Abstract
Hypothesis
Cochlear implantation may cause an increase in the number of macrophages in the human cochlea similar to previous findings in the vestibular endorgans.
Background
Macrophages play a key role in both an inflammatory response and homeostatic maintenance. Recently an increase in the prevalence of macrophages was demonstrated in the human vestibular endorgans after implantation. However, the prevalence of macrophages in the cochlea after implantation is unclear. The aim of this study was to compare the distribution and prevalence of macrophages in implanted human cochleae and the contralateral unimplanted ears.
Methods
The prevalence of macrophages in the cochlea in ten human subjects who had undergone unilateral cochlear implantation was studied by light microscopy using anti-Iba1 immunostaining. The densities of macrophages in the osseous spiral lamina (OSL) and Rosenthal’s canal (RC) in implanted cochleae were compared with the contralateral unimplanted ears. The distribution of macrophage morphology (amoeboid, transitional and ramified) was also compared.
Results
There were activated and phagocytosing macrophages within the fibrotic sheath surrounding the electrode track and within fibrous tissue with lymphocytic infiltration in implanted ears. The densities of macrophages in OSL and RC in implanted ears were significantly greater than in unimplanted ears in some areas. There was also a difference in the prevalence of macrophage phenotype between the OSL and RC.
Conclusion
An increase in the density of macrophages in the cochlea after cochlear implantation was demonstrated. Both phagocytosis and anti-inflammatory activity of macrophages were suggested by the distribution and prevalence of macrophages in the implanted cochlea.
Keywords: Cochlear implant, Macrophage, Temporal bone pathology, Osseous spiral lamina, Spiral ganglion cell, Human
1. Introduction
Macrophages play an important role in the immune responses during foreign body reaction, inflammation, wound healing, and disease progression (1–4). Macrophages have been demonstrated in the animal (5) and human inner ear (6). Recently we reported that macrophages were greater in number and demonstrated more activated morphology in the human vestibular endorgans after cochlear implantation in the implanted ear compared to the unimplanted ear (7). Animal studies have also demonstrated the acute effect of implantation on the cochlea one month postoperatively (8) including an increase in the number of macrophages in the lateral cochlear wall, organ of Corti (OC), dendritic processes, Rosenthal’s canal, and fibrous tissue surrounding the electrode. Although the presence of macrophages in the human cochlea following cochlear implantation has been demonstrated (9, 10), the distribution and prevalence of macrophages in the cochlea following cochlear implantation has not been fully described in the human. We hypothesized that macrophages in the cochlea after cochlear implantation may be more numerous and more highly activated than in the opposite unimplanted ear. In this report, we undertook a histopathological analysis of the cochlea in the same 10 patients in the previous study of the vestibular system (7). The aim of the study was to evaluate the effects of cochlear implantation on the cochlear immune system by quantification and characterization of macrophages in implanted and contralateral unimplanted ears using light-microscopy and anti-Iba1 immunostaining.
2. Materials and Methods
2.1. Selection of cases
Twenty temporal bones from ten patients who in life had undergone unilateral cochlear implantation from the collection of the Otopathology Laboratory of the Massachusetts Eye and Ear were studied. The demographic and clinical data for these 10 cases are shown in the supplemental Table and in the previously published study (7). This study was approved by the Institutional Review Board of the Massachusetts Eye and Ear.
2.2. Histologic Preparation
The methods used for histologic preparation and 2-dimensional graphic reconstruction have been described in previous studies (6, 11, 12).
2.3. Immunohistochemistry methods
A total of 22 modiolar sections from10 patients were selected for immunostaining. In cases 2 and 10, two sections in each ear were used to evaluate Rosenthal’s canal of implanted ears. An antibody against ionized calcium-binding adaptor molecule 1 (Iba1), made in rabbit, (Wako chemicals USA, Inc, Richmond, VA) at a dilution of 1:200 to 1:2000 was used to immunostain macrophages in the same manner as previous reported (6).
2.4. Non-quantitative assessment of macrophages
2.4.1. Organ of Corti, stria vascularis, and spiral ligament
The distribution of anti-Iba1 immunostained macrophages containing nuclei in the organ of Corti (OC), stria vascularis (SV), and spiral ligament (SL) in the lower basal, upper basal, lower middle, upper middle and apical turns was examined in implanted and unimplanted ears in all 10 subjects using an Olympus BX51 light microscope at 40×.
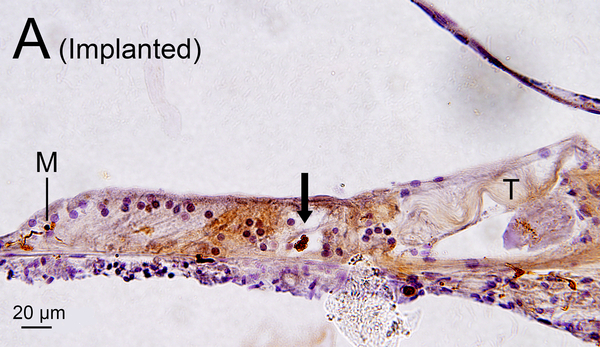
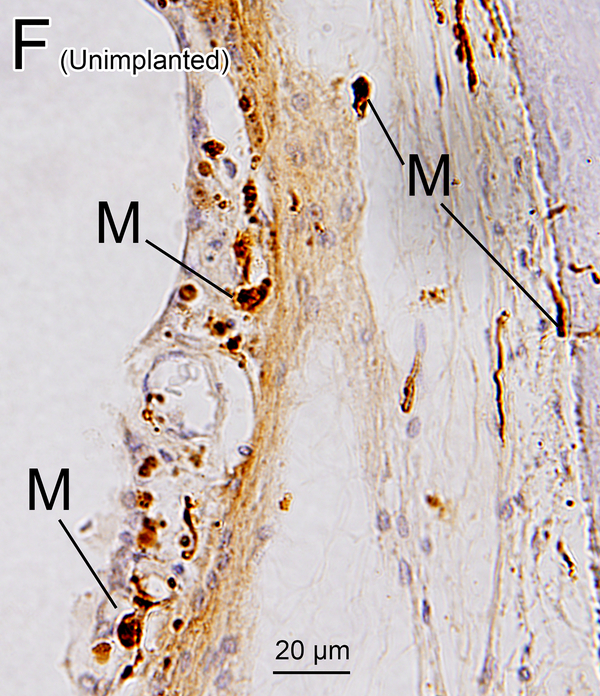
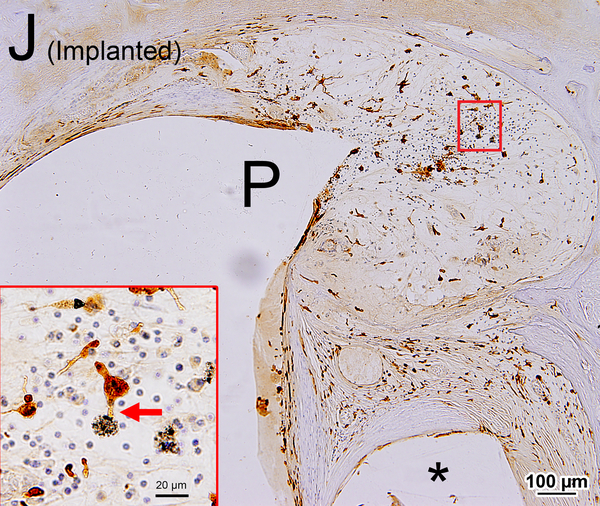
In the implanted ears, macrophages in the fibrous sheath surrounding the electrode track and fibrous tissue demonstrating lymphocytic infiltration in the perilymphatic scalae were also examined (Fig. 1A, B).
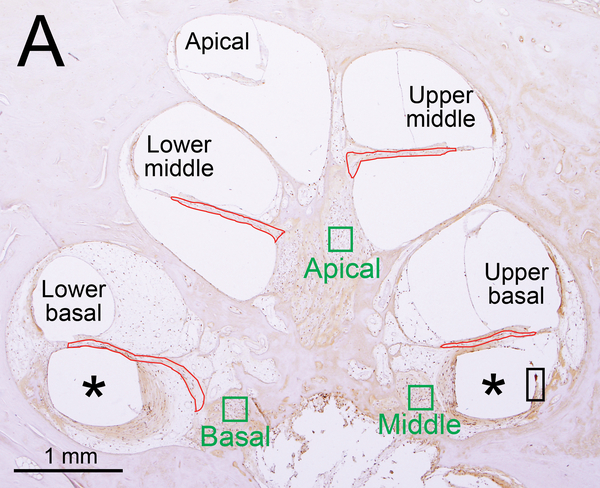
Figure 1. Iba1- immunostaining and H&E staining of cochlea: quantification and subtyping of macrophages.
(A) Midmodiolar section of right implanted cochlea of case 3 with Iba1 immunostaining. The prevalence of Iba1-positive macrophages was evaluated in the organ of Corti, stria vascularis, and spiral ligament in five cochlear segments. A fibrous sheath enveloped the electrode track (*) within the scala tympani from lower to upper basal turns and the “sheath” boxed in black is shown in Fig. 2I. Four segments of the osseous spiral lamina are outlined in red. Three segments of Rosenthal’s canal are outlined in green boxes, each measuring 200×200 μm2.
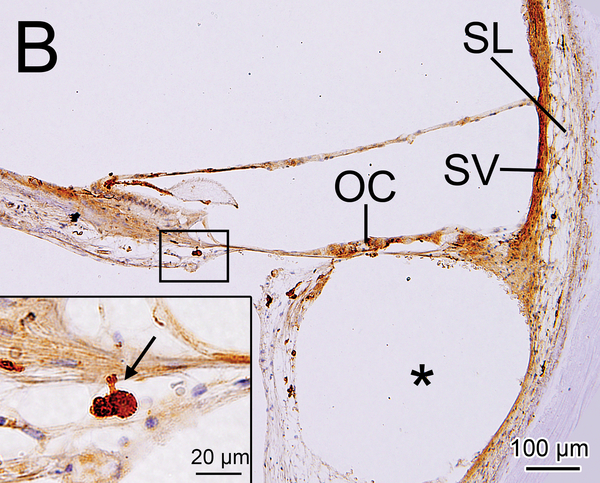
(B) Upper basal turn of right implanted cochlea of case1 immunostained by Iba1. The organ of Corti (OC), stria vascularis (SV) and spiral ligament (SL) were degenerated. A fibrous sheath enveloped the electrode track (*) within the scala tympani. The boxed area near the habenula perforata is shown in the inset. A process of a macrophage (arrow) extended to the distal OSL.
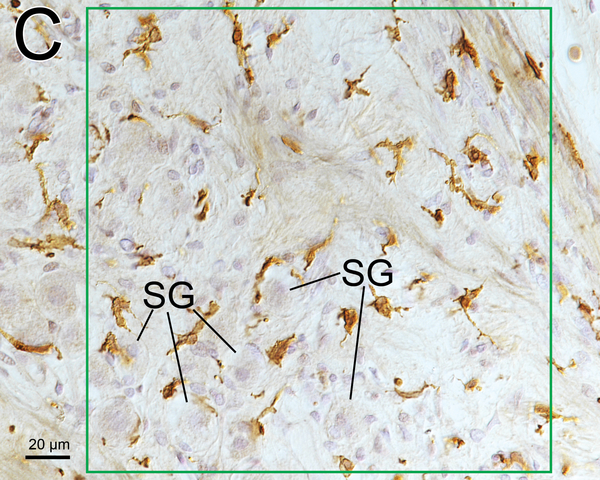
(C) Higher magnification of the area of the middle RC by Normarski (different interference contrast) microscopy shown in Fig.1A. The counting frame was placed in the RC where the density of SG cells was highest. Some spiral ganglion cells (SG) were wrapped by macrophages.
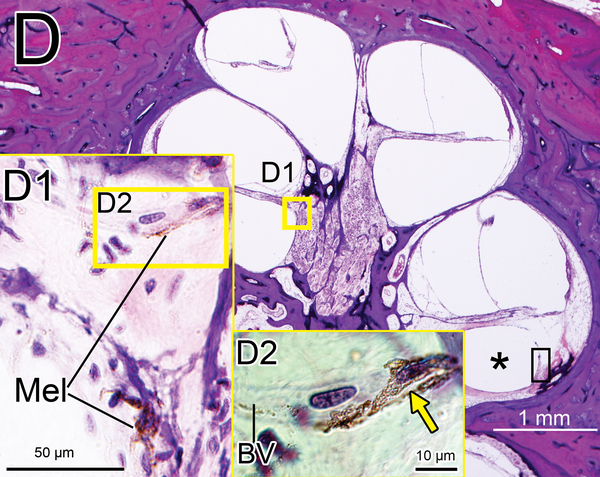
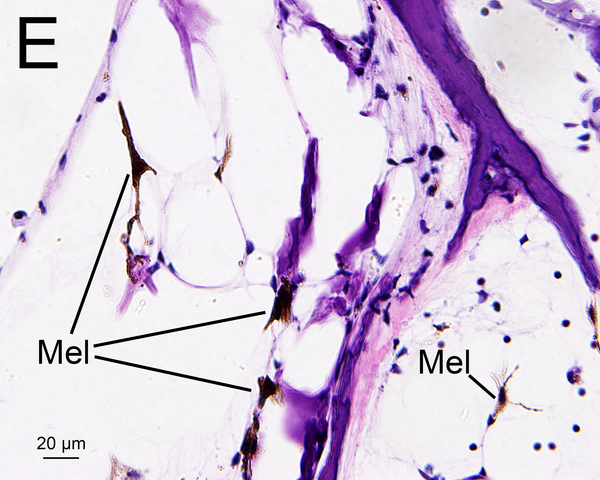
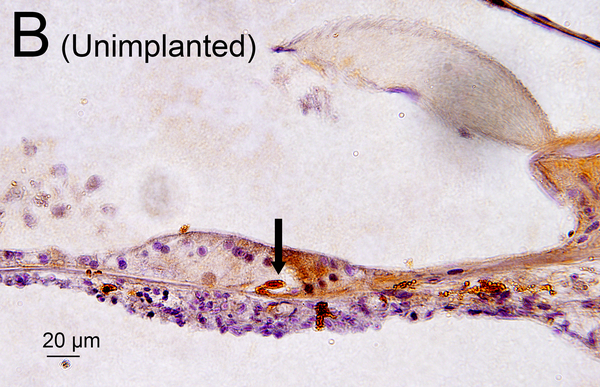
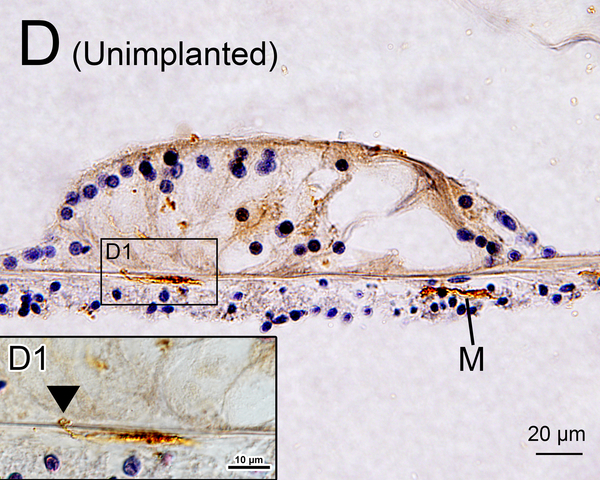
(D) Midmodiolar section of right implanted ear of case 3 adjacent to the section shown in Fig. 1A with H&E staining. (D1): A higher magnification of the boxed area in OSL of lower middle turn. A few dark macrophage-like melanocytes (Mel) were shown. (D2): A higher magnification by Normarski microscopy. A melanocyte surrounded the small blood vessel (BV) in OSL (yellow arrow). The area of the “sheath” boxed in black is shown in Fig. 2H.
(E) Section of the modiolus of the implanted left ear of case 7 demonstrated the presence of melanocytes (Mel) with H&E staining.
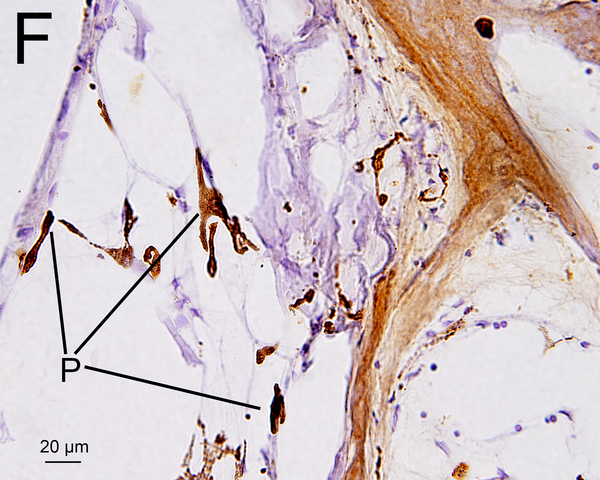
(F) An adjacent sections to that shown in Fig. 1E with Iba1 immunostainning. Some Iba1-positive cells (P) may be melanocytes as shown in Fig. 1E.
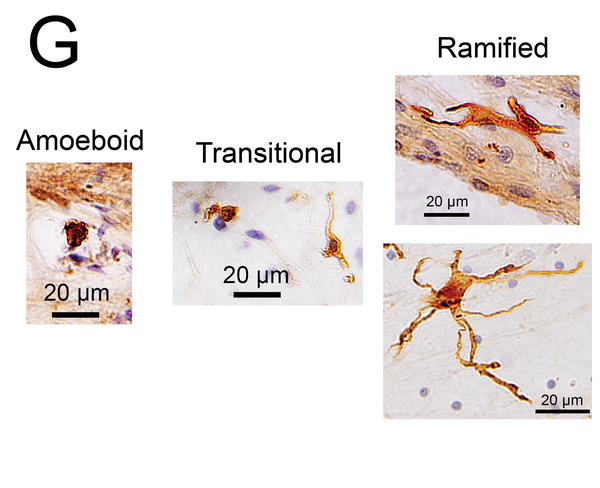
(G) Macrophage phenotypes with Iba1 immunostaining. The amoeboid type has few processes, a relatively round cell body and a large nucleus. The ramified type has long and some branching processes. The transitional type displays intermediate morphology.
2.5. Quantitative assessment of macrophages
Only Iba1-positive macrophages containing nuclei were counted for the quantitative assessments in this study.
2.5.1. Along dendritic processes in the osseous spiral lamina
Iba1-positive macrophages located along dendritic processes in the osseous spiral lamina (OSL) were counted using an Excelis HD camera & monitoring system (AccuScope, Inc., Commack, NY), coupled to an Olympus BX51 light microscope. To capture the images at 40× (1920 × 1080 pixels), imaging software (Capta Vision: AccuScope Inc.) was used, and the images were displayed on a LED monitor (VH27, Hewlett-Packard). Macrophages in the OSL in lower basal, upper basal, lower middle, and upper middle turns in the implanted and in the unimplanted ears were compared. The area of each OSL was outlined using CaptaVision imaging software (Fig. 1A). The densities of Iba1-positive macrophage cells in the OSLs of four segments were expressed as the number of macrophages per 40,000 μm2.
2.5.2. Rosenthal’s canal
Counting frames (200 × 200μm2) were outlined in the basal, middle and apical segments of Rosenthal’s canal (RC) where the density of spiral ganglion (SG) cells was highest using Capta Vision at 20× (Fig. 1A, C) and displayed on the LED monitor. The numbers of macrophages and SG cells within the counting frames were counted. The densities of macrophages and SG cells were expressed as the number per 40,000 μm2.
The number of SG cells “wrapped” by macrophages (SG cells surrounded along more than 50% of their circumference) was also counted.
2.5.3. Melanocytes
Melanocytes were occasionally found in the OSL and in RC in hematoxylin and eosin (H&E) (Fig. 1D, 1E) (13, 14). Melanocytes are derived from neural crest cells and are negative for Iba1 staining. However, a few a few Iba1 positive cells in the OSL and modiolus (Fig 1F) looked morphologically similar to melanocytes in an adjacent HE section (Fig 1E). They may be a confounding factor in quantifying macrophages in the cochlea. Previous studies of Iba1-positive macrophages in animals and humans did not distinguish between macrophages and melanocytes (8, 15, 16). In an attempt to resolve this confounding factor, in the study reported herein the numbers of melanocytes in an adjacent H&E section was also counted in the same manner as for Iba1-positive cells. The “corrected” densities of Iba1-positive macrophages were calculated by subtracting the numbers of melanocytes.
2.5.4. Macrophage morphology
Macrophages were morphologically classified into three types: amoeboid, transitional and ramified (17, 18) (Fig. 1G). The amoeboid type was defined by demonstrating a cell body with an amoeba-like shape and only few branched processes. The ramified type was defined by demonstrating a cell body with highly branched long processes. The transitional type included all other macrophages.
2.6. Statistical Analysis
The paired-t test or Wilcoxon matched-pairs signed rank test was used to statistically compare data from implanted and unimplanted ears. To evaluate the correlation of the number of macrophages with the number of spiral ganglion cells, Pearson’s or Spearman’s coefficients of correlation test was used. All statistical analyses were performed using GraphPad software (GraphPad Prism version 7.02; GraphPad Software, Inc., LaJolla, CA). p < 0.05 considered significant.
3. Results
3.1. Clinical variables
The demographics are presented in the supplemental Table and in a previous report (7). There were no re-implantation cases. In Case 9, residual hearing after implantation was lost in a delayed fashion as previously described (19). In Case 10, the cochlear implantation included a positioner as well as an electrode (20).
3.2. Non-quantitative assessment of macrophages
3.2.1. Organ of Corti, stria vascularis and spiral ligament
The OC remained in only a few specimens in the basal turn of the cochlea in both implanted and unimplanted ears, probably due to the etiology of deafness. Macrophages were found in 28.6% of the remaining OCs in the implanted ears and in 26.7% of the remaining OCs in the unimplanted ears (Table 1A). The finding of Iba1-positive cells present in the tunnel of Corti or at the base of Hensen cells (Fig 2A, 2B) were similar to a previous report (6). Macrophages were commonly seen under the basilar membrane in the perilymphatic compartment. Some processes of macrophages seemed to penetrate through the basilar membrane (Fig 2C, 2D).
TABLE 1A.
Presence of Iba1-positive macrophages in the surviving cochlear segments.
| Implanted Case | OC Present/Survived | SV Present/Survived | SL Present/Survived | Unimplanted Case | OC Present/Survived | SV Present/Survived | SL Present/Survived |
|---|---|---|---|---|---|---|---|
| 1 | 0/2 | 2/2 | 5/5 | 1 | 0/1 | 4/4 | 5/5 |
| 2 | 0/1 | 3/3 | 5/5 | 2 | 1/1 | 4/4 | 5/5 |
| 3 | 2/5 | 5/5 | 5/5 | 3 | 0/5 | 5/5 | 5/5 |
| 4 | 2/5 | 5/5 | 5/5 | 4 | 1/3 | 5/5 | 5/5 |
| 5 | 1/3 | 3/3 | 5/5 | 5 | 0/3 | 0/0 | 5/5 |
| 6 | 0/2 | 0/1 | 5/5 | 6 | 2/5 | 0/2 | 5/5 |
| 7 | 2/4 | 3/3 | 5/5 | 7 | 3/3 | 3/3 | 5/5 |
| 8 | 0/3 | 3/3 | 5/5 | 8 | 0/5 | 5/5 | 5/5 |
| 9 | 1/4 | 2/4 | 5/5 | 9 | 1/1 | 5/5 | 5/5 |
| 10 | 0/0 | 0/0 | 0/0 | 10 | 0/3 | 5/5 | 5/5 |
| Rate Present | 28.6% | 89.7% | 100.0% | Rate Present | 26.7% | 94.7% | 100.0% |
OC: Organ of Corti; SV: Stria vascularis; SL: Spiral ligament
Figure 2. Iba1-immunostaining of cochlear elements in implanted (A, C, E, G-J) and unimplanted (B, D, F) ears and H&E staining (H).
(A) OC of upper middle turn of implanted left ear of case 7. The tectorial membrane (T) was degenerated and adherent to OC. Macrophages were seen in the tunnel of Corti (arrow) and among Hensen cells (M).
(B) OC of lower middle turn of unimplanted right ear of case 7. A macrophage was seen in the tunnel of Corti (arrow) as was also seen in the implanted ear (Fig. 2A).
(C) OC of lower middle turn of implanted right ear of case 3 (Also shown in Fig. 1 A). Macrophages (M) were seen beneath the basilar membrane. (C1): A higher magnification (Normarski microscopy). A process of a macrophage (arrowhead) extended into the OC.
(D) OC of lower middle turn of unimplanted right ear of case 4. There were a few macrophages (M) seen beneath the basilar membrane. (D1): A higher magnification (Normarski microscopy). A process of a macrophage (arrowhead) extended into the OC.
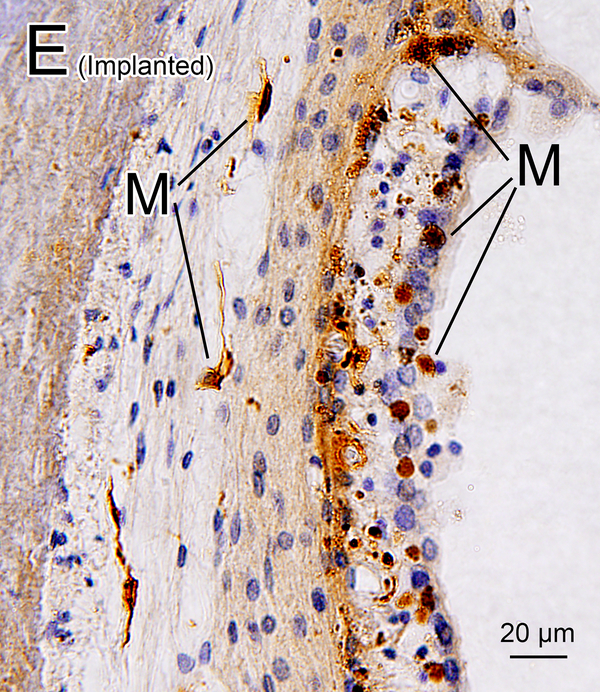
(E), (F) Lateral wall of lower middle turn of implanted left (E) and unimplanted right (F) ear of case 4. There were several macrophages (M) seen in the SV and SL in both ears.
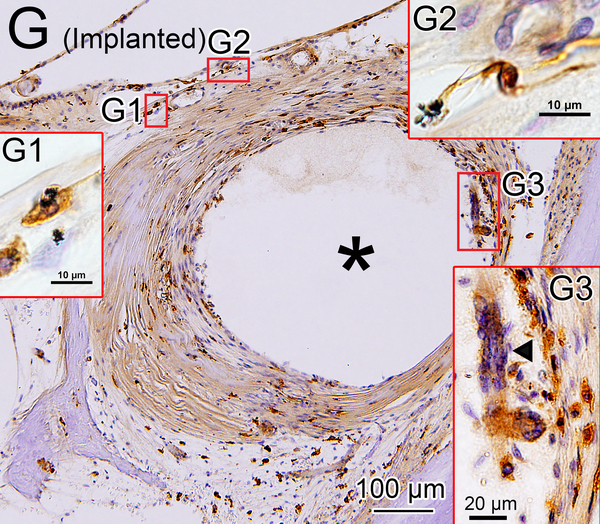
(G) Iba1-immunostaining of the fibrous sheath surrounding the electrode track (*) in implanted left ear of case 2. Boxed areas are shown by Nomarski microscopy at high magnification (G1-G3). Abundant amoeboid and transitional macrophages were seen. Phagocytosing macropages were seen in the outer layer of the fibrous sheath. (G1): Black particulate material phagocytized by a macrophage. (G2): Long process of macrophage was in contact with black particulate material. (G3): There was a multinuclear FBGC (arrowhead) in inner layer of the sheath.
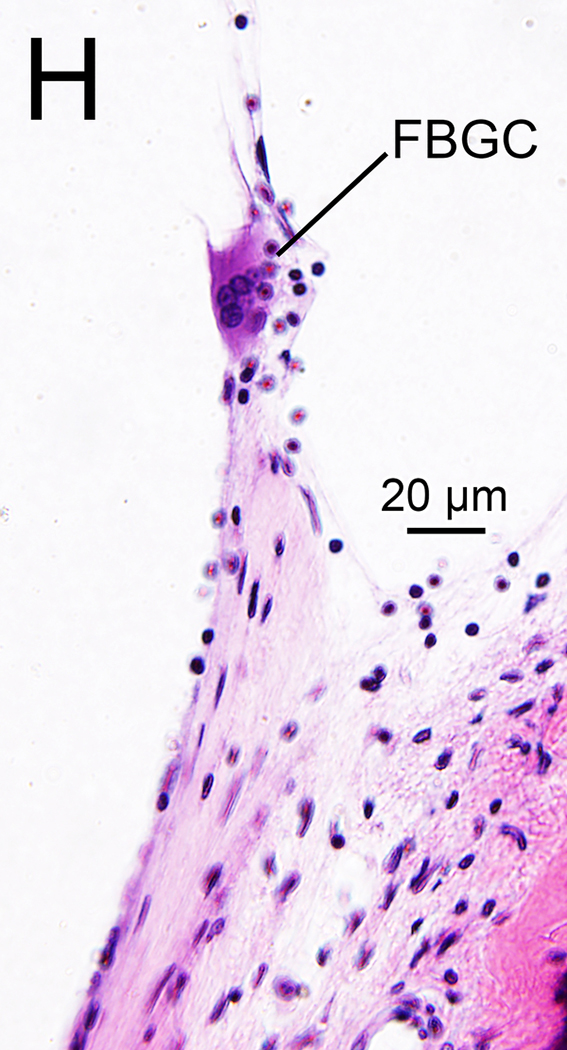
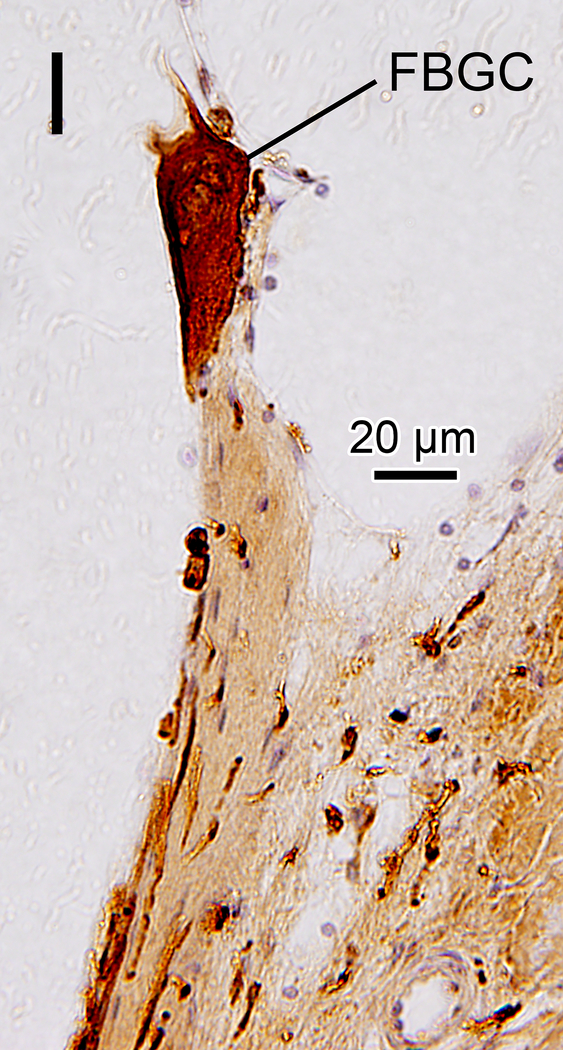
(H), (I) Typical multinucleated FBGC seen in adjacent sections stained by H&E (H) and Iba1 (I) are seen.
(J) Iba1-immunostaining of a lymphocytic infiltration in the lower basal turn of implanted right ear of case 10. A larger track caused by positioner (P) and a smaller track caused by electrode (*) were seen. There were many macrophages within the lymphocytic infiltration in the scala vestibuli. The boxed area is shown at high magnification. A process of a macrophage is seen in contact with foreign body material (red arrow).
In the basal turn of implanted ears, the electrode caused loss of some or all of the SV. In other cases, the SV was missing in both ears due to the etiology of hearing loss. In the remaining SV, there were some macrophages around the blood vessels in both implanted and unimplanted ears (Fig 2E, 2F). Macrophages were seen in 86.9% of remaining SV in implanted ears and in 97.4% of remaining SV in unimplanted ears (Table 1A).
In the basal turn of implanted ears, the SL was sometimes displaced, degenerated, or destroyed by the electrode or by fibrotic tissue and calcification surrounding electrode. However, there were several macrophages found in the SL throughout the cochleae in both implanted and unimplanted ears (Table 1A).
3.2.2. Fibrous electrode sheath and areas with lymphocytic infiltration and particulate foreign bodies
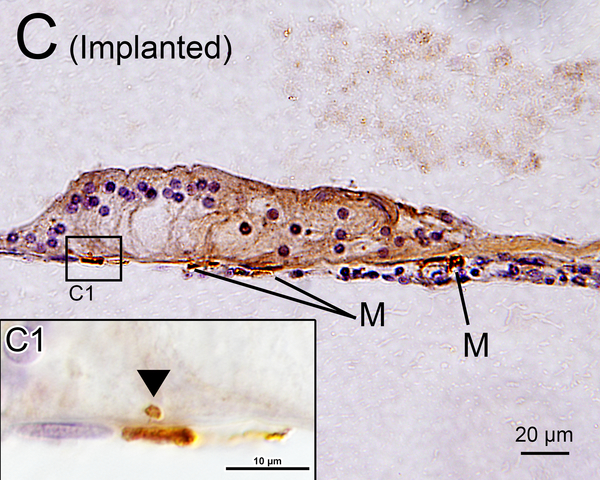
In nine of ten implanted ears a fibrous sheath surrounded the electrode track (Table 1B). Many macrophages were found within the fibrous tissue (Fig. 2G). The fibrous sheath of case 2 in the implanted ear also contained foreign bodies. There were many amoeboid and transitional macrophages but seldom ramified macrophages near the foreign bodies and in the inner fibrous layer adjacent to the electrode track. Some macrophages contained opaque particles consistent with phagocytized foreign material (Fig. 2G1, 2G2). Multinucleated foreign body giant cells (FBGCs) were common in the inner layer of the fibrous sheath (Fig. 2G3, 2H, 2I) adjacent to the electrode track.
TABLE 1B.
Presence of FS and LI.
| Implanted Case | FS | LI |
|---|---|---|
| 1 | + | − |
| 2 | + | + |
| 3 | + | + |
| 4 | + | + |
| 5 | + | − |
| 6 | + | − |
| 7 | + | − |
| 8 | + | − |
| 9 | − | − |
| 10 | + | + |
| Rate Present | 90.0% | 40.0% |
+: Present; −: Absent; FS: Fibrous sheath; LI: Lymphocytic infiltration
In four of ten implanted ears, a lymphocytic infiltration was found in the loose areolar fibrous tissue in the scala tympani or scala vestibuli (Table 1B), and many amoeboid and transitional macrophages were seen near particulate foreign material. In these areas, some of the macrophages seemed to be in the process of phagocytosis of the foreign body particles (Fig. 2J).
3.3. Quantitative assessment of macrophages
3.3.1. Along dendritic processes (OSL)
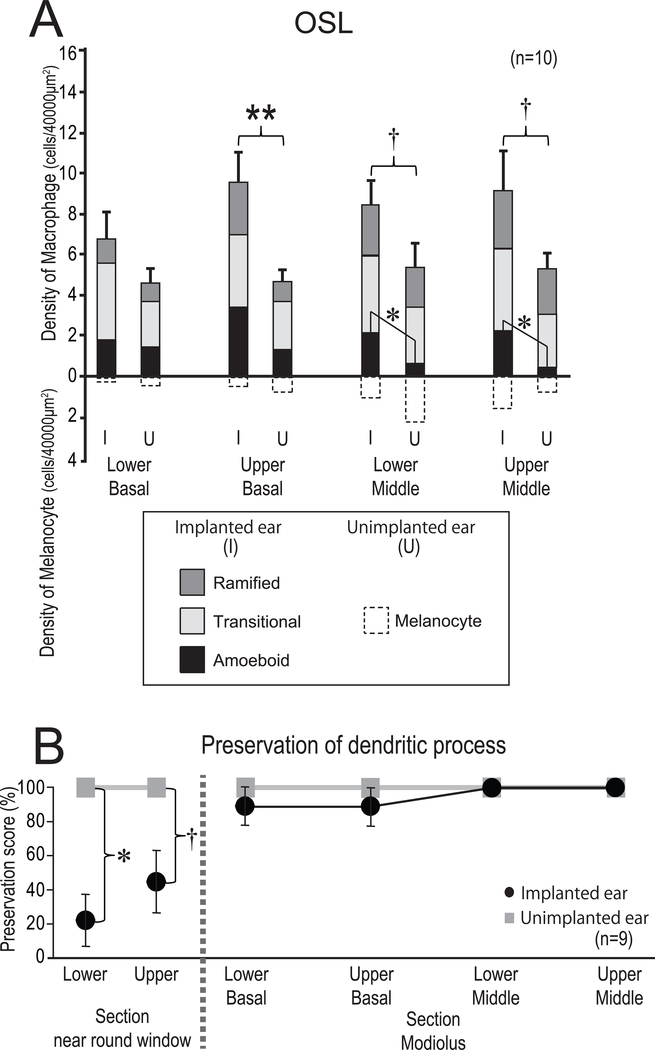
Macrophages were frequently seen in the OSL in both implanted and unimplanted ears. In Fig. 3A, the average densities of macrophages in the OSL in four cochlear segments are shown. There tended to be more macrophages in implanted than unimplanted ears in the lower and upper middle turns (both; p = 0.08). In the upper basal turn, there were significantly more macrophages in the implanted than in the unimplanted ears (p < 0.01). The prevalence of amoeboid macrophages in the OSL in implanted ears tended to be greater than in the contralateral unimplanted ears. The densities of amoeboid macrophages in lower and upper middle turns in the implanted ears were significantly greater than in the unimplanted ears (p < 0.05).
Figure 3. Density and phenotype of macrophages in OSL (A) and preservation of neuronal fibers in OSL (B).
(A) The mean densities of macrophages and melanocytes in four segments of the OSL (lower basal, upper basal, lower middle, upper middle turn) of implanted (I) and unimplanted (U) ears are shown. The error bars indicate the standard errors of total density. Amoeboid, transitional and ramified types of macrophages are shown in gradation. A paired-t test or Wilcoxon matched-pairs signed rank test was used for statistical analysis between I and U ears. The asterisks (*) indicate the statistical significance in total density (**: p < 0.01). The daggers (†) indicate a significant tendency (†: 0.05 < p < 0.1). The asterisks (*) indicate the statistical significance in the phenotype of macrophages (*: p < 0.05).
(B) The mean preservation of neuronal dendritic process in the OSL evaluated by anti-neurofilament immunostaining in the sections of modiolus and basal turn near the round window [Data of 9 of 10 cases as provided in a previous study by Kamakura et al. (11)]. Presence or absence of dendritic process with neurofilament staining in OSL was represented by preservation score of 100 or 0 (%). Black circles and gray squares indicate implanted and unimplanted ears, respectively. The section of basal turn near round window where there was significant difference in the preservation of dendritic process between implanted and unimplanted ears (Wilcoxon matched-pairs signed rank test) was not included in current study.
Since preservation of dendritic processes may influence the density of macrophages, the preservation of neuronal dendritic processes of OSL is shown Fig. 3B (Data provided by Kamakura et al. (11)). There was no difference in the preservation of neuronal dendritic processes between implanted and unimplanted ears in the modiolar section of this study.
3.3.2. Rosenthal’s canal
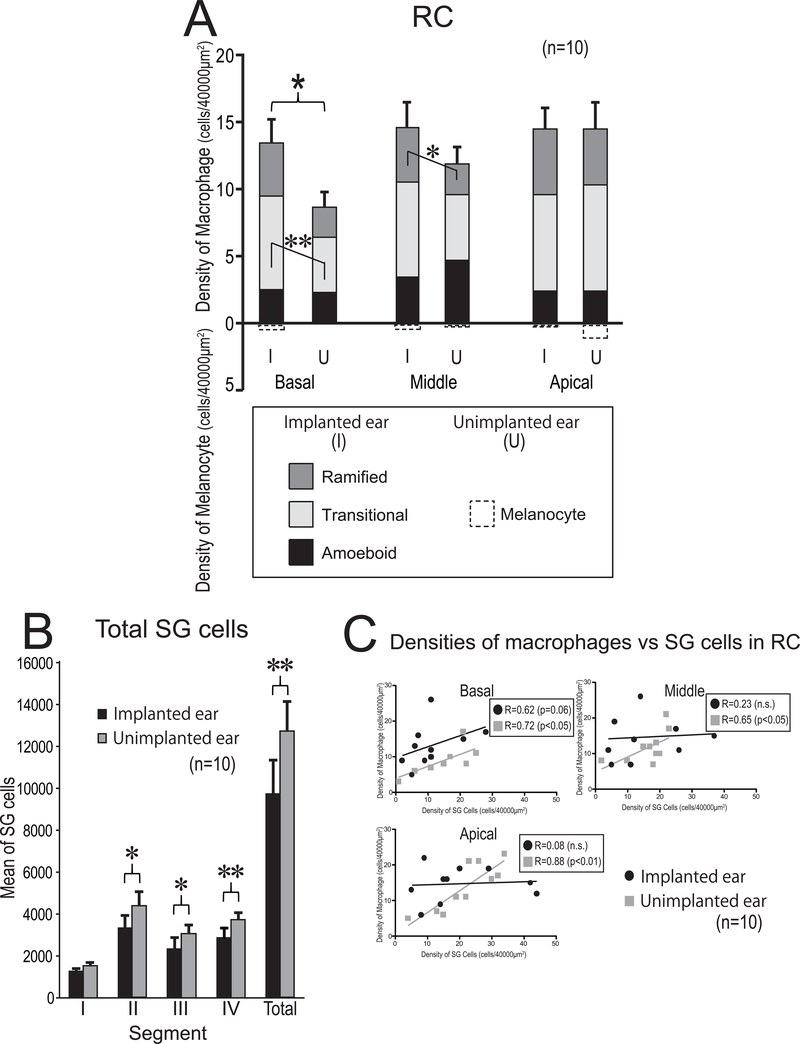
The densities of macrophages in the three segments of RC are displayed in Fig. 4A. There tended to be more macrophages in implanted than unimplanted ears. In the basal RC, there were significantly more macrophages in implanted than in unimplanted ears (p < 0.05). The density of transitional macrophages in the implanted ears was significantly greater than in unimplanted ears (p < 0.01). In the middle RC, the density of ramified macrophages in the implanted ears was significantly greater than in unimplanted ears (p < 0.05). There was no significant difference in the densities of amoeboid macrophages in all segments of RC between implanted and unimplanted ears.
Figure 4. Density and phenotype of macrophages (A), SG cell counts (B), and Correlation between macrophages vs SG cells (C).
(A) The mean densities of macrophages and melanocytes in three segments of RC (basal, middle, apical turn) of implanted (I) and unimplanted (U) ears. A paired-t test or Wilcoxon matched-pairs signed rank test was used for statistical analysis between I and U ears. The asterisks (*) or (*) indicate the statistical significance in total density (*: p < 0.05) or in phenotype of macrophage (*: p < 0.05, **: p < 0.01), respectively.
(B) The mean of total and segmental SG cell counts in 10 cases. The error bars indicated the standard errors. Black and gray column indicate implanted and unimplanted ears, respectively. The asterisks (*) indicate the statistical significance between implanted and unimplanted ears (*: p < 0.05, **: p < 0.01, paired-t test or Wilcoxon matched-pairs signed rank test).
(C) Correlation between densities of macrophages vs SG cells. Scatter plot of densities of macrophages verses SG cell in three segments of RC are shown. Black circles and gray squares indicate implanted and unimplanted ears, respectively. (Pearson’s or Spearman’s coefficients of correlation test).
The mean of the total number of SG cells in 10 cases as determined by 2D reconstruction are shown in Fig. 4B. The total number of SG cells in implanted ears was significantly less than in unimplanted ears. The correlation between the density of SG cells and macrophages in captured counting frames was done in the three segments of RC (Fig. 4C). The density of macrophages in unimplanted ears showed significant positive correlation with the density of SG cells. However, in the implanted ear there was no significant correlation between the density of SG cells and macrophages.
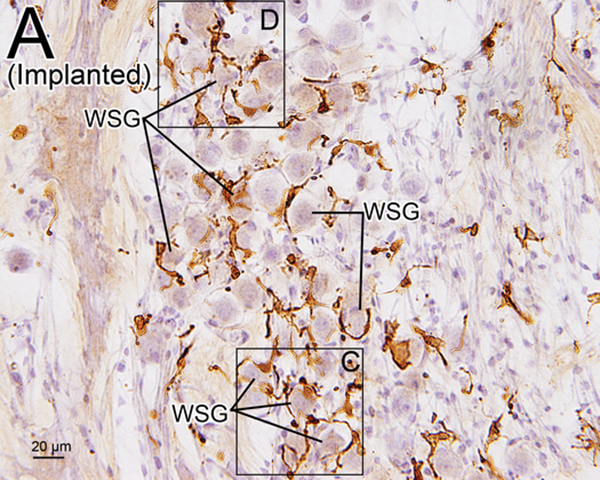
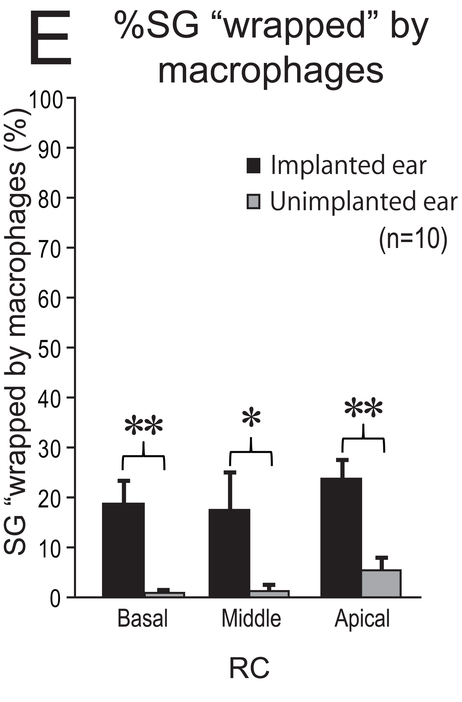
Some macrophages seemed to be “wrapping” the SG cells (Fig. 5). This finding was more commonly seen in implanted ears than in unimplanted ears. The percentage of SG cells “wrapped” by macrophages was significantly greater in implanted ears than in unimplanted ears in the basal (p < 0.01), middle (p < 0.05), and apical (p < 0.01) segments of RC (Fig 5E).
Figure 5. Iba1-immunostaining of RC (A-D) and %SG “wapped” by macrophages (E).
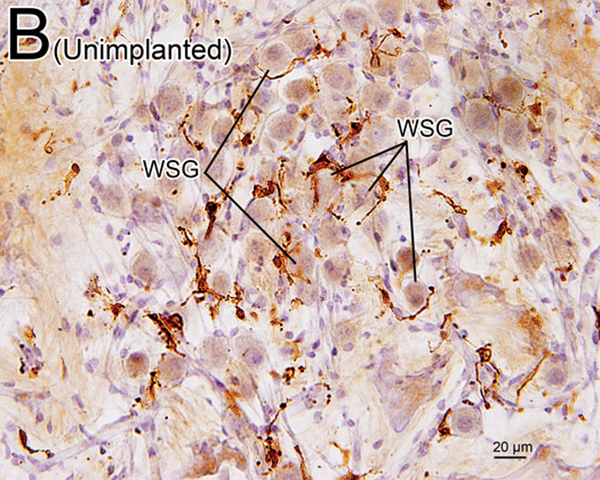
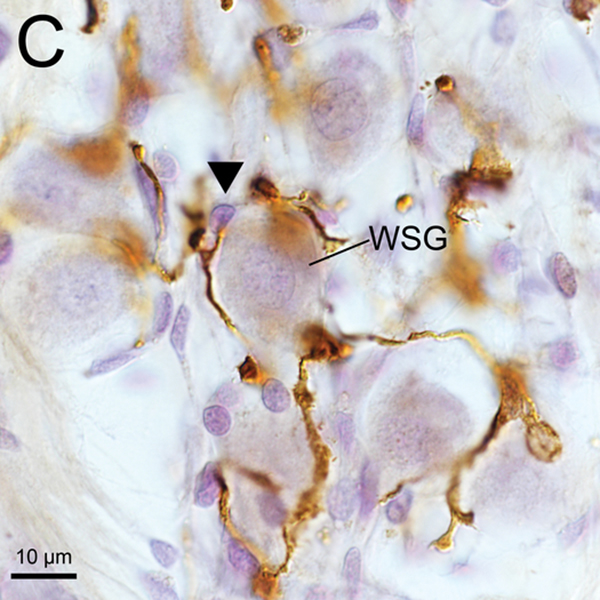
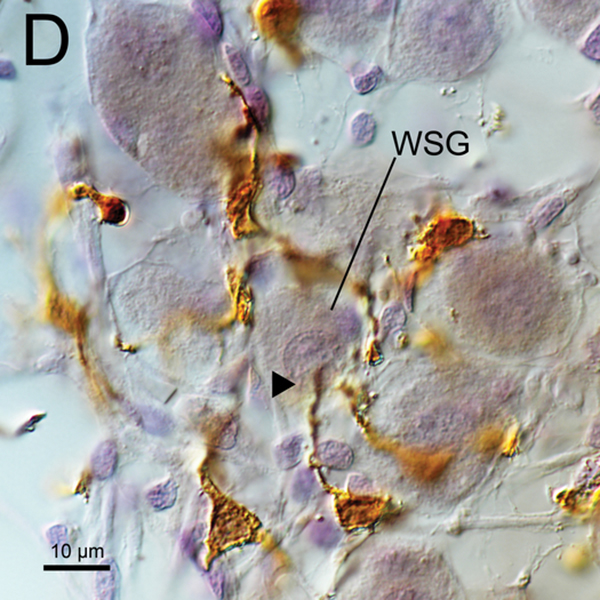
(A) Iba1-immunostaining of apical RC in implanted right ear of case 7. There were many transitional and ramified macrophages with long processes. Some SG cells (WSG) were wrapped by macrophages.
(B) Iba1-immunostaining of apical RC in unimplanted left ear of case 7. There were also many transitional and ramified macrophages with long processes. The percentage of SG cells (WSG) wrapped by macrophages was less than in the implanted ear of (A).
(C), (D) A higher magnification of wrapped SG cells (WSG) by Normarski microscopy shown in Fig5A. Long processes of macrophages were in close contact with the nucleus of one satellite cell and one SG cell (arrowheads).
(E) The mean %SG “wapped” by macrophages in RC in 10 cases. Black and gray column indicate implanted and unimplanted ear, respectively. The error bars indicated the standard errors. The asterisks (*) indicate the statistical significance between implanted and unimplanted ears (*: p < 0.05, **: p < 0.01, Wilcoxon matched-pairs signed rank test).
4. Discussion
4.1. Iba1- positive macrophages
In this study, the distribution and prevalence of macrophages in the human cochlea following cochlear implantation were examined using immunostaining using an anti-Iba1 antibody. Infiltration of many macrophages into the fibrous sheath and in tissue with lymphocytic infiltration was demonstrated in implanted ears. Although there were also many macrophages in OSL and Rosenthal’s canal in both ears, the number of macrophages in the implanted ears was significantly greater than in the unimplanted ears in some segments. These results suggest several effects on the immune system of cochlea caused by cochlear implantation. Although the pathogenesis of the immunologic function of macrophages in the cochlea was not directly tested in this study, possible immunologic activities are suggested by the findings as discussed below.
4.2. Non-Quantitative assessment of macrophages
4.2.1. Organ of Corti, stria vascularis and spiral ligament
In the OC in the normal human, although macrophages were commonly present beneath the basilar membrane, the prevalence of macrophages in the OC was infrequent (6). This study demonstrated that the presence of macrophages in the OC in implanted and unimplanted ears was infrequent and similar to reported finding in normal ears (6). In acute inflammation, cochlear macrophages were commonly found in the OC following operative trauma, noise trauma, and ototoxic damage (15, 18, 21, 22). Although cochlear implantation is associated with reduced preservation of cochlear hair cells (11), this study demonstrated no difference in the distribution of macrophages in the OC between implanted and unimplanted ears after 12 to 210 months after implantation, suggesting saturation of macrophage activation in the OC. Some macrophages were present beneath the basilar membrane and their processes extended into the OC (Fig. 2C, 2D). This observation agreed with the prevalence of macrophages found in vestibular endorgans (7). These macrophages may be involved in the maintenance and homeostasis of the OC.
Macrophages in the SV were seen near blood vessels in both implanted and unimplanted ears. There were several macrophages in the SL throughout the cochlea in both implanted and unimplanted ears. These observations were also reported in normal cochleae (6). There was no difference in the prevalence of macrophages between implanted and unimplanted ears in both SV and SL, perhaps due to the etiology of hearing loss and prior degeneration and loss of cochlear elements.
4.2.2. Fibrotic sheath and lymphocytic infiltration
The presence of a fibrous sheath surrounding the electrode, new bone formation, and lymphocytic infiltration following cochlear implantation were previously reported in the basal turn of the cochlea in several histopathologic studies (23–25). In the current study, some foreign body particles were seen within the fibrous sheath (Fig 2G). The morphology of most macrophages within the fibrotic sheath was consistent with amoeboid or transitional types and seldom the ramified type. The macrophage morphology suggests activation of phagocytosis (26). Some multinucleated FBGCs which were likely formed by fusion of macrophages in the state of chronic inflammation (27) were stained by Iba1 (Fig 2I).
There were many macrophages in the areas of lymphocytic infiltration. Some macrophages were seen in the process of phagocytosis of foreign body particles (Fig 2 J). Antigen presentation to T-cells is also an important role of macrophages (2, 28, 29). Considering T-cells were identified within the tissue with lymphocytic infiltration as reported in a previous study (9), macrophages might play a role in antigen presentation. Reduction of foreign body debris during implantation surgery may result in a decrease of the foreign body response.
4.3. Quantitative assessment of macrophages
4.3.1. Along dendritic processes (OSL)
Distortion and fracture of the OSL has been reported as common secondary to insertional trauma of the implant electrode (24, 30, 31). In the present study, in the upper basal turn of OSL, the density of macrophages in implanted ears was significantly greater than in unimplanted ears. In the lower and upper middle turns, there tended to be more macrophages in implanted than unimplanted ears. The densities of amoeboid macrophages in the lower and upper middle turns were significantly greater in implanted ears than in unimplanted ears. These results are consistent with an increase of immune activity in OSL in the implanted ears and similar to the results of our previous study in the vestibular endorgans (6).
Kamakura et al. (11) evaluated the preservation of neuronal dendritic processes of spiral ganglion cells in the OSL after cochlear implantation. Although significant loss of dendritic processes along the electrode track in implanted ears compared to the unimplanted ear was found, in the upper OSL apical to the electrode, dendritic processes were preserved. Our results demonstrated significant increases in the prevalence of total and amoeboid macrophages in the upper turn where dendritic neuronal fibers were preserved (Fig. 3B). Kaur et al. (32) pointed out a preservation effect of macrophages on the dendritic neuronal fibers after loss of hair cells. Therefore, other possible roles of macrophages are suggested such as resolution of inflammation and surveillance, which is consistent with the finding of macrophages with extended process (Fig. 1B).
4.3.2. Rosenthal’s Canal
The density of macrophages in RC was positively correlated with the density of SG cells in unimplanted ears. However, in the implanted ears, macrophages were consistently present in spite of only a few remaining SG cells. The density of macrophages in implanted ears was significantly greater than in unimplanted ears in basal RC. This result suggests an increase of immune activity in RC in the implanted ears.
One possible function of macrophages in RC is phagocytosis since SG cells were significantly less in implanted ears compared to unimplanted ears. However, there was no difference in density of amoeboid macrophage between implanted and unimplanted ears. On the contrary, the density of ramified macrophages in implanted ears was significantly greater than in unimplanted ears in the middle RC. Ramified macrophages with long processes play a role in surveillance function, the rescue of neurons, and preservation of neuronal function (1, 33–35). Shifting the phenotype of macrophages from M1 (pro-inflammatory) to M2 (anti-inflammatory) macrophage was observed following electrical stimulation of peripheral nerve in the rat (36). Electrical stimulation by the cochlear implant might be involved in an increase in the density of M2 macrophages which is consistent with less phagocytotic activity and decrease amoeboid morphology found in implanted ears. Thus, the increase of ramified and transitional macrophages in implanted ears suggests a role in maintenance and preservation of the SG cells. This hypothesis is supported by the previous reports suggesting that macrophages participate in the survival of spiral ganglion neurons (32), and microglia have been shown to promote a neurotrophic factor (37) or insulin-like growth factor1 (IGF1) (38).
Interestingly, SG cells in RC in implanted ears were more frequently “wrapped” by macrophages. This wrapping coincides with the distribution of more transitional and ramified macrophages with long processes in the RC of implanted ears. Immunostaining for Iba1 revealed that some macrophages made contact with satellite cells surrounding the SG cells and infrequently with SG cells directly (Fig. 5C, D). SG cells are surrounded by satellite glial cells and non-myelinated Schwann cells. Unfortunately, this light-microscopic study could not distinguish between these two cell types. Although contact of macrophages with non-myelinated Schwann cells has been reported in the human (16), their investigations placed emphasis on the direct contact of macrophages with the axon and soma of SG cells. Considering that non-myelinated Schwann cells protect the SG cells (39) and express neurotrophic factors such as brain-derived neurotrophic factor (BDNF) and neuotrophin-3 (NT-3) (40), and that satellite glial cells may play a role in prevention of degeneration of the SG cells (41), these macrophages contacting satellite cells may indirectly contribute the preservation of SG cells or, perhaps “wrapping” is a first step to engulfing the sick and dying SG cells.
5. Conclusion
This study demonstrated the contribution of macrophages to a foreign body response and an increase in the density of macrophages in the OSL and Rosenthal’s canal after cochlear implantation. Our findings suggest that activation of the immune system after cochlear implantation is evident within the cochlea similar to the finding in vestibular endorgans (7). Analysis of phenotypes of macrophages in the OSL demonstrated an increase in amoeboid macrophages after implantation whereas there was an increase in the density of transitional or ramified macrophages in RC. This difference in prevalence of macrophage phenotypes between OSL and RC may reflect the multi-functional activities of macrophage including inflammatory and anti-inflammatory processes. Reduction of the foreign body response induced by macrophages and enhancing the anti-inflammatory effect of macrophages may protect residual hearing after cochlear implantation.
Supplementary Material
Acknowledgements
We thank Diane Jones, Barbara Burgess, and Meng Yu Zhu for their expert preparation of the temporal bone specimens, and Garyfallia Pagonis for technical assistance in creating digitized images of the temporal bone sections. This work was supported by grants #U24-DC013983 and R01-DC000152–34 from the National Institute of Deafness and Other Communication Disorders (NIDCD).
(source of support)
Grants #U24DC013983 and R01DC000152-34, National Institute on Deafness and Other Communication Disorders (NIH)
Abbreviation list
- OSL
osseous spiral lamina
- RC
Rosenthal’s canal
- OC
organ of Corti
- Iba1
ionized calcium-binding adaptor molecule 1
- SV
stria vascularis
- SL
spiral ligament
- SG
spiral ganglion
- H&E
hematoxylin and eosin
- FBGC
foreign body giant cell
- IGF1
insulin-like growth factor1
- BDNF
brain-derived neurotrophic factor
- NT-3
neuotrophin-3
Footnotes
(disclosure)
All authors declare no conflicts of interests related to this manuscript.
Bibliography
- 1.Nimmerjahn A, Kirchhoff F, Helmchen F. Neuroscience: Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science (308): 1314–1318. 2005. doi: 10.1126/science.1110647 [DOI] [PubMed] [Google Scholar]
- 2.Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4(3):281–286. http://www.ncbi.nlm.nih.gov/pubmed/16101534. [DOI] [PubMed] [Google Scholar]
- 3.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011, 11: 723–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vijaya Bhaskar TB, Ma N, Lendlein A, Roch T. The interaction of human macrophage subsets with silicone as a biomaterial. Clin Hemorheol Microcirc. 2015, 61: 119–133. [DOI] [PubMed] [Google Scholar]
- 5.Hirose K, Discolo CM, Keasler JR, Ransohoff R. Mononuclear phagocytes migrate into the murine cochlea after acoustic trauma. J Comp Neurol. 2005;489(2):180–94. [DOI] [PubMed] [Google Scholar]
- 6.O’Malley JT, Nadol JB, McKenna MJ. Anti CD163+, Iba1+, and CD68+ Cells in the Adult Human Inner Ear: Normal Distribution of an Unappreciated Class of Macrophages/Microglia and Implications for Inflammatory Otopathology in Humans. Otol Neurotol. 2016;37(1):99–108. doi: 10.1097/MAO.0000000000000879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okayasu T, O’Malley JT, Nadol JB Jr. Density of macrophages immunostained with anti-Iba1 antibody in the vestibular endorgans after cochlear implantation in the human. Otol Neurotol. 2019. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bas E, Goncalves S, Adams M, Dinh CT, Bas JM, Van De Water TR, Eshraghi AA. Spiral ganglion cells and macrophages initiate neuro-inflammation and scarring following cochlear implantation. Front Cell Neurosci. 2015;9:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nadol JB, O’Malley JT, Burgess BJ, Galler D. Cellular immunologic responses to cochlear implantation in the human. Hear Res. 2014. doi: 10.1016/j.heares.2014.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Malley JT, Burgess BJ, Galler D, Nadol JB. Foreign body response to silicone in cochlear implant electrodes in the human. Otol Neurotol. 2017;38(7):970–977. doi: 10.1097/MAO.0000000000001454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamakura T, O’Malley JT, Nadol JB Jr. Preservation of Cells of the Organ of Corti and Innervating Dendritic Processes Following Cochlear Implantation in the Human: An Immunohistochemical Study. Otol Neurotol. 2018;39(3):284–293. doi: 10.1097/MAO.0000000000001686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merchant S and Nadol JB Jr. Schuknecht’s pathology of the ear, 3rd edn Shelton, People’s Medical Publishing House, 2010, pp 34–40. [Google Scholar]
- 13.Zhang W, Dai M, Fridberger A, Hassan A, Degagne J, Neng L, Zhang F, He W, Ren T, Trune D, Auer M, Shi X. Perivascular-resident macrophage-like melanocytes in the inner ear are essential for the integrity of the intrastrial fluid-blood barrier. Proc Natl Acad Sci U S A. 2012;109(26):10388–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts DS, Linthicum FH Jr. Distribution of melanocytes in the human cochlea. Otol Neurotol. 2015;36(3):e99–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okano T, Nakagawa T, Kita T, et al. Bone marrow-derived cells expressing Iba1 are constitutively present as resident tissue macrophages in the mouse cochlea. J Neurosci Res. 2008;86(8):1758–67. doi: 10.1002/jnr.21625 [DOI] [PubMed] [Google Scholar]
- 16.Liu W, Molnar M, Garnham C, Benav H, Rask-Andersen H. Macrophages in the human cochlea: Saviors or predators-A study using super-resolution immunohistochemistry. Front Immunol. 2018, 9: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torres-Platas SG, Comeau S, Rachalski A, et al. Morphometric characterization of microglial phenotypes in human cerebral cortex. J Neuroinflammation. 2014;11:12. doi: 10.1186/1742-2094-11-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirose K, Rutherford MA, Warchol ME. Two cell populations participate in clearance of damaged hair cells from the sensory epithelia of the inner ear. Hear Res. 2017; 352: 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quesnel AM, Nakajima HH, Rosowski JJ, Hansen MR, Gantz BJ, Nadol JB Jr. Delayed loss of hearing after hearing preservation cochlear implantation: human temporal bone pathology and implications for etiology. Hear Res 2016;333: 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamakura T, Nadol JB Jr. Cochlear Histopathology as observed in two patients with a cochlear implant electrode with positioner. Otol Neurotol. 2016; 37: 642–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ladrech S, Wang J, Simonneau L, Puel JL, Lenoir M. Macrophage contribution to the response of the rat organ of Corti to amikacin. J Neurosci Res. 2007;85(9):1970–9. doi: 10.1002/jnr.21335 [DOI] [PubMed] [Google Scholar]
- 22.Frye MD, Yang W, Zhang C, Xiong B, Hu BH. Dynamic activation of basilar membrane macrophages in response to chronic sensory cell degeneration in aging mouse cochleae. Hear Res. 2017;344:125–134. doi: 10.1016/j.heares.2016.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seyyedi M, Nadol JB. Intracochlear inflammatory response to cochlear implant electrodes in humans. Otol Neurotol. 2014;35(9):1545–1551. doi: 10.1097/MAO.0000000000000540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamakura T, Nadol JB. Correlation between word recognition score and intracochlear new bone and fibrous tissue after cochlear implantation in the human. Hear Res. 2016;339:132–141. doi: 10.1016/j.heares.2016.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishai R, Herrmann BS, Nadol JB, Quesnel AM. The pattern and degree of capsular fibrous sheaths surrounding cochlear electrode arrays. Hear Res. 2017;348:44–53. doi: 10.1016/j.heares.2017.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fredelius L, Rask-Andersen H. The role of macrophages in the disposal of degeneration products within the organ of corti after acoustic overstimulation. Acta Otolaryngol. 1990;109(1–2):76–82. doi: 10.3109/00016489009107417 [DOI] [PubMed] [Google Scholar]
- 27.McNally AK and Anderson JM. Macrophage fusion and multinucleated giant cells of inflammation. Adv Exp Med Biol. 2011;713:97–111. [DOI] [PubMed] [Google Scholar]
- 28.Yang W, Vethanayagam RR, Dong Y, Cai Q, Hu BH. Activation of the antigen presentation function of mononuclear phagocyte populations associated with the basilar membrane of the cochlea after acoustic overstimulation. Neuroscience. 2015;10;303:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kämpfe Nordström C, Danckwardt-Lillieström N, Laurell G, Liu W, Rask-Andersen H. The Human Endolymphatic Sac and Inner Ear Immunity: Macrophage Interaction and Molecular Expression. Front Immunol. 2018;9:3181. doi: 10.3389/fimmu.2018.03181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nadol JB Jr, Shiao JY, Burgess BJ, Ketten DR, Eddington DK, Gantz BJ, Kos I, Montandon P, Coker NJ, Roland JT Jr, Shallop JK. Histopathology of cochlear implants in humans. Ann Otol Rhinol Laryngol. 2001;110(9):883–91. [DOI] [PubMed] [Google Scholar]
- 31.Trakimas DR, Kozin ED, Ghanad I, Nadol JB, Remenschneider AK. Human otopathologic findings in cases of folded cochlear implant electrodes. Otol Neurotol. 2018;39(8):970–978. doi: 10.1097/MAO.0000000000001886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaur T, Zamani D, Tong L, et al. Fractalkine Signaling Regulates Macrophage Recruitment into the Cochlea and Promotes the Survival of Spiral Ganglion Neurons after Selective Hair Cell Lesion. J Neurosci. 2015;35(45):15050–15061. doi: 10.1523/JNEUROSCI.2325-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29(43):13435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parakalan R, Jiang B, Nimmi B, et al. Transcriptome analysis of amoeboid and ramified microglia isolated from the corpus callosum of rat brain. BMC Neurosci. 2012;13(1). doi: 10.1186/1471-2202-13-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma SF, Chen YJ, Zhang JX, et al. Adoptive transfer of M2 macrophages promotes locomotor recovery in adult rats after spinal cord injury. Brain Behav Immun. 2015;45:157–170. doi: 10.1016/j.bbi.2014.11.007 [DOI] [PubMed] [Google Scholar]
- 36.McLean NA, Verge VMK. Dynamic impact of brief electrical nerve stimulation on the neural immune axis—polarization of macrophages toward a pro-repair phenotype in demyelinated peripheral nerve. Glia. 2016;64(9):1546–1561. doi: 10.1002/glia.23021 [DOI] [PubMed] [Google Scholar]
- 37.Hickman S, Izzy S, Sen P, Morsett L, El Khoury J. Microglia in neurodegeneration. Nat Neurosci. 2018;21(10):1359–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ueno M, Fujita Y, Tanaka T, et al. Layer v cortical neurons require microglial support for survival during postnatal development. Nat Neurosci. 2013;16(5):543–551. doi: 10.1038/nn.3358 [DOI] [PubMed] [Google Scholar]
- 39.Liu W, Edin F, Atturo F, Rieger G, Löwenheim H, Senn P, Blumer M, Schrott-Fischer A, Rask-Andersen H, Glueckert R. The pre- and post-somatic segments of the human type I spiral ganglion neurons-structural and functional considerations related to cochlear implantation. Neuroscience. 2015;284:470–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hansen MR, Vijapurkar U, Koland JG, Green SH. Reciprocal signaling between spiral ganglion neurons and Schwann cells involves neuregulin and neurotrophins. Hear Res. 2001;161(1–2):87–98. doi: 10.1016/S0378-5955(01)00360-4 [DOI] [PubMed] [Google Scholar]
- 41.Liu W, Glueckert R, Linthicum FH, et al. Possible role of gap junction intercellular channels and connexin 43 in satellite glial cells (SGCs) for preservation of human spiral ganglion neurons: A comparative study with clinical implications. Cell Tissue Res. 2014;355(2):267–278. doi: 10.1007/s00441-013-1735-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.