Abstract
Aim:
To conduct an evidence synthesis of normative reference values for bladder function parameters in women.
Methods:
We conducted a systematic review and meta-analysis of studies reporting bladder function parameters obtained from non-invasive tests in healthy women. Seven databases were searched for relevant studies from inception through December 2018, with manual searching of reference lists. We included English language articles that provided quantitative data on urination frequency, voided and postvoid residual volumes, and uroflowmetry results in women without lower urinary tract symptoms. Study selection, data extraction, and quality assessment were undertaken by at least two independent reviewers. Random effects meta-analytic models were used to derive study-level pooled mean estimates and 95% confidence intervals.
Results:
A total of 24 studies (N=3,090 women, age range: 18-91 years) met eligibility criteria. Pooled mean estimates of bladder function parameters were: 6.6 daytime voids (95% CI 6.2, 7.0), 0.4 nighttime voids (95% CI 0.0, 0.8), 1577 ml for 24-hour voided volume (95% CI 1428,1725); 12 mL for post-void residual volume (95% CI 4, 20); and 28 mL/sec for maximum flow rate (95% CI 27,30). Between-study heterogeneity was high for all outcomes (I2 = 61.1-99.6%), but insufficient data were available to explore reasons for this high heterogeneity (e.g., differences by age).
Conclusion:
Although summary mean estimates of bladder function parameters were calculated, the wide heterogeneity across studies precludes generalization of these estimates to all healthy women. Further research is needed to determine normative reference values within specific groups, such as those defined by age.
Keywords: Voiding, urinary frequency, urinary volume, uroflowmetry, reference values
1. Introduction
Established in 2015, the mission of the Prevention of Lower Urinary Tract Symptoms (PLUS) Research Consortium is to identify promising approaches for promoting bladder health and preventing lower urinary tract symptoms (LUTS) and conditions in girls and women.1,2 Bladder health is defined as a complete state of physical, mental, and social well-being related to bladder function, and not merely the absence of LUTS.2 This definition highlights not only the importance of bladder function, but also the subjective experiences associated with well-being. Early work of the PLUS Research Consortium has focused on developing the concept of bladder health in the context of established LUTS (unhealthy bladder) terminology and proposing novel definitions by bladder function (storage, emptying, and bioregulatory).2,3 In addition to recognizing the need for healthy bladder terminology, the Consortium also recognized the importance of corresponding reference values for bladder function in asymptomatic or healthy women. Although extensive studies have been conducted on objective measures of bladder function in women with LUTS, limited normative data exist in women without LUTS, either by non-invasive or invasive testing. This information is important to help clinicians and women determine whether objective bladder function values are within “normal” limits or signify a possible bladder disorder such as overactive bladder that may need further evaluation and management.
Three prior literature reviews published in 20124,5 and 20166 examined normative bladder function reference value ranges in healthy or asymptomatic adult women using noninvasive or invasive tests. Because of the limited number of available studies at that time, data were qualitatively summarized in a narrative fashion, without the use of meta-analytic techniques to create summary estimates for various bladder function parameters. In addition, these reviews had methodological limitations including use of limited search strategies; lack of clear eligibility criteria related to inclusion of healthy or asymptomatic women; and the use of clinical data or anecdotal evidence to interpret the results. Since these reviews were published, several original studies have been published increasing the evidence regarding objective bladder function measures in women without LUTS. Therefore, the aim of this paper was to conduct a systematic review and meta-analysis to describe normative reference values of non-invasive bladder function tests assessing urination frequency, voided volume, postvoid residual volume, and uroflowmetry results in healthy or asymptomatic women. We focused on non-invasive bladder function tests as these tests are more generalizable to population-based research and can be used more practically in clinical practice.
2. Materials and Methods
2.1. Protocol and Registration
This systematic review and meta-analysis conform to the Preferred Reported Items for Systematic Reviews and Meta-analyses (PRISMA) Guidelines7 and the reporting of Meta-analysis of Observational Studies in Epidemiology.8 The review protocol was registered in PROSPERO, an international prospective register of systematic reviews (CRD420160498528).
2.2. Search Strategy
Informed by an initial scoping search of PubMed, a medical librarian searched published literature for records discussing bladder function measurements and reference values in healthy women from database inception through December 2018. The librarian created search strategies using a combination of keywords and controlled vocabulary in seven electronic databases: Ovid Medline 1946-, Embase 1947-, Scopus 1923-, EbscoHost CINAHL Plus 1937-, Cochrane Database of Systematic Reviews (CDSR), Cochrane Central Register of Controlled Trials (CENTRAL), Database of Abstracts of Reviews of Effects (DARE), NHS Economic Evaluation Database (EED), and Clinicaltrials.gov 1997- (see Appendix 1). Animal studies were excluded using the human filter recommended in the Cochrane Handbook for Systematic Reviews of Interventions.9 Search strategies were completed in February 2017 and executed again in December 2018, with a total of 12,515 results were exported to EndNote. Duplicates were removed using the automatic duplicate finder in EndNote. Reference lists from relevant articles and systematic reviews were searched manually, identifying six additional studies and resulting in a total of 7,571 unique references. Fully reproducible search strategies for each database can be found in Supplemental Table 1.
2.2. Eligibility Criteria
Studies included in this review and meta-analysis were required to meet the following criteria: 1) community-residing adult women (defined as inclusion criteria or mean/median age ≥18 years) without urological symptoms or disorders or defined as healthy volunteers; 2) non-invasive bladder function measurement; 3) observational studies (cross-sectional, case-control, or longitudinal studies; randomized controlled trials if at least one of the study arms met all inclusion criteria); and 4) full-text, peer-reviewed article published in an English language journal. Studies were excluded if they comprised: 1) women unselected with respect to LUTS (i.e., without consideration for their LUTS status); 2) pregnant women and women within first six months of postpartum; 3) women with urinary tract infections, urinary incontinence, overactive bladder, interstitial cystitis/bladder pain syndrome, pelvic organ prolapse, or congenital urinary tract abnormalities; and 4) individuals with cognitive or developmental disabilities, spinal cord injury, or progressive neurological conditions (e.g., dementia, multiple sclerosis, Parkinson’s disease). Studies were also excluded if they focused solely on individuals with diabetes, end-stage kidney disease or kidney transplants, individuals in active cancer treatment, those using catheters, hospitalized with acute illness or enrolled in a palliative care or hospice program; those residing in a supervised living facility; and those involving voiding positions with unnatural posture and/or straining (e.g., forward sitting position with straining). Studies including both sexes and/or clinical populations were included if they performed subgroup analyses for women meeting the above criteria. Only studies that provided outcomes with reported measures of central tendency and statistical dispersion were included in the meta-analysis.
2.3. Study Selection
Study selection was performed by applying eligibility criteria in three stages, following established guidelines for systematic reviews.9 Titles were assessed initially for eligibility. After removal of records meeting exclusion criteria, the remaining records were reviewed again for eligibility, first by abstract and then by full text article. At each stage, records were reviewed independently by a minimum of two reviewers. Disagreements were resolved by a third reviewer and reasons for exclusion were recorded. Studies included in the meta-analysis had to report a measure of central tendency with a standard deviation or standard error for at least one of the outcome variables (e.g., urination frequency, voided or postvoid volume, or uroflowmetry results).
2.3. Data Extraction
Two reviewers independently extracted data from each article using a specifically developed and piloted data extraction form using REDCap (Research Electronic Data Capture) electronic data capture tools9 hosted in the Washington University School of Medicine Institute for Informatics, Informatics Core Services. Data extracted from eligible articles included: study-level details (author, year, journal, country, and study design); study eligibility (age eligibility and description of sample as healthy, normal, or asymptomatic with definition); and patient characteristics (age, race, menopause status, body mass index, hysterectomy status, use of diuretics, tobacco smoking, and comorbidities). Outcomes and their method of measurement were also extracted and included: urination frequency (daytime, nighttime, 24-hour); urine volume (daytime, nighttime, and 24-hour volumes; mean, maximum, and minimum voided volumes; and postvoid residual volume), uroflowmetry parameters (maximum flow rate, mean flow rate, time to maximum flow, flow time, and voided volume); and pad test weights. Quality appraisal criteria were also included on the data extraction form. Extracted information was compared using REDCap to identify discrepancies between reviewers. All discrepancies were resolved through consensus or consultation with a third reviewer when needed.
2.4. Assessment of Methodological Quality
We were unable to identify a critical appraisal instrument for studies focused on normative reference values. Guided by the approach of Sorel et al.,6 in their review of uroflowmetry in healthy women, we created several questions to assess the methodological quality of the reference values from each study. Our modified instrument included two items evaluating the characteristics of the study sample and two evaluating the quality of data collection. Specifically, we assessed whether: women with LUTS or other urological disorders were explicitly excluded; and the age of the sample was precisely specified with mean or median and standard deviation or standard error. A precise measurement of age was used as a quality criterion for two reasons: age is a strong risk factor for LUTS, and bladder function may vary over the life course affecting normative reference values. We also assessed whether a standardized method was used to assess self-reported bladder function outcomes (e.g., bladder diary) and to measure non-invasive laboratory tests of bladder function (e.g., uroflowmetry, bladder ultrasonography, and pad tests). Two reviewers independently assessed these criteria using a rating scale of “yes,” “no,” “unclear,” and “not applicable.” Discordant responses were adjudicated by discussion with reviewers.
2.5. Data Synthesis and Analysis
Meta-analysis was performed using the R software version 3.4.3. Means and 95% confidence intervals (CIs) of bladder function parameters (urination frequency, voided and postvoid residual volumes, and uroflowmetry results) were computed using random-effects models. Forest plots were constructed for each bladder function parameter to examine and display study-level data. The I2 statistic was used to describe the percentage of the variation across studies that is due to between-study differences, rather than chance.10 Common cut-off points for low (I2 =25%), moderate (I2 =50%), and high degrees of heterogeneity (I2 =75% or higher) were used. For studies that reported bladder function parameters for multiple groups,12-14 pooled mean estimates and standard deviations were obtained via weighting by group sample size. The DerSimonian-Laird15 random effects method was used to compute the overall estimate of each bladder function parameter. In addition to 95% confidence intervals for the overall mean estimate of each bladder function parameter which explain how precisely the overall mean has been estimated across studies, we estimated 90% normative reference values for individual participants using the fixed-effects models under the log-normal distribution assumption. These normative reference values explain how widely bladder function parameters vary across individual participants, i.e., 90% of participants would have bladder function parameter values within the interval if the model assumptions were valid. With the exception of urination frequency, means and standard deviations of pooled estimates and normative reference values for voided and postvoid residual volumes and uroflowmetry results were rounded to integer numbers, as these numbers are more easily interpretable in clinical practice. Subgroup analyses to examine reasons for study heterogeneity could not be performed because of insufficient data reported on age, body mass index, fluid intake (amount or type), parity, hormonal status, medications, and medical comorbidities.
3. RESULTS
3.1. Study Identification
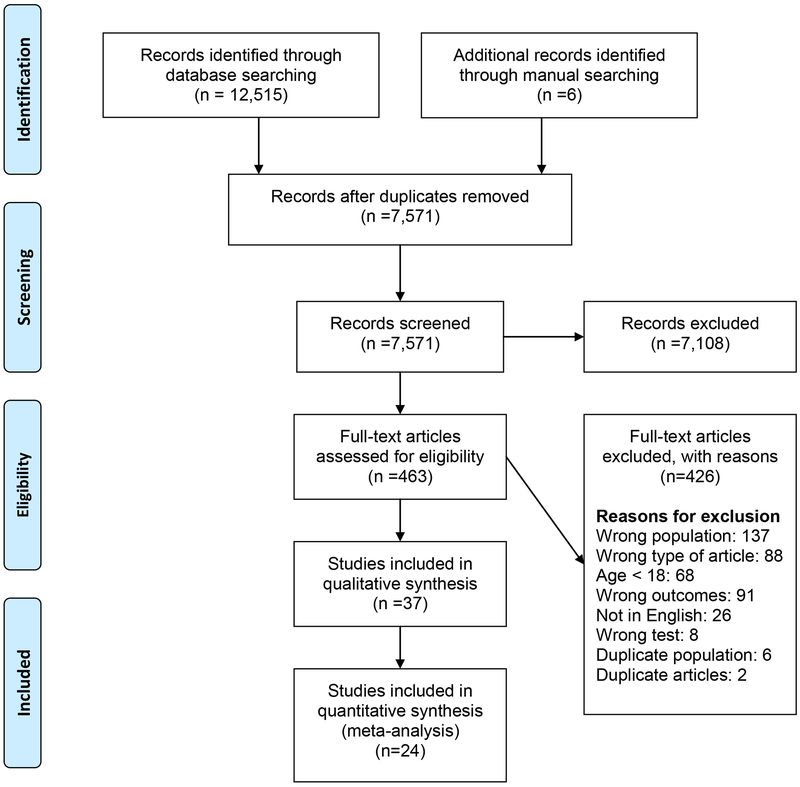
A total of 12,515 articles (Ovid Medline, n=2694; Embase, n=4472; Cochrane databases, n=622; Scopus, n=3898; CINAHL, n=803; and ClinicalTrials.gov, n=26) were identified through the initial database search, and an updated search through December 2015. Six additional articles were identified from a manual search of reference lists from relevant studies and reviews (Figure 1). After removing duplicates, 463 full-text articles were reviewed, 37 of which met initial eligibility criteria (published from 1979 to 2018).12-14,16-52 Of these, only 24 studies12-14, 16-19, 21-35 provided sufficient quantitative data to be included in the meta-analyses. Findings from two publications that involved the same study population19,20 were summarized as one study.19
Figure 1:
Study Selection
3.2. Characteristics of Included Studies
Table 1 summarizes characteristics of the 24 studies included in the meta-analysis. These studies included 3,090 women ranging in age from 18 to 91 years. Of these studies, the majority (n=21; 88%) included women without urinary symptoms or voiding difficulty, and/or urological or pelvic floor disorders, treatment, or surgery,12-14,16-19,22-25, 27-29,31,33-37 or those who reported normal micturition.30 The remaining three studies (13%) included healthy volunteers,26 healthy women without any gynecological history,32 and those without urinary tract infections (only criteria).21 Three studies (13%) explicitly required a negative urinalysis or urine culture.21,23,29 Twelve studies (50%) excluded women on: 1) anticholinergic or other medications that could affect the lower urinary tract,12,19,25, 27,28,31,33,36 2) any medications,14,16,18 or 3) medications other than hormone replacement therapy.31 Several studies excluded women with either specific chronic diseases or symptoms (n=8; 33%),12-14,25,27,31,36,37 (e.g., diabetes, neurological diseases, hypertension), or those working night shifts (n=3, 13%).19,25,27 One study recruited only nulliparous women,16 four studies (17%) excluded women with pelvic prolapse,12,22,25,27 and five studies (21%) excluded pregnant or postpartum women.17,23,25,27,36 One study excluded women with poor knee range of motion making it difficult to toilet.36
Table 1.
Characteristics of Studies Included in the Quantitative and/or Qualitative Analyses
| First Author | Sample Size Meeting Inclusion Criteria |
Country | Age Inclusion Criteria |
Age of Participants |
Definition of Healthy Population |
Voiding Frequency Outcomesa |
Voided Volume (VV) Outcomesa |
Uroflow and Postvoid Residual Volume Outcomesa |
|---|---|---|---|---|---|---|---|---|
| Ahmed17 | 12 | USA | Not specified | Not specified | Non-pregnant, no reported voiding difficulty or treatment for LUT condition | Qmax, Qave, Tvv, VV-U | ||
| Altun18 | 36 | Turkey | 17-24 yrs | Not specified | Nursing students without clinical voiding dysfunction and use of medicines, vitamins, or supplements | VVmax24-D, VVmax-C | ||
| Amundsen19 (Also Parsons20) | 161 | USA, UK | Not specified | Mean: 48.1 Range: 19.6-81.8 |
No bladder symptoms, previous urological or continence surgery, medications influencing bladder habits and night-shift sleep pattern, and UI reported on diary | FD, FN, F24 | V24-D, VN-D, VD-D, VV24-D, VVmin24-D, VVmax24-D, BC-D, VN-C, VD-C, VV24-C, VVmin-C, VVmax-C | |
| Barapatre12 | 333 | India | Not specified | Mean (SD): 33.9 (8.9) Range: 13-47 |
No LUTS or UTI ≤ 30 days, pelvic prolapse, physical or surgical therapy on LUT, cystoscopy, neurological disease, diabetes, prolapsed disc, spinal trauma, or medications affecting LUT function | Qmax, Qave, TQmax, Tvv, VV-U, PVR-ND | ||
| Boyko21 | 352 | USA | 55-75 yrs | Not specified | No UTI in past month, UTI diagnosis or urine culture with 105 uropathic organisms/ml | PVR-U | ||
| Devreese22 | 21 | Belgium | Not specified | Mean (SD): 33.6 (8.6) Range: 21-49 |
No pelvic floor problems such as incontinence or surgery of the urethra, uterus, rectum, or pelvic floor; gynecologist confirmed absence of prolapse | Qmax, Qave, TQmax, Tvv, VV-U | ||
| De Wachter16 | 15 | Belgium | 18-24 yrs | Mean: 21 Range: 18-24 |
Nulliparous women, in good health and no urological history or symptoms | FD, FN, F24 | VV24-D, VVN-D, VVD-D, VV24-C, VVN-C, VVD-C | |
| Drach23 | 121 | USA | Not specified | Mean (SD): 33 (13) | Female patients who attended gynecologic or urologic clinics without history of infection for > 1 year, no symptoms of LUT disease, >3 months post-partum, with normal urinalysis | Qmax, VV-U | ||
| Fantl24 | 60 | USA | Not specified | Mean (SD): 38.8 (13.8) | General good health, absence of past or actual gynecologic or urologic symptoms and/or disease | VV-U | ||
| FitzGerald25 | 300 | USA | Adults | Range: 18-91 | Ambulatory, non-pregnant women who self-reported normal LUT function, no symptoms of UI or voiding difficulty, pelvic organ prolapse or pelvic pain, current or past use of medications with primary anticholinergic effect, relevant neurologic disorder, previous urogynecologic surgery, current pessary use, or working primarily at night | FD, FN, F24 | V24-D, VV24-D, VVmax24-D, VV24-C, VVmax-C | |
| Gärtner26 | 36 | Czech Republic | 25-54 yrs | Not specified | Healthy volunteers | Qmax, Tvv, VV-U | ||
| Haliloglu27 | 119 | Turkey | Not specified | Range: 18-74 | Ambulatory, non-pregnant women without UI or voiding difficulty; no pelvic organ prolapse, pelvic pain, current or past use of medications with primary anticholinergic effect; no hormone replacement therapy; no relevant neurological disorder (e.g., multiple sclerosis, Parkinson’s disease); no previous surgery for incontinence or pelvic organ prolapse; no current vaginal pessary use; not working primarily at night; no diabetes | F24 | V24-D, VV24-D, VVN-D, VVmin24-D, VVmax24-D, VV24-C, VVN-C, VVmin-C, VVmax-C | |
| Huang28 | 68 | Taiwan | 19-66 yrs | Mean (SD): 39 (13.8) Range: 19-66 |
No known urological disease or symptoms or taking medications that affect voiding | FD, FN, F24 | V24-D, VN-D, VD-D, VV24-D, VVN-D, VVD-D, VN-C, VD-C, VV24-C, VVN-C, VVD-C | |
| Kassis29 | 33 | Canada | 25-56 yrs | Mean: 39.7 Range: 25-56 |
No urinary symptoms and negative urine culture | FD, FN | V24-D, VN-D, VD-D, VV24-D, VVN-D, VVD-D, VN-C, VD-C, VVN-C, VVD-C | |
| Kumar13 | 299 | India | >15 yrs | Not specified | Healthy without urological complaints or history of neurological disorders | Qmax, Qave, TQmax, Tvv, VV-U | ||
| Larsson30 | 151 | Sweden | Not specified | Mean: 43 | Self-reported normal micturition | FN, F24 | V24-D, VV24-D, VVmax24-D, VV24-C, VVmax-C | |
| Pauwels31 | 24 | Belgium | Not specified | Mean (SD): 49 (6) Range: 38-60 |
No history/symptoms of urological, gynecologic, gastrointestinal, or neurological pathology, abdominal or pelvic surgery, or medications other than hormone replacement therapy | FD, FN, F24 | VV24-D, VVN-D, VVD-D, VV24-C, VVN-C, VVD-C | |
| Porru32 | 34 | Italy | Not specified | Mean: 50 Range: 28-75 |
Healthy women without any gynecological history | Qmax, Qave, TQmax, Tvv, VV-U | ||
| Suebnukanwattan14 | 70 | Thailand | 18-60 yrs | Mean (SD): 31.7 (14.8) | Healthy without a history of urinary symptoms, bladder cancer or surgery, urethral stricture or surgery, urinary tract stone or any operation involving the urinary system. No medical conditions known to affect normal voiding such as diabetes mellitus, hypertension, or hyperthyroidism, and no medication within one week before the study | Qmax, Qave, Tvv, VV-U, PVR-U | ||
| Ünsal33 | 36 | Turkey | Not specified | Mean: 32 Range: 21-44 |
Healthy without presence of urinary disorder or taking medications known to interfere with LUT function | Qmax, Qave, VV-U, PVR-U | ||
| Van Haarst34 | 592 | Netherlands | ≥20 yrs | Not specified | No reported voiding complaints and not under treatment for urological symptoms | FD, FN, F24 | VV24-D, VVD-D, VVN-D | |
| Wyndaele35 | 10 | Belgium | Not specified | Mean: 24 Range: 19-28 |
University students with complete absence of urological, gynecological, sexual, and anorectal history, and without symptoms or signs of disease | Qave, TQmax | ||
| Yang36 | 45 | Taiwan | Not specified | Mean (SD): 23.2 (4.6) | Score of ≤7 on IPSS, BMI <24.5 kg/m2, not pregnant or having given birth, not taking any medication, particularly α-adrenergic stimulants or anticholinergic drugs, which could interfere with LUT function, not diagnosed with a chronic disease, such as hypertension, diabetes mellitus, UTI, not having moderate to severe voiding symptoms or past abdominal surgery, and not poor knee range of motion, making it difficult to crouch over a toilet seat. | Qmax, Qave, TQmax, Tvv, VV-U, PVR-U | ||
| Yu37 | 162 | Taiwan | Not specified | Mean (SD): 60.9 (9.5) | Had not sought treatment for voiding dysfunction; no co-existing medical factors affecting voiding function | Qmax, PVR-U |
Italicized outcomes indicate inclusion in meta-analysis
Abbreviations:
LUT= lower urinary tract; LUTS = lower urinary tract symptoms; UTI = urinary tract infection; UI = urinary incontinence; BMI = body mass index
Urination Frequency: FD = Frequency- daytime; FN = Frequency- nighttime; F24 = Frequency- 24h; IPPS = International Prostate Symptom Score
Voided and Postvoid Residual Volumes: V24-D = Volume- 24h via diary; VN-D = Volume-nighttime via diary; VD-D = volume- daytime via diary; VV24-D = mean voided volume 24h via diary; VVN-D = Nighttime mean voided volume via diary; VVD-D = Daytime mean voided volume via diary; VVNoc-D = Mean nocturnal volume via diary; VVmin24-D = 24h Minimum Voided Volume via diary; VVmax24-D = Maximum Voided Volume 24h via diary; BC-D = Bladder Capacity (not ultrasound) 24h via diary; VN-C = Volume-nighttime via collection; VD-C = Volume-daytime via collection; VV24-C = Volume - Mean void via collection; VVN-C = Volume - Nighttime mean void via collection; VVD-C = Volume - Daytime mean void via collection; VVmin-C = Volume - Min void via collection; VVmax-C = Volume - Max void via collection; V24-C = Volume 24 hour via collection; PVR-U = PVR (ultrasound); PVR-ND = PVR (technique not defined)
Uroflowmetry Parameters: Qmax = Uroflow - Max flow rate (Qmax); Qave = Uroflow - Mean flow rate (Qave); TQmax = Uroflow - Time to max flow (TQMax); Tvv = Uroflow - Flow Time (Tvv); VV-U = Uroflow - Voided volume; FR-O = Flow Rate (not via uroflow)
Studies were conducted in 12 countries, with five studies from the United States,17,21,23-25 one study from the United States and the United Kingdom,19 four from Belgium,16,22,31,35 three each from Taiwan8,36.37 and Turkey,18,27,33 two from India,12,13 and one study each from Canada,29 Czech Republic,26 Italy,32 Netherlands,34 Sweden,30 and Thailand.14
Nine studies included at least one urination frequency parameter,16,19,25,27-31,34 10 included at least one voided volume parameter,16,18,19,25,27-31,34 six included postvoid residual volume12,14,21,33,36,37 and 13 included one or more uroflowmetry result.12,13,17,22-24,26,32,33,35-37 No study included pad test weights among women meeting our eligibility criteria. Sample sizes ranged from 10 to 592 in the urination frequency and voided volume studies,16,19,25,27-29,30,31,34 36 to 352 in the post-void residual studies,12,14,21,33,36 and 10 to 333 in the uroflowmetry studies.12-14,17,22,23,24,26,32,33,35,36,37
3.3. Methodological Quality
Table 2 summarizes the key measures of methodological quality for studies included in the meta-analysis. Overall, 12 studies (50%) met all applicable quality criteria.12,14,19,22,24,25,27-30,33,36 Sixteen studies (67%) included women who were explicitly documented as not having urological symptoms or disorders;13,14,16,17-19,22-25,27-29,31,33-36 five studies (21%) included women described as “healthy” but either did not provide eligibility criteria or lacked a clear operational definition of the term.21,26,30,32,37 Seventeen studies (71%) characterized age in a precise manner using a measure of central tendency and dispersion12,14,18,19,21,22,24,25,27-33,36,37 All studies used a standardized method for assessing bladder function by either a bladder diary and/or a noninvasive laboratory test, e.g., uroflowmetry or bladder ultrasonography.
Table 2.
Summary of Methodological Quality of Studies Included in Meta-analysis
| First Author | Sample | Standardized Outcome Measurement |
||
|---|---|---|---|---|
| Excluded Symptomatic Women |
Characterized Age in Precise Manner1 |
Self-report Instrument2 |
Noninvasive Laboratory Test3 |
|
| Ahmed17 | Y | N | NA | Y |
| Altun18 | U | Y | NA | Y |
| Amundsen19 | Y | Y | NA | Y |
| Barapatre12 | Y | Y | Y | NA |
| Boyko21 | U | Y | Y | NA |
| Devreese22 | Y | Y | Y | NA |
| De Wachter16 | Y | N | NA | Y |
| Drach23 | Y | N | Y | NA |
| Fantl24 | Y | Y | Y | NA |
| FitzGerald25 | Y | Y | NA | Y |
| Gärtner26 | U | N | Y | NA |
| Haliloglu27 | Y | Y | NA | Y |
| Huang28 | Y | Y | Y | NA |
| Kassis29 | Y | Y | NA | Y |
| Kumar13 | U | N | Y | NA |
| Larsson30 | Y | Y | NA | Y |
| Pauwels31 | U | Y | NA | Y |
| Porru32 | U | Y | Y | NA |
| Suebnukanwattan14 | Y | Y | Y | NA |
| Ünsal33 | Y | Y | Y | NA |
| van Haarst34 | Y | N | Y | NA |
| Wyndaele35 | Y | N | N | Y |
| Yang36 | Y | Y | Y | NA |
| Yu37 | N | Y | Y | NA |
Ratings: Y = yes; N= no, U=unclear; NA = not applicable
= Mean or median with standard deviation or standard error.
= diary
= uroflowmetry, postvoid residual volume, 24-hour urine volume
3.4. Synthesis of Results
Table 3 presents the results from the meta-analyses by bladder function parameter. Summary outcome data from individual studies are provided in Supplemental Tables 2-4. Overall, there was insufficient data to conduct subgroup analyses exploring possible reasons for study heterogeneity, such as differences by age, country, body mass index, fluid intake amount or type, parity, medications, and medical comorbidities.
Table 3.
Summary of Pooled Bladder Function Measurements with Normative Reference Values
| Studies (N) |
Total Sample |
Mean Range of Individual Studies |
Overall Estimate (95% CI) |
I2 % |
90% Normative Reference Value1 |
|
|---|---|---|---|---|---|---|
| Urination Frequencies2 | ||||||
| Daytime frequency | 5 | 869 | 5.6-7.2 | 6.6 (6.2, 7.0) | 85.9 | 4-10 |
| Nighttime frequency | 5 | 869 | 0.1-1.0 | 0.4 (0.0, 0.8) | 99.0 | 0-2 |
| 24-hour frequency | 7 | 1406 | 5.8-8.3 | 7.0 (6.3, 7.7) | 97.5 | 4-12 |
| Voided and Postvoid Residual Volumes3 | ||||||
| Total daytime voided volume | 4 | 854 | 273-1261 | 945 (289, 1601) | 99.6 | 136-1718 |
| Mean daytime voided volume | 4 | 140 | 207-289 | 230 (203,258) | 61.1 | 108-418 |
| Total nighttime voided volume | 4 | 854 | 347-468 | 401 (343, 459) | 91.5 | 152-743 |
| Mean nighttime voided volume | 5 | 259 | 155-450 | 317 (225, 408) | 97.1 | 44-588 |
| 24-hour voided volume | 7 | 1424 | 1256-1759 | 1577 (1428, 1725) | 94.0 | 761-3042 |
| Mean voided volume | 7 | 838 | 216-294 | 245 (226, 265) | 85.7 | 116-428 |
| Maximum voided volume | 5 | 767 | 362-514 | 425 (363, 487) | 95.6 | 184-761 |
| Minimum voided volume | 2 | 280 | 81-193 | 137 (27, 246) | 98.9 | 34-325 |
| Postvoid residual volume | 4 | 503 | 1-20 | 12 (4, 20) | 99.9 | 1-56 |
| Uroflowmetry Parameters3,4,5 | ||||||
| Maximum flow rate (Qmax) | 11 | 1169 | 20-49 | 29 (26, 32) | 97.9 | 12-44 |
| Mean flow rate (Qave) | 9 | 860 | 9-24 | 15 (12, 18) | 99.3 | 6-26 |
| Time to maximum flow (TQMax) | 5 | 399 | 7-15 | 10 (8, 12) | 90.4 | 3-20 |
| Flow time (Tvv) | 7 | 517 | 24-38 | 28 (23, 33) | 94.6 | 13-64 |
| Voided volume (VV) | 11 | 1067 | 224-409 | 324 (299, 350) | 99.2 | 121-638 |
Reference values rounded to whole numbers
Frequency counts rounded to tenths
Volumes reported in milliliters
Flow rates in milliliters per seconds
Flow time in seconds
3.4.1. Urination Frequency
Five studies provided usable data on urination frequency during daytime and/or nighttime sleeping hours,16,19,28,29,34 and seven reported mean 24-hour urination frequency.16,19,25,27,28,30,34 Pooled overall estimates (95% confidence intervals [95%] ) were 6.6 (6.2, 7.0) for daytime frequency, 0.4 (0.0, 0.8) for nighttime frequency, and 7.0 (6.3, 7.7) for 24-hour frequency. In all pooled analyses, we found a high degree of heterogeneity in outcomes with I2 values ranging from 85.9% (daytime frequency) to 99.0% (nighttime frequency). With the exception of urination frequency during sleeping hours, normative reference ranges varied considerably. Reference ranges calculated for daytime frequency ranged from 4-10, from 0 to 2 during sleeping hours, and 24-hour frequency ranged from 4-12.
3.4.2. Voided and Postvoid Residual Volumes
Based on voided volumes collected from bladder diaries, data were pooled from seven studies16,19,25,27-30,34 reporting 24-hour voided volume; seven16,19,25,27,28,30,31 mean voided volume; five18,19,25,30,27 for maximum voided volume; two19,27 for minimum voided volume; four19,28,29,34 for total daytime voided volume; four16,28,29,31 for mean daytime voided volume; four19,28,29,34 for the total volume voided during sleeping hours; and five16,27-29,31 for mean nighttime voided volume. Overall pooled estimates were 1577 (1428, 1725) for 24-hour volume and 425 (362, 487) and for maximum voided volume. Four studies13,19,31,32 provided pooled data on mean postvoid residual volume collected by bladder ultrasonography with an overall pooled estimate of 12 (4, 20). We found a high degree of heterogeneity (I2) for voided volumes ranging from 61.1% for mean daytime voided volume to 99.6% for total daytime voided volume, and 99.9% for postvoid residual volume. Normative reference values varied considerably for voided volumes; values calculated for postvoid residual volume were less variable.
3.4.3. Uroflowmetry Results
We pooled data from 11 studies12-14,17,22,23,26,32,33,35,36 reporting maximum flow rate (Qmax) and voided volume following uroflowmetry,12-14,17,2-24,26,32,33,36 nine studies 12-14,17,22,32,33,35,36 reporting mean flow rate (Qave), five13,22,32,35,36 reporting time to maximum flow (TQMax), and seven13,14,16,17,26,32,36 reporting flow time (Tvv) from a non-instrumented flow test. Overall pooled estimates were 29 (26, 32) mL/sec for Qmax, 15 (12,18) mL/sec for Qave, 10 (8, 12) secs for TQMax), and 28 (23, 33) secs for flow time. There was a high degree of heterogeneity for bladder parameters collected from all uroflow studies with I2 ranging from 90.4% for time to maximum flow to > 99% for mean flow rate and voided volume. With the exception of voided volumes, most normative reference values for uroflow parameters were within relatively narrow ranges.
4. Discussion
4.1. Summary of Evidence
This systematic review and meta-analysis provides an in-depth synthesis and evaluation of bladder function parameters from 22 studies involving non-invasive tests for urination frequencies, voided and postvoid residual volumes, and uroflowmetry parameters in healthy or asymptomatic women. To our knowledge, this is the first meta-analysis to provide summary mean estimates and normative reference value ranges based on data from women without LUTS or urologic disorders. Most of what is known about normative reference values for bladder function in healthy populations is based on clinical populations with LUTS53 or clinical judgment.4 Our review provides 90% normative reference values for various bladder function parameters in asymptomatic adult females estimated by fixed-effects models under a log-normal distribution assumption.
We found a wide range of normative reference values for daytime and 24-hour frequencies and for most measures of voided volume. Of note, the calculated reference values for 24-hour frequency which varied from 4 to 11 voids in asymptomatic women challenges the commonly used frequency criteria for diagnosing overactive bladder of 9 or more voids per 24-hours. Our wider range of normative reference values are in contrast with a prior review3 that suggested daytime frequency ranged from 6-7 voids in healthy women versus our findings of 4-10 voids, and nighttime frequency from 0-1 voids versus 0-2 voids, respectively. Our results also differ from this prior review regarding 24-hour voided volume (732-2,929 mLs vs 1,400-1,800 mLs, respectively), mean daytime voided volume (108-418 mLs vs 200-250 mLs, respectively), and mean nighttime voided volume (44-588 mLs) vs 300-400 mLs, respectively). These differences might be attributed the inclusion of a greater number of studies and overall larger sample size, and/or use of different methods to calculate reference ranges. As expected, postvoid residual volumes (1-56 mLs) in this meta-analysis of asymptomatic women are lower than the 100-150 mL or one-third of total bladder volume that is customarily used to determine incomplete bladder emptying in clinical practice.
Non-instrumented uroflowmetry results can often be difficult to interpret as a single value because maximum and average urine flow rates are directly proportional to the voided volume. As voided volume increases uroflow rates also increase. In this meta-analysis, the maximal and mean urine flow rates were 29 mL/sec and 15 mL/sec, respectively, with time to maximum flow of 10 sec and with an average voided volume of 324 mL. These uroflow parameters are quite similar to those values found in a previous review6 which reported a maximal flow rate of 23.5±10 mL/sec, mean flow rate of 13±6 mL/s, time to maximum flow of 8±6 sec, and an average voided volume of 338±161 mL in healthy women. Based on our normative reference value calculations, maximal and mean urine flow rates ranged from 12 to 44 mL/sec and 6 to 26 mL/sec, respectively, with a time to voided volume of 3 to 20 sec and an average voided volume of 121 to 638 mL.
Our results on normative reference values for all bladder function parameters should be interpreted cautiously because of differences in eligibility criteria used across included studies, particularly with respect to age and definitions of healthy or asymptomatic women. We found marked heterogeneity of results from studies included in the meta-analysis on all bladder function outcomes. Given the high I2 values and the wide confidence intervals, along with the relatively wide variations in calculated reference values for most bladder function parameters, it is difficult to determine with accuracy whether these reference ranges describe “healthy” bladder function in women. However, these reference ranges may indicate normal variability which is greater than previously known. Variables known to affect bladder function such as age, fluid and caffeine intake, health and functional status, exercise status, medications, chronic disease, menstrual status, cultural norms, and long-standing toileting habits likely influenced these results. Unfortunately, we were not able to take these into account in our analyses. Differences across studies might also be explained by errors in reporting urination frequencies and voided volumes on bladder diaries which rely on self-report. However, even with a larger number of studies, a more accurate method of assessment, and a sample of carefully selected healthy women, it may not be possible to provide one single estimate of bladder function for all women because of the many factors that influence these values. Instead, future studies should report reference values stratified by factors known or believed to influence bladder function.
4.2. Strengths and Limitations
There are several limitations to this review. First, we found relatively few eligible studies for bladder function parameters, but particularly for urination frequency, voided volumes, and postvoid residual volumes. Second, the pooled sample size for each outcome assessed was relatively low, which contributed to the wide confidence intervals. Third, there was high heterogeneity across studies for all outcomes, with few studies providing sufficient information on sample characteristics (e.g., parity, hormonal status, fluid intake, or health status) to help identify sources of heterogeneity. Although voiding outcomes may differ by country because of the availability of clean drinking water, cultural norms, and availability of bathrooms, we were to examine the influence of country because of the small number of studies with these outcomes. The wide confidence intervals and high heterogeneity across studies makes it difficult to precisely estimate normative reference values. Fourth, we were unable to estimate reference values within specific age groups. The use of broad age groups fails to consider how bladder function may change across the life span, particularly with menopause and aging. Age is a known factor that can affect bladder function such as uroflowmetry results in symptomatic women.54 And finally, we only include journal articles published in the English language as full-text articles, thereby omitting any studies published or reported in other languages, and studies available only in the grey literature such as abstracts and unpublished reports.
Despite these limitations, the study has several strengths. A comprehensive search in seven electronic databases along with manual searching yielded additional studies not identified in previous reviews,4-6 with seven of these included in the quantitative synthesis.4-6 We also applied strict eligibility criteria to identify a healthy or asymptomatic female population without known urological that may affect bladder function as measured by noninvasive tests. Our eligibility criteria for the quantitative analysis required outcomes reported with a measure of central tendency along with a standard deviation or standard error. This allowed us to conduct meta-analyses on urination frequencies and voided and postvoid residual volumes in women without LUTS or urological disorders, and to update previous reviews on uroflowmetry parameters. Based on the data, we also calculated normative reference values for bladder function parameters which have not been reported previously in women without LUTS.
4.3. Conclusions
There appears to be a wide range of normality in bladder function in healthy women that affects urination frequency, voided volumes, and some uroflowmetry results. We also found a narrower range of normality for postvoid residual volumes. However, there are insufficient data available to define reference values for noninvasive measures of bladder function in “healthy” women. In this meta-analysis of 24 studies involving 3,090 women, we found a limited number of studies with a relatively small overall sample size for most outcomes in which to calculate reference value ranges for normal bladder function. The substantial variability across studies makes it difficult to accurately define normative reference values for women in various age groups. Study heterogeneity might be attributed to differing definitions of a “healthy” population, and varying sample populations which were poorly characterized by age and provided little information on additional factors that might potentially influence bladder function parameters. The high level of heterogeneity in the results found in this review highlights the need for future research with larger well-screened populations that explicitly exclude those with LUTS and urological disorders and medications that can affect bladder function to better define reference values for bladder function in healthy women. Future studies should also provide more detail on demographic and clinical characteristics that could potentially affect bladder function and conduct analyses based on well-defined age groups. Having a well-characterized population would aid subgroup analyses such as those groups defined by parity, hormonal status, medications, and certain chronic diseases or gynecological surgeries. Finally, studies should consider what “healthy” bladder function is within the context of each woman’s environment and lifestyle habits.
Supplementary Material
Acknowledgment
We appreciate the contributions of Melissa Milbrandt who assisted in the data extraction.
The Prevention of Lower Urinary Tract Symptoms (PLUS) Research Consortium is supported by the National Institutes of Health (NIH) through cooperative agreements (grants U01DK106786, U01DK106853, U01DK106858, U01DK106898, U01DK106893, U01DK106827, U01DK106908, and U01DK106892). Additional funding from: National Institute on Aging, NIH Office on Research in Women’s Health and Office of Behavioral and Social Science. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The Prevention of Lower Urinary Tract Symptoms (PLUS) Research Consortium Centers and Investigators:
Loyola University Chicago
Multi-Principal Investigators: Linda Brubaker, MD, MS; Elizabeth R. Mueller, MD, MSME; Colleen M. Fitzgerald, MD, MS. Investigators: Cecilia T. Hardacker, MSN, RN, CNL; Jennifer M. Hebert-Beirne, PhD, MPH; Missy Lavender, MBA; David A. Shoham, PhD
University of Alabama at Birmingham
Principal Investigator: Kathryn L. Burgio, PhD. Investigators: Cora E. Lewis, MD; Alayne D. Markland, DO, MSc; Gerald McGwin, PhD; Beverly R. Williams, PhD
University of California San Diego
Principal Investigator: Emily S. Lukacz, MD. Investigators: Sheila Gahagan, MD, MPH; D. Yvette LaCoursiere, MD, MPH; Jesse Nodora, DrPH
University of Michigan ∣ Ann Arbor MI
Principal Investigator: Janis M. Miller, PhD, MSN. Investigators: Lawrence Chin-I An, MD; Lisa Kane Low, PhD
University of Minnesota ∣ Minneapolis MN
Multi-Principal Investigators: Bernard L. Harlow, PhD, Multi-PI; Kyle D. Rudser, PhD. Investigators: Sonya S. Brady, PhD; John Connett, PhD; Melissa L. Constantine, PhD, MPAff; Haitao Chu, MD, PhD; Cynthia S. Fok, MD, MPH; Todd Rockwood, PhD
University of Pennsylvania ∣ Philadelphia PA
Principal Investigator: Diane K. Newman, DNP, ANP-BC, FAAN. Investigators: Amanda Berry, MSN, CRNP; C. Neill Epperson, MD; Kathryn H. Schmitz, PhD, MPH, FACSM, FTOS; Ariana L. Smith, MD; Ann E. Stapleton, MD; Jean Wyman, PhD, RN, FAAN
Washington University in St. Louis ∣ St. Louis MO
Principal Investigator: Siobhan Sutcliffe, PhD, ScM, MHS. Investigators: Colleen McNicholas, DO, MSc; Aimee S. James, PhD, MPH; Jerry L. Lowder, MD, MSc; Mary Townsend, ScD
Yale University ∣ New Haven CT
Principal Investigator: Leslie Rickey, MD. Investigators: Deepa Camenga, MD, MHS; Toby Chai, MD; Jessica B. Lewis, LMFT, MPhil
Steering Committee Chair: Mary H. Palmer, PhD, University of North Carolina
NIH Program Office: National Institute of Diabetes and Digestive and Kidney Diseases, Division of Kidney, Urologic, and Hematologic Diseases, Bethesda, MD
NIH Project Scientist: Tamara Bavendam MD, MS; Project Officer: Ziya Kirkali, MD; Scientific Advisors: Chris Mullins, PhD and Jenna Norton, MPH
Contributor Information
Jean F. Wyman, School of Nursing, University of Minnesota, Minneapolis, MN.
Jincheng Zhou, Division of Biostatistics, School of Public Health, University of Minnesota, Minneapolis, MN.
D. Yvette LaCoursiere, Department of Obstetrics, Gynecology and Reproductive Sciences, University of California San Diego, LaJolla, CA.
Alayne D. Markland, Department of Medicine, Division of Gerontology, Geriatrics, and Palliative Care, University of Alabama at Birmingham, Birmingham/Atlanta Geriatrics Research, Education, and Clinical Center, Birmingham, AL.
Elizabeth R. Mueller, Departments of Urology and Obstetrics/Gynecology, Loyola University, Chicago, Loyola University Medical Center, Maywood, IL.
Laura Simon, Bernard Becker Medical Library, Washington University in St. Louis, St. Louis, MO.
Ann Stapleton, Department of Medicine, University of Washington, Seattle, WA.
Carolyn R.T. Stoll, Division of Public Health Sciences, Department of Surgery, Washington University in St. Louis, St. Louis, MO.
Haitao Chu, Division of Biostatistics, School of Public Health, University of Minnesota, Minneapolis, MN.
Siobhan Sutcliffe, Division of Public Health Sciences, Department of Surgery, Washington University in St. Louis, St. Louis, MO.
References
- 1.Harlow BL, Bavendam TG, Palmer MH, et al. The Prevention of Lower Urinary Tract Symptoms (PLUS) Research Consortium: A transdisciplinary approach toward promoting bladder health and preventing lower urinary tract symptoms in women across the life course. J Womens Health. 2017; 27(3):283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lukacz E, Bavendam T, Berry A, et al. A novel research definition of bladder health in women and girls: Implications for research and public health promotion. J Womens Health. 2018; 27(8):974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lowder JL, Bavendam RG, Berry A, Brady SS, Fitzgerald CM, Fok CS, et al. Terminology for bladder health research in women and girls: Prevention of Lower Urinary Tract Symptoms transdisciplinary consortium definitions. Neururol Urodyn, 2019. April 8. doi: 10.1002/nqu.23985 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al Afraa T, Mahfouz W, Campeau L, Cortes J. Normal lower urinary tract assessment in women: 1. Uroflowmetry and post-void residual, pad tests, and bladder diaries. Int Urogynecol J. 2012; 23(6): 681–685. [DOI] [PubMed] [Google Scholar]
- 5.Mahfouz W, Al Afraa T, Campeau L, Corcos J. Normal urodynamic parameters in women: part II—invasive urodynamics. Int Urogynecol J. 2012; 23(3): 269–277. [DOI] [PubMed] [Google Scholar]
- 6.Sorel MR, Reitsma HJB, Rosier PFWM, Bosch RJLHR, de Kort LMO. Uroflowmetry in healthy women: A systematic review. Neurourol Urodyn. 2017; 36(4): 953–959. [DOI] [PubMed] [Google Scholar]
- 7.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Sys Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stroup DF, Berline JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000; 283(15):2008–2012. [DOI] [PubMed] [Google Scholar]
- 9.Higgins JPT, Green S, Eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org. [Google Scholar]
- 10.Harris PA, Taylor R, Thiele R, et al. Research electronic data capture (REDCap) – A metadata-driven methodology and workflow process for providing translational research informatics support, J Biomed Inform. 2009; 42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins J, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002; 21(11): 1539–1558. [DOI] [PubMed] [Google Scholar]
- 12.Barapatre Y, Agarwal MM, Singh SK, Sharma SK, Mavuduru R, Mete UK, et al. Uroflowmetry in healthy women: Development and validation of flow-volume and corrected flow-age nomograms. Neurourol Urodyn. 2009; 28:1003–1009. [DOI] [PubMed] [Google Scholar]
- 13.Kumar V, Dhabalia JV, Nelivigi GG, Punia MS, Suryavanshi M. Age, gender, and voided volume dependency of peak urinary flow rate and uroflowmetry nomogram in the Indian population. Ind J Urol. 2009; 25:461–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suebnukanwattana T, Chaikomin R, Soontrapa S, Lohsiriwat S, Tantiwongse A. Uroflowmetry in normal Thai subjects. J Med Assoc Thai. 2003; 86:353–360. [PubMed] [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986; 7(3):177–188. [DOI] [PubMed] [Google Scholar]
- 16.De Wachter S, Wyndaele JJ. Frequency-volume charts: A tool to evaluate bladder sensation. Neurourol Urodyn. 2003; 22:638–642. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed S, McNanley A, Perevich M, Glantz JC, Buchsbaum G. Uroflow measurements in healthy female volunteers. Female Pelvic Med Reconstr Surg. 2010; 16:327–330. [DOI] [PubMed] [Google Scholar]
- 18.Altun I, Cinar ND. Self-reported quantity of normal maximal voided volume in healthy young. Int J Urol Nurs. 2013; 7:111–113. [Google Scholar]
- 19.Amundsen CL, Parsons M, Tissot B, Cardozo L, Diokno A, Coats AC. Bladder diary measurements in asymptomatic females: Functional bladder capacity, frequency, and 24-hr volume. Neurourol Urodyn. 2007; 26:341–349. [DOI] [PubMed] [Google Scholar]
- 20.Parsons M, Tissot W, Cardozo L, Diokno A, Amundsen CL, Coats AC. Normative bladder diary measurements: night versus day. Neurourol Urodyn. 2007; 26:465–473. [DOI] [PubMed] [Google Scholar]
- 21.Boyko EJ, Fihn SD, Scholes D, Chen CL, Normand EH, Yarbro P. Diabetes and the risk of acute urinary tract infection among postmenopausal women. Diabetes Care. 2002; 25:1778–1783. [DOI] [PubMed] [Google Scholar]
- 22.Devreese AMN G, Staes F, Vereecken RL, De Weerdt W, Stappaerts K. Do posture and straining influence urinary-flow parameters in normal women? Neurourol Urodyn: 2000; 19:3–8. [DOI] [PubMed] [Google Scholar]
- 23.Drach GW, Ignatoff J, Layton T. Peak urinary flow rate: observations in female subjects and comparison to male subjects. J Urol. 1979; 122:215–219. [DOI] [PubMed] [Google Scholar]
- 24.Fantl JA, Smith PJ, Schneider V, Hurt WG, Dunn LJ. Fluid weight uroflowmetry in women. Am J Obstet Gynecol. 1983; 145:1017–1024. [DOI] [PubMed] [Google Scholar]
- 25.FitzGerald MP, Stablein U, Brubaker L. Urinary habits among asymptomatic women. Am J Obstet Gynecol. 2002; 187:1384–1388. [DOI] [PubMed] [Google Scholar]
- 26.Gärtner M, Krhut J, Hurtik P, Burda M, Zvarova K, Zvara P. Evaluation of voiding parameters in healthy women using sound analysis. Low Urin Tract Symptoms. 2018. 10(1):12–16. [DOI] [PubMed] [Google Scholar]
- 27.Haliloglu B, Peker H, Ilter E, Celik A, Kucukasci M, Bozkurt S. Fluid intake and voiding parameters in asymptomatic Turkish women. Int Urogynecol J. 2012; 23:791–795. [DOI] [PubMed] [Google Scholar]
- 28.Huang YH, Lin ATL, Chen KK, Chang LS. Voiding pattern of healthy Taiwanese women. Urol Int. 2006; 77:322–326. [DOI] [PubMed] [Google Scholar]
- 29.Kassis A, Schick E. Frequency-volume chart pattern in a healthy female population. Br J Urol. 1993; 72:708–710. [DOI] [PubMed] [Google Scholar]
- 30.Larsson G, Victor A. Micturition patterns in a healthy female population, studied with a frequency/volume chart. Scan J Urol Nephrol. 1988; 114 Suppl: 53–57. [PubMed] [Google Scholar]
- 31.Pauwels E, De Wachter S, Wyndaele JJ. Normality of bladder filling studied in symptom-free middle-aged women. J Urol. 2004; 171:1567–1570. [DOI] [PubMed] [Google Scholar]
- 32.Porru D, Scarpa RM, Onnis P, Lavra S, Delisa A, Usai E. Urinary symptoms in women with gynecological disorders: The role of symptom evaluation and home uroflowmetry. Arch Esp Urol.1998; 51:843–848. [PubMed] [Google Scholar]
- 33.Ünsal A, Çimentepe E. Voiding position does not affect uroflowmetric parameters and post-void residual urine volume in healthy volunteers. Scand J Urol Nephrol. 2004; 38:469–471. [DOI] [PubMed] [Google Scholar]
- 34.Van Haarst EP, Heldeweg EA, Newling DW, Schlatmann TJ. The 24-h frequency-volume chart in adults reporting no voiding complaints: Defining reference values and analysing variables. BJU Int. 2004; 93:1257–1261. [DOI] [PubMed] [Google Scholar]
- 35.Wyndaele J-J. Normality in urodynamics studied in healthy adults. J Urol, 1999; 161: 889–902. [PubMed] [Google Scholar]
- 36.Yang KN, Chen SC, Chen SY, Chang CH, Wu HC, Chou EC. Female voiding postures and their effects on micturition. Int Urogynecol J. 2010; 21:1371–1376. [DOI] [PubMed] [Google Scholar]
- 37.Yu H, Lee W, Liu S, Tai T, Wu H, Chen J. Unrecognized voiding difficulty in female type 2 diabetic patients in the diabetes clinic: a prospective case-control study. Diabetes Care. 2004; 27:988–989. [DOI] [PubMed] [Google Scholar]
- 38.Andersson L, Haraldsson B, Johansson C, Barregard L. Methodological issues on the use of urinary alpha-1-microglobuline in epidemiological studies. Nephrol Dial Transplant. 2008; 23:1252–1256. [DOI] [PubMed] [Google Scholar]
- 39.Armstrong LEJ, Evan C, Munoz CX, Swokla B, Le Bellego L, Jimenez L, et al. Hydration biomarkers and dietary fluid consumption of women. J Acad Nutr Diet. 2012; 112:1056–1061. [DOI] [PubMed] [Google Scholar]
- 40.Bing MH, Jennum PM, Moller LA, Mortensen S, Lose G. Obstructive sleep apnea in a Danish population of men and women aged 60-80 years with nocturia. J Clin Sleep Med. 2012; 8:515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brostrom S, Jennum P, Lose G. Short-term reproducibility of cystometry and pressure-flow micturition studies in health women. Neurourol Urodyn, 2002; 21:457–460. [DOI] [PubMed] [Google Scholar]
- 42.Cartwright R, Panayi D, Cardozo L, Khullar V. Reliability and normal ranges for the Patient’s Perception of Intensity of Urgency Scale in asymptomatic women. BJU Int. 2010; 105:832–836. [DOI] [PubMed] [Google Scholar]
- 43.Fagerstrom P, Sallsten G, Akerstrom M, Haraldsson B, Barregard L. Urinary albumin excretion in healthy adults: A cross sectional study of 24-hour versus timed overnight samples and impact of GFR and other personal characteristics. BMC Nephrol. 2015; January 24, 168. doi: 10.1186/1471-2369-16-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glauser A, Hochreiter W, Jaeger P, Hess B. Determinants of urinary excretion of Tamm-Horsfall protein in non-selected kidney stone formers and healthy subjects. Nephrol Dial Transplant. 2000; 15:1580–1587. [DOI] [PubMed] [Google Scholar]
- 45.Haylen BT, Asby D, Sutherst JR et al. Maximum and average urine flow rates in normal male and female populations—the Liverpool nomograms. Br J Urol, 1989; 64:30–38. [DOI] [PubMed] [Google Scholar]
- 46.Huang AJ, Brown JS, Boyko EJ, Moore EE, Scholes D, Walter LC, Lin F, Vittinghoff E, Fihn SD. Clinical significance of postvoid residual volume in older ambulatory women. J Am Geriatr Soc. 2011; 59:1452–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karantanis E, O’Sullivan R, Moore KH. The 24-hour pad test in continent women and men: Normal values and cyclical alterations. BJOG. 2003;110:567–571. [PubMed] [Google Scholar]
- 48.Kobwitaya K, Bunyavejchevin S. 24-hour pad tests in thai continent women. J Med Assoc Thail. 2015;98:123–128. [PubMed] [Google Scholar]
- 49.Pfisterer MH, Griffiths DJ, Rosenberg L, Schaefer W, Resnick NM. Parameters of bladder function in pre-, peri-, and postmenopausal continent women without detrusor overactivity. Neurourol Urodyn. 2007; 26:356–361. [DOI] [PubMed] [Google Scholar]
- 50.Rane A, Corstiaans A. Does micturition improve in the squatting position? J Obstetr Gynaecol. 2008; 28:317–319. [DOI] [PubMed] [Google Scholar]
- 51.Singh SP, Pandey DN, Bhushan S, Seth P, Sisodia AK. Study of the intake of fluid, output and titratable acidity of urine in medical students. Indian J Physiol Pharmacol. 1977; 21:369–373. [PubMed] [Google Scholar]
- 52.Weykamp CW, Penders TJ, Schmidt NA, Borburgh AJ, Van De Calseyde JF, Wolthers BJ. Steroid profile for urine: reference values. Clin Chem. 1989; 35:2281–2284. [PubMed] [Google Scholar]
- 53.Abrams P, Feneley R, Torrens M. Urodynamics. Springer-Verlag, New York, 1983. [Google Scholar]
- 54.Zimmern P, Litman HJ, Nager CW, Lemack GE, Richter HE, Sirls L, et al. Effect of aging on storage and voiding function in women with stress–predominant urinary incontinence. J Urol. 2014; 192(2):464–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.