Abstract
Background:
The benefit of thymectomy in reducing requirement for corticosteroids, symptom severity, need for immunosuppression, and hospitalization rates in patients with seropositive generalized myasthenia has recently been established. It is unclear whether this benefit applies to patients with myasthenia and purely ocular manifestations (ocular myasthenia gravis, OMG).
Methods:
We conducted a retrospective single-center cohort study of patients with OMG. Patients were included if their diagnosis was confirmed by acetylcholine receptor or muscle-specific kinase (MuSK) antibodies, abnormal electrophysiology, or a positive edrophonium test and at least one year of clinical follow-up. At each visit, the presence and severity of ocular and generalized symptoms was ascertained using a 4-point scale. Prednisone dose, steroid-sparing agent use, and need for intravenous immunoglobulin (IVIg) or plasmapheresis were recorded. The effect of thymectomy on time-weighted prednisone dose and symptom severity score was assessed using linear regression models. To adjust for non-randomization of thymectomy, we employed inverse probability weighting using a propensity score model derived from the pre-thymectomy observation period for thymectomy patients and a 6-month lead-in period for non-thymectomy patients that incorporated age, sex, acetylcholine receptor antibody seropositivity, disease severity (as defined by both symptom severity and treatment requirement), and treating physician preferences.
Results:
Eighty two patients (30 with thymectomy, 52 non-thymectomy) were included. In unadjusted analyses, time-weighted daily prednisone dose was 2.9 mg higher with thymectomy compared to non-thymectomy (95% CI: 0.2–5.7); but, after inverse probability weighting, this was no longer statistically significant (difference = 1.7 mg, 95% CI: −0.8 to 4.2). There was no statistically significant difference in symptom severity score (adjusted difference = 0.35, 95% CI: −0.02 to 0.72) or risk of generalization (p=0.22).
Conclusion:
In this retrospective study that used statistical techniques to account for non-randomization, no significant differences in prednisone dose or symptom severity after thymectomy in ocular myasthenia were demonstrated.
Keywords: ocular myasthenia, myasthenia gravis, thymectomy, propensity score, inverse probability weighting
Introduction
Myasthenia gravis (MG) is an autoimmune neuromuscular disorder that causes fatigable muscle weakness. The thymus gland is believed to play a role in the pathophysiology of MG, as thymoma, thymic hyperplasia and other thymic pathologic abnormalities are frequently found in MG patients with acetylcholine receptor autoantibodies1. Recently, a randomized controlled trial reported that transsternal thymectomy reduced average prednisone dose, need for immunosuppressive medication, and frequency of hospitalization in patients with non-thymomatous MG2. Patients in this trial also noted improved quality of life. However, the trial only included patients with generalized myasthenia gravis (GMG) and excluded those with purely ocular disease (ocular myasthenia gravis, OMG).
The evidence for thymectomy as an effective treatment for OMG is limited to single-center case series3–8. Determining the true clinical benefit reported in these studies is challenging because they use different, subjectively defined outcomes of “remission” and “improvement” as defined by symptomatology, clinical findings, medication requirement, or a combination of the three3–8. A meta-analysis of 640 patients from 26 studies found a pooled remission rate of 50% in OMG patients undergoing thymectomy but was limited by significant heterogeneity (individual study remission rates ranged from 17% to 86%) and publication bias within the literature9. The patients in these studies also underwent transsternal thymectomies, which are associated with greater morbidity and need for inpatient hospitalization compared to newer non-invasive laparoscopic or transcervical approaches. Virtually all prior studies lacked an appropriate control group and relied on comparisons to remission rates from historical controls, who may be different in terms of symptom severity or other characteristics compared to patients who are referred for surgery. One study examined a cohort of treatment-naïve OMG patients who were offered thymectomy and either accepted (n=47) or refused (n=62) surgery but did not account for differences in pre-thymectomy disease severity or other factors that may have influenced the decision for or against surgery10.
To evaluate the potential benefit from thymectomy in patients with OMG, and to account for the lack of randomization in our study, we performed inverse probability weighting with a propensity score model derived from baseline pre-thymectomy data in a cohort of patients with OMG.
Methods
This was a retrospective single-center cohort study of adult patients with OMG. The study was approved by the Institutional Review Board at the University of Pennsylvania. Patients who were seen by a neuro-ophthalmologist at the University of Pennsylvania between January 1, 2010 (when electronic medical records were first available) and April 1, 2017 and received an ICD-9 or ICD-10 diagnosis for MG were identified for potential inclusion in the study. Additional patients were identified through a single neuro-ophthalmologist’s patient database (GTL) that included patients seen between 1997 and 2017. Patients were included if clinical MG symptoms were purely ocular (i.e., no generalized MG symptoms) at the time of diagnosis; the diagnosis was confirmed by the presence of acetylcholine receptor antibodies, electrodecrement on nerve conduction study (NCS) with repetitive stimulation, abnormal jitter on single fiber electromyography (sfEMG), or a positive edrophonium test; and they had at least one year of clinical follow-up. Patients were excluded if they had comorbid thyroid eye disease, other efferent neuro-ophthalmic disorders, or thymoma. We chose to limit our analysis to transcervical rather than trans-sternal thymectomy since this is the surgical approach of choice at our institution, where trans-sternal thymectomy is almost never performed unless there is a clinical suspicion for malignant thymoma or the patient’s anatomy does not allow for a transcervical approach.
Patients meeting inclusion criteria underwent further chart review, and each neuro-ophthalmology clinic visit was recorded as a cohort study visit. At the initial clinical encounter (corresponding to the baseline study visit), information regarding age of diagnosis, sex, race/ethnicity, presenting symptoms (diplopia, ptosis, or both), duration of symptoms at diagnosis, acetylcholine receptor antibody titer and other diagnostic test results, chest imaging results, and treating neuro-ophthalmologist were collected. At each visit, information regarding MG symptom severity and treatment was also abstracted. MG symptom severity was graded on a 4-point ordinal scale based on frequency: none, occurs but not daily, daily but not constant, or constant. Minimal-manifestation status was defined “no symptoms or functional limitations from myasthenia gravis, but there may be some weakness on examination of some muscles”2. The presence of generalized myasthenic symptoms was also recorded. Treatment information was recorded as prednisone dose (mg/day), pyridostigmine use, steroid-sparing agent, intravenous immune globulin (IVIg), plasmapheresis, inpatient hospitalization (excluding hospitalization for thymectomy), and intubation for myasthenic crisis. For symptom severity and prednisone dose, time-weighted averages were calculated by multiplying the score or dose at each visit by the amount of elapsed time between that visit and the previous visit.
Inverse probability weighting was used to adjust for non-randomization of thymectomy11–13. To do this, a propensity score model was first developed using data from baseline and pre-thymectomy follow-up visits to predict the probability that an individual patient would receive thymectomy. For patients who underwent thymectomy, data from the baseline visit and each follow-up visit prior to thymectomy was incorporated into the propensity score model. For patients who did not receive thymectomy, an arbitrary lead-in period of 6 months was used. The propensity score model incorporated the following variables: age, sex, treating neuro-ophthalmologist, acetylcholine receptor antibody titer, time-weighted prednisone dose, time-weighted symptom severity, time spent in minimal-manifestation status, and any history of steroid-sparing agent use. Muscle-specific kinase (MuSK) antibody titer was not included as a covariate in the propensity score model because it was rarely obtained and no patient in either group were seropositive.
Next, inverse probability weighting of the cohort was performed for the purposes of analysis. Weight is calculated as the 1/p(thymectomy) for thymectomy patients and 1/[1-p(thymectomy)] for non-thymectomy patients. Thus, patients who had a probability of thymectomy close to 50% were weighted equally, and patients who had probabilities of thymectomy at either extreme (very low or very high) were weighted more heavily if they received thymectomy or did not receive thymectomy, respectively (Figure 1). Weights are standardized to average 1.0 over the entire sample. The final result is a virtual population of equal size to the actual population but whose probability of thymectomy clusters around 50% (in other words, where thymectomy is pseudo-randomized). Linear regression models were used to assess the effect of thymectomy on cumulative time-weighted prednisone dose, cumulative time on any prednisone, cumulative time on any steroid-sparing agent, cumulative time-weighted symptom severity, cumulative time in minimal-manifestation status, and cumulative time with generalized symptoms. Models for medication use were adjusted for symptom severity, and models for symptom severity were adjusted for medication use to account for confounding by indication. A Cox proportional hazard model was used to determine the effect of thymectomy on first generalization of MG symptoms. Since data from both observational and interventional studies suggests that thymectomy may be more effective in patients with acetylcholine receptor antibodies and when performed earlier in the course of disease14, we also performed exploratory analyses stratified by acetylcholine receptor antibody status (positive or negative) and timing of thymectomy. Because practice patterns of the treating neuro-ophthalmologist were identified a priori as a potential source of confounding for both thymectomy and medical management, a sensitivity analysis was performed that was limited to patients treated by a single neuro-ophthalmologist who referred the greatest number of patients for thymectomy. Statistical significance was defined at the p<0.05 level. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Figure 1:
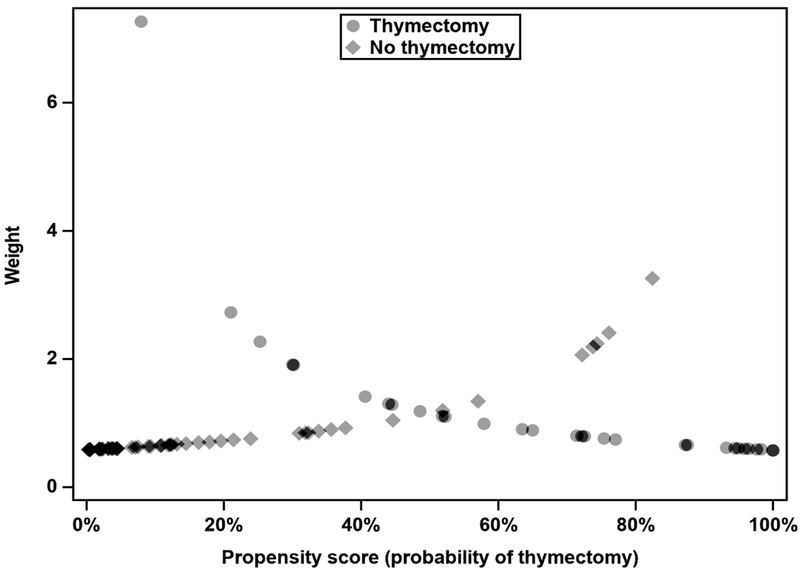
Scatterplot of sample weighting by propensity score for thymectomy
Note that for predicted probabilities of thymectomy near 0.5, thymectomy and non-thymectomy patients have similar weights. At higher probabilities of thymectomy, non-thymectomy patients are up-weighted and thymectomy patients are down-weighted, and vice versa for lower probabilities of thymectomy.
Results
Of the initial 311 patients identified for potential study inclusion, 9 were excluded due to lack of confirmatory diagnostic testing, 48 because of the presence of generalized symptoms at diagnosis, 10 for comorbid thyroid eye disease and 1 for a comorbid vasculopathic third nerve palsy, 7 because of thymoma, 1 due to transsternal thymectomy approach, 37 due to inadequate data, and 115 for having less than one year of clinical follow-up. One patient was additionally excluded due to a history of comorbid systemic lupus erythematosus complicating prednisone dosing. This yielded a final sample size of 82 patients.
Of these 82 patients, 34 were offered thymectomy as part of their care and 48 were not. Reasons that were cited in the charts for not offering thymectomy included older age (n=10), mild symptoms (n=2), patient preference (n=1), and other medical comorbidities (n=1). Four patients were offered thymectomy but ultimately did not receive it due to patient preference (n=3) or concerns regarding medical comorbidities and neck anatomy potentially requiring conversion from transcervical to transsternal approach (n=1). Ultimately, 30 patients underwent thymectomy and 52 were managed with medication alone. Of the 115 patients who were excluded due to insufficient follow-up duration, 11 underwent thymectomy.
Baseline demographic and clinical data from the lead-in period (pre-thymectomy for thymectomy patients, first six months of observation for non-thymectomy patients) is presented in Table 1. Younger age of onset and the practice pattern of the treating neuro-ophthalmologist were significantly associated with a greater probability of thymectomy; prednisone dose was also noticeably higher in patients who underwent thymectomy compared to those who did not, though this was not statistically significant. The median propensity score for patients who underwent thymectomy was 0.66 (IQR: 0.49–0.91), and for patients who did not undergo thymectomy, it was 0.09 (IQR: 0.03–0.28). A box-plot of predicted probability of thymectomy according to the propensity score model for thymectomy and non-thymectomy patients is shown in Figure 2.
Table 1:
Baseline demographic and clinical data from lead-in period
| Thymectomy (n=30) | Non-thymectomy (n=51) | p-value | ||
|---|---|---|---|---|
| Age at diagnosis (years) | 49.4 (15.2) | 65.8 (11.9) | <0.001 | |
| Sex (female) | 15 (50%) | 20 (40%) | 0.38 | |
| Race/ethnicity | Caucasian | 23 (96%) | 36 (86%) | 0.59 |
| African-American | 1 (4%) | 4 (10%) | ||
| Asian-American | 0 (0%) | 1 (2%) | ||
| Other/unknown | 0 (0%) | 1 (2%) | ||
| Treating neuro-ophthalmologist | 1 | 25 (83%) | 26 (52%) | |
| 2 | 4 (13%) | 23 (46%) | ||
| 3 | 1 (1%) | 1 (2%) | ||
| Acetylcholine receptor binding antibody positive (>0.4 nmol/L) | 18 (58%) | 35 (70%) | 0.27 | |
| Symptom duration at diagnosis (months) | 7.2 (17.5) | 4.7 (7.5) | 0.38 | |
| Symptom severity (time-weighted) | 1.19 (0.89) | 1.08 (0.77) | 0.55 | |
| Prednisone dose (time-weighted) | 3.11 (5.56) | 1.12 (3.52) | 0.053 | |
| Steroid-sparing agent | 4 (13%) | 3 (6%) | 0.26 | |
Summary statistics are shown as mean and standard deviation for continuous variables and number and frequency for categorical variables. P-values are for t-test for continuous variables and chi-squared test for categorical variables.
Figure 2:
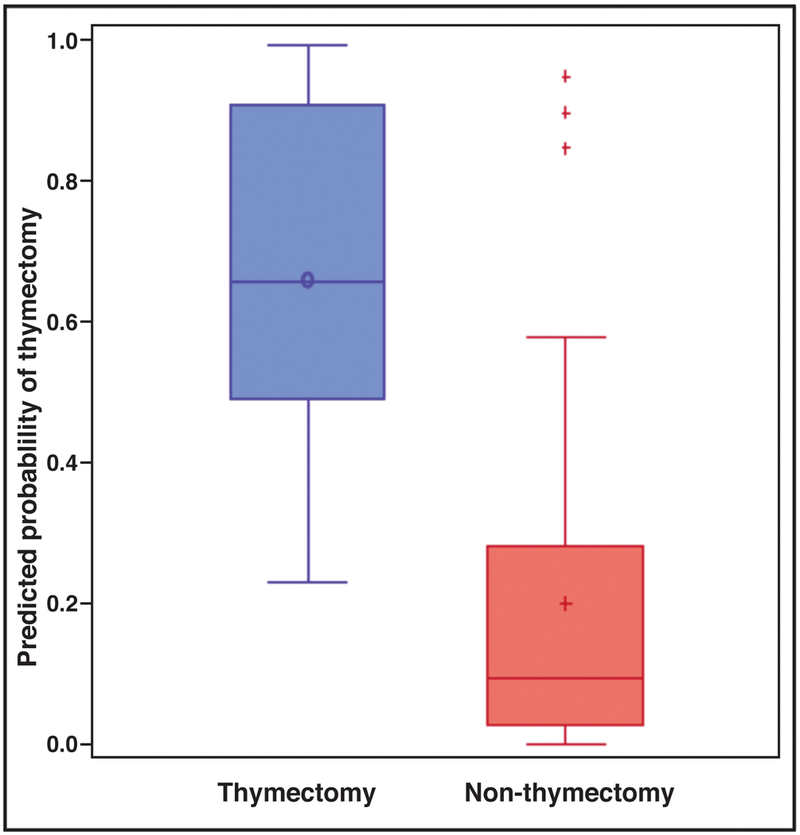
Boxplot of predicted probability of thymectomy according to propensity score model
The horizontal line inside each box indicates the median, and the icon inside each box (hollow circle for thymectomy, plus-sign for non-thymectomy) indicates the mean.
During the follow-up period (total 300 person-years, mean 3.7 years/person), the mean time-weighted prednisone dose was 6.69 mg/day for patients who underwent thymectomy and 3.75 mg/day for patients who did not undergo thymectomy (p=0.04). After adjusting for symptom severity and inverse probability weighting for propensity score, this difference decreased and was no longer statistically significance (mean difference 1.7 mg/day, 95% CI: −0.8 to 4.2). Unadjusted mean time-weighted symptom severity score was 1.02 for thymectomy and 0.86 for non-thymectomy (p=0.41). After adjustment, this difference increased in favor of non-thymectomy and became statistically significant (mean difference, 95% CI: 0.04 to 0.76). The unadjusted proportion of the follow-up period spent in minimal-manifestation status was similar for thymectomy (44%) and non-thymectomy (50%) (p=0.5). After adjustment, this difference increased from 0.06 to 0.18 in favor of non-thymectomy and became statistically significant (p=0.046). These outcomes were similar when examined at 3, 6, and 12 month follow-up intervals (Table 2) and did not change when thymectomy was restricted to within 6 months after diagnosis. The frequency of generalized symptoms and steroid-sparing agent use were similar for thymectomy and non-thymectomy in both unadjusted and adjusted analyses.
Table 2:
Steroid and symptom severity outcomes for thymectomy vs. non-thymectomy at 3, 6, and 12 month follow-up intervals.
| Follow-up interval | Thymectomy (mean, SD) | Non-thymectomy (mean, SD) | Adjusted difference (95% CI) | |
|---|---|---|---|---|
| Time-weighted prednisone dose (mg/day) | 3 months | 7.41 (10.12) | 4.63 (9.46) | 0.8 (−3.2 to 4.8) |
| 6 months | 6.98 (9.24) | 4.83 (8.96) | 0.3 (−3.5 to 4.1) | |
| 12 months | 6.70 (7.97) | 4.44 (7.95) | 0.8 (−2.6 to 4.1) | |
| Time-weighted symptom severity | 3 months | 1.20 (0.84) | 1.06 (0.91) | 0.23 (−0.15 to 0.61) |
| 6 months | 1.08 (0.78) | 1.03 (0.88) | 0.10 (−0.26 to 0.45) | |
| 12 months | 0.90 (0.74) | 0.80 (0.77) | 0.06 (−0.25 to 0.38) |
A positive difference indicates that the value is greater for thymectomy than non-thymectomy, and a negative difference indicates that the value is smaller for thymectomy than non-thymectomy.
Generalization occurred in 9 patients (30%) who underwent thymectomy and 10 (20%) patients who were managed with medication alone (adjusted hazard ratio 1.86, 95% CI: 0.68–5.08). Survival curve for generalization of ocular MG is shown in Figure 3. Inpatient hospitalization for myasthenic symptoms were rare (n=2 for thymectomy and n=1 for non-thymectomy) and deemed too infrequent for statistical analysis. There were no intubations for myasthenic crisis in the cohort.
Figure 3:
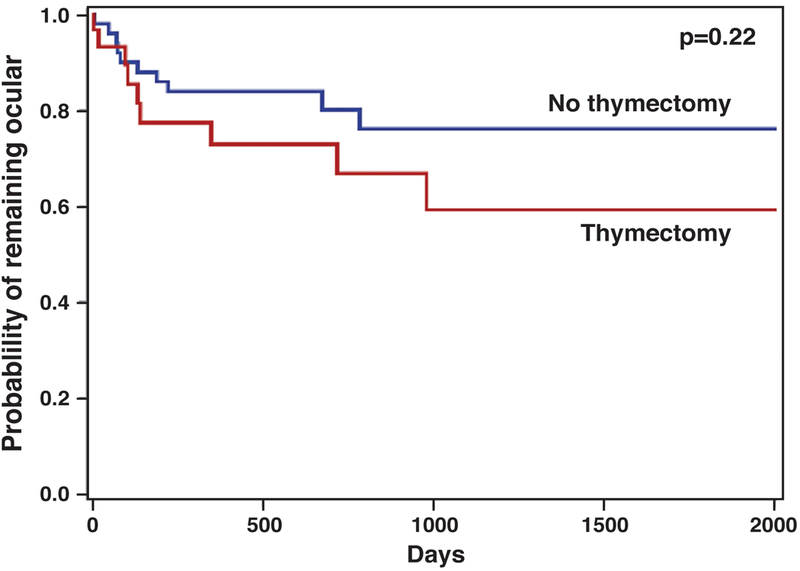
Kaplan-Meier curve for generalization of ocular myasthenia
P-value is for hazard ratio using adjusted Cox proportional hazard model.
When stratified by acetylcholine receptor titer, thymectomy was associated with a tendency towards greater prednisone dose (mean adjusted difference 2.4 mg/day, 95% CI: −0.4 to 5.1) and symptom severity score (mean adjusted difference 0.51, 95% 0.10–0.92) in seropositive patients. However, for seronegative patients, prednisone dose (mean adjusted difference −0.5 mg/day, 95% CI: −5.5 to 4.5) and symptom severity (mean adjusted difference 0.08, 95% CI: −0.59 to 0.76) were essentially equal for thymectomy and non-thymectomy patients.
When the analysis was restricted to a single neuro-ophthalmologist who was responsible for 25 (83%) of thymectomy referrals and 26 (52%) of non-thymectomy patients, there was no difference in propensity score-weighted prednisone dose or symptom severity outcomes either overall or when thymectomy was restricted to within 6 months of diagnosis (Table 3).
Table 3:
Steroid and symptom severity outcomes for thymectomy vs. non-thymectomy restricted to a single treating neuro-ophthalmologist.
| All thymectomy (n=25) vs. all non-thymectomy (n=26) | Thymectomy within 6 months (n=14) vs. all non-thymectomy (n=26) | |
|---|---|---|
| Prednisone dose (weighted difference in mg/day) | 1.9 (95% CI: −1.3 to 5.2) | 0.9 (95% CI: −2.8 to 4.6) |
| Symptom severity (weighted difference) | 0.36 (95% CI: −0.07 to 0.58) | 0.41 (−0.07 to 0.89) |
A positive difference indicates that the value is greater for thymectomy than non-thymectomy, and a negative difference indicates that the value is smaller for thymectomy than non-thymectomy.
Discussion
In this retrospective cohort study, we used propensity score modeling with inverse probability weighting to assess the effect of transcervical thymectomy on prednisone dose and symptom severity in ocular MG. Overall, there was no clear association between thymectomy and myasthenia outcomes, with a trend towards thymectomy being associated with greater prednisone dose and symptom severity primarily in unadjusted analyses.
The lack of benefit associated with thymectomy in this study contrasts with previous literature suggesting an increased likelihood of disease remission with thymectomy9. Much of this is attributable to differences in methodology, specifically the inclusion of a reference group and stricter outcome definitions in our study. However, even when looking solely at patients who underwent thymectomy, prior case series have reported remission rates of as high as 70%3,8 and generalization rates of less than 2%6, whereas our thymectomy cohort experienced less sustained minimal-manifestation status and greater generalization. This suggests the presence of other underlying differences between these populations causing selection bias.
The paradoxical association between thymectomy and worse outcomes in this study likely reflects confounding by indication: namely, patients with more severe disease and higher prednisone requirement were more likely to be referred for thymectomy. We attempted to adjust for this using a propensity score model with inverse probability weighting, but this has several limitations. First, we were unable to account for medical comorbidities (e.g. diabetes), which may have affected both prednisone dosing and thymectomy decision, and the complexity surrounding differences in physician referral patterns was also difficult to capture. Second, for a propensity score-based approach to be effective, there needs to be sufficient overlap in propensity score distributions between the two groups15. In our study, however, the distributions were somewhat divergent (Figure 1) such that patients who underwent thymectomy had a very high predicted probability of thymectomy, and those who did not had a very low predicted probability of thymectomy. This makes adjustment, either through propensity score matching (an approach that has been previously used to study thymectomy in generalized myasthenia16) or inverse probability weighting, challenging because even after adjustment, the propensity score distributions will not fully overlap, which effectively translates into residual confounding. Specifically, in our cohort there were very few non-thymectomy patients with predicted probability of thymectomy near 50%, and the few that were had to be heavily weighted. Thus, while our results do not provide evidence to support transcervical thymectomy for OMG, we are unable to draw definitive conclusions regarding treatment efficacy from this study.
Another limitation of our study was outcome assessment. While time-weighted prednisone dose is objective and easily calculated, symptom severity is inherently subjective and may be difficult to capture from a retrospective review of patient charts (as opposed to prospectively collected patient questionnaires). Furthermore, there were strong floor effects for symptom severity among both thymectomy and non-thymectomy groups; symptom severity can range from 0 to 4, yet for both thymectomy and non-thymectomy, unadjusted means were around 1, which corresponds to ocular symptoms that occur but not daily. By all accounts, this is considered a favorable outcome, and many physicians would not escalate care for ptosis that occurs just once or twice per week. The large degree of success with medical management in our cohort thus made it difficult to detect a difference with thymectomy.
We limited our study to transcervical thymectomy, since very few patients at our institution undergo trans-sternal thymectomy unless there is concern for malignant thymoma or the patient’s anatomy does not allow for a transcervical approach. The lower surgical morbidity of transcervical thymectomy compared to a trans-sternal approach also changes the risk-benefit ratio for thymectomy in OMG. This approach contrasts with the MGTX trial, in which extended trans-sternal thymectomies were performed. In observational studies, transcervical and trans-sternal thymectomy have generally yielded similar clinical outcomes8,17,18. Because there is less direct visualization of the mediastinum in a minimally invasive approach, it is possible for residual thymus gland to be present after transcervical thymectomy19. However, this may occur after trans-sternal thymectomy as well20, and because the presence of residual thymus gland is not routinely assessed after thymectomy with either approach, its actual prevalence and clinical significance remains unclear. Of note, a small number of patients underwent thymectomy but lacked sufficient follow-up duration to be included in the analysis. Most of these were due to censoring at the time of analysis rather than loss to clinical follow-up, and given their small number (less than 10% of all patients who were excluded due to insufficient follow-up), we do not think that these patients were lost to follow-up due to exceptionally positive outcomes from thymectomy and thus that their exclusion biased results towards the null hypothesis. If non-thymectomy patients were lost to follow-up for this reason, it would actually have biased our results in favor of thymectomy.
The strengths of this study include the use of clearly defined, quantifiable outcomes that have been validated in the clinical trial setting2 and the inclusion of an appropriate reference group, which many studies of thymectomy in ocular myasthenia lack. However, methodologic challenges pertaining to non-random treatment allocation are difficult to overcome, and outcomes developed for generalized myasthenia may not be entirely adaptable to ocular myasthenia. Randomized controlled trials that include both standardized treatment protocols and outcomes developed specifically for OMG are needed to determine the optimal treatment approach for these patients.
Acknowledgements
The authors wish to acknowledge Drs. Joel Cooper and Larry Kaiser for performing some of the thymectomies for these patients.
Funding: Dr. Hamedani receives grant funding from the NIH (NINDS T32 NS061779–10)
Footnotes
Conflicts of interest: none
References
- 1.Drachman DB. Myasthenia gravis. N Engl J Med. 1994;330(25):1797–1810. doi: 10.1056/NEJM199406233302507. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe GI, Kaminski HJ, Aban IB, Minisman G, Kuo H-C, Marx A, Ströbel P, Mazia C, Oger J, Cea JG, Heckmann JM, Evoli A, Nix W, Ciafaloni E, Antonini G, Witoonpanich R, King JO, Beydoun SR, Chalk CH, Barboi AC, Amato AA, Shaibani AI, Katirji B, Lecky BRF, Buckley C, Vincent A, Dias-Tosta E, Yoshikawa H, Waddington-Cruz M, Pulley MT, Rivner MH, Kostera-Pruszczyk A, Pascuzzi RM, Jackson CE, Garcia Ramos GS, Verschuuren JJGM, Massey JM, Kissel JT, Werneck LC, Benatar M, Barohn RJ, Tandan R, Mozaffar T, Conwit R, Odenkirchen J, Sonett JR, Jaretzki A, Newsom-Davis J, Cutter GR, MGTX Study Group. Randomized Trial of Thymectomy in Myasthenia Gravis. N Engl J Med. 2016;375(6):511–522. doi: 10.1056/NEJMoa1602489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C-S, Hsu H-S, Huang B-S, Lee H-C, Kao K-P, Hsu W-H, Huang M-H. Factors influencing the outcome of transsternal thymectomy for myasthenia gravis. Acta Neurol Scand. 2005;112(2):108–114. doi: 10.1111/j.1600-0404.2005.00424.x. [DOI] [PubMed] [Google Scholar]
- 4.Liu Z, Feng H, Yeung S-CJ, Zheng Z, Liu W, Ma J, Zhong F-T, Luo H, Cheng C. Extended transsternal thymectomy for the treatment of ocular myasthenia gravis. Ann Thorac Surg. 2011;92(6):1993–1999. doi: 10.1016/j.athoracsur.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura H, Taniguchi Y, Suzuki Y, Tanaka Y, Ishiguro K, Fukuda M, Hara H, Mori T. Delayed remission after thymectomy for myasthenia gravis of the purely ocular type. J Thorac Cardiovasc Surg. 1996;112(2):371–375. doi: 10.1016/S0022-5223(96)70264-7. [DOI] [PubMed] [Google Scholar]
- 6.Roberts PF, Venuta F, Rendina E, De Giacomo T, Coloni GF, Follette DM, Richman DP, Benfield JR. Thymectomy in the treatment of ocular myasthenia gravis. J Thorac Cardiovasc Surg. 2001;122(3):562–568. doi: 10.1067/mtc.2001.116191. [DOI] [PubMed] [Google Scholar]
- 7.Schumm F, Wiethölter H, Fateh-Moghadam A, Dichgans J. Thymectomy in myasthenia with pure ocular symptoms. J Neurol Neurosurg Psychiatry. 1985;48(4):332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shrager JB, Deeb ME, Mick R, Brinster CJ, Childers HE, Marshall MB, Kucharczuk JC, Galetta SL, Bird SJ, Kaiser LR. Transcervical thymectomy for myasthenia gravis achieves results comparable to thymectomy by sternotomy. Ann Thorac Surg. 2002;74(2):320–326; discussion 326–327. [DOI] [PubMed] [Google Scholar]
- 9.Zhu K, Li J, Huang X, Xu W, Liu W, Chen J, Chen P, Feng H. Thymectomy is a beneficial therapy for patients with non-thymomatous ocular myasthenia gravis: a systematic review and meta-analysis. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol. 2017;38(10):1753–1760. doi: 10.1007/s10072-017-3058-7. [DOI] [PubMed] [Google Scholar]
- 10.Mineo TC, Ambrogi V. Outcomes after thymectomy in class I myasthenia gravis. J Thorac Cardiovasc Surg. 2013;145(5):1319–1324. doi: 10.1016/j.jtcvs.2012.12.053. [DOI] [PubMed] [Google Scholar]
- 11.Mansournia MA, Altman DG. Inverse probability weighting. BMJ. 2016;352:i189. doi: 10.1136/bmj.i189. [DOI] [PubMed] [Google Scholar]
- 12.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127(8 Pt 2):757–763. [DOI] [PubMed] [Google Scholar]
- 13.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiol Camb Mass. 2000;11(5):550–560. [DOI] [PubMed] [Google Scholar]
- 14.Gilhus NE, Skeie GO, Romi F, Lazaridis K, Zisimopoulou P, Tzartos S. Myasthenia gravis - autoantibody characteristics and their implications for therapy. Nat Rev Neurol. 2016;12(5):259–268. doi: 10.1038/nrneurol.2016.44. [DOI] [PubMed] [Google Scholar]
- 15.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661–3679. doi: 10.1002/sim.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnett C, Katzberg HD, Keshavjee S, Bril V. Thymectomy for non-thymomatous myasthenia gravis: a propensity score matched study. Orphanet J Rare Dis. 2014;9:214. doi: 10.1186/s13023-014-0214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bril V, Kojic J, Ilse WK, Cooper JD. Long-term clinical outcome after transcervical thymectomy for myasthenia gravis. Ann Thorac Surg. 1998;65(6):1520–1522. [DOI] [PubMed] [Google Scholar]
- 18.Calhoun RF, Ritter JH, Guthrie TJ, Pestronk A, Meyers BF, Patterson GA, Pohl MS, Cooper JD. Results of transcervical thymectomy for myasthenia gravis in 100 consecutive patients. Ann Surg. 1999;230(4):555–559; discussion 559–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Austin EH, Olanow CW, Wechsler AS. Thymoma following transcervical thymectomy for myasthenia gravis. Ann Thorac Surg. 1983;35(5):548–550. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg M, Jáuregui WO, De Vega ME, Herrera MR, Roncoroni AJ. Recurrence of thymic hyperplasia after thymectomy in myasthenia gravis. Its importance as a cause of failure of surgical treatment. Am J Med. 1983;74(1):78–82. [DOI] [PubMed] [Google Scholar]


