Abstract
Objective
To assess the performance of thiol Michael photocurable composites based on ester-free thiols and vinyl sulfonamides of varying monomer structures and varied filler loadings and to contrast the properties of the prototype composites with conventional BisGMA-TEGDMA methacrylate composite.
Methods
Synthetic divinyl sulfonamides and ester-free tetrafunctional thiol monomers were utilized for thiol-Michael composite development with the incorporation of thiolated microfiller. Polymerization kinetics was investigated using FTIR spectroscopy. Resin viscosities were assessed with rheometry. Water uptake properties were assessed according to standardized methods. Thermomechanical properties were analyzed by dynamic mechanical analysis. Flexural modulus/strength and flexural toughness were measured on a universal testing machine in three-point bending testing mode.
Results
The vinyl sulfonamide-based thiol-Michael resin formulation demonstrated a wide range of viscosities with a significant increase in the functional group conversion when compared to the BisGMA-TEGDMA system. The two different types of vinyl sulfonamide under investigation demonstrated significant differences towards the water sorption. Tertiary vinyl sulfonamide did not undergo visible swelling whereas the secondary vinyl sulfonamide composite swelled extensively in water. With the introduction of rigid monomer into the polymer matrix the glass transition temperature increased and so increased the toughness. Glassy thiol-Michael composites were obtained by ambient curing.
Keywords: vinyl sulfonamide, thermosets, thiol-Michael, photoinitiation, step-growth polymerization, toughness, hydrolytic degradation, ester free, composites
1. Introduction
Significant progress has been made in recent years towards the development of resin-based composites for dental applications that consist of more durable materials and show long-term viability.1, 2 In widespread use are conventional resin-based dental restorative composites, which are formulated from inorganic fillers dispersed in a polymer matrix comprised of two or more dimethacrylate-based functional monomeric mixtures. Incorporation of inorganic filler in the matrix plays a vital role in the enhancement of the mechanical performance including properties such as hardness, reduced thermal diffusivity, and wear resistance, along with improved biocompatibility and moisture resistance.3–5 These inorganic fillers are mostly silica/glass particles and are typically used in high volume fraction in dental composites (for instance, ~50vol% of 0.4–1.0μm size filler).6 While the increase in filler loading assists in improving the mechanical properties, extensive filler loading can hinder the polymerization as a result of a substantial decrease in monomer mobility and increased light attenuation. Hence, various factors governing the influence of fillers have been investigated, such as loading, particle size, shape and silanization.7–15
The most widely used monomers are BisGMA (2,2-bis[4-(2’-hydroxy-3’-methacryloxy-propoxy)phenyl]propane) with TEGDMA (triethylene glycol dimethacrylate) as a diluent, which readily undergo radical-mediated photoinitiated crosslinking polymerization.16–18 In practice, this conventional curing of methacrylate-based composite has been associated with high polymerization shrinkage stress,19 brittleness,20 monomer toxicity as well as the formation of biofilms21. Most importantly, the free-radical photopolymerization of BisGMA generates highly crosslinked glassy networks; however this resulting structure has been shown to be structurally heterogeneous and highly brittle, as well as prone to hydrolytic and enzymatic degradation in moist environments due to the presence of ester functionalities.22 Many efforts have been undertaken to overcome the inherent disadvantages associated with methacrylates. For example, approaches have included soft-start polymerization with a step-wise modulation of light energy,23 alternate polymerization mechanisms,24 as well as monomer chemistry alterations.25
Several alternate polymerization reactions have gained attention in the development of dental resins. Ring opening polymerizations of spiro orthocarbonate,26 oxirane,27 silorane,28, 29 epoxy or cyclic monomers30 have been applied to counter polymerization shrinkage. Also, a significant amount of research has been focused on utilizing thiol-ene step growth polymerizations, as they facilitate rapid polymerization and high functional group conversion, produce uniform and tough network polymers that are not inhibited by oxygen.31, 32 Additionally, the photoinitiated copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) step-growth polymerization has been developed, with the capability to produce low-shrinkage dental resin composites with enhanced mechanical performance owing to the presence of the triazole rings.33, 34 Recently, ether-based di-styrenyl monomers and hydrolytically stable acrylamides have been incorporated into methacrylate dental resin to enhance long-term performance and durability. 35–37
In another recent advance, the thiol-Michael step-growth polymerization was proposed for dental composites, as a potential replacement for free-radical polymerization.38 As a step-growth process, the thiol-Michael polymerization proved to proceed rapidly, which allowed for rapid and very homogeneous network formation, delayed gelation and therefore decreased polymerization stress.39 These improved kinetic and mechanical properties are expected to contribute to greater longevity of these materials in the oral environment.
Another approach to resin-based composite modification is to create materials that actively interact with the oral biome rather than current materials, which are largely biologically inert. Nikaido et al. demonstrated that the incorporation of a sulfonamide derivative co-initiator into the resin composite may introduce greater affinity of the material to dentin.40 Recent work and many commercial pharmaceuticals have shown biological activity associated with the sulfonamide moiety.41, 42 Hence, the inclusion of this structure into the organic matrix of dental materials could enhance not only their mechanical strength but also their inherent antimicrobial properties. A bacteria-resistant material could be important for limiting caries formation, in particular carious secondary decay.
The current work is a continuation of our previous report on thiol-Michael dental composites. Herein, we introduced innovations into the structures/reactivities of the thiol-Michael monomers, and for the first time developed and assessed thiol-vinyl sulfonamide silica-filled composite materials. Although these materials still require continued optimization, they exhibit promising properties that could be beneficial for a new generation of non-ester-containing dental composites. The polymerization kinetics and (thermo-) mechanical properties of the vinyl sulfonamide-based resins and composites were investigated, and the experimental formulations were designed with varying silica microfillers. For dental restorative applications, Elastic modulus, flexural strength, and flexural toughness were examined and compared to BisGMA-TEGDMA based dimethacrylate composites as a control.
2. Materials and methods
2.1. Materials
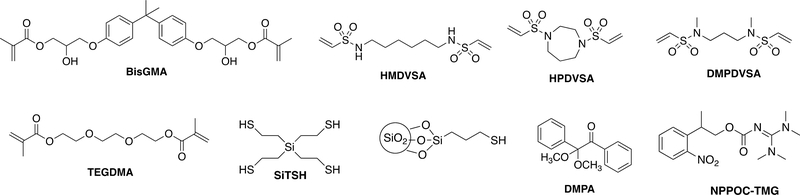
Hexamethylenediamine, N,N′-dimethyl-1,6-diaminohexane, N,N′-dimethyl-1,3-propanediamine, Homopiperazine, 2,2-dimethoxy-2-phenyl phenylacetophenone (DMPA), anhydrous dichloromethane were purchased from Sigma Aldrich. 2-Chloroethane sulfonyl chloride was purchased from TCI Chemicals and triethylamine was purchased from Fisher Scientific. (3-Mercaptopropyl) trimethoxysilane was purchased from Gelest, Inc. NPPOC-TMG and SiTSH were synthesized according to previously reported procedures.32 BisGMA/TEGDMA (70/30) comonomer mixture was used as donated by ESSTECH. Fusion silane coupling agent was used as received from George Taub Products & Fusion Co., Inc. Schott glass (mean particle size of 0.4 μm) with both untreated surface and surface treated with 3-(trimethoxysilyl)propyl methacrylate were used as received from ESSTECH. All other chemicals were of reagent grade and used without further purification.
2.2. Methods
2.2.1. BisGMA/TEGDMA composite preparation
BisGMA/TEGDMA (70:30 weight ratio) mixture with 1wt% DMPA was prepared by physical mixing. Methacrylated microfillers (Schott, 0.4 μm) were added to the resin mixture and blended in a speedmixer (DAC 150 FVZ, Flakteck) to ensure uniform dispersion of the filler.
2.2.2. Vinyl sulfonamide composite preparation
1:1 stoichiometric mixture of vinyl sulfonamide and thiol (per functional group concentration) with 2wt% NPPOC-TMG as a photocatalyst were prepared. Dichloromethane was added to homogenize the mixture and later removed in vacuo. Thiolated microfiller (Schott, 0.4 μm) was added to the resin mixture and blended in a speedmixer (DAC150FVZ, Flakteck) to ensure uniform dispersion of filler.
2.2.3. Filler Functionalization
Glass particles (20g, Schott, 0.4 μm) were placed in a glass tube and dried for 3.5 h at 165 °C in vacuo using a Buchi drying glass oven. To a round bottom flask containing 20g of dried glass particle was added 400mL of anhydrous toluene, 6mL of (3-mercaptopropyl) trimethoxysilane, and 0.4g of n-propylamine. The reaction mixture was kept stirring at room temperature for 24 h for silanization. After particle functionalization, the liquid suspension was centrifuged, and the solid glass particles obtained were washed with toluene (4x) followed by washing with dichloromethane (3x). The washed filler particles were dried under vacuum overnight at 70°C. Thermogravimetry (TGA) and diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) were used to analyze the functionalized particles.
2.2.4. Fourier transform infrared spectroscopy
Reaction kinetics were analyzed using a FTIR spectrometer (Nicolet 8700) to monitor the real-time functional group conversion in transmission mode. Samples were sandwiched between two NaCl windows with a spacer of 30 μm and placed into a horizontal transmission apparatus. The final resin conversions were calculated per the absorbance peak area of the vinyl functional group, around 6,160 cm−1 in the near-infrared spectrum.
2.2.5. Dynamic mechanical analysis
Polymer network properties were tested using a TA Instruments Q800 dynamic mechanical analyzer in multifrequency strain mode with a sinusoidal stress of 1 Hz frequency and a heating rate of 3 °C min−1. The glass transition temperature(s) (Tg) were assigned as the temperature(s) at the maxima of the tan δ curve. Tg half widths were taken as the widths of the tan delta peaks at half maximum values. The rectangular dimensions of each sample specimen were 0.25 × 5 × 25mm (t × w × l). No thermal treatment was conducted, all the samples were UV-cured at ambient and tested within 24 h after curing.
2.2.6. Three-point flexural test
Flexural strength, Elastic modulus and toughness were measured via a tensile test (MTS 858 Mini Bionix II) with a strain rate of 1 mm min−1 and a span of 20 mm. Flexural modulus was determined from the slope between 0.5 and 1% strain. Flexural strength, σ, was calculated from σ =3FL/2BH2, where F is the maximum load, and L (length), H (height), B (breath) are the sample dimensions. Ten replicates were made for each sample. The rectangular dimensions of each sample specimen were 2 × 2 × 25 mm (t × w × l).
2.2.7. Viscosity Measurement
Rheometry (ARES, TA Instruments) was used to measure the viscosity of the monomers in the torque ranges from 2 × 10−6 to 2 × 10−2 N m, using 500μm thick samples placed 8mm diameter quartz plates.
2.2.8. Thermal gravimetric analysis
Thermogravimetric analysis (TGA Pyris 1, PerkinElmer) was used to analyze the functionalized fillers. Each sample was run in a nitrogen atmosphere (20 ml /min) from 50°C to 850°C at a heating rate of 10°C/ min.
2.2.9. Quantification of bioluminescence by biofilm-associated Streptococcus mutans
Mature biofilms were formed using a firefly luciferase expressing strain of Streptococcus mutans on thiol-Michael resins (SiTSH/HPDVSA and SiTSH/HMDVSA) and control discs (BisGMA:TEGDMA). The discs were incubated in the presence of Streptococcus mutans for a total of 24 h to allow for the development of mature biofilms. After incubation, all discs were transferred to fresh BHI + 1% sucrose and incubated for one hour to allow for repletion of ATP. To measure luciferase activity, 2mM D luciferin was added and luminescence was measured. After luciferase activity was measured, biofilms on each resin were disrupted through sonication and agitation; the number of viable bacteria within the biofilm was enumerated.
2.2.10. Water sorption
Water sorption tests were performed using disc shaped specimens of resin system of 0.5 mm in thickness and 15 mm in diameter. The samples were placed in distilled water at 37 °C and the change in mass was recorded every 24 h. Finally, the samples were dried in an oven at 37 °C for 24 h cycles until the measured mass remained constant.
2.2.11. Statistical Analysis
Statistical analysis of the experiments was performed via one-way analysis of variance (ANOVA), and multiple pair-wise comparisons were conducted via a Tukey’s post-hoc test with a significance level of 0.05. The number of repetitions for each experiment were as follows: dynamic mechanical analysis (n = 3), water sorption (n = 5), three-point flexural test (n = 5), and viscosity measurement (n = 3).
3. Results and discussion
Three different vinyl sulfonamides (secondary and tertiary) were utilized in the thiol-Michael addition polymerization with an ester-free tetrathiol (SiTSH) and investigated for potential use as dental restorative resins and composites. The monomer structures used are presented in Fig 1. The silane-based tetrathiol (SiTSH) was chosen as the multi-thiol component due to its reactivity, low viscosity and enhanced hydrolytic stability.32 The vinyl sulfonamide-based monomers were synthesized from inexpensive commercial difunctional amines. Recently, the use of vinyl sulfonamide monomers has been investigated in crosslinked networks formed by photo-, and thermal thiol-Michael addition polymerization.43 Depending on the sulfonamide core type some of these new photopolymers were found to exhibit impressive hydrolytic properties when compared to their acrylate analogues. Tunable reactivity and the resistance to hydrolytic degradation of these functional groups make them promising candidates as resin components of dental composites. Herein, multiple formulations of thiol-Michael resins with varying microfiller loadings were investigated. In order to assess the handling characteristic of the material prior to curing, the viscosities of the unpolymerized composite pastes were measured for both the thiol-Michael selected compositions (SiTSH/HMDVSA and SiTSH/DMPDVSA) as well as the dimethacrylate reference formulation composed of BisGMA (70%) and TEGDMA (30%). The broad range of variations observed in relatively low viscosity thiol-Michael resin and composite systems suggests a convenient adjustment of the inorganic filler loadings in composite mixtures, which allows for an easy handling of the material and prevention of filler sedimentation. The effect of microfiller loading on the initial composite viscosity is summarized in Table 1.
Fig1.
Chemical structures for the difunctional vinyl sulfonamide, tetra(2-mercaptoethyl)silane (SiTSH), BisGMA, TEGDMA, photoinitiator, base, and thiol silane filler utilized for this study.
Table 1:
The viscosity of unpolymerized Thiol-Michael and BisGMA/TEGMA composite pastes with variable microfiller loading including 0%, 20%, 40%, and 60%, as measured at a shear rate of 200s−1. The letters indicate statistically significant differences (p<0.05) via a one-way ANOVA and a Tukey’s post-hoc test (capital letters for row and lowercase for column).
| Filler Loading (%) | Viscosity (Pa. s) | ||
|---|---|---|---|
| BisGMA/TEGMA | HMDVSA/SiTSH. | DMPDVSA/SiTSH | |
| 0 |
0.8±0.1A,a | 1.1±0.1A,a | 0.2±0.1 A,a |
| 20 |
1.5±0.4A,a | 2.4±0.6B,a | 0.2±0.1C,a |
| 40 |
2.5±0.4A,a | 2.7±0.2A,a | 0.5±0.2B,a |
| 60 | 29±1A,b | 9.6±0.3B,a | 66±2C,b |
Although resins activated by visible light are required for clinical dental applications, the thiol-Michael composite mixtures employed here were initiated with an ultraviolet irradiance in the presence of suitable UV-sensitive photobase. As demonstrated in the literature, visible light responsive photobase systems have successfully been introduced into thiol-Michael photopolymerizations, and adopting these for use in dental curing, where the visible wavelengths offer significantly greater light penetration compared with UV, would be an obvious next implementation step.44, 45 In the meantime, synthetically non-demanding, and efficient NPPOC-TMG UV photobase was selected for this study, which exhibits high efficiency after exposure.46 Initially, the kinetic experiments were performed on unfilled thiol-Michael formulations and the BisGMA-TEGDMA resin, by utilizing a stoichiometric mixture (1:1) of vinyl sulfonamide and thiol (per functional group concentration) in the presence of 2wt% of NPPOC-TMG with UV light of 320–390 nm (100mW/cm2) and 1wt% of DMPA with UV light of 32–390nm (50mW/cm2), respectively.
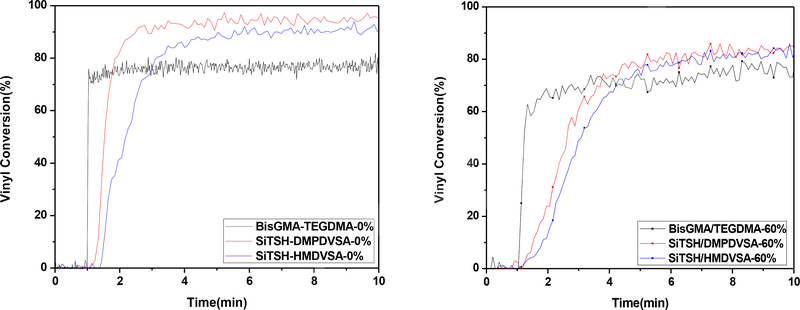
The kinetic studies demonstrated that the thiol-Michael formulation based on vinyl sulfonamide resulted in higher overall conversions (>90%) when compared to the conventional BisGMA-TEGDMA formulation. This significant difference in the conversion could be attributed to the advantage of delayed gelation associated with the step-growth thiol-Michael polymerization compared to chain growth polymerization for BisGMA-TEGDMA (Fig 2). The vitrification for the thiol-Michael polymers does not occur until higher conversions which affects the mobility of the reactive oligomers, resulting in overall higher conversions. However, the increase in the filler loading leads to a slower polymerization. This outcome may be attributed to the increase in the viscosity. Despite the relatively slower polymerization, the thiol-Michael formulation resulted in 85% conversion as compared to 70% for BisGMA-TEGDMA.
Fig2.
Polymerization kinetics of thiol-Michael composites and BisGMA/TEGDMA composites with 0% and 60% microfiller loadings, as measured by FTIR. Each mixture was irradiated for 10min at ambient temperature with 100mW/cm2 of 320–390 nm light following 1min in the dark to establish a baseline.
Thermomechanical properties of both the thiol-Michael and BisGMA-TEGDMA composites, such as Tg and storage modulus at 40°C, are listed in Table 2. Each sample was cured at ambient temperature by using 2wt% NPPOC-TMG for 10 min. The thiol-Michael and BisGMA-TEGDMA composites with different filler loading have statistically similar Tg values within each group, suggesting that the functional group conversions are nearly equivalent despite the different filler loadings. This phenomenon indicated that the progressive enhancement in the storage modulus with increasing filler content is almost exclusively based on the increased filler incorporation.
Table 2:
Storage Modulus at 40°C and glass transition temperature (Tg) for Thiol Michael composite and BisGMA/TEGMA composite with varying microfiller loadings as measured by DMA. The letters indicate statistically significant differences (p<0.05) via a one-way ANOVA and a Tukey’s post-hoc test (capital letters for row and lowercase for column).
| BisGMA/TEGMA | HMDVSA/SiTSH | DMPDVSA/SiTSH | ||||
|---|---|---|---|---|---|---|
| Filler Loading (%) | Tg (°C) | E’ @ 40°C(GPa) | Tg (°C) | E’ @ 40°C(GPa) | Tg (°C) | E’ @ 40°C(GPa) |
| 0 | 163±2A,a | 1.6±1 A,a | 57±1B,a | 1.43±0.01A,a | 57.5±0.3B,a | 0.6±0.7A,a |
| 20 | 171±1A,b | 2.5±0.1A,a,b | 60± 2B,b | 1.5±0.4A,a | 57±1C,a | 1.4±0.5A,a,b |
| 40 | 173±2A,b | 2.6±0.3A,a,b | 66±1B,c | 2.1±0.1B,b | 62±3B,b | 1.8±0.0B,b |
| 60 | 176±1A,c | 4.4±0.3A,b | 65±1B,c | 3.4±0.1B,c | 69±1C,b | 4.7±0.1A,c |
As demonstrated earlier the tertiary vinyl sulfonamides are not affected in acidic or basic conditions;43 herein, the water sorption and solubility were examined for the unfilled thiol-Michael resin and compared to the conventional methacrylate dental material. As expected, the tertiary vinyl sulfonamide did not undergo visible swelling whereas the secondary vinyl sulfonamide composite swelled extensively in water. This high uptake of water could be attributed to the presence of secondary amide protons and their sensitivity toward basic hydrolytic degradation (Table 3). The tertiary vinyl sulfonamide presence in the resin system leads to a significant reduction in water uptake; it is worth noting that the presence of such functional group could be exceptionally beneficial from the standpoint of prolonged exposure to moist conditions that results in the deterioration of ester-based material properties.
Table 3:
The results from water sorption tests of BisGMA/TEGDMA and thiol-Michael resin systems. The letters indicate statistically significant differences (p<0.05) via a one-way ANOVA and a Tukey’s post-hoc test
| Samples | BisGMA/TEGMA | HMDVSA/SiTSH | DMPDVSA/SiTSH |
|---|---|---|---|
| Solubility (μg/mm3) | 0.8±0.1A | 0.56±0.07B | 0 |
| Sorption (μg/mm3) | 58±5A | 180±4B | 0.4±0.1C |
In general, the introduction of a rigid monomer into the polymer matrix tends to increase the glass transition temperature, resulting in tougher and more robust materials. Therefore, the toughness of the formulated resin would increase as a result of the favorable combination of the rigid cyclic vinyl sulfonamide and the flexibility of the alkyl chain. To exploit such efficacy, we formulated thiol-Michael composite pastes with cyclic tertiary vinyl sulfonamide, HPDVSA, and an ester-free tetrathiol, SiTSH. Mechanical properties including elastic modulus, flexural strength, and flexural toughness of the thiol-Michael composites and BisGMA-TEGDMA resins/composites were further analyzed via three-point bend tests performed on a universal testing machine (MTS) and presented in Table 4. Although exhibiting lower values of modulus and flexural strength, the toughness of the thiol-Michael composite systems was observed to be higher than that for the BisGMA-TEGDMA composite. This behavior suggests that the incorporation of the vinyl sulfonamide functionality into the network has a reinforcing effect on otherwise glassy materials.
Table 4:
Comparison of resin and composite flexural elasticity, flexural strength, flexural toughness from the three-point bend testing. The letters indicate statistically significant differences (p<0.05) via a one-way ANOVA and a Tukey’s post-hoc test (capital letters for row and lowercase for column).
| BisGMA/TEGMA | HMDVSA/SiTSH | HPDVSA/SiTSH | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Filler Loading (%) | Flexural Elasticity [GPa] | Flexural strength [MPa] | Toughness [MJm−3] | Flexural Elasticity [GPa] | Flexural strength [MPa] | Toughness [MJm−3] | Flexural Elasticity [GPa] | Flexural strength [MPa] | Toughness [MJm−3] |
| 0 | 3.1±0.4A,a | 92±25A,a | 1.68±0.84A,a | 0.26±0.08B,a | 14.3±6.8B,a | 2.02±0.99A,a,b | 0.43±0.12B,a | 18.2±5.7B,a | 1.6±1.2A,a |
| 20 | 3.5±0.6A,a | 59±18A,b | 0.58±0.35A,b | 1.11±0.48B,a | 36.5±10.9B,b | 2.62±1.02B,a | 0.43±0.26C,a | 16.6±5.7C,a | 1.5±1.0A,a |
| 40 | 4.8±0.7A,b | 77±11A,b | 0.64±0.18A,b | 2.53±0.70B,b | 59.6±13.5A,c | 2.05±0.83B,a,b | 2.91±0.85B,b | 63.7±8.3A,b | 1.5±0.4B,a |
| 60 | 7.6±1.1A,c. | 97±20A,a | 0.65±0.20A,b | 3.81±1.03B,c | 58.1±8.7B,c | 1.14±0.32A,b | |||
Recent work on commercial pharmaceuticals has shown biological activity associated with a ring-structured vinyl sulfonamide moiety.41 The inclusion of this structure into the organic matrix of dental materials was anticipated to enhance the antimicrobial properties. A bacteria resistant material could be important for caries formation, in particular carious secondary decay. To evaluate the efficacy of the resin system, the luciferase activity and viable count analysis of bacteria associated with the biofilms grown on thiol-Michael resins was performed47, and it was found that there is no statistical difference in the luminescence as compared to BisGMA-TEGDMA (See supporting information for more details, FigS5 and Fig S6). Although, no significant improvement in the antimicrobial performance was detected, extended studies would be more appropriate in the detailed assessment of the composite biological activity. This study demonstrates; however, that this approach does not introduce any negative bacterial growth effects, and therefore could be considered for the designing of composite materials.
4. Conclusions
In summary, ester-free thiol-Michael resin composites were prepared and evaluated for their potential as dental composite materials. Vinyl sulfonamide containing thermosetting composites were formed via Thiol-Michael polymerization which exhibits higher overall conversions and is devoid of the presence of hydrolytically susceptible ester moieties. The study proves that the tertiary vinyl sulfonamide exhibited impressive hydrolytic properties when compared to a methacrylate-based system. Further, the incorporation of a structurally rigid monomer increased the composite toughness and the glass transition temperature of the resulting composite material. Although well documented in other biological systems, the biological activity of the sulfonamide moiety still requires further in-depth testing in dental composites. This study demonstrates that such a functional group could be incorporated into the design of new materials as a promising alternative for dental restoration with impressive hydrolytic properties.
Supplementary Material
Significance.
Employing the newly developed step-growth thiol-Michael resins in dental composites will provide structural uniformity, improved stability and lower water sorption.
Acknowledgments
The authors acknowledge financial support from the National Institutes of Health (NIH: 1U01DE023777-01).
Footnotes
Appendix A. Supplementary Data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cramer NB, Stansbury JW, Bowman CN. Recent Advances and Developments in Composite Dental Restorative Materials. J Dent Res 2011;90:402–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anseth KS, Newman SM, Bowman CN. Polymeric dental composites: Properties and reaction behavior of multimethacrylate dental restorations. Adv Polym Sci 1995;122:177–217. [Google Scholar]
- 3.Ferracane JL. Current trends in Dental Composites. Crit Rev Oral Biol Med 1995;6:302–318. [DOI] [PubMed] [Google Scholar]
- 4.Moszner N, Salz U. New developments of polymeric dental composites. Prog Polym Sci 2001;26:535–576. [Google Scholar]
- 5.Habib E, Wang RL, Wang YZ, Zhu MF, Zhu XX. Inorganic Fillers for Dental Resin Composites: Present and Future. ACS Biomater Sci Eng 2016;2:1–11. [DOI] [PubMed] [Google Scholar]
- 6.Anusavice KJ SC, Rawls HR. Phillips’ science of dental materials. 12 ed: Elsevier Health Sciences; 2003. [Google Scholar]
- 7.Topcu FT, Erdemir U, Sahinkesen G, Yildiz E, Uslan I, Acikel C. Evaluation of Microhardness, Surface Roughness, and Wear Behavior of Different Types of Resin Composites Polymerized With Two Different Light Sources. J Biomed Mater Res B Appl Biomater. 2010;92B:470–478. [DOI] [PubMed] [Google Scholar]
- 8.Shah PK, Stansburya JW. Role of filler and functional group conversion in the evolution of properties in polymeric dental restoratives. Dent Mater 2014;30:586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beun S, Glorieux T, Devaux J, Vreven J, Leloup G. Characterization of nanofilled compared to universal and microfilled composites. Dent Mater 2007;23:51–59. [DOI] [PubMed] [Google Scholar]
- 10.Matinlinna JP, Lassila LVJ, Ozcan M, Yli-Urpo A, Vallittu PK. An introduction to silanes and their clinical applications in dentistry. Int J Prosthodont 2004;17:155–164. [PubMed] [Google Scholar]
- 11.Bowen RL. Properties of a Silica-reinforced Polymer for Dental Restorations. J Am Dent Assoc 1963;66:57–64. [DOI] [PubMed] [Google Scholar]
- 12.Bowen RL. Effect of Particle Shape + size Distribution in Reinforced Polymer. J Am Dent Assoc 1964;69:481–95. [DOI] [PubMed] [Google Scholar]
- 13.Masouras K, Silikas N, Watts DC. Correlation of filler content and elastic properties of resin-composites. Dent Mater 2008;24:932–939. [DOI] [PubMed] [Google Scholar]
- 14.Ikejima I, Nomoto R, McCabe JF. Shear punch strength and flexural strength of model composites with varying filler volume fraction, particle size and silanation. Dent Mater 2003;19:206–211. [DOI] [PubMed] [Google Scholar]
- 15.Xu HHK, Quinn JB, Smith DT, Antonucci JM, Schumacher GE, Eichmiller FC. Dental resin composites containing silica-fused whiskers - effects of whisker-to-silica ratio on fracture toughness and indentation properties. Biomaterials 2002;23:735–742. [DOI] [PubMed] [Google Scholar]
- 16.Reed BB, Choi K, Dickens SH, Stansbury JW. Effect of resin composition on kinetics of dimethacrylate photopolymerization. Abstracts of Papers of the American Chemical Society. 1997;214:41-POLY. [Google Scholar]
- 17.Pereira SG, Osorio R, Toledano M, Nunes TG. Evaluation of two Bis-GMA analogues as potential monomer diluents to improve the mechanical properties of light-cured composite resins. Dent Mater 2005;21:823–830. [DOI] [PubMed] [Google Scholar]
- 18.Srivastava R, Liu JH, He CX, Sun YY. BisGMA analogues as monomers and diluents for dental restorative composite materials. Mater Sci Eng C Mater Biol Appl 2018;88:25–31. [DOI] [PubMed] [Google Scholar]
- 19.Braga RR, Ballester RY, Ferracane JL. Factors involved in the development of polymerization shrinkage stress in resin-composites: A systematic review. Dent Mater 2005;21:962–970. [DOI] [PubMed] [Google Scholar]
- 20.Matsukawa S, Hayakawa T, Nemoto K. Development of High-toughness Resin for Dental applications. Dent Mater 1994;10:343–346. [DOI] [PubMed] [Google Scholar]
- 21.Busscher HJ, Rinastiti M, Siswomihardjo W, van der Mei HC. Biofilm Formation on Dental Restorative and Implant Materials. J Dent Res 2010;89:657–665. [DOI] [PubMed] [Google Scholar]
- 22.Santerre JP, Shajii L, Leung BW. Relation of dental composite formulations to their degradation and the release of hydrolyzed polymeric-resin-derived products. Crit Rev Oral Biol Med 2001;12:136–151. [DOI] [PubMed] [Google Scholar]
- 23.Yap AUJ, Ng SC, Kiow KS. Soft-start polymerization: Influence on effectiveness of cure and post-gel shrinkage. Oper Dent 2001;26:260–266. [PubMed] [Google Scholar]
- 24.Hamano N, Chiang YC, Nyamaa I, et al. Repair of silorane-based dental composites: Influence of surface treatments. Dent Mater 2012;28:894–902. [DOI] [PubMed] [Google Scholar]
- 25.Khatri CA, Stansbury JW, Schultheisz CR, Antonucci JM. Synthesis, characterization and evaluation of urethane derivatives of Bis-GMA. Dent Mater 2003;19:584–588. [DOI] [PubMed] [Google Scholar]
- 26.Sun X, Li YC, Xiong J, Hu XY, Chen JH. Shrinkage properties of a modified dental resin composites containing a novel spiro-orthocarbonate expanding monomer. Mater Lett. 2011;65:3586–3589. [Google Scholar]
- 27.Danso R, Hoedebecke B, Whang K, et al. Development of an oxirane/acrylate interpenetrating polymer network (IPN) resin system. Dent Mater 2018;34:1459–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weinmann W, Thalacker C, Guggenberger R. Siloranes in dental composites. Dent Mater 2005;21:68–74. [DOI] [PubMed] [Google Scholar]
- 29.Eick JD, Kotha SP, Chappelow CC, et al. Properties of silorane-based dental resins and composites containing a stress-reducing monomer. Dent Mater 2007;23:1011–1017. [DOI] [PubMed] [Google Scholar]
- 30.Contreras PP, Tyagi P, Agarwal S. Low volume shrinkage of polymers by photopolymerization of 1,1-bis(ethoxycarbonyl)-2-vinylcyclopropanes. Polym Chem. 2015;6:2297–2304. [Google Scholar]
- 31.Lu H, Carioscia JA, Stansbury JW, Bowman CN. Investigations of step-growth thiol-ene polymerizations for novel dental restoratives. Dent Mater 2005;21:1129–1136. [DOI] [PubMed] [Google Scholar]
- 32.Podgorski M, Becka E, Claudino M, et al. Ester-free thiol-ene dental restoratives-Part A: Resin development. Dent Mater 2015;31:1255–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song HB, Sowan N, Shah PK, et al. Reduced shrinkage stress via photo-initiated copper(I)-catalyzed cycloaddition polymerizations of azide-alkyne resins. Dent Mater 2016;32:1332–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song HB, Wang X, Patton JR, Stansbury JW, Bowman CN. Kinetics and mechanics of photo-polymerized triazole-containing thermosetting composites via the copper(I)-catalyzed azide-alkyne cycloaddition. Dent Mater 2017;33:621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang XH, Huyang G, Palagummi SV, et al. High performance dental resin composites with hydrolytically stable monomers. Dent Mater 2018;34:228–237. [DOI] [PubMed] [Google Scholar]
- 36.Moszner N, Fischer UK, Angermann J, Rheinberger V. Bis-(acrylamide)s as new cross-linkers for resin-based composite restoratives. Dent Mater 2006;22:1157–1162. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez-Bonet A, Kaufman G, Yang Y, et al. Preparation of Dental Resins Resistant to Enzymatic and Hydrolytic Degradation in Oral Environments. Biomacromolecules 2015;16:3381–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang S, Podgorski M, Zhang X, et al. Dental Restorative Materials Based on Thiol-Michael Photopolymerization. J Dent Res 2018;97:530–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kolb HC, Finn MG, Sharpless KB. Click chemistry: Diverse chemical function from a few good reactions. Angew Chem Int Ed 2001;40:2004–2021. [DOI] [PubMed] [Google Scholar]
- 40.Nikaido T, Podszun W, Muller M, Nakabayashi N. Effect of Sulfonamides and 4-met on Adhesion to Tooth Substrates. Dent Mater 1990;6:78–82. [DOI] [PubMed] [Google Scholar]
- 41.Ozbek N, Katircioglu H, Karacan N, Baykal T. Synthesis, characterization and antimicrobial activity of new aliphatic sulfonamide. Bioorg Med Chem 2007;15:5105–5109. [DOI] [PubMed] [Google Scholar]
- 42.Kwon Y, Song J, Lee H, et al. Design, Synthesis, and Biological Activity of Sulfonamide Analogues of Antofine and Cryptopleurine as Potent and Orally Active Antitumor Agents. J Med Chem 2015;58:7749–7762. [DOI] [PubMed] [Google Scholar]
- 43.Sinha J, Podgorski M, Huang SJ, Bowman CN. Multifunctional monomers based on vinyl sulfonates and vinyl sulfonamides for crosslinking thiol-Michael polymerizations: monomer reactivity and mechanical behavior. Chem Commun 2018;54:3034–3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suyama K, Araki H, Shirai M. Quaternary ammonium salt as DBU - Generating photobase generator. J Photopolym Sci Tec 2006;19:81–84. [Google Scholar]
- 45.Suyama K, Shirai M. Photobase generators: Recent progress and application trend in polymer systems. Prog Polym Sci 2009;34:194–209. [Google Scholar]
- 46.Xi W, Krieger M, Kloxin CJ, Bowman CN. A new photoclick reaction strategy: photo-induced catalysis of the thiol-Michael addition via a caged primary amine. Chem Commun 2013;49:4504–4506. [DOI] [PubMed] [Google Scholar]
- 47.Zajdowicz S, Song HB, Baranek A, Bowman CN. Evaluation of biofilm formation on novel copper-catalyzed azide-alkyne cycloaddition (CuAAC)-based resins for dental restoratives. Dent Mater 2018;34:657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.