Abstract
Rationale and objective:
The pathogenesis of disordered mineral metabolism in chronic kidney disease (CKD) is largely informed by cross-sectional human studies and longitudinal animal studies. We sought to characterize the longitudinal evolution of disordered mineral metabolism during the course of CKD.
Study design:
Retrospective analysis nested in a cohort study.
Setting & Participants:
Participants of the Chronic Renal Insufficiency Cohort (CRIC) Study who had up to 5 serial annual measurements of estimated glomerular filtration rate (eGFR), fibroblast growth factor 23 (FGF-23), parathyroid hormone (PTH), serum phosphate, and serum calcium and who subsequently reached end stage kidney disease (ESKD) during follow-up (n=847).
Exposure:
Years prior to ESKD.
Outcomes:
Serial FGF-23, PTH, serum phosphate, and serum calcium.
Analytical Approach:
To assess longitudinal dynamics of disordered mineral metabolism in human CKD, we used “ESKD-anchored longitudinal analyses” to express time as years before ESRD, enabling assessments of mineral metabolites spanning 8 years of CKD progression prior to ESKD.
Results:
Mean concentrations of FGF-23 rose markedly as time before ESRD decreased, while PTH and phosphate rose modestly and levels of calcium declined minimally. Compared to other mineral metabolites, FGF-23 demonstrated the highest rate of change (velocity: 1st derivative of the function of concentration over time) and magnitude of acceleration (2nd derivative). These changes became evident approximately 5 years prior to ESRD and persisted without deceleration through ESRD onset. Rates of changes in PTH and phosphate increased modestly and without marked acceleration around the same time, with modest deceleration immediately prior to ESRD, when use of active vitamin D and phosphate binders increased.
Limitations:
Individuals who entered the CRIC Study at early stages of CKD and who did not progress to ESRD were not studied.
Conclusions:
Among patients with progressive CKD, levels of FGF23 begin to rise 5 years prior to ESRD and continue to rapidly accelerate until transition to ESRD.
Keywords: chronic kidney disease (CKD), disordered mineral metabolism, end-stage renal disease (ESRD), fibroblast growth factor 23 (FGF-23), parathyroid hormone (PTH), CKD progression, incident kidney failure, biomarker, phosphate, calcium, kidney function, longitudinal trends, serial measurements
Introduction
Disordered mineral metabolism is a nearly universal complication of chronic kidney disease (CKD) that has been linked to increased risks of cardiovascular disease events, progression of CKD to end stage renal disease (ESRD) and mortality.1–4 Understanding how changes in markers of mineral metabolism evolve over time will provide insights into the pathogenesis of disordered mineral metabolism in CKD, enable epidemiologic analyses that relate temporal trends to risks of adverse events and facilitate identification of high-risk patterns of change that can be targeted for intervention in future studies.
Using cross-sectional data from the Chronic Renal Insufficiency Cohort (CRIC) Study, we previously reported that elevated fibroblast growth factor 23 (FGF-23) was more prevalent at higher estimated glomerular filtration rate (eGFR) than secondary hyperparathyroidism or hyperphosphatemia.1 Our findings suggested that FGF-23 excess preceded the onset of secondary hyperparathyroidism and hyperphosphatemia in CKD. Although longitudinal data from animal studies are supportive,5,6 long-term serial data within individual patients with progressive CKD are lacking. To determine the longitudinal evolution of disordered mineral metabolism during the course of human CKD, we examined a subset of CRIC Study participants who developed ESRD, and we looked back at their repeated annual measurements of mineral metabolites using “ESRD-anchored longitudinal analyses.” We hypothesized that, in the prelude to ESRD, levels of FGF23 would rise more rapidly compared to changes in other mineral metabolites.
Methods
The CRIC Study
The CRIC Study is a prospective observational cohort study of patients with CKD conducted in the United States across 7 clinical centers, comprising 13 recruitment sites.7 During Phase I (June 2003 – August 2008), 3,939 individuals aged 21 to 74 years with an eGFR of 20–70 ml/min/1.73m2 were enrolled. Blacks and individuals with diabetes were oversampled, and 12% of participants are Hispanic. Exclusion criteria included inability to consent, institutionalization, enrollment in other studies, pregnancy, New York Heart Association class III–IV heart failure, HIV, cirrhosis, myeloma, polycystic kidney disease, renal cancer, recent chemotherapy or immunosuppressive therapy, organ transplantation, or prior treatment with dialysis for one month. The protocol was approved by institutional review boards at each study site, and all participants provided written informed consent.
Study population and study design
The CRIC Mineral Metabolism Subcohort was designed to evaluate serial levels of markers of mineral metabolism and relate their changes over a maximum of 5 yearly time points to risks of progression to ESRD and mortality through case-cohort studies.8
To capture mineral metabolism evolution in the period up to ESRD, we studied 847 CRIC Study participants who had serial measurements of mineral metabolism markers and who progressed to ESRD during follow up. In ESRD-anchored longitudinal analyses, we expressed time as years before ESRD instead of years of the CRIC annual study visits. Therefore, we set the time at ESRD onset to be time=0; time prior to ESRD transition had a negative value. Anchoring follow up time to time of ESRD onset, allowed us to have adequate coverage with at least 69 participants at each time point for each analyte over 8 annual time points prior to ESRD onset.
Measurements of markers of mineral metabolism
The central CRIC Study laboratory measured levels of FGF-23 in duplicate using a second-generation carboxy-terminal assay (Immutopics, San Clemente, CA) in stored frozen plasma samples collected at the baseline and subsequent annual visits. The mean intra-assay coefficient of variation for paired assays was <6.5%. The laboratory also measured annual calcium, phosphate and PTH levels. PTH levels were measured using the total PTH assay, which includes the 1–84 PTH molecule and 7–84 fragments assay (Scantibodies, Santee, CA). Calcitriol levels were measured at two time points (corresponding to CRIC Study follow up visits at years 1 and 4) in the University of Washington using a multiplex HPLC-mass spectrometry assay on a Xevo TQ spectrometer (Waters Corp., Milford, MA).9
Assessment of covariates
Demographic and clinical data were ascertained by interview, questionnaire and physical examination at annual in-person visits. eGFR was calculated using the CKD-EPI formula.10
ESRD status and timing of onset were ascertained by self-report, review of medical records and linkage with the United States Renal Data System (USRDS), a national data registry on ESRD population in the United States.
Statistical analysis
We used descriptive statistics to characterize the study population and the distribution of eGFR and markers of mineral metabolism across time in relation to the number of years prior to ESRD onset. We applied natural log-transformation for PTH and FGF-23, which were not normally distributed.
In ESRD-anchored longitudinal analyses, we assessed the dynamic patterns of change in markers of mineral metabolism prior to ESRD transition. We used a modified version of an integrated approach previously developed for analysis of hormonal changes across menopausal transition.11 First, to examine the magnitudes of mineral metabolism abnormalities at any given point of time prior to ESRD, we plotted cubic spline smoothed population mean levels of mineral metabolites across time in relation to the number of years prior to ESRD onset. Next, we estimated instantaneous rates of change of mineral metabolites (“velocity”) with first-order derivatives and acceleration with second-order derivatives. We used the ‘npregfast’ package12 in R, which uses cubic spline smoothing approach to plot these estimates and to obtain their 95% confidence intervals (CI) through bootstrapping. When the 95% CI in acceleration excluded the null value of zero, we inferred that there was an increase in the rate of change and assumed that there may be a significant change-point in the corresponding population mean. We tested this assumption with piecewise linear mixed models that tested for significant differences in slopes between the two segments separated by the tested change-point. Since linear mixed models allow for missing data, we studied all available individuals.
To test whether dynamic changes in mineral metabolism are starker in certain CRIC participants, we examined longitudinal evolution of disordered mineral metabolism within subgroups of gender, race, and history of diabetes. Because use of phosphate binders and nutritional and active vitamin D may affect levels of mineral metabolites,13–15 we performed sensitivity analyses that only used data prior to exposure of participants to these medications. Since death precludes the onset of ESRD, we evaluated population means of mineral metabolites as functions of years until death in 719 CRIC Study participants who were included in the case-cohort study of mortality.8
All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC), and R version 3.4.0 (April 21, 2017; http://cran.r-project.org). All statistical tests were two-sided, and p-values < 0.05 were considered statistically significant.
Results
The study population consisted of 847 individuals who all progressed to ESRD during follow-up. At enrollment, the study participants demonstrated advanced CKD, as evidenced by low eGFR and high UACR, and high prevalence of comorbidities and risk factors for CKD progression (Table 1). Less than 10% of the participants were prescribed nutritional vitamin D, phosphate binders or active vitamin D at enrollment (Table 1).
Table 1.
Characteristics at enrollment of the 847 participants who progressed to ESRD during follow up.
| Characteristic | Values |
|---|---|
| Age, years | 55.5 ± 11.6 |
| Female, % | 40.0 |
| Black, % | 52.3 |
| Hispanic, % | 17.4 |
| Current smoking, % | 16.3 |
| BMI, kg/m2 | 32.6 ± 8.2 |
| SBP, mmHg | 138.6 ± 24.0 |
| Hypertension, % | 93.7 |
| Diabetes, % | 65.4 |
| Heart failure, % | 12.9 |
| Stroke, % | 11.3 |
| Peripheral vascular disease, % | 9.8 |
| Coronary artery disease, % | 23.9 |
| Phosphate binder use (%) | 8.0 |
| Nutritional vitamin D use (%) | 7.7 |
| Active vitamin D use (%) | 5.2 |
| eGFR, ml/min/1.73m2 | 33.9 ± 11.1 |
| UACR, mg/g | 786.3 (232.9 – 1978.3) |
| Hemoglobin, g/dL | 11.9 ± 1.8 |
| Serum albumin, g/dL | 3.7 ± 0.5 |
| Calcium, mg/dL | 9.0 ± 0.5 |
| Phosphate, mg/dL | 4.0 ± 0.8 |
| PTH, pg/mL | 88.0 (52.0 – 144.1) |
| FGF-23, RU/mL | 207.2 (140.9 – 334.7) |
Values are means ± standard deviation (SD), medians [interquartile range].
Abbreviations: BMI, body mass index; SBP, systolic blood pressure; eGFR, estimated glomerular filtration rate; UACR, urine albumin-creatinine ratio; PTH, parathyroid hormone; FGF-23, fibroblast growth factor 23; RU, reference units.
Serial measures of mineral metabolism prior to progression to ESRD
To investigate evolution of mineral metabolism as functions of individual patients’ variable rates of CKD progression in ESRD-anchored longitudinal analyses, we expressed time as years before ESRD onset rather than anchoring time to the CRIC Study annual visits. This allowed us to estimate longitudinal rates of change in mineral metabolites, assess how rapidly changes developed during a time frame when eGFR declined progressively (Figure 1A), and determine when change-points occurred in relation to years prior to ESRD.
Figure 1. Serial eGFR and levels of mineral metabolites prior to ESRD onset.
A) Mean (standard deviation) in eGFR prior to ESRD transition;
B) Box plots of ln(FGF-23), ln(PTH), phosphate, and calcium levels prior to ESRD transition;
C) Repeated measures of eGFR, FGF-23, PTH, phosphate, and calcium prior to ESRD transition.
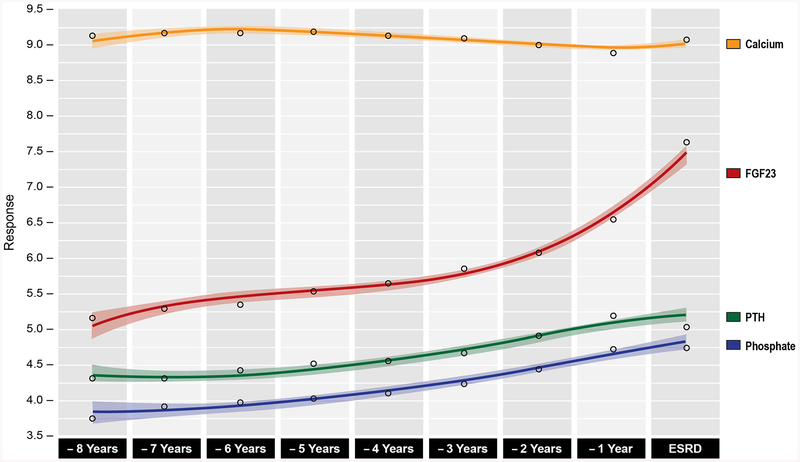
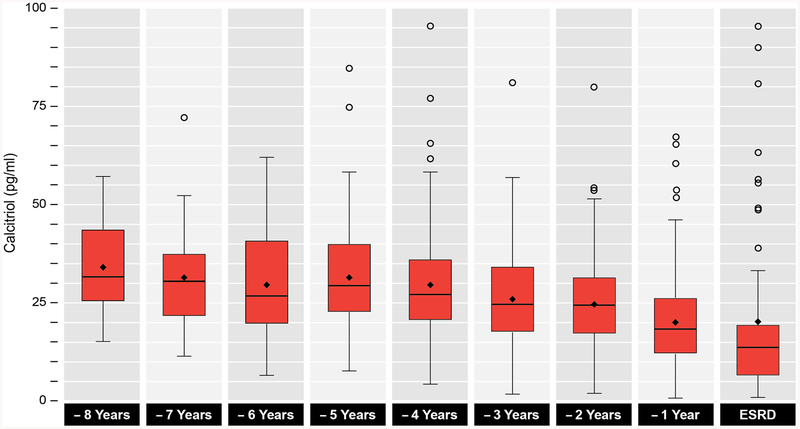
Overall, levels of FGF-23 displayed a steeper rise over time compared to more modest elevations in levels of PTH and phosphate (Figures 1B, 1C and 2). Levels of calcium were mostly stable (Figures 1B, 1C and 2), while calcitriol steadily declined as ESRD approached (Figure 3).
Figure 2. Population mean levels of mineral metabolites prior to ESRD onset.
Cubic spline smoothed population mean levels (with 95% confidence intervals) in ln(FGF-23) (red), ln(PTH) (green), phosphate (blue), and calcium (orange) levels prior to ESRD transition. Shaded areas represent 95% confidence intervals.
Figure 3. Serial calcitriol concentrations prior to ESRD onset.
Box plots of calcitriol levels prior to ESRD transition.
Rates of change and acceleration in mineral metabolites prior to progression to ESRD
We calculated the first and second derivatives of the longitudinal functions of FGF-23, PTH, phosphate, and calcium concentrations over time as CKD progressed to ESRD to estimate rates of change (concentration per year: “velocity”) and acceleration (change in velocity) of each metabolite. Consistent with the observation of marked elevation of FGF-23 prior to ESRD transition (Figures 1 and 2), the rate of change and acceleration of FGF-23 also increased; the rate of precipitous FGF-23 rise occurred at approximately 5 years prior to ESRD onset (Figure 4A), when the acceleration curve and its 95% confidence intervals exceeded zero. Thereafter, there was no detectable deceleration in the FGF23 function over time.
Figure 4. Rates of change and acceleration in mineral metabolites prior to ESRD transition.
First and second derivatives of A) FGF-23; B) PTH; C) phosphate; and D) calcium estimated by cubic spline approach. First derivative denotes rate of change and second derivative represents acceleration. Values are shown as estimated rate of change (blue) and acceleration (red), and shaded areas represent 95% confidence intervals.
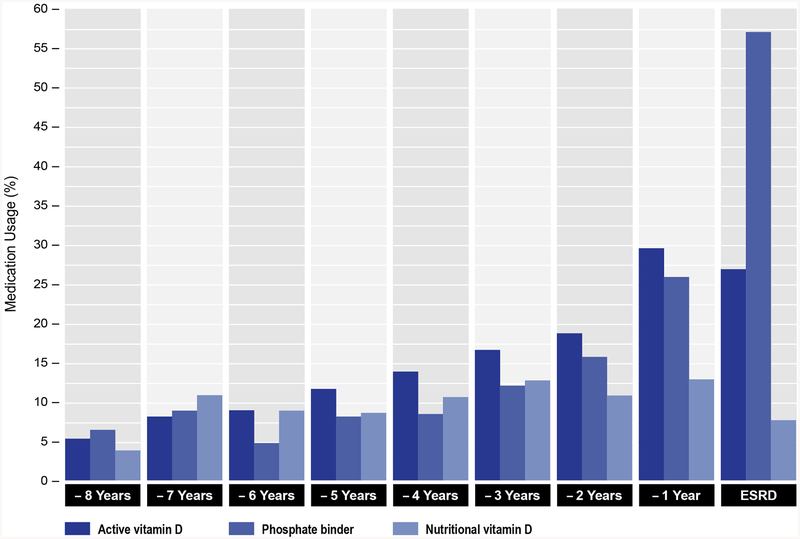
The rate of change and acceleration curves increased slightly for PTH at approximately 5 years prior to ESRD (Figure 4B). The rate of change of PTH increased more noticeably at approximately 4 years prior to ESRD, but acceleration remained minimal. The rate of change in PTH decreased at approximately 2.5 years prior to ESRD, possibly due to increasing use of activated vitamin D (Figure 5) as CKD neared ESRD. The rate of change of serum phosphate increased modestly but steadily prior to ESRD with only negligible acceleration (Figure 4C). Serum calcium levels remained relatively stable early in the course of CKD, with declining rate of change evident at approximately 4 years. The rate of change in calcium was accompanied by a flat acceleration, until approximately 2.7 years prior to ESRD (Figure 4D). Around this time point, the rate of change for calcium started to reverse direction, suggesting that the increasing use of phosphate binders and activated vitamin D might have also affected serum calcium levels.
Figure 5. Use of phosphate binders and nutritional and active vitamin D prior to ESRD onset.
Percentage of use of phosphate binders and nutritional and active vitamin D by years prior to ESRD onset.
Change-points and change in slope of mineral metabolites prior to progression to ESRD
We identified statistically significant change-points in the mineral metabolites’ longitudinal functions by examining the time when the 95% CI for the acceleration curves of each mineral metabolite excluded 0. Using these pre-determined change-points, we determined the slopes before and after the change-points and tested for significance of the changes in slopes with piecewise linear mixed models (Table 2). This step demonstrated that the timing of significant change-points was similar for FGF-23, PTH and serum phosphate at approximately 5 years prior to ESRD, however, the magnitude of the change in slope was highest for FGF-23. Based on changes in acceleration, the change-point for serum calcium was estimated to occur at 2.7 years prior to ESRD, although the slopes before and after this time point did not differ significantly (Table 2).
Table 2.
Change-points and change in slope of mineral metabolites prior to progression to ESRD.
| Mineral metabolite | Change-point | Slope before change-point | Slope after change-point | Change of slope | %change of slope | p-value |
|---|---|---|---|---|---|---|
| Ln(FGF-23, RU/mL/y) | −5.1 | 0.032 (−0.027 – 0.092) | 0.372 (0.242 – 0.503) | 0.340 (0.269 – 0.411) | 1055.3 | <0.001 |
| Ln(PTH, pg/mL/y) | −4.9 | 0.089 (0.046 – 0.132) | 0.179 (0.084 – 0.273) | 0.090 (0.038 – 0.142) | 101 | <0.001 |
| Phosphate, mg/dL/y | −5.2 | 0.056 (−0.003 – 0.116) | 0.186 (0.055 – 0.316) | 0.129 (0.058 – 0.200) | 229 | <0.001 |
| Calcium, mg/dL/y | −2.7 | −0.014 (−0.030 – 0.001) | −0.011 (−0.057 – 0.036) | 0.004 (−0.027 – 0.035) | 26.7 | 0.8 |
P values were obtained from piecewise linear mixed models that were used to test for significant differences in slopes between the two segments separated by change-points that where identified when the 95% CI in acceleration curves excluded the null value of zero.
Sensitivity and subgroup analyses
Plots of population means of mineral metabolites were qualitatively similar across strata of gender, race, and history of diabetes (Figure S1–S3).
Population means of mineral metabolites restricted to observations prior to initiation of phosphate binders and nutritional and active vitamin D were qualitatively similar to overall results (Figure 6). However, the peak magnitude of the FGF-23 rise immediately prior to ESRD was attenuated, whereas serial changes in PTH, phosphate and serum calcium were accentuated (Figure 6).
Figure 6. Population mean levels of mineral metabolites restricted to observations prior to initiation of phosphate binders and nutritional and active vitamin D.
Cubic spline smoothed population mean levels (with 95% confidence intervals) in ln(FGF-23) (red), ln(PTH) (green), phosphate (blue), and calcium (orange) levels prior to ESRD transition. Shaded areas represent 95% confidence intervals. The table below the plot depicts the number of participants with available measurements of FGF-23, PTH, phosphate, and calcium by years prior to ESRD transition. Repeated measures values are shown as median (interquartile range) for FGF-23 and PTH and mean ± standard deviation for phosphate and calcium.
Plots of serial levels of mineral metabolism markers anchored to years prior to death, rather than years prior to ESRD, demonstrated rising levels of FGF-23, but stable trends for other parameters in 719 participants who were included the case-cohort study of mortality (Figure S4).
Discussion
Cross-sectional human studies and longitudinal animal studies suggest that elevation in FGF-23 is one of the earliest detectable changes in mineral metabolism in CKD.1,5,6 In this longitudinal study of CRIC Study participants who progressed to ESRD, we examined the dynamics of FGF-23, PTH, phosphate, and calcium concentrations for up to 8 years leading up to ESRD. We found that as eGFR declined progressively, levels of FGF-23 rose markedly and persistently, with the greatest rate of change and the highest magnitude of acceleration compared to other mineral metabolites. Changes in FGF-23, but also in PTH and phosphate, albeit to lower magnitudes and without marked acceleration, were evident at approximately 5 years prior to ESRD, when the mean eGFR was 33 ml/min/1.73m2. The results support the findings from prior cross-sectional human studies and longitudinal animal studies and yield insights into the CKD population that is likely to experience dynamic changes in mineral metabolism in the course of progressive CKD.
For our ESRD-anchored longitudinal analyses, we leveraged an integrated approach previously developed for comprehensive analysis of dynamic hormonal changes around the menopausal transition.11 We aimed to capture dynamic pathophysiologic effects by evaluating participants who progressed to ESRD during follow-up and by expressing time as years before ESRD onset instead of years of the CRIC Study annual visits. By anchoring time to a discrete clinical event in the course of progressive CKD rather than the study’s operational design, we reduced noise that would be introduced by evaluating a blend of participants with different baseline severity of CKD and different rates of CKD progression. Furthermore, anchoring to onset of ESRD enabled the maximum of 5 time points of blood sampling per individual participant to span 8 years of pre-ESRD time, which allowed us to detect dynamic changes in mineral metabolites beginning 5 years prior to ESRD transition.
Importantly, although our study population had lower mean eGFR at enrollment compared to the entire CRIC Study cohort (34 vs. 45 ml/min/1.73m2), our time frame included a 3-year period of relative stability (between 8 and 5 years prior to ESRD). The period of stability prior to dynamic change in individuals who progressed to ESRD suggests that our chosen study population and time frame were optimal for detecting dynamic changes in mineral metabolism over time.
Abnormalities of mineral metabolism intensified approximately 5 years prior to the onset of ESRD, when the mean baseline eGFR was 33 ml/min/1.73m2. We interpret the finding of nearly identical change points for serum phosphate, PTH, and FGF-23 to likely represent interdependency between our parameters of interest, which is consistent with the well-described endocrine feedback loops involved in the regulation of mineral metabolism. Although the timing for dynamic changes in FGF-23, PTH and phosphate was nearly identical, we found that FGF-23 demonstrated the greatest rate of change and the highest magnitude of acceleration compared to other mineral metabolites. Whereas PTH and phosphate changes decelerated modestly immediately prior to ESRD, the acceleration in FGF-23 increased unabated during the 5-year prelude to ESRD. Infrequent use of phosphate binders and active vitamin D and the observational design of our study limited our ability to define the impact of medications on mineral metabolism dynamics. Nevertheless, based on known effects of phosphate binders and active vitamin D13–15 and our data showing their use increased in the time period immediately prior to ESRD, we hypothesize that the medications might have affected dynamic trends in all mineral metabolites during the late prelude interval. The subtle differences we observed between the plots of population mean levels in all participants (Figure 2) versus plots from data restricted to observations prior to initiation of phosphate binders and nutritional and active vitamin D (Figure 6) suggest that increased use of binders and active vitamin D attenuated the increases in phosphate and PTH and accelerated the rise in FGF-23 immediately prior to ESRD onset.
Our data further show that the magnitude of the change in slope before and after the change-point of ~5 years was highest for FGF-23 (Table 2), supporting the conclusion that while all mineral metabolism parameters change at the same time before ESRD, the evolution of FGF-23 is most marked. Consistent with the timing of the rise in FGF-23, calcitriol levels declined around 5 years prior to ESRD (Figure 3). We also confirm that hyperphosphatemia and hypocalcemia are late developments,16,17 occurring at 1 year prior to ESRD onset (Figure 1C). Taken together, our data broadly support the findings from prior cross-sectional human studies and longitudinal animal studies.1,5,6,18,19
The major strengths of our study are the longitudinal design with a sizable population of patients with progressive CKD who had serial yearly measurements of mineral metabolites and who were followed until onset of ESRD. In an adaptation of a method developed for studying the prelude to menopause,11 we applied ESRD-anchored longitudinal modeling as a novel method of CKD research. Our integrated analytic approach incorporated population means together with rates of change and acceleration and allowed us to detect change-points, which we subsequently tested with piecewise linear mixed models.
Limitations of our study include inability to assess changes in levels of α-Klotho and intact FGF-23, measurements of which are not available in the CRIC Study. Serial levels of urinary phosphate excretion, dietary phosphate intake, 25-hydroxyvitamin D or iron status, which preferentially affect carboxy-terminal FGF-23 levels, are also not available. Because etiology of CKD was not ascertained in the CRIC Study, we were not able to examine heterogeneity by CKD subtype. However, we did not find any differences in population means in subgroups of gender, race, and history of diabetes. Since our study population included American patients with CKD, whose characteristics differ from other CKD populations globally, our results may not be fully generalizable to all individuals with CKD across the world. Because we only studied participants who progressed to ESRD during follow up, our analysis excluded many individuals who entered the CRIC Study at early stages of CKD and who did not progress to ESRD during follow up. In addition to potential selection bias, this may have impeded our ability to detect earlier change-points in mineral metabolites. However, we were able to examine a period of 8 years prior to ESRD, which included several years before and after our determined change-points. Therefore, our approach allowed for detection of important pathophysiologic trends. Although our current study did not include evaluations of risk or causality, analyses of population mean levels of mineral metabolites prior to death support the significance of our findings by confirming the previously reported relationship between rising FGF23 levels and mortality.8
Disordered mineral metabolism is a progressive disorder that develops early in the course of CKD, continues through the period of ESRD, and often persists into the post-transplantation period.20 Based on a cross-sectional analysis of the CRIC Study, we previously concluded that patients with eGFR less than 60 ml/min/1.73m2 could be targeted for clinical trials testing interventions for disordered mineral metabolism.1 Our findings from ESRD-anchored longitudinal analyses suggest that a critical period in the pathogenesis is approximately 5 years prior to ESRD, or at CKD stage 3b. Although the necessity to intervene is debated by guideline groups,21,22 our current results would suggest that it is prudent to consider close surveillance of disordered mineral metabolism in patients with CKD stage 3b. Since this population is likely to experience dynamic changes in markers of mineral metabolism over time, clinical investigators may opt to target patients with CKD stage 3b for future trials aimed at testing novel interventions for disordered mineral metabolism.
Supplementary Material
Figure S1. Population mean levels of mineral metabolites prior to ESRD, by sex.
Figure S2. Population mean levels of mineral metabolites prior to ESRD, by race.
Figure S3. Population mean levels of mineral metabolites prior to ESRD, by presence of diabetes at baseline.
Figure S4. Population mean levels of mineral metabolites prior to death.
Acknowledgements:
The authors thank the participants, investigators, and staff of the CRIC study for their time and commitment.
Support: This work was supported by grants R01DK102438 (TI), R01DK110087 (TI), R01DK099199 (IdB), R01DK111952 (JS), R01DK081374 (MW), R01DK076116 (MW), R01DK094796 (MW), K24DK093723 (MW), U01DK099930 (MW, TI), and P30DK114857 from the National Institutes of Health, and a Strategically Focused Research Network Center Grant on Health Disparities from the American Heart Association (MW, TI). Funding for the CRIC Study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003; Johns Hopkins University UL1 TR-000424; University of Maryland GCRC M01 RR-16500; Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research; Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433; University of Illinois at Chicago CTSA UL1RR029879; Tulane COBRE for Clinical and Translational Research in Cardiometabolic Diseases P20 GM109036; and Kaiser Permanente NIH/NCRR UCSF-CTSI UL1 RR-024131. None of the funders of this study had any role in the current study design; collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
Financial Disclosure: T.I. received grant support from Shire and honoraria from Bayer and Eli Lilly. M.W. has served as a consultant or received honoraria from Akebia, Amag, Amgen, Ardelyx, Diasorin, Japanese Torii Tobacco, Keryx, Luitpold, Sanofi, and Pharmacosmos and received grant support from Shire. The remaining authors declare that they have no relevant financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Isakova T, Wahl P, Vargas GS, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79(12):1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Isakova T, Xie H, Yang W, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305(23):2432–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scialla JJ, Astor BC, Isakova T, Xie H, Appel LJ, Wolf M. Mineral metabolites and CKD progression in African Americans. J Am Soc Nephrol. 2013;24(1):125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scialla JJ, Xie H, Rahman M, et al. Fibroblast growth factor-23 and cardiovascular events in CKD. J Am Soc Nephrol. 2014;25(2):349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasegawa H, Nagano N, Urakawa I, et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int. 2010;78(10):975–980. [DOI] [PubMed] [Google Scholar]
- 6.Stubbs JR, He N, Idiculla A, et al. Longitudinal evaluation of FGF23 changes and mineral metabolism abnormalities in a mouse model of chronic kidney disease. J Bone Miner Res. 2012;27(1):38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) study: design and methods. J Am Soc Nephrol. 2003;14(7 Suppl 2):S148–153. [DOI] [PubMed] [Google Scholar]
- 8.Isakova T, Cai X, Lee J, et al. Longitudinal FGF23 Trajectories and Mortality in Patients with CKD. J Am Soc Nephrol. 2018;29(2):579–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Boer IH, Sachs MC, Chonchol M, et al. Estimated GFR and circulating 24,25-dihydroxyvitamin D3 concentration: a participant-level analysis of 5 cohort studies and clinical trials. Am J Kidney Dis. 2014;64(2):187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng H, Sowers M, Randolph JF Jr., Harlow SD. An Integrated Quantitative Methodology to Longitudinally Characterize Complex Dynamic Processes Associated with Ovarian Aging and the Menopausal Transition. J Syst Cybern Inf. 2011;9(3):13–21. [PMC free article] [PubMed] [Google Scholar]
- 12.Sestelo M, Villanueva NM, Meira-Machado L, Roca-Pardinas J. npregfast: An R Package for Nonparametric Estimation and Inference in Life Sciences. Journal of Statistical Software. 2017;82(12):1–27. [Google Scholar]
- 13.Isakova T, Xie H, Barchi-Chung A, et al. Fibroblast growth factor 23 in patients undergoing peritoneal dialysis. Clin J Am Soc Nephrol. 2011;6(11):2688–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wesseling-Perry K, Pereira RC, Sahney S, et al. Calcitriol and doxercalciferol are equivalent in controlling bone turnover, suppressing parathyroid hormone, and increasing fibroblast growth factor-23 in secondary hyperparathyroidism. Kidney Int. 2011;79(1):112–119. [DOI] [PubMed] [Google Scholar]
- 15.Burnett-Bowie SA, Leder BZ, Henao MP, Baldwin CM, Hayden DL, Finkelstein JS. Randomized trial assessing the effects of ergocalciferol administration on circulating FGF23. Clin J Am Soc Nephrol. 2012;7(4):624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71(1):31–38. [DOI] [PubMed] [Google Scholar]
- 17.Craver L, Marco MP, Martinez I, et al. Mineral metabolism parameters throughout chronic kidney disease stages 1–5--achievement of K/DOQI target ranges. Nephrol Dial Transplant. 2007;22(4):1171–1176. [DOI] [PubMed] [Google Scholar]
- 18.Shigematsu T, Kazama JJ, Yamashita T, et al. Possible involvement of circulating fibroblast growth factor 23 in the development of secondary hyperparathyroidism associated with renal insufficiency. Am J Kidney Dis. 2004;44(2):250–256. [DOI] [PubMed] [Google Scholar]
- 19.Gutierrez O, Isakova T, Rhee E, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16(7):2205–2215. [DOI] [PubMed] [Google Scholar]
- 20.Wolf M, Weir MR, Kopyt N, et al. A Prospective Cohort Study of Mineral Metabolism After Kidney Transplantation. Transplantation. 2016;100(1):184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isakova T, Nickolas TL, Denburg M, et al. KDOQI US Commentary on the 2017 KDIGO Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Am J Kidney Dis. 2017;70(6):737–751. [DOI] [PubMed] [Google Scholar]
- 22.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2017;7(1–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Population mean levels of mineral metabolites prior to ESRD, by sex.
Figure S2. Population mean levels of mineral metabolites prior to ESRD, by race.
Figure S3. Population mean levels of mineral metabolites prior to ESRD, by presence of diabetes at baseline.
Figure S4. Population mean levels of mineral metabolites prior to death.