Abstract
A hallmark feature of Alzheimer’s disease (AD) and other tauopathies is the misfolding, aggregation and cerebral accumulation of tau deposits. Compelling evidence indicates that misfolded tau aggregates are neurotoxic, producing synaptic loss and neuronal damage. Misfolded tau aggregates are able to spread the pathology from cell-to-cell by a prion like seeding mechanism. The factors implicated in the initiation and progression of tau misfolding and aggregation are largely unclear. In this study, we evaluated the effect of DNA extracted from diverse prokaryotic and eukaryotic cells in tau misfolding and aggregation. Our results show that DNA from various, unrelated gram-positive and gram-negative bacteria results in a more pronounced tau misfolding compared to eukaryotic DNA. Interestingly, a higher effect in promoting tau aggregation was observed for DNA extracted from certain bacterial species previously detected in the brain, CSF or oral cavity of patients with AD. Our findings indicate that microbial DNA may play a previously overlooked role in the propagation of tau protein misfolding and AD pathogenesis, providing a new conceptual framework that positions the compromised blood-brain and intestinal barriers as important sources of microbial DNA in the CNS, opening novel opportunities for therapeutic interventions.
Subject terms: Alzheimer's disease, Neurodegeneration
Introduction
The pathogenesis of certain neurodegenerative and autoimmune diseases is characterized by the misfolding and aggregation of proteins with prion-like properties, such as β-amyloid (Aβ) and tau in Alzheimer’s disease (AD), alpha-synuclein (α-syn) in Parkinson’s disease, TDP-43 and SOD1 in amyotrophic lateral sclerosis, and IAPP in type 2 diabetes1–5. Despite the fact that these proteins are associated with different diseases, they all accumulate in tissues in the form of toxic misfolded protein aggregates that have the capacity to spread among cells and tissues during the pathological progression1–4. Indeed, studies with tau, Aβ, and α-syn have shown that inoculation with tissue homogenates or misfolded proteins obtained from hosts afflicted with neurodegenerative diseases results in the induction of the disease pathology in the recipient cellular or animal models1–4. Moreover, in animals not genetically programmed to spontaneously develop the disease, pathological induction has reportedly resulted in completely de novo diseases that are more akin to infectious prions6,7.
AD is a devastating degenerative disorder of the brain for which there is no effective treatment or accurate pre-clinical diagnosis. The major neuropathological changes in the brain of AD patients are neuronal death, synaptic alterations, brain inflammation and the presence of protein aggregates in the form of extracellular amyloid plaques, composed of Aβ, and intracellular neurofibrillary tangles (NFTs), composed of hyperphosphorylated tau8. For many years it was thought that Aβ aggregation was the most important pathological event in the disease, but recent findings suggest that tau hyperphosphorylation, misfolding and oligomerization might play a major role in AD pathogenesis8,9. Indeed, all AD clinical trials based on Aβ as a therapeutic target have failed so far10. In addition, the burden of NFTs correlates well with the severity of dementia in AD8. Finally, increasing evidence from in vitro and in vivo studies in experimental animal models provides a compelling case for tau as a promising therapeutic target11. The current view is that Aβ pathology is the primary driving force of the disease initiation, but this is accomplished by induction of changes in tau protein leading to the neurodegenerative cascade8. Tau aggregates do not accumulate only in AD, but are a common feature of several neurodegenerative disorders, termed tauopathies12. In other tauopathies, such as Progressive supranuclear palsy, Chronic traumatic encephalopathy, Corticobasal degeneration, Pick’s disease and Frontotemporal dementia, tau leads to neurodegeneration in the absence of amyloid plaques. Pathological tau is characterized by the formation of both intra- and extracellular misfolded forms, indicating that the seeding agent could be localized outside as well as within neurons13,14.
The misfolding and aggregation of proteins into prion-like amyloid aggregates is not restricted to human diseases, but it has been observed in diverse organisms and in many cases leading not to disease, but actually to a beneficial activity for the cell3,15. Functional prion-like protein aggregates have been described in various organisms from bacteria to humans, and participate in diverse of functions including biofilm development, regulation of water surface tension, modulation of cytotoxicity, formation of spider webs, eggshell protection, promoting viral infection, modulation of melanin biosynthesis, regulation of memory formation, epigenetic factors and storage of peptide hormones3,15. It has been proposed that functional prion-like amyloid aggregates may promote disease-associated aggregates through a cross-seeding mechanism3,16. Indeed, a recent study reported that bacterial amyloids may play a role in α-syn aggregation17. This is interesting considering that several lines of evidence indicate that intestinal bacteria play a key role in the pathogenic cascade of both PD and AD18,19. Supporting the hypothesis that microorganisms play role in AD pathogenesis, HHV-1, Porphyromonas gingivalis, Candida spp., Escherichia coli, Chlamydophila pneumoniae, and Borrelia burgdorferi have been detected in the cerebrospinal fluid (CSF) and postmortem brains of individuals with AD20–25. Moreover, the cell wall components of C.pneumoniae, an obligate intracellular bacteria, and E. coli, which can act as a facultative intracellular parasite, have been found within neurons, suggesting that some of these bacteria could directly invade neurons26,27.
The mechanisms and factors responsible for initiating the protein misfolding process in AD remain poorly understood. Various molecules have been shown to bind Aβ and tau and be able to initiate and/or promote protein misfolding and aggregation28. Among them, nucleic acids (including DNA and RNA) have been shown to bind with relatively high affinity to various misfolded proteins and in some cases to promote or stabilize the aggregates29,30. Extracellular bacterial DNA induces prion aggregation within microbial biofilm matrices and plays the critical role of converting bacterial amyloid into highly ordered, aggregated cross-β structures enabling unique structural properties of microbial biofilms31,32. A recent report identified the association between bacterial extracellular DNA and the formation of heat-resistant protein fractions within different proteins – even those lacking prion domains33. We hypothesize that DNA can act as an efficient promoter for protein misfolding in AD pathogenesis. To test this hypothesis, we focused on tau, an intracellular protein that has been shown to bind tightly to DNA and RNA and protect nucleic acids from degradation34,35. Moreover, in AD brains nucleic acids have been detected attached to NFTs and intracellular inclusions primarily composed of tau36. Using an in vitro tau aggregation assay, we observed that DNA derived from different bacterial cells induced a robust promotion of tau misfolding and aggregation. The effect was eliminated by cleaving the DNA with DNAse I.
Results
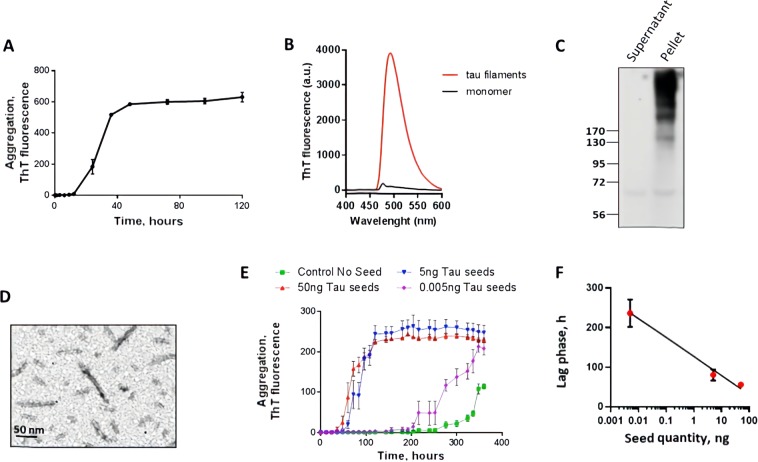
Tau misfolding and aggregation follows a seeding nucleation mechanism that can be modeled in vitro using purified recombinant tau protein incubated in the presence of heparin at physiological pH and temperature37,38. For our experiments, we used full-length human tau protein containing 4 microtubule binding repeats (4 R) and 2 N-terminal inserts (2 N). The 2N4R tau is the largest form of tau and the most active in promoting microtubule assembly12,13. In most tauopathies the 4R tau is the predominant form of the protein in the aggregates12,13. Incubation of tau at a concentration of 50 µM in 10 mM HEPES, pH 7.4 containing 100 mM NaCl and 12.5 µM heparin at 37 °C with constant agitation led to the formation of amyloid aggregates, detectable by the fluorescence emission of thioflavin T (ThT) (Fig. 1A). ThT is an amyloid-binding molecule, which emits fluorescence when bound to the aggregates and is widely used to characterize the kinetic of amyloid formation39. Under these conditions, tau form ThT-positive aggregates (Fig. 1A,B, Supplementary Fig. S1) with a lag phase of around 15 h (Fig. 1A). To measure the amount of aggregated tau, we centrifuged the samples and evaluated the tau signal in pellet and supernatant by western blot. As shown in Fig. 1C, most of the protein was recovered in the pellet and appears as a smear of high molecular weight bands. This result suggests that, under the conditions used the majority of tau was forming part of large aggregates. To use this aggregated material as seeds, we sonicated the preparation in order to generate seeding-competent short fibrils, as described in recent publications to produce tau pre-formed fibrils (tau-PFF)40,41. Analysis by transmission electron microscopy (TEM), revealed that this preparation contains short unbranched amyloid-like fibrils of different sizes and the expected width of ~10 nm (Fig. 1D). One of the typical biological activities of tau aggregates is their ability to seed aggregation of monomeric tau. To test this property, we incubated tau at a lower concentration (22 µM), with less heparin (4.4 µM) and lower temperature (20 °C) in order to slow down spontaneous aggregation and observe a clear seeding activity (Fig. 1E). Addition of different quantities of PFF tau aggregates (sonicated fibrils) accelerated tau aggregation in a concentration-dependent manner (Fig. 1E). Indeed, the lag phase was directly proportional to the logarithmic amount of tau aggregates added as seeds (Fig. 1F). The lag phase was measured as the time in which aggregation begins, which is defined as the moment when the ThT fluorescence reaches a threshold of 40 fluorescence units.
Figure 1.
Tau seeding aggregation assay. (A) Full-length Tau seeds were prepared by incubating tau monomer (50 µM) with 12.5 µM heparin in 10 mM HEPES pH 7.4, 100 mM NaCl for 5 days at 37 °C with shaking. Aggregation was monitored by ThT fluorescence. (B) Tau aggregates exhibit the typical ThT fluorescence spectrum with a maximum around 495 nm when excited at 435 nm. (C) The aggregation state was further confirmed by sedimentation followed by western blot, showing that the majority of tau appeared in the pellet in the form of large molecular weight bands. (D) the morphological characteristics of the tau aggregates were studied by transmission electron microscopy after negative staining with uranyl acetate. (E) The Tau aggregation assay was performed on 96 well plates using 22 µM Tau monomer, 4.4 µM heparin, 10 µM Thioflavin T, using cyclic agitation (1 min shaking at 500 rpm followed by 29 min without shaking). Aggregation was followed over time by ThT fluorescence using a plate spectrofluorometer (excitation: 435; emmision: 485). Graph show the mean and SD of three replicates. (F) Relationship between the quantity of tau oligomers and the Tau-PMCA signal (time to reach 50% aggregation).
Using this tau aggregation assay, we examined whether tau fibril formation could be accelerated in the presence of extracellular DNA from various species including bacteria, yeast and human. For the experiments, monomeric tau was incubated with preparations containing 100 ng of DNA extracted from different bacterial species including Pseudomonas aeruginosa (PA), Tetzosporium hominis (TH), Tetzerella alzheimeri (TA), Escherichia coli ATCC 25922 (EC25), Escherichia coli ATCC 472217 (EC47), Porphyromonas gingivalis (PG), Borrelia burgdorferi (BB). We also incubated tau with the same amount of DNA extracted from Candida albicans (CA) and human samples. The results showed that DNA from various (but not all) bacterial species significantly promoted tau aggregation (Fig. 2A). Conversely, addition of eukaryotic DNA, such as from yeast or human cells, had a much lower effect in promoting tau aggregation. To compare the magnitude of the effect of these different DNA extracts on the kinetic of aggregation, we measured the lag phase, defined as the time at which aggregation begins, which is experimentally determined as the time when ThT fluorescence reaches a value >40 fluorescent units (equivalent to ~2-folds the background levels after subtraction of the blank)42. Comparisons of the lag phases indicate that the largest promoting effect (shorter lag phase) was obtained in the presence of Tetzerella alzheimeri, Escherichia coli ATCC 25922, and Escherichia coli 472217 (Fig. 2B). Interestingly, Tetzerella alzheimeri (VT-16-1752 gen.nov, sp.nov) is a new species which was isolated from the oral cavity of a patient with AD. Moderate promoting effect was observed with Porphyromonas gingivalis and Borrelia burgdorferi, and no significant effect was detectable for Pseudomonas aeruginosa and Tetzosporium hominis43 (Fig. 2B).
Figure 2.
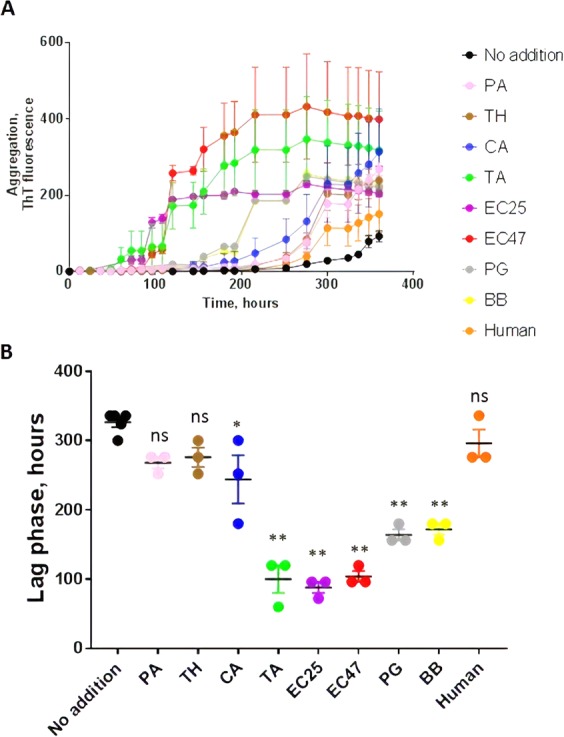
Effect of DNA extracted from diverse sources on tau aggregation. To study the effect of DNA on tau aggregation, monomeric tau (22 µM) under the conditions described in Fig. 1E, was incubated with preparations containing 100 ng of DNA extracted from different bacterial species including Pseudomonas aeruginosa (PA), Tetzosporium hominis (TH), Tetzerella alzheimeri (TA), Escherichia coli ATCC 25922 (EC25), Escherichia coli ATCC 472217 (EC47), Porphyromonas gingivalis (PG), Borrelia burgdorferi (BB). We also incubated tau with same amount of DNA extracted from Candida albicans (CA) and human samples. In all experiments the signal at time zero, corresponding to buffer + DNA + heparin + ThT + monomeric tau was substracted from the values. (A) tau aggregation was monitored over time by ThT fluorescence. Data corresponds to the average ± standard error of experiments done in triplicate (except for control without seeds that was performed in quintuplicate). (B) The lag phase, estimated as the time in which ThT fluorescence was higher than the threshold of 40 arbitrary units, was calculated for each experiment. The points represent the values obtained in each of the replicates. Data was analyzed by one-way ANOVA, followed by Tukey multiple comparison post-test. *P < 0.01; **P < 0.001.
We then aimed to confirm whether the promoting activity of bacterial DNA is dose dependent. We found that DNA of E. coli ATCC 25922 and P. gingivalis at concentrations of 1000 to 10 ng significantly accelerated Tau aggregation relative to controls. The promoting activity of E. coli ATCC 25922 (Fig. 3) and especially P. gingivalis (Fig. 4) was lower than that of tau seeds. A dose dependent effect was more clearly observed only for addition of P. gingivalis DNA, perhaps because of the higher efficiency of E. coli ATCC 25922, which may require lower concentrations to observe a dose-dependency.
Figure 3.
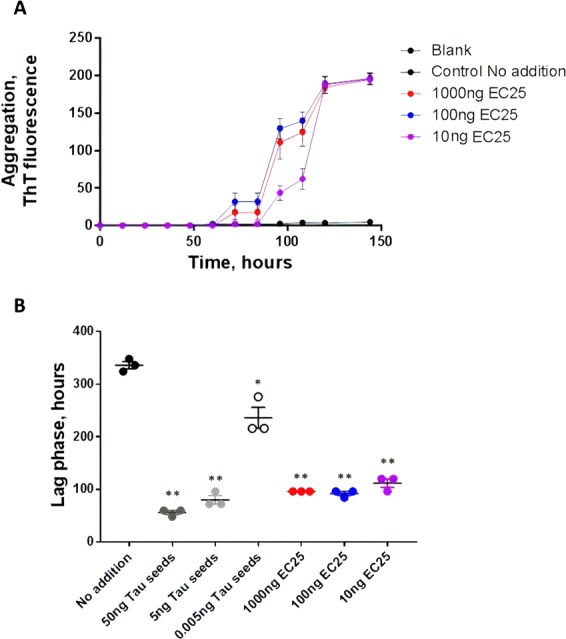
Influence of different concentration of E. coli ATCC 25922 DNA on tau aggregation. To study whether the promoting effect of E. coli DNA can be observed at different concentrations of DNA, we incubated monomeric tau under the conditions described above (Figs. 1E and 2) with 1000, 100 and 10 ng of DNA extracted from E. coli ATCC 25922. (A) tau aggregation was monitored overtime by ThT fluorescence. Data corresponds to the average ± standard error of experiments done in triplicate. (B) The lag phase, estimated as the time in which ThT fluorescence was higher than the threshold of 40 arbitrary units, was calculated for each experiment. The points represent the values obtained in each of the replicates. Data was analyzed by one-way ANOVA, followed by Tukey multiple comparison post-test. *P < 0.01; **P < 0.001.
Figure 4.
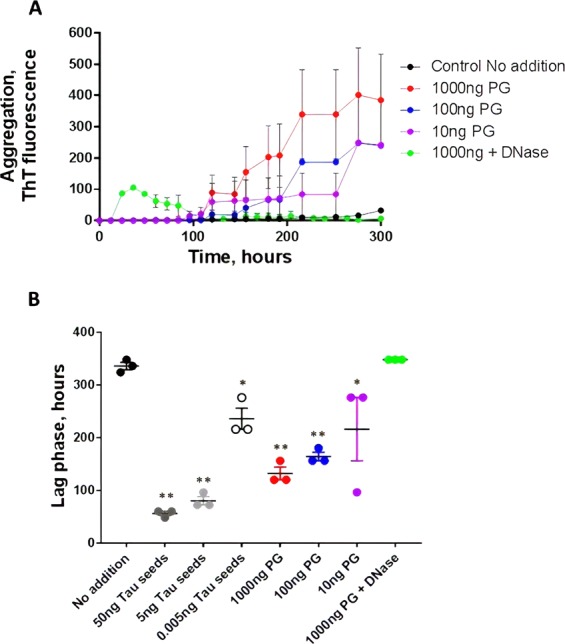
Dose-dependent effect of DNA from Porphyromonas gingivalis on tau aggregation. Monomeric tau was incubated under the conditions described above (Figs. 1E and 2) with 1000, 100 and 10 ng of DNA extracted from P. gingivalis. Сontrol probes of 1000 ng of P. gingivalis DNA were treated with DNase I to remove DNA from the sample. (A) tau aggregation was monitored overtime by ThT fluorescence. Data corresponds to the average ± standard error of experiments done in triplicate. (B) The lag phase, estimated as the time in which ThT fluorescence was higher than the threshold of 40 arbitrary units, was calculated for each experiment. The points represent the values obtained in each of the replicates. Data was analyzed by one-way ANOVA, followed by Tukey multiple comparison post-test. *P < 0.05; **P < 0.001.
Notably treatment of P. gingivalis DNA samples with DNAse I (Fig. 4A, green line) led to a complete prevention of Tau aggregation, pointing out the specificity of the DNA effect on protein misfolding. This result strongly indicate that the promoting effect is due to DNA and not other small contaminant that might be present in the preparation.
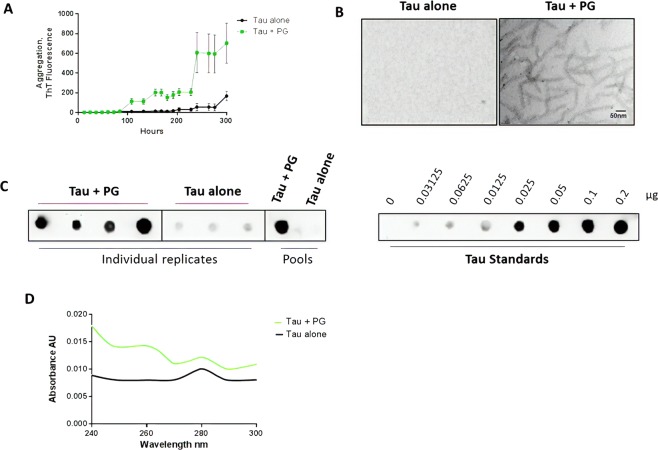
To confirm the effect of DNA on promoting tau misfolding and aggregation, we employed two additional methodologies to examine the presence and quantity of tau aggregates after incubation with DNA. For these studies we incubated monomeric tau with 1000 ng of P. gingivalis DNA and measured formation of amyloid-like aggregates by sedimentation assay and TEM, as well as by the ThT assay. The result of the ThT assay (Fig. 5A) was very similar as that described in Fig. 4A, indicating the reproducibility of the result. Aliquots were taken after 300 h of incubation and analyzed by TEM after negative staining. The study showed that in the presence of P. gingivalis DNA tau formed an abundant amount of ~10 nm amyloid-like fibrils as observed by TEM, whereas in the absence of DNA, no fibrils were observed (Fig. 5B). Tau aggregation was also monitored by a sedimentation assay employing centrifugation to separate soluble from aggregated tau and measuring the amount of protein in pellet and supernatant by a dot blot assay. The result indicate that after 300 hours of incubation in the presence of P. gingivalis a large amount of tau was found as aggregates in the pellet fraction, whereas in the absence of DNA, a small amount of the protein was aggregated (Fig. 5C, left panel). To measure the extent of aggregation in a more quantitative manner, we compared the dot blot signal with that obtained in the same membrane with different concentrations of recombinant monomeric tau (Fig. 5C, right panel). Considering the amount loaded in the blots and the dilution used, we estimated that >80% of tau protein was detectable in the pellet fraction (aggregated) for the experiment done in the presence of DNA, whereas only ~10% of tau incubated alone was aggregated. Finally, to analyze whether DNA was interacting with tau aggregates, we measured the UV absorbance of the pellet fraction. The data showed that in the tubes incubated with DNA, a peak at ~260 nm was observed, in addition to the protein peak at 280 nm, suggesting the presence of DNA in this fraction (Fig. 5D). Overall, these results fully support our experiments using ThT fluorescence, and strongly indicate that bacterial DNA promotes tau misfolding and aggregation.
Figure 5.
Effect of DNA from Porphyromonas gingivalis on tau aggregation measured by TEM and sedimentation assay. Monomeric tau was incubated under the conditions described above (Figs. 1E, 2 and 4) with 1000 ng of DNA extracted from P. gingivalis. (A) tau aggregation was monitored overtime by ThT fluorescence. Data corresponds to the average ± standard error of experiments done in triplicate. (B) Aliquots taken after 300 h of incubation were taken and loaded into TEM grids and stained with uranyl acetate, as indicated in methods. Scale bar corresponds to 50 nm. (C) The aggregation state was further confirmed by sedimentation followed by dot blot. The left panel shows the signal obtained in the pellet fraction after 300 h of incubation for individual wells or the pool of the samples in the presence and absence of DNA. For this experiment, the pellet was resuspended in 200 µl of the same buffer used for aggregation (10 mM Hepes pH 7.4, 100 mM NaCl) and 2 µl of a 8-fold dilution of this sample was loaded in the membrane. The right panel shows the dot blot signal of distinct concentrations of recombinant monomeric tau. (D) The UV spectra of solubilized pellet was measured between 240 and 300 nm for the pool of replicates incubated alone or in the presence of 1000 ng of PG DNA.
Analyses of the DNA sizes used in the study via electrophoresis showed no visible differences between DNA from different microorganisms consisting of bands with sizes ranging from 20 to 30 kb. The only difference was noticed in the human DNA, which, in addition to 20–30 kb bands, had larger fragments at the start (Fig. 6).
Figure 6.
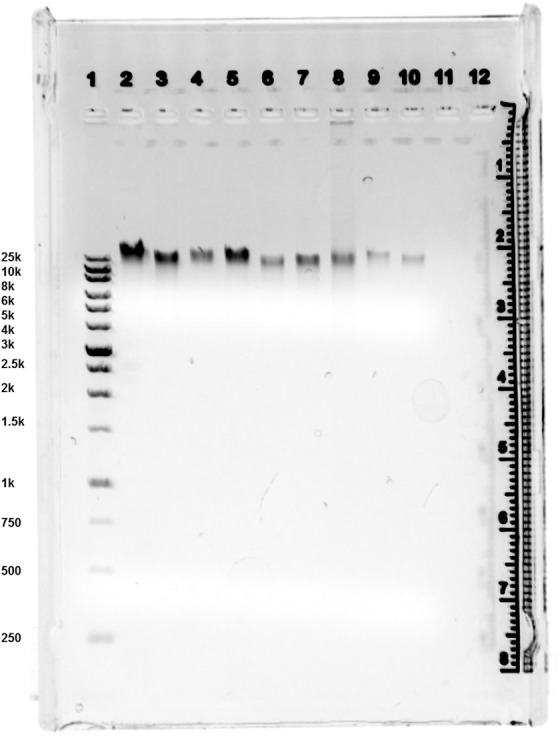
Agarose gel electrophoresis images of DNA. Lane 1, XL 1 kb Plus DNA Marker; Lane 2 DNA P. aeruginosa; Lane 3 DNA T. hominis; Lane 4 DNA T. alzheimeri; Lane 5 DNA C. albicans; Lane 6 DNA E. coli ATCC 472217; Lane 7 DNA E. coli ATCC 25922; Lane 8 Human DNA; Lane 9 DNA B. burgdorferi; Lane 10 DNA P. gingivalis.
Discussion
In addition to being one of the most devastating diseases of the 21st century, AD remains incurable. The cognitive symptoms and neurodegeneration appear to be mostly related to the extensive synaptic dysfunction and neuronal death observed in the brain8. In turn, neuronal loss and synaptic damage appears to be mediated by the progressive misfolding, aggregation and deposition of Aβ and tau proteins forming protein aggregates able to spread from cell-to-cell by a prion-like mechanism1,3,4. Genetics alone cannot account for the complex process of protein misfolding, aggregation and subsequent neurodegeneration observed in AD, particularly because the large majority of the cases are not associated to genetic mutations. Thus, it is likely that diverse environmental factors and age-related abnormalities play an important role on the initiation of the pathological abnormalities44,45. In this sense, various studies have shown that bacterial infection, as well as alterations in the intestinal microbiome may be implicated in the AD pathology18,46,47.
Here, we report the first evidence for the capacity of extracellular DNA from certain bacterial species to substantially promote tau misfolding and aggregation. The promoting effect of DNA on tau aggregation was observed in a wide range of concentrations from 10 to 1000 ng. The use of these concentrations were informed by the range of cerebrospinal fluid DNA concentrations observed in patients with different diseases: 1–600 ng/mL48–50. The sources of bacterial and fungal DNA were selected based on the literature and personal data that showed associations of certain microorganisms with AD. Among the bacteria previously cultivated directly from the brains of patients with AD, or those whose components (such as nucleic acids, lipopolysaccharides, enzymes) were identified in the CSF, amyloid plaques, or brains of patients with AD, we used the DNA from B. burgdorferi, P. gingivalis, C. albicans, and E. coli20,51–53.
Our data indicate that DNA from various, unrelated gram-positive and gram-negative bacteria significantly accelerated Tau aggregation. One of the best promoters was DNA from E. coli species, which is interesting for several reasons. First, Zhan et al. demonstrated that some strains of E. coli (such as K99) were detectable immunocytochemically in brain parenchyma and vessels in AD patients more frequently compared to control brains26. Second, E. coli and P. gingivalis are known to share properties of facultative intracellular parasites and be localized within hippocampal neurons51,54; the latter finding is significant, as the hippocampus is extensively damaged in AD55. The intracellular localization of E. coli introduces unique possibilities regarding the interaction of bacterial DNA with tau proteins inside the neuron; e.g. DNA can be secreted via transportation to the outer membrane or released following prophage induction and directly access the host neuron’s cytosol, where tau is normally present. Of note, in brains fo patients with AD, P. gingivalis is also localized intracellularly; therefore, as in the case of E. coli the same processes for the intracellular interaction of its DNA with tau are applicable for this microorganism51. While Tau is normally considered an intracellular protein, Tau aggregates are observed in the extracellular space as well56–58. As of today, the nature and role of these extracellular Tau aggregates remains unknown.
Multiple lines of evidence indicate that the oral microbiome is implicated in AD development23,24,46,58–60. Orally localized bacteria may gain access to the brain via multiple pathways. As a peripheral chronic infection, periodontitis can trigger the onset of proinflammatory signaling cascades, weakening the blood-brain barrier (BBB) and resulting in direct CNS colonization by bacteria46,60,61. Moreover, the neurotropism of spirochetal periopathogens enables their spread through cranial nerves and propagation along the olfactory and trigeminal tracts23,24,62. The present study has found that the DNA of P. gingivalis, a causative agent of chronic periodontitis that has recently been isolated from the brain of people afflicted with AD63 and is believed to be involved in the disease pathogenesis, triggers Tau misfolding. Moreover, the DNA of other oral bacteria were also found to trigger Tau aggregation: Tetzerella alzheimeri VT-16-1752 gen. nov., sp. nov., belonging to Brucellaceae that was isolated from the prediodontal pocket of a patient with AD (complete genome sequence has been deposited in GenBank under the accession no. RQUW00000000). Notably, the DNA of T. alzheimeri (together with that of E. coli) evinced the highest promoting activity relative to other bacteria. The reason for the differential effect of DNA from distinct species of bacteria on Tau misfolding is currently unclear. Analysis of the CG/AT ratio between species that affect tau aggregation compared with those that do not produce any effect showed no statistically significant differences. The analysis of the sizes of DNA molecules used in the study revealed no correlation between the sizes of DNA bands and different effects of DNA from different microorganisms on tau aggregation. Further studies need to be done to investigate the molecular mechanism by which DNA from some species of bacteria produce effect while others do not alter tau aggregation.The findings obtained in the present work indicate that DNA may play a previously overlooked role in the propagation of tau protein misfolding and AD pathogenesis, providing a new conceptual framework that positions the compromised blood brain and intestinal barriers as important sources of microbial DNA in the brain; indeed, altered gut permeability and disrupted BBB could precede AD development64. Moreover, we have recently described a clinical case showing improvement of cognition in an AD patient following therapy with deoxyribonuclease I enzyme, that cleaves cell-free DNA65,66.
Previous studies have shown that various other cellular component including RNA (but not DNA), lipids, heparin and other poly-anions promote protein misfolding and aggregation67,68. However, to the best of our knowledge, this is the first study to outline a universal seeding effect of DNA isolated from different microorganisms on Tau protein aggregation. We found that DNA from various species of bacteria, some of which were previously identified in the CSF and brains of patients with AD, can lead to tau protein misfolding and aggregation, suggesting their potential role in the initiation and progression of pathological abnormalities responsible for AD.
Future studies should further investigate the possible role of DNA as an initial seeding factor for protein misfolding using cellular and in vivo models as well as the effect of DNA on inducing misfolding of other proteins, including those associated with neurodegeneration, autoimmune diseases, and cancer. Moreover, subsequent studies should explore the targeting of DNA as a therapeutic strategy to prevent tau aggregation.
Materials and Methods
Sources and procedures for DNA extraction
Extracellular DNA was extracted from the matrix of P. aeruginosa ATCC 27853, E. coli ATCC 25922, Escherichia coli 472217, Porphyromonas gingivalis, Borrelia burgdorferi.
Tetzerella alzheimeri VT-16-1752, Tetzosporium hominis and Candida albicans.
All bacterial strains were subcultured from freezer stocks onto Columbia agar plates (Oxoid, UK) and incubated at 37 °C for 48 h, fungal strain was subcultured from freezer stocks onto Sabouraud dextrose agar (Oxoid, UK) and incubated at 30 °C for 48 h.
To extract the extracellular DNA, bacterial and fungal cells were separated from the matrix by centrifugation at 5000 g for 10 min at 4 °C. The supernatant was aspirated and filtered through a 0.2-μm-pore-size cellulose acetate filter (Millipore Corporation, USA). Extracellular DNA was extracted by using a DNeasy kit (Qiagen). Human genomic DNA (Roche Cat#11691112001) was purchased from Sigma (Sigma-Aldrich).
A part of the DNA probes were treated with 100 units of DNase I (Sigma- Aldrich, USA) for 20 minutes at 37 °C in order to degrade DNA in the probes.
Tau expression and purification
For these studies we used full-length Tau containing 4 microtubule-binding domains and 2 N-terminal inserts, with its 2 cysteines residues (C291, C322) replaced by serines38 to prevent formation of covalent dimers and aggregates. The plasmid encoding Tau40 was kindly provided by Dr. Martin Margittai. Expression and purification was done using previously described procedures38. Briefly, the plasmid was transformed into BL21 (DE3) Escherichia coli bacteria (New England BioLabs, catalog # C2527H) and grown overnight in 30 μg/ml kanamycin Terrific Broth (TB) at 37 °C with agitation. The culture was then diluted 1:20 and grown until the optical density 600 nm reached 0.6. One mM isopropropyl-β-D-thiogalactopyranoside (IPTG) was added to induce protein expression, and then the cultures were grown at 37 °C with agitation for 6 hours. Bacteria were collected by centrifugation at 3,000 × g and pellets stored frozen at −20 °C until lysis.
Pellets were thawed and resuspended in 20 mM PIPES pH 6.5, 500 mM NaCl, with protease inhibitor cocktail complete (Roche) and sonicated with a 1/2” probe (S-4000, Misonix), then heated at 95 °C for 20 minutes. Lysates were centrifuged at 15,000 × g for 20 min at 4 °C twice to remove cell debris. To precipitate proteins, ammonium sulfate (Sigma) was added at 55% w/v and incubated at room temperature for 1 hour with a magnetic stirrer. Precipitated protein was recovered by centrifugation at 15,000 × g at room temperature, and pellets were stored at −20 °C.
Tau protein was then purified by Cation Exchange Chromatography, pellets were dissolved in water (>18.2 MΩ cm) and then the solution was filtered through 0.2 µm filter. The sample was applied to a Hitrap SP HP column and eluted in a linear salt gradient (50–1000 mM NaCl, 20 mM PIPES pH 6.5). The content of tau protein in the fractions was followed by SDS-PAGE and Blue Coomasie staining. Fractions containing tau protein were pooled and dialysed overnight 1:100 in 10 mM HEPES buffer pH 7.4, 100 mM NaCl.
Tau protein was concentrated in Amicon centrifugal filters 10 kDa MWCO and finally filtered through 100 kDa Amicon filter (Millipore) to remove pre-formed aggregates, aliquoted and stored at −80 °C. Protein concentration was determined using BCA protein Assay (Thermo Scientific).
Preparation of tau seeds
To prepare aggregated tau for seeding experiments, monomeric aggregate-free tau was incubated at a concentration of 50 µM, containing 25 µM heparin (Average MW 18,000, Sigma) in 10 mM HEPES buffer pH 7.4, 100 mM NaCl for 5 days at 37 °C with constant shaking at 500 rpm in a thermomixer (Eppendorf). The formation of amyloid filaments was followed by Thioflavin T (ThT) fluorescence from samples taken from replicate tubes.
Tau filaments (2.3 mg/ml) were sonicated to prepare seeds (tau-PFF) by diluting the filaments to 0.1 mg/ml in 10 mM HEPES buffer pH 7.4, 100 mM NaCl and sonicated inside an Eppendorf tube in a floating rack using a microplate horn sonicator (S-400 Misonix) with settings of 30 seconds of total sonication time with pulses of 1 s ON − 1 s OFF at Amp 30.
Tau aggregation assay
Solutions of 22 µM aggregate-free tau40 in 100 mM HEPES pH 7.4, 100 mM NaCl (200 µl total volume) supplemented with 4.4 µM heparin were placed in opaque 96-wells plates and incubated alone or in the presence of tau-PFF used as seeds to trigger tau aggregation or in the presence of distinct concentrations of DNA. Samples were incubated in the presence of 10 μM Thioflavin T (ThT) and subjected to cyclic agitation (1 min at 500 rpm followed by 29 min without shaking) using an Eppendorf thermomixer, at a constant temperature of 20 °C. At various time points, ThT fluorescence was measured in the plates at 485 nm after excitation at 435 nm using a plate spectrofluorometer.
Transmission electron microscopy (TEM)
An aliquot of 10 µl of reaction sample was placed onto Formvar-coated 200-mesh copper grids for 5 min, washed at least three times with distilled water, and then negatively stained with 2% uranyl acetate for 1 min. Grids were examined by electron microscopy (H-7600, Hitachi, Japan) operated at an accelerating voltage of 80 kV.
Analysis of the amount of aggregated tau by sedimentation
To measure the quantity of tau remaining soluble and forming part of aggregates after incubation, we centrifuged samples at 100,000 × g for 1 h at 4 °C. The material in supernatant and pellet was analyzed by either western blot or dot blot. For western blots, samples were treated with NuPAGE LDS buffer at 100 °C for 10 min, and then samples were resolved by 12% Bis-Tris gels (Invitrogen). Proteins were electrophoretically transferred to nitrocellulose membranes (Amersham Biosciences, Germany). Membranes were blocked with 5% w/v nonfat dry milk in Tris-buffered saline-Tween 20 and developed with the anti-tau ab64193 antibody from Abcam. For dot blot, the pellet was resuspended on the same volume of the buffer used for aggregation. Aliquots corresponding to a 8-fold dilution of this sample were leaded onto nitrocellulose membranes (2 µl per sample) membrane together with different concetrations of recombinant full length monomeric tau. The membrane was blocked with 5% non-fat dry milk in PBS 0.1% Tween 20 for 1 hour, incubated at 4 °C overnight with the anti-tau antibody ab64193(Abcam) at 1:4,000 dilution, followed by anti-rabbit peroxidase-conjugated secondary antibody (1:5,000) for 1 hour. The membrane was developed with ECL detection reagents on a ChemiDoc imaging instrument (Bio-Rad).
DNA characterization with electrophoresis
Th sizes of DNA from different bacteria, fungi, and humans were analyzed with 1% agarose gel stained with ethidium bromide (Bio-Rad Laboratories, Hercules, CA, USA). Images were obtained using an Amersham Imager 680 (GE Healthcare, Buckinghamshire, UK).
Statistical analysis
The significance of the differences in aggregation kinetics in the presence of different samples was analyzed by one-way ANOVA, followed by the Tukey’s multiple comparison post-test. To compare the effect of different samples, we estimated the lag phase, which corresponds to the time in which aggregation begins, defined as the moment when the ThT fluorescence reaches a threshold of 40 fluorescence units (around 2x the blank signal) after subtraction of the blank containing the buffer and ThT but not tau. The level of significance was set at P < 0.05. Statistical tests were performed using the Graph Pad Prism 5.0 software.
Supplementary information
Author contributions
G.T. and V.T. designed experiments. M.P. and N.M. performed the experiments. S.P. and C.S. supervised the people performing the experiments. C.S., V.T. and G.T. supervised data analysis, analyzed data and wrote the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-59364-x.
References
- 1.Frost B, Diamond M. Prion-like mechanisms in neurodegenerative diseases. Nature Reviews Neuroscience. 2009;11:155–159. doi: 10.1038/nrn2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olanow CW, Prusiner S. Is Parkinson’s disease a prion disorder? Proceedings of the National Academy of Sciences. 2009;106:12571–2. doi: 10.1073/pnas.0906759106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soto C. Transmissible Proteins: Expanding the Prion Heresy. Cell. 2012;149:968–977. doi: 10.1016/j.cell.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jucker M, Walker L. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature. 2013;501:45–51. doi: 10.1038/nature12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stefani M, Dobson C. Protein aggregation and aggregate toxicity: new insights into protein folding, misfolding diseases and biological evolution. Journal of molecular medicine. 2003;81:678–99. doi: 10.1007/s00109-003-0464-5. [DOI] [PubMed] [Google Scholar]
- 6.Luk K, et al. Pathological -Synuclein Transmission Initiates Parkinson-like Neurodegeneration in Nontransgenic Mice. Science. 2012;338:949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morales R, Duran-Aniotz C, Castilla J, Estrada L, Soto C. De novo induction of amyloid-β deposition in vivo. Molecular Psychiatry. 2011;17:1347–1353. doi: 10.1038/mp.2011.120. [DOI] [PubMed] [Google Scholar]
- 8.Nelson P, et al. Correlation of Alzheimer Disease Neuropathologic Changes With Cognitive Status: A Review of the Literature. Journal of Neuropathology & Experimental Neurology. 2012;71:362–381. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giacobini E, Gold G. Alzheimer disease therapy—moving from amyloid-β to tau. Nature Reviews. Neurology. 2013;9:677–686. doi: 10.1038/nrneurol.2013.223. [DOI] [PubMed] [Google Scholar]
- 10.Cummings J, Lee G, Ritter A, Zhong K. Alzheimer’s disease drug development pipeline: 2018. Alzheimer’s & Dementia: Translational Research & Clinical Interventions. 2018;4:195–214. doi: 10.1016/j.trci.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong C, Iqbal K. Hyperphosphorylation of Microtubule-Associated Protein Tau: A Promising Therapeutic Target for Alzheimer Disease. Current Medicinal Chemistry. 2008;15:2321–2328. doi: 10.2174/092986708785909111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arendt T, Stieler J, Holzer M. Tau and tauopathies. Brain Research Bulletin. 2016;126:238–292. doi: 10.1016/j.brainresbull.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Buée L, Delacourte A. Comparative Biochemistry of Tau in Progressive Supranuclear Palsy, Corticobasal Degeneration, FTDP-17 and Pick’s Disease. Brain Pathology. 1999;9:681–693. doi: 10.1111/j.1750-3639.1999.tb00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghetti B, et al. Invited review: Frontotemporal dementia caused bymicrotubule-associated protein taugene (MAPT) mutations: a chameleon for neuropathology and neuroimaging. Neuropathology and Applied Neurobiology. 2015;41:24–46. doi: 10.1111/nan.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halfmann R, Alberti S, Lindquist S. Prions, protein homeostasis, and phenotypic diversity. Trends in Cell Biology. 2010;20:125–133. doi: 10.1016/j.tcb.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedland R, Chapman M. The role of microbial amyloid in neurodegeneration. PLOS Pathogens. 2017;13:e1006654. doi: 10.1371/journal.ppat.1006654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen, S. et al. Exposure to the Functional Bacterial Amyloid Protein Curli Enhances Alpha-Synuclein Aggregation in Aged Fischer 344 Rats and Caenorhabditis elegans. Scientific Reports6 (2016). [DOI] [PMC free article] [PubMed]
- 18.Bhattacharjee, S. & Lukiw, W. Alzheimer’s disease and the microbiome. Frontiers in Cellular Neuroscience7 (2013). [DOI] [PMC free article] [PubMed]
- 19.Tetz, G., Brown, S., Hao, Y. & Tetz, V. Parkinson’s disease and bacteriophages as its overlooked contributors. Scientific Reports8 (2018). [DOI] [PMC free article] [PubMed]
- 20.Miklossy J. Alzheimer’s disease - a neurospirochetosis. Analysis of the evidence following Koch’s and Hill’s criteria. Journal of Neuroinflammation. 2011;8:90. doi: 10.1186/1742-2094-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pisa, D., Alonso, R., Fernández-Fernández, A., Rábano, A. & Carrasco, L. Polymicrobial Infections In Brain Tissue From Alzheimer’s Disease Patients. Scientific Reports7 (2017). [DOI] [PMC free article] [PubMed]
- 22.Carter C. Genetic, Transcriptome, Proteomic, and Epidemiological Evidence for Blood-Brain Barrier Disruption and Polymicrobial Brain Invasion as Determinant Factors in Alzheimer’s Disease. Journal of Alzheimer’s Disease Reports. 2017;1:125–157. doi: 10.3233/ADR-170017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riviere G, Riviere K, Smith K. Molecular and immunological evidence of oral Treponema in the human brain and their association with Alzheimer’s disease. Oral Microbiology and Immunology. 2002;17:113–118. doi: 10.1046/j.0902-0055.2001.00100.x. [DOI] [PubMed] [Google Scholar]
- 24.Olsen I, Singhrao S. Can oral infection be a risk factor for Alzheimer’s disease? Journal of Oral Microbiology. 2015;7:29143. doi: 10.3402/jom.v7.29143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beffert U, Bertrand P, Champagne D, Gauthier S, Poirier J. HSV-1 in brain and risk of Alzheimer’s disease. The Lancet. 1998;351:1330–1331. doi: 10.1016/S0140-6736(05)79057-7. [DOI] [PubMed] [Google Scholar]
- 26.Zhan X, et al. Gram-negative bacterial molecules associate with Alzheimer disease pathology. Neurology. 2016;87:2324–2332. doi: 10.1212/WNL.0000000000003391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhan, X., Stamova, B. & Sharp, F. Lipopolysaccharide Associates with Amyloid Plaques, Neurons and Oligodendrocytes in Alzheimer’s Disease Brain: A Review. Frontiers in Aging Neuroscience10 (2018). [DOI] [PMC free article] [PubMed]
- 28.Zhang, X., Fu, Z., Meng, L., He, M. & Zhang, Z. The Early Events That Initiate β-Amyloid Aggregation in Alzheimer’s Disease. Frontiers in Aging Neuroscience10 (2018). [DOI] [PMC free article] [PubMed]
- 29.Cordeiro Y, Macedo B, Silva J, Gomes M. Pathological implications of nucleic acid interactions with proteins associated with neurodegenerative diseases. Biophysical Reviews. 2014;6:97–110. doi: 10.1007/s12551-013-0132-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silva J, Cordeiro Y. The “Jekyll and Hyde” Actions of Nucleic Acids on the Prion-like Aggregation of Proteins. Journal of Biological Chemistry. 2016;291:15482–15490. doi: 10.1074/jbc.R116.733428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz K, Ganesan M, Payne D, Solomon M, Boles B. Extracellular DNA facilitates the formation of functional amyloids in Staphylococcus aureus biofilms. Molecular Microbiology. 2015;99:123–134. doi: 10.1111/mmi.13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tetz G, Brown SM, Hao Y, Tetz V. Type 1 Diabetes: an Association Between Autoimmunity the Dynamics of Gut Amyloid-producing E. coli and Their Phages. Scientific reports. 2019;9:1–11. doi: 10.1038/s41598-018-37186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tetz V, Tetz G. Bacterial DNA induces the formation of heat-resistant disease-associated proteins in human plasma. Scientific Reports. 2019;9:1–10. doi: 10.1038/s41598-018-37186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Violet, M. et al. A major role for Tau in neuronal DNA and RNA protection in vivo under physiological and hyperthermic conditions. Frontiers in Cellular Neuroscience8 (2014). [DOI] [PMC free article] [PubMed]
- 35.Camero S, et al. Tau Protein Provides DNA with Thermodynamic and Structural Features which are Similar to those Found in Histone-DNA Complex. Journal of Alzheimer’s Disease. 2014;39:649–660. doi: 10.3233/JAD-131415. [DOI] [PubMed] [Google Scholar]
- 36.Berger J, Müller C. Protein–nucleic acid interactions: some assembly required. Current Opinion in Structural Biology. 2007;17:77–79. doi: 10.1016/j.sbi.2007.01.010. [DOI] [Google Scholar]
- 37.Crespo R, Koudstaal W, Apetri A. In Vitro Assay for Studying the Aggregation of Tau Protein and Drug Screening. JoVE. 2018;20:e58570. doi: 10.3791/58570. [DOI] [PubMed] [Google Scholar]
- 38.Meyer V, Dinkel P, Rickman Hager E, Margittai M. Amplification of Tau Fibrils from Minute Quantities of Seeds. Biochemistry. 2014;53:5804–5809. doi: 10.1021/bi501050g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hudson S, Ecroyd H, Kee T, Carver J. The thioflavin T fluorescence assay for amyloid fibril detection can be biased by the presence of exogenous compounds. FEBS Journal. 2009;276:5960–5972. doi: 10.1111/j.1742-4658.2009.07307.x. [DOI] [PubMed] [Google Scholar]
- 40.Guo J, Lee V. Seeding of Normal Tau by Pathological Tau Conformers Drives Pathogenesis of Alzheimer-like Tangles. Journal of Biological Chemistry. 2011;286:15317–15331. doi: 10.1074/jbc.M110.209296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iba M, et al. Synthetic Tau Fibrils Mediate Transmission of Neurofibrillary Tangles in a Transgenic Mouse Model of Alzheimer’s-Like Tauopathy. Journal of Neuroscience. 2013;33:1024–1037. doi: 10.1523/JNEUROSCI.2642-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salvadores N, Shahnawaz M, Scarpini E, Tagliavini F, Soto C. Detection of Misfolded Aβ Oligomers for Sensitive Biochemical Diagnosis of Alzheimer’s Disease. Cell Reports. 2014;7:261–268. doi: 10.1016/j.celrep.2014.02.031. [DOI] [PubMed] [Google Scholar]
- 43.Tetz G, Tetz V. Draft Genome Sequence of Tetzosporium hominis VT-49 gen. nov., sp. nov., Isolated from the Dental Decay Plaque of a Patient with Periodontitis. Genome Announcements. 2018;6:e01541–17. doi: 10.1128/genomeA.01541-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yegambaram M, Manivannan BG, Beach T, Halden R. Role of environmental contaminants in the etiology of Alzheimer’s disease: a review. Current Alzheimer Research. 2015;1:116–46. doi: 10.2174/1567205012666150204121719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grant W, Campbell A, Itzhaki R, Savory J. The significance of environmental factors in the etiology of Alzheimer’s disease. Journal of Alzheimer’s Disease. 2002;4:179–189. doi: 10.3233/JAD-2002-4308. [DOI] [PubMed] [Google Scholar]
- 46.Shoemark D, Allen S. The Microbiome and Disease: Reviewing the Links between the Oral Microbiome, Aging, and Alzheimer’s Disease. Journal of Alzheimer’s Disease. 2014;43:725–738. doi: 10.3233/JAD-141170. [DOI] [PubMed] [Google Scholar]
- 47.Singhrao S, Harding A, Poole S, Kesavalu L, Crean S. Porphyromonas gingivalisPeriodontal Infection and Its Putative Links with Alzheimer’s Disease. Mediators of Inflammation. 2015;2015:1–10. doi: 10.1155/2015/137357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Connolly I, et al. A pilot study on the use of cerebrospinal fluid cell-free DNA in intramedullary spinal ependymoma. Journal of Neuro-Oncology. 2017;135:29–36. doi: 10.1007/s11060-017-2557-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glebova K, Konorova I, Poleshchuk V, Baidakova G, Veiko N. Properties of Extracellular DNA from the Cerebrospinal Fluid and Blood Plasma during Parkinson’s Disease. Bulletin of Experimental Biology and Medicine. 2014;156:826–828. doi: 10.1007/s10517-014-2461-9. [DOI] [PubMed] [Google Scholar]
- 50.Bennett, J., Keeney, P. & Brohawn, D. RNA Sequencing Reveals Small and Variable Contributions of Infectious Agents to Transcriptomes of Postmortem Nervous Tissues From Amyotrophic Lateral Sclerosis, Alzheimer’s Disease and Parkinson’s Disease Subjects, and Increased Expression of Genes From Disease-Activated Microglia. Frontiers in Neuroscience13 (2019). [DOI] [PMC free article] [PubMed]
- 51.Dominy S, et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Science Advances. 2019;5:eaau3333. doi: 10.1126/sciadv.aau3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alonso R, Pisa D, Aguado B, Carrasco L. Identification of Fungal Species in Brain Tissue from Alzheimer’s Disease by Next-Generation Sequencing. Journal of Alzheimer’s Disease. 2017;58:55–67. doi: 10.3233/JAD-170058. [DOI] [PubMed] [Google Scholar]
- 53.Zhan, X., Stamova, B. & Sharp, F. Lipopolysaccharide Associates with Amyloid Plaques, Neurons and Oligodendrocytes in Alzheimer’s Disease Brain: A Review. Frontiers in Aging Neuroscience10 (2018). [DOI] [PMC free article] [PubMed]
- 54.Zhao W, Liu D, Chen Y. Escherichia coli hijack Caspr1 receptor to invade cerebral vascular and neuronal hosts. Microbial Cell. 2018;5:418–420. doi: 10.15698/mic2018.09.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ball M, et al. A new definition of alzheimer’s disease: a hippocampal dementia. The Lancet. 1985;325:14–16. doi: 10.1016/S0140-6736(85)90965-1. [DOI] [PubMed] [Google Scholar]
- 56.Frost B, Jacks RL, Diamond MI. Propagation of tau misfolding from the outside to the inside of a cell. Journal of Biological Chemistry. 2009;284:12845–12852. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Medina M, Avila J. The role of extracellular Tau in the spreading of neurofibrillary pathology. Frontiers in cellular neuroscience. 2014;8:113. doi: 10.3389/fncel.2014.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holmes BB, Diamond MI. Prion-like properties of Tau protein: the importance of extracellular Tau as a therapeutic target. Journal of Biological Chemistry. 2014;289:19855–19861. doi: 10.1074/jbc.R114.549295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Olsen I, Singhrao S. Can oral infection be a risk factor for Alzheimer’s disease? Journal of Oral Microbiology. 2015;7:29143. doi: 10.3402/jom.v7.29143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aguayo S, Schuh C, Vicente B, Aguayo L. Association between Alzheimer’s Disease and Oral and Gut Microbiota: Are Pore Forming Proteins the Missing Link? Journal of Alzheimer’s Disease. 2018;65:29–46. doi: 10.3233/JAD-180319. [DOI] [PubMed] [Google Scholar]
- 61.Sheets S, Potempa J, Travis J, Casiano C, Fletcher H. Gingipains from Porphyromonas gingivalis W83 Induce Cell Adhesion Molecule Cleavage and Apoptosis in Endothelial Cells. Infection and Immunity. 2005;73:1543–1552. doi: 10.1128/IAI.73.3.1543-1552.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramya V, Bhuvaneshwarri B, Paddmanabhan P, Manisundar N. Alziemer’s Disease and Periodontal Disease Bidirectional Interrelationships. Biosciences Biotechnology Research Asia. 2014;11:259–261. doi: 10.13005/bbra/1264. [DOI] [Google Scholar]
- 63.Poole S, Singhrao S, Kesavalu L, Curtis M, Crean S. Determining the Presence of Periodontopathic Virulence Factors in Short-Term Postmortem Alzheimer’s Disease Brain Tissue. Journal of Alzheimer’s Disease. 2013;36:665–677. doi: 10.3233/JAD-121918. [DOI] [PubMed] [Google Scholar]
- 64.Carter C. Genetic, Transcriptome, Proteomic, and Epidemiological Evidence for Blood-Brain Barrier Disruption and Polymicrobial Brain Invasion as Determinant Factors in Alzheimer’s Disease. Journal of Alzheimer’s Disease Reports. 2017;1:125–157. doi: 10.3233/ADR-170017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tetz, V. & Tetz, G. Effect of deoxyribonuclease I treatment for dementia in end-stage Alzheimer’s disease: a case report. Journal of Medical Case Reports10 (2016). [DOI] [PMC free article] [PubMed]
- 66.Smalheiser N. Mining Clinical Case Reports to Identify New Lines of Investigation in Alzheimer’s Disease: The Curious Case of DNase I. Journal of Alzheimer’s Disease Reports. 2019;3:71–76. doi: 10.3233/ADR-190100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mandelkow EM, Mandelkow E. Biochemistry and Cell Biology of Tau Protein in Neurofibrillary Degeneration. Cold Spring Harb Perspect Med. 2012;2:a006247. doi: 10.1101/cshperspect.a006247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kampers T, Friedhoff P, Biernat J, Mandelkow EM, Mandelkow E. RNA stimulates aggregation of microtubule-associated protein tau into Alzheimer-like paired helical filaments. FEBS Lett. 1996;399:344–349. doi: 10.1016/S0014-5793(96)01386-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.