Abstract
Objective:
It is unclear why Black smokers in the United States have elevated risk of some tobacco-related diseases compared to White smokers. One possible causal mechanism is differential intake of tobacco toxicants but results across studies are inconsistent. Thus, we examined racial differences in biomarkers of toxic volatile organic compounds (VOCs) present in tobacco smoke.
Method:
We analyzed baseline data collected from 182 Black and 184 White adult smokers who participated in a randomized clinical trial in 2013–2014 at 10 sites across the U.S. We examined differences in urinary levels of 10 VOC metabolites, total nicotine equivalents (TNE), and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), controlling for covariates such as cigarettes per day (CPD), as well as differences in VOCs per TNE to assess the extent to which tobacco exposure, and not metabolic factors, accounted for racial differences.
Results:
Concentration of metabolites of acrolein, acrylonitrile, ethylene oxide, and methylating agents were significantly higher in Blacks compared to Whites when controlled for covariates. Other than the metabolite of methylating agents, VOCs per TNE did not differ between Blacks and Whites. Concentrations of TNE/CPD and VOCs/CPD were significantly higher in Blacks. Menthol did not contribute to racial differences in VOC levels.
Conclusion:
For a given level of CPD, Black smokers likely take in higher levels of acrolein, acrylonitrile, and ethylene oxide than White smokers. Our findings are consistent with Blacks taking in more nicotine and toxicants per cigarette smoked, which may explain their elevated disease risk relative to other racial groups.
Keywords: racial differences, tobacco-related disparities, volatile organic compounds, acrolein
INTRODUCTION
Tobacco smoking is a major contributor to racial disparities related to lung cancer and cardiovascular diseases in the United States.1 The leading type of cancer death in the U.S. is lung cancer,2 of which about 80% of cases are attributed to smoking.3 Despite similar smoking prevalence and the fact that African Americans (Blacks) smoke fewer cigarettes per day (CPD) and on fewer days of the month compared to Whites,4–6 several studies have found higher lung cancer risk among Black smokers compared to White smokers.7–10 For example, the Multiethnic Cohort (MEC) study found that, for the same number of CPD, Blacks had higher lung cancer risk compared to Whites.11,12 Smoking is also a major risk factor for cardiovascular diseases, and compared to Whites, Blacks have higher prevalence of cardiovascular diseases.13
A proposed hypothesis for higher tobacco-related disease risk for Blacks relative to Whites is that at any given level of cigarettes smoked per day, systemic exposure to nicotine and toxicants is higher for Black smokers than it is for White smokers. Early support for this hypothesis came from a study in a controlled research setting which found that Blacks took in about 30% more nicotine per cigarette compared to Whites.14 These findings have been supported by some observational studies. The MEC study found that Black smokers, with a median of 10 CPD, had higher levels of urinary total nicotine equivalents (TNE, the molar sum of nicotine and its metabolites) than White smokers who consumed a median of 20 CPD.15 Interestingly, at fixed TNE, indicating similar daily nicotine intake and toxicant exposure, a recent publication from the MEC study showed no difference in cancer risk between Blacks and Whites,12 suggesting that differences in exposure to toxicants explain differential lung cancer risk between these two racial groups.
Indeed, the MEC study found that Blacks had significantly higher levels of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), a metabolite of the tobacco-specific nitrosamine (TSNA), 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), than Whites after controlling for age, sex, race/ethnicity and urinary creatinine levels.16 A large body of laboratory data17 and some human epidemiology studies18 provide evidence that NNK is an important contributor to lung cancer in smokers. Nonetheless, studies do not consistently show that Black smokers have higher NNAL levels compared to White smokers. An analysis of nationally representative data from the National Health and Nutrition Examination Survey (NHANES) from 2007–2010 found higher urinary NNAL levels in Whites compared to Blacks along the CPD spectrum even though the corresponding serum cotinine levels were higher in Blacks.19
Tobacco smoke contains numerous toxic and carcinogenic volatile organic compounds (VOCs),20 some of which are emitted at up to 1000-fold higher levels than TSNAs such as NNK.21 An analysis of 3R4F reference cigarettes reported NNK yields of 0.24 μg/cigarette in contrast to 1,3-butadiene, acrolein, acrylonitrile and benzene yields of 63.8, 154, 31.9, and 97.6 μg/cigarette, respectively.22 Given their inherent toxicity and relatively high levels in tobacco smoke, VOCs are important contributors to tobacco-related cancer and non-cancer disease risk.23–25 Benzene and 1,3-butadiene, whose primary sources of exposure in the U.S. population are cigarette smoke and vehicle exhaust,26 are known to cause hematological malignancies.27,28 Acrolein, an abundant VOC in tobacco smoke, is a potent cardiopulmonary toxicant,29 and contributes as much as 88.5% of the non-cancer hazard index of tobacco smoke.25
Intake of VOCs can be measured using mercapturic acid metabolites formed from glutathione (GSH) S-conjugates and excreted in urine30; racial differences have been reported previously but not consistently in the same direction. Phenyl mercapturic acid (PMA), the benzene metabolite, was significantly higher among Black smokers compared to White smokers,31 while levels of 3-hydroxypropyl mercapturic acid (3-HPMA), a metabolite of acrolein, and 3-hydroxy-1-methylpropyl mercapturic acid (HPMMA), a metabolite of crotonaldehyde, were not significantly different between Blacks and Whites in the same study (biomarker levels were controlled for age, sex, race/ethnicity and creatinine levels).32 An analysis of NHANES 2011–2012 found that White smokers had higher levels of 3-HPMA (acrolein) and 4-hydroxy-2-buten-1-yl-mercapturic acid (MHBMA-3), a 1,3-butadiene metabolite, compared to Black smokers,33 similar to results from a cross-sectional study of smokers in 31 U.S. states.34 Given conflicting findings from previous studies, we simultaneously measured and compared ten VOC metabolites in spot urine samples collected from Black and White non-treatment-seeking smokers enrolled in a randomized clinical trial at ten sites across the U.S.35
MATERIALS AND METHODS
Study
The current study is an analysis of baseline data collected from a sample of Black and White smokers who participated in a randomized clinical trial of reduced nicotine content cigarettes from June 2013 and July 2014 at 10 sites across the U.S.35 The study was approved by the institutional review board at each study site.
Participants
Participants in the parent study were recruited through flyers, direct mailings, television and radio announcements. Participants had to be 18 years or older, smoked at least five CPD, and had expired carbon monoxide levels of more than 8 ppm or a urinary cotinine level of more than 100 ng/ml. Exclusion criteria have been described previously.35 Participants provided written informed consent before enrollment and were financially compensated for their time.
Our analysis of left-over urine samples was limited to 366 Black and White smokers (of an overall total of 839 enrolled in the parent study) who were at least 40 years old. The final sample size was determined, in part, by financial resources available to perform the VOC assays. Further, using unpublished mean TNE from all Blacks and all Whites in the parent study and assuming that the ratio of VOC biomarker levels between Blacks and Whites would be similar to that of TNE, a priori analysis showed that a sample size of 366 achieved >80% power to detect at least a 15% difference in mean biomarker levels between Blacks and Whites. We restricted our analysis to only those 40 years and over since smoking prevalence is highest in this age group4 and risk of tobacco-related diseases increases with age.2
Measures
Demographic information including age, gender, income, employment status and education were collected using standardized questionnaires. Information on smoking behavior included average CPD during the 2-week baseline period (presented as CPD), and type of cigarette smoked (menthol or non-menthol). We used the Fagerström Test of Cigarette Dependence (FTCD), which includes time to first cigarette after waking (TFC),36 to assess the level of tobacco dependence.
Analytical chemistry
Nicotine Biomarkers
We obtained the saliva ratio of 3′-hydroxycotinine to cotinine (or nicotine metabolite ratio, NMR) and urinary concentrations of total nicotine equivalents (TNE) and total NNAL from the parent study. The NMR is a measure of the extent of CYP2A6-mediated nicotine metabolism.37 TNE was computed as the molar sum of total concentrations of nicotine, cotinine, and 3’-hydroxycotinine and nicotine N-oxide. Liquid chromatography with tandem mass spectrometry (LC-MS/MS) was used for these analyses and the methods have been described previously.15,38,39
Volatile Organic Compounds
We measured mercapturic acid metabolites of VOCs in urine samples stored at −20 °C using LC-MS/MS by a method previously described in the online supplementary materials of a manuscript by Jacob and colleagues.40 The data in the supplementary materials were acquired before Alwis and colleagues41 reported MHBMA-3 as a major 1,3-butadiene metabolite. Subsequently, and for the current paper, we measured MHBMA-1 and MHBMA-2 (summed, see definition of acronym below) and MHBMA-3. Therefore, we report data on precision and accuracy for the analytes reported, compiled from quality control (QC) data of the analytical runs carried out for the current study in Supplementary Table S1.
The mercapturic acid metabolites measured were as follows, shown as the mercapturic acid metabolite [abbreviation, parent compound(s), limit of quantitation (LOQ), and percent below LOQ]: 2-hydroxypropylmercapturic acid [2-HPMA, propylene oxide, 0.5 ng/mL, 0%]; 3-hydroxypropylmercapturic acid [3-HPMA, acrolein, 1 ng/mL, 0%]; 2-carbamoylethylmercapturic acid [AAMA, acrylamide, 0.5 ng/mL, 0%]; 2-cyanoethylmercapturic acid [CNEMA, acrylonitrile, 0.5 ng/mL, 0%]; 2-hydroxyethylmercapturic acid [HEMA, acrylonitrile, vinyl chloride, ethylene oxide, 0.5 ng/mL, 4.1%]; 3-hydroxy-1-methylpropylmercapturic acid [HPMMA, crotonaldehyde, 1 ng/mL, 6.8%]; sum of isomers 1-hydroxy-3-buten-2-yl-mercapturic acid and 2-hydroxy-3-buten-1-yl-mercapturic acid [MHBMA-1+2, 1,3-butadiene, 0.1 ng/mL, 6.3%]; 4-hydroxy-2-buten-1-yl-mercapturic acid [MHBMA-3, 1,3-butadiene, 0.1 ng/mL, 44.5%]; methylmercapturic acid [MMA, methylating agents such as 4-(methylnitrosamino)-1-(3-pyridyl)-1- butanone (NNK), N-nitrosodimethylamine (NDMA), and endogenous methylating agents, 5 ng/mL, 17.2%]; and phenylmercapturic acid [PMA, benzene, 0.1 ng/mL, 2.7%].
Statistical analysis
We first computed univariate statistics by race for demographic characteristics, smoking behavior, tobacco dependence, and NMR. Differences between races (unadjusted for covariates) were assessed using Mann-Whitney U test for continuous variables and chi-square for categorical variables. Biomarker levels below the LOQ were replaced with LOQ/√2.
Variables such as race, age, sex, and body mass index (BMI) influence creatinine excretion,42 and would be sources of bias if concentrations of urinary biomarkers in spot urine samples are normalized by urinary creatinine levels (i.e. biomarker concentration ÷ creatinine concentration). As shown in Supplementary Table S2, Whites had significantly higher creatinine-normalized biomarker levels than Blacks because Whites had lower creatinine levels in their urine, 0.76 (0.68–0.85) vs 1.00 (0.91–1.11) (GM, 95% CI). As a result, to control for urine dilution and to avoid the inherent bias introduced by creatinine-normalization when examining race and sex differences, we used an approach described by O’Brien and colleagues, which controls the covariate-independent, short-term multiplicative effect of hydration on urinary dilution.43
Following “Method 3” in the O’Brien manuscript, we first fit a model for ln(creatinine) as a function of the covariates that directly and chronically affect creatinine levels, namely age, sex, race, and BMI. We obtained the covariate-adjusted standardized biomarker concentration by dividing the unadjusted urinary biomarker concentration by the ratio of the observed creatinine concentration to the fitted creatinine concentration. We present concentrations of urinary biomarkers unadjusted for urinary creatinine concentration (unit: mass/mL), those normalized for creatinine concentration (mass/mg creatinine), and the covariate-adjusted standardized biomarker concentrations (mass/mL) by race in Supplementary Table S2. P values for comparisons by race are based on univariate analyses. For Blacks, all three forms of urinary concentrations were similar since the geometric means of the covariate-adjusted concentrations and the absolute creatinine concentration were 1.00 (0.91–1.10) and 1.00 (0.91–1.11), respectively. On the other hand, for Whites, the geometric mean of the covariate-adjusted concentrations were similar to the uncorrected concentrations but the creatinine-normalized concentrations were generally higher; while the geometric mean of the creatinine ratio for Whites was 1.00 (0.90–1.11), the geometric mean of the absolute creatinine levels was 0.76 (0.68–0.85).
We examined differences in natural log-transformed covariate-adjusted standardized biomarker concentrations across race using linear regression models; biomarker levels were log-normally distributed. The dependent variable was natural log-transformed covariate-adjusted standardized urinary levels of NNAL, TNE, or mercapturic acid metabolites (each biomarker outcome was modeled separately). Race was the independent variable and covariates included gender (women or men), age group (40–49 years and ≥50 years), CPD (1–10 CPD, 11–20 CPD, and >20 CPD), the number of cigarettes smoked by the time of their visit on the study day (continuous variable), menthol use (yes or no), NMR quartiles, education (less than high school, high school, or at least some college).43 We included a race-by-gender interaction term in all models. The variance inflation factor for variables in the models was about 2 or smaller, indicating no threat of high multicollinearity. Further, we examined the effect of menthol on biomarker levels in similar linear regression models that were stratified by race.
In another set of linear regression models, we entered the ratio of log-transformed non-creatinine-corrected mercapturic acid concentrations (or raw concentrations) to non-creatinine-corrected TNE concentration as dependent variables. The independent variables included only race, gender, and the race-by-gender interaction term. The ratios are independent of creatinine levels and were used to address the question of whether racial differences in VOC biomarker levels were related to differences in extent of intake of the parent compounds (in which case the ratios would be similar by race) or to differences in metabolic conversion of VOCs to mercapturic acids (in which case the ratios would differ by race).
The final set of linear regression models included the ratios of log-transformed covariate-adjusted standardized biomarker concentrations to number of cigarettes smoked per day (biomarker/CPD) as the dependent variables to test racial differences in intake of nicotine and toxicants per each cigarette smoked. Independent variables were race, gender, menthol, education, age group and creatinine; multiple comparisons between the three CPD groups were adjusted by Bonferroni’s method.
We carried out all analyses using SAS v. 9.4 (SAS Institute, Inc., Cary, NC, USA) and we considered statistical tests to be statistically significant at two-tailed ≤ 0.05.
RESULTS
Participant characteristics
Of the 839 randomized participants in the parent study, we included 184 of 428 (43%) Whites and 182 of 321 (57%) Blacks. Baseline characteristics of the 366 participants that we included in this study are presented in Table 1. Among Blacks and Whites, 50% of the sample were women. BMI, prevalence of menthol use, percent who smoked within 5 minutes of waking, and urinary creatinine levels were significantly higher in Blacks than Whites. Level of education, CPD, and NMR were significantly lower in Blacks compared to Whites. Mean age, age distribution, mean FTCD, and percent positive tests for cannabis use were not significantly different between Blacks and Whites.
TABLE 1.
Demographic information, smoking history, and tobacco dependence of 182 Black and 184 White smokers, June 2013 to July 2014 at 10 sites across the U.S.
| Variable | Blacks | Whites | p value |
|---|---|---|---|
| N (%) | 182 (49.7%) | 184 (50.3%) | |
| Sex | |||
| Women (n, %) | 91 (50%) | 92 (50%) | 1.0 |
| Men (n, %) | 91 (50%) | 92 (50%) | |
| Age (mean, SD) | 51.0 (6.0) | 51.4 (6.7) | 0.806 |
| Age group | |||
| 40–50 (n, %) | 89 (48.9%) | 89 (48.4%) | 1.0 |
| >50 (n, %) | 93 (51.1%) | 95 (51.6%) | 0.002 |
| Body mass index (BMI) (kg/m2) | 30.7 (7.6) | 28.7 (7.1) | |
| Education | 0.018 | ||
| less than high school (n, %) | 31 (17.0%) | 19 (10.3%) | |
| high school graduate (n, %) | 66 (36.3%) | 53 (28.8%) | |
| At least some college (n, %) | 85 (46.7%) | 112 (60.9%) | |
| Mentholated cigarettes | <0.001 | ||
| No (n, %) | 21 (11.5%) | 116 (63.0%) | |
| Yes (n, %) | 161 (88.5%) | 68 (37.0%) | |
| Time to first cigarette (min) | 0.016 | ||
| Within 5 min (n, %) | 109 (59.9%) | 87 (47.3%) | |
| After 5 min (n, %) | 73 (40.1%) | 97 (52.7%) | <0.001 |
| Cigarettes per day, CPD (mean, SD) | 14.6 (6.7) | 19.3 (9.3) | |
| CPD category | <0.001 | ||
| 1–10 (n, %) | 48 (26.4%) | 21 (11.4%) | |
| 11–20 (n, %) | 108 (59.3%) | 106 (57.6%) | |
| >20 (n, %) | 26 (14.3%) | 57 (31.0%) | |
| FTCD (mean, SD) | 5.6 (2.0) | 5.7 (2.0) | 0.396 |
| Saliva NMR (mean, SD) | 0.28 (0.18) | 0.36 (0.28) | 0.007 |
| 11-nor-9-carboxy-THC test | |||
| Negative (n, %) | 159 (87.9%) | 159 (86.4%) | 0.755 |
| Positive (n, %) | 22 (12.1%) | 25 (13.6%) | <0.001 |
| Urinary creatinine (mg/mL) (mean, SD) | 1.23 (0.73) | 0.96 (0.59) |
Notes: CPD = average cigarettes per day over 2-week baseline period; FTCD = Fagerström Test of Cigarette Dependence; NMR = nicotine metabolite ratio (ratio of 3´-hydroxycotinine to cotinine); NMR quartile 1 = 0.17; NMR median = 0.27; NMR quartile 3 = 0.39; THC = tetrahydrocannabinol. ignificant differences are in bold.
Correlations between VOC metabolites and CPD, TNE, and NNAL
Correlations between CPD and VOC metabolite concentrations (non-creatinine corrected) were weak while correlations between the two tobacco-specific biomarkers (NNAL and TNE) and 9 of 10 VOC metabolites (non-creatinine corrected) were moderate to high in each racial group (Table 2). NNAL and TNE were not significantly correlated with MMA (methylating agents) for Blacks and 2-HPMA (propylene oxide) for Whites.
TABLE 2.
Pearson correlation coefficients between concentrations of mercapturic acid metabolites and cigarette per day (CPD) and concentrations of tobacco-specific biomarkers by race in 182 Black and 184 White smokers, June 2013 to July 2014 at 10 sites across the U.S.
| 2-HPMA | 3-HPMA | AAMA | CNEMA | HEMA | HPMMA | MHBMA-1+2 | MHBMA-3 | MMA | PMA | |
|---|---|---|---|---|---|---|---|---|---|---|
| Blacks (n = 182) | ||||||||||
| CPD | 0.02 | 0.28*** | 0.17* | 0.24** | 0.20** | 0.17* | 0.19** | 0.10 | 0.02 | 0.19* |
| NNAL | 0.35*** | 0.70*** | 0.69*** | 0.80*** | 0.47*** | 0.60*** | 0.46*** | 0.37*** | 0.11 | 0.64*** |
| TNE | 0.32*** | 0.77*** | 0.78*** | 0.78*** | 0.46*** | 0.64*** | 0.53*** | 0.32*** | 0.13 | 0.64*** |
| Whites (n = 184) | ||||||||||
| CPD | −0.07 | 0.14 | 0.02 | 0.14 | 0.10 | 0.08 | 0.18* | 0.09 | 0.04 | 0.13 |
| NNAL | 0.11 | 0.56*** | 0.44*** | 0.71*** | 0.45*** | 0.44*** | 0.51*** | 0.44*** | 0.36*** | 0.52*** |
| TNE | 0.19 | 0.76*** | 0.66*** | 0.74*** | 0.53*** | 0.61*** | 0.60*** | 0.58*** | 0.32*** | 0.57*** |
p <0.05
p < 0.01
p < 0.001
Biomarker concentrations were raw concentrations (not adjusted for creatinine or covariates); CPD is average cigarettes per day over 2-week baseline period; TNE = total nicotine equivalents; NNAL = 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol; 2-HPMA = 2-hydroxypropylmercapturic acid (propylene oxide); 3-HPMA = 3-hydroxypropylmercapturic acid (acrolein); AAMA = 2-carbamoylethylmercapturic acid (acrylamide); CNEMA = 2-cyanoethylmercapturic acid (acrylonitrile); HEMA = 2-hydroxyethylmercapturic acid (acrylonitrile, vinyl chloride, ethylene oxide); HPMMA = 3-hydroxy-1-methyl-propylmercapturic acid (crotonaldehyde); MHBMA-1+2 = sum of isomers 1-hydroxy-3-buten-2-yl-mercapturic acid and 2-hydroxy-3-buten-1-yl-mercapturic acid (1,3-butadiene); MHBMA-3 = 4-hydroxy-2-buten-1-yl-mercapturic acid (1,3-butadiene); MMA = methylmercapturic acid (methylating agents); and, PMA = phenylmercapturic acid (benzene).
Racial differences in concentrations of TNE, NNAL, and VOC metabolites
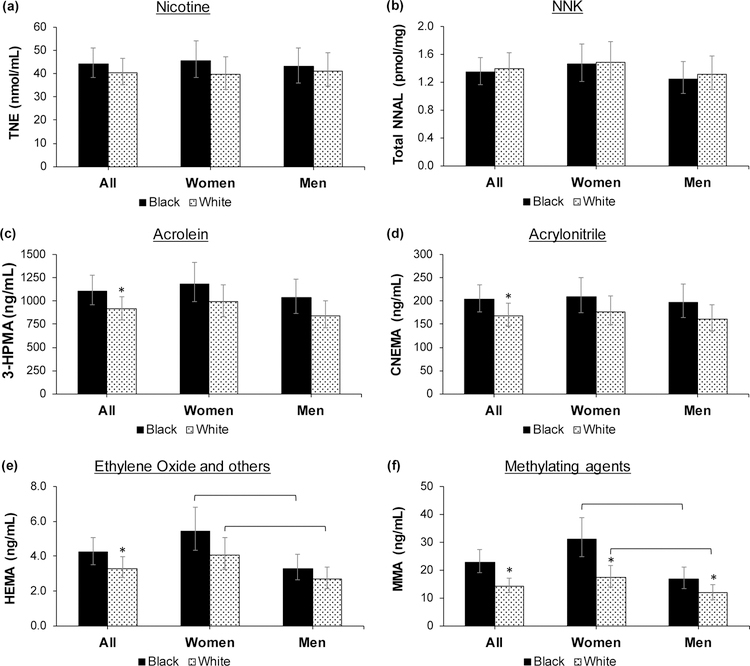
We present model-predicted means of urinary concentrations of TNE, NNAL, and VOC metabolites in Table 3. Urinary concentrations of TNE and NNAL were not significantly different between Blacks and Whites, with Black to White ratios of 1.10 and 0.96, respectively. Concentrations of 3-HPMA (acrolein), CNEMA (acrylonitrile), HEMA (acrylonitrile, vinyl chloride, ethylene oxide), and MMA (methylating agents) were significantly higher in Blacks compared to Whites, with ratios of 1.21, 1.20, 1.28, and 1.60, respectively. MHBMA-1+2 (1,3-butadiene) was, on average, 32% higher in Blacks compared to Whites (ratio of 1.32) but this difference was not statistically significant (p = 0.059). The race-by-gender interaction terms were not significant in any of the models. However, within the same race, HEMA and MMA levels were significantly higher among women compared to men (Figure 1). Inclusion of site in the models (since the study was multi-site) or exclusion of all participants who had a positive tetrahydrocannabinol (THC) test (which was not different by race) did not alter the ratios of VOC biomarker levels in Blacks compared to Whites.
TABLE 3.
Model-predicted means of concentrations of total nicotine equivalents (TNE), 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), and mercapturic acid metabolites of volatile organic compounds for 182 Black and 184 White smokers, June 2013 to July 2014 at 10 sites across the U.S.
| Biomarker | Blacks | Whites | Ratio | p value |
|---|---|---|---|---|
| TNE (nmol/mL) | 44.3 (38.5 – 50.9) | 40.4 (35.1 – 46.4) | 1.10 (0.92 – 1.31) | 0.312 |
| NNAL (pmol/mL) | 1.35 (1.17 – 1.56) | 1.40 (1.21 – 1.62) | 0.96 (0.80 – 1.16) | 0.707 |
| 2-HPMA (ng/mL) | 64.6 (55.7 – 75.0) | 56.1 (48.4 – 64.9) | 1.15 (0.95 – 1.39) | 0.143 |
| 3-HPMA (ng/mL) | 1108 (963 – 1275) | 914 (796 – 1050) | 1.21 (1.01 – 1.45) | 0.035 |
| AAMA (ng/mL) | 200 (181 – 221) | 192 (174 – 212) | 1.04 (0.92 – 1.19) | 0.525 |
| CNEMA (ng/mL) | 203 (176 – 235) | 169 (146 – 195) | 1.20 (1.00 – 1.45) | 0.048 |
| HEMA (ng/mL) | 4.24 (3.53 – 5.08) | 3.31 (2.77 – 3.96) | 1.28 (1.01 – 1.61) | 0.038 |
| HPMMA (ng/mL) | 177 (126 – 248) | 174 (125 – 244) | 1.01 (0.66 – 1.57) | 0.950 |
| MHBMA-1+2 (ng/mL) | 1.35 (1.08 – 1.69) | 1.02 (0.82 – 1.28) | 1.32 (0.99 – 1.76) | 0.059 |
| MHBMA-3 (ng/mL) | 0.14 (0.12 – 0.16) | 0.12 (0.11 – 0.14) | 1.15 (0.96 – 1.38) | 0.121 |
| MMA (ng/mL) | 22.9 (19.2 – 27.5) | 14.3 (12.0 – 17.1) | 1.60 (1.27 – 2.02) | <0.001 |
| PMA (ng/mL) | 1.31 (1.08 – 1.58) | 1.15 (0.96 – 1.39) | 1.13 (0.89 – 1.45) | 0.311 |
Notes: Model-predicted means are back-transformed least square means. Participants were at least 40 years old and were enrolled in a randomized clinical trial of reduced nicotine content cigarettes between June 2013 and July 2014 at 10 sites across the U.S. Concentrations of urinary metabolites were entered in the models as covariate-adjusted standardized concentrations (ref 43); independent variables included race, sex, cigarettes per day, menthol, education, age group, nicotine metabolite ratio (NMR) quartile, and a race-by-sex interaction term; models with mercapturic acids also included CPDvisit (number of cigarettes smoked by the time of assessment on the day of the study visit). TNE = total nicotine equivalents (nmol/mL); NNAL = 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (pmol/mL); the unit for concentrations of VOC metabolites is ng/mL; 2-HPMA = 2-hydroxypropylmercapturic acid (propylene oxide); 3-HPMA = 3-hydroxypropylmercapturic acid (acrolein); AAMA = 2-carbamoylethylmercapturic acid (acrylamide); CNEMA = 2-cyanoethylmercapturic acid (acrylonitrile); HEMA = 2-hydroxyethylmercapturic acid (acrylonitrile, vinyl chloride, ethylene oxide); HPMMA = 3-hydroxy-1-methyl-propylmercapturic acid (crotonaldehyde); MHBMA −1+2 = sum of isomers 1-hydroxy-3-buten-2-yl-mercapturic acid and 2-hydroxy-3-buten-1-yl-mercapturic acid (1,3-butadiene); MHBMA-3 = 4-hydroxy-2-buten-1-yl-mercapturic acid (1,3-butadiene); MMA = methylmercapturic acid (methylating agents); and, PMA = phenylmercapturic acid (benzene). Significant differences are in bold.
FIGURE 1:
Comparison of levels of biomarkers of nicotine intake and toxicant exposure controlling for cigarettes per day by race, and for women and men of each race. Participants were a subset of Black and White smokers who participated in a randomized clinical trial of reduced nicotine content cigarettes between June 2013 and July 2014 at 10 sites across the U.S. Concentrations were controlled for race, gender, cigarettes per day, menthol, education, age group, nicotine metabolite ratio (NMR) quartile, and a race-by-gender interaction term; models with mercapturic acids also included number of cigarettes smoked by the time of assessment on the day of the study visit. The metabolites measured are on the y-axis and the parent compounds are presented as the title of each graph. * = significant racial differences; square brackets = significant sex differences within each race; TNE = total nicotine equivalents (nmol/mL); NNAL = 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol; NNK = 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone; 3-HPMA = 3-hydroxypropylmercapturic acid; CNEMA = 2-cyanoethylmercapturic acid; HEMA = 2-hydroxyethylmercapturic acid (acrylonitrile, vinyl chloride, ethylene oxide); MMA = methylmercapturic acid.
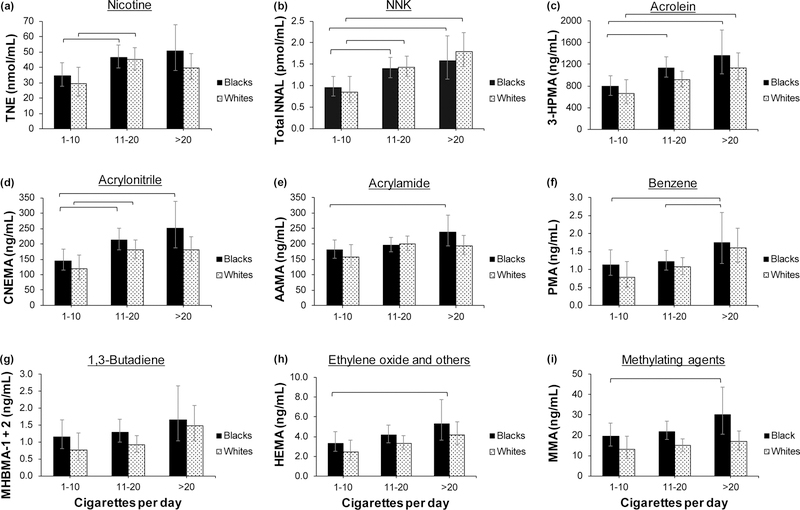
We examined differences in biomarker levels across categories of CPD, first with race as a covariate in one set of models and then stratified by race in other models. The results of the two sets of models were similar. The CPD effect in models with race as a covariate was significant for TNE (p = 0.002), NNAL (p <0.001), 3-HPMA (p < 0.001), AAMA (p = 0.03), CNEMA (p < 0.001), HEMA (p = 0.02), MHBMA-1+2 (p = 0.04), and PMA (p = 0.006). Figure 2 shows model-predicted biomarker levels across CPD categories stratified by race, indicating that levels of VOC metabolites generally increased with the number of cigarettes smoked per day for both Blacks and Whites.
FIGURE 2.
Comparison of model-predicted biomarker levels across categories of cigarettes per day (CPD) by race. The study included a subset of Black and White smokers over the age of 40 who participated in a randomized clinical trial of reduced nicotine content cigarettes between June 2013 and July 2014 at 10 sites across the U.S. Concentrations are model-predicted means, controlling for the effects of gender, menthol, education, and age group. The metabolites measured are on the y-axis and the parent compounds are presented as the title of each graph. Square brackets = significant differences between CPD categories within each race. TNE = total nicotine equivalents (nmol/mL); NNAL = 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol; NNK = 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone; 3-HPMA = 3-hydroxypropylmercapturic acid; CNEMA = 2-cyanoethylmercapturic acid; AAMA = 2-carbamoylethylmercapturic acid; PMA = phenylmercapturic acid; MHBMA-1+2 = sum of isomers 1-hydroxy-3-buten-2-yl-mercapturic acid and 2-hydroxy-3-buten-1-yl-mercapturic acid; HEMA = 2-hydroxyethylmercapturic acid (acrylonitrile, vinyl chloride, ethylene oxide); MMA = methylmercapturic acid.
Racial differences in the ratios of concentrations of VOC metabolites to TNE and to CPD
We present model-predicted means of the ratios of biomarker levels to TNE and to CPD in Table 4. The ratio of NNAL to TNE was significantly lower in Blacks compared to Whites while MMA to TNE was significantly higher in Blacks; the ratios of the other VOCs to TNE did not differ significantly by race. The ratios of all biomarker levels to CPD were significantly higher in Blacks compared to Whites, except NNAL and HMPMA, which were not significantly different.
TABLE 4.
Model-predicted means of the ratios of concentrations of mercapturic acid metabolites of volatile organic compounds (VOCs) to total nicotine equivalents (TNE) and to cigarettes per day (CPD) for 182 Black and 184 White smokers, June 2013 to July 2014 at 10 sites across the U.S.
| Biomarker | Blacks | Whites | Ratio | p value |
|---|---|---|---|---|
| A. Ratios of concentrations of biomarkers to TNE levels | ||||
| NNAL/TNE | 0.027 (0.025 – 0.030) | 0.032 (0.030 – 0.035) | 0.84 (0.74 – 0.96) | 0.009 |
| 2-HPMA/TNE | 1.5 (1.3 – 1.7) | 1.5 (1.3 – 1.7) | 1.04 (0.86 – 1.26) | 0.665 |
| 3-HPMA/TNE | 24.7 (22.2 – 27.5) | 22.5 (20.3 – 25.1) | 1.10 (0.94 – 1.27) | 0.232 |
| AAMA/TNE | 5.0 (4.6 – 5.5) | 4.7 (4.3 – 5.1) | 1.07 (0.94 – 1.22) | 0.294 |
| CNEMA/TNE | 4.5 (4.1 – 4.9) | 4.3 (4.0 – 4.7) | 1.03 (0.92 – 1.16) | 0.569 |
| HEMA/TNE | 0.090 (0.078 – 0.104) | 0.080 (0.070 – 0.093) | 1.12 (0.91 – 1.37) | 0.287 |
| HPMMA/TNE | 5.1 (4.0 – 6.7) | 4.7 (3.6 – 6.1) | 1.10 (0.76 – 1.59) | 0.606 |
| MHBMA-1+2/TNE | 0.030 (0.026 – 0.035) | 0.028 (0.024 – 0.033) | 1.06 (0.85 – 1.32) | 0.612 |
| MHBMA-3/TNE | 0.0030 (0.0027 – 0.0034) | 0.0032 (0.0029 – 0.0036) | 0.93 (0.78 – 1.11) | 0.424 |
| MMA/TNE | 0.49 (0.41 – 0.58) | 0.34 (0.29 – 0.40) | 1.42 (1.12 – 1.80) | 0.004 |
| PMA/TNE | 0.028 (0.025 – 0.032) | 0.029 (0.026 – 0.034) | 0.96 (0.80 – 1.17) | 0.709 |
| B. Ratios of concentrations of biomarkers to CPD | ||||
| TNE/CPD | 3.09 (2.67 – 3.57) | 2.39 (2.08 – 2.76) | 1.29 (1.08 – 1.54) | 0.006 |
| NNAL/CPD | 0.092 (0.080 – 0.107) | 0.085 (0.073 – 0.098) | 1.09 (0.91 – 1.31) | 0.342 |
| 2-HPMA/CPD | 4.49 (3.82 – 5.27) | 3.35 (2.86 – 3.93) | 1.34 (1.09 – 1.64) | 0.005 |
| 3-HPMA/CPD | 75.9 (65.8 – 87.6) | 55.9 (48.6 – 64.3) | 1.36 (1.13 – 1.63) | 0.001 |
| AAMA/CPD | 13.9 (12.3 – 15.6) | 11.5 (10.3 – 12.9) | 1.21 (1.04 – 1.40) | 0.013 |
| CNEMA/CPD | 13.9 (12.0 – 16.2) | 10.3 (8.9 – 11.9) | 1.35 (1.12 – 1.63) | 0.002 |
| HEMA/CPD | 0.292 (0.242 – 0.352) | 0.200 (0.167 – 0.241) | 1.46 (1.15 – 1.85) | 0.002 |
| HPMMA/CPD | 12.4 (8.8 – 17.6) | 10.2 (7.3 – 14.3) | 1.22 (0.79 – 1.89) | 0.377 |
| MHBMA-1+2/CPD | 0.092 (0.074 – 0.116) | 0.063 (0.050 – 0.078) | 1.48 (1.11 – 1.97) | 0.008 |
| MHBMA-3/CPD | 0.010 (0.008 – 0.011) | 0.007 (0.006 – 0.008) | 1.35 (1.11 – 1.64) | 0.002 |
| MMA/CPD | 1.59 (1.31 – 1.92) | 0.86 (0.72 – 1.04) | 1.84 (1.45 – 2.34) | <0.001 |
| PMA/CPD | 0.090 (0.074 – 0.109) | 0.070 (0.058 – 0.085) | 1.29 (1.01 – 1.65) | 0.045 |
Notes: Model-predicted means are back-transformed least square means. Participants were at least 40 years old and were enrolled in a randomized clinical trial of reduced nicotine content cigarettes between June 2013 and July 2014 at 10 sites across the U.S. independent variables for A and B included race, gender, and a race-by-gender interaction term; models for C included race, gender, menthol, education, age group and race-by-gender interaction. TNE = total nicotine equivalents (nmol/mL); NNAL = 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (pmol/mL); the unit for concentrations of VOC metabolites is ng/mL; 2-HPMA = 2-hydroxypropylmercapturic acid (propylene oxide); 3-HPMA = 3-hydroxypropylmercapturic acid (acrolein); AAMA = 2-carbamoylethylmercapturic acid (acrylamide); CNEMA = 2-cyanoethylmercapturic acid (acrylonitrile); HEMA = 2-hydroxyethylmercapturic acid (acrylonitrile, vinyl chloride, ethylene oxide); HPMMA = 3-hydroxy-1-methyl-propylmercapturic acid (crotonaldehyde); MHBMA-1+2 = sum of isomers 1-hydroxy-3-buten-2-yl-mercapturic acid and 2-hydroxy-3-buten-1-yl-mercapturic acid (1,3-butadiene); MHBMA-3 = 4-hydroxy-2-buten-1-yl-mercapturic acid (1,3-butadiene); MMA = methylmercapturic acid (methylating agents); and, PMA = phenylmercapturic acid (benzene). Significant differences are in bold.
Differences in TNE, NNAL, and VOC metabolites across menthol use
We explored differences in biomarker levels and biomarkers levels per CPD by menthol use stratified by race (Table 5). The average HEMA level (acrylonitrile, vinyl chloride, ethylene oxide) was significantly higher in Black non-menthol users compared to Black menthol users (p = 0.026). The average HEMA level normalized by CPD was also significantly higher in Black non-menthol users compared to Black menthol users (p = 0.035). The average levels of TNE, NNAL, and other VOC metabolites were not significantly different between Black non-menthol and menthol users. There were no significant differences across White non-menthol versus menthol users for any of the biomarkers.
TABLE 5:
Comparison of model-predicted means of concentrations of total nicotine equivalents (TNE), 4-(methylnitrosamino)-1-(3)pyridyl-1-butanonol (NNAL), and mercapturic acid metabolites of volatile organic compounds (VOCs), and concentrations normalized by cigarettes per day (CPD) across menthol use stratified by race for 182 Black and 184 White smokers, June 2013 to July 2014 at 10 sites across the U.S.
| Blacks | Whites | |||||
|---|---|---|---|---|---|---|
| Non-menthol (n = 21) | Menthol (n = 161) | p value | Non-menthol (n = 116) | Menthol (n = 68) | p value | |
| A. Biomarker | ||||||
| TNE (nmol/mL) | 46.5 (32.7 – 66.3) | 40.5 (32.7 – 46.4) | 0.444 | 41.9 (34.4 – 51.0) | 48.1 (38.0 – 61.0) | 0.218 |
| NNAL (pmol/mL) | 1.41 (0.93 – 2.14) | 1.09 (0.93 – 1.28) | 0.230 | 1.56 (1.30 – 1.87) | 1.53 (1.23 – 1.89) | 0.833 |
| 2-HPMA (ng/mL) | 76.9 (53.0 – 111.7) | 56.6 (53.0 – 65.4) | 0.111 | 61.4 (50.1 – 75.2) | 60.5 (47.0 – 77.7) | 0.902 |
| 3-HPMA (ng/mL) | 1346 (944 – 1918) | 963 (944 – 1103) | 0.067 | 948 (788 – 1140) | 1036 (825 – 1302) | 0.418 |
| AAMA (ng/mL) | 205.2 (158.8 – 265.3) | 206.6 (158.8 – 228.0) | 0.960 | 181.9 (158.5 – 208.8) | 197.5 (166.5 – 234.2) | 0.316 |
| CNEMA (ng/mL) | 230.4 (163.2 – 325.3) | 177.3 (163.2 – 202.4) | 0.140 | 173.8 (142.2 – 212.4) | 174.6 (136.3 – 223.8) | 0.969 |
| HEMA (ng/mL) | 6.21 (3.75 – 10.28) | 3.47 (3.75 – 4.21) | 0.026 | 3.71 (2.97 – 4.62) | 3.74 (2.85 – 4.92) | 0.940 |
| HPMMA (ng/mL) | 181.0 (71.0 – 461.4) | 172.1 (71.0 – 246.6) | 0.916 | 168.7 (109.2 – 260.4) | 219.0 (128.0 – 374.5) | 0.312 |
| MHBMA-1,2 (ng/mL) | 1.76 (1.00 – 3.10) | 1.17 (1.00 – 1.46) | 0.164 | 1.06 (0.79 – 1.43) | 1.04 (0.72 – 1.51) | 0.919 |
| MHBMA-3 (ng/mL) | 0.113 (0.078 – 0.165) | 0.115 (0.078 – 0.133) | 0.925 | 0.149 (0.124 – 0.178) | 0.130 (0.104 – 0.163) | 0.217 |
| MMA (ng/mL) | 29.6 (17.2 – 50.9) | 17.4 (17.2 – 21.4) | 0.057 | 17.9 (14.7 – 21.8) | 16.5 (12.9 – 21.1) | 0.502 |
| PMA (ng/mL) | 1.63 (1.02 – 2.61) | 1.17 (1.02 – 1.40) | 0.173 | 1.19 (0.91 – 1.54) | 1.15 (0.83 – 1.60) | 0.857 |
| B. Biomarker/CPD | ||||||
| TNE/CPD | 3.43 (2.43 – 4.85) | 3.05 (2.67 – 3.49) | 0.511 | 2.38 (1.97 – 2.89) | 2.75 (2.18 – 3.47) | 0.194 |
| NNAL/CPD | 0.11 (0.07 – 0.16) | 0.08 (0.07 – 0.10) | 0.270 | 0.09 (0.07 – 0.10) | 0.09 (0.07 – 0.11) | 0.913 |
| 2-HPMA/CPD | 5.63 (3.83 – 8.28) | 4.27 (3.68 – 4.95) | 0.163 | 3.48 (2.84 – 4.26) | 3.46 (2.69 – 4.44) | 0.956 |
| 3-HPMA/CPD | 98.5 (69.2 – 140.1) | 72.5 (63.4 – 83.1) | 0.092 | 53.8 (44.9 – 64.4) | 59.3 (47.5 – 74.0) | 0.363 |
| AAMA/CPD | 15.0 (11.5 – 19.6) | 15.6 (14.0 – 17.3) | 0.795 | 10.3 (9.0 – 11.8) | 11.3 (9.5 – 13.4) | 0.271 |
| CNEMA/CPD | 16.9 (12.0 – 23.8) | 13.4 (11.7 – 15.2) | 0.186 | 10.0 (7.8 – 12.7) | 10.0 (7.8 – 12.7) | 0.912 |
| HEMA/CPD | 0.45 (0.27 – 0.76) | 0.26 (0.22 – 0.32) | 0.035 | 0.21 (0.17 – 0.26) | 0.21 (0.16 – 0.28) | 0.888 |
| HPMMA/CPD | 13.2 (5.2 – 33.6) | 13.0 (9.1 – 18.5) | 0.964 | 9.6 (6.2 – 14.8) | 12.5 97.3 – 21.5) | 0.300 |
| MHBMA-1,2/CPD | 0.13 (0.07 – 0.23) | 0.09 (0.07 – 0.11) | 0.195 | 0.06 (0.04 – 0.08) | 0.06 (0.04 – 0.09) | 0.956 |
| MHBMA-3/CPD | 0.008 (0.006 – 0.012) | 0.009 (0.008 – 0.010) | 0.806 | 0.008 (0.007 – 0.010) | 0.007 (0.006 – 0.009) | 0.252 |
| MMA/CPD | 2.17 (1.25 – 3.76) | 1.31 (1.06 – 1.62) | 0.076 | 1.01 (0.83 – 1.24) | 0.95 (0.74 – 1.21) | 0.549 |
| PMA/CPD | 0.12 (0.07 – 0.19) | 0.09 (0.07 – 0.11) | 0.218 | 0.07 (0.05 – 0.09) | 0.07 (0.05 – 0.09) | 0.896 |
Notes: Participants were at least 40 years old and were enrolled in a randomized clinical trial of reduced nicotine content cigarettes between June 2013 and July 2014 at 10 sites across the U.S. Independent variables for models in A included menthol, gender, menthol-by-gender interaction term, age group, CPD, CPDvisit in models with VOCs, NMR quartiles, education, and creatinine concentration; models for B included menthol, gender, menthol-by-gender interaction term, age group, CPDvisit in models with VOCs, NMR quartiles, education, and creatinine concentration. TNE = total nicotine equivalents (nmol/mL); NNAL = 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (pmol/mL); units of VOC metabolites are ng/mL; 2-HPMA = 2-hydroxypropylmercapturic acid (propylene oxide); 3-HPMA = 3-hydroxypropylmercapturic acid (acrolein); AAMA = 2-carbamoylethylmercapturic acid (acrylamide); CNEMA = 2-cyanoethylmercapturic acid (acrylonitrile); HEMA = 2-hydroxyethylmercapturic acid (acrylonitrile, vinyl chloride, ethylene oxide); HPMMA = 3-hydroxy-1-methyl-propylmercapturic acid (crotonaldehyde); MHBMA-1+2 = sum of isomers 1-hydroxy-3-buten-2-yl-mercapturic acid and 2-hydroxy-3-buten-1-yl-mercapturic acid (1,3-butadiene); MHBMA-3 = 4-hydroxy-2-buten-1-yl-mercapturic acid (1,3-butadiene); MMA = methylmercapturic acid (methylating agents); and, PMA = phenylmercapturic acid (benzene). Significant differences are in bold.
DISCUSSION
Understanding the causal pathways as to why Blacks have disproportionately higher rates of some tobacco-related diseases compared to other racial-ethnic groups may potentially lead to novel preventive and therapeutic interventions. In this study, urinary metabolites of acrolein, acrylonitrile, ethylene oxide, and methylating agents were significantly higher in Black smokers compared to White smokers after controlling for covariates such as CPD. These results indicate that Black smokers have higher intake of toxic and carcinogenic VOCs from each cigarette smoked, which may contribute to increased risk of smoking-related diseases. These findings are consistent with a few previous studies showing higher levels of some VOC metabolites in Black smokers compared to White smokers.31,32
Acrolein is a major tobacco toxicant; exposure to acrolein leads to extensive cardiovascular injury in animal models44,45 and is associated with increased risk of cardiovascular disease in humans.29 Although acrolein has not been shown to be a lung carcinogen, acrolein likely contributes to lung carcinogenesis by inducing mutations that have been found in lung cancer mutational hotspots in the p53 gene (p53 is a tumor suppressor protein involved in regulating a wide array of signaling pathways) and by inhibiting cellular repair capacity to remove DNA adducts of other toxicants.46 Benzo[a]pyrene, a known human carcinogen found in tobacco smoke at much lower levels than acrolein, produces this spectrum of mutations in p53.47 Acrolein-induced oxidative stress and inflammation may also play a role in the etiology of lung cancer and cardiopulmonary diseases in humans. Ours is among the first studies suggesting higher acrolein intake in Black smokers relative to White smokers, with the implication of higher risk of cardiopulmonary disease risks among Blacks smokers relative to White smokers. Other studies have reported higher acrolein exposure (3-HPMA) in White smokers compared to Black smokers, but the bias introduced by creatinine normalization33 and not controlling for differences in CPD across races34 could have influenced these findings.
The International Agency for Research on Cancer (IARC) has classified acrylonitrile as a Group 2B carcinogen (i.e., possibly carcinogenic to humans)48 and ethylene oxide as Group 1 (carcinogenic to humans).49 Although studies have reported inconsistent findings regarding acrylonitrile-associated lung cancer risk,50 workers exposed to acrylonitrile had significantly increased risk of lung cancer independent of smoking and with some indication of a dose-response relationship in one study.51 Ethylene oxide, which has not yet been implicated in lung cancer development, is associated with lymphatic and hematopoietic cancers; it is a direct-acting alkylating agent and evidence suggests its carcinogenicity operates through a genotoxic mechanism.49 Further, although not statistically significant, likely due to a lack of statistical power, the 1,3-butadiene metabolite, MHBMA-1+2, was 1.32 times higher in Blacks compared to Whites, with a larger average difference than the other VOC metabolites except for MMA. 1,3-Butadiene is a known human carcinogen.52 It should be noted that the relative abundance of the three 1,3-butadiene metabolite isomers differed from another publication,41 the reasons for which are still unknown.
The largest difference between Blacks and Whites was seen for MMA, the metabolite of methylating agents, and is a potentially important finding of our study. While tobacco smoke contains several constituents that are known to act as methylating agents, a prior study found that urinary MMA levels were not associated with tobacco smoke exposure.53 Consistent with the conclusions of that study, we found weak correlations between MMA levels and levels of TNE and NNAL, which are tobacco-specific. It seems MMA is related to other sources of methylating agents, including dietary and environmental, and that intake from these sources may differ by race.54,55
Regardless of the source, higher exposure to methylating agents among Blacks compared to Whites has implications for health disparities. Aberrations in the DNA methylation system, hypermethylation of genes, and other epigenetic changes are associated with cancer and other diseases such as asthma.56,57 Interestingly, methylation of DNA at CpG sites (CpG, cytosine nucleotide followed by a guanine nucleotide) enhances acrolein-DNA binding at these sites.46 The role of dietary and environmental sources of methylating agents, their interaction with tobacco smoke constituents, and contribution to smoking-related disease risk warrant further study.
Since mercapturic acid metabolites are products of glutathione S-transferase (GST)-mediated detoxification reactions, racial differences in glutathione S-transferase genotypes, which have been observed,31,58 might contribute to differences in mercapturic acid levels between Blacks and Whites. Studies have shown significant GSTT1 genotype effect on PMA levels, the benzene metabolite, such that people with GSTT1-null status have reduced levels of PMA in urine compared to those with normal-function alleles.31,59 An important limitation of our study is that we did not have GST genotype to examine whether the racial differences in mercapturic acid levels observed were attributed to differences in intake of VOCs and not to metabolic differences. While not statistically significant, we found that TNE levels, which are independent of differences in metabolic pathways, were 1.10 times higher in Blacks compared to Whites, suggestive of a general trend of higher tobacco constituent intake in Blacks.
To gain further insight into whether the observed racial difference in VOC metabolites was driven by differences in intake of VOCs or to differences in VOC metabolism, we examined VOC metabolite levels normalized to TNE. It is known that nicotine and toxicants are emitted in mainstream smoke at relatively constant ratios (i.e. yields are highly correlated, R2 > 0.98 from one study60). Thus, it can be assumed that if there is no significant racial difference in VOC metabolism between racial groups, then the average VOC metabolite/TNE would be equal between the racial groups. Indeed, mercapturic acid metabolites normalized per TNE were not significantly different by race, except for MMA, indicating that differences in intake of VOCs and not differences in VOC metabolism explains the racial differences in levels of VOC metabolites observed.
Higher intake of some VOCs among Blacks compared to Whites might be related to differences in the cigarette products used and/or smoking behavior. Use of menthol, which is more prevalent among Blacks, is frequently offered as an explanation for smoking-related health disparities. Other than HEMA, metabolite of acrylonitrile, vinyl chloride and ethylene oxide, which was higher in Black non-menthol smokers compared to Black menthol smokers, levels of VOC metabolites were not significantly different across menthol use among Black or White smokers, consistent with other studies.61
We did not find a significant effect of the rate of nicotine metabolism, measured by the NMR, on racial differences in VOC metabolites, possibly because the main nicotine-metabolizing enzymes, such as CYP2A6, are not known to be involved in the metabolism of the VOCs measured.59 We found no effect of education, as a proxy for socioeconomic status (SES), independent of race on toxicant biomarker levels, and this may be due to a higher proportion of Blacks with less years of education. Although cannabis smoking is a source of the same VOCs measured in tobacco smoke,62 prevalence of cannabis use (positive THC test) did not differ by race in this study; cannabis use did not alter the magnitude of differences in VOC metabolite levels between Blacks and Whites. Finally, while secondhand smoke exposes nonsmokers to significant levels of VOCs,63 the levels are proportionately much lower than that from smoking and are unlikely to explain racial differences among smokers. It is possible that other environmental sources can contribute to racial differences in VOC intake, but we are unable to conduct source apportionment due to limited data.
The generalizability of our findings may be limited by the exclusion of people who smoke less than five CPD – a growing number of smokers in the U.S.,64 and our enrollment of participants drawn from a clinical trial. As stated before, we are limited by a lack of GST genotype data to tease out the potential contribution of metabolic differences to the observed racial differences in biomarker levels. Nevertheless, VOCs normalized to TNE did not vary across race, suggesting that while we cannot rule out metabolic differences, differential intake of tobacco smoke constituents across race may explain, in part, our observations. Finally, we used an improved method to correct for the effect of urine diluteness on biomarker levels measured in spot urine samples across individuals.43
Our findings indicate that for a given level of cigarette consumption per day, Black smokers have higher average levels of biomarkers of acrolein, acrylonitrile, ethylene oxide, and possibly 1,3-butadiene, than White smokers, suggestive of higher intake of these constituents per cigarette smoked. MMA was significantly higher in Blacks compared to Whites, however this may not be related to smoking as a prior study demonstrated that smoking cessation did not lower urinary MMA levels.53 Our findings provide evidence to support the biological plausibility of reported elevated lung cancer and cardiopulmonary disease risks among Black smokers compared to White smokers for a given level of cigarette consumption. Why Black smokers take in more smoke and more toxicants per cigarettes than White smokers remains an unresolved question.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Lisa Yu, Kristina Bello, and Lawrence Chan for lab analyses and all members of the Center for the Evaluation of Nicotine in Cigarettes for data collection. This work was supported by grant U54 DA031659 (Donny/Hatsukami) from the National Institute on Drug Abuse and the Food and Drug Administration Center for Tobacco Products; grants DA02277 (N.L. Benowitz), DA012393 (Reese Jones, not a co-author), and R25DA035163 (James Sorensen, not a co-author) from the National Institute on Drug Abuse; grant P30AG15272 (E. J. Pérez-Stable) from the National Institute on Aging; grant S10RR026437 from the National Center for Research Resources; grant 22FT-0067 (G. St.Helen) from the California Tobacco Related Disease Research Program and a Resource Allocation Program (RAP) grant from the University of California, San Francisco (G. St.Helen). The contents and views in this manuscript are those of the authors and should not be construed to represent the views of the National Institutes of Health, the Food and Drug Administration, or any of the sponsoring organizations or agencies of the U.S. government.
Footnotes
CONFLICT OF INTEREST
NL Benowitz is a consultant to Pfizer and Achieve Life Sciences, companies that market or are developing smoking cessation medications, and has served as a paid expert witness in litigation against tobacco companies. The other authors declare no conflict of interest.
REFERENCES
- 1.U.S. National Cancer Institute. A Socioecological approach to addressing tobacco related health disparities. National Cancer Institute Tobacco Control Monograph 22. NIH Publication No. 17-CA-8035A In: U.S. Department of Health and Human Services, and National Institutes of Health N.C.I. (eds): Bethesda, MD. , 2017. [Google Scholar]
- 2.Siegel RL, Miller KD, and Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018: 68(1): 7–30. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Jacobs EJ, Newton CC, Feskanich D, Freedman ND, Prentice RL, et al. Deaths due to cigarette smoking for 12 smoking-related cancers in the United States. JAMA internal medicine 2015: 175(9): 1574–1576. [DOI] [PubMed] [Google Scholar]
- 4.Jamal A, Phillips E, Gentzke AS, Homa DM, Babb SD, King BA, et al. Current Cigarette Smoking Among Adults—United States, 2016. MMWR 2018: 67(2): 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trinidad DR, Perez-Stable EJ, Emery SL, White MM, Grana RA, and Messer KS. Intermittent and light daily smoking across racial/ethnic groups in the United States. Nicotine Tob Res 2009: 11(2): 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trinidad DR, Perez-Stable EJ, White MM, Emery SL, and Messer K. A nationwide analysis of US racial/ethnic disparities in smoking behaviors, smoking cessation, and cessation-related factors. Am J Public Health 2011: 101(4): 699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris RE, Zang EA, Anderson JI, and Wynder EL. Race and sex differences in lung cancer risk associated with cigarette smoking. Int J Epidemiol 1993: 22(4): 592–599. [DOI] [PubMed] [Google Scholar]
- 8.Gadgeel SM, Severson RK, Kau Y, Graff J, Weiss LK, and Kalemkerian GP. Impact of race in lung cancer: analysis of temporal trends from a surveillance, epidemiology, and end results database. Chest 2001: 120(1): 55–63. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz AG, and Swanson GM. Lung carcinoma in African Americans and whites. Cancer 1997: 79(1): 45–52. [DOI] [PubMed] [Google Scholar]
- 10.Stellman SD, Chen Y, Muscat JE, Djordjevic IV, Richie JP, Lazarus P, et al. Lung cancer risk in white and black Americans. Ann Epidemiol 2003: 13(4): 294–302. [DOI] [PubMed] [Google Scholar]
- 11.Haiman CA, Stram DO, Wilkens LR, Pike MC, Kolonel LN, Henderson BE, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med 2006: 354(4): 333–342. [DOI] [PubMed] [Google Scholar]
- 12.Stram DO, Park S, Haiman CA, Murphy SE, Patel Y, Hecht SS, et al. Racial/Ethnic Differences in Lung Cancer Incidence in the Multiethnic Cohort Study: An Update. JNCI: Journal of the National Cancer Institute 2019. [DOI] [PMC free article] [PubMed]
- 13.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics—2016 update. Circulation 2016: 133(4): e38–e360. [DOI] [PubMed] [Google Scholar]
- 14.Pérez-Stable EJ, Herrera B, Jacob P, and Benowitz NL. Nicotine metabolism and intake in black and white smokers. JAMA 1998: 280(2): 152–156. [DOI] [PubMed] [Google Scholar]
- 15.Murphy SE, Park SS, Thompson EF, Wilkens LR, Patel Y, Stram DO, et al. Nicotine N-glucuronidation relative to N-oxidation and C-oxidation and UGT2B10 genotype in five ethnic/racial groups. Carcinogenesis 2014: 35(11): 2526–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park SL, Carmella SG, Ming X, Vielguth E, Stram DO, Le Marchand L, et al. Variation in levels of the lung carcinogen NNAL and its glucuronides in the urine of cigarette smokers from five ethnic groups with differing risks for lung cancer. Cancer Epidemiology and Prevention Biomarkers 2015: 24(3): 561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol 1998: 11(6): 559–603. [DOI] [PubMed] [Google Scholar]
- 18.Yuan JM, Koh WP, Murphy SE, Fan Y, Wang R, Carmella SG, et al. Urinary levels of tobacco-specific nitrosamine metabolites in relation to lung cancer development in two prospective cohorts of cigarette smokers. Cancer Res 2009: 69(7): 2990–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rostron B NNAL exposure by race and menthol cigarette use among US smokers. Nicotine Tob Res 2013: 15(5): 950–956. [DOI] [PubMed] [Google Scholar]
- 20.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nature Reviews Cancer 2003: 3(10): 733–744. [DOI] [PubMed] [Google Scholar]
- 21.Roemer E, Schramke H, Weiler H, Buettner A, Kausche S, Weber S, et al. Mainstream smoke chemistry and in vitro and in vivo toxicity of the reference cigarettes 3R4F and 2R4F. Beiträge zur Tabakforschung/Contributions to Tobacco Research 2014: 25(1): 316–335. [Google Scholar]
- 22.Schaller JP, Keller D, Poget L, Pratte P, Kaelin E, McHugh D, et al. Evaluation of the Tobacco Heating System 2.2. Part 2: Chemical composition, genotoxicity, cytotoxicity, and physical properties of the aerosol. Regul Toxicol Pharmacol 2016: 81 Suppl 2: S27–S47. [DOI] [PubMed] [Google Scholar]
- 23.Fowles J, and Dybing E. Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke. Tob Control 2003: 12(4): 424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie J, Marano KM, Wilson CL, Liu H, Gan H, Xie F, et al. A probabilistic risk assessment approach used to prioritize chemical constituents in mainstream smoke of cigarettes sold in China. Regul Toxicol Pharmacol 2012: 62(2): 355–362. [DOI] [PubMed] [Google Scholar]
- 25.Haussmann H-J. Use of hazard indices for a theoretical evaluation of cigarette smoke composition. Chem Res Toxicol 2012: 25(4): 794–810. [DOI] [PubMed] [Google Scholar]
- 26.Perbellini L, Veronese N, and Princivalle A. Mercapturic acids in the biological monitoring of occupational exposure to chemicals. J Chromatogr B 2002: 781(1–2): 269–290. [DOI] [PubMed] [Google Scholar]
- 27.Hoffmann D, Brunnemann KD, and Hoffmann I. Significance of benzene in tobacco carcinogenesis. Advances in Modern Environmental Toxicology Benzene: Occupational and Environmental Hazards-Scientific Update Princeton: Princeton Scientific Publishing Co 1989.
- 28.Grosse Y, Baan R, Straif K, Secretan B, El Ghissassi F, Bouvard V, et al. Carcinogenicity of 1, 3-butadiene, ethylene oxide, vinyl chloride, vinyl fluoride, and vinyl bromide Elsevier, 2007. [DOI] [PubMed] [Google Scholar]
- 29.DeJarnett N, Conklin DJ, Riggs DW, Myers JA, O’Toole TE, Hamzeh I, et al. Acrolein exposure is associated with increased cardiovascular disease risk. Journal of the American Heart Association 2014: 3(4): e000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding YS, Blount BC, Valentin-Blasini L, Applewhite HS, Xia Y, Watson CH, et al. Simultaneous determination of six mercapturic acid metabolites of volatile organic compounds in human urine. Chem Res Toxicol 2009: 22(6): 1018–1025. [DOI] [PubMed] [Google Scholar]
- 31.Haiman CA, Patel YM, Stram DO, Carmella SG, Chen M, Wilkens LR, et al. Benzene uptake and glutathione S-transferase T1 status as determinants of S-phenylmercapturic acid in cigarette smokers in the Multiethnic Cohort. PloS one 2016: 11(3): e0150641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park SL, Carmella SG, Chen M, Patel Y, Stram DO, Haiman CA, et al. Mercapturic acids derived from the toxicants acrolein and crotonaldehyde in the urine of cigarette smokers from five ethnic groups with differing risks for lung cancer. PloS one 2015: 10(6): e0124841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jain RB. Distributions of selected urinary metabolites of volatile organic compounds by age, gender, race/ethnicity, and smoking status in a representative sample of US adults. Environmental toxicology and pharmacology 2015: 40(2): 471–479. [DOI] [PubMed] [Google Scholar]
- 34.Roethig HJ, Munjal S, Feng S, Liang Q, Sarkar M, Walk R-A, et al. Population estimates for biomarkers of exposure to cigarette smoke in adult US cigarette smokers. Nicotine Tob Res 2009: 11(10): 1216–1225. [DOI] [PubMed] [Google Scholar]
- 35.Donny EC, Denlinger RL, Tidey JW, Koopmeiners JS, Benowitz NL, Vandrey RG, et al. Randomized trial of reduced-nicotine standards for cigarettes. N Engl J Med 2015: 373(14): 1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heatherton TF, Kozlowski LT, Frecker RC, and Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict 1991: 86(9): 1119–1127. [DOI] [PubMed] [Google Scholar]
- 37.Dempsey D, Tutka P, Jacob P, Allen F, Schoedel K, Tyndale RF, et al. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin Pharmacol Ther 2004: 76(1): 64–72. [DOI] [PubMed] [Google Scholar]
- 38.Murphy SE, Wickham KM, Lindgren BR, Spector LG, and Joseph A. Cotinine and trans 3 ‘-hydroxycotinine in dried blood spots as biomarkers of tobacco exposure and nicotine metabolism. Journal of Exposure Science and Environmental Epidemiology 2013: 23(5): 513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carmella SG, Ming X, Olvera N, Brookmeyer C, Yoder A, and Hecht SS. High throughput liquid and gas chromatography–tandem mass spectrometry assays for tobacco-specific nitrosamine and polycyclic aromatic hydrocarbon metabolites associated with lung cancer in smokers. Chem Res Toxicol 2013: 26(8): 1209–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacob P, Raddaha AHA, Dempsey D, Havel C, Peng M, Yu L, et al. Comparison of Nicotine and Carcinogen Exposure with Water pipe and Cigarette Smoking. Cancer Epidemiology Biomarkers & Prevention 2013: 22(5): 765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alwis KU, Blount BC, Britt AS, Patel D, and Ashley DL. Simultaneous analysis of 28 urinary VOC metabolites using ultra high performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry (UPLC-ESI/MSMS). Anal Chim Acta 2012: 750: 152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, and Pirkle JL. Urinary creatinine concentrations in the US population: Implications for urinary biologic monitoring measurements. Environ Health Perspect 2005: 113(2): 192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Brien KM, Upson K, Cook NR, and Weinberg CR. Environmental chemicals in urine and blood: improving methods for creatinine and lipid adjustment. Environ Health Perspect 2016: 124(2): 220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hazari MS, Griggs J, Winsett DW, Haykal-Coates N, Ledbetter A, Costa DL, et al. A single exposure to acrolein desensitizes baroreflex responsiveness and increases cardiac arrhythmias in normotensive and hypertensive rats. Cardiovascular toxicology 2014: 14(1): 52–63. [DOI] [PubMed] [Google Scholar]
- 45.Ismahil MA, Hamid T, Haberzettl P, Gu Y, Chandrasekar B, Srivastava S, et al. Chronic oral exposure to the aldehyde pollutant acrolein induces dilated cardiomyopathy. American Journal of Physiology-Heart and Circulatory Physiology 2011: 301(5): H2050–H2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feng Z, Hu W, Hu Y, and Tang M-s. Acrolein is a major cigarette-related lung cancer agent: Preferential binding at p53 mutational hotspots and inhibition of DNA repair. Proceedings of the National Academy of Sciences 2006: 103(42): 15404–15409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Denissenko MF, Pao A, Tang M-s, and Pfeifer GP. Preferential formation of benzo [a] pyrene adducts at lung cancer mutational hotspots in P53. Science 1996: 274(5286): 430–432. [DOI] [PubMed] [Google Scholar]
- 48.Smith C, Perfetti T, Rumple M, Rodgman A, and Doolittle D. “IARC Group 2B carcinogens” reported in cigarette mainstream smoke. Food Chem Toxicol 2001: 39(2): 183–205. [DOI] [PubMed] [Google Scholar]
- 49.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC monographs on the evaluation of carcinogenic risks to humans. Volume 97. 1, 3-butadiene, ethylene oxide and vinyl halides (vinyl fluoride, vinyl chloride and vinyl bromide). IARC Monogr Eval Carcinog Risks Hum 2008: 97: 3. [PMC free article] [PubMed] [Google Scholar]
- 50.Marsh GM, Youk AO, and Collins JJ. Reevaluation of lung cancer risk in the acrylonitrile cohort study of the National Cancer Institute and the National Institute for Occupational Safety and Health. Scand J Work Environ Health 2001: 5–13. [DOI] [PubMed]
- 51.Scélo G, Constantinescu V, Csiki I, Zaridze D, Szeszenia-Dabrowska N, Rudnai P, et al. Occupational exposure to vinyl chloride, acrylonitrile and styrene and lung cancer risk (Europe). Cancer Causes Control 2004: 15(5): 445–452. [DOI] [PubMed] [Google Scholar]
- 52.Arrazola RA, Singh T, Corey CG, Husten CG, Neff LJ, Apelberg BJ, et al. Tobacco use among middle and high school students—United States, 2011–2014. MMWR 2015: 64(14): 381–358. [PMC free article] [PubMed] [Google Scholar]
- 53.Scherer G, Urban M, Hagedorn H-W, Serafin R, Feng S, Kapur S, et al. Determination of methyl-, 2-hydroxyethyl-and 2-cyanoethylmercapturic acids as biomarkers of exposure to alkylating agents in cigarette smoke. J Chromatogr B 2010: 878(27): 2520–2528. [DOI] [PubMed] [Google Scholar]
- 54.Lim U, and Song M-A. Dietary and lifestyle factors of DNA methylation. Cancer epigenetics Springer, 2012, pp 359–376. [DOI] [PubMed] [Google Scholar]
- 55.Gee GC, and Payne-Sturges DC. Environmental health disparities: a framework integrating psychosocial and environmental concepts. Environ Health Perspect 2004: 112(17): 1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robertson KD. DNA methylation and human disease. Nature Reviews Genetics 2005: 6: 597. [DOI] [PubMed] [Google Scholar]
- 57.Miller RL, and Ho S-m. Environmental epigenetics and asthma: current concepts and call for studies. Am J Respir Crit Care Med 2008: 177(6): 567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nelson HH, Wiencke JK, Christiani DC, Cheng T, Zuo Z-F, Schwartz BS, et al. Ethnic differences in the prevalence of the homozygous deleted genotype of glutathione S-transferase theta. Carcinogenesis 1995: 16(5): 1243–1246. [DOI] [PubMed] [Google Scholar]
- 59.Dougherty D, Garte S, Barchowsky A, Zmuda J, and Taioli E. NQO1, MPO, CYP2E1, GSTT1 and GSTM1 polymorphisms and biological effects of benzene exposure—a literature review. Toxicol Lett 2008: 182(1–3): 7–17. [DOI] [PubMed] [Google Scholar]
- 60.Charles FS, Cook C, and Clayton P. The linear relationship between cigarette tar and nicotine yields: regulatory implications for smoke constituent ratios. Regul Toxicol Pharmacol 2011: 59(1): 143–148. [DOI] [PubMed] [Google Scholar]
- 61.Tobacco Products Scientific Advisory Committee. Menthol cigarettes and public health: review of the scientific evidence and recommendations US Food and Drug Administration: Washington, DC, 1974. [Google Scholar]
- 62.Wei B, Wang L, and Blount BC. Analysis of cannabinoids and their metabolites in human urine. Anal Chem 2015: 87(20): 10183–10187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Helen G St., Jacob P 3rd, Peng M, Dempsey DA, Hammond SK, and Benowitz NL. Intake of toxic and carcinogenic volatile organic compounds from secondhand smoke in motor vehicles. Cancer Epidemiol Biomarkers Prev 2014: 23(12): 2774–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pierce JP, White MM, and Messer K. Changing age-specific patterns of cigarette consumption in the United States, 1992–2002: association with smoke-free homes and state-level tobacco control activity. Nicotine Tob Res 2009: 11(2): 171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.