) | 29.17 [12.60, 45.74] |
| 17 Blood urea nitrogen (µmol/L) | 1 | 134 | Mean Difference (IV, Fixed, 95% CI) | 18.45 [12.76, 24.14] |
| 18 Mortality | 1 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.34, 1.90] |
Comparison 2. Oral ibuprofen versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to close a patent ductus arteriosus after single or 3 doses | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.11, 0.62] |
Comparison 3. Intravenous or oral ibuprofen versus intravenous or oral indomethacin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to close a patent ductus arteriosus (PDA) (after single or 3 doses) | 24 | 1590 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.92, 1.24] |
| 2 All‐cause mortality | 10 | 697 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.54, 1.17] |
| 3 Neonatal mortality (during first 28/30 days of life) | 4 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.59, 2.11] |
| 4 Reopening of the ductus arteriosus | 7 | 305 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.83, 2.99] |
| 5 Need for surgical closure of the PDA | 16 | 1275 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.81, 1.39] |
| 6 Need for re‐treatment with indomethacin or ibuprofen to close the PDA | 7 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.76, 1.90] |
| 7 Duration of ventilator support (days) | 6 | 471 | Mean Difference (IV, Fixed, 95% CI) | ‐2.35 [‐3.71, ‐0.99] |
| 8 Duration of need for supplementary oxygen (days) | 6 | 556 | Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐1.66, 0.99] |
| 9 Pulmonary haemorrhage | 4 | 303 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.40, 2.04] |
| 10 Pulmonary hypertension | 2 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.53 [0.15, 81.11] |
| 11 Chronic lung disease (at 28 days) | 5 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.93, 1.55] |
| 12 Chronic lung disease (at 36 weeks' postmenstrual age) | 3 | 357 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.77, 1.61] |
| 13 Chronic lung disease (age not stated) | 3 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.82, 1.12] |
| 14 Intraventricular haemorrhage (any grade) | 7 | 524 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.61, 1.31] |
| 15 Intraventricular haemorrhage (grades III and IV) | 10 | 798 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.68, 1.63] |
| 16 Periventricular leukomalacia (cystic) | 6 | 573 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.67, 2.30] |
| 17 Necrotising enterocolitis (any stage) | 18 | 1292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.49, 0.94] |
| 18 Intestinal perforation | 5 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.20, 1.14] |
| 19 Gastrointestinal bleed | 7 | 514 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.55, 1.61] |
| 20 Time to full enteral feeds | 4 | 413 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐1.89, 3.29] |
| 21 Time to regain birth weight (days) | 2 | 188 | Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐2.59, 2.22] |
| 22 Retinopathy of prematurity | 7 | 581 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.60, 1.10] |
| 23 Sepsis | 7 | 735 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.84, 1.76] |
| 24 Oliguria (urine output < 1 mL/kg/hour) | 6 | 576 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.14, 0.54] |
| 25 Serum/plasma creatinine levels (μmol/L) 72 hours after treatment | 11 | 918 | Mean Difference (IV, Fixed, 95% CI) | ‐8.12 [‐10.81, ‐5.43] |
| 26 Increase in serum/plasma creatinine levels (mg/dL) following treatment | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐15.91 [‐31.78, ‐0.04] |
| 27 Duration of hospitalisation (days) | 4 | 368 | Mean Difference (IV, Fixed, 95% CI) | ‐0.69 [‐4.54, 3.16] |
| 28 Significant decrease in urine output (> 20% decrease in urine output after starting therapy) | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.30, 0.87] |
| 29 Daily urine output mL/kg/hr | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | 0.59 [0.45, 0.73] |
| 30 Serum bilirubin (µmol/L) after treatment | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | 12.65 [9.96, 15.34] |
| 31 Platelet count (x109/L) | 1 | 200 | Mean Difference (IV, Fixed, 95% CI) | 72.0 [58.07, 85.93] |
3.25. Analysis.
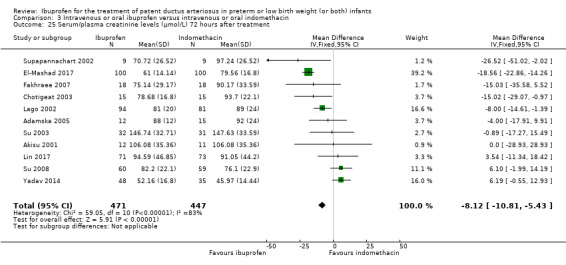
Comparison 3 Intravenous or oral ibuprofen versus intravenous or oral indomethacin, Outcome 25 Serum/plasma creatinine levels (μmol/L) 72 hours after treatment.
Comparison 4. Oral ibuprofen versus intravenous (IV) or oral indomethacin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to close a patent ductus arteriosus (PDA) (after 3 doses) | 8 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.73, 1.27] |
| 2 All‐cause mortality | 4 | 165 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.10 [‐0.20, ‐0.00] |
| 3 Neonatal mortality (during first 28/30 days of life) | 2 | 66 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [‐0.12, 0.18] |
| 4 Reopening of the ductus arteriosus | 1 | 20 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.17, 0.17] |
| 5 Need for surgical closure of the PDA | 4 | 174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.50, 1.74] |
| 6 Pulmonary haemorrhage | 1 | 21 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.22 [‐0.51, 0.07] |
| 7 Pulmonary hypertension | 1 | 83 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.05, 0.05] |
| 8 Chronic lung disease (at 28 days) | 1 | 30 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.07 [‐0.42, 0.29] |
| 9 Chronic lung disease (age not stated) | 1 | 18 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.44, 0.44] |
| 10 Intraventricular haemorrhage (any grade) | 3 | 77 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.03 [‐0.22, 0.16] |
| 11 Intraventricular haemorrhage (grades III and IV) | 2 | 124 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.04 [‐0.14, 0.05] |
| 12 Periventricular leukomalacia (cystic) | 1 | 41 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.05 [‐0.18, 0.08] |
| 13 Necrotising enterocolitis (any stage) | 7 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.23, 0.73] |
| 14 Intestinal perforation | 2 | 62 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.10 [‐0.25, 0.04] |
| 15 Gastrointestinal bleed | 3 | 85 | Risk Difference (M‐H, Fixed, 95% CI) | 0.07 [‐0.05, 0.18] |
| 16 Retinopathy of prematurity | 2 | 71 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.18, 0.17] |
| 17 Sepsis | 2 | 53 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [‐0.22, 0.28] |
| 18 Oliguria (urine output < 1 mL/kg/hour) | 1 | 36 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.10, 0.10] |
| 19 Serum/plasma creatinine levels (µmol/L) 72 hours after treatment | 5 | 190 | Mean Difference (IV, Fixed, 95% CI) | ‐0.51 [‐6.04, 5.01] |
| 20 Duration of hospital stay (days) | 1 | 83 | Mean Difference (IV, Fixed, 95% CI) | 4.55 [‐3.61, 12.71] |
Comparison 5. Oral ibuprofen versus intravenous (IV) ibuprofen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to close a patent ductus arteriosus (after single or 3 doses) | 5 | 406 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.26, 0.56] |
| 2 Mortality (during first 28/30 days of life) | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.50, 2.55] |
| 3 Mortality (during hospital stay) | 2 | 188 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.38, 1.82] |
| 4 Mean plasma cystatin‐C (mg/L) after treatment | 1 | 102 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.37, ‐0.13] |
| 5 Need for surgical closure of the ductus | 5 | 406 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.14, 1.21] |
| 6 Duration of ventilatory support | 2 | 134 | Mean Difference (IV, Fixed, 95% CI) | 0.54 [‐0.01, 1.10] |
| 7 Duration of hospitalisation (days) | 3 | 236 | Mean Difference (IV, Fixed, 95% CI) | ‐2.51 [‐5.21, 0.19] |
| 8 Pneumothorax | 2 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.11, 1.54] |
| 9 Pulmonary haemorrhage | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.52] |
| 10 Pulmonary hypertension | 2 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Chronic lung disease (at 36 weeks' postmenstrual age or at discharge) | 3 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.56, 1.20] |
| 12 Intraventricular haemorrhage (any grade) | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.59, 2.00] |
| 13 Periventricular leukomalacia | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.15, 6.67] |
| 14 Necrotising enterocolitis (any stage) | 3 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.35, 2.15] |
| 15 Intestinal perforation | 2 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.48] |
| 16 Gastrointestinal bleed | 2 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.89 [0.12, 69.24] |
| 17 Sepsis | 3 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.54, 1.25] |
| 18 Retinopathy of prematurity that required laser treatment | 2 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.26, 1.34] |
| 19 Serum/plasma creatinine levels (μmol/L) after treatment | 2 | 170 | Mean Difference (IV, Fixed, 95% CI) | ‐22.47 [‐32.40, ‐12.53] |
| 20 Oliguria (Urine output < 1 mL/kg/hour) | 4 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.66] |
| 21 Mental Developmental Index (Bayley II) at 18‐24 months | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | ‐9.0 [‐23.89, 5.89] |
| 22 Psychomotor Developmental Index at 18‐24 months | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | 5.0 [‐7.67, 17.67] |
| 23 Moderate/severe cerebral palsy at 18‐24 months | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.24, 7.48] |
| 24 Blindness at 18‐24 months | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Deafness at 18‐24 months | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6. High‐dose (oral or IV) versus standard‐dose ibuprofen (oral or IV).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to close a patent ductus arteriosus after 3 doses of ibuprofen | 3 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.22, 0.61] |
| 2 Reopening after second course of ibuprofen | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.39, 10.22] |
| 3 Need for surgical closure | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.15, 6.71] |
| 4 Mortality during hospital stay | 2 | 155 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.58, 1.79] |
| 5 Urine output on day 3 of treatment (mL/kg/hour) | 2 | 130 | Mean Difference (IV, Fixed, 95% CI) | 0.21 [‐0.43, 0.85] |
| 6 Oliguria (< 1 mL/kg/hour during 24 hours) | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.27, 8.43] |
| 7 Intraventricular haemorrhage (any grade) | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.21, 2.16] |
| 8 Intraventricular haemorrhage (grades III and IV) | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.10, 2.56] |
| 9 Periventricular leukomalacia | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.27, 8.43] |
| 10 Retinopathy of prematurity (any stage) | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.27, 3.69] |
| 11 Retinopathy of prematurity (stage 3 or 4) | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.06] |
| 12 Necrotising enterocolitis | 2 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.40, 2.50] |
| 13 Chronic lung disease (at 36 weeks' postmenstrual age) | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.6 [0.85, 3.02] |
| 14 Sepsis | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.51, 1.68] |
| 15 Hospital stay (days) | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | 21.0 [‐1.44, 43.44] |
| 16 Oliguria (< 0.5 mL/kg/hour) after onset of treatment | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.44, 5.63] |
| 17 Gastrointestinal bleed | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.58, 3.86] |
| 18 Platelet count (x 109/L) after treatment | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐29.0 [‐74.83, 16.83] |
| 19 Serum creatinine (µmol/L) after treatment | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 8.84 [‐4.41, 22.09] |
Comparison 7. Early versus expectant administration of intravenous ibuprofen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Days on supplemental oxygen during the first 28 days | 1 | 105 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [0.04, 3.96] |
| 2 Days on supplemental oxygen | 1 | 105 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐8.20, 12.20] |
| 3 Days on mechanical ventilation first 28 days | 1 | 105 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐0.58, 4.58] |
| 4 Days on mechanical ventilation | 1 | 105 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐6.98, 4.98] |
| 5 Chronic lung disease (at 36 weeks' postmenstrual age (PMA)) | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.57, 1.75] |
| 6 Mortality or chronic lung disease (at 36 weeks' PMA) | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.59, 1.67] |
| 7 Mortality during hospital stay | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.19, 2.10] |
| 8 Pneumothorax | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.30, 5.35] |
| 9 Intraventricular haemorrhage (grades III and IV) | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.29, 2.25] |
| 10 Periventricular leukomalacia | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.30, 5.35] |
| 11 Necrotising enterocolitis (requiring surgery) | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.36 [0.48, 11.63] |
| 12 Intestinal perforation | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.09, 2.47] |
| 13 Sepsis | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.58, 1.41] |
| 14 Retinopathy of prematurity (stage 3 or 4) | 1 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.49, 5.03] |
Comparison 8. Echocardiographically (ECHO)‐guided intravenous ibuprofen treatment versus standard intravenous ibuprofen treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to close a patent ductus arteriosus (PDA) | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.44, 3.91] |
| 2 Reopening of PDA | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [0.25, 20.13] |
| 3 Number of ibuprofen doses | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐1.25 [‐1.70, ‐0.80] |
| 4 Mortality during hospital stay | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.14, 2.25] |
| 5 Bronchopulmonary dysplasia (supplemental oxygen at 36 weeks' postmenstrual age) | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.53, 3.44] |
| 6 Necrotising enterocolitis | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.08, 1.86] |
| 7 Intraventricular haemorrhage (grade II and III) | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.60, 3.74] |
| 8 White matter damage | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.40, 8.74] |
| 9 Oliguria (urine output < 1 mL/kg/hour) | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.31 [0.29, 97.57] |
| 10 Serum/plasma creatinine (µmol/L) after treatment | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐11.49 [‐29.88, 6.90] |
| 11 Laser therapy for retinopathy of prematurity | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [0.50, 10.05] |
Comparison 9. Continuous infusion of ibuprofen versus intermittent boluses of ibuprofen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to close a patent ductus arteriosus (PDA) after 1 course of ibuprofen | 1 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.88, 1.58] |
| 2 Reopening of PDA | 1 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [0.33, 28.47] |
| 3 Need for surgical ligation | 1 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.08, 0.94] |
| 4 Mortality (in hospital) | 1 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.07, 15.87] |
| 5 Chronic lung disease (at 36 weeks' postmenstrual age) | 1 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.55, 2.20] |
| 6 Retinopathy of prematurity (any stage) | 1 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.39, 1.19] |
| 7 Retinopathy of prematurity (stage 3 or 4) | 1 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.04, 3.16] |
| 8 Intraventricular haemorrhage (any grade) | 1 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.25, 2.15] |
| 9 Intraventricular haemorrhage (grade III and IV) | 1 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.15] |
| 10 Periventricular leukomalacia (cystic) | 1 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.45] |
| 11 Necrotising enterocolitis | 1 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.12, 1.60] |
| 12 Isolated intestinal perforation | 1 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.19, 21.82] |
| 13 Oliguria (urine output ≤ 1 mL/kg/hour) | 1 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.45] |
| 14 Serum/plasma creatinine after treatment (µmol/L) | 1 | 111 | Mean Difference (IV, Fixed, 95% CI) | 2.10 [‐4.92, 9.12] |
| 15 Gastrointestinal haemorrhage | 1 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.16, 1.59] |
Comparison 10. Rectal ibuprofen versus oral ibuprofen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to close a PDA after 3 doses | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.28, 2.49] |
| 2 Need for surgical ligation | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.15, 6.72] |
| 3 Plasma creatinine (µmol/L | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐6.18 [‐7.22, ‐5.14] |
| 4 Plasma bilirubin (µmol/L) after treatment | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | 7.01 [‐11.23, 25.25] |
| 5 Urine output (mL/kg/hr) after treatment | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐0.45, 0.01] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adamska 2005.
| Methods | Single centre, randomised controlled trial conducted in one NICU in Warsaw, Poland. Study period: not stated | |
| Participants | 35 preterm (< 33 weeks' gestation and BW < 1500 grams) infants with a PDA diagnosed by Doppler ECHO Ibuprofen: 16 infants, mean (SD) GA 27.7 (1.8) weeks; BW 1074 (264) grams; 9 boys, 7 girls Indomethacin: 19 infants, mean (SD) GA 27.6 (2.0) weeks; BW 1003 (192) grams; 11 boys, 8 girls |
|
| Interventions | Ibuprofen: 3 doses given at 24‐hour intervals (10, 5 and 5 mg/kg IV) Indomethacin: 3 doses given at 24‐hour intervals (0.2 mg/kg/dose IV) |
|
| Outcomes | Primary outcome: ductal closure Other outcomes: need for surgical ligation, IVH, PVL, NEC, intestinal perforation, oliguria, time to full oral feeds, CLD (at 28 days of age), pulmonary haemorrhage, pulmonary hypertension, duration of mechanical ventilation, and days in supplemental oxygen |
|
| Notes | Study published in Polish. No information about funding of the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information other than "...randomly assigned" |
| Allocation concealment (selection bias) | Low risk | Allocation was done in a blinded fashion |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Ibuprofen and indomethacin were given at the same time intervals |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Staff and researchers were blinded to the group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up ‐ yes |
| Selective reporting (reporting bias) | Unclear risk | 27 infants (12 received ibuprofen and 15 received indomethacin) were treated as per protocol. In the remaining 8 infants, treatment was stopped due to adverse effects. In the ibuprofen group, the reasons to stop treatment were pulmonary haemorrhage (3/16 infants) and pulmonary hypertension (1/16); in the indomethacin group, it was increased serum creatinine and urea nitrogen concentrations (3/19) and IVH (grade IV) (1/19). The protocol was not available to us so we cannot ascertain if there were any deviations from the protocol or not |
| Other bias | Low risk | Appeared free of other bias |
Akar 2017.
| Methods | Randomised controlled trial in the NICU of Zekai Tahir Burak Maternity Teaching Hospital, Ankara, Turkey. Study period: January 2009 to February 2010 | |
| Participants | Newborns of < 32 weeks' PMA, birth weight < 1500 grams, and postnatal age 48 to 96 hours with PDA | |
| Interventions | IV Ibuprofen 10 mg/kg initial dose followed by 5 mg/kg after 24 and 48 hours PO ibuprofen 10 mg/kg initial dose followed by 5 mg/kg after 24 and 48 hours |
|
| Outcomes | The primary outcome of the study was the effect of different forms of ibuprofen treatment on the antioxidant and oxidant status of the patients Secondary outcomes were the relationship between pretreatment total antioxidant capacity and total oxidant status levels and the success rate of PDA closure and need for surgical ligation |
|
| Notes | We included PDA closure rates and need for surgical ligation in the analyses. "This research received no specific grant from any funding agency, commercial, or not for profit sectors" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned. Details not provided |
| Allocation concealment (selection bias) | Low risk | Patients were allocated to treatment groups using cards in sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The treatment was known to the caregivers as oral or IV ibuprofen were administered |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The treatment was known to the caregivers as oral or IV ibuprofen were administered. It is not stated that the ECHOs were conducted blinded to the treatments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes were reported on all randomised infants |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available to us, so we could not judge if there were any deviations or not |
| Other bias | Low risk | Appeared free of other bias |
Akisu 2001.
| Methods | Single centre, randomised controlled trial conducted in one NICU in Izmir, Turkey. Study period: July 1988 to January 2000 | |
| Participants | 23 infants < 35 weeks' GA with ECHO‐confirmed PDA Ibuprofen: 12 infants, mean (SD) GA 32.1 (1.2) weeks; BW 1706 (187) grams; 5 girls, 7 boys; 9 born by C/S, 2 born vaginally, 10 had RDS, 7 received surfactant. PDA was diagnosed on day 3.9 (0.5) Indomethacin: 11 infants, mean (SD) GA 31.9 (1.3) weeks; BW 1645 (190) grams; 6 girls, 5 boys; 8 born by C/S, 3 born vaginally, 8 had RDS, 7 received surfactant. PDA diagnosed on day 3.5 (0.6) |
|
| Interventions | Ibuprofen: via an oro‐gastric tube (10 mg/kg as the initial dose followed by 5 mg/kg 24 and 48 hours later) Indomethacin: via an oro‐gastric tube (0.2 mg/kg for 3 doses at 12‐hour intervals) 2 neonates in the ibuprofen group and 3 in the indomethacin group required a second treatment with the same drug |
|
| Outcomes | PDA closure; diuresis; serum creatinine; thrombocyte count; gastrointestinal haemorrhage; IVH; sepsis; mortality | |
| Notes | Study published in Turkish. No information provided about funding of the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Single centre, randomised controlled trial. No other information provided |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment ‐ no information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Indomethacin and ibuprofen were administered at different time points |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Indomethacin and ibuprofen were administered at different time points |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all randomised infants |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available to us so we could not ascertain if there were deviations from the protocol |
| Other bias | Low risk | Appeared free of other bias |
Aly 2007.
| Methods | Single centre, randomised controlled trial conducted in Cairo, Egypt. Study period: not stated | |
| Participants | 21 preterm infants (< 35 weeks' gestation) aged 2 to 7 days with respiratory distress and PDA diagnosed by Doppler ECHO Ibuprofen (oral): 12 infants, mean (SD) GA 31.2 (2.5) weeks; BW 1521 (398) grams; 8 boys, 4 girls Indomethacin (IV): 9 infants, mean (SD) GA 32.9 (1.6) weeks; BW 1884 (485) grams; 4 boys, 5 girls |
|
| Interventions | Ibuprofen: initial oral dose of 10 mg/kg, followed by 2 doses orally of 5 mg/kg after 24 and 48 hours Indomethacin: IV as 3 doses of 0.2 mg/kg at 12‐hour intervals |
|
| Outcomes | Primary outcome: ductal closure Secondary outcomes: biochemical tests (serum creatinine), pulmonary haemorrhage, gastrointestinal bleed, NEC, gastrointestinal perforation, and increase in serum creatinine following treatment |
|
| Notes | No information about funding of the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description provided |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes were used for random assignment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Ibuprofen was given orally, whereas indomethacin was given IV. Ibuprofen and indomethacin were given at different time points |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Ibuprofen was given orally, whereas indomethacin was given IV. Ibuprofen and indomethacin were given at different time points. ECHOs were performed by an experienced paediatric cardiologist, and it is stated that he was blinded to the treatments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all randomised infants |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available to us so we could not ascertain if there were deviations from the protocol |
| Other bias | Low risk | Appeared free of other bias |
Aranda 2009.
| Methods | Multicentre, randomised controlled trial conducted in 11 centres in the USA. Study period: March 2002 to March 2005 | |
| Participants | 136 preterm infants (BW 500 to 1000 grams; PMA < 30 weeks) with evidence of ductal shunting by ECHO Mean (SD) GA 26.2 (1.4) weeks, BW 798 (130.3) grams, 51% boys, 49% girls |
|
| Interventions | Ibuprofen: 68 infants, IV as 3‐day treatment course of 10 mg/kg, 5 mg/kg and 5 mg/kg Placebo: 68 infants, saline |
|
| Outcomes | Proportion of infants who required rescue treatment for PDA (indomethacin or surgery), died or dropped out on or prior to study day 14, mortality, NEC, IVH, pulmonary haemorrhage, pulmonary hypertension, ROP, BPD (supplemental oxygen at 28 days), BPD (supplemental oxygen at 36 weeks' PMA), PVL | |
| Notes | This study was published in abstract form in 2005, but was published in a complete report in 2009. This study was supported by National Institues of Health grant 5‐U01HD‐37261‐01. Funding and Study Sponsor: Ross Laboratories, Columbus, Ohio in collaboration with the NICHD Pediatric Pharmacology Research Unit Network | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation was implemented using a dynamic allocation method of biased coin randomisation, balancing within BW (500 to 750 grams and 751 to 1000 grams), within each site, and in the study overall |
| Allocation concealment (selection bias) | Low risk | The coded vials of study drug or placebo contained indistinguishable colourless solutions dispensed by the blinded research pharmacists of the participating sites |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The coded vials of study drug or placebo contained indistinguishable colourless solutions dispensed by the blinded research pharmacists of the participating sites |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The coded vials of study drug or placebo contained indistinguishable colourless solutions dispensed by the blinded research pharmacists of the participating sites. Outcome assessors were blinded to the group assignments |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The outcome of BPD (supplemental oxygen at 36 weeks' PMA) was not ascertained in the whole sample as randomised. The denominator in the ibuprofen group was 46 infants and in the placebo group it was 52, which is too low when accounting for mortality |
| Selective reporting (reporting bias) | Unclear risk | See incomplete data. The trials was registered with clinicaltrials.gov: ID # NCT00440804 |
| Other bias | Low risk | Appeared free of other bias |
Bagnoli 2013.
| Methods | Single centre, randomised controlled trial conducted in Siena, Italy. Study period: January 2006 to December 2010 | |
| Participants | 134 preterm newborns with ECHO‐confirmed PDA (PMA < 32 weeks, BW < 1500 grams, postnatal age > 72 hours | |
| Interventions | Ibuprofen: 67 infants, 3‐day treatment course of ibuprofen 10 mg/kg, 5 mg/kg and 5 mg/kg given IV over 10 minutes Placebo: 67 infants, 0.9% NaCl given IV |
|
| Outcomes | Failure to close a PDA, need for surgical ligation of the PDA, oliguria, NEC, creatinine and BUN before and after treatment, mortality at 28 days of life | |
| Notes | Dr. Annalisa Rossetti provided additional outcome data and information about the conduct of the trial that were not in the published report. No information on funding provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The randomisation sequence was manually generated (according to an internal protocol) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "This trial was a randomised, placebo‐controlled, double‐blind parallel design study...". No other detailed information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "This trial was a randomised, placebo‐controlled, double‐blind parallel design study...". No other detailed information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data reported for all randomised infants |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available to us so we could not judge if there were any deviations from the protocol |
| Other bias | Low risk | Appeared free of other bias |
Bravo 2014.
| Methods | Single centre, randomised placebo‐controlled, double‐blind trial conducted in Madrid, Spain. Study period: 11 months | |
| Participants | 49 preterm infants with ECHO‐confirmed PDA measuring ≥ 1.5 mm (PMA 24 to 34 weeks) | |
| Interventions | Infants with PDA ≥ 1.5 mm received the first dose of ibuprofen (10 mg/kg) and were then randomised to receive either standard treatment (21 infants) or ECHO‐guided treatment (28 infants) Standard treatment: 2 additional doses of ibuprofen 5 mg/kg at 24‐hour intervals after the initial dose of 10 mg/kg, independently of ductal size, as long as additional doses were not contraindicated ECHO: additional doses of ibuprofen (5 mg/kg at 24‐hour intervals) only if the PDA was still ≥ 1.5 mm at the time of the corresponding ibuprofen dose. A decision on whether to treat the PDA when the diameter was < 1.5 mm in the ECHO group was made on the basis of previous reports using the same approach with indomethacin. Additional ibuprofen doses were administered only when the PDA was > 1.5 mm 24 hours after a complete ibuprofen course (therapeutic failure), or when a reopening was documented because a diameter ≥ 1.5 mm has been correlated with pulmonary overflow, as small, nonsymptomatic PDA do not seem to play an important role in the pathogenesis of PDA‐related morbidity |
|
| Outcomes | Primary outcome: reopening of PDA Secondary outcomes: failure to close a PDA, number of ibuprofen doses used, need for surgical ligation, mortality, BPD (need for supplemental oxygen at 36 weeks' PMA), IVH (grade II and III), PVL, oliguria (urine output < 1 mL/kg/hour), creatinine after treatment and laser therapy for ROP |
|
| Notes | Dr. Bravo provided additional information regarding the methods and the outcomes of the trial. The study received financial support from the Spanish Fondo de Investigacion Sanitaria, grant CM07/00111, and the scientific advice of the SAMID network (RD08/0072/0018) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque envelopes that contained the allocation written on a card inside |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Healthcare providers were not blinded to the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | ECHOs were conducted by the same examiner, who was blind to the patient group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data provided for all randomised infants |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available to us so we could not judge if there were any deviations from the protocol |
| Other bias | Low risk | Appeared free of other bias |
Cherif 2008.
| Methods | Single centre, randomised controlled trial. Conducted in the NICU of the Neonatal and Maternity Center of Tunis, Tunis, Tunisia. Study period: one year, January 2007 to December 2007 | |
| Participants | 64 VLBW infants with ECHO‐confirmed PDA, PMA < 32 weeks, BW < 1500 grams, postnatal age 48 to 96 hours, respiratory distress requiring > 25% oxygen supplementation and ECHO evidence of significant left‐to‐right shunting across PDA | |
| Interventions | Ibuprofen (oral): 32 infants, oral ibuprofen 10 mg/kg as the initial dose Ibuprofen (IV): 32 infants, IV ibuprofen 10 mg/kg as the initial dose After the first dose of treatment in both groups, ECHO evaluation was performed to determine the need for a second or a third dose. In each group, in case the ductus was still open after the third dose, IV ibuprofen (an initial dose of 10 mg/kg followed by 2 doses of 5 mg/kg each, after 24 and 48 hours) as a non‐randomised rescue treatment was given. If this therapy did not promote ductal closure and the infant continued to receive mechanical ventilation, surgical ligation of the ductus was performed |
|
| Outcomes | PDA closure rate, need for surgical ligation, rate of reopening of the ductus, oliguria, increase in serum creatinine level > 16 mg/dL, change in creatinine concentrations, IVH grades I‐II and grades III‐IV, PVL, NEC, bowel perforation, sepsis, duration of intubation, survival at 1 month, and duration of hospital stay | |
| Notes | No information on funding provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Low risk | Infants were randomly assigned to a treatment group by means of cards in sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Health care providers were not blinded to treatment groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Physicians performing ECHO and making the decision for second and third dose administration were unaware of assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all enrolled infants |
| Selective reporting (reporting bias) | Low risk | The protocol was available to us. Trial number: NCT00642330. There did not seem to be any definitive deviations from the protocol |
| Other bias | Low risk | Appeared free of other bias |
Chotigeat 2003.
| Methods | Single centre, randomised, controlled trial conducted in Bangkok, Thailand. Study period: 1 January 2001 to 31 May 2002 | |
| Participants | 30 preterm infants (GA ≤ 35 weeks, postnatal age ≤ 10 days) with an ECHO‐confirmed PDA Ibuprofen: 15 infants, mean (SD) GA 30.8 (2.3) weeks; BW 1412 (354) grams Indomethacin: 15 infants, mean (SD) GA 29.9 (2.9) weeks; BW 1434 (421) grams |
|
| Interventions | Ibuprofen: orally as a 3‐day treatment course every 24 hours Indomethacin: IV at 12‐hour intervals The doses of ibuprofen and indomethacin were not stated |
|
| Outcomes | PDA closure, need for surgical ligation, NEC | |
| Notes | No information on funding provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | The infants were assigned to treatment group by random number |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Ibuprofen was given orally and indomethacin was given IV |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Ibuprofen was given orally and indomethacin was given IV. It is not stated that ECHOs were conducted by physicians blinded to the treatment the infant received |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported on all randomised infants |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available to us and we could not ascertain whether there were deviations from the protocol |
| Other bias | Low risk | Appeared free of other bias |
Dani 2012.
| Methods | Multicentre, randomised, controlled trial conducted in four NICUs (Florence, Turin, Bozen, Milan) in Italy: Study period July 2007 to June 2009 | |
| Participants | 70 infants with PMA < 29 weeks, ECHO evidence of significant PDA, aged 12 to 24 hours and RDS necessitating respiratory support | |
| Interventions | High‐dose ibuprofen: 35 infants, mean (SD) PMA 25.6 (1.8) weeks; BW 781 (225) grams) randomised to high‐dose ibuprofen 20‐10‐10 mg/kg/day Standard‐dose ibuprofen: 35 infants, mean (SD) PMA 26.0 (1.7) weeks; BW 835 (215) grams) randomised to standard‐dose IV ibuprofen 10‐5‐5 mg/kg/day |
|
| Outcomes | Ductal closure, serum creatinine on day 3 of treatment, oliguria (≤ 1 mL/kg/hour during a 24‐hour collection period), peak total serum bilirubin during the first week of life, IVH (all grades and grades III‐IV), PVL (all grades), ROP (all stages, stage > 2), NEC, BPD (oxygen requirement at 36 weeks' PMA), sepsis, mortality and hospital stay (days) | |
| Notes | No information on funding provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It was not stated if clinical staff members were blinded to the dose of ibuprofen the infant received |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | ECHO studies were performed by physicians, who were blinded as to the infants' treatment assignments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all enrolled infants |
| Selective reporting (reporting bias) | Low risk | The trial was registered with ClinicalTrials.gov under identifier NCT01243996. There did not seem to have been any deviations from the published protocol |
| Other bias | Low risk | Appeared free of other bias |
Demir 2017.
| Methods | Randomised controlled study conducted in the Yuzuncu Yil University, NICU, Van, Turkey. Study period: January 2014 to July 2015 | |
| Participants | Infants < 32 weeks' PMA and with birth weights < 1500 grams and with a haemodynamically significant PDA | |
| Interventions | A total of three ibuprofen doses were administered; the initial dose was 10 mg/kg and the following two doses at 24 and 48 hours were 5 mg/kg. Both rectal and oral ibuprofen were given via an oro‐gastric tube, which was flushed with 1 to 2 ml of sterile water to ensure the delivery of the drug | |
| Outcomes | Failure to close the PDA, need for a 2nd course, need for surgical ligation. Plasma creatinine (mg/dL) after treatment, urine output after treatment, plasma bilirubin (mg/dL) after treatment | |
| Notes | "This manuscript presents independent research funded by Office of Scientific Research Projects of Yuzuncu Yil Unicersity for Health research and Patient Benefit program (Grant reference number 2014‐TF‐B184)" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | "The patients were randomised into treatment groups using numbered cards in sealed envelopes which are not opaque" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The infants received either rectal or oral ibuprofen and the administration methods were known to the caregivers. The authors stated: "The limitations of our study included that it was not a double‐blind study and the sample size was relatively small" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The infants received either rectal or oral ibuprofen and the administration methods were known to the caregivers. It was not stated whether the cardiologist performing the ECHOs was blinded to the groups or not |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all randomised infants. Two infants in the oral ibuprofen group and one infant in the rectal ibuprofen group died before completed treatment; they were not included in a intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not state if there were any deviations or not |
| Other bias | Low risk | Appeared free of other bias |
Ding 2014.
| Methods | Randomised controlled trial at Provincial Hospital affiliated to Shandong University, Jinan, China. Study period: July 2011 to December 2011. | |
| Participants | Preterm infants with a PDA | |
| Interventions | Oral ibuprofen 10 mg/kg, followed by 5 mg/kg after 24 and 48 H, and the placebo group received the same volume of 5% glucose | |
| Outcomes | PDA closure at 7 days after treatment | |
| Notes | We did not include N‐terminal pro‐brain natriuretic peptide as an outcome. No information on funding provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | Randomly placed into two groups. No other information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | ECHOs were performed by a senior paediatric attending physician, who was unaware of the infants' treatment schedule |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported on all randomised infants |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge if there were any deviations or not |
| Other bias | Unclear risk | Inclusion criteria not well defined and study design not well described |
El‐Mashad 2017.
| Methods | Randomised controlled trial in the NICU of Tanta University Hospital, Pediatric department, Tanta University Hospital, Tanta, Egypt. Study period: January 2012 to December 2015 | |
| Participants | Preterm neonates with PMA < 28 weeks or birth weight < 1500 grams in the first 2 weeks of life with haemodynamically significant PDA (hs‐PDA) diagnosed with ECHO and clinical examination | |
| Interventions | Experimental intervention: Group I (paracetamol group): 100 neonates received 15 mg/kg IV infusion paracetamol over 30 min followed by 15 mg/kg/6 H IV infusion for 3 days. Group II (Ibuprofen group): 100 neonates received 10mg/kg IV infusion ibuprofen followed by 5 mg/kg/day for 2 days Group III (Indomethacin group): 100 neonates received 0.2 mg/kg indomethacin IV infusion over 30 min for three doses 12 H apart |
|
| Outcomes | Primary: Failure to close the PDA Secondary: Surgical ligation, ROP, GI bleeding, NEC, pulmonary haemorrhage, IVH, sepsis, daily urine output, serum creatinine, serum bilirubin, and platelet count |
|
| Notes | This was a three arm trial comparing paracetamol to ibuprofen and indomethacin. For this review, we compared the results in the ibuprofen group to the indomethacin group. Funding: "None to declare" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation software was used for random sequence generation |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The neonates were enrolled into the respective group by the doctor on duty, who was not blinded and not part of the study. Drugs were given at different times and duration |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | ECHOs were performed by a paediatric cardiologist, who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported on all randomised infants |
| Selective reporting (reporting bias) | Unclear risk | The study was not registered in a trials registry so we could not tell if there were any deviations from the protocol |
| Other bias | Low risk | Appeared free of other bias |
Erdeve 2012.
| Methods | Single centre, randomised controlled trial, conducted in Ankara, Turkey. Study period: January 2010 to February 2011 | |
| Participants | 80 infants with PMA ≤ 28 weeks, BW < 1000 grams, postnatal age 48 to 96 hours and with ECHO‐confirmed significant PDA | |
| Interventions | Ibuprofen (oral): 36 infants Ibuprofen (IV): 34 infants Both at a dose of 10 mg/kg followed by 5 mg/kg at 24 and 48 hours. 4 infants in the oral group and 6 in the IV group were excluded because of mortality before complete treatment course |
|
| Outcomes | Primary outcome: PDA closure rate Secondary outcomes: mortality, need for re‐treatment or surgical treatment of the PDA, duration of ventilation, duration of hospital stay, increase in serum bilirubin level after treatment, plasma creatinine after the first course of treatment, rate of ductal reopening, pneumothorax, pulmonary haemorrhage, pulmonary hypertension, BPD (supplemental oxygen at 36 weeks' PMA), IVH (grades I‐IV), NEC, ROP and ROP requiring laser treatment |
|
| Notes | 4 infants in the oral group and 6 in the IV group were excluded because of mortality before complete treatment course. They were not included in an ITT analysis for the outcome of mortality. We did include these deaths in our analysis of mortality. No information on funding provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Ibuprofen was administered either orally or IV, which would have been known to the caregivers |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A paediatric cardiologist was blinded to the treatment group to determine the success of the treatment and the need for a second course via the same route |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4 infants in the oral group and 6 in the IV group were excluded because of mortality before complete treatment course. They were not included in an ITT analysis for the outcome of mortality. We did include these deaths in our analysis of mortality |
| Selective reporting (reporting bias) | Low risk | Study protocol was available to us. Trial registration # NCT01261117. There did not seem to have been any deviations from the protocol |
| Other bias | Low risk | Appeared free of other bias |
Fakhraee 2007.
| Methods | Single centre randomised controlled trial in Tehran, Iran. Study period: June 2003 to June 2004 | |
| Participants | 36 preterm infants PMA < 34 weeks, aged ≤ 14 days, platelet count > 100,000/μL, serum creatinine ≤ 1.6 mg/dL, absence of clinical manifestations of abnormal clotting function, absence of grades III‐IV IVH. Colour Doppler ECHO evidence of significant PDA Ibuprofen: 18 infants, mean (SD) PMA 31.5 (1.4) weeks; BW 1658 (387) grams Indomethacin: 18 infants, mean (SD) PMA 30.9 (2.0) weeks; BW 1522 (358) grams Study period: June 2003 to June 2004 |
|
| Interventions | Ibuprofen: orally as a suspension at a first dose of 10 mg/kg, followed at an interval of 24 hours by 2 doses of 5 mg/kg Indomethacin: orally 3 times at 0.2 mg/kg/dose at intervals of 24 hours |
|
| Outcomes | Ductal closure, need for re‐treatment, reopening of the duct, mortality during the first 30 days of life, maximum serum BUN and creatinine levels, NEC, IVH (grades III‐IV), oliguria | |
| Notes | No information on funding provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description provided |
| Allocation concealment (selection bias) | Unclear risk | No description provided. "The enrolled patients randomly received either oral ibuprofen or oral indomethacin" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | ECHOs were performed by a paediatric cardiologist, who was blinded to the infants' treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes were reported for all enrolled infants |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available to us, so we could not judge if there were any deviations |
| Other bias | Low risk | Appeared free of other bias |
Fesharaki 2012.
| Methods | Randomised controlled clinical trial in NICU of Vali‐ye‐Asr Hospital, Tehran, Iran. Study period 1387 to 1389 years (Shia calendar). | |
| Participants | 60 infants with ECHO‐confirmed PDA, PMA from 29 weeks to 35 weeks six days at birth, birth weight between 1000 to 2500g, age 72 hours to 120 hours and presence of a PDA confirmed by ECHO | |
| Interventions | Oral loading dose ibuprofen: 10 mg/kg on first day, followed by 2 doses of 5 mg/kg in the next 2 days Oral loading dose ibuprofen: 15 mg/kg on first day followed by 2 doses of 7.5 mg/kg in next 2 days |
|
| Outcomes | PDA closure rates, high BUN and creatinine (levels not provided), urine output < 0.5 mL/kg after onset of treatment and gastrointestinal bleed | |
| Notes | 30 (100%) infants in 15‐mg/kg group and 23 (76.7%) infants in 10 mg/kg group had successful PDA closure with no need for surgery (P value = 0.011) Dr. Fatemeh Nayeri (corresponding author) kindly provided us with an English translation of the article in January 2013. We are still awaiting some clarifications regarding the trial. In email dated 24 May 2014, we asked for clarification regarding how the randomisation sequence was generated and how the infants were allocated to 1 of the 2 groups. We asked if it was possible for the investigators and the clinicians to determine the difference between the 2 dosing regimens? As of 17 August 2014, we have not received a response Article in Persian. No information on funding provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided regarding sequence generation |
| Allocation concealment (selection bias) | Unclear risk | "...divided into two groups of 30 by randomisation" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data provided for all 60 randomised infants |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available to us, so we could not judge if there were any deviations |
| Other bias | Low risk | Appeared free of other bias |
Gimeno Navarro 2005.
| Methods | Single centre, randomised controlled trial, conducted in a NICU in Valencia, Spain. Study period: January 2003 to July 2004 | |
| Participants | 47 ventilated, preterm infants (< 34 weeks' GA) with a haemodynamically significant PDA, confirmed by ECHO in the first week of life and who required respiratory support Ibuprofen: median (25th and 75th centiles) GA 28 (24, 31) weeks; mean (SD) BW 1.169 (490) grams Indomethacin: median (25th and 75th centiles) GA 28.5 (27, 30) weeks; mean (SD) BW 1.206 (513) grams |
|
| Interventions | Ibuprofen: 23 infants, ibuprofen 10 mg/kg IV, followed by 2 doses of ibuprofen IV every 24 hours Indomethacin: 24 infants indomethacin 0.2 mg/kg/dose IV every 12 hours for a total of 3 doses |
|
| Outcomes | Primary outcome: pharmacological ductal closure Other outcomes: mortality, ductal reopening, need for surgical ligation, NEC, isolated bowel perforation, intestinal haemorrhage, pulmonary haemorrhage, CLD (supplemental oxygen at 28 days), IVH (grades III‐IV), days on assisted ventilation, days in supplemental oxygen, days in NICU |
|
| Notes | Study published in Spanish. For the 2014 update of this review, we used Google Translate for Business (Translator Toolkit Website Translator Global Market Finder). No information on funding provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation ‐ sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Indomethacin and ibuprofen were given at different time points |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | We could not find information whether the cardiologist performing the ECHOs was blinded to the treatment or not |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up ‐ yes Outcomes reported on all randomised infants |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available to us, so we could not judge if there were any deviations |
| Other bias | Low risk | Appeared free of other bias |
Gokmen 2011.
| Methods | Single centre, randomised, controlled trial conducted at Zekai Tahir Burak Maternity Teaching Hospital, Ankara, Turkey. Study period: January 2010 to February 2011 | |
| Participants | 108 VLBW infants with PDA | |
| Interventions | Ibuprofen (IV): 54 infants, IV ibuprofen at an initial dose of 10 mg/kg, followed by 5 mg/kg at 24 and 48 hours Ibuprofen (oral): 54 infants, oral ibuprofen at an initial dose of 10 mg/kg, followed by 5 mg/kg at 24 and 48 hours 6 infants (4 in the IV group and 2 in the oral group) died before they completed the treatment and were excluded from the analyses |
|
| Outcomes | Renal tolerance, mean plasma creatinine after treatment, urine output after treatment, cystatin‐C levels, failure to close a PDA, need for second course of ibuprofen, oliguria, hospital stay, NEC, gastrointestinal bleed, sepsis, pneumothorax, BPD (supplemental oxygen at 36 weeks' PMA or at discharge, which ever came first, ROP requiring laser treatment, and mortality during hospital stay | |
| Notes | In 2013, a follow‐up study of this trial was published. 57 children (56%) of the original 102 infants enrolled in this study were followed to an age of 18 to 24 months corrected age; 30 infants in the oral ibuprofen group and 27 infants in the IV ibuprofen group were assessed for long‐term outcomes. The following outcomes were reported; Mental (MDI) and Psychomotor (PDI) Developmental Index on Bayley Scales of Infant Development II, moderate/severe cerebral palsy with functional deficits that required rehabilitation services, bilateral hearing loss (requiring amplification), blindness in either eye, MDI < 70 and PDI < 70. Information on funding was not provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Low risk | Infants were assigned randomly using cards in sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Ibuprofen was given either orally or IV and this would have been known to staff |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | ECHOs were performed by a paediatric cardiologist, who was blinded to the treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 infants (4 in the IV ibuprofen group and 2 in the oral group) died before they completed their treatment. These infants were not included in an ITT analysis. We included them in our analyses |
| Selective reporting (reporting bias) | Unclear risk | The protocol for this study was not available to us and we could not ascertain whether there were deviations from the protocol |
| Other bias | Low risk | Appeared free of other bias |
Hammerman 2008.
| Methods | Single centre, randomised, controlled trial, conducted in Jerusalem, Israel. Study period: February 2002 to December 2006 | |
| Participants | 64 preterm (PMA ≤ 33 weeks, BW ≤ 1750 grams) infants with PDA | |
| Interventions | Ibuprofen: 32 infants, ibuprofen 10 mg/kg IV followed by 2 doses of 5 mg/kg at 24‐hour intervals Indomethacin: 31 infants, continuous IV infusion of indomethacin for 36 hours at a rate of 17 μg/kg/hour |
|
| Outcomes | Ductal closure, need for surgical ligation, need for re‐treatment with either indomethacin or ibuprofen, need for surgical treatment, mortality, BPD (age not stated), IVH (grades III‐IV), ROP, and NEC | |
| Notes | The outcomes of BPD (age not stated), NEC, IVH (III‐IV) and ROP (3‐4) were reported in graphic form only and the numbers had to be estimated from the graph. No information on funding provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was based on computer‐generated random numbers without sub stratification |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Because the methods of drug administration were clearly different, the study could not be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The cardiologist performing the ECHOs was blinded to study group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 infant assigned to the ibuprofen group was withdrawn by his parents before he started therapy, and he was not included in the analyses |
| Selective reporting (reporting bias) | Low risk | The protocol was available to us. Trial registration # NCT00485160. There were no deviations from the protocol |
| Other bias | Low risk | Appeared free of other bias |
Lago 2002.
| Methods | Two centre, randomised, controlled trial conducted in Padova and Treviso, Italy: Study period: January 1998 to December 2000 | |
| Participants | 175 preterm infants with ECHO‐confirmed PDA were enrolled Ibuprofen: 94 infants, mean (SD) GA 28 (2) weeks; BW 1126 (412) grams; 52 boys, 42 girls Indomethacin: 81 infants, mean (SD) GA 29 (3) weeks; BW 1214 (427) grams; 43 boys, 38 girls |
|
| Interventions | Ibuprofen: IV 10 mg/kg as the initial dose followed by 5 mg/kg each at 24 and 48 hours Indomethacin: IV 0.2 mg/kg for 3 doses at 12‐hour intervals |
|
| Outcomes | PDA closure, serum creatinine, and oliguria | |
| Notes | An interim report with 153 infants enrolled (ibuprofen group 82 infants and indomethacin group 71 infants) has been published (Lago 2001). Zanardo 2005 represented a subpopulation of this study and examined the effect of ibuprofen and indomethacin on urinary antidiuretic hormone excretion. No information on funding provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description provided |
| Allocation concealment (selection bias) | Low risk | Cards in sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Ibuprofen and indomethacin were administered at different times |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were not assessed blinded to group assignments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all randomised infants |
| Selective reporting (reporting bias) | Unclear risk | The authors did not comment on the imbalance in the numbers enrolled in the ibuprofen group (94 infants) versus the indomethacin group (81 infants) The protocol was not available to us, so we could not ascertain if there were deviations from the protocol |
| Other bias | Low risk | Appeared free of other bias |
Lago 2014.
| Methods | Single centre double‐blind randomised controlled trial conducted at the NICU of the Padua University Hospital, Padua, Italy Study period: February 2008 to June 2010 |
|
| Participants | 112 preterm infants < 32 weeks' PMA with haemodynamically significant PDA on ECHO. Informed consent was withdrawn from 1 infant in the continuous ibuprofen group | |
| Interventions | Ibuprofen (standard treatment): 56 infants, bolus of IV ibuprofen of 10 mg 5 mg and 5 mg administered over 15 minutes, 24 hours apart Ibuprofen (infusion): 55 infants, continuous infusion of ibuprofen of 10 mg, 5 mg and 5 mg given over 24 hours, and boluses of equal volumes of 5% dextrose administered over 15 minutes, 24 hours apart |
|
| Outcomes | PDA closure rate after 2 standard‐dose ibuprofen courses, PDA closure after first ibuprofen course, reopening of PDA, need for surgical ligation, oliguria (urine output ≤ 1 mL/kg/hour, creatinine after treatment, gastrointestinal haemorrhage, intestinal perforation during ibuprofen treatment, NEC during ibuprofen treatment, BPD (at 36 weeks' PMA), ROP (all stages and stage ≥ 3, IVH (all grades and grades III‐IV), cystic PVL, NEC, isolated bowel perforation, mortality, and duration of hospital stay | |
| Notes | No information on funding provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Eligible infants were randomised by the hospital pharmacist to receive in a 1 : 1 ratio either standard treatment (bolus) or continuous infusion of ibuprofen. Throughout the study, the hospital pharmacist kept the randomisation list inaccessible to the clinical investigators and NICU personnel |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Ibuprofen was given as continuous infusion or as intermittent boluses, which must have been known to the caregivers |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not stated whether the cardiologist performing the ECHOs was blinded to the treatments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were presented for all randomised infants, except for 1 infant in the continuous ibuprofen group, for whom the parents withdrew informed consent |
| Selective reporting (reporting bias) | Unclear risk | The protocol for this study was not available to us and we could not ascertain whether there were deviations from the protocol |
| Other bias | Low risk | Appeared free of other bias |
Lin 2012.
| Methods | Single centre, randomised controlled trial at the Maternal and Child health Hospital of Xiamen City, Xiamen, Fujian, China. Study period: Not stated in the abstract written in English. | |
| Participants | 64 symptomatic VLBW infants with a PDA confirmed by bedside colour Doppler ultrasound were enrolled within 24 hours after birth | |
| Interventions | Ibuprofen: 32 infants, oral ibuprofen within 24 hours after birth at 10 mg/kg, followed 24 hours later by a second dose of 5 mg/kg and 48 hours later by a third dose of 5 mg/kg Placebo: 32 infants, placebo (normal saline) at 1 mL/kg, followed 24 hours later by a second dose of 0.5 mL/kg and 48 hours later by a third dose of 0.5 mL/kg |
|
| Outcomes | PDA closure, PVL, BPD, duration of ventilator support, duration of hospital stay, IVH, pulmonary haemorrhage, NEC, adverse effects From the abstract, we were only able to calculate the rates for PDA closure in the 2 groups. P values were provided for some of the other outcomes, and we quoted them in the results section |
|
| Notes | This study was published in Chinese and we could only understand the abstract, which was published in English. No information on funding provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided in the abstract |
| Allocation concealment (selection bias) | Unclear risk | Infants were randomly divided into 2 groups |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It is possible that the study was blinded as a placebo was used in the control group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | We could not judge from the abstract |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | We could not judge from the abstract |
| Selective reporting (reporting bias) | Unclear risk | We could not judge from the abstract |
| Other bias | Unclear risk | We could not judge from the abstract |
Lin 2017.
| Methods | Randomised controlled trial conducted in two NICUs in John Stroge's Hospital of Cook County, Chicago, USA and in Chaina Medical University Hospital, Taiwan. Study period: not stated | |
| Participants | Inclusion criteria: : (1) preterm infants with a birth weight < 1,000 g; (2) a radiographic figure of respiratory distress syndrome; (3) requirement for mechanical ventilation, and (4) echocardiography proven and clinically significant PDA |
|
| Interventions | Infants assigned to ibuprofen were given an initial dose of 10 mg/kg (1.0 mL/kg) followed by 2 doses of 5 mg/kg (0.5 mL/kg) at 24‐hour intervals IV Infants assigned to the indomethacin group were given an initial dose of 0.2 mg/kg (1.0 mL/kg) followed by 2 doses of 0.1 mg/kg (0.5 mL/kg) at 24‐hour intervals IV |
|
| Outcomes | Primary outcomes – renal function and ductal response; reopening, persistent ductal closure, and surgical ligation. NEC, BPD (age not stated), IVH (≥ grade 2) | |
| Notes | The study was funded by Lundbeck (Ovation) Pharmaceuticals (Chicago, Ill, USA) and the National Science Council (NSC 95‐2314‐B‐039‐032‐MY2), Taipei, Taiwan | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Both medications were clear and indistinguishable, and for each administration a similar volume was infused continuously over a period of 15 min. During the study, only the primary investigator in each participating hospital was aware of the content of the medication, while the other medical and nursing staff responsible for daily care were blinded to the medication administered. The primary investigator was either the chief or the consultant of the NICU, who was rarely involved in direct patient care |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The ECHOs were read by a paediatric cardiologist who was blinded to the study medication |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcome group; before the data were analysed, two infants with a birthweight of < 500 g and four infants with intractable respiratory failure and with severe IVH were excluded – thus not intention‐to‐treat analysis. These infants fulfilled inclusion criteria |
| Selective reporting (reporting bias) | Unclear risk | The trial was registered as NCT01758913, but only in 2013. The study took place between 2007 and 2012 and therefore the study was completed before it was registered. Thus we cannot ascertain whether there were any deviations from the original protocol. |
| Other bias | Low risk | Appeared free of other bias |
Mosca 1997.
| Methods | Single centre, randomised, controlled trial conducted in Milan, Italy. Study period: not stated | |
| Participants | 16 infants receiving mechanical ventilation (< 31 weeks' GA) with ECHO evidence of PDA were randomised Ibuprofen: 8 neonates, median and range GA 29 (27 to 31) weeks, BW 855 (620 to 1620) grams , postnatal age 24 (10 to 53) hours, 4 boys, 4 girls Indomethacin: 8 neonates, median and range GA 28 (25 to 300) weeks, BW 820 (600 to 1390) grams, postnatal age 29 (5 to 120) hours, 5 boys, 3 girls |
|
| Interventions | Ibuprofen: IV 10 mg/kg infused over 1 minute as a first dose and a second and third dose administered at 24‐hour intervals provided that no significant adverse effect was observed Indomethacin: IV 0.2 mg/kg infused over 1 minute and a second and third dose of 0.1 mg/kg were administered at 24‐hour intervals provided no significant adverse effects were observed |
|
| Outcomes | PDA closure, cerebral blood flow velocity, near‐infrared spectroscopy was used to measure changes in cerebral blood volume and in oxidised cytochrome oxidase concentration | |
| Notes | The results of this study were reported in abstract form with the same number of infants enrolled (Mosca 1996). Whether there was any overlap with an additional study was unclear (Mosca 1997a) No information on funding provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | The infants were randomised. No other information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinicians were aware of group assignments |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not stated whether the cardiologist performing the ECHOs was blinded to the treatments or not |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported on all randomised infants |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available to us so we could not ascertain if there were deviations from the protocol |
| Other bias | Low risk | Appeared free of other bias |
Patel 1995.
| Methods | Single centre, randomised, controlled trial without the use of a placebo in the UK. Study period: not stated | |
| Participants | 33 infants with a median GA of 26 weeks (range 23 to 28) were enrolled. All infants had ECHO‐confirmed PDA | |
| Interventions | Ibuprofen: 12 infants, ibuprofen 5 mg/kg Ibuprofen: 6 infants, ibuprofen 10 mg/kg Indomethacin: 15 infants, indomethacin 0.1 mg/kg The drugs were infused IV over 15 minutes |
|
| Outcomes | PDA closure rate, near‐infrared spectroscopy was used to observe the effect of treatment on cerebral perfusion, indicated by changes in cerebral blood volume, and cerebral mitochondrial oxygenation, determined by the change in concentration of oxidised cytochrome aa3 | |
| Notes | Published as a letter to the editor. This study was supported by the British Heart Foundation and Hammamatsu Photonics KK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The intervention was not blinded to caregivers |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The intervention was not blinded to outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up ‐ yes |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available to us so we could not ascertain if there were deviations from the protocol |
| Other bias | Low risk | Appeared free of other bias |
Patel 2000.
| Methods | 4 centre, randomised controlled trial in 4 NICUs (Hammersmith, Queen Charlotte's, St George's and St Mary's Hospitals, London, UK). Study period: January 1966 to December 1996 | |
| Participants | 33 preterm infants with a haemodynamically significant PDA diagnosed clinically and on ECHO criteria Ibuprofen: 18 infants, median (range) GA 26.0 (23.9 to 35.00) weeks; BW 790 (620 to 2780) grams; postnatal age 8 (3 to 20) days; 9 boys, 9 girls Indomethacin: 15 infants, median (range) GA 26.7 (23.2 to 30.0) weeks; BW 838 (458 to 1377) grams; postnatal age 7 (3 to 21) days; 7 boys, 8 girls. |
|
| Interventions | Ibuprofen: 10 mg/kg IV as the initial dose followed by 5 mg/kg at 24 and 48 hours after the initial dose Indomethacin: 0.2 mg/kg as initial dose. 2 further doses were administered after 12 and 24 hours: infants aged 2 to 7 days at the time of the first dose received 0.2 mg/kg and infants ≥ 8 days received 0.25 mg/kg To prevent identification of the drug administered from the timing schedule, all infants received a fourth dose containing 0.9% saline: in the indomethacin group, 48 hours after the first dose and, in the ibuprofen group, 12 hours after the first dose; IV infusions of all drugs were performed over 15 minutes using an infusion pump |
|
| Outcomes | PDA closure rate, near‐infrared spectroscopy was used to measure changes in cerebral blood volume, cerebral blood flow, and cerebral oxygen delivery | |
| Notes | Merckle GmbH donated the ibuprofen used in the study. No information on other funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Low risk | The Pharmacy Department at Queen Charlotte's Hospital performed randomisation in blocks of 12 for each hospital and provided all trial medication. All other personnel were blinded to the identity of the drug administered |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | See allocation concealment and intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See allocation concealment and intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up ‐ yes |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available to us so we could not ascertain if there were deviations from the protocol |
| Other bias | Low risk | Appeared free of other bias |
Pezzati 1999.
| Methods | Single centre, randomised controlled trial, conducted in Firenze, Italy. Study period: not stated | |
| Participants | 17 preterm infants (< 33 weeks' GA) Ibuprofen: 9 infants, mean (SD) GA 29.1 (2.2) weeks; BW 1151 (426) grams Indomethacin: 8 infants, mean (SD) GA 29.5 (2.6) weeks; BW 1277 (440) grams |
|
| Interventions | Ibuprofen: 10 mg/kg given as a continuous infusion over 15 minutes Indomethacin: 0.2 mg/kg as a continuous infusion over 15 minutes Regardless of ductal closure after the first dose, all infants received a second and third dose of indomethacin (0.1 mg/kg) or ibuprofen (5 mg/kg) at 24‐hour intervals |
|
| Outcomes | Primary outcome: mesenteric and renal blood flow velocity Secondary outcomes: ductal closure, ductal reopening, and NEC |
|
| Notes | No information on funding provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | Infants were randomly assigned ‐ no further information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Infants were randomly assigned to receive either IV ibuprofen or IV indomethacin infusions over 15 minutes (see Interventions) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not stated whether the cardiologist performing the ECHOs was blinded to the treatments or not |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported on all randomised infants |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available to us so we could not ascertain if there were deviations from the protocol |
| Other bias | Low risk | Appeared free of other bias |
Pistulli 2014.
| Methods | Single centre, randomised controlled trial, conducted in the NICU of the University Hospital for Obstetrics and Gynecology, Koço Gliozheni, Tirana, Albania. Study period: January 2010 to December 2012 | |
| Participants | 80 preterm infants with a PMA 28 to 32 weeks, BW ≤ 2000 grams, postnatal age 48 to 96 hours, RDS treated with mechanical ventilation with additional oxygen requirements above 30% and PDA documented by ECHO Ibuprofen (oral): 44 infants randomised, 7 infants were excluded because of mortality before complete treatment course and 1 infant was excluded because of pulmonary haemorrhage (total 8 infants). Outcomes reported for 36 infants Ibuprofen (IV): 36 infants randomised, 3 infants were excluded because of gastrointestinal bleed and 1 infant was excluded because only 2 doses of ibuprofen were administered (total 4 infants). Outcomes reported for 32 infants |
|
| Interventions | Ibuprofen (oral): 10 mg/kg given via an oro‐gastric tube, flushed with 1 mL of sterile water Ibuprofen (IV): 10 mg/kg given via IV route infused over a 15‐minute period with a syringe pump, and the line was subsequently flushed with saline ECHO was performed again 24 hours after each ibuprofen dose. When the PDA was still haemodynamically significant, as demonstrated by ECHO, and there was no evidence of deterioration in brain ultrasonography, a second dose of ibuprofen 5 mg/kg was administered. A third equivalent dose was given after another 24 hours, if deemed necessary |
|
| Outcomes | Failure to close a PDA (after single or 3 doses), need for surgical ligation, oliguria, and mean plasma creatinine on day 3 of treatment | |
| Notes | No information on funding provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Ibuprofen was given via an oro‐gastric tube in one group and by IV in the other group, so the mode of drug delivery must have been known to caregivers |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | It was not stated that ECHOs were performed by a paediatric cardiologist blinded to the groups |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In the oral ibuprofen group, 7 infants were excluded because of mortality before complete treatment course and 1 infant was excluded because of pulmonary haemorrhage. Outcomes reported for 36 infants in the oral ibuprofen group. In the IV ibuprofen group, 3 infants were excluded because of gastrointestinal bleed and 1 infant was excluded because only 2 doses of ibuprofen were administered. Outcomes reported for 32 infants |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available to us so we could not ascertain if there were deviations from the protocol |
| Other bias | Unclear risk | Appeared free of other bias |
Plavka 2001.
| Methods | Three centre, randomised controlled trial in 3 NICUs in the Czech Republic. Study period: not stated | |
| Participants | 41 preterm infants with clinical and ECHO signs of PDA were randomised Ibuprofen: 21 infants, mean (SD) GA 27.6 (2.3) weeks; BW 929 (213) grams Indomethacin: 20 infants, mean (SD) GA 26.9 (1.7) weeks; BW 902 (211) grams |
|
| Interventions | Ibuprofen: IV 8 mg/kg every 24 hours for 3 doses Indomethacin: IV 0.2 mg/kg every 24 hours for 3 doses If PDA persisted, treatment was repeated at half dose every 24 hours for 6 doses. Persistent PDA was ligated |
|
| Outcomes | Cerebral blood flow velocities, blood pressure, serum creatinine, mortality, and ductal reopening | |
| Notes | Published in abstract form only. No information on funding provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up ‐ yes |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available to us so we could not ascertain if there were deviations from the protocol |
| Other bias | Low risk | Appeared free of other bias |
Pourarian 2008.
| Methods | One centre, randomised controlled trial, conducted in Shiraz, Republic of Iran. Study period: a 6‐month period in 2001 | |
| Participants | 20 preterm infants with ECHO‐confirmed PDA Ibuprofen: 10 infants, mean (SD) PMA 31.3 (4.4) weeks; BW 1860 (402) grams Indomethacin: 10 infants, mean (SD) PMA 33.2 (3.1) weeks; BW 1720 (630) grams |
|
| Interventions | Ibuprofen: oral suspension containing 100 mg/5 mL was given as an initial dose of 10 mg/kg, followed by 2 further doses of 5 mg/kg at 24‐hour intervals Indomethacin: powder content of an indomethacin 25 mg capsule was freshly prepared by dissolving in 25 mL distilled water. This was given orally as 0.2 mg/kg for 3 doses at 24‐hour intervals Administration of the second or third doses of each drug was dependent on achievement of ductal closure after the initial doses |
|
| Outcomes | Primary outcome: ductal closure Secondary outcomes: need for surgical closure, NEC, change in mean serum creatinine levels before and after treatment, increase in BUN level > 14 µmol/L, and thrombocytopenia < 50,000 mm3 |
|
| Notes | No information on funding provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | High risk | "As soon as the diagnosis (of PDA) was made for the 1st eligible baby, he/she was enrolled to the ibuprofen group and then the next eligible baby was assigned to the indomethacin group, and so on". This statement clearly indicated that the infants were not allocated to the two groups in a concealed manner |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The researchers were aware of group assignment ‐ see allocation concealment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The researchers were aware of group assignment ‐ see allocation concealment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Results for all randomised infants were reported |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available to us so we could not ascertain if there were deviations from the protocol |
| Other bias | Low risk | Study appeared free of other bias |
Pourarian 2015.
| Methods | Randomised controlled trial conducted in the Neonatology Research Center, Shiraz Univeristy Medical Sciences, Shiraz, Iran. Study period: April 2012 to May 2013 | |
| Participants | Preterm infants with PMA < 37 weeks and postnatal age 3 to 7 days with ECHO diagnosis of a haemodynamically significant PDA | |
| Interventions | Group I (high‐dose ibuprofen group) received 20 mg/kg PO ibuprofen as first dose followed by 10 mg/kg/dose after 24 and 48 hours Group II (normal‐dose ibuprofen group) received 10 mg/kg PO ibuprofen as first dose followed by 5 mg/kg/dose after 24 and 48 hours |
|
| Outcomes | Primary: Failure to close the PDA after the first course and after the second course. Secondary: Surgical ligation, ROP, bleeding disorders, GI bleeding, NEC, pulmonary haemorrhage, IVH (all grades) mortality, oliguria, serum BUN (mmol/L) after treatment, serum creatinine (mg/dL) after treatment, urine output (mL/kg/hr) after treatment, platelet count | |
| Notes | No external funding was secured for this study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Low risk | Cards in sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The cardiologist performing the ECHOs was unaware of the infants' treatment assignments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data provided for all randomised infants |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us so we could not judge if there were any deviations from the protocol or not |
| Other bias | Low risk | Appeared free of other bias |
Salama 2008.
| Methods | Single centre, randomised controlled trial conducted in Doha, State of Qatar. Study period: January 2005 to March 2007 | |
| Participants | 41 preterm infants (PMA, 34 weeks, BW < 2500 grams) diagnosed with haemodynamically significant PDA confirmed by ECHO Ibuprofen: 21 infants, mean (SD) PMA 27.7 (2.5); BW 1094 (480) grams Indomethacin: 20 infants, mean (SD) PMA 27.8 (2.8) weeks; BW 1050 (440) grams |
|
| Interventions | Ibuprofen: oral 10 mg/kg on the first day followed by 5 mg/kg for 2 more days. Ibuprofen was mixed with 0.5 mL of milk before its administration via an oro‐gastric tube Indomethacin: IV 3 doses of 0.2 mg/kg/dose every 24 hours |
|
| Outcomes | Primary outcome: complete closure of the PDA Secondary outcomes: need for surgical ligation, bowel perforation, and mortality |
|
| Notes | No information on funding provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was conducted according to a pre designed simple block randomisation table 'A' for indomethacin and 'B' for ibuprofen (AABABBBBAA, BBBAAABBA, AABBABABAAB, etc.) |
| Allocation concealment (selection bias) | Unclear risk | No description of possible concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Indomethacin was given IV and ibuprofen was given via an oro‐gastric tube |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "The paediatric cardiologist was aware of patient's group allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported on all randomised infants |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available to us so we could not ascertain if there were deviations from the protocol |
| Other bias | Low risk | Appeared free of other bias |
Sosenko 2012.
| Methods | Single centre, double‐blind, randomised controlled trial conducted in Miami, Florida, US. Study period January 2008 to August 2010 | |
| Participants | Infants born with BW 500 to 1250 g and PMA 23 to 32 weeks, who were > 24 hours old but ≤ 14 days old and who had ECHO for subtle PDA symptoms (metabolic acidosis, murmur, bounding pulses) | |
| Interventions | 'Early' treatment: 54 infants, blinded ibuprofen 'Expectant' management: 51 infants, blinded placebo If the PDA became haemodynamically significant (pulmonary haemorrhage, hypotension, respiratory deterioration), infants received open‐label ibuprofen. Infants with haemodynamically significant PDA at enrolment were excluded from the study The dosing schedule for ibuprofen was an initial dose of 10 mg/kg, followed by 2 doses of 5 mg/kg each, every 24 hours, by slow IV infusion; dosing of placebo involved equivalent volumes of dextrose by slow IV infusion on the same schedule |
|
| Outcomes | Days on supplemental oxygen during the first 28 days of life, mortality during hospital stay, supplemental oxygen at 36 weeks' PMA, intestinal perforation, NEC requiring surgery, IVH (grades III‐IV), PVL, sepsis and ROP (stage ≥ 3) | |
| Notes | After 105 of 168 infants were enrolled, the study drug (NeoProfen) was recalled by the manufacturer and was no longer available in the US. The study was supported by an unrestricted grant from Ovation (now Lundbeck) Pharmaceuticals and University of Miami | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Clinicians, investigators, and nursing staff were blinded to the study group to which the infant was assigned and the medication the infant was receiving. Only the neonatal pharmacists were aware of the study group of each infant and were responsible for preparing the "blinded" ibuprofen or "blinded" placebo study drug |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As per above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all enrolled infants |
| Selective reporting (reporting bias) | Low risk | This study was registered, #NCT00802685, and there did not seem to be any deviations from the protocol, except that the study had to be stopped when the study drug was no longer available |
| Other bias | Low risk | Appeared free of other bias |
Su 2003.
| Methods | Single centre, randomised controlled trial conducted in Taichung, Taiwan. Study period: January 2001 to December 2002 | |
| Participants | 63 preterm infants with GA ≤ 32 weeks and BW ≤ 1500 grams and with ECHO evidence of a PDA were randomised between 2 and 7 days of age Ibuprofen: 32 infants, mean (SD) GA 28.7 (2.2) weeks; BW 1134 (200) grams Indomethacin: 31 infants, mean (SD) GA 28.2 (2.4) weeks; BW 1110 (244) grams |
|
| Interventions | Ibuprofen: IV 10 mg/kg initially, followed by 5 mg/kg after 24 and 48 hours Indomethacin: IV 0.2 mg/kg every 12 hours for 3 doses |
|
| Outcomes | Rate of PDA closure, rate of reopening of the duct, mortality, gastric bleeding, IVH, PVL, NEC, BPD at 36 weeks' GA, duration of mechanical ventilation, time to full oral feeds, and length of hospital stay | |
| Notes | No information on funding provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | Patients were randomly placed into two groups |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Ibuprofen and indomethacin were administered at different times |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | ECHOs were performed by a senior paediatric attending physician, who was unaware of the infants treatment schedule |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all infants randomised |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available to us so we could not ascertain if there were deviations from the protocol |
| Other bias | Low risk | Appeared free of other bias |
Su 2008.
| Methods | Single centre, randomised controlled trial conducted in Taichung, Taiwan. Study period: February 2004 to October 2006 | |
| Participants | 119 infants with ECHO evidence of a significant PDA Ibuprofen: 60 infants, median (range) PMA 25 (23 to 28) weeks; BW 825 (550 to 990) grams Indomethacin: 59 infants, median (range) PMA 25 (23 to 28) weeks; BW 762 (540 to 980) grams |
|
| Interventions | Ibuprofen: IV 10 mg/kg initially followed by 5 mg/kg at 24‐hour intervals Indomethacin: IV 0.2 mg/kg as the initial dose and then 0.1 mg/kg in infants < 48 hours old, 0.2 mg/kg in infants > 48 hours at 24‐hour intervals as indicated by PDA flow pattern |
|
| Outcomes | Primary outcome: PDA closure Secondary outcomes: need for surgical ligation, mortality within 30 days, NEC, CLD at 36 weeks' GA, IVH, PVL, ROP, BPD at 36 weeks' PMA, oliguria, post‐treatment serum creatinine, hospital stay, duration of mechanical ventilation, days to full enteral feeds, and gastric bleeding |
|
| Notes | Study included a sample size calculation. No information on funding provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | According to a random number table sequence, which had been prepared by a study assistant who was not involved in the care of infants |
| Allocation concealment (selection bias) | Low risk | The attending doctors were unaware of the drug used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The attending doctors were unaware of the drug used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The attending doctors were unaware of the drug used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was complete follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available to us so we could not ascertain if there were deviations from the protocol |
| Other bias | Low risk | Appeared free of other bias |
Supapannachart 2002.
| Methods | Single centre, randomised, controlled trial conducted in Bangkok, Thailand. Study period: 1 April 2000 to 31 August 2001 | |
| Participants | 18 preterm infants (< 34 weeks' GA) with symptomatic PDA Ibuprofen: 9 infants, mean (SD) GA 30.1 (2.7); BW 1447 (39) g; 8 boys, 1 girl Indomethacin: 9 infants, mean (SD) GA 30.4 (2.6); BW 1432 (531) g; 6 boys, 3 girls |
|
| Interventions | Ibuprofen: orally 10 mg/kg/dose for 3 doses at 24‐hour intervals Indomethacin: oral or IV 0.2 mg/kg/dose for 3 doses given at 12‐hour intervals |
|
| Outcomes | PDA closure rate, duration of ventilatory support, CLD (age not stated), IVH (grade not stated), NEC, and mortality | |
| Notes | No information on funding provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used for allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Ibuprofen and indomethacin were given at different times |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Ibuprofen and indomethacin were given at different times |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all infants randomised |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available to us so we could not ascertain if there were deviations from the protocol |
| Other bias | Low risk | Appeared free of other bias |
Van Overmeire 1997.
| Methods | Single centre, randomised controlled trial conducted in Antwerp, Belgium Study period: not stated Blinding of randomisation ‐ yes Blinding of intervention ‐ no Complete follow‐up ‐ yes Blinding of outcome measurement(s) ‐ no |
|
| Participants | 40 preterm infants (GA 33 weeks) were randomised Ibuprofen: 20 infants, mean (SD) GA 29.0 (2.4) weeks; BW 1270 (450) grams; surfactant use 15 Indomethacin: 20 infants, mean (SD) GA 28.7 (1.9) weeks; BW 1210 (360) grams, surfactant use 19 |
|
| Interventions | Ibuprofen: IV 10 mg/kg as the initial dose followed by 5 mg/kg 24 and 48 hours later Indomethacin: IV 0.2 mg/kg every 12 hours for 3 doses Both drugs were infused over 15 minutes |
|
| Outcomes | PDA closure rate, PDA ligation rate, mortality, sepsis, NEC, age to regain BW, and ROP | |
| Notes | It is possible that there was overlap between this study and a report in abstract form with 28 infants enrolled (Van Overmeire 1996). No information on funding provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Ibuprofen and indomethacin were given at different times |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Ibuprofen and indomethacin were given at different times. It was not stated whether the ECHOs were performed by a physician blinded to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all infants randomised |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available to us so we could not ascertain if there were deviations from the protocol |
| Other bias | Low risk | Appeared free of other bias |
Van Overmeire 2000.
| Methods | Multicentre, randomised controlled trial without the use of a placebo conducted in 5 NICUs in Belgium (2 hospitals in Antwerp, 1 hospital each in Ghent, Bruges, and Rocourt) Study period: not stated Blinding of randomisation ‐ yes Blinding of intervention ‐ no Complete follow‐up ‐ yes Blinding of outcome measurement(s) ‐ no |
|
| Participants | 148 infants with PMA 24 to 32 weeks, who had RDS and ECHO‐confirmed PDA were randomised Ibuprofen: 74 infants, mean (SD) GA 29.0 (2.3) weeks; BW 1230 (390) grams; surfactant treatment 56 Indomethacin: 74 infants, mean (SD) GA 29.0 (2.1) weeks; BW 1230 (380) grams; surfactant treatment 63 |
|
| Interventions | Ibuprofen: IV 10 mg/kg as the initial dose, followed at 24‐hour intervals by 2 doses of 5 mg/kg Indomethacin: IV 0.2 mg/kg every 12 hours |
|
| Outcomes | PDA closure rate, oliguria, PDA ligation rate, mortality by 30 days, NEC, localised bowel perforation, sepsis, PVL, CLD at 28 days, time to regain BW, time to full enteral feeding | |
| Notes | We believe this study has been reported in abstract form when 103 preterm infants were enrolled (Van Overmeire 1998), but we have not been able to verify this with the authors. No information on funding provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes were used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Ibuprofen and indomethacin were given at different times |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | ECHOs were performed by physicians who were unaware of the infants' treatment assignments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all randomised infants |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available to us so we could not ascertain if there were deviations from the protocol |
| Other bias | Low risk | Appeared free of other bias |
Yadav 2014.
| Methods | Study was conducted in two tertiary care institutions in New Delhi, Northern India. Study period: March 2010 to May 2012 | |
| Participants | 83 preterm infants < 37 weeks' PMA, BW < 2500 grams with haemodynamically significant PDA confirmed by ECHO | |
| Interventions | Ibuprofen: 48 infants, 3 doses of oral ibuprofen suspension 10, 5, 5 mg/kg every 24 hours. The drug was given via the oro‐gastric route, followed by 0.5 mL of distilled water Indomethacin: 35 infants, 3 doses indomethacin 0.20 to 0.25 mg/kg every 24 hours depending on the GA (initial dose was 0.2 mg/kg, subsequent doses 2 to 7 days of age were 0.2 mg/kg/dose every 24 hours for 2 doses, 7 days of age 0.25 mg/kg/dose every 24 hours for 2 doses) |
|
| Outcomes | Failure to close a PDA, surgical ligation, oliguria, NEC, IVH, gastrointestinal bleed, mortality, hospital stay, serum creatinine, and PPHN | |
| Notes | No information on funding provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Randomisation was carried out by investigators not involved in the study. Sequentially numbered opaque sealed envelopes containing the code for intervention were used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The major limitation of our study was that the clinician was not blinded to the drug administered" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "The major limitation of our study was that the clinician was not blinded to the drug administered" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all randomised infants |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available to us so we could not ascertain if there were deviations from the protocol |
| Other bias | Low risk | Appeared free of other bias |
BPD: bronchopulmonary dysplasia BUN: blood urea nitrogen BW: birth weight CLD: chronic lung disease C/S: caesarean section ECHO: echocardiographically/echocardiography GA: gestational age GI: gastrointestinal H: hour ITT: intention‐to‐treat IV: intravenous IVH: intraventricular haemorrhage MDI: Mental Developmental Index (Bayley Scales of Infant Development) min: minute(s) NaCI: Normal saline NEC: necrotising enterocolitis NICU: neonatal intensive care unit PDA: patent ductus arteriosus PDI: Psychomotor Developmental Index (Bayley Scales of Infant Development) PMA: postmenstrual age PO: per os ‐ orally PPHN: persistent pulmonary hypertension of the newborn PVL: periventricular leukomalacia RDS: respiratory distress syndrome ROP: retinopathy of prematurity SD: standard deviation VLBW: very low birth weight (< 1500 g)
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alipour 2016 | The effects of oral ibuprofen on medicinal closure of patent ductus arteriosus in full‐term neonates in the second postnatal week. The study was not conducted in preterm infants |
| Amoozgar 2010 | A randomised controlled study of ibuprofen in term neonates |
| Cherif 2007 | Evaluated the use of oral ibuprofen for closure of PDA, but did not include a control group |
| Desfrere 2005 | A dose‐finding study |
| Kalani 2016 | This study compared early ibuprofen with indomethacin administration to prevent intraventricular haemorrhage among preterm infants |
PDA: patent ductus arteriosus
Characteristics of ongoing studies [ordered by study ID]
ACTRN12616000195459.
| Trial name or title | Early pharmacological treatment with supportive care versus supportive care alone in preterm infants with a patent ductus arteriosus |
| Methods | Randomised controlled trial |
| Participants | Preterm infants less than 29 weeks' PMA, PDA diameter > 1.5 mm, postnatal age 0 to 72 hours |
| Interventions | Both commonly used NSAID preparations will be eligible and can be used according to current local guidelines. The standard recommended dose and interval were Indomethacin IV 0.2‐0.1‐0.1 mg/kg with 24 hour intervals and Ibuprofen IV 10‐5‐5 mg/kg with 24 hour intervals combined with supportive care. Placebo (comparable volume as 0.9% saline in 24 hour intervals) combined with supportive care. Supportive care included optimising airway pressure, careful fluid management with or without the use of diuretics as per current standard practice. No directive guideline was provided with this study, as none of these supportive care measures have been rigorously tested. |
| Outcomes | Primary: composite chronic lung disease or death, or both, at 36 weeks' corrected PMA |
| Starting date | June 2016 |
| Contact information | Dr Koert de Waal, John Hunter Children's Hospital, Department of Newborn Care, Lookout Road, New Lambton NSW 2305, Australia Phone +61 2 49855537. Email: koert.dewaal@hnehealth.nsw.gov.au |
| Notes | Anticipated date of last recruitment March 2018 |
ChiCTR‐TRC‐14004719.
| Trial name or title | Developmental pharmacokinetics and pharmacodynamics of chiral ibuprofen associated with the CYP2C8/9 gene polymorphism in premature infants with PDA |
| Methods | Randomised controlled trial |
| Participants | Preterm newborns, with PDA |
| Interventions | Objectives of the study: study the pharmacodynamic process by oral S‐ibuprofen and ibuprofen to determine the superiority of administration of S‐ibuprofen. "The first day the experimental group given 10 mg/kg, next 5 mg/kg, 5 mg/kg third regimen" "The first day the control group given 10 mg/kg, next 5 mg/kg, 5 mg/kg third regimen" |
| Outcomes | Plasma concentrations of ibuprofen. Clinical outcomes not stated |
| Starting date | Registered May 2014 |
| Contact information | Li Zhiping, 399 Wanyuan Road, Minhang, Shanghai, China. Email: zhipinglifudan@yeah.net |
| Notes | As of November 2016, the study was ongoing. This study is also registered as ChiCTR‐TRC‐14004559 |
EUCTR2016‐002974‐11‐ES.
| Trial name or title | Phase III, randomised, multi centre, double‐blind clinical trial to evaluate two echo‐guided administration regimens of ibuprofen in the treatment of patent ductus arteriosus: impact on intestinal prognosis |
| Methods | Randomised controlled trial |
| Participants | Preterm infants less than 33 weeks' PMA with a PDA ≥ 1.5 mm and medical decision to start drug treatment |
| Interventions | Two ECHO‐guided administration regimens of ibuprofen in the treatment of patent ductus arteriosus |
| Outcomes | Primary outcome: Incidence of NEC or API, defined as the presence of intestinal pneumatosis, pneumoperitoneum, or air in portal vein at time of discharge of Neonatology Department or at completed 40 weeks' PMA (whichever comes first). Secondary outcomes: numerous clinical and laboratory findings |
| Starting date | September 2016 |
| Contact information | Mara Carmen Bravo Laguna, Hospital Universitario La Paz, Paseo de la Castellana 261, Madrid, 28046, Spain. Email: herranz.estelles@gmail.com |
| Notes | As of February 4, 2018, the trial was ongoing |
IRCT201205029611N1.
| Trial name or title | High‐dose oral ibuprofen in PDA closure in premature infants |
| Methods | Randomised controlled trial |
| Participants | Preterm neonates with < 37 weeks' PMA and postnatal age of 3 to 7 days, who will be admitted at the neonatal intensive care unit with diagnosis of PDA |
| Interventions | Intervention is treatment of the case group with high‐dose regimen of oral ibuprofen in 3 doses.The first dose is 20 mg/kg and the 2nd and 3rd doses are 10 mg/kg using a 24‐hour interval. The control group will be given 3 doses of standard regimen of oral ibuprofen with initial dose of 10 mg/kg followed by two doses of 5 mg/kg each, 24 and 48 hours later |
| Outcomes | PDA closure, side effects |
| Starting date | August 2015 |
| Contact information | Dr.Faranak, Hafez Hospital, NICU, Shiraz, Iran |
| Notes | Recruitment was expected to end in Decemeber, 2013 |
IRCT2015111024977N1.
| Trial name or title | Comparison of oral ibuprofen and intravenous indomethacin for the treatment of patent ductus arteriosus |
| Methods | Randomised controlled trial |
| Participants | Preterm infants: PMA between 29 weeks and six days to 35 weeks and six days, between 1000 and 2500 grams birth weight, postnatal age 72 to 120 hours |
| Interventions | In the intervention group: treatment with ibuprofen dose: 10 mg per kg and then to 5 mg per kg at 24 and 48 hours after birth. In the comparison group: treatment with Indomethacin dose: 0.2 milligrams per kilogram at 24 and 48 hours after birth |
| Outcomes | Primary outcomes: thrombocytopenia, pulmonary haemorrhage, increased creatinine, increased bilirubin Secondary outcomes: closure of PDA |
| Starting date | August 2014 |
| Contact information | Seyedeh Masoomeh Mohaqeqi Kamal, Qom University of Medical Sciences, Iran. Phone +98 25 3663 3608. Email address ho.mohaghegh@uswr.ac.ir |
| Notes | Registered while recruiting. Recruitment completed |
ISRCTN13281214.
| Trial name or title | Closing patent ductus arteriosus in preterm babies by using a risk‐based score |
| Methods | Randomised controlled trial |
| Participants | Preterm infants less than 29 weeks' PMA |
| Interventions | Infants in the intervention arm will receive intravenous Ibuprofen (5 mg/1mL) at a dose of 10 mg/kg (2 mL/kg), followed by 2 doses of 5 mg/kg (1 mL/kg) 24 hours apart administered as a short infusion over 15 minutes Infants in the control group will receive an intravenous dose of placebo (normal saline) at a volume equivalent to that in the intervention group (2 mL/kg 1st dose; 1 mL/kg 2nd & 3rd doses) |
| Outcomes | The patency of the ductus will be assessed 24 hours after the last ibuprofen dose using echocardiography. If the PDA remains open (PDA diameter > 1.5 mm), then a second course of ibuprofen will be given. No further doses of ibuprofen will be administered The patency of the ductus will be assessed 24 hours after the last placebo dose using echocardiography. If the PDA remains open (defined as any identifiable flow on colour Doppler), then a second course of placebo will be given Primary outcome: chronic lung disease, defined as the need for oxygen at 36 weeks' corrected age, or death, or both, before discharge. This will be assessed prior to hospital discharge at 36 weeks' corrected age Secondary outcomes: numerous clinical and laboratory outcomes |
| Starting date | September 2016 |
| Contact information | Dr Afif El‐Khuffash, Department of Neonatology, The Rotunda Hospital Dublin 1, Ireland |
| Notes | Overall trial end date: August 2018. Has been entered as EUCTR2015‐004526‐33‐IE too |
NCT01149564.
| Trial name or title | Comparison of oral and intravenous ibuprofen for PDA treatment in premature infants |
| Methods | Double‐blind, randomised controlled trial |
| Participants | 70 extremely preterm infants with ECHO‐confirmed PDA |
| Interventions | Oral or IV ibuprofen 10 mg/kg (1 mL) and then 5 mg/kg at 24‐hour intervals as indicated by ECHO PDA flow pattern |
| Outcomes | Primary outcome: effectiveness and safety Secondary outcome: complications |
| Starting date | December 2009 |
| Contact information | Bai‐Horng Su, M.D., Ph.D., Medical University Hospital, Taichung, Taiwan, 404 Phone: 886‐4‐22052121 ext 2061 bais@ms49.hinet.net |
| Notes | ClinicalTrials.gov identifier: NCT01149564 As of June 23rd, 2010, the recruitment was unknown |
NCT01630278.
| Trial name or title | Early ibuprofen treatment of patent ductus arteriosus (PDA) in premature infants (TRIOCAPI) |
| Methods | Randomised controlled trial |
| Participants | 385 very premature (PMA ≤ 28 weeks) infants with a large ductus, selected by an early ECHO |
| Interventions | Ibuprofen or placebo before 12 hours of life. Follow‐up will include repeated ECHO and cranial ultrasound at 36 hours, 14 days and 36 weeks of postconceptional age |
| Outcomes | Primary outcome: 2‐year survival without cerebral palsy Secondary outcomes: ASQ (Ages and Stages Questionnaire) score at 2 years; incidence of other prematurity‐related morbidities (pulmonary, digestive, neurological, renal) To compare the outcome between the large and the small ductus groups and outcomes according to the McNamara stage at surgical ligation |
| Starting date | March 2012 |
| Contact information | Prof. Véronique Gournay, Nantes University Hospital Phone +33 2 40 08 77 84; veronique.gournay@chu‐nantes.fr |
| Notes | ClinicalTrials.gov identifier: NCT01630278 As of October 13, 2017, the study was active but not recruiting |
NCT01758913.
| Trial name or title | Closure of patent ductus arteriosus with indomethacin or ibuprofen in extreme low‐birth‐weight infants |
| Methods | Randomised controlled trial |
| Participants | Selection criteria: preterm infants with birth weight < 1000 g, radiographic diagnosis of respiratory distress syndrome, requirement of mechanical ventilation, and ECHO and clinical evidence of significant PDA |
| Interventions | Indomethacin: 56 infants, indomethacin 0.2 mg/kg, 0.1 mg/kg and 0.1 mg/kg in 24‐hour interval Ibuprofen: 54 infants, ibuprofen 10 mg/kg, 5 mg/kg and 5 mg/kg in 24‐hour interval |
| Outcomes | Serum electrolytes, creatinine, renal function (urine output, glomerular filtration rate, fractional excretion of sodium and potassium, osmolar clearance and free water clearance, urinary prostaglandin excretion), pulmonary outcome, and mortality |
| Starting date | February 2007 |
| Contact information | Tsu‐Fu Yeh, M.D., Ph.D., Taipei Medical University |
| Notes | ClinicalTrials.gov identifier: NCT01758913 As of January 3, 2013, the recruitment for this study was completed |
NCT02128191.
| Trial name or title | No treatment versus ibuprofen treatment for patent ductus arteriosus in preterm infants |
| Methods | Randomised controlled trial |
| Participants | Infants with a gestational age of ≤ 30 weeks or birth weight of ≤ 1250 grams confirmed to have haemodynamically significant PDA during day of life 7 to 14 |
| Interventions | Ibuprofen: initial dose of oral ibuprofen 10 mg/kg, followed by 2 doses of 5 mg/kg 24 and 48 hours later Saline: normal saline followed by second and third dose 24 and 48 hours later, at equal volume to ibuprofen |
| Outcomes | Incidence of moderate‐to‐severe bronchopulmonary dysplasia or mortality at 36 weeks' PMA (time frame 36 weeks' PMA) |
| Starting date | July 2014 |
| Contact information | Contact: Se In Sung, M.D.; phone: 82‐2‐3410‐1775; sein.sung@samsung.com |
| Notes | ClinicalTrials.gov Identifier: NCT02128191 As of October 14, 2016, this study was still recruiting |
NCT02884219.
| Trial name or title | Multicenter, randomised non‐inferiority trial of early treatment versus expectative management of patent ductus arteriosus in preterm infants (BeNeDuctus Trial ‐ Belgium Netherlands Ductus Trial) |
| Methods | Randomised controlled trial |
| Participants | Preterm infants < 28 weeks' PMA with a PDA (PDA diameter > 1.5 mm) and ductal (predominantly) left‐to‐right shunt |
| Interventions | Active comparator: early treatment with cyclooxygenase inhibitors: treatment of PDA that starts within the first 3 days of life using cyclooxygenase‐inhibitors (Ibuprofen or Indomethacin) Sham comparator: expectative treatment: expectative PDA management is characterised as 'watchful waiting'. No intervention is initiated with the intention to close a PDA |
| Outcomes | Primary outcome: composite of mortality, and/or NEC, and/or BPD Secondary outcomes:
|
| Starting date | December 2016 |
| Contact information | Contact: Willem P de Boode, MD PhD; +31 24 361 44 30; email: willem.deboode@radboudumc.nl |
| Notes | Expected end date: July 2019 |
API: Defined as the presence of intestinal pneumatosis, pneumoperitoneum, or air in the portal vein ASQ: Acoustic structure quantification BSID‐III‐NL: Bayley Scales of Infant Development III (Nl ‐ Netherlands) ECHO: echocardiographically/echocardiography IV: intravenous mm: millimetre NEC: Necrotizing enterocolitis NSAID: non‐steroidal anti‐inflammatory drug PDA: patent ductus arteriosus PMA: postmenstrual age.
Differences between protocol and review
We added additional comparisons and outcomes in previus updates (see Primary outcomes; Secondary outcomes). For the update in 2012, we included studies that compared the effectiveness of oral ibuprofen with placebo, studies that compared oral ibuprofen with IV ibuprofen, studies that compared high‐dose ibuprofen versus standard‐dose ibuprofen and studies that compared 'early' ibuprofen treatment versus expectant management for closure of PDA. For the update in 2015, we included studies that compared ECHO‐guided ibuprofen treatment versus standard ibuprofen treatment and studies that compared continuous infusion of ibuprofen versus standard boluses of ibuprofen. For this update in 2018, we included studies that administered ibuprofen rectally.
Contributions of authors
Arne Ohlsson ‐ developed and wrote the text of the protocol and the review; identified eligible trials for inclusion, performed data abstraction and analyses; and performed the updates of the review in 2005, 2008, 2010, 2013, 2014, and 2017.
Rajneesh Walia ‐ developed and wrote the text of the protocol; and contributed to the updates in 2013, 2014 and 2017.
Sachin Shah ‐ identified eligible trials for inclusion, performed data abstraction and analyses; edited the text of the review; and performed the updates in 2013, 2014 and 2017.
Sources of support
Internal sources
Department of Paediatrics, Mount Sinai Hospital, Toronto, Ontario, Canada.
External sources
No sources of support supplied
Declarations of interest
Arne Ohlsson ‐ none known.
Rajneesh Walia ‐ none known.
Sachin Shah ‐ none known.
Deceased
Edited (no change to conclusions)
References
References to studies included in this review
Adamska 2005 {published data only}
- Adamska E, Helwich E, Rutkowska M, Zacharska E, Piotrowska A. Comparison of the efficacy of ibuprofen and indomethacin in the treatment of patent ductus arteriosus in prematurely born infants [Porownanie ibuprofenu i indometacyny w leczeniu przetrwalego przewodu tetniczego u noworodkow urodzonych przedwczesnie]. Medycyna Wieku Rozwojowego 2005;9(3 Pt 1):335‐54. [PUBMED: 16547381] [PubMed] [Google Scholar]
Akar 2017 {published data only}
- Akar M, Yildirim TG, Sandal G, Bozdag S, Erdeve O, Altug N, et al. Does ibuprofen treatment in patent ductus arteriosus alter oxygen free radicals in premature infants?. Cardiology in the Young 2017;27(3):507–11. [PUBMED: 27319277] [DOI] [PubMed] [Google Scholar]
Akisu 2001 {published data only}
- Akisu M, Ozyurek AR, Dorak C, Parlar A, Kultursay N. Enteral ibuprofen versus indomethacin in the treatment of patent ductus arteriosus in preterm newborn infants [Premature bebeklerde patent duktus arteriozusun tedavisinde enteral ibuprofen ve indometazinin etkinligi ve guvenilirligi]. Cocuk Sagligi ve Hastaliklari Dergisi 2001;44(1):56‐60. [Google Scholar]
Aly 2007 {published data only}
- Aly H, Lotfy W, Badrawi N, Ghawas M, Abdel‐Meguid IE, Hammad TA. Oral ibuprofen and ductus arteriosus in premature infants: a randomized pilot study. American Journal of Perinatology 2007;24(5):267‐70. [PUBMED: 17484080] [DOI] [PubMed] [Google Scholar]
- Lotfy W, Badrawi N, Ghawas M, Ehsan E, Aly H. Oral ibuprofen solution (O) is efficacious for the treatment of patent ductus arteriosus (PDA) in premature infants: a randomized controlled trial. Pediatric Academic Societies Annual Meeting; 2005 May 14‐17; Washington DC, United States. 2005. [PAS2005:1410]
Aranda 2009 {published data only}
- Aranda JV. Multicentre randomized double‐blind placebo controlled trial of ibuprofen L‐Lysine intravenous solution (IV Ibuprofen) in premature infants for the early treatment of patent ductus arteriosus (PDA). Pediatric Academic Societies Annual Meeting; 2005 May 14‐17; Washington DC, United States. 2005.
- Aranda JV, Clyman R, Cox B, Overmeire B, Wozniak P, Sosenko I, et al. A randomized, double‐blind, placebo‐controlled trial of intravenous ibuprofen L‐lysine for the early closure of non‐symptomatic patent ductus arteriosus within 72 hours of birth in extremely low‐birth‐weight infants. American Journal of Perinatology 2009;26(3):235‐45. [DOI: 10.1055/s-0028-1103515; PUBMED: 19067286] [DOI] [PubMed] [Google Scholar]
Bagnoli 2013 {published data only}
- Bagnoli F, Rossetti A, Messina G, Mori A, Casucci M, Tomasini B. Treatment of patent ductus arteriosus (PDA) using ibuprofen: renal side‐effects in VLBW and ELBW newborns. Journal of Maternal‐Fetal & Neonatal Medicine 2013;26(4):423‐9. [DOI: 10.3109/14767058.2012.733775; PUBMED: 23057804] [DOI] [PubMed] [Google Scholar]
Bravo 2014 {published data only}
- Bravo MC, Cabaňas F, Riera J, Pérez‐Fernández E, Quero J, Pérez‐Rodriguez J, et al. Randomized controlled clinical trial of standard versus echocardiographically guided ibuprofen treatment for patent ductus arteriosus in preterm infants: a pilot study. Journal of Maternal‐Fetal & Neonatal Medicine 2014;27(9):904‐9. [DOI: 10.3109/14767058.2013.846312; PUBMED: 24047189] [DOI] [PubMed] [Google Scholar]
Cherif 2008 {published data only}
- Cherif A, Khrouf N, Jabnoun S, Mokrani C, Amara MB, Guellouze N, et al. Randomized pilot study comparing oral ibuprofen with intravenous ibuprofen in very low birth weight infants with patent ductus arteriosus. Pediatrics 2008;122(6):e1256‐61. [PUBMED: 19047225] [DOI] [PubMed] [Google Scholar]
Chotigeat 2003 {published data only}
- Chotigeat U, Jirapapa K, Layangkool T. A comparison of oral ibuprofen and intravenous indomethacin for closure of patent ductus arteriosus in preterm infants. Chotmaihet Thangphaet [Journal of the Medical Association of Thailand] 2003;86(Suppl 3):S563‐9. [PUBMED: 14700149] [PubMed] [Google Scholar]
Dani 2012 {published data only}
- Dani C, Vangi V, Bertini G, Pratesi S, Lori I, Favelli F, et al. High‐dose ibuprofen for patent ductus arteriosus in extremely preterm infants: a randomized controlled study. Clinical Pharmacology and Therapeutics 2012;91(4):590‐6. [PUBMED: 22089267] [DOI] [PubMed] [Google Scholar]
Demir 2017 {published data only}
- Demir N, Peker E, Ece I, Balahoroglu R, Tuncer O. Efficacy and safety of rectal ibuprofen for ductus arteriosus closure in very low birth weight infants. European Journal of Pediatrics 2016;175(11):1705‐6. [DOI] [PubMed] [Google Scholar]
- Demir N, Peker E, Ece I, Balahoroglu R, Tuncer O. Efficacy and safety of rectal ibuprofen for patent ductus arteriosus closure in very low birth weight preterm infants. Journal of Maternal‐Fetal & Neonatal Medicine 2017;30(17):2119–25. [DOI: 10.1080/14767058.2016.1238897; PUBMED: 28052714] [DOI] [PubMed] [Google Scholar]
- Peker E, Demir N, Ece I, Balahorotlu R, Tuncer O. Efficacy and safety of rectal ibuprofen for ductus arteriosus closure in very low birth weight infants. Journal of Maternal‐Fetal & Neonatal Medicine 2016;29(Suppl 1):114. [DOI] [PubMed] [Google Scholar]
Ding 2014 {published data only}
- Ding YJ, Han B, Yang B, Zhu M. NT‐proBNP plays an important role in the effect of ibuprofen on preterm infants with patent ductus arteriosus. European Review for Medical and Pharmacological Sciences 2014;18(18):2596‐8. [PUBMED: 25317790] [PubMed] [Google Scholar]
El‐Mashad 2017 {published data only}
- El‐Mashad AE, El‐Mahdy H, Amrousy DE, Elgendy M. Comparative study of the efficacy and safety of paracetamol, ibuprofen, and indomethacin in closure of patent ductus arteriosus in preterm neonates. European Journal of Pediatrics 2017;176(2):233‐40. [DOI: 10.1007/s00431-016-2830-7; PUBMED: 28004188] [DOI] [PubMed] [Google Scholar]
Erdeve 2012 {published data only}
- Erdeve O, Yurttutan S, Altug N, Ozdemir R, Gokmen T, Dilmen U, et al. Oral versus intravenous ibuprofen for patent ductus arteriosus closure: a randomised controlled trial in extremely low birthweight infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2012;97(4):F279‐83. [DOI: 10.1136/archdischild-2011-300532; PUBMED: 22147286] [DOI] [PubMed] [Google Scholar]
Fakhraee 2007 {published data only}
- Fakhraee SH, Badiee Z, Mojtahedzadeh S, Kazemian M, Kelishadi R. Comparison of oral ibuprofen and indomethacin therapy for patent ductus arteriosus in preterm infants. Zhongguo Dang Dai Er Ke za Zhi [Chinese Journal of Contemporary Pediatrics] 2007;9(5):399‐403. [PUBMED: 17937843] [PubMed] [Google Scholar]
Fesharaki 2012 {published data only}
- Fesharaki HJ, Nayeri FS, Asbaq PA, Amini E, Sedagat M. Different doses of ibuprofen in the treatment of patent ductus arteriosus: a randomized controlled trial. Tehran University Medical Journal 2012;70(8):488‐93. [Google Scholar]
Gimeno Navarro 2005 {published data only}
- Gimeno Navarro A, Cano Sanchez A, Fernandez Gilino C, Carrasco Moreno JI, Izquierdo Macian I, Gutierrez Laso A, et al. Ibuprofen versus indomethacin in the treatment of patent ductus arteriosus in preterm infants [Ibuprofeno frente a indometacina en el tratamiento del conducto arterioso persistente del prematuro]. Anales de Pediatria 2005;63(3):212‐8. [PUBMED: 16219273] [DOI] [PubMed] [Google Scholar]
Gokmen 2011 {published data only}
- Eras Z, Gokmen T, Erdeve O, Ozyurt BM, Saridas B, Dilmen U. Impact of oral versus intravenous ibuprofen on neurodevelopmental outcome: a randomized controlled parallel study. American Journal of Perinatology 2013;30(10):857‐62. [DOI: 10.1055/s-0033-1333667; PUBMED: 23359230] [DOI] [PubMed] [Google Scholar]
- Gokmen T, Erdeve O, Altug N, Oguz SS, Uras N, Dilmen U. Efficacy and safety of oral verus intravenous ibuprofen in very low birth weight infants with patent ductus arteriosus. Journal of Pediatrics 2011;158(4):549‐54; Erratum in: Journal of Pediatrics; 2012;160(1):181. [10.1016/j.jpeds.2010.10.008; PUBMED: 21094951] [DOI] [PubMed] [Google Scholar]
Hammerman 2008 {published data only}
- Hammerman C, Shchors I, Jacobson S, Schimmel MS, Bromiker R, Kaplan M, et al. Ibuprofen versus continuous indomethacin in premature neonates with patent ductus arteriosus: is the difference in the mode of administration?. Pediatric Research 2008;64(3):291‐7. [DOI: 10.1203/PDR.0b013e31817d9bb0; PUBMED: 18458658] [DOI] [PubMed] [Google Scholar]
Lago 2002 {published data only}
- Lago P, Bettiol T, Salvadori S, Pitassi I, Vianello A, Chiandetti L, et al. Safety and efficacy of ibuprofen versus indomethacin in preterm infants treated for patent ductus arteriosus: a randomised controlled trial. European Journal of Pediatrics 2002;161(4):202‐7. [PUBMED: 12014386] [DOI] [PubMed] [Google Scholar]
- Lago P, Salvadori S, Bettiol T, Pitassi I, Chiandetti L, Saia OS. Effects of indomethacin and ibuprofen on renal function in preterm infants treated for patent ductus arteriosus: a randomized controlled trial. Pediatric Research 2001;49:375A. [DOI] [PubMed] [Google Scholar]
- Zanardo V, Vedovato S, Lago P, Piva D, Faggian D, Chiozza L. Effects of ibuprofen and indomethacin on urinary antidiuretic hormone excretion in preterm infants treated for patent ductus arteriosus. Fetal Diagnosis and Therapy 2005;20(6):534‐9. [DOI: 10.1159/000088046; PUBMED: 16260891] [DOI] [PubMed] [Google Scholar]
Lago 2014 {published data only}
- Lago P, Salvadori S, Opocher F, Ricato S, Chiandetti L, Frigo AC. Continuous infusion of ibuprofen for treatment of patent ductus arteriosus in very low birth weight infants. Neonatology 2014;105(1):46‐54. [DOI: 10.1159/000355679; PUBMED: 24281435] [DOI] [PubMed] [Google Scholar]
Lin 2012 {published data only}
- Lin XZ, Chen HQ, Zheng Z, Li YD, Lai JD, Huang LH. Therapeutic effect of early administration of oral ibuprofen in very low birth weight infants with patent ductus arteriosus. Zhongguo Dang Dai Er Ke za Zhi [Chinese Journal of Contemporary Pediatrics] 2012;14(7):502‐5. [PUBMED: 22809601] [PubMed] [Google Scholar]
Lin 2017 {published data only}
- Lin YJ, Chen CM, Rehan VK, Florens A, Wu SY, Tsai ML, et al. Randomized trial to compare renal function and ductal response between Indomethacin and ibuprofen treatment in extremely low birth weight infants. Neonatology 2017;111(3):195–202. [DOI: 10.1159/000450822; PUBMED: 27842315] [DOI] [PubMed] [Google Scholar]
Mosca 1997 {published data only}
- Mosca F, Bray M, Lattanzio M, Fumagalli M, Colnaghi M, Castoldi F, et al. Comparison of the effects of ibuprofen and indomethacin on PDA closure and cerebral perfusion and oxygenation. Pediatric Research 1997;41:165A. [Google Scholar]
- Mosca F, Bray M, Lattanzio M, Fumagalli M, Colnaghi MR, Compagnoni G. Comparison of the effects of indomethacin (INDO) and ibuprofen (IBU) on cerebral perfusion and oxygenation in preterm infants. Pediatric Research 1996;39:231A. [Google Scholar]
- Mosca F, Bray M, Lattanzio M, Fumagalli M, Tosetto C. Comparative evaluation of the effects of indomethacin and ibuprofen on cerebral perfusion and oxygenation in preterm infants with patent ductus arteriosus. Journal of Pediatrics 1997;131(4):549‐54. [PUBMED: 9386657] [DOI] [PubMed] [Google Scholar]
Patel 1995 {published data only}
- Patel J, Marks KA, Roberts I, Azzopardi D, Edwards AD. Ibuprofen treatment of patent ductus arteriosus. Lancet 1995;346(8969):255. [PUBMED: 7616831] [DOI] [PubMed] [Google Scholar]
Patel 2000 {published data only}
- Patel J, Roberts I, Azzopardi D, Hamilton P, Edwards AD. Randomized double‐blind controlled trial comparing the effects of ibuprofen with indomethacin on cerebral hemodynamics in preterm infants with patent ductus arteriosus. Pediatric Research 2000;47(1):36‐42. [PUBMED: 10625080] [DOI] [PubMed] [Google Scholar]
Pezzati 1999 {published data only}
- Pezzati M, Bertini G, Vangi V, Biagiotti R, Cianciulli D, Rubaltelli FF. Mesenteric and renal perfusion in preterm infants with PDA: indomethacin vs ibuprofen. Pediatric Research 1999;45:218A. [DOI] [PubMed] [Google Scholar]
- Pezzati M, Vangi V, Biagiotti R, Bertini G, Cianciulli D, Rubatelli FF. Effects of indomethacin and ibuprofen on mesenteric and renal blood flow in preterm infants with patent ductus arteriosus. Journal of Pediatrics 1999;135(6):733‐8. [PUBMED: 10586177] [DOI] [PubMed] [Google Scholar]
Pistulli 2014 {published data only}
- Hoxha A, Gjyzari A, Tushe E. Renal effects of ibuprofen during the treatment of patent ductus arteriosus in low birth weight premature infants. Nephrology Dialysis Transplantation Conference. 50th ERA‐EDTA Congress; 2013 May 18‐21; Istanbul, Turkey. 2013.
- Pistulli E, Hamiti A, Buba S, Hoxha A, Kelmendi N, Vyshka G. The association between patent ductus arteriosus and perinatal infection in a group of low birth weight preterm infants. Iranian Journal of Pediatrics 2014;24(1):42‐8. [PUBMED: 25793044] [PMC free article] [PubMed] [Google Scholar]
- Prifti E, Enkeleda P, Rubena M, Alketa H. The impact of antenatal corticosteroids on PDA in low birth weight infants. Journal of Perinatal Medicine 2013;41:s1. [Google Scholar]
- Qosja A, Kuneshka N, Tushe E, Teneqexhi L. Oral versus intravenous ibuprofen for patent ductus arteriosus closure. Giornale Italiano di Cardiologia 2012;13(Suppl 1):15S‐6S. [Google Scholar]
Plavka 2001 {published data only}
- Plavka R, Svihovec P, Borek I, Biolek J, Kostirova M, Liska K, et al. Ibuprofen vs. indomethacin in the treatment of patent ductus arteriosus (PDA) in very premature neonates. Pediatric Research 2001;49:375A. [Google Scholar]
Pourarian 2008 {published data only}
- Pourarian SH, Pishva N, Madani A, Rastegari M. Comparison of oral ibuprofen and indomethacin on closure of patent ductus arteriosus in preterm infants. Eastern Mediterranean Health Journal 2008;14(2):360‐5. [PUBMED: 18561728] [PubMed] [Google Scholar]
Pourarian 2015 {published data only}
- Pourarian S, Takmil F, Cheriki S, Amoozgar H. The effect of oral high‐dose ibuprofen on patent ductus arteriosus closure in preterm infants. American Journal of Perinatology 2015;32(12):1158‐63. [DOI: 10.1055/s-0035-1551671; PUBMED: 26007314] [DOI] [PubMed] [Google Scholar]
Salama 2008 {published data only}
- Salama H, Alsisi A, Al‐Rifai H, Shaddad A, Samawal L, Habboub L, et al. A randomized controlled trial on the use of oral ibuprofen to close patent ductus arteriosus in premature infants. Journal of Neonatal‐Perinatal Medicine 2008;1(3):153‐8. [Google Scholar]
Sosenko 2012 {published data only}
- Sosenko IR, Fajardo MF, Claure N, Bancalari E. Timing of patent ductus arteriosus treatment and respiratory outcome in premature infants: a double‐blind randomized controlled trial. Journal of Pediatrics 2012;160(6):929‐35. [PUBMED: 22284563] [DOI] [PubMed] [Google Scholar]
Su 2003 {published data only}
- Su PH, Chen JY, Su CM, Huang TC, Lee HS. Comparison of ibuprofen and indomethacin therapy for patent ductus arteriosus in preterm infants. Pediatrics International 2003;45(6):665‐70. [PUBMED: 14651538] [DOI] [PubMed] [Google Scholar]
Su 2008 {published data only}
- Su BH, Lin HC, Chiu HY, Hsieh HY, Chen HH, Tsai YC. Comparison of ibuprofen and indomethacin for early‐targeted treatment of patent ductus arteriosus in extremely premature infants: a randomised controlled trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2008;93(2):F94‐9. [DOI: 10.1136/adc.2007.120584; PUBMED: 17768157] [DOI] [PubMed] [Google Scholar]
Supapannachart 2002 {published data only}
- Supapannachart S, Limrungsikul A, Khowsathit P. Oral ibuprofen and indomethacin for treatment of patent ductus arteriosus in premature infants: a randomized trial at Ramathibodi Hospital. Chotmaihet Thangphaet [Journal of the Medical Association of Thailand] 2002;85(Suppl 4):S1252‐8. [PUBMED: 12549803] [PubMed] [Google Scholar]
Van Overmeire 1997 {published data only}
- Overmeire B, Follens I, Hartmann S, Creten WL, Acker KJ. Treatment of patent ductus arteriosus with ibuprofen. Archives of Disease in Childhood. Fetal and Neonatal Edition 1997;76(3):F179‐84. [PUBMED: 9175948] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overmeire B, Follens I, Hartmann S, Mahieu L, Reempts PJ. Intravenous ibuprofen (IBU) for the treatment of patent ductus arteriosus (PDA) in preterm infants with respiratory distress syndrome (RDS). Pediatric Research 1996;39:250A. [Google Scholar]
Van Overmeire 2000 {published data only}
- Pezzati M, Vangi V, Biagiotti R, Bertini G, Cianciulli D, Rubaltelli FF. Effects of indomethacin and ibuprofen on mesenteric and renal blood flow in preterm infants with patent ductus arteriosus. Journal of Pediatrics 1999;135(6):733‐8. [PUBMED: 10586177] [DOI] [PubMed] [Google Scholar]
- Overmeire B, Langhendries JP, Vanhaesebrouck P, Lecoutere D, Broek H. Ibuprofen for early treatment of patent ductus arteriosus, a randomized multicentre trial. Pediatric Research 1998;43:200A. [Google Scholar]
- Overmeire B, Smets K, Lecoutere D, Broek H, Weyler J, Groote K, et al. A comparison of ibuprofen and indomethacin for closure of patent ductus arteriosus. New England Journal of Medicine 2000;343(10):674‐81. [DOI: 10.1056/NEJM200009073431001; PUBMED: 10974130] [DOI] [PubMed] [Google Scholar]
Yadav 2014 {published data only}
- Yadav S, Agarwal S, Maria A, Dudeja A, Dubey NK, Anand P, et al. Comparison of oral ibuprofen with oral indomethacin for PDA closure in Indian preterm neonates: a randomized controlled trial. Pediatric Cardiology 2014;35(5):824‐30. [DOI: 10.1007/s00246-014-0861-2; PUBMED: 24435507] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alipour 2016 {published data only}
- Alipour MR, Shamsi MM, Namayandeh SM, Pezeshkpour Z, Rezaeipour F, Sarebanhassanabadi M. The effects of oral ibuprofen on medicinal closure of patent ductus arteriosus in full‐term neonates in the second postnatal week. Iranian Journal of Pediatrics 2016;26(4):e5807. [DOI: 10.5812/ijp.5807; PUBMED: 27729962] [DOI] [PMC free article] [PubMed] [Google Scholar]
Amoozgar 2010 {published data only}
- Amoozgar H, Ghodstehrani M, Pishva N. Oral ibuprofen and ductus arteriosus closure in full‐term neonates: a prospective case‐control study. Pediatric Cardiology 2010;31(1):40‐3. [DOI: 10.1007/s00246-009-9542-y; PUBMED: 19841966] [DOI] [PubMed] [Google Scholar]
Cherif 2007 {published data only}
- Cherif A, Jabnoun S, Khrouf N. Oral ibuprofen in early curative closure of patent ductus arteriosus in very premature infants. American Journal of Perinatology 2007;24(6):339‐45. [DOI: 10.1055/s-2007-981853; PUBMED: 17564958] [DOI] [PubMed] [Google Scholar]
Desfrere 2005 {published data only}
- Desfrere L, Zohar S, Morville P, Brunhes A, Chevret S, Pons G, et al. Dose‐finding study of ibuprofen in patent ductus arteriosus using the continual reassessment method. Journal of Clinical Pharmacy and Therapeutics 2005;30(2):121‐32. [DOI: 10.1111/j.1365-2710.2005.00630.x; PUBMED: 15811164] [DOI] [PubMed] [Google Scholar]
Kalani 2016 {published data only}
- Kalani M, Shariat M, Khalesi N, Farahani Z, Ahmadi S. A comparison of early ibuprofen and indomethacin administration to prevent intraventricular hemorrhage among preterm infants. Acta Medica Iranica 2016;54(12):788‐92. [PUBMED: 28120591] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12616000195459 {published data only}
- ACTRN12616000195459. Early pharmacological treatment with supportive care versus supportive care alone in preterm infants with a patent ductus arteriosus. anzctr.org.au/ACTRN12616000195459.aspx (first received 08 February 2016).
ChiCTR‐TRC‐14004719 {published data only}
- ChiCTR‐TRC‐14004559. Comparison of the dose effect of oral ibuprofen suspension for PDA treatment in premature infants [Developmental pharmacokinetics and pharmacodynamics of chiral ibuprofen associated with the CYP2C8/9 gene polymorphism]. chictr.org.cn/showproj.aspx?proj=5014 (first received 15 April 2014).
EUCTR2016‐002974‐11‐ES {published data only}
- EUCTR2016‐002974‐11‐ES. Clinical trial to evaluate the impact on the intestinal prognosis of 2 ibuprofen administration regimens for the treatment of patent ductus arteriosus, guided by echocardiography [Phase III, randomized, multicenter, double‐blind clinical trial to evaluate two echo‐guided administration regimens of ibuprofen in the treatment of patent ductus arteriosus: impact on intestinal prognosis]. clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2016‐002974‐11 (first received 26 September 2016).
IRCT201205029611N1 {published data only}
- IRCT201205029611N1. High dose oral ibuprofen in PDA closure in premature infants. en.irct.ir/trial/10178 (first received 25 August 2014).
IRCT2015111024977N1 {published data only}
- IRCT2015111024977N1. Comparison of oral Ibuprofen and intravenous indomethacin for the treatment of patent ductus arteriosus [Comparison of oral Ibuprofen and intravenous indomethacin for the treatment of patent ductus arteriosus in preterm infants]. en.irct.ir/trial/20973 (first received 16 December 2015).
ISRCTN13281214 {published data only}
- ISRCTN13281214. Closing patent ductus arteriosus in preterm babies by using a risk‐based score. isrctn.com/ISRCTN13281214?q=ISRCTN13281214&filters=&sort=&offset=1&totalResults=1&page=1&pageSize=10&searchType=basic‐search (first received 1 July 2016).
NCT01149564 {published data only}
- NCT01149564. Comparison of oral and intravenous ibuprofen for PDA treatment in premature infants. clinicaltrials.gov/show/NCT01149564 (first received 23 June 2010).
NCT01630278 {published data only}
- NCT01630278. Early ibuprofen treatment of patent ductus arteriosus (PDA) in premature infants (TRIOCAPI) [Impact of early targeted ibuprofene treatment of patent ductus arteriosus (PDA) on long term neurodevelopmental outcome in very premature infants (TRIOCAPI)]. clinicaltrials.gov/show/NCT01630278 (first received 28 June 2012).
NCT01758913 {published data only}
- NCT01758913. Closure of patent ductus arteriosus with indomethacin or ibuprofen in extreme low birth weight infants [Pharmacological closure of patent ductus arteriosus in extreme low birth weight infants. A comparison of efficacy, side effects and outcomes between indomethacin and ibuprofen]. clinicaltrials.gov/show/NCT01758913 (first received 1 January 2013).
NCT02128191 {published data only}
- NCT02128191. No treatment versus ibuprofen treatment for patent ductus arteriosus in preterm infants [Efficacy and safety of no treatment compared with oral ibuprofen treatment for patent ductus arteriosus in preterm infants: a randomized, double‐blind, placebo‐controlled, non‐inferiority clinical trial]. clinicaltrials.gov/show/NCT02128191 (first received 1 May 2014).
NCT02884219 {published data only}
- NCT02884219. Early treatment versus expectative management of PDA in preterm infants [Multi‐center, randomized non‐inferiority trial of early treatment versus expectative management of patent ductus arteriosus in preterm infants (BeNeDuctus Trial ‐ Belgium Netherlands Ductus Trial)]. clinicaltrials.gov/ct2/show/record/NCT02884219 (first received 30 August 2016).
Additional references
Amendolia 2012
- Amendolia B, Lynn M, Bhat V, Ritz SB, Aghai ZH. Severe pulmonary hypertension with therapeutic L‐lysine ibuprofen in 2 preterm infants. Pediatrics 2012;129(5):e1360‐3. [DOI: 10.1542/peds.2011-0117; PUBMED: 22492771] [DOI] [PubMed] [Google Scholar]
Bellini 2006
- Bellini C, Campone F, Serra G. Pulmonary hypertension following L‐lysine ibuprofen therapy in a preterm infant with patent ductus arteriosus. Canadian Medical Association Journal 2006;174(13):1843‐4. [DOI: 10.1503/cmaj.051446; PUBMED: 16785458] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bravo 2014a
- Bravo MC, Cordeiro M, Deiros L, Pérez‐Rodríguez J. Lethal pulmonary hypertension associated with ibuprofen treatment in a very low birth weight infant. Journal of Paediatrics and Child Health 2014;50(1):85‐6. [DOI: 10.1111/jpc.12445; PUBMED: 24397458] [DOI] [PubMed] [Google Scholar]
Chemtob 1990
- Chemtob S, Behary K, Rex J, Varma DR, Aranda JV. Prostanoids determine the range of cerebral blood flow autoregulation of newborn piglets. Stroke 1990;21(5):777‐84. [PUBMED: 2339458] [DOI] [PubMed] [Google Scholar]
Chemtob 1991
- Chemtob S, Beharry K, Barna T, Varma DR, Aranda JV. Differences in the effects in the newborn piglet of various nonsteroidal antiinflammatory drugs on cerebral blood flow but not on cerebrovascular prostaglandins. Pediatric Research 1991;30(1):106‐11. [DOI: 10.1203/00006450-199107000-00021; PUBMED: 1891274] [DOI] [PubMed] [Google Scholar]
Coceani 1979
- Coceani F, White E, Bodach E, Olley PM. Age‐dependent changes in the responses of the lamb ductus arteriosus to oxygen and ibuprofen. Canadian Journal of Physiology and Pharmacology 1979;57(8):825‐31. [PUBMED: 497895] [DOI] [PubMed] [Google Scholar]
Coceani 2005
- Coceani F, Barogi S, Brizza F, Ackerley C, Seidlitz E, Kelsey L, et al. Cyclooxygenase isoenzymes and patency of ductus arteriosus. Prostaglandins, Leukotrienes, and Essential Fatty Acids 2005;72(2):71‐7. [DOI: 10.1016/j.plefa.2004.10.004; PUBMED: 15626588] [DOI] [PubMed] [Google Scholar]
Cotton 1978a
- Cotton RB, Stahlman MT, Kovar I, Catterton WZ. Medical management of small preterm infants with symptomatic patent ductus arteriosus. Journal of Pediatrics 1978;92(3):467‐73. [PUBMED: 632994] [DOI] [PubMed] [Google Scholar]
Cotton 1978b
- Cotton RB, Stahlman MT, Bender HW, Graham TP, Catterton WZ, Kovar I. Randomized trial of early closure of symptomatic patent ductus arteriosus in small preterm infants. Journal of Pediatrics 1978;93(4):647‐51. [PUBMED: 702245] [DOI] [PubMed] [Google Scholar]
Edwards 1990
- Edwards AD, Wyatt JS, Richardson C, Potter A, Cope M, Delpy DT, et al. Effects of indomethacin on cerebral haemodynamics in very preterm infants. Lancet 1990;335(8704):1491‐5. [PUBMED: 1972434] [DOI] [PubMed] [Google Scholar]
ElHassan 2014
- ElHassan NO, Bird TM, King AJ, Ambadwar PB, Jaquiss RD, Kaiser JR, et al. Variation and comparative effectiveness of patent ductus arteriosus pharmacotherapy in extremely low birth weight infants. Journal of Neonatal‐Perinatal Medicine 2014;7(3):229‐35. [DOI: 10.3233/NPM-14814015; PUBMED: 25322995] [DOI] [PubMed] [Google Scholar]
Ellison 1983
- Ellison RC, Pecham GJ, Lang P, Talner NS, Lerer TJ, Lin L, et al. Evaluation of the preterm infant for patent ductus arteriosus. Pediatrics 1983;71(3):364‐72. [PUBMED: 6338474] [PubMed] [Google Scholar]
Fowlie 2010
- Fowlie PW, Davis PG, McGuire W. Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD000174.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gournay 2002
- Gournay V, Savagner C, Thirez G, Kuster A, Roze JC. Pulmonary hypertension after ibuprofen prophylaxis in very preterm infants. Lancet 2002;359(9316):1486‐8. [PUBMED: 11988250] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 30 November 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Grosfeld 1983
- Grosfeld JL, Kamman K, Gross K, Cikrit D, Ross D, Wolfe M, et al. Comparative effects of indomethacin, prostaglandin E1, and ibuprofen on bowel ischaemia. Journal of Pediatric Surgery 1983;18(6):738‐42. [PUBMED: 6686609] [DOI] [PubMed] [Google Scholar]
Hardy 1996
- Hardy P, Peri KG, Lahaie I, Varma DR, Chemtob S. Increased nitric oxide synthesis and action preclude choroidal vasoconstriction to hyperoxia in newborn pigs. Circulation Research 1996;79(3):504‐11. [PUBMED: 8781483] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI: 10.1136/bmj.327.7414.557; PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from training.cochrane.org/handbook.
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;5:13. [DOI: 10.1186/1471-2288-5-13; PUBMED: 15840177] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ito 1994
- Ito K, Niida Y, Sato J, Owada E, Ito K, Umetsu M. Pharmacokinetics of mefenamic acid in preterm infants with patent ductus arteriosus. Acta Paediatrica Japonica; Overseas Edition 1994;36(4):387‐91. [PUBMED: 7942001] [DOI] [PubMed] [Google Scholar]
Kaplan 1994
- Kaplan BS, Restaino I, Raval DS, Gottlieb RP, Bernstein J. Renal failure in the neonate associated with in utero exposure to non‐steroidal anti‐inflammatory agents. Pediatric Nephrology (Berlin, Germany) 1994;8(6):700‐4. [PUBMED: 7696108] [DOI] [PubMed] [Google Scholar]
Kim 2016
- Kim SY, Shin SH, Kim HS, Jung YH, Kim EK, Choi JH. Pulmonary arterial hypertension after ibuprofen treatment for patent ductus arteriosus in very low birth weight infants. Journal of Pediatrics 2016;179:49‐53.e1. [DOI: 10.1016/j.jpeds.2016.08.103; PUBMED: 27692860] [DOI] [PubMed] [Google Scholar]
Lee 2000
- Lee SK, McMillan DD, Ohlsson A, Pendray M, Synnes A, Whyte R, et al. Variations in practice and outcomes in the Canadian NICU Network: 1996‐1997. Pediatrics 2000;106(5):1070‐9. [PUBMED: 11061777] [DOI] [PubMed] [Google Scholar]
Mahony 1982
- Mahony L, Carnero V, Brett C, Heymann MA, Clyman RI. Prophylactic indomethacin therapy for patent ductus arteriosus in very‐low‐birth‐weight infants. New England Journal of Medicine 1982;306(9):506‐10. [DOI: 10.1056/NEJM198203043060903; PUBMED: 7035955] [DOI] [PubMed] [Google Scholar]
Malikiwi 2015
- Malikiwi A, Roufaeil C, Tan K, Sehgal A. Indomethacin vs ibuprofen: comparison of efficacy in the setting of conservative therapeutic approach. European Journal of Pediatrics 2015;174(5):615‐20. [DOI: 10.1007/s00431-014-2441-0; PUBMED: 25344763] [DOI] [PubMed] [Google Scholar]
Mathew 1998
- Mathew R. Development of the pulmonary circulation: metabolic aspects. In: Polin RA, Fox WW editor(s). Fetal and Neonatal Physiology. Vol. 1, Philadelphia: W.B. Saunders Company, 1998:924‐9. [Google Scholar]
Mitra 2016
- Mitra S, Florez ID, Tamayo ME, Aune D, Mbuagbaw L, Veroniki AA, et al. Effectiveness and safety of treatments used for the management of patent ductus arteriosus (PDA) in preterm infants: a protocol for a systematic review and network meta‐analysis. BMJ Open 2016;6(7):e011271. [DOI: 10.1136/bmjopen-2016-011271; PUBMED: 27456327] [DOI] [PMC free article] [PubMed] [Google Scholar]
Naulty 1978
- Naulty CM, Horn S, Conry J, Avery GB. Improved lung compliance after ligation of patent ductus arteriosus in hyaline membrane disease. Journal of Pediatrics 1978;93(4):682‐4. [PUBMED: 702251] [DOI] [PubMed] [Google Scholar]
Neumann 2012
- Neumann R, Schulzke SM, Bührer C. Oral ibuprofen versus intravenous ibuprofen or intravenous indomethacin for the treatment of patent ductus arteriosus in preterm infants: a systematic review and meta‐analysis. Neonatology 2012;102(1):9‐15. [DOI: 10.1159/000335332; PUBMED: 22414850] [DOI] [PubMed] [Google Scholar]
Niopas 1994
- Niopas I, Mamzoridi K. Determination of indomethacin and mefenamic acid in plasma performance liquid chromatography. Journal of Chromatography. B, Biomedical Applications 1994;656(2):447‐50. [PUBMED: 7987501] [DOI] [PubMed] [Google Scholar]
Ohlsson 1993
- Ohlsson A, Bottu J, Govan J, Ryan ML, Fong K, Myhr T. The effect of indomethacin on cerebral blood flow velocities in very low birth weight neonates with patent ductus arteriosus. Developmental Pharmacology and Therapeutics 1993;20(1‐2):100‐6. [PUBMED: 7924757] [DOI] [PubMed] [Google Scholar]
Ohlsson 2000
- Ohlsson A. Back to the drawing board. Pediatric Research 2000;47(1):4‐5. [PUBMED: 10625075] [DOI] [PubMed] [Google Scholar]
Ohlsson 2011
- Ohlsson A, Shah SS. Ibuprofen for the prevention of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD004213.pub3] [DOI] [PubMed] [Google Scholar]
Ohlsson 2018
- Ohlsson A, Shah PS. Paracetamol (acetaminophen) for patent ductus arteriosus in preterm or low birth weight infants.. Cochrane Database Systematic Reviews 2018, (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
Oncel 2016
- Oncel MY, Erdeve O. Oral medications regarding their safety and efficacy in the management of patent ductus arteriosus. World Journal of Clinical Pediatrics 2016;5(1):75‐81. [DOI: 10.5409/wjcp.v5.i1.75; PUBMED: 26862505] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pellicer 1999
- Pellicer A, Aparicio M, Cabanas F, Valverde E, Quero J, Stiris TA. Effect of the cyclo‐oxygenase blocker ibuprofen on cerebral blood volume and cerebral blood flow during normocarbia and hypercarbia in newborn piglets. Acta Pediatrica 1999;88(1):82‐8. [PUBMED: 10090554] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rodriguez‐Castano 2016
- Rodriguez‐Castano MJ, Aleo E, Arruza L. Oral sildenafil for severe pulmonary hypertension developing after ibuprofen use in a neonate. Indian Pediatrics 2016;53(4):349‐50. [PUBMED: 27156554] [DOI] [PubMed] [Google Scholar]
Sakhalkar 1992
- Sakhalkar VS, Merchant RH. Therapy of symptomatic patent ductus arteriosus in preterms with mefenemic acid and indomethacin. Indian Pediatrics 1992;29(3):313‐8. [PUBMED: 1612672] [PubMed] [Google Scholar]
Schmidt 2001
- Schmidt B, Davis P, Moddeman D, Ohlsson A, Roberts RS, Saigal S, et al. Trial of Indomethacin Prophylaxis in Preterms Investigators. Long‐term effect of indomethacin prophylaxis in extremely‐low‐birth‐weight infants. New England Journal of Medicine 2001;344(26):1966‐72. [DOI: 10.1056/NEJM200106283442602; PUBMED: 11430325] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. gdt.guidelinedevelopment.org/app/handbook/handbook.html (accessed prior to 31 August 2018).
Sehgal 2013
- Sehgal A, Kumarshingri PS. Pulmonary hypertension in an infant treated with ibuprofen. Indian Journal of Pediatrics 2013;80(8):697‐9 (Epub 2012 Jul 29). [DOI: 10.1007/s12098-012-0829-2; PUBMED: 22843343] [DOI] [PubMed] [Google Scholar]
Seyberth 1983
- Seyberth HW, Rascher W, Hackenthal R, Wille L. Effect of prolonged indomethacin therapy on renal function and selected vasoactive hormones in very low birth weight infants with symptomatic ductus arteriosus. Journal of Pediatrics 1983;103(6):979‐84. [PUBMED: 6358443] [DOI] [PubMed] [Google Scholar]
Stefano 1991
- Stefano JL, Abbasi S, Pearlman SA, Spear ML, Esterly KL, Bhutani VK. Closure of the ductus arteriosus with indomethacin in ventilated neonates with respiratory distress syndrome: effects on pulmonary compliance and ventilation. American Review of Respiratory Disease 1991;143(2):236‐9. [DOI: 10.1164/ajrccm/143.2.236; PUBMED: 1990934] [DOI] [PubMed] [Google Scholar]
Uchiyama 2011
- Uchiyama A, Nagasawa H, Yamamoto Y, Tatebayashi K, Suzuki H, Yamada K, et al. Clinical aspects of very‐low‐birthweight infants showing reopening of ductus arteriosus. Pediatrics International 2011;53(3):322‐7. [DOI: 10.1111/j.1442-200X.2010.03251.x; PUBMED: 20854286] [DOI] [PubMed] [Google Scholar]
Van Overmeire 1998
- Overmeire B, Langhendries JP, Vanhasebrouck P, Lecoutere D, Broek H. Ibuprofen for early treatment of patent ductus arteriosus, a randomized multicentre trial. Pediatric Research 1998;43:200A. [Google Scholar]
Varvarigou 1996
- Varvarigou A, Bardin CL, Beharry K, Chemtob S, Papageorgiou A, Aranda JV. Early ibuprofen administration to prevent patent ductus arteriosus in premature newborn infants. JAMA 1996;275(7):539‐44. [PUBMED: 8606475] [PubMed] [Google Scholar]
Weir 1999
- Weir FJ, Ohlsson A, Myhr TL, Fong K, Ryan ML. A patent ductus arteriosus is associated with reduced middle cerebral artery blood flow velocity. European Journal of Pediatrics 1999;158(6):484‐7. [PUBMED: 10378397] [DOI] [PubMed] [Google Scholar]
Wolf 1989
- Wolf WM, Snover DC, Leonard AS. Localized intestinal perforation following intravenous indomethacin in premature infants. Journal of Pediatric Surgery 1989;24(4):409‐10. [PUBMED: 2732888] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Ohlsson 2003
- Ohlsson A, Walia R, Shah S. Ibuprofen for the treatment of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD003481] [DOI] [PubMed] [Google Scholar]
Ohlsson 2005
- Ohlsson A, Walia R, Shah S. Ibuprofen for the treatment of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD003481.pub2] [DOI] [PubMed] [Google Scholar]
Ohlsson 2008
- Ohlsson A, Walia R, Shah S. Ibuprofen for the treatment of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD003481.pub3] [DOI] [PubMed] [Google Scholar]
Ohlsson 2010
- Ohlsson A, Walia R, Shah S. Ibuprofen for the treatment of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.CD003481.pub4] [DOI] [PubMed] [Google Scholar]
Ohlsson 2013
- Ohlsson A, Walia R, Shah S. Ibuprofen for the treatment of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD003481.pub5] [DOI] [PubMed] [Google Scholar]
Ohlsson 2015
- Ohlsson A, Walia R, Shah SS. Ibuprofen for the treatment of patent ductus arteriosus in preterm or low birth weight (or both) infants. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD003481.pub6] [DOI] [PubMed] [Google Scholar]


