Abstract
Rationale & Objective:
The PRESERVE trial used a 2x2 factorial design to compare intravenous saline with intravenous sodium bicarbonate and oral N-acetylcysteine with placebo for the prevention of 90-day major adverse kidney events and death (MAKE-D) and contrast-associated acute kidney injury (CA-AKI) among patients with chronic kidney disease undergoing angiography. In this ancillary study, we evaluated the predictive capacities of pre-angiography injury and repair proteins in urine and plasma for MAKE-D, CA-AKI, and their impact on trial design.
Study Design:
Longitudinal analysis
Setting and Participants:
A subset of participants from the PRESERVE trial.
Exposures:
Injury (KIM-1, NGAL, IL-18) and repair (MCP-1, UMOD, and YKL-40) proteins in urine and plasma 1-2 hours pre-angiography.
Outcomes:
MAKE-D and CA-AKI.
Analytic Approach:
We analyzed the associations of pre-angiography biomarkers with MAKE-D and with CA-AKI. We evaluated whether the biomarkers could enrich the MAKE-D event rate and improve future clinical trial efficiency through an online biomarker prognostic enrichment tool available at prognosticenrichment.com.
Results:
We measured plasma biomarkers in 916 participants and urine biomarkers in 797 participants. After adjusting for urinary albumin-creatinine ratio and baseline eGFR, pre-angiography levels of four plasma (KIM-1, NGAL, UMOD, YKL-40) and three urine (NGAL, IL-18, YKL-40) biomarkers were associated with MAKE-D. Only plasma KIM-1 was significantly associated with CA-AKI after adjustment. Biomarkers provided modest discriminatory capacity for MAKE-D. Screening patients using the 50th percentile of pre-angiography plasma KIM-1 or YKL-40 would have reduced the required sample size by 30% (~2000 participants).
Limitations:
Evaluation of prognostic enrichment does not account for changing trial costs, time needed to screen patients, or loss to follow up. Most participants were male, limiting the generalizability of our findings.
Conclusions:
Pre-angiography levels of injury and repair biomarkers modestly predict the development of MAKE-D and can be used to improve the efficiency of future CA-AKI trials.
Keywords: acute kidney injury (AKI); contrast-associated acute kidney injury (CA-AKI); contrast-induced acute kidney injury (CI-AKI); contrast media; prognostic biomarker, tubular injury; urinary biomarkers; plasma biomarkers; MAKE-D; angiography, event rate, enrollment criteria, clinical trial design
INTRODUCTION
Contrast-associated acute kidney injury (CA-AKI) is one of the most studied forms of acute kidney injury (AKI) due to the potential for prevention. The timing of nephrotoxic insult is more predictable with CA-AKI than in other clinical settings such as sepsis. In 2015, approximately 40% of ongoing clinical trials for the prevention of AKI were in the setting of contrast administration.1 Contrast media are used widely in various clinical settings. More than 75 million patients undergo procedures with contrast media every year.2 CA-AKI encompasses a third of all acute kidney injury cases3 with incidence estimates for high risk patients as high as 25%.4,5 Furthermore, CA-AKI is independently associated with cardiovascular events, increased hospital stay, and mortality.6–8
Recent guidelines to minimize the risk of CA-AKI recommended pre-procedure intravenous sodium chloride or sodium bicarbonate with consideration for concurrent use of oral N-acetylcysteine (NAC) in patients at increased risk.9 These guidelines were informed by multiple clinical trials that evaluated these specific interventions. However, these studies were limited in sample size, resulting in equipoise on comparison of sodium bicarbonate with sodium chloride and the efficacy of NAC.1 With 5,177 randomized patients, the recently completed PRESERVE trial was a multi-site study uniquely powered to compare intravenous saline with intravenous sodium bicarbonate and NAC with oral placebo through a 2×2 factorial design with patient-centered, clinical outcomes.10
The PRESERVE trial, like many other clinical trials in CA-AKI, enrolled patients with decreased eGFR to enrich event rates. However, the use of serum creatinine-based criteria, such as standard GFR estimation equations, for trial enrollment has been shown to yield heterogeneous study populations that consist of patients with varying risks for the event of interest.11,12 Serum creatinine has long been considered an imperfect gold standard in the field of kidney diseases as it may not always reflect true injury13 and does not capture subclinical disease.13,14 As the cost of clinical trials escalates, improving the efficiency by identifying a high-risk population likely to benefit from an intervention has become an important goal. Sensitive novel biomarkers of injury and repair can help screen individuals who are more likely to experience events of interest that constitute study outcomes, thereby lending prognostic value to clinical trials.
Prior prospective cohort studies have demonstrated that serum and urine biomarkers can be used to stratify a patient’s risk for AKI.15–17 In the setting of CA-AKI, studies have suggested that biomarkers may have predictive and diagnostic potential.18–21 However, these studies were limited in size and were primarily single-center studies or lacked follow up for serious adverse clinical outcomes.18–21 As a result, there is a lack of information on how urine and plasma biomarkers can help risk stratify patients for CA-AKI and associated adverse events, inform the design of clinical trials, and advance the development of therapies. We undertook this sub-study of the PRESERVE trial to assess the predictive capacity of pre-angiography plasma and urine biomarkers for CA-AKI and 90-day major adverse kidney events and death (MAKE-D), and to examine their utility in enhancing clinical trial design.
METHODS
Study Design
The design of the PRESERVE trial has been described previously.10 The trial was a 2-by-2 factorial design comparing intravenous isotonic sodium bicarbonate with intravenous isotonic saline and oral NAC with oral placebo in patients with chronic kidney disease (estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2 and diabetes or <45 ml/min/1.73 m2 with or without diabetes) who were undergoing angiography.
The primary outcome of the trial was a composite (MAKE-D) within 90 days. Adverse kidney events included the need for dialysis or persistent decrease in kidney function. The latter was defined as a ≥ 50% increase in serum creatinine at 90 days after angiography, confirmed by subsequent testing within 14 days of the initial measurement. The secondary outcome was CA-AKI, defined as an increase in serum creatinine of ≥25% or ≥0.5 mg/dL (44 μmol/L) from baseline at 3 to 5 days after angiography. The trial had a target sample size of 8,680 patients22 but was terminated after randomizing 5,177 patients following a prespecified interim analysis that indicated it was unlikely to show an effect of treatment assignment on study outcomes.10
The PRESERVE trial and this ancillary biomarker study were approved by the Veteran’s Affairs Central Institutional Review Board and all appropriate study site ethics and regulatory committees. Written informed consent was obtained from all participants.
Sub-Study Sample Collection and Biomarker Measurement
19 participating centers collected additional plasma and urine samples 1 to 2 hours pre-angiography as part of an ancillary study. Patients were asked at the time of their enrollment if they were also interested in participating in the ancillary study. The samples were aliquoted and stored at −80C until biomarker measurements.
We measured the plasma and urine samples for injury (KIM-1 [kidney injury marker 1], NGAL [neutrophil gelatinase-associated lipocalin], IL-18 [interleukin 18]) and repair (MCP-1 [monocyte chemoattractant protein 1], UMOD [uromodulin], and YKL-40 [chitinase-3-like protein 1]) proteins, the methods of which have been described previously.15,22 Urine and plasma NGAL, IL-18, KIM-1, YKL-40, UMOD, and MCP-1 were measured using a multiplex assay (Meso Scale Diagnostics [MSD] LLC, Rockville, MD, USA). This assay used patterned arrays and an electrochemiluminescence detection method, which was quantified using the MSD Quickplex SQ 120 instrument. Biomarkers were measured in duplicate and averaged. All personnel measuring the biomarkers were blinded to clinical outcomes.
Statistical Analysis
We compared demographic characteristics and clinical variables by 90-day MAKE-D status using t-tests for normally distributed continuous variables, the Wilcoxon rank-sum test for variables without a normal distribution, and the chi square test for categorical variables. We compared pre-angiography biomarker levels by MAKE-D and CA-AKI. We also reported p-values adjusted for urinary albumin-creatinine ratio (UACR) and baseline eGFR using van Elteren tests, which are a stratified extension of the Wilcoxon rank-sum test. In supplementary analyses, we corrected urine biomarker measurements for urine creatinine. We plotted receiver operator characteristic curves and calculated areas under the curve (c-statistics) for each pre-operative plasma and urine biomarker for MAKE-D and CA-AKI. Statistical analyses were run on SAS software, version 9.4 (SAS Institute) and Stata version 14 (StataCorp LLC).
To examine the potential prognostic enrichment of pre-angiography plasma and urine biomarkers in designing clinical trials for CA-AKI, we used the Biomarker Prognostic Enrichment Tool (BioPET) at prognosticenrichment.com, the methods of which have been described previously.23 BioPET was designed such that a user provides the expected event rate in the non-intervention group without enrichment, treatment efficacy, statistical testing parameters, and costs to generate quantitative analyses including the event rate among biomarker-positive patients, sample size, total screened, and total costs. For event rate, BioPET simulates data for 500,000 hypothetical patients. At each screening threshold, the proportion of events is determined among all patients who exceed the threshold. BioPET calculates sample size based on the desired power, Type I error rate, event rates with and without intervention for a two-sided test. BioPET derives the corresponding number of participants that would need to be screened to achieve the enriched sample size at the desired threshold. Trial costs and percent reduction in trial costs assume fixed costs throughout the duration of the trial.
For BioPET, we selected two biomarkers with the strongest predictive capacity based on AUC. We then used parameters as indicated from the original PRESERVE study design: predicted primary event rate (8.7%) in the non-intervention group (i.e., IV isotonic saline and placebo), anticipated percent reduction in event rate under treatment (25%), Type 1 error rate (2.5%), and power of 90% for two-sided alternative hypothesis testing.24 We then compared the enriched event rate and trial sample size to those of the original trial design. To examine trial costs associated with the use of prognostic biomarkers in clinical trial design, we set the cost to screen a patient at $500 and the cost of retaining a patient through the trial to be $10,000. A screening cost of $500 is consistent with the expected costs for blood and urine processing for screening.25 Trial cost per patient is appropriate for a clinical trial in AKI that does not require multiple years of follow up.23,25
RESULTS
Biomarker Associations with MAKE-D and CA-AKI
From 19 centers, we measured plasma biomarkers from 916 participants and urine biomarkers from 797 participants. Patients who developed the primary outcome of MAKE-D were more likely to have higher UACRs and lower baseline eGFRs but were otherwise similar to patients who did not develop MAKE-D (Table 1). The overall patients in this sub-study had comparable demographic, clinical, and procedural characteristics to the overall PRESERVE study (Table S1). Among pre-angiography plasma biomarkers, KIM-1, NGAL, and YKL-40 were significantly higher among those who experienced MAKE-D compared to those who did not experience MAKE-D (Table 2). Pre-angiography plasma UMOD was significantly lower among those with MAKE-D compared to those who did not experience MAKE-D. Among pre-angiography urine biomarkers, NGAL, IL-18, and YKL-40 were significantly higher among those who experienced MAKE-D compared to those who did not. For CA-AKI, only pre-angiography plasma KIM-1 was significantly higher among patients who experienced the event compared to those who did not (Table 3). After adjustment for UACR and baseline eGFR, plasma biomarker findings remained consistent but urine NGAL and MCP-1 were no longer significantly associated with MAKE-D. Urine creatinine-corrected results for urine biomarkers can be seen in Table S2. Urine creatinine-corrected IL-18, MCP-1, and YKL-40 were significantly associated with MAKE-D after adjustment for UACR and baseline eGFR. No urine creatinine-corrected biomarkers were associated with CA-AKI.
Table 1.
DemoaraDhic. Clinical, and Procedural Characteristics of Patients bv MAKE-D Status
| MAKE-D | P-value | ||
|---|---|---|---|
| No (n=862) | Yes (n=60) | ||
| Demographic Characteristics | |||
| Age-years | 70 ± 8 | 71 ± 8 | 0.3 |
| Male sex, no. (%) | 838 (97%) | 58 (97%) | 0.7 |
| Race/Ethnicity, no. (%) | 0.09 | ||
| White | 674 (78%) | 44 (75%) | |
| Black | 137 (16%) | 7 (12%) | |
| Hispanic | 28 (3%) | 4 (7%) | |
| Other | 22 (3%) | 4 (7%) | |
| Clinical Characteristics | |||
| Weight –kg | 100 ± 22 | 102 ± 26 | 0.7 |
| baseline serum creatinine -mg/dL | 1.6 [1.3-1.7] | 1.6 [1.2-2.0] | 0.9 |
| UACR Categories, | 0.002 | ||
| <30 mg/g | 356 (44%) | 15 (27%) | |
| 30-300 mg/g | 269 (33%) | 17 (30%) | |
| >300 mg/g | 181 (22%) | 24 (43%) | |
| Baseline eGFR, mL/min/1.73 m2 | 0.01 | ||
| [15-30) | 58 (7%) | 11 (18%) | |
| [30-45) | 296 (35%) | 17 (28%) | |
| >45 | 489 (58%) | 32 (53%) | |
| Diabetes, no. (%) | 708 (82%) | 52 (87%) | 0.5 |
| Procedural Characteristics | |||
| Coronary Procedure, no. (%) | 758 (88%) | 54 (90%) | 0.8 |
| Percutaneous Intervention, no. (%) | 244 (28%) | 11 (18%) | 0.1 |
| LVEDP - mmHg | 19 ± 8 | 20 ± 8 | 0.6 |
| Trial arm, no. (%) | 0.5 | ||
| Saline + Placebo | 200 (23%) | 11 (18%) | |
| Saline + NAC | 224 (26%) | 12 (20%) | |
| Sodium Bicarbonate + Placebo | 223 (26%) | 19 (32 %) | |
| Sodium Bicarbonate + NAC | 215 (25%) | 18 (30%) | |
Values for continuous variables presented as mean ± sd or median [interquartile]; for categorical variables, as count (percentage).
Abbreviations: eGFR, estimated glomerular filtration rate; IQR, inter quartile range; UACR, Urinary Albumin-Creatinine Ratio; NAC, N-acetylcysteine; LVEDP, Left ventricular end diastolic pressure
Table 2.
Pre-Angiography Plasma and Urine Biomarker Levels by MAKE-D Status
| Plasma Biomarker | Urine Biomarker | |||||||
|---|---|---|---|---|---|---|---|---|
| Median [IQR], pg/mL | P | Median [IQR, pg/mL | P | |||||
| MAKE-D | No MAKE-D | Unadj | Adj* | MAKE-D | No MAKE-D | Unadj | Adj* | |
| KIM-1 | 504 (306, 948) | 321 (189, 585) | <0.001 | 0.04 | 1804 (961,3558) | 1392 (720, 2743) | 0.06 | 0.2 |
| NGAL | 259 (209, 420) | 228 (173, 296) | 0.001 | 0.04 | 37 (16, 97) | 23 (11, 49) | 0.009 | 0.09 |
| IL-18 | 332 (238, 446) | 318 (246, 408) | 0.5 | 0.9 | 33 (16, 54) | 21 (12, 37) | 0.003 | 0.01 |
| MCP-1 | 229 (167, 280) | 217 (181,270) | 0.9 | 0.9 | 289 (159, 565) | 205 (118, 363) | 0.01 | 0.1 |
| UMOD | 59 (41,81) | 71 (51, 97) | 0.006 | 0.01 | 2300 (1462, 3642) | 2484 (1554, 3944) | 0.5 | 0.9 |
| YKL-40 | 141987 (98750, 284819) | 91371 (53545, 174700) | <0.001 | 0.001 | 902 (272, 4474) | 394 (127, 1184) | <0.001 | 0.02 |
Urine creatinine-corrected results in Table S1
Adjusted for Urinary Albumin-Creatinine Ratio and baseline eGFR
Adj, adjusted; unadj, unadjusted; KIM-1 [kidney injury marker 1], NGAL [neutrophil gelatinase-associated lipocalin], IL-18 [interleukin 18], MCP-1 [monocyte chemoattractant protein 1], UMOD [uromodulin], and YKL-40 [chitinase-3-like protein 1]; MAKE-D, major adverse kidney events and death.
Table 3.
Pre-Angiography Plasma and Urine Biomarker Levels by CA-AKI Status
| Plasma Biomarker | Urine Biomarker | |||||||
|---|---|---|---|---|---|---|---|---|
| Median [IQR], pg/mL | P | Median [IQR], pg/mL | P | |||||
| CA-AKI | No CA-AKI | Unadj | Adj* | CA-AKI | No CA-AKI | Unadj | Adj* | |
| KIM-1 | 430 (295, 713) | 319 (190, 593) | 0.002 | 0.004 | 1460 (767, 2633) | 1419 (731, 2805) | 0.9 | 0.9 |
| NGAL | 232 (168, 297) | 230 (175, 299) | 0.9 | 0.6 | 20 (9, 41) | 24 (12, 51) | 0.4 | 0.3 |
| IL-18 | 347 (265, 447) | 316 (245, 408) | 0.09 | 0.1 | 22 (10, 38) | 21 (12, 38) | 0.8 | 0.9 |
| MCP-1 | 218 (168, 265) | 218 (181, 271) | 0.5 | 0.3 | 183 (115, 302) | 210 (119, 374) | 0.3 | 0.4 |
| UMOD | 68 (49, 87) | 71 (50, 97) | 0.3 | 0.3 | 2776 (1683, 4606) | 2453 (1548, 3862) | 0.2 | 0.3 |
| YKL-40 | 108251 (54743, 205041) | 94598 (54166, 178090) | 0.4 | 0.5 | 400 (137, 1202) | 406 (137, 1255) | 0.9 | 0.7 |
Urine creatinine-corrected results in Table S1
Adjusted for Urinary Albumin-Creatinine Ratio and baseline eGFR
Adj, adjusted; unadj, unadjusted; KIM-1 [kidney injury marker 1], NGAL [neutrophil gelatinase-associated lipocalin], IL-18 [interleukin 18], MCP-1 [monocyte chemoattractant protein 1], UMOD [uromodulin], and YKL-40 [chitinase-3-like protein 1].
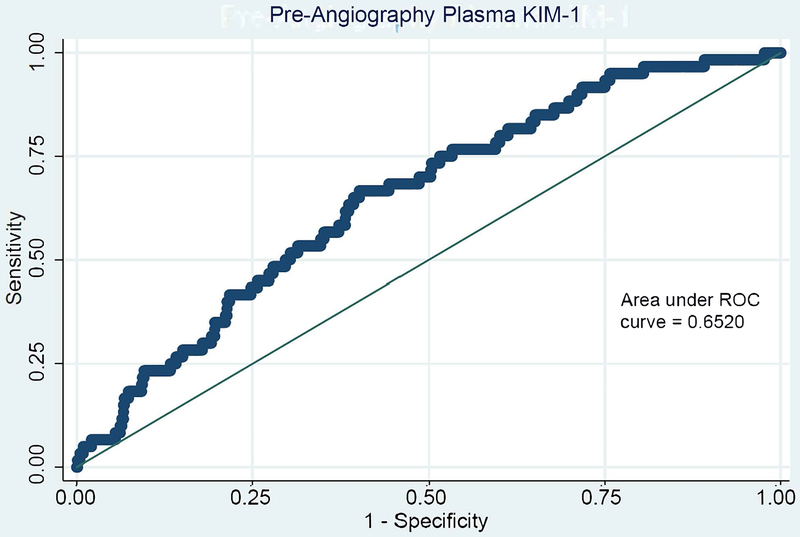
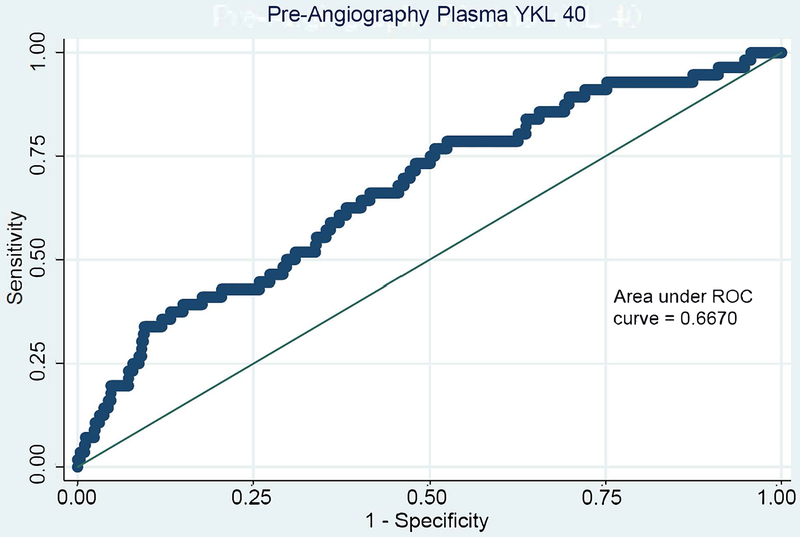
C-statistics for all pre-angiography plasma and urine biomarkers for both MAKE-D and CA-AKI are shown in Table S3. Plasma YKL-40 and plasma KIM-1 show the strongest discrimination for the primary outcome with modest areas under the curve (c-statistics) of 0.66 (95% CI, 0.59-0.73) and 0.65 (95% CI, 0.58-0.72), respectively. Receiver operator characteristic curves are shown for plasma KIM-1 and YKL-40 in Figure 1.
Figure 1. Receiver Operator Curves of Pre-Angiography Plasma KIM-1 (A) and Plasma YKL-40 (B) for MAKE-D.
Table S2 includes the areas under the receiver operator curves for all plasma and urine pre-angiography biomarkers for the outcomes MAKE-D and CA-AKI
Findings from the Biomarker Prognostic Enrichment Tool
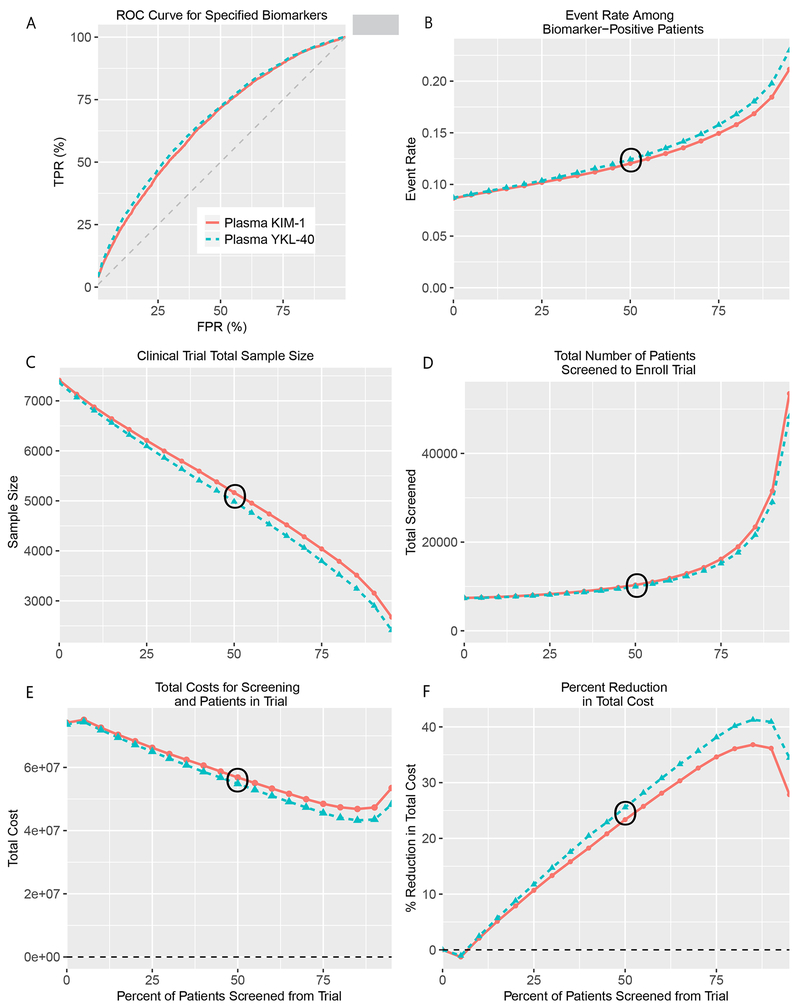
Findings from the Biomarker Prognostic Enrichment Tool demonstrated that inclusion of plasma KIM-1 or plasma YKL-40 could have prognostically enriched the PRESERVE Trial (Figure 2). Without biomarkers, the event rate of the placebo group during the original trial design was estimated to be 8.7%; enriching patients from the trial with plasma KIM-1 or YKL-40 levels above the 50th percentile would have increased the primary trial event rate to 12% (Figure 2, Panel B). Similarly, incorporating the 50th percentile of these biomarker levels to screen patients would have reduced the necessary sample size from 7,376 to 5191 and 4992 patients for plasma KIM-1 and YKL-40, respectively (Figure 2 Panel C). To achieve the enriched sample size target through biomarker screening, more patients would have had to be screened. Screening out 50% of patients using pre-angiography plasma KIM-1 and YKL-40 levels would have required a total of 10,382 and 9,984 to be screened for the trial, respectively. Overall, incorporation of the 50th percentile of plasma KIM-1 or YKL-40 would have required screening ~3,000 more patients than the original trial (Figure 2 Panel D). Given the costs associated with screening and maintaining the patients in the trial, the percent reduction in total trial costs is not uniform depending on the percentage of patients screened (Figure 2 Panel E). For both plasma KIM-1 and YKL-40, prognostic enrichment yielded a reduction in total costs when the biomarker threshold screened at least 5% of patients from the trial. The greatest expected cost reduction (37-40%) occurred when using the 85th percentile of either biomarker to screen patients (Figure 2 Panel F). Detailed information for each of the panels in Figure 2 can be found in Table S4 and Table S5.
Figure 2. Incorporation of Pre-Angiography Plasma YKL-40 or KIM-1 in the PRESERVE Trial Design with the Biomarker Prognostic Enrichment Tool (BioPET).
BioPET enrichment tool analysis after inputting the area under the curve (c-statistic) for plasma YKL-40 (0.66) and KIM-1 (0.65). With the exception of Panel A, all graphs have a horizontal axis of the percent of patients screened from the trial. Panel A. displays the receiver operating characteristic curve for YKL-40 and KIM-1 generated from BioPET tool. Panel B displays the simulated data for 500,000 hypothetical patients. At each screening threshold, the proportion of events is determined among all patients who exceed the threshold. Panels C shows changes in sample size based on the desired power, Type I error rate, event rates with and without intervention for a two-sided test. Panel D indicates the corresponding number of participants that would need to be screened to achieve the enriched sample size at the desired threshold. Panels E and F show trial costs and percent reduction in trial costs, respectively, and were calculated by setting the cost of screening to $500 and the cost of enrolling and retaining a patient to $10,000.
DISCUSSION
In this sub-study of the PRESERVE trial, we demonstrated that four plasma and three urine pre-angiography biomarkers had modest discriminatory predictive capacity for the primary trial outcome, MAKE-D. Despite these modest associations, the biomarkers have considerable potential in enriching the event rate and reducing the number of patients needed to achieve the same power for clinical trials in the setting of CA-AKI. In particular, incorporation of the 50th percentile of pre-angiography plasma KIM-1 or YKL-40 in the PRESERVE trial would have increased the absolute event rate by more than 3%, for a relative increase of nearly 40%, and reduced the sample size by ~2,000 patients.
Our findings of the predictive capacity of biomarkers among patients from the PRESERVE trial are largely consistent with those of prior single-center, prospective cohorts that have recorded comparable elevations in pre-angiography urine NGAL and IL-18 by CA-AKI status.20 Current literature supports YKL-40 and KIM-1 as biomarkers of repair and injury, respectively. YKL-40 is produced in response to cellular damage in a range of inflammatory cells26–28 with cytoprotective effects in the setting of kidney transplantation.29 Injury proteins like KIM-1 are sensitive and specific markers that are upregulated during kidney tubular injury.26,30,31 Patients with elevated levels of injury markers prior to contrast exposure may have had existing subclinical injury or have been predisposed to experience kidney injury following exposure. Further studies are needed to fully understand the capacity of novel biomarkers to characterize the repair potential of the kidney, develop prognostic tools, and inform point of care testing.
Prognostic biomarkers as defined by the U.S. Food and Drug Administration can be used “to identify likelihood of a clinical event, disease recurrence or progression in patients who have the disease or medical condition of interest.”33 Prognostic biomarkers such as albuminuria, reduced eGFR, and total kidney volume have been identified and successfully incorporated in the design of clinical trials for kidney diseases outside of AKI.34–36 The vast majority of clinical trials in AKI still rely on elevated serum creatinine or decreased eGFR, which can arise from a variety of etiologies and result in a heterogeneous study population, complicating the development of therapies. Urine and plasma biomarkers have been shown to have prognostic potential in various clinical settings of AKI. 18–21,25 A prior study examined the effects of kidney injury biomarkers and cardiopulmonary bypass time, a known renal insult, on enriching a hypothetical clinical trial for AKI after surgery in the TRIBE cohort.25 In this setting of cardiac surgery, clinical trial enrichment through plasma NGAL, urine IL-18, cardiopulmonary bypass time (CBP), or a combination of the three would be predicted to increase AKI rates by 60-280% and reduce trial costs by 34-55%.25
In the PRESERVE trial, biomarkers such as plasma YKL-40 and KIM-1 may serve as prognostic biomarkers by providing much-needed granularity in identifying persons at highest risk for CA-AKI and serious adverse longer-term outcomes. In addition, injury proteins can capture persons who may have subclinical AKI with normal levels of creatinine.37 Both possibilities suggest that current inclusion criteria for clinical trials in CA-AKI may not be appropriately capturing those at risk. Enrollment practices based on current inclusion criteria require a larger sample size to achieve the event rates needed to evaluate a therapy.25
Our sub-study leveraged the large sample size of the PRESERVE trial to assess the prognostic potential of urine and plasma biomarkers, and evaluate their potential impact on clinical trial design. While the PRESERVE trial found no benefit of intravenous isotonic sodium bicarbonate compared with intravenous isotonic sodium chloride or NAC compared with placebo for the prevention of MAKE-D or CA-AKI, the incorporation of prognostic plasma or urine biomarkers would have reduced the number of patients necessary for the trial. Enriching clinical trials with prognostic biomarkers will inevitably reduce a trial’s generalizability. However, clinical trials, particularly early-phase clinical trials in settings such as CA-AKI, benefit from high-risk study populations who are more likely to experience adverse kidney outcomes with contrast media to advance the development of therapies.25
There are several limitations worth noting in this analysis. First, the BioPET clinical enrichment tool does not account for changing screening and retention costs for the trial, the amount of time needed to screen an increased number of patients, and does not assess for expected loss to follow up in assessing the enrichment potential of prognostic biomarkers in the design of clinical trials. These characteristics present additional considerations for investigators in designing clinical trials. Second, the majority of patients of the PRESERVE trial were male, which limits the generalizability of our findings. Furthermore, our findings need to be validated through other studies to ensure consistency of observed associations and their subsequent impact on clinical trial design.
In conclusion, our study shows that some injury and repair proteins have modest predictive capacities. However, these biomarkers can guide the design of clinical trials in the setting of CA-AKI.
Supplementary Material
Table S1. Demographic, clinical, and procedural characteristics of the PRESERVE trial and substudy.
Table S2. Creatinine-corrected urine pre-angiography biomarkers by MAKE-D and CA-AKI status.
Table S3. AUCs for pre-angiography plasma and urine biomarkers for MAKE-D and CA-AKI.
Table S4. BioPET analysis of pre-angiography plasma KIM-1.
Table S5. BioPET analysis of pre-angiography plasma YKL-40.
Acknowledgments
Support: This study was supported by the U.S. Department of Veterans Affairs Office of Research and Development (VA CSP #578 PRESERVE Trial; PI: S.D. Weisbord, Co-PI: P. M. Palevsky), the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK098214 Biomarker Collection and Analysis in the PRESERVE Trial Cohort; MPI’s: Weisbord, Palevsky and Parikh), and the National Health and Medical Research Council of Australia. C.R. Parikh was additionally supported by the George M. O’Brien Kidney Center (P30DK079310) and the National Institutes of Health (R01HL085757). Funding source had no role in the study design, data collection, analysis, reporting, or decision to submit for publication.
Financial Disclosure: C.R. Parikh has received consulting fees from Renalytix, and serves on the Data and Safety Monitoring Board of Genfit and Abbott. P.M. Palevsky receives consulting fees and advisory committee fees from Durect, Novartis, and HealthSpan Dx, serves on the Data and Safety Monitoring Board of Baxter, and serves as a member of an end-point adjudication committee from GE Healthcare. S.D. Weisbord has received consulting fees and advisory fees from Durect and Saghmos Therapeutics. The remaining authors declare that they have no relevant financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Supplementary Material Descriptive Text for Online Delivery
Disclaimer: The opinions in this article are those of the authors and do not represent the views of the United States government or the Department of Veterans Affairs.
Prior Presentation: Parts of this study were presented in poster form at the American Society of Nephrology Kidney Week, San Diego, CA, 23-28 October 2018.
Received February 7, 2019. Evaluated by 2 external peer reviewers and a statistician, with editorial input from an Acting Editor-in-Chief (Abhijit Kshirsagar, MD, MPH). Accepted in revised form June 20, 2019. The involvement of an Acting Editor-in-Chief to handle the peer-review and decision-making processes was to comply with AJKD’s procedures for potential conflicts of interest for editors, described in the Information for Authors & Journal Policies.
References
- 1.Faubel S, Chawla LS, Chertow GM, Goldstein SL, Jaber BL, Liu KD. Ongoing Clinical Trials in AKI. Clin J Am Soc Nephrol. 2012;7. doi: 10.2215/CJN.12191111 [DOI] [PubMed] [Google Scholar]
- 2.Christiansen C X-ray contrast media—an overview. Toxicology. 2005;209(2):185–187. doi: 10.1016/J.TOX.2004.12.020 [DOI] [PubMed] [Google Scholar]
- 3.Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930–936. doi: 10.1053/AJKD.2002.32766 [DOI] [PubMed] [Google Scholar]
- 4.Rudnick MR, Goldfarb S, Tumlin J. Contrast-induced nephropathy: is the picture any clearer? Clin J Am Soc Nephrol. 2008;3(1):261–262. doi: 10.2215/CJN.04951107 [DOI] [PubMed] [Google Scholar]
- 5.Reese M Nephrotoxicity of Contrast Media Principal Discussant: ARNOLD S. BERNS. Vol 36; 1989. doi: 10.1038/ki.1989.254 [DOI] [Google Scholar]
- 6.McCullough PA. Radiocontrast-induced acute kidney injury. Nephron Physiol. 2008;109(4):p61–72. doi: 10.1159/000142938 [DOI] [PubMed] [Google Scholar]
- 7.McCullough PA, Wolyn R, Rocher LL, Levin RN, O’Neill WW. Acute Renal Failure After Coronary Intervention: Incidence, Risk Factors, and Relationship to Mortality. Am J Med. 1997;103(5):368–375. doi: 10.1016/S0002-9343(97)00150-2 [DOI] [PubMed] [Google Scholar]
- 8.Weisbord SD, Chen H, Stone RA, et al. Associations of Increases in Serum Creatinine with Mortality and Length of Hospital Stay after Coronary Angiography. J Am Soc Nephrol. 2006;17(10):2871–2877. doi: 10.1681/ASN.2006030301 [DOI] [PubMed] [Google Scholar]
- 9.KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. 2012;2(1). doi: 10.1159/000339789 [DOI] [Google Scholar]
- 10.Weisbord SD, Gallagher M, Jneid H, et al. Outcomes after Angiography with Sodium Bicarbonate and Acetylcysteine. N Engl J Med. 2018;378(7):603–614. doi: 10.1056/NEJMoa1710933 [DOI] [PubMed] [Google Scholar]
- 11.Waikar SS, Betensky RA, Emerson SC, Bonventre JV. Imperfect Gold Standards for Kidney Injury Biomarker Evaluation. J Am Soc Nephrol. 2012;23(1):13–21. doi: 10.1681/ASN.2010111124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin J, Fernandez H, Shashaty MGS, et al. False-Positive Rate of AKI Using Consensus Creatinine-Based Criteria. Clin J Am Soc Nephrol. 2015;10(10):1723–1731. doi : 10.2215/CJN.02430315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moledina DG, Hall IE, Thiessen-Philbrook H, et al. Performance of Serum Creatinine and Kidney Injury Biomarkers for Diagnosing Histologic Acute Tubular Injury. Am J Kidney Dis. 2017;70(6):807–816. doi: 10.1053/J.AJKD.2017.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koyner JL, Garg AX, Coca SG, et al. Biomarkers predict progression of acute kidney injury after cardiac surgery. J Am Soc Nephrol. 2012;23(5):905–914. doi: 10.1681/ASN.2011090907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parikh CR, Devarajan P, Zappitelli M, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after pediatric cardiac surgery. J Am Soc Nephrol. 2011;22(9):1737–1747. doi: 10.1681/ASN.2010111163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huen SC, Parikh CR. Molecular phenotyping of clinical AKI with novel urinary biomarkers. Am J Physiol Physiol. 2015;309(5):F406–F413. doi: 10.1152/ajprenal.00682.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coca SG, Nadkarni GN, Garg AX, et al. First Post-Operative Urinary Kidney Injury Biomarkers and Association with the Duration of AKI in the TRIBE-AKI Cohort. Latus J, ed. PLoS One. 2016;11(8):e0161098. doi: 10.1371/journal.pone.0161098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaker O, El-Shehaby A, El-Khatib M. Early Diagnostic Markers for Contrast Nephropathy in Patients Undergoing Coronary Angiography. Angiology. 2010;61(8):731–736. doi: 10.1177/0003319710373093 [DOI] [PubMed] [Google Scholar]
- 19.Malyszko J, Bachorzewska-Gajewska H, Poniatowski B, Malyszko JS, Dobrzycki S. Urinary and Serum Biomarkers after Cardiac Catheterization in Diabetic Patients with Stable Angina and without Severe Chronic Kidney Disease. Ren Fail. 2009;31(10):910–919. doi: 10.3109/08860220903216113 [DOI] [PubMed] [Google Scholar]
- 20.Ling W, Zhaohui N, Ben H, et al. Urinary IL-18 and NGAL as Early Predictive Biomarkers in Contrast-Induced Nephropathy after Coronary Angiography. Nephron Clin Pract. 2008;108(3):c176–c181. doi: 10.1159/000117814 [DOI] [PubMed] [Google Scholar]
- 21.Bachorzewska-Gajewska H, Malyszko J, Sitniewska E, et al. Could Neutrophil-Gelatinase-Associated Lipocalin and Cystatin C Predict the Development of Contrast-Induced Nephropathy after Percutaneous Coronary Interventions in Patients with Stable Angina and Normal Serum Creatinine Values? Kidney Blood Press Res. 2007;30(6):408–415. doi: 10.1159/000109102 [DOI] [PubMed] [Google Scholar]
- 22.Parikh CR, Thiessen-Philbrook H, Garg AX, et al. Performance of kidney injury molecule-1 and liver fatty acid-binding protein and combined biomarkers of AKI after cardiac surgery. Clin J Am Soc Nephrol. 2013;8(7):1079–1088. doi: 10.2215/CJN.10971012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerr KF, Roth J, Zhu K, et al. Evaluating biomarkers for prognostic enrichment of clinical trials. Clin Trials. 2017;14(6):629–638. doi: 10.1177/1740774517723588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weisbord SD, Gallagher M, Kaufman J, et al. Prevention of Contrast-Induced AKI: A Review of Published Trials and the Design of the Prevention of Serious Adverse Events following Angiography (PRESERVE) Trial. Clin J Am Soc Nephrol. 2013;8(9):1618–1631. doi: 10.2215/CJN.11161012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parikh CR, Moledina DG, Coca SG, Thiessen-Philbrook HR, Garg AX. Application of new acute kidney injury biomarkers in human randomized controlled trials. Kidney Int. 2016;89(6):1372–1379. doi: 10.1016/j.kint.2016.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre J V. Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62(1):237–244. doi: 10.1046/j.1523-1755.2002.00433.x [DOI] [PubMed] [Google Scholar]
- 27.Zhou Y, Peng H, Sun H, et al. Chitinase 3-like 1 suppresses injury and promotes fibroproliferative responses in Mammalian lung fibrosis. Sci Transl Med. 2014;6(240):240ra76. doi: 10.1126/scitranslmed.3007096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt IM, Hall IE, Kale S, et al. Chitinase-Like Protein Brp-39/YKL-40 Modulates the Renal Response to Ischemic Injury and Predicts Delayed Allograft Function. J Am Soc Nephrol. 2013;24(2):309–319. doi: 10.1681/ASN.2012060579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puthumana J, Hall IE, Reese PP, et al. YKL-40 Associates with Renal Recovery in Deceased Donor Kidney Transplantation. J Am Soc Nephrol. 2017;28(2):661–670. doi: 10.1681/ASN.2016010091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ichimura T, Bonventre J V, Bailly V, et al. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273(7):4135–4142. http://www.ncbi.nlm.nih.gov/pubmed/9461608. Accessed December 26, 2018. [DOI] [PubMed] [Google Scholar]
- 31.Jee Ko G, Grigoryev DN, Linfert D, et al. Transcriptional analysis of kidneys during repair from AKI reveals possible roles for NGAL and KIM-1 as biomarkers of AKI-to-CKD transition Downloaded from. Am J Physiol Ren Physiol. 2010;298:1472–1483. doi: 10.1152/ajprenal.00619.2009.-Acute [DOI] [PubMed] [Google Scholar]
- 32.Ko GJ, Grigoryev DN, Linfert D, et al. Transcriptional analysis of kidneys during repair from AKI reveals possible roles for NGAL and KIM-1 as biomarkers of AKI-to-CKD transition. Am J Physiol Physiol. 2010;298(6):F1472–F1483. doi: 10.1152/ajprenal.00619.2009 [DOI] [PubMed] [Google Scholar]
- 33.U.S. Department of Health and Human Services Food and Drug Administration C for DE and R. Guidance for Industry and FDA Staff Qualification Process for Drug Development Tools; 2014. http://www.fda.gov/cder/guidance/index.htm. Accessed November 5, 2018.
- 34.Fried LF, Duckworth W, Zhang JH, et al. Design of combination angiotensin receptor blocker and angiotensin-converting enzyme inhibitor for treatment of diabetic nephropathy (VA NEPHRON-D). Clin J Am Soc Nephrol. 2009;4(2):361–368. doi: 10.2215/CJN.03350708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Zeeuw D, Akizawa T, Audhya P, et al. Bardoxolone Methyl in Type 2 Diabetes and Stage 4 Chronic Kidney Disease. N Engl J Med. 2013;369(26):2492–2503. doi: 10.1056/NEJMoa1306033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torres VE, Abebe KZ, Chapman AB, et al. Angiotensin Blockade in Late Autosomal Dominant Polycystic Kidney Disease. N Engl J Med. 2014;371(24):2267–2276. doi: 10.1056/NEJMoa1402686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haase M, Kellum JA, Ronco C. Subclinical AKI—an emerging syndrome with important consequences. Nat Rev Nephrol. 2012;8(12):735–739. doi: 10.1038/nrneph.2012.197 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Demographic, clinical, and procedural characteristics of the PRESERVE trial and substudy.
Table S2. Creatinine-corrected urine pre-angiography biomarkers by MAKE-D and CA-AKI status.
Table S3. AUCs for pre-angiography plasma and urine biomarkers for MAKE-D and CA-AKI.
Table S4. BioPET analysis of pre-angiography plasma KIM-1.
Table S5. BioPET analysis of pre-angiography plasma YKL-40.