Abstract
The Cy/+ rat has been characterized as a progressive model of chronic kidney disease-mineral bone disorder (CKD-MBD). We aimed to determine the effect of kidney disease progression on intestinal phosphorus absorption and whole-body phosphorus balance in this model. N=48 Cy/+ (CKD) and N=48 normal littermates (NL) rats were studied at two ages: 20-weeks and 30-weeks, to model progressive kidney function decline at approximately 50 and 20% of normal kidney function. Sodium-dependent and sodium-independent intestinal phosphorus absorption efficiency were measured by the in situ jejunal ligated loop method using 33P radioisotope. Our results show that CKD rats had slightly higher sodium-dependent phosphorus absorption compared to NL rats, and absorption decreased from 20- to 30-weeks. These results are in contrast to plasma 1,250H2D, which was lower in CKD rats. Gene expression of the major intestinal phosphorus transporter, NaPi-2b, was not different between CKD and NL rats in the jejunum but was lower in CKD rats versus NL rats in the duodenum. Jejunal ligated loop phosphorus absorption results are consistent with percent net phosphorus absorption results obtained from metabolic balance: higher net percent phosphorus absorption values in CKD rats compared with NL, and lower values in 30-week-olds compared with 20-week-olds. Phosphorus balance was negative (below zero) in CKD rats, significantly lower in 30-week-old rats compared with 20-week-old rats, and lower in CKD rats compared with NL rats at both ages. These results demonstrate no reduction in intestinal phosphorus absorption with progression of CKD despite lower 1,250H2D status when assessed by an in situ ligated loop test, which is in contrast to the majority of in vitro studies, and if confirmed in further studies, could challenge the physiological relevance of in vitro findings.
Keywords: Disorders of calcium/phosphate metabolism, nutrition, genetic animal models, animal models, PTH/Vit D/FGF23
Introduction
Chronic kidney disease-mineral bone disorder (CKD-MBD) is characterized by biochemical abnormalities related to calcium and phosphorus metabolism, including elevated fibroblast growth factor 23 (FGF23), parathyroid hormone (PTH), and serum phosphate, and lower serum 1,25-dihydroxyvitamin D3 (1,25D) and calcium, bone abnormalities, and vascular or other soft tissue calcification (1). Phosphorus and calcium regulating hormones (FGF23, 1,25D, PTH) change in early stages of the disease to maintain serum mineral concentrations (2, 3). However, these hormonal alterations have secondary consequences that contribute to the elevated risk for cardiovascular events, bone fragility fractures, and death (4–6). Because phosphorus dysregulation is a central driver of these adverse events, interventions aimed at maintaining phosphorus homeostasis have been of interest, including targeting the intestinal absorption of dietary phosphorus (7).
Gaps exist in understanding the hormonal regulation of intestinal phosphorus absorption in CKD. 1,25D is a recognized positive regulator of the main known intestinal phosphorus transporter, sodium phosphate cotransporter-2b (NaPi-2b) and active intestinal phosphorus absorption (8). In CKD, 1,25D is decreased via elevated FGF23 (10) and reduced nephron mass (9), and therefore active intestinal phosphorus absorption is expected to be lower as kidney function declines. However, literature on this topic is mixed. In humans, reduced phosphorus absorption has been demonstrated in patients with end-stage kidney disease in older metabolic balance studies as well as radioisotope phosphorus tracers (11–13) and in patients on hemodialysis by a triple-lumen perfusion technique (14). These findings have been supported in some but not all studies in experimental rat models: Peerce et al. (15) showed decreased sodium-dependent jejunal brush border membrane vesicle (BBMV) phosphorus uptake in 5/6 nephrectomized rats versus age-matched controls, and we (16) previously found a reduction in active phosphorus transport by Ussing chamber in Cy/+ CKD rats at 21-weeks of age compared to normal rats, though this effect appeared to be driven by the groups of rats treated with phosphate binders. In contrast, sodium-dependent jejunal BBMV phosphorus uptake was not different in 5/6 nephrectomized rats versus sham-operated rats in two additional studies (17, 18).
Additional assessment techniques that are more physiologic include the in situ ligated loop method. Marks et al. (19) also found no difference in 5/6 nephrectomized versus sham-operated rats using the in situ ligated loop method in the jejunum or duodenum, and gene expression of the major intestinal phosphorus transporter, NaPi-2b, was also not downregulated with 5/6 nephrectomy. This was despite significantly lower 1,25D levels in the 5/6 nephrectomized rats. Further, in rats with adenine-induced CKD, the mild-CKD and severe CKD rats had similar appearance of 33P into serum over 2 hours after an oral gavage compared with controls (20). It is unclear whether differences in these rat experiments are the result of different stages of severity of the disease, or methodological differences in the in vitro vs in situ/in vivo absorption assessment techniques. This question is important to resolve, because if intestinal phosphorus absorption is in fact reduced with CKD, approaches targeting active intestinal phosphorus transport may be less effective than anticipated. If intestinal phosphorus absorption is not reduced with disease, then this suggests further complexity than described in current models of phosphorus regulation in CKD.
There are additional unanswered questions in regard to phosphorus homeostasis with CKD-MBD progression. Given that changes to tight junction proteins are observed in CKD (21, 22), and given that paracellular transport of intestinal phosphorus is not well understood, it is plausible that sodium-independent absorption could also change with disease progression. To our knowledge, only one study has assessed this using BBMV uptake and found no difference in sodium-independent phosphorus absorption in 5/6 nephrectomized rats compared to controls (18). Additionally, it is unclear whether whole-body phosphorus retention increases with CKD-MBD progression, as a driver or consequence of disease. Certainly, elevated serum phosphate appears in later stages of disease, but serum phosphate does not necessarily reflect whole-body status. Human balance studies have shown that moderate-stage CKD patients on average have whole-body phosphorus balance around zero (23), but large variability existed with some patients having negative, neutral, or positive phosphorus balance (24).
In this study we aimed to determine the effects of kidney disease progression in the Cy/+ rat model of progressive CKD-MBD (25) on intestinal phosphorus absorption as measured by the in situ ligated loop absorption method, as well as whole-body phosphorus balance, biochemistries of phosphorus and calcium metabolism, and gene expression of the major intestinal phosphorus transporters in the jejunum and duodenum. We hypothesized that absorption would be similar to controls at a moderate stage of kidney disease but decreased at the later stage, corresponding to a reduction in 1,25D, NaPi-2b expression, and also higher phosphorus balance.
Materials and Methods
Animals
Male rats (N = 96) were obtained from the Cy rat colony at the Indiana University School of Medicine. Forty-eight heterozygous Cy/+ (CKD) and forty-eight normal Cy +/+ (NL) littermate controls were block randomized using Excel to 20- or 30-week-old age groups in a 2×2 factorial design (N = 24 rats per age x genotype). Half of the rats in each group were block randomized to the sodium-dependent phosphorus absorption test outcome or the sodium-independent phosphorus absorption test outcome, as these were mutually exclusive procedures. Rats were fed standard, unautoclaved rat chow containing 0.7% phosphorus, 1.0% Ca, and vitamin D3 1000 IU/kg (Envigo Teklad 2018, Madison, WI) and water ad libitum until 16 weeks of age (“baseline”), at which time they were switched to an ad libitum purified casein-based diet (0.7% phosphorus and 0.7% calcium, TD.04539, Envigo Teklad, Madison, WI) that has been shown to accelerate kidney function decline relative to a grain-based diet likely due to the increased phosphorus bioavailability (25), until sacrifice at either 20- or 30-weeks. Rats were housed individually in shoe-box cages until five days prior to sacrifice when they were transferred to wire-bottom metabolic cages and phosphorus and calcium balance was performed during the last four days prior to sacrifice. Body weights were taken weekly. One rat in the CKD 30-week group was euthanized due to extreme weight loss from kidney disease prior to reaching its assigned age and was not included. One rat assigned to a 30-week group was accidentally placed into the metabolic balance period as described below at 20-weeks, therefore its balance outcomes were included at both ages. The light-dark cycle was maintained from 6AM-6PM. Average room temperature was ~21.5°C (SD 1.0°C) and humidity 46% (SD 7%) This protocol was approved by the Purdue University Animal Care and Use Committee.
Intestinal Phosphorus Absorption Efficiency
Intestinal phosphorus absorption efficiency was determined by in situ jejunal ligated loop absorption tests as described previously (26), with the exception of being fasted on the day of sacrifice from midnight until morning for the ligated loop absorption test. Half (N = 12) of the rats in each age x genotype group were randomly assigned to the “sodium-dependent” absorption test using a transport buffer containing (mmol/L): 16 Na-N-2-hydroxyethylpiperazine-N0–2-ethanesulfonicacid or 4-(2-Hydroxyethyl)piperazin-1-ylethanesulfonic acid, 140 NaCl or ChCl, 3.5 KCl, 0.1 KH2PO4, and ~5 uCi 33P (33P-orthophosphoric acid, PerkinElmer, Waltham, MA), pH 7.4, and the other half (N = 12) to the “sodium-independent” absorption test using a transport buffer containing (mmol/L): 4-(2-Hydroxyethyl)piperazin-1-ylethanesulfonic acid, 140 ChCl, 3.5 KCl, 0.1 KH2PO4, and ~5 uCi 33P, pH 7.4. After injection of the transport buffer (0.5 mL), blood (0.5 mL/sampling) was collected at 5, 10, 15, and 30 min post-injection in lithium heparin tubes and centrifuged at 5000 RPM for 10 minutes (Labofuge A 2502, Baxter Scientific Products, McGaw Park, IL) to separate plasma. Jejunal loops were removed, the lengths measured, placed in a 20 mL scintillation vial containing 6 mL Soluene-350 (Perkin Elmer, Waltham, MA) and heated until dissolved (up to 3 days) at 45°C in an oven. After the loop was removed, a final blood draw was taken from the jugular catheter for biochemical measures, or by cardiac puncture when necessary, and rats were sacrificed by exsanguination followed by cardiac excision.
Absorption of 33P was evaluated two ways: 1) area under the curve (AUC) was calculated for plasma 33P activity over 30 minutes, and 2) percent intestinal phosphorus absorption efficiency over 30 minutes was calculated as:
1 minus (33P activity remaining in digested loop) / (Total 33P activity in 0.5mL dose) X 100 In addition, because absorption without sodium in the buffer may not be truly sodium- independent due to endogenous secretions into the intestine (27), an “exclusively” sodium-dependent component was calculated from each rat as:
(33P absorption (from loop or AUC)) - (average absorption for the corresponding rat’s group given the absorption buffer without sodium)
The percent sodium-dependency of absorption was calculated as:
(average absorption for the group given the sodium-containing absorption buffer - average absorption for the group given the sodium-free buffer)/(sodium-containing buffer group).
Phosphorus and Calcium Balance and Net Absorption
Over the four days prior to sacrifice, urine and feces were collected as previously described (26) to assess balance and net absorption of phosphorus and calcium. Feces and diet were ashed in a muffle furnace (Thermolyne Sybron Type 30400, Dubuque, IA) for 10 days at 600°C and diluted 1400X and diet 140X with 2% nitric acid. Urine was diluted 11X with 2% nitric acid. Phosphorus and calcium in urine, feces, and diet were quantified by inductively coupled plasma-optical emission spectrophotometry (ICP-OES; Optima 4300DV, Perkin Elmer, Shelton, CT). Urine creatinine was determined by colorimetric method (Quantichrom, BioAssay Systems, Hayward, CA). Four-day phosphorus balance was calculated as dietary phosphorus intake (mg/d) - urine and fecal phosphorus excretion (mg/d), and net phosphorus absorption (%) as phosphorus intake (mg/d) - fecal excretion (mg/d) / phosphorus intake (mg/d) X 100. Calcium balance and net calcium absorption were calculated similarly.
Intestinal Phosphorus Transporter Gene Expression
After the completion of the ligated loop absorption tests and the removal of the radioactive jejunal loop, approximately 8 cm of jejunum distal to the ligated loop and the duodenum distal to the pylorus up to the ligated loop were removed and cut open. The mucosal layers were scraped, and mucosa from each intestinal segment was placed into TRI Reagent (Fisher Scientific, Hampton, NH) and flash frozen in liquid nitrogen for later mRNA quantification by RT-PCR. The left kidney was removed, and flash frozen wrapped in foil for later mRNA quantification. Upon thawing, kidneys were weighed and cut into thirds cross-sectionally. The cortex was removed, mixed thoroughly with a scalpel, and a sample homogenized in TRI Reagent (Fisher Scientific, Hampton, NH).
Gene expression of intestinal NaPi2b and PiT1 using real-time PCR was performed as previously described (26). NaPi2a and NaPi2c were assessed in the renal cortex (Applied Biosystems Rn00564677_m1 and Rn00595128_m1).
Plasma Biochemistries
Plasma stored at −80C was thawed and analyzed for phosphorus, calcium, creatinine, and blood urea nitrogen (BUN) concentration by colorimetric methods (phosphorus and calcium: Pointe Scientific, Inc., Canton, MI; creatinine and BUN: Quantichrom, BioAssay Systems, Hayward, CA). Intact PTH (iPTH) and intact FGF23 (iFGF23) were measured by enzyme-linked immunosorbent assay (Alpco, Salem, NH; Quidel, San Diego, CA), and 1,25D by enzyme immunoassay (Immunodiagnostic Systems, The Boldons, UK).
Statistics
A sample size of n = 12 rats/group was determined to be sufficient to detect a 30% difference between groups for phosphorus absorption (β = 0.80, α = 0.05) based on means and standard deviations reported by Marks et al. (19). For metabolic balance, biochemical, and gene expression outcomes, data from n = 24 rats per disease*age group were analyzed except where exceptions are noted. One baseline BUN, one final PTH, and one final FGF23 value were excluded because of implausible values. Baseline biochemistries were not available for all rats, and therefore baseline to final changes were not assessed. All outcomes were assessed for normality, and creatinine clearance, baseline and final PTH, and gene expression for duodenal NaPi-2b and PiT-1, and kidney NaPi-2a and NaPi-2c were log transformed prior to analysis because of non-normal distribution of residuals, and reported p-values reflect transformed values. Two-way ANOVA was performed for all outcomes with main effects for age and disease and their interaction, with Tukey post-hoc comparisons. Linear regression was used to compare relationships between absorption and creatinine clearance, BUN, and kidney weight. Statistical significance was set at α < 0.05. Statistical Analysis Software version 9.4 (SAS Institute, Cary, NC) was used for all two-way ANOVA comparisons, and OriginPro 2018 (OriginLab, Northampton, MA) for regressions. Results are reported as mean ± SEM unless otherwise indicated.
Results
The baseline blood and urine values at 16 weeks of age between animals randomized to the 20- or 30-week age groups are shown in Table 1 and demonstrate similar lab values and expected differences between CKD and NL animals. The laboratory tests at the time of sacrifice showed evidence of progressive CKD-MBD that was more advanced at 30-weeks compared to 20-weeks of age. There were no differences in plasma calcium or phosphorus at baseline or final draws. Final 1,25D was lower in CKD rats compared to NL and lower in 30-week-olds compared to 20-week-olds. However, Tukey’s post-hoc tests revealed the only difference in pairwise comparisons was for 30-week-old CKD rats that had lower 1,25D than all other groups, despite a non-significant p = 0.07 for the interaction (Table 2).
Table 1:
Baseline blood biochemistries at 16-weeks of age
| 20-week-old group At 16-weeks-old |
30-week-old group At 16-weeks-old |
P-Value for NL v. CKD |
|||
|---|---|---|---|---|---|
| NL | CKD | NL | CKD | ||
|
Plasma P (mg/dL) |
5.7 (0.3) n = 20 |
6.0 (0.2) n = 19 |
5.6 (0.2) n = 15 |
5.8 (0.3) n = 15 |
0.30 |
|
Plasma Ca (mg/dL) |
6.7 (0.4) n = 20 |
6.4 (0.3) n = 19 |
7.1 (0.4) n = 15 |
6.1 (0.3) n = 15 |
0.09 |
|
Plasma Creatinine (mg/dL) |
0.39 (0.02) n=18 |
0.55 (0.01) n=18 |
0.30 (0.02) n=15 |
0.52 (0.03) n=18 |
<0.0001 |
|
BUN (mg/dL) |
18.3 (0.8) n=20 |
35.5 (0.7) n=19 |
19.2 (0.8) n = 14 |
34.1 (0.8) n = 15 |
<0.0001 |
|
Plasma PTH (pg/mL) |
315.1 (30.0) n = 17 |
444.2 (72.2) n = 19 |
328.5 (27.0) n = 15 |
336.8 (18.2) n = 18 |
0.052 |
|
Plasma iFGF23 (pg/mL) |
441.6 (21.1) n = 15 |
745.1 (26.6) n = 15 |
375.9 (8.2) n = 16 |
721.1 (24.1) n = 15 |
<0.0001 |
Baseline blood biochemistries at 16 weeks of age in groups randomized to 20- and 30-week groups.
ANOVA p-values for the main effect of disease (PDisease) are shown, and means and (SEM) are shown for each group. Plasma creatinine was higher in CKD vs NL. Plasma PTH did not differ between groups. iFGF23 was higher in CKD rats vs NL. Plasma 1,25D was lower in CKD vs NL.
Table 2:
Final weights and blood and urine biochemistries†
| 20-weeks-old | 30-weeks-old | P-Values | ||||||
|---|---|---|---|---|---|---|---|---|
| NL | CKD | NL | CKD | ANOVA Model |
Age (20 vs 30) |
Disease (CKD vs NL) |
Age x Disease |
|
|
Body weight
(g) |
487.9 (5.4) b
n = 24 |
460.2 (6.1) c
n = 24 |
546.3 (7.7)a
n = 24 |
511.0 (5.9) b
n = 23 |
< 0.0001 | < 0.0001 | < 0.0001 | 0.55 |
|
Kidney
weight (g) |
1.7 (0.02) c
n = 22 |
2.1 (0.05) b
n = 22 |
1.8 (0.03) c
n = 24 |
2.7 (0.09) a
n = 23 |
< 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
|
Plasma P (mg/dL) |
6.1 (0.4) n = 22 |
6.0 (0.3) n = 21 |
6.6 (0.4) n = 22 |
6.2 (0.4) n = 21 |
0.74 | 0.42 | 0.56 | 0.63 |
|
Plasma Ca (mg/dL) |
5.5 (0.2) n = 22 |
5.6 (0.2) n = 21 |
6.3 (0.2) n = 22 |
5.7 (0.4) n = 21 |
0.12 | 0.09 | 0.37 | 0.14 |
|
Plasma Creatinine (mg/dL) |
0.45 (0.02) c
n = 23 |
0.78 (0.03) b
n = 23 |
0.45 (0.02) c
n = 23 |
0.92 (0.06) a
n = 22 |
<0.0001 | 0.06 | <0.0001 | 0.056 |
|
Urine Creatinine (mg/day)* |
14.4 (0.7) bc
n = 24 |
12.3 (0.5) c
n = 24 |
17.4 (0.6) a
n = 24 |
14.8 (0.5) b
n = 23 |
<0.0001 | <0.0001 | 0.0001 | 0.72 |
|
Creatinine
Clearance (mL/min) |
2.55 (0.36) a
n = 24 |
1.18 (0.07) b
n = 24 |
2.85 (0.24) a
n = 23 |
1.35 (0.10) b
n = 22 |
<0.0001 | 0.08 | <0.0001 | 0.50 |
|
Plasma BUN (mg/dL) |
15.0 (0.4) c
n = 22 |
32.3 (0.7) b
n = 21 |
15.7 (0.9) c
n = 22 |
37.0 (1.3) a
n = 21 |
<0.0001 | 0.003 | <0.0001 | 0.02 |
|
Hematocrit
(%) |
49.5 (10.1) a
n = 24 |
43.3 (9.2) b
n = 22 |
49.5 (10.1) a
n = 24 |
42.4 (8.8) b
n = 23 |
<0.0001 | 0.53 | <0.0001 | 0.07 |
|
Plasma PTH (pg/mL) |
773.4 (69.9) b
n = 24 |
873.8 (68.0) b
n = 24 |
877.1 (64.8) b
n = 24 |
1849.2 (239.2) a
n = 22 |
<0.0001 | <0.0001 | <0.0001 | 0.008 |
|
Plasma
iFGF23 (pg/mL) |
527.0 (24.5) c
n = 24 |
841.0 (49.7) b
n = 24 |
527.0 (24.5) c
n = 24 |
2345.8 (276.9) a
n = 22 |
<0.0001 | <0.0001 | <0.0001 | <0.0001 |
|
Plasma
1,25D (pmol/L) |
278.6 (37.4) a
n = 24 |
259.2 (36.3) a n = 24 |
230.5 (37.4) a n = 24 |
96.1 (22.1) b n = 23 |
0.0005 | 0.001 | 0.02 | 0.07 |
Final weights, and blood and urine biochemistries.
ANOVA p-values for the overall model (PModel), main effect of age (PAge), main effect of disease (PDisease), and interaction of age and disease (PAxD) are shown, and means and (SEM) are shown for each group. Different letters represent group differences.
average 24 hour urine creatinine (mg/day) over 4-day balance period.
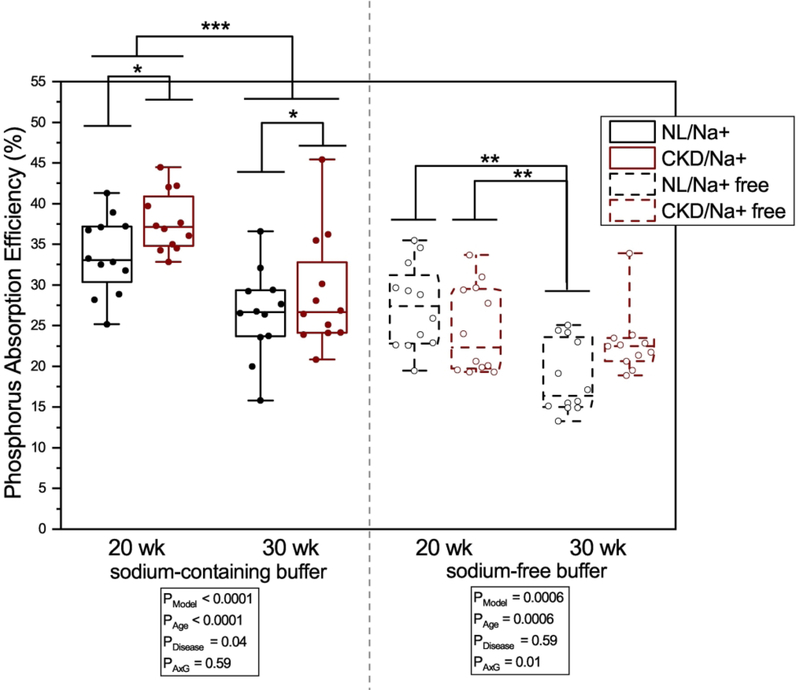
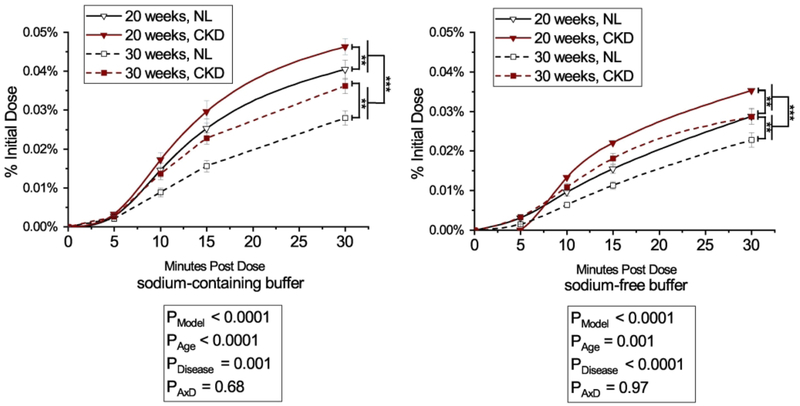
The percent of 33P intestinal phosphorus absorption efficiency, as assessed by disappearance of 33P radioactivity from the intestinal loop with the sodium-containing absorption buffer at 30 minutes was higher in 20-week-old rats compared with 30-week-olds (35.7 ± 0.9 % vs 27.7 ± 1.3 %, p < 0.0001), and higher in CKD rats compared with NL (33.3 ± 1.4 % vs 30.1 ± 1.3 %, p = 0.04, interaction p = 0.59) (Figure 1). For 33P intestinal absorption efficiency with the sodium-free buffer, there was a significant age x disease interaction (p = 0.01), where 20-week old CKD and NL rats had higher absorption efficiency than 30-week old NL rats (24.5 ± 1.6 % and 27.3 ± 1.5 % vs 18.5 ± 1.3 %, p = 0.02 and p = 0.0003) (Figure 1). Phosphorus absorption by plasma AUC had a similar pattern; absorption with the sodium-containing buffer was higher in 20-week old rats compared with 30-week-olds (0.7 ± 0.04 vs 0.5 ± 0.03, p < 0.0001), and higher in CKD rats compared with NL (0.7 ± 0.03 vs 0.5 ± 0.04, p = 0.001interaction p = 0.68) (Figure 2). Similarly, AUC absorption with the sodium-free buffer was higher in 20-week-old rats compared with 30-week-old rats (0.5 ± 0.03 vs 0.4 ± 0.02, p = 0.001), and higher in CKD compared with NL (0.5 ± 0.03 vs 0.4 ± 0.02, p < 0.0001; interaction p = 0.97) (Figure 2). The sodium-dependency of phosphorus absorption varied from 21–35% for CKD rats and 19–33% depending on age and method of determination (i.e. label disappearance from loop or appearance into plasma) (Supplemental Table 1). There were no strong relationships between absorption outcomes and creatinine clearance, BUN, or kidney weight (all R2 < 0.06, Supplemental Figure 2).
Figure 1.
Percent jejunal phosphorus absorption efficiency by age (20- or 30-weeks) and disease (CKD or NL) with sodium-containing and sodium-free absorption buffers. Phosphorus absorption efficiency was calculated as 1-(33P activity remaining in jejunal loop)/(Total 33P activity in dose) after 30 minutes post 33P injection into the jejunal loop. Means and standard error bars are shown for each group. For rats given the sodium-containing absorption buffer (left), there was a main effect for age where 20-week-old rats had higher absorption efficiency compared to both 30-week-olds, and CKD rats had higher absorption efficiency compared to NL, with no significant age x disease interaction.
For the sodium-free absorption buffer (right), there was a significant age x disease interaction, where 30-week NL rats had a lower absorption efficiency vs 20-week NL and 20-week CKD rats. Sodium-containing buffer: CKD rats are shown with black bars and white dots, NL rats are shown with white bars and black dots. Sodium-free buffer: CKD rats are shown with black bars with white hashes and white dots, NL rats are shown with white bars with black hashes and black dots. ANOVA p-values for the overall model (PModel), main effect of age (PAge), main effect of disease (PDisease), and interaction of age and disease (PAxD) are shown. n=24 NL, n=24 CKD for sodium-containing buffer; n=24 NL, n=23 CKD for sodium-free buffer, * p < 0.05, ** p < 0.01, *** p < 0.0001.
Figure 2.
Jejunal phosphorus absorption in plasma over 30 minutes by age (20- or 30-weeks) and disease (CKD or NL) with a sodium-containing absorption buffer (left) or sodium-free buffer (right). Phosphorus absorption from plasma was calculated as the area under the curve of the % of initial dose of 33P of each timepoint over the 30 minute test. Means and standard error bars are shown for each group. For rats given either the sodium-containing or sodium-free absorption buffer, there was a main effect for age where 20-week-old rats had higher absorption efficiency compared to both 30-week-olds, and CKD rats had higher balance compared to NL, with no significant age x disease interaction. Absorption with sodium-containing buffer (left): CKD rats are shown with black lines and black triangles, NL rats are shown with dashed lines and white squares. Absorption with sodium-free buffer: CKD rats are shown with black lines with black triangles, NL rats are shown with dashed lines with black dashes and white squares. ANOVA p-values for the overall model (PModel), main effect of age (PAge), main effect of disease (PDisease), and interaction of age and disease (PAxD) are shown. n=24 NL, n=24 CKD for sodium-containing buffer; n=24 NL, n=23 CKD for sodium-free buffer, ** p < 0.01, *** p < 0.0001.
Body weight at sacrifice was lower in CKD rats compared with NL and in 20-week-olds vs 30-week-olds (Table 2), however body weight was not a significant covariate when tested in models for absorption results (all p > 0.08). The mean (SD) of the length of the loops was 5.5 ± 0.7 cm, and was only a significant covariate (p = 0.04) in the statistical model for phosphorus absorption using the sodium-containing buffer, but inclusion as a covariate did not affect the results for effects disease or age; length of the loop was not a significant covariate in other models for the other outcomes of absorption into plasma or from loops (all p > 0.07). We also analyzed absorption buffer pH as a covariate (28) because of an inadvertent error where some rats received absorption buffers that had not been pH-adjusted to 7.4 as per protocol. This affected N=55/96 rats, ranging from 4 to 10 animals in each group of 12; the difference in pH between the sodium-containing vs. sodium-free buffer was 9.4 vs. 5.5. pH was a significant covariate in the statistical model for absorption from loops with the sodium-free buffer (p = 0.04) but did not change disease and age effects and was not a significant covariate in other models (all p > 0.34). Additionally, a subanalysis of rats with only the per protocol buffers with pH adjusted to 7.4 (N=41, 2 to 8 per group) yielded similar results for main effects, interactions, and group differences as the full set of data reported here.
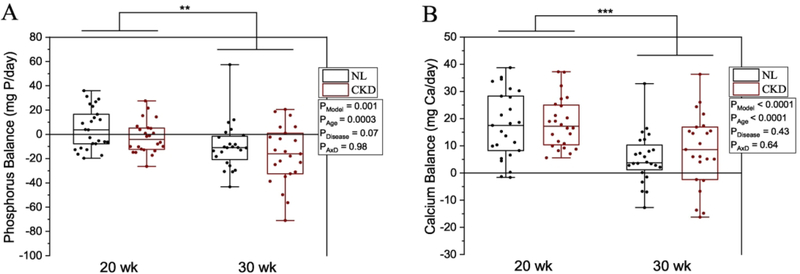
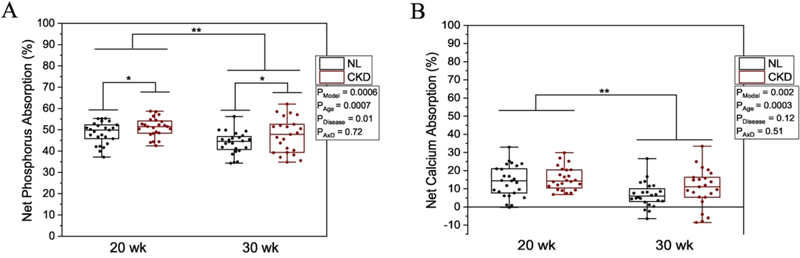
Phosphorus and calcium balance and percent net absorption were higher in 20-week-olds compared with 30-week-olds (Figure 3 & 4). Phosphorus balance was numerically lower but not statistically significant (p = 0.07), and percent net phosphorus absorption was higher in CKD rats versus NL (p = 0.01) (Figures 3A, 4A), but calcium balance and percent net calcium absorption were not different between CKD and NL (Figures 3B, 4B). Individual components of phosphorus and calcium balance are given in Supplemental Tables 2 and 3.
Figure 3.
A) Phosphorus balance by age (20- or 30-weeks) and disease (CKD or NL). There was a main effect for age where 20-week-old rats had a more positive phosphorus balance compared to both 30-week-olds, and CKD rats tended to have a more negative balance compared to NL, with no significant age x disease interaction. B) Calcium balance by age and disease. There was a main effect for age where 20-week-old rats had a more positive calcium balance compared to 30-week-olds, but there was no significant effect of disease and no significant age x disease interaction. Balance for each mineral was calculated as intake − fecal + urine. Means and standard error bars are shown for each group. The CKD group is shown in black bars and white circles, and NL white bars with black circles. ANOVA p-values for the overall model (PModel), main effect of age (PAge), main effect of disease (PDisease), and interaction of age and disease (PAxD) are shown. n=49 NL and n=47 CKD for both phosphorus and calcium, * p < 0.05, ** p < 0.01, *** p < 0.0001.
Figure 4.
A) Percent net phosphorus absorption by age (20- or 30-weeks) and disease (CKD or NL). There was a main effect for age where 20-week-old rats had higher phosphorus absorption compared to 30-week-olds, and a main effect of disease, where CKD rats had higher phosphorus absorption, while no significant age x disease interaction. B) Percent net calcium absorption by age and disease. There was a main effect for age where 20-week-old rats had higher calcium balance compared to 30-week-olds, with no difference by disease and no significant age x disease interaction. Percent net absorption for each mineral was calculated as (intake - fecal)/intake. Means and standard error bars are shown for each group. The CKD group is shown in black bars and white circles, and NL white bars with black circles. ANOVA p-values for the overall model (PModel), main effect of age (PAge), main effect of disease (PDisease), and interaction of age and disease (PAxD) are shown. n=49 NL and n=47 CKD for both phosphorus and calcium, * p < 0.05, ** p < 0.01.
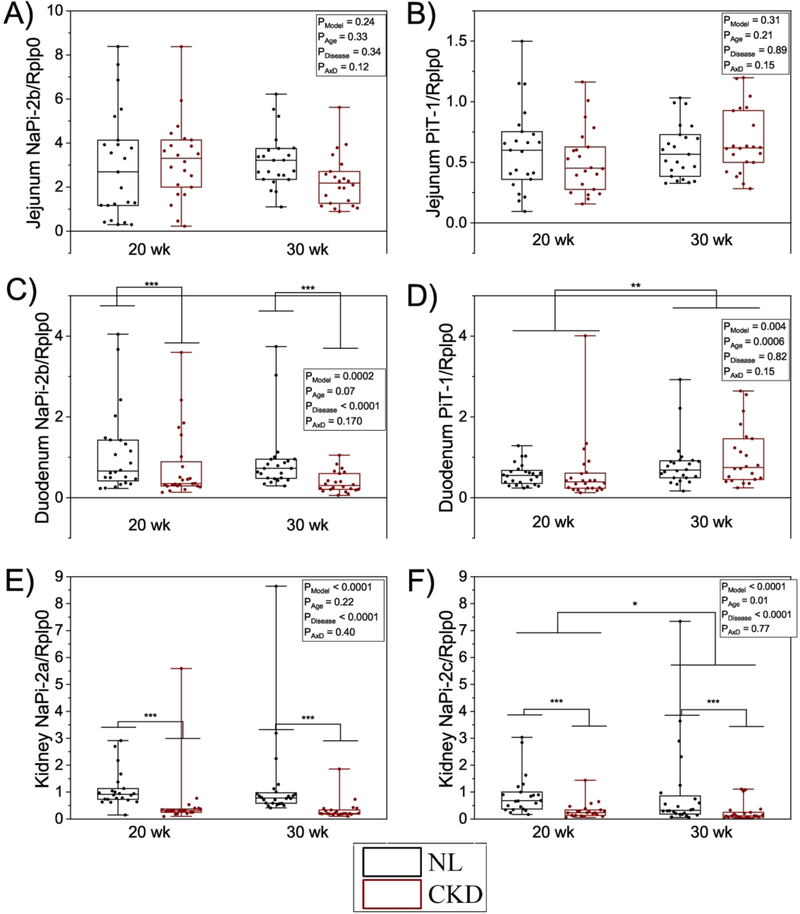
NaPi-2b mRNA did not differ by groups in the jejunum, but in the duodenum it was lower in CKD rats compared with NL rats, while there was no difference between 20- and 30- week-olds (Figure 5). There were no effects of age or disease on PiT-1 mRNA in the jejunum, but expression was increased at 30-weeks vs 20 in the duodenum (Figure 5). In the kidney, both NaPi-2a and NaPi-2c were lower in CKD rats compared with NL, and NaPi-2c was lower at 30- weeks vs 20-weeks (Figure 5).
Figure 5.
RNA expression of NaPi-2b, PiT-1 in jejunum and duodenum, and NaPi-2a and NaPi- 2c in kidney. A) NaPi-2b mRNA was not different between groups in the jejunum. B) PiT-1 mRNA was not different between groups in the jejunum. C) NaPi-2b mRNA was lower in CKD rats vs NL in the duodenum. D) PiT-1 mRNA was not different between groups in the duodenum. E) NaPi-2a was lower in CKD rats vs NL in the kidney. F) NaPi-2c was lower in CKD rats vs NL in the kidney. Outliers are excluded above 2 SD of the mean. The CKD group is shown in black bars and white circles, and NL white bars with black circles. ANOVA p-values for the overall model (PModel), main effect of age (PAge), main effect of disease (PDisease), and interaction of age and disease (PAxD) are shown. n=46 NL and n=44 CKD for jejunum NaPi-2b and PiT-1, n=48 NL and n=46 CKD for duodenum NaPi-2b and PiT-1, n=41 NL and n=44 CKD for kidney NaPi-2a, n=42 NL and n=44 CKD for kidney NaPi-2c, * p < 0.05, ** p < 0.01, *** p < 0.0001.
Discussion
In this study, 20-week-old rats had higher intestinal phosphorus absorption efficiency compared with 30-week-old rats as measured by in situ jejunal ligated loop. This corresponds with the higher net phosphorus absorption from metabolic balance and greater positive overall whole-body phosphorus balance observed in 20-week-old versus 30-week-old rats. Interestingly, the results in the NL animals in the present study are in contrast with our previous study in healthy Sprague Dawley male rats, in which we observed no age difference in jejunal phosphorus absorption efficiency, net phosphorus absorption, nor balance between 20-week-old and 30-week-old rats using similar methods (26). This may be due, however, to a difference in rat strain. The Cy rats obtained from the inbred IUSM colony have a Han:Sprague Dawley background (29), which may have different physiologic adaptations with age compared to commercial Sprague-Dawley rats (Envigo). However, in both studies, the age effects on absorption mirrored the age effects on 1,25D: no difference in 1,25D or absorption in 20-week-old versus 30-week-old Sprague-Dawley rats; higher 1,25D and higher absorption in 20-week- old versus 30-week-old Cy/+ rats and normal littermates in the present study. This is in agreement with the classic understanding of a positive relationship between 1,25D and intestinal phosphorus absorption.
Notably, in CKD rats, intestinal phosphorus absorption efficiency was slightly but statistically higher compared with NL, which runs in contrast to the expected effect of lower 1,25D levels in CKD compared with NL. Similarly, percent net phosphorus absorption from metabolic balance was higher in CKD rats versus NL, in line with previous ligated loop absorption findings. Marks et al. (30) observed no statistical difference in phosphorus absorption using the ligated loop in 5/6 nephrectomized CKD Sprague-Dawley rats in either the jejunum or duodenum (but numerically higher in the CKD rats in the jejunum, as in our study), despite lower 1,25D in CKD rats. Recently, Turner et al. (20) also showed no difference in phosphorus absorption in adenine-induced mild-CKD or advanced CKD compared to controls using an in vivo oral gavage method in Sprague-Dawley rats. Our data suggests increased phosphorus absorption with CKD in this model, which is unexpected given the low 1,25D levels. Albeit the increase is numerically small, and this finding requires replication. Overall, our data show that, CKD does not result in a physiological adaptation to limit intestinal phosphorus absorption even when 1,25D is low.
In vitro studies contrast in jejunal brush border membrane vesicle (BBMV) uptake in 5/6 nephrectomized rats finding either decreased uptake compared with age-matched controls or no change compared with sham surgery (17, 18). Interestingly, despite showing a reduction in sodium-dependent phosphorus uptake, Peerce et al. found no difference in the percentage of net phosphorus absorbed from metabolic balance in CKD rats (15). Further, Marks et al. (30) observed no difference in uptake as assessed by the everted gut sac technique in 5/6 nephrectomized rats compared with sham-operated. In vitro techniques assess uptake into enterocytes, whereas the in situ and in vivo assessments include basolateral transport into circulation. These methodological differences could illuminate whether basolateral regulation can explain different outcomes, although the inconsistencies within in vitro results generally rules this out.
We observed some differences with age between 20- and 30-weeks and between CKD and NL when assessing phosphorus transport with a sodium-free absorption buffer, although it is unclear whether these reflect true sodium-independent transport with the ligated loop technique. Endogenous sodium secretion into the ligated loop during the test may contribute to sodium- dependent absorption (27). Thus, it is unlikely that we are able to observe true sodium-independent absorption pathways with this technique compared to completely controlled closed-system in vitro methods. In support of this, it would be expected that since sodium-dependent absorption is higher in CKD, sodium-independent absorption would be lower consequent to the lower phosphate concentration gradient, however this is not consistently observed in our data. This is also reflected in the much lower estimations of sodium-dependency when using the ligated loop (32±8% in the jejunum) or oral gavage (no evidence of sodium-dependency) (27, 31) compared to in vitro techniques (73±5% using the everted sleeve) (27) using the same phosphate concentration. This may be explained in part by the fact that luminal phosphate concentration in vivo is ~1.5–40 mM in the proximal intestine depending on measurement technique (27, 32), which would result in a higher proportion of passive transport estimated from in vivo compared with in vitro techniques, despite utilizing the same low phosphate concentration transport buffer. Our finding of sodium-dependency estimated by appearance into plasma in NL rats of 33% is in alignment of the finding of 32% by Marks et al. in healthy Sprague Dawley rats using the ligated loop method (27). We also found the same absorption patterns (higher in 20-weeks versus 30-weeks and in CKD rats versus NL) when using the sodium-free absorption buffer for appearance of 33P into plasma that we observed with the sodium-containing buffer. This may reflect a small amount of sodium-dependent transport (due to endogenous secretion) in the sodium-free test, although this pattern was not observed for absorption when assessed by disappearance of radioactivity from the loop over 30 minutes. A previous study found no difference in sodium-independent phosphorus uptake in 5/6th nephrectomized CKD rats versus controls in vitro (18); our results contradict this in situ. To further explore this question with our data, we calculated an “exclusively” sodium-dependent phosphorus absorption component by subtracting the absorption with the sodium-containing buffer for each rat from the absorption average of the corresponding group given the sodium-free buffer. This “exclusively” sodium-dependent component was higher in 20-week-old CKD rats compared with 20-week NL and 30-week CKD rats for absorption efficiency as measured by disappearance from the loop (p = 0.04 and p = 0.02), but not different when assessed as plasma AUC (Supplemental Figure 1). By any measure, phosphorous absorption efficiency in CKD rats was not lower than NL rats.
We observed no difference in NaPi-2b mRNA between CKD and NL rats in the jejunum, but lower NaPi-2b in CKD rats compared with NL in the duodenum. No CKD differences in PiT-1 mRNA were observed in either intestinal segment. Similarly, Marks et al. (19) observed no difference in either duodenum nor jejunum NaPi-2b mRNA expression between 5/6th nephrectomized compared with sham-operated rats. However, we have previously (16) observed lower NaPi-2b mRNA in the duodenum and jejunum, but not ileum, in the same Cy/+ rat model compared with NL controls of similar age to the 20-week animals in the present study. Interestingly, despite differential changes in NaPi-2b expression, the CKD rats in all three studies consistently had 1,25D ~40–50 pg/mL and normal/controls ~≥ 100 pg/mL. It is notable that in all studies, the absorption outcomes generally follow NaPi-2b mRNA expression outcomes. While we did not measure transporter protein expression, this question is important to address in future experiments. Further, although the ligated loop is not ideal to assess sodium-independent transport, future studies should also examine the modulation of tight junctions, including protein expression therein, with emerging evidence showing changes in response to intestinal manipulations that alter phosphate transport such as NaPi-2b knockout (32) and sodium/hydrogen exchanger isoform 3 (NHE3) inhibition (33).
There are several limitations with our model and method. First, the Cy/+ rat is a model of autosomal dominant polycystic kidney disease (29), and although the responsible gene mutation is known (34), it is unclear if this specific genetic defect can explain our results, although our findings are similar to that of Marks et al. (19). Furthermore, we have extensively characterized the Cy/+ rat as a model of progressive CKD-MBD, observing biochemical and tissue changes similar to the human disease (16, 25). Second, the interpretation of absorption using the ligated loop assumes that differences in renal filtration and tissue distribution within the 30 minute timeframe of the test will not significantly affect plasma 33P. Recently, Zelt et al. (35) performed experiments in nephrectomized rats that address these questions. While kidney filtration was only different between pre- and post-nephrectomy beyond a 30-minute period, suggesting that this does not affect our interpretation, the nephrectomized group experienced differences in tissue distribution 30 minutes post IV infusion of 33P. Their finding of ~75% clearance of circulating phosphate within 30 minutes indicates that plasma 33P does not reflect an accumulation of absorbed 33P, and this may affect our measurement of absorption into plasma. However, a strength of our study is the measurement of absorption by both appearance into plasma as well as disappearance from the loop, the latter likely independent of clearance into other tissues, which both provided similar results. Third, the inadvertent error of the lack of Ph adjustment in some of the absorption buffers would be expected to change our results, but inclusion as a covariate and a per protocol sub analysis do not suggest this had a large impact on our overall findings; we surmise that the luminal environment may have buffered toward a neutral pH with the in vivo test. Candeal et al. (28) showed that total sodium-dependent jejunal phosphate BBMV uptake increased as pH decreased from 8.5 to 6, despite decreasing divalent phosphate concentrations favored by NaPi-2b. However, Lee et al., using a Ussing chamber, showed that phosphate flux decreased from a pH of 6 to 7.4, but increased from 7.4 to 8.5 (36). Our pH range of 5.5, 7.4, and 9.4 permit an exploration of this pattern; interestingly, we found small but generally consistent absorption patterns that match those of Lee et al. (Supplemental Table 4). Both sodium-dependent and -independent transport may be altered by pH (28, 33, 37), but it is unclear which is relatively more important in vivo. However, based on the error in pH adjustment, our results may be expected to slightly overestimate absorption with both the sodium-containing and sodium-free buffer compared to if all rats had been administered the correct pH of 7.4. Such differences appear similar to the magnitude of changes in absorption between CKD and NL groups, so some caution is needed when considering how this affects results. Finally, although our 1,25D measures decreased in the 30-week CKD group consistent with other animal studies (16, 19), but without a corresponding decrease in intestinal phosphorus absorption, it is possible that a more severe reduction in 1,25D may produce reduced absorption using the ligated loop model. In humans, CKD patients with a GFR < 20 ml/min have 1,25D of ~15 pg/mL, whereas GFR > 80 ml/min were ~ 45 pg/mL (2). It is unclear precisely how 1,25D concentrations in different stages of the human disease relate to those in the rat. However, our observations together with Marks et al. (19) of no change or a slight increase in absorption efficiency with kidney disease using the ligated loop, versus other studies showing a decrease in transport with the Ussing chamber (16), despite similar 1,25D concentrations in diseased rats among studies, suggests that methodological differences in how transport is measured may explain the different results.
While patients with end-stage kidney disease (11–13) and on dialysis (14) appear to have reduced absorption of dietary phosphorus compared to healthy individuals, whether it is reduced in moderate stages of the disease is unresolved. Our results, together with others, suggest that absorption is relatively unchanged as the disease progresses to later stages in animal models. Thus, interventions that target active intestinal phosphorus transport may be as fruitful as one would postulate if human transport parallels rodent models. Although 1,25D decreases markedly with disease progression, we observed no reduction in phosphorus absorption, despite the well-documented effect of 1,25D on increasing absorption via increased NaPi-2b. Future work is needed to assess whether a bottom threshold exists for effects of low 1,25D on phosphorus absorption in CKD, or if additional regulators are responsible for maintaining phosphorus absorption at normal levels in CKD despite declining 1,25D. Our findings further suggest that intestinal phosphorus absorption efficiency may even be increased in moderate stages of the disease, but this requires additional confirmation. These results underscore the need for further in vivo absorption studies in both animal models and clinical research in patients with CKD.
Supplementary Material
Acknowledgements
This work was supported through NIH K01 DK102864 (KMHG), the Ralph W. and Grace M. Showalter Research Trust Fund (KMHG), and a USDA NIFA Hatch Project (grant #1008923, KMHG). SMM was supported by NIH R01 DK11087103 and a Veterans Affairs Merit Award (BX001471). AB was supported by NIH T32 (AR065971–04).
We thank Dr. James Fleet for his scientific and technical guidance, and Courtney Nelson and Rebecca Lapides for their technical assistance.
Dr. Moe reports grants from Chugai, grants from Keryx, during the conduct of the study; personal fees from Amgen, outside the submitted work.
Dr. Hill Gallant reports grants from NIH NIDDK K01 DK102864, grants from USDA NIFA 1008923, during the conduct of the study; personal fees from Relypsa, Inc, grants from Chugai Pharmaceutical Co., Ltd, personal fees from Tricida, Inc, outside the submitted work.
Dr. Biruete reports personal fees from Amgen, grants from Keryx, outside the submitted work.
Footnotes
Disclosures
Dr. Vorland has nothing to disclose.
Ms. Lachcik has nothing to disclose.
Ms. Srinivasan has nothing to disclose.
Dr. Chen has nothing to disclose.
Data Sharing
The data and diet datasheet that support the findings of this study are openly available in OSF at https://doi.org/10.17605/OSF.IO/BUECO.
Contributor Information
Colby J. Vorland, Department of Nutrition Science, Purdue University, West Lafayette, IN 47907.
Annabel Biruete, Division of Nephrology, Department of Medicine, Indiana University School of Medicine, Indianapolis, IN 46202.
Pamela J. Lachcik, Department of Nutrition Science, Purdue University, West Lafayette, IN 47907.
Shruthi Srinivasan, Division of Nephrology, Department of Medicine, Indiana University School of Medicine, Indianapolis, IN 46202.
Neal X. Chen, Division of Nephrology, Department of Medicine, Indiana University School of Medicine, Indianapolis, IN 46202.
Sharon M. Moe, Division of Nephrology, Department of Medicine, Indiana University School of Medicine, Indianapolis, IN 46202; Department of Anatomy and Cell Biology, Indiana University School of Medicine, Indianapolis, IN 46202; Department of Medicine, Roudebush Veterans Affairs Medical Center, Indianapolis, IN 46202.
Kathleen M. Hill Gallant, Department of Nutrition Science, Purdue University, West Lafayette, IN 47907; Division of Nephrology, Department of Medicine, Indiana University School of Medicine, Indianapolis, IN 46202.
References
- 1.Moe S, Drueke T, Cunningham J, Goodman W, Martin K, Olgaard K, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2006;69(11):1945–53. [DOI] [PubMed] [Google Scholar]
- 2.Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71(1):31–8. [DOI] [PubMed] [Google Scholar]
- 3.Isakova T, Wahl P, Vargas GS, Gutierrez OM, Scialla J, Xie H, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79(12):1370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382(9889):339–52. [DOI] [PubMed] [Google Scholar]
- 5.Miller PD. Chronic kidney disease and the skeleton. Bone Res. 2014;2:14044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, et al. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17(7):2034–47. [DOI] [PubMed] [Google Scholar]
- 7.Vervloet MG, Sezer S, Massy ZA, Johansson L, Cozzolino M, Fouque D, et al. The role of phosphate in kidney disease. Nat Rev Nephrol. 2017;13(1):27–38. [DOI] [PubMed] [Google Scholar]
- 8.Marks J, Debnam ES, Unwin RJ. Phosphate homeostasis and the renal-gastrointestinal axis. Am J Physiol Renal Physiol. 2010;299(2):F285–96. [DOI] [PubMed] [Google Scholar]
- 9.Gutiérrez OM. Fibroblast growth factor 23 and disordered vitamin D metabolism in chronic kidney disease: updating the “trade-off’ hypothesis. Clinical Journal of the American Society of Nephrology. 2010;5(9):1710–6. [DOI] [PubMed] [Google Scholar]
- 10.Wolf M. Forging forward with 10 burning questions on FGF23 in kidney disease. Journal of the American Society of Nephrology. 2010:ASN. 2009121293. [DOI] [PubMed] [Google Scholar]
- 11.Coburn JW, Brickman AS, Hartenbower DL, Norman AW. Intestinal phosphate absorption in normal and uremic man: effects of 1,25(OH)2-vitamin D3 and 1alpha(OH)-vitamin D3. Adv Exp Med Biol. 1977;81:549–57. [DOI] [PubMed] [Google Scholar]
- 12.Farrington K, Mohammed MN, Newman SP, Varghese Z, Moorhead JF. Comparison of radioisotope methods for the measurement of phosphate absorption in normal subjects and in patients with chronic renal failure. Clin Sci (Lond). 1981;60(1):55–63. [DOI] [PubMed] [Google Scholar]
- 13.Stanbury SW, Lumb GA. Metabolic studies of renal osteodystrophy. I. Calcium, phosphorus and nitrogen metabolism in rickets, osteomalacia and hyperparathyroidism complicating chronic uremia and in the osteomalacia of the adult Fanconi syndrome. Medicine (Baltimore). 1962;41:1–34. [PubMed] [Google Scholar]
- 14.Davis GR, Zerwekh JE, Parker TF, Krejs GJ, Pak CY, Fordtran JS. Absorption of phosphate in the jejunum of patients with chronic renal failure before and after correction of vitamin D deficiency. Gastroenterology. 1983;85(4):908–16. [PubMed] [Google Scholar]
- 15.Peerce BE, Weaver L, Clarke RD. Effect of 2’-phosphophloretin on renal function in chronic renal failure rats. Am J Physiol Renal Physiol. 2004;287(1):F48–56. [DOI] [PubMed] [Google Scholar]
- 16.Moe SM, Radcliffe JS, White KE, Gattone VH 2nd, Seifert MF, Chen X, et al. The pathophysiology of early-stage chronic kidney disease-mineral bone disorder (CKD-MBD) and response to phosphate binders in the rat. J Bone Miner Res. 2011;26(11):2672–81. [DOI] [PubMed] [Google Scholar]
- 17.Loghman-Adham M. Renal and intestinal Pi transport adaptation to low phosphorus diet in uremic rats. J Am Soc Nephrol. 1993;3(12):1930–7. [DOI] [PubMed] [Google Scholar]
- 18.Loghman-Adham M, Szczepanska-Konkel M, Dousa TP. Phosphate transport in brush border membranes from uremic rats. Response to phosphonoformic acid. J Am Soc Nephrol. 1992;3(6):1253–9. [DOI] [PubMed] [Google Scholar]
- 19.Marks J, Churchill LJ, Srai SK, Biber J, Murer H, Jaeger P, et al. Intestinal phosphate absorption in a model of chronic renal failure. Kidney Int. 2007;72(2):166–73. [DOI] [PubMed] [Google Scholar]
- 20.Turner ME, White CA, Hopman WM, Ward EC, Jeronimo PS, Adams MA, et al. Impaired Phosphate Tolerance Revealed With an Acute Oral Challenge. J Bone Miner Res. 2018;33(1):113–22. [DOI] [PubMed] [Google Scholar]
- 21.Vaziri ND, Yuan J, Nazertehrani S, Ni Z, Liu S. Chronic kidney disease causes disruption of gastric and small intestinal epithelial tight junction. Am J Nephrol. 2013;38(2):99–103. [DOI] [PubMed] [Google Scholar]
- 22.Vaziri ND, Yuan J, Rahimi A, Ni Z, Said H, Subramanian VS. Disintegration of colonic epithelial tight junction in uremia: a likely cause of CKD-associated inflammation. Nephrol Dial Transplant. 2012;27(7):2686–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill KM, Martin BR, Wastney ME, McCabe GP, Moe SM, Weaver CM, et al. Oral calcium carbonate affects calcium but not phosphorus balance in stage 3–4 chronic kidney disease. Kidney Int. 2013;83(5):959–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stremke ER, McCabe LD, McCabe GP, Martin BR, Moe SM, Weaver CM, et al. Twenty-Four-Hour Urine Phosphorus as a Biomarker of Dietary Phosphorus Intake and Absorption in CKD A Secondary Analysis from a Controlled Diet Balance Study. Clinical Journal of the American Society of Nephrology. 2018:CJN. 00390118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moe SM, Chen NX, Seifert MF, Sinders RM, Duan D, Chen X, et al. A rat model of chronic kidney disease-mineral bone disorder. Kidney Int. 2009;75(2):176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vorland CJ, Lachcik PJ, Aromeh LO, Moe SM, Chen NX, Gallant KM Hill. Effect of dietary phosphorus intake and age on intestinal phosphorus absorption efficiency and phosphorus balance in male rats. PLOS ONE. 2018;13(11):e0207601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marks J, Lee GJ, Nadaraja SP, Debnam ES, Unwin RJ. Experimental and regional variations in Na+-dependent and Na+-independent phosphate transport along the rat small intestine and colon. Physiol Rep. 2015;3(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Candeal E, Caldas YA, Guillen N, Levi M, Sorribas V. Intestinal phosphate absorption is mediated by multiple transport systems in rats. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2017;312(4):G355–G66. [DOI] [PubMed] [Google Scholar]
- 29.Cowley BD Jr, Gudapaty S, Kraybill AL, Barash BD, Harding MA, Calvet JP, et al. Autosomal-dominant polycystic kidney disease in the rat. Kidney Int. 1993;43(3):522–34. [DOI] [PubMed] [Google Scholar]
- 30.Marks J, Churchill L, Srai S, Biber J, Murer H, Jaeger P, et al. Intestinal phosphate absorption in a model of chronic renal failure. Kidney international. 2007;72(2):166–73. [DOI] [PubMed] [Google Scholar]
- 31.Williams KB, DeLuca HF. Characterization of intestinal phosphate absorption using a novel in vivo method. Am J Physiol Endocrinol Metab. 2007;292(6):E1917–21. [DOI] [PubMed] [Google Scholar]
- 32.Ikuta K, Segawa H, Sasaki S, Hanazaki A, Fujii T, Kushi A, et al. Effect of Npt2b deletion on intestinal and renal inorganic phosphate (Pi) handling. Clin Exp Nephrol. 2017. [DOI] [PubMed] [Google Scholar]
- 33.King AJ, Siegel M, He Y, Nie B, Wang J, Koo-McCoy S, et al. Inhibition of sodium/hydrogen exchanger 3 in the gastrointestinal tract by tenapanor reduces paracellular phosphate permeability. Science translational medicine. 2018;10(456):eaam6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagao S, Morita M, Kugita M, Yoshihara D, Yamaguchi T, Kurahashi H, et al. Polycystic kidney disease in Han: SPRD Cy rats is associated with elevated expression and mislocalization of SamCystin. American Journal of Physiology-Renal Physiology. 2010;299(5):F1078–F86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zelt JG, Svajger BA, Quinn K, Turner ME, Laverty KJ, Shum B, et al. Acute Tissue Mineral Deposition in Response to a Phosphate Pulse in Experimental CKD. Journal of Bone and Mineral Research. 2019;34(2):270–81. [DOI] [PubMed] [Google Scholar]
- 36.Lee D, Walling MW, Corry DB. Phosphate transport across rat jejunum: influence of sodium, pH, and 1, 25-dihydroxyvitamin D3. American Journal of Physiology-Gastrointestinal and Liver Physiology. 1986;251(1):G90–G5. [DOI] [PubMed] [Google Scholar]
- 37.Knöpfel T, Himmerkus N, Günzel D, Bleich M, Hernando N, Wagner CA. Paracellular transport of phosphate along the intestine. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.