Abstract
The functional information on heat-shock proteins (Hsp) and heat-shock promoters from an important agricultural insect pest, Spodoptera frugiperda, is still lacking. We conducted a genome-wide identification of Hsp genes and identified a total of 21 genes belonging to four major insect Hsp families (small heat-shock proteins, Hsp60, Hsp70, and Hsp90) in S. frugiperda. Expression of most of S. frugiperda (SfHsp) genes could be detected in Sf9 cells, embryos and larval tissues of S. frugiperda. The heat-inducible activity of heat-shock promoters from several SfHsp genes was tested in Sf9 cells and embryos. The promoter of SfHsp70D showed the high constitutive activity in cell line and embryos, while the activity of SfHsp20.15 and SfHsp20.71 promoters was most dramatically induced in Sf9 cells and embryos. In embryos, the heat-induced activity of SfHsp20.71 and SfHsp70D promoters outperformed commercially used ie1 and ie2 promoters. The heat-induced activity of SfHsp70D and SfHsp19.07 promoters were more robust than ie2 promoter in Sf9 cells. These SfHsp promoters with high basal activity or with heat-induced activity from low basal activity, could be used in S. frugiperda or other lepidopteran insects for many applications including transgenesis and genome editing.
Subject terms: Zoology, Entomology
Introduction
Heat-shock proteins (Hsp) are abundant and ubiquitously expressed in insects playing important roles in enhancing abiotic and biotic stress tolerance, as well as regulating normal development1,2. Based on their molecular mass and function, insect Hsp can be divided into four major families, small heat-shock proteins, Hsp60, Hsp70, and Hsp 902. Expression of Hsp from a wide range of insect species have been reported to be induced and modulated by abiotic stressors, including extreme temperature3–5, ultraviolet radiation6,7, pesticides8,9, heavy metals10,11, desiccation12–14, starvation15,16, and anoxia/hypoxia17–19, as well as several biotic insults, including parasites20,21, pathogens22,23, and high population density24. In recent years, the availability of both transcriptome and genome data greatly contributed to the identification of an increasing number of Hsp genes from diverse insect species and promoted their functional studies5,9,25–27. However, information of Hsp genes from a destructive insect pest, Spodoptera frugiperda, is still limited23,28.
The stress-inducible expression of Hsp gene is conferred by binding of heat-shock factor (HSF) to heat-shock elements (HSEs), which consists of arrays of the 5-bp unit NGAAN arranged as inverted repeats in the promoter region29. Since exposure to high temperature is likely the simpler way to achieve inducible expression of insect Hsp genes, the promoters of insect Hsp genes are good candidates to drive the expression of foreign genes by heat-shock. Analysis of heat-shock promoters is still limited to a few model insect species. Promoters of Hsp26, Hsp68, Hsp70, and Hsp82, from Drosophila melanogaster have been used to drive the heat-shock induced expression of transposases facilitating the germline transformation in insects including those belong to order Coleoptera30,31, Diptera32–36, Hymenoptera37, and Lepidoptera38–41. The promoter of D. melanogaster Hsp70 was also successfully used to establish a transient expression system for foreign protein production in Sf9 cells42. Two promoters of Hsp70 genes from Aedes aegypti showed robust heat-inducible activity in transgenic mosquitoes. Recently, promoter of Hsp68 from Tribolium castaneum was used to improve germline transformation in this insect43. Identification and analysis of heat-shock promoters form other insect species could benefit the conditional expression of foreign genes in insects and cell lines developed from insects.
In this work, we identified multiple Hsp genes from S. frugiperda, and analyzed their heat-inducible expression in Sf9 cells, embryos, and different tissues of larvae. The potential promoters from several highly induced Hsp genes were cloned into the luciferase expression vector and evaluated their activity in Sf9 cells and embryos. Several promoters with activity in Sf9 cells and embryos were identified. The promoters with strong heat-inducible activity could be used for expression of proteins as well as for the development of transgenic and genome editing methods in this and other lepidopteran insects.
Results
SfHsp genes and their promoters
By blasting the Transcriptome Shotgun Assembly of S. frugiperda available at NCBI, orthologs of 21 heat-shock protein genes were identified and named as SfHsp11.2, SfHsp15.82, SfHsp19.07, SfHsp19.35, SfHsp19.66, SfHsp19.74, SfHsp20.15, SfHsp20.71, SfHsp21.37, SfHsp21.38, SfHsp21.96, SfHsp24.35, SfHsp26.61, SfHsp29.00, SfHsp60, SfHsp70A, SfHsp70B, SfHsp70C, SfHsp70D, SfHsp75, SfHsp83, and SfHsp97 based on the predicted molecular weights of proteins encoded by these genes (The accession numbers of these genes are shown in Table S1). Phylogenetic analysis showed that heat-shock proteins are conserved among lepidopteran insects (Figs. S1 and S2).
Three types of HSEs, tail-tail, head-head, and step/gap44, were identified within the 2 kb-long potential promoter regions of 11 SfHsp genes (SfHsp19.74, SfHsp20.15, SfHsp20.71, SfHsp21.37, SfHsp29.00, SfHsp70A, SfHsp70B, SfHsp70C, SfHsp70D, SfHsp83, and SfHsp97). No HSEs were found in the promoter region of SfHsp19.66. One or two types of HSEs were detected in the promoter regions of other Hsp genes. Maximum number of HSEs, 26 HSEs, were identified in the potential promoter of SfHsp70D (Table 1). Promoter sequence of SfHsp20.71 gene with potential HSE marked are shown in Fig. S3.
Table 1.
Number of HSEs within 2 kb sequence upstream of ATG.
| Tail-tail | Head-head | Step/gap | Total | |
|---|---|---|---|---|
| SfHsp11.2 | 0 | 2 | 4 | 6 |
| SfHsp15.82 | 6 | 0 | 2 | 8 |
| SfHsp19.07 | 0 | 8 | 4 | 12 |
| SfHsp19.35 | 0 | 1 | 4 | 5 |
| SfHsp19.66 | 0 | 0 | 0 | 0 |
| SfHsp19.74 | 2 | 6 | 4 | 12 |
| SfHsp20.15 | 4 | 4 | 3 | 11 |
| SfHsp20.71 | 6 | 4 | 8 | 18 |
| SfHsp21.37 | 6 | 6 | 5 | 17 |
| SfHsp21.38 | 2 | 0 | 3 | 5 |
| SfHsp21.96 | 0 | 0 | 4 | 4 |
| SfHsp24.35 | 0 | 0 | 1 | 1 |
| SfHsp26.61 | 0 | 4 | 0 | 4 |
| SfHsp29.00 | 6 | 4 | 2 | 12 |
| SfHsp60 | 2 | 4 | 3 | 9 |
| SfHsp70A | 6 | 6 | 3 | 15 |
| SfHsp70B | 4 | 4 | 3 | 11 |
| SfHsp70C | 4 | 4 | 4 | 12 |
| SfHsp70D | 8 | 10 | 8 | 26 |
| SfHsp75 | 2 | 0 | 0 | 2 |
| SfHsp83 | 8 | 8 | 3 | 19 |
| SfHsp97 | 2 | 5 | 4 | 11 |
The sequences of 15 bp length HSEs are NTTCNNGAANNNNNN for Tail-tail type, NGAANNTCCNNNNNN for Head-head type, and NTTCNNNNNNNTTCN for Step/gap type. N is any nucleotide.
Heat-shock induced expression of SfHsp genes
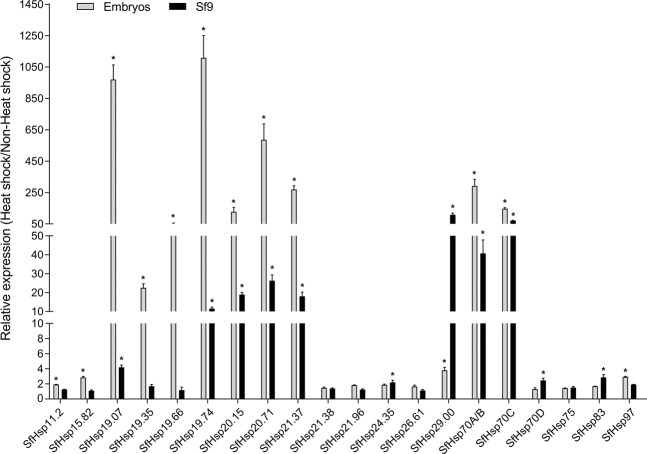
Heat-shock response of SfHsp genes was first investigated in the embryos within 2 hr after oviposition and ovary-derived cell line, Sf9. Due to the presence of multiple melting peaks of SfHsp60 amplification found in the melt curve analysis (data not shown), this gene was not included in the expression studies. The nucleotide sequences of SfHsp70A and SfHsp70B are highly similar. The same primers were used for the analysis of SfHsp70A and SfHsp70B expression. All SfHsp genes were expressed in embryos and Sf9 cells (Fig. S4), and most of the SfHsp genes were induced by heat-shock (Fig. 1). In embryos, expression of SfHsp19.74 was up-regulated by 1,108.49-fold. Expression of SfHsp19.07, SfHsp20.71, SfHsp70A/B, SfHsp21.37, SfHsp70C, and SfHsp20.15 were up-regulated by 970-, 585-, 291-, 269-, 146-, and 127-fold, respectively. The mRNA levels of SfHsp19.35 was induced by 22-fold. Other SfHsp genes showed less than four-fold increase in their mRNA levels in heat-shocked embryos. In Sf9 cells, mRNA levels of Sf29.00 increased the most, by 108-fold after heat-shock. The mRNA levels of SfHsp19.74, SfHsp20.15, SfHsp20.71, SfHsp21.37, SfHsp70A/B, and SfHsp70C were increased by 11.52- to 71.07-fold, respectively (Fig. 1). It appears that the heat-shock response of SfHsp genes in embryos is more pronounced than in the cell line.
Figure 1.
Heat-shock induced expression of SfHsp genes in Sf9 cells and embryos. The Sf9 cells and embryos were exposed to 37 °C for 1 hr, then let them recover at 27 °C for 1 hr. Cells and fresh embryos kept at 27 °C were used as non-heat-shock control. Total RNA was isolated, converted to cDNA and used in RT-qPCR to quantify mRNA levels. 28 s rRNA gene was used as the reference gene. Fold induction of heat-shock treatment over non-heat-shock control was calculated by the 2−∆∆Ct method. Mean ± SD (n = 3) are shown. Data were analyzed using independent samples t-test built-in SPSS software. *Significantly different at p < 0.05.
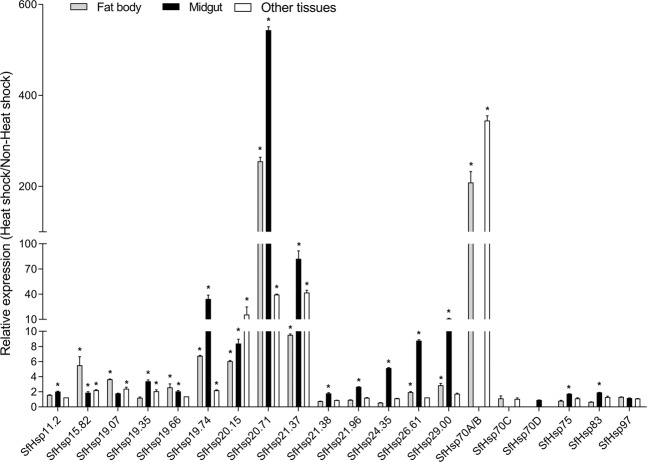
Expression of SfHsp genes after heat-shock was also determined in the midgut, fat body, and other tissues from 6th instar larvae. The mRNA of SfHsp70A/B and SfHsp70C were not detected in the midgut, while SfHsp70D was expressed only in the midgut (Fig. S5). All other SfHsp genes were expressed in all tested tissues. Most of the SfHsp genes showed heat-shock response in different tissues tested (Fig. 2). In the fat body, expression levels of SfHsp20.71 and SfHsp70A/B were induced by 255.08- and 208.46-fold, respectively. Expression of SfHsp15.82, SfHsp19.74, SfHsp20.15, and SfHsp21.37 were up-regulated by 5.51-, 6.74, 6.05-, and 9.52-fold, respectively. Other SfHsp genes showed less than 4-fold heat-shock induction. In the midgut, SfHsp20.71 mRNA displayed the most remarkable increase of 543-fold, and expression of SfHsp21.37 was also enhanced by 82-fold. Expression of SfHsp19.74, SfHsp20.15, SfHsp24.35, SfHsp26.61, and SfHsp29.00 increased by 5- to 34-fold. In other mixed tissues, only four SfHsp genes were induced by heat-shock (SfHsp70A/B at 344-fold, SfHsp20.15 at 16-fold, SfHsp20.71 at 40-fold, and SfHsp21.37 at 42-fold).
Figure 2.
Heat-shock induced expression of SfHsp genes in larval tissues. The 6th instar larvae were exposed to 37 °C for 1 hr, then let them recover at 27 °C for 1 hr. Fat body, midgut, and the rest of the tissues were dissected and used for quantifying mRNA levels as described in Fig. 1 legend.
Analysis of promoter activity
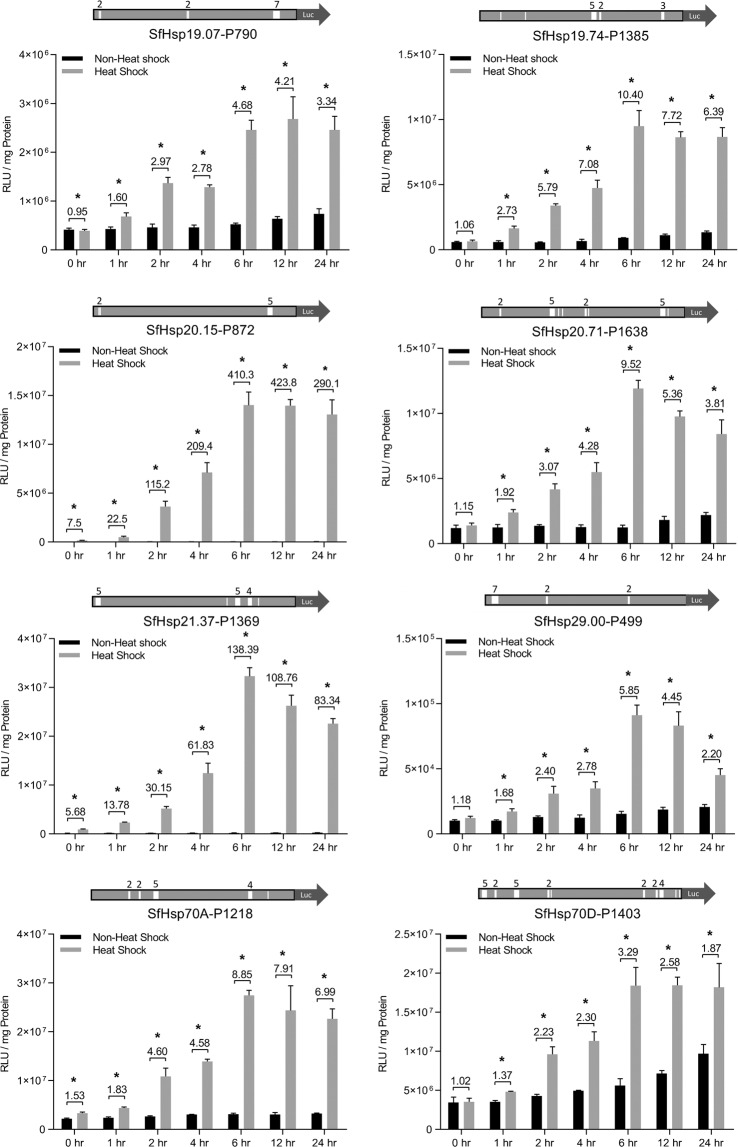
Based on the heat-shock response of SfHsp genes, potential promoters of seven highly induced genes (SfHsp19.07, SfHsp19.74, SfHsp20.15, SfHsp20.71, SfHsp21.37, SfHsp29.00, and SfHsp70A) were chosen to conduct the promoter activity test. The potential promoter of SfHsp70D, containing the maximum number of HSEs, was also included in the promoter activity test. The nucleotide sequences upstream to the ATG, harboring most of the HSEs in the potential promoters, were amplified, yielding 790 bp (14 HSEs) for SfHsp19.07, 1385 bp (12 HSEs) for SfHsp19.74, 872 bp (7 HSEs) for SfHsp20.15, 1638 bp (18 HSEs) for SfHsp20.71, 1369 bp (16 HSEs) for SfHsp21.37, 499 bp (11 HSEs) for SfHsp29.00, 1218 bp (14 HSEs) for SfHsp70A, and 1403 bp (25 HSEs) for SfHsp70D fragments. These fragments were cloned into pGL5luc vector to obtain SfHsp-promoter-pGL5luc plasmids.
In Sf9 cells, a time-course measurement of the luciferase activity was carried out at 0, 1, 2, 4, 6 12, and 24 hr post-heat-shock. All the eight constructs supported an increase in the luciferase activity from 0 hr to 6 hr post-heat-shock (Fig. 3). The maximum activity was maintained at 12 and 24 hr after heat-shock (Fig. 3). The rank of basal activities of eight promoters is SfHsp70D-P1403 > SfHsp70A-P1218 > SfHsp20.71-P1638 > SfHsp19.74-P1385 > SfHsp19.07-P790 > SfHsp21.37-P1369 > SfHsp20.15-P872 > SfHsp29.00-P499. The rank of heat-shock induced activities of eight promoters at 6 hr post heat-shock is SfHsp21.37-P1369 > SfHsp70A-P1218 > SfHsp70D-P1403 > SfHsp20.15-P872 > SfHsp20.71-P1638 > SfHsp19.74-P1385 > SfHsp19.07-P790 > SfHsp29.00-P499. At 6 hr post-heat-shock, SfHsp20.15-P872-pGL5luc and SfHsp21.37-P1369-pGL5luc constructs showed 410- and 138-fold heat-induced enhanced of the luciferase activity, respectively. The heat-induced luciferase activity of the other six constructs increased by 3- to 10-fold (Fig. 3).
Figure 3.
Time-course assay of S. frugiperda heat-shock promoters in Sf9 cells. Structure of each heat-shock promoter construct is shown on the top of each panel, with a solid rectangle of light gray representing heat-shock promoter, and solid arrow of dark gray indicating the open reading frame of firefly luciferase. The approximate locations of HSEs are indicated with white vertical bars, with the numbers above representing the number of HSEs in each HSEs cluster. 100 ng of heat-shock promoter construct or pGL5luc vector was transfected into Sf9 cells. At 48 hr post-transfection, the cells were exposed to 37 °C for 1 hr, followed by recovery at 27 °C for 0, 1, 2, 4, 6, 12, and 24 hr. The cells were harvested, lysed, and the luciferase activity and protein concentration were determined. Transfected cells kept at 27 °C were used as non-heat-shock control. Numbers on the top indicate the increase in fold induction after heat-shock. Mean ± SD (n = 5) are shown. Data were analyzed using independent samples t-test built in SPSS software. *Significantly different at p < 0.05.
To examine the luciferase activity of SfHsp-promoter-pGL5luc constructs in embryos, the constructs were injected into embryos along with an EGFP expression vector. In preliminary experiments, we found that, at 24 hr post-injection, only the living fertile embryos showed the visible transient expression of EGFP, which facilitated the selection of embryos for luciferase activity test. The presence of EGFP had no effect on luciferase activity. Unlike in cell lines, promoter of SfHsp20.15-P872 showed only 3.3-fold heat-inducible activity in embryos (Fig. 4). SfHsp20.71-P1638-pGL5luc construct showed the highest (50-fold increase) luciferase activity after heat-shock. Promoters of SfHsp19.74-P1385 and SfHsp19.07-P790 also displayed strong heat-inducible activity at 20.92- and 12.68-fold increase, respectively. The luciferase activity of the other four constructs was increased by 1.32- to 5.49-fold (Fig. 4). Similar relativity activity of these promoters was observed when the luciferase activity was normalized with EGFP activity from a co-transfected construct (Fig. S6). These data showed that the promoter of SfHsp20.71-P1638 could be used to drive controlled expression of endogenous or exogenous genes in embryos.
Figure 4.
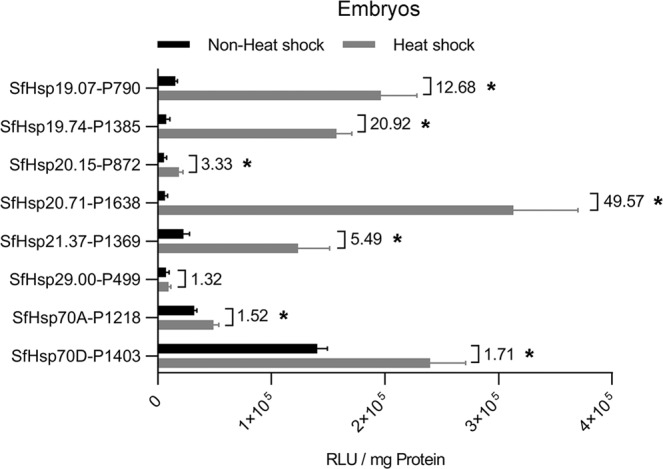
Promoter activity assay in embryos. A mixture containing 1.0 μg/μL of promoter construct or pGL5luc vector and 0.5 μg/μL of pBac-hr5/ie1-EGFP-SV40 plasmid was injected into eggs within 2 hr after oviposition. At 24 hr post-injection, the eggs were exposed to 37 °C for 1 hr, then kept at 27 °C for 6 hr. The EGFP expressing eggs were collected, and the luciferase activity and protein concentration were determined. Injected eggs kept at 27 °C were used as non-heat-shock control. Numbers on the top indicate the fold induction after heat-shock. Mean ± SD (n = 5, Sf9; n = 3, embryos) are shown. Data were analyzed using independent samples t-test built-in SPSS software. *Significantly different at p < 0.05.
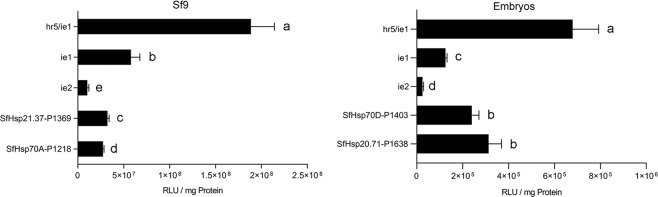
Four constructs with the highest heat-inducible activity, SfHsp21.37-P1369-pGL5luc, and SfHsp70A-P1218-pGL5luc in Sf9 cells and SfHsp70D-P1403-pGL5luc and SfHsp20.71-P1638-pGL5luc in embryos were selected for comparing their performance with the promoters (ie1, hr5/ie1, and ie2) currently used for expression of genes in insect cells. These constructs along with hr5/ie1-pGL5luc, ie1-pGL5luc, and ie2-pGL5luc were transfected into cells or injected into embryos. The luciferase activity was measured at 6 hr post-heat-shock as described above. All SfHsp promoter constructs tested showed higher activity than ie2 construct. However, they displayed lower activity than hr5/ie1 construct in cell line and embryos. The luciferase activity of both SfHsp-promoter constructs was lower than that of ie1 construct in Sf9 cells and embryos (Fig. 5).
Figure 5.
Comparison of highly heat-inducible S. frugiperda heat-shock promoters with commercially used promoters. The top two heat-shock promoter constructs that showed heat-inducible activity in Sf9 cells or embryos and ie1, ie2, and hr5/ie1 constructs were transfected into Sf9 cells or injected into embryos. The transfected cells and injected eggs were processed as described in Fig. 4 legend. Mean ± SD (n = 5) are shown. Different letters beside each column indicate significant differences (at p < 0.05) among multiple groups, which were determined using one-way ANOVA followed by the Tukey HSD test.
Discussion
Since the increase in synthesis of heat-shock proteins (Hsp) after heat-shock were reported in D. melanogaster45, a large number of Hsp genes have been identified in many insect species as important modulators of survival under environmental stresses1, as well as crucial regulators of normal development and diapause2. Inducible expression of insect Hsp genes has been extensively studied; however, functional information on heat-shock promoters is still lacking. In this study, genome-wide identification of S. frugiperda Hsp genes, as well as functional analysis of heat-inducible promoters were conducted.
Similar to the findings in other insects5,25,27, the expression of several Hsp genes was induced by heat-shock in S. frugiperda cell line, embryos, and larval tissues. Since heat-inducible expression of Hsp gene was achieved by HSF binding to the HSEs located in the promoters of Hsp genes29, the variation of heat-inducibility among SfHsp gene is likely associated with the number of HSEs in their promoters. We found that most SfHsp genes possessing more than 10 HSEs in their promoters were significantly up-regulated in at least one tested system after heat-shock at 37 °C. However, to our surprise, SfHsp83 containing 19 HSEs displayed quite low heat-inducibility in cell line and embryos (Table 1). The previous study found that the induction of the SfHsp90 (SfHsp83 in this study) occurred at 42 °C, but not at 37 °C28. Expression of SfHsp83 might be induced at a higher temperature. It is interesting that induction of non-HSE containing SfHsp19.66 was detected in embryos. These data suggest that the heat-shock induction of SfHsp genes may depend not only on temperature and number of HSEs, but also on other factors yet to be identified. Promoters of ie1 and ie2 are most commonly used in transient gene expression studies in insect cells46–50. However, lower expression of foreign proteins in insect cells was observed when using ie1/ie2-based transient gene expression systems51–53. Recently, a Drosophila Hsp70 promoter based transient gene expression system was established to produce foreign proteins by heat-shock in Sf9 cells42. These heat-inducible SfHsp promoters could be used for establishing novel heat-induced transient gene expression system in lepidopteran cell lines.
In early embryos, promoters of SfHsp70 and SfHsp20.71, showed the highest basal activity and heat-inducible activity, respectively (Fig. 4). These promoters are more active than the commercially used ie2 promoter (Fig. 5). In insect transgenic studies, helper plasmids containing promoters of Drosophila Hsp genes have been widely used for successful germline transformation in many insect species54. However, there is no report about using endogenous heat-shock promoters for germline transformation in lepidopteran insects, which is mainly due to lack of functional information on lepidopteran heat-shock promoters. Promoters of SfHsp70 and SfHsp20.71 could be used for driving expression of transposases in germline transformation of S. frugiperda.
Conclusion
In conclusion, we identified and characterized multiple Hsp genes from an important lepidopteran pest, S. frugiperda. The heat-inducible activity of several SfHsp promoters was also analyzed. We identified several promoters with strong heat-inducible activity, which could be used for protein production, as well as for development of transgenic and genome editing methods in this insect. Because of conservation among lepidopteran Hsp genes, promoters of SfHsp genes could also function in other lepidopteran insects and therefore could be used to generate transgenic insects.
Methods
Insect and cells
The laboratory strain of S. frugiperda was purchased from Benzon Research Inc. (Pennsylvania, USA). Adults were fed with 10% sucrose solution. The eggs laid on paper towel were collected, and larvae were reared on artificial diet purchased from Southland Product Inc. (Arkansas, USA). Sf9 cells were maintained at 27 °C in Sf-900 II medium (Thermo Fisher, USA).
Identification and analysis of SfHsp genes
The putative SfHsp genes were identified from Transcriptome Shotgun Assembly of S. frugiperda available from NCBI using nucleotide sequences of S. litura Hsp genes as queries. Their deduced amino acid sequences were subjected to the non-redundant database on NCBI to confirm homology with other insect Hsp proteins. To analyze the relationship of small Hsp genes among lepidopteran insects, a phylogenetic tree was constructed based on the amino acid sequences of small Hsp genes from B. mori, Danaua plexippus, P. xylostella, S. frugiperda, and S. litura. Another phylogenetic tree was also constructed based on the amino acid sequences of Hsp60, Hsp70, Hsp75, Hsp83, and Hsp97 from B. mori, D. melanogaster, T. castaneum, S. frugiperda, and S. litura. Phylogenetic trees were obtained by MEGA755 using the neighbor-joining method with a bootstrap test of 1,000 replicates.
The putative promoter sequences were obtained from Whole-genome shotgun contigs of S. frugiperda available from NCBI using nucleotide sequences of identified SfHsp genes as queries. The consensus heat-shock elements (HSEs) present in the 2 kb putative promoter region upstream to the ATG site were identified as described previously44.
Heat-shock assays of SfHsp genes
Eggs, Sf9 cells and 6th instar larvae were exposed to 37 °C for 1 hour, then recovered at 27 °C for 1 hr. Cells were directly subjected to RNA extraction using TRI reagent (Molecular Research Center Inc., USA). Larval tissues including midgut, fat body, and the remaining tissues were dissected for RNA preparation. Cells and animals maintained at 27 °C were used as non-heat-shock controls. Each treatment included three biological replicates. The tissues and cells were stored in −80 °C until RNA extraction. Total RNA was extracted using TRisol reagent (MRC laboratories, Cincinnati, OH). Complementary DNAs (cDNAs) were synthesized from 1.0 μg total RNA using the M-MLV reverse transcriptase kit (Invitrogen, USA) and stored at −20 °C. Using 20-fold diluted cDNAs as templates, real-time PCR reactions were conducted in a 10-μL total reaction volume containing 5 μL of 2xSYBR Mixture (BioRad, USA), 0.4 μL of each primer, 0.8 μL of cDNA, and 3.2 μL of double-distilled water. The reaction conditions were as follows: 95 °C for 2 min, 40 cycles of 95 °C for 10 s, and 60 °C for 1 min, then followed by a dissociation analysis. For each gene, the reactions included three technical replicates. Basal expression levels of SfHsp genes were represented as fold change over the expression levels of reference gene 28 s rRNA. Fold induction were calculated with the 2−∆∆Ct method56 between treatment and control samples for each biological replicate. Primers were generated by online tool, Primer3 (http://bioinfo.ut.ee/primer3-0.4.0/), and listed in Table S2.
SfHsp promoter-reporter constructs
Sequences containing most of the HSEs in putative promoters of SfHsp19.07, SfHsp19.74, SfHsp20.15, SfHsp20.71, SfHsp21.37, SfHsp29.00, SfHsp70A, and SfHsp70D, were amplified from genomic DNA using Prime STAR GXL DNA Polymerase (TakaRa, Japan). Promoter sequences of ie1 (immediate-early gene 1 of AcMNPV) and hr5/ie1 were amplified using pBac-hr5/ie1-EGFP-SV40 plasmid as a template, and promoter sequence of ie2 (immediate-early gene 2 of OpMNPV) was amplified from pIZT/V5-His vector (Invitrogen, USA). All amplified products were cloned into pGL5luc upstream of the firefly luciferase open reading frame, yielding plasmids of SfHsp19.07-P790-pGL5luc, SfHsp19.74-P1385-pGL5luc, SfHsp20.15-P872-pGL5luc, SfHsp20.71-P1638-pGL5luc, SfHsp21.37-P1369-pGL5luc, SfHsp19.00-P499-pGL5luc, SfHsp70A-P1218-pGL5luc, SfHsp70D-P1403-pGL5luc, hr5/ie1-pGL5luc, ie1-pGL5luc, and ie2-pGL5luc. Primers used in the preparation of these constructs are listed in Table S2.
Reporter assays
The cells were seeded into 96-well culture plates at a density of 2 × 105 cells per ml and incubated at 27 °C overnight for transfection. Sf9 cells were transfected with 100 ng of promoter construct or pGL5luc vector per well using 0.8 μL of Cellfectin II reagent (Thermo Fisher, USA) in 50 μL of Sf-900 II medium (Thermo Fisher, USA). Four hours post-transfection, the medium was removed and replaced with 100 μL of fresh medium. At 48 hours post-transfection, the cells were exposed to 37 °C for 1 hr. The cells were recovered at 27 °C for 0, 1, 2, 4, 6, 12, and 24 hr, then harvested for luciferase activity assay. The medium was removed, and cells were washed with 100 μL of 1xPBS, then 100 μL of ice-cold lysis buffer was added into each well. The plates were placed on a shaker for 20 min at room temperature. 20 μL and 10 μL of cell lysate were used for the luciferase activity assay and protein concentration assay, respectively, as described57. Five replicates for each construct were performed.
Eggs were collected within 2 hr after oviposition and aligned on glass slides. A mixture containing 1.0 μg/μL of promoter construct or pGL5luc vector and 0.5 μg/μL of pBac-hr5/ie1-EGFP-SV40 plasmid was injected into aligned eggs. At 24 hr post-injection, eggs were exposed to 37 °C for 1 hr, then kept at 27 °C for 6 hr. The EGFP expressing eggs were collected and randomly divided into 3 groups with 20–30 eggs in each group. The pooled eggs in each group were homogenized with 200 μL of ice-cold lysis buffer, then centrifuged at 15,000xg for 30 min at 4 °C. 20 μL and 10 μL of supernatant extract were used for the luciferase activity assay and protein concentration determination respectively, as described57.
Statistical analysis
For statistical analysis, IBM SPSS Statistic 25 was used. All data were shown as mean ± SD (standard deviation). The significant difference between two groups was analyzed using independent samples t-test; p < 0.05 was considered statistically significant. Significant differences among multiple groups were analyzed using one-way ANOVA followed by the Tukey HSD test.
Supplementary information
Acknowledgements
This project was supported by the National Institute of Food and Agriculture of US Department of Agriculture, HATCH Project 2351177000 and Agriculture and Food Research Initiative Competitive Grant no. 2019-67013-29351.
Author contributions
X.C., A.T. and S.R.P. designed the experiments. X.C. performed the experiments. X.C. and A.T. analyzed the data. X.C. and S.R.P. wrote the manuscript. All authors read and approved the final manuscript.
Data availability
All data generated or analyzed during this study are included in this published article and in additional information files.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-59197-8.
References
- 1.Zhao L, Jones WA. Expression of heat-shock protein genes in insect stress responses. Invertebr. Surviv. J. 2012;9:93–101. [Google Scholar]
- 2.King AM, MacRae TH. Insect heat-shock proteins during stress and diapause. Annu. Rev. Entomol. 2015;60:59–75. doi: 10.1146/annurev-ento-011613-162107. [DOI] [PubMed] [Google Scholar]
- 3.Colinet H, Lee SF, Hoffmann A. Temporal expression of heat-shock genes during cold stress and recovery from chill coma in adult Drosophila melanogaster. FEBS J. 2010;77:174–185. doi: 10.1111/j.1742-4658.2009.07470.x. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Q, Denlinger DL. Molecular characterization of heat-shock protein 90, 70 and 70 cognate cDNAs and their expression patterns during thermal stress and pupal diapause in the corn earworm. J. Insect Physiol. 2010;56:138–150. doi: 10.1016/j.jinsphys.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Quan G, Duan J, Ladd T, Krell PJ. Identification and expression analysis of multiple small heat-shock protein genes in spruce budworm, Choristoneura fumiferana (L.) Cell Stress. Chaperones. 2018;23:141–154. doi: 10.1007/s12192-017-0832-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen TTA, Michaud D, Cloutier C. A proteomic analysis of the aphid Macrosiphum euphorbiae under heat and radiation stress. Insect Biochem. Mol. Biol. 2009;39:20–30. doi: 10.1016/j.ibmb.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Sang W, et al. The involvement of heat-shock protein and cytochrome P450 genes in response to UV-A exposure in the beetle Tribolium castaneum. J. Insect Physiol. 2012;58:830–836. doi: 10.1016/j.jinsphys.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Sonoda S, Ashfaq M, Tsumuki H. A comparison of heat-shock protein genes from cultured cells of the cabbage armyworm, Mamestra brassicae, in response to heavy metals. Arch. Insect Biochem. 2007;65:210–222. doi: 10.1002/arch.20178. [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Zhang Y. Identification of multiple small heat-shock protein genes in Plutella xylostella (L.) and their expression profiles in response to abiotic stresses. Cell Stress. Chaperones. 2015;20:23–35. doi: 10.1007/s12192-014-0522-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shu Y, Du Y, Wang J. Molecular characterization and expression patterns of Spodoptera litura heat-shock protein 70/90, and their response to zinc stress. Comp. Biochem. Physiol. A. 2011;158:102–110. doi: 10.1016/j.cbpa.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, et al. Cloning and expression pattern of heat-shock protein genes from the endoparasitoid wasp, Pteromalus puparum in response to environmental stresses. Arch. Insect Biochem. 2012;79:247–263. doi: 10.1002/arch.21013. [DOI] [PubMed] [Google Scholar]
- 12.Hayward SA, Rinehart JP, Denlinger DL. Desiccation and rehydration elicit distinct heat-shock protein transcript responses in flesh fly pupae. J. Exp. Biol. 2004;207:963–971. doi: 10.1242/jeb.00842. [DOI] [PubMed] [Google Scholar]
- 13.Sinclair BJ, Gibbs AG, Roberts SP. Gene transcription during exposure to, and recovery from, cold and desiccation stress in Drosophila melanogaster. Insect Mol. Biol. 2007;16:435–443. doi: 10.1111/j.1365-2583.2007.00739.x. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen AD, et al. Effects of desiccation and starvation on thermal tolerance and the heat-shock response in forest ants. J. Comp. Physiol. B. 2017;187:1107–1116. doi: 10.1007/s00360-017-1101-x. [DOI] [PubMed] [Google Scholar]
- 15.Paim RM, et al. Functional evaluation of Heat-shock Proteins 70 (HSP70/HSC70) on Rhodnius prolixus (Hemiptera, Reduviidae) physiological responses associated with feeding and starvation. Insect Biochem. Mol. Biol. 2016;77:10–20. doi: 10.1016/j.ibmb.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Tian L, et al. Starvation-, thermal-and heavy metal-associated expression of four small heat-shock protein genes in Musca domestica. Gene. 2018;642:268–276. doi: 10.1016/j.gene.2017.11.041. [DOI] [PubMed] [Google Scholar]
- 17.Liu G, Roy J, Johnson EA. Identification and function of hypoxia-response genes in Drosophila melanogaster. Physiol. Genomics. 2006;25:134–141. doi: 10.1152/physiolgenomics.00262.2005. [DOI] [PubMed] [Google Scholar]
- 18.Azad P, Ryu J, Haddad GG. Distinct role of Hsp70 in Drosophila hemocytes during severe hypoxia. Free. Radic. Biol. Med. 2011;51:530–538. doi: 10.1016/j.freeradbiomed.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michaud MR, et al. Heat-shock response to hypoxia and its attenuation during recovery in the flesh fly, Sarcophaga crassipalpis. J. Insect Physiol. 2011;57:203–210. doi: 10.1016/j.jinsphys.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Hao YJ, et al. Transcripts analysis of the entomopathogenic nematode Steinernema carpocapsae induced in vitro with insect haemolymph. Mol. Biochem. Parasit. 2010;169:79–86. doi: 10.1016/j.molbiopara.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burke GR, Moran NA. Responses of the pea aphid transcriptome to infection by facultative symbionts. Insect Mol. Biol. 2011;20:357–365. doi: 10.1111/j.1365-2583.2011.01070.x. [DOI] [PubMed] [Google Scholar]
- 22.Hong SM, et al. Efficient soluble protein production on transgenic silkworms expressing cytoplasmic chaperones. Appl. Microbiol. Biotech. 2010;87:2147–2156. doi: 10.1007/s00253-010-2617-0. [DOI] [PubMed] [Google Scholar]
- 23.Lyupina YV, et al. An important role of the heat-shock response in infected cells for replication of baculoviruses. Virology. 2010;406:336–341. doi: 10.1016/j.virol.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 24.Chapuis MP, Simpson SJ, Blondin L, Sword GA. Taxa-specific heat shock proteins are over-expressed with crowding in the Australian plague locust. J. Insect Physiol. 2011;57:1562–1567. doi: 10.1016/j.jinsphys.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen AD, Gotelli NJ, Cahan SH. The evolution of heat-shock protein sequences, cis-regulatory elements, and expression profiles in the eusocial Hymenoptera. BMC Evol. Biol. 2016;16:15. doi: 10.1186/s12862-015-0573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang B, et al. Response of heat-shock protein genes of the oriental fruit moth under diapause and thermal stress reveals multiple patterns dependent on the nature of stress exposure. Cell Stress. Chaperones. 2016;21:653–663. doi: 10.1007/s12192-016-0690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen B, Feder ME, Kang L. Evolution of heat‐shock protein expression underlying adaptive responses to environmental stress. Mol. Ecol. 2018;27:3040–3054. doi: 10.1111/mec.14769. [DOI] [PubMed] [Google Scholar]
- 28.Landais I, et al. Characterization of the cDNA encoding the 90 kDa heat-shock protein in the Lepidoptera Bombyx mori and Spodoptera frugiperda. Gene. 2001;271:223–231. doi: 10.1016/S0378-1119(01)00523-6. [DOI] [PubMed] [Google Scholar]
- 29.Sorger PK. Heat-shock factor and the heat-shock response. Cell. 1991;65:363–366. doi: 10.1016/0092-8674(91)90452-5. [DOI] [PubMed] [Google Scholar]
- 30.Lorenzen MD, et al. piggyBac‐mediated germline transformation in the beetle Tribolium castaneum. Insect Mol. Biol. 2003;12:433–440. doi: 10.1046/j.1365-2583.2003.00427.x. [DOI] [PubMed] [Google Scholar]
- 31.Kuwayama H, Yaginuma T, Yamashita O, Niimi T. Germ‐line transformation and RNAi of the ladybird beetle, Harmonia axyridis. Insect Mol. Biol. 2006;15:507–512. doi: 10.1111/j.1365-2583.2006.00665.x. [DOI] [PubMed] [Google Scholar]
- 32.Handler AM, Harrell RA. Germline transformation of Drosophila melanogaster with the piggyBac transposon vector. Insect Mol. Biol. 1999;8:449–457. doi: 10.1046/j.1365-2583.1999.00139.x. [DOI] [PubMed] [Google Scholar]
- 33.Handler AM. Prospects for using genetic transformation for improved SIT and new biocontrol methods. Genetica. 2002;116:137–149. doi: 10.1023/A:1020924028450. [DOI] [PubMed] [Google Scholar]
- 34.Labbé GM, Nimmo DD, Alphey L. piggybac- and PhiC31-mediated genetic transformation of the Asian tiger mosquito, Aedes albopictus (Skuse) PLoS Negl. Trop. D. 2010;4:e788. doi: 10.1371/journal.pntd.0000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raphael KA, et al. Germ-line transformation of the Queensland fruit fly, Bactrocera tryoni, using a piggyBac vector in the presence of endogenous piggyBac elements. Genetica. 2011;139:91–97. doi: 10.1007/s10709-010-9500-x. [DOI] [PubMed] [Google Scholar]
- 36.Schetelig MF, Handler AM. Germline transformation of the spotted wing drosophilid, Drosophila suzukii, with a piggyBac transposon vector. Genetica. 2013;141:189–193. doi: 10.1007/s10709-013-9717-6. [DOI] [PubMed] [Google Scholar]
- 37.Sumitani M, et al. Germline transformation of the sawfly, Athalia rosae (Hymenoptera: Symphyta), mediated by a piggyBac-derived vector. Insect Biochem. Mol. Biol. 2003;33:449–458. doi: 10.1016/S0965-1748(03)00009-2. [DOI] [PubMed] [Google Scholar]
- 38.Peloquin JJ, Thibault ST, Staten R, Miller TA. Germ‐line transformation of pink bollworm (Lepidoptera: Gelechiidae) mediated by the piggyBac transposable element. Insect Mol. Biol. 2000;9:323–333. doi: 10.1046/j.1365-2583.2000.00194.x. [DOI] [PubMed] [Google Scholar]
- 39.Marcus JM, Ramos DM, Monteiro A. Germline transformation of the butterfly Bicyclus anynana. Proc. Roy. Soc. Lond. B Biol. Sci. 2004;271:S263–265. doi: 10.1098/rsbl.2004.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferguson HJ, et al. Genetic transformation of the codling moth, Cydia pomonella L., with piggyBac EGFP. Transgenic Res. 2011;20:201–214. doi: 10.1007/s11248-010-9391-8. [DOI] [PubMed] [Google Scholar]
- 41.Martins S, et al. Germline transformation of the diamondback moth, Plutella xylostella L., using the piggyBac transposable element. Insect Mol. Biol. 2012;21:414–421. doi: 10.1111/j.1365-2583.2012.01146.x. [DOI] [PubMed] [Google Scholar]
- 42.Chang JC, et al. Transient expression of foreign genes in insect cells (Sf9) for protein functional assay. J. Vis. Exp. 2018;132:e56693. doi: 10.3791/56693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eckermann KN, et al. Hyperactive piggyBac transposase improves transformation efficiency in diverse insect species. Insect Biochem. Mol. Biol. 2018;98:16–24. doi: 10.1016/j.ibmb.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 44.Carpenetti TL, Aryan A, Myles KM, Adelman ZN. Robust heat‐inducible gene expression by two endogenous hsp70‐derived promoters in transgenic Aedes aegypti. Insect Mol. Biol. 2012;21:97–106. doi: 10.1111/j.1365-2583.2011.01116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tissiéres A, Mitchell HK, Tracy UM. Protein synthesis in salivary glands of Drosophila melanogaster: relation to chromosome puffs. J. Mol. Biol. 1974;84:389–398. doi: 10.1016/0022-2836(74)90447-1. [DOI] [PubMed] [Google Scholar]
- 46.Hegedus DD, et al. A series of broad host range shuttle vectors for constitutive and inducible expression of heterologous proteins in insect cell lines. Gene. 1998;207:241–249. doi: 10.1016/S0378-1119(97)00636-7. [DOI] [PubMed] [Google Scholar]
- 47.Condreay JP, Witherspoon SM, Clay WC, Kost TA. Transient and stable gene expression in mammalian cells transduced with a recombinant baculovirus vector. Proc. Natl Acad. Sci. USA. 1999;96:127–132. doi: 10.1073/pnas.96.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu YC. Baculoviral vectors for gene delivery: a review. Curr. Gene Ther. 2008;8:54–65. doi: 10.2174/156652308783688509. [DOI] [PubMed] [Google Scholar]
- 49.Radner S, et al. Transient transfection coupled to baculovirus infection for rapid protein expression screening in insect cells. J. Struct. Biol. 2012;179:46–55. doi: 10.1016/j.jsb.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 50.Li J, et al. FOXA transcriptional factor modulates insect susceptibility to Bacillus thuringiensis Cry1Ac toxin by regulating the expression of toxin-receptor ABCC2 and ABCC3 genes. Insect Biochem. Mol. Biol. 2017;88:1–11. doi: 10.1016/j.ibmb.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 51.Clem RJ, Miller LK. Control of programmed cell death by the baculovirus genes p35 and iap. Mol. Cell Biol. 1994;14:5212–5222. doi: 10.1128/MCB.14.8.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao YG, Eggleston P. Comparative analysis of promoters for transient gene expression in cultured mosquito cells. Insect Mol. Biol. 1999;8:31–38. doi: 10.1046/j.1365-2583.1999.810031.x. [DOI] [PubMed] [Google Scholar]
- 53.Leu JH, Kuo YC, Kou GH, Lo CF. Molecular cloning and characterization of an inhibitor of apoptosis protein (IAP) from the tiger shrimp, Penaeus monodon. Dev. Comp. Immunol. 2008;32:121–133. doi: 10.1016/j.dci.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 54.Gregory M, Alphey L, Morrison NI, Shimeld SM. Insect transformation with piggyBac: getting the number of injections just right. Insect Mol. Biol. 2016;25:259–271. doi: 10.1111/imb.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 57.Kalsi M, Palli SR. Transcription factors, CncC and Maf, regulate expression of CYP6BQ genes responsible for deltamethrin resistance in Tribolium castaneum. Insect Biochem. Mol. Biol. 2015;65:47–56. doi: 10.1016/j.ibmb.2015.08.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and in additional information files.