Abstract
Computed tomography (CT) assessment of the cross-sectional area of the erector spinae muscles (ESMCSA) can be used to evaluate sarcopenia and cachexia in patients with lung diseases. This study aimed to confirm whether serial changes in ESMCSA are associated with survival in patients with idiopathic pulmonary fibrosis (IPF). Data from consecutive patients with IPF who were referred to a single centre were retrospectively reviewed. We measured the ESMCSA at the level of the 12th thoracic vertebra on CT images at referral and 6 months later (n = 119). The follow-up time was from 817–1633 days (median, 1335 days) and 59 patients (49.6%) died. A univariate Cox regression analysis showed that the decline in % predicted forced vital capacity (FVC) (Hazard ratios [HR] 1.041, 95% confidence interval [CI] 1.013–1.069, P = 0.004), the decline in body mass index (BMI) (HR 1.084, 95% CI 1.037–1.128; P < 0.001) and that in ESMCSA (HR 1.057, 95% CI 1.027–1.086; P < 0.001) were prognostic factors. For multivariate analyses, the decline in ESMCSA (HR 1.039, 95% CI 1.007–1.071, P = 0.015) was a significant prognostic factor, while those in % FVC and BMI were discarded. Early decrease in ESMCSA may be a useful predictor of prognosis in patients with IPF.
Subject terms: Respiratory tract diseases, Medical research
Introduction
Idiopathic pulmonary fibrosis (IPF) is a fibrotic pulmonary disease which leads to the death of most patients1,2. The prognosis for IPF patients is poor at 3 to 4 years1–4. The disease is characterised by decreased lung volumes and reduced gas exchange, and it is associated with symptoms of progressive dyspnoea, cough and reduced exercise capacity. Several prognostic factors for IPF are known, including dyspnoea score, pulmonary function, oxygen desaturation during exercise, and fibrotic changes on high resolution computed tomography (HRCT)5. Other prognostic factors have been reported, such as pathological findings6,7, serum biomarkers8,9, St. George’s Respiratory Questionnaire score10, fibrosis score11, normal lung volume on HRCT12,13 and mean pulmonary arterial pressure14. Among pulmonary function variables, a decline in forced vital capacity (FVC) is widely known as a prognostic factor15,16.
Cachexia is a complex metabolic syndrome. It is associated with underlying diseases and is characterised by loss of muscle with or without loss of fat mass in cancer and chronic diseases such as congestive heart failure, chronic kidney disease and chronic obstructive pulmonary disease (COPD)17,18. Loss of skeletal muscle in cancer patients can potentially be due to anorexia and early satiety, reduced muscle protein synthesis, and/or increased muscle protein breakdown19. Sarcopenia is a syndrome characterised by a progressive and generalised loss of skeletal muscle mass and strength, and it carries a risk of poor outcomes such as physical disability, poor quality of life and death20.
Assessment of the cross-sectional area of the erector spinae muscles (ESMCSA) from chest computed tomography (CT) scans has been used to evaluate sarcopenia and cachexia in patients with chronic lung disease21,22. Compared with healthy individuals, ESMCSA is decreased in patients with IPF, idiopathic pleuroparenchymal fibroelastosis (iPPEE) and chronic obstructive pulmonary disease (COPD)21,22. ESMCSA assessed by chest CT is an independent prognostic factor for patients with COPD21. Miller et al. reported that height-adjusted ESMCSA was significantly associated with 30-day mortality and length of hospital stay after lobectomy in patients with lung cancer23. Suzuki et al. also revealed that a smaller ESMCSA in patients with IPF was associated with a poor prognosis22.
We queried whether the survival of patients with IPF decreases with a decrease in muscle mass. Resultantly, we hypothesised that a relative decline in ESMCSA over the course of 6 months is associated with decreased survival in patients with IPF. We measured the ESMCSA at the level of the spinous process of 12th thoracic vertebra on CT images at baseline (n = 144) and 6 months later (n = 119) in patients with IPF.
Results
Patient characteristics and ESMCSA
The clinical characteristics and ESMCSA of 119 patients who underwent HRCT after 6 months are shown in Table 1. The baseline ESMCSA was 34.2 [27.7–40.0] cm2 whereas ESMCSA after 6 months was 31.6 [25.0–37.0] cm2.
Table 1.
Characteristics and ESMCSA of patients available for CT after 6 months.
| Variable | 0 month | After 6 months |
|---|---|---|
| Total, n | 119 | 119 |
| Age, years | 67.0 [61.0–71.0] | |
| Sex, Female, n (%) | 21 (17.6%) | |
| Never smoker, n (%) | 25 (21.0%) | |
| Ex-smoker, n (%) | 81 (68.1%) | |
| Current smoker, n (%) | 13 (10.9%) | |
| Smoking history, pack-years | 36.0 [5.0–55.5] | |
| Body mass index, kg/m2 | 23.4 [21.7–25.2] | 23.1 [21.4–25.4] |
| Biopsy-proven IPF, n (%) | 62 (52.1%) | |
| FVC, % predicted | 84.2 [70.4–96.5] | 78.1 [67.5–96.3] |
| FEV1/FVC, % | 85.8 [81.3–90.6] | 86.2 [79.7–90.7] |
| DLCO, % predicted* | 60.7 [48.8–76.7] | 58.6 [45.3–69.6] |
| Distance walked during 6MWT, m | 589 [524–645] | |
| Lowest SpO2 during 6MWT, % | 85.0 [80.0–89.0] | |
| ESMCSA, cm2 | 34.2 [27.7–40.0] | 31.6 [25.0–37.0] |
Data are presented as median [interquartile range] or n (%). Abbreviations: FVC, forced vital capacity; FEV1, forced expiratory volume in 1.0 second; DLCO, diffuse capacity of the lung for carbon monoxide; 6MWT, 6-minute walk test; SpO2, percutaneous oxygen saturation; ESMCSA, cross-sectional area of elector spine muscles. *We analysed using n = 114, because 5 cases were missing.
The correlation of ESMCSA values in the 119 patients between trained individuals were as follows; r = 0.951(95% confidence intervals [CI] 0.913–0.965), P < 0.001. The Bland-Altman analysis revealed that the agreement between two individuals was excellent (0.934).
The clinical characteristics and ESMCSA of 144 patients at baseline, 119 patients who underwent HRCT after 6 months, and 25 patients who did not undergo HRCT after 6 months are shown in Supplemental Table 1. We could not analyse CT in 25 patients after 6 months. Eleven of the 25 patients died; 5 were transferred; 3 were unchanged in pulmonary function test; 2 were out of timing; 1 was rejected; and 1 experienced acute exacerbation. There were significant differences in age (P = 0.001), body mass index (BMI) (P = 0.039), %FVC (P < 0.001), % deffuse capacity of the lung for carbon monoxide (DLCO) (P = 0.008), distance walked during the 6 minute walk test (6MWT) (P < 0.001), lowest SpO2 during the 6MWT (P = 0.046) and ESMCSA (P = 0.009) between patients who underwent HRCT after 6 months and those who did not.
The distributions of ESMCSA declines
The distribution of the relative decline in ESMCSA is shown in Fig. 1. We compared the relative decline in %FVC with that in ESMCSA to determine which was greater. We observed that the relative decline in ESMCSA (6.2 [0.5–11.8] %) was significantly greater than that in %FVC (1.4 [−3.1%–5.0] %) (Student’s t-test, P < 0.001).
Figure 1.
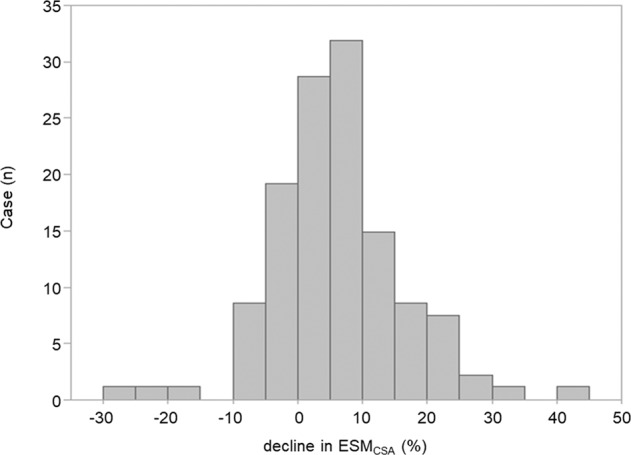
The distribution histogram of the decline in ESMCSA. The horizontal axis represents the relative decline in ESMCSA and the vertical axis represents the number of patients.
Correlations between the decline in ESMCSA and other clinical parameters
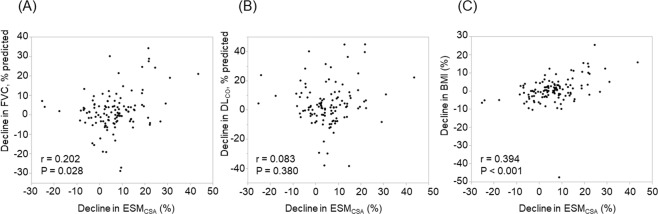
The correlations between the relative decline in ESMCSA and other clinical parameters were as follows (Table 2); baseline %FVC (r = −0.285 [−0.464–−0.089], P = 0.002), lowest SpO2 during 6MWT (r = −0.290 [−0.458– −0.108], P = 0.002), the relative decline in %FVC (r = 0.202 [0.022–0.358], P = 0.028), and the relative decline in BMI (r = 0.394 [0.217–0.552], P < 0.001). Figure 2 shows the correlations of relative decline in ESMCSA with the relative decline in %FVC, the relative decline in %DLCO, and the relative decline in BMI.
Table 2.
Correlations between the decline in the ESMCSAs and clinical parameters.
| Variables | r | 95%CI | P-value |
|---|---|---|---|
| Age, year | −0.044 | −0.244–0.156 | 0.637 |
| Baseline body mass index, kg/m2 | −0.175 | −0.357–0.020 | 0.057 |
| Baseline FVC, % predicted | −0.285 | −0.464–−0.089 | 0.002 |
| Baseline FEV1/FVC, % | 0.187 | −0.009–0.369 | 0.042 |
| Baseline DLCO, % predicted | −0.172 | −0.337–0.017 | 0.063 |
| Baseline distance walked during 6MWT, m | −0.171 | −0.341–0.019 | 0.064 |
| Baseline lowest SpO2 during 6MWT, % | −0.290 | −0.458–−0.101 | 0.002 |
| Relative decline in FVC, % | 0.202 | 0.022–0.358 | 0.028 |
| Relative decline in DLCO, %* | 0.083 | −0.096–0.267 | 0.377 |
| Relative decline in body mass index, % | 0.394 | 0.217–0.552 | <0.001 |
Abbreviations: ESMCSA;, cross-sectional areas of elector spine muscles; FVC, forced vital capacity; FEV1, forced expiratory volume in 1.0 second; DLCO, diffuse capacity of the lung for carbon monoxide; 6MWT, 6-minute walk test; SpO2, percutaneous oxygen saturation.
*We analysed using n = 114 because 5 cases were missing.
Figure 2.
The correlations of decline in ESMCSA with declines in FVC, DLCO and BMI. The correlations of relative decline in ESMCSA with relative declines in FVC (A), DLCO(B) and BMI(C) are shown.
The ESMCSA at baseline in 144 patients was 33.3 [25.9–39.1] cm2. The correlations between the ESMCSA at baseline and other clinical parameters are shown in Supplemental Table 2.
Prognostic survey
Fifty-nine of 119 patients (49.6%) died during the study period. The follow-up time of 119 patients was 1335 [817–1633] days. Four cases were lost to follow-up.
Uni- and multivariate Cox regression analyses
Hazard ratios (HRs) and 95% CIs in the cohort that was available for 6 months of follow-up (n = 119) on univariate and multivariate Cox regression analyses are shown in Table 3. The univariate Cox regression analysis showed that the relative decline in BMI (HR 1.084, 95% CI 1.037–1.128, P < 0.001), the relative decline in % FVC (HR 1.041, 95% CI 1.013–1.069, P = 0.004), and the relative decline in ESMCSA (HR 1.057, 95% CI 1.027–1.086, P < 0.001) were prognostic factors. On multivariate analyses, the relative decline in ESMCSA (HR 1.039, 95% CI 1.007–1.071, P = 0.015) was a significant prognostic factor, while the relative decline BMI (HR 1.036, 95% CI 0.986–1.088, P = 0.163) and the relative decline in %FVC (HR 1.021, 95% CI 0.992–1.050, P = 0.155) were not.
Table 3.
Prediction of mortality by uni- and multivariate Cox-proportion analyses in the patients available for CT after 6 months (n = 119).
| Predictor | HR | 95% CI | P-value |
|---|---|---|---|
| Univariate analysis | |||
| Age | 0.994 | 0.964–1.028 | 0.749 |
| Sex, female | 0.602 | 0.263–1.203 | 0.185 |
| Relative decline in body mass index, % | 1.084 | 1.037–1.128 | <0.001 |
| Relative decline in FVC, % predicted | 1.041 | 1.013–1.069 | 0.004 |
| Relative decline in DLCO, % predicted* | 1.013 | 0.991–1.034 | 0.248 |
| Relative decline in ESMCSA, % | 1.057 | 1.027–1.086 | <0.001 |
| Multivariate analysis | |||
| Relative decline in body mass index, % | 1.036 | 0.986–1.088 | 0.163 |
| Relative decline in FVC, % predicted | 1.021 | 0.992–1.050 | 0.155 |
| Relative decline in ESMCSA, % | 1.039 | 1.007–1.071 | 0.015 |
Abbreviations: HR, hazard ratio; CI, confidence interval; FVC, forced vital capacity; DLCO, diffuse capacity of the lung for carbon monoxide; ESMCSA, cross-sectional area of erector spinae muscles.
*We analysed using n = 114, because 5 cases were missing.
HRs and 95% CI for each parameter on uni- and multivariate Cox regression analyses in the baseline cohort (n = 144) are shown in Supplemental Table 3.
Kaplan–Meier curves, log-rank tests and number at risk
Kaplan–Meier curves of the cohort available for 6 months of follow-up (n =119) are shown in Fig. 3. To determine an optimal cutoff value for predicting 3-year mortality, we constructed receiver operator characteristic (ROC) analysis. A cutoff value of 10.5% (AUC = 0.734, specificity: 0.752, sensitivity: 0.553) was identified. The median survival times were as follows: the relative decline in ESMCSA ≥ 10.5%, 602 [480–1269] days; the relative decline in ESMCSA < 10.5%, 1431 [1130–1743] days. The relative decline in ESMCSA ≥ 10.5% had a significantly poorer prognosis (P < 0.001, log-rank test).
Figure 3.
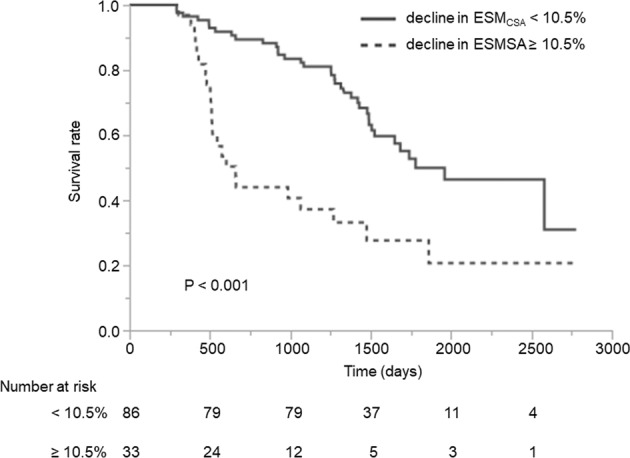
Kaplan–Meier curves and log-rank test. Kaplan–Meier survival curves stratified by the relative decline in ESMCSA at 6 months (n = 119). The cutoff value was set at 10.5%. The patients with a relative decline in ESMCSA more than 10.5% had significantly poorer survival (P < 0.001 by log-rank test).
Characteristics with IPF patients with or without ESMCSA decline
The clinical characteristics of patients with IPF with (≥10.5%) or without (<10.5%) ESMCSA decline are shown in Table 4. There were significant difference in baseline BMI (P = 0.049), baseline %FVC (P = 0.002), baseline forced expiratory volume in 1 second (FEV1)/FVC (P = 0.002), baseline lowest SpO2 during 6MWT (P = 0.004), ESMCSA after 6 months (P < 0.001), survival time (P < 0.001), the relative decline in ESMCSA (P < 0.001), the relative decline in %FVC (P = 0.003), the relative decline in %DLCO (P = 0.004) and the relative decline in BMI (P < 0.001). During the 6 months, there were 7 unexpected hospitalisations (3 acute exacerbations, 2 infections and 2 instances of worsening IPF). Pirfenidone was prescribed to 28 patients during the 6 months whereas nintedanib was prescribed to none (Table 4). The number of cancer and chronic heart failure comorbidities, as well as, the Charlson comorbidity index of the study population, are also shown in Table 4.
Table 4.
Characteristics with IPF patients with or without ESMCSA decline.
| Variable | With ESMCSA decline (≥10.5%) | Without ESMCSA decline (<10.5%) | P-value |
|---|---|---|---|
| Total, n | 33 | 86 | |
| Age, years | 65.0 [60.0–71.0] | 67.0 [62.0–70.8] | 0.744 |
| Sex, Female, n (%) | 6 (18.2%) | 15 (17.4%) | |
| Baseline body mass index, kg/m2 | 22.6 [21.5–24.1] | 24.0 [21.8–26.0] | 0.049 |
| Baseline FVC, % predicted, % | 73.0 [64.9–86.6] | 87.2 [72.7–99.7] | 0.002 |
| Baseline FEV1/ FVC, % | 89.7 [84.8–94.0] | 85.5 [79.1–89.2] | 0.002 |
| Baseline DLCO, % predicted, % | 60.0 [43.6–76.8] | 60.8 [49.4–76.5] | 0.690 |
| Baseline distance walked during 6MWT, m | 558 [482–627] | 593 [536–649] | 0.123 |
| Baseline lowest SpO2 during 6MWT, % | 80.0 [77.0–86.5] | 87.0 [82.0–90.0] | 0.004 |
| Baseline ESMCSA, cm2 | 34.2 [28.2–37.5] | 34.3 [27.3–40.7] | 0.495 |
| ESMCSA after 6 months, cm2 | 27.4 [23.5–31.5] | 33.6 [27.4–39.5] | <0.001 |
| Survival time, day | 602 [480–1269] | 1430 [1130–1743] | <0.001 |
| Death during observation period, n (%) | 23 (69.7%) | 36 (41.9%) | |
| Relative decline in ESMCSA, % | 15.7 [13.9–21.8] | 3.0 [−2.2–6.9] | <0.001 |
| Relative decline in %FVC, % | 4.0 [−1.5–12.8] | 0.3 [−3.5–4.1] | 0.003 |
| Relative decline in %DLCO, % | 6.4 [2.1–14.5] | 1.4 [−4.2–7.8] | 0.004 |
| Relative decline in body mass index, % | 3.2 [−1.0–10.5] | −0.1 [−3.3–2.2] | <0.001 |
| Corticosteroid treatment, n (%) | 5 (15.2%) | 6 (7.0%) | |
| Pirfenidone treatment, n (%) | 12 (35.3%) | 16 (18.6%) | |
| Hospitalisation during 6 months, n (%) | 4 (12.1%) | 3 (3.5%) | |
| Acute exacerbations, n (%) | 0 (0%) | 3 (3.5%) | |
| Infections, n (%) | 2 (6%) | 0 (0%) | |
| Worsening of IPF | 2 (6%) | 0 (0%) | |
| Charlson comorbidity index | 1.0 [1.0–1.0] | 1.0 [1.0–1.0] | |
| Chronic heart failure, n (%) | 2 (6%) | 2 (6%) | |
| Cancers, n (%) | 1 (3%) | 2 (6%) |
Data are presented as median [interquartile range] or n (%). Abbreviations: ESMCSA, cross-sectional area of elector spine muscles; FVC, forced vital capacity: FEV1, forced expiratory volume in 1.0 second; DLCO, diffuse capacity of the lung for carbon monoxide; 6MWT; 6-minute walk test, SpO2; percutaneous oxygen saturation, IPF, idiopathic pulmonary fibrosis. *We analysed using n = 114, because 5 cases were missing.
Decline in ESMCSA and effects of treatment with corticosteroids
No patients had been treated with corticosteroids at baseline, but 11 patients were treated with corticosteroids 6 months later. We compared the relative decline in ESMCSA between the patients treated with corticosteroids and those who were not. In the patients treated with corticosteroids, the relative decline in ESMCSA was 9.6 [3.5–16.8] % after 6 months. In the patients not treated with corticosteroids, the relative decline in ESMCSA was 6.0 [−0.2–10.6] % after 6 months. There was no statistical difference (P = 0.096) between the two groups (Wilcoxon rank-sum test).
Discussion
Baseline ESMCSA is a prognostic factor in patients with IPF22,24. Here, we demonstrated that the serial change in ESMCSA is also a prognostic factor in patients with IPF. On multivariate analysis, the decline in ESMCSA was a significant prognostic factor, while those of predicted %FVC and BMI were not.
It is widely known that the decline in FVC is a strong prognostic factor15,16. Measurements of serial changes in FVC are used as a gold standard in clinical trials25–28. Interestingly, in the present study, the decline in ESMCSA (HR 1.039, 95% CI 1.007–1.071, P = 0.015) was a significant prognostic factor in the multivariate analyses, while the decline in %FVC was not. This result may indicate that the decline in ESMCSA is a stronger prognostic factor than the decline in %FVC. The decline in ESMCSA was correlated with the decline in %FVC. However, the decline in ESMCSA (6.2 [0.5–11.8] %) was significantly greater than that in %FVC (1.4 [−3.1–5.0] %). It is possible that this result influences the data of multivariate Cox-proportion analysis. We did not evaluate the results of 6MWT because the missing data of 6 months later were not small. Further studies are needed to verify whether the decline in ESMCSA is useful for predicting mortality as compared with serial changes in the lowest SpO2 and the distance walked during the 6MWT.
Cachexia and sarcopenia have been extensively studied in patients with lung cancer and COPD. Evan’s diagnostic criteria for cachexia include evaluation items such as muscle mass, fatigue and weight loss. In an attempt to include a wider evaluation of the burden of cachexia, diagnostic criteria based on an assessment of laboratory data and symptoms in addition to weight have been proposed17. The criteria included weight loss ≥5% in 12 months or low BMI (<20 kg/m2) with 3 of 5 of the following features: decrease muscle strength, fatigue, anorexia, low fat-free mass index, of abnormal biochemistry (increased inflammatory markers, anaemia and low serum albumin). We reason that cachexia would be associated with the survival in IPF.
Kinsey et al. reported that a smaller pectoralis muscle area, as measured on CT images obtained at the time of diagnosis of non-small cell lung cancer, was associated with poorer overall survival29. McDonald et al. reported that patients with a smaller pectoralis muscle area on CT scan tended to have a more severe expiratory air flow obstruction, lower quality of life scores and diminished exercise capacity, as compared with people with a lower BMI30. The measurement of the psoas muscle at the L3 or L4 level on CT images is frequently used for evaluating sarcopenia31. Canvasser et al. reported a strong correlation between the paraspinous muscle area at Th12 and the total psoas area at L4 (r = 0.72, P < 0.001), and both were associated with lower mortality rates after surgery32. Those authors suggest that measuring the area of the erector spinae muscles at the Th12 level might be useful for evaluating skeletal muscles in patients with lung disease who have not undergone an abdominal CT. However, no data on the pectoralis muscles exist in patients with IPF presently. In patients with IPF, chest CT scans are regularly taken in clinical practice, along with chest X-rays and pulmonary function tests. Based on these and our results, measuring erector spinae muscles (ESMs) by chest CT images would be useful in evaluating sarcopenia and cachexia in patients with IPF.
Loss of skeletal muscle mass is considered to be induced by systemic inflammation, inactivity, malnutrition and enhanced energy expenditure17–20. In the present study, the decline in ESMCSA was weakly correlated with the decline in BMI (r = 0.394 [0.2170.552], P < 0.001), baseline lowest SpO2 during 6MWT (r = −0.290 [−0.458–−0.101], P = 0.002), baseline %FVC (r = −0.285 [−0.464–−0.089], P = 0.002) and the decline in %FVC (r = 0.202 [0.0220.358], P = 0.028). In a previous report, ESMCSA was weakly correlated with percentage of predicted FEV1 (r = 0.31, P < 0.0004) in patients with COPD21. Impaired pulmonary function, exercise-induced hypoxemia and nutrition status might influence the decline in ESMCSA. However, further studies are needed to understand the mechanisms underlying muscle loss in IPF.
Only 11 patients were treated with corticosteroids in this study, and there was no significant difference in the decline in ESMCSA. However, we cannot rule out the possibility that muscle atrophy due to the side effects of corticosteroids affected the ESMCSA. Further studies are needed to explore this possibility. Moreover, the influence of appetite loss side effects of anti-fibrotic drugs on the ESMCSA should be investigated in future studies.
Recently, several studies have reported the association between lung disease and ESMCSA. The ESMCSA was significantly associated with health-related quality of life and prognostic physiological parameters in patients with Mycobacterium avium complex lung disease33. A smaller ESMCSA was significantly associated with a lower level of activities of daily living at the end of treatment for pneumonia34.
The present study has the following limitations. First, in the study, the results were obtained by a retrospective analysis of all Japanese patients from a single centre. The sample size was small and there was no replication cohort. Further studies are required to confirm our observations in other external validation cohorts. Second, the diagnoses of IPF were according to the 2011 international guideline. The diagnosis of patients who initially presented before 2011 was confirmed by MDD according to the 2011 guidelines. In these patients, the diagnosis may be more accurate due to a consideration of the MDD time course. The limitation is that the MDD diagnosis before and after 2011 may not be the same. Third, 25 of 144 patients (17.4%) were not available for CT images to compare the changes at 6 months. Eleven patients died within 6 months, and CT images of the rest of the patients were not available for several reasons. Fourth, in the present study, approximately 16.7% (30 of 180 patients) of the CT of patients with IPF excluded the 12th thoracic spinous process level. This might be because the lungs of patients with IPF are smaller than those with COPD. This is another limitation of the present study. Fifth, we did not adjust for treatment in the survival analysis. The reason was that there was bias because a little evidence in anti-fibrotic drugs for IPF existed before 2014.
In conclusion, we have demonstrated that a smaller ESMCSA is a prognostic predictor in patients with IPF. A reduced ESMCSA after 6 months was an independent prognostic predictor in patients with IPF.
Methods
This single centre retrospective study was performed in accordance with the amended Declaration of Helsinki. The ethical review board of Tosei General Hospital, which contributed cases to this study, provided approval for the study (approval number 745). The study was also approved by the ethical review board of the Nagoya City University Hospital (approval number 60-18-0210), where the CT analyses were performed. Given that the data were analysed anonymously, the ethical review boards did not ask for the patients’ approval or informed consent. The opt-out document can be found on the website of Tosei General Hospital.
Patients
During the period of June 2008 to July 2013, the clinical records of 180 consecutive patients with IPF, referred to the Tosei General Hospital without corticosteroid or anti-fibrotic drug pre-treatments, were retrospectively reviewed. IPF was diagnosed by multidisciplinary discussion (MDD) according to 2011 international guidelines1. The diagnosis of patients who initially presented before 2011 was confirmed by MDD according to the 2011 guidelines before May 2015.
The following patients were excluded: one patient, who experienced acute exacerbations at the initial visit; patients who had other comorbidities, such as lung cancer (n = 1), infectious diseases or congested cardiac failure at the initial presentation; thirty patients, whose chest CT lacked the level of the spinous process of the 12th thoracic vertebrae and four patients, who underwent lung transplantation because due to the small number of patients and the consideration of death and lung transplantation as different outcomes.
Ultimately a total of 144 patients were enrolled. After approximately 6 months, Chest CT images were available for 119 patients. The interval between performing the CT at baseline and the second time was 190 [169–217] days.
Besides, the duration between the day of performing the chest CT on the first visit to the last visit or death was recorded. Other cases were contacted to confirm their life-or-death status by telephone.
Computed tomography
All patients underwent CT scan using a commercially available CT scanner (Aquilion, Toshiba Medical Systems, Tokyo, Japan) with a high-frequency algorithm. HRCT images were obtained without intravenous contrast and with the patient in the supine position at full inspiration. HRCT images with 0.5-mm-thick slices at 0.5-mm intervals were used for the analysis.
Derivation of ESMCSA by imaging analysis software
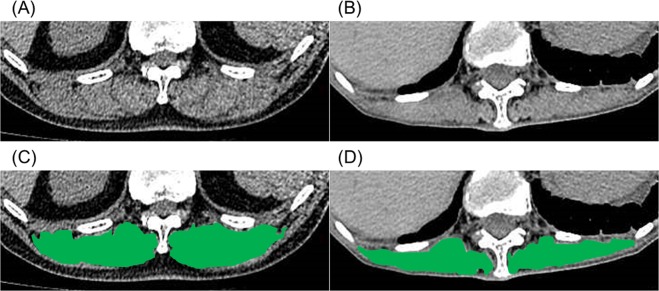
SYNAPSE VINCENT (Fujifilm Medical Systems, Tokyo, Japan) CT imaging analysis software was used for the derivation of ESMCSA. ESMCSA was calculated manually according to a previously published method21. Briefly, ESMCSA was measured on a single-slice axial CT image at the level of the spinous process of the 12th thoracic vertebra. For the quantitative analysis of the ESMs, chest HRCT images were reconstructed using the mediastinal window settings (window level, 40 HU; window width, 300 HU). The left and right ESMs were identified and manually shaded and the ESM area was reported as the sum of the right and left ESMs. All CT analyses were independently performed by trained individuals (ANa and HO) who were blinded to the patients’ clinical information. The average values of ESMCSA of ANa and HO were used in this study. Figure 4 shows the images of the ESMCSA in two patients with IPF. The correlation of the values of ESMCSA between trained individuals and the agreement of two individuals were analysed using the Spearman’s correlation test and the Bland-Altman analysis, respectively.
Figure 4.
The cross-sectional area of the erector spinae muscles. Representative computed tomographic images used to measure the cross-sectional area of the erector spinae muscles (A,B). The cross-sectional areas of the erector spinae muscles are in green (C,D). The sums of the areas of the erector spinae muscles were 55.8 cm2 (A,C), and 14.9 cm2 (B,D).
Pulmonary function tests and six-minute walk test
All patients underwent pulmonary function tests by spirometry (CHESTAC-55 V; Chest, Tokyo, Japan), according to the American Thoracic Society and European Respiratory Society (ATS/ERS) criteria35. The DLCO was also measured (CHESTAC-55 V). The values of FVC, FEV1 and DLCO were measured according to ATS/ERS recommendations36. We calculated %FEV1, %FVC and %DLCO based on the patients’ height, age and sex per the Japanese guidelines37. We then conducted 6MWTs without supplemental oxygen in accordance with ATS guidelines38.
The comorbidities and the Charlson comorbidity index
The Charlson comorbidity index was calculated according to a previously reported method39.
Statistical analyses
Continuous variables were presented as medians and interquartile ranges. Categorical variables were presented as numbers and percentages. The differences between patients who underwent HRCT after 6 months and those who did not were analysed using the Student’s t-test or the Wilcoxon rank-sum test. Spearman’s rank correlation coefficients were used to test for correlations between ESMCSA and clinical parameters and those between the relative decline in ESMCSA and clinical parameters. Univariate and multivariate Cox regression analyses were performed to evaluate the relationship between each variable and mortality. We included factors with P-values < 0.05 in the univariate analysis for the multivariate analyses. Survival times were estimated using the Kaplan–Meier method and compared with the log-rank test. To determine the optimal cutoff value for predicting 3-year mortality, we constructed ROC curves. P-values less than 0.05 were considered significant. The statistical analyses were conducted using JMP statistical software (version 14; SAS Institution Japan Ltd, Japan). Since we could not analyse the r (95%CI) with JMP statistical software (version 14), we analysed r (95%CI) using SPSS (version 26; IBM, Japan).
Supplementary information
Acknowledgements
The authors would like to thank Enago (www.enago.jp) for the English language review.
Author contributions
A.Na. and H.O. equally contributed to the study. A.Na. and H.O. drafted the submitted article and were responsible for the integrity of the data and the accuracy of the data analysis. Y.Ka. and A.Ni. contributed to the study design, and interpretation of the manuscript. H.T., Y.Ko., T.M., M.Y. and T.F. contributed to the study design, data accumulation and interpretation of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-59100-5.
References
- 1.Raghu G, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N. Engl. J. Med. 2018;378:1811–1823. doi: 10.1056/NEJMra1705751. [DOI] [PubMed] [Google Scholar]
- 3.Ley B, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann. Intern. Med. 2012;156:684–691. doi: 10.7326/0003-4819-156-10-201205150-00004. [DOI] [PubMed] [Google Scholar]
- 4.Natsuizaka M, et al. Epidemiologic survey of Japanese patients with idiopathic pulmonary fibrosis and investigation of ethnic differences. Am. J. Respir. Crit. Care Med. 2014;190:773–779. doi: 10.1164/rccm.201403-0566OC. [DOI] [PubMed] [Google Scholar]
- 5.Sumikawa H, et al. Computed tomography findings in pathological usual interstitial pneumonia: relationship to survival. Am. J. Respir. Crit. Care Med. 2008;177:433–439. doi: 10.1164/rccm.200611-1696OC. [DOI] [PubMed] [Google Scholar]
- 6.Enomoto N, et al. Amount of elastic fibers predicts prognosis of idiopathic pulmonary fibrosis. Respir. Med. 2013;107:1608–1616. doi: 10.1016/j.rmed.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Harada T, Watanabe K, Nabeshima K, Hamasaki M, Iwasaki H. Prognostic significance of fibroblastic foci in usual interstitial pneumonia and non-specific interstitial pneumonia. Respirology. 2013;18:278–283. doi: 10.1111/j.1440-1843.2012.02272.x. [DOI] [PubMed] [Google Scholar]
- 8.Tajiri M, et al. Serum level of periostin can predict long-term outcome of idiopathic pulmonary fibrosis. Respir. Investig. 2015;53:73–81. doi: 10.1016/j.resinv.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Song JW, et al. Blood biomarkers MMP-7 and SP-A: predictors of outcome in idiopathic pulmonary fibrosis. Chest. 2013;143:1422–1429. doi: 10.1378/chest.11-2735. [DOI] [PubMed] [Google Scholar]
- 10.Furukawa T, et al. The St. George’s Respiratory Questionnaire as a prognostic factor in IPF. Respir. Res. 2017;18:18. doi: 10.1186/s12931-017-0503-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oda K, et al. High-resolution CT scoring system-based grading scale predicts the clinical outcomes in patients with idiopathic pulmonary fibrosis. Respir Res. 2014;15:10. doi: 10.1186/1465-9921-15-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohkubo H, et al. A volumetric computed tomography analysis of the normal lung in idiopathic pulmonary fibrosis: The relationship with the survival. Intern. Med. 2018;57:929–937. doi: 10.2169/internalmedicine.9508-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohkubo H, Nakagawa H, Niimi A. Computer-based quantitative computed tomography image analysis in idiopathic pulmonary fibrosis: A mini review. Respir Investig. 2018;56:5–13. doi: 10.1016/j.resinv.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Teramachi R, et al. Progression of mean pulmonary arterial pressure in idiopathic pulmonary fibrosis with mild to moderate restriction. Respirology. 2017;22:986–990. doi: 10.1111/resp.12986. [DOI] [PubMed] [Google Scholar]
- 15.Zappala CJ, et al. Marginal decline in forced vital capacity is associated with a poor outcome in idiopathic pulmonary fibrosis. Eur. Respir. J. 2010;35:830–836. doi: 10.1183/09031936.00155108. [DOI] [PubMed] [Google Scholar]
- 16.Paterniti MO, et al. Acute exacerbation and decline in forced vital capacity are associated with increased mortality in idiopathic pulmonary fibrosis. Ann. Am. Thorac. Soc. 2017;9:1395–1402. doi: 10.1513/AnnalsATS.201606-458OC. [DOI] [PubMed] [Google Scholar]
- 17.Evans WJ, et al. Cachexia: a new definition. Clin. Nutr. 2008;27:793–799. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 18.Morley JE, Thomas DR, Wilson MM. Cachexia: pathophysiology and clinical relevance. Am. J. Clin. Nutr. 2006;83:735–743. doi: 10.1093/ajcn/83.4.735. [DOI] [PubMed] [Google Scholar]
- 19.Durham WJ, Dillon EL, Sheffield-Moore M. Inflammatory burden and amino acid metabolism cancer cachexia. Curr. Opin. Clin. Nutr. Metab. Care. 2009;12:72–77. doi: 10.1097/MCO.0b013e32831cef61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cruz-Jentoft AJ, et al. European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis. Report of the European Working Group on Sarcopenia in older people. Age Aging. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanimura K, et al. Quantitative assessment of erector spinae muscles in patients with chronic obstructive pulmonary disease. Novel chest computed tomography-derived index for prognosis. Ann. Am. Thorac. Soc. 2016;13:334–341. doi: 10.1513/AnnalsATS.201507-446OC. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki Y, et al. Distinct profile and prognostic impact of body composition changes in idiopathic pulmonary fibrosis and idiopathic pleuroparenchymal fibroelastosis. Sci. Rep. 2018;8:14074. doi: 10.1038/s41598-018-32478-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller JA, et al. Sarcopenia is a predictor of outcomes after lobectomy. J. Thorac. Dis. 2018;10:432–440. doi: 10.21037/jtd.2017.12.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moon SW, et al. Thoracic skeletal muscle quantification: low muscle mass is related with worse prognosis in idiopathic pulmonary fibrosis patients. Respir. Res. 2019;20:35. doi: 10.1186/s12931-019-1001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richeldi L, et al. INPULSIS Trial Investigators: Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 26.King TE, Jr., et al. ASCEND Study Group: A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 27.Lederer DJ, et al. Sensitivity Analyzes of the change in FVC in a Phase 3 Trial of Pirfenidone for idiopathic pulmonary fibrosis. Chest. 2018;148:196–201. doi: 10.1378/chest.14-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.du Bois RM, et al. Forced vital capacity in patients with idiopathic pulmonary fibrosis: test properties and minimal clinically important difference. Am. J. Respir. Crit. Care Med. 2011;184:1382–1389. doi: 10.1164/rccm.201105-0840OC. [DOI] [PubMed] [Google Scholar]
- 29.Kinsey CM, et al. Lower pectoralis muscle area is associated with worse overall survival in non-small cell lung cancer. Cancer Epidemiol. Biomarkers Prev. 2017;26:38–43. doi: 10.1158/1055-9965.EPI-15-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDonald ML, et al. Quantitative computed tomography measures of pectoralis muscle area and disease severity in chronic obstructive pulmonary disease. A cross-sectional study. Ann. Am. Thorac. Soc. 2014;11:326–334. doi: 10.1513/AnnalsATS.201307-229OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boutin RD, Yao L, Canter RJ, Lenchik L. Sarcopenia: Current concepts and imaging implications. AJR Am. J. Roentgenol. 2015;205:W255–266. doi: 10.2214/AJR.15.14635. [DOI] [PubMed] [Google Scholar]
- 32.Canvasser LD, et al. Paraspinous muscle as a predictor of surgical outcome. J. Surg. Res. 2014;192:76–81. doi: 10.1016/j.jss.2014.05.057. [DOI] [PubMed] [Google Scholar]
- 33.Asakura T, et al. Quantitative assessment of erector spinae muscles in patients with Mycobacterium avium complex lung disease. Respir. Med. 2018;145:66–72. doi: 10.1016/j.rmed.2018.10.023. [DOI] [PubMed] [Google Scholar]
- 34.Minegishi Y, et al. Smaller erector spinae muscle size is associated with inability to recover activities of daily living after pneumonia treatment. Respir. Investig. 2019;57:191–197. doi: 10.1016/j.resinv.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Laszlo G. Standardisation of lung function testing: helpful guidance from the ATS/ERS Task Force. Thorax. 2006;61:744–746. doi: 10.1136/thx.2006.061648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macintyre N, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720–735. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 37.Sasaki E, Nakamura M, Kida K. [Reference values for spirogram and blood gas analysis in Japanese non-smoking healthy adults] [in Japanese] Nihon Kokyuki Gakkai Zassi. 2001;39:S1–S17. [Google Scholar]
- 38.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 39.Quan H, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011;2173:676–82. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.