Abstract
Background and aims
The long-term associations between zero, minimal coronary artery calcium (CAC) and cause-specific mortality are currently unknown, particularly after accounting for competing risks with other causes of death.
Methods
We evaluated 66,363 individuals from the CAC Consortium (mean age 54 years, 33% women), a multi-center, retrospective cohort study of asymptomatic individuals undergoing CAC scoring for clinical risk assessment. Baseline evaluations occurred between 1991 and 2010.
Results
Over a mean of 12 years of follow-up, individuals with CAC=0 (45% prevalence, mean age 45 years) had stable low rates of coronary heart disease (CHD) death, cardiovascular disease (CVD) death (ranging 0.32 to 0.43 per 1,000 person-years), and all-cause death (1.38 to 1.62 per 1,000 person-years). Cancer was the predominant cause of death in this group, yet rates were also very low (0.47 to 0.79 per 1,000 person-years). Compared to CAC=0, individuals with CAC 1–10 had an increased multivariable-adjusted risk of CVD death only under age 40. Individuals with CAC>10 had multivariable-adjusted increased risks of CHD death, CVD death and all-cause death at all ages, and a higher proportion of CVD deaths.
Conclusions
CAC=0 is a frequent finding among individuals undergoing CAC scanning for risk assessment and is associated with low rates of all-cause death at 12 years of follow-up. Our results support the emerging consensus that CAC=0 represents a unique population with favorable all-cause prognosis who may be considered for more flexible treatment goals in primary prevention. Detection of any CAC in young adults could be used to trigger aggressive preventive interventions.
Keywords: cancer, cardiovascular disease, competing risks, coronary artery calcium, mortality, risk
Graphical Abstract
Introduction
There is increasing interest in zero coronary artery calcium (CAC) as a marker of sustained good health [1,2]. Prior studies have reported very low rates of coronary heart disease (CHD) events, cardiovascular disease (CVD) events, and all-cause mortality in the presence of a CAC score of zero (CAC=0) [3–12]. Indeed, CAC=0 appears to be the single strongest “negative risk factor” for incident CVD [13]. Consistent with this, recent guidelines from the American College of Cardiology/American Heart Association (ACC/AHA) have endorsed CAC=0 as a powerful marker of low CVD risk among individuals with borderline/intermediate risk estimations [14].
Intriguingly, recent reports have also linked the absence of CAC with low rates of cancer and other non-CVD events such as incident chronic obstructive lung disease, chronic kidney disease, hip fracture, and dementia [15]. This has led to the hypothesis that the absence of CAC may be a marker of “healthy aging”. Supporting this view, even minimal CAC (scores of 1–10) have been associated with higher CVD events and all-cause mortality as compared to CAC=0 [9,10].
However, currently there are little data available on the long-term associations of CAC=0, CAC 1–10, and higher CAC scores, particularly after accounting for competing risks with other cause-specific mortality. The predominant cause of the infrequent deaths in people with CAC=0 is unknown, and the impact of increasing CAC scores on CVD versus non-CVD causes of death remains unclear—as well as the importance of age and sex on these relationships.
Given the complicated association of CAC with risk of multiple diseases, we sought to conduct a competing risk analysis studying zero and minimal CAC within the CAC Consortium, a large cohort with long follow-up for cause-specific death [16]. Such data may be important for estimating prognosis and informing preventive strategies among individuals at the low end of the risk spectrum.
Materials and methods
The CAC consortium
The characteristics of the CAC Consortium have been described elsewhere [16–18]. Briefly, this was a multi-center, retrospective cohort study of 66,636 consecutive individuals undergoing clinical CAC scoring for CVD risk assessment purposes in 4 high-volume US centers. All participants were free of established CVD at cohort entry, defined as the date of the baseline CAC examination. Baseline data including demographic characteristics, cardiovascular risk factors, and baseline CAC scores were obtained at cohort entry between 1991 and 2010, and follow-up information was obtained through June 2014 [16]. For the present analysis, all 66,363 individuals from the CAC Consortium were included.
Research ethics
Written informed consent for participation in research was collected at all centers prior to the baseline CAC scan. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, and institutional review board approval for coordinating center activities including death ascertainment and death certificate collection was obtained at the Johns Hopkins Hospital (Baltimore [MD], USA).
Baseline evaluation
All individuals from the CAC Consortium underwent a baseline computed tomographic (CT) scan for CAC quantification. This included individuals scanned using electron beam tomography (93%), as well as in later years, individuals scanned using multi-detector CT (7%). A common standard non-contrast cardiac-gated CT scanning protocol was used across sites, adapted to each CT scanner technology. CAC was scored using the Agatston method [19]. For the present analyses, participants were categorized into three groups: CAC=0, minimal CAC (1–10), and CAC>10 [9,10].
In each center, data on self-reported cardiovascular risk factors, treatment use, and laboratory test results were collected as part of the routine clinical visit associated with the referral for CAC testing and/or from a semi-structured in-person interview. Details on the definitions used for each cardiovascular risk factor [16–18] are summarized in the Supplementary Methods.
Outcome definitions and ascertainment
Mortality was assessed via linkage of patient records with the Social Security Administration Death Master File using a previously validated algorithm [16]. Death certificates were obtained from the National Death Index, and the underlying cause of death was categorized into common causes of death using the International Classification of Diseases, versions 9 and 10 codes as previously described [16].
The 4 primary outcomes for the present study were all-cause, CHD, CVD, and cancer mortality, all assessed over a mean of 12 years follow-up (maximum follow-up across sites ranging from 13.6 to 22.5 years). Additional study outcomes included stroke mortality, heart failure mortality, other circulatory disorder mortality (non-CHD, non-stroke), total non-CVD mortality (death from all causes except for CVD), and pulmonary death.
Statistical analyses
The incidence proportion (%) at a mean of 12 years of follow-up of all-cause and cause-specific death was calculated overall and by baseline CAC burden. All-cause and cause-specific incident mortality rates during follow-up were also calculated at each year of follow-up, expressed per 1,000 patient-years.
Multivariable-adjusted Cox proportional hazards regression models were used to assess the associations between increasing baseline CAC burden (CAC 1–10 and CAC>10, respectively, compared to CAC=0) and all-cause mortality. For cause-specific death outcomes, competing risks regression using Fine and Gray models [20] were used to examine the associations between CAC and CHD death, CVD death, and cancer death, respectively, accounting for competing risks with other causes of death. These results were presented using sub-distribution hazard ratios (SHR) and 95% confidence intervals (CI). Two regression models were used: Model 1 was unadjusted, and Model 2 adjusted for age, sex, hypertension, current smoking, diabetes, dyslipidemia, and family history of CHD. The Proportional Hazards assumption was met up to 15 years of follow-up, with slight deviance from proportionality after 15 years due in part to small numbers.
Likelihood ratio tests for all-cause death were conducted for interaction terms by age, sex, and by three clinical characteristics considered markers of increased CVD risk by recent ACC/AHA guidelines: diabetes, current tobacco use, and family history of CVD [14]. The death rates of the latter subgroups were also described.
Two sensitivity analyses were conducted. First, to account for potential residual confounding by age, a sensitivity analysis adjusted for age2 rather than linear age. Second, in a complete-case analysis restricted to the 65% study participants in whom information on race/ethnicity was available we further adjusted for this covariate.
Finally, exploratory analyses were also pursued, assessing the multivariable-adjusted associations between CAC categories and other relevant causes of death: stroke, heart failure, other circulatory causes, any non-CVD cause, and pulmonary death.
All analyses were conducted using Stata version 15 [21]. A threshold of <0.05 was used to define statistical significance.
Results
Baseline characteristics
The mean age of the 66,363 study participants was 54.5 years, 33% were women, and the vast majority in whom race/ethnicity data were available were non-Hispanic Whites (89.1%) (Table 1). Dyslipidemia was the most prevalent cardiovascular risk factor (56.8%) while diabetes was the least (6.8%). Median estimated 10-year ASCVD risk using the ACC/AHA Pooled Cohort Equations was 4.4%.
Table 1.
Baseline characteristics of the study population.
| Total N=66,636 | Baseline CAC score |
|||
|---|---|---|---|---|
| =0 N=29,757 | 1–10 N=7,808 | >10 N=29,071 | ||
| Age, years | 54.4 (10.6) | 49.9 (9.2) | 52.7 (9.3) | 59.5 (10.0) |
| Women | 22,003 (33.0) | 13,230 (44.5) | 2,153 (27.6) | 6,620 (22.8) |
| Race/Ethnicity (N=42,964) | ||||
| Non-Hispanic White | 38,277 (89.1) | 16,933 (88.7) | 4,308 (87.5) | 17,036 (89.9) |
| Asian | 1,621 (3.8) | 794 (4.2) | 181 (3.7) | 646 (3.4) |
| Black | 977 (2.3) | 429 (2.3) | 140 (2.8) | 408 (2.2) |
| Hispanic | 1,349 (3.1) | 620 (3.3) | 188 (3.8) | 541 (2.9) |
| Hypertension | 20,625 (31.0) | 6,782 (22.8) | 2,291 (29.3) | 11,552 (39.7) |
| Diabetes | 4,503 (6.8) | 1,163 (3.9) | 464 (5.9) | 2,876 (9.9) |
| Dyslipidemia | 37,861 (56.8) | 15,112 (50.8) | 4,466 (57.2) | 18,283 (62.9) |
| Current smoking | 6,400 (9.6) | 2,646 (8.9) | 718 (9.2) | 3,036 (10.4) |
| Family history of CHD | 30,721 (45.6) | 13,567 (45.6) | 3,719 (47.6) | 13,435 (46.2) |
| 10-Year ASCVD riska | 4.4 (1.9, 9.2) | 2.4 (1.2, 4.7) | 4.0 (2.0, 7.5) | 7.9 (4.1, 14.8) |
| ASCVD riska categories | ||||
| <5% | 36,793 (55.2) | 22,882 (76.9) | 4,688 (60.0) | 9,223 (31.7) |
| 5–7.5% | 8,939 (13.4) | 3,181 (10.7) | 1,163 (14.9) | 4,595 (15.8) |
| 7.5–20% | 15,665 (23.5) | 3,264 (11.0) | 1,679 (21.5) | 10,722 (36.9) |
| >20% | 5,239 (7.86) | 430 (1.45) | 278 (3.56) | 4,531 (15.6) |
Estimated using the Pooled Cohort Equations
Categorical variables presented as number (percentage), and continuous variables presented as mean (standard deviation) or median (interquartile range). All p values for the comparison across CAC categories were <0.001 except for family history of CHD (0.01)
ASCVD = atherosclerotic cardiovascular disease risk; CAC = coronary artery calcium; CHD = coronary heart disease
At baseline, 44.7% participants had CAC=0, 11.7% had CAC 1–10, and 43.6% had CAC>10. Individuals with higher CAC scores were significantly older, more likely to be male, had a greater burden of traditional CVD risk factors, and had a higher average 10-year estimated ASCVD risk.
Incident Deaths during Follow-Up
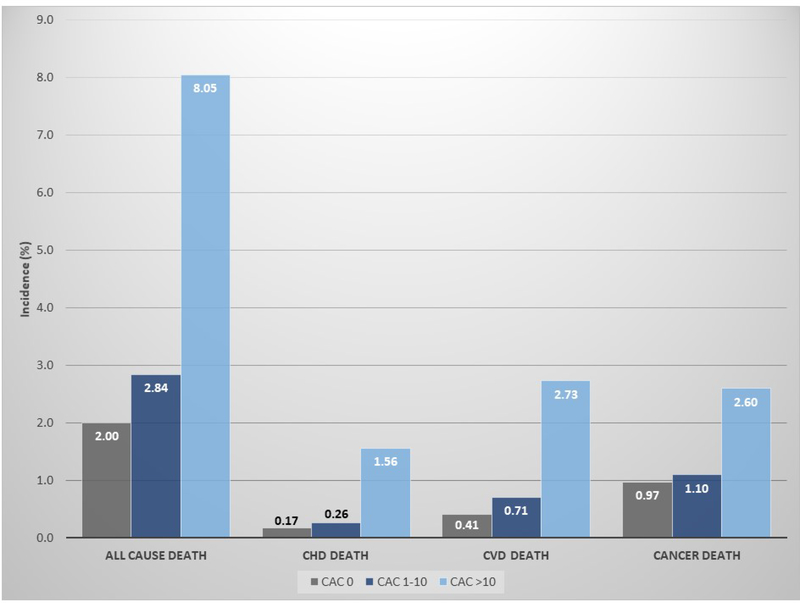
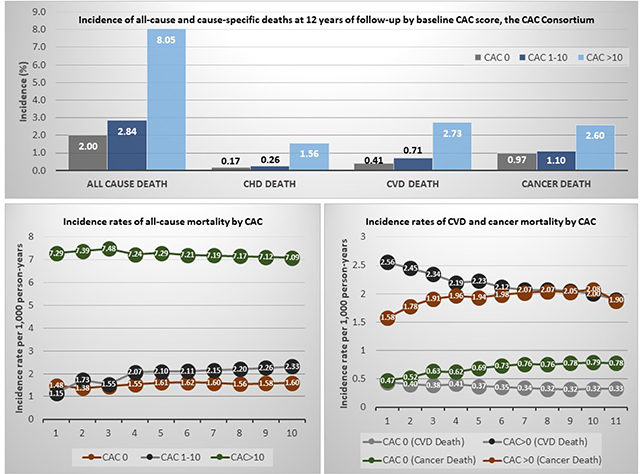
Over a 12-year mean follow-up, 3,158 deaths occurred. The lowest all-cause and cause-specific crude mortality risks were observed among individuals with CAC=0 (0.17% for CHD mortality, 0.41% for CVD mortality, and 0.97% for cancer mortality) (Figure 1). All risks of death were slightly higher for individuals in the CAC 1–10 group, and substantially higher for those with CAC>10. The proportion of deaths due to CHD and other CVD causes increased with increasing CAC scores, while the proportion of cancer deaths decreased (Supplementary Figure 1).
Figure 1.
Incidence proportion of all-cause and cause-specific deaths at 12 years of follow-up, by baseline CAC score.
Results presented as incidence proportions, in %. CAC = coronary artery calcium; CHD = coronary heart disease; CVD = cardiovascular disease
Trends in All-Cause and Cause-Specific Death Rates over Time
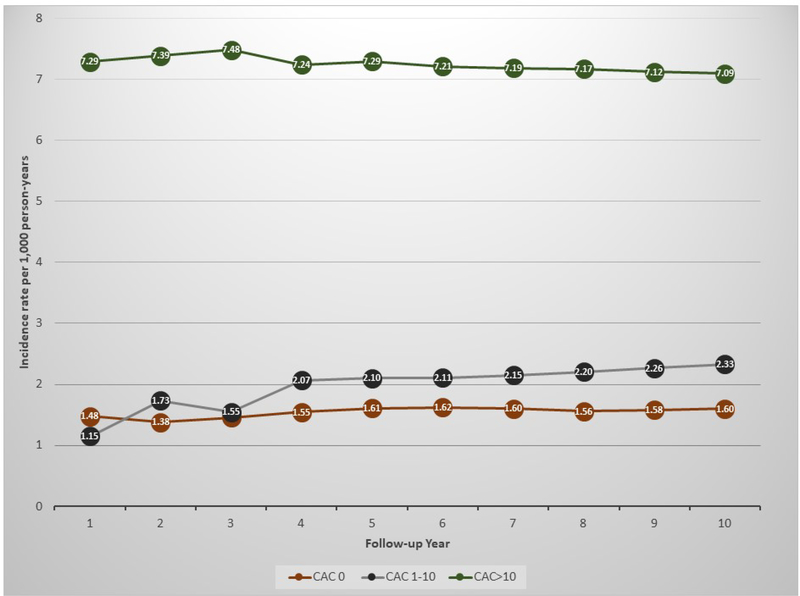
Among individuals with CAC=0, crude incidence rates of all-cause death were stable and low over time and were the lowest across all study groups, ranging 1.38 to 1.62 per 1,000 person-years (Figure 2 and Supplementary Figure 2). Death rates were slightly higher among individuals with CAC 1–10, although the highest rates were observed among individuals with CAC >10, approximately 4-fold higher than those of CAC=0.
Figure 2.
Incidence rates of all-cause mortality during follow-up, by baseline CAC score.
Results are presented as incidence rates between baseline and up to each year of follow-up, per 1,000 person-years. The X axis presents number of years of follow-up. CAC = coronary artery calcium
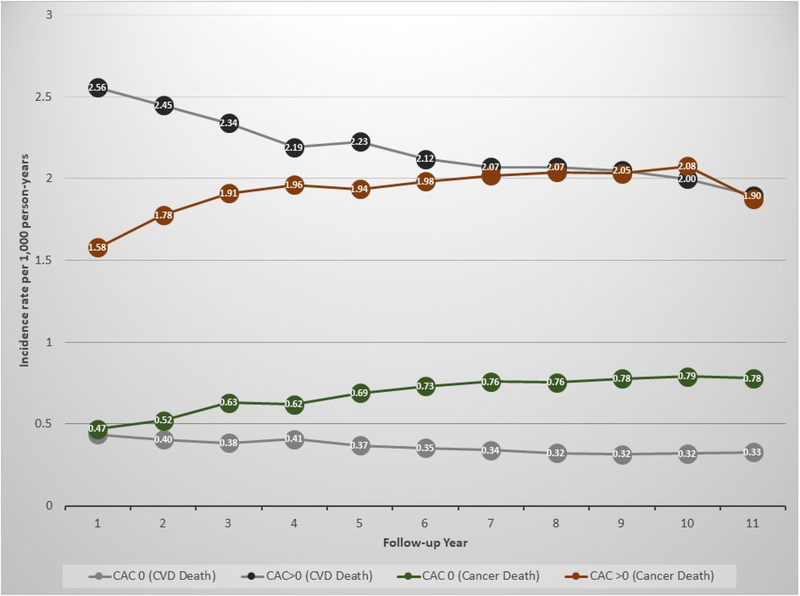
In both individuals with CAC=0 and CAC>0, there was a progressive decline in the rates of CVD death over time, the largest absolute decreases observed among individuals with CAC>0 and the largest relative decreases in those with CAC=0 (Figure 3). In parallel, there was a progressive increase in the incidence of cancer death rates in both study groups. Specifically, among individuals with CAC=0, at the end of follow-up cancer death rates were roughly 2.4-fold higher than those for CVD death.
Figure 3.
Incidence rates of CVD death and cancer death during follow-up, by baseline CAC score.
Results are presented as incidence rates between baseline and up to each year of follow-up, per 1000 person-years. The X axis presents number of years of follow-up. CAC = coronary artery calcium; CVD = cardiovascular disease
Associations between Baseline CAC, All-Cause and Cause-Specific Mortality
In unadjusted analyses, compared to individuals with CAC=0, those with CAC 1–10 had a 1.4-fold increased risk of death from any cause, while those with CAC>10 had a 4-fold increased risk (Table 2). After adjusting for traditional risk factors, there was no longer an independent association between CAC 1–10 and all-cause death, while individuals with CAC>10 still had a 1.6-fold increased risk of all-cause death compared to those with CAC=0.
Table 2.
Associations between baseline CAC burden, all-cause and cause-specific mortality, overall and by age strata.
| Overall | Age strata | |||||
|---|---|---|---|---|---|---|
| <40 years (N=4,855) | 40–50 years (N=17,802) | 50–60 years (N=24,838) | 60–70 years (N=13,418) | ≥70 years (N=5,723) | ||
| All-cause death | ||||||
| CAC=0 (Ref.) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| CAC 1–10 | 1.04 (0.89, 1.22) | 2.91 (1.41, 6.00) | 0.80 (0.52, 1.23) | 1.20 (0.92, 1.58) | 0.98 (0.74, 1.31) | 0.97 (0.68, 1.40) |
| CAC>10 | 1.63 (1.48, 1.81) | 2.90 (1.36, 6.21) | 1.81 (1.37, 2.38) | 1.77 (1.47, 2.13) | 1.43 (1.19, 1.72) | 1.80 (1.44, 2.25) |
| CVD death | ||||||
| CAC=0 (Ref.) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| CAC 1–10 | 1.22 (0.88, 1.68) | 4.45 (1.34, 14.85) | 1.17 (0.56, 2.42) | 1.18 (0.59, 2.34) | 1.07 (0.57, 2.01) | 1.15 (0.59, 2.26) |
| CAC>10 | 2.31 (1.88, 2.85) | 3.88 (0.92, 16.37) | 1.87 (1.11, 3.16) | 3.17 (2.07, 4.86) | 2.11 (1.39, 3.19) | 2.33 (1.52, 3.56) |
| CHD death | ||||||
| CAC=0 (Ref.) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| CAC 1–10 | 1.00 (0.59, 1.68) | 1.25 (0.14, 10.91) | 1.67 (0.57, 4.91) | 1.74 (0.66, 4.58) | 0.42 (0.12, 1.47) | 0.66 (0.21, 2.09) |
| CAC>10 | 2.83 (2.07, 3.86) | 4.12 (0.66, 25.85) | 2.97 (1.32, 6.69) | 5.08 (2.68, 9.63) | 1.89 (1.08, 3.31) | 2.43 (1.33, 4.46) |
| Cancer death | ||||||
| CAC=0 (Ref.) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| CAC 1–10 | 0.88 (0.69, 1.12) | - | 0.76 (0.34, 1.70) | 0.91 (0.62, 1.35) | 0.88 (0.58, 1.34) | 0.78 (0.42, 1.45) |
| CAC>10 | 1.19 (1.02, 1.40) | 1.64 (0.14, 19.00) | 1.34 (0.79, 2.23) | 1.08 (0.82, 1.41) | 1.07 (0.82, 1.40) | 1.23 (0.84, 1.78) |
Results presented as hazard ratios from Cox proportional hazards regression analyses (all-cause death), and sub-distribution hazard ratios from Fine and Gray analyses accounting for competing risks (cause-specific death)
Analyses adjusted for age, sex, hypertension, hyperlipidemia, family history of CHD, diabetes, and current smoking status
CAC = coronary artery calcium; CHD = coronary heart disease; CVD = cardiovascular disease; Ref. = reference
In competing risk Fine and Gray analyses, there was a strong multivariable-adjusted association between CAC>10 (as compared to CAC=0) and CVD death (SHR 2.31, 95% CI 1.88, 2.85). The association was even stronger between CAC>10 (as compared to CAC=0) and CHD death (SHR 2.83, 95% CI 2.07, 3.86). On the other hand, the association between CAC>10 and cancer death was much weaker (SHR 1.19, 95% CI 1.02, 1.40). For CAC 1–10, all multivariable-adjusted 95% CIs included 1.00.
Subgroup analyses
There was a statistically significant interaction between CAC and age for all-cause death (p value 0.002). In stratified analyses, strong multivariable-adjusted associations were observed between CAC>10 (as compared to CAC=0), all-cause death, CVD death and CHD death in all age strata (Table 2). The strongest associations with all-cause death and CVD death were observed in the <40 years group, although confidence intervals were wide. Strong multivariable-adjusted associations were also observed between CAC 1 – 10 (as compared to CAC=0), all-cause and CVD death among individuals <40 years, while these associations tended to be progressively weaker and non significant in increasing age strata.
Interaction tests by sex, diabetes, current tobacco use, and family history of CVD yielded non-significant results (p value of all likelihood ratio tests >0.05, Supplementary Tables 1 and 2). For any CAC strata, higher all-cause death rates were observed in individuals with diabetes, current tobacco users, and with a family history of CVD as compared to the overall study population (Supplementary Figure 3). However, CVD death rates remained very low in CAC=0 participants from the three subgroups, the highest being observed in diabetic patients (0.76 per 1,000 person-years).
Sensitivity and exploratory analyses
The results of the sensitivity analysis adjusting for age2 were consistent with those from the main analysis (Supplementary Table 3). Similarly, analyses further adjusting for race/ethnicity did not significantly alter the main results (data not shown).
Compared to CAC=0, CAC>10 was associated with a significantly higher risk of all evaluated causes of death including stroke, heart failure, other circulatory disease, total non-CVD death, and pulmonary death (Supplementary Table 4). Very strong multivariable adjusted associations were also observed between CAC 1–10 (as compared to CAC=0), heart failure death and pulmonary death, although for the former outcome the 95% CIs were wide.
Discussion
In the first large competing risks analysis of zero and minimal CAC and long-term cause-specific death, including 66,363 apparently healthy individuals undergoing clinical CAC scanning, we demonstrated that those with CAC=0 (representing 45% of the study population) had stable low rates of CHD death, CVD death and all-cause death at 12 years of follow-up. Cancer was the predominant cause of the infrequent deaths in this group. Individuals with CAC 1–10 had a greater incidence of deaths from CHD and CVD than CAC=0 participants, although in multivariable-adjusted competing risk models a persistent relationship with increased CVD mortality was only present among individuals <40 years old. Individuals with CAC>10 had multivariable-adjusted increased risks of CHD, CVD and all-cause mortality compared to those with CAC=0, particularly among participants <40 years; with a greater proportion of deaths due to CVD vs. non-CVD causes. CVD death even rates remained low in the presence of CAC= 0 in diabetics, current smokers, and individuals with a family history of CVD.
Our results extend the work of prior studies examining the association of zero CAC, minimal (i.e., 1–10) CAC, and all-cause mortality. Our group previously demonstrated a very low death rate —approximately 0.5% over 5 years— in a subset of the CAC Consortium population with CAC=0 [3]. More recently, Valenti et al. also found a very low mortality rate in asymptomatic individuals with CAC=0 undergoing CAC scoring in a single US center extending to 15-year follow-up [4]. However, these two prior studies only assessed all-cause rather than case-specific mortality. Our present analysis adds to the existing literature by showing that among individuals undergoing clinical CAC scanning who are found to have CAC=0, death is a rare event at 12 years of follow-up, CHD and CVD deaths are both very infrequent, and cancer is the leading cause of the infrequent deaths in this overall-healthy group.
Our observations are also consistent with prior studies showing low risk of CHD and CVD events among individuals with CAC=0. For example, Budoff et al. observed very low risk of CHD events amongst participants from the Multi-Ethnic Study of Atherosclerosis (MESA) with CAC=0, with a 3-fold higher death rate for those with minimal CAC 1–10 [9]. Silverman et al. demonstrated very low rates of percutaneous coronary intervention and coronary artery bypass surgery over 8.5 years of follow-up in CAC=0 MESA participants [11]. Expanding on non-CHD CVD endpoints, Gibson et al. also demonstrated a low risk of stroke in MESA participants with zero CAC [12]. Of note, as compared to the analysis by Budoff et al., in our study a multivariable-adjusted association between CAC 1–10 (as compared to CAC=0) and increased risk of CVD death was observed only in young adults. Potential explanations to this discrepancy include an older mean age in MESA and other baseline differences between the two study populations, consideration only of fatal events in the present analysis, and use of competing risk modelling to account for competing causes of death in the present study.
The present results have important clinical implications. First, they provide further support to recent US clinical practice guideline recommendations, which in recent years have given increasing recognition to the “power of zero” [22]. This includes the 2017 guidelines from the Society of Cardiovascular Computed Tomography [23], which articulated CAC=0 as a clinically actionable result, driving an enhanced clinician-patient risk discussion, with potential for selecting more flexible preventive treatment goals amongst these very low risk individuals. Subsequently, the 2018 and 2019 ACC/AHA Cholesterol and Prevention of CVD guidelines brought CAC=0 to the forefront as a highly valuable tool for downgrading risk estimates in patients who would otherwise be considered candidates for chronic statin therapy [14,24]. The fact that in our cohort almost half of the study population had a CAC score of zero (which is consistent with reports from other cohorts [3–10,25]) supports the potential usefulness of CAC=0 in a broad borderline/intermediate risk group.
Second, although cancer was the predominant cause of death among individuals with CAC=0 from our study, death from cancer or any other cause was a rare event at 12 years of follow-up in this healthy group. Ours is not the first study to describe the low risk of non-CVD events among individuals with zero CAC. In MESA, Handy et al. reported low 10-year rates of incident cancer, chronic kidney disease, chronic obstructive pulmonary disease, hip fracture, and dementia-related hospitalizations in participants with CAC=0 [15]. These observations are consistent with the understanding that CAC serves as integrator of most upstream risk exposures and of vulnerability to their effects. Individuals with CAC=0, and particularly those with persistent CAC=0 over time [26,27] represent a unique group of overall “healthy agers”, largely resilient to atherosclerosis and to other pathological mechanisms of disease, deserving further study.
Third, the fact that CAC 1–10 was associated with increased mortality (as compared to CAC=0) only in young adults highlights the importance of considering not only absolute but also relative scores (within age and sex strata) when interpreting a CAC score in a given patient [28]. While in elderly individuals a CAC score of 1–10 represents a relatively low burden within the distribution for this age group [1,28] (i.e., below the 25th percentile of the CAC distribution for men ages 75–84 years included in MESA [28]) the same score in a 35–45 year-old individual identifies a marked risk increase compared to age- and sex-matched peers. Importantly, our findings for the subgroup <40 years of age, in which multivariable-adjusted associations between any CAC, CVD and all-cause death were particularly strong, suggest that early, aggressive lifestyle and even pharmacological interventions in those with any detectable CAC could be highly beneficial. Detection of higher CAC burden (i.e., CAC>10) in these individuals could trigger even stronger consideration of those interventions. Nevertheless, in both instances evidence from randomized trials is needed to better understand the actual value of CAC guiding preventive therapy allocation.
Strengths of the present study include large sample size, long mean follow-up, and ascertainment of cause-specific mortality. These also allowed to examine additional causes of death including stroke, heart failure, and pulmonary disease. In addition, a key strength and novel contribution to the CAC=0 literature is the use of competing risk Fine and Gray modeling, which provides more accurate estimates of cause-specific risk in the presence of competing events in prognostic studies. Recent international epidemiological data have shown that cancer deaths have become twice as frequent as CVD deaths in developed countries [29], which stresses the need to appropriately account for competing risks with cancer death in cardiovascular epidemiological analyses in Western countries.
Study Limitations
This was a clinical population of asymptomatic individuals referred for CAC scoring. While this may reduce generalizability of our results to certain unselected populations, our study should be highly generalizable to persons commonly referred for CAC scoring in clinical practice. Specifically, it is possible that young adults undergoing CAC assessment for early ASCVD risk stratification represent a selected subgroup with a high burden of specific risk factors, such as hypercholesterolemia or family history of ASCVD. Once again, while our results may have a limited generalizability in unselected populations, they can be used to inform clinician-patient discussions of young adults undergoing CAC scoring in the US. Also, inclusion of a mostly White patient population in the CAC Consortium may also limit generalizability of the present findings to other racial/ethnic groups, although prior studies have shown that CAC=0 is as predictive of optimal prognosis in other racial/ethnic groups [3–13,28].
Second, information on treatment initiation after CAC scoring was not available. While a limitation, subsequent treatment with pharmacotherapies such as statins would be generally expected to yield a conservative bias in the CHD/CVD analyses, as patients with CAC>0 would be more likely to be treated with those therapies, reducing their likelihood of developing incident events and death.
Third, consistent with the changing epidemiology of CVD vs. cancer mortality [29], there were likely cohort effects within our broad study period, with higher CVD death rates among participants enrolled earlier in our study. This likely explains, at least partly, why CHD and CVD mortality in CAC=0 drifted down with time over our study, while cancer mortality rose.
Fourth, it is possible that some individuals with CAC=0 included in the CAC Consortium had been referred for CT assessment for other, non-coronary health concerns, their CAC score being assessed as part of the same exam. This would explain the counterintuitive, slightly higher death rates (particularly cancer death) observed for CAC=0 as compared to CAC 1–10 during the first year of follow-up. This may have yielded a conservative bias when comparing individuals with CAC=0 (who would be at increased risk of death) to those with minimal CAC.
Finally, there were likely some missed deaths in the CAC Consortium. Our prior analyses have suggested that mortality rates may be 15–30% higher than we report, due to limitations inherent in vital status ascertainment in the US [16]. However, this phenomenon should be non-differential across causes of death, which would be expected to bias the results towards the null. Moreover, even accounting for missed events, individuals with CAC=0 would still have a highly favorable prognosis (<3 deaths per 1000 patient-years).
Conclusions
We have shown that zero CAC, which is a frequent finding in a clinical referral population, is associated with stable very low rates of CHD and CVD mortality over 12-year mean follow-up, with cancer as the predominant (twice as likely) cause among the infrequent deaths in these individuals. Our results support the emerging consensus that CAC=0 identifies a unique population with highly favorable all-cause prognosis, who may be considered for more flexible treatment goals in primary prevention. On the other hand, individuals <40 years with minimal CAC are at increased risk, and should be considered a distinct risk group in which early, aggressive preventive interventions could be considered. At any age, a CAC score >10 is associated with a markedly increased risk of death from any cause compared to CAC=0 individuals, with CVD death more common than cancer death. Further research with even longer follow-up is needed to better understand the mechanisms underlying the “healthy aging” observed among individuals with CAC=0, as well as their lifetime trajectory.
Supplementary Material
HIGHLIGHTS.
We evaluated 66,363 individuals from the Coronary Artery Calcium (CAC) Consortium
CAC=0 participants had stable low 12-year rates of cardiovascular death
Cancer was the predominant cause of the infrequent deaths in the CAC=0 group
CAC 1–10 was associated with higher risk of cardiovascular death at ages <40
CAC>10 was associated with higher risk of cardiovascular and all-cause death
Acknowledgments
FINANCIAL SUPPORT
Dr. Blaha has received support from NIH award L30 HL110027 for this project.
Footnotes
CONFLICTS OF INTEREST
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Mortensen MB, Fuster V, Muntendam P, Mehran R, Baber U, Sartori S, Falk E. Negative Risk Markers for Cardiovascular Events in the Elderly. J Am Coll Cardiol. 2019;74:1–11. [DOI] [PubMed] [Google Scholar]
- 2.Blaha MJ, Blankstein R, Nasir K. Coronary Artery Calcium Scores of Zero and Establishing the Concept of Negative Risk Factors. J Am Coll Cardiol. 2019;74:12–14. [DOI] [PubMed] [Google Scholar]
- 3.Blaha M, Budoff MJ, Shaw LJ, Khosa F, Rumberger JA, Berman D, Callister T, Raggi P, Blumenthal RS, Nasir K. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging. 2009;2:692–700. [DOI] [PubMed] [Google Scholar]
- 4.Valenti V, Hartaigh BO, Cho I, Schulman-Marcus J, Gransar H, Heo R, Truong Q, Shaw LJ, Knapper J, Kelkar AA, Sciarretta S, Chang HJ, Callister TQ, Min JK. Absence of Coronary Artery Calcium Identifies Asymptomatic Diabetic Individuals at Low Near-Term But Not Long-Term Risk of Mortality: A 15-Year Follow-Up Study of 9715 Patients. Circ Cardiovasc Imaging. 2016;9:e003528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarwar A, Shaw LJ, Shapiro MD, Blankstein R, Hoffman U, Cury RC, Abbara S, Brady TJ, Budoff MJ, Blumenthal RS, Nasir K. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging. 2009;2:675–688 [DOI] [PubMed] [Google Scholar]
- 6.Martin SS, Blaha MJ, Blankstein R, Agatston AA, Rivera JJ, Virani VS, Ouyang P, Jones SR, Blumenthal RS, Budoff MJ, Nasir K. Dyslipidemia, Coronary Artery Calcium, and Incident Atherosclerotic Cardiovascular Disease: Implications for Statin Therapy from the Multi-Ethnic Study of Atherosclerosis. Circulation. 2014;129:77–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silverman MG, Blaha MJ, Krumholz HM, Budoff MJ, Blanstein R, Sibley CT, Agatston AS, Blumenthal RS, Nasir K. Impact of Coronary Artery Calcium on Coronary Heart Disease Events in Individuals at the Extremes of Traditional Risk Factor Burden: The Multi-Ethnic Study of Atherosclerosis (MESA). EurHeart J. 2014; 35:2232–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nasir K, Bittencourt M, Blaha MJ, Budoff MJ, Blankstein RB, Agatston A, Shaw LJ, Sibley CT, Blumenthal RS, Krumholz HM. Implications of Coronary Artery Calcium Testing Among Statin Candidates According to American College of Cardiology/American Heart Association Cholesterol Management Guidelines. J Am Coll Cardiol. 2015;66:1657–1668. [DOI] [PubMed] [Google Scholar]
- 9.Budoff MJ, McClelland RL, Nasir K, Greenland P, Kronmal RA, Kondos GT, Shea S, Lima JA, Blumenthal RS. Cardiovascular events with absent or minimal coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA). Am Heart J. 2009;158:554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joshi PH, Blaha MJ, Budoff MJ, Miedema MD, McClelland RL, Lima JAC, Agatston AS, Blankstein R, Blumenthal RS, Nasir K. The 10-Year Prognostic Value of Zero and Minimal CAC. JACC Cardiovasc Imaging. 2017;10:957–958. [DOI] [PubMed] [Google Scholar]
- 11.Silverman MG, Harkness JR, Blankstein R, Budoff MJ, Agatston AS, Carr JJ, Lima JA, Blumenthal RS, Nasir K, Blaha MJ. Baseline subclinical atherosclerosis burden and distribution are associated with frequency and mode of future coronary revascularization: multi-ethnic study of atherosclerosis. JACC Cardiovasc Imaging. 2014;7:476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibson AO, Blaha MJ, Arnan MK, Sacco RL, Szklo M, Herrington DM, Yeboah J. Coronary artery calcium and incident cerebrovascular events in an asymptomatic cohort. The MESA Study. JACC Cardiovasc Imaging. 2014;7:1108–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blaha MJ, Cainzos-Achirica M, Greenland P, McEvoy JW, Blankstein R, Budoff MJ, Dardari Z, Sibley CT, Burke GL, Kronmal RA, Szklo M, Blumenthal RS, Nasir K. Role of Coronary Artery Calcium Score of Zero and Other Negative Risk Markers for Cardiovascular Disease: The Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2016;133:849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Muñoz D, Smith SC Jr, Virani SS, Williams KA Sr, Yeboah J, Ziaeian B. 2019. ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. Circulation. 2019 March 17: 10.1161/CIR.0000000000000678. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Handy CE, Desai CS, Dardari ZA, Al-Mallah MH, Miedema MD, Ouyang P, Budoff MJ, Blumenthal RS, Nasir K, Blaha MJ. The Association of Coronary Artery Calcium With Noncardiovascular Disease: The Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc Imaging. 2016;9:568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blaha MJ, Whelton SP, Al Rifai M, Dardari ZA, Shaw LJ, Al-Mallah MH, Matsushita K, Rumberger JA, Berman DS, Budoff MJ, Miedema MD, Nasir K. Rationale and design of the coronary artery calcium consortium: A multicenter cohort study. J Cardiovasc Comput Tomogr. 2017;11:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw LJ, Min JK, Nasir K, Xie JX, Berman DS, Miedema MD, Whelton SP, Dardari ZA, Rozanski A, Rumberger J, Bairey Merz CN, Al-Mallah MH, Budoff MJ, Blaha MJ. Sex differences in calcified plaque and long-term cardiovascular mortality: observations from the CAC Consortium. Eur Heart J. 2018;39:3727–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orimoloye OA, Budoff MJ, Dardari ZA, Mirbolouk M, Uddin SMI, Berman DS, Rozanski A, Shaw LJ, Rumberger JA, Nasir K, Miedema MD, Blumenthal RS, Blaha MJ. Race/Ethnicity and the Prognostic Implications of Coronary Artery Calcium for All-Cause and Cardiovascular Disease Mortality: The Coronary Artery Calcium Consortium. J Am Heart Assoc. 2018;7:e010471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- 20.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association, Vol. 94, No. 446 (June, 1999), pp. 496–509. [Google Scholar]
- 21.StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC. [Google Scholar]
- 22.Nasir K Message for 2018 Cholesterol Management Guidelines Update: Time to Accept the Power of Zero. J Am Coll Cardiol. 2018;72:3243–3245. [DOI] [PubMed] [Google Scholar]
- 23.Hecht H, Blaha MJ, Berman DS, Nasir K, Budoff M, Leipsic J, Blankstein R, Narula J, Rumberger J, Shaw LJ. Clinical indications for coronary artery calcium scoring in asymptomatic patients: Expert consensus statement from the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr. 2017;11:157–168. [DOI] [PubMed] [Google Scholar]
- 24.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC Jr, Sperling L, Virani SS, Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenland P, Bonow RO. How low-risk is a coronary calcium score of zero? The importance of conditional probability. Circulation. 2008;117:1627–1629. [DOI] [PubMed] [Google Scholar]
- 26.Lehmann N, Erbel R, Mahabadi AA, Rauwolf M, Mohlenkamp S, Moebus S, Kälsch H, Budde T, Schmermund A, Stang A, Führer-Sakel D, Weimar C, Roggenbuck U, Dragano N, Jockel KH; Heinz Nixdorf Recall Study Investigators. Value of Progression of Coronary Artery Calcification for Risk Prediction of Coronary and Cardiovascular Events: Result of the HNR Study (Heinz Nixdorf Recall). Circulation. 2018;137:665–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hecht HS. A zero coronary artery calcium score: priceless. J Am Coll Cardiol. 2010;55:1118–1120. [DOI] [PubMed] [Google Scholar]
- 28.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2006;113:30–37. [DOI] [PubMed] [Google Scholar]
- 29.Yusuf S, Joseph P, Rangarajan S, Islam S, Mente A, Hystad P, Brauer M, Kutty VR, Gupta R, Wielgosz A, AlHabib KF, Dans A, Lopez-Jaramillo P, Avezum A, Lanas F, Oguz A, Kruger IM, Diaz R, Yusoff K, Mony P, Chifamba J, Yeates K, Kelishadi R, Yusufali A, Khatib R, Rahman O, Zatonska K, Iqbal R, Wei L, Bo H, Rosengren A, Kaur M, Mohan V, Lear SA, Teo KK, Leong D, O’Donnell M, McKee M, Dagenais G Modifiable risk factors, cardiovascular disease, and mortality in 155,722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet. 2019; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.