Abstract
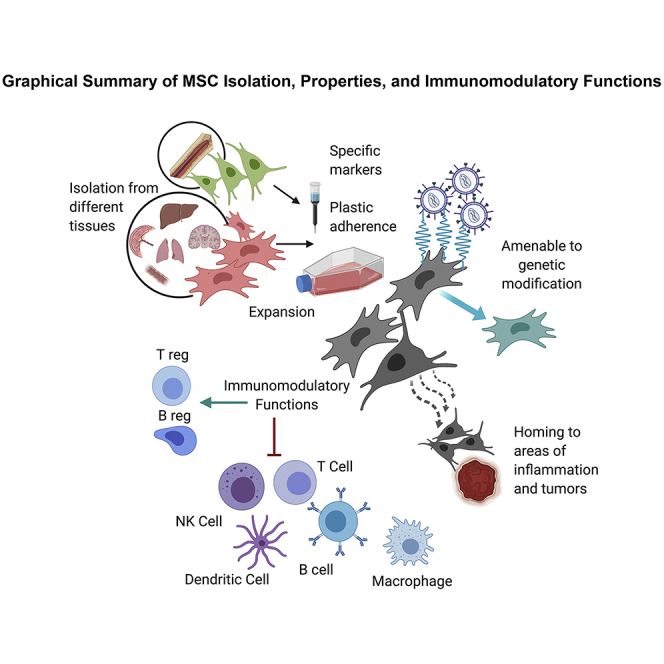
Mesenchymal stromal cells (MSCs) possess several fairly unique properties that, when combined, make them ideally suited for cellular-based immunotherapy and as vehicles for gene and drug delivery for a wide range of diseases and disorders. Key among these are: (1) their relative ease of isolation from a variety of tissues; (2) the ability to be expanded in culture without a loss of functionality, a property that varies to some degree with tissue source; (3) they are relatively immune-inert, perhaps obviating the need for precise donor/recipient matching; (4) they possess potent immunomodulatory functions that can be tailored by so-called licensing in vitro and in vivo; (5) the efficiency with which they can be modified with viral-based vectors; and (6) their almost uncanny ability to selectively home to damaged tissues, tumors, and metastases following systemic administration. In this review, we summarize the latest research in the immunological properties of MSCs, their use as immunomodulatory/anti-inflammatory agents, methods for licensing MSCs to customize their immunological profile, and their use as vehicles for transferring both therapeutic genes in genetic disease and drugs and genes designed to destroy tumor cells.
Graphical Abstract
Main Text
Mesenchymal Stromal Cells (MSCs): Discovery, Origin, and Basic Biology
The existence of non-hematopoietic stem cells within the bone marrow (BM) was first postulated in 1867 by the German pathologist Julius Cohnheim, who made the remarkable demonstration that the BM gives rise to circulating cells, including stromal cells, and that these cells can then migrate to sites of injury and inflammation within the body, exit the bloodstream, enter the affected tissue, and participate in the process of wound healing, a rather controversial notion at the time, and even to this day.1, 2, 3, 4 It would be nearly 100 years from this remarkable discovery before Tavassoli and Crosby5 would provide further evidence for the existence of these non-hematopoietic stem cells by showing that transplanting intact pieces of BM into extramedullary sites in rodents not only reconstituted hematopoiesis, but also led to the formation of structures reminiscent of the native BM, and that Herzog and Bucala6 would put forth the idea of circulating “fibrocytes.”
Despite the existence in the literature of these seminal studies, however, Friedenstein is generally credited with the definitive discovery of marrow stromal/stem cells, as a result of a series of publications in the 1970s. In these reports, Friedenstein et al.7 performed elegant studies demonstrating that the BM contained a plastic-adherent fibroblastoid cell subpopulation that possessed colony-forming potential, had the ability to differentiate into osteoblasts in vitro, and was capable of transferring the hematopoietic microenvironment to ectopic sites following transplantation, thereby establishing the concept that the marrow microenvironment resided within the so-called stromal cells of the BM. It was not until 1991, however, that Simmons and Torok-Storb8 developed the Stro-1 antibody to identify these cells and that Caplan9 attributed properties of true stem cells to MSCs. This finding led him to coin the term “mesenchymal stem cell,” which he defined as “stromal cells that are capable of differentiating through a series of separate and unique lineage transitions into a variety of end-stage phenotypes.”9 This far-sighted hypothesis was provided with solid scientific support a few years later, when the first detailed description of the trilineage potential of MSCs was published.10
Since these ground-breaking studies, great strides have been made in both our understanding of these cells and their potential therapeutic uses.11 MSCs are now known to be a key part of the highly specialized BM microenvironment/niche that maintains hematopoietic stem/progenitor cells (HSCs) and regulates hematopoiesis.4,12, 13, 14 MSCs actively participate in maintaining the critical balance between self-renewal and differentiation of HSCs via both direct cell-cell interactions and by secreting cytokines to exert paracrine effects.15,16 Despite their essential role within the BM, MSCs are very rare, being present at a frequency of only about 1 in 10,000 nucleated cells shortly after birth and declining thereafter as a function of age.17 In addition to their role in regulating hematopoiesis, MSCs also serve as progenitors for mesodermal tissues,10,18, 19, 20 giving rise, in the presence of the appropriate stimuli, to bone, cartilage, and fat.10
Sources of MSCs and Tissue Repair
Although much work to date has focused on MSCs isolated from adult murine and human BM, it is important to realize that tissue-specific MSCs, or pericytes, are now known to be widely distributed in perivascular regions of almost all tissues throughout the body, where they are thought to play an important role in tissue homeostasis, physiological remodeling, injury repair, and tissue regeneration throughout the life of the individual.21, 22, 23, 24, 25 Indeed, our group and others have successfully isolated MSCs from numerous tissues, including brain, liver, lung, fetal blood, umbilical cord blood, amniotic fluid, placenta, kidney, and liposuction material.18,20,26, 27, 28, 29, 30 However, even though MSCs from each of these various tissues appear similar with respect to phenotype and overall differentiative potential, differences exist in the protein and transcriptomic profiles, as well as in the secretome and global microRNA (miRNA) expression profile of MSCs, such that each tissue’s MSCs possess a molecular fingerprint indicative of their tissue of origin,24,28,31, 32, 33, 34, 35, 36, 37 and we and others have provided experimental evidence that these differences likely reflect differing biological properties/potential in vitro and in vivo.38, 39, 40, 41
Based on their widespread distribution and ability to mediate repair in a wide range of injuries and diseases, it is intriguing to speculate that MSCs may in fact represent a latent pool of stem/progenitor cells, distributed ubiquitously throughout the body,42 potentially capable of migrating to sites of injury/inflammation and generating tissue-specific cells and/or releasing paracrine factors to repair the damage in question.43 Indeed, MSCs have been proven to have the ability to migrate and seed specifically into damaged tissue sites, where they can replace damaged or diseased cells via differentiation/ reprogramming in situ44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54 (even into cells of endodermal and ectodermal derivation, albeit at low frequencies55, 56, 57, 58), and to secrete cytokines, proteolytic enzymes, and angiogenic factors that serve to stimulate the proliferation and survival of endogenous cells within the local tissue while inhibiting apoptosis and fibrosis.44,59, 60, 61, 62, 63, 64, 65 Scadden and colleagues66 provided evidence in a model of type 1 diabetes that MSCs may actually be mobilized from the marrow in response to inflammation, adding further credence to this claim. This ability to reprogram to adopt alternate cellular fates and thereby repair damaged tissue has, however, been questioned by some in the field, as has the ability of these cells to engraft long-term in human recipients.67 Using a fetal sheep model, our group was the first to show that human MSCs engraft in multiple tissues following in utero transplantation, and they possess the ability to reprogram and/or differentiate to give rise to a wide variety of tissue-specific cells in this non-injury setting, in the absence of cellular fusion or donor-to-host mitochondrial/membrane transfer.55,68 Our work in the fetal sheep model agrees with clinical observations made by Fisk and colleagues,69,70 who used X-Y fluorescence in situ hybridization (FISH) to demonstrate decade-long persistence of MSCs of fetal (male) origin within tissues of the mother. Thus, within the fetal milieu, there is very strong evidence to support the engraftability and broad differentiative potential of MSCs.
Isolation of MSCs
The most straightforward method to obtain MSCs is to exploit their plastic adherence and their ability to be passaged with trypsin. This simple approach yields a relatively morphologically homogeneous population of fibroblastic cells within only two to three culture passages.10,71,72 However, “MSCs” derived in this way represent a highly heterogeneous population of cells with multiple distinct phenotypic and biological properties, only a small percentage of which are true mesenchymal stem/progenitor cells.73 In addition, studies have provided evidence for the existence of specific subpopulations, each with its own distinct differentiative preference toward specific lineages, in addition to true MSCs that possess multilineage differentiative potential.74 This heterogeneity creates a lack of consistency and has confounded comparison of results obtained in different laboratories. To further complicate matters, the conditions used during culture expansion can also exert a marked effect on the phenotype and functionality of the final cell product, as can their cryopreservation.75, 76, 77, 78
For clinical applications, it is essential to start with a well-defined cell population, including validated functionality. However, unlike the hematopoietic system,79, 80, 81, 82 there is no widely accepted and straightforward in vivo assay to quantify the stemness/multipotency of MSCs, making it difficult to convincingly distinguish primitive MSCs from progenitors and more differentiated stromal elements.83 Bianco et al.67 and Keating84 developed a model in which MSC potency could be assayed by transplanting a clonal population of MSCs and assessing the formation of an ectopic marrow niche that could support hematopoiesis in vivo, but this system has not seen widespread use in the field. To overcome the lack of a simple in vivo readout for potency, ever-increasing numbers of studies have used surface markers in an effort to identify antigens that are unique to MSCs, thereby allowing their isolation to relative purity, and to catalog specific subsets of MSCs with respect to proliferation and survival rates, immunomodulatory features, and their differentiation bias.3,74 These efforts to define an MSC-specific marker have, however, thus far been largely unsuccessful;83 while a diverse range of antigens have been found to be expressed on the surface of MSCs, including CD29, CD44, CD54 (intercellular adhesion molecule 1 [ICAM-1]), CD73, CD90, CD105, CD106 (vascular cell adhesion molecule 1 [VCAM-1]), and Stro-1,18,20,74,83,85, 86, 87, 88 none of these has proven to be unique to these cells. Due to this lack of unique markers, and in an effort to achieve comparable and unambiguous results with respect to MSC functionality and efficacy between various groups, the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy (ISCT) proposed a minimal set of standard criteria to be used to define human MSCs,11,18,20,89 and these are still considered the reference/benchmark for characterizing these cells at the end of their in vitro expansion. These criteria include: (1) plastic adherence; (2) expression of CD105, CD73, and CD90; (3) the absence of the hematopoietic markers CD45, CD34, CD11b, CD14, CD19, CD79a, and histocompatibility leukocyte antigen-DR isotype (HLA-DR); and (4) the ability to differentiate into chondrocytes, osteoblasts, and adipocytes in vitro, when provided with the appropriate stimuli.18,74,90 In addition, the absence of CD31 (platelet endothelial cell adhesion molecule [PECAM]) is also considered to be important, to exclude confusion with phenotypically very similar endothelial cells. Recently, efforts have been undertaken to establish monitoring of CD142/tissue factor, as both a phenotypic marker and safety criterion for MSC products, as MSCs expressing high levels of this molecule can trigger the instant blood-mediated inflammatory reaction (IBMIR), leading to rapid elimination of the infused cells and loss of therapeutic effect.77,91
A critical caveat to this set of ISCT standards, however, is that these criteria are based on the features of MSCs that have been culture-expanded in vitro, and they may not accurately reflect the properties that MSCs possess in vivo within the BM and other tissues. Moreover, it is important to realize that even MSCs that meet the above minimal criteria often represent a mixture of cells with diverse phenotypes, biological activities, and corresponding therapeutic potential,74,92,93 and that these properties can be dramatically altered by cryopreservation, negatively affecting therapeutic outcome.77,78,91 For example, the expression of molecules such as CXC chemokine receptor (CXCR)4, platelet-derived growth factor (PDGF) receptor, and VCAM-1 that play a vital role in MSC biology/function have been shown to be restricted to specific subsets of MSCs.94, 95, 96 Selecting for the fraction of MSCs that express CXCR4, or forced overexpression of CXCR4, led to a marked enhancement in tissue repair in multiple injury models, including myocardial infarction,97 stroke,98,99 acute kidney injury,100 and early liver regeneration,101 as well as augmented homing to the BM.99,100 Likewise, the subpopulation of MSCs expressing high levels of the Stro-1 antigen was shown to possess high growth capacity and enhanced trafficking and tissue repair abilities. These studies led to Stro-1 being proposed as a critical marker to assess MSC functional potency.55,102, 103, 104, 105 Studies have reported similar findings for subsets of MSCs expressing CD105, CD106, CD146, and CD271.95,106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122
Collectively, these studies provide compelling evidence that it may be possible to develop far more effective therapies by using specific subpopulations of MSCs that exhibit an enhanced ability to provide the function most appropriate for the condition to be treated.
Immunological Properties of MSCs and Their Use to Modulate Immunity
MSCs are fairly unique cells from an immunological standpoint, in that they express only HLA-I antigens on their surface, but lack expression of HLA-II and the co-stimulatory molecules CD80 and CD86 that are required for Т lymphocyte activation.84,123, 124, 125, 126, 127, 128 As a result, MSCs are not very good targets for lysis by cytotoxic T cells, nor do they efficiently induce the proliferation of allogeneic lymphocytes when used as stimulators in a traditional mixed lymphocyte reaction. These properties led to the general consensus that MSCs enjoy a relatively immune-inert status in vivo, a premise that is supported by an accumulating body of evidence showing that MSCs can be transplanted across allogeneic barriers without eliciting a robust immune response.129, 130, 131, 132, 133, 134, 135 Note, however, that this important issue is still the subject of intense investigation,130, 131, 132, 133, 134, 135, 136 as rodent transplantation studies have indicated that allogeneic MSCs can, in fact, elicit an immune response in vivo,90 inducing allospecific CD4+ and CD8+ memory T cells137,138 and the formation of alloantibodies.132,139,140 Similarly, we and others have shown that human MSCs can, under certain conditions, serve as effective targets for lysis by natural killer (NK) cells.141, 142, 143, 144 This is clearly an area that merits further study, as the true immune status of allogeneic MSCs is obviously of critical importance for their safe (and effective) clinical use.
In addition to their interesting “hypoimmune” nature, a wealth of data has now provided irrefutable proof that MSCs have highly potent immunomodulatory/immune-dampening properties both in vitro and in vivo.84,125,145, 146, 147, 148 Since Bartholomew et al.149 showed in 2002 that MSCs had the ability to suppress a mixed lymphocyte response in vitro and prevent rejection in a baboon skin allograft model in vivo, countless studies have shown that MSCs can act upon both the innate and adaptive arms of the immune system and target virtually all immune cells, impairing the proliferation and/or functionality of T, B, and NK cells in response to mitogens, alloantigens, and activating antibodies, both in vitro and in vivo.60, 61, 62,83,86,123,124,145,149, 150, 151, 152, 153, 154, 155, 156, 157, 158, 159 These effects appear to be mediated both by direct contact with target cells160, 161, 162 and by the release of myriad soluble molecules.127,163, 164, 165, 166, 167, 168, 169 These findings have generated tremendous enthusiasm and hundreds of clinical trials to test their potential as immunotherapeutics to treat diseases involving immune dysregulation, such as autoimmune disorders, inflammatory bowel disease (IBD), type 1 diabetes, type 2 diabetes, arthritis, ischemia-reperfusion injury, and to thwart the immunological complications that arise following the transplantation of HSCs, solid organs, and vascularized composite allografts (VCAs).90,125,135,143,170, 171, 172, 173, 174, 175, 176, 177, 178, 179, 180, 181, 182, 183, 184, 185, 186, 187, 188, 189, 190, 191, 192, 193, 194, 195, 196, 197
Of particular clinical interest, MSCs also exhibit a remarkably potent ability to skew the balance between effector/memory T cells and CD4+FoxP3+ regulatory T cells (Tregs), polarizing both naive and memory T cells toward a Treg phenotype in vitro and tipping the alloimmune response toward tolerance and long-term allograft acceptance in vivo.90,125,145,149,156,160,198, 199, 200, 201
MSCs can also induce the formation of non-traditional CD8+ Tregs that can act to suppress allogeneic lymphocyte proliferation86,156,202,203 and stimulate the differentiation of B cells into regulatory B cells (Bregs), which further aid the process of tolerance induction.204 In addition to their effects on regulatory T and B cell populations, MSCs also efficiently target and modulate memory T cells, potently suppressing the in vitro proliferation of human memory T cells in response to alloantigens or cytokines205, 206, 207 and the proliferation and cytotoxic function of memory T cells against alloantigens of both minor and major histocompatibility complexes in vivo in mice.125,205,208
MSCs also exert marked suppressive effects on antigen-presenting cells (APCs). Looking specifically at the “professional” APCs, dendritic cells (DCs), co-culture with MSCs has been shown to affect DC maturation, differentiation, and functionality with respect to antigen presentation.146,209 Specifically, when in the presence of MSCs, DCs were unable to respond to maturation signals and failed to upregulate expression of HLA-DR, CD80, and CD86.209, 210, 211, 212, 213 Moreover, the presence of MSCs resulted in a shift in the cytokine profile of the DCs such that the levels of the inflammatory cytokines tumor necrosis factor (TNF)-α, interferon (IFN)-γ, and interleukin (IL)-12 were all decreased, while expression of the anti-inflammatory cytokine IL-10 was upregulated.150,209 As a result of these alterations, MSC-exposed DCs were no longer able to activate effector Т cells, but instead stimulated the proliferation of Тregs. Under particular conditions, MSCs have also been shown to skew the inflammatory phenotype of macrophages (another APC) by converting pro-inflammatory M1-type cells into a more anti-inflammatory M2-type subset, adding yet another layer of complexity to their immunomodulatory repertoire.214, 215, 216
The mechanisms by which MSCs exert these varied effects on multiple immune effector lineages are not at all straightforward, and a wide range of molecules/pathways have been implicated. Some of the major players in this ever-growing list include: transforming growth factor (TGF)-β1, hepatocyte growth factor (HGF), prostaglandin E2 (PGE2), indoleamine 2,3-dioxygenase (IDO), inducible nitric oxide synthase (iNOS), leukemia inhibitory factor (LIF), HLA-G, heme oxygenase-1 (HO-1), insulin growth factor (IGF), IGF-binding protein (IGFBP),217 TNF-stimulated gene 6 (TSG-6), IL-10, the semaphorins (in particular semaphorin-3a218,219), the galectins (specifically Gal-1, Gal-3, and Gal-9219, 220, 221, 222, 223), erythropoietin-producing hepatocellular (Eph) receptor tyrosine kinase-B/Eph family receptor-interacting protein (ephrin)-B, glycoprotein A repetitions predominant (GARP; a receptor for latent TGF-β), and even purinergic signaling.59, 60, 61, 62,124,161,224, 225, 226, 227, 228, 229, 230, 231, 232, 233, 234, 235, 236, 237, 238, 239, 240, 241, 242, 243
Of particular interest in this long list are three molecules/pathways that were initially discovered for their role in promoting maternal tolerance to the fetus which, immunologically speaking, represents a haplo-identical allograft during pregnancy. The first of these is IDO, the enzyme that catalyzes the rate-limiting step in the pathway that breaks down tryptophan into kynurenine. IDO is now recognized to mediate immunosuppression and to play a key role in the generation of immune tolerance in many settings aside from pregnancy,244 primarily via inducing the generation of Tregs and tolerogenic DCs.182,245 The IDO pathway represents one of the main mechanisms by human MSCs mediate immunosuppression.246 This is in marked contrast to murine MSCs, which work primarily through iNOS, and rat MSCs, which work through HO-1.246, 247, 248 These key species differences highlight the care that must be taken when performing studies with MSCs in rodents and trying to directly extrapolate the findings to the human setting. To further complicate matters, the mechanism by which human MSCs exert their immunomodulatory effects has also been demonstrated to depend on the tissue from which the MSCs are derived. MSCs from the BM and umbilical blood suppressed T cells by inducing cell cycle arrest, while MSCs from adipose tissue and umbilical cord inhibited T cell proliferation by inducing apoptosis.249
Interestingly, Chen et al.250 found that dexamethasone inhibits the expression of iNOS in mouse MSCs and IDO in human MSCs, and thereby abolishes the immunomodulatory and therapeutic effects of MSCs from both species. This finding is of great clinical importance, as it suggests that concurrent treatment of patients with steroids would likely interfere with any therapeutic effects that would be mediated by infused MSCs. It also provides a possible explanation for why the outcomes of studies using MSCs in similar disease settings have often been contradictory.
HLA-G is another molecule that was initially described for its involvement in fetal-maternal tolerance and is now recognized for its ability to affect the function of diverse immune cell populations and to induce several subsets of suppressive/regulatory cells.251 Specifically, HLA-G is thought to regulate the cytokine balance by polarizing the T helper (Th)1/Th2 balance in favor of Th2 with increased IL-10 secretion.252 While subpopulations of MSCs express both the membrane-bound (HLA-G1) and soluble (HLA-G5) forms of HLA-G, Giuliani et al.253 provided evidence that it is the surface expression of the HLA-G1 isoform that is responsible for the T cell inhibition by MSCs. Siegel et al.254 made the significant observation that both HLA-G1 and HLA-G5 are downregulated by MSCs during culture expansion, underscoring the importance of using MSCs at relatively low passage number if one wishes to maximize their immunomodulatory properties. We and others have shown that the immunosuppressive effects of human MSCs can be enhanced by engineering them to stably produce HLA-G1 via lentiviral transduction.255,256 Quite intriguingly, when other vector systems were used to deliver the HLA-G1 gene, its immunomodulatory benefits were lost.256 In lieu of genetic modification, one can imagine that by selecting a subpopulation of MSCs that express high levels of HLA-G1, it should be possible to ensure that potent immunosuppressive effects are achieved upon infusion.235
The third molecule that MSCs have co-opted from fetomaternal tolerance during pregnancy is LIF.257, 258, 259 MSCs express high levels of LIF,260,261 and these levels increase during co-culture with lymphocytes. Data from Najar et al.262 and Nasef et al.263 have demonstrated that LIF has the ability both to induce direct inhibition of effector T cells and to promote the generation of Tregs, thereby playing a pivotal role in MSC immunomodulation. Subsequent studies have indicated that LIF likely exerts these immune-dampening effects, at least in part, through its ability to modulate HLA-G production by MSCs.264
In an effort to make sense of this complex array of immunoregulatory pathways, Nasef et al.265 recently proposed two distinct mechanisms by which MSCs can tip the balance in favor of T cell tolerance. The first of these relies on the induction of the tolerogenic genes IDO, LIF, and HLA-G, and it takes place in a contact-independent manner. The second mechanism requires direct contact between the MSCs and the target T cells, and it involves the modulation of IL-10 and TGF-β gene expression within the T cells.
Decrypting how all of these MSC-derived regulatory mediators act in concert with one another will make it possible to better define the regulatory network by which MSCs tune the immune microenvironment and provide fundamental information for developing more clinically effective MSC-based immunotherapies. It is quite likely that our current imperfect knowledge of MSC immunobiology can explain why the results of clinical trials to date have been inconsistent and why conclusive proof of efficacy often remains elusive.146,152,246,266,267
To aid the reader in navigating the myriad factors and many effects that MSCs exert on the immune system and the range of immune-related therapeutic targets being considered, a summary appears in Table 1, including citations of salient studies.
Table 1.
Immunomodulatory Effects, Mechanisms, and Therapeutic Uses of MSCs
| MSCs | Representative References |
|---|---|
| Effect | |
| Suppress mixed lymphocyte reaction (MLR) | 149 |
| Impair proliferation and/or functionality of: | |
| T cells | 123,124,149,150,152,205, 206, 207, 208 |
| B cells | 158,159 |
| NK cells | 153 |
| DCs | 146,151,157,209, 210, 211, 212, 213 |
| Skew the balance of T cells toward FoxP3+ Tregs | 90,125,145,149,155,156,160,198, 199, 200, 201 |
| Induce formation of non-traditional CD8+ Tregs | 86,156,202,203 |
| Stimulate Bregs | 204 |
| Skew macrophages toward an anti-inflammatory M2 phenotype | 214, 215, 216 |
| Soluble Factor Produced by MSCs to Modulate Immunity | |
| TGFb1 | 265 |
| HGF | 167 |
| PGE2 | 165 |
| IDO (human) | 244,246,267 |
| iNOS (mouse) | 162,164 |
| HO (rat) | 239,267 |
| LIF | 261, 262, 263 |
| HLA-G | 253, 254, 255, 256 |
| IGF/IGFBP | 217 |
| TSG-6 | 266 |
| IL-10 | 265 |
| Semaphorins | 218,219 |
| Galectins | 161,219, 220, 221, 222, 223 |
| Ephrin B | 230 |
| GARP | 228 |
| Adenosine | 224,225 |
| Disease/Therapeutic Target | |
| Inflammatory bowel disease (IBD) | 3 |
| Type 1 and type 2 diabetes | 177,314, 315, 316, 317, 318 |
| Arthritis | 180 |
| Ischemia/reperfusion injury | 96,100 |
| To thwart immune response to transplanted: | |
| HSCs | 109,129 |
| Solid organs | 90,149,176,181,182 |
| Vascularized composite allografts (VCAs) | 126,171,172,183 |
Tailoring the Immunomodulatory Properties of MSCs
DCs and macrophages serve as conventional immunocompetent “tissue sentinels,” but evidence is increasing to suggest that MSCs also participate in the process of immunosurveillance.248 It is critical to realize that MSCs are not static and they do not constitutively express all of their myriad immunomodulatory functions discussed in the preceding section. Rather, MSCs can actively sense the surrounding microenvironment and modulate, accordingly, the function of various immune cells within the host, dependent upon the prevailing immunological milieu.268,269 The surrounding microenvironment can influence the immunologic phenotype and immunomodulatory behavior of MSCs.270 When presented with inflammatory stimuli, such as the proinflammatory cytokines TNF-α and INF-γ, MSCs are induced to adopt an immunosuppressive phenotype. Conversely, when inflammation is absent, MSCs tend to exist in a proinflammatory state.269,270 This ability to adapt to their local surroundings has led some to describe MSCs as “environmentally responsive therapeutics.”4,269,271 Indeed, for MSCs to exert their multiple therapeutic effects, the communication of MSCs with the environment upon arrival to the injured site is essential.
Interestingly, to produce optimal immunomodulation, MSCs require priming with a combination of pro-inflammatory cytokines, specifically IFN-γ together with either TNF-α or IL-1.272 In response to this priming, MSCs switch their secretome toward an anti-inflammatory and pro-trophic phenotype, producing high levels of immunoregulatory factors, cell-mobilization factors, and growth factors that work together to facilitate tissue repair by resident cells.11,270,273, 274, 275 Priming of MSCs with the pro-inflammatory cytokines IFN-γ and TNF-α also induces upregulation of chemokine receptors such as CXCR3 and CC chemokine receptor 5 (CCR5),276 enabling these primed MSCs to sense the chemoattractant gradient and more efficiently home to sites of injury,277 and the adhesion molecules ICAM-1 and VCAM-1, which potentiates the accumulation of immune cells in close proximity to MSCs, thereby enhancing their immunosuppressive effects.162,278
One must exercise great care, however, when attempting to augment the immunomodulatory properties of MSCs by priming them with pro-inflammatory cytokines, as data indicate that the concentration and duration of exposure to a given cytokine can dramatically influence the biological response of MSCs,270 with rapid intense exposure of MSCs to high concentrations of pro-inflammatory cytokines producing a very different response compared to prolonged exposure at lower concentrations.279, 280, 281 For example, the effect of IFN-γ on MSC expression of HLA-DR is bimodal. HLA-DR expression is induced at low IFN-γ concentrations, inducing MSCs to adopt a pro-inflammatory phenotype that enables them to uptake, process, and present soluble exogenous antigens through their major histocompatibility complex (MHC) class II molecules, leading to the activation of naive CD4+ T cells and induction of CD8+ T cells in vitro and in vivo.86,136,282, 283, 284, 285, 286, 287, 288, 289, 290, 291, 292 In contrast, HLA-DR expression is downregulated at high IFN-γ levels, thereby stripping MSCs of their ability to act as APCs and triggering a tolerogenic phenotype.282,285,287,293,294
Sivanathan et al.295 showed that IL-17A priming of MSCs may represent a novel immunomodulatory strategy and an alternative to IFN-γ to enhance the immunosuppressive properties of MSCs while maintaining their native immune-inert state. MSCs primed with IL-17, unlike those primed with IFN-γ, showed no induction or upregulation of MHC class I, MHC class II, or of the T cell costimulatory molecule CD40 that could have the potential to negatively affect their fate/survival in vivo.
Mimicking infection in vitro using agonists to activate specific Toll-like receptors (TLRs) has also been shown to modulate the functions and responses of MSCs.4,296,297 This should not be surprising, since the activation of TLRs expressed on the surface of MSCs by their corresponding ligands present at the site of tissue injury/inflammation is thought to be one of the major factors influencing the biological functionality of MSCs in vivo.298 In humans, 10 functional TLRs have been described.299 These receptors are expressed on immune cells and non-immune cells such as MSCs.297 In nature, TLRs are activated by pathogen-associated molecular patterns (PAMPs), which are derived from microbial structures300, released by normal cells in response to ischemia, tissue damage, and trauma. The TLRs are traditionally divided into two subgroups depending on their subcellular localization and the nature of the PAMP ligands they sense. TLRs 1, 2, 4, 5, 6, and 10 are expressed at the cell surface and recognize microbial membrane components, while TLRs 3, 7, 8, and 9 are expressed only in intracellular membrane compartments (endoplasmic reticulum, lysosomes, and endosomes) and recognize viral nucleic acids. In human MSCs, expression of many of these TLRs has been shown to be dependent on their tissue of origin, and to be markedly altered by environmental conditions such as inflammation.290,296,301, 302, 303, 304, 305, 306, 307 Indeed, many of the immunomodulatory properties MSCs exhibit following cytokine priming can be recapitulated by adding agonists to specific TLRs.290 Importantly, activation of TLRs on MSCs does not induce the expression of HLA-I, HLA-II, CD80, or CD86, and, consequently, TLRs do not alter the immunogenicity of MSCs.302,308,309
Waterman et al.310 reported a new paradigm for MSC immunomodulatory functions by showing that they can be specifically polarized by downstream TLR signaling, analogous to that described for the monocyte/macrophage lineage. They showed that MSCs primed with the TLR4 agonist lipopolysaccharide (LPS) adopted a pro-inflammatory phenotype (MSC1), and they produced mediators such as macrophage-inflammatory protein (MIP)-1α and MIP-1β, regulated on activation, normal T cell expressed and secreted (RANTES), CXC chemokine ligand (CXCL)9, and CXCL10 that are able to induce T lymphocyte activation. In contrast, MSCs primed with the TLR3 agonist poly(I:C) adopted an immunosuppressive/tolerogenic phenotype (MSC2), expressing factors known to play a key role in the T cell-inhibiting effects of MSCs such as IDO, PGE2, NO, TGF-β, HGF, and HO-1. Giuliani et al.311 furthered these studies by showing that exposure to certain TLR ligands can modulate the surface expression and secretion of MICA (MHC class I polypeptide-related sequence A) by primed MSCs, which can protect primed MSCs against activated NK cells and inhibit cytolytic functions of NK cells. In other related work, Lombardo et al.302 showed that activation of TLRs 2, 3, 4, and 9 on human adipose-derived (hAD)-MSCs induced molecules in the nuclear factor κB (NF-κβ) pathway, including manganese superoxide dismutase (MnSOD), and that expression of MnSOD provided better engraftment and induced the survival of hAD-MSCs in inflammatory conditions or injured tissues.
These collective findings led Waterman et al.292 to propose that MSCs should be skewed toward the desired MSC1 or MSC2 phenotype prior to infusion in order to ensure that they produce the desired immune actions. However, things may not be as neat and simple as they appear with this new paradigm, as the molecular mechanisms underlying MSC polarization into these two distinct phenotypes remain unclear, as do the effects of TLR-priming MSCs on T lymphocyte functions.303,306,308,310 Moreover, studies by other groups have suggested that the time of exposure to TLR ligands and the concomitant presence of other cytokines are likely to add layers of complexity to this regulatory pathway.248
MSCs as Antigen-Specific Immunotherapies
Broad-based non-specific immunosuppression is far from optimal for treating autoimmune diseases and other disorders that involve immune dysregulation due to the unacceptably high toxicity and risk of opportunistic infection. MSCs have been tested for their ability to modulate adaptive immunity non-specifically,312, 313, 314, 315, 316 but if it were possible to exploit the marked immunomodulatory effects of MSCs with their ability to serve as unconventional APCs upon activation/priming,136,282, 283, 284, 285 MSCs could theoretically become an antigen-specific therapy,317 a holy grail in the field of immunotherapy.318 van Megen et al.317 provided in vitro evidence that peptide-pulsed activated human MSCs can inhibit antigen-specific responses, thus taking a critical step toward the clinical translation of MSCs as an adaptive, antigen-specific immunotherapy for treating autoimmunity. Interesting, HLA class II matching with the recipient was found to be required to deliver adaptive immune alterations, implying that the suppressive licensing by MSCs is a direct consequence of peptide presentation on the appropriate HLA restriction elements to the T cell. However, matching the MSCs for one HLA haplotype with the T cell donor was sufficient for antigen-specific inhibition, increasing the number of recipients who could potentially be treated with a given off-the-shelf MSC-based product.136 Intriguingly, the authors also found that while activation and peptide-pulsing of human MSCs resulted in inhibition of T cells, performing the same procedure with mouse MSCs resulted in the activation of T cells,285 again underscoring the species-specific differences that exist between the MSCs of mice and humans with respect to their immunomodulatory properties and the importance of using an appropriate model when aiming to translate research findings to the clinic.
MSCs as Vehicles for Gene Delivery
MSCs possess tremendous therapeutic potential due to their ability to home to sites of injury within the body, mediate potent immunomodulation to restore homeostasis, and both give rise to tissue-specific cells and release trophic factors that trigger the tissue’s own endogenous repair pathways.43,55,68,166 However, these properties are just the beginning of the therapeutic applications for MSCs.319, 320, 321 By using gene transfer to engineer MSCs, it is possible to either augment their innate production of specific desired proteins or to enable them to express proteins they normally do not, and it is possible to greatly broaden the clinical utility of MSCs. MSCs possess several qualities that make them ideal vehicles for gene delivery.43,55,63,68,71,150,322 First, they can be transduced at high efficiency with all of the major viral-based vectors, including adeno-associated virus (AAV),323,324 adenovirus,325, 326, 327 lentiviruses,328, 329, 330, 331, 332, 333 and the murine retroviruses,327,334, 335, 336, 337 and robustly produce a wide range of cytoplasmic, membrane-bound, and secreted proteins. Following transduction, the gene-modified MSCs can be selected and extensively expanded in vitro to generate adequate numbers for transplantation. This is in marked contrast to other cells being used as gene delivery vehicles, such as HSCs, which cannot be expanded in vitro without loss of in vivo functionality. The immune-inert nature of MSCs (as discussed in detail in preceding sections) also represents a significant strength, as it may enable MSCs expressing a “foreign” protein to go undetected by the recipient’s immune system, and the use of allogeneic “off-the-shelf” gene-modified MSCs should be possible. In our opinion, these features combine to make MSCs one of the most promising populations for use in cell-based approaches to gene therapy.
Despite their many advantages as gene delivery vehicles, however, few studies have thus far explored the potential of using gene-modified MSCs to treat genetic diseases. One disease that we and others have spent many years investigating with regard to the potential of MSCs as cellular vehicles for delivering a therapeutic gene is hemophilia A.338, 339, 340, 341, 342, 343, 344 Both hemophilia A and B are rather unique genetic diseases, because the missing coagulation factor (FVIII or FIX, respectively) does not need to be expressed in either a cell- or tissue-specific manner to produce phenotypic correction. The endothelial cells of the liver sinusoids are thought to be the primary natural site of FVIII synthesis.345 However, expression of FVIII in other tissues exerts no deleterious effects, as is evidenced by low levels of endogenous expression of FVIII in multiple tissues throughout the body.343,346, 347, 348 To be therapeutic, FVIII simply has to be expressed in cells with ready access to the circulation, so that it can be secreted into the bloodstream and exert its appropriate clotting activity. Hemophilia A is also unique in that very low levels of FVIII are actually required to exert a pronounced therapeutic benefit. Levels of FVIII of only 2%–3% of normal would convert a hemophilia A patient from a severe, life-threatening phenotype to a moderate phenotype, greatly improving their quality of life.
FVIII is a challenging protein to express, as it is large and needs to undergo complex post-translational modifications to fold properly and exert procoagulant activity. As such, forced overexpression of FVIII can often place an undue amount of stress on the endoplasmic reticulum and trigger the unfolded protein response (UPR).349,350 We previously showed that MSCs/pericytes form various tissues of the body endogenously produce and secrete fully functional FVIII, albeit at low levels,343 thus establishing that these cells possess the requisite machinery to express, process, and secrete FVIII. In support of this supposition, we and others have also shown that MSCs can be transduced with FVIII-expressing viral vectors and secrete high levels of FVIII protein339,340,342,351 that has a specific activity, relative electrophoretic mobility, and proteolytic activation pattern that is virtually identical to that of FVIII produced by commercial cell lines.340
Given the widespread distribution and engraftment of MSCs following systemic infusion, their ability to efficiently process and secrete high amounts of biologically active FVIII, and their documented ability to migrate to sites of injury and inflammation within the body, we performed a pilot study evaluating the ability of haploidentical (paternal) BM-derived MSCs transduced with a lentiviral vector driving constitutive expression of FVIII to correct two pediatric sheep with severe hemophilia A342,352. At the time of MSC administration (via ultrasound-guided intraperitoneal injection), both animals had received multiple infusions of human FVIII protein to treat spontaneous bleeding events, they had low-titer inhibitors to FVIII, and the rapidly progressing hemarthroses of their legs had rendered them nearly immobile. Within days following the infusion of FVIII-expressing MSCs, the hemarthroses resolved, both sheep regained the ability to stand, and they subsequently returned to normal levels of activity/movement. All spontaneous bleeding events also ceased.
At roughly 6 months after MSC infusion, the animals were euthanized and their tissues collected for analysis. The haploidentical FVIII-expressing MSCs were found in almost all tissues examined but were present in the highest numbers in the joints that had been bleeding at the time of infusion. These findings illustrate several key aspects that support the value of MSCs as vehicles for gene delivery. The first of these is the fact that the haploidentical MSCs were able to engraft and persist in this large animal model system following postnatal infusion, supporting the assertion that MSCs are indeed relatively immune-inert and can be transplanted across allogeneic barriers. The second finding of note is that the MSCs that were infused into the peritoneal cavity migrated to and engrafted predominantly in the joints with active bleeds, establishing that MSCs can sense and are drawn to the injury and inflammation present in the context of hemarthroses. The third and perhaps most remarkable observation is the cessation of bleeding and the resolution of the hemarthroses in animals who both had inhibitors to FVIII. This finding supports our assertion that the immune-inert nature of MSCs can be exploited to deliver an immunogenic transgene and achieve durable expression without rejection of the transgene-expressing cells. It also suggests that FVIII-expressing MSCs could potentially serve as a novel immune-evading treatment for hemophilia A patients with inhibitors.
These promising results in the context of hemophilia A provide a critical proof of principle that MSCs can be used as vehicles to deliver therapeutic gene products to numerous tissues in the body, and that this approach could thus provide a permanent cure for a diverse range of diseases.
MSCs for Cancer Immunotherapy
Cancer represents a condition in which there is a state of chronic inflammation and the forming tumor creates a selective need for new cells, much as occurs during development or following injury. A wealth of data now supports the extraordinary ability of MSCs to “sense” this need and migrate to the forming tumor following intravenous administration, likely due to the inflammatory mediators present at the site of a tumor.353, 354, 355, 356, 357, 358, 359 Once they arrive at the tumor, however, MSCs appear to integrate and contribute to the newly forming supportive “stroma” of the tumor.59, 60, 61, 62,359, 360, 361, 362, 363, 364 This property constitutes a serious risk, since infused MSCs could actually provide support, contribute to the growing tumor, and dampen tumor immunity through their immunomodulatory properties.192,361,362 Clearly, these are not desirable outcomes in the clinical treatment of cancer. However, this tumor-homing propensity could be harnessed to achieve a powerful and unique means of selectively delivering chemotherapeutics, cytokines, and the genes for drug-activating enzymes to tumor cells in vivo.359,364, 365, 366, 367, 368, 369, 370
At the present time, the utility of many of the most promising biological agents for cancer therapy is limited by their short in vivo half-life and the pronounced toxicity as a result of their inability to distinguish between tumor cells and all of the normal, non-malignant cells within the body. Given their ability to selectively migrate to the tumor site, using MSCs to deliver these cancer therapeutics could solve both problems, as the MSCs would ensure the therapeutic/toxic payload is only unloaded within the tumor. This should greatly increase the intratumoral concentration of the agent, boosting its therapeutic effects while simultaneously lowering systemic toxicity.371,372
The tumor-homing abilities of MSCs are not limited to solid tumors and the primary tumor mass. On the contrary, studies have now shown that this tumor affinity of MSCs also confers them with the ability to actively seek out metastases, even when they are located at sites far removed from the primary tumor.322,364,373,374 Given the difficulty and poor clinical outcomes that are often achieved using traditional approaches such as surgery and radiotherapy/chemotherapy to treat tumors that are highly invasive or prone to metastasis, this property of MSCs holds great potential for tackling these difficult malignancies.364
Looking first at the use of MSCs to deliver chemotherapeutics directly to the tumor, an extensive body of work has demonstrated that human and mouse MSCs have the ability to take up chemotherapeutics such as paclitaxel and gemcitabine.375, 376, 377, 378, 379 Interestingly, these highly toxic agents had little effect on the viability, migration, cell cycle, or differentiation potential of MSCs,380 enabling them to be used as “Trojan horses,”381 to selectively deliver chemotherapeutic agents to tumors in vivo, to then act, in effect, as tumor-resident pharmacologic pumps.382 While this approach was successful, more recent work has shown that the efficiency of uptake and the resultant therapeutic efficacy can be greatly enhanced if the chemotherapy drugs are first loaded into nanoparticles (NPs) which are then taken up by the MSCs, creating so-called “nanoengineered MSCs.”383 When MSCs were nanoengineered to carry paclitaxel and infused intravenously in an orthotopic human lung tumor model, they selectively homed to the tumor sites, where they were retained, thereby creating cellular drug depots that released the drug over an extended time period.380,383,384 This was in marked contrast to free paclitaxel-loaded NPs, which predominantly accumulated in the liver and spleen following intravenous injection. Importantly, the use of the nanoengineered MSCs led to more effective inhibition of tumor growth and superior survival than did either standard solution or NP-encapsulated forms of paclitaxel, despite significantly lower total doses of paclitaxel being used. The ability to greatly lower the dose administered also mitigated the common toxic side effects of paclitaxel such as leukopenia, greatly improving safety and tolerability. Collectively, these studies provided compelling evidence to support the clinical utility of MSCs as delivery vehicles for chemotherapeutic agents.
The first MSC-based gene therapy for cancer began roughly 17 years ago when human MSCs were engineered to express IFN-β in an effort to activate the antigen-presenting properties of MSCs and thereby induce an immune response to the tumor. This approach was shown to enable successful targeted delivery of this potent immune-stimulating agent to orthotopic tumors in metastatic breast and melanoma cancer models.359,364 IFN-β-transduced MSCs significantly inhibited tumor growth in severe combined immunodeficiency (SCID) mouse xenograft models of human melanoma and established MDA-231 or A375SM pulmonary metastases, and the survival of animals was prolonged,359 while the intravenous infusion of recombinant IFN-β produced minimal benefits in this same model.
Similar highly promising results were obtained385 with human MSCs engineered to express and secrete IFN-γ, one of the most important molecules in suppressing cancer development and progression.386 Despite the positive effects of IFN-γ on cancer cells, systemic administration is associated with significant side effects, including nausea, depression, fever, and leukopenia.387 As with the studies using IFN-β-transduced MSCs,359,364 the engineered MSCs delivered IFN-γ locally into the tumor, thereby eliminating systemic toxicities and activating the innate immune system, which decreased tumor growth and increased overall survival in a challenging model of neuroblastoma, characterized by liver and lung metastases.
TNF-related apoptosis-inducing ligand (TRAIL/CD253) is another cytokine whose gene has been inserted into MSCs to treat and eliminate tumors.363,373,374,388 TRAIL can have potent anti-cancer effects, because it induces apoptosis in cells that express the death receptors TRAIL-R1 and TRAIL-R2, but not the decoy receptors TRAIL-R3 or TRAIL-R4. Since many tumor cells express the TRAIL death receptors in the absence of the decoy receptors, they are highly vulnerable to TRAIL-induced apoptosis.389 Quite fortuitously, MSCs express very low levels of the TRAIL death receptors and normal levels of the decoy receptors. As such, TRAIL-transduced MSCs are resistant to TRAIL-induced apoptosis and can thus continuously deliver TRAIL to tumor cells in vivo. Indeed, human BM-MSCs that were virally transduced to overexpress TRAIL exhibited potent antitumor effects when tested in murine orthotopic tumor models.366
Gene-directed enzyme prodrug therapy (GDEPT), or suicide gene therapy, is another approach to cancer treatment in which MSCs have featured prominently for several years. GDEPT is a two-step process. In the first step, one transfers a gene encoding a prodrug-activating enzyme to the tumor, ideally in a selective fashion. In the second step, an inactive prodrug is systemically administered, but is only activated into cytotoxic metabolites locally within the tumor cells expressing this enzyme.390, 391, 392 To maximize the benefit of this approach, it is essential that the cytotoxic metabolites are able to diffuse through the cell membrane, since expression of the transgene does not occur in all tumor cells. This so-called “bystander” effect results in the death of not only the tumor cells in which the metabolites are formed but also the neighboring tumor cells that do not express the transgene.393 In addition to this direct effect of the toxic metabolites, the dying tumor cells can induce a host immune response mediated by NK cells, T cells, and macrophages, accompanied by increased levels of various cytokines, further enhancing the therapeutic effects of GDEPT.394, 395, 396, 397
Two of the most common prodrug-activating enzyme and prodrug combinations employed thus far include:
-
(1)
The thymidine kinase gene from herpes simplex virus (HSV-TK) combined with ganciclovir (GCV).398,399 GCV is a nontoxic purine analog that HSV-TK phosphorylates to a monophosphate form.400 Host cell kinases then complete the conversion to the active triphosphate form, which inhibits DNA synthesis, leading to induction of apoptosis.
-
(2)
The cytosine deaminase (CD) gene from E. coli combined with 5-fluorocytosine (5-FC).401, 402, 403, 404, 405, 406 CD catalyzes the hydrolytic deamination of the non-toxic 5-FC molecule into 5-fluorouracil (5-FU), which is then transformed within cells into other cytotoxic metabolites that are incorporated into DNA and RNA, leading to cell cycle arrest and apoptosis.407
Both of these combinations have been tested successfully in vitro and preclinically in animals bearing a variety of human tumors, and these studies have shown that the active triphosphate form of GCV and 5-FU both diffuse freely across cell membranes to exert a strong bystander effect.408, 409, 410, 411, 412, 413, 414, 415, 416, 417, 418, 419 Unfortunately, however, the therapeutic success of this approach has been fairly limited, largely due to lack of specificity and low efficiency of direct gene delivery to the tumor cells in vivo.420,421
To overcome these issues, investigators have turned to MSCs to achieve the promise of GDEPT.422,423 MSCs can be transduced at high efficiency in vitro with viral vectors encoding the prodrug-activating enzyme. Upon intravenous infusion, the engineered MSCs home to the target tumor, the inactive prodrug is administered systemically, and the tumor-resident MSCs activate the prodrug to its cytotoxic metabolites, which are then pumped out into the local microenvironment killing neighboring tumor cells.390,391,424,425 A number of in vitro and in vivo studies have demonstrated the efficacy and potency of this MSC-based approach to cancer immunotherapy against a wide variety of human tumors.382,423,426, 427, 428, 429, 430, 431, 432, 433, 434
Perhaps the most recent and innovative approach to using MSCs as cancer immunotherapeutics has arisen in the field of bispecific antibodies (bsAbs).435 A number of studies have demonstrated that primary human T cells engaged with bsAbs can drive a profound anti-tumor reaction, both in vitro and in vivo.436,437 However, to sustain clinically relevant plasma levels, continuous delivery of bsAbs is necessary, due to their short half-lives in vivo and the rapidity with which they are cleared from the circulation.438,439 Using MSCs as cellular bsAb production factories would enable the continuous production and secretion of bsAbs continuously in the patient’s body.322,440 Studies exploring this tactic have demonstrated that gene-modified MSCs are able to express a CD33-CD3 specific bsAb at high levels and mediate efficient lysis of acute myelogenous leukemia (AML) blasts by human primary T cells of both healthy donors and AML patients. While still relatively early in development, these initial studies highlight the vast potential of combining bsAb with MSCs to achieve potent anti-tumor effects.
Concluding Remarks
Since their initial identification as cells contributing to the hematopoietic niche within the BM, MSCs have received an ever-increasing amount of attention, mainly for reasons completely independent of their hematopoiesis-supporting properties. There are currently more than 800 human trials listed on ClinicalTrials.gov that employ MSCs for regenerative medicine and as modulators of the immune system.77,441 By virtue of the fact that their surrounding milieu can “license” MSCs, it is possible to tailor these cells to either inhibit or to stimulate an immune response, making them a unique and valuable tool in the immunotherapy arsenal. This remarkable immunological plasticity enables MSCs to be used to dampen aberrant immune responses in autoimmune disease, help to prevent rejection following solid organ or hematopoietic cell transplantation, deliver highly immunogenic therapeutic transgene products such as FVIII for treating genetic diseases, and to selectively target tumor cells for immune elimination. It is truly an exciting time in the MSC field, with each month seeing new and highly promising therapeutic uses for these versatile cells. We envision that the coming years will see the immunomodulatory properties of MSCs forming the basis for mainline therapy for a wide range of inherited and acquired disorders, enabling the successful treatment, and perhaps cure, of many diseases and forms of cancer for which current therapeutic strategies are ineffective.
Acknowledgments
G.A.P. and C.D.P. are supported by the following NIH grants from the NHLBI: HL130856, HL135853, HL148681, and by grant NNJ16ZSA001N-TRIRT from the Translational Research Institute for Space Health through Cooperative Agreement NNX16AO69A with NASA. The Graphical Abstract was created using BioRender software.
References
- 1.(1968). Julius Cohnheim (1839–1884) experimental pathologist. JAMA 206, 1561–1562. [PubMed]
- 2.Cohnheim J. Über Entzündung und Eiterung [Inflammation and suppuration] Path. Anat. Physiol. Klin. Med. 1867;40:1–79. [Google Scholar]
- 3.Mao F., Tu Q., Wang L., Chu F., Li X., Li H.S., Xu W. Mesenchymal stem cells and their therapeutic applications in inflammatory bowel disease. Oncotarget. 2017;8:38008–38021. doi: 10.18632/oncotarget.16682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Najar M., Krayem M., Meuleman N., Bron D., Lagneaux L. Mesenchymal stromal cells and Toll-like receptor priming: a critical review. Immune Netw. 2017;17:89–102. doi: 10.4110/in.2017.17.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tavassoli M., Crosby W.H. Transplantation of marrow to extramedullary sites. Science. 1968;161:54–56. doi: 10.1126/science.161.3836.54. [DOI] [PubMed] [Google Scholar]
- 6.Herzog E.L., Bucala R. Fibrocytes in health and disease. Exp. Hematol. 2010;38:548–556. doi: 10.1016/j.exphem.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedenstein A.J., Chailakhyan R.K., Latsinik N.V., Panasyuk A.F., Keiliss-Borok I.V. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Simmons P.J., Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- 9.Caplan A.I. Mesenchymal stem cells. J. Orthop. Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 10.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 11.Najar M., Raicevic G., Crompot E., Fayyad-Kazan H., Bron D., Toungouz M., Lagneaux L. The immunomodulatory potential of mesenchymal stromal cells: a story of a regulatory network. J. Immunother. 2016;39:45–59. doi: 10.1097/CJI.0000000000000108. [DOI] [PubMed] [Google Scholar]
- 12.Anthony B.A., Link D.C. Regulation of hematopoietic stem cells by bone marrow stromal cells. Trends Immunol. 2014;35:32–37. doi: 10.1016/j.it.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fajardo-Orduña G.R., Mayani H., Montesinos J.J. Hematopoietic support capacity of mesenchymal stem cells: biology and clinical potential. Arch. Med. Res. 2015;46:589–596. doi: 10.1016/j.arcmed.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Frenette P.S., Pinho S., Lucas D., Scheiermann C. Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu. Rev. Immunol. 2013;31:285–316. doi: 10.1146/annurev-immunol-032712-095919. [DOI] [PubMed] [Google Scholar]
- 15.Diaz de la Guardia R., Lopez-Millan B., Lavoie J.R., Bueno C., Castaño J., Gómez-Casares M., Vives S., Palomo L., Juan M., Delgado J. Detailed characterization of mesenchymal stem/stromal cells from a large cohort of AML patients demonstrates a definitive link to treatment outcomes. Stem Cell Reports. 2017;8:1573–1586. doi: 10.1016/j.stemcr.2017.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Konopleva M., Konoplev S., Hu W., Zaritskey A.Y., Afanasiev B.V., Andreeff M. Stromal cells prevent apoptosis of AML cells by up-regulation of anti-apoptotic proteins. Leukemia. 2002;16:1713–1724. doi: 10.1038/sj.leu.2402608. [DOI] [PubMed] [Google Scholar]
- 17.Galotto M., Berisso G., Delfino L., Podesta M., Ottaggio L., Dallorso S., Dufour C., Ferrara G.B., Abbondandolo A., Dini G. Stromal damage as consequence of high-dose chemo/radiotherapy in bone marrow transplant recipients. Exp. Hematol. 1999;27:1460–1466. doi: 10.1016/s0301-472x(99)00076-4. [DOI] [PubMed] [Google Scholar]
- 18.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop Dj., Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 19.García-Castro J., Trigueros C., Madrenas J., Pérez-Simón J.A., Rodriguez R., Menendez P. Mesenchymal stem cells and their use as cell replacement therapy and disease modelling tool. J. Cell. Mol. Med. 2008;12(6B):2552–2565. doi: 10.1111/j.1582-4934.2008.00516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horwitz E.M., Le Blanc K., Dominici M., Mueller I., Slaper-Cortenbach I., Marini F.C., Deans R.J., Krause D.S., Keating A., International Society for Cellular Therapy Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–395. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 21.Crisan M., Yap S., Casteilla L., Chen C.W., Corselli M., Park T.S., Andriolo G., Sun B., Zheng B., Zhang L. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Bianco P. “Mesenchymal” stem cells. Annu. Rev. Cell Dev. Biol. 2014;30:677–704. doi: 10.1146/annurev-cellbio-100913-013132. [DOI] [PubMed] [Google Scholar]
- 23.Bianco P., Robey P.G. Stem cells in tissue engineering. Nature. 2001;414:118–121. doi: 10.1038/35102181. [DOI] [PubMed] [Google Scholar]
- 24.Sacchetti B., Funari A., Remoli C., Giannicola G., Kogler G., Liedtke S., Cossu G., Serafini M., Sampaolesi M., Tagliafico E. No identical “mesenchymal stem cells” at different times and sites: human committed progenitors of distinct origin and differentiation potential are incorporated as adventitial cells in microvessels. Stem Cell Reports. 2016;6:897–913. doi: 10.1016/j.stemcr.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esteves C.L., Donadeu F.X. Pericytes and their potential in regenerative medicine across species. Cytometry A. 2018;93:50–59. doi: 10.1002/cyto.a.23243. [DOI] [PubMed] [Google Scholar]
- 26.Almeida-Porada G., El Shabrawy D., Porada C., Zanjani E.D. Differentiative potential of human metanephric mesenchymal cells. Exp. Hematol. 2002;30:1454–1462. doi: 10.1016/s0301-472x(02)00967-0. [DOI] [PubMed] [Google Scholar]
- 27.Delo D.M., De Coppi P., Bartsch G., Jr., Atala A. Amniotic fluid and placental stem cells. Methods Enzymol. 2006;419:426–438. doi: 10.1016/S0076-6879(06)19017-5. [DOI] [PubMed] [Google Scholar]
- 28.in ’t Anker P.S., Noort W.A., Scherjon S.A., Kleijburg-van der Keur C., Kruisselbrink A.B., van Bezooijen R.L., Beekhuizen W., Willemze R., Kanhai H.H., Fibbe W.E. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica. 2003;88:845–852. [PubMed] [Google Scholar]
- 29.Via A.G., Frizziero A., Oliva F. Biological properties of mesenchymal stem cells from different sources. Muscles Ligaments Tendons J. 2012;2:154–162. [PMC free article] [PubMed] [Google Scholar]
- 30.Zuk P.A., Zhu M., Ashjian P., De Ugarte D.A., Huang J.I., Mizuno H., Alfonso Z.C., Fraser J.K., Benhaim P., Hedrick M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Götherström C., West A., Liden J., Uzunel M., Lahesmaa R., Le Blanc K. Difference in gene expression between human fetal liver and adult bone marrow mesenchymal stem cells. Haematologica. 2005;90:1017–1026. [PubMed] [Google Scholar]
- 32.Garol N.J., Yamagami T., Osborne C., Porada C.D., Zanjani E.D., Almeida-Porada G. Tissue-specific molecular signature may explain differentiative bias of human MSC from different tissues. Blood. 2007;110:1918. [Google Scholar]
- 33.Kern S., Eichler H., Stoeve J., Klüter H., Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 34.Lee R.H., Kim B., Choi I., Kim H., Choi H.S., Suh K., Bae Y.C., Jung J.S. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell. Physiol. Biochem. 2004;14:311–324. doi: 10.1159/000080341. [DOI] [PubMed] [Google Scholar]
- 35.Mazhari S., Desai J., Chamberlain J., Porada C., Zanjani E.D., Almeida-Porada G. Proteomic analysis reveals intrinsic differences between phenotypically identical mesenchymal stem cells. Blood. 2005;106:395. [Google Scholar]
- 36.Mazhari S.M., Porada C.D., Chamberlain J., Zanjani E.D., Almeida-Porada G. Characterization of membrane proteins of mesenchymal stem cells from human liver. Exp. Hematol. 2006;34:80. [Google Scholar]
- 37.Reinisch A., Etchart N., Thomas D., Hofmann N.A., Fruehwirth M., Sinha S., Chan C.K., Senarath-Yapa K., Seo E.Y., Wearda T. Epigenetic and in vivo comparison of diverse MSC sources reveals an endochondral signature for human hematopoietic niche formation. Blood. 2015;125:249–260. doi: 10.1182/blood-2014-04-572255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clark E.A., Kalomoiris S., Nolta J.A., Fierro F.A. Concise review: microRNA function in multipotent mesenchymal stromal cells. Stem Cells. 2014;32:1074–1082. doi: 10.1002/stem.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El Omar R., Beroud J., Stoltz J.F., Menu P., Velot E., Decot V. Umbilical cord mesenchymal stem cells: the new gold standard for mesenchymal stem cell-based therapies? Tissue Eng. Part B Rev. 2014;20:523–544. doi: 10.1089/ten.TEB.2013.0664. [DOI] [PubMed] [Google Scholar]
- 40.Almeida-Porada M.G., Chamberlain J., Frias A., Porada C.D., Zanjani E.D. Tissue of origin influences in vivo differentiative potential of mesenchymal stem cells. Blood. 2003;102:1304. [Google Scholar]
- 41.Chamberlain J., Frias A., Porada C., Zanjani E.D., Almeida-Porada G. Neural generation in vivo differs with route of administration and source of mesenchymal stem cells. Exp. Hematol. 2005;33:47a. [Google Scholar]
- 42.Almeida-Porada M.G., Porada C., ElShabrawy D., Simmons P.J., Zanjani E.D. Human marrow stromal cells (MSC) represent a latent pool of stem cells capable of generating long-term hematopoietic cells. Blood. 2001;98:713. [Google Scholar]
- 43.Porada C.D., Almeida-Porada G. Mesenchymal stem cells as therapeutics and vehicles for gene and drug delivery. Adv. Drug Deliv. Rev. 2010;62:1156–1166. doi: 10.1016/j.addr.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Q.Q., Yan L., Wang C.Z., Wang W.H., Shi H., Su B.B., Zeng Q.H., Du H.T., Wan J. Mesenchymal stem cells alleviate TNBS-induced colitis by modulating inflammatory and autoimmune responses. World J. Gastroenterol. 2013;19:4702–4717. doi: 10.3748/wjg.v19.i29.4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dai W., Hale S.L., Martin B.J., Kuang J.Q., Dow J.S., Wold L.E., Kloner R.A. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: short- and long-term effects. Circulation. 2005;112:214–223. doi: 10.1161/CIRCULATIONAHA.104.527937. [DOI] [PubMed] [Google Scholar]
- 46.Hofstetter C.P., Schwarz E.J., Hess D., Widenfalk J., El Manira A., Prockop D.J., Olson L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc. Natl. Acad. Sci. USA. 2002;99:2199–2204. doi: 10.1073/pnas.042678299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Horwitz E.M., Gordon P.L., Koo W.K., Marx J.C., Neel M.D., McNall R.Y., Muul L., Hofmann T. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc. Natl. Acad. Sci. USA. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iso Y., Spees J.L., Serrano C., Bakondi B., Pochampally R., Song Y.H., Sobel B.E., Delafontaine P., Prockop D.J. Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochem. Biophys. Res. Commun. 2007;354:700–706. doi: 10.1016/j.bbrc.2007.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee R.H., Pulin A.A., Seo M.J., Kota D.J., Ylostalo J., Larson B.L., Semprun-Prieto L., Delafontaine P., Prockop D.J. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee R.H., Seo M.J., Pulin A.A., Gregory C.A., Ylostalo J., Prockop D.J. The CD34-like protein PODXL and α6-integrin (CD49f) identify early progenitor MSCs with increased clonogenicity and migration to infarcted heart in mice. Blood. 2009;113:816–826. doi: 10.1182/blood-2007-12-128702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mahmood A., Lu D., Lu M., Chopp M. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery. 2003;53:697. doi: 10.1227/01.neu.0000079333.61863.aa. 702, discussion 702–703. [DOI] [PubMed] [Google Scholar]
- 52.Pittenger M., Vanguri P., Simonetti D., Young R. Adult mesenchymal stem cells: potential for muscle and tendon regeneration and use in gene therapy. J. Musculoskelet. Neuronal Interact. 2002;2:309–320. [PubMed] [Google Scholar]
- 53.Rasulov M.F., Vasilchenkov A.V., Onishchenko N.A., Krasheninnikov M.E., Kravchenko V.I., Gorshenin T.L., Pidtsan R.E., Potapov I.V. First experience of the use bone marrow mesenchymal stem cells for the treatment of a patient with deep skin burns. Bull. Exp. Biol. Med. 2005;139:141–144. doi: 10.1007/s10517-005-0232-3. [DOI] [PubMed] [Google Scholar]
- 54.Sakaida I., Terai S., Yamamoto N., Aoyama K., Ishikawa T., Nishina H., Okita K. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology. 2004;40:1304–1311. doi: 10.1002/hep.20452. [DOI] [PubMed] [Google Scholar]
- 55.Chamberlain J., Yamagami T., Colletti E., Theise N.D., Desai J., Frias A., Pixley J., Zanjani E.D., Porada C.D., Almeida-Porada G. Efficient generation of human hepatocytes by the intrahepatic delivery of clonal human mesenchymal stem cells in fetal sheep. Hepatology. 2007;46:1935–1945. doi: 10.1002/hep.21899. [DOI] [PubMed] [Google Scholar]
- 56.Phinney D.G., Prockop D.J. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 57.Wu Y., Chen L., Scott P.G., Tredget E.E. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 58.Wu Y., Zhao R.C., Tredget E.E. Concise review: bone marrow-derived stem/progenitor cells in cutaneous repair and regeneration. Stem Cells. 2010;28:905–915. doi: 10.1002/stem.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kalluri R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer. 2016;16:582–598. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 60.Poggi A., Giuliani M. Mesenchymal stromal cells can regulate the immune response in the tumor microenvironment. Vaccines (Basel) 2016;4:E41. doi: 10.3390/vaccines4040041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Poggi A., Musso A., Dapino I., Zocchi M.R. Mechanisms of tumor escape from immune system: role of mesenchymal stromal cells. Immunol. Lett. 2014;159:55–72. doi: 10.1016/j.imlet.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 62.Turley S.J., Cremasco V., Astarita J.L. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat. Rev. Immunol. 2015;15:669–682. doi: 10.1038/nri3902. [DOI] [PubMed] [Google Scholar]
- 63.Caplan A.I. Why are MSCs therapeutic? New data: new insight. J. Pathol. 2009;217:318–324. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kachgal S., Putnam A.J. Mesenchymal stem cells from adipose and bone marrow promote angiogenesis via distinct cytokine and protease expression mechanisms. Angiogenesis. 2011;14:47–59. doi: 10.1007/s10456-010-9194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prockop D.J. “Stemness” does not explain the repair of many tissues by mesenchymal stem/multipotent stromal cells (MSCs) Clin. Pharmacol. Ther. 2007;82:241–243. doi: 10.1038/sj.clpt.6100313. [DOI] [PubMed] [Google Scholar]
- 66.Ferraro F., Lymperi S., Méndez-Ferrer S., Saez B., Spencer J.A., Yeap B.Y., Masselli E., Graiani G., Prezioso L., Rizzini E.L. Diabetes impairs hematopoietic stem cell mobilization by altering niche function. Sci. Transl. Med. 2011;3:104ra101. doi: 10.1126/scitranslmed.3002191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bianco P., Cao X., Frenette P.S., Mao J.J., Robey P.G., Simmons P.J., Wang C.Y. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat. Med. 2013;19:35–42. doi: 10.1038/nm.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Colletti E.J., Airey J.A., Liu W., Simmons P.J., Zanjani E.D., Porada C.D., Almeida-Porada G. Generation of tissue-specific cells from MSC does not require fusion or donor-to-host mitochondrial/membrane transfer. Stem Cell Res. (Amst.) 2009;2:125–138. doi: 10.1016/j.scr.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Campagnoli C., Roberts I.A., Kumar S., Bennett P.R., Bellantuono I., Fisk N.M. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98:2396–2402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 70.O’Donoghue K., Chan J., de la Fuente J., Kennea N., Sandison A., Anderson J.R., Roberts I.A., Fisk N.M. Microchimerism in female bone marrow and bone decades after fetal mesenchymal stem-cell trafficking in pregnancy. Lancet. 2004;364:179–182. doi: 10.1016/S0140-6736(04)16631-2. [DOI] [PubMed] [Google Scholar]
- 71.Kassem M. Mesenchymal stem cells: biological characteristics and potential clinical applications. Cloning Stem Cells. 2004;6:369–374. doi: 10.1089/clo.2004.6.369. [DOI] [PubMed] [Google Scholar]
- 72.Luria E.A., Panasyuk A.F., Friedenstein A.Y. Fibroblast colony formation from monolayer cultures of blood cells. Transfusion. 1971;11:345–349. doi: 10.1111/j.1537-2995.1971.tb04426.x. [DOI] [PubMed] [Google Scholar]
- 73.Wilson A., Hodgson-Garms M., Frith J.E., Genever P. Multiplicity of mesenchymal stromal cells: finding the right route to therapy. Front. Immunol. 2019;10:1112. doi: 10.3389/fimmu.2019.01112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mo M., Wang S., Zhou Y., Li H., Wu Y. Mesenchymal stem cell subpopulations: phenotype, property and therapeutic potential. Cell. Mol. Life Sci. 2016;73:3311–3321. doi: 10.1007/s00018-016-2229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Andrzejewska A., Catar R., Schoon J., Qazi T.H., Sass F.A., Jacobi D., Blankenstein A., Reinke S., Krüger D., Streitz M. Multi-parameter analysis of biobanked human bone marrow stromal cells shows little influence for donor age and mild comorbidities on phenotypic and functional properties. Front. Immunol. 2019;10:2474. doi: 10.3389/fimmu.2019.02474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boland L.K., Burand A.J., Boyt D.T., Dobroski H., Di L., Liszewski J.N., Schrodt M.V., Frazer M.K., Santillan D.A., Ankrum J.A. Nature vs. nurture: defining the effects of mesenchymal stromal cell isolation and culture conditions on resiliency to palmitate challenge. Front. Immunol. 2019;10:1080. doi: 10.3389/fimmu.2019.01080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moll G., Ankrum J.A., Kamhieh-Milz J., Bieback K., Ringdén O., Volk H.D., Geissler S., Reinke P. Intravascular mesenchymal stromal/stem cell therapy product diversification: time for new clinical guidelines. Trends Mol. Med. 2019;25:149–163. doi: 10.1016/j.molmed.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 78.Moll G., Geißler S., Catar R., Ignatowicz L., Hoogduijn M.J., Strunk D., Bieback K., Ringdén O. Cryopreserved or fresh mesenchymal stromal cells: only a matter of taste or key to unleash the full clinical potential of MSC therapy? Adv. Exp. Med. Biol. 2016;951:77–98. doi: 10.1007/978-3-319-45457-3_7. [DOI] [PubMed] [Google Scholar]
- 79.Dick J.E., Guenechea G., Gan O.I., Dorrell C. In vivo dynamics of human stem cell repopulation in NOD/SCID mice. Ann. N Y Acad. Sci. 2001;938:184–190. doi: 10.1111/j.1749-6632.2001.tb03588.x. [DOI] [PubMed] [Google Scholar]
- 80.McDermott S.P., Eppert K., Lechman E.R., Doedens M., Dick J.E. Comparison of human cord blood engraftment between immunocompromised mouse strains. Blood. 2010;116:193–200. doi: 10.1182/blood-2010-02-271841. [DOI] [PubMed] [Google Scholar]
- 81.McKenzie J.L., Gan O.I., Doedens M., Dick J.E. Reversible cell surface expression of CD38 on CD34-positive human hematopoietic repopulating cells. Exp. Hematol. 2007;35:1429–1436. doi: 10.1016/j.exphem.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 82.Spangrude G.J., Heimfeld S., Weissman I.L. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 83.Poggi A., Varesano S., Zocchi M.R. How to hit mesenchymal stromal cells and make the tumor microenvironment immunostimulant rather than immunosuppressive. Front. Immunol. 2018;9:262. doi: 10.3389/fimmu.2018.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10:709–716. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 85.Conget P.A., Minguell J.J. Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J. Cell. Physiol. 1999;181:67–73. doi: 10.1002/(SICI)1097-4652(199910)181:1<67::AID-JCP7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 86.Le Blanc K., Tammik C., Rosendahl K., Zetterberg E., Ringdén O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp. Hematol. 2003;31:890–896. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 87.Lv F.J., Tuan R.S., Cheung K.M., Leung V.Y. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32:1408–1419. doi: 10.1002/stem.1681. [DOI] [PubMed] [Google Scholar]
- 88.Rostovskaya M., Anastassiadis K. Differential expression of surface markers in mouse bone marrow mesenchymal stromal cell subpopulations with distinct lineage commitment. PLoS ONE. 2012;7:e51221. doi: 10.1371/journal.pone.0051221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Krampera M., Galipeau J., Shi Y., Tarte K., Sensebe L., MSC Committee of the International Society for Cellular Therapy (ISCT) Immunological characterization of multipotent mesenchymal stromal cells—the International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy. 2013;15:1054–1061. doi: 10.1016/j.jcyt.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 90.Casiraghi F., Perico N., Remuzzi G. Mesenchymal stromal cells for tolerance induction in organ transplantation. Hum. Immunol. 2018;79:304–313. doi: 10.1016/j.humimm.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 91.Caplan H., Olson S.D., Kumar A., George M., Prabhakara K.S., Wenzel P., Bedi S., Toledano-Furman N.E., Triolo F., Kamhieh-Milz J. Mesenchymal stromal cell therapeutic delivery: translational challenges to clinical application. Front. Immunol. 2019;10:1645. doi: 10.3389/fimmu.2019.01645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Phinney D.G. Functional heterogeneity of mesenchymal stem cells: implications for cell therapy. J. Cell. Biochem. 2012;113:2806–2812. doi: 10.1002/jcb.24166. [DOI] [PubMed] [Google Scholar]
- 93.Tormin A., Brune J.C., Olsson E., Valcich J., Neuman U., Olofsson T., Jacobsen S.E., Scheding S. Characterization of bone marrow-derived mesenchymal stromal cells (MSC) based on gene expression profiling of functionally defined MSC subsets. Cytotherapy. 2009;11:114–128. doi: 10.1080/14653240802716590. [DOI] [PubMed] [Google Scholar]
- 94.Iinuma S., Aikawa E., Tamai K., Fujita R., Kikuchi Y., Chino T., Kikuta J., McGrath J.A., Uitto J., Ishii M. Transplanted bone marrow-derived circulating PDGFRα+ cells restore type VII collagen in recessive dystrophic epidermolysis bullosa mouse skin graft. J. Immunol. 2015;194:1996–2003. doi: 10.4049/jimmunol.1400914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mabuchi Y., Morikawa S., Harada S., Niibe K., Suzuki S., Renault-Mihara F., Houlihan D.D., Akazawa C., Okano H., Matsuzaki Y. LNGFR+THY-1+VCAM-1hi+ cells reveal functionally distinct subpopulations in mesenchymal stem cells. Stem Cell Reports. 2013;1:152–165. doi: 10.1016/j.stemcr.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Seeger F.H., Rasper T., Koyanagi M., Fox H., Zeiher A.M., Dimmeler S. CXCR4 expression determines functional activity of bone marrow-derived mononuclear cells for therapeutic neovascularization in acute ischemia. Arterioscler. Thromb. Vasc. Biol. 2009;29:1802–1809. doi: 10.1161/ATVBAHA.109.194688. [DOI] [PubMed] [Google Scholar]
- 97.Cheng Z., Ou L., Zhou X., Li F., Jia X., Zhang Y., Liu X., Li Y., Ward C.A., Melo L.G., Kong D. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol. Ther. 2008;16:571–579. doi: 10.1038/sj.mt.6300374. [DOI] [PubMed] [Google Scholar]
- 98.Wang Z., Wang Y., Wang Z., Gutkind J.S., Wang Z., Wang F., Lu J., Niu G., Teng G., Chen X. Engineered mesenchymal stem cells with enhanced tropism and paracrine secretion of cytokines and growth factors to treat traumatic brain injury. Stem Cells. 2015;33:456–467. doi: 10.1002/stem.1878. [DOI] [PubMed] [Google Scholar]
- 99.Yu Q., Liu L., Lin J., Wang Y., Xuan X., Guo Y., Hu S. SDF-1α/CXCR4 axis mediates the migration of mesenchymal stem cells to the hypoxic-ischemic brain lesion in a rat model. Cell J. 2015;16:440–447. doi: 10.22074/cellj.2015.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu H., Liu S., Li Y., Wang X., Xue W., Ge G., Luo X. The role of SDF-1-CXCR4/CXCR7 axis in the therapeutic effects of hypoxia-preconditioned mesenchymal stem cells for renal ischemia/reperfusion injury. PLoS ONE. 2012;7:e34608. doi: 10.1371/journal.pone.0034608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Du Z., Wei C., Yan J., Han B., Zhang M., Peng C., Liu Y. Mesenchymal stem cells overexpressing C-X-C chemokine receptor type 4 improve early liver regeneration of small-for-size liver grafts. Liver Transpl. 2013;19:215–225. doi: 10.1002/lt.23577. [DOI] [PubMed] [Google Scholar]
- 102.Samsonraj R.M., Rai B., Sathiyanathan P., Puan K.J., Rötzschke O., Hui J.H., Raghunath M., Stanton L.W., Nurcombe V., Cool S.M. Establishing criteria for human mesenchymal stem cell potency. Stem Cells. 2015;33:1878–1891. doi: 10.1002/stem.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bensidhoum M., Chapel A., Francois S., Demarquay C., Mazurier C., Fouillard L., Bouchet S., Bertho J.M., Gourmelon P., Aigueperse J. Homing of in vitro expanded Stro-1− or Stro-1+ human mesenchymal stem cells into the NOD/SCID mouse and their role in supporting human CD34 cell engraftment. Blood. 2004;103:3313–3319. doi: 10.1182/blood-2003-04-1121. [DOI] [PubMed] [Google Scholar]
- 104.Martens T.P., See F., Schuster M.D., Sondermeijer H.P., Hefti M.M., Zannettino A., Gronthos S., Seki T., Itescu S. Mesenchymal lineage precursor cells induce vascular network formation in ischemic myocardium. Nat. Clin. Pract. Cardiovasc. Med. 2006;3(Suppl 1):S18–S22. doi: 10.1038/ncpcardio0404. [DOI] [PubMed] [Google Scholar]
- 105.Psaltis P.J., Paton S., See F., Arthur A., Martin S., Itescu S., Worthley S.G., Gronthos S., Zannettino A.C. Enrichment for STRO-1 expression enhances the cardiovascular paracrine activity of human bone marrow-derived mesenchymal cell populations. J. Cell. Physiol. 2010;223:530–540. doi: 10.1002/jcp.22081. [DOI] [PubMed] [Google Scholar]
- 106.Busser H., Najar M., Raicevic G., Pieters K., Velez Pombo R., Philippart P., Meuleman N., Bron D., Lagneaux L. Isolation and characterization of human mesenchymal stromal cell subpopulations: comparison of bone marrow and adipose tissue. Stem Cells Dev. 2015;24:2142–2157. doi: 10.1089/scd.2015.0172. [DOI] [PubMed] [Google Scholar]
- 107.Cuthbert R.J., Giannoudis P.V., Wang X.N., Nicholson L., Pawson D., Lubenko A., Tan H.B., Dickinson A., McGonagle D., Jones E. Examining the feasibility of clinical grade CD271+ enrichment of mesenchymal stromal cells for bone regeneration. PLoS ONE. 2015;10:e0117855. doi: 10.1371/journal.pone.0117855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Quirici N., Soligo D., Bossolasco P., Servida F., Lumini C., Deliliers G.L. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp. Hematol. 2002;30:783–791. doi: 10.1016/s0301-472x(02)00812-3. [DOI] [PubMed] [Google Scholar]
- 109.Kuçi S., Kuçi Z., Kreyenberg H., Deak E., Pütsch K., Huenecke S., Amara C., Koller S., Rettinger E., Grez M. CD271 antigen defines a subset of multipotent stromal cells with immunosuppressive and lymphohematopoietic engraftment-promoting properties. Haematologica. 2010;95:651–659. doi: 10.3324/haematol.2009.015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Arufe M.C., De la Fuente A., Fuentes I., de Toro F.J., Blanco F.J. Chondrogenic potential of subpopulations of cells expressing mesenchymal stem cell markers derived from human synovial membranes. J. Cell. Biochem. 2010;111:834–845. doi: 10.1002/jcb.22768. [DOI] [PubMed] [Google Scholar]
- 111.Gaebel R., Furlani D., Sorg H., Polchow B., Frank J., Bieback K., Wang W., Klopsch C., Ong L.L., Li W. Cell origin of human mesenchymal stem cells determines a different healing performance in cardiac regeneration. PLoS ONE. 2011;6:e15652. doi: 10.1371/journal.pone.0015652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Conconi M.T., Burra P., Di Liddo R., Calore C., Turetta M., Bellini S., Bo P., Nussdorfer G.G., Parnigotto P.P. CD105+ cells from Wharton’s jelly show in vitro and in vivo myogenic differentiative potential. Int. J. Mol. Med. 2006;18:1089–1096. [PubMed] [Google Scholar]
- 113.Iohara K., Imabayashi K., Ishizaka R., Watanabe A., Nabekura J., Ito M., Matsushita K., Nakamura H., Nakashima M. Complete pulp regeneration after pulpectomy by transplantation of CD105+ stem cells with stromal cell-derived factor-1. Tissue Eng. Part A. 2011;17:1911–1920. doi: 10.1089/ten.TEA.2010.0615. [DOI] [PubMed] [Google Scholar]
- 114.Li N., Wang C., Jia L., Du J. Heart regeneration, stem cells, and cytokines. Regen. Med. Res. 2014;2:6. doi: 10.1186/2050-490X-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ranganath S.H., Levy O., Inamdar M.S., Karp J.M. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10:244–258. doi: 10.1016/j.stem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Roura S., Farré J., Soler-Botija C., Llach A., Hove-Madsen L., Cairó J.J., Gòdia F., Cinca J., Bayes-Genis A. Effect of aging on the pluripotential capacity of human CD105+ mesenchymal stem cells. Eur. J. Heart Fail. 2006;8:555–563. doi: 10.1016/j.ejheart.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 117.Schäffler A., Büchler C. Concise review: adipose tissue-derived stromal cells—basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25:818–827. doi: 10.1634/stemcells.2006-0589. [DOI] [PubMed] [Google Scholar]
- 118.Yang Z.X., Han Z.B., Ji Y.R., Wang Y.W., Liang L., Chi Y., Yang S.G., Li L.N., Luo W.F., Li J.P. CD106 identifies a subpopulation of mesenchymal stem cells with unique immunomodulatory properties. PLoS ONE. 2013;8:e59354. doi: 10.1371/journal.pone.0059354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gronthos S., Zannettino A.C., Hay S.J., Shi S., Graves S.E., Kortesidis A., Simmons P.J. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J. Cell Sci. 2003;116:1827–1835. doi: 10.1242/jcs.00369. [DOI] [PubMed] [Google Scholar]
- 120.Gomes J.P., Coatti G.C., Valadares M.C., Assoni A.F., Pelatti M.V., Secco M., Zatz M. Human adipose-derived CD146+ stem cells increase life span of a muscular dystrophy mouse model more efficiently than mesenchymal stromal cells. DNA Cell Biol. 2018;37:798–804. doi: 10.1089/dna.2018.4158. [DOI] [PubMed] [Google Scholar]
- 121.Hörl S., Ejaz A., Ernst S., Mattesich M., Kaiser A., Jenewein B., Zwierzina M.E., Hammerle S., Miggitsch C., Mitterberger-Vogt M.C. CD146 (MCAM) in human cs-DLK1−/cs-CD34+ adipose stromal/progenitor cells. Stem Cell Res. (Amst.) 2017;22:1–12. doi: 10.1016/j.scr.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 122.Wangler S., Menzel U., Li Z., Ma J., Hoppe S., Benneker L.M., Alini M., Grad S., Peroglio M. CD146/MCAM distinguishes stem cell subpopulations with distinct migration and regenerative potential in degenerative intervertebral discs. Osteoarthritis Cartilage. 2019;27:1094–1105. doi: 10.1016/j.joca.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 123.Andreeva E., Bobyleva P., Gornostaeva A., Buravkova L. Interaction of multipotent mesenchymal stromal and immune cells: bidirectional effects. Cytotherapy. 2017;19:1152–1166. doi: 10.1016/j.jcyt.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 124.Di Nicola M., Carlo-Stella C., Magni M., Milanesi M., Longoni P.D., Matteucci P., Grisanti S., Gianni A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 125.Krampera M., Glennie S., Dyson J., Scott D., Laylor R., Simpson E., Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 126.Plock J.A., Schnider J.T., Schweizer R., Zhang W., Tsuji W., Waldner M., Solari M.G., Marra K.G., Rubin J.P., Gorantla V.S. The influence of timing and frequency of adipose-derived mesenchymal stem cell therapy on immunomodulation outcomes after vascularized composite allotransplantation. Transplantation. 2017;101:e1–e11. doi: 10.1097/TP.0000000000001498. [DOI] [PubMed] [Google Scholar]
- 127.Parekkadan B., Milwid J.M. Mesenchymal stem cells as therapeutics. Annu. Rev. Biomed. Eng. 2010;12:87–117. doi: 10.1146/annurev-bioeng-070909-105309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yagi H., Soto-Gutierrez A., Parekkadan B., Kitagawa Y., Tompkins R.G., Kobayashi N., Yarmush M.L. Mesenchymal stem cells: mechanisms of immunomodulation and homing. Cell Transplant. 2010;19:667–679. doi: 10.3727/096368910X508762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Le Blanc K., Ringdén O. Immunobiology of human mesenchymal stem cells and future use in hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2005;11:321–334. doi: 10.1016/j.bbmt.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 130.Ankrum J.A., Ong J.F., Karp J.M. Mesenchymal stem cells: immune evasive, not immune privileged. Nat. Biotechnol. 2014;32:252–260. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Panés J., García-Olmo D., Van Assche G., Colombel J.F., Reinisch W., Baumgart D.C., Dignass A., Nachury M., Ferrante M., Kazemi-Shirazi L., ADMIRE CD Study Group Collaborators Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. Lancet. 2016;388:1281–1290. doi: 10.1016/S0140-6736(16)31203-X. [DOI] [PubMed] [Google Scholar]
- 132.Poncelet A.J., Nizet Y., Vercruysse J., Hiel A.L., Saliez A., Gianello P. Inhibition of humoral response to allogeneic porcine mesenchymal stem cell with 12 days of tacrolimus. Transplantation. 2008;86:1586–1595. doi: 10.1097/TP.0b013e31818bd96f. [DOI] [PubMed] [Google Scholar]
- 133.Wang D., Zhang H., Liang J., Li X., Feng X., Wang H., Hua B., Liu B., Lu L., Gilkeson G.S. Allogeneic mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus: 4 years of experience. Cell Transplant. 2013;22:2267–2277. doi: 10.3727/096368911X582769c. [DOI] [PubMed] [Google Scholar]
- 134.Wang D., Zhang H., Liang J., Wang H., Hua B., Feng X., Gilkeson G.S., Farge D., Shi S., Sun L. A long-term follow-up study of allogeneic mesenchymal stem/stromal cell transplantation in patients with drug-resistant systemic lupus erythematosus. Stem Cell Reports. 2018;10:933–941. doi: 10.1016/j.stemcr.2018.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xu J., Wang D., Liu D., Fan Z., Zhang H., Liu O., Ding G., Gao R., Zhang C., Ding Y. Allogeneic mesenchymal stem cell treatment alleviates experimental and clinical Sjögren syndrome. Blood. 2012;120:3142–3151. doi: 10.1182/blood-2011-11-391144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Berglund A.K., Fortier L.A., Antczak D.F., Schnabel L.V. Immunoprivileged no more: measuring the immunogenicity of allogeneic adult mesenchymal stem cells. Stem Cell Res. Ther. 2017;8:288. doi: 10.1186/s13287-017-0742-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Eliopoulos N., Stagg J., Lejeune L., Pommey S., Galipeau J. Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood. 2005;106:4057–4065. doi: 10.1182/blood-2005-03-1004. [DOI] [PubMed] [Google Scholar]
- 138.Zangi L., Margalit R., Reich-Zeliger S., Bachar-Lustig E., Beilhack A., Negrin R., Reisner Y. Direct imaging of immune rejection and memory induction by allogeneic mesenchymal stromal cells. Stem Cells. 2009;27:2865–2874. doi: 10.1002/stem.217. [DOI] [PubMed] [Google Scholar]
- 139.Badillo A.T., Beggs K.J., Javazon E.H., Tebbets J.C., Flake A.W. Murine bone marrow stromal progenitor cells elicit an in vivo cellular and humoral alloimmune response. Biol. Blood Marrow Transplant. 2007;13:412–422. doi: 10.1016/j.bbmt.2006.12.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Beggs K.J., Lyubimov A., Borneman J.N., Bartholomew A., Moseley A., Dodds R., Archambault M.P., Smith A.K., McIntosh K.R. Immunologic consequences of multiple, high-dose administration of allogeneic mesenchymal stem cells to baboons. Cell Transplant. 2006;15:711–721. doi: 10.3727/000000006783981503. [DOI] [PubMed] [Google Scholar]
- 141.Crop M.J., Korevaar S.S., de Kuiper R., IJzermans J.N., van Besouw N.M., Baan C.C., Weimar W., Hoogduijn M.J. Human mesenchymal stem cells are susceptible to lysis by CD8+ T cells and NK cells. Cell Transplant. 2011;20:1547–1559. doi: 10.3727/096368910X564076. [DOI] [PubMed] [Google Scholar]
- 142.English K., Mahon B.P. Allogeneic mesenchymal stem cells: agents of immune modulation. J. Cell. Biochem. 2011;112:1963–1968. doi: 10.1002/jcb.23119. [DOI] [PubMed] [Google Scholar]
- 143.Prigione I., Benvenuto F., Bocca P., Battistini L., Uccelli A., Pistoia V. Reciprocal interactions between human mesenchymal stem cells and γδ T cells or invariant natural killer T cells. Stem Cells. 2009;27:693–702. doi: 10.1634/stemcells.2008-0687. [DOI] [PubMed] [Google Scholar]
- 144.Soland M.A., Bego M.G., Colletti E., Porada C.D., Zanjani E.D., St Jeor S., Almeida-Porada G. Modulation of human mesenchymal stem cell immunogenicity through forced expression of human cytomegalovirus us proteins. PLoS ONE. 2012;7:e36163. doi: 10.1371/journal.pone.0036163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Galleu A., Riffo-Vasquez Y., Trento C., Lomas C., Dolcetti L., Cheung T.S., von Bonin M., Barbieri L., Halai K., Ward S. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci. Transl. Med. 2017;9:eaam7828. doi: 10.1126/scitranslmed.aam7828. [DOI] [PubMed] [Google Scholar]
- 146.Yu Y., Liao L., Shao B., Su X., Shuai Y., Wang H., Shang F., Zhou Z., Yang D., Jin Y. Knockdown of microRNA Let-7a improves the functionality of bone marrow-derived mesenchymal stem cells in immunotherapy. Mol. Ther. 2017;25:480–493. doi: 10.1016/j.ymthe.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Singer N.G., Caplan A.I. Mesenchymal stem cells: mechanisms of inflammation. Annu. Rev. Pathol. 2011;6:457–478. doi: 10.1146/annurev-pathol-011110-130230. [DOI] [PubMed] [Google Scholar]
- 148.Wolf D., Wolf A.M. Mesenchymal stem cells as cellular immunosuppressants. Lancet. 2008;371:1553–1554. doi: 10.1016/S0140-6736(08)60666-2. [DOI] [PubMed] [Google Scholar]
- 149.Bartholomew A., Sturgeon C., Siatskas M., Ferrer K., McIntosh K., Patil S., Hardy W., Devine S., Ucker D., Deans R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp. Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 150.Aggarwal S., Pittenger M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 151.Chiesa S., Morbelli S., Morando S., Massollo M., Marini C., Bertoni A., Frassoni F., Bartolomé S.T., Sambuceti G., Traggiai E., Uccelli A. Mesenchymal stem cells impair in vivo T-cell priming by dendritic cells. Proc. Natl. Acad. Sci. USA. 2011;108:17384–17389. doi: 10.1073/pnas.1103650108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Glennie S., Soeiro I., Dyson P.J., Lam E.W., Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821–2827. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- 153.Hu C.D., Kosaka Y., Marcus P., Rashedi I., Keating A. Differential immunomodulatory effects of human bone marrow-derived mesenchymal stromal cells on natural killer cells. Stem Cells Dev. 2019;28:933–943. doi: 10.1089/scd.2019.0059. [DOI] [PubMed] [Google Scholar]
- 154.Le Blanc K., Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat. Rev. Immunol. 2012;12:383–396. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- 155.Luz-Crawford P., Kurte M., Bravo-Alegría J., Contreras R., Nova-Lamperti E., Tejedor G., Noël D., Jorgensen C., Figueroa F., Djouad F., Carrión F. Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res. Ther. 2013;4:65. doi: 10.1186/scrt216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Prevosto C., Zancolli M., Canevali P., Zocchi M.R., Poggi A. Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica. 2007;92:881–888. doi: 10.3324/haematol.11240. [DOI] [PubMed] [Google Scholar]
- 157.Ramasamy R., Fazekasova H., Lam E.W., Soeiro I., Lombardi G., Dazzi F. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation. 2007;83:71–76. doi: 10.1097/01.tp.0000244572.24780.54. [DOI] [PubMed] [Google Scholar]
- 158.Carreras-Planella L., Monguió-Tortajada M., Borràs F.E., Franquesa M. Immunomodulatory effect of MSC on B cells is independent of secreted extracellular vesicles. Front. Immunol. 2019;10:1288. doi: 10.3389/fimmu.2019.01288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lu D., Ma T., Zhou X., Jiang Y., Han Y., Li H. B lymphocytes are the target of mesenchymal stem cells immunoregulatory effect in a murine graft-versus-host disease model. Cell Transplant. 2019;28:1279–1288. doi: 10.1177/0963689719860127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.English K., Ryan J.M., Tobin L., Murphy M.J., Barry F.P., Mahon B.P. Cell contact, prostaglandin E2 and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25High forkhead box P3+ regulatory T cells. Clin. Exp. Immunol. 2009;156:149–160. doi: 10.1111/j.1365-2249.2009.03874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gieseke F., Böhringer J., Bussolari R., Dominici M., Handgretinger R., Müller I. Human multipotent mesenchymal stromal cells use galectin-1 to inhibit immune effector cells. Blood. 2010;116:3770–3779. doi: 10.1182/blood-2010-02-270777. [DOI] [PubMed] [Google Scholar]
- 162.Ren G., Zhang L., Zhao X., Xu G., Zhang Y., Roberts A.I., Zhao R.C., Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 163.Akiyama K., Chen C., Wang D., Xu X., Qu C., Yamaza T., Cai T., Chen W., Sun L., Shi S. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 2012;10:544–555. doi: 10.1016/j.stem.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sato K., Ozaki K., Oh I., Meguro A., Hatanaka K., Nagai T., Muroi K., Ozawa K. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109:228–234. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- 165.Spaggiari G.M., Abdelrazik H., Becchetti F., Moretta L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood. 2009;113:6576–6583. doi: 10.1182/blood-2009-02-203943. [DOI] [PubMed] [Google Scholar]
- 166.Caplan A.I., Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9:11–15. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Doorn J., Moll G., Le Blanc K., van Blitterswijk C., de Boer J. Therapeutic applications of mesenchymal stromal cells: paracrine effects and potential improvements. Tissue Eng. Part B Rev. 2012;18:101–115. doi: 10.1089/ten.TEB.2011.0488. [DOI] [PubMed] [Google Scholar]
- 168.English K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunol. Cell Biol. 2013;91:19–26. doi: 10.1038/icb.2012.56. [DOI] [PubMed] [Google Scholar]
- 169.Wang S., Qu X., Zhao R.C. Clinical applications of mesenchymal stem cells. J. Hematol. Oncol. 2012;5:19. doi: 10.1186/1756-8722-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Munir H., McGettrick H.M. Mesenchymal stem cell therapy for autoimmune disease: risks and rewards. Stem Cells Dev. 2015;24:2091–2100. doi: 10.1089/scd.2015.0008. [DOI] [PubMed] [Google Scholar]
- 171.Kuo Y.R., Chen C.C., Goto S., Lee I.T., Huang C.W., Tsai C.C., Wang C.T., Chen C.L. Modulation of immune response and T-cell regulation by donor adipose-derived stem cells in a rodent hind-limb allotransplant model. Plast. Reconstr. Surg. 2011;128:661e–672e. doi: 10.1097/PRS.0b013e318230c60b. [DOI] [PubMed] [Google Scholar]
- 172.Kuo Y.R., Chen C.C., Shih H.S., Goto S., Huang C.W., Wang C.T., Chen C.L., Wei F.C. Prolongation of composite tissue allotransplant survival by treatment with bone marrow mesenchymal stem cells is correlated with T-cell regulation in a swine hind-limb model. Plast. Reconstr. Surg. 2011;127:569–579. doi: 10.1097/PRS.0b013e318200a92c. [DOI] [PubMed] [Google Scholar]
- 173.Luk F., de Witte S.F., Korevaar S.S., Roemeling-van Rhijn M., Franquesa M., Strini T., van den Engel S., Gargesha M., Roy D., Dor F.J. Inactivated mesenchymal stem cells maintain immunomodulatory capacity. Stem Cells Dev. 2016;25:1342–1354. doi: 10.1089/scd.2016.0068. [DOI] [PubMed] [Google Scholar]
- 174.Bernardo M.E., Ball L.M., Cometa A.M., Roelofs H., Zecca M., Avanzini M.A., Bertaina A., Vinti L., Lankester A., Maccario R. Co-infusion of ex vivo-expanded, parental MSCs prevents life-threatening acute GVHD, but does not reduce the risk of graft failure in pediatric patients undergoing allogeneic umbilical cord blood transplantation. Bone Marrow Transplant. 2011;46:200–207. doi: 10.1038/bmt.2010.87. [DOI] [PubMed] [Google Scholar]
- 175.Forbes G.M., Sturm M.J., Leong R.W., Sparrow M.P., Segarajasingam D., Cummins A.G., Phillips M., Herrmann R.P. A phase 2 study of allogeneic mesenchymal stromal cells for luminal Crohn’s disease refractory to biologic therapy. Clin. Gastroenterol. Hepatol. 2014;12:64–71. doi: 10.1016/j.cgh.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 176.Franquesa M., Hoogduijn M.J., Reinders M.E., Eggenhofer E., Engela A.U., Mensah F.K., Torras J., Pileggi A., van Kooten C., Mahon B., MiSOT Study Group Mesenchymal Stem Cells in Solid Organ Transplantation (MiSOT) fourth meeting: lessons learned from first clinical trials. Transplantation. 2013;96:234–238. doi: 10.1097/TP.0b013e318298f9fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hu J., Yu X., Wang Z., Wang F., Wang L., Gao H., Chen Y., Zhao W., Jia Z., Yan S., Wang Y. Long term effects of the implantation of Wharton’s jelly-derived mesenchymal stem cells from the umbilical cord for newly-onset type 1 diabetes mellitus. Endocr. J. 2013;60:347–357. doi: 10.1507/endocrj.ej12-0343. [DOI] [PubMed] [Google Scholar]
- 178.Le Blanc K., Frassoni F., Ball L., Locatelli F., Roelofs H., Lewis I., Lanino E., Sundberg B., Bernardo M.E., Remberger M., Developmental Committee of the European Group for Blood and Marrow Transplantation Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 179.Wang Y., Zhang A., Ye Z., Xie H., Zheng S. Bone marrow-derived mesenchymal stem cells inhibit acute rejection of rat liver allografts in association with regulatory T-cell expansion. Transplant. Proc. 2009;41:4352–4356. doi: 10.1016/j.transproceed.2009.08.072. [DOI] [PubMed] [Google Scholar]
- 180.El-Jawhari J.J., El-Sherbiny Y.M., Jones E.A., McGonagle D. Mesenchymal stem cells, autoimmunity and rheumatoid arthritis. QJM. 2014;107:505–514. doi: 10.1093/qjmed/hcu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Obermajer N., Popp F.C., Johnson C.L., Benseler V., Dahlke M.H. Rationale and prospects of mesenchymal stem cell therapy for liver transplantation. Curr. Opin. Organ Transplant. 2014;19:60–64. doi: 10.1097/MOT.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 182.Popp F.C., Eggenhofer E., Renner P., Slowik P., Lang S.A., Kaspar H., Geissler E.K., Piso P., Schlitt H.J., Dahlke M.H. Mesenchymal stem cells can induce long-term acceptance of solid organ allografts in synergy with low-dose mycophenolate. Transpl. Immunol. 2008;20:55–60. doi: 10.1016/j.trim.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 183.Schneeberger S., Gorantla V.S., Brandacher G., Zeevi A., Demetris A.J., Lunz J.G., Metes D.M., Donnenberg A.D., Shores J.T., Dimartini A.F. Upper-extremity transplantation using a cell-based protocol to minimize immunosuppression. Ann. Surg. 2013;257:345–351. doi: 10.1097/SLA.0b013e31826d90bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zappia E., Casazza S., Pedemonte E., Benvenuto F., Bonanni I., Gerdoni E., Giunti D., Ceravolo A., Cazzanti F., Frassoni F. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 185.Ciccocioppo R., Bernardo M.E., Sgarella A., Maccario R., Avanzini M.A., Ubezio C., Minelli A., Alvisi C., Vanoli A., Calliada F. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn’s disease. Gut. 2011;60:788–798. doi: 10.1136/gut.2010.214841. [DOI] [PubMed] [Google Scholar]
- 186.Dazzi F., Krampera M. Mesenchymal stem cells and autoimmune diseases. Best Pract. Res. Clin. Haematol. 2011;24:49–57. doi: 10.1016/j.beha.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 187.Tan J., Wu W., Xu X., Liao L., Zheng F., Messinger S., Sun X., Chen J., Yang S., Cai J. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial. JAMA. 2012;307:1169–1177. doi: 10.1001/jama.2012.316. [DOI] [PubMed] [Google Scholar]
- 188.von Dalowski F., Kramer M., Wermke M., Wehner R., Röllig C., Alakel N., Stölzel F., Parmentier S., Sockel K., Krech M. Mesenchymal stromal cells for treatment of acute steroid-refractory graft versus host disease: clinical responses and long-term outcome. Stem Cells. 2016;34:357–366. doi: 10.1002/stem.2224. [DOI] [PubMed] [Google Scholar]
- 189.Ghannam S., Pène J., Moquet-Torcy G., Jorgensen C., Yssel H. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J. Immunol. 2010;185:302–312. doi: 10.4049/jimmunol.0902007. [DOI] [PubMed] [Google Scholar]
- 190.Spaggiari G.M., Capobianco A., Abdelrazik H., Becchetti F., Mingari M.C., Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 191.Perico N., Casiraghi F., Todeschini M., Cortinovis M., Gotti E., Portalupi V., Mister M., Gaspari F., Villa A., Fiori S. Long-term clinical and immunological profile of kidney transplant patients given mesenchymal stromal cell immunotherapy. Front. Immunol. 2018;9:1359. doi: 10.3389/fimmu.2018.01359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Shi Y., Du L., Lin L., Wang Y. Tumour-associated mesenchymal stem/stromal cells: emerging therapeutic targets. Nat. Rev. Drug Discov. 2017;16:35–52. doi: 10.1038/nrd.2016.193. [DOI] [PubMed] [Google Scholar]
- 193.Le Blanc K., Rasmusson I., Sundberg B., Götherström C., Hassan M., Uzunel M., Ringdén O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 194.Sun L., Wang D., Liang J., Zhang H., Feng X., Wang H., Hua B., Liu B., Ye S., Hu X. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum. 2010;62:2467–2475. doi: 10.1002/art.27548. [DOI] [PubMed] [Google Scholar]
- 195.Vojtassák J., Danisovic L., Kubes M., Bakos D., Jarábek L., Ulicná M., Blasko M. Autologous biograft and mesenchymal stem cells in treatment of the diabetic foot. Neuroendocrinol. Lett. 2006;27(Suppl 2):134–137. [PubMed] [Google Scholar]
- 196.Zhang Z., Lin H., Shi M., Xu R., Fu J., Lv J., Chen L., Lv S., Li Y., Yu S. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J. Gastroenterol. Hepatol. 2012;27(Suppl 2):112–120. doi: 10.1111/j.1440-1746.2011.07024.x. [DOI] [PubMed] [Google Scholar]
- 197.Syed B.A., Evans J.B. Stem cell therapy market. Nat. Rev. Drug Discov. 2013;12:185–186. doi: 10.1038/nrd3953. [DOI] [PubMed] [Google Scholar]
- 198.Casiraghi F., Azzollini N., Cassis P., Imberti B., Morigi M., Cugini D., Cavinato R.A., Todeschini M., Solini S., Sonzogni A. Pretransplant infusion of mesenchymal stem cells prolongs the survival of a semiallogeneic heart transplant through the generation of regulatory T cells. J. Immunol. 2008;181:3933–3946. doi: 10.4049/jimmunol.181.6.3933. [DOI] [PubMed] [Google Scholar]
- 199.Casiraghi F., Perico N., Remuzzi G. Mesenchymal stromal cells to promote solid organ transplantation tolerance. Curr. Opin. Organ Transplant. 2013;18:51–58. doi: 10.1097/MOT.0b013e32835c5016. [DOI] [PubMed] [Google Scholar]
- 200.Maccario R., Podestà M., Moretta A., Cometa A., Comoli P., Montagna D., Daudt L., Ibatici A., Piaggio G., Pozzi S. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica. 2005;90:516–525. [PubMed] [Google Scholar]
- 201.Xu D.M., Yu X.F., Zhang D., Zhang M.X., Zhou J.F., Tan P.H., Ding Y.C. Mesenchymal stem cells differentially mediate regulatory T cells and conventional effector T cells to protect fully allogeneic islet grafts in mice. Diabetologia. 2012;55:1091–1102. doi: 10.1007/s00125-011-2433-9. [DOI] [PubMed] [Google Scholar]
- 202.La Rocca G., Lo Iacono M., Corsello T., Corrao S., Farina F., Anzalone R. Human Wharton’s jelly mesenchymal stem cells maintain the expression of key immunomodulatory molecules when subjected to osteogenic, adipogenic and chondrogenic differentiation in vitro: new perspectives for cellular therapy. Curr. Stem Cell Res. Ther. 2013;8:100–113. doi: 10.2174/1574888x11308010012. [DOI] [PubMed] [Google Scholar]
- 203.Mukonoweshuro B., Brown C.J., Fisher J., Ingham E. Immunogenicity of undifferentiated and differentiated allogeneic mouse mesenchymal stem cells. J. Tissue Eng. 2014;5 doi: 10.1177/2041731414534255. 2041731414534255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Franquesa M., Mensah F.K., Huizinga R., Strini T., Boon L., Lombardo E., DelaRosa O., Laman J.D., Grinyó J.M., Weimar W. Human adipose tissue-derived mesenchymal stem cells abrogate plasmablast formation and induce regulatory B cells independently of T helper cells. Stem Cells. 2015;33:880–891. doi: 10.1002/stem.1881. [DOI] [PubMed] [Google Scholar]
- 205.Engela A.U., Baan C.C., Litjens N.H., Franquesa M., Betjes M.G., Weimar W., Hoogduijn M.J. Mesenchymal stem cells control alloreactive CD8+ CD28− T cells. Clin. Exp. Immunol. 2013;174:449–458. doi: 10.1111/cei.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Reading J.L., Yang J.H., Sabbah S., Skowera A., Knight R.R., Pinxteren J., Vaes B., Allsopp T., Ting A.E., Busch S. Clinical-grade multipotent adult progenitor cells durably control pathogenic T cell responses in human models of transplantation and autoimmunity. J. Immunol. 2013;190:4542–4552. doi: 10.4049/jimmunol.1202710. [DOI] [PubMed] [Google Scholar]
- 207.Reading J.L., Vaes B., Hull C., Sabbah S., Hayday T., Wang N.S., DiPiero A., Lehman N.A., Taggart J.M., Carty F. Suppression of IL-7-dependent effector T-cell expansion by multipotent adult progenitor cells and PGE2. Mol. Ther. 2015;23:1783–1793. doi: 10.1038/mt.2015.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Karlsson H., Samarasinghe S., Ball L.M., Sundberg B., Lankester A.C., Dazzi F., Uzunel M., Rao K., Veys P., Le Blanc K. Mesenchymal stem cells exert differential effects on alloantigen and virus-specific T-cell responses. Blood. 2008;112:532–541. doi: 10.1182/blood-2007-10-119370. [DOI] [PubMed] [Google Scholar]
- 209.Jiang X.X., Zhang Y., Liu B., Zhang S.X., Wu Y., Yu X.D., Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 210.Djouad F., Charbonnier L.M., Bouffi C., Louis-Plence P., Bony C., Apparailly F., Cantos C., Jorgensen C., Noël D. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells. 2007;25:2025–2032. doi: 10.1634/stemcells.2006-0548. [DOI] [PubMed] [Google Scholar]
- 211.English K., Barry F.P., Mahon B.P. Murine mesenchymal stem cells suppress dendritic cell migration, maturation and antigen presentation. Immunol. Lett. 2008;115:50–58. doi: 10.1016/j.imlet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 212.Nauta A.J., Kruisselbrink A.B., Lurvink E., Willemze R., Fibbe W.E. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J. Immunol. 2006;177:2080–2087. doi: 10.4049/jimmunol.177.4.2080. [DOI] [PubMed] [Google Scholar]
- 213.Zhang W., Ge W., Li C., You S., Liao L., Han Q., Deng W., Zhao R.C. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev. 2004;13:263–271. doi: 10.1089/154732804323099190. [DOI] [PubMed] [Google Scholar]
- 214.Németh K., Leelahavanichkul A., Yuen P.S., Mayer B., Parmelee A., Doi K., Robey P.G., Leelahavanichkul K., Koller B.H., Brown J.M. Bone marrow stromal cells attenuate sepsis via prostaglandin E2-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Selleri S., Bifsha P., Civini S., Pacelli C., Dieng M.M., Lemieux W., Jin P., Bazin R., Patey N., Marincola F.M. Human mesenchymal stromal cell-secreted lactate induces M2-macrophage differentiation by metabolic reprogramming. Oncotarget. 2016;7:30193–30210. doi: 10.18632/oncotarget.8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zhang Q.Z., Su W.R., Shi S.H., Wilder-Smith P., Xiang A.P., Wong A., Nguyen A.L., Kwon C.W., Le A.D. Human gingiva-derived mesenchymal stem cells elicit polarization of M2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28:1856–1868. doi: 10.1002/stem.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Miyagawa I., Nakayamada S., Nakano K., Yamagata K., Sakata K., Yamaoka K., Tanaka Y. Induction of regulatory T cells and its regulation with insulin-like growth factor/insulin-like growth factor binding protein-4 by human mesenchymal stem cells. J. Immunol. 2017;199:1616–1625. doi: 10.4049/jimmunol.1600230. [DOI] [PubMed] [Google Scholar]
- 218.Kumanogoh A., Kikutani H. Immunological functions of the neuropilins and plexins as receptors for semaphorins. Nat. Rev. Immunol. 2013;13:802–814. doi: 10.1038/nri3545. [DOI] [PubMed] [Google Scholar]
- 219.Lepelletier Y., Lecourt S., Renand A., Arnulf B., Vanneaux V., Fermand J.P., Menasché P., Domet T., Marolleau J.P., Hermine O., Larghero J. Galectin-1 and semaphorin-3A are two soluble factors conferring T-cell immunosuppression to bone marrow mesenchymal stem cell. Stem Cells Dev. 2010;19:1075–1079. doi: 10.1089/scd.2009.0212. [DOI] [PubMed] [Google Scholar]
- 220.Najar M., Raicevic G., Id Boufker H., Stamatopoulos B., De Bruyn C., Meuleman N., Bron D., Toungouz M., Lagneaux L. Modulated expression of adhesion molecules and galectin-1: role during mesenchymal stromal cell immunoregulatory functions. Exp. Hematol. 2010;38:922–932. doi: 10.1016/j.exphem.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 221.Liu G.Y., Xu Y., Li Y., Wang L.H., Liu Y.J., Zhu D. Secreted galectin-3 as a possible biomarker for the immunomodulatory potential of human umbilical cord mesenchymal stromal cells. Cytotherapy. 2013;15:1208–1217. doi: 10.1016/j.jcyt.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 222.Sioud M., Mobergslien A., Boudabous A., Fløisand Y. Evidence for the involvement of galectin-3 in mesenchymal stem cell suppression of allogeneic T-cell proliferation. Scand. J. Immunol. 2010;71:267–274. doi: 10.1111/j.1365-3083.2010.02378.x. [DOI] [PubMed] [Google Scholar]
- 223.Gieseke F., Schütt B., Viebahn S., Koscielniak E., Friedrich W., Handgretinger R., Müller I. Human multipotent mesenchymal stromal cells inhibit proliferation of PBMCs independently of IFNγR1 signaling and IDO expression. Blood. 2007;110:2197–2200. doi: 10.1182/blood-2007-04-083162. [DOI] [PubMed] [Google Scholar]
- 224.Kerkelä E., Laitinen A., Räbinä J., Valkonen S., Takatalo M., Larjo A., Veijola J., Lampinen M., Siljander P., Lehenkari P. Adenosinergic immunosuppression by human mesenchymal stromal cells requires co-operation with T cells. Stem Cells. 2016;34:781–790. doi: 10.1002/stem.2280. [DOI] [PubMed] [Google Scholar]
- 225.Amarnath S., Foley J.E., Farthing D.E., Gress R.E., Laurence A., Eckhaus M.A., Métais J.Y., Rose J.J., Hakim F.T., Felizardo T.C. Bone marrow-derived mesenchymal stromal cells harness purinergenic signaling to tolerize human Th1 cells in vivo. Stem Cells. 2015;33:1200–1212. doi: 10.1002/stem.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Bassi E.J., de Almeida D.C., Moraes-Vieira P.M., Câmara N.O. Exploring the role of soluble factors associated with immune regulatory properties of mesenchymal stem cells. Stem Cell Rev Rep. 2012;8:329–342. doi: 10.1007/s12015-011-9311-1. [DOI] [PubMed] [Google Scholar]
- 227.Bunnell B.A., Betancourt A.M., Sullivan D.E. New concepts on the immune modulation mediated by mesenchymal stem cells. Stem Cell Res. Ther. 2010;1:34. doi: 10.1186/scrt34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Carrillo-Galvez A.B., Cobo M., Cuevas-Ocaña S., Gutiérrez-Guerrero A., Sánchez-Gilabert A., Bongarzone P., García-Pérez A., Muñoz P., Benabdellah K., Toscano M.G. Mesenchymal stromal cells express GARP/LRRC32 on their surface: effects on their biology and immunomodulatory capacity. Stem Cells. 2015;33:183–195. doi: 10.1002/stem.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Najar M., Raicevic G., Jebbawi F., De Bruyn C., Meuleman N., Bron D., Toungouz M., Lagneaux L. Characterization and functionality of the CD200-CD200R system during mesenchymal stromal cell interactions with T-lymphocytes. Immunol. Lett. 2012;146:50–56. doi: 10.1016/j.imlet.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 230.Nguyen T.M., Arthur A., Hayball J.D., Gronthos S. EphB and Ephrin-B interactions mediate human mesenchymal stem cell suppression of activated T-cells. Stem Cells Dev. 2013;22:2751–2764. doi: 10.1089/scd.2012.0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Barbet R., Peiffer I., Hatzfeld A., Charbord P., Hatzfeld J.A. Comparison of gene expression in human embryonic stem cells, hESC-derived mesenchymal stem cells and human mesenchymal stem cells. Stem Cells Int. 2011;2011:368192. doi: 10.4061/2011/368192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Li M.O., Wan Y.Y., Sanjabi S., Robertson A.K., Flavell R.A. Transforming growth factor-β regulation of immune responses. Annu. Rev. Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 233.Bouffi C., Bony C., Courties G., Jorgensen C., Noël D. IL-6-dependent PGE2 secretion by mesenchymal stem cells inhibits local inflammation in experimental arthritis. PLoS ONE. 2010;5:e14247. doi: 10.1371/journal.pone.0014247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Selmani Z., Naji A., Zidi I., Favier B., Gaiffe E., Obert L., Borg C., Saas P., Tiberghien P., Rouas-Freiss N. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26:212–222. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 235.Selmani Z., Naji A., Gaiffe E., Obert L., Tiberghien P., Rouas-Freiss N., Carosella E.D., Deschaseaux F. HLA-G is a crucial immunosuppressive molecule secreted by adult human mesenchymal stem cells. Transplantation. 2009;87(9, Suppl):S62–S66. doi: 10.1097/TP.0b013e3181a2a4b3. [DOI] [PubMed] [Google Scholar]
- 236.Allard D., Allard B., Gaudreau P.O., Chrobak P., Stagg J. CD73-adenosine: a next-generation target in immuno-oncology. Immunotherapy. 2016;8:145–163. doi: 10.2217/imt.15.106. [DOI] [PubMed] [Google Scholar]
- 237.Barnas J.L., Simpson-Abelson M.R., Brooks S.P., Kelleher R.J., Jr., Bankert R.B. Reciprocal functional modulation of the activation of T lymphocytes and fibroblasts derived from human solid tumors. J. Immunol. 2010;185:2681–2692. doi: 10.4049/jimmunol.1000896. [DOI] [PubMed] [Google Scholar]
- 238.Ino Y., Yamazaki-Itoh R., Oguro S., Shimada K., Kosuge T., Zavada J., Kanai Y., Hiraoka N. Arginase II expressed in cancer-associated fibroblasts indicates tissue hypoxia and predicts poor outcome in patients with pancreatic cancer. PLoS ONE. 2013;8:e55146. doi: 10.1371/journal.pone.0055146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Mougiakakos D., Jitschin R., Johansson C.C., Okita R., Kiessling R., Le Blanc K. The impact of inflammatory licensing on heme oxygenase-1-mediated induction of regulatory T cells by human mesenchymal stem cells. Blood. 2011;117:4826–4835. doi: 10.1182/blood-2010-12-324038. [DOI] [PubMed] [Google Scholar]
- 240.Ohta A. A metabolic immune checkpoint: adenosine in tumor microenvironment. Front. Immunol. 2016;7:109. doi: 10.3389/fimmu.2016.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Uyttenhove C., Pilotte L., Théate I., Stroobant V., Colau D., Parmentier N., Boon T., Van den Eynde B.J. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat. Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 242.Vig M., Srivastava S., Kandpal U., Sade H., Lewis V., Sarin A., George A., Bal V., Durdik J.M., Rath S. Inducible nitric oxide synthase in T cells regulates T cell death and immune memory. J. Clin. Invest. 2004;113:1734–1742. doi: 10.1172/JCI20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Young A., Mittal D., Stagg J., Smyth M.J. Targeting cancer-derived adenosine: new therapeutic approaches. Cancer Discov. 2014;4:879–888. doi: 10.1158/2159-8290.CD-14-0341. [DOI] [PubMed] [Google Scholar]
- 244.Katz J.B., Muller A.J., Prendergast G.C. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol. Rev. 2008;222:206–221. doi: 10.1111/j.1600-065X.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- 245.Ge W., Jiang J., Arp J., Liu W., Garcia B., Wang H. Regulatory T-cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3-dioxygenase expression. Transplantation. 2010;90:1312–1320. doi: 10.1097/TP.0b013e3181fed001. [DOI] [PubMed] [Google Scholar]
- 246.Meisel R., Zibert A., Laryea M., Göbel U., Däubener W., Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 247.Ren G., Su J., Zhang L., Zhao X., Ling W., L’huillie A., Zhang J., Lu Y., Roberts A.I., Ji W. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27:1954–1962. doi: 10.1002/stem.118. [DOI] [PubMed] [Google Scholar]
- 248.Dazzi F., Lopes L., Weng L. Mesenchymal stromal cells: a key player in “innate tolerance”? Immunology. 2012;137:206–213. doi: 10.1111/j.1365-2567.2012.03621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Carrade Holt D.D., Wood J.A., Granick J.L., Walker N.J., Clark K.C., Borjesson D.L. Equine mesenchymal stem cells inhibit T cell proliferation through different mechanisms depending on tissue source. Stem Cells Dev. 2014;23:1258–1265. doi: 10.1089/scd.2013.0537. [DOI] [PubMed] [Google Scholar]
- 250.Chen X., Gan Y., Li W., Su J., Zhang Y., Huang Y., Roberts A.I., Han Y., Li J., Wang Y., Shi Y. The interaction between mesenchymal stem cells and steroids during inflammation. Cell Death Dis. 2014;5:e1009. doi: 10.1038/cddis.2013.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Carosella E.D., Gregori S., Rouas-Freiss N., LeMaoult J., Menier C., Favier B. The role of HLA-G in immunity and hematopoiesis. Cell. Mol. Life Sci. 2011;68:353–368. doi: 10.1007/s00018-010-0579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Kanai T., Fujii T., Kozuma S., Yamashita T., Miki A., Kikuchi A., Taketani Y. Soluble HLA-G influences the release of cytokines from allogeneic peripheral blood mononuclear cells in culture. Mol. Hum. Reprod. 2001;7:195–200. doi: 10.1093/molehr/7.2.195. [DOI] [PubMed] [Google Scholar]
- 253.Giuliani M., Fleury M., Vernochet A., Ketroussi F., Clay D., Azzarone B., Lataillade J.J., Durrbach A. Long-lasting inhibitory effects of fetal liver mesenchymal stem cells on T-lymphocyte proliferation. PLoS ONE. 2011;6:e19988. doi: 10.1371/journal.pone.0019988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Siegel G., Schäfer R., Dazzi F. The immunosuppressive properties of mesenchymal stem cells. Transplantation. 2009;87(9, Suppl):S45–S49. doi: 10.1097/TP.0b013e3181a285b0. [DOI] [PubMed] [Google Scholar]
- 255.Yang H.M., Sung J.H., Choi Y.S., Lee H.J., Roh C.R., Kim J., Shin M., Song S., Kwon C.H., Joh J.W., Kim S.J. Enhancement of the immunosuppressive effect of human adipose tissue-derived mesenchymal stromal cells through HLA-G1 expression. Cytotherapy. 2012;14:70–79. doi: 10.3109/14653249.2011.613926. [DOI] [PubMed] [Google Scholar]
- 256.Boura J.S., Vance M., Yin W., Madeira C., Lobato da Silva C., Porada C.D., Almeida-Porada G. Evaluation of gene delivery strategies to efficiently overexpress functional HLA-G on human bone marrow stromal cells. Mol. Ther. Methods Clin. Dev. 2014;2014:14041. doi: 10.1038/mtm.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Thellin O., Coumans B., Zorzi W., Igout A., Heinen E. Tolerance to the foeto-placental “graft”: ten ways to support a child for nine months. Curr. Opin. Immunol. 2000;12:731–737. doi: 10.1016/s0952-7915(00)00170-9. [DOI] [PubMed] [Google Scholar]
- 258.Metcalfe S.M., Watson T.J., Shurey S., Adams E., Green C.J. Leukemia inhibitory factor is linked to regulatory transplantation tolerance. Transplantation. 2005;79:726–730. doi: 10.1097/01.tp.0000149324.42994.38. [DOI] [PubMed] [Google Scholar]
- 259.Nicola N.A., Babon J.J. Leukemia inhibitory factor (LIF) Cytokine Growth Factor Rev. 2015;26:533–544. doi: 10.1016/j.cytogfr.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Jansen B.J., Gilissen C., Roelofs H., Schaap-Oziemlak A., Veltman J.A., Raymakers R.A., Jansen J.H., Kögler G., Figdor C.G., Torensma R., Adema G.J. Functional differences between mesenchymal stem cell populations are reflected by their transcriptome. Stem Cells Dev. 2010;19:481–490. doi: 10.1089/scd.2009.0288. [DOI] [PubMed] [Google Scholar]
- 261.Majumdar M.K., Thiede M.A., Haynesworth S.E., Bruder S.P., Gerson S.L. Human marrow-derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages. J. Hematother. Stem Cell Res. 2000;9:841–848. doi: 10.1089/152581600750062264. [DOI] [PubMed] [Google Scholar]
- 262.Najar M., Raicevic G., Boufker H.I., Fayyad-Kazan H., De Bruyn C., Meuleman N., Bron D., Toungouz M., Lagneaux L. Adipose-tissue-derived and Wharton’s jelly-derived mesenchymal stromal cells suppress lymphocyte responses by secreting leukemia inhibitory factor. Tissue Eng. Part A. 2010;16:3537–3546. doi: 10.1089/ten.TEA.2010.0159. [DOI] [PubMed] [Google Scholar]
- 263.Nasef A., Mazurier C., Bouchet S., François S., Chapel A., Thierry D., Gorin N.C., Fouillard L. Leukemia inhibitory factor: role in human mesenchymal stem cells mediated immunosuppression. Cell. Immunol. 2008;253:16–22. doi: 10.1016/j.cellimm.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 264.Bamberger A.M., Jenatschke S., Schulte H.M., Löning T., Bamberger M.C. Leukemia inhibitory factor (LIF) stimulates the human HLA-G promoter in JEG3 choriocarcinoma cells. J. Clin. Endocrinol. Metab. 2000;85:3932–3936. doi: 10.1210/jcem.85.10.6849. [DOI] [PubMed] [Google Scholar]
- 265.Nasef A., Chapel A., Mazurier C., Bouchet S., Lopez M., Mathieu N., Sensebé L., Zhang Y., Gorin N.C., Thierry D., Fouillard L. Identification of IL-10 and TGF-β transcripts involved in the inhibition of T-lymphocyte proliferation during cell contact with human mesenchymal stem cells. Gene Expr. 2007;13:217–226. doi: 10.3727/000000006780666957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Choi H., Lee R.H., Bazhanov N., Oh J.Y., Prockop D.J. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-κB signaling in resident macrophages. Blood. 2011;118:330–338. doi: 10.1182/blood-2010-12-327353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Su J., Chen X., Huang Y., Li W., Li J., Cao K., Cao G., Zhang L., Li F., Roberts A.I. Phylogenetic distinction of iNOS and IDO function in mesenchymal stem cell-mediated immunosuppression in mammalian species. Cell Death Differ. 2014;21:388–396. doi: 10.1038/cdd.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Fayyad-Kazan M., Fayyad-Kazan H., Lagneaux L., Najar M. The potential of mesenchymal stromal cells in immunotherapy. Immunotherapy. 2016;8:839–842. doi: 10.2217/imt-2016-0037. [DOI] [PubMed] [Google Scholar]
- 269.Bernardo M.E., Fibbe W.E. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13:392–402. doi: 10.1016/j.stem.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 270.Wang Y., Chen X., Cao W., Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat. Immunol. 2014;15:1009–1016. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- 271.Murphy M.B., Moncivais K., Caplan A.I. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp. Mol. Med. 2013;45:e54. doi: 10.1038/emm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Shi Y., Cao J., Wang Y. Rethinking regeneration: empowerment of stem cells by inflammation. Cell Death Differ. 2015;22:1891–1892. doi: 10.1038/cdd.2015.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Redondo-Castro E., Cunningham C., Miller J., Martuscelli L., Aoulad-Ali S., Rothwell N.J., Kielty C.M., Allan S.M., Pinteaux E. Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro. Stem Cell Res. Ther. 2017;8:79. doi: 10.1186/s13287-017-0531-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Najar M., Raicevic G., Fayyad-Kazan H., Bron D., Toungouz M., Lagneaux L. Mesenchymal stromal cells and immunomodulation: A gathering of regulatory immune cells. Cytotherapy. 2016;18:160–171. doi: 10.1016/j.jcyt.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 275.Najar M., Krayem M., Merimi M., Burny A., Meuleman N., Bron D., Raicevic G., Lagneaux L. Insights into inflammatory priming of mesenchymal stromal cells: functional biological impacts. Inflamm. Res. 2018;67:467–477. doi: 10.1007/s00011-018-1131-1. [DOI] [PubMed] [Google Scholar]
- 276.Hemeda H., Jakob M., Ludwig A.K., Giebel B., Lang S., Brandau S. Interferon-gamma and tumor necrosis factor-α differentially affect cytokine expression and migration properties of mesenchymal stem cells. Stem Cells Dev. 2010;19:693–706. doi: 10.1089/scd.2009.0365. [DOI] [PubMed] [Google Scholar]
- 277.Barry F.P., Murphy J.M. Mesenchymal stem cells: clinical applications and biological characterization. Int. J. Biochem. Cell Biol. 2004;36:568–584. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 278.Ren G., Zhao X., Zhang L., Zhang J., L’Huillier A., Ling W., Roberts A.I., Le A.D., Shi S., Shao C., Shi Y. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J. Immunol. 2010;184:2321–2328. doi: 10.4049/jimmunol.0902023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Daxecker H., Raab M., Markovic S., Karimi A., Griesmacher A., Mueller M.M. Endothelial adhesion molecule expression in an in vitro model of inflammation. Clin. Chim. Acta. 2002;325:171–175. doi: 10.1016/s0009-8981(02)00244-9. [DOI] [PubMed] [Google Scholar]
- 280.Hoogduijn M.J., Popp F., Verbeek R., Masoodi M., Nicolaou A., Baan C., Dahlke M.H. The immunomodulatory properties of mesenchymal stem cells and their use for immunotherapy. Int. Immunopharmacol. 2010;10:1496–1500. doi: 10.1016/j.intimp.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 281.Kyurkchiev D., Bochev I., Ivanova-Todorova E., Mourdjeva M., Oreshkova T., Belemezova K., Kyurkchiev S. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J. Stem Cells. 2014;6:552–570. doi: 10.4252/wjsc.v6.i5.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Chan J.L., Tang K.C., Patel A.P., Bonilla L.M., Pierobon N., Ponzio N.M., Rameshwar P. Antigen-presenting property of mesenchymal stem cells occurs during a narrow window at low levels of interferon-γ. Blood. 2006;107:4817–4824. doi: 10.1182/blood-2006-01-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.François M., Romieu-Mourez R., Stock-Martineau S., Boivin M.N., Bramson J.L., Galipeau J. Mesenchymal stromal cells cross-present soluble exogenous antigens as part of their antigen-presenting cell properties. Blood. 2009;114:2632–2638. doi: 10.1182/blood-2009-02-207795. [DOI] [PubMed] [Google Scholar]
- 284.Sánchez-Abarca L.I., Alvarez-Laderas I., Díez Campelo M., Caballero-Velázquez T., Herrero C., Muntión S., Calderón C., García-Guerrero E., Sánchez-Guijo F., Del Cañizo C. Uptake and delivery of antigens by mesenchymal stromal cells. Cytotherapy. 2013;15:673–678. doi: 10.1016/j.jcyt.2013.01.216. [DOI] [PubMed] [Google Scholar]
- 285.Stagg J., Pommey S., Eliopoulos N., Galipeau J. Interferon-γ-stimulated marrow stromal cells: a new type of nonhematopoietic antigen-presenting cell. Blood. 2006;107:2570–2577. doi: 10.1182/blood-2005-07-2793. [DOI] [PubMed] [Google Scholar]
- 286.Morandi F., Raffaghello L., Bianchi G., Meloni F., Salis A., Millo E., Ferrone S., Barnaba V., Pistoia V. Immunogenicity of human mesenchymal stem cells in HLA-class I-restricted T-cell responses against viral or tumor-associated antigens. Stem Cells. 2008;26:1275–1287. doi: 10.1634/stemcells.2007-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Romieu-Mourez R., François M., Boivin M.N., Stagg J., Galipeau J. Regulation of MHC class II expression and antigen processing in murine and human mesenchymal stromal cells by IFN-γ, TGF-β, and cell density. J. Immunol. 2007;179:1549–1558. doi: 10.4049/jimmunol.179.3.1549. [DOI] [PubMed] [Google Scholar]
- 288.Cresswell P., Ackerman A.L., Giodini A., Peaper D.R., Wearsch P.A. Mechanisms of MHC class I-restricted antigen processing and cross-presentation. Immunol. Rev. 2005;207:145–157. doi: 10.1111/j.0105-2896.2005.00316.x. [DOI] [PubMed] [Google Scholar]
- 289.Lin M.L., Zhan Y., Villadangos J.A., Lew A.M. The cell biology of cross-presentation and the role of dendritic cell subsets. Immunol. Cell Biol. 2008;86:353–362. doi: 10.1038/icb.2008.3. [DOI] [PubMed] [Google Scholar]
- 290.Romieu-Mourez R., François M., Boivin M.N., Bouchentouf M., Spaner D.E., Galipeau J. Cytokine modulation of TLR expression and activation in mesenchymal stromal cells leads to a proinflammatory phenotype. J. Immunol. 2009;182:7963–7973. doi: 10.4049/jimmunol.0803864. [DOI] [PubMed] [Google Scholar]
- 291.Ströjby S., Eberstål S., Svensson A., Fritzell S., Bexell D., Siesjö P., Darabi A., Bengzon J. Intratumorally implanted mesenchymal stromal cells potentiate peripheral immunotherapy against malignant rat gliomas. J. Neuroimmunol. 2014;274:240–243. doi: 10.1016/j.jneuroim.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 292.Waterman R.S., Henkle S.L., Betancourt A.M. Mesenchymal stem cell 1 (MSC1)-based therapy attenuates tumor growth whereas MSC2-treatment promotes tumor growth and metastasis. PLoS ONE. 2012;7:e45590. doi: 10.1371/journal.pone.0045590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Fu X., Chen Y., Xie F.N., Dong P., Liu W.B., Cao Y., Zhang W.J., Xiao R. Comparison of immunological characteristics of mesenchymal stem cells derived from human embryonic stem cells and bone marrow. Tissue Eng. Part A. 2015;21:616–626. doi: 10.1089/ten.tea.2013.0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Chan W.K., Lau A.S., Li J.C., Law H.K., Lau Y.L., Chan G.C. MHC expression kinetics and immunogenicity of mesenchymal stromal cells after short-term IFN-γ challenge. Exp. Hematol. 2008;36:1545–1555. doi: 10.1016/j.exphem.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 295.Sivanathan K.N., Rojas-Canales D.M., Hope C.M., Krishnan R., Carroll R.P., Gronthos S., Grey S.T., Coates P.T. Interleukin-17A-induced human mesenchymal stem cells are superior modulators of immunological function. Stem Cells. 2015;33:2850–2863. doi: 10.1002/stem.2075. [DOI] [PubMed] [Google Scholar]
- 296.Hwa Cho H., Bae Y.C., Jung J.S. Role of Toll-like receptors on human adipose-derived stromal cells. Stem Cells. 2006;24:2744–2752. doi: 10.1634/stemcells.2006-0189. [DOI] [PubMed] [Google Scholar]
- 297.Pevsner-Fischer M., Morad V., Cohen-Sfady M., Rousso-Noori L., Zanin-Zhorov A., Cohen S., Cohen I.R., Zipori D. Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood. 2007;109:1422–1432. doi: 10.1182/blood-2006-06-028704. [DOI] [PubMed] [Google Scholar]
- 298.Shirjang S., Mansoori B., Solali S., Hagh M.F., Shamsasenjan K. Toll-like receptors as a key regulator of mesenchymal stem cell function: an up-to-date review. Cell. Immunol. 2017;315:1–10. doi: 10.1016/j.cellimm.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 299.Kawai T., Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 300.Delarosa O., Dalemans W., Lombardo E. Toll-like receptors as modulators of mesenchymal stem cells. Front. Immunol. 2012;3:182. doi: 10.3389/fimmu.2012.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Krampera M., Sartoris S., Liotta F., Pasini A., Angeli R., Cosmi L., Andreini A., Mosna F., Bonetti B., Rebellato E. Immune regulation by mesenchymal stem cells derived from adult spleen and thymus. Stem Cells Dev. 2007;16:797–810. doi: 10.1089/scd.2007.0024. [DOI] [PubMed] [Google Scholar]
- 302.Lombardo E., DelaRosa O., Mancheño-Corvo P., Menta R., Ramírez C., Büscher D. Toll-like receptor-mediated signaling in human adipose-derived stem cells: implications for immunogenicity and immunosuppressive potential. Tissue Eng. Part A. 2009;15:1579–1589. doi: 10.1089/ten.tea.2008.0340. [DOI] [PubMed] [Google Scholar]
- 303.Opitz C.A., Litzenburger U.M., Lutz C., Lanz T.V., Tritschler I., Köppel A., Tolosa E., Hoberg M., Anderl J., Aicher W.K. Toll-like receptor engagement enhances the immunosuppressive properties of human bone marrow-derived mesenchymal stem cells by inducing indoleamine-2,3-dioxygenase-1 via interferon-β and protein kinase R. Stem Cells. 2009;27:909–919. doi: 10.1002/stem.7. [DOI] [PubMed] [Google Scholar]
- 304.Tomchuck S.L., Zwezdaryk K.J., Coffelt S.B., Waterman R.S., Danka E.S., Scandurro A.B. Toll-like receptors on human mesenchymal stem cells drive their migration and immunomodulating responses. Stem Cells. 2008;26:99–107. doi: 10.1634/stemcells.2007-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Wang X., Cheng Q., Li L., Wang J., Xia L., Xu X., Sun Z. Toll-like receptors 2 and 4 mediate the capacity of mesenchymal stromal cells to support the proliferation and differentiation of CD34+ cells. Exp. Cell Res. 2012;318:196–206. doi: 10.1016/j.yexcr.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 306.Raicevic G., Najar M., Stamatopoulos B., De Bruyn C., Meuleman N., Bron D., Toungouz M., Lagneaux L. The source of human mesenchymal stromal cells influences their TLR profile as well as their functional properties. Cell. Immunol. 2011;270:207–216. doi: 10.1016/j.cellimm.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 307.Raicevic G., Rouas R., Najar M., Stordeur P., Boufker H.I., Bron D., Martiat P., Goldman M., Nevessignsky M.T., Lagneaux L. Inflammation modifies the pattern and the function of Toll-like receptors expressed by human mesenchymal stromal cells. Hum. Immunol. 2010;71:235–244. doi: 10.1016/j.humimm.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 308.Liotta F., Angeli R., Cosmi L., Filì L., Manuelli C., Frosali F., Mazzinghi B., Maggi L., Pasini A., Lisi V. Toll-like receptors 3 and 4 are expressed by human bone marrow-derived mesenchymal stem cells and can inhibit their T-cell modulatory activity by impairing Notch signaling. Stem Cells. 2008;26:279–289. doi: 10.1634/stemcells.2007-0454. [DOI] [PubMed] [Google Scholar]
- 309.Zhang L., Liu D., Pu D., Wang Y., Li L., He Y., Li Y., Li L., Qiu Z., Zhao S., Li W. The role of Toll-like receptor 3 and 4 in regulating the function of mesenchymal stem cells isolated from umbilical cord. Int. J. Mol. Med. 2015;35:1003–1010. doi: 10.3892/ijmm.2015.2106. [DOI] [PubMed] [Google Scholar]
- 310.Waterman R.S., Tomchuck S.L., Henkle S.L., Betancourt A.M. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS ONE. 2010;5:e10088. doi: 10.1371/journal.pone.0010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Giuliani M., Bennaceur-Griscelli A., Nanbakhsh A., Oudrhiri N., Chouaib S., Azzarone B., Durrbach A., Lataillade J.J. TLR ligands stimulation protects MSC from NK killing. Stem Cells. 2014;32:290–300. doi: 10.1002/stem.1563. [DOI] [PubMed] [Google Scholar]
- 312.Ezquer F., Ezquer M., Contador D., Ricca M., Simon V., Conget P. The antidiabetic effect of mesenchymal stem cells is unrelated to their transdifferentiation potential but to their capability to restore Th1/Th2 balance and to modify the pancreatic microenvironment. Stem Cells. 2012;30:1664–1674. doi: 10.1002/stem.1132. [DOI] [PubMed] [Google Scholar]
- 313.Favaro E., Carpanetto A., Caorsi C., Giovarelli M., Angelini C., Cavallo-Perin P., Tetta C., Camussi G., Zanone M.M. Human mesenchymal stem cells and derived extracellular vesicles induce regulatory dendritic cells in type 1 diabetic patients. Diabetologia. 2016;59:325–333. doi: 10.1007/s00125-015-3808-0. [DOI] [PubMed] [Google Scholar]
- 314.Favaro E., Carpanetto A., Lamorte S., Fusco A., Caorsi C., Deregibus M.C., Bruno S., Amoroso A., Giovarelli M., Porta M. Human mesenchymal stem cell-derived microvesicles modulate T cell response to islet antigen glutamic acid decarboxylase in patients with type 1 diabetes. Diabetologia. 2014;57:1664–1673. doi: 10.1007/s00125-014-3262-4. [DOI] [PubMed] [Google Scholar]
- 315.Madec A.M., Mallone R., Afonso G., Abou Mrad E., Mesnier A., Eljaafari A., Thivolet C. Mesenchymal stem cells protect NOD mice from diabetes by inducing regulatory T cells. Diabetologia. 2009;52:1391–1399. doi: 10.1007/s00125-009-1374-z. [DOI] [PubMed] [Google Scholar]
- 316.Zanone M.M., Favaro E., Miceli I., Grassi G., Camussi E., Caorsi C., Amoroso A., Giovarelli M., Perin P.C., Camussi G. Human mesenchymal stem cells modulate cellular immune response to islet antigen glutamic acid decarboxylase in type 1 diabetes. J. Clin. Endocrinol. Metab. 2010;95:3788–3797. doi: 10.1210/jc.2009-2350. [DOI] [PubMed] [Google Scholar]
- 317.van Megen K.M., van ’t Wout E.T., Lages Motta J., Dekker B., Nikolic T., Roep B.O. Activated mesenchymal stromal cells process and present antigens regulating adaptive immunity. Front. Immunol. 2019;10:694. doi: 10.3389/fimmu.2019.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Roep B.O., Wheeler D.C.S., Peakman M. Antigen-based immune modulation therapy for type 1 diabetes: the era of precision medicine. Lancet Diabetes Endocrinol. 2019;7:65–74. doi: 10.1016/S2213-8587(18)30109-8. [DOI] [PubMed] [Google Scholar]
- 319.Morizono K., De Ugarte D.A., Zhu M., Zuk P., Elbarbary A., Ashjian P., Benhaim P., Chen I.S., Hedrick M.H. Multilineage cells from adipose tissue as gene delivery vehicles. Hum. Gene Ther. 2003;14:59–66. doi: 10.1089/10430340360464714. [DOI] [PubMed] [Google Scholar]
- 320.Ozawa K., Sato K., Oh I., Ozaki K., Uchibori R., Obara Y., Kikuchi Y., Ito T., Okada T., Urabe M. Cell and gene therapy using mesenchymal stem cells (MSCs) J. Autoimmun. 2008;30:121–127. doi: 10.1016/j.jaut.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 321.Reiser J., Zhang X.Y., Hemenway C.S., Mondal D., Pradhan L., La Russa V.F. Potential of mesenchymal stem cells in gene therapy approaches for inherited and acquired diseases. Expert Opin. Biol. Ther. 2005;5:1571–1584. doi: 10.1517/14712598.5.12.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Hamada H., Kobune M., Nakamura K., Kawano Y., Kato K., Honmou O., Houkin K., Matsunaga T., Niitsu Y. Mesenchymal stem cells (MSC) as therapeutic cytoreagents for gene therapy. Cancer Sci. 2005;96:149–156. doi: 10.1111/j.1349-7006.2005.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Kumar S., Mahendra G., Nagy T.R., Ponnazhagan S. Osteogenic differentiation of recombinant adeno-associated virus 2-transduced murine mesenchymal stem cells and development of an immunocompetent mouse model for ex vivo osteoporosis gene therapy. Hum. Gene Ther. 2004;15:1197–1206. doi: 10.1089/hum.2004.15.1197. [DOI] [PubMed] [Google Scholar]
- 324.Stender S., Murphy M., O’Brien T., Stengaard C., Ulrich-Vinther M., Søballe K., Barry F. Adeno-associated viral vector transduction of human mesenchymal stem cells. Eur. Cell. Mater. 2007;13:93. doi: 10.22203/ecm.v013a10. 99, discussion 99. [DOI] [PubMed] [Google Scholar]
- 325.Bosch P., Fouletier-Dilling C., Olmsted-Davis E.A., Davis A.R., Stice S.L. Efficient adenoviral-mediated gene delivery into porcine mesenchymal stem cells. Mol. Reprod. Dev. 2006;73:1393–1403. doi: 10.1002/mrd.20593. [DOI] [PubMed] [Google Scholar]
- 326.Bosch P., Stice S.L. Adenoviral transduction of mesenchymal stem cells. Methods Mol. Biol. 2007;407:265–274. doi: 10.1007/978-1-59745-536-7_18. [DOI] [PubMed] [Google Scholar]
- 327.Roelants V., Labar D., de Meester C., Havaux X., Tabilio A., Gambhir S.S., Di Ianni M., Bol A., Bertrand L., Vanoverschelde J.L. Comparison between adenoviral and retroviral vectors for the transduction of the thymidine kinase PET reporter gene in rat mesenchymal stem cells. J. Nucl. Med. 2008;49:1836–1844. doi: 10.2967/jnumed.108.052175. [DOI] [PubMed] [Google Scholar]
- 328.Fan L., Lin C., Zhuo S., Chen L., Liu N., Luo Y., Fang J., Huang Z., Lin Y., Chen J. Transplantation with survivin-engineered mesenchymal stem cells results in better prognosis in a rat model of myocardial infarction. Eur. J. Heart Fail. 2009;11:1023–1030. doi: 10.1093/eurjhf/hfp135. [DOI] [PubMed] [Google Scholar]
- 329.Meyerrose T.E., Roberts M., Ohlemiller K.K., Vogler C.A., Wirthlin L., Nolta J.A., Sands M.S. Lentiviral-transduced human mesenchymal stem cells persistently express therapeutic levels of enzyme in a xenotransplantation model of human disease. Stem Cells. 2008;26:1713–1722. doi: 10.1634/stemcells.2008-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Wang F., Dennis J.E., Awadallah A., Solchaga L.A., Molter J., Kuang Y., Salem N., Lin Y., Tian H., Kolthammer J.A. Transcriptional profiling of human mesenchymal stem cells transduced with reporter genes for imaging. Physiol. Genomics. 2009;37:23–34. doi: 10.1152/physiolgenomics.00300.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Xiang J., Tang J., Song C., Yang Z., Hirst D.G., Zheng Q.J., Li G. Mesenchymal stem cells as a gene therapy carrier for treatment of fibrosarcoma. Cytotherapy. 2009;11:516–526. doi: 10.1080/14653240902960429. [DOI] [PubMed] [Google Scholar]
- 332.Zhang X.Y., La Russa V.F., Bao L., Kolls J., Schwarzenberger P., Reiser J. Lentiviral vectors for sustained transgene expression in human bone marrow-derived stromal cells. Mol. Ther. 2002;5:555–565. doi: 10.1006/mthe.2002.0585. [DOI] [PubMed] [Google Scholar]
- 333.Zhang X.Y., La Russa V.F., Reiser J. Transduction of bone-marrow-derived mesenchymal stem cells by using lentivirus vectors pseudotyped with modified RD114 envelope glycoproteins. J. Virol. 2004;78:1219–1229. doi: 10.1128/JVI.78.3.1219-1229.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Gnecchi M., Melo L.G. Bone marrow-derived mesenchymal stem cells: isolation, expansion, characterization, viral transduction, and production of conditioned medium. Methods Mol. Biol. 2009;482:281–294. doi: 10.1007/978-1-59745-060-7_18. [DOI] [PubMed] [Google Scholar]
- 335.Meyerrose T.E., De Ugarte D.A., Hofling A.A., Herrbrich P.E., Cordonnier T.D., Shultz L.D., Eagon J.C., Wirthlin L., Sands M.S., Hedrick M.A., Nolta J.A. In vivo distribution of human adipose-derived mesenchymal stem cells in novel xenotransplantation models. Stem Cells. 2007;25:220–227. doi: 10.1634/stemcells.2006-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Piccoli C., Scrima R., Ripoli M., Di Ianni M., Del Papa B., D’Aprile A., Quarato G., Martelli M.P., Servillo G., Ligas C. Transformation by retroviral vectors of bone marrow-derived mesenchymal cells induces mitochondria-dependent cAMP-sensitive reactive oxygen species production. Stem Cells. 2008;26:2843–2854. doi: 10.1634/stemcells.2007-0885. [DOI] [PubMed] [Google Scholar]
- 337.Sales V.L., Mettler B.A., Lopez-Ilasaca M., Johnson J.A., Jr., Mayer J.E., Jr. Endothelial progenitor and mesenchymal stem cell-derived cells persist in tissue-engineered patch in vivo: application of green and red fluorescent protein-expressing retroviral vector. Tissue Eng. 2007;13:525–535. doi: 10.1089/ten.2006.0128. [DOI] [PubMed] [Google Scholar]
- 338.Chiang G.G., Rubin H.L., Cherington V., Wang T., Sobolewski J., McGrath C.A., Gaffney A., Emami S., Sarver N., Levine P.H. Bone marrow stromal cell-mediated gene therapy for hemophilia A: in vitro expression of human factor VIII with high biological activity requires the inclusion of the proteolytic site at amino acid 1648. Hum. Gene Ther. 1999;10:61–76. doi: 10.1089/10430349950019192. [DOI] [PubMed] [Google Scholar]
- 339.Chuah M.K., Brems H., Vanslembrouck V., Collen D., VandenDriessche T. Bone marrow stromal cells as targets for gene therapy of hemophilia A. Hum. Gene Ther. 1998;9:353–365. doi: 10.1089/hum.1998.9.3-353. [DOI] [PubMed] [Google Scholar]
- 340.Doering C.B. Retroviral modification of mesenchymal stem cells for gene therapy of hemophilia. Methods Mol. Biol. 2008;433:203–212. doi: 10.1007/978-1-59745-237-3_12. [DOI] [PubMed] [Google Scholar]
- 341.Ohmori T., Mizukami H., Katakai Y., Kawai S., Nakamura H., Inoue M., Shu T., Sugimoto H., Sakata Y. Safety of intra-articular transplantation of lentivirally transduced mesenchymal stromal cells for haemophilic arthropathy in a non-human primate. Int. J. Hematol. 2018;108:239–245. doi: 10.1007/s12185-018-2465-8. [DOI] [PubMed] [Google Scholar]
- 342.Porada C.D., Sanada C., Kuo C.J., Colletti E., Mandeville W., Hasenau J., Zanjani E.D., Moot R., Doering C., Spencer H.T. Phenotypic correction of hemophilia A in sheep by postnatal intraperitoneal transplantation of FVIII-expressing MSC. Exp. Hematol. 2011;39:1124–1135.e4. doi: 10.1016/j.exphem.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Sanada C., Kuo C.J., Colletti E.J., Soland M., Mokhtari S., Knovich M.A., Owen J., Zanjani E.D., Porada C.D., Almeida-Porada G. Mesenchymal stem cells contribute to endogenous FVIII:c production. J. Cell. Physiol. 2013;228:1010–1016. doi: 10.1002/jcp.24247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Van Damme A., Chuah M.K., Dell’accio F., De Bari C., Luyten F., Collen D., VandenDriessche T. Bone marrow mesenchymal cells for haemophilia A gene therapy using retroviral vectors with modified long-terminal repeats. Haemophilia. 2003;9:94–103. doi: 10.1046/j.1365-2516.2003.00709.x. [DOI] [PubMed] [Google Scholar]
- 345.Fahs S.A., Hille M.T., Shi Q., Weiler H., Montgomery R.R. A conditional knockout mouse model reveals endothelial cells as the principal and possibly exclusive source of plasma factor VIII. Blood. 2014;123:3706–3713. doi: 10.1182/blood-2014-02-555151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Hollestelle M.J., Thinnes T., Crain K., Stiko A., Kruijt J.K., van Berkel T.J., Loskutoff D.J., van Mourik J.A. Tissue distribution of factor VIII gene expression in vivo—a closer look. Thromb. Haemost. 2001;86:855–861. [PubMed] [Google Scholar]
- 347.Jacquemin M., Neyrinck A., Hermanns M.I., Lavend’homme R., Rega F., Saint-Remy J.M., Peerlinck K., Van Raemdonck D., Kirkpatrick C.J. FVIII production by human lung microvascular endothelial cells. Blood. 2006;108:515–517. doi: 10.1182/blood-2005-11-4571. [DOI] [PubMed] [Google Scholar]
- 348.Shahani T., Lavend’homme R., Luttun A., Saint-Remy J.M., Peerlinck K., Jacquemin M. Activation of human endothelial cells from specific vascular beds induces the release of a FVIII storage pool. Blood. 2010;115:4902–4909. doi: 10.1182/blood-2009-07-232546. [DOI] [PubMed] [Google Scholar]
- 349.Brown H.C., Gangadharan B., Doering C.B. Enhanced biosynthesis of coagulation factor VIII through diminished engagement of the unfolded protein response. J. Biol. Chem. 2011;286:24451–24457. doi: 10.1074/jbc.M111.238758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Malhotra J.D., Miao H., Zhang K., Wolfson A., Pennathur S., Pipe S.W., Kaufman R.J. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc. Natl. Acad. Sci. USA. 2008;105:18525–18530. doi: 10.1073/pnas.0809677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Chuah M.K., Van Damme A., Zwinnen H., Goovaerts I., Vanslembrouck V., Collen D., VandenDriessche T. Long-term persistence of human bone marrow stromal cells transduced with factor VIII-retroviral vectors and transient production of therapeutic levels of human factor VIII in nonmyeloablated immunodeficient mice. Hum. Gene Ther. 2000;11:729–738. doi: 10.1089/10430340050015626. [DOI] [PubMed] [Google Scholar]
- 352.Porada C.D., Sanada C., Long C.R., Wood J.A., Desai J., Frederick N., Millsap L., Bormann C., Menges S.L., Hanna C. Clinical and molecular characterization of a re-established line of sheep exhibiting hemophilia A. J. Thromb. Haemost. 2010;8:276–285. doi: 10.1111/j.1538-7836.2009.03697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Chamberlain G., Wright K., Rot A., Ashton B., Middleton J. Murine mesenchymal stem cells exhibit a restricted repertoire of functional chemokine receptors: comparison with human. PLoS ONE. 2008;3:e2934. doi: 10.1371/journal.pone.0002934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Wu Y., Zhao R.C. The role of chemokines in mesenchymal stem cell homing to myocardium. Stem Cell Rev. Rep. 2012;8:243–250. doi: 10.1007/s12015-011-9293-z. [DOI] [PubMed] [Google Scholar]
- 355.Lourenco S., Teixeira V.H., Kalber T., Jose R.J., Floto R.A., Janes S.M. Macrophage migration inhibitory factor-CXCR4 is the dominant chemotactic axis in human mesenchymal stem cell recruitment to tumors. J. Immunol. 2015;194:3463–3474. doi: 10.4049/jimmunol.1402097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Bexell D., Gunnarsson S., Tormin A., Darabi A., Gisselsson D., Roybon L., Scheding S., Bengzon J. Bone marrow multipotent mesenchymal stroma cells act as pericyte-like migratory vehicles in experimental gliomas. Mol. Ther. 2009;17:183–190. doi: 10.1038/mt.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Kidd S., Caldwell L., Dietrich M., Samudio I., Spaeth E.L., Watson K., Shi Y., Abbruzzese J., Konopleva M., Andreeff M., Marini F.C. Mesenchymal stromal cells alone or expressing interferon-beta suppress pancreatic tumors in vivo, an effect countered by anti-inflammatory treatment. Cytotherapy. 2010;12:615–625. doi: 10.3109/14653241003631815. [DOI] [PubMed] [Google Scholar]
- 358.Spaeth E., Klopp A., Dembinski J., Andreeff M., Marini F. Inflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cells. Gene Ther. 2008;15:730–738. doi: 10.1038/gt.2008.39. [DOI] [PubMed] [Google Scholar]
- 359.Studeny M., Marini F.C., Champlin R.E., Zompetta C., Fidler I.J., Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-β delivery into tumors. Cancer Res. 2002;62:3603–3608. [PubMed] [Google Scholar]
- 360.Kidd S., Spaeth E., Dembinski J.L., Dietrich M., Watson K., Klopp A., Battula V.L., Weil M., Andreeff M., Marini F.C. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells. 2009;27:2614–2623. doi: 10.1002/stem.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Quante M., Tu S.P., Tomita H., Gonda T., Wang S.S., Takashi S., Baik G.H., Shibata W., Diprete B., Betz K.S. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257–272. doi: 10.1016/j.ccr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Ren G., Zhao X., Wang Y., Zhang X., Chen X., Xu C., Yuan Z.R., Roberts A.I., Zhang L., Zheng B. CCR2-dependent recruitment of macrophages by tumor-educated mesenchymal stromal cells promotes tumor development and is mimicked by TNFα. Cell Stem Cell. 2012;11:812–824. doi: 10.1016/j.stem.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Grisendi G., Bussolari R., Cafarelli L., Petak I., Rasini V., Veronesi E., De Santis G., Spano C., Tagliazzucchi M., Barti-Juhasz H. Adipose-derived mesenchymal stem cells as stable source of tumor necrosis factor-related apoptosis-inducing ligand delivery for cancer therapy. Cancer Res. 2010;70:3718–3729. doi: 10.1158/0008-5472.CAN-09-1865. [DOI] [PubMed] [Google Scholar]
- 364.Studeny M., Marini F.C., Dembinski J.L., Zompetta C., Cabreira-Hansen M., Bekele B.N., Champlin R.E., Andreeff M. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J. Natl. Cancer Inst. 2004;96:1593–1603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 365.Hu Y.L., Fu Y.H., Tabata Y., Gao J.Q. Mesenchymal stem cells: a promising targeted-delivery vehicle in cancer gene therapy. J. Control. Release. 2010;147:154–162. doi: 10.1016/j.jconrel.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 366.Shah K. Mesenchymal stem cells engineered for cancer therapy. Adv. Drug Deliv. Rev. 2012;64:739–748. doi: 10.1016/j.addr.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Kim S.M., Lim J.Y., Park S.I., Jeong C.H., Oh J.H., Jeong M., Oh W., Park S.H., Sung Y.C., Jeun S.S. Gene therapy using TRAIL-secreting human umbilical cord blood-derived mesenchymal stem cells against intracranial glioma. Cancer Res. 2008;68:9614–9623. doi: 10.1158/0008-5472.CAN-08-0451. [DOI] [PubMed] [Google Scholar]
- 368.Ren C., Kumar S., Chanda D., Chen J., Mountz J.D., Ponnazhagan S. Therapeutic potential of mesenchymal stem cells producing interferon-α in a mouse melanoma lung metastasis model. Stem Cells. 2008;26:2332–2338. doi: 10.1634/stemcells.2008-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Uchibori R., Okada T., Ito T., Urabe M., Mizukami H., Kume A., Ozawa K. Retroviral vector-producing mesenchymal stem cells for targeted suicide cancer gene therapy. J. Gene Med. 2009;11:373–381. doi: 10.1002/jgm.1313. [DOI] [PubMed] [Google Scholar]
- 370.Dwyer R.M., Khan S., Barry F.P., O’Brien T., Kerin M.J. Advances in mesenchymal stem cell-mediated gene therapy for cancer. Stem Cell Res. Ther. 2010;1:25. doi: 10.1186/scrt25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Nelson D., Fisher S., Robinson B. The “Trojan Horse” approach to tumor immunotherapy: targeting the tumor microenvironment. J. Immunol. Res. 2014;2014:789069. doi: 10.1155/2014/789069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Niess H., Thomas M.N., Schiergens T.S., Kleespies A., Jauch K.W., Bruns C., Werner J., Nelson P.J., Angele M.K. Genetic engineering of mesenchymal stromal cells for cancer therapy: turning partners in crime into Trojan horses. Innov Surg Sci. 2016;1:19–32. doi: 10.1515/iss-2016-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Loebinger M.R., Eddaoudi A., Davies D., Janes S.M. Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer. Cancer Res. 2009;69:4134–4142. doi: 10.1158/0008-5472.CAN-08-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Sasportas L.S., Kasmieh R., Wakimoto H., Hingtgen S., van de Water J.A., Mohapatra G., Figueiredo J.L., Martuza R.L., Weissleder R., Shah K. Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proc. Natl. Acad. Sci. USA. 2009;106:4822–4827. doi: 10.1073/pnas.0806647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Girdlestone J. Mesenchymal stromal cells with enhanced therapeutic properties. Immunotherapy. 2016;8:1405–1416. doi: 10.2217/imt-2016-0098. [DOI] [PubMed] [Google Scholar]
- 376.Pessina A., Bonomi A., Coccè V., Invernici G., Navone S., Cavicchini L., Sisto F., Ferrari M., Viganò L., Locatelli A. Mesenchymal stromal cells primed with paclitaxel provide a new approach for cancer therapy. PLoS ONE. 2011;6:e28321. doi: 10.1371/journal.pone.0028321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Pessina A., Leonetti C., Artuso S., Benetti A., Dessy E., Pascucci L., Passeri D., Orlandi A., Berenzi A., Bonomi A. Drug-releasing mesenchymal cells strongly suppress B16 lung metastasis in a syngeneic murine model. J. Exp. Clin. Cancer Res. 2015;34:82. doi: 10.1186/s13046-015-0200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Conforti A., Biagini S., Starc N., Proia A., Pessina A., Alessandri G., Locatelli F., Bernardo M.E. Human mesenchymal stromal cells primed with paclitaxel, apart from displaying anti-tumor activity, maintain their immune regulatory functions in vitro. Cytotherapy. 2014;16:868–870. doi: 10.1016/j.jcyt.2014.01.414. [DOI] [PubMed] [Google Scholar]
- 379.Bonomi A., Sordi V., Dugnani E., Ceserani V., Dossena M., Coccè V., Cavicchini L., Ciusani E., Bondiolotti G., Piovani G. Gemcitabine-releasing mesenchymal stromal cells inhibit in vitro proliferation of human pancreatic carcinoma cells. Cytotherapy. 2015;17:1687–1695. doi: 10.1016/j.jcyt.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 380.Wang X., Gao J., Ouyang X., Wang J., Sun X., Lv Y. Mesenchymal stem cells loaded with paclitaxel-poly(lactic-co-glycolic acid) nanoparticles for glioma-targeting therapy. Int. J. Nanomedicine. 2018;13:5231–5248. doi: 10.2147/IJN.S167142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Willmon C., Harrington K., Kottke T., Prestwich R., Melcher A., Vile R. Cell carriers for oncolytic viruses: Fed Ex for cancer therapy. Mol. Ther. 2009;17:1667–1676. doi: 10.1038/mt.2009.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Eltoukhy H.S., Sinha G., Moore C.A., Sandiford O.A., Rameshwar P. Immune modulation by a cellular network of mesenchymal stem cells and breast cancer cell subsets: implication for cancer therapy. Cell. Immunol. 2018;326:33–41. doi: 10.1016/j.cellimm.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 383.Sadhukha T., O’Brien T.D., Prabha S. Nano-engineered mesenchymal stem cells as targeted therapeutic carriers. J. Control. Release. 2014;196:243–251. doi: 10.1016/j.jconrel.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 384.Layek B., Sadhukha T., Panyam J., Prabha S. Nano-engineered mesenchymal stem cells increase therapeutic efficacy of anticancer drug through true active tumor targeting. Mol. Cancer Ther. 2018;17:1196–1206. doi: 10.1158/1535-7163.MCT-17-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Relation T., Yi T., Guess A.J., La Perle K., Otsuru S., Hasgur S., Dominici M., Breuer C., Horwitz E.M. Intratumoral delivery of interferonγ-secreting mesenchymal stromal cells repolarizes tumor-associated macrophages and suppresses neuroblastoma proliferation in vivo. Stem Cells. 2018;36:915–924. doi: 10.1002/stem.2801. [DOI] [PubMed] [Google Scholar]
- 386.Hui L., Chen Y. Tumor microenvironment: sanctuary of the devil. Cancer Lett. 2015;368:7–13. doi: 10.1016/j.canlet.2015.07.039. [DOI] [PubMed] [Google Scholar]
- 387.Parker B.S., Rautela J., Hertzog P.J. Antitumour actions of interferons: implications for cancer therapy. Nat. Rev. Cancer. 2016;16:131–144. doi: 10.1038/nrc.2016.14. [DOI] [PubMed] [Google Scholar]
- 388.Menon L.G., Kelly K., Yang H.W., Kim S.K., Black P.M., Carroll R.S. Human bone marrow-derived mesenchymal stromal cells expressing S-TRAIL as a cellular delivery vehicle for human glioma therapy. Stem Cells. 2009;27:2320–2330. doi: 10.1002/stem.136. [DOI] [PubMed] [Google Scholar]
- 389.Pan G., O’Rourke K., Chinnaiyan A.M., Gentz R., Ebner R., Ni J., Dixit V.M. The receptor for the cytotoxic ligand TRAIL. Science. 1997;276:111–113. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- 390.Amara I., Touati W., Beaune P., de Waziers I. Mesenchymal stem cells as cellular vehicles for prodrug gene therapy against tumors. Biochimie. 2014;105:4–11. doi: 10.1016/j.biochi.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 391.Greco O., Dachs G.U. Gene directed enzyme/prodrug therapy of cancer: historical appraisal and future prospectives. J. Cell. Physiol. 2001;187:22–36. doi: 10.1002/1097-4652(2001)9999:9999<::AID-JCP1060>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 392.Touati W., Beaune P., de Waziers I. Cancer gene therapy: the new targeting challenge. In: Ozdemir O., editor. Current Cancer Treatment—Novel Beyond Conventional Approaches. InTech; 2011. [Google Scholar]
- 393.Bi W.L., Parysek L.M., Warnick R., Stambrook P.J. In vitro evidence that metabolic cooperation is responsible for the bystander effect observed with HSV tk retroviral gene therapy. Hum. Gene Ther. 1993;4:725–731. doi: 10.1089/hum.1993.4.6-725. [DOI] [PubMed] [Google Scholar]
- 394.Gagandeep S., Brew R., Green B., Christmas S.E., Klatzmann D., Poston G.J., Kinsella A.R. Prodrug-activated gene therapy: involvement of an immunological component in the “bystander effect”. Cancer Gene Ther. 1996;3:83–88. [PubMed] [Google Scholar]
- 395.Kroemer G., Galluzzi L., Kepp O., Zitvogel L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 396.Kuriyama S., Tsujinoue H., Yoshiji H. Immune response to suicide gene therapy. Methods Mol. Med. 2004;90:353–369. doi: 10.1385/1-59259-429-8:353. [DOI] [PubMed] [Google Scholar]
- 397.Touati W., Tran T., Seguin J., Diry M., Flinois J.P., Baillou C., Lescaille G., Andre F., Tartour E., Lemoine F.M. A suicide gene therapy combining the improvement of cyclophosphamide tumor cytotoxicity and the development of an anti-tumor immune response. Curr. Gene Ther. 2014;14:236–246. doi: 10.2174/1566523214666140424152734. [DOI] [PubMed] [Google Scholar]
- 398.Elshami A.A., Saavedra A., Zhang H., Kucharczuk J.C., Spray D.C., Fishman G.I., Amin K.M., Kaiser L.R., Albelda S.M. Gap junctions play a role in the “bystander effect” of the herpes simplex virus thymidine kinase/ganciclovir system in vitro. Gene Ther. 1996;3:85–92. [PubMed] [Google Scholar]
- 399.Fillat C., Carrió M., Cascante A., Sangro B. Suicide gene therapy mediated by the herpes simplex virus thymidine kinase gene/ganciclovir system: fifteen years of application. Curr. Gene Ther. 2003;3:13–26. doi: 10.2174/1566523033347426. [DOI] [PubMed] [Google Scholar]
- 400.Aghi M., Hochberg F., Breakefield X.O. Prodrug activation enzymes in cancer gene therapy. J. Gene Med. 2000;2:148–164. doi: 10.1002/(SICI)1521-2254(200005/06)2:3<148::AID-JGM105>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 401.Crystal R.G., Hirschowitz E., Lieberman M., Daly J., Kazam E., Henschke C., Yankelevitz D., Kemeny N., Silverstein R., Ohwada A. Phase I study of direct administration of a replication deficient adenovirus vector containing the E. coli cytosine deaminase gene to metastatic colon carcinoma of the liver in association with the oral administration of the pro-drug 5-fluorocytosine. Hum. Gene Ther. 1997;8:985–1001. doi: 10.1089/hum.1997.8.8-985. [DOI] [PubMed] [Google Scholar]
- 402.King I., Bermudes D., Lin S., Belcourt M., Pike J., Troy K., Le T., Ittensohn M., Mao J., Lang W. Tumor-targeted Salmonella expressing cytosine deaminase as an anticancer agent. Hum. Gene Ther. 2002;13:1225–1233. doi: 10.1089/104303402320139005. [DOI] [PubMed] [Google Scholar]
- 403.Chaszczewska-Markowska M., Stebelska K., Sikorski A., Madej J., Opolski A., Ugorski M. Liposomal formulation of 5-fluorocytosine in suicide gene therapy with cytosine deaminase—for colorectal cancer. Cancer Lett. 2008;262:164–172. doi: 10.1016/j.canlet.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 404.Hanna N.N., Mauceri H.J., Wayne J.D., Hallahan D.E., Kufe D.W., Weichselbaum R.R. Virally directed cytosine deaminase/5-fluorocytosine gene therapy enhances radiation response in human cancer xenografts. Cancer Res. 1997;57:4205–4209. [PubMed] [Google Scholar]
- 405.Hirschowitz E.A., Ohwada A., Pascal W.R., Russi T.J., Crystal R.G. In vivo adenovirus-mediated gene transfer of the Escherichia coli cytosine deaminase gene to human colon carcinoma-derived tumors induces chemosensitivity to 5-fluorocytosine. Hum. Gene Ther. 1995;6:1055–1063. doi: 10.1089/hum.1995.6.8-1055. [DOI] [PubMed] [Google Scholar]
- 406.Kato H., Koshida K., Yokoyama K., Mizokami A., Namiki M. Potential benefits of combining cytosine deaminase/5-fluorocytosine gene therapy and irradiation for prostate cancer: experimental study. Int. J. Urol. 2002;9:567–576. doi: 10.1046/j.1442-2042.2002.00513.x. [DOI] [PubMed] [Google Scholar]
- 407.Gopinath P., Ghosh S.S. Implication of functional activity for determining therapeutic efficacy of suicide genes in vitro. Biotechnol. Lett. 2008;30:1913–1921. doi: 10.1007/s10529-008-9787-1. [DOI] [PubMed] [Google Scholar]
- 408.Ichikawa T., Tamiya T., Adachi Y., Ono Y., Matsumoto K., Furuta T., Yoshida Y., Hamada H., Ohmoto T. In vivo efficacy and toxicity of 5-fluorocytosine/cytosine deaminase gene therapy for malignant gliomas mediated by adenovirus. Cancer Gene Ther. 2000;7:74–82. doi: 10.1038/sj.cgt.7700086. [DOI] [PubMed] [Google Scholar]
- 409.Amano S., Li S., Gu C., Gao Y., Koizumi S., Yamamoto S., Terakawa S., Namba H. Use of genetically engineered bone marrow-derived mesenchymal stem cells for glioma gene therapy. Int. J. Oncol. 2009;35:1265–1270. doi: 10.3892/ijo_00000443. [DOI] [PubMed] [Google Scholar]
- 410.Bak X.Y., Yang J., Wang S. Baculovirus-transduced bone marrow mesenchymal stem cells for systemic cancer therapy. Cancer Gene Ther. 2010;17:721–729. doi: 10.1038/cgt.2010.32. [DOI] [PubMed] [Google Scholar]
- 411.Conrad C., Hüsemann Y., Niess H., von Luettichau I., Huss R., Bauer C., Jauch K.W., Klein C.A., Bruns C., Nelson P.J. Linking transgene expression of engineered mesenchymal stem cells and angiopoietin-1-induced differentiation to target cancer angiogenesis. Ann. Surg. 2011;253:566–571. doi: 10.1097/SLA.0b013e3181fcb5d8. [DOI] [PubMed] [Google Scholar]
- 412.Ďuriniková E., Kučerová L., Matúšková M. Mesenchymal stromal cells retrovirally transduced with prodrug-converting genes are suitable vehicles for cancer gene therapy. Acta Virol. 2014;58:1–13. doi: 10.4149/av_2014_01_3. [DOI] [PubMed] [Google Scholar]
- 413.Kim S.W., Kim S.J., Park S.H., Yang H.G., Kang M.C., Choi Y.W., Kim S.M., Jeun S.S., Sung Y.C. Complete regression of metastatic renal cell carcinoma by multiple injections of engineered mesenchymal stem cells expressing dodecameric TRAIL and HSV-TK. Clin. Cancer Res. 2013;19:415–427. doi: 10.1158/1078-0432.CCR-12-1568. [DOI] [PubMed] [Google Scholar]
- 414.Li S., Gu C., Gao Y., Amano S., Koizumi S., Tokuyama T., Namba H. Bystander effect in glioma suicide gene therapy using bone marrow stromal cells. Stem Cell Res. (Amst.) 2012;9:270–276. doi: 10.1016/j.scr.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 415.Matuskova M., Hlubinova K., Pastorakova A., Hunakova L., Altanerova V., Altaner C., Kucerova L. HSV-tk expressing mesenchymal stem cells exert bystander effect on human glioblastoma cells. Cancer Lett. 2010;290:58–67. doi: 10.1016/j.canlet.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 416.Niess H., Bao Q., Conrad C., Zischek C., Notohamiprodjo M., Schwab F., Schwarz B., Huss R., Jauch K.W., Nelson P.J., Bruns C.J. Selective targeting of genetically engineered mesenchymal stem cells to tumor stroma microenvironments using tissue-specific suicide gene expression suppresses growth of hepatocellular carcinoma. Ann. Surg. 2011;254:767. doi: 10.1097/SLA.0b013e3182368c4f. 774, discussion 774–775. [DOI] [PubMed] [Google Scholar]
- 417.Ryu C.H., Park K.Y., Kim S.M., Jeong C.H., Woo J.S., Hou Y., Jeun S.S. Valproic acid enhances anti-tumor effect of mesenchymal stem cell mediated HSV-TK gene therapy in intracranial glioma. Biochem. Biophys. Res. Commun. 2012;421:585–590. doi: 10.1016/j.bbrc.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 418.Zhang T.Y., Huang B., Yuan Z.Y., Hu Y.L., Tabata Y., Gao J.Q. Gene recombinant bone marrow mesenchymal stem cells as a tumor-targeted suicide gene delivery vehicle in pulmonary metastasis therapy using non-viral transfection. Nanomedicine (Lond.) 2014;10:257–267. doi: 10.1016/j.nano.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 419.Zischek C., Niess H., Ischenko I., Conrad C., Huss R., Jauch K.W., Nelson P.J., Bruns C. Targeting tumor stroma using engineered mesenchymal stem cells reduces the growth of pancreatic carcinoma. Ann. Surg. 2009;250:747–753. doi: 10.1097/SLA.0b013e3181bd62d0. [DOI] [PubMed] [Google Scholar]
- 420.Braybrooke J.P., Slade A., Deplanque G., Harrop R., Madhusudan S., Forster M.D., Gibson R., Makris A., Talbot D.C., Steiner J. Phase I study of MetXia-P450 gene therapy and oral cyclophosphamide for patients with advanced breast cancer or melanoma. Clin. Cancer Res. 2005;11:1512–1520. doi: 10.1158/1078-0432.CCR-04-0155. [DOI] [PubMed] [Google Scholar]
- 421.Xu F., Li S., Li X.L., Guo Y., Zou B.Y., Xu R., Liao H., Zhao H.Y., Zhang Y., Guan Z.Z., Zhang L. Phase I and biodistribution study of recombinant adenovirus vector-mediated herpes simplex virus thymidine kinase gene and ganciclovir administration in patients with head and neck cancer and other malignant tumors. Cancer Gene Ther. 2009;16:723–730. doi: 10.1038/cgt.2009.19. [DOI] [PubMed] [Google Scholar]
- 422.Kim N., Cho S.G. Clinical applications of mesenchymal stem cells. Korean J. Intern. Med. (Korean. Assoc. Intern. Med.) 2013;28:387–402. doi: 10.3904/kjim.2013.28.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Kosaka H., Ichikawa T., Kurozumi K., Kambara H., Inoue S., Maruo T., Nakamura K., Hamada H., Date I. Therapeutic effect of suicide gene-transferred mesenchymal stem cells in a rat model of glioma. Cancer Gene Ther. 2012;19:572–578. doi: 10.1038/cgt.2012.35. [DOI] [PubMed] [Google Scholar]
- 424.Sherman L.S., Shaker M., Mariotti V., Rameshwar P. Mesenchymal stromal/stem cells in drug therapy: new perspective. Cytotherapy. 2017;19:19–27. doi: 10.1016/j.jcyt.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 425.Dachs G.U., Hunt M.A., Syddall S., Singleton D.C., Patterson A.V. Bystander or no bystander for gene directed enzyme prodrug therapy. Molecules. 2009;14:4517–4545. doi: 10.3390/molecules14114517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.You M.H., Kim W.J., Shim W., Lee S.R., Lee G., Choi S., Kim D.Y., Kim Y.M., Kim H., Han S.U. Cytosine deaminase-producing human mesenchymal stem cells mediate an antitumor effect in a mouse xenograft model. J. Gastroenterol. Hepatol. 2009;24:1393–1400. doi: 10.1111/j.1440-1746.2009.05862.x. [DOI] [PubMed] [Google Scholar]
- 427.Kievit E., Nyati M.K., Ng E., Stegman L.D., Parsels J., Ross B.D., Rehemtulla A., Lawrence T.S. Yeast cytosine deaminase improves radiosensitization and bystander effect by 5-fluorocytosine of human colorectal cancer xenografts. Cancer Res. 2000;60:6649–6655. [PubMed] [Google Scholar]
- 428.Khatri A., Zhang B., Doherty E., Chapman J., Ow K., Pwint H., Martiniello-Wilks R., Russell P.J. Combination of cytosine deaminase with uracil phosphoribosyl transferase leads to local and distant bystander effects against RM1 prostate cancer in mice. J. Gene Med. 2006;8:1086–1096. doi: 10.1002/jgm.944. [DOI] [PubMed] [Google Scholar]
- 429.Ramnaraine M., Pan W., Goblirsch M., Lynch C., Lewis V., Orchard P., Mantyh P., Clohisy D.R. Direct and bystander killing of sarcomas by novel cytosine deaminase fusion gene. Cancer Res. 2003;63:6847–6854. [PubMed] [Google Scholar]
- 430.Shi D.Z., Hu W.X., Li L.X., Chen G., Wei D., Gu P.Y. Pharmacokinetics and the bystander effect in CD:UPRT/5-FC bi-gene therapy of glioma. Chin. Med. J. (Engl.) 2009;122:1267–1272. [PubMed] [Google Scholar]
- 431.Kucerova L., Matuskova M., Pastorakova A., Tyciakova S., Jakubikova J., Bohovic R., Altanerova V., Altaner C. Cytosine deaminase expressing human mesenchymal stem cells mediated tumour regression in melanoma bearing mice. J. Gene Med. 2008;10:1071–1082. doi: 10.1002/jgm.1239. [DOI] [PubMed] [Google Scholar]
- 432.Altanerova V., Cihova M., Babic M., Rychly B., Ondicova K., Mravec B., Altaner C. Human adipose tissue-derived mesenchymal stem cells expressing yeast cytosinedeaminase:uracil phosphoribosyltransferase inhibit intracerebral rat glioblastoma. Int. J. Cancer. 2012;130:2455–2463. doi: 10.1002/ijc.26278. [DOI] [PubMed] [Google Scholar]
- 433.Cavarretta I.T., Altanerova V., Matuskova M., Kucerova L., Culig Z., Altaner C. Adipose tissue-derived mesenchymal stem cells expressing prodrug-converting enzyme inhibit human prostate tumor growth. Mol. Ther. 2010;18:223–231. doi: 10.1038/mt.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Kucerova L., Altanerova V., Matuskova M., Tyciakova S., Altaner C. Adipose tissue-derived human mesenchymal stem cells mediated prodrug cancer gene therapy. Cancer Res. 2007;67:6304–6313. doi: 10.1158/0008-5472.CAN-06-4024. [DOI] [PubMed] [Google Scholar]
- 435.Aliperta R., Cartellieri M., Feldmann A., Arndt C., Koristka S., Michalk I., von Bonin M., Ehninger A., Bachmann J., Ehninger G. Bispecific antibody releasing-mesenchymal stromal cell machinery for retargeting T cells towards acute myeloid leukemia blasts. Blood Cancer J. 2015;5:e348. doi: 10.1038/bcj.2015.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Feldmann A., Arndt C., Töpfer K., Stamova S., Krone F., Cartellieri M., Koristka S., Michalk I., Lindemann D., Schmitz M. Novel humanized and highly efficient bispecific antibodies mediate killing of prostate stem cell antigen-expressing tumor cells by CD8+ and CD4+ T cells. J. Immunol. 2012;189:3249–3259. doi: 10.4049/jimmunol.1200341. [DOI] [PubMed] [Google Scholar]
- 437.Stamova S., Cartellieri M., Feldmann A., Bippes C.C., Bartsch H., Wehner R., Schmitz M., von Bonin M., Bornhäuser M., Ehninger G. Simultaneous engagement of the activatory receptors NKG2D and CD3 for retargeting of effector cells to CD33-positive malignant cells. Leukemia. 2011;25:1053–1056. doi: 10.1038/leu.2011.42. [DOI] [PubMed] [Google Scholar]
- 438.Schlereth B., Quadt C., Dreier T., Kufer P., Lorenczewski G., Prang N., Brandl C., Lippold S., Cobb K., Brasky K. T-cell activation and B-cell depletion in chimpanzees treated with a bispecific anti-CD19/anti-CD3 single-chain antibody construct. Cancer Immunol. Immunother. 2006;55:503–514. doi: 10.1007/s00262-005-0001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Stork R., Zettlitz K.A., Müller D., Rether M., Hanisch F.G., Kontermann R.E. N-glycosylation as novel strategy to improve pharmacokinetic properties of bispecific single-chain diabodies. J. Biol. Chem. 2008;283:7804–7812. doi: 10.1074/jbc.M709179200. [DOI] [PubMed] [Google Scholar]
- 440.Compte M., Nuñez-Prado N., Sanz L., Alvarez-Vallina L. Immunotherapeutic organoids: a new approach to cancer treatment. Biomatter. 2013;3:e23897. doi: 10.4161/biom.23897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Pittenger M.F., Discher D.E., Péault B.M., Phinney D.G., Hare J.M., Caplan A.I. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen. Med. 2019;4:22. doi: 10.1038/s41536-019-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


