Abstract
Alzheimer’s disease (AD) and frontotemporal dementia (FTD) are the most common neurodegenerative early-onset dementias. Despite the fact that both conditions have a very distinctive clinical pattern, they present with an overlap in their cognitive and behavioral features that may lead to misdiagnosis or delay in diagnosis. The current review intends to summarize briefly the main differences at the clinical, neuropsychological, and behavioral levels, in an attempt to suggest which aspects would facilitate an adequate diagnosis in a clinical setting, especially in Latin American and low- and middle-income countries, where the resources needed for a differential diagnosis (such as MRI or biomarkers) are not always available. A timely diagnosis of AD and FTD have significant implications for the medical management and quality of life of patients and careers.
Keywords: Alzheimer’s disease, cognitive neuropsychology, differential diagnosis, frontotemporal dementia, young onset dementia
INTRODUCTION
Dementia poses an increasing challenge for global public health. Worldwide, the estimated number of patients was 46.8 million in 2015. This number is expected to double every 20 years, reaching 74.7 million in 2030 and 131.5 million in 2050 [1]. In Latin American countries, this number is predicted to increase 4-fold [1–3]. Furthermore, the Alzheimer’s Disease International report informed the highest global prevalence of dementia in Latin American countries (8.4%) after North Africa/Middle East (8.7%) in people>60 years [1, 4].
Young-onset dementia (YOD) is conventionally thought to include patients with onset before 65 years of age [5] and is an important and under-recognized condition among the total dementia population, with adverse impact both on the individual and wider society [6], mainly because of the progression that the YOD has, as well as the high level of caregiver burden. Some studies have shown that in the case of Alzheimer’s disease (AD), younger age is related to a more progressive course and shorter survival [7, 8]. On the other hand, the YOD caregivers are affected more directly in their daily lives as it concerns the spousal relationship. On the NeedYD Study, authors found that YOD caregivers feel less vital, more exhausted, have more complaints related to pain, and have more problems with their mental health (such as feeling nervous or depressed). They also feel limited in their social functioning and social activities [9]. There are considerable delays in diagnosing YOD compared to late-onset dementia. Time to diagnosis for YOD is 4.4 years versus 2.8 years for late-onset dementia [6,10]. These delays undoubtedly have significant repercussions for affected individuals and their families.
The main causes of YOD are AD, followed by vascular dementia and frontotemporal dementia (FTD) [5, 6, 10]. FTD accounts for 10% of all confirmed YOD [11,12], and it is most often diagnosed between the ages of 45 and 65, but it can also affect younger and older people [13].
Both AD and FTD have heterogeneous and atypical manifestations, meaning the diagnosis can turn into a complex challenge to achieve. On the one hand, FTD can be divided into a behavioral variant (bvFTD) and a language variant, which is classified as a primary progressive aphasia (PPA) in their two forms, semantic variant and nonfluent/agrammatic variant PPA [14]. On the other, AD has other atypical forms of presentations such as a behavioral/dysexecutive variant (bdAD), a logopenic variant of primary progressive aphasia (lvPPA), and a posterior cortical atrophy (PCA) [14].
Differential diagnosis between YOD, especially FTD and AD, is clinically important because these disorders are associated with different medical, psychological, and social needs of patients and caregivers. Furthermore, AD and FTD have different implications with respect to heritability, disease progression, and life expectancy [15]. Besides facilitating proper and tailored care, early diagnosis will provide higher benefit from the future therapies delaying disease progression.
Unfortunately, to establish the differential diagnosis between AD and bvFTD proves to be difficult. First, YOD patients often initially present with atypical symptoms. For instance, there are consistent differences between clinical findings in early onset AD (EOAD) and late-onset AD (LOAD), where the onset of EOAD is more likely to be marked by atypical symptoms, and cognitive assessments point to poorer executive, visuospatial functioning and praxis, with less marked memory impairment [16]. Turning to bvFTD, there is significant evidence that demonstrates that some bvFTD patients show memory impairment similar to AD in episodic memory tests, which is in line with a hippocampal affectation profile [17, 18]. Therefore, the diagnosis of YOD continues to be a challenge despite considerable improvement in the quality of neuropsychology, neuroimaging and biomarker assays [5, 10].
This clinical overlap may make it challenging to distinguish between the two entities, which often implies a delay in diagnosis [6, 10]. In this respect, the INSPIRED study, which aimed to determine which factors were associated with the timeframe of symptom onset to dementia diagnosis, shows that compared with EOAD patients, those with FTD did not have a longer timeframe for dementia diagnosis, but the time from the first to the final type of dementia diagnosis was more than double [19]. In the same way, misdiagnosis or delayed incorrect diagnosis contributes to the burden of family caregivers and reduces the family caregivers’ possibilities to seek supportive resources, support, and management [20]. To address this challenge, several studies have suggested that memory clinics might hasten the correct dementia diagnosis in YOD [6, 12, 19], because of the higher number of dementia specialists (neurologist, old age/geriatric psychiatrist, or geriatrician) and multi-disciplinary teams, who might confirm the diagnosis at an earlier stage as well as offer early treatment and guidance.
Nevertheless, most Latin American countries have outdated health systems which are not currently able to offer the resource-intensive and multidisciplinary programs that people with dementia and their caregivers require [21]. Moreover, some Latin American countries have a limited number of private and public memory clinics, since public policies have recently taken in account the importance of dementia diagnosis and treatment in the population [3]. Even though the acceptance of international recommendations on dementia by local scientific/clinical/academic communities is increasing, it does not receive systematic support from relevant stakeholders [22]. As a consequence, there is limited access to new technologies which could improve the possibility to establish a differential diagnosis, such as MRI, neuroimaging markers (e.g., amyloid/tau-PET and FDG-PET) and biomarkers. Under these circumstances, the priority should be to timely diagnose using affordable neuropsychological and clinical assessments, as well as appropriate patient care [22].
This review aims to give some key points in order to guide the differential diagnosis between typical and behavioral/dysexecutive variant of AD and bvFTD with a clinical standpoint, which could be very helpful especially for low-and middle-income countries.
METHOD
We searched PubMed until October 2019, using the search terms [Young / Early Onset Dementia]; [Frontotemporal Dementia]; [Frontal variant of Alzheimer Disease] and [Behavioral Dysexecutive variant of Alzheimer Disease], and [Differential diagnosis Alzheimer Disease and Frontotemporal dementia]. We identified additional studies by hand-searching references’ lists of critical papers on the matter.
CLINICAL PROFILES OF AD AND BVFTD
As it is widely known, typical AD (tAD) is characterized by an insidious and progressive decline of episodic memory and other cognitive domains, such as visuospatial skills, language, or executive function, among others [23].
However, as mentioned above, there are different presentations beyond the typical amnestic AD, which are non-amnestic variants with the same underlying neuropathology.
bdAD, also referred to as frontal AD, can present with a predominance of behavioral and/or executive dysfunction, with personality and behavioral changes such as disinhibition, apathy or compulsiveness. This variant may mimic that of bvFTD, turning the differential diagnostic into a major challenge [24, 25]. Recently, the diagnostic criteria for AD have been revised to include different auxiliary methods and to facilitate this task [23]; however, there still are problems in the clinical setting.
It is important to consider that significant differences between EOAD and LOAD have been described in the past few years [16, 24, 26]. Overall, patients with EOAD, compared to patients with LOAD, have a more aggressive clinical course, better memory recognition and semantic memory [24], but they tend to have worse attention, executive functions, and visuospatial skills [16, 24, 26, 27]. This is consistent with atypical presentations of AD. In fact, about 22% to 64% of EOAD are non-amnestic variant phenotypes [24, 28]. It is unclear the proportion of bdAD in this group, but it has been reported that the most common variant may be lvPPA and PCA [24].
bvFTD on its part has been characterized by a remarkable change in personality with prominent apathy and disinhibition, accompanied by a lack of empathy and insight, stereotypical behaviors and changes in eating habits (e.g., the development of a ‘sweet tooth’), obsessive-compulsive behaviors, yet with relative preservation of other cognitive areas such as visuospatial function and memory in the early stages [12, 29]. However, a subset of patients with bvFTD exhibit early problems with episodic memory [17], which in some occasions can easily be confused with AD, because they show the same type of memory loss. The diagnostic criteria currently used are those proposed by Raskovsky et al., which even though do not consider the memory impairment in bvFTD, have demonstrated to be more sensitive than previous criteria [30].
CLINICAL EXAMINATION
History
In order to differentiate between AD and bvFTD, a combination of the medical history obtained from the patient and a reliable informant (including questions concerning comorbidities, educational level, and family disease history), as well as a neurological examination, are necessary to complete the full clinical workout.
The first step to perform a clinical interview is a detailed history, with particular attention to identifying the onset of the illness, the timing, order, and progression of the symptoms. This information should be elicited from patients and a reliable informant. To allow patients engagement is important not to talk in front of them as if they were not there. It is also desirable to include them in the general conversation when they enter the office. The informant should be interviewed alone during part of the assessment to facilitate disclosure, as there is often a reluctance to describe handicaps and misbehaviors in front of the patients. It is important not only to focus on the main complaint but also how they deviate from a patient’s lifelong temperament, behavior, habits, and more subtle personality changes such as the insidious coarsening of conduct and habits, self-neglect, and abandonment of work, social routines, and relationships. This distinction is important because of the high overlap between primary psychiatric disorders and bvFTD [31]. The current evidence on cognition, neuropsychiatric symptoms, and activities of daily living to distinguish those syndromes will be reviewed in the following sections.
Neuropsychological evaluation
Although the description of these symptoms appears to show very different clinical profiles, the differential diagnosis can become very challenging considering that: 1) a considerable percentage of bvFTD show memory deficits; 2) a substantial percentage of AD show behavioral changes and executive dysfunction, especially the behavioral/dysexecutive variants. This raises the question as to how current tools may allow a differentiation between tAD, bdAD, and bvFTD, considering that there is a major overlap for cognition and behavior on a clinical level.
General cognitive screening
The most widely used tool for the evaluation of global cognitive impairment is the Mini-Mental State Examination (MMSE) [32], which shows overall good detection of memory deficits but usually does not detect early cases of bvFTD [33].
Another global scale widely used is the Addenbrooke’s Cognitive Examination Revised (ACE-R), which unlike the MMSE, offers a statistical value (VLOM coefficient) for the differentiation of different types of dementia. It has been reported that the ACE-R can detect impairment in 90% of the bvFTD cases [34]. The ACE-R has recently been updated to a new version, the ACE-III, showing similar psychometric properties and diagnostic utility to detect different types of dementia as its precursor [35].
Other tests are the Frontal Assessment Battery [36] and the INECO Frontal Screening [37]. Both may be valuable for distinguishing tAD and bvFTD, since they were proposed for a rapid assessment of executive dysfunction in dementia and have been reported to be effective in differential diagnosis [38,39]. Leslie et al. also developed a new tool to assess executive functions, the FRONTIER Executive Screen, which combines three measures: verbal fluency, inhibitory control, and working memory. This test demonstrated to have solid discriminative validity to differentiate bvFTD from AD [40].
Executive function
To date, executive dysfunction is considered as part of the current neuropsychological profile for bvFTD [29, 30]. However, bdAD and tAD also have this deficit [25, 41]. In a retrospective study with neuropathologically verified patients with bvFTD, bdAD, and tAD, investigators reported that bdAD had worse executive functioning than bvFTD and tAD. Similar results were reported by Reul et al. [42] where patients with AD were significantly more impaired in executive function than bvFTD. In consequence, several studies have attempted to investigate which executive tools would be more accurate to reach diagnosis [43–47]. However, due to the variety of proposed tests, it becomes difficult to decide which one is the most useful in a clinical setting. In this regard, Hornberger et al. determined that patients with bvFTD were consistently impaired on the Hayling Test of Inhibitory Control, Digit Span Backwards, Letter fluency, and Trail Making Test B when compared to controls and phenocopy subjects [48]. In a longitudinal study comparing AD and bvFTD patients by neuropsychological testing, 86% of the patients were correctly classified as bvFTD based on their performance on The Hayling Test and Digit Span Backwards [49] (Table 1).
Table 1.
Summary of recommended cognitive tests for the differential diagnosis between tAD, bdAD, and bvFTD
| Cognitive domain | Sub-domain | Test | Performance |
|---|---|---|---|
| Cognitive screening | General screening | ACE-III | tAD < bdAD < bvFTD |
| Executive function | FAB | bvFTD ~ bdAD < tAD | |
| Executive function | FRONTIER Executive Screen | bvFTD ~ bdAD < tAD | |
| Executive function | INECO Frontal Screening | bvFTD ~ bdAD < tAD | |
| Executive function | Inhibitory control | Hayling Test | bvFTD < bdAD < tAD |
| Working Memory | Digit Span Backwards | bvFTD ~ bdAD < tAD | |
| Cognitive flexibility | Letter Fluency | bvFTD < bdAD < tAD | |
| Trail Making Test -B | bvFTD ~ bdAD < tAD | ||
| Memory | Episodic Memory, verbal recall | FCSRT | tAD ~ bdAD < bvFTD |
| Social Cognition | ToM | Reading the mind in the eyes | bvFTD < bdAD < tAD |
| ToM | Faux Pas | bvFTD < bdAD < tAD | |
| Emotion recognition | Ekman 60 Faces Test | bvFTD < bdAD < tAD | |
| Visuospatial function | Topographical memory | Supermarket task | tAD < bdAD ~ bvFTD |
| Language | Object Naming | Boston Naming Test | tAD < bdAD ~ bvFTD |
| Semantic paraphasia | Boston Naming Test; Spontaneous speech | tAD < bdAD < bvFTD | |
| Semantic fluency | Semantic fluency | bdAD < bvFTD < tAD | |
ACE-III, Addenbrooke’s Cognitive Examination-III; bdAD, behavioral/dysexecutive variant Alzheimer’s disease; bvFTD, behavioral variant frontotemporal dementia; FAB, Frontal Assessment Battery; FCSRT, Free and Cued Selective Reminding Test; tAD, typical Alzheimer’s disease; ToM, Theory of Mind.
Memory impairment
The current diagnostic criteria for bvFTD considers memory impairment with relative preservation [30] and when bvFTD patients presents with memory impairment, it is often attributed to prefrontal dysfunction. Conversely, there is significant evidence that a subset of bvFTD patients present memory impairment similar to AD in episodic memory tests [18]. Moreover, Bertoux et al. demonstrated that memory performance in bvFTD is independent of executive function, showing storage and consolidation deficits characteristic of hippocampal involvement, even at early stages of the disease [17].
The most widely episodic memory test used that allows identifying hippocampal affectation (storage deficits) as well as deficits in encoding and retrieval is the Free and Cued Selective Reminding Test, which is based on a semantic cueing method that controls the effective encoding of 16 words or pictures and facilitates retrieval by semantic cueing [50]. This test has been proven to be an effective tool to distinguish between AD and cognitively healthy controls [50], detect AD at its early stages [50–52], and identify patients with mild cognitive impairment who are at a higher risk for developing AD [53].
A comparative neuroimaging study in AD versus bvFTD patients, using whole brain voxel-based morphometry analyses demonstrated that common brain regions including right anterior hippocampus, right temporal and frontal lobes, and left paracingulate gyrus are implicated in episodic retrieval in both groups [54]. Also, when comparing bvFTD, tAD, and bdAD, all three groups had comparable memory profiles in standard episodic memory tests, where bdAD patients had similar memory performance than tAD patients, and worse than bvFTD [25]. Besides that, voxel-based morphometry analyses revealed that bvFTD and bdAD groups had a common pattern of atrophy in prefrontal and medial temporal lobe [55]. These findings are strongly supported by studies in brain imaging that showed medial temporal shrinkage in bvFTD as well as neuropathological findings demonstrating significant hippocampal atrophy in early stages of the disease, with the presence of TDP-43 inclusions in cases with severe memory impairment [51, 56].
Social cognition
Social cognition, defined as the ability to recognize how other people are feeling and make judgements based on their inferred thoughts [57], includes domains such as the theory of mind (ToM), emotion recognition, empathy, social cooperation, and moral cognition. ToM (the ability to infer the beliefs, intentions, and mental states of others) is classically impaired in bvFTD [58]. Several studies in bvFTD have reported impairment on the ability to understand sarcasm [59], as well as on ToM on tests such as “reading the mind in the eyes” and “faux pas” [60, 61]. Concerning recognition of emotions, patients with bvFTD are severely impaired on the task of identifying the facial expressions of basic emotions including happiness, sadness, disgust, fear, surprise, and anger [62]. It has been described that negative emotions seem to be more affected, specifically fear, sadness, anger, and disgust [63–66]. Moreover, patients with bvFTD show reduced empathic concern when observing others in pain, which has been associated with grey matter atrophy in the orbitofrontal cortex [67,68], along with abnormal moral judgments [69, 70].
In AD, failures of social cognition have also been found, but they seem to be less pronounced than in bvFTD. Social cognition decline is related with the progression of the disease and secondary to more global cognitive deficits and other cognitive domains such as memory, attention, and executive function [71]. A recent study shows that more than half of ToM variance in AD can be explained by executive function and attention impairment as well as memory deficits, which was in stark contrast with what was observed in bvFTD, where ToM emerged largely independent of executive performance as well as general cognition and memory processing [71]. Bora et al. concluded in a meta-analysis study that individuals with bvFTD exhibited significantly poorer performance across both facial emotion recognition and ToM tasks compared to those with AD, despite the presence of worse MMSE performance in the AD group [72]. For ToM tasks, they suggested that faux pas and sarcasm tests have the greatest discriminatory potential between bvFTD and AD (Table 1), whereas false belief tasks are less reliable in distinguishing both pathologies. In this regard, another study concluded that social cognition assessment is currently one of the best cognitive domains to discriminate AD from bvFTD clinically, even when either condition presents with severe amnesia [73]. Furthermore, Gossinketal. [74] found that Ekman 60 Faces test can distinguish bvFTD successfully from other neurodegenerative diseases and psychiatric disorders. Those social cognition deficits also appear to influence other aspects of cognition, such as social status influence on decision making [75] and emotional enhancement of memory [76–78].
Visuospatial function
Broadly defined, the visuospatial function is the ability to specify the parts and overall configuration of a percept, appreciate its position in space, integrate a coherent spatial framework, and perform mental operations on spatial concepts [79]. Visuospatial dysfunction is among the earliest manifestations of AD, eventually affecting 20%−43% of patients [80], where the main changes occur in medial and lateral parietal lobe structure [79, 81]. In contrast, visuospatial abilities appear to be relatively preserved in the early stages of bvFTD, likely explained by the relative sparing of posterior brain structures by the disease [79, 82, 83]. Consequently, tests of visuospatial abilities may prove to be more accurate in differentiating AD and bvFTD than other cognitive tests [79].
The visuospatial function is commonly conceptualized in three components: visual perception, construction, and visual memory. To assess visual perception, the Visual Object and Space Perception Battery (VOSP) [84] may provide a great opportunity to evaluate this domain, independent of language and motor function [79]; however, it is time consuming and its performance requires intact attention, which is often compromised in AD [79]. Although evidence is limited, the “cube analysis” subtest may be helpful in discriminating AD from bvFTD, especially when the disease severity is controlled [85, 86].
To assess construction abilities, the drawing tasks, such as the Clock Drawing Test and the Rey-Osterrieth Complex Figure (ROCF) [87] test, are commonly used, although they do not appear to differentiate between bvFTD and AD because of the higher interference of executive function, attention and motor skills in this ability [79].
Lastly, there are some test widely used to assess visual memory, such as the delayed recall component of the ROCF (after 3 or 45 min) and the Benton Visual Retention Test [88], but the first one is subject to the same confounds as the copy component and impulsivity may impair the performance of the second one [79]. A recent meta-analysis study suggested that topographical memory tasks may be able to distinguish AD from bvFTD in a clinical setting [79]. In this line, a further study suggests that spatial orientation and visual memory assessed by a novel virtual supermarket task allows to discriminate AD and bvFTD patients in an early stage, where the retrosplenial cortex emerges as a critical biomarker to assess spatial orientation deficits in AD [89].
Language
Language impairment in AD primarily occurs because of a decline in semantic and pragmatic levels of language processing [90]. Some problems such as naming disorders, impaired auditory and written comprehension, fluent but empty speech, and semantic paraphasia are typical language deficits in AD; however, repetition abilities and articulation remain relatively intact [91]. In the EOAD, language impairment involves lexical retrieval problems, loss of verbal fluency, and breakdown in the comprehension of higher-order written and spoken languages [90].
Even if though language in bvFTD is initially spared, some patients with this form of dementia may present difficulties to name action words. Such a deficit has been shown to be associated with executive abilities and may not reflect defective verb processing [41]. Alternatively, in relation to their apathy, patients with bvFTD may not participate in communication, and thus may be perceived with a reduced spontaneous speech output. A further major point to consider is that social and emotional aspects of speech may be impaired in bvFTD, with an inability to understand the subtleties and context of conversations [92]. Fluency may also be helpful in differentiating both disorders. While semantic (category) fluency is usually impaired to a greater degree in bdAD, phonemic (letter) fluency is more affected in bvFTD [91, 92]. Interestingly, the use of profanity during verbal fluency testing has been shown to be more suggestive of bvFTD than tAD or bdAD [92].
Neuropsychiatric features
Neuropsychiatric symptoms are common in the course of dementia [93, 94]. They can be present at prodromal stages of dementia, such as mild cognitive impairment, and may even increase the conversion risk to dementia [95]. Additionally, a mild behavioral impairment (MBI) may be considered as an early manifestation of a neurodegenerative disease such as bvFTD, when this is not explained by a previous psychiatric condition. A few years ago, Taragano et al. [96] and Ismail et al. [95] revised the MBI criteria, which determined that behavioral changes have to persist for at least 6 months to be considered as MBI. The MBI criteria features were divided into five subcategories: motivation, affective regulation, impulse control, social cognition, and perception/thought content, that may help detect the early onset of dementia such as bvFTD. A checklist with the core features of MBI has been developed to facilitate diagnosis [97].
Patients with bvFTD frequently present with psychiatric symptoms at onset or during the course of their illness [31]. Distinguishing behavioral features from primary psychiatric disorders, such as major depression, bipolar disorder, obsessive-compulsive disorder, autism spectrum disorders, and schizophrenia, remain challenging [98].
To date, apathy and agitation/aggression have been reported in both AD and bvFTD. Nonetheless, some neuropsychiatric symptoms have been more associated with each one. For AD, it has been described that patients may experience a higher frequency of depression, a situation that has not been linked to bvFTD [99–101]. In fact, in bvFTD, it is unusual for patients to complain of sadness, despair, or anxiety or indeed to acknowledge any suffering or handicap due to their lack of insight [31].
Regarding bvFTD, the principal behavioral symptoms include a disruptive change in personality characterized by impulsiveness, disinhibited behavior, inappropriateness, and a combination of obsessions/compulsions and ritualistic acts, collecting or hoarding, hyperorality, and utilization behaviors [29, 102]. In a recent study, criminal behavior was shown to be recurrent in patients with bvFTD compared to patients with AD, and delinquency was considered an early manifestation of FTD disorder [103]
When comparing bvFTD with tAD and bdAD, bvFTD patients’ eating habits may be significantly altered, with hyperphagia, food rituals, and a tendency to seek out and eat one type of food, such as high-carbohydrate meals (e.g., ‘sweet tooth’), with resultant weight gain. This contrasts with the typical weight loss of bdAD patients, which is more related to depression [92]. Moreover, several studies have reported that personality and behavioral changes, including loss of empathy, are more prevalent and severe in bvFTD [25, 31, 92] and these symptoms are more related to caregiver’s burden [25, 31, 92]. It is important to highlight that recognizing behavioral changes may not be obvious, as dementia tends to insidiously exaggerate pre-morbid personality traits. Whereas irritability, depression, and emotional lability may be more common in AD, constricted affect and apathy are more typical in bvFTD [92].
As mentioned above, bvFTD affects multiple levels of social behavior, and some characteristics that occur in daily life might not appear in the clinical and neuropsychological assessment, such as a decrease in conventional tact and poor manners when reaching for items during meals, appropriately excusing themselves when necessary, engaging in eating off tablemates’ plates or uncouthly eating with their hands [92, 104]. Moreover, it is not uncommon for patients with FTD to have problems at work, lose their jobs, and cause financial instability in the household [12]. In the same line, there is social awkwardness, tactlessness, decreased propriety and manners, disagreeableness, and absence of “you” statements or questions during conversation. Those characteristics are neither present in tAD or bdAD [104]. Table 2 summarizes the core differences between these diseases.
Table 2.
Comparison of the core neuropsychiatric and behavioral changes present in behavioral variant frontotemportal dementia (bvFTD), typical AD (tAD), and behavioral/dysexecutive variant of AD (bdAD)
| Neuropsychiatric Symptom |
tAD | bdAD | bvFTD |
|---|---|---|---|
| Depression | ++++ | ++ | + |
| Disinhibition | ++ | +++ | +++++ |
| Apathy | +++ | ++++ | +++++ |
| Loss of Empathy | + | +++ | +++++ |
| Perseverative | ** | +++ | ++++ |
| Hyperorality | + | ++ | +++++ |
| Irritability | ** | ++++ | ++ |
| Lack of awareness | ++ | +++ | +++++ |
| Agitation/aggression | **** | +++ | ++ |
| Delusions | ** | + | |
Appears in advanced stages of dementia.
Several informant-based scales have been developed in order to assess neuropsychiatric symptoms. The Neuropsychiatric Inventory Questionnaire [105, 106] aims to identify the presence of hallucinations, delusions, depression, apathy, and other signs that occur in both pathologies. The Cambridge Behavioral Inventory [107] is used to assess behavioral and cognitive changes along with daily life activities as well. The Frontal Systems Behavioural Scale [108] evaluates executive dysfunction, which may facilitate the differential diagnosis in AD and FTD. Another useful diagnostic test is the Frontal Behavioral Inventory, which is sensitive in differentiating between FTD and AD [109]. Finally, the Frontotemporal Rating Scale is a dementia staging tool based on clinical judgement aimed at assessing changes on neuropsychiatric symptoms and performance on ADL. This scale is useful to distinguish rates of disease progression in different subtypes of FTD and AD [110].
Functional impairment in the activities of daily living
The diagnostic guidelines for both diseases emphasize the requirement for the presence of a disabling functional impairment on the activities of daily living (ADL). In both pathologies, instrumental ADL (IADL) are affected earlier than basic ADL (BADL). Complex IADL, such as travel or employment, are affected in the mild stage of AD and bvFTD [111], whereas BADLs, such as personal hygiene and grooming remain intact. Classical progression in bvFTD and AD is characterized by a decline in everyday life abilities, progressively affecting less complex IADL and BADL [112]. Functional impairment in bvFTD is greater and decline faster than that observed in AD and is associated with involvement of different neural correlates [113]. Studies with the Disability Assessment Scale revealed that impairment in functionality affects differentially the three components of performance, e.g., planning, initiation, and execution. In both AD and bvFTD, there is no simple relationship between the degree of cognitive and functional impairment, suggesting that functional impairment could be also associated with another dimension of the dementia syndrome, such as social and behavioral disturbance [112]. Conversely, in a recent study, Amanzio et al. found that the disabilities in IADL are associated with reduced awareness of their own deficits in bvFTD patients, which are related to medial prefrontal cortex atrophy, where the mid-cingulate cortices, dorsal anterior insula, and cuneus play an important role [114]. In turn, patients with EOAD, especially the autosomal dominant variants, had a more aggressive course and non-cognitive neurological symptoms, such as myoclonus, seizures, hallucinations (PSEN1 and PSEN2 mutation), as well as hemorrhage, stroke-like episodes, leukoen-cephalopathy, and even cortical calcification (APP mutation), which also affects their functionality of ADL [115]. In addition, a comparative study found that bvFTD patients had their IADL more impaired than AD patients, most especially in activities related to bills, shopping, taxes, meal preparation, and hobbies [116].
Caregiver burden
As in all types of dementia, caregiver burden is also present in AD and bvFTD, which is especially associated with neuropsychiatric symptoms. In this respect, some studies have found that bvFTD caregivers reported higher general burden than those who care for AD patients [117, 118]. In fact, behavioral changes rather than the level of disability appeared to be correlated with caregiver distress and burden in bvFTD [119]. Furthermore, bvFTD caregivers were less satisfied with the patient as a care recipient and with themselves as a caregiver [118]. In the same line, Wong et al. have found that bvFTD caregivers experience greater strain and distress, more depressive symptoms, and lower perceived control than EOAD caregivers [120]. Additionally, Mioshi et al. [121] have found a strong relationship between depression and stress in bvFTD caregivers, where despite other studies, caregiver burden is not explained by the neuropsychiatric symptoms or the functional disability, but by depression symptoms and other characteristics relative to the caregiver itself, where bvFTD caregivers were also significantly more depressed than AD caregivers [121].
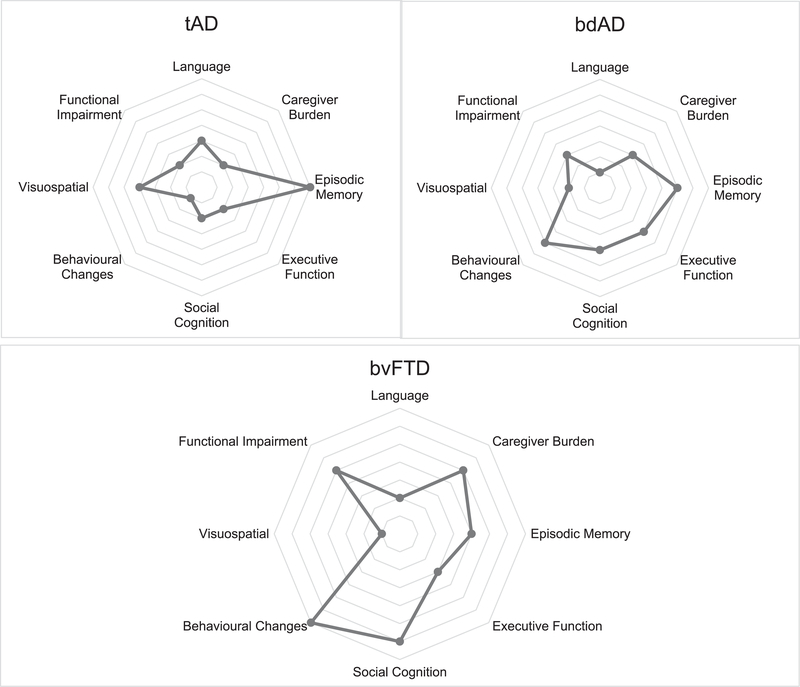
As a summary, Fig. 1 shows a comparative profile of the clinical features explained above, that could help for the differential diagnosis between tAD, bdAD, and bvFTD.
Fig. 1.
Neuropsychological and clinical signatures of tAD, bdAD, and bvFTD. Each point of the target shows a particular cognitive or clinical domain. The distance along the radial dimension indicates the level of impaired or decline function. Loss of function is indicated by a major distance from the central point, which corresponds to the cognitive/clinical domain in reference. The neuropsychological profile of a particular disease is evident in the pattern of decline of cognitive and clinical features: the differential loss of function across cognitive domains.
DIAGNOSTIC FORMULATION
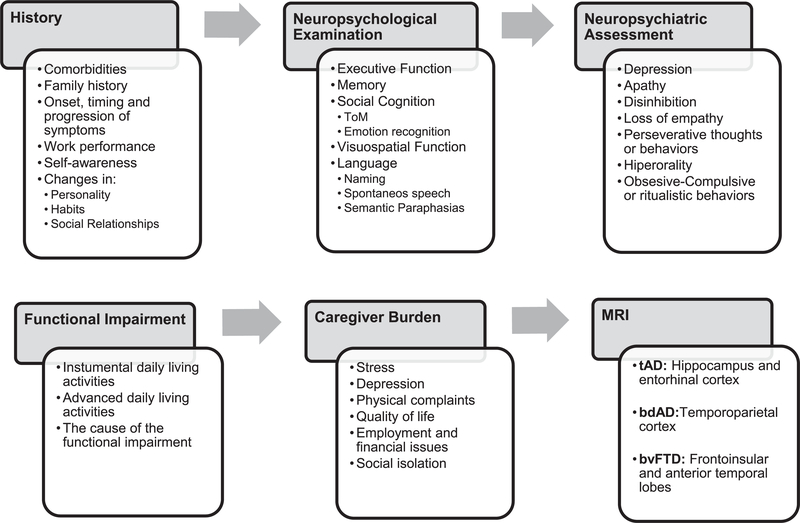
We propose starting the diagnostic process with extensive history-taking and neuropsychological testing, as well as the assessment of functionality on IADL and caregiver burden, followed by a brain MRI scan, if it is possible. The objective remains to detect the core cognitive and behavioral symptoms, functionality on ADL and integrated them in a probabilistic model to determine the most likely neuropathology underlying the clinical syndrome to facilitate the differential diagnosis.
Considering the foregoing, it is important to note the core symptoms that could contribute to the differential diagnosis and which of them usually occur simultaneously across the diseases (Figs. 1 and 2; Table 2) as well as the instruments proposed for their accurate assessment (Table 1). In this regard, it seems that both memory and executive function can be impaired in AD and bvFTD, with differences in their severity; however, the involvement of memory and executive function cannot be always useful for the differentiation of both diseases because of their high overlap. On the contrary, deficits in social cognition, visual orientation, semantic versus verbal fluency as well as lack of insight and behavioral symptoms such as hyperorality, personality changes, loss of empathy, the absence or presence of depression, the rate of progression on their functional impairment and the consecutive caregiver burden in the early stages of the disease can help to differentiate tAD and bdAD from bvFTD and guide the respective clinical picture into a more certain diagnostic formulation (Fig. 2).
Fig. 2.
Summary of the recommended key points to be assessed in each stage of the clinical examination.
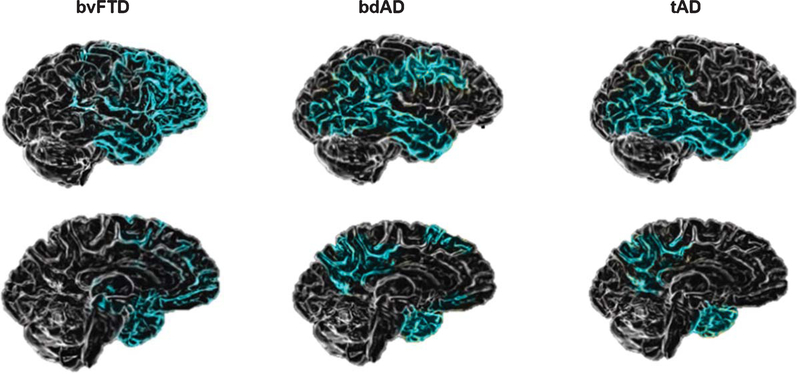
Ossenkoppele et al. [25] suggest that the key diagnostic features to distinguish bdAD from bvFTD are the magnitude of memory impairment and brain atrophy pattern, were they differentiate two groups, wherein bvFTD patients showed characteristic atrophy in anterior brain regions and the bdAD showed a classical AD pattern involving wide regions of the temporoparietal cortex (Figs. 2 and 3).
Fig. 3.
Patterns of brain atrophy in bvFTD, bdAD, and tAD. Image modified from Elahi and Miller, 2017 [14]. The brain images show patterns of atrophy on structural neuroimaging observed across the different clinical syndromes. In bvFTD, the main atrophy is localized into right frontal structures. In the bdAD, voxel-based morphometric studies reveal temporoparietal atrophy with relative preservation of frontal grey matter. In tAD atrophy is first noted in the medial temporal lobes and gradually spreads toward broader temporoparietal and posterior cingulate cortices.
DISCUSSION AND CONCLUSION
The differential diagnosis of bvFTD and AD is often challenging given the variety of clinical presentations. To date, it is not possible to determine the clinical profile of the patients based only on a specific neuropsychological tool. In contrast, a combination of different instruments including measures of executive function and social cognition, neuropsychiatric symptoms, caregiver burden and functional impairment in daily life will help recognize patients with AD and bvFTD. Moreover, there is a lack of research aimed at studying the clinical differentiation between tAD and bdAD. As a consequence, although the respective discriminability of most of the cognitive tests and scales mentioned in this review may be inaccurate, they can still be helpful to distinguish tAD and bdAD from bvFTD. Current evidence has demonstrated that patients with AD might manifest behavioral changes, being generally less marked than bvFTD [122]. In turn, bvFTD patients might present significant amnestic disorder even at early stages of disease. In the same line, executive function is neither particularly nor exclusively impaired. These findings lead to consider the impairment of other cognitive domains apart from memory and executive function, such as visuospatial function, language, and social cognition.
The main novelty of this review is to offer a comparative clinical overview that may aid the differential diagnosis between AD and bvFTD. This contrast can be particularly useful for clinical settings from low-and middle-income Latin American countries, where there is an unfortunate limited access to neuroimaging and biomarker techniques [22].
Moreover, there is a need for widening transcultural research in dementia with the purpose of exploring the interlink between the main symptoms and cultural factors. Indeed, the detection of behavioral changes is likely to vary according to the culture in reference [123], as well as the performance in different cognitive tests. In addition, while most of the tests and scales revised here have been validated in some Latin American countries such as Argentina, Brazil, Chile, Colombia, Cuba, México, and Peru, the cultural differences and presence of aboriginal communities variability across the level of educations of the countries of Latin America pose a major challenge for timely diagnosis [22]. Hence, the adaptation and validation of the different cognitive tests to the local culture is required in order to make an accurate diagnosis.
As explained before, it is important to reduce diagnosis time in YOD, especially when it comes to differentiating FTD and AD, since there are different prognoses for each disease, as along with distinct medical, psychological, and social needs for the patients and their caregivers. Above all, it is necessary to consider that it is exhausting and difficult for families to wait for a final diagnosis and face an uncertain future since the progression of dementia in YOD patients appears to be highly variable [74]. Thus, tailored plans and treatment options can mitigate suffering, reduce and control neuropsychiatric symptoms to improve patients’ quality of life as well as for the people who live in their surroundings and care for them [12].
In other words, considering that the patients with YOD are often employed, many of them might be the main earners among the members of their families and will often have parenting roles. Appropriate early diagnosis and support can enable patients not only many to continue participating and contributing to society, but also to postpone their institutionalization and reduce the associated caregiver burden [5]. This is an important aspect for offering nonpharmacological treatment for patients and contributing to savings for their families and the community, which can result in fewer patients residing in costly nursing homes.
Given the inherent complexity of dementia syndromes, a better knowledge on their clinical profiles might be one of the main factors that may shape the correct diagnosis [124]. As a matter of fact, a lack of specialists in mental health for the elderly, limited training and low confidence of physicians contributes to dementia underdiagnosis [124, 125]. For this reason, there is an important need for training across different levels of the health care system, particularly at the primary care level. Also, qualified staff and investment in infrastructure and technology will be vital to achieve earlier diagnosis and more effective future therapeutic interventions. In the same way, although there is local awareness regarding the importance of harmonizing diagnostic procedures in some Latin American countries, this has not reached a regional level yet [22].
Finally, multiple factors such as resources, culture, language, and stigmas affect the accurate diagnosis [22, 124]. New challenges need to be integrated into this matter, in order to contribute to better comprehension and knowledge about dementia and their different types, especially in public health centers.
ACKNOWLEDGMENTS
AS, PL, and GM are supported by CONICYT / FONDECYT / 1160940, CONICYT / FONDAP /15150012, AS is partially supported by CONICYT/ FONDEF/ID18I10113. GM and CMN are sponsored by the programme of scholarships ‘Becas Chile’ from CONICYT. AI is partially supported by grants from CONICET, CONICYT/FONDECYT Regular (1170010 and 1171200), FONCyT-PICT 2017-1818, FONCyT-PICT 2017-1820, FONDAP 15150012, INECO Foundation, the Interamerican Development Bank (IDB), GBHI ALZ UK-20-639295, and R01 AG057234.
Footnotes
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/19-0924r2).
REFERENCES
- [1].Prince MJ, Wimo A, Guerchet MM, Ali GC, Wu Y-T, Prina M (2015) World Alzheimer Report 2015 The global impact of dementia: An analysis of prevalence, incidence, cost and trends. Alzheimer’s Disease International, London. [Google Scholar]
- [2].Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP (2013) The global prevalence of dementia: A systematic review and metaanalysis. Alzheimers Dement 9,63–75 e62. [DOI] [PubMed] [Google Scholar]
- [3].Baez S, Ibanez A (2016) Dementia in Latin America: An emergent silent tsunami. Front Aging Neurosci 8, 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wortmann M (2012) Dementia: A global health priority-highlights from an ADI and World Health Organization report. Alzheimers Res Ther 4, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rossor MN, Fox NC, Mummery CJ, Schott JM, Warren JD (2010) The diagnosis of young-onset dementia. Lancet Neurol 9, 793–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Venkataraman AV, Perry RJ, Malhotra PA (2018) Young onset dementia In Reference Module in Neuroscience and Biobehavioral Psychology. Elsevier. [Google Scholar]
- [7].Gerritsen AAJ, Bakker C, Verhey FRJ, Bor H, Pijnenburg YAL, de Vugt ME, Koopmans R (2018) The progression of dementia and cognitive decline in a Dutch 2-year cohort study of people with young-onset dementia. J Alzheimers Dis 63,343–351. [DOI] [PubMed] [Google Scholar]
- [8].Gerritsen AAJ, Bakker C, Verhey FRJ, Pijnenburg YAL, Millenaar JK, de Vugt ME, Koopmans R (2019) Survival and life-expectancy in a young-onset dementia cohort with six years of follow-up: The NeedYD-study. Int Psychogeriatr, doi: 10.1017/S1041610219000152 [DOI] [PubMed] [Google Scholar]
- [9].Millenaar JK, de Vugt ME, Bakker C, van Vliet D, Pijnenburg YA, Koopmans RT, Verhey FR (2016) The impact of young onset dementia on informal caregivers compared with late onset dementia: Results fromthe NeedYD Study. Am J Geriatr Psychiatry 24, 467–474. [DOI] [PubMed] [Google Scholar]
- [10].van Vliet D, de Vugt ME, Bakker C, Pijnenburg YA, Vernooij-Dassen MJ, Koopmans RT, Verhey FR (2013) Time to diagnosis in young-onset dementia as compared with late-onset dementia. Psychol Med 43, 423–432. [DOI] [PubMed] [Google Scholar]
- [11].Karageorgiou E, Miller BL (2014) Frontotemporal lobar degeneration: A clinical approach. Semin Neurol 34, 189–201. [DOI] [PubMed] [Google Scholar]
- [12].Rosness TA, Engedal K, Chemali Z (2016) Frontotemporal dementia: An updated clinician’s guide. J Geriatr Psychiatry Neurol 29, 271–280. [DOI] [PubMed] [Google Scholar]
- [13].Onyike CU, Diehl-Schmid J (2013) The epidemiology of frontotemporal dementia. Int Rev Psychiatry 25, 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Elahi FM, Miller BL (2017) A clinicopathological approach to the diagnosis of dementia. Nat Rev Neurol 13, 457–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Steketee R (2016) Advanced MR Neuroimaging in early stage presenile dementia (thesis). Erasmus University Rotterdam. [Google Scholar]
- [16].Tellechea P, Pujol N, Esteve-Belloch P, Echeveste B, Garcia-Eulate MR, Arbizu J, Riverol M (2018) Early-and late-onset Alzheimer disease: Are they the same entity? Neurologia 33, 244–253. [DOI] [PubMed] [Google Scholar]
- [17].Bertoux M, Ramanan S, Slachevsky A, Wong S, Henriquez F, Musa G, Delgado C, Flanagan E, Bottlaender M, Sarazin M, Hornberger M, Dubois B (2016) So close yet so far: Executive contribution to memory processing in behavioral variant frontotemporal dementia. J Alzheimers Dis 54, 1005–1014. [DOI] [PubMed] [Google Scholar]
- [18].Pennington C, Hodges JR, Hornberger M (2011) Neural correlates of episodic memory in behavioral variant frontotemporal dementia. J Alzheimers Dis 24, 261–268. [DOI] [PubMed] [Google Scholar]
- [19].Draper B, Cations M, White F, Trollor J, Loy C, Brodaty H, Sachdev P, Gonski P, Demirkol A, Cumming RG, With-all A (2016) Time to diagnosis in young-onset dementia and its determinants: The INSPIRED study. Int J Geriatr Psychiatry 31, 1217–1224. [DOI] [PubMed] [Google Scholar]
- [20].Rasmussen H, Hellzen O, Stordal E, Enmarker I (2019) Family caregivers experiences of the pre-diagnostic stage in frontotemporal dementia. Geriatr Nurs 40, 246–251. [DOI] [PubMed] [Google Scholar]
- [21].Custodio N, Wheelock A, Thumala D, Slachevsky A (2017) Dementia in Latin America: Epidemiological evidence and implications for public policy. Front Aging Neurosci 9, 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Parra MA, Baez S, Allegri R, Nitrini R, Lopera F, Slachevsky A, Custodio N, Lira D, Piguet O, Kumfor F, Huepe D, Cogram P, Bak T, Manes F, Ibanez A (2018) Dementia in Latin America: Assessing the present and envisioning the future. Neurology 90, 222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr., Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH (2011) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mendez MF (2017) Early-onset Alzheimer disease. Neurol Clin 35, 263–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ossenkoppele R, Pijnenburg YA, Perry DC, Cohn-Sheehy BI, Scheltens NM, Vogel JW, Kramer JH, van der Vlies AE, La Joie R, Rosen HJ, van der Flier WM, Grinberg LT, Rozemuller AJ, Huang EJ, van Berckel BN, Miller BL, Barkhof F, Jagust WJ, Scheltens P, Seeley WW, Rabinovici GD (2015) The behavioural/dysexecutive variant of Alzheimer’s disease: Clinical, neuroimaging and pathological features. Brain 138, 2732–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Palasi A, Gutierrez-Iglesias B, Alegret M, Pujadas F, Olabarrieta M, Liebana D, Quintana M, Alvarez-Sabin J, Boada M (2015) Differentiated clinical presentation of early and late-onset Alzheimer’s disease: Is 65 years of age providing a reliable threshold? J Neurol 262, 1238–1246. [DOI] [PubMed] [Google Scholar]
- [27].Joubert S, Gour N, Guedj E, Didic M, Gueriot C, Koric L, Ranjeva JP, Felician O, Guye M, Ceccaldi M (2016) Early-onset and late-onset Alzheimer’s disease are associated with distinct patterns of memory impairment. Cortex 74, 217–232. [DOI] [PubMed] [Google Scholar]
- [28].Panegyres PK, Chen HY (2013) Differences between early and late onset Alzheimer’s disease. Am J Neurodegener Dis 2, 300–306. [PMC free article] [PubMed] [Google Scholar]
- [29].Piguet O, Hornberger M, Mioshi E, Hodges JR (2011) Behavioural-variant frontotemporal dementia: Diagnosis, clinical staging, and management. Lancet Neurol 10, 162–172. [DOI] [PubMed] [Google Scholar]
- [30].Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EG, Onyike CU, Hillis AE, Josephs KA, Boeve BF, Kertesz A, Seeley WW, Rankin KP, Johnson JK, Gorno-Tempini ML, Rosen H, Prioleau-Latham CE, Lee A, Kipps CM, Lillo P, Piguet O, Rohrer JD, Rossor MN, Warren JD, Fox NC, Galasko D, Salmon DP, Black SE, Mesulam M, Weintraub S, Dickerson BC, Diehl-Schmid J, Pasquier F, Deramecourt V, Lebert F, Pijnenburg Y, Chow TW, Manes F, Grafman J, Cappa SF, Freedman M, Grossman M, Miller BL (2011) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134, 2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ducharme S, Dickerson BC (2015) The neuropsychiatric examination of the young-onset dementias. Psychiatr Clin North Am 38, 249–264. [DOI] [PubMed] [Google Scholar]
- [32].Folstein MF, Folstein SE, McHugh PR (1975) Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12, 189–198. [DOI] [PubMed] [Google Scholar]
- [33].Villarejo A, Puertas-Martin V (2011) [Usefulness of short tests in dementia screening]. Neurologia 26, 425–433. [DOI] [PubMed] [Google Scholar]
- [34].Mioshi E, Dawson K, Mitchell J, Arnold R, Hodges JR (2006) The Addenbrooke’s Cognitive Examination Revised (ACE-R): A brief cognitive test battery for dementia screening. Int J Geriatr Psychiatry 21, 1078–1085. [DOI] [PubMed] [Google Scholar]
- [35].Hsieh S, Schubert S, Hoon C, Mioshi E, Hodges JR (2013) Validation of the Addenbrooke’s Cognitive Examination III in frontotemporal dementia and Alzheimer’s disease. Dement Geriatr Cogn Disord 36, 242–250. [DOI] [PubMed] [Google Scholar]
- [36].Dubois B, Slachevsky A, Litvan I, Pillon B (2000) The FAB: A Frontal Assessment Battery at bedside. Neurology 55, 1621–1626. [DOI] [PubMed] [Google Scholar]
- [37].Torralva T, Roca M, Gleichgerrcht E, Lopez P, Manes F (2009) INECO Frontal Screening (IFS):A brief, sensitive, and specific tool to assess executive functions in dementia. J Int Neuropsychol Soc 15, 777–786. [DOI] [PubMed] [Google Scholar]
- [38].Gleichgerrcht E, Roca M, Manes F, Torralva T (2011) Comparing the clinical usefulness of the Institute of Cognitive Neurology (INECO) Frontal Screening (IFS) and the Frontal Assessment Battery (FAB) in frontotemporal dementia. J Clin Exp Neuropsychol 33, 997–1004. [DOI] [PubMed] [Google Scholar]
- [39].Slachevsky A, Villalpando JM, Sarazin M, Hahn-Barma V, Pillon B, Dubois B (2004) Frontal assessment battery and differential diagnosis of frontotemporal dementia and Alzheimer disease. Arch Neurol 61, 1104–1107. [DOI] [PubMed] [Google Scholar]
- [40].Leslie FV, Foxe D, Daveson N, Flannagan E, Hodges JR, Piguet O (2016) FRONTIER Executive Screen: A brief executive battery to differentiate frontotemporal dementia and Alzheimer’s disease. J Neurol Neurosurg Psychiatry 87, 831–835. [DOI] [PubMed] [Google Scholar]
- [41].Harciarek M, Cosentino S (2013) Language, executive function and social cognition in the diagnosis of frontotemporal dementia syndromes. Int Rev Psychiatry 25, 178–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Reul S, Lohmann H, Wiendl H, Duning T, Johnen A (2017) Can cognitive assessment really discriminate early stages of Alzheimer’s and behavioural variant frontotemporal dementia at initial clinical presentation? Alzheimers Res Ther 9, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Stopford CL, Thompson JC, Neary D, Richardson AM, Snowden JS (2012) Working memory, attention, and executive function in Alzheimer’s disease and frontotemporal dementia. Cortex 48, 429–446. [DOI] [PubMed] [Google Scholar]
- [44].Franceschi M, Caffarra P, Savare R, Cerutti R, Grossi E, Tol Research G (2011) Tower of London test: A comparison between conventional statistic approach and modelling based on artificial neural network in differentiating fronto-temporal dementia from Alzheimer’s disease. Behav Neurol 24, 149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Harciarek M, Jodzio K (2005) Neuropsychological differences between frontotemporal dementia and Alzheimer’s disease: A review. Neuropsychol Rev 15, 131–145. [DOI] [PubMed] [Google Scholar]
- [46].Giovagnoli AR, Erbetta A, Reati F, Bugiani O (2008) Differential neuropsychological patterns of frontal variant frontotemporal dementia and Alzheimer’s disease in a study of diagnostic concordance. Neuropsychologia 46, 1495–1504. [DOI] [PubMed] [Google Scholar]
- [47].Baudic S, Barba GD, Thibaudet MC, Smagghe A, Remy P, Traykov L (2006) Executive function deficits in early Alzheimer’s disease and their relations with episodic memory. Arch Clin Neuropsychol 21, 15–21. [DOI] [PubMed] [Google Scholar]
- [48].Hornberger M, Piguet O, Kipps C, Hodges JR (2008) Executive function in progressive and nonprogressive behavioral variant frontotemporal dementia. Neurology 71, 1481–1488. [DOI] [PubMed] [Google Scholar]
- [49].Hornberger M, Savage S, Hsieh S, Mioshi E, Piguet O, Hodges JR (2010) Orbitofrontal dysfunction discriminates behavioral variant frontotemporal dementia from Alzheimer’s disease. Dement Geriatr Cogn Disord 30, 547–552. [DOI] [PubMed] [Google Scholar]
- [50].Delgado C, Munoz-Neira C, Soto A, Martinez M, Henriquez F, Flores P, Slachevsky A (2016) Comparison of the psychometric properties of the “word” and “picture” versions of the Free and Cued Selective Reminding Test in a Spanish-speaking cohort of patients with mild Alzheimer’s disease and cognitively healthy controls. Arch Clin Neuropsychol 31, 165–175. [DOI] [PubMed] [Google Scholar]
- [51].Petersen RC, Smith GE, Ivnik RJ, Kokmen E, Tangalos EG (1994) Memory function in very early Alzheimer’s disease. Neurology 44, 867–872. [DOI] [PubMed] [Google Scholar]
- [52].Grober E, Buschke H, Crystal H, Bang S, Dresner R(1988) Screening for dementia by memory testing. Neurology 38, 900–903. [DOI] [PubMed] [Google Scholar]
- [53].Sarazin M, Berr C, De Rotrou J, Fabrigoule C, Pasquier F, Legrain S, Michel B, Puel M, Volteau M, Touchon J, Verny M, Dubois B (2007) Amnestic syndrome of the medial temporal type identifies prodromal AD: A longitudinal study. Neurology 69, 1859–1867. [DOI] [PubMed] [Google Scholar]
- [54].Irish M, Piguet O, Hodges JR, Hornberger M (2014) Common and unique gray matter correlates of episodic memory dysfunction in frontotemporal dementia and Alzheimer’s disease. Hum Brain Mapp 35, 1422–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wong S, Bertoux M, Savage G, Hodges JR, Piguet O, Hornberger M (2016) Comparison of prefrontal atrophy and episodic memory performance in dysexecutive Alzheimer’s disease and behavioral-variant frontotemporal dementia. J Alzheimers Dis 51, 889–903. [DOI] [PubMed] [Google Scholar]
- [56].Kril JJ, Halliday GM (2011) Pathological staging of frontotemporal lobar degeneration. J Mol Neurosci 45, 379–383. [DOI] [PubMed] [Google Scholar]
- [57].Kumfor F, Honan C, McDonald S, Hazelton JL, Hodges JR, Piguet O (2017) Assessing the “social brain” in dementia: Applying TASIT-S. Cortex 93, 166–177. [DOI] [PubMed] [Google Scholar]
- [58].Le Bouc R, Lenfant P, Delbeuck X, Ravasi L, Lebert F, Semah F, Pasquier F (2012) My belief or yours? Differential theory of mind deficits in frontotemporal dementia and Alzheimer’s disease. Brain 135, 3026–3038. [DOI] [PubMed] [Google Scholar]
- [59].Kipps CM, Nestor PJ, Acosta-Cabronero J, Arnold R, Hodges JR (2009) Understanding social dysfunction in the behavioural variant of frontotemporal dementia: The role of emotion and sarcasm processing. Brain 132, 592–603. [DOI] [PubMed] [Google Scholar]
- [60].Gregory C, Lough S, Stone V, Erzinclioglu S, Martin L, Baron-Cohen S, Hodges JR (2002) Theory of mind in patients with frontal variant frontotemporal dementia and Alzheimer’s disease: Theoretical and practical implications. Brain 125, 752–764. [DOI] [PubMed] [Google Scholar]
- [61].Torralva T, Kipps CM, Hodges JR, Clark L, Bekinschtein T, Roca M, Calcagno ML, Manes F (2007) The relationship between affective decision-making and theory of mind in the frontal variant of fronto-temporal dementia. Neuropsychologia 45, 342–349. [DOI] [PubMed] [Google Scholar]
- [62].Diehl-Schmid J, Pohl C, Ruprecht C, Wagenpfeil S, Foerstl H, Kurz A (2007) The Ekman 60 Faces Test as a diagnostic instrument in frontotemporal dementia. Arch Clin Neuropsychol 22, 459–464. [DOI] [PubMed] [Google Scholar]
- [63].Fernandez-Duque D, Black SE (2005) Impaired recognition of negative facial emotions in patients with frontotemporal dementia. Neuropsychologia 43, 1673–1687. [DOI] [PubMed] [Google Scholar]
- [64].Kumfor F, Piguet O (2012) Disturbance of emotion processing in frontotemporal dementia: A synthesis of cognitive and neuroimaging findings. Neuropsychol Rev 22, 280–297. [DOI] [PubMed] [Google Scholar]
- [65].Kumfor F, Irish M, Hodges JR, Piguet O (2013) Discrete neural correlates for the recognition of negative emotions: Insights from frontotemporal dementia. PLoS One 8, e67457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Lough S, Kipps CM, Treise C, Watson P, Blair JR, Hodges JR (2006) Social reasoning, emotion and empathy in frontotemporal dementia. Neuropsychologia 44, 950–958. [DOI] [PubMed] [Google Scholar]
- [67].Baez S, Manes F, Huepe D, Torralva T, Fiorentino N, Richter F, Huepe-Artigas D, Ferrari J, Montanes P, Reyes P, Matallana D, Vigliecca NS, Decety J, Ibanez A (2014) Primary empathy deficits in frontotemporal dementia. Front Aging Neurosci 6, 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Baez S, Morales JP, Slachevsky A, Torralva T, Matus C, Manes F, Ibanez A (2016) Orbitofrontal and limbic signatures of empathic concern and intentional harm in the behavioral variant frontotemporal dementia. Cortex 75, 20–32. [DOI] [PubMed] [Google Scholar]
- [69].Baez S, Couto B, Torralva T, Sposato LA, Huepe D, Montanes P, Reyes P, Matallana D, Vigliecca NS, Slachevsky A, Manes F, Ibanez A (2014) Comparing moral judgments of patients with frontotemporal dementia and frontal stroke. JAMA Neurol 71, 1172–1176. [DOI] [PubMed] [Google Scholar]
- [70].Baez S, Kanske P, Matallana D, Montanes P, Reyes P, Slachevsky A, Matus C, Vigliecca NS, Torralva T, Manes F, Ibanez A (2016) Integration of intention and outcome for moral judgment in frontotemporal dementia: Brain structural signatures. Neurodegener Dis 16, 206–217. [DOI] [PubMed] [Google Scholar]
- [71].Ramanan S, de Souza LC, Moreau N, Sarazin M, Teixeira AL, Allen Z, Guimaraes HC, Caramelli P, Dubois B, Hornberger M, Bertoux M (2017) Determinants of theory of mind performance in Alzheimer’s disease: A data-mining study. Cortex 88, 8–18. [DOI] [PubMed] [Google Scholar]
- [72].Bora E, Walterfang M, Velakoulis D (2015) Theory of mind in behavioural-variant frontotemporal dementia and Alzheimer’s disease: A meta-analysis. J Neurol Neurosurg Psychiatry 86, 714–719. [DOI] [PubMed] [Google Scholar]
- [73].Bertoux M, de Souza LC, O’Callaghan C, Greve A, Sarazin M, Dubois B, Hornberger M (2016) Social cognition deficits: The key to discriminate behavioral variant frontotemporal dementia from Alzheimer’s disease regardless of amnesia? J Alzheimers Dis 49, 1065–1074. [DOI] [PubMed] [Google Scholar]
- [74].Gossink F, Schouws S, Krudop W, Scheltens P, Stek M, Pijnenburg Y, Dols A (2018) Social cognition differentiates behavioral variant frontotemporal dementia from other neurodegenerative diseases and psychiatric disorders. Am J Geriatr Psychiatry 26, 569–579. [DOI] [PubMed] [Google Scholar]
- [75].O’Callaghan C, Bertoux M, Irish M, Shine JM, Wong S, Spiliopoulos L, Hodges JR, Hornberger M (2016) Fair play: Social norm compliance failures in behavioural variant frontotemporal dementia. Brain 139, 204–216. [DOI] [PubMed] [Google Scholar]
- [76].Torralva T, Roca M, Gleichgerrcht E, Bekinschtein T, Manes F (2009) A neuropsychological battery to detect specific executive and social cognitive impairments in early frontotemporal dementia. Brain 132, 1299–1309. [DOI] [PubMed] [Google Scholar]
- [77].Kumfor F, Irish M, Hodges JR, Piguet O (2014) Frontal and temporal lobe contributions to emotional enhancement of memory in behavioral-variant frontotemporal dementia and Alzheimer’s disease. Front Behav Neurosci 8, 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Kumfor F, Irish M, Hodges JR, Piguet O (2013) The orbitofrontal cortex is involved in emotional enhancement of memory: Evidence from the dementias. Brain 136, 2992–3003. [DOI] [PubMed] [Google Scholar]
- [79].Salimi S, Irish M, Foxe D, Hodges JR, Piguet O, Burrell JR (2018) Can visuospatial measures improve the diagnosis of Alzheimer’s disease? Alzheimers Dement (Amst) 10, 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Quental NBM, Brucki SMD, Bueno OFA (2009) Visuospatial function in early Alzheimer’s disease: Preliminary study. Dement Neuropsychol 3, 234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Geldmacher DS (2003) Visuospatial dysfunction in the neurodegenerative diseases. Front Biosci 8, e428–436. [DOI] [PubMed] [Google Scholar]
- [82].Cronin-Golomb A (2011) Visuospatial function in Alzheimer’s disease and related disorders In The Handbook of Alzheimer’s Disease and Other Dementias, First Edition Budson AE, Kowall NW, eds. Blackwell Publishing Ltd, UK, pp. 457–482. [Google Scholar]
- [83].Weintraub S, Wicklund AH, Salmon DP (2012) The neuropsychological profile of Alzheimer disease. Cold Spring Harb Perspect Med 2, a006171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Warrington EK, James M (1991) The visual object and space perception battery, Pearson. [Google Scholar]
- [85].Siri S, Benaglio I, Frigerio A, Binetti G, Cappa SF (2001) A brief neuropsychological assessment for the differential diagnosis between frontotemporal dementia and Alzheimer’s disease. Eur J Neurol 8, 125–132. [DOI] [PubMed] [Google Scholar]
- [86].Salimi S, Irish M, Foxe D, Hodges JR, Piguet O, Burrell JR (2019) Visuospatial dysfunction in Alzheimer’s disease and behavioural variant frontotemporal dementia. J Neurol Sci 402, 74–80. [DOI] [PubMed] [Google Scholar]
- [87].Osterrieth PA (1944) Le test de copie d’une figure complexe. Arch Psychol 30, 206–356. [Google Scholar]
- [88].Benton AL (1992) Benton Visual Retention Test: Manual, Psychological Corporation. [Google Scholar]
- [89].Tu S, Wong S, Hodges JR, Irish M, Piguet O, Hornberger M (2015) Lost in spatial translation-A novel tool to objectively assess spatial disorientation in Alzheimer’s disease and frontotemporal dementia. Cortex 67, 83–94. [DOI] [PubMed] [Google Scholar]
- [90].Ferris SH, Farlow M (2013) Language impairment in Alzheimer’s disease and benefits of acetylcholinesterase inhibitors. Clin Interv Aging 8, 1007–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Szatloczki G, Hoffmann I, Vincze V, Kalman J, Pakaski M (2015) Speaking in Alzheimer’s disease, is that an early sign? Importance of changes in language abilities in Alzheimer’s disease. Front Aging Neurosci 7, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Sawyer RP, Rodriguez-Porcel F, Hagen M, Shatz R, Espay AJ (2017) Diagnosing the frontal variant of Alzheimer’s disease: A clinician’s yellow brickroad. J Clin Mov Disord 4, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Ford AH (2014) Neuropsychiatric aspects of dementia. Maturitas 79,209–215. [DOI] [PubMed] [Google Scholar]
- [94].Selbaek G, Engedal K, Benth JS, Bergh S (2014) The course of neuropsychiatric symptoms in nursing-home patients with dementia over a 53-month follow-up period. Int Psychogeriatr 26, 81–91. [DOI] [PubMed] [Google Scholar]
- [95].Ismail Z, Smith EE, Geda Y, Sultzer D, Brodaty H, Smith G, Aguera-Ortiz L, Sweet R, Miller D, Lyketsos CG, ISTAART Neuropsychiatric Symptoms Professional Interest Area (2016) Neuropsychiatric symptoms as early manifestations of emergent dementia: Provisional diagnostic criteria for mild behavioral impairment. Alzheimers Dement 12, 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Taragano FE, Allegri RF, Krupitzki H, Sarasola DR, Serrano CM, Lon L, Lyketsos CG (2009) Mild behavioral impairment and risk of dementia: A prospective cohort study of 358 patients. J Clin Psychiatry 70, 584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Ismail Z, Aguera-Ortiz L, Brodaty H, Cieslak A, Cummings J, Fischer CE, Gauthier S, Geda YE, Herrmann N, Kanji J, Lanctot KL, Miller DS, Mortby ME, Onyike CU, Rosenberg PB, Smith EE, Smith GS, Sultzer DL, Lyketsos C, NPS Professional Interest Area of the International Society of to Advance Alzheimer’s Research and Treatment (NPS-PIA of ISTAART) (2017) The Mild Behavioral Impairment Checklist (MBI-C): A rating scale for neuropsychiatric symptoms in pre-dementia populations. J Alzheimers Dis 56, 929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Flora G EV, Yolande P, Annemiek D (2018) Neuropsychiatry in clinical practice: The challenge of diagnosing behavioral variant frontotemporal dementia. J Neuropsychiatry 2, 4. [Google Scholar]
- [99].Echavarri C, Burgmans S, Uylings H, Cuesta MJ, Peralta V, Kamphorst W, Rozemuller AJ, Verhey FR (2013) Neuropsychiatric symptoms in Alzheimer’s disease and vascular dementia. J Alzheimers Dis 33, 715–721. [DOI] [PubMed] [Google Scholar]
- [100].Fernandez-Martinez M, Castro J, Molano A, Zarranz JJ, Rodrigo RM, Ortega R (2008) Prevalence of neuropsychiatric symptoms in Alzheimer’s disease and vascular dementia. Curr Alzheimer Res 5, 61–69. [DOI] [PubMed] [Google Scholar]
- [101].Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S (2002) Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: Results from the cardiovascular health study. JAMA 288, 1475–1483. [DOI] [PubMed] [Google Scholar]
- [102].Slachevsky A, Munoz-Neira C, Nunez-Huasaf J, Stern TA, Blesius CR, Atri A (2011) Late-onset cinephilia and compulsive behaviors: Harbingers of frontotemporal dementia. Prim Care Companion CNS Disord 13, PCC.10f01115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Liljegren M, Naasan G, Temlett J, Perry DC, Rankin KP, Merrilees J, Grinberg LT, Seeley WW, Englund E, Miller BL (2015) Criminal behavior in frontotemporal dementia and Alzheimer disease. JAMA Neurol 72, 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Mendez MF, Fong SS, Shapira JS, Jimenez EE, Kaiser NC, Kremen SA, Tsai PH (2014) Observation of social behavior in frontotemporal dementia. Am J Alzheimers Dis Other Demen 29, 215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, Lopez OL, DeKosky ST (2000) Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci 12, 233–239. [DOI] [PubMed] [Google Scholar]
- [106].Musa G, Henriquez F, Munoz-Neira C, Delgado C, Lillo P, Slachevsky A (2017) Utility of the Neuropsychiatric Inventory Questionnaire (NPI-Q) in the assessment of a sample of patients with Alzheimer’s disease in Chile. Dement Neuropsychol 11, 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Wedderburn C, Wear H, Brown J, Mason SJ, Barker RA, Hodges J, Williams-Gray C (2008) The utility of the Cambridge Behavioural Inventory in neurodegenerative disease. J Neurol Neurosurg Psychiatry 79, 500–503. [DOI] [PubMed] [Google Scholar]
- [108].Grace J, Stout JC, Malloy PF (1999) Assessing frontal lobe behavioral syndromes with the frontal lobe personality scale. Assessment 6, 269–284. [DOI] [PubMed] [Google Scholar]
- [109].Konstantinopoulou E, Aretouli E, Ioannidis P, Karacostas D, Kosmidis MH (2013) Behavioral disturbances differentiate frontotemporal lobar degeneration subtypes and Alzheimer’s disease: Evidence from the Frontal Behavioral Inventory. Int J Geriatr Psychiatry 28, 939–946. [DOI] [PubMed] [Google Scholar]
- [110].Mioshi E, Hsieh S, Savage S, Hornberger M, Hodges JR (2010) Clinical staging and disease progression in frontotemporal dementia. Neurology 74, 1591–1597. [DOI] [PubMed] [Google Scholar]
- [111].Wicklund AH, Johnson N, Rademaker A, Weitner BB, Weintraub S (2007) Profiles of decline in activities of daily living in non-Alzheimer dementia. Alzheimer Dis Assoc Disord 21, 8–13. [DOI] [PubMed] [Google Scholar]
- [112].Mioshi E, Kipps CM, Dawson K, Mitchell J, Graham A, Hodges JR (2007) Activities of daily living in frontotemporal dementia and Alzheimer disease. Neurology 68, 2077–2084. [DOI] [PubMed] [Google Scholar]
- [113].Mioshi E, Hodges JR, Hornberger M (2013) Neural correlates of activities of daily living in frontotemporal dementia. J Geriatr Psychiatry Neurol 26, 51–57. [DOI] [PubMed] [Google Scholar]
- [114].Amanzio M, D’Agata F, Palermo S, Rubino E, Zucca M, Galati A, Pinessi L, Castellano G, Rainero I (2016) Neural correlates of reduced awareness in instrumental activities of daily living in frontotemporal dementia. Exp Gerontol 83, 158–164. [DOI] [PubMed] [Google Scholar]
- [115].Wu L, Rosa-Neto P, Hsiung G-YR, Sadovnick AD, Masellis M, Black SE, Jia J, Gauthier S (2012) Early-onset familial Alzheimer’s disease (EOFAD). Can J Neurol Sci 39, 436–445. [DOI] [PubMed] [Google Scholar]
- [116].Giebel CME, Knopman DS, Khondoker M(2017) Decline in instrumental activities of daily living is more marked in behavioural variant frontotemporal dementia than in Alzheimer’s disease. Alzheimers Dement 13, 385. [Google Scholar]
- [117].Riedijk SR, De Vugt ME, Duivenvoorden HJ, Niermeijer MF, Van Swieten JC, Verhey FR, Tibben A (2006) Caregiver burden, health-related quality of life and coping in dementia caregivers: A comparison of frontotemporal dementia and Alzheimer’s disease. Dement Geriatr Cogn Disord 22, 405–412. [DOI] [PubMed] [Google Scholar]
- [118].de Vugt ME, Riedijk SR, Aalten P, Tibben A, van Swieten JC, Verhey FR (2006) Impact of behavioural problems on spousal caregivers: A comparison between Alzheimer’s disease and frontotemporal dementia. Dement Geriatr Cogn Disord 22, 35–41. [DOI] [PubMed] [Google Scholar]
- [119].Boutoleau-Bretonniere C, Vercelletto M, Volteau C, Renou P, Lamy E (2008) Zarit burden inventory and activities of daily living in the behavioral variant of frontotemporal dementia. Dement Geriatr Cogn Disord 25, 272–277. [DOI] [PubMed] [Google Scholar]
- [120].Wong C, Merrilees J, Ketelle R, Barton C, Wallhagen M, Miller B (2012) The experience of caregiving: Differences between behavioral variant of frontotemporal dementia and Alzheimer disease. Am J Geriatr Psychiatry 20, 724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Mioshi E, Bristow M, Cook R, Hodges JR (2009) Factors underlying caregiver stress in frontotemporal dementia and Alzheimer’s disease. Dement Geriatr Cogn Disord 27, 76–81. [DOI] [PubMed] [Google Scholar]
- [122].Blennerhassett R, Lillo P, Halliday GM, Hodges JR, Kril JJ (2014) Distribution of pathology in frontal variant Alzheimer’s disease. J Alzheimers Dis 39, 63–70. [DOI] [PubMed] [Google Scholar]
- [123].Papatriantafyllou JD, Viskontas IV, Papageorgiou SG, Miller BL, Pavlic D, Bingol A, Yener G (2009) Difficulties in detecting behavioral symptoms of frontotemporal lobar degeneration across cultures. Alzheimer Dis Assoc Disord 23, 77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Olavarria L, Mardones C, Delgado C, Slachevsky Ch A (2016) [Chilean healthcare professionals perception of knowledge about dementia]. Rev Med Chil 144, 1365–1368. [DOI] [PubMed] [Google Scholar]
- [125].Gleichgerrcht E, Flichtentrei D, Manes F (2011) How much do physicians in Latin America know about behavioral variant frontotemporal dementia? J Mol Neurosci 45, 609–617. [DOI] [PubMed] [Google Scholar]