Abstract
Cholangiocytes are the target of a group of chronic liver diseases termed the cholangiopathies, in which cholangiocytes react to exogenous and endogenous insults leading to disease initiation and progression. In primary sclerosing cholangitis (PSC), the focus of this review, the cholangiocyte response to genetic or environmental insults can lead to a heterogeneous response; that is, a subpopulation acquires a ductular reactive and proliferative phenotype, while another subpopulation undergoes senescence and growth arrest. Both ductular reactive cholangiocytes and senescent cholangiocytes can modify the periductal microenvironment through their ability to secrete various cytokines, chemokines, and growth factors, initiating and perpetuating inflammatory and pro-fibrotic responses. This review discusses the similarities and differences, the inter-relationships, and the potential pathogenic roles of these reactive proliferative and senescent cholangiocyte subpopulations in PSC.
Cholangiocytes are the target of a category of chronic liver diseases referred to as cholangiopathies (1). Based on their etiology, the cholangiopathies are classified into genetic (i.e., polycystic liver disease), infectious (i.e., AIDS cholangitis), drug- or toxin-induced, immune-mediated (i.e., primary biliary cholangitis [PBC]), idiopathic (i.e., primary sclerosing cholangitis [PSC]) and malignant (i.e., cholangiocarcinoma). Regardless of their cause, the cholangiopathies share a common pathophysiological phenotype, characterized by varying degrees of cholestasis, ductopenia, bile duct hyperplasia, portal and periportal inflammation, and fibrosis, which can progress to cirrhosis and end-stage liver disease. Liver transplantation is currently the best curative option for patients with end-stage liver disease, as therapeutic options for these diseases are limited and often ineffective. Herein, we will focus on PSC to support our hypothesis that aberrant, heterogeneous cholangiocyte phenotypes contribute to disease pathogenesis and progression.
PSC is an example of a cholangiopathy affecting both large and small bile ducts, and is associated with chronic, progressive biliary inflammation and fibrosis, with high risk for the development of cholangiocarcinoma (2). PSC is characterized by periductal fibrosis leading to large bile duct biliary strictures that causes the characteristic “beaded” appearance of the biliary ducts, with alternating strictures and dilatations detectable by cholangiography. Histopathologically, PSC displays fibro-inflammatory destruction of interlobular bile ducts, often with concentric or “onion skinning” fibrosis. The pathogenesis of PSC is still unclear, although both genetic and environmental factors have been found to contribute to the development and progression of the disease (2). However, we and others have recognized that altered cholangiocyte phenotypes may initiate and perpetuate inflammatory cascades, resulting in the recruitment and activation of immune cells and fibroblasts in the periductal stroma, leading to the characteristic fibro-inflammatory phenotype of this disease (3).
In the adult liver, the biliary epithelium has a limited replication rate, as do other mature liver cells. However, cholangiocyte proliferation is often observed following liver injury, where cholangiocytes can also act as facultative liver stem cells to regenerate the liver parenchyma, if hepatocyte proliferation is impaired (4). In the cholangiopathies, the ability of cholangiocytes to proliferate is particularly important to maintain the ductal mass and prevent ductopenia. However, proliferating cholangiocytes often display increased secretion of pro-inflammatory cytokines (tumor necrosis factor alpha [TNFα], interleukin [IL-6]), chemokines (monocyte chemoattractant protein 1 [MCP1], IL-8) and growth factors (platelet derived growth factor [PDGF], vascular endothelial growth factor [VEGF]), acting in both autocrine and paracrine manner to promote biliary remodeling and regulating the immune response. This link between proliferation and inflammation is an evolutionary conserved process, as transient or self-limited inflammatory cascades aid regeneration responses (5). For example, the pro-inflammatory cytokine IL-6 is mitogenic for cholangiocytes (6). The acquisition of this “activated’ or “reactive” phenotype constitutes the initial cholangiocyte response to endogenous or exogenous insults (7). Through the secretion of these pro-inflammatory mediators and growth factors, either in their soluble form or as extracellular vesicle cargo, the reactive cholangiocytes coordinate injury repair by modulating the recruitment, activation and function of a variety of cells, including hepatocytes, resident and recruited macrophages, hepatic stellate cells (HSCs), portal fibroblasts (PFs), neutrophils and lymphocytes. In the case of a moderate and/or acute injury, damaged cholangiocytes undergo cell death either by apoptosis (most frequently) or necrosis, triggering an immune response mediated by the release of apoptotic bodies or damage-associated molecular patterns (DAMPs), respectively, whereas proliferating cholangiocytes counteract the cell loss and repair the injury. In these instances, the pro-inflammatory response is transient and quickly resolved. However, in the presence of chronic injury, or in susceptible individuals, this reparative process becomes ineffective, generating a persistent inflammatory response, aberrant bile duct proliferation and angiogenesis, and liver fibrosis, a phenomenon known as the ductular reaction. In addition, a subpopulation of damaged cholangiocytes has been shown to acquire a senescent phenotype characterized by irreversible cell cycle withdrawal, altered gene expression, mitochondrial dysfunction, secretion of bioactive molecules, and up-regulation of anti-apoptotic pathways (8). Senescence likely reflects an evolutionary process to limit the risk of carcinogenesis (9). Cellular senescence can be triggered by several stressor events, including repeated cell division and strong mitogenic signals, telomere shortening, DNA damage, and chromatin remodeling, which activate the p53 and p16INK4a tumor suppressor pathways that initiate the senescence response (10). Acute, short-lived senescence is a physiological cellular checkpoint that targets a specific cell population, usually damaged and/or unwanted cells, with the aim of preventing the proliferation of cells with potentially oncogenic DNA damage, marking them for elimination by immune cells, and mobilizing progenitor cells to re-establish cell numbers (11). However, chronic senescence develops from repeated, prolonged exposure to stressors, and results in an accumulation of senescent cells due to the impaired ability of these cells to be removed and replaced. Moreover, senescent cells frequently exhibit a hypersecretory state known as the senescence-associated secretory phenotype (SASP), composed of cytokines, chemokines, growth factors, proteases, and extracellular matrix factors, which can reinforce and maintain the process of senescence and be detrimental to the ability of tissues to function normally. Interestingly, senescent cholangiocytes and ductular reactive cells have very similar secretory profiles, which contribute to the exacerbation of the fibro-inflammatory process and disease progression (12). We hypothesize that aberrant proliferation and senescence represent opposite fates of the reactive cholangiocyte in response to a chronic injury. Herein, we expand on this hypothesis, review the evidence supporting it, and discuss the contribution of both ductular reactive cholangiocytes and senescent cholangiocytes to the pathogenesis of PSC. Due to a lack of information regarding ductular reactive cells and senescent cholangiocytes in extrahepatic bile ducts, our review is limited to the function and interaction of ductular reactive cells and senescent cholangiocytes within the hepatic parenchyma.
The Proliferative Cholangiocyte Compartment in PSC: Ductular Reactive Cholangiocytes (DRCs)
Within the ductular reaction, proliferating biliary epithelial cell-marker positive cells can be recognized in irregularly shaped structures within the portal mesenchyme (non-invasive ductular reaction), sometimes extending from the edge of the portal space into the hepatic parenchyma, in a portal-to-portal pattern, closely associated with the fibrotic scar (minimally invasive to invasive ductular reaction) (13). The origin of these cholangiocyte-like cells is still debated and likely depends on the nature of the liver injury. Indeed, in different pathologic settings, they have been shown to originate from reactive cholangiocytes, hepatic and biliary progenitor/stem cells (HPCs), or transdifferentiated hepatocytes/hybrid hepatocytes (Sox9+HNF4α+)(14). This heterogeneity has rendered the identification of common, specific markers of DRCs quite challenging, as different DRCs can express different markers depending on their origin and the nature of the injury (15). On the other hand, frequently used markers to identify DRCs, such as Epithelial Cell Adhesion Molecule (EpCAM), Sex-Determining Region Y-Box 9 (SOX9), Cytokeratin-7 (CK7) and -19 (CK19), lack specificity, as they are not exclusively expressed by DRCs, but also by normal, and in some cases, senescent cholangiocytes. Nevertheless, recent lineage-tracing studies have demonstrated that DRCs in biliary injury are mainly derived from the expansion of proliferative cholangiocytes (particularly, of a select population with persistent proliferative activity) and/or activation of biliary tree progenitor/stem cells in the peribiliary gland niche (13, 16, 17). This etiology-dependent origin of DRCs likely affects their interaction with non-parenchymal cells within the ductular reaction, as demonstrated by a recent study that compared the transcriptomic profile of DRCs/HPCs from HCV-infected and PSC livers, as models for hepatocellular or biliary injury, respectively. The study identified significant differences in the expression of over 300 genes between the DRCs of the two diseases, and defined how these differences influence the recruiting and homing of inflammatory cells (15).
DRCs/progenitor cells are intimately associated with other cells, including HSCs and macrophages, and the extracellular matrix, within the ductular reaction micro-niche. Signaling coming from HSCs and macrophages, as well as individual matrix components, have been shown to activate different signaling pathways in progenitor cells and influence their fate. Morphogenic signals such as Wnt/β-catenin, Hedgehog, Notch, TWEAK/Fn14, and, more recently, YAP/Hippo pathways, have all been implicated in DRC activation and expansion (18, 19). In particular, macrophage-derived TWEAK induces progenitor cell expansion and biliary duct hyperplasia in healthy mice, and progenitor cell expansion in mouse models of cholestasis is prevented in Fn14-deficient mice or by neutralization of TWEAK (20, 21). Increased Fn14 expression is seen in DRC from human chronic liver diseases associated with intense ductular reaction, including PSC (3, 21). A recent study has demonstrated that PSC patient livers exhibit elevated levels of lymphotoxin β and nuclear RELB, the functional subunit of the transcription factor NF-κB activated through the non-canonical pathway by several members of the TNF family, including lymphotoxin β and TWEAK, and that RELB activation drives ductular reaction, progenitor cell activation and biliary fibrosis (22). Interestingly, cholangiocytes and DRCs in the liver of PSC patients display reduced expression of cellular inhibitor of apoptosis 1 and 2 (cIAP1, cIAP2), which are targeted for degradation following exposure to TWEAK (23), and transient depletion of cIAP1 and 2 in cholangiocytes results in non-canonical activation of NF-κB and generation of a fibro-inflammatory biliary phenotype in mice (3).
In human liver diseases, the magnitude of the DRC population correlates with the severity of fibrosis, implicating a cross-talk between DRCs and pro-fibrogenic cells; however, the question of whether fibrosis is the cause or the result of the activation and expansion of DRCs remains unanswered (14, 24, 25). In chronic liver diseases such as PSC, the development of liver fibrosis is promoted by collagen type-1-producing myofibroblasts, derived from either PFs, HSCs, or bone marrow-derived fibrocytes (26, 27). PFs were initially believed to be the major contributors to peribiliary fibrosis, while HSCs contribute to parenchymal fibrosis (28). However, both PFs and HSCs have now been shown to be the source of myofibroblasts in cholestatic liver injury, with PFs also being able to directly activate HSCs (29). Indeed, it was recently demonstrated that in the Mdr2−/− mouse, a murine model of sclerosing cholangitis, and other models of cholestatic liver injury, activated PFs and HSCs, but not bone marrow-derived fibrocytes, contribute to liver fibrosis, with activated PFs predominately localized to the peribiliary area, and activated HSCs found within the portal and sinusoidal areas (27). Abundant DRCs and extensive fibrosis are features of late stage PSC, as well as older Mdr2−/− mice, although whether fibrosis precedes and supports the DRC proliferation, or DRCs promote the development of fibrosis, remains to be clarified. Interestingly, genetic deletion of the receptor for tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) exacerbates the ductular reaction and causes parenchymal, bridging fibrosis in the Mdr2−/− mouse (Mdr2−/−Tr−/−), suggesting that TRAIL pro-apoptotic signaling may restrain the expansion of DRCs, which, in turn, can influence HSC activation. Indeed, these mice exhibit expansion of a sub-population of restorative macrophages (MerTK+CD206+Lgals2+) expressing TRAIL, pointing to an on-going compensatory process to restrain the number of DRCs (30–32).
The Non-Proliferative Cholangiocyte Compartment in PSC: Senescent Cholangiocytes (SCs)
Cholangiocyte senescence is a key contributor to the pathogenesis of PSC, although there are currently no available data on the relationship of cholangiocyte senescence and prognosis of the disease. In end-stage PSC, a large number of cholangiocytes express high levels of senescence markers, including p16INK4a and the DNA-damage marker yH2A.x, suggesting a pathogenic role for DNA damage-induced cholangiocyte senescence in the development and progression of PSC (33). PSC cholangiocytes also highly express components of the SASP, including cytokines and chemokines (IL-6, IL-8, MCP1, plasminogen activator inhibitor-1 [PAI-1]), growth factors (PDGF, epithelial growth factor [EGF]) and extracellular matrix proteins (fibronectin)(33). Additionally, transcriptomic analysis of cholangiocytes isolated from PSC patient explant tissue indicated robust expression of numerous cytokines, chemokines, growth factors, and markers of extracellular vesicles, indicating a hypersecretory nature of these cells (34). Likewise, cholangiocytes in the Mdr2−/− mouse and in vitro models of cholangiocyte senescence (normal human cholangiocytes exposed to the microbial insult lipopolysaccharide, or to damaging agents, such as hydrogen peroxide and γ-irradiation) also express elevated levels of the senescence markers p16INK4a, yH2A.x, and senescence-associated (SA)-beta galactosidase, and SASP components (33, 35). Moreover, cultured PSC cholangiocytes and normal cholangiocytes induced to senescence demonstrate increased secretion of cytokines and chemokines in addition to increased secretion of exosomes enriched in growth factors (34). While the molecular mechanisms accounting for the senescent cholangiocyte phenotype in PSC are still unclear, recent data suggest that a transcription factor, ETS1, likely is central to the process (36, 37). Further analysis based on co-culture experiments indicates that cultured senescent cholangiocytes can induce senescence in target cells, underscoring the significant role SASP plays in promoting and exacerbating cellular senescence (33). Indeed, SASP modulates the immune surveillance of senescent cells, and can attract immune cells, especially macrophages, to senescent cells for their elimination (38, 39). Although the involvement of other cell types cannot be ruled out, in vitro co-culture experiments demonstrated the ability of senescent cholangiocyte (but not normal cholangiocyte) conditioned media to activate the monocytic cell line THP-1, and induce their migration, suggesting that monocyte-attractant chemokines are secreted by senescent cholangiocytes; furthermore, THP-1 cells preferentially migrated toward and interacted with the SCs in monolayer, as well as in 3D-organoid cultures, compared to normal cholangiocyte and organoid cultures (39, 40). Importantly, this process could be abrogated by blocking key cholangiocyte SASP cytokines, such as IL-6, IL-8, and MCP1 (39). During the progression of PSC, infiltration of immune cells can be observed during early stages, with increased portal inflammation by end stage. Consistently, we have recently demonstrated a significant, progressive accumulation of infiltrating, pro-inflammatory macrophages, as well as pro-fibrogenic macrophages, in the peribiliary area in liver specimens from PSC patients, with similar findings corroborated in the Mdr2−/− mouse (39).
Senescent cholangiocyte SASP can also affect progression of peribiliary fibrosis. Notably, in an in vitro co-culture system, senescent cholangiocyte conditioned media activates the hepatic stellate cell line LX-2, and this activation is abrogated by blocking PDGF secretion from senescent cholangiocytes (41). In addition, secretin stimulation has been shown to drive cholangiocyte senescence, and HSC activation is induced when cells are incubated with cholangiocyte supernatant from secretin-treated wild type mice or bile duct ligated (BDL) mice, in which cholangiocyte senescence is also increased. Secretin-induced senescent cholangiocytes modulate liver fibrosis via TGFβ1 secretion, which also promotes cholangiocyte senescence in an autocrine loop (42, 43). Thus, the persistence of cholangiocyte senescence (likely due to their apoptosis resistance) and associated SASP may have deleterious effects on liver fibrosis by promoting activation and propagation of myofibroblasts.
Therapeutically Targeting of Reactive Cholangiocytes
The development in recent years of a class of small molecules aiming to eliminate senescent cells (senolytics) or inhibit SASP (senomorphics) has paved the way for studying the role of senescent cells in multiple diseases and the potential impact of reducing senescent cell burden on disease progression. Several preclinical studies have shown that senolytic agents can ameliorate the phenotype of senescence-related diseases (8, 9). In a mouse model of idiopathic pulmonary fibrosis, the combination of two senolytic drugs effectively reduced the number of senescent cells in the lung, and diminished lung inflammation and fibrosis, suggesting that senotherapeutics could be beneficial in the treatment of senescence-associated fibro-inflammatory diseases (44). Experimentally-induced SCs, as well as cholangiocytes from PSC patients and Mdr2−/− mice, over-express the anti-apoptotic protein Bcl-xL, and exhibit dependence on Bcl-xL for survival, which can be exploited therapeutically (37, 41). Indeed, in pre-clinical studies, treatment with the selective Bcl-xL inhibitor A-1331852 or the Bcl-xL/Bcl-2/Bcl-W inhibitor navitoclax (ABT-263) induced apoptosis of cultured senescent cholangiocytes, but not normal cholangiocytes. More importantly, treatment of Mdr2−/− mice with A-1331852 or navitoclax reduced the number of p16INK4a-positive cholangiocytes, in addition to decreasing the expression of activated fibroblasts, reducing levels of liver cytokine and chemokine expression, and reducing the overall level of peribiliary fibrosis (41). Clinical trials to test the use of navitoclax in cancer therapy have been limited by the development of severe thrombocytopenia, as Bcl-xL is required for platelet survival; however, because senescent cells do not replicate and accumulate slowly in the tissue, their elimination could be achieved by intermittent dosing, thereby limiting the toxic side effects of the treatment. Similarly, experimentally-induced DRCs, but not normal cholangiocytes, display a selective dependence on the anti-apoptotic protein Mcl-1, and pharmacologic targeting of DRCs by the Mcl-1 inhibitor S63845 effectively eliminates the parenchymal fibrosis in Mdr2−/−Tr−/− mice, supporting the hypothesis that DRCs actively participate in the development of parenchymal fibrosis in cholestatic injury (45). Thus, pharmacological targeting of SCs or DRCs may represent an effective therapeutic avenue for the treatment of PSC and other cholestatic liver diseases.
PSC – One Target Cell, Divergent Pathways
Based on the above, we would propose that, while cholangiocytes are the target cells in PSC, the cholangiocyte response in PSC is not uniform. During PSC disease progression, it appears that cholangiocytes take divergent pathways in response to molecular cues, as one subset undertakes a ductular reactive and proliferative pathway, while another subset undergoes senescence and growth arrest. This concept is based on the notion that cholangiocytes are not a homogenous population of cells that respond equally to an injurious agent, that the environmental cues influencing cholangiocyte fate vary depending on the cholangiocyte subpopulation and the context of cell-cell and cell-extracellular matrix communication in the peribiliary milieu, and that the state of PSC disease progression is dependent on the dynamics involved with both these pathways.
We further propose that SCs do not deviate from their biliary point of origin; that these cells were meant to be molecular beacons indicating the location of severe damage above the threshold of routine reactive cholangiocyte response, a signal that an alternate reparative response is required. As evolving data suggest that activated PFs are the main fibroblast cell type recruited to the site of periductular injury, it could be postulated that the senescent cell SASP likely maintains an exacerbated activated PF presence around the bile ducts, leading to the onion skin-like appearance of periductular fibrosis and, in extreme cases, ductopenia. Due to their resistance to apoptosis, the persistence of SCs and SASP, and the continued secretion of “seek and repair” signals, maintain a pathological state in which resolution to a homeostatic state remains elusive.
On the other hand, cholangiocytes undergoing ductular reaction proliferate beyond the original ductule structure and into the parenchyma in an aberrant attempt to repair and restore biliary structural integrity. We would suggest that the ductular reactive secretory phenotype likely attracts and activates HSCs, which reside mainly in the parenchyma, to provide a foundation for the ductular reactive cholangiocytes and HSCs to amass. This influx of HSCs expectedly contributes to the bridging parenchymal fibrosis seen in advanced stages of PSC. In addition to attracting HSCs, cytokines secreted by DRCs could be attracting a unique subset of immune cells that could either provide additional signals to sustain this anomalous reparative state of ductular reactive cholangiocytes, or alternatively, attempt to halt progression of uncontrolled cholangiocyte proliferation, but fail due to expression of immune evasive molecules on DRCs.
Taken together, the divergent cholangiocyte fates result in two unique molecular responses leading to recruitment and interaction of distinct cell types for each respective cholangiocyte subset (Fig. 1). However, why cholangiocytes are predisposed to choose one path over the other remains unclear. Both ductular reaction and senescence likely start as part of a beneficial reaction (injury repair), but become deleterious in conditions of chronic injury. Persistence of damaging SCs or aberrantly proliferating DRCs not only are major contributors to the fibro-inflammatory disease progression of PSC, but also generate a favorable setting for the development of biliary cancer. Unfortunately, most of the work to date interrogating cholangiocyte senescence or ductular reaction has been done in end-stage PSC explant liver tissue; thus, neither the state of cholangiocyte senescence or ductular reaction in earlier stages of PSC, nor the exact onset of cholangiocyte senescence (whether acute or chronic) or ductular reaction is known. Similarly, how the chronological events of ductular reaction and senescence unfold over the course of PSC disease progression is also not fully understood. While there is accumulation of peribiliary fibrosis and immune cell infiltration in early-stage PSC, whether this is linked to the onset of cholangiocyte senescence in PSC or ductular reaction needs to be determined. However, our and other’s data are consistent with the hypothesis we propose; namely, that SCs and PFs participate mainly in the development and persistence of the peribiliary fibrosis, whereas DRCs and HSC would be involved in the formation of the parenchymal fibrosis. Ultimately, understanding why cholangiocytes undergo divergent and distinct responses in PSC disease progression can help provide better therapeutic insights in the treatment for, and potentially, origin of this disease.
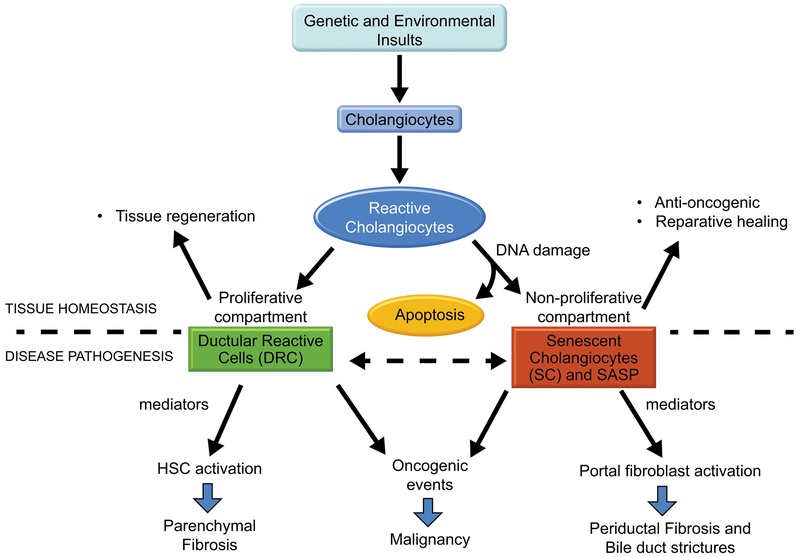
Figure 1. Divergent cholangiocyte fates in the pathogenesis of PSC.
Genetic and environmental insults targeting the cholangiocytes result in their acquisition of a reactive phenotype characterized by increased proliferation and upregulation of pro-inflammatory mediators. In a physiologic context, the expansion of this proliferative compartment and the cross-talk with recruited and resident cells, including hepatocytes, leukocytes and fibroblasts (known as the ductular reaction), promote the repair of the damaged epithelium and tissue homeostasis. During this process, cells that have sustained unrepairable DNA damage, either as a direct consequence of the insult or of their proliferative activity, are generally eliminated by apoptosis. Among these damaged cells, those that have developed resistance to apoptosis acquire a senescent phenotype characterized by growth arrest, which prevents the propagation of potentially oncogenic mutations. Senescent cells are also frequently hypersecretory and release pro-inflammatory cytokines, chenokines, growth factors and extracellular matrix proteins aimed to promote the reparative process. In the presence of a chronic injury, the secretory state of both the proliferative and the senescent cholangiocyte compartment leads to persistent inflammation, aberrant fibrogenic response and progressive liver injury, with increased oncogenic risk. Based on experimental data and supported by their anatomical location, we propose that, in PSC, senescent cholangiocytes and portal fibroblasts participate mainly in the development and persistence of the peribiliary fibrosis, whereas ductular reactive cells and hepatic stellate cells would be involved in the formation of the parenchymal fibrosis.
Acknowledgments
This work was supported by NIH grant DK57993 (to NFL) and the Chris M. Carlos and Catharine Nicole Jockisch Carlos Endowment Fund in PSC.
REFERENCES
- 1.Lazaridis KN, LaRusso NF. The Cholangiopathies. Mayo Clin Proc 2015;90:791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lazaridis KN, LaRusso NF. Primary Sclerosing Cholangitis. N Engl J Med 2016;375:1161–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guicciardi ME, Krishnan A, Bronk SF, Hirsova P, Griffith TS, Gores GJ. Biliary tract instillation of a SMAC mimetic induces TRAIL-dependent acute sclerosing cholangitis-like injury in mice. Cell Death Dis 2017;8:e2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raven A, Lu WY, Man TY, Ferreira-Gonzalez S, O’Duibhir E, Dwyer BJ, Thomson JP, et al. Cholangiocytes act as facultative liver stem cells during impaired hepatocyte regeneration. Nature 2017;547:350–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karin M, Clevers H. Reparative inflammation takes charge of tissue regeneration. Nature 2016;529:307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yokomuro S, Tsuji H, Lunz JG 3rd, Sakamoto T, Ezure T, Murase N, Demetris AJ. Growth control of human biliary epithelial cells by interleukin 6, hepatocyte growth factor, transforming growth factor beta1, and activin A: comparison of a cholangiocarcinoma cell line with primary cultures of non-neoplastic biliary epithelial cells. Hepatology 2000;32:26–35. [DOI] [PubMed] [Google Scholar]
- 7.O’Hara SP, Tabibian JH, Splinter PL, LaRusso NF. The dynamic biliary epithelia: molecules, pathways, and disease. J Hepatol 2013;58:575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirkland JL, Tchkonia T. Cellular Senescence: A Translational Perspective. EBioMedicine 2017;21:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Deursen JM. Senolytic therapies for healthy longevity. Science 2019;364:636–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest 2013;123:966–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman J, Fielder E, Passos JF. Mitochondrial dysfunction and cell senescence: deciphering a complex relationship. FEBS Lett 2019;593:1566–1579. [DOI] [PubMed] [Google Scholar]
- 12.Banales JM, Huebert RC, Karlsen T, Strazzabosco M, LaRusso NF, Gores GJ. Cholangiocyte pathobiology. Nat Rev Gastroenterol Hepatol 2019;16:269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clerbaux LA, Manco R, Van Hul N, Bouzin C, Sciarra A, Sempoux C, Theise ND, et al. Invasive Ductular Reaction Operates Hepatobiliary Junctions upon Hepatocellular Injury in Rodents and Humans. Am J Pathol 2019;189:1569–1581. [DOI] [PubMed] [Google Scholar]
- 14.Sato K, Marzioni M, Meng F, Francis H, Glaser S, Alpini G. Ductular Reaction in Liver Diseases: Pathological Mechanisms and Translational Significances. Hepatology 2019;69:420–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Govaere O, Cockell S, Van Haele M, Wouters J, Van Delm W, Van den Eynde K, Bianchi A, et al. High-throughput sequencing identifies aetiology-dependent differences in ductular reaction in human chronic liver disease. J Pathol 2019;248:66–76. [DOI] [PubMed] [Google Scholar]
- 16.Kamimoto K, Kaneko K, Kok CY, Okada H, Miyajima A, Itoh T. Heterogeneity and stochastic growth regulation of biliary epithelial cells dictate dynamic epithelial tissue remodeling. Elife 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carpino G, Nevi L, Overi D, Cardinale V, Lu WY, Di Matteo S, Safarikia S, et al. Peribiliary gland niche participates in biliary tree regeneration in mouse and in human primary sclerosing cholangitis. Hepatology 2019. [DOI] [PubMed] [Google Scholar]
- 18.Fabris L, Spirli C, Cadamuro M, Fiorotto R, Strazzabosco M. Emerging concepts in biliary repair and fibrosis. Am J Physiol Gastrointest Liver Physiol 2017;313:G102–G116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Planas-Paz L, Sun T, Pikiolek M, Cochran NR, Bergling S, Orsini V, Yang Z, et al. YAP, but Not RSPO-LGR4/5, Signaling in Biliary Epithelial Cells Promotes a Ductular Reaction in Response to Liver Injury. Cell Stem Cell 2019;25:39–53 e10. [DOI] [PubMed] [Google Scholar]
- 20.Bird TG, Lu WY, Boulter L, Gordon-Keylock S, Ridgway RA, Williams MJ, Taube J, et al. Bone marrow injection stimulates hepatic ductular reactions in the absence of injury via macrophage-mediated TWEAK signaling. Proc Natl Acad Sci U S A 2013;110:6542–6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jakubowski A, Ambrose C, Parr M, Lincecum JM, Wang MZ, Zheng TS, Browning B, et al. TWEAK induces liver progenitor cell proliferation. J Clin Invest 2005;115:2330–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elssner C, Goeppert B, Longerich T, Scherr AL, Stindt J, Nanduri LK, Rupp C, et al. Nuclear Translocation of RELB Is Increased in Diseased Human Liver and Promotes Ductular Reaction and Biliary Fibrosis in Mice. Gastroenterology 2019;156:1190–1205 e1114. [DOI] [PubMed] [Google Scholar]
- 23.Vince JE, Chau D, Callus B, Wong WW, Hawkins CJ, Schneider P, McKinlay M, et al. TWEAK-FN14 signaling induces lysosomal degradation of a cIAP1-TRAF2 complex to sensitize tumor cells to TNFalpha. J Cell Biol 2008;182:171–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams MJ, Clouston AD, Forbes SJ. Links between hepatic fibrosis, ductular reaction, and progenitor cell expansion. Gastroenterology 2014;146:349–356. [DOI] [PubMed] [Google Scholar]
- 25.Clouston AD, Jonsson JR, Powell EE. Hepatic progenitor cell-mediated regeneration and fibrosis: chicken or egg? Hepatology 2009;49:1424–1426. [DOI] [PubMed] [Google Scholar]
- 26.Lua I, Li Y, Zagory JA, Wang KS, French SW, Sevigny J, Asahina K. Characterization of hepatic stellate cells, portal fibroblasts, and mesothelial cells in normal and fibrotic livers. J Hepatol 2016;64:1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishio T, Hu R, Koyama Y, Liang S, Rosenthal SB, Yamamoto G, Karin D, et al. Activated hepatic stellate cells and portal fibroblasts contribute to cholestatic liver fibrosis in MDR2 knockout mice. J Hepatol 2019;71:573–585. [DOI] [PubMed] [Google Scholar]
- 28.Karin D, Koyama Y, Brenner D, Kisseleva T. The characteristics of activated portal fibroblasts/myofibroblasts in liver fibrosis. Differentiation 2016;92:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwaisako K, Jiang C, Zhang M, Cong M, Moore-Morris TJ, Park TJ, Liu X, et al. Origin of myofibroblasts in the fibrotic liver in mice. Proc Natl Acad Sci U S A 2014;111:E3297–3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krishnan A, Katsumi T, Guicciardi ME, Bronk S, Azad A, Gores GJ. Death receptor deficiency augments ductular reaction and hepatic fibrosis in the Mdr2−/− mouse model of sclerosing cholangitis. Hepatology 2018;68:829A. [Google Scholar]
- 31.Triantafyllou E, Pop OT, Possamai LA, Wilhelm A, Liaskou E, Singanayagam A, Bernsmeier C, et al. MerTK expressing hepatic macrophages promote the resolution of inflammation in acute liver failure. Gut 2018;67:333–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cartland SP, Genner SW, Martinez GJ, Robertson S, Kockx M, Lin RC, O’Sullivan JF, et al. TRAIL-Expressing Monocyte/Macrophages Are Critical for Reducing Inflammation and Atherosclerosis. iScience 2019;12:41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tabibian JH, O’Hara SP, Splinter PL, Trussoni CE, LaRusso NF. Cholangiocyte senescence by way of N-ras activation is a characteristic of primary sclerosing cholangitis. Hepatology 2014;59:2263–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tabibian JH, Trussoni CE, O’Hara SP, Splinter PL, Heimbach JK, LaRusso NF. Characterization of cultured cholangiocytes isolated from livers of patients with primary sclerosing cholangitis. Lab Invest 2014;94:1126–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tabibian JH, O’Hara SP, Trussoni CE, Tietz PS, Splinter PL, Mounajjed T, Hagey LR, et al. Absence of the intestinal microbiota exacerbates hepatobiliary disease in a murine model of primary sclerosing cholangitis. Hepatology 2016;63:185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Hara SP, Splinter PL, Trussoni CE, Pisarello MJ, Loarca L, Splinter NS, Schutte BF, et al. ETS Proto-oncogene 1 Transcriptionally Up-regulates the Cholangiocyte Senescence-associated Protein Cyclin-dependent Kinase Inhibitor 2A. J Biol Chem 2017;292:4833–4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Hara SP, Splinter PL, Trussoni CE, Guicciardi ME, Splinter NP, Al Suraih MS, Nasser-Ghodsi N, et al. The transcription factor ETS1 promotes apoptosis resistance of senescent cholangiocytes by epigenetically up-regulating the apoptosis suppressor BCL2L1. J Biol Chem 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoenicke L, Zender L. Immune surveillance of senescent cells--biological significance in cancer- and non-cancer pathologies. Carcinogenesis 2012;33:1123–1126. [DOI] [PubMed] [Google Scholar]
- 39.Guicciardi ME, Trussoni CE, Krishnan A, Bronk SF, Lorenzo Pisarello MJ, O’Hara SP, Splinter PL, et al. Macrophages contribute to the pathogenesis of sclerosing cholangitis in mice. J Hepatol 2018;69:676–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loarca L, De Assuncao TM, Jalan-Sakrikar N, Bronk S, Krishnan A, Huang B, Morton L, et al. Development and characterization of cholangioids from normal and diseased human cholangiocytes as an in vitro model to study primary sclerosing cholangitis. Lab Invest 2017;97:1385–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moncsek A, Al-Suraih MS, Trussoni CE, O’Hara SP, Splinter PL, Zuber C, Patsenker E, et al. Targeting senescent cholangiocytes and activated fibroblasts with B-cell lymphoma-extra large inhibitors ameliorates fibrosis in multidrug resistance 2 gene knockout (Mdr2(−/−)) mice. Hepatology 2018;67:247–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu N, Meng F, Zhou T, Venter J, Giang TK, Kyritsi K, Wu C, et al. The Secretin/Secretin Receptor Axis Modulates Ductular Reaction and Liver Fibrosis through Changes in Transforming Growth Factor-beta1-Mediated Biliary Senescence. Am J Pathol 2018;188:2264–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou T, Wu N, Meng F, Venter J, Giang TK, Francis H, Kyritsi K, et al. Knockout of secretin receptor reduces biliary damage and liver fibrosis in Mdr2(−/−) mice by diminishing senescence of cholangiocytes. Lab Invest 2018;98:1449–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ, Oberg AL, et al. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun 2017;8:14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Azad A, Katsumi T, Bronk SF, Krishnan A, Hirsova P, Kostallari E, Guicciardi ME, et al. Therapeutic targeting of ductular reactive cells in cholestatic liver disease. Hepatology 2019;70:165A. [Google Scholar]