Abstract
As a consequence of the obesity epidemic and increasing incidence of metabolic syndrome fatty liver disease now affects a large portion of the world’s population. Left untreated, fatty liver disease can progress to more severe pathologic conditions such as cirrhosis and liver cancer. In an effort to probe the pathophysiology of fatty liver disease and its progression, research over the last decade has led to the engineering of in vitro models of the liver to aid in drug discovery and study of liver pathophysiology. In this review, we discuss advances in modeling liver tissue and the latest developments in understanding disease etiology and treatment from the perspective of engineered in vitro models spanning from conventional planar, static monolayer cultures to those based on the more recently developed bioprinted and liver-on-a-chip platforms. These technologies promise to transform basic biological research, the pharmaceutical industry, and clinical medicine of the liver.
Introduction
To study the liver, it is crucial to develop reproducible and accurate models that can replicate its architecture, regenerative capacity, and response to injury. Such a model is currently in desperate need as non-alcoholic fatty liver disease (NAFLD) is one of the fastest growing disease worldwide. NAFLD constitutes a spectrum beginning with fat accumulation in the liver (steatosis), progressing through hepatocyte injury and an inflammatory response, features of non-alcoholic steatohepatitis (NASH), followed by a reactive deposition of collagen and formation of excess fibrous connective tissue (fibrosis), and ultimately irreversible scarring (cirrhosis) (1). Because of the complexity of the pathophysiology, as well as the role of multiple cell types and environmental and stromal effects, current models of hepatic steatosis, steatohepatitis, and fibrosis are lacking.
Though invaluable, animal models of NAFLD have several limitations, such as being low-throughput and lacking features of steatohepatitis and inflammatory infiltrates (2). Planar in vitro models do not replicate complex liver structures, including cell-cell interactions important to the generation of fibrosis. Although using primary human cells is ideal, limited availability and concern for reproducibility due to heterogeneity limit their application. Biomimetic in vitro models are needed to investigate mechanisms of liver fibrosis and perform higher-throughput screening of drugs to decrease fat accumulation or prevent inflammatory or fibrotic reactions to steatosis. In vitro models have been developed (3, 4), including three-dimensional (3D) hepatic spheroids (5, 6) and bioprinted 3D models (7, 8), but these are static and lack multicellular interactions, proper functional recapitulation, and in situ bioanalysis capacity and dynamic cues that might improve model relevance.
In vitro models of liver diseases
Two-dimensional (2D) cultures of hepatic cell lines have long been used to model diseases and develop drugs (9). Examples include planar monocultures of hepatic stellate cells with or without the incorporation of soft matrices such as polyacrylamide gels (10), but transcriptional profiles of these cells do not appear to correlate with human cirrhotic tissues (11). It has been difficult to translate findings from 2D monolayers, as they do not replicate native features such as dynamic physicochemical signals and microenvironmental architectures found within hepatic lobule. There is usually rapid loss of hepatocellular function. However, in some cases micropatterned co-cultures remain stable for weeks and retain some liver-specific functions (12). Nevertheless, these models in general have a limited ability to reproduce gene expression and metabolic activity seen in vivo.
Hepatic spheroids recreate 3D liver tissues resembling native structure and function, including fat accumulation and collagen deposition (5, 6). However, they are typically static and limited in architecture and incorporation of multiple cell types. To improve this, researchers developed 3D models using human cells, including microfluidic, microphysiological platforms, referred to as “liver-on-a-chip” models (5, 13, 14).
Liver-on-a-chip models
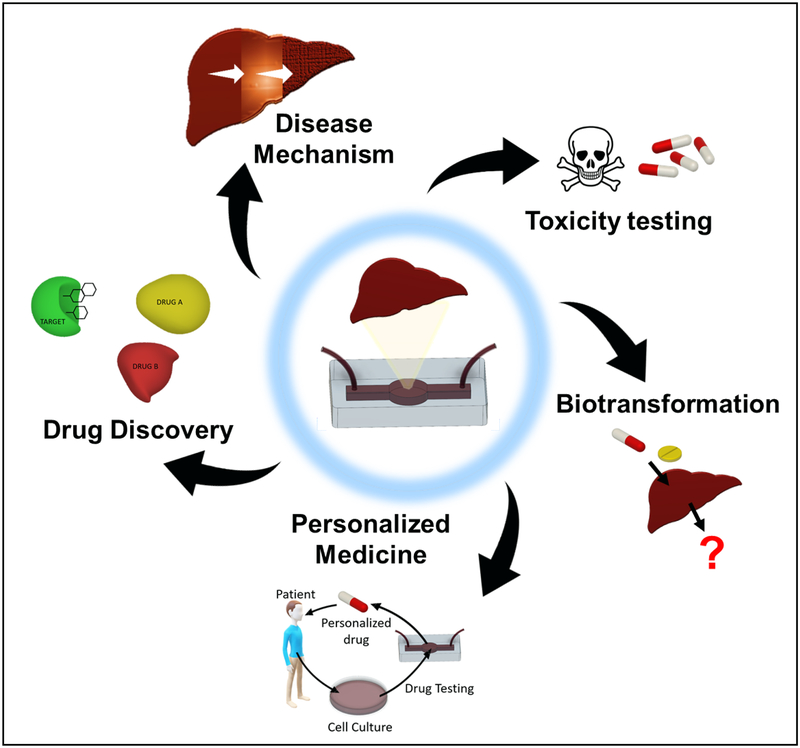
Liver-on-a-chip is a 3D in vitro hepatic microphysiological system aiming to recreate the conditions of liver tissue on a microscopic scale (15). An ideal liver-on-a-chip is a higher-throughput system capable of mimicking conditions of hepatocytes and the dynamic physicochemical hepatic environment (Figure 1).
Figure 1.
Overview of various medical applications of liver-on-a-chip and liver-disease-on-a-chip systems. These systems can be used to discover new drugs, study their biotransformation, and test their toxicities. Chips provide a means to study the mechanisms and progression of liver diseases in vitro and to test drugs to inhibit or treat them. Chips, where the patients’ own cells are incorporated, can also be used for personalized medicine to generate models for individualized treatment testing.
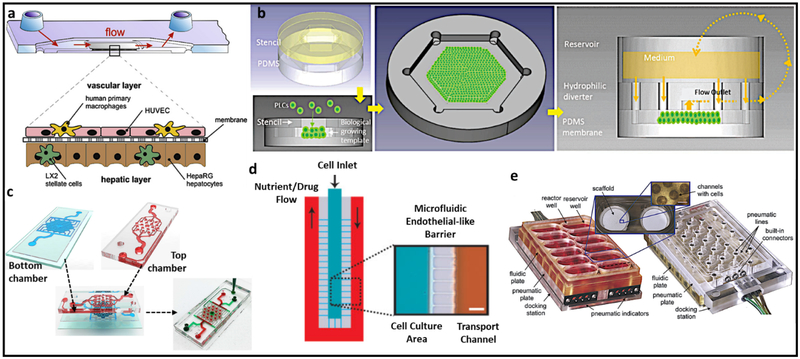
One such device is a microtissue platform integrating non-parenchymal cells (NPCs) in a vascular layer made of HUVECs and tissue macrophages, Kupffer cells, and an opposing hepatic layer made of hepatic stellate cells (HSCs) co-cultured with hepatocytes (HepaRG) (Figure 2a) (16). Luminescence-based sensors integrated into the device at inlets and outlets of perfusion channels allowed real-time measurement of oxygen consumption. Cells exhibited clear differentiation and structural reorganization, including polarization but were functionally stable only for 4 days. Another design recapitulates a multiscale structural hierarchy featuring drug clearance and zonal physiology for long-term culture (Figure 2b) (17). However, due to the absence of NPCs important for normal liver physiology, this model did not adequately reflect the physiological regulation of hepatocellular function and polarization.
Figure 2.
a) A microfluidic perfused three-dimensional human liver model. b) Scaffold-free liver-on-a-chip model with multiscale organotypic cultures. c) VLSLL-on-a-chip model showing the fabrication of liver lobules. d) An artificial liver sinusoid with a microfluidic endothelial-like barrier for primary hepatocyte culture. e) Perfused multiwell plate for 3D liver tissue engineering. Reprinted a) with permission from Elsevier (16), b) and d) from John Wiley & Sons (17) and (18), respectively, c) from IOP publishing (19), and e) from Royal Society of Chemistry (20). VLSLL: Very large-scale liver-lobule, HUVEC: Human umbilical vein endothelial cells, PDMS: Polydimethylsiloxane, PLCs: Primary liver cells.
The very large-scale liver-lobule (VLSLL)-on-a-chip model consists of an integrated network of hexagonal tissue-culture chambers with a separate seed-feed network, mimicking the infrastructure of the hepatic lobule with its central vein (Figure 2c) (19). The VLSLL-on-a-chip led to bile-canaliculi network formation. Figure 2d shows a system for the creation of a biologically inspired artificial liver sinusoid using a microfluidic endothelial-like barrier with mass transport properties similar to liver acini (18). In both models, diffusion channels mimicking endothelial layers restrained hepatocytes and protected them from sheer stress, while allowing diffusion of nutrients. However, these abiological endothelial-like barriers cannot recapitulate cell–cell interactions and drug metabolism in vivo. Yet another device that maintains 3D tissue cultures with constant perfusion integrates multiple fluidically isolated bioreactors into an array in a multi-well plate format with each bioreactor containing a scaffold for the formation of hundreds of 3D microscale tissue units (Figure 2e) (20). Here, hepatocytes and liver sinusoidal endothelial cells co-cultured with continuous media circulation formed functional liver-like tissues. Similar to static multiwell plates, the bioreactors in this system are fluidically isolated and cannot combine two or more separate tissue culture wells. (Table 1)
Table 1.
An overview of important characteristics of some liver-on-a-chip systems discussed in Figure 2.
| Model (Ref.) | Hepatocyte | NPCs present? | Cellular Polarization | Oxygen Diffusion | Timeline (Weeks) | Remarks |
|---|---|---|---|---|---|---|
| (16) | HepaRG cells | Yes | Yes | Yes | <1 | CYP450(+), bile canaliculi-like structures. Recapitulates physiological function. |
| (17) | Primary rat hepatocytes | Only HSCs | No | Yes | 2 | Multiscale structural hierarchy in a scaffold-free condition. Does not recapitulate full function. |
| (18) | HepG2/hiCell hepatocytes* | No | Yes | Yes | 2 – 3 | Scalable, functional bile- canaliculi network in hiCell hepatocytes. Does not recapitulate full function. |
| (19) | Rat and human primary hepatocyte | No | No | No | 1 | Sustaining of hepatocytes without an ECM coating. Does not recapitulate full function. |
| (20) | Rat primary hepatocyte | Yes | Yes | Yes | 2 | Generation of 3D microscale tissue units in hundreds. Recapitulates physiological function. |
human-induced pluripotent cell-derived hepatocytes
Liver disease-on-a-chip models
Based on liver-on-a-chip systems, liver-disease-on-a-chip models provide a platform for in vitro drug testing. In a recent study, free fatty acids (FFAs) oleic acid (OA) and palmitic acid (PA) were used to induce steatosis in primary rat hepatocytes (21). OA, PA, and their mixtures led to increased accumulation of intracellular triacylglycerols (TAG), but PA led to a dose-dependent cytotoxic effect associated with an increase in reactive oxygen species (ROS) and decreased albumin production (21). With this in mind, NAFLD-on-a-chip was created with a central chamber containing HepG2 cells surrounded by closely spaced, parallel microchannels to simulate endothelial cells. To minimize shear stress, media flowed through a separate chamber connected to the central chamber by microchannels. Cultures were supplemented with OA, and PA for up to 48 hours to induce NAFLD. Pathogenesis was characterized using intracellular triglyceride accumulation, cell viability, and cellular levels of ROS (22).
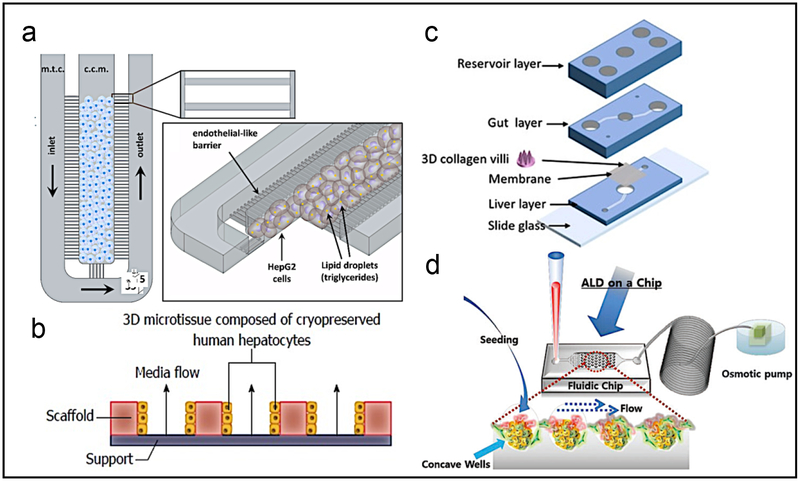
In another FFA-based NAFLD model, twelve individual bioreactors with a built-in pump to control the flow of media creating an oxygen gradient were used to simulate in vivo conditions (Figure 3a,b) (23). Devices were seeded with primary human hepatocytes and supplemented with OA and PA for 14 days. There was intracellular accumulation of TAG and upregulation of key genes such as CYP2E1 and CYP7A1 along with production of proteins linked with fibrosis, liver injury, and inflammation. This model was then used to screen drugs such as pioglitazone and metformin, showing decreased TAG accumulation. While hepatocytes responded to high concentrations of FFAs by reducing the activity of metabolic enzymes such as CYP3A4 and expressing several adipokines, they lacked many features of NAFLD/NASH such as expression of inflammatory cytokines, likely due to the absence of NPCs.
Figure 3.
a) Schematics of microarchitecture and geometric configuration of the NAFLD-on-a-chip device. b) Chip mimicking the early stages of NAFLD by treatment with FFA. c) The gut-liver chip to mimic the absorption and accumulation of fatty acids in the intestines and the liver. d) A reversibly-and irreversibly-injured ALD model where the development of fibrosis can be seen. Reprinted a) with permission from Public Library of Science (22), b) from Baishideng Publishing Group (23), c) from John Wiley & Sons (24), and d) from Royal Society of Chemistry (25). ALD: Alcoholic liver disease, FFA: Free fatty acids.
Adipose tissues and intestines contribute to NASH/NAFLD through dysregulated lipolysis (26) or altered intestinal permeability (27). To study interactions between liver and other organs and the dynamic responses to drugs, multi-organ platforms have been developed connecting different organs-on-a-chip. For example, one device consisting of two chambers separated by a porous membrane with a collagen scaffold on the intestinal side, Caco-2 cells were seeded in the intestinal chamber and HepG2 cells in the hepatic chamber (Figure 3c) (24). Cells were perfused with media containing OA and PA for 24 hours after perfusing serum-free media for 24 hours. Steatosis was quantified after treatment with α-lipolic acid (ALA), butyrate, and tumor necrosis factor (TNF)-α. ALA decreased TAG accumulation in both chips and 2D cultures (24, 28). Butyrate decreased steatosis by enhancing intestinal barrier function and decreasing TAG accumulation in hepatocytes (29, 30). TNF-α induced steatosis by affecting intestines, increasing steatosis in chip-based studies, but not in 2D monolayers.
To study the effect of alcohol on livers, hepatocyte/HSC spheroids in perfused and static environments were tested for the ability to recover in regular media after alcohol-induced injury (Figure 3d) (25). They have been shown to develop complex extracellular matrix support and hepatic structures such as bile canaliculi, tight junctions, desmosomes, lipid storage, as well as improved cytochrome P450 function and expression compared to monocultures (31). At higher concentrations, damage to spheroids was irreversible in 3 days. However, at lower concentrations in co-culture spheroids, there was significantly higher activation of HSCs compared to hepatocytes alone. There was also improvement in hepatocyte function in co-culture spheroids. This model was able to recreate in vitro the ability of HSCs to reconstitute the extracellular matrix after injury.
A liver-on-a-chip with four sensors to measure oxygen, lactate, glucose, and temperature was used to estimate the central drug flux to determine the mechanism of injury of valproate and stavudine (32). Both drugs led to dose-dependent damage due to lipid accumulation in this translational demonstration. The lack of a multicellular architecture makes it unclear whether these devices can mimic the mechanism and toxicological endpoints of in vivo responses to drugs. More recently, a 3D liver-sinusoid-on-a-chip consisting of four cell lines (HepG2, LX-2, EAhy926c, and U937) developed to study the pathophysiological process of hepatic NPCs in ALD under physiological flow was able to recreate alcohol injury and permitted the study of the intercellular communication between different cell types cells during ALD (33).
Commercially available models
There are a number of commercially available liver-on-a-chip models for research and drug development (Table 2). Insphero incorporates primary hepatocytes, Kupffer cells, and sinusoidal endothelial cells to recapitulate the native liver microenvironment in spheroid-based models (34). Ideal for long-term treatment regimens needed to gauge the efficacy of compounds, these models are geared for use in high-throughput screening. Hepregen (BioIVT) offers customized 3D liver disease models for in-depth in vitro modeling for drug development (35). 3D Biomatrix provides hanging drop plates, allowing users to customize their own spheroids (36). This will help develop specific spheroids for different types of liver diseases. Organovo provides bioprinted human liver tissue models (37). These models remain functional and stable for more than 28 days and can be used for predictive toxicology endpoints, gene and protein expression profiling, and histological tissue assessment.
Table 2.
An overview of some commercially available liver-on-a-chip systems
| Static In-vitro Liver models | Dynamic In-vitro Liver models | ||||||
|---|---|---|---|---|---|---|---|
| Company | Technology | Product | Ref. | Company | Technology | Product | Ref. |
| Insphero | Liver Spheroid |  |
(34) | Emulate | PDMS liver-on-a-chip |  |
(38) |
| Hepregen (BioIVT) | Micropatterning |  |
(42) | ApreX | Origami liver-on-a-chip |  |
(43) |
| 3D Biomatrix | 3D Hanging Drop |  |
(44) | Mimetas | Organoplate |  |
(45) |
| Organovo | 3D Bioprinting |  |
(46) | CnBio | Transwell + Scaffold |  |
(47) |
Emulate, uses PDMS-based chips available in three different cell configurations: co-culture, tri-culture, and quad-culture allowing incorporation of different cell types (38). ApreX’s origami liver-on-a-chip involves folding adhesive sheets to create microfluidic devices specifically to study drug toxicity in humans (39). Mimetas’ Organoplate is a higher-throughput platform, as it has an array of microfluidic channels supporting up to 96 tissues in a single plate; it is compatible with standard microtiter plate readers and microscopes (40). CN Bio’s multi-well chip uses a transwell setup; it provides the physiological and mechanical microenvironment of the liver in a multi-well format and enables extended maintenance of phenotypically intact 3D primary human hepatocytes (41). These models provide readily-available liver-on-a-chip systems to study hepatopathologies such as hepatic steatosis and to develop viable treatments.
Conclusions
Several 3D in vitro models for the liver have been developed and are available for use. These models are valuable tools to study the progression of liver disease, facilitate drug discovery, and enable toxicity testing. However, this facet of bioengineering is still in its infancy, and more work is needed to develop representative models to study pathologies such as steatosis and NASH. Future directions may focus on the development of devices including all cell types in the liver. Additionally, real-time monitoring instead of end-point analyses would provide a better understanding of the dynamic changes in these devices (14). With refinement and improvement, the liver-on-a-chip holds much promise in the investigation and eventual cure of liver disease.
Box: Key components of organ-on-a-chip devices for improved applications in disease modeling, drug screening, and personalized medicine.
| Microfluidic bioreactors | Physiologically relevant cues for emulating and promoting microtissue functions
|
| Biomimetic tissue microarchitectures | Cells and ECMs arranged in biomimetic microarchitectures for emulating and promoting microtissue functions
|
| Controlling units | Microfluidic pumps, actuators, valves, and other units for proper routing of the media/liquids within the single- or multi-chip platforms |
| Biosensors | Ideally microfluidics-integrated bioanalysis units (optical, physical, chemical) for in situ, on-chip, continual, and non-invasive monitoring of microtissue behaviors and drug interactions |
Footnotes
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record.
References
- 1.Bedossa P. Pathology of non-alcoholic fatty liver disease. Liver Int 2017;37 Suppl 1:85–89. [DOI] [PubMed] [Google Scholar]
- 2.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. 2005;41:1313–1321. [DOI] [PubMed] [Google Scholar]
- 3.van Grunsven LA. 3D in vitro models of liver fibrosis. Adv Drug Deliv Rev 2017;121:133–146. [DOI] [PubMed] [Google Scholar]
- 4.Cole BK, Feaver RE, Wamhoff BR, Dash A. Non-alcoholic fatty liver disease (NAFLD) models in drug discovery. Expert Opin Drug Discov 2018;13:193–205. [DOI] [PubMed] [Google Scholar]
- 5.Kozyra M, Johansson I, Nordling Å, Ullah S, Lauschke VM, Ingelman-Sundberg MJSr. Human hepatic 3D spheroids as a model for steatosis and insulin resistance. 2018;8:14297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pingitore P, Sasidharan K, Ekstrand M, Prill S, Lindén D, Romeo SJIjoms . Human Multilineage 3D Spheroids as a Model of Liver Steatosis and Fibrosis. 2019;20:1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DE C, DG N, D B, SC P, AE C. Modeling NAFLD Using 3D Bioprinted Human Liver Tissue In: The Liver Meeting, American Association for the Study of Liver Diseases. Washington, DC; 2017. [Google Scholar]
- 8.AE C, DE C, CC G, RN H, JT I, Norona, LM, et al. 3D Bioprinted Human Liver Tissue for Modeling Progressive Liver Disease In: World Preclinical Congress. Boston, MA; 2017. [Google Scholar]
- 9.Janorkar AV, King KR, Megeed Z, Yarmush ML. Development of an in vitro cell culture model of hepatic steatosis using hepatocyte-derived reporter cells. Biotechnology and bioengineering 2009;102:1466–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mannaerts I, Leite SB, Verhulst S, Claerhout S, Eysackers N, Thoen LF, Hoorens A, et al. The Hippo pathway effector YAP controls mouse hepatic stellate cell activation. J Hepatol 2015;63:679–688. [DOI] [PubMed] [Google Scholar]
- 11.Sancho-Bru P, Bataller R, Gasull X, Colmenero J, Khurdayan V, Gual A, Nicolás JM, et al. Genomic and functional characterization of stellate cells isolated from human cirrhotic livers. J Hepatol 2005;43:272–282. [DOI] [PubMed] [Google Scholar]
- 12.Khetani SR, Bhatia SNJNb. Microscale culture of human liver cells for drug development. 2008;26:120. [DOI] [PubMed] [Google Scholar]
- 13.Massa S, Seo J, Arneri A, Bersini S, Cha B-H, Antona S, Enrico A, et al. Bioprinted 3D vascularized tissue model for drug toxicity analysis. Biomicrofluidics 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang YS, Aleman J, Shin SR, Kilic T, Kim D, Mousavi Shaegh SA, Massa S, et al. Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proceedings of the National Academy of Sciences 2017;114:E2293–E2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Materne E-M, Tonevitsky AG, Marx U. Chip-based liver equivalents for toxicity testing – organotypicalness versus cost-efficient high throughput. Lab on a Chip 2013;13:3481–3495. [DOI] [PubMed] [Google Scholar]
- 16.Rennert K, Steinborn S, Gröger M, Ungerböck B, Jank A-M, Ehgartner J, Nietzsche S, et al. A microfluidically perfused three dimensional human liver model. Biomaterials 2015;71:119–131. [DOI] [PubMed] [Google Scholar]
- 17.Weng Y-S, Chang S-F, Shih M-C, Tseng S-H, Lai C-H. Scaffold-Free Liver-On-A-Chip with Multiscale Organotypic Cultures. Advanced Materials 2017;29:1701545. [DOI] [PubMed] [Google Scholar]
- 18.Lee PJ, Hung PJ, Lee LP. An artificial liver sinusoid with a microfluidic endothelial-like barrier for primary hepatocyte culture. Biotechnology and Bioengineering 2007;97:1340–1346. [DOI] [PubMed] [Google Scholar]
- 19.Amin AB, Jannick T, Jurgita P, Stefan W, Caroline BA, Mattias G. Design and fabrication of a scalable liver-lobule-on-a-chip microphysiological platform. Biofabrication 2017;9:015014. [DOI] [PubMed] [Google Scholar]
- 20.Domansky K, Inman W, Serdy J, Dash A, Lim MHM, Griffith LG. Perfused multiwell plate for 3D liver tissue engineering. Lab on a Chip 2010;10:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moravcová A, Červinková Z, Kučera O, Mezera V, Rychtrmoc D, Lotková H. The effect of oleic and palmitic acid on induction of steatosis and cytotoxicity on rat hepatocytes in primary culture. Physiological Research 2015;64:S627–623. [DOI] [PubMed] [Google Scholar]
- 22.Gori M, Simonelli MC, Giannitelli SM, Businaro L, Trombetta M, Rainer A. Investigating Nonalcoholic Fatty Liver Disease in a Liver-on-a-Chip Microfluidic Device. PLOS ONE 2016;11:e0159729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kostrzewski T, Cornforth T, Snow SA, Ouro-Gnao L, Rowe C, Large EM, Hughes DJ. Three-dimensional perfused human in vitro model of non-alcoholic fatty liver disease. World journal of gastroenterology 2017;23:204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SY, Sung JH. Gut–liver on a chip toward an in vitro model of hepatic steatosis. Biotechnology and Bioengineering 2018;115:2817–2827. [DOI] [PubMed] [Google Scholar]
- 25.Lee J, Choi B, No DY, Lee G, Lee S-r, Oh H, Lee S-H. A 3D alcoholic liver disease model on a chip. Integrative Biology 2016;8:302–308. [DOI] [PubMed] [Google Scholar]
- 26.Stojsavljević S, Palčić MG, Jukić LV, Duvnjak LS, Duvnjak MJWjogW. Adipokines and proinflammatory cytokines, the key mediators in the pathogenesis of nonalcoholic fatty liver disease. 2014;20:18070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santis AJRorct. Intestinal permeability in non-alcoholic fatty liver disease: the gut-liver axis. 2014;9:141–147. [DOI] [PubMed] [Google Scholar]
- 28.Park K-G, Min A-K, Koh EH, Kim HS, Kim M-O, Park H-S, Kim Y-D, et al. Alpha-lipoic acid decreases hepatic lipogenesis through adenosine monophosphate-activated protein kinase (AMPK)-dependent and AMPK-independent pathways. Hepatology 2008;48:1477–1486. [DOI] [PubMed] [Google Scholar]
- 29.Peng L, He Z, Chen W, Holzman IR, Lin J. Effects of Butyrate on Intestinal Barrier Function in a Caco-2 Cell Monolayer Model of Intestinal Barrier. Pediatric Research 2007;61:37. [DOI] [PubMed] [Google Scholar]
- 30.Peng L, Li Z-R, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. The Journal of nutrition 2009;139:1619–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas RJ, Bhandari R, Barrett DA, Bennett AJ, Fry JR, Powe D, Thomson BJ, et al. The Effect of Three-Dimensional Co-Culture of Hepatocytes and Hepatic Stellate Cells on Key Hepatocyte Functions in vitro. Cells Tissues Organs 2005;181:67–79. [DOI] [PubMed] [Google Scholar]
- 32.Ehrlich A, Tsytkin-Kirschenzweig S, Ioannidis K, Ayyash M, Riu A, Note R, Ouedraogo G, et al. Microphysiological flux balance platform unravels the dynamics of drug induced steatosis. Lab on a Chip 2018;18:2510–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deng J, Chen Z, Zhang X, Luo Y, Wu Z, Lu Y, Liu T, et al. A liver-chip-based alcoholic liver disease model featuring multi-non-parenchymal cells. 2019;21:57. [DOI] [PubMed] [Google Scholar]
- 34.Mikos AG, Sarakinos G, Leite SM, Vacanti JP, Langer R. Laminated three-dimensional biodegradable foams for use in tissue engineering. Biomaterials 1993;14:323–330. [DOI] [PubMed] [Google Scholar]
- 35.McCann JT, Marquez M, Xia YN. Highly porous fibers by electrospinning into a cryogenic liquid. Journal of the American Chemical Society 2006;128:1436–1437. [DOI] [PubMed] [Google Scholar]
- 36.Li D, Wang YL, Xia YN. Electrospinning of polymeric and ceramic nanofibers as uniaxially aligned arrays. Nano Letters 2003;3:1167–1171. [Google Scholar]
- 37.Li D, McCann JT, Xia YN. Use of electrospinning to directly fabricate hollow nanofibers with functionalized inner and outer surfaces. Small 2005;1:83–86. [DOI] [PubMed] [Google Scholar]
- 38.EmulateBio. Liver chip. In: Emulate bio; 2019. [Google Scholar]
- 39.Li D, Babel A, Jenekhe SA, Xia YN. Nanofibers of conjugated polymers prepared by electrospinning with a two-capillary spinneret. Advanced Materials 2004;16:2062-+. [Google Scholar]
- 40.McCann JT, Marquez M, Xia YN. Melt coaxial electrospinning: A versatile method for the encapsulation of solid materials and fabrication of phase change nanofibers. Nano Letters 2006;6:2868–2872. [DOI] [PubMed] [Google Scholar]
- 41.Li D, Xia YN. Direct fabrication of composite and ceramic hollow nanofibers by electrospinning. Nano Letters 2004;4:933–938. [Google Scholar]
- 42.BioIVT. Liver disease modeling. In; 2019.
- 43.ApreX. ApreX Biotech Spinoff Uses Origami for Drug Development. In: Northeastern University. [Google Scholar]
- 44.3DBiomatrix. 3D Biomatrix, LLC is a life sciences company developing and marketing revolutionary solutions for three dimensional (3D) cell cultures. In; 2019.
- 45.Mimetas. Human liver models. In: Mimetas. [Google Scholar]
- 46.Organovo. 3D human liver tissue testing services. In: Organovo. [Google Scholar]
- 47.CnBio. Liver Chip. In: CnBio Innovations. [Google Scholar]