Abstract
Background:
Objective gait evaluation in humans is used as a predictive disability outcome measure as well as an indicator for intervention effectiveness. Parallel methods of gait analysis in nonhuman primate models are essential for clinical translation. The goal of this study was to first assess whether marmosets’ gait data could be reliably collected in a Noldus CatWalk XT10.6 and second, establish a testing protocol to assess gait and the intraindividual variability during repeated testing.
New Method:
The CatWalk, originally developed for rodents, was modified and used to assess gait in eight adult common marmoset monkeys across multiple days and trials. Data was first analyzed to identify valid runs. Repeated measures ANOVA was completed for the following gate measures: mean base of support, average stride length, average swing time, and average stance time.
Results:
Raters had a high level of concurrence of usable data across all trials with successful trials including four consecutive hindfoot footfalls, during a continuous, uninterrupted segment of walking. A significant main effect of time (p<0.000) but not rater (p=0.98) was present with significant interactions for time by subject (p<0.000), but not rater per subject (p=0.538), time (p=0.186), or three-way interaction (p=0.297).
Comparison with Existing Method(s):
Gait has been assessed using force-plate and video data. The CatWalk allowed reproducible, automated and translational locomotor data to be collected at multiple time points with detailed analyses that identified a diagonal gait pattern.
Conclusions:
The CatWalk system, similar to those used in humans, can be effectively used to quantify spatiotemporal characteristics of gait in the common marmoset.
Keywords: common marmosets, monkeys, gait, locomotion, stride, stance
Graphical abstract
Partial screenshot of the CatWalk Noldus XT run visualization screen during acquisition of a common marmoset run, showing the right front (RF) and left hindlimb (LH) identified.

1. INTRODUCTION
Measurable changes in locomotor performance among humans have been long associated with normal aging as well as numerous neurodevelopmental and neurological presentations such as Wolfram syndrome (Pickett, Duncan et al. 2012), stroke (Patterson, Parafianowicz et al. 2008), Parkinson’s disease (PD;(Baltadjieva, Giladi et al. 2006, Ellis, Cavanaugh et al. 2016)) and Alzheimer’s disease (AD; (Cedervall, Halvorsen et al. 2014, Rucco, Agosti et al. 2017)). Relatively recent methodological advances in the study of human gait have allowed for translation from the laboratory into clinical settings. The use of portable computerized walkway systems, such as the GAITRite Walkway System™ and the Protokinetics Zeno™ Mat, has enabled spatiotemporal gait analysis to be completed quickly, accurately and without undue burden in clinical settings. Their application includes longitudinal tracking of disease progression and evaluation of clinical interventions (Duncan and Earhart 2012, Patterson, Mansfield et al. 2015, O'Dowd, Galna et al. 2017, Rozanski, Wong et al. 2019).
The importance of unbiased gait evaluation is well showcased in individuals with PD, whose early changes in gait may be predictive of future clinical decline in cognitive domains (Morris, Lord et al. 2017) as well as disability (Ellis, Cavanaugh et al. 2016). Changes in measures of gait performance have also been used as a marker of effectiveness following interventions for individuals with PD, including physical therapy (Morris, Martin et al. 2010), exercise (Shulman, Katzel et al. 2013) and tango dance (Duncan and Earhart 2012). Thus, measurement and tracking of decline in gait performance for individuals with PD has the potential to be used for clinical assessment of disease progression and optimization of pharmacological treatments and targeted rehabilitation.
Spatiotemporal variables such as cadence (steps per minute), step length (distance from the heel of one foot to the heel the next), stride length (distance from the heel of one foot the heel of the same foot on the next step, base of support (distance between the feet) and double support time (time spent with both feet on the ground) are commonly used to assess parkinsonian gait in clinical and research settings (Hackney and Earhart 2009, Hill, Campbell et al. 2013, Pilgram, Earhart et al. 2016, Myers, McNeely et al. 2018). Recent evidence suggests that variability of these measures may offer the most informative use of the data in human participants (Bryant, Rintala et al. 2011, Lord, Baker et al. 2011, Bryant, Rintala et al. 2016). These variables are easily measured in a time and cost-effective manner, but do require instrumentation for precision and accuracy.
Animal models are critical for development and preclinical testing of novel disease-modifying strategies. Establishing and using preclinical outcome measures similar to the ones used in humans offers a means of examining disease models across species using a common point of inference. Computerized systems for measuring locomotion in animals that are equivalent to human ones are needed to maximize the translational value of the results. An emerging tool for quantifying gait parameters in animal models is the Noldus CatWalk XT10.6 gait analysis system. Originally designed for rodents, it has been used to automate the quantification of locomotion in rat models of neurological disease including PD, Huntington’s disease and stroke (Vandeputte, Taymans et al. 2010), bilateral parkinsonian presentations (Westin, Janssen et al. 2012), multiple sclerosis (Herold, Kumar et al. 2016) and spinal cord injury (Hamers, Lankhorst et al. 2001). By standardizing the quantification of locomotion, it is possible to compare and contrast multiple details including base of support, swing time, stance time, stride length, gait pattern, and footfall intensity.
Studies in nonhuman primates (NHPs) are essential to assess efficacy and safety of first-in-class and invasive therapies (Capitanio and Emborg 2008), as compared to rodents, NHPs have more complex neuroanatomy and behavior. The common marmoset (Callithrix jacchus) has become a prime NHP species for behavioral neuroscience research (Prins, Pohlmeyer et al. 2017), especially for the development of genetic models of neurodegenerative human diseases (Tokuno, Watson et al. 2015). The mechanics of marmoset gait have previously been described using force-plate and video data (Schmitt 2003, Shimada et al 2017). Kinematic and kinetic movement analysis applying these methods is comprehensive and informative, yet the use of separate recording systems requires extensive instrumentation, data processing and analysis. If the CatWalk gait analysis system could be adapted for use with marmosets and potentially other NHPs, reproducible, automated and translational locomotor data could be collected at multiple time points in the natural lifespan, in various models of disease and throughout the course of potential interventions.
Here we report our efforts to automate the quantification of locomotion in common marmosets using the Noldus CatWalk XT10.6 gait analysis system. After performing systematic modifications to the apparatus to adjust to marmoset anatomy and movement characteristics, we first (Experiment 1) focused on the acquisition technique and protocol development. Based on the findings, we further optimized the acquisition methods and performed data collection (Experiment 2) in a small population of adult common marmoset monkeys to assess the reliability and reproducibility of the procedure and processing stream across multiple days within and across individual NHPs.
2. MATERIALS AND METHODS
2.1. Subjects
Eight healthy adult common marmoset monkeys (Callithrix jacchus; 4 females, 4 males, 2-8.5 y.o., 0.41-0.59 kg) (Table 1) were used in this project. Animal procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals Eighth Edition (NRC 2011) and with the approval of the Institutional Animal Care and Use Committee (IACUC) of the University of Wisconsin-Madison (G005208). All efforts were made to minimize the number of animals used and to ameliorate any distress caused by the experimental procedures outlined in this report.
Table 1.
Demographic information for the eight marmosets who completed the study.
| Monkey | Gender | Age (years) |
Starting weight (Kg) |
Ending weight (Kg) |
|
|---|---|---|---|---|---|
|
Experiment 1 |
Cj1 | male | 8.5 | 0.412 | 0.414 |
| Cj2 | male | 3.5 | 0.444 | 0.444 | |
| Cj3 | female | 3.3 | 0.452 | 0.436 | |
| Cj4 | female | 2.3 | 0.593 | 0.597 | |
|
Experiment 2 |
Cj5 | male | 2.6 | 0.413 | 0.418 |
| Cj6 | female | 2.0 | 0.530 | 0.520 | |
| Cj7 | male | 8.4 | 0.507 | 0.507 | |
| Cj8 | female | 7.5 | 0.419 | 0.409 |
Animals were housed in pairs and maintained on a 12-hr light/dark schedule, with temperature and relative humidity ranges of 24–30°C and 30–70% respectively. Water was continuously provided and feedings (Mazuri Callitrichid High Fiber Diet, Land O Lakes, Mazuri, Brentwood, MO) were twice daily ad lib, supplemented once daily with a small portion of fruit, vegetables, or mealworms. General health monitoring during the study included condition, appearance, weight, food intake and feces output.
2.2. Gait monitoring system
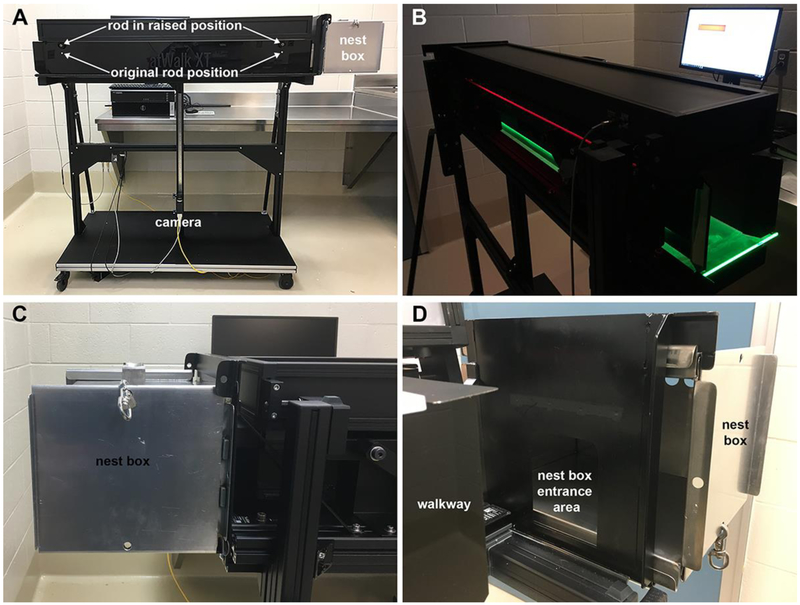
The Noldus CatWalk XT10.6 system was used to assess gait. As the system was originally developed for use with rodents (e.g.: (Tatenhorst, Eckermann et al. 2016)) modifications were necessary prior to use with common marmosets (Figure 1). The original CatWalk testing environment consisted of a 130cm long corridor, which connected to a goal box. To allow for minimal handling, ease of transport and standardization of the trial initiation and termination procedure, the original goal box was removed and replaced with a lightweight marmoset transport/nest box. The removable nest box was secured to the apparatus by adding tracks for sliding and locking the upper and lower rims of the transport cage in place.
Figure 1:
CatWalk Noldus XT system environment. (A) CatWalk system with nest box in place. (B) CatWalk’s walkway with floor illuminated and computer monitor placement. (C) Articulation of the nest box to the walkway. (D) View with tunnel walls retracted to show the entrance to the nest box from the walkway
The lateral walls of the CatWalk corridor are solid black and can be repositioned. The dark walls limit distractions from outside the testing environment and direct animal's movement over the plate floor. By repositioning the lateral walls, the corridor width can be adjusted to the size of the animal. Optimal corridor width is not constrictive but limits the lateral movement of the animal in an attempt to elicit uniform and replicable gait patterns within and between subjects. Due to the range of animal’s weights and to minimize sources of variations between trials, a width of 10.8cm was selected to accommodate the size of the largest marmoset subject. The floor of the corridor is made of transparent scratch resistant glass, which allows for data collection from a high-speed digital camera located beneath the floor. The distance between the high-speed camera and the floor is adjustable and defines the section of the corridor that is used for data collection. The camera was placed at 66.5cm from the floor plate. This distance allowed approximately 50-60% of the walkway glass to be visualized for gait measurement. The camera was connected to a data collection computer, which operated the Noldus Software and controlled data acquisition and storage. Following the CatWalk XT software instructions, the selected detection settings were: camera gain, 16.02dB; green intensity threshold, 0.14; red ceiling light, 17.7v; green walkway light, 16.0v.
The technology used by the gait analysis system is based on processing changes in light reflection within the glass floor. During a trial, green LED light is focused into the glass plate and is internally reflected by the glass, except in those areas where the animal touches the glass plate (Figure 1B). In the contact area the light escapes and is reflected by the body part (typically the marmoset’s hands and feet). If other body areas (e.g.: elbows, abdomen) contact the glass, they can be easily identified after acquisition and analyzed separately. The high-speed camera (100 Hz) positioned underneath the glass plate captures the illuminated hands/feet area, the location in 2-dimensional space and the time of the data acquisition. Intensity differences on the walkway can be observed based upon the values of the recorded light signal. The CatWalk XT software processes the acquired data. Individual footsteps can be identified using an automated or manual technique. The established automated algorithms were based on the gait patterns of rodent subjects and the footfalls are labeled as right hind, left hind, right front and left front. To identify marmoset’s footfalls, the automated gait analysis was applied first, followed by a fully manual method of fore- and hind-foot identification for side-by-side comparison.
2.3. Procedure for data collection
During data collection, the CatWalk system (including walkway and computer) was located in a quiet, secured room, adjacent to the animals’ home room. On experimental days, animals had regular meals and water ad libitum. The animals were transported in pairs when possible from their home cages to the behavioral testing room, in their home cage nest/transport box.
In this report, a run is defined as the period of time lapsed when a monkey was placed in the CatWalk to traverse the walkway and data was collected. A trial corresponds to the given number of runs done sequentially with the same subject in the same day. We planned for 4 trials with 3 completed runs each on 4 different days over a 2-week period in Experiment 1. In Experiment 2 we collected 4 completed runs per trial keeping the same number of trials.
Before each individual trial, the surface of the glass walkway was carefully cleaned with glass cleaner. Up to 3 small desirable, edible treats (e.g. mealworms, marshmallow pieces, cereal, crackers, etc.) were placed inside the previously cleaned and secured goal/nest box at the end of the walkway opposite where the animal would begin the trial.
To begin each trial run, an investigator trained in marmoset handling, gently took one of the subjects out of his/her nest/transport cage. Simultaneously, a second investigator set the system for data acquisition using the programmed definition for “rat” animal type. After ensuring that the animal and computer were ready, the lights of the room were turned off and the monkey was placed in the walkway and acquisition program started. The subject crossed the distance of the corridor at his/her own self-selected speed and entered the modified marmoset goal nest box. Once the animal was inside the goal nest box, the door of the box was slid back in place and the box disengaged from the CatWalk system before removing the animal. The goal box was then placed again on the exit side of the walkway and the animal gently carried back to the entry side of the walkway by an investigator for the next run. After the completion of the final run in each trial, the animal was again taken out of the disengaged goal box and placed back in his/her own transport cage. Here the marmoset waited until his/her next run or his/her partner completed the trial-run, to be returned to the home cage, where positive rewards (e.g. Ensure) were presented.
During initial pilot runs, we tried placing a door at the entry side of the CatWalk after the animal was released for a run; this was to discourage the animal from turning back to the entry. We realized that it was less distracting for the animals if a known, gentle handler simply placed the animals directly in the walkway and silently stood there.
2.4. Experiment 1
The primary goals of Experiment 1 were to determine if the marmosets could be tested in the altered CatWalk system and if the data could be collected in a similar and reproducible fashion on multiple days to collect useable data for analysis.
2.4.1. Performance of experimental trials
Four subjects completed Experiment 1 (see Table 1 for demographic data). Four total data collection trials were conducted over a two-week period with two trials occurring per week. Weekly trials were conducted at least one day apart from each other and trial data were collected at the same time of the day (1-2 PM) for all animals. Each trial aimed to acquire data from three completed runs per animal. Each animal performed runs until three successful runs were completed. A run was labeled unsuccessful if any of the following occurred: 1) the subject failed to complete the run; 2) an event occurred in the testing room or the nearby area that clearly altered the experimental set-up (i.e. lights were turned on in the room, a loud noise occurred in the hallway or another researcher entered the room and disrupted the run); or 3) when the acquisition program was not initiated before the animal entered the corridor. Data collection for three completed runs lasted approximately five minutes per individual subject.
2.4.2. Gait data analysis
Collected data was analyzed off site using either a Dell 7520 mobile workstation laptop computer or a Dell workstation with a Dual Intel® Xeon® E5-2630 v2 Processor. Data were first analyzed to decide which runs were considered valid. Two independent raters, one trained on human gait (AB) and the other an expert in marmoset behavior (NSD), watched video of each recorded run while simultaneously viewing the footfall data recorded by the CatWalk system. They subjectively evaluated if the subject’s performance met the following criteria: 1) completed four consecutive hind-limb footfalls; 2) without stopping or becoming visibly distracted; and 3) did not leap or bound during these consecutive footfalls. These criteria were established using common criteria for the assessment of human gait (Pickett, Duncan et al. 2012, Pilgram, Earhart et al. 2016). Four consecutive footfalls with one pair of limbs (fore or hind) allows for analysis of a minimum of two gait cycles for each run. As gait is a behavioral measure that is defined by cycles (i.e. a walking pattern is a repeated right, left, right, left pattern), not a single step, inclusion of a single repetition detracts from the goal of the analysis. If these criteria were met, the individual steps of each run were counted. Any partial hand- or footprints at the beginning or end of the run were not included in the analysis.
2.4.3. Statistical analysis
Analysis of Experiment 1 focused on an overall quality assessment of the methodology and an examination of the concurrence between the accepted and not accepted trials by two blinded raters. Additionally, the CatWalk automated algorithms versus manual identification of individual step identification were compared for accuracy to assess the usability of the automated analysis for data collected on common marmosets. Statistical analysis was limited to descriptive statistics.
2.5. Experiment 2
Based upon the findings from Experiment 1, the CatWalk apparatus was further altered prior to Experiment 2. The connecting rods that set the width of the walkway walls were raised 3.8cm on both ends of the walkway to allow the animals to enter and leave the walkway without ducking (Figure 1D). This modification was made after observing the decreased velocity of the animals as they approached the goal box on the majority of trials in Experiment 1. Additionally, the total number of completed runs collected per trial was increased from three to four in order to ensure enough valid runs to include in the analysis.
2.5.1. Performance of experimental trials
Four subjects completed Experiment 2 (see Table 1 for demographic data). Similar to Experiment 1, four trials were conducted over a two-week period with two trials occurring each week. Weekly trials were conducted at least one day apart from each other and at the same time of the day (1-2 PM). For Experiment 2, a trial consisted of four successful runs per animal. The animals continued performing runs until data from 4 successful runs were collected. An unsuccessful trial was defined as described in Experiment 1. Data collection for four completed runs lasted approximately seven minutes per individual subject.
2.5.2. Gait data analysis
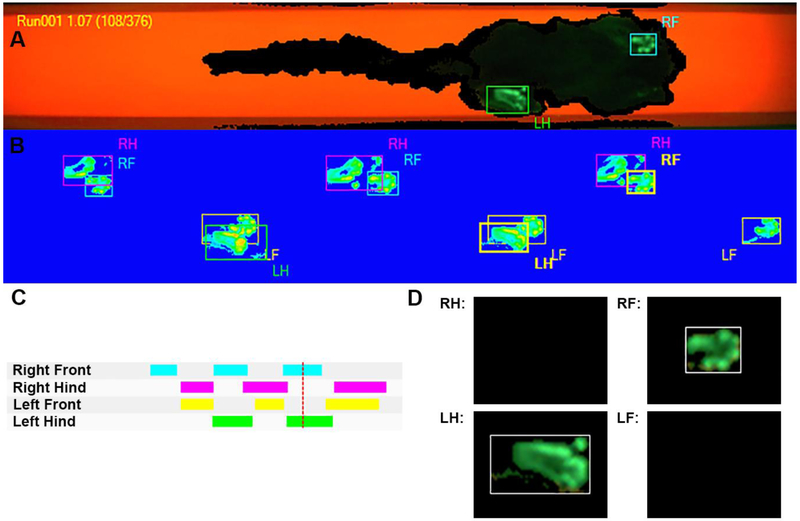
Data were first analyzed to decide which trials were considered valid. The same two independent raters from Experiment 1 watched each recorded run in Experiment 2 (both video and footfall data) and subjectively evaluated if the subject’s performance met the previously listed criteria of valid run. If these criteria were met, the individual steps of each run were first counted and then analyzed further to examine spatiotemporal characteristics of gait. When it was appropriate, a run could be clipped to remove a non-valid portion, but only segments with four consecutive footfalls were included in the data analysis. For example, if the run was valid for the first four steps of each limb but at hind-limb step-five the animal became distracted and stopped, the last portion of the trial was removed and only the first four steps analyzed (Figure 2).
Figure 2:
Screenshot of the catwalk Noldus XT run visualization screen during acquisition of a common marmoset run, showing the right front (RF) and left hindlimb (LH) identified. B) All the identified footfalls for that run. (C) Graphical gait sequence. (D) Pressure maps for the two identified limbs. The red line in C refers to the time of the collection of the pressure maps identified in A and shown in D.
Automated algorithms were applied for each individual run to determine location and timing of individual contact points and attempt to identify foot- and hand-falls. As mentioned above, the algorithms were originally established for use with rodents, thus a manual analysis was also performed on each trial by the two different raters. Each rater grouped the identified contact points into logical footfall patterns and then identified each footfall as right or left and hind or front. The first and last step of each run was excluded. Each valid run for each animal at each time point was grouped into a trial. The footfall data were used to calculate gait characteristics based on the combined run data for each day. That is, data from all valid runs completed during one testing session were combined to establish the behavioral measures for that animal on that day. A minimum of two runs were necessary to consider a trial valid.
Stepping patterns were analyzed to determine the percent of the trial that exhibited a normal step sequence pattern (Regularity Index) and the order of the individual steps. The Footfall Pattern visualization tool within CatWalk and the step sequence output allowed for quantification and visualization of the timing and order of the footfalls. A four-step sequence was counted as “alternate” when two ipsilateral steps precede two contralateral steps. Two “alternate” patterns are possible, Aa and Ab. Aa is Right-Front -> Right-Hind -> Left-Front -> Left-Hind. Ab is Right-Hind -> Right-Front -> Left-Hind -> Left-Front. A four-step sequence is counted as “cruciate” when the sequence alternates, contralateral, ipsilateral, contralateral. Two “cruciate” patters are possible, Ca and Cb. Ca is Right-Front -> Left-Front -> Right-Hind -> Left-Hind. Cb is Left-Front -> Right-Front -> Left-Hind -> Right-Hind. Finally, a “Rotate” pattern was identified when a four-step sequence progressed from front ipsi- to front contra- to hind contra and hind ipsilateral. The two “rotate” patterns are Ra and Rb. Ra is Right-Front -> Left-Front -> Left-Hind -> Right-Hind. Rb is Left-Front -> Right-Front -> Right-Hind -> Left-Hind.
Coordination of the limbs relative to each other was analyzed using the coupling variable. Coupling is defined as the extent to which the target limb is related or coupled to the gait cycle of the anchor limb and is quantified by the percentage of the gait cycle relative to the anchor limb. If two limbs were exactly temporally matched with regard to when they contact and lift off from the surface of the walkway, they would be 100% coupled. In contrast, if the target limb contacts the surface at the exact midpoint of the anchor limb gait cycle, the coupling value would be 50. Negative values are not possible, therefore values over 100% can occur in situations where the reference limb contacts the surface prior to the moment the anchor contacts the surface in the given gait cycle.
Data from the hind limbs were selected for analysis as they most closely couple with human gait characteristics. Spatiotemporal gait analysis of individual footfalls was completed using the CatWalk software. Similar to human gait analysis (Pickett, Duncan et al. 2012, Peterson, Pickett et al. 2014, Pilgram, Earhart et al. 2016), data extrapolated included: hind limb base of support (cm), average hind limb stride length (cm), average hind limb swing time (s) and duty cycle (%). Duty cycle, defined as the percentage of the gait cycle in which the specified limb was in contact with the ground, was selected instead of stance time to allow for contextualization of the duration relative to the gait cycle rather than absolute time.
2.5.3. Statistical analysis
A rater (2) by time (4) repeated measures ANOVA was performed for the gait measures of average base of support (BOS), average stride length (Stride), average swing time (Swing) and average stance time (Stance). Stride, Swing and Stance were averaged across the right and left hind limbs. Statistical analysis was performed using SPSS 25.0. A rater by time comparison is presented using means and standard error measures. Given the limited sample size, data are discussed using descriptive methods.
3. RESULTS
A brief series of pilot trials with the four initial subjects were performed a month prior to the experimental series to determine settings and modifications and evaluate if they were useful for data collection of marmoset runs. During this pilot series, it was found that although the animals were not habituated, they readily entered and traversed the walkway to the goal/nest box in the majority of the runs in these trials. Because of this, it was determined that habituation was not necessary prior to experimental sessions. It should be noted that at the WNPRC marmoset colony, the animals are accustomed from birth to being gently handled and that the investigators handling the animals during the trials have extensive experience in marmoset handling and care. For both experiments, animal health was not affected, e.g. weight remained stable during the testing period (Table 1).
3.1. Experiment 1
Raters independently rated the 48 runs collected as part of experiment 1. The two raters agreed with 100% concurrence across all trials for all subjects on which runs included usable data, following the agreed criteria for a useable run or portion of a run (four consecutive hind-limb footfalls, without stopping or becoming visibly distracted; and without leaping or bounding during the consecutive footfalls).
Experiment 1 resulted in 9 of the 16 trials containing a full set of three useable runs from all four monkeys. No useable runs were found in two of the trials. In one case, the subject (male 3.5 years, Cj2) appeared to be repeatedly distracted and was unable to complete a straight pass through the corridor without stopping. In a second case, the animal (female 3.3 years, Cj3) bounded/leapt during the execution of every pass.
Automated analysis results were visually examined and found to be an inaccurate representation of the gait pattern. For most subjects, the average number of footfalls identified by the automated analysis was more than double that of the manual identification. It was concluded that the automated analysis alone was not suitable for continued examination. Instead, we used the program to first identify all contact points, and then a rater manually confirmed, deleted or added appropriate footfalls.
3.2. Experiment 2
Raters 1 and 2 again rated all trials collected as part of experiment 2 using the established criteria for inclusion. The two raters agreed on the useable runs with a high level of concurrence across all trials (94%). Experiment 2 generated 10 of 16 trials with 75% or more useable data (out of four runs that were considered valid at the time of acquisition). One animal (male 2.6 years, Cj5) accounted for 4 of the 6 data sets with 50% or fewer useable trials. This subject generated 2 out of 4 good runs on day 1, 1 out of 4 on day 2, 1 out of 4 on day 3 and 0 out of 4 on day 4.
Runs scored as usable by each rater were included in the analysis. As one subject (male, 2.6 y.o., Cj5) failed to have any valid runs at one time point (due to multiple bounds and leaps) and only one valid sample at two other time points, it was removed from further analysis. A significant main effect of time (p-value < 0.001) but not rater (p-value = 0.98) was present. Significant interactions were present for time by subject (p-value < 0.001) but not for rater by subject (p-value = 0.538), rater by time (p-value = 0.186) or the three-way interaction of rater by time by subject (p-value =0.297). Means and standard deviations are shown in Table 2.
Table 2:
Means and standard deviation (S.D.) for the measures of base of support, swing time, stride length and stance time obtained by two raters over four trial-sets during Experiment 2.
| Rater 1 | Rater 2 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monkey | Day 1 | Day 2 | Day 3 | Day 4 | Day 1 | Day 2 | Day 3 | Day 4 | |||||||||
| Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. | ||
| Base of Support | Cj7 | 4.53 | 0.27 | 5.99 | 0.25 | 5.94 | 0.14 | 5.83 | 0.16 | 4.53 | 0.27 | 5.99 | 0.25 | 5.91 | 0.18 | 5.84 | 0.51 |
| Cj8 | 5.35 | 0.39 | 4.78 | 0.13 | 4.84 | 0.18 | 4.65 | 0.26 | 5.37 | 0.42 | 4.78 | 0.13 | 4.84 | 0.18 | 4.65 | 0.26 | |
| Cj6 | 4.75 | 0.35 | 4.94 | 0.81 | 5.03 | 0.23 | 5.26 | 0.24 | 4.85 | 0.51 | 4.94 | 0.81 | 4.85 | 0.51 | 5.26 | 0.24 | |
| Total | 4.92 | 0.47 | 5.14 | 0.69 | 5.19 | 0.50 | 5.17 | 0.53 | 4.96 | 0.52 | 5.14 | 0.69 | 5.11 | 0.57 | 5.17 | 0.57 | |
| Swing | Cj7 | 0.16 | 0.01 | 0.17 | 0.02 | 0.18 | 0.01 | 0.19 | 0.01 | 0.14 | 0.01 | 0.15 | 0.01 | 0.16 | 0.01 | 0.16 | 0.01 |
| Cj8 | 0.17 | 0.01 | 0.22 | 0.03 | 0.23 | 0.01 | 0.28 | 0.01 | 0.17 | 0.02 | 0.19 | 0.04 | 0.20 | 0.01 | 0.26 | 0.01 | |
| Cj6 | 0.16 | 0.02 | 0.14 | 0.02 | 0.17 | 0.01 | 0.18 | 0.02 | 0.14 | 0.02 | 0.13 | 0.01 | 0.16 | 0.02 | 0.16 | 0.01 | |
| Total | 0.16 | 0.01 | 0.18 | 0.04 | 0.19 | 0.03 | 0.22 | 0.05 | 0.15 | 0.02 | 0.16 | 0.04 | 0.17 | 0.03 | 0.20 | 0.05 | |
| Stride | Cj7 | 37.37 | 0.19 | 29.91 | 3.55 | 27.25 | 1.22 | 27.38 | 3.18 | 37.29 | 0.31 | 29.91 | 3.55 | 27.28 | 1.26 | 28.27 | 1.20 |
| Cj8 | 22.46 | 3.73 | 22.88 | 3.72 | 21.14 | 2.10 | 18.91 | 1.81 | 23.79 | 1.79 | 22.88 | 3.72 | 21.14 | 2.10 | 18.91 | 1.81 | |
| Cj6 | 30.72 | 0.45 | 30.78 | 2.56 | 25.29 | 3.11 | 22.66 | 3.26 | 30.73 | 0.39 | 30.78 | 2.56 | 25.22 | 3.20 | 22.66 | 3.26 | |
| Total | 29.29 | 6.60 | 27.60 | 4.80 | 24.23 | 3.38 | 22.43 | 4.21 | 29.77 | 5.73 | 27.60 | 4.80 | 24.21 | 3.40 | 22.66 | 4.38 | |
| Stance | Cj7 | 0.07 | 0.00 | 0.13 | 0.02 | 0.14 | 0.04 | 0.17 | 0.01 | 0.07 | 0.01 | 0.14 | 0.01 | 0.14 | 0.03 | 0.13 | 0.06 |
| Cj8 | 0.16 | 0.03 | 0.21 | 0.05 | 0.23 | 0.01 | 0.28 | 0.01 | 0.14 | 0.02 | 0.19 | 0.04 | 0.20 | 0.01 | 0.26 | 0.01 | |
| Cj6 | 0.09 | 0.06 | 0.13 | 0.03 | 0.14 | 0.02 | 0.17 | 0.03 | 0.11 | 0.03 | 0.12 | 0.02 | 0.15 | 0.02 | 0.16 | 0.02 | |
| Total | 0.11 | 0.06 | 0.16 | 0.05 | 0.17 | 0.05 | 0.21 | 0.06 | 0.11 | 0.03 | 0.15 | 0.04 | 0.17 | 0.04 | 0.19 | 0.06 |
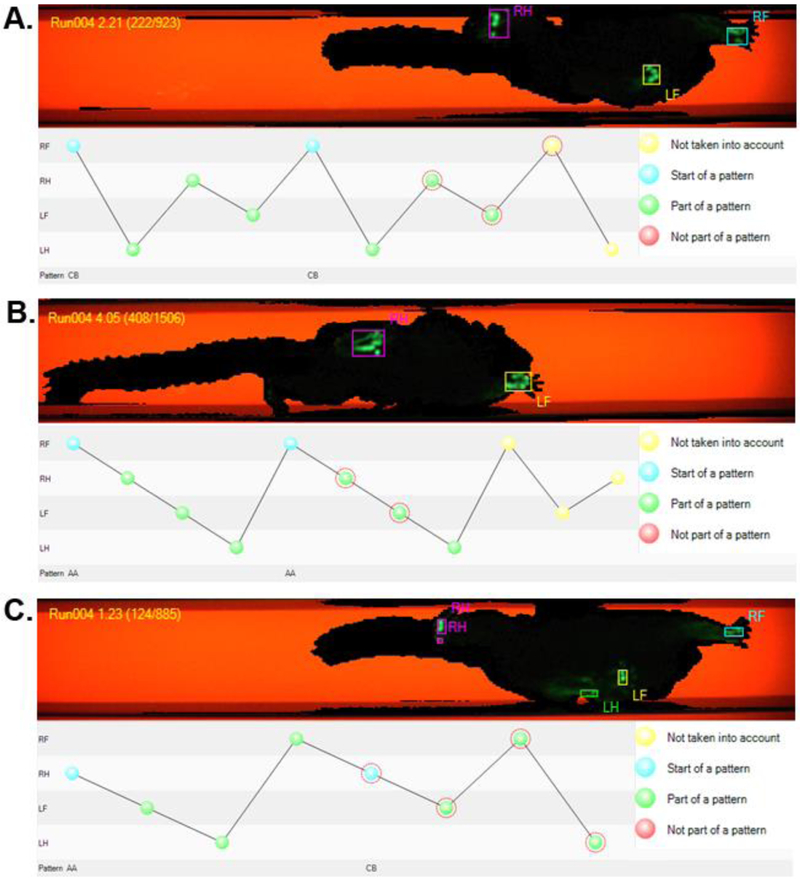
As the data from the two raters did not differ, a single agreed upon data set was used for further analysis. This decision was made to maximize the utility of the data by reducing the variability introduced by the two-raters. The average velocity across all trials for all subjects was 75.2 cm/s. Average velocities were 95.09 cm/s, 83.57 cm/s, 66.26 cm/s and 55.89 cm/s from the first to last days respectively, showing a 59% decrease in speed over the duration of the testing cycle. Step sequence regularity index across all trials for all subjects was 98.07 +/− 4.59, with 10 of the 12 trials resulting in index values of 100. In total, 52.3% of the trials demonstrated an alternate step pattern while 47.7% showed a cruciate step pattern (0% rotate). (Figure 3).
Figure 3:
Analysis of the marmoset step sequence. Examples of a Cruciate (A) and Alternate (B) step sequence pattern. Markers highlighted by the red fiducial lines in the plot correspond to the hands and feet outlined in the image above. (C) In some cases, the subject appears to switch gait patterns mid-run. RF = Right Front, RH = Right Hind, LF = Left Front, LH = Left Hind, CB = Cruciate Pattern B, AA = Alternate Pattern A.
Subsequent analysis revealed numerous runs with differing step patterns within the same run (for example see Figure 3c). In these cases, the subject appears to switch gait patterns mid-run. However, further analysis revealed that while these runs do show two or more patterns within the same run, this is usually due to either simultaneous placement of the diagonal limbs (e.g. RF-LH) or placement of the diagonal limb within 1 to 3 frames (1/100 to 3/100 of a second) of the initial limb placement. In the case of a simultaneous footfall, the CatWalk software identifies the limb that was first labeled in the analysis of the run as the first footfall. This tight coupling of the diagonal limb pair limits the extent to which the step pattern analysis tool is useful for analysis of common marmoset gait
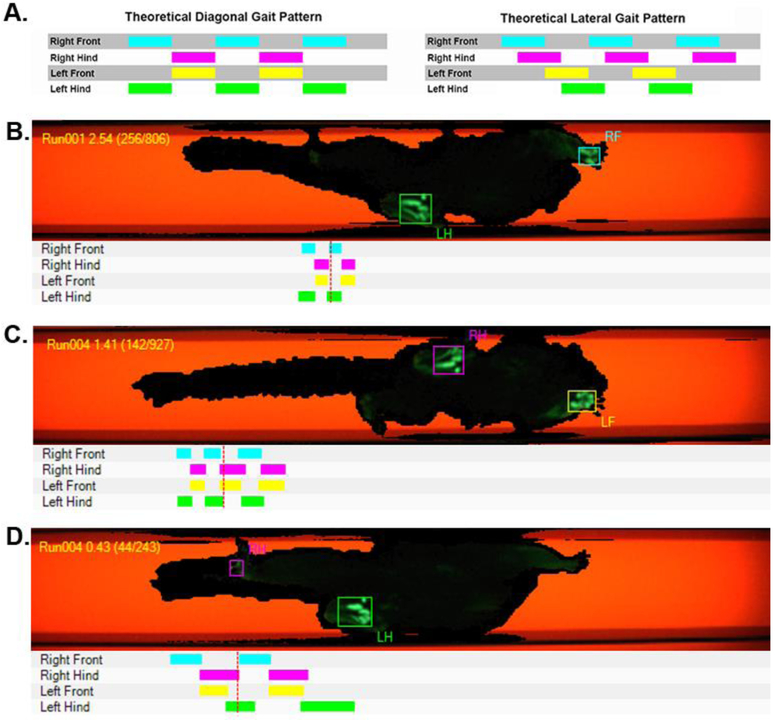
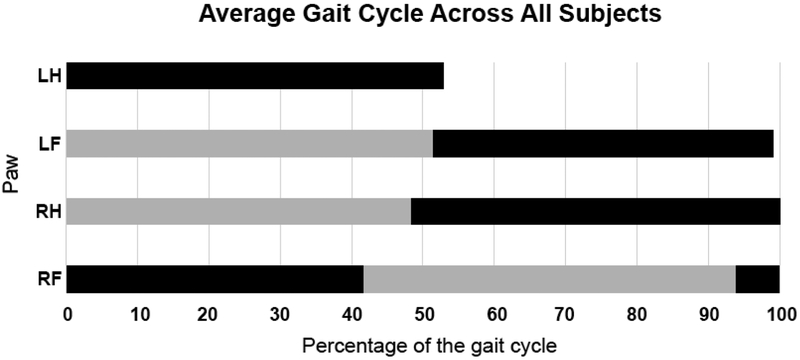
To better quantify the extent to which the limbs were coupled and explore the coordination of the limbs relative to each other we examined visual representations of the runs (Figure 4) and coupling values of the limbs. Visual inspection of the trials resulted in a classification of a diagonal gait pattern in 100% of the runs. Coupling values for the individual limbs for each subject are presented in Table 2. A representation of an average gait cycle for the full experiment was built by combining the coupling value for each of the three target limbs (left front, right hind, right front) with the duty cycle value for that limb. The average gait cycle across all animals for all trials clearly indicates a horizontal gait pattern (Figure 5), which can be seen in the tight coupling of the left hind and right front limbs as well as the right hind and left front limbs.
Figure 4.
Diagonal gait pattern. (A) Idealized versions of a diagonal and lateral gait pattern based on the timing of the initial paw contacts with the recording areas. Examples are shown of the video (top) and limb strike patterns (bottom) for (B) a diagonal right front, left hind contact pattern, (C) a diagonal left front, right hind contact pattern and (D) an atypically-paired but still diagonal right hind, left hind contact pattern. The diagonal pattern was observed in all runs of all trials of all subjects.
Figure 5.
Average limb phase for each step across all subjects and all runs. Black bars represent the average duty cycle for each limb. Grey bars represent the coupling of the indicated limb to the left hind limb (anchor). LH: left hind (foot), LF: left front (hand), RH: right hind (foot), RF: right front (hand)
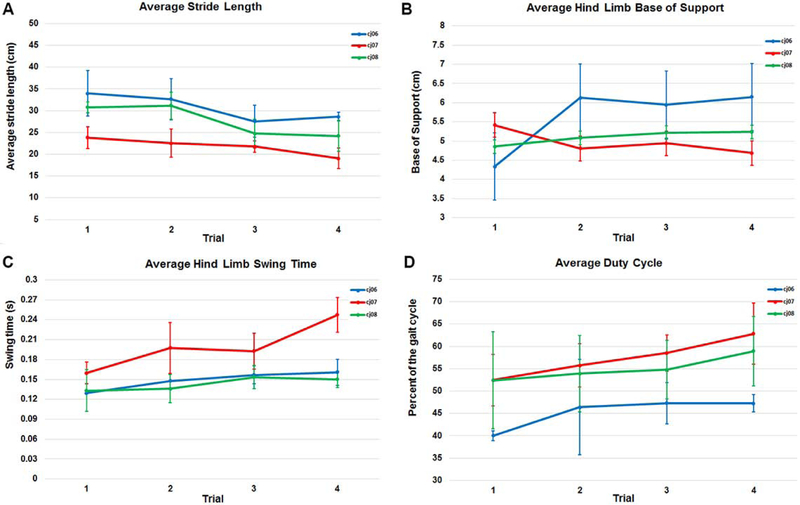
Spatiotemporal measures for the average of the right and left sides were examined at the four time points for each of the three subjects (Figure 6).
Figure 6:
Spatiotemporal gait measures obtained in Experiment 2 including (A) Stride length, (B) Base of support, (C) Swing time, and (D) Duty cycle. Stride length, base of support and swing time are the compost averages of the left and right sides.
4. DISCUSSION
It has been well established that NHPs have a great deal to offer neuroscience research (Capitanio and Emborg 2008, Izpisua Belmonte, Callaway et al. 2015), yet robust, unbiased behavioral measures focused on performance of common everyday tasks, particularly those that translate to a human model are lacking. Establishing and using variables that match measures used in patients offer means of examining pathologies across species using a common point of inference. Here we explored the use of a gait analysis system, similar to those used in humans, for the quantification of gait measures in the common marmoset.
One frequently discussed point during the establishment of the testing and analysis protocol was the extent to which the standard operating procedure for NHPs species should mirror that of humans. On the one hand, this methodological approach is being considered in order to examine behavioral measures that may be more sensitive to change in translational models of disease. On the other hand, the common marmoset is not a human and the quadrupedal gait patterns observed as the subjects traverse the CatWalk corridor are dissimilar in many ways to the bipedal gait of humans. That said, gait, is common to both species in a number of ways. For ambulatory individuals, gait is an activity that is experienced daily and is a common component of many activities of daily living. Although data collection requires modification of the typical environment, the execution of the task can be done in a manner similar to that observed in the natural environment. Additionally, many of the logistical considerations that drive the collection of human gait data hold true for the measurement of NHP data. For example, the established protocol requires that the subject complete four consecutive steps with their hind limbs for a trial or portion of a trial to be valid. While this is a consideration that is based on human gait analysis methods, allowing for data to be included that are derived from less than four consecutive steps results in the inclusion of a single data point for measures such as stride from the side that has two steps and no stride measures from the side with only one step. This issue is particularly impactful when considering pathologies such as PD, in which the disease typically presents unilaterally and progresses differently on the left and right sides.
Marmosets differ from most other nonhuman primates and humans in that they possess claws on their hands and feet instead of nails (except for the big toe) and are arboreal with leaping, jumping, and climbing often their main locomotion pattern in the trees (Schiel and Souto 2017). In spite of this difference, we validated that marmoset locomotion parameters can be measured on a flat substrate using the CatWalk and valid trials can be identified with a high degree of accuracy by trained raters. These parameters matched those commonly used in clinical settings analyzing locomotion of normal versus patients with specific neuromotor deficits (Patterson, Parafianowicz et al. 2008, Pickett, Duncan et al. 2012, Shulman, Katzel et al. 2013, Morris, Lord et al. 2017, Rucco, Agosti et al. 2017). In that regard, our study found that the gait pattern of the marmosets walking on a flat surface fit the normal primate diagonal sequence. These findings contrast two previous works which described marmoset gait as a lateral sequence (Schmitt 2003, Shimada et al 2017), typically found in non-primate mammals (e.g., horses). The different results can probably be explained by technical differences. For gait analysis, Schmitt et al relied upon the collection of simultaneous video from two synchronized cameras and two force plates. Similarly, Shimada et al data collection depended on a four-camera analysis system coupled with two custom force plates. In both reports, the gait pattern was defined by observation of the videos. Video analysis can be subjective and open to interpretation. Additionally, the use of force plates and a limited data collection window likely limits the number of full gait cycles that can be observed and allows for misinterpretation of limb pairing. The video examples presented by Schmitt et al and Shimada et al seemed to us similar to the primate gait pattern included in this report. The CatWalk system analyzes the gait pattern in real time including the force data for each limb, which showed unmistakably that marmosets use a diagonal sequence gait pattern in those conditions.
Dissimilar from humans in most cases, common marmosets are much less compliant with the established protocol. The CatWalk corridor guides the subjects across the measurement area and into the nestbox, yet some subjects failed to conform to a standard gait pattern. Jumps or leaps were sometimes observed during the testing sessions as well as some smaller events, which were not detected visually, but later during data analysis. Although for this report we focused our analysis on the more optimal walking runs to facilitate comparison with human gait, we noticed that when a marmoset leaps and bounds in the CatWalk, it covers the walkway in fewer steps and keeps both front or both hind limbs on the substrate at the same time. Again, the argument could be made that, jumping, leaping and running are common to marmoset gait however, inclusion of such gait patterns would limit the extent to which data could be compared across models (e.g.: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) model of PD) or to develop and test treatments longitudinally. Even within our selective sample, the variability within and between subjects was greatly affected by how the subjects elected to cross the walkway during a given run. This variability is evident in the changes in velocity that occurred over the four-day testing window as well as in the inter-subject variability of the base of support, stride length, swing time, and duty cycle between the testing sessions. Given this variability, future work should explore the possibility of a greater number of runs and/or a longer data collection area.
In Experiment 1 and 2, one subject failed to complete any valid trials during at least one data collection session and repeatedly performed poorly with regard to the established trial inclusion criteria. Both individuals were younger (3.3 and 2.6 years of age at the time of testing) but were not the youngest subjects and were of different genders. Based on these findings we are unable to suggest a type or demographic of common marmoset that may perform better on the CatWalk, but it does appear that some subjects may not be well suited for testing in this environment.
Given the variability of the data across testing days and after discussing the observation notes taken during the study, we propose the following testing procedures be considered: 1) Screen the animals before assigning them to a project that will depend on the CatWalk gait data as a primary outcome measure. 2) Habituate marmosets to handling by a familiar investigator. 3) Although food reward was available, it did not seem to be the primary motivator, thus fasting is not recommended. 4) All data collection points of a study (e.g.: baseline, post-drug, follow-up) should be conducted at the same time of day. 5) Environmental noise and overall distractions should be minimized during data collection; testing environment should remain the same across experimental days. We recommend that the walkway is checked, and clean as needed, between runs of the same and different subjects, as spots in the walkway can be distracting for some monkeys. Some individual variability is unavoidable, such as monkeys’ preference for leaping over walking, or different levels of curiosity that distracts them from completing the run. For the analysis, identification of valid runs and proper footfalls is a critical step in the process of obtaining reliable data.
A limitation of this study was the length of the CatWalk walkway relative to the step length of the subjects. The longer the instrumented recording area, the more steps that can be recorded while moving at a consistent velocity. Additionally, the more steps that can be recorded at each time point, the less variability in the dataset. It will be beneficial to increase the length of the walkway before the future application of this tool for the assessment of variability (both intra- and inter-limb) within the gait cycle. Another issue was the limited length of the instrumented area between the nestbox and the CatWalk, which prevented the animals from walking at a consistent speed when they entered and exited the recording area. In human trials, a one to three meter space is marked off the end of the mat to allow for acceleration and deceleration at the beginning and end of each run. Future work with the CatWalk may attempt to design a gait analysis area with a larger clear glass recording area to facilitate longer data acquisition periods on each pass. A longer walkway would also allow for a more clear translation from the non-human primate model to the human model of pathology as gait analysis in studies involving humans typically examines multiple gait cycles. Another issue was the marmosets’ wide range of velocities while crossing the walkway, which increased the variability in the collected dataset. Although this variance in velocity can be corrected to a certain extent, future work may explore means to better standardize the speed at which the subjects transverse the walkway.
5. Conclusions
The CatWalk system, similar to those used in humans, can be effectively used to quantify spatiotemporal characteristics of gait in the common marmoset after it is retrofitted to this species.
Table 3.
Interlimb coordination measured by coupling of the reference limb to the anchor limb.
| Subject | Trial | LH->RF Mean |
+/− | LH->RH Mean |
+/− | LH->LF Mean |
+/− |
|---|---|---|---|---|---|---|---|
| cj6 | 1 | 100 | 18.45 | 36.96 | 15.37 | 56.52 | 12.30 |
| cj6 | 2 | 102.02 | 8.01 | 54.72 | 7.65 | 51.33 | 7.56 |
| cj6 | 3 | 103.28 | 5.63 | 54.48 | 11.28 | 44.26 | 4.27 |
| cj6 | 4 | 97.52 | 7.27 | 45.25 | 3.14 | 46.41 | 4.61 |
| cj7 | 1 | 100.66 | 6.37 | 48.13 | 4.16 | 48.75 | 3.78 |
| cj7 | 2 | 98.40 | 4.14 | 46.69 | 3.64 | 47.84 | 4.65 |
| cj7 | 3 | 99.89 | 3.75 | 48.24 | 4.09 | 49.00 | 3.40 |
| cj7 | 4 | 95.19 | 3.84 | 46.80 | 3.69 | 43.82 | 2.99 |
| cj8 | 1 | 10.40 | 9.28 | 45.59 | 14.28 | 61.15 | 3.47 |
| cj8 | 2 | 106.53 | 6.06 | 46.82 | 3.02 | 60.76 | 8.74 |
| cj8 | 3 | 104.87 | 9.75 | 52.77 | 10.91 | 53.15 | 5.62 |
| cj8 | 4 | 106.73 | 11.61 | 54.19 | 9.67 | 54.03 | 7.61 |
Highlights.
The Noldus CatWalk XT10.6 was modified for use in common marmoset monkeys.
It allowed unbiased automated quantification of marmosets’ gait.
The data acquisition required minimal animal habituation.
Completion of four consecutive hind-limb footfalls defined a successful run.
A diagonal gait pattern was observed in all subjects.
Acknowledgments
We gratefully acknowledge the dedicated animal care and veterinary staff at the Wisconsin National Primate Research Center for their technical support. This research was supported by grants NIH P51OD011106, NIH UL1TR000427, NIH UL1TR002373, NIH R24OD019803, NIH KL2TR002374, and the University of Wisconsin–Madison Office of Vice Chancellor for Research and Graduate Education, and Department of Medical Physics.
Footnotes
All authors have contributed to this manuscript and have reviewed this document prior to submission.
Competing interests
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baltadjieva R, Giladi N, Gruendlinger L, Peretz C and Hausdorff JM (2006). "Marked alterations in the gait timing and rhythmicity of patients with de novo Parkinson's disease." Eur J Neurosci 24(6): 1815–1820. [DOI] [PubMed] [Google Scholar]
- Bryant MS, Rintala DH, Hou JG, Charness AL, Fernandez AL, Collins RL, Baker J, Lai EC and Protas EJ (2011). "Gait variability in Parkinson's disease: influence of walking speed and dopaminergic treatment." Neurol Res 33(9): 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant MS, Rintala DH, Hou JG, Collins RL and Protas EJ (2016). "Gait variability in Parkinson's disease: levodopa and walking direction." Acta Neurol Scand 134(1): 83–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP and Emborg ME (2008). "Contributions of non-human primates to neuroscience research." Lancet 371(9618): 1126–1135. [DOI] [PubMed] [Google Scholar]
- Cedervall Y, Halvorsen K and Aberg AC (2014). "A longitudinal study of gait function and characteristics of gait disturbance in individuals with Alzheimer's disease." Gait Posture 39(4): 1022–1027. [DOI] [PubMed] [Google Scholar]
- Duncan RP and Earhart GM (2012). "Randomized controlled trial of community-based dancing to modify disease progression in Parkinson disease." Neurorehabil Neural Repair 26(2): 132–143. [DOI] [PubMed] [Google Scholar]
- Ellis TD, Cavanaugh JT, Earhart GM, Ford MP, Foreman KB, Thackeray A, Thiese MS and Dibble LE (2016). "Identifying clinical measures that most accurately reflect the progression of disability in Parkinson disease." Parkinsonism Relat Disord 25: 65–71. [DOI] [PubMed] [Google Scholar]
- Hackney ME and Earhart GM (2009). "Backward walking in Parkinson's disease." Mov Disord 24(2): 218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamers FP, Lankhorst AJ, van Laar TJ, Veldhuis WB and Gispen WH (2001). "Automated quantitative gait analysis during overground locomotion in the rat: its application to spinal cord contusion and transection injuries." J Neurotrauma 18(2): 187–201. [DOI] [PubMed] [Google Scholar]
- Herold S, Kumar P, Jung K, Graf I, Menkhoff H, Schulz X, Bähr M and Hein K (2016). "CatWalk gait analysis in a rat model of multiple sclerosis." BMC Neurosci 17(1): 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill KK, Campbell MC, McNeely ME, Karimi M, Ushe M, Tabbal SD, Hershey T, Flores HP, Hartlein JM, Lugar HM, Revilla FJ, Videen TO, Earhart GM and Perlmutter JS (2013). "Cerebral blood flow responses to dorsal and ventral STN DBS correlate with gait and balance responses in Parkinson's disease." Exp Neurol 241: 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izpisua Belmonte JC, Callaway EM, Caddick SJ, Churchland P, Feng G, Homanics GE, Lee KF, Leopold DA, Miller CT, Mitchell JF, Mitalipov S, Moutri AR, Movshon JA, Okano H, Reynolds JH, Ringach D, Sejnowski TJ, Silva AC, Strick PL, Wu J and Zhang F (2015). "Brains, genes, and primates." Neuron 86(3): 617–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord S, Baker K, Nieuwboer A, Burn D and Rochester L (2011). "Gait variability in Parkinson's disease: an indicator of non-dopaminergic contributors to gait dysfunction?" J Neurol 258(4): 566–572. [DOI] [PubMed] [Google Scholar]
- Morris ME, Martin CL and Schenkman ML (2010). "Striding out with Parkinson disease: evidence-based physical therapy for gait disorders." Phys Ther 90(2): 280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R, Lord S, Lawson RA, Coleman S, Galna B, Duncan GW, Khoo TK, Yarnall AJ, Burn DJ and Rochester L (2017). "Gait Rather Than Cognition Predicts Decline in Specific Cognitive Domains in Early Parkinson's Disease." J Gerontol A Biol Sci Med Sci 72(12): 1656–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers PS, McNeely ME, Pickett KA, Duncan RP and Earhart GM (2018). "Effects of exercise on gait and motor imagery in people with Parkinson disease and freezing of gait." Parkinsonism Relat Disord 53: 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dowd S, Galna B, Morris R, Lawson RA, McDonald C, Yarnall AJ, Burn DJ, Rochester L and Anderson KN (2017). "Poor Sleep Quality and Progression of Gait Impairment in an Incident Parkinson's Disease Cohort." J Parkinsons Dis 7(3): 465–470. [DOI] [PubMed] [Google Scholar]
- Patterson KK, Mansfield A, Biasin L, Brunton K, Inness EL and McIlroy WE (2015). "Longitudinal changes in poststroke spatiotemporal gait asymmetry over inpatient rehabilitation." Neurorehabil Neural Repair 29(2): 153–162. [DOI] [PubMed] [Google Scholar]
- Patterson KK, Parafianowicz I, Danells CJ, Closson V, Verrier MC, Staines WR, Black SE and McIlroy WE (2008). "Gait asymmetry in community-ambulating stroke survivors." Arch Phys Med Rehabil 89(2): 304–310. [DOI] [PubMed] [Google Scholar]
- Peterson DS, Pickett KA, Duncan R, Perlmutter J and Earhart GM (2014). "Gait-related brain activity in people with Parkinson disease with freezing of gait." PLoS One 9(3): e90634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett KA, Duncan RP, Hoekel J, Marshall B, Hershey T, Earhart GM and Washington G University Wolfram Study (2012). "Early presentation of gait impairment in Wolfram Syndrome." Orphanet J Rare Dis 7: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilgram LM, Earhart GM and Pickett KA (2016). "Impact of limiting visual input on gait: Individuals with Parkinson disease, age-matched controls, and healthy young participants." Somatosens Mot Res 33(1): 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins NW, Pohlmeyer EA, Debnath S, Mylavarapu R, Geng S, Sanchez JC, Rothen D and Prasad A (2017). "Common marmoset (Callithrix jacchus) as a primate model for behavioral neuroscience studies." J Neurosci Methods 284: 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozanski GM, Wong JS, Inness EL, Patterson KK and Mansfield A (2019). "Longitudinal change in spatiotemporal gait symmetry after discharge from inpatient stroke rehabilitation." Disabil Rehabil: 1–7. [DOI] [PubMed] [Google Scholar]
- Rucco R, Agosti V, Jacini F, Sorrentino P, Varriale P, De Stefano M, Milan G, Montella P and Sorrentino G (2017). "Spatio-temporal and kinematic gait analysis in patients with Frontotemporal dementia and Alzheimer's disease through 3D motion capture." Gait Posture 52: 312–317. [DOI] [PubMed] [Google Scholar]
- Schiel N and Souto A (2017). "The common marmoset: An overview of its natural history, ecology and behavior." Dev Neurobiol 77(3): 244–262. [DOI] [PubMed] [Google Scholar]
- Schmitt D (2003). "Evolutionary implications of the unusual walking mechanics of the common marmoset (C. jacchus)." Am J Phys Anthropol 122(1): 28–37. [DOI] [PubMed] [Google Scholar]
- Shimada H, Kanai R, Kondo T, Yoshino-Saito K, Uchida A, Nakamura M, Ushiba J, Okano H and Ogihara N (2017). "Three-dimensional kinematic and kinetic analysis of quadrupedal walking in the common marmoset (Callithrix jacchus)." Neuroscience research, 125: 11–20. [DOI] [PubMed] [Google Scholar]
- Shulman LM, Katzel LI, Ivey FM, Sorkin JD, Favors K, Anderson KE, Smith BA, Reich SG, Weiner WJ and Macko RF (2013). "Randomized clinical trial of 3 types of physical exercise for patients with Parkinson disease." JAMA Neurol 70(2): 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatenhorst L, Eckermann K, Dambeck V, Fonseca-Ornelas L, Walle H, Lopes da Fonseca T, Koch JC, Becker S, Tönges L, Bähr M, Outeiro TF, Zweckstetter M and Lingor P (2016). "Fasudil attenuates aggregation of α-synuclein in models of Parkinson's disease." Acta Neuropathol Commun 4: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuno H, Watson C, Roberts A, Sasaki E and Okano H (2015). "Marmoset neuroscience." Neurosci Res 93: 1–2. [DOI] [PubMed] [Google Scholar]
- Vandeputte C, Taymans JM, Casteels C, Coun F, Ni Y, Van Laere K and Baekelandt V (2010). "Automated quantitative gait analysis in animal models of movement disorders." BMC Neurosci 11: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin JE, Janssen ML, Sager TN and Temel Y (2012). "Automated gait analysis in bilateral parkinsonian rats and the role of L-DOPA therapy." Behav Brain Res 226(2): 519–528. [DOI] [PubMed] [Google Scholar]