Abstract
Background:
From 2003–2015, only 1 biologic was approved for treatment of moderate-severe asthma in the United States. Since 2015, 4 new asthma biologics were approved by the United States Food and Drug Administration.
Objective:
To describe trends and disparities of asthma biologic use in the United States from 2003–2018
Methods:
We conducted a retrospective analysis using a cohort developed from the OptumLabs Data Warehouse. Prevalent and incident asthma biologic users were identified, and characteristics of users and non-users were analyzed using regression analysis. Clinician prescribing behavior was described.
Results:
Use of biologic medications remains uncommon among individuals with asthma, with prevalence peaking in 2006 at 3 in 1000 individuals with asthma. Several factors are associated with a higher likelihood of asthma biologic use: middle age, higher income, commercial insurance, and access to a specialist. Most clinicians (65%)in the cohort prescribed only 1 biologic.
Conclusions:
We report low overall use of asthma biologics and evidence of disparities in access to asthma biologics.
Keywords: asthma, anti-asthma agents, biological products, asthma biologics, trends, disparities, utilization, adherence, epidemiology
INTRODUCTION
Asthma affects 8–9% of the US population, disproportionately affecting people with lower income (1). The impact of day-to-day symptoms, the frequency and severity of disease exacerbations, and objective measures of lung function are used to classify asthma into mild, moderate, or severe cases (2). A new class of drugs, asthma biologics, has emerged as a treatment option for patients with moderate-severe asthma. Multiple clinical trials have shown that asthma biologics are effective for reducing asthma exacerbation rates and the need to use systemic corticosteroids. Currently, asthma biologics cost 30,000 US Dollars or more annually. (3) Prior work has shown evidence of suboptimal patient selection (i.e. patients with mild disease or non-Th2 endotypes), with overall very low use of asthma biologics and evidence suggesting many patients did not have an adequate trial of other treatments before starting asthma biologics (4). From 2003–2015, only 1 biologic was approved for treatment of moderate-severe asthma. Since 2015, 4 new asthma biologics were approved by the Food and Drug Administration (FDA). Little is known about the trends of asthma biologic use, the potential barriers to their use, and the prescribing pattern of clinicians. The purpose of this study is to describe trends and disparities of asthma biologic use in the United States from 2003–2018.
METHODS
Data.
We conducted a retrospective analysis using a cohort developed from administrative data from the OptumLabs Data Warehouse (OLDW), a database that includes over 110 million commercially insured and Medicare Advantage enrollees in the United States (5). The Mayo Clinic Institutional Review Board exempted this study from review because the study used preexisting, de-identified data.
Asthma Population.
We identified all individuals in the OLDW from January 2003 to June 2018 who met a modified Healthcare Effectiveness Data and Information Set (HEDIS) definition (6) for asthma (See Online Repository Text) and had at least 6 months of continuous enrollment in both medical and pharmacy coverage after meeting the definition. We identified 2.01 million unique individuals who contributed 20.49 million person-quarters to the asthma population in this study.
Prevalent Biologic Users.
From the asthma population, we identified individuals with at least one claim for an asthma biologic medication (benralizumab, dupilumab, mepolizumab, omalizumab, or reslizumab) in pharmacy or medical claims (codes in Table E1). We identified 6,641 unique individuals who contributed 44,033 person-quarters of use to the prevalent cohort.
Incident Biologic Users.
Incident use was defined as the first use by an individual with asthma of an asthma biologic medication, with no such use in the prior 6 months. With this definition, an individual may contribute multiple incident episodes in the study frame if they used more than one asthma biologic medication. We identified 4,779 individuals who contributed to the total numerator of incident users (123 individuals contributed multiple incident episodes).
Statistical Analysis.
Both prevalent and incident uses were graphed by quarter over the course of the study as a rate per 1,000 people with asthma. We conducted a logistic regression analysis on the prevalence cohort, using multiple individual characteristics [age, sex, race/ethnicity, region, income, insurance type (commercial, Medicare Advantage, and high deductible insurance plan), and use of allergy or pulmonology specialist care] as independent variables and use of an asthma biologic medication as the dependent variable. High deductible insurance plans were defined as having an individual deductible >$1,300 or family deductible >$2,600. Adjusted odds ratios with 95% confidence intervals were calculated for each independent variable.
Prescribing clinicians were identified using a unique identifier within OLDW. We assigned the prescribing clinician by looking back 90 days before the incident biologic administration. We then assigned a clinician most likely to responsible for the prescription by first selecting allergist, then pulmonologist, and then family medicine. A family medicine provider was defined in this study as family practice, family practice/clinic, family practice specialist, and internal medicine specialist. We described their prescribing patterns as the number of patients using biologics per person with asthma treated by each clinician. Reimbursement methods (medicine versus pharmacy benefit) were described as a percentage of biologic administrations. Patients with Medicare Advantage before 2006 were excluded from the reimbursement analysis given the program began January 1, 2006.
RESULTS
Individuals.
Prevalent omalizumab use peaked in Q1 of 2006 at 2.95 per 1,000 individuals with asthma. Prevalent use of dupilumab peaked in Q2 2018 at 0.07, while mepolizumab and reslizumab peaked in Q1 of 2018 at 0.64 and 0.09, respectively. Benralizumab use is not reported because the use was lower than the allowable threshold to ensure the data remain de-identifiable. Incident omalizumab use peaked in Q2 of 2004 at 0.95 per 1,000 individuals with asthma. Dupilumab incidence peaked in Q1 of 2018 at 0.03, reslizumab peaked in Q4 of 2017 at 0.03, while mepolizumab peaked in Q3 of 2017 at 0.15 incident users per 1,000 (Figure 1 A and B). Overall incidence and prevalence of asthma biologics have increased since the introduction of 4 new drugs, but remain below historic highs in 2004 and 2006, respectively.
Figure 1.
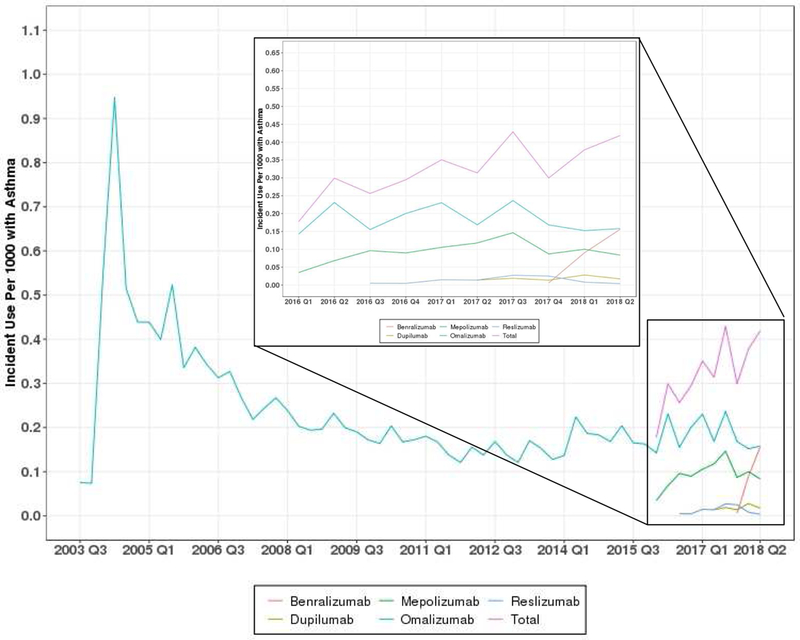
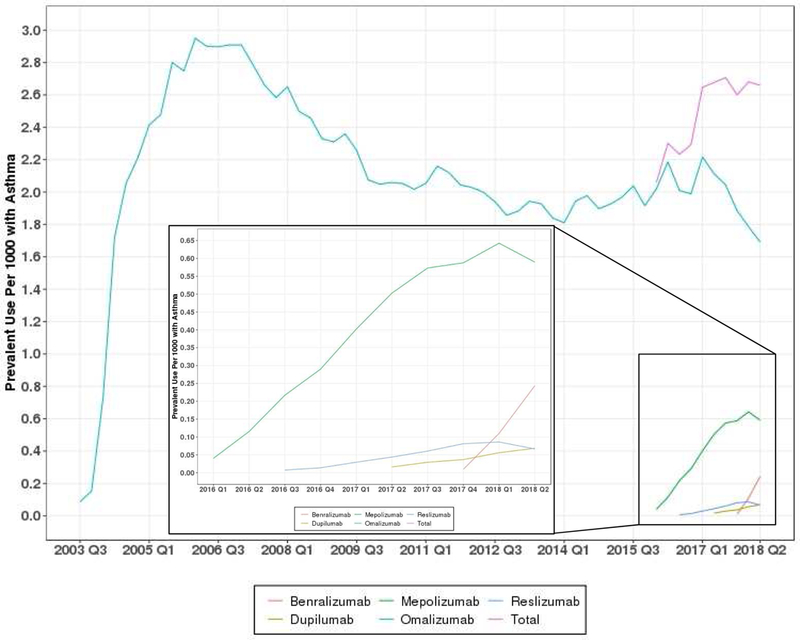
Prevalent and Incident Use of Asthma Biologics, 2003–2018
The characteristics of the individuals in the study are shown in Table 1. The majority of asthma biologic users are between 18–64 years old, female, white, living in the South and Midwest, have annual household income >$75,000 per year, and have seen a specialist. We compared individuals with asthma who received biologics to those who did not using a logistic regression analysis (Figure 2). Age (highest use for 18–64 year-olds), race/ethnicity (highest use in Asian ethnicity), annual household income (higher for >$40,000), region (highest in West), insurance type (highest in commercial), and specialist access (highest for patients who see a specialist) were all factors significantly associated with asthma biologic use.
TABLE 1.
Demographics of Study Population
| Biologic - Commercial | Biologic-Medicare | No Biologic - Commercial | No Biologic - Medicare | |
|---|---|---|---|---|
| Number of individuals | N=5,544 | N=746 | N=1,570,372 | N=431,058 |
| Age, Mean (SD) | 41.4 (15.0) | 65.5 (10.4) | 31.4 (20.9) | 70.2 (10.2) |
| Age Group | ||||
| 0–5 | 68 (1.23%) | <11 | 223,671 (14.24%) | 113 (0.03%) |
| 6–11 | 185 (3.34%) | <11 | 197,897 (12.60%) | 14 (0.00%) |
| 12–17 | 306 (5.52%) | <11 | 140,186 (8.93%) | 0 (0.00%) |
| 18–64 | 4,883 (88.08%) | > 239 | 965,204 (61.46%) | 89,839 (20.84%) |
| 65+ | 102 (1.84%) | 502 (67.29%) | 43,414 (2.76%) | 341,092 (79.13%) |
| Sex | ||||
| Missing | 0 (0.00%) | 0 (0.00%) | 89 (0.01%) | 0 (0.00%) |
| Female | 3,380 (60.97%) | 492 (65.95%) | 841,398 (53.58%) | 273,711 (63.50%) |
| Male | 2,164 (39.03%) | 254 (34.05%) | 728,885 (46.41%) | 157,347 (36.50%) |
| Race | ||||
| Missing | 267 (4.82%) | 29 (3.89%) | 93,465 (5.95%) | 21,943 (5.09%) |
| Asian | 177 (3.19%) | 22 (2.95%) | 51,670 (3.29%) | 9,057 (2.10%) |
| Black | 416 (7.50%) | 116 (15.55%) | 123,040 (7.84%) | 66,799 (15.50%) |
| Hispanic | 440 (7.94%) | 74 (9.92%) | 141,746 (9.03%) | 36,730 (8.52%) |
| White | 4,244 (76.55%) | 505 (67.69%) | 1,160,451 (73.90%) | 296,529 (68.79%) |
| Region | ||||
| Missing | 11 (0.20%) | 0 (0.00%) | 3,233 (0.21%) | 394 (0.09%) |
| Midwest | 1,310 (23.63%) | 201 (26.94%) | 436,568 (27.80%) | 119,569 (27.74%) |
| Northeast | 544 (9.81%) | 92 (12.33%) | 174,029 (11.08%) | 71,407 (16.57%) |
| South | 2,697 (48.65%) | 379 (50.80%) | 707,981 (45.08%) | 200,351 (46.48%) |
| West | 982 (17.71%) | 74 (9.92%) | 248,561 (15.83%) | 39,337 (9.13%) |
| Income | ||||
| Missing | 1,124 (20.27%) | 61 (8.18%) | 423,682 (26.98%) | 65,982 (15.31%) |
| <$40,000 | 536 (9.67%) | 271 (36.33%) | 154,335 (9.83%) | 180,716 (41.92%) |
| $40,000-$74,999 | 1,045 (18.85%) | 217 (29.09%) | 279,881 (17.82%) | 106,897 (24.80%) |
| $75,000–124,999 | 1,348 (24.31%) | 142 (19.03%) | 339,711 (21.63%) | 57,223 (13.28%) |
| $125,000-$199,999 | 857 (15.46%) | 39 (5.23%) | 209,040 (13.31%) | 14,971 (3.47%) |
| $200,000+ | 634 (11.44%) | 16 (2.14%) | 163,723 (10.43%) | 5,269 (1.22%) |
| Specialist Access | ||||
| No | 212 (3.82%) | 28 (3.75%) | 874,331 (55.68%) | 190,092 (44.10%) |
| Yes | 5,332 (96.18%) | 718 (96.25%) | 696,041 (44.32%) | 240,966 (55.90%) |
NOTE: Excludes 218 people with missing insurance info
Figure 2.
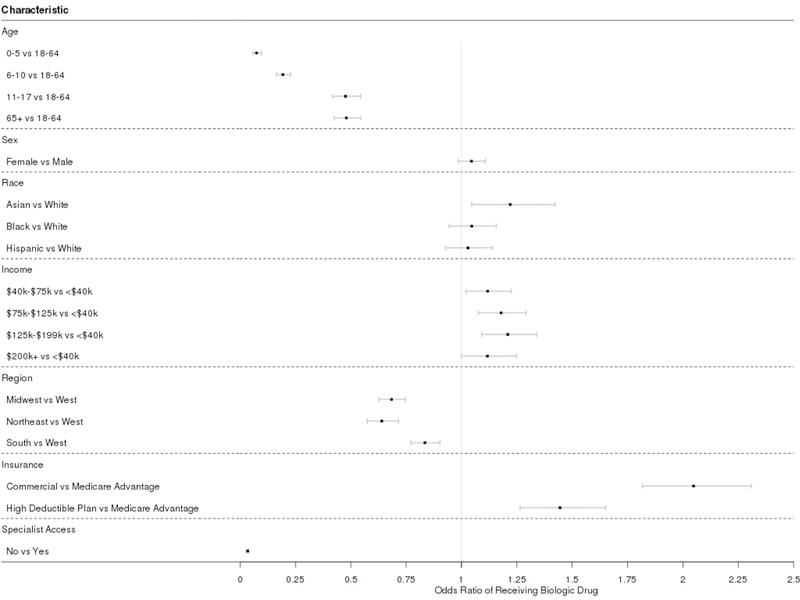
Odds ratios (adjusted) indicating likelihood of a given group being a biologic user
Clinicians:
We were able to match 4,327 of the 4,779 incident biologic prescriptions to a unique provider. This included a total of 2,358 providers, of whom 56% are allergists, 35% are pulmonologists, and the remaining 9% are defined family practice providers in this study. There were 1.84 incident administrations per clinician among clinicians who prescribed 1 or more asthma biologic. Nearly two-thirds (65%) of the providers in our cohort had only 1 incident biologic prescription during the study period. A small proportion of clinicians (9.5%; n=225 of 2,358 clinicians prescribing any biologic) prescribed 29.5% (n=1,412) of the 4,779 incident biologic uses.
Reimbursement method:
The majority of administrations were paid for through the medical benefit, but in 9.1% of asthma biologic administrations, the medication was paid for through the pharmacy benefit, suggesting patients purchased the drug separately and brought it to their physician’s office for administration. This strategy has become more common for omalizumab from 2009 (4.1%) to 2017 (15.9%) and is more common among those with Medicare Advantage (27.0%) than either those with high deductible plans (5.9%) and other commercial insurance (7.4%).
DISCUSSION
Analysis of asthma biologic use from 2003–2018 reveals an increase in overall asthma biologic use after 2015 compared to 2009–2014. However, asthma biologic use after 2015 remains below historic highs in 2004–2006. This study expands upon the previous finding of low overall omalizumab use by finding similar low utilization rates for the 4 more recently introduced asthma biologics (4). The percentage of individuals with severe asthma who would be eligible to use asthma biologics (7,8) is likely to be several times higher than the use rates we identified in this study. This suggests that asthma biologics are not being used by many individuals who have frequent exacerbations and would benefit by having them reduced.
We describe potential disparities in access to asthma biologic drugs by age, gender, race/ethnicity, income, insurance type, and specialty clinician access. We identified higher annual income as a significant factor in patients’ likelihood of using asthma biologics. These findings could be explained by the high prices for asthma biologics, and, given the strong association between specialist access and use of biologics, a concentration of specialists in wealthier geographic locations. A December 2018 report from the Institute for Clinical and Economic Review (ICER) (3) found that current prices would need to be reduced substantially to properly align the benefits of these drugs with their cost. Future efforts to reduce barriers to accessing asthma biologics must take drug cost into account.
Individuals in our sample were much more likely to receive asthma biologics if they were seen by a specialist. The administrative expertise to purchase and administer asthma biologics is considerable (9). Like chemotherapy and other expensive specialty drugs, biologics can be challenging for small-to medium-sized physician practices to provide. The most common model for reimbursement is “buy and bill,” where the physician purchases the drug directly, then submits a claim to the patient’s medical benefit part of their health insurance after administering (10). The high cost of acquiring and storing drugs, and the risk that some may expire before being used, can prevent smaller practices from providing these drugs to their patients. The vast majority of patients receive these drugs in a physician’s office (currently, only dupilumab can be self-administered by patients). The implications of a shift to home delivery of asthma biologics for a clinician include (1) a different challenge to measure adherence to the treatments (2) a need to develop follow-up assessments to determine treatment effectiveness and (3) financial implications for the work that clinicians do to prescribe and monitor patients on biologics.
Unlike oncology practices, where a substantial proportion of revenue may come from buy and bill drugs, most physicians treating people with asthma may lack the personnel, facilities, and knowledge to provide biologics. “White-bagging” programs where the drugs are paid for through the pharmacy benefit and delivered to physician offices just in time for treatment may allow more access to biologics for patients seen in smaller practices (11,12). In our sample, we found that over the entire study period, about 1 out of every 11 administrations was paid for through the pharmacy benefit and may have represented either brown bagging (where the patient brings the drug) or white bagging. White and brown bagging became more common over the course of the study, nearly quadrupling from 2009 to 2017 for omalizumab. More than one-quarter of Medicare Advantage beneficiary administrations were paid for through the pharmacy benefit, which is substantially higher than for any other insurance type. This suggests that medical providers, insurers, or pharmacies may preferentially steer patients with Medicare Advantage to white or brown bagging programs based on various and potentially competing financial interests. However, brown/white-bagging may not be a good solution for improving patient access to asthma biologics. Brown bagging may not be as safe as white-bagging or buy and bill, as the proper handling of the medicine cannot be ensured. Physicians may not be able to recoup the costs of administering biologics if their only reimbursement is the small payment for administration of an injected medication. Those costs could include working with the patient’s insurance company to obtain and renew prior authorization for the treatment every 6–12 months, coordinating delivery of the drug with patient scheduling, monitoring the patient after treatment, and so on. Furthermore, shifting drug reimbursement from the patient’s medical benefit to the pharmacy benefit may substantially affect patient out-of-pocket costs, exacerbating the affordability issue. For example, patients with Medicare Part D coverage may face the coverage gap (“donut hole”), making them responsible for 25% of brand name or 37% of generic drug costs when paid through the pharmacy benefit, vs. the 20% coinsurance they would pay for treatment through the medical benefit.
Another key aspect of delivering asthma biologics to individuals in the US is the rules set for drug coverage by payers. All 5 asthma biologics are subject to medical necessity review, with reauthorization required every 6–12 months. Prior authorization procedures represent a substantial burden for physicians, with 86% of physician practices surveyed by the AMA reporting a high or extremely high burden for staff and physicians and 91% reporting a significant negative impact of the prior authorization process on clinical outcomes. (14) Attention to the impact of payer coverage policies and how they are implemented are also likely to be very important for crafting more efficient ways to delivery asthma biologics to individuals who would benefit.
A survey of individuals with asthma conducted by The Asthma and Allergy Foundation of America revealed that the top two criteria for choosing asthma treatment options were effectiveness (82%) and cost (52%) (15). In addition, individuals with asthma reported a general lack of knowledge about asthma biologics. Given concerns about high cost of asthma biologics and the limited use of asthma biologics by a small concentration of specialists, it may not be surprising that we found low overall use of asthma biologics.
In summary, we report low overall use of asthma biologics, evidence of disparities in access to asthma biologics, and a small concentration of asthma biologic prescriptions among a small group of clinicians. The main policy implication of these findings is a call to reform the current system that delivers asthma biologics to people who could benefit from them. Further research is needed on asthma biologic eligibility, clinician variation in asthma biologic use, payer variation in coverage policies, and the effects of asthma biologic price.
Supplementary Material
Highlights Box:
What is already known about this topic?
Asthma biologics are used infrequently in the US population.
What does this article add to our knowledge?
There are barriers to accessing asthma biologics that need to be recognized.
How does this study impact current management guidelines?
Clinicians may need additional logistical support to deliver asthma biologics to patients as recommended by guidelines.
Acknowledgments
Funding: National Institutes of Health, National Heart, Lung, and Blood Institute (NIH R21 HL140287) and the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery.
Abbreviations:
- (FDA)
Food and Drug Administration
- (HEDIS)
Healthcare Effectiveness Data and Information Set
- (OLDW)
OptumLabs Data Warehouse
- (US)
United States
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: No authors have any disclosures to make.
REFERENCES
- 1.Jones K. Asthma and Injustice on Chicago’s Southeast Side. Health Aff (Millwood) 2016;35(5):928–31. [DOI] [PubMed] [Google Scholar]
- 2.Global Initiative for Asthma. https://ginasthma.org/ Accessed March 25, 2019.
- 3.Biologic Therapies for Treatment of Asthma Associated with Type 2 Inflammation: Effectiveness, Value, and Value-Based Price Benchmarks https://icer-review.org/wp-content/uploads/2018/04/ICER_Asthma_Final_Report_122018-1.pdf Accessed March 26, 2019.
- 4.Jeffery MM, Shah ND, Karaca-Mandic P, Ross JS, Rank MA. Trends in omalizumab utilization for asthma: Evidence of suboptimal patient selection. J Allergy Clin Immunol Pract 2018;6(5):1568–1577. [DOI] [PubMed] [Google Scholar]
- 5.Wallace PJ, Shah ND, Dennen T, Bleicher PA, Crown WH. Optum Labs: building a novel node in the learning health system. Health Aff (Millwood) 2014;33:1187–94. [DOI] [PubMed] [Google Scholar]
- 6.Schatz M, Zeiger RS, Yang SJ, Chen W, Crawford WW, Saigian SG, Allen-Ramey F. Persistent asthma defined using HEDIS versus survey criteria. Am J Manag Care 2010;16(11):e281–8. [PubMed] [Google Scholar]
- 7.Albers FC, Mullerova H, Gunsoy NB, Shin SY, Nelsen LM, Bradford ES, et al. Biologic treatment eligibility for real-world patients with severe asthma: The IDEAL study. J Asthma 2018;55(2):152–160. [DOI] [PubMed] [Google Scholar]
- 8.Aubier M, Thabut G, Fabry-Vendrand C. Characteristics of patients with severe, uncontrolled, eosinophilic asthma enrolled in a French cohort. J Asthma Allergy 2018;11:217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. [March 26, 2019];Biologics buy and bill – buyer beware. https://college.acaai.org/advocacy/advocacy-insider/biologics-buy-and-bill-buyer-beware Accessed.
- 10.Polite B, Conti RM, Ward JC. Reform of the Buy-and-Bill System for Outpatient Chemotherapy Care Is Inevitable: Perspectives from an Economist, a Realpolitik, and an Oncologist. Am Soc Clin Oncol Educ Book 2015:e75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Medical Association Council on Medical Service. 2016”Medication ‘Brown Bagging’ (Resolution 827-i-15). CMS Report 10-A-16 https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/about-ama/councils/Council%20Reports/council-on-medical-service/a16-cms-report10.pdf
- 12.Polite BN, Ward JC, Cox JV, Morton RF, Hennessy J, Page RD, Conti RM. Payment for oncolytics in the United States: a history of buy and bill and proposals for reform. J Oncol Pract 2014. November;10(6):357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. [March 26, 2019]; https://www.uhcprovider.com/content/dam/provider/docs/public/policies/comm-medical-drug/respiratory-interleukins.pdf and https://www.uhcprovider.com/content/dam/provider/docs/public/policies/comm-medical-drug/xolair-omalizumab.pdf Accessed.
- 14.American Medical Association. 2018 AMA Prior Authorization (PA) Physician Survey 2019. Available at https://www.ama-assn.org/system/files/2019-02/prior-auth-2018.pdf; accessed May 20, 2019
- 15.My Life with Asthma in 2017. https://www.aafa.org/media/1684/my-life-with-asthma-in-2017-survey-findings-report.pdf Accessed March 26, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


