Abstract
Theory about the conceptual basis of psychiatric disorders has long emphasized negative emotionality. More recent ideas emphasize roles for positive emotionality and impulsivity as well. This article examines impulsive responses to positive and negative emotions, which have been labelled as urgency. Urgency is conceptually and empirically distinct from other forms of impulsivity. A large body of work indicates that Urgency is more robustly related to psychopathology than are other forms of impulsivity. Researchers have considered four neurocognitive models of urgency: excessive emotion generation, poor emotion regulation, risky decision-making, and poor cognitive control. Little evidence supports emotion generation or risky decision-making as the core issues driving urgency. Rather, urgency appears related to dysfunction in key hubs implicated in the integration of cognitive control and emotion regulation (e.g., the orbitofrontal cortex and anterior insula), expressed as response inhibition deficits that emerge most robustly in high arousal contexts. These neurocognitive processes appear remarkably parallel for positive and negative urgency. We provide methodological suggestions and theoretical hypotheses to guide future research.
Keywords: impulsivity, emotion, urgency, psychopathology, cognition, imaging
This article focuses on trait-like tendencies to respond impulsively to emotion states, referred to as emotion-related impulsivity. This literature first centered on impulsive reactions to negative emotions (1) but later broadened to incorporate impulsive reactions to positive emotions (2–3). Work in this domain rapidly demonstrated the power of integrating the study of emotion with that of impulse. The focus on this nexus has emerged in parallel with a growing body of work in neuroscience on the interface of emotion and cognitive control (4–6). Collectively, this work is beginning to transform psychopathology research to focus on integrating these domains (7–8).
Even though this section of the issue focuses on positive emotions, we consider impulsive responses to both positive and negative emotions, because the body of work pertaining to negative emotion is much larger, and the parallel findings provide a foundation for understanding responses to positive emotion. Indeed, one of our conclusions is that the valence of the emotion is often less important than the impulsive responsivity per se.
Definitions and Measures
Close study of emotion-related impulsivity began with the development of the Urgency scale in 2001, now referred to as Negative Urgency (NU). NU items cover impulsive responses to negative emotion (e.g., “When I am upset I often act without thinking.”) NU is one subscale of the UPPS, which is an abbreviation for Urgency, (low) Perseverance, (low) Premeditation, and Sensation-Seeking (1). NU is not merely a tendency toward negative emotionality, in that it shows divergent validity with such constructs as distress tolerance and neuroticism (8–9). The idea that some people experience impulsivity triggered by emotions rapidly became influential.
NU references mostly negative emotions. The Positive Urgency (PU) measure, developed a few years later, references impulsiveness during states of positive emotions (3). Whether measured using self-report or interview, NU and PU are highly correlated (r’s = .46 to .49, N’s = 183, 1886) and form a higher-order factor (10, replicated in 11). Hence, problems with impulsive responding to emotion appear not to be specific to the valence of emotion. Rather people who have problematic responsivity to negative emotions tend to report problematic responses to positive emotions.
Urgency is consistently found to be distinct from other forms of impulsivity that do not reference emotion, including self-reported lack of perseverance or premeditation, r’s < .35 [2, 10–11], and laboratory tasks involving impulsivity measured outside of emotion contexts (12–13). This suggests that problems of constraint over emotion are separable.
Links of Emotion-Related Impulsivity with Psychopathology
Across hundreds of studies, NU relates robustly to a broad range of psychopathologies (14). Beyond effects for externalizing disorders (r=.34, or Hedges g =.74), NU also correlates with depressive symptoms and diagnoses (14–19), (r=.45, Hedges g = 1.00[14]), and these effects withstand control for comorbid externalizing syndromes (20). NU also is elevated among persons with schizophrenia (21). Beyond syndromes, NU is related to interview and self-report measures of aggression (20–22), suicidal ideation and attempts (14,23–25), and self-harm (24–28).
The development of the PU scale was inspired by clinical observations that many impulsive symptoms involve positive affectivity, such as drinking and gambling (3). Indeed, PU appears significantly more elevated than NU for persons with a history of mania (29). Nonetheless, effect sizes for PU are comparable to those of NU across a range of internalizing and externalizing disorders (14,17,20, 30–31). Effect sizes for PU even parallel those of NU for lifetime major depressive disorder (32–33). High PU is also related to suicidality and self-harm (14). The counterintuitive findings linking depression and suicidality to impulsive reactions to positive emotions point to the importance of poor constraint over emotion as the core concern.
These findings do not appear to be artifactual (8). Despite concerns that the effects could reflect some form of bias in self-evaluation, parent-, interviewer-, and self-ratings of Urgency each show robust effects on psychopathology (2,34–35,14). Contrary to the idea that ratings simply reflect memories of symptoms, NU predicts onset and worse course of alcohol and smoking problems (36–37), as well as risk of suicide attempt (38) and self-harm in longitudinal studies (39). PU also predicts a worse course of illegal drug use, risky sexual behavior, and alcohol use (40–43). Also supporting scale validity, laboratory and experience sampling studies confirm that the scales predict emotion-related changes in behavior. That is, NU predicts symptom worsening (e.g., dietary lapses, drinking) during negative emotion states (44–46), and PU predicts more alcohol use during positive emotion states (47).
Emotion-related impulsivity appears more predictive than studying either emotion or impulsivity in isolation. Effect sizes for NU and PU are much larger than those for other forms of impulsivity measured on the UPPS [mean r<.15 for Premeditation, Perseverence, and Sensation-Seeking] (14). The observed effect sizes for NU with internalizing and externalizing disorders (Hedges g = .75 to 1.00) are also substantively larger than those observed for behavioral impulsivity measures, such as the go/no-go task (generally < .50) (48) or delay discounting tasks (r with addictive behavior = .14) (49). Effects of NU and PU consistently emerge after controlling for other forms of impulsivity or emotionality (3,8,14,44).
The robust and transdiagnostic mental health effects have led to the argument that emotion-related impulsivity may help explain the p-factor, a general vulnerability to psychopathology (50). Other risk factors, such as approach motivation and threat sensitivity, may shape which specific symptoms emerge in the context of this failure of constraint (see 51).
Neurocognitive Correlates of Urgency
In the search for basic mechanisms, researchers have considered four domains: emotion generation, emotion regulation, risky decision-making, and response inhibition. We consider behavioral and neuroimaging findings jointly within domain (52). We highlight the few studies probing how emotion and cognition interact, using valenced stimuli or mood inductions. We also integrate relevant findings from task-free methodologies (e.g. positron emission topography (PET), structural MRI, and resting-state functional connectivity (RSFC).
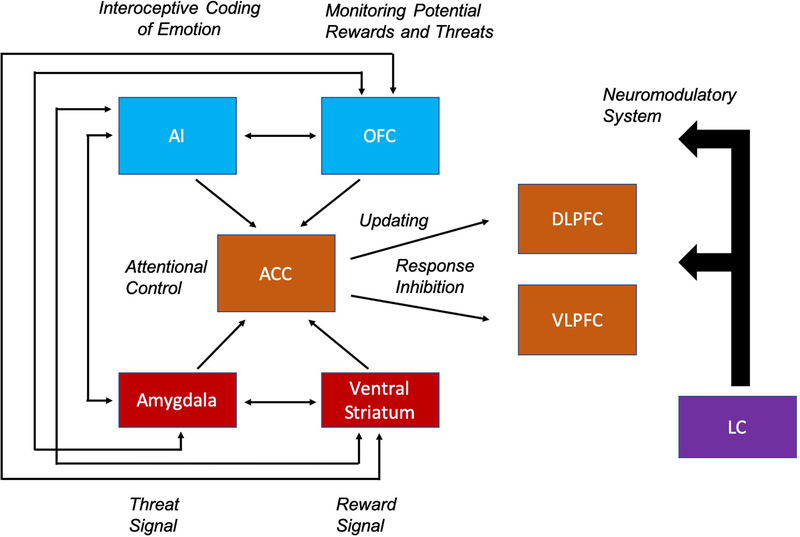
Figure 1 provides a schematic of key neural nodes involved in the generation and control of emotion. Although each region is clearly involved in many task contexts, the figure highlights key functions implicated in the interface of emotion and impulsivity. We attend closely to findings regarding the orbitofrontal cortex (OFC) and anterior insula (AI), as they are integrative hubs activated by emotion generation, emotion regulation, and cognitive control tasks (5,52–54).
Figure 1.
Key neural regions involved in the integration of emotion and cognition, adapted from Pessoa, 2009 (142). This includes regions that contribute to emotion generation (143,144) (Red) and cognitive control regions (Orange) involved in attentional control, response inhibition, and the updating of information (145–147). Regions that integrate affective and motivational signals (Blue) are hubs in both the emotion generation and control processes (53,54,71). Neuromodulators (Purple), such as NE projecting from the LC may affect the rest of the network via state-dependent shifts in arousal (55). Arrows represent the hypothesized functional interactions among regions of interest.
The locus coeruleus (LC) exerts neuromodulatory effects across this network. The LC is the dominant source of norepinephrine (NE) in the brain, and dynamic fluctuations in NE correlate with subjective arousal (55), which is present with intense positive and negative emotions (56).
Given the history of inflated effect sizes for neuroimaging correlates of individual difference variables (57–58), we prioritize replicated findings and theoretically-guided patterns, not effect sizes. Tables 1–4 provide the broader set of imaging and urgency studies.
Table 1.
Summary of Task-Based fMRI Studies
| Study | Task | Emotion Stimuli | Sample Size by Group | Findings with High Negative Urgency |
Findings with High Positive Urgency | Other Findings |
|---|---|---|---|---|---|---|
| Emotion Generation | ||||||
| Cyders et al., 2014 (64) | Passive Smelling | Alcohol/Juice/Sham (olfactory) | HC: 27 | Alcohol > Sham: ↑ OFC | Null |
|
| Cyders et al., 2015 (63) | Passive Viewing | IAPS (Positive, Negative, Neutral) | HC: 27a | Negative > Neutral: ↑ lateral OFC, ↑ amygdala | Null | NU mediated relationship between amygdala & OFC and risk-taking |
| Eiler et al., 2014 (67) | Passive Smelling | Food/Non-food (olfactory) | Normal Weight: 25 Obese: 25 |
Normal Weight: Null Obese (Food > No Food): ↑ OFC |
N/A | |
| Chase et al., 2017(69) | Card Guessing | Expectancy Cue | Psychological Distress: 48 HC: 52 |
Whole Sample (Uncertain Reward Anticipation): ↑ VLPFC, ↑ ventral striatum |
Whole Sample (Uncertain Reward Anticipation): ↑ VLPFC, ↑ ventral striatum |
|
| Emotion Regulation | ||||||
| Albein-Urios et al., 2012 (79) | Cognitive Reappraisal (83) | IAPS (Negative, Neutral) | HC: 18 CD: 17 |
Whole sample (Maintain > Observe): ↑ DLPFC HC (Reappraise> Maintain): ↓ VLPFC ⬄ amygdala CD (Maintain > Observe): ↑ DLPFC ⬄ AI/OFC |
N/A | |
| Albein-Urios et al., 2013 (78) | Cognitive Reappraisal (83) | IAPS (Negative, Neutral) | HC: 21 CD: 17 CD+PD: 18 |
HC: Null CD: Null CD + PD (Reappraise > Maintain): ↑ amygdala |
N/A | |
| Risky Decision-Making | ||||||
| Smith et al., 2018 (92) | Virtual Dating | Avatars of virtual dates | HC: 107 | Sexual > Conversational Decision: ↑ AI | Sexual Decision > Conversation Decision: ↑ AI | |
| Xiao et al., 2013 (93) | Iowa Gambling Task | Win/Loss Feedback | Never Drink: 14 Binge Drink: 14 |
Whole sample (IGT > Control task): ↑ AI, ↓ OFC |
N/A | |
| Xue et al., 2010 (94) | Modified Cups Task | Win/Loss Feedback | HC: 14 | Trial after Safe Bet: ↑ AI | N/A | ↑ AI activation on current trial predicted risky decision on the subsequent trial |
| Response Inhibition | ||||||
| Barkley-Levenson et al., 2018 (105) | Color-Word Stroop | None | No History of Risky Sex: 33 History Risky Sex: 72 |
Null | Null | ACCb, DLPFCb, frontal poleb & AI activation mediated relationship between NU and risk-taking |
| Chester et al., 2016 (106) | Go/NoGo – IAPS images displayed behind targets | IAPS (Positive, Negative, Neutral) | High Urgency: 40 Low Urgency: 38 |
NoGo > Go on Negative Trials: ↑ AI, dorsal striatum, VLPFC NoGo > Go on Neutral and Positive Trials: Null |
N/A | AI mediated relationship between NU and alcohol consumption |
| Tervo-Clemmens et al., 2017 (107) | Antisaccade | Reward Cue (50% of trials) | HC: 116 | Null | Rewarded Antisaccade: ↑ SEF Unrewarded Antisaccade: Null |
|
| Wilbertz et al., 2014 (108) | Stop Signal Task | Reward Stop Signal (50% of Stop trials) | High Impulsivity: 24 Low Impulsivity: 25 |
Rewarded Stop > Go: ↓ VLPFC Unrewarded Stop > Go: ↓ VLPFC |
N/A | ↑ VS after successful reward trial predicted increased performance for low and medium, but not high Urgency participants. |
ACC, anterior cingulate cortex; AI, anterior insula; CD, cocaine dependent; DLPFC, dorsolateral prefrontal cortex; HC, healthy control; IAPS, International Affective Picture System; IGT, Iowa Gambling Task; NU, negative urgency; OFC, orbitofrontal cortex; PD, personality disorder (cluster B); SEF, supplementary eye field; VLPFC, ventrolateral prefrontal cortex; VS, ventral striatum.
Same sample used in Cyders et al., 2014 and Cyders et al., 2015
These regions also mediated the relationship found between Positive Urgency and risk-taking.
Represents a positive correlation with Urgency
Represents a negative correlation with Urgency
Represents bidirectional functional connectivity
– Study did not include Positive Urgency in analyses
Table 4.
Summary of PET and MRS Studies
| Study | Sample Size by Group | Imaging Method | Findings with High Negative Urgency |
Findings with High Positive Urgency |
|---|---|---|---|---|
| Boy et al., 2011 (80) | HC: 12 (Cohort 1) HC: 13 (Cohort 2) |
MRS: GABA-edited MEGA-PRESS spectra | ↓ GABA in DLPFCa | N/A |
| Mick et al., 2017 (65) | HC: 19 GD:15 |
PET: {11C]Ro15–4513 |
HC: Null GD: ↑ GABA-A receptor availability in amygdala, hippocampus and ventral striatum |
N/A |
| Clark et al., 2012 (69) | HC: 9 PG: 9 |
PET: [11C]-raclopride |
HC: Null PG: ↓ D2/D3 receptor binding potential in striatum |
HC: Null PG: ↓ D2/D3 receptor binding potential in striatum |
D2/D3, dopamine receptor D2/D3; DLPFC, dorsolateral prefrontal cortex; GABA, gamma-amino butyric acid; GD, gambling disorder; HC, healthy control; MEGA-PRESS,
MEshcher-GArwood Point RESolved Spectroscopy; MRS, magnetic resonance spectroscopy; PET, positron emission tomography; PG, pathological gambling.
Finding from cohort 1 replicated in cohort 2
Represents a positive correlation with Urgency
Represents a negative correlation with Urgency
– Study did not include Positive Urgency in analyses
Emotion Generation.
Persons with NU and PU do not show elevated subjective, facial behavior, psychophysiological, or cortisol responses to standardized emotion-relevant stimuli or mood induction procedures (12,44,46–47,59), stressors such as speech tasks (60,61), or failed trials on cognitive laboratory tasks (62).
As shown in Table 1, several fMRI studies have considered passive observations of valenced stimuli. Both of the studies of PU were limited by small samples and by use of stimuli that evoked more negative than positive emotion (63–64). Unsurprisingly, PU was not related significantly to neural activation profiles in these studies. Consistent with the null effects for behavioral reactivity, NU related to increased amygdala activity in only one of three fMRI studies to probe passive observation of valenced stimuli (63). NU, however, did correlate with higher GABA-A binding availability in the amygdala (65), which has been related to heightened behavioral reactivity to emotion and acute stress (66). In contrast to the mostly null findings regarding emotion generation or amygdala reactivity, all three passive observation studies found that NU related to increased OFC activation to valenced (vs. neutral) stimuli (63,64,67).
Potential monetary reward may evoke more positive emotion than positive pictures do, of import for PU. In one large study (N=100), high NU and PU both related to increased ventral striatum (VS) and ventrolateral prefrontal cortex (VLPFC) activation during uncertain monetary reward anticipation (68). Findings with different imaging modalities also implicate the VS. NU and PU related to D2/D3 receptor binding potential in VS (69). NU (PU not measured) also correlated with GABA-A receptor availability and gray matter volume in the VS (65,70).
Taken together, Urgency appears linked to OFC responses to valenced stimuli and VS and VLPFC responses to reward expectancy. OFC and VLPFC are implicated in many task contexts, including representation, monitoring, and updating of stimulus-outcome relationships in the context of goal pursuit (53,71,75). Aberrant function in these regions may reflect greater subjective evaluation of cues of potential threat and reward, which could influence updating and learning processes.
Emotion Regulation.
Rather than emotion reactivity, high NU relates to poor emotion regulation, including less reappraisal and perspective-taking, and more rumination (76). Two fMRI studies have used a cognitive reappraisal task (77). In one, NU correlated with higher amygdala activation when directed to reappraise, suggesting less down-regulation of emotion (78). In the other study, profiles differed by group, but in aggregate, NU correlated with increased dorsolateral prefrontal cortex (DLPFC) activation during the sustain condition (79), suggesting ineffective recruitment of cognitive control networks when instructed to modulate emotion. One report linked NU to lower GABA levels in DLPFC (80), which could contribute to these DLPFC inefficiencies during emotion regulation. No data is available concerning PU and neural correlates of emotion regulation.
Risky Decision-Making.
More than 100 behavioral studies have considered risky decision-making tasks such as the Balloon Analog Risk Task (81), the Iowa Gambling Task (82), and Delay of Gratification tasks. NU and PU are not significantly related to such measures, r’s ≤.13, in meta-analyses (12–13), or recent findings (83–88 but see 89).
Evidence is mixed about whether higher arousal conditions trigger risky decision-making for those with high Urgency. In one meta-analysis, NU correlated with less ability to delay gratification for actual payments, r=.24, but not for hypothetical rewards, r=.03 (13 but see 90–91 for nonreplications). In one study, PU related to greater risk taking after (though not before) a mood induction (47 but see 61,88 for nonreplications).
In the fMRI studies of risky decision making, higher anterior insula (AI) activation, consistent with greater prioritization of interoceptive cues (54), was correlated with NU (92–94) and PU (92). Consistent with the importance of emotional arousal in Urgency, higher AI activation was observed in decisions involving higher arousal or risk, such as sex vs. conversation (92) and uncertain vs. certain decisions (93).
In sum, although Urgency does not consistently relate to performance on the laboratory measures being used to assess risky decision-making. fMRI findings link Urgency to elevated AI during risky decision-making in higher arousal/risk conditions. Given evidence that AI lesions relate to diminished risky decision-making (95), dynamic fluctuations in AI might help explain the rash behavior observed with high PU and NU.
Response Inhibition.
Consistent with theory (96–98), findings of meta-analyses indicate that NU and PU relate to poorer performance on tasks involving response inhibition, including go/no-go, go-stop, and antisaccade. These effects appear specific, and are not observed with other facets of executive function, including attention, planning, or time estimation tasks (12, 13, 88). One meta-analysis showed correlations with shifting tasks (13), consistent with models of response inhibition and shifting as closely related facets of executive function (99).
Focusing on response inhibition, the correlations with Urgency are not universally observed. These effects are generally small among student and community samples, mean r=.11 for NU, and r=.14 for PU, but more robust in the 4 available clinical samples, r=.34 for NU (no PU effects available; 88). Response inhibition deficits might be present only for those with severe urgency. In two studies with college students, we found the expected curvilinear pattern, in which little link with response inhibition was observed at the low end of Urgency, but response inhibition deficits emerged at higher levels (33,88). These effects were observed for PU and a composite of PU and NU (33,88).
Some have suggested the importance of distinguishing between early- and late-stage response inhibition, which differentially involve withholding initiation of a prepotent response versus stopping a response already underway (27–28). In one meta-analysis, NU related to deficits on late-stage response inhibition (stop-signal) task but not early-stage response inhibition (13). Nonetheless, when both tasks were administered in the same sample, poorer performance on both the early and late response inhibition measures correlated with PU and NU (28).
Given that emotion triggers symptoms for those with highUurgency, we hypothesized that small increases in arousal might trigger declines in response inhibition for those with high urgency. We used pupil dilation, which is innervated by the LC and correlates closely with subjective arousal (55), to measure trial-by-trial arousal during a response inhibition (antisaccade) task. Persons with higher emotion-related impulsivity showed decay in response inhibition after even minor increases in arousal (pupil); those with low emotion-related impulsivity did not (62). Hence emotional arousal (tied to positive and negative emotions) may interfere with response inhibition for those with high emotion-related impulsivity. Declines in cognitive control with extreme elevations of NE are normative (100–104); those with higher urgency may show more fragility in response inhibition with these dynamic fluctuations in NE.
Consistent with the role of emotional arousal, fMRI findings have linked NU and PU to activation of regions involved in response inhibition only when valenced stimuli were incorporated into the task, and not in response to neutral stimuli (105–108). In the two fMRI studies of early-stage response inhibition that included valenced stimuli, NU and PU related to increased activity in cognitive control regions, including AI, dorsal caudate, and VLPFC (106), as well as supplementary eye field during an antisaccade task (107). Activation of these regions was correlated to NU in the presence of negative stimuli (106), and to PU in the presence of positive stimuli (107).
In contrast, in an fMRI study of late response inhibition, NU related to less activity in VLPFC (108). Although more studies are needed to test the replicability of this divergence, these results suggest a compensatory response that enables early-stage response inhibition, and diminished activation to late-stage response inhibition task demands in people with high urgency.
Intriguingly, VS activation after a rewarded “Stop” trial in this study correlated with better task performance for people with lower, but not higher Urgency (108). This highlights reinforcement signaling in the VS, alongside NE-based arousal, as a dynamic circuit that integrates with response inhibition networks and may be disrupted in those with high Urgency.
Of clinical relevance, atypical activations of canonical response inhibition regions appear to statistically mediate relationships of NU and PU with real-world outcomes, such as alcohol consumption and risky sexual behavior (105–106). In addition, in structural MRI studies (Table 3), NU was tied to decreased grey matter volume or cortical thickness in several cognitive control and integration hub regions shown in Figure 1, complementing the pattern of results from fMRI (21,109–1110).
Table 3.
Summary of Structural MRI Studies
| Study | Sample Size by Group | Quantitative Method | Findings with High Negative Urgency |
Findings with High Positive Urgency |
|---|---|---|---|---|
| Hoptman et al., 2014 (21) | SCZ: 33 HC: 31 |
Cortical Thickness |
SCZ: ↓ frontal pole, ↓ medial OFC HC: Null |
SCZ: ↓ frontal pole, ↓ rACC HC: Null |
| Wang et al., 2017 (109) | Obese: 31 Normal Weight: 49 |
Gray Matter Volume |
Obese: Null Normal Weight: ↓ AI |
Null |
| Ruiz de Lara et al., 2018 (110) | HC: 25 GD: 25 |
Gray Matter Volume |
HC: Null GD: ↓ VLPFC |
N/A |
| Muhlert & Lawrence, 2015 (70) | HC: 152 | Gray Matter Volume |
↓ DMPFC ↓ temporal pole ↓ ventral striatum |
N/A |
| Moreno-Lopez et al., 2012 (139) | HC: 38 CD: 38 |
Gray Matter Volume |
HC: ↓ SFG CD: ↑ SFG |
Null |
| Albein-Urios et al., 2013 (140) | HC: 34 CD + PD: 32 CD: 44 |
Gray Matter Volume | HC: Null CD + PD: ↑ rolandic operculum CD: ↓ rolandic operculum Whole group: ↓ temporal pole |
Null |
AI, anterior insula; CD, cocaine dependent; DMPFC, dorsomedial prefrontal cortex; GD, gambling disorder; HC, healthy control; OFC, orbitofrontal cortex; PD, personality disorder (cluster B); rACC, rostral anterior cingulate cortex; SCZ, schizophrenia; SFG, superior frontal gyrus; VLPFC, ventrolateral prefrontal cortex.
Represents a positive correlation with Urgency
Represents a negative correlation with Urgency
– Study did not include Positive Urgency in analyses
Functional Connectivity.
RSFC, a technique to index synchrony between brain regions in the absence of task performance, has been interpreted as the intrinsic and modal functional state of neural networks (111). Because motion may produce systematic Type 1 errors in RSFC (112–114), we only include studies that took recommended steps to reduce these artifacts (115). As shown in Table 2, RSFC findings published before newer standards for cluster-defining primary thresholds in multiple comparisons corrections (116,117) had less conservative analyses (21).
Table 2.
Summary of Resting-State Functional Connectivity Studies
| Study | Sample Size by Group | Scan Time | Motion Correction | Correction for Multiple Comparisons | Findings with High Negative Urgency | Findings with High Positive Urgency |
|---|---|---|---|---|---|---|
| Hoptman et al., 2014 (21) | HC: 31 SCZ: 33 |
6 min | Motion correction, denoising, mean FD included as covariate | Primary threshold: z=2.3 Cluster-level correction: p=0.05 using GRF |
SCZ: ↓ lateral OFC → MFG (left) ↓ medial OFC → SFG ↓ medial OFC → rACC ↓ rACC → SFG ↑ lateral OFC → IFG/MFG (right) ↑ lateral OFC → PCC ↑ medial OFC → precuneus ↑ medial OFC → cuneus ↑ frontal pole → SPL HC: ↓ lateral OFC → insula ↓ frontal pole → insula ↓ frontal pole → postcentral gyrus ↑ frontal pole → putamen ↑ lateral OFC → STG |
SCZ: ↓ rACC → frontal pole ↑ frontal pole → occipital gyrus HC: ↓ lateral OFC → insula ↑ rACC→ IPL ↑ rACC→ parahippocampal gyrus ↑ frontal pole → putamen |
| Golchert et al., 2017 (119) | HC: 112 (MPI-S) HC: 92 (NKI-RS) |
MPI-S: 15.5 min NKI-RS: 10 min | Motion correction, denoising, mean FD included as covariate | Primary threshold: z=3.1 Cluster-level correction: pFWE < .05 |
Null | ↓ subgenual ACC → retrosplenial cortex |
| Zhao et al., 2017 (120) * | HC: 85 | 10.83 min | Motion correction, scrubbing FD > 0.3mm |
Primary threshold: p<0.001 Cluster-level correction: pFWE < 0.05 |
↓ PCC/precuneus → VTA, thalamus, LN, medial GP, putamen, SN and caudate ↓ PCC/precuneus → precentral gyrus |
|
| Zhu et al., 2017 (118) | HC: 26 AD: 25 |
5 min | Motion correction, scrubbing FD > 0.5mm or > 5% change global signal intensity | Primary threshold: pFWE < 0.05; Results correction: p < 0.05 bonferroni |
AD: ↓ amygdala ⬄ striatum ↓ OFC ⬄ ECN ↓ OFC ⬄ DMN HC: Null |
AD: Null HC: ↓ anterior DMN ⬄ anterior DMN |
| Um et al., 2019 (121) | HC: 62 TU: 34 |
5 min | Motion correction, denoising, motion parameters included as covariates, scrubbing FD > 0.3mm | Primary threshold: p = .001; Cluster-level threshold pFWE < .05. |
Whole group: ↑ AI → dACC TU: ↓ NAcc → dACC, DLPFC HC: ↑ NAcc → dACC, DLPFC |
N/A |
ACC, anterior cingulate cortex; AD, alcohol dependence; AI, anterior insula; dACC, dorsal anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; DMN, default mode network; ECN, executive control network; FD, framewise displacement; FWE, family-wise error rate; GP, globus pallidus; HC, healthy control; IFG, inferior frontal gyrus; IPL, inferior parietal lobule LN, lentiform nucleus; MFG, middle frontal gyrus; MPI-S, Max Planck Institute Sample; NAcc, nucleus accumbens; NKI-RS, Nathan Kline Institute – Rockland Sample; OFC, orbitofrontal cortex; PCC, posterior cingulate cortex; rACC, rostral anterior cingulate cortex; SCZ, schizophrenia; SFG, superior frontal gyrus; SN, substantia nigra; SPL, superior parietal lobule; STG, superior temporal gyrus TU, tobacco user; VTA, ventral tegmental area.
Used an Urgency score that combined PU and NU.
Represents a positive correlation with Urgency
Represents a negative correlation with Urgency
Represents bidirectional functional connectivity
Represents directional (seeded) functional connectivity
– Study did not include Positive Urgency in analyses
Multiple studies indicate that NU and PU are related to weaker RSFC involving cognitive control regions, but the specific profiles vary across studies. NU and PU have been related to weaker RSFC of cognitive control regions with OFC (21,118) and with default-mode network (DMN) (118,119). Findings regarding other RSFC correlates of Urgency have been even more mixed, including those involving emotion generation regions (118,120,121). Perhaps different network disturbances can contribute to Urgency in an equifinal way. It is also possible that RSFC is too unconstrained, and standard correlational and “seed-to-voxel” approaches may capture only a small slice of the complex functional connectivity profile in any one study.
Treatment Development
Multiple authors have emphasized the need to develop treatments to address Urgency (122,123). Urgency, however, may be a difficult treatment target. For example, high NU predicted poorer outcome across six studies of cognitive therapy for alcohol use disorder (124). Similarly, NU predicted less ability to implement coping to improve diet after a single-session intervention (125). These findings suggest that interventions may need to be tailored to address NU. We briefly consider treatment development work targeting emotion regulation, cognitive control, and their combination.
Several findings show promise for emotion regulation approaches. In secondary analyses of interventions focused on improving emotion regulation, NU predicted positive outcomes (126). One study tested a 9-week dialectical behavior therapy group to enhance emotion regulation in a school setting as a preventative approach to tendencies to engage in rash behavior in response to emotion. Pre-post scores on a measure of risky behavior during positive and negative emotion states declined, particularly for students with higher NU scores at baseline (127). In another study, college students received a single session of emotion management training or impulsivity reduction training. Emotion management training was more powerful than impulsivity training in reducing NU scores (128).
Cognitive training also appears promising. In one study, adults with high emotion-related impulsivity were assigned to either immediate cognitive training (response inhibition on the Go/No-go task and working memory on the PASAT) or a waitlist. Emotion-related impulsivity scores declined after 6 sessions of training, but not in the waitlist condition (129).
One intervention for emotion-related impulsivity and aggression targeted emotion and cognitive control. To address emotionality, participants were taught to identify anger and related triggers, and then learned self-calming skills such as relaxation. To remediate deficits in cognitive control during high arousal, participants were taught to pre-plan how they would cope with anger using implementation intentions, a well-validated approach to diminishing impulsive actions (130). Aggression and self-harm declined significantly in the active intervention but not the waitlist condition (131).
Overall, these early treatment development findings support the idea that addressing problems of executive control during periods of high arousal can help alleviate emotion-related impulsivity and its consequences. This fits with the mechanisms being identified in the basic research on emotion-related impulsivity. Given the transdiagnostic effects of emotion-related impulsivity, one goal will be to test whether targeting emotion-related impulsivity can reduce symptoms transdiagnostically.
Conclusion
The Urgency scales are well-validated as related to a broad range of psychopathologies and behavioral problems, with effect sizes larger than those for measures of emotionality or impulsivity alone. Despite a long history of focus on negative emotionality, the effects for PU are as large as those for NU.
Work on the neurocognitive correlates of Urgency is accelerating, and mostly falls within four domains: emotion generation, emotion regulation, risky decision-making and response inhibition. Some models have achieved little support. Urgency does not appear related to emotion generation, across behavioral, psychophysiological or neural indices. Behavioral performance on the tasks used to capture risky decision-making does not appear to be consistent correlates of Urgency, although elevated anterior insula is observed during such tasks, particularly when higher risk decisions are being made.
Across behavior and imaging methods, evidence supports difficulties in response inhibition (but not facets of executive function such as attention problems) for both PU and NU. Given that reappraisal rests on similar cognitive control circuitry to response inhibition (132), it is unsurprising that Urgency also relates to less use of this emotion regulation strategy and atypical neural responses when instructed to regulate emotion. Smaller effect sizes are observed for healthy (as compared to clinical) samples in behavioral studies of response inhibition (88). Consistent with basic findings, early intervention work suggests the merit of targeting response inhibition and emotion regulation deficits.
In addition to canonical cognitive control regions, key regions at the interface of cognition × emotion, OFC and AI, are tied to Urgency across task contexts. OFC and AI lesions have both been tied to impulsive action (71,95,133). Structural deficits in OFC were observed in one severe clinical sample. RSFC findings, albeit more mixed and more evident in severe clinical samples, also highlight the importance of weakened communication between OFC and key regions involved in cognitive control. Together, findings suggest these regions play a central role in the phenotype.
The stronger clinical versus community profile fits models of primary (generalized and structural) versus secondary (triggered and dynamic) cognitive control deficits (134). There is no clear evidence of reproducible structural correlates in community samples. Rather it appears that constraint circuits are perturbed by high emotion/arousal. That is, some perturbations may magnify deficits among those with high Urgency in the community samples—response inhibition deficits appear to decay with valenced stimuli, mood inductions, and increases in norepinephrine levels (pupil), and fail to show normative improvement with higher ventral striatum activation. AI, which has been related to response inhibition (135) appears to be more powerfully related to Urgency in high risk contexts, possibly indicating increased prioritization of interoceptive emotion cues. More research is needed to understand how these dynamic fluctuations interact with abnormal function of cognitive control regions and OFC.
Despite the importance of Urgency in mental health outcomes, behavioral and imaging research remains limited. Many studies rely on behavioral tasks with poor reliability (136–137). Latent models are needed to control for task unreliability and overlap in functions probed by the behavioral measures, as a way to evaluate specificity of executive function (99). Neuroimaging studies of PU are sparse—in each neurocognitive domain, only one PU study used adequate positive stimuli. Many fMRI studies rely on small sample sizes. Many RSFC findings have not replicated, perhaps because the standard correlational statistics used do not capture dynamic processes of urgency. Community samples may not include many highly impulsive individuals; future community-based studies will need to adequately sample those with higher Urgency, and to consider potential curvilinear effects in which neurocognitive deficits may only be observed as Urgency is more severe.
Taking into account the strengths and limitations, the profile of findings suggests several hypotheses for future research:
In community samples, behavioral and neural correlates of NU and PU will be more robustly observed when the system is perturbed, through techniques such as experimental manipulation of emotional arousal.
The dynamic nature of the deficits calls for imaging techniques designed to capture temporally instable processes, such as dynamic functional connectivity.
Findings regarding the OFC and AI suggest the importance of using tasks that effectively probe the function of these regions, such as updating and shifting stimulus-outcome operations during goal pursuit, and responses to interoceptive cues of arousal.
Although we hope these ideas will help guide future research, it may be that more than one process will culminate, in an equifinal manner, in Urgency. Indeed, promising but currently underexplored findings of reinforcement signaling in VS, GABA binding in amygdala, and RSFC of DMN may prove key in future models. Given the complex nature of the neural circuits underpinning the deficits, best-suited techniques will be those that assess the architecture of multiple brain circuits, such as graph theory and structural equation modeling (138,139).
In sum, poor control over positive (as well as negative) emotions appears to be a major risk factor for a very broad range of psychopathologies. This suggests that positive affectivity is as important for understanding psychopathology as is negative affectivity. PU correlates highly with NU, and the behavioral and neural correlates of PU and NU are largely parallel. Given the overlap, those studying problematic outcomes of emotionality would do well to consider the idea that the real culprit may be the loss of control over high emotion states generally, rather than positive or negative emotion specifically. The PU and NU scales may provide a quick tool to capture problems of cognitive control and behavioral problems in contexts of high emotional arousal, and appear related to disturbances in OFC, AI, and cognitive control circuitry in such contexts. As such, these brief, easily administered scales may provide a method to bridge these neural indicators to transdiagnostic psychopathology outcomes in future research.
Acknowledgements
Preparation of this manuscript was supported by National Institute of Mental Health (NIMH) R01 110477 to Johnson and Carver. No other grants supported the preparation of this manuscript.
The contents of this review are original and have not been published previously, although we have discussed the strength of effects for psychopathology, and the behavioral findings regarding response inhibition in:
Carver CS, Johnson SL. (2018). Impulsive reactivity to emotion and vulnerability to psychopathology. Am Psychol.73:1067–1078. doi:10.1037/amp0000387
Footnotes
Disclosures
Drs. Johnson and Carver have received support from National Institute of Mental Health (NIMH) R01 110477. The authors have no other financial interests or conflicts of interest to report.
References
- 1.Whiteside SP, Lynam DR. (2001). The five factor model and impulsivity: Using a structural model of personality to understand impulsivity. Personality and Individual Differences 30:669–689. doi: 10.1016/S0191-8869(00)00064-7 [DOI] [Google Scholar]
- 2.Cyders MA, Smith GT. (2007). Mood-based rash action and its components: Positive and Negative Urgency. Personality and Individual Differences 43:839–850. doi: 10.1016/j.paid.2007.02.008 [DOI] [Google Scholar]
- 3.Cyders MA, Smith GT, Spillane NS, Fischer S, Annus AM, Peterson C. (2007). Integration of impulsivity and positive mood to predict risky behavior: Development and validation of a measure of positive urgency. Psychol Assess 19:107–118. doi: 10.1037/1040-3590.19.1.107 [DOI] [PubMed] [Google Scholar]
- 4.Phillips ML, Ladouceur CD, Drevets WC. (2008). A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry 13:833–857. doi: 10.1038/mp.2008.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips ML, Drevets WC, Rauch SL, Lane R. (2003). Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biological Psychiatry 54 (5):504–514. doi: 10.1016/S0006-3223(03)00168-9 [DOI] [PubMed] [Google Scholar]
- 6.Ochsner KN, Gross JJ. (2005). The cognitive control of emotion. Trends in Cognitive Sciences 9:242–249. doi: 10.1016/j.tics.2005.03.010 [DOI] [PubMed] [Google Scholar]
- 7.Carver CS, Johnson SL. (2018). Impulsive reactivity to emotion and vulnerability to psychopathology. American Psychologist 73:1067–1078. doi: 10.1037/amp0000387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cyders MA, Smith GT. (2008). Emotion-based dispositions to rash action: Positive and Negative urgency. Psychol Bulletin 134:807–828. doi: 10.1037/a0013341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaiser AJ, Milich R, Lynam DR, Charnigo RJ. (2012). Negative urgency, distress tolerance, and substance abuse among college students. Addict Behav 37:1075–1083. doi: 10.1016/j.addbeh.2012.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith GT, Fischer S, Cyders MA, Annus AM, Spillane NS, & McCarthy DM (2007). On the validity and utility of discriminating among impulsivity-like traits. Assessmen. 14:155–170. Doi: 10.1177/1073191106295527 [DOI] [PubMed] [Google Scholar]
- 11.Carver CS, Johnson SL, Joormann J, Kim Y, Nam JY. (2011). Serotonin transporter polymorphism interacts with childhood adversity to predict aspects of impulsivity. Psychol Science 22:589–595. doi: 10.1177/0956797611404085 [DOI] [PubMed] [Google Scholar]
- 12.Cyders MA, Coskunpinar A. (2011). Measurement of constructs using self-report and behavioral lab tasks: Is there overlap in nomothetic span and construct representation for impulsivity? Clin Psychol Review 31 (6):965–982. doi: 10.1016/j.cpr.2011.06.001 [DOI] [PubMed] [Google Scholar]
- 13.Sharma L, Markon KE, Clark LA. (2014). Toward a theory of distinct types of “impulsive” behaviors: A meta-analysis of self-report and behavioral measures. Psychol Bulletin 140 (2):374–408. doi: 10.1037/a0034418 [DOI] [PubMed] [Google Scholar]
- 14.Berg JM, Latzman RD, Bliwise NG, Lilienfeld SO. (2015). Parsing the heterogeneity of impulsivity: A meta-analytic review of the behavioral implications of the UPPS for psychopathology. Psychol Assessment 27:1129–1146. doi: 10.1037/pas0000111 [DOI] [PubMed] [Google Scholar]
- 15.Gunn RL, Jackson KM, Borsari B, Metrik J. (2018). Negative urgency partially accounts for the relationship between major depressive disorder and marijuana problems. Borderline Personal Disord Emot Dysregulation 5:10. doi: 10.1186/s40479-018-0087-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.d’Acremont M, Van der Linden M, D’Acremont M, Van der Linden M. (2007). How is impulsivity related to depression in adolescence? Evidence from a French validation of the cognitive emotion regulation questionnaire. J Adolescence 30:271–282. doi: 10.1016/j.adolescence.2006.02.007 [DOI] [PubMed] [Google Scholar]
- 17.King KM, Karyadi KA, Luk JW, Patock-Peckham JA. (2011). Dispositions to rash action moderate the associations between concurrent drinking, depressive symptoms, and alcohol problems during emerging adulthood. Psychol Addict Behavior 25:446–454. doi: 10.1037/a0023777 [DOI] [PubMed] [Google Scholar]
- 18.Pang RD, Farrahi L, Glazier S, Sussman S, Leventhal AM. (2014). Depressive symptoms, negative urgency and substance use initiation in adolescents. Drug Alcohol Depend 144:225–230. doi: 10.1016/j.drugalcdep.2014.09.771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez VM, Reynolds B, Skewes MC. (2011). Role of impulsivity in the relationship between depression and alcohol problems among emerging adult college drinkers. Exp Clin Psychopharmacol. 19:303–313. doi: 10.1037/a0022720 [DOI] [PubMed] [Google Scholar]
- 20.Johnson SL, Carver CS, Joormann J. (2013). Impulsive responses to emotion as a transdiagnostic vulnerability to internalizing and externalizing symptoms. J Affect Disord 150:872–878. doi: 10.1016/j.jad.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 21.Hoptman MJ, Antonius D, Mauro CJ, Parker EM, Javitt DC. (2014). Cortical thinning, functional connectivity, and mood-related impulsivity in schizophrenia: Relationship to Aggressive attitudes and behavior. Am J Psychiatry 171:939–948. doi: 10.1176/appi.ajp.2014.13111553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson SL, Carver CS. (2016). Emotion-relevant impulsivity predicts sustained anger and aggression after remission in bipolar I disorder. J Affect Disord 189:169–175. doi: 10.1016/j.jad.2015.07.050 [DOI] [PubMed] [Google Scholar]
- 23.Auerbach RP, Stewart JG, Johnson SL. (2017). Impulsivity and suicidality in adolescent inpatients. J Abnorm Child Psychol 45:91–103. doi: 10.1007/s10802-016-0146-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson SL, Carver CS, Tharp JA. (2017). Suicidality in Bbipolar Ddisorder: The role of emotion-triggered impulsivity. Suicide Life-Threat Behav 47:177–192. doi: 10.1111/sltb.12274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lynam DR, Miller JD, Miller DJ, Bornovalova MA, Lejuez CW. (2011). Testing the relations between impulsivity-related traits, suicidality, and nonsuicidal self-injury: a test of the incremental validity of the UPPS model. Personal Disord 2:151–160. doi: 10.1037/a0019978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterson CM, Fischer S. (2012). A prospective study of the influence of the UPPS model of impulsivity on the co-occurrence of bulimic symptoms and non-suicidal self-injury. Eat Behav 13:335–341. doi: 10.1016/j.eatbeh.2012.05.007 [DOI] [PubMed] [Google Scholar]
- 27.Allen KJD, Hooley JM. (2015). Inhibitory control in people who self-injure: Evidence for impairment and enhancement. Psychiatry Res 225: 631–637. doi: 10.1016/j.psychres.2014.11.033 [DOI] [PubMed] [Google Scholar]
- 28.Allen KJD, Jill Miranda Hooley DP. (2019. preprint). Negative Emotional Response Inhibition: Support for a cognitive mechanism underlying negative urgency in nonsuicidal self-injury. doi: 10.31219/OSF.IO/DTXRB [DOI] [PubMed] [Google Scholar]
- 29.Muhtadie L, Johnson SL, Carver CS, Gotlib IH, Ketter TA. (2014). A profile approach to impulsivity in bipolar disorder: the key role of strong emotions. Acta Psychiatr Scand 129:100–108. doi: 10.1111/acps.12136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marmorstein NR. (2013). Associations between dispositions to rash action and internalizing and externalizing symptoms in children. J Clin Child Adolesc Psychol 42:131–138. doi: 10.1080/15374416.2012.734021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson SL, Tharp JA, Peckham AD, Carver CS, Haase CM. (2017). A path model of different forms of impulsivity with externalizing and internalizing psychopathology: Towards greater specificity. Br J Clin Psychol 56:235–252. doi: 10.1111/bjc.12135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carver CS, Johnson SL, Joormann J. (2013). Major depressive disorder and impulsive reactivity to emotion: Toward a dual-process view of depression. Br J Clin Psychol 52:285–299. doi: 10.1111/bjc.12014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dekker MR, Johnson SL. (2018). Major depressive disorder and emotion-related Impulsivity: Are both related to cognitive inhibition? Cognit Ther Res 42:398–407. doi: 10.1007/s10608-017-9885-2 [DOI] [Google Scholar]
- 34.Zapolski TCB, Stairs AM, Settles RF, Combs JL, Smith GT. (2010). The measurement of dispositions to rash action in children. Assessment 17 (1):116–125. doi: 10.1177/1073191109351372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zapolski TC, Smith GT. (2013). Comparison of parent versus child-report of child impulsivity traits and prediction of outcome variables. Journal of Psychopathology and Behavioral Assessment 35: 301–313. doi: 10.1007/s10862-013-9349-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riley EN, Rukavina M, Smith GT. (2016). The reciprocal predictive relationship between high-risk personality and drinking: An 8-wave longitudinal study in early adolescents. J Abnorm Psychol 125 (6):798–804. doi: 10.1037/abn0000189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Webb Hooper M, Carver CS. (2016). Reflexive reaction to feelings predicts failed smoking cessation better than does lack of general self-control. J Consult Clin Psychol 84 (7):612–618. doi: 10.1037/ccp0000109 [DOI] [PubMed] [Google Scholar]
- 38.Kasen S, Cohen P, Chen H. (2011). Developmental course of impulsivity and capability from age 10 to age 25 as related to trajectory of suicide attempt in a community cohort. Suicide Life Threat Beha. 41 (2):180–192. doi: 10.1111/j.1943-278X.2011.00017.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riley EN, Combs JL, Jordan CE, Smith GT. (2015). Negative urgency and lack of Perseverance: Identification of differential pathways of onset and maintenance risk in the longitudinal prediction of nonsuicidal self-injury. Behav Ther 46:439–448. doi: 10.1016/j.beth.2015.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zapolski TCB, Cyders MA, Smith GT. (2009). Positive urgency predicts illegal drug use and risky sexual behavior. Psychol Addict Behav 23 (2):348–354. doi: 10.1037/a0014684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Milich R, Bonsu JA, Lynam DR, Kaiser A, Charnigo RJ. (2016). Impulsive personality and alcohol use: Bidirectional relations over one year. J Stud Alcohol Drugs 77 (3):473–482. doi: 10.15288/jsad.2016.77.473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lopez-Vergara HI, Spillane NS, Merrill JE, Jackson KM. (2016). Developmental trends in alcohol use initiation and escalation from early to middle adolescence: Prediction by Urgency and trait affect. Psychol Addict Behav 30:578–587. doi: 10.1037/adb0000173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Settles RE, Zapolski TCB, Smith GT. (2014). Longitudinal test of a developmental model of the transition to early drinking. J Abnorm Psychol 123 (1):141–151. doi: 10.1037/a0035670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cyders MA, Coskunpinar A. (2010). Is urgency emotionality? Separating urgent behaviors from effects of emotional experiences. Pers Individ Differences. 48 (7):839–844. doi: 10.1016/j.paid.2010.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manasse SM, Crochiere RJ, Dallal DH, Lieber EW, Schumacher LM, Crosby RD, …, Forman EM (2018). A multimodal investigation of impulsivity as a moderator of the relation between momentary elevations in negative internal states and subsequent dietary lapses. Appetite 127:52–58. doi: 10.1016/j.appet.2018.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.VanderVeen JD, Plawecki MH, Millward JB, Hays J, Kareken DA, O’Connor S, Cyders MA. (2016). Negative urgency, mood induction, and alcohol seeking behaviors. Drug and Alcohol Dependence 165:151–158. doi: 10.1016/j.drugalcdep.2016.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cyders MA, Zapolski TCB, Combs JL, Settles RF, Fillmore MT, Smith GT. (2010). Experimental effect of Positive Urgency on negative outcomes from risk taking and on increased alcohol consumption. Psychol Addict Behav 24 (3):367–375. doi: 10.1037/a0019494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wright L, Lipszyc J, Dupuis A, Thayapararajah SW, Schachar R. (2014). Response inhibition and psychopathology: A meta-analysis of go/no-go task performance. Journal of Abnormal Psychology 123: 429–439. doi: 10.1037/a0036295 [DOI] [PubMed] [Google Scholar]
- 49.Amlung M, Vedelago L, Acker J, Balodis I, MacKillop J. (2017). Steep delay discounting and addictive behavior: a meta-analysis of continuous associations. Addiction 112:51–62. doi: 10.1111/add.13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carver CS, Johnson SL, Timpano KR. (2017). Toward a functional view of the P factor in psychopathology. Clin Psychol Sci 5 (5):880–889. doi: 10.1177/2167702617710037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carver CS, Johnson SL, Joormann J. (2009). Two-mode models of self-regulation as a tool for conceptualizing effects of the serotonin system in normal behavior and diverse disorders. Curr Dir Psychol Sci 18:195–199. doi: 10.1111/j.1467-8721.2009.01635.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pessoa L (2019). Embracing integration and complexity: placing emotion within a science of brain and behaviour. Cognition and Emotion 33 (1):55–60. doi: 10.1080/02699931.2018.1520079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hiser J, Koenigs M. (2018). The multifaceted role of the ventromedial prefrontal cortex in emotion, decision making, social cognition, and psychopathology. Biological Psychiatry 83:638–647. doi: 10.1016/j.biopsych.2017.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Craig AD. (2009). How do you feel--now? The anterior insula and human awareness. Nature Reviews Neuroscience 10 (1):59–70. doi: 10.1038/nrn2555 [DOI] [PubMed] [Google Scholar]
- 55.Joshi S, Li Y, Kalwani RM, Gold JI. (2016). Relationships between pupil diameter and neuronal activity in the locus coeruleus, colliculi, and cingulate cortex. Neuron 89 (1):221–234. doi: 10.1016/j.neuron.2015.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Russell JA (1980). A circumplex model of affect. Journal of Personality and Social Psychology 39:1161–1178.doi: 10.1037/h0077714 [DOI] [PubMed] [Google Scholar]
- 57.Vul E, Harris C, Winkielman P, Pashler H. (2009). Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspectives on Psychological Science 4 (3), 274–290. doi: 10.1111/j.1745-6924.2009.01125 [DOI] [PubMed] [Google Scholar]
- 58.Reddan MC, Lindquist MA, Wager TD. (2017). Effect size estimation in neuroimaging. JAMA Psychiatry 74 (3):207–208. doi: 10.1001/jamapsychiatry.2016.3356 [DOI] [PubMed] [Google Scholar]
- 59.Johnson SL, Haase CM, Beermann U, Sanchez AH, Tharp JA, Lwi SJ, …, Nguyen NK. (2017). Positive urgency and emotional reactivity: Evidence for altered responding to positive stimuli. Emotion 17 (3):442–449. doi: 10.1037/emo0000240 [DOI] [PubMed] [Google Scholar]
- 60.Owens MM, Amlung MT, Stojek M, MacKillop J. (2018). Negative urgency moderates reactivity to laboratory stress inductions. Journal of Abnormal Psychology 127 (4):385–393. doi: 10.1037/abn0000350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wise RJ, Phung AL, Labuschagne I, Stout JC. (2015). Differential effects of social stress on laboratory-based decision-making are related to both impulsive personality traits and gender. Cognition and Emotion 29 (8):1475–1485. doi: 10.1080/02699931.2014.989815 [DOI] [PubMed] [Google Scholar]
- 62.Pearlstein JG, Johnson SL, Modavi K, Peckham AD, Carver CS. (2019). Neurocognitive mechanisms of emotion related impulsivity: The role of arousal. Psychophysiology 56: e13293. doi: 10.1111/psyp.13293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cyders MA, Dzemidzic M, Eiler WJ, Coskunpinar A, Karyadi KA, Kareken DA. (2015). Negative urgency mediates the relationship between amygdala and orbitofrontal cortex activation to negative emotional stimuli and general risk-taking. Cerebral Cortex 25 (11): 4094–4102. doi: 10.1093/cercor/bhu123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cyders MA, Dzemidzic M, Eiler WJ, Coskunpinar A, Karyadi K, Kareken DA. (2014). Negative urgency and ventromedial prefrontal cortex responses to alcohol cues: fMRI evidence of emotion-based impulsivity. Alcoholism: Clinical and Experimental Research 38 (2):409–417. doi: 10.1111/acer.12266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mick I, Ramos AC, Myers J, Stokes PR, Chandrasekera S, Erritzoe D, …, Galduróz JC. (2017). Evidence for GABA A receptor dysregulation in gambling disorder: Correlation with impulsivity. Addiction Biology 22:1601–1609. doi: 10.1111/adb.12457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jie F, Yin G, Yang W, Yang M, Gao S, Lv J, Li B. (2018). Stress in regulation of GABA amygdala system and relevance to neuropsychiatric diseases. Frontiers in Neuroscience 12:562. doi: 10.3389/fnins.2018.00562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eiler WJ, Dzemidzic M, Case KR, Armstrong CL, Mattes RD, Cyders MA …, Kareken DA. (2014). Ventral frontal satiation-mediated responses to food aromas in obese and normal-weight women. The American Journal of Clinical Nutrition 99:1309–1318. doi: 10.3945/ajcn.113.080788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chase HW, Fournier JC, Bertocci MA, Greenberg T, Aslam H, Stiffler R, …, Phillips ML. (2017). A pathway linking reward circuitry, impulsive sensation-seeking and risky decision-making in young adults: identifying neural markers for new interventions. Translational Psychiatry 7:e1096. doi: 10.1038/tp.2017.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Clark L, Stokes PR, Wu K, Michalczuk R, Benecke A, Watson BJ, …, Lingford-Hughes AR. (2012). Striatal dopamine D2/D3 receptor binding in pathological gambling is correlated with mood-related impulsivity. Neuroimage 63 (1):40–46. doi: 10.1016/j.neuroimage.2012.06.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Muhlert N, Lawrence AD. (2015). Brain structure correlates of emotion-based rash impulsivity. Neuroimage. 115 (1):138–146. doi: 10.1016/j.neuroimage.2015.04.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kringelbach ML, Rolls ET. (2004). The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Progress in Neurobiology 72:341–372. doi: 10.1016/j.pneurobio.2004.03.006 [DOI] [PubMed] [Google Scholar]
- 72.Boorman ED, Rajendran VG, O’Reilly JX, & Behrens TE. (2016). Two anatomically and computationally distinct learning signals predict changes to stimulus-outcome associations in hippocampus. Neuron 89(6): 1343–1354. doi: 10.1016/j.neuron.2016.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, & Andrews C. (2001). Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience 4(1): 95–102. doi: 10.1038/82959 [DOI] [PubMed] [Google Scholar]
- 74.Beer JS, John OP, Scabini D, & Knight RT. (2006). Orbitofrontal cortex and social behavior: integrating self-monitoring and emotion-cognition interactions. Journal of Cognitive Neuroscience 18: 871–879. doi: 10.1162/jocn.2006.18.6.871 [DOI] [PubMed] [Google Scholar]
- 75.Badre D, & Wagner AD. (2007). Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia 45: 2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015 [DOI] [PubMed] [Google Scholar]
- 76.King KM, Feil MC, Halvorson MA. (2018). Negative Urgency is correlated with the use of reflexive and disengagement emotion regulation strategies. Clin Psychol Science 6 (6):822–834. doi: 10.1177/2167702618785619 [DOI] [Google Scholar]
- 77.Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S,Gabrieli JDE, & Gross JJ. (2004). For better or for worse: Neuralsystems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage 23: 483–499. doi: 10.1016/j.neuroimage.2004.06.030 [DOI] [PubMed] [Google Scholar]
- 78.Albein-Urios N, Verdejo-Román J, Soriano-Mas C, Asensio S, Martínez-González JM, Verdejo-García A. (2013). Cocaine users with comorbid Cluster B personality disorders show dysfunctional brain activation and connectivity in the emotional regulation networks during negative emotion maintenance and reappraisal. European Neuropsychopharmacology 23:1698–1707. doi: 10.1016/j.euroneuro.2013.04.012 [DOI] [PubMed] [Google Scholar]
- 79.Albein Urios N, Verdejo Román J, Asensio S, Soriano-Mas C, Martínez‐González JM, Verdejo-García A. (2012). Re-appraisal of negative emotions in cocaine dependence: Dysfunctional corticolimbic activation and connectivity. Addiction Biology 19:415–426. doi: 10.1111/j.1369-1600.2012.00497 [DOI] [PubMed] [Google Scholar]
- 80.Boy F, Evans CJ, Edden RA, Lawrence AD, Singh KD, Husain M, Sumner P. (2011). Dorsolateral prefrontal γ-aminobutyric acid in men predicts individual differences in rash impulsivity. Biological Psychiatry 70:866–872. doi: 10.1016/j.biopsych.2011.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, …, Brown RA. (2002). Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART). Journal of Experimental Psychology: Applied 8 (2):75–84. doi: 10.1037/1076-898X.8.2.75 [DOI] [PubMed] [Google Scholar]
- 82.Bechara A, Damasio H, Tranel D, Damasio AR. (1997). Deciding advantageously before knowing the advantageous strategy. Science 275 (5304):1293–1295. doi: 10.1126/science.275.5304.1293 [DOI] [PubMed] [Google Scholar]
- 83.Bayard S, Raffard S, Gely-Nargeot MC. (2011). Do facets of self-reported impulsivity predict decision-making under ambiguity and risk? Evidence from a community sample. Psychiatry Research 190:322–326. doi: 10.1016/j.psychres.2011.06.013 [DOI] [PubMed] [Google Scholar]
- 84.Stojek MM, Fischer S, Murphy CM, MacKillop J. (2014). The role of impulsivity traits and delayed reward discounting in dysregulated eating and drinking among heavy drinkers. Appetite 80:81–88. doi: 10.1016/j.appet.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.VanderBroek-Stice L, Stojek MK, Beach SR, MacKillop J. (2017). Multidimensional assessment of impulsivity in relation to obesity and food addiction. Appetite 112:59–68. doi: 10.1016/j.appet.2017.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Steward T, Mestre-Bach G, Fernández-Aranda F, Granero R, Perales JC, Navas JF, …, Menchón JM. (2017). Delay discounting and impulsivity traits in young and older gambling disorder patients. Addict Behavior 71:96–103. doi: 10.1016/j.addbeh.2017.03.001 [DOI] [PubMed] [Google Scholar]
- 87.Harden KP, Kretsch N, Mann FD, Herzhoff K, Tackett JL, Steinberg L, Tucker-Drob EM. (2017). Beyond dual systems: A genetically-informed, latent factor model of behavioral and self-report measures related to adolescent risk-taking. Dev Cogn Neuroscience 25:221–234. doi: 10.1016/j.dcn.2016.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Johnson SL, Tharp JA, Peckham AD, Sanchez AH, Carver CS. (2016). Positive urgency is related to difficulty inhibiting prepotent responses. Emotion 16:750–759. doi: 10.1037/emo0000/182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.MacLean RR, Wilson SJ, Pincus AL, Smyth JM, Geier CF. (2017). Extending the Balloon Analogue Risk Task to assess naturalistic risk taking via a mobile platform. J Psychopathol Behav Assess 40:107–116. doi: 10.1007/s10862-017-9628-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.MacKillop J, Weafer J, Gray J, Oshri A, Palmer A, Wit H. (2016). The latent structure of impulsivity: Impulsive choice, impulsive action, and impulsive personality traits. Psychopharmacology 233:3361–3370. doi: 10.1007/s00213-016-4372-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.MacKillop J, Miller JD, Fortune E, Maples J, Lance CE, Campbell WK, Goodie AS. (2014). Multidimensional examination of impulsivity in relation to disordered gambling. Exp Clin Psychopharmacol 22:176–185. doi: 10.1037/a0035874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Smith BJ, Xue F, Droutman V, Barkley-Levenson E, Melrose AJ, Miller LC, …, Godoy CG. (2017). Virtually ‘in the heat of the moment’: Insula activation in safe sex negotiation among risky men. Social Cognitive and Affective Neuroscience 13 (1):80–91. doi: 10.1093/scan/nsx137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xiao L, Bechara A, Gong Q, Huang X, Li X, Xue G, …, Jia Y. (2013). Abnormal affective decision making revealed in adolescent binge drinkers using a functional magnetic resonance imaging study. Psychology of Addictive Behaviors 27 (2):443–454. doi: 10.1037/a0027892 [DOI] [PubMed] [Google Scholar]
- 94.Xue G, Lu Z, Levin IP, Bechara A. (2010). The impact of prior risk experiences on subsequent risky decision-making: the role of the insula. Neuroimage 50:709–716. doi: 10.1016/j.neuroimage.2009.12.097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Clark L, Studer B, Bruss J, Tranel D, & Bechara A. (2014). Damage to insula abolishes cognitive distortions during simulated gambling. Proceedings of the National Academy of Sciences 111: 6098–6103. doi: 10.1073/pnas.1322295111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bari A, Robbins TW. (2013). Inhibition and impulsilvity: Behavioral and neural basis of response control. Prog Neurobiol 108: 44–79. doi: 10.1016/j.pneurobio.2013.06.005 [DOI] [PubMed] [Google Scholar]
- 97.Carver CS, Johnson SL, Joormann J. (2008). Serotonergic function, two-mode models of self-regulation, and vulnerability to depression: What depression has in common with impulsive aggression. Psychol Bulletin. 134:912–943. doi: 10.1037/a0013740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.MacDonald KB. (2008). Effortful control, explicit processing, and the regulation of human evolved predispositions. Psychol Review 15 (4):1012–31. doi: 10.1037/a0013327 [DOI] [PubMed] [Google Scholar]
- 99.Friedman NP, Miyake A. (2017). Unity and diversity of executive functions: Individual differences as a window on cognitive structure. Cortex. 86:186–204. doi: 10.1016/j.cortex.2016.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Birnbaum S, Gobeske KT, Auerbach J, Taylor JR, Arnsten AF. (1999). A role for norepinephrine in stress-induced cognitive deficits: alpha-1-adrenoceptor mediation in the prefrontal cortex. Biol Psychiatry 46 (9):1266–1274. doi: 10.1016/S0006-3223(99)00138-9 [DOI] [PubMed] [Google Scholar]
- 101.Shields GS, Sazma MA, Yonelinas AP. (2016). The effects of acute stress on core executive functions: A meta-analysis and comparison with cortisol. Neurosci Biobehav Rev 68:651–668. doi: 10.1016/j.neubiorev.2016.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Alexander JK, Hillier A, Smith RM, Tivarus ME, Beversdorf DQ. (2007). Beta-adrenergic modulation of cognitive flexibility during stress. J Cogn Neurosci 19 (3):468–478. doi: 10.1162/jocn.2007.19.3.468 [DOI] [PubMed] [Google Scholar]
- 103.Arnsten AFT. (2015). Stress weakens prefrontal networks: molecular insults to higher cognition. Nat Neurosci 18 (10):1376–1385. doi: 10.1038/nn.4087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Arnsten AFT. (2009). Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 10 (6):410–422. doi: 10.1038/nrn2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Barkley-Levenson E, Xue F, Droutman V, Miller LC, Smith BJ, Jeong D, …, Read SJ. (2018). Prefrontal cortical activity during the Stroop Task: New insights into the why and the who of real-world risky sexual behavior. Annals of Behavioral Medicine 52:367–379. doi. 10.1093/abm/kax019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chester DS, Lynam DR, Milich R, Powell DK, Andersen AH, DeWall CN. (2016). How do negative emotions impair self-control? A neural model of Negative urgency. NeuroImage 132:43–50. doi: 10.1016/j.neuroimage.2016.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tervo-Clemmens B, Quach A, Luna B, Foran W, Chung T, De Bellis MD, Clark DB. (2017). Neural correlates of rewarded response inhibition in youth at risk for problematic alcohol use. Frontiers in Behavioral Neuroscience 11:205. doi: 10.3389/fnbeh.2017.00205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wilbertz T, Deserno L, Horstmann A, Neumann J, Villringer A, Heinze HJ, …, Schlagenhauf F. (2014). Response inhibition and its relation to multidimensional impulsivity. Neuroimage 103:241–248. doi: 10.1016/j.neuroimage.2014.09.021 [DOI] [PubMed] [Google Scholar]
- 109.Wang H, Wen B, Cheng J, Li H. (2017). Brain structural differences between normal and obese adults and their links with lack of Perseverance, Negative urgency, and Sensation seeking. Scientific Reports 7:40595. doi: 10.1038/srep40595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ruiz de Lara CM, Navas JF, Soriano-Mas C, Sescousse G, Perales JC. (2018). Regional grey matter volume correlates of gambling disorder, gambling-related cognitive distortions, and emotion-driven impulsivity. International Gambling Studies 18 (2):195–216. doi: 10.1080/14459795.2018.1448427 [DOI] [Google Scholar]
- 111.Cole MW, Bassett DS, Power JD, Braver TS, Petersen SE. (2014). Intrinsic and task-evoked network architectures of the human brain. Neuron 83:238–251. doi: 10.1016/j.neuron.2014.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, …, Gur RE. (2012). Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage 60 (1):623–632. doi: 10.1016/j.neuroimage.2011.12.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Van Dijk KR, Sabuncu MR, Buckner RL. (2012). The influence of head motion on intrinsic functional connectivity MRI. Neuroimage 59 (1), 431–438. doi: 10.1016/j.neuroimage.2011.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. (2014). Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage 84:320–341. doi: 10.1016/j.neuroimage.2013.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Woo CW, Krishnan A, & Wager TD. (2014). Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage 91: 412–419. doi: 10.1016/j.neuroimage.2013.12.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Eklund A, Nichols TE, & Knutsson H. (2016). Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences 113: 7900–7905. doi: 10.1073/pnas.1602413113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhu X, Cortes CR, Mathur K, Tomasi D, Momenan R. (2017). Model‐free functional connectivity and impulsivity correlates of alcohol dependence: a resting‐state study. Addiction Biology 22:206–217. doi: 10.1111/adb.12272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Golchert J, Smallwood J, Jefferies E, Liem F, Huntenburg JM, Falkiewicz M, …, Margulies DS. (2017). In need of constraint: Understanding the role of the cingulate cortex in the impulsive mind. NeuroImage 146:804–813. doi: 10.1016/j.neuroimage.2016.10.041 [DOI] [PubMed] [Google Scholar]
- 120.Zhao J, Tomasi D, Wiers CE, Shokri-Kojori E, Demiral ŞB, Zhang Y, …, Wang GJ. (2017). Correlation between traits of emotion-based impulsivity and intrinsic default-mode network activity. Neural Plasticity 9297621. doi: 10.1155/2017/9297621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Um M, Hummer TA, Cyders MA. (2019). Relationship of Negative urgency to cingulo-insular and cortico-striatal resting state functional connectivity in tobacco use. Brain Imaging and Behavior 1–12. doi: 10.1007/s11682-019-00136-1 (E-publication ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zapolski TCB, Settles RE, Cyders MA, Smith GT. (2010). Borderline personality disorder, bulimia nervosa, antisocial personality disorder, ADHD, substance use: Common threads, common treatment needs, and the nature of impulsivity. Indep Pract. 30 (1):20–23. [PMC free article] [PubMed] [Google Scholar]
- 123.Johnson SL, Zisser MR, Sandel DB, Fernandez E, Carver CS. (2019). Tendencies to respond to emotion states in an impulsive manner: What tools might work? Adv Cogn Ther. Published online. [Google Scholar]
- 124.Hershberger AR, Um M, Cyders MA. (2017). The relationship between the UPPS P impulsive personality traits and substance use psychotherapy outcomes: A meta-analysis. Drug Alcohol Depend 178:408–416. doi: 10.1016/j.drugalcdep.2017.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Churchill S, Jessop DC. (2011). Too impulsive for implementation intentions? Evidence that impulsivity moderates the effectiveness of an implementation intention intervention. Psychol Heal 26 (5):517–530. doi: 10.1080/08870441003611536 [DOI] [PubMed] [Google Scholar]
- 126.Um M, Hershberger AR, Whitt ZT, Cyders MA. (2018). Recommendations for applying a multi-dimensional model of impulsive personality to diagnosis and treatment. Borderline Personal Disord Emot Dysregulation 5:6. doi: 10.1186/s40479-018-0084-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zapolski TCB, Smith GT. (2017). Pilot study: Implementing a brief DBT skills program in schools to reduce health risk behaviors among early adolescents. J Sch Nursing 33 (3):198–204. doi: 10.1177/1059840516673188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Weiss NH, Tull MT, Davis LT, Searcy J, Williams I, Gratz KL. (2015). A preliminary experimental investigation of emotion dysregulation and impulsivity in risky behaviours. Behav Change 32:127–142. doi: 10.1017/bec.2015.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Peckham AD, Johnson SL. (2018). Cognitive control training for emotion-related impulsivity. Behav Res Ther 105:17–26. doi: 10.1016/j.brat.2018.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gollwitzer P (2006). Implementation intentions and goal achievement: A meta analysis of effects and processes. Advances in Experimental Social Psychology 38:69–119. doi: 10.1016/S0065-2601(06)38002-1 [DOI] [Google Scholar]
- 131.Johnson SL, Zisser M, Sandel D, et al. (2019). Emotion-related impulsivity and aggression: An online intervention. Unpublished manuscript. [Google Scholar]
- 132.Ochsner KN, Silvers JA, Buhle JT. (2012). Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences 1251 (1):1–24. doi: 10.1111/j.1749-6632.2012.06751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bechara A, Damasio AR, Damasio H, & Anderson SW. (1994). Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 50(1–3), 7–15. doi: 10.1016/0010-0277(94)90018-3 [DOI] [PubMed] [Google Scholar]
- 134.Cole MW, Repovš G, & Anticevic A (2014). The frontoparietal control system: A central role in mental health. The Neuroscientist 20: 652–664. doi: 10.1177/1073858414525995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wager TD, Sylvester CYC, Lacey SC, Nee DE, Franklin ME, Jonides J (2005). Common and unique components of response inhibition revealed by fMRI NeuroImage 27: 323–340. [DOI] [PubMed] [Google Scholar]
- 136.Enkavi AZ, Eisenberg IW, Bissett PG, Mazza GL, MacKinnon DP, Marsch LA, Poldrack RA. (2019). Large-scale analysis of test-retest reliabilities of self-regulation measures. Proc Natl Acad Sci USA 116 (12):5472–5477. doi: 10.1073/pnas.1818430116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Weafer J, Baggott MJ, De Wit H. (2013). Test-retest reliability of behavioral measures of impulsive choice, impulsive action, and inattention. Exp Clin Psychopharmacology 21 (6):475–81. doi: 10.1037/a0033659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Termenon M, Jaillard A, Delon-Martin C, & Achard S. (2016). Reliability of graph analysis of resting state fMRI using test-retest dataset from the Human Connectome Project. Neuroimage 142: 172–187. doi: 10.1016/j.neuroimage.2016.05.062 [DOI] [PubMed] [Google Scholar]
- 139.Cooper SR, Jackson JJ, Barch DM, & Braver TS. (2019). Neuroimaging of individual differences: A latent variable modeling perspective. Neuroscience & Biobehavioral Reviews 98: 29–46. doi: 10.1016/j.neubiorev.2018.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Moreno-López L, Catena A, Fernández-Serrano MJ, Delgado-Rico E, Stamatakis EA, Pérez-García M, Verdejo-García A. (2012). Trait impulsivity and prefrontal gray matter reductions in cocaine dependent individuals. Drug and Alcohol Dependence 125 (3):208–214. doi: 10.1016/j.drugalcdep.2012.02.012 [DOI] [PubMed] [Google Scholar]
- 141.Albein-Urios N, Martinez-Gonzalez JM, Lozano Ó, Moreno-López L, Soriano-Mas C, Verdejo-Garcia A. (2013). Negative urgency, disinhibition and reduced temporal pole gray matter characterize the comorbidity of cocaine dependence and personality disorders. Drug and Alcohol Dependence 132 (1–2):231–237. doi: 10.1016/j.drugalcdep.2013.02.008 [DOI] [PubMed] [Google Scholar]
- 142.Pessoa L (2009). How do emotion and motivation direct executive control?. Trends in Cognitive Sciences 13 (4):160–166. doi: 10.1016/j.tics.2009.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.LeDoux J (2007). The amygdala. Current Biology 17:868–874. doi: 10.1016/j.cub.2007.08.005 [DOI] [PubMed] [Google Scholar]
- 144.Fliessbach K, Weber B, Trautner P, Dohmen T, Sunde U, Elger CE, Falk A. (2007). Social comparison affects reward-related brain activity in the human ventral striatum. Science 318 (5854):1305–1308. doi: 10.1126/science.1145876 [DOI] [PubMed] [Google Scholar]
- 145.MacDonald AW, Cohen JD, Stenger VA, & Carter CS. (2000). Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288(5472), 1835–1838. doi: 10.1126/science.288.5472.1835 [DOI] [PubMed] [Google Scholar]
- 146.Petrides M (2005). Lateral prefrontal cortex: architectonic and functional organization. Philosophical Transactions of the Royal Society B: Biological Sciences 360(1456), 781–795. doi: 10.1098/rstb.2005.1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shenhav A, Botvinick MM, Cohen JD. (2013). The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron 79 (2):217–240. doi: 10.1016/j.neuron.2013.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]