Abstract
Background
Respiratory syncytial virus (RSV)- and rhinovirus (RV)-induced bronchiolitis are associated with an increased risk of asthma, but more detailed information is needed on virus types.
Objective
To study whether RSV or RV types are differentially associated with the future use of asthma control medication.
Methods
Over 2 consecutive winter seasons (2008-2010), we enrolled 408 children hospitalized for bronchiolitis at age less than 24 months into a prospective, 3-center, 4-year follow-up study in Finland. Virus detection was performed by real-time reverse transcription PCR from nasal wash samples. Four years later, we examined current use of asthma control medication.
Results
A total of 349 (86%) children completed the 4-year follow-up. At study entry, the median age was 7.5 months, and 42% had RSV, 29% RV, 2% both RSV and RV, and 27% non-RSV/-RV etiology. The children with RV-A (adjusted hazard ratio, 2.3; P = .01), RV-C (adjusted hazard ratio, 3.5; P < .001), and non-RSV/-RV (adjusted hazard ratio, 2.0; P = .004) bronchiolitis started the asthma control medication earlier than did children with RSV bronchiolitis. Four years later, 27% of patients used asthma control medication; both RV-A (adjusted odds ratio, 3.0; P = .03) and RV-C (adjusted odds ratio, 3.7; P < .001) etiology were associated with the current use of asthma medication. The highest risk was found among patients with RV-C, atopic dermatitis, and fever (adjusted odds ratio, 5.0; P = .03).
Conclusions
Severe bronchiolitis caused by RV-A and RV-C was associated with earlier initiation and prolonged use of asthma control medication. The risk was especially high when bronchiolitis was associated with RV-C, atopic dermatitis, and fever.
Key words: Asthma development, Bronchiolitis, Respiratory syncytial virus, Rhinovirus, Wheeze, Wheezing
Abbreviations used: aHR, Adjusted hazard ratio; aOR, Adjusted odds ratio; HR, Hazard ratio; OR, Odds ratio; RSV, Respiratory syncytial virus; RV, Rhinovirus
What is already known about this topic? Respiratory syncytial virus (RSV)- and rhinovirus (RV)-induced bronchiolitis are associated with an increased risk of asthma.
What does this article add to our knowledge? Compared with children with RSV-induced bronchiolitis, children with RV-A– or RV-C–induced bronchiolitis start asthma control medication earlier and are more likely to use it 4 years later. The risk is especially high among patients with RV-C, atopic dermatitis, and fever.
How does this study impact current management guidelines? Secondary prevention strategies targeting rhinoviruses, especially RV-C, might help to prevent childhood asthma.
Introduction
Bronchiolitis is an infection of small airways causing breathing difficulties in young children.1 It is one of the leading reasons for hospitalization among infants without underlying illness.1, 2, 3 Severe bronchiolitis is also considered a risk factor for future asthma.4, 5 Respiratory syncytial virus (RSV) is the most frequent cause of bronchiolitis, but other viruses, especially rhinovirus (RV), are also detected.3, 6 In follow-up studies, RV-induced bronchiolitis/early wheezing illness has been highly associated with the development of asthma.7, 8, 9, 10, 11 Whether it is the RV infection itself or the immunologic responses of the child, which primarily enhance the development, is still partly unknown. Furthermore, it is unclear whether the RV types involved with bronchiolitis have different risks associated with the later development of asthma.
RVs cause 20% to 40% of bronchiolitis and 50% to 80% of wheezing episodes and asthma exacerbations in children.10, 12, 13 RV types are classified into 3 species: RV-A, RV-B, and RV-C.14 RV-A and RV-C cause more severe respiratory illness than RV-B and are associated with wheezing in early childhood,14 as well as exacerbations of asthma.15, 16, 17, 18, 19 Cadherin-related family member 3 (a receptor for RV-C) and atopy have been shown to be risk factors for the development of early-onset asthma.20 Cadherin-related family member 3 mediates RV-C binding and replication in airway epithelia, and polymorphism in gene encoding the protein has been linked with increased susceptibility to asthma.21, 22 We aimed to study whether RSV or RV types are differently associated with the use of long-term asthma control medication during the 4 years after severe bronchiolitis during infancy. Our study hypothesis was that RV-A or RV-C etiology of severe bronchiolitis is associated with increased need for the long-term asthma control medication.
Methods
Study subjects
The study subjects consisted of the 408 participants of the 30th Multicenter Airway Research Collaboration (MARC-30) Finland study, which is a prospective, multicenter cohort study of the Emergency Medicine Network (EMNet, Boston, Mass). The children were recruited from the pediatric wards or intensive care units of Kuopio, Tampere, and Turku University Hospitals in Finland during the winter seasons 2008 to 2010 (from November 1 until March 31).23 The children were hospitalized for bronchiolitis and were younger than 24 months on admission. The diagnosis of bronchiolitis was clinical and made according to the American Academy of Pediatrics guidelines including children with acute respiratory illness with some combination of rhinitis, cough, tachypnea, wheezing, crackles, and/or retractions.1 No initial exclusions were made on the basis of history of wheezing or use of asthma medication before hospitalization. In addition, we identified a subgroup of 204 infants who were younger than 12 months with no history of wheezing (strict bronchiolitis criteria).24, 25 The exclusion criteria were previous enrollment or transfer to a participating hospital more than 48 hours after the original admission time. Because all the children were hospitalized, they were defined as having a severe bronchiolitis. The Institutional Ethical Board of Turku University Hospital approved the study. The institutional ethical boards of other participating hospitals confirmed this approval. The parents or guardians gave written informed consent to the study.
Data collection
During hospitalization, the guardians were interviewed using a structured questionnaire, including demographic, environmental, and baseline medical data. Also, data on signs, symptoms, and management of the current infection were collected.23 Four to 5 years after hospitalization, study questionnaires modified from the International Study of Asthma and Allergy in Childhood questionnaires were sent to all participants. The questionnaires consisted of inquiries on respiratory symptoms and need, prescription, and use of asthma medication during the follow-up period after the index hospitalization. If the parents did not respond within 6 months, participants were contacted by telephone and interviewed. If regular use of inhaled corticosteroids or leukotriene receptor antagonists ever was reported, the patient's files (ie, hospital records or inquiries sent to private sector pediatricians) of reported medical encounters were reviewed for the exact prescription time of medication.
Nasopharyngeal wash samples
Nasopharyngeal wash samples were collected at the study entry using a standardized protocol.23, 26 The same collection equipment (Medline Industries, Mundelein, Ill) was used by all hospitals. The samples were collected within 24 hours after the child's arrival at the ward or in the intensive care unit by instilling 1 mL of normal saline into each naris and suctioned. Thereafter, the samples were immediately refrigerated and stored at −80°C within 24 hours until further analyses.
Virology testing
Viral detection was performed by using singleplex or duplex 2-step real-time PCR. Real-time reverse transcription PCR was used for the detection of RNA respiratory viruses (RSV types A and B; rhinovirus types A, B, and C; parainfluenza virus types 1, 2, and 3; influenza virus types A and B and 2009 novel H1N1; human metapneumovirus; coronaviruses NL-63, HKU1, OC43, and 229E; and enteroviruses) and real-time PCR for detection of DNA pathogens (adenovirus, Mycoplasma pneumoniae, and Bordetella pertussis). Details of the primers and probes have been described earlier.23 All PCR assays were tested in duplicate, and samples with incongruent values (1 well positive) were retested. For the analyses, children were divided into 3 groups on the basis of virology testing: RSV (including coinfection with viruses other than RV), RV (including coinfection with viruses other than RSV), and non-RSV/-RV (including coinfection with viruses other than RSV or RV).27, 28, 29, 30
Main outcomes and clinical definitions
The time to initiation of long-term asthma control (inhaled corticosteroid or leukotriene receptor antagonist) medication was defined as time from the discharge of index hospitalization until the prescription of at least a 1-month period of asthma control medication. The children with reported use of asthma control medication before index hospitalization were excluded from the analyses. The current use of long-term asthma control medication was defined as the prescription of regular asthma control medication within the previous 12 months. If use or prescription of any asthma control medication was reported in the parental interview, the patient's medical files were reviewed for the exact beginning of medication use. Atopic dermatitis was defined as a parent-reported atopic dermatitis diagnosed by a physician. No IgE or other immunologic testing was done. Fever was defined as temperature higher than 37.5°C (99.5°F) during the severe bronchiolitis episode. In the case of missing data of initiation time of medication or some of the confounders used in multivariable analyses, the child in question was excluded from that particular analysis.
Statistical methods
Unadjusted analyses for categorical data were done using Pearson χ2 test, and for continuous data using Kruskal-Wallis test. Multivariable analyses were done using binary logistic and Cox regression and the results were presented as odds ratios (ORs) and hazard ratios (HRs), respectively, with 95% confidence intervals. The potential confounding factors were first tested in unadjusted analyses. The nonviral factors were then selected by the backwards stepwise method, and variables with P less than .05 were included in the final adjusted models. The tested variables were age at index hospitalization (<12 vs ≥12 months), sex, parental history of asthma, gestational weeks at birth (<37 vs ≥37 weeks), comorbid medical disorders, history of wheezing, history of atopic dermatitis, siblings, exposure to smoking during pregnancy or early childhood, breast-feeding, length of index hospitalization greater than or equal to 3 days, use of systemic corticosteroids during index hospitalization (yes vs no), and study center (Turku, Tampere vs Kuopio). Analyses were conducted with IBM SPSS Statistics for Windows version 24.0 (IBM Corp, Armonk, NY).
Results
Study cohort
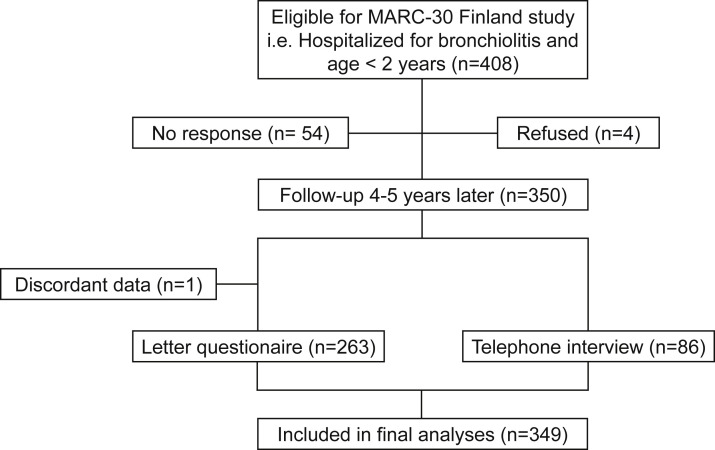
Among the 408 infants followed for 4 years after hospitalization, 264 returned the questionnaire and 86 underwent telephonic interviews (Figure 1 ). However, 1 child had discordant data; thus, taken together, 349 children (86%) had sufficient follow-up data to be included in this cohort study.
Figure 1.
Study flow chart. MARC-30, 30th Multicenter Airway Research Collaboration.
Virus etiology
At the time of hospitalization 145 (42%) patients had RSV, 101 (29%) had RV, 8 (2%) had both RSV and RV, and 95 (27%) were negative for both viruses (non-RSV/-RV) (Table I ). Of all patients, 73 (21%) had RV-C, 24 (7%) had RV-A, 3 (1%) had RV-B, and 1 (0%) had both RV-B and RV-C (Table I).
Table I.
Patient characteristics of the 349 study children at study entry
| Characteristic | No. of children (%) |
|---|---|
| Age at study entry (mo), median (range) | 7.5 (23.7) |
| Sex: male | 223 (64) |
| Parental history of asthma | 81 of 346 (23) |
| Prematurity | 46 of 343 (13) |
| Comorbid medical disorder | 40 (12) |
| History of wheezing | 128 (37) |
| History of atopic dermatitis | 103 (30) |
| Siblings | 243 (70) |
| Exposure to smoking during pregnancy or early childhood | 51 of 346 (15) |
| Breast-feeding | 321 of 348 (92) |
| Hospitalization ≥3 d | 108 (31) |
| ICU stay | 12 (3) |
| Systemic corticosteroid | 73 of 348 (21) |
| Virus positive | 302 (87) |
| RSV∗ | 145 (42) |
| RV† | 101 (29) |
| RV-A | 24 (7) |
| RV-B | 3 (1) |
| RV-C | 73 (21) |
| RV-B + RV-C | 1 (0) |
| RV + RSV‡ | 8 (2) |
| Non-RSV/-RV | 95 (27) |
| Human metapneumovirus | 17 (5) |
| Parainfluenzavirus 1 | 12 (3) |
| Parainfluenzavirus 3 | 7 (2) |
| Coronavirus | 5 (1) |
| Adenovirus | 2 (1) |
| Influenzavirus A | 3 (1) |
| H1N1-virus | 3 (1) |
| Enterovirus | 1 (0) |
| Parainfluenzavirus 2 | 1 (0) |
| Negative for tested viruses | 44 (13) |
ICU, Intensive care unit.
Including coinfection with viruses other than RV.
Including coinfection with viruses other than RSV.
Including coinfection with viruses other than RV or RSV.
Patient characteristics
The median age at study entry was 7.5 months, 64% were males, 23% had parental asthma, 13% were born preterm, 12% had some comorbid medical disorder, 37% had a history of wheezing, and 30% had a history of atopic dermatitis (Table I). The baseline characteristics were similar in the dropout group, except for including less males (48%) and more children with early childhood smoking exposure (25%).
When children were compared in relation to the virus type (Table II ), some between-group differences were seen. Children with RSV were younger and had less comorbid medical disorders, but needed longer hospitalizations and more often intensive care unit care, whereas history of wheezing or atopic dermatitis and use of systemic corticosteroids during initial hospitalization were more common among the other children. Patient characteristics of children with RV-A and RV-C were similar.
Table II.
Patient characteristics at study entry according to virus type
| Characteristic | No. of children (%) |
P value | |||
|---|---|---|---|---|---|
| RSV∗ (n = 145) | RV-A† (n = 24) | RV-C† (n = 73) | Non-RSV/-RV‡ (n = 95) | ||
| Age at study entry (mo), median (range) | 3.7 (23.2) | 13.2 (17.3) | 13.6 (23.5) | 9.8 (23.5) | <.001 |
| Sex: male | 86 (59) | 16 (67) | 46 (63) | 64 (67) | .62 |
| Parental history of asthma | 38 (27) | 3 (13) | 13 (18) | 26 (27) | .22 |
| Prematurity | 19 (14) | 4 (17) | 8 (11) | 14 (15) | .87 |
| Comorbid medical disorder | 8 (6) | 4 (17) | 14 (19) | 12 (13) | .02 |
| History of wheezing | 27 (19) | 14 (58) | 42 (58) | 40 (42) | <.001 |
| History of atopic dermatitis | 30 (21) | 10 (42) | 27 (37) | 33 (35) | .02 |
| Siblings | 67 (81) | 16 (67) | 35 (48) | 118 (71) | <.001 |
| Exposure to smoking during pregnancy or early childhood | 24 (17) | 5 (21) | 6 (9) | 15 (16) | .34 |
| Breast-feeding | 132 (92) | 23 (96) | 67 (92) | 87 (92) | .91 |
| Hospitalization ≥3 d | 60 (41) | 6 (25) | 14 (19) | 24 (25) | .003 |
| ICU stay | 11 (9) | 0 (0) | 0 (0) | 1 (1) | .02 |
| Systemic corticosteroid | 10 (7) | 8 (33) | 27 (37) | 25 (26) | <.001 |
ICU, Intensive care unit.
P values are from Pearson χ2 or Kruskal-Wallis test.
Bold indicates statistical significance (P < .05).
Including coinfection with viruses other than RV.
Including coinfection with viruses other than RSV.
Including coinfection with viruses other than RV or RSV.
Time to initiation of asthma control medication
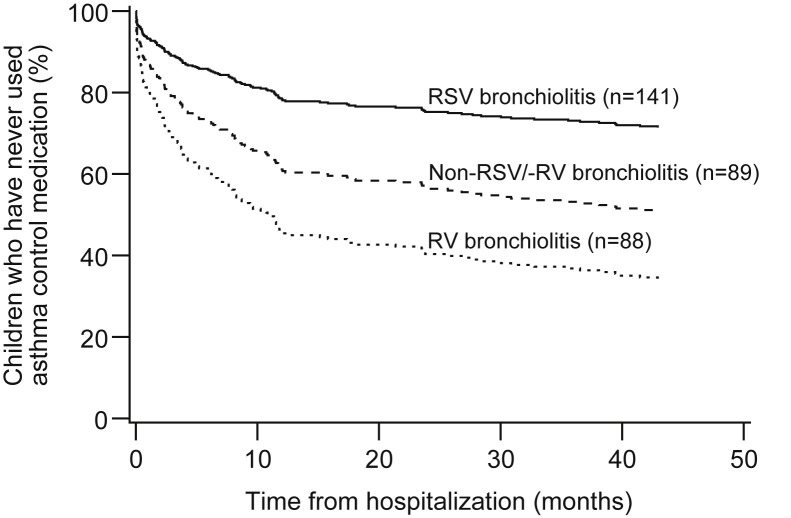
Of the 319 patients with data adequate for analyses of initiation time of medication, 145 (45%) reported prescription of asthma control medication within 48 months after hospitalization (Table III ). Asthma control medication was started a mean of 4.6 months after hospitalization in patients with RV, 6.1 months in patients with non-RSV/-RV, and 15.4 months in patients with RSV bronchiolitis (see Figure E1 in this article's Online Repository at www.jaci-inpractice.org). Patients with RV (adjusted P < .001) and non-RSV/-RV (adjusted P = .003) bronchiolitis started asthma control medication significantly earlier than did those with RSV bronchiolitis (Table III and Figure E1). When RV types were analyzed separately, asthma control medication was started significantly earlier after RV-C (3.7 months, adjusted P < .001) and RV-A bronchiolitis (8.7 months, adjusted P = .01) (Table III and Figure 2 ).
Table III.
Viral risk factors for the time to initiation of asthma control medication
| Viral risk factor | No. of children in analyses∗ (n = 319) | No. of children who have started medication† (n = 145) | Unadjusted analysis |
Multivariable analysis‡ |
||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | aHR | 95% CI | P value | |||
| RSV | 142/141 | 36 (25%/26%) | 1 | 1 | ||||
| RV | 88 | 65 (74%) | 5.14 | 3.41-7.75 | <.001 | 3.19 | 2.03-5.01 | <.001 |
| Non-RSV/-RV | 89 | 44 (49%) | 2.51 | 1.62-3.90 | <.001 | 2.02 | 1.27-3.20 | .003 |
| RSV | 142/141 | 36 (25%/26%) | 1 | 1 | ||||
| RV-A | 19 | 14 (74%) | 4.16 | 2.24-7.74 | <.001 | 2.30 | 1.19-4.44 | .01 |
| RV-C | 65 | 48 (74%) | 5.45 | 3.52-8.43 | <.001 | 3.48 | 2.17-5.59 | <.001 |
| Non-RSV/-RV | 89 | 44 (49%) | 2.52 | 1.62-3.92 | <.001 | 1.99 | 1.25-3.17 | .004 |
CI, Confidence interval.
HRs and 95% CIs are from Cox regression.
Bold indicates statistical significance (P < .05).
Numbers apply to both analyses, unless 2 figures are presented, whereupon they apply to unadjusted and multivariable analysis, respectively.
Percentages apply to both analyses, unless 2 figures are presented, whereupon they apply to unadjusted and multivariable analysis, respectively.
Analyses are adjusted for history of wheezing, history of atopic dermatitis, siblings, length of hospital stay, use of systemic corticosteroids, and study center.
Figure E1.
Children who never used asthma control medication during the 4-year follow-up period after hospitalization for bronchiolitis: effect of viral etiology. Analysis of the main study population. P < .001 for RSV vs RV and P = .003 for RSV vs non-RSV/-RV. The figure and P value are from adjusted Cox regression analysis.
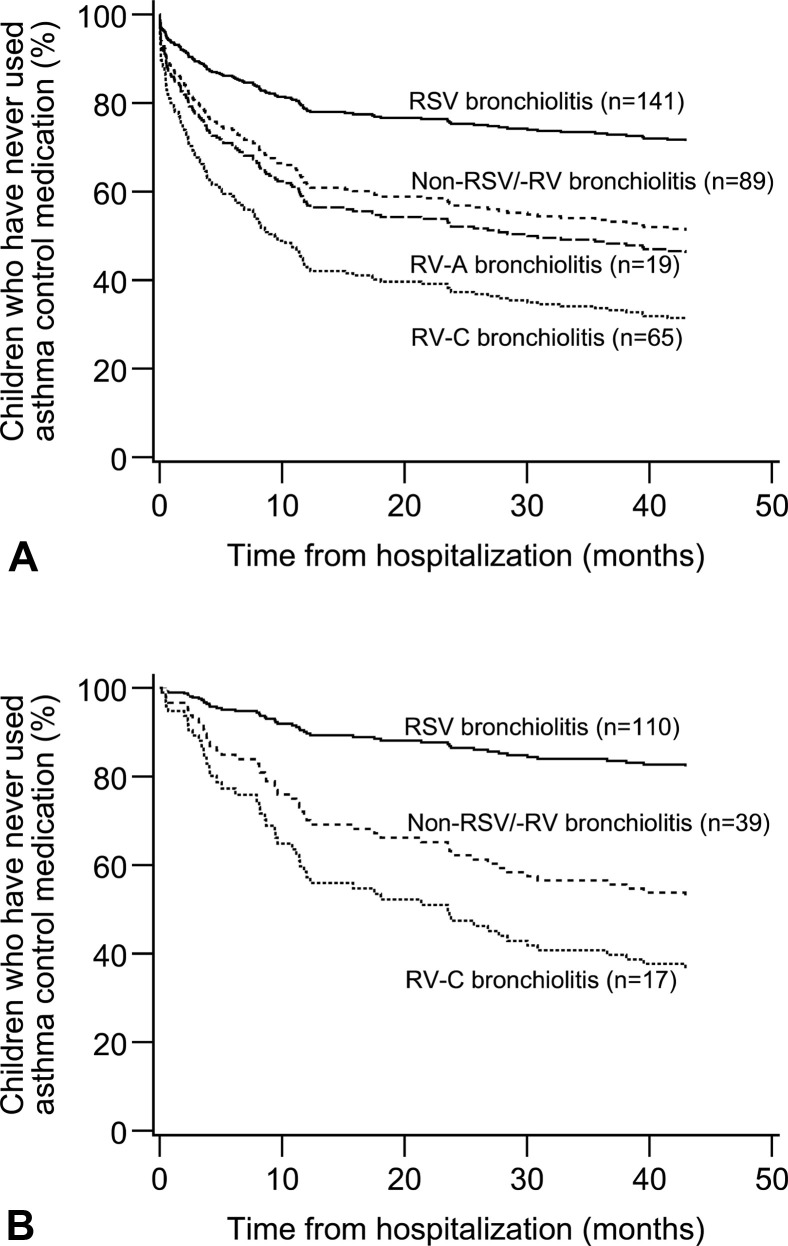
Figure 2.
Use of asthma control medication during 4-year follow-up after bronchiolitis: effect of viral etiology. (A) All children. RSV vs RV-A, RV-C, or non-RSV/-RV (all P ≤ .001). (B) Children with bronchiolitis by strict criteria. RV-A excluded (n = 2). RSV vs RV-C or non-RSV/-RV (both P ≤ .001). The figures are from Cox regression.
All 68 children with detailed data on medication use during the 48-month follow-up (45% of the children) had been using inhaled corticosteroids. Furthermore, 37% of children with RSV, 50% of children with RV, and 31% of children with non-RSV/-RV bronchiolitis had used a leukotriene receptor antagonist at some point during the follow-up (P = .42). No difference (P = .94) was found in the use of leukotriene receptor antagonist between RV-A (50% of the children) and RV-C (52% of the children).
Use of asthma control medication 4 years after severe bronchiolitis
Four years after bronchiolitis, 47 (47%) patients with RV, 24 (26%) patients with non-RSV/-RV, and 21 (15%) patients with RSV bronchiolitis had used asthma control medication during the past 12 months (Table IV ). Patients with RV infection were more likely to use asthma control medication compared with those with RSV infection (adjusted P < .001) (Table IV). When RV types were compared separately, both RV-A (42%, adjusted P = .03) and RV-C (48%, adjusted P < .001) were associated with current use of asthma control medication (Table IV and Figure 3 ). In unadjusted analyses, patients negative for both viruses used asthma control medication more than did patients with RSV (P = .04), but the difference was no longer significant after adjustment (P = .22) (Table IV and Figure 3).
Table IV.
Viral risk factors for the use of asthma control medication 4 y after severe bronchiolitis
| Viral risk factor | No. of children in analyses | No. of children using medication (%) | Unadjusted analysis |
Multivariable analysis∗ |
||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | aOR | 95% CI | P value | |||
| RSV | 145 | 21 (15%) | 1 | 1 | ||||
| RV | 101 | 47 (47%) | 5.14 | 2.81-9.42 | <.001 | 3.67 | 1.88-7.19 | <.001 |
| Non-RSV/-RV | 94 | 24 (26%) | 2.02 | 1.05-3.90 | .04 | 1.53 | 0.77-3.05 | .22 |
| RSV | 145 | 21 (15%) | 1 | 1 | ||||
| RV-A | 24 | 10 (42%) | 4.22 | 1.66-10.73 | .003 | 3.02 | 1.12-8.17 | .03 |
| RV-C | 73 | 35 (48%) | 5.44 | 2.83-10.44 | <.001 | 3.72 | 1.80-7.66 | <.001 |
| Non-RSV/-RV | 94 | 24 (26%) | 2.02 | 1.05-3.90 | .04 | 1.50 | 0.75-2.99 | .22 |
CI, Confidence interval.
ORs and 95% CIs are from logistic regression.
Bold indicates statistical significance (P < .05).
Analyses are adjusted for age, history of atopic dermatitis, siblings, and study center.
Figure 3.
Children who had used asthma control medication during past 12 months at the time of 4-year follow-up after bronchiolitis: effect of viral etiology. Figure presents separately the outcome for all children with bronchiolitis and children with bronchiolitis by strict criteria. The cases with RV-A infection were excluded from the latter (n = 2).
Other risk factors for the use of asthma control medication
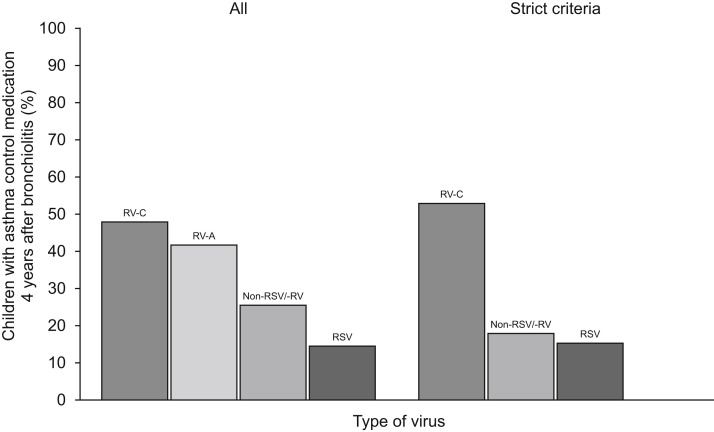
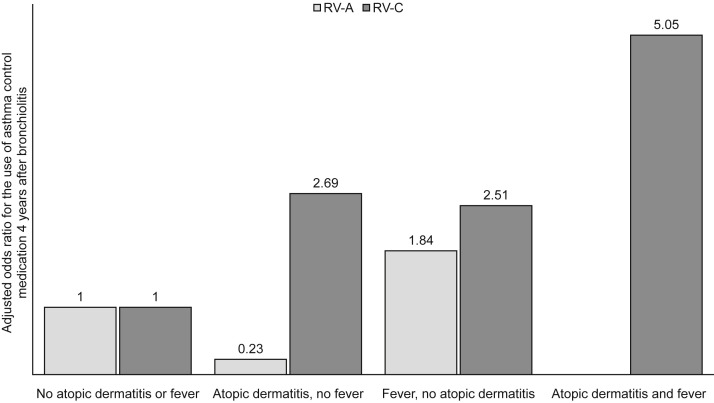
We also analyzed nonviral risk factors at the study entry for the initiation and the current use of asthma control medication (see Table E1 in this article's Online Repository at www.jaci-inpractice.org). History of wheezing (adjusted HR [aHR], 1.7; P = .01), atopic dermatitis (aHR, 1.8; P =.002), and use of systemic corticosteroid during the index hospitalization (aHR, 1.8; P = .005) were independent risk factors in multivariable analysis for initiation of asthma control medication in addition to bronchiolitis caused by RV-A (aHR, 2.1; P = .04), RV-C (aHR, 3.3; P < .001), or non-RSV/-RV (aHR, 1.8; P = .02), whereas the length of hospital stay of more than 3 days was not (aHR, 0.7; P = .04) (Table E1). Respectively, for the current use of asthma medication 4 years after bronchiolitis, only history of atopic dermatitis (adjusted OR [aOR], 2.1; P = .01) in addition to bronchiolitis caused by RV-A (aOR, 2.8; P = .048) or RV-C (aOR, 3.6; P = .001) were significant risk factors (Table E1). When children with RV-C infection were analyzed separately, those with both history of atopic dermatitis and fever at the time of hospitalization were more likely to use asthma medication 4 years after bronchiolitis (65%; aOR, 5.0; P = .03) compared with children with only a history of atopic dermatitis (50%; aOR, 2.7; P = .19), with fever (54%; aOR, 2.5; P = .16), or neither (33%) (Figure 4 ).
Figure 4.
Current use of asthma control medication 4 years after hospitalization for bronchiolitis: the effect of history of atopic dermatitis and fever on the current use of asthma control medication 4 years after hospitalization for bronchiolitis. Figures are aORs from logistic regression and presented separately for patients with RV-A and patients with RV-C.
Study outcomes using strict bronchiolitis criteria
Characteristics of the 175 children with bronchiolitis defined by strict criteria (age <12 months and the first wheeze) are presented in Table E2 in this article's Online Repository at www.jaci-inpractice.org. Of them, 111 had bronchiolitis induced by RSV, 17 had RV-C infection, and 2 tested positive for RV-A (Table E2). Both RV-C (adjusted P < .001) and non-RSV/-RV (adjusted P = .001) bronchiolitis were associated with earlier prescription of asthma control medication compared with RSV bronchiolitis (see Table E3 in this article's Online Repository at www.jaci-inpractice.org). RV-C bronchiolitis also increased the use of asthma medication 4 years later after the hospitalization compared with RSV (adjusted P = .009) (see Table E4 in this article's Online Repository at www.jaci-inpractice.org).
Discussion
Our study has 4 clinically interesting findings. First, among children hospitalized for bronchiolitis before age 2 years, bronchiolitis caused by RV-A or RV-C was associated with an earlier initiation of asthma control medication. Second, 4 years after RV-A or RV-C bronchiolitis, 40% to 50% of patients were still using asthma control medication compared with 15% of patients after RSV and 26% of patients after non-RSV/-RV bronchiolitis. Third, patients with RV-C bronchiolitis, atopic dermatitis, and fever had the highest risk for the development of asthma. Fourth, bronchiolitis caused by RV-C was also associated with an earlier initiation of asthma control medication in children with their initial wheezing episode in the first year of life.
Several factors can contribute to the linkage of RV-induced wheezing in infancy with the risk of childhood asthma. The inflammatory response caused by different RVs might damage the airways, although RSV could cause even more structural damage to airway epithelium than RV.11 However, changes in epithelial barrier function even before the infection can increase the risk of severe RV bronchiolitis, and host factors such as atopy and low IFN responses could have additive effects. In addition, risk genes such as cadherin-related family member 3 (encoding a receptor for RV-C) and 17q21 can predispose children to more severe RV-C infections and development of asthma.8, 11, 21, 22, 31 Finally, RV infection can inhibit IFN responses and potentiate TH2-cell–driven inflammation and production of cytokines, which promote airway obstruction and wheeze.10 Atopic characteristics are not seen as a risk factor for RSV-induced wheezing and asthma, which is more linked to young age and low lung function, indicating that the timing of RSV infection may be important for the future development of respiratory illnesses.11 Similarly, genetic factors can modify the risk of RSV bronchiolitis and development of asthma.10
Our finding of strong association of both RV-A– and RV-C–induced bronchiolitis with the use of asthma control medication 4 years later is consistent with previous RV studies. In a recent meta-analysis including 15 original articles, the association between RV-induced wheezing and later development of asthma was confirmed.32 In our previous report, the link between RV-A or RV-C and recurrences of wheezing was already evident at 12-month follow-up.27 Interestingly, in our study and others, the need for long-term asthma control medication typically develops within 2 years after RV-induced bronchiolitis.28, 33, 34
Early-life RV wheezing illnesses and aeroallergen sensitization increase the risk of asthma.10, 11 In a high-risk birth cohort, the persistence of asthma at age 13 years has been shown to be strongly and additively associated with RV wheezing illness and aeroallergen sensitization in early life.8 In accordance, we show that histories of wheezing, atopic dermatitis, and RV-A– and RV-C–induced bronchiolitis were independently associated with the initiation and the current use of asthma control medications 4 years later. Data from these studies suggest that atopic airways are more likely to develop obstructive changes after RV-related wheeze. Also, severe febrile viral respiratory infections in infancy and early atopy are known risk factors for persistent wheeze and asthma.35 Similarly, in our study, the occurrence of RV-C bronchiolitis, atopic dermatitis, and fever together was an especially important marker of risk for wheeze and asthma later in childhood.
Because bronchiolitis is defined differently in the United States and in Europe, we also analyzed the data using strict (European) criteria for bronchiolitis, for example, age less than 12 months and the first wheezing episode.1, 24, 25 Using these strict criteria for bronchiolitis, RV-C bronchiolitis in the first year of life was strongly associated with increased use of asthma medication 4 years later, whereas RV-A bronchiolitis was very rare in this age group. This is in agreement with our previous report on recurrence of wheezing within 12-month follow-up.27
The strengths of the study are the prospective design and longitudinal follow-up with high follow-up rates (86%). In this real-life study, only hospitalized patients with bronchiolitis who were younger than 2 years were included. We also conducted analyses separately for children with bronchiolitis by strict criteria, making the population more uniform and considering the possible effect of previous wheezing illnesses. In addition, the different viruses were evaluated at the time of bronchiolitis, and the later use of asthma medication was carefully characterized using registry data in addition to parental reporting. However, no further specific tests to confirm allergic sensitization were performed. One limitation is that the cohort includes only patients with severe bronchiolitis cases hospitalized before age 2 years. The role of viral infections may differ in children with milder forms of bronchiolitis.
Conclusions
It will be a challenge to prevent the development of asthma. To achieve that, we should reevaluate the diagnosis of bronchiolitis, which is generally considered to be a uniform entity. However, our data suggest that bronchiolitis is instead a disease with subgroups that have different risk factors and genetic and pathogenetic etiologies that lead to dissimilar responses to treatment and distinct prognosis.10 Our previous trials have shown that systemic corticosteroid during the first RV-induced wheezing episode reduced recurrences and need for asthma control medication although in those trials RVs were not subtyped.29, 33, 36 If these results are confirmed in further trials, testing for viral etiology of bronchiolitis could be recommended in forthcoming treatment guidelines to better estimate the future risk for developing asthma. This study also suggests that a preventive strategy targeting either RV-A or especially RV-C, and the inflammatory response associated with these viruses, might help to prevent at least some types of childhood asthma.
Footnotes
This study was supported by the Sigrid Jusélius Foundation, the Paulo Foundation, the Foundation for Pediatric Research, and the Allergy Research Foundation, all in Helsinki, Finland.
Conflicts of interest: The authors declare that they have no relevant conflicts of interest.
Online Repository
Table E1.
Risk factors at study entry for the initiation and use of asthma control medication 4 y after bronchiolitis
| Risk factor | Time to initiation of asthma control medication |
|||||
|---|---|---|---|---|---|---|
| Unadjusted analysis |
Multivariable analysis |
|||||
| HR | 95% CI | P value | aHR | 95% CI | P value | |
| Age at admission >12 mo | 2.41 | 1.75-3.33 | <.001 | 1.00 | 0.64-1.56 | .98 |
| Male | 1.70 | 1.19-2.43 | .004 | 1.33 | 0.91-1.95 | .14 |
| Parental history of asthma | 0.79 | 0.53-1.19 | .27 | 0.94 | 0.60-1.47 | .77 |
| Prematurity | 1.05 | 0.66-1.66 | .84 | 1.24 | 0.75-2.05 | .41 |
| Comorbid medical disorder | 1.75 | 1.08-2.83 | .02 | 1.08 | 0.61-1.91 | .80 |
| History of wheezing | 2.96 | 2.14-4.08 | <.001 | 1.66 | 1.13-2.44 | .01 |
| History of atopic dermatitis | 2.38 | 1.72-3.30 | <.001 | 1.77 | 1.24-2.56 | .002 |
| Siblings | 0.58 | 0.42-0.80 | .001 | 0.82 | 0.57-1.19 | .30 |
| Exposure to smoking during pregnancy or early childhood | 0.69 | 0.42-1.12 | .13 | 0.67 | 0.38-1.16 | .15 |
| Breast-feeding | 0.64 | 0.38-1.08 | .09 | 0.58 | 0.33-1.02 | .06 |
| Hospitalization ≥3 d | 0.63 | 0.43-0.91 | .02 | 0.65 | 0.42-0.99 | .04 |
| Systemic corticosteroid | 3.06 | 2.17-4.32 | <.001 | 1.84 | 1.20-2.82 | .005 |
| RV-A compared with RSV | 4.16 | 2.24-7.74 | <.001 | 2.08 | 1.04-4.17 | .04 |
| RV-C compared with RSV | 5.45 | 3.52-8.43 | <.001 | 3.34 | 2.03-5.47 | <.001 |
| Non-RSV/-RV compared with RSV | 2.51 | 1.62-3.90 | <.001 | 1.77 | 1.11-2.84 | .02 |
| Risk factor | Use of asthma control medication 4 y after bronchiolitis |
|||||
|---|---|---|---|---|---|---|
| Unadjusted analysis |
Multivariable analysis |
|||||
| OR | 95% CI | P value | aOR | 95% CI | P value | |
| Age at admission >12 mo | 2.49 | 1.53-4.05 | <.001 | 1.25 | 0.66-2.36 | .50 |
| Male | 1.46 | 0.88-2.42 | .15 | 1.37 | 0.77-2.45 | .28 |
| Parental history of asthma | 1.47 | 0.86-2.52 | .16 | 1.74 | 0.92-3.28 | .09 |
| Prematurity | 1.08 | 0.54-2.17 | .82 | 1.26 | 0.57-2.82 | .57 |
| Comorbid medical disorder | 1.54 | 0.76-3.08 | .23 | 0.94 | 0.42-2.13 | .89 |
| History of wheezing | 2.31 | 1.43-3.75 | .001 | 1.11 | 0.59-2.07 | .75 |
| History of atopic dermatitis | 2.40 | 1.46-3.94 | .001 | 2.13 | 1.20-3.78 | .01 |
| Siblings | 0.45 | 0.27-0.73 | .001 | 0.62 | 0.35-1.11 | .11 |
| Exposure to smoking during pregnancy or early childhood | 1.03 | 0.53-2.01 | .93 | 1.10 | 0.49-2.47 | .82 |
| Breast-feeding | 0.61 | 0.27-1.37 | .23 | 0.59 | 0.22-1.59 | .30 |
| Hospitalization ≥3 d | 0.82 | 0.48-1.38 | .45 | 0.99 | 0.54-1.83 | .97 |
| Systemic corticosteroid | 2.29 | 1.33-3.94 | .003 | 1.48 | 0.75-2.91 | .26 |
| RV-A compared with RSV | 4.22 | 1.66-10.73 | .003 | 2.84 | 1.01-7.99 | .048 |
| RV-C compared with RSV | 5.44 | 2.83-10.44 | <.001 | 3.56 | 1.63-7.77 | .001 |
| Non-RSV/-RV compared with RSV | 2.02 | 1.05-3.90 | .04 | 1.36 | 0.66-2.80 | .41 |
CI, Confidence interval.
HRs and their 95% CIs are from Cox regression; ORs and their 95% CIs are from logistic regression.
Bold indicates statistical significance (P < .05).
Table E2.
Patient characteristics of the 175 study children with bronchiolitis defined by strict criteria at study entry
| Characteristic | No. of children (%) |
|---|---|
| Age at study entry (mo), median (range) | 3.7 (11.7) |
| Sex: male | 106 (61) |
| Parental history of asthma | 36 of 173 (21) |
| Prematurity | 23 of 172 (13) |
| Comorbid medical disorder | 11 (6) |
| History of atopic dermatitis | 30 (17) |
| Siblings | 133 (76) |
| Exposure to smoking during pregnancy or early childhood | 28 (16) |
| Breast-feeding | 155 of 174 (89) |
| Hospitalization ≥3 d | 64 (37) |
| ICU stay | 12 (7) |
| Systemic corticosteroid | 12 of 174 (7) |
| Virus positive | 157 (90) |
| RSV∗ | 111 (63) |
| RV† | 19 (11) |
| RV-A | 2 (1) |
| RV-B | 0 (0) |
| RV-C | 17 (10) |
| RV-B + RV-C | 0 (0) |
| RV + RSV‡ | 6 (3) |
| Non-RSV/-RV | 39 (22) |
| Human metanpneumovirus | 10 (6) |
| Parainfluenzavirus 1 | 4 (2) |
| Parainfluenzavirus 3 | 3 (2) |
| Adenovirus | 1 (1) |
| Coronavirus HKU1 | 1 (1) |
| Coronavirus NL63 | 1 (1) |
| Influenza virus A | 1 (1) |
| Negative for tested viruses | 18 (10) |
ICU, Intensive care unit.
Including coinfection with viruses other than RV.
Including coinfection with viruses other than RSV.
Including coinfection with viruses other than RV or RSV.
Table E3.
Viral risk factors for the time to initiation of asthma control medication among children with bronchiolitis defined by strict criteria
| Viral risk factor | No. of children in analyses∗ | No. of children who have started medication | Unadjusted analysis |
Multivariable analysis† |
||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | aHR | 95% CI | P value | |||
| RSV | 111/110 | 24 (22%) | 1 | 1 | ||||
| RV | 19 | 12 (63%) | 5.34 | 2.66-10.72 | <.001 | 4.29 | 2.01-9.16 | <.001 |
| Non-RSV/-RV | 39 | 16 (41%) | 2.35 | 1.25-4.42 | .008 | 3.19 | 1.58-6.41 | .001 |
| RSV | 111/110 | 24 (22%) | 1 | 1 | ||||
| RV-A | 2 | 1 (50%) | 2.78 | 0.38-20.60 | .32 | 1.67 | 0.21-12.99 | .63 |
| RV-C | 17 | 11 (65%) | 5.83 | 2.84-11.95 | <.001 | 5.13 | 2.31-11.41 | <.001 |
| Non-RSV/-RV | 39 | 16 (41%) | 2.35 | 1.25-4.42 | .008 | 3.26 | 1.62-6.57 | .001 |
CI, Confidence interval.
HRs and 95% CI are from Cox regression.
Bold indicates statistical significance (P < .05).
Numbers apply to both analyses, unless 2 figures are presented, whereupon they apply to unadjusted and multivariable analysis, respectively.
Analyses are adjusted for history of atopic dermatitis, siblings, use of systemic corticosteroids, and study center.
Table E4.
Viral risk factors for the use of asthma control medication 4 y after severe bronchiolitis among children with bronchiolitis defined by strict criteria
| Viral risk factor | No. of children in analyses∗ | No. of children using medication† (%) | Unadjusted analysis |
Multivariable analysis‡ |
||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | aOR | 95% CI | P value | |||
| RSV | 111/109 | 17 (15%/16%) | 1 | 1 | ||||
| RV | 19 | 9 (47%) | 4.98 | 1.76-14.05 | .002 | 4.07 | 1.30-12.76 | .02 |
| Non-RSV/-RV | 39 | 7 (18%) | 1.21 | 0.46-3.18 | .70 | 0.99 | 0.34-2.92 | .99 |
| RSV | 111/109 | 17 (15%/16%) | 1 | 1 | ||||
| RV-A | 2 | 0 (0%) | — | — | — | — | — | — |
| RV-C | 17 | 9 (53%) | 6.22 | 2.11-18.38 | .001 | 4.93 | 1.49-16.30 | .009 |
| Non-RSV/-RV | 39 | 7 (18%) | 1.21 | 0.46-3.18 | .70 | 1.00 | 0.34-2.92 | .99 |
CI, Confidence interval.
ORs and 95% CIs are from logistic regression.
Bold indicates statistical significance (P < .05).
Numbers apply to both analyses, unless 2 figures are presented, whereupon they apply to unadjusted and multivariable analysis, respectively.
Percentages apply to both analyses, unless 2 figures are presented, whereupon they apply to unadjusted and multivariable analysis, respectively.
Analyses are adjusted for parental history of asthma, history of atopic dermatitis, siblings, and study center.
References
- 1.Ralston S.L., Lieberthal A.S., Meissner H.C., Alverson B.K., Baley J.E., Gadomski A.M. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134:e1474–e1502. doi: 10.1542/peds.2014-2742. [DOI] [PubMed] [Google Scholar]
- 2.Hasegawa K., Tsugawa Y., Brown D.F., Mansbach J.M., Camargo C.A. Trends in bronchiolitis hospitalizations in the United States, 2000-2009. Pediatrics. 2013;132:28–36. doi: 10.1542/peds.2012-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meissner H.C. Viral bronchiolitis in children. N Engl J Med. 2016;374:62–72. doi: 10.1056/NEJMra1413456. [DOI] [PubMed] [Google Scholar]
- 4.Balekian D.S., Linneman R.W., Hasegawa K., Ravi T., Camargo C.A. Cohort study of severe bronchiolitis during infancy and risk of asthma by age 5 years. J Allergy Clin Immunol Pract. 2017;5:92–96. doi: 10.1016/j.jaip.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Gaffin J.M., Phipatanakul W. The calculated risk of childhood asthma from severe bronchiolitis. J Allergy Clin Immunol Pract. 2017;5:97–98. doi: 10.1016/j.jaip.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mansbach J.M., Piedra P.A., Teach S.J., Sullivan A.F., Forgey T., Clark S. Prospective multicenter study of viral etiology and hospital length of stay in children with severe bronchiolitis. Arch Pediatr Adolesc Med. 2012;166:700–706. doi: 10.1001/archpediatrics.2011.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carroll K.N., Wu P., Gebretsadik T., Griffin M.R., Dupont W.D., Mitchel E.F. The severity-dependent relationship of infant bronchiolitis on the risk and morbidity of early childhood asthma. J Allergy Clin Immunol. 2009;123:1055–10561.e1. doi: 10.1016/j.jaci.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubner F.J., Jackson D.J., Evans M.D., Gangnon R.E., Tisler C.J., Pappas T.E. Early life rhinovirus wheezing, allergic sensitization, and asthma risk at adolescence. J Allergy Clin Immunol. 2017;139:501–507. doi: 10.1016/j.jaci.2016.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jartti T., Korppi M. Rhinovirus-induced bronchiolitis and asthma development. Pediatr Allergy Immunol. 2011;22:350–355. doi: 10.1111/j.1399-3038.2011.01170.x. [DOI] [PubMed] [Google Scholar]
- 10.Jartti T., Smits H.H., Bønnelykke K., Cavkaytar O., Elenius V., Konradsen J.R. Bronchiolitis needs a revisit: distinguishing between virus entities and their treatments. Allergy. 2019;74:40–52. doi: 10.1111/all.13624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jartti T., Gern J.E. Role of viral infections in the development and exacerbation of asthma in children. J Allergy Clin Immunol. 2017;140:895–906. doi: 10.1016/j.jaci.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marguet C., Lubrano M., Gueudin M., Le Roux P., Deschildre A., Forget C. In very young infants severity of acute bronchiolitis depends on carried viruses. PLoS One. 2009;4:e4596. doi: 10.1371/journal.pone.0004596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Midulla F., Scagnolari C., Bonci E., Pierangeli A., Antonelli G., De Angelis D. Respiratory syncytial virus, human bocavirus and rhinovirus bronchiolitis in infants. Arch Dis Child. 2010;95:35–41. doi: 10.1136/adc.2008.153361. [DOI] [PubMed] [Google Scholar]
- 14.McIntyre C.L., Knowles N.J., Simmonds P. Proposals for the classification of human rhinovirus species A, B and C into genotypically assigned types. J Gen Virol. 2013;94:1791–1806. doi: 10.1099/vir.0.053686-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Souëf P. Viral infections in wheezing disorders. Eur Respir Rev. 2018;27:170133. doi: 10.1183/16000617.0133-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng S.Y., Wang L.L., Ren L., Luo J., Liao W., Liu E.M. Epidemiological analysis and follow-up of human rhinovirus infection in children with asthma exacerbation. J Med Virol. 2018;90:219–228. doi: 10.1002/jmv.24850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bizzintino J., Lee W.M., Laing I.A., Vang F., Pappas T., Zhang G. Association between human rhinovirus C and severity of acute asthma in children. Eur Respir J. 2011;37:1037–1042. doi: 10.1183/09031936.00092410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Annamalay A.A., Jroundi I., Bizzintino J., Khoo S.K., Zhang G., Lehmann D. Rhinovirus C is associated with wheezing and rhinovirus A is associated with pneumonia in hospitalized children in Morocco. J Med Virol. 2017;89:582–588. doi: 10.1002/jmv.24684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cox D.W., Bizzintino J., Ferrari G., Khoo S.K., Zhang G., Whelan S. Human rhinovirus species C infection in young children with acute wheeze is associated with increased acute respiratory hospital admissions. Am J Respir Crit Care Med. 2013;188:1358–1364. doi: 10.1164/rccm.201303-0498OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanazawa J., Masuko H., Yatagai Y., Sakamoto T., Yamada H., Kaneko Y. Genetic association of the functional CDHR3 genotype with early-onset adult asthma in Japanese populations. Allergol Int. 2017;66:563–567. doi: 10.1016/j.alit.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 21.Bochkov Y.A., Watters K., Ashraf S., Griggs T.F., Devries M.K., Jackson D.J. Cadherin-related family member 3, a childhood asthma susceptibility gene product, mediates rhinovirus C binding and replication. Proc Natl Acad Sci U S A. 2015;112:5485–5490. doi: 10.1073/pnas.1421178112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bønnelykke K., Sleiman P., Nielsen K., Kreiner-Møller E., Mercader J.M., Belgrave D. A genome-wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nat Genet. 2014;46:51–55. doi: 10.1038/ng.2830. [DOI] [PubMed] [Google Scholar]
- 23.Jartti T., Aakula M., Mansbach J.M., Piedra P.A., Bergroth E., Koponen P. Hospital length-of-stay is associated with rhinovirus etiology of bronchiolitis. Pediatr Infect Dis J. 2014;33:829–834. doi: 10.1097/INF.0000000000000313. [DOI] [PubMed] [Google Scholar]
- 24.Megalaa R., Perez G.F., Kilaikode-Cheruveettara S., Kotwal N., Rodriguez-Martinez C.E., Nino G. Clinical definition of respiratory viral infections in young children and potential bronchiolitis misclassification. J Investig Med. 2018;66:46–51. doi: 10.1136/jim-2017-000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hancock D.G., Charles-Britton B., Dixon D.L., Forsyth K.D. The heterogeneity of viral bronchiolitis: a lack of universal consensus definitions. Pediatr Pulmonol. 2017;52:1234–1240. doi: 10.1002/ppul.23750. [DOI] [PubMed] [Google Scholar]
- 26.Hasegawa K., Jartti T., Mansbach J.M., Laham F.R., Jewell A.M., Espinola J.A. Respiratory syncytial virus genomic load and disease severity among children hospitalized with bronchiolitis: multicenter cohort studies in the United States and Finland. J Infect Dis. 2015;211:1550–1559. doi: 10.1093/infdis/jiu658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergroth E., Aakula M., Korppi M., Remes S., Kivistö J.E., Piedra P.A. Post-bronchiolitis use of asthma medication: a prospective 1-year follow-up study. Pediatr Infect Dis J. 2016;35:363–368. doi: 10.1097/INF.0000000000001017. [DOI] [PubMed] [Google Scholar]
- 28.Lukkarinen M., Koistinen A., Turunen R., Lehtinen P., Vuorinen T., Jartti T. Rhinovirus-induced first wheezing episode predicts atopic but not nonatopic asthma at school age. J Allergy Clin Immunol. 2017;140:988–995. doi: 10.1016/j.jaci.2016.12.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lehtinen P., Ruohola A., Vanto T., Vuorinen T., Ruuskanen O., Jartti T. Prednisolone reduces recurrent wheezing after a first wheezing episode associated with rhinovirus infection or eczema. J Allergy Clin Immunol. 2007;119:570–575. doi: 10.1016/j.jaci.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dumas O., Mansbach J.M., Jartti T., Hasegawa K., Sullivan A.F., Piedra P.A. A clustering approach to identify severe bronchiolitis profiles in children. Thorax. 2016;71:712–718. doi: 10.1136/thoraxjnl-2016-208535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calişkan M., Bochkov Y.A., Kreiner-Møller E., Bønnelykke K., Stein M.M., Du G. Rhinovirus wheezing illness and genetic risk of childhood-onset asthma. N Engl J Med. 2013;368:1398–1407. doi: 10.1056/NEJMoa1211592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu L., Pan Y., Zhu Y., Song Y., Su X., Yang L. Association between rhinovirus wheezing illness and the development of childhood asthma: a meta-analysis. BMJ Open. 2017;7:e013034. doi: 10.1136/bmjopen-2016-013034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koistinen A., Lukkarinen M., Turunen R., Vuorinen T., Vahlberg T., Camargo C.A. Prednisolone for the first rhinovirus-induced wheezing and 4-year asthma risk: a randomized trial. Pediatr Allergy Immunol. 2017;28:557–563. doi: 10.1111/pai.12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leino A., Lukkarinen M., Turunen R., Vuorinen T., Söderlund-Venermo M., Vahlberg T. Pulmonary function and bronchial reactivity 4 years after the first virus-induced wheezing. Allergy. 2019;74:518–526. doi: 10.1111/all.13593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kusel M.M., Kebadze T., Johnston S.L., Holt P.G., Sly P.D. Febrile respiratory illnesses in infancy and atopy are risk factors for persistent asthma and wheeze. Eur Respir J. 2012;39:876–882. doi: 10.1183/09031936.00193310. [DOI] [PubMed] [Google Scholar]
- 36.Jartti T., Nieminen R., Vuorinen T., Lehtinen P., Vahlberg T., Gern J. Short- and long-term efficacy of prednisolone for first acute rhinovirus-induced wheezing episode. J Allergy Clin Immunol. 2015;135:691–698.e9. doi: 10.1016/j.jaci.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]