Abstract
Several behavioural tests have been developed to study and measure emotionally charged or emotionally neutral memories and how these may be affected by pharmacological, dietary or environmental manipulations. In this review, we describe the experimental paradigms used in preclinical studies to unravel the brain circuits involved in the recognition and memorization of environmentally salient stimuli devoid of strong emotional value. In particular, we focus on the modulatory role of the brain histaminergic system in the elaboration of recognition memory that is based on the judgement of the prior occurrence of an event, and it is believed to be a critical component of human declarative memory. The review also addresses questions that may help improve the treatment of impaired declarative memory described in several affective and neuropsychiatric disorders such as ADHD, Alzheimer's disease and major neurocognitive disorder.
Linked Articles
This article is part of a themed section on New Uses for 21st Century. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v177.3/issuetoc
Abbreviations
- ADHD
attention deficit hyperactivity syndrome
- GSK‐3β
glycogen synthase kinase‐3β
- APP
amyloid precursor protein gene
- DBB
dried bonito broth
- FSL
Flinders Sensitive Line
- HDC‐KO
histidine decarboxylase knockout
- nAChR
nicotinic ACh receptor
- TMN
tuberomamillary nucleus
- VGAT
vesicular GABA transporter
- VLPO
ventrolateral preoptic nucleus
Introduction
Despite the early diffidence in accepting the existence of a brain histaminergic system, its cardinal role in regulating several aspects of behaviour and arousal are now well established. The investigation of the histaminergic neuronal system lagged behind the exploration of other aminergic neurotransmitter systems, as the methods available were useful for characterizing brain catecholaminergic and serotonergic neurons, but were not suitable for visualizing histamine due to a strong interference by the ubiquitous spermidine (Hakanson et al., 1972). Thus, while disturbances of dopaminergic, noradrenergic and serotonergic systems were soon hypothesized to be implicated in affective states, the failure to demonstrate histamine localization in the brain greatly limited the understanding and acceptance of this neuronal system (Haas et al., 2008). However, in the early 1980s, two major discoveries convinced the scientific community of the existence of a central histaminergic system: the pharmacological identification of H3 autoreceptors (Arrang et al., 1983) and the direct evidence of histaminergic neurons after the development of antibodies against histamine (Panula et al., 1984) and histidine decarboxylase (Watanabe et al., 1984). The brain histaminergic system is well conserved throughout many species, from zebrafish (Sundvik and Panula, 2012) to humans (Panula et al., 1990), and in all species studied so far, histaminergic projections cover all encephalic areas although with different densities in different brain regions (Inagaki et al., 1988a; Panula et al., 1989).
Histamine is implicated in arousal, awakening and maintenance of wakefulness and has a pivotal role in the maintenance of high vigilance that is required for cognitive processes (Thakkar, 2011). Not surprisingly, current research is providing evidence that malfunctioning of the histaminergic system is associated with neuropathological disorders (reviewed in Shan et al., 2017). For instance, deficits in the histaminergic system are associated with neuropsychiatric disorders such as Gilles de la Tourette's syndrome (Ercan‐Sencicek et al., 2010; Karagiannidis et al., 2013). As proof of concept, the group of Pittenger found that histidine decarboxylase knockout (HDC‐KO) mice exhibit stereotypic locomotor behaviours that replicate the core phenomenology of Tourette's syndrome (Rapanelli et al., 2017). Although many questions remain about the involvement of this histaminergic system in autism spectrum disorder, recent studies have suggested a potential involvement of histamine in this pathology (Fernandez et al., 2012; Wright et al., 2017).
Four histamine receptors have been cloned (H1–H4), and the H1–3 receptors have been unequivocally found in distinctive patterns in the brain. The role of the H4 receptor in cognitive processes is still a matter of debate. The H3 receptor has been the focus of much research in the last 20 years as preclinical studies have demonstrated procognitive and awakening properties of H3 antagonists (reviewed in Passani and Blandina, 2011; Schlicker and Kathmann, 2017).
In this review, we summarize the main findings describing the role of the brain histaminergic system and its receptors in recognition memory. Experimental paradigms that are used in preclinical studies to unravel the brain circuits involved in the animal equivalent of declarative memory in humans will be covered in this review, as well as the few reported cases of brain histamine involvement in human studies.
The brain histaminergic system
We will briefly summarize the fundamental characteristics of the brain histaminergic system. For more detailed information, we refer the readers to other extensive reviews (Haas et al., 2008; Panula and Nuutinen, 2013; Schlicker and Kathmann, 2017).
In vertebrates, the source of brain histamine in physiological conditions is mainly attributed to neurones residing in the tuberomamillary nuclei (TMN) in the posterior hypothalamus and different species differ in their number of histaminergic neurons and density of innervation (Ekstrom et al., 1995; Eriksson et al., 1998; Panula et al., 1984; Takeda et al., 1984). In rodents, a detailed mapping of TMN provided a subdivision of histaminergic neurons in distinct subpopulations. In the rat brain, histaminergic neuronal somata are grouped within the TMN in five clusters, E1–E5, each of which sends overlapping projections throughout the neuroaxis (Moriwaki et al., 2015). A similar pattern of distribution has been reported in the brains of other mammals and non‐mammalian vertebrates (Sundvik and Panula, 2012; Wada et al., 1991).
Mast cells are another source of histamine in the brain of some species. For instance, in birds, immature mast cells infiltrate the CNS and undergo in situ differentiation within the neuropile (Zhuang et al., 1999), and massive mast cell migrations into the brain have been associated with courting behaviour in doves (Silverman et al., 1994). Also, brain mast cells and histamine release have been implicated in the modulation of anxiety‐like behaviour and learning (Nautiyal et al., 2008). Other possible sources of histamine in the brain may include microglia and microvascular endothelial cells (Katoh et al., 2001; Yamakami et al., 2000).
The main terminal areas of the histaminergic projections from the TMN are different in different species, but they cover essentially the whole CNS (Inagaki et al., 1988b; Panula et al., 1989). Retrograde tracing studies have shown that histaminergic neurons are a relatively uniform cell group, as they do not segregate according to projection areas (Inagaki et al., 1990; Köhler et al., 1985). However, functional differences exist among TMN neurones, suggesting that they do not belong to a homogeneous population but are organized in distinct functional subpopulations (Blandina et al., 2012). Both neuroanatomical and functional observations in our and others' laboratories point to this conclusion. By using the double‐probe microdialysis technique, we discriminated groups of histaminergic neurons impinging on different brain regions (Giannoni et al., 2009); furthermore, immunohistochemical staining with anti‐H3 receptor antibodies demonstrated that histaminergic neuronal populations differ significantly in the expression level of this receptor (Blandina et al., 2012). Recent chemo‐ and optogenetic studies have demonstrated the existence of separable behavioural effects as a consequence of TMN excitation of specific brain circuits (Fujita et al., 2017; Rapanelli et al., 2017). However, despite the accepted notion that histaminergic neurons may be functionally heterogeneous, the identification of specific subpopulations of histaminergic neurons that modulate the different phases of memorization has been elusive.
Histamine receptors
The basic homeostatic and higher functions, including cognition, regulated by brain histamine are due to the action of at least three metabotropic receptors: H1, H2 and H3 receptors, which are expressed at different densities in different brain areas. The H4 receptor has been detected in the brain, but its function is not quite clear yet (Schneider and Seifert, 2016). All metabotropic histamine receptors (H1–4) belong to the rhodopsin‐like family of GPCRs. The action of H1–2 receptors is usually excitatory (Panula et al., 2015). H1 receptors are found at particularly high density in brain regions concerned with arousal and nutritional state control. Brain‐penetrating antihistamines as well as antidepressant and antipsychotics that activate the H1 receptor cause sedative and metabolic side effects (Provensi et al., 2016a). H2 agonists potentiate hippocampal synaptic transmission and increase the firing of many types of neurons (Selbach et al., 1997). H3 receptors are both presynaptic autoreceptors that inhibit histamine synthesis and release from histaminergic neurons, and heteroreceptors mediating the release of other neurotransmitters (Panula et al., 2015). Therefore, by blocking H3 receptors, histamine release as well as the release of other neurotransmitters is augmented in brain regions crucial for maintenance of alertness and storage of information (Brown et al., 2001).
Histamine functions in the CNS
Histamine exerts several functions in the brain, and only some aspects that are relevant to cognition are briefly reviewed here. Histamine is the major wake‐promoting neuromodulator in the CNS and histaminergic neurons with their extensive networks sustain wake and arousal by modulating and interacting with different brain circuitries (Thakkar, 2011). The hypothesis that histaminergic neurons are involved in brain arousal is supported by several studies. Earlier works showed that H1 receptor‐KO mice have an impaired sleep–wake cycle, and in these animals, the wake‐promoting effect of H3 antagonists is abolished (Huang et al., 2006; Lin et al., 2002). Furthermore, c‐Fos activation was observed in the TMN during waking (Lin et al., 2000; Nelson et al., 2002, 2003; Scammell et al., 2000; Sherin et al., 1998). More recently, lesion studies demonstrated that inactivation of the TMN with the GABA agonist muscimol induces long‐lasting, non‐rapid eye movement sleep (Xie et al., 2017), and optogenetic activation of a subpopulation of TMN neurons induces wakefulness (Fujita et al., 2017). Histamine maintains wakefulness through direct projections of the TMN to the thalamus and the cortex and indirectly through activation of other, cholinergic (Khateb et al., 1990, 1995; Xu et al., 2004) and aminergic (Brown et al., 2001; Korotkova et al., 2002, 2005) ascending arousal systems. The activity of TMN neurons varies according to the state of wakefulness: it is low during quiet waking, moderate during active waking and highest during attentive waking (Takahashi et al., 2006). The transition between wakefulness and sleep and vice versa requires the regulation of antagonism between sleep‐promoting neurons in the ventrolateral preoptic nucleus (VLPO) that provide GABA‐ and galanin‐mediated inhibition of histaminergic neurons, and brainstem cholinergic and monoaminergic neurons (reviewed in Benarroch, 2010). Furthermore, histaminergic and orexinergic neurons cooperate in the hypothalamic control of the sleep–wake states exerting a distinct but complementary and synergistic control of wakefulness (Anaclet et al., 2009). Release of histamine from TMN neurons can disinhibit histaminergic neurons and suppress the activity of sleep‐active VLPO neurons to promote histaminergic neuronal firing and arousal (Williams et al., 2014). Cortical histamine release is also stimulated by an appetitive state most likely related to increased behavioural activation during active food searching (Riveros et al., 2015; Valdés et al., 2010). Histaminergic neurons express the glutamic acid decarboxylase (GAD) enzymes, GABA (Airaksinen et al., 1992; Kukko‐Lukjanov and Panula, 2003; Takeda et al., 1984; Trottier et al., 2002) and the vesicular GABA transporter (VGAT/VIAAT) (Yu et al., 2015). Recently, it was demonstrated that histamine and GABA co‐transmission in the neocortex and striatum are necessary for appropriate wakefulness. By ablating the VGAT gene on murine histaminergic neurons, Yu and colleagues observed an increase in general activity and sustained wakefulness. The intriguing proposal of the authors states that wake‐active TMN neurons ‘generate a paracrine GABAergic signal that serves to provide a brake on over‐activation, but could also increase the precision of neocortical processing’ (Yu et al., 2015). However, this is not a general feature of histaminergic neurons; in fact, TMN neurons are heterogeneous in this respect as well, as not all of them co‐transmit GABA. Optogenetic stimulation of histaminergic neuron projections to the hypothalamic preoptic nucleus produces only histamine release which in turn stimulates local GABAergic neurons to induce a net inhibitory effect (Williams et al., 2014). In addition to GABA, a subpopulation of histaminergic neurons co‐expresses thyrotropin releasing hormone (TRH) and galanin (Airaksinen et al., 1992; Chotard et al., 2002), but the physiological relevance is not clear. Other functions of histamine may affect learning and memory processes such as thermoregulation, energy expenditure and feeding (reviewed in Tabarean, 2016 and Provensi et al., 2016a). A novel role of histamine was described in the cerebellar nuclei, where histamine selectively depolarizes output projections and improves cerebellar nuclei‐mediated motor balance and coordination (Zhang et al., 2016) that may contribute to the exploratory locomotor activity induced by activation of H2 receptors (Mohsen et al., 2014).
Different types of memory and memory modulation by histamine
The term memory covers three important aspects of information processing: encoding, storage (consolidation) and recall or retrieval. Encoding is the acquisition of an engram, or specific information, and, therefore, entails learning. During consolidation, the engram is stored for variable periods of time as short‐term or long‐term memories; the former may last hours or days, the latter may last forever. Retrieval is the process of accessing the engram. In animal studies, retrieval is the only measurable behavioural expression of an acquired and stored memory. During retrieval, the engram may become labile and can be reconsolidated (Josselyn et al., 2015). As time passes, even the most consolidated memories may disappear, a process called forgetting (Davis and Zhong, 2017). All these related but dissociable events involve the elaboration of disparate learning situations requiring different degrees of activation in distinct brain region at different times (Passani et al., 2007). It is not surprising that such important and dynamic processes are modulated by several neurotransmitter systems including histamine and by disparate brain circuitries that become active at different time points during the many phases of memorization.
Emotionally charged events are often remembered more accurately and more vividly than events devoid of an emotional component. Furthermore, emotionally neutral situations usually lead to the creation of shorter lasting memories of the event (Reisberg and Heuer, 2005). Several behavioural tests have been developed to study and measure emotionally charged or emotionally neutral memories and how these may be affected by pharmacological, dietary or environmental manipulations. Emotionally charged memories will be dealt with in another chapter of this issue. Recognition memory is based on the judgement of the prior occurrence of an event, and it is believed to be a critical component of human declarative memory, a kind of memory we use to answer questions like ‘where?’, ‘who?’ and ‘when?’ (Winters et al., 2008). Although highly conserved among species, the expression of declarative memories varies greatly among different species (Paul et al., 2009). Declarative memory in humans, for example, is formulated through language and other explicit representations. Animals, on the other hand, cannot represent such knowledge verbally or symbolically, and different tests have been used as models of episodic memory tasks in rodents (reviewed in Fouquet et al., 2010). The most used tasks are the object recognition task and variations thereof (Ennaceur and Delacour, 1988; Leger et al., 2013). The comparability of these episodic memory tests in animals with human episodic memory tests is limited; however, it appears that similar brain regions do support this type of memory both in humans and animals (Allen and Fortin, 2013). Therefore, such preclinical tests are still widely used to address questions that may help improve the treatment of impaired declarative memory described in several affective and neuropsychiatric disorders such as attention‐deficit hyperactivity syndrome (ADHD), Alzheimer's disease and major neurocognitive disorders.
The novel object recognition (Figure 1A) and the novel spatial location tests (Figure 1B) rely on the motivational strength of novelty, as they are based on the natural tendency of rodents to search and explore novel objects or the new location where an object has been displaced. These procedures have become popular methods for studying emotionally neutral memories as they do not require punishments, food or water restriction, and several behavioural endpoints can be rapidly obtained, including general activity, reactivity to novelty, and learning (Blaser and Heyser, 2015). Experimental animals usually remember objects previously encountered in an open arena and their location and spend more time exploring new objects or their new location. Usually, this type of memory is labile and does not last for more than 6–12 h. However, long habituation sessions before the training may produce a longer lasting memory for the novel object that persists days after the test (da Silveira et al., 2013).
Figure 1.

Schematic representation of the novel object recognition and novel spatial location paradigm.
Another paradigm is based on social recognition, a fundamental behaviour to form and consolidate social groups; hence, it is important for reproduction, species survival and the establishment of dominance hierarchies. In addition to these forms of long‐term social recognition, rodents are also known to form transient, short‐term memories of recently encountered individuals (Thor and Holloway, 1982; Winslow and Insel, 2004). In rodents, social recognition memory is tested by using their instinctive tendency to investigate unfamiliar conspecifics with respect to familiar ones (van der Kooij and Sandi, 2012). Social recognition relies largely on odour recognition whereas other sensory inputs are considered much less important (Wacker and Ludwig, 2012). In the most used experimental design, the habituation/dishabituation paradigm (Figure 2A), an adult rodent is exposed to an unfamiliar, juvenile subject. The two animals engage in a series of social investigative behaviours to become acquainted with each other. During the subsequent encounter, the retention trial, the juvenile subject is ‘recognized’ and will be investigated for a shorter period of time. This decrease in social investigation upon repeated encounters can be interpreted as an index for social recognition. Usually, this is a form of short‐term memory with a limited duration (30 min to 2 h) in individually housed mice and rats (Burman and Mendl, 2000; Lemaire, 2003; Okuyama et al., 2016). Another version of the social recognition paradigm is the social discrimination test (Figure 2B; Engelmann et al., 1995) that shares the initial phase with the habituation/dishabituation paradigm. However, during the retention test, the familiar juvenile and a novel conspecific are simultaneously presented to the adult animal, and the time it spends exploring each conspecific is recorded. Again, if the animal recognizes the familiar juvenile, it will spend more time exploring the novel one.
Figure 2.
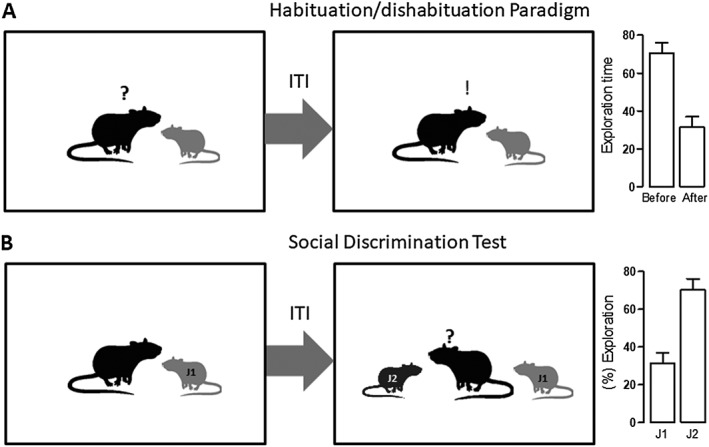
Schematic representation of two paradigms used to evaluate social recognition.
Histamine and social recognition memory
As previously mentioned, social memory refers to the ability to remember the identity of a conspecific, which is fundamental to the building of social relationships and survival. Neurotransmitters such as noradrenaline, dopamine and acetylcholine (Griffin and Taylor, 1995; Di Cara et al., 2007; Deiana et al., 2011) and hormones such as oxytocin (Raam et al., 2017; Lin et al., 2018) have been suggested to play key roles in social discrimination and memory. Early work by Philippu and colleagues showed that histamine is also involved in this type of memory, as an increased histamine concentration in the brain improved short‐term recognition memory, whereas depletion of neuronal histamine had an amnesic effect (Prast et al., 1996). Social recognition is disrupted by ageing as well; adult rats recognize a juvenile for long periods of time, whereas aged rats hardly retain the information for longer than 30 min (Markham and Juraska, 2007). The H3 antagonist ABT‐239 that does not significantly improve social memory in adult rats improved recall in aged rats to the extent that their performance was comparable to that of adult rats, without altering exploratory behaviour (Fox et al., 2005). Other recently synthesized H3 antagonists were also found to enhance short‐term memory in the rat social recognition memory model (Hudkins et al., 2014). Using a protocol entailing re‐exposure of the adult rat to the same juvenile 90 min after the first encounter, Kraus and colleagues suggested that histaminergic neurotransmission within the nucleus accumbens facilitated short‐term social memory without influencing cholinergic and glutamatergic transmission (Kraus et al., 2013). Of note, one of the components of the storage site of social memory appears to be the ventral hippocampus and its projections to the nucleus accumbens shell (Okuyama et al., 2016). Another study used the social discrimination protocol (see above) to show that recognition consolidation is mediated by H2 receptors in both the amygdala and dorsal hippocampus, as rats injected with the H2 antagonist ranitidine spent a similar length of time exploring the novel and familiar juveniles, and the H2 agonist dimaprit reversed this effect (Garrido Zinn et al., 2016). Nevertheless, H2 receptor activation in the infralimbic cortex does not appear to participate in the consolidation of social recognition memory (Cavalcante et al., 2017). Kraus and colleagues had previously reported that an infusion of famotidine, another H2 antagonist, did not affect the thioperamide‐induced facilitatory effect on recognition memory (Kraus et al., 2013). These apparently contrasting results could be related to differences in the injection site. Famotidine was administered into the brain ventricular system, whereas ranitidine was given directly into the BLA or CA1 at very similar dosages; thus, it is conceivable that the final concentration of famotidine within these specific structures was not sufficient to prevent thioperamide's effects.
The histaminergic system and object recognition memory: pharmacological studies in rodents
Early work by the group of Blandina et al. (1996) described the effects of systemic administration of the H3 agonists imetit and R‐α‐methylhistamine prior to the acquisition session in the object discrimination test. As expected for a short inter‐trial interval (60 min), control rats remembered the previous encounter with the familiar object and spent significantly more time exploring the novel one. However, the memory of H3 agonist‐treated rats was impaired as they showed no significant differences in the time spent exploring either object (Blandina et al., 1996). Furthermore, scopolamine‐induced memory impairment was prevented by pretreatment with the H3 antagonists thioperamide or clobenpropit (Giovannini et al., 1999). These results suggest a localization of H3 receptors on presynaptic histaminergic terminals, where their activation reduces histamine synthesis and release.
Using a slightly different procedure, Ghi et al. (1999) explored sexual differences in the object recognition task with different inter‐trial intervals and the promnesic effects of H3 receptor antagonism. They found that female rats preserved their memory for the novel object for longer periods of time with respect to males. In this context, the H3 antagonist thioperamide preserved the ability to discriminate the familiar and novel objects when administered 40 min before the retention test, hence suggesting that histaminergic modulation of recognition memory facilitates memory retrieval (Ghi et al., 1999). Nowadays, most experimental observations agree that H3 antagonists prolong recognition memory and prevent anterograde or retrograde, pharmacologically‐induced memory impairment. Bongers et al. (2004), however, observed that systemic treatment with the H3 antagonists thioperamide and clobenpropit caused memory impairments (Bongers et al., 2004). The discrepancy may be explained by the fact that the study was performed in mice rather than rats, with long habituation and acquisition sessions and using high doses of H3 antagonists that are known to produce an inverted U‐shaped dose–response curve in the object recognition test (Pascoli et al., 2009).
An open issue regarding the efficacy of H3 antagonists on recognition memory is the specific memory phase affected by these compounds. Some studies report the effects of pharmacological treatments administered before acquisition or retention sessions or both, others before habituation. Two studies specifically investigated the effects of drug treatments given at different time‐points during the object recognition paradigm. In one study, memory facilitation occurred when the H3 antagonist ciproxifan was given before the retention session, whereas administration before or immediately after training was ineffective (Pascoli et al., 2009). The other study reported that the same compound prevented time‐induced memory impairment when administered shortly after training as well as before retention sessions, but not when given 1 h before acquisition (Trofimiuk and Braszko, 2014). These results are in agreement with the majority of studies indicating that H3 receptor blockade facilitates retrieval; in other words, it facilitates the expression of the memory. However, there is experimental evidence that acute H3 antagonist injection before acquisition has a beneficial effect on recognition memory, but this was mostly using experimental designs that entail short inter‐trial intervals (5–180 min). Considering the high occupancy of brain H3 receptors that is rapidly achieved after systemic administration and their low elimination rate (Le et al., 2008; Sakurai et al., 1994), it is conceivable that when using short inter‐trial intervals, the drugs may affect all phases of the mnemonic process.
Recent evidence of the relevance of histamine neurotransmission in object recognition was provided by Sadek's group who showed that acute systemic post‐training administration of a newly synthesized H3 antagonist ameliorated the performance of rats with cognitive impairments induced by dizocilpine (MK‐801; Alachkar et al., 2017). The same group also demonstrated the efficacy of another H3 antagonist, which counteracted the MK‐801‐induced mnemonic deficit when injected shortly before the retrieval session (Eissa et al., 2018). The authors suggested that the postsynaptic receptor that mediates the promnesic effect is the H2 receptor without providing information of what brain structure is primarily responsible for the effect.
As mentioned above, H3 receptor antagonists increase histamine release as well as the release of other neurotransmitters in brain regions crucial for the maintenance of alertness and storage of information. In keeping with this hypothesis, we demonstrated that administration of the non‐imidazole H3 antagonist, ABT‐239, to wild‐type mice before training and retention test improved memory in the object recognition paradigm; the efficacy of ABT‐239 on memory was not observed in the brain of histamine‐depleted mice, suggesting that endogenous histamine is crucial for the mnemonic effects of H3R ligands (Provensi et al., 2016b).
The results summarized so far support the concept that memory improvement generated by H3R antagonists is caused by increased endogenous histaminergic tone and subsequent activation of post‐synaptic histaminergic receptors. Indeed, several studies point towards this conclusion as, for instance, intra‐hippocampal infusion of the H1R antagonist pyrilamine 30–120 min after training impaired recognition memory, whereas no effects on retention were observed when the drug was infused immediately or 360 min after acquisition (da Silveira et al., 2013). Similar findings were observed when the H2R antagonist ranitidine or the H3R agonist imetit were directly delivered into the CA1 region of the hippocampus (da Silveira et al., 2013).
Nozawa and colleagues in Japan performed a very curious study. They investigated the effects of oral administration of dried bonito broth (DBB) to rodents in several learning paradigms, including the object recognition. DBB is a hot‐water extract of dried bonito (skipjack tuna) muscle, which is ubiquitous in the Japanese diet, enhancing the taste and flavour of dishes that presents high levels of the histamine precursor, histidine (Fuke and Konosu, 1991). Oral administration of 1.6 g·kg−1 of DBB to mice significantly increased not only plasma histidine levels but also histamine levels in the hypothalamus. This diet prevented time‐induced natural forgetting to the same extent as thioperamide and L‐histidine acute injections (Nozawa et al., 2014). These results suggest that histamine participates in the DBB‐induced procognitive effects and contributes to understanding previously reported mood improvement (Nozawa et al., 2008) and better performance in calculation tasks observed in humans (Kuroda et al., 2007).
The histaminergic system and object recognition memory: studies in genetically modified mice
Results obtained with genetically modified mice lacking components of the histaminergic system have significantly increased our understanding of the histaminergic system. Comprehensive overviews of the behavioural phenotypes of these mice can be found in Schneider et al. (2014a,b). In line with the findings observed using classical pharmacological approaches, the results obtained using knockout models depend on the task and the type of memory being evaluated. Regarding specifically the novel object recognition, it was reported that the performance of histamine‐deficient mice, both males (Acevedo et al., 2006a) and females (Acevedo et al., 2006b), did not differ significantly from age‐matched wild‐type mice. In contrast, Dere and co‐workers described a poorer performance for HDC‐KO male mice in a non‐reinforced object exploration task (Dere et al., 2003). These discrepancies could be ascribed to considerable differences in the tasks used: in the first studies, a classical, one‐trial novel object recognition is used; the latter paper describes a very complex protocol in which the mice were presented to four objects and were demanded to discriminate among the objects based on the temporal sequence of presentation. In this case, memory retention was evaluated by the number of contacts with each object: wild‐type mice explored for a longer time, whereas HDC‐KO were unable to differentiate the objects' order (Dere et al., 2003). It is important to note that the sum of contacts observed in HDC‐KO animals was lower than those of wild types, which may have contributed to the results observed.
Several reports suggest that H1R as well as H2R deficiency impairs learning and memory, including object recognition (Dai et al., 2007). Mice lacking the H1R display also episodic‐like memory impairments as evaluated in a complex spatial and temporal object recollection task (Dere et al., 2008). More recently, the same group observed that wild‐type mice preferentially explore the objects based on the temporal order of presentation, but H1R‐KO are completely unable to maintain temporal or order information (Zlomuzica et al., 2013). Interestingly, these animals did not develop a conditioned place‐preference induced by novel objects, even though they still explored novel objects, suggesting that although motivation to explore novel objects was unchanged, their reinforcing value was probably diminished (Zlomuzica et al., 2008). Mice lacking H2R function exhibit selective cognitive deficits along with an impairment of hippocampal LTP (Dai et al., 2007).
Although pharmacological studies have shown consistently that H3R modulation results in alterations of the mnemonic processes (either improvement or impairment depending on the test), H3R deficiency does not cause significant impairments in several memory tasks (Toyota et al., 2002) including the object recognition (Rizk et al., 2004). Recent findings indicate that also the ablation of H4R did not result in significant alterations of recognition memory (Sanna et al., 2017).
The histaminergic system and recognition memory: novel object versus novel location recognition memory
Despite several studies reporting the participation of the central histaminergic system in the modulation of spatial memories, studies using the spatial version of the object recognition task are scarce. As previously mentioned, the novel object location test relies on a rodent's innate preference for novelty. The major difference between the two versions of the test occurs on the day of testing: in the object recognition test, one of the familiar objects presented during the acquisition session is replaced with a new one in the same location, while in the novel object location version, one of the familiar objects is moved to a new position in the arena. Therefore, animals that remember the original training experience will preferentially explore the object in the new location relative to the non‐displaced object (Weible et al., 2009; Vogel‐Ciernia and Wood, 2014). Acevedo and co‐workers demonstrated sex‐ and age‐dependent alterations in cognitive performance in HDC‐KO mice. Female histamine‐deficient mice did not differ from wild‐type controls in the novel location recognition task throughout lifespan (Acevedo et al., 2006b). On the other hand, while male HDC‐KO mice showed impaired novel location recognition in adulthood, middle‐aged animals did not differ from age‐matched controls in this test (Acevedo et al., 2006a). These results underscore the influence of gender and age in behaviour experiments. The spatial impairment observed in young male HDC‐KO mice is in agreement with results showing spatial memory facilitation following histamine central administration (Chen et al., 2001; Huang et al., 2003) but is more difficult to reconcile with the better performance of young HDC‐KO than age‐matched controls in the hidden platform session using the Morris water maze (Dere et al., 2003). However, it is important to note that these two tests are based on a different number of training and testing trials and involve different motivational factors; thus, histamine‐deficient mice could either remember the location of the hidden platform better or might be more motivated to find it (Acevedo et al., 2006a). Novel spatial location memory was also unaffected in H1R‐KO mice (Zlomuzica et al., 2008). To the best of our knowledge, there are no reports describing the consequences of H2R, H3R and H4R silencing using the new object location memory test.
Histamine and stress: effects on recognition memory
Memory processes can be profoundly affected by stress, although the impact of diverse stressful stimuli on cognitive functions is not the same: while moderate stress can facilitate learning, excessive stress (acute or chronic) leads to impairments of memory function (Sandi and Pinelo‐Nava, 2007). Trofimiuk and Braszko (2014) observed that daily restrained rats for 21 days were unable to differentiate the novel from the familiar objects when the retention session was performed 24 h after the acquisition. Acute ciproxifan treatment counteracted the deleterious effects of chronic restrain stress on long‐term recognition memory (Trofimiuk and Braszko 2014). Prolonged exposure to stress evokes profound structural, physiological and molecular changes in brain areas related to cognition and emotions and increases the risks of developing neuropsychiatric disorders, such as depression (Schneiderman et al., 2005; McEwen and Gianaros, 2010). The Flinders Sensitive Line (FSL) is a suitable rat model to study emotional and cognitive deficits of depression‐like symptoms because this inbred rat model shows impaired emotional and recognition memory (Eriksson et al., 2012; Gomez‐Galan et al., 2013; Overstreet and Wegener, 2013). The behavioural repertoire of FSL rats acutely treated with the H3R antagonist clobenpropit or saline was compared with that of Sprague–Dawley (SD) rats treated with saline. During the test session, performed 24 h after training, SD rats preferentially explored the novel objects, whereas saline‐treated FSL rats showed no object preference. Treatment of FSL rats with clobenpropit increased the recognition index to the same level observed in SD rats, indicating that the drug treatment restored recognition memory (Femenia et al., 2015).
Perinatal asphyxia is a severe condition associated with obstetric complications during labour and delivery with high mortality. It may affect virtually any organ, but hypoxic–ischaemic encephalopathy is the most studied clinical condition as it is burdened with the most severe sequelae (Antonucci et al., 2014). Animal studies confirmed that perinatal asphyxia results in cognitive impairments in adulthood in several models (Boksa et al., 1995; Simola et al., 2008) and, among others, alteration of neurotransmitters in the hypothalamus (Kohlhauser et al., 1999). Flores‐Balter and co‐workers evaluated neurochemical and behavioural consequences of perinatal asphyxia in adulthood with particular attention to the histaminergic system. Interestingly, asphyxia‐exposed rats exhibited an impaired performance in the object recognition test, which was correlated with a decreased number of ventral TMN neurons and marked reduction of histidine decarboxylase expression in the hypothalamus. Acute treatment with the H3R antagonist thioperamide dose‐dependently reverted perinatal asphyxia‐induced recognition memory impairment in adulthood (Flores‐Balter et al., 2016).
Some evidence suggests that prenatally or immediately postnatal exposure to methamphetamine, a highly addictive amphetamine‐like psychomotor stimulant widely used worldwide (Courtney and Ray, 2014), may result in long‐term hippocampus‐dependent spatial learning and memory deficits in rodents (Williams et al., 2003a,b). As histamine mediates some effects of methamphetamine when given acutely in adulthood (Kubota et al., 2002; Dai et al., 2004; Kitanaka et al., 2007), Raber's group examined whether histamine could also contribute to the long‐term cognitive deficits observed in early‐life methamphetamine‐exposed mice. Mice received the treatments from post‐natal days 11 to 20 and were tested at 3 months of age using a complex novel location and novel object recognition test. When a known object was moved to a novel location, control animals (saline treated males and females) recognized the novel spatial arrangement of familiar objects. Methamphetamine‐ and thioperamide‐treated females showed impairments in novel location recognition. In contrast to females, no cognitive deficits were observed in males, suggesting that females might be more susceptible. Finally, co‐administration of the H3R agonist immepip prevented methamphetamine‐induced spatial recognition deficit in females (Acevedo et al., 2007, 2008; Acevedo and Raber, 2011). Accordingly, the same group demonstrated that methamphetamine administration to neonatal mice increased brain histamine levels and activated the hypothalamic–pituitary–adrenal axis; both effects were more pronounced in female than male mice (Acevedo et al., 2008). Moreover, the same treatment reduced levels of the dendritic marker microtubule‐associated protein 2 in the CA3 region of the hippocampus and the enthorhinal cortex. Such reduction was not observed in mice receiving immepip along with methamphetamine, and the animals did not show cognitive impairments, suggesting that these brain areas are particularly important for the long‐term effects of methamphetamine on cognitive function (Acevedo et al., 2008). These data support a role for histamine in the effects of methamphetamine on the developing brain.
Efficacy of histamine ligands in animal models of cognitive disorders
The preclinical results showing procognitive effects of H3R antagonists raised great expectations on the translational values of these compounds, given the encouraging results obtained in preclinical models of cognitive disorders. As an example, the administration of SAR110894, a potent H3R antagonist, 1 h before the acquisition session significantly attenuated impaired short‐term episodic memory performance in the object recognition task of rodents that received i.c.v. injection of the Aβ23‐35 amino acid sequence of the amyloid peptide (Chen et al., 1996; Olariu et al., 2001; Griebel et al., 2012). Long‐term treatment with the same H3R antagonist inhibited τ pathology and prevented cognitive deficits in a τ transgenic mouse model (THY‐Tau22) by reducing τ hyperphosphorylation in the hippocampus, decreasing the formation of neurofibrillary tangles in the cortex, hippocampus and amygdala and macrophage inflammatory protein 1‐α mRNA expression. SAR110894 also prevented episodic memory deficits, and this effect persisted after treatment washout (Delay‐Goyet et al., 2016). Similar procognitive effects were observed when the H3R antagonist ciproxifan was administered to mice that express a mutant form of the human amyloid precursor protein gene (APPTg2576) associated with familiar early‐onset Alzheimer's disease. Treatment with ciproxifan 30 min before the retention session of the object recognition test prevented the discrimination deficits observed in 12‐ to 14‐month‐old APPTg2576 animals (Bardgett et al., 2011). Using the same animal model, Bitner and co‐workers demonstrated that the treatment with another H3R antagonist, ABT‐239, normalized the hyperactivation of hippocampal glycogen synthase kinase‐3β (GSK‐3β) and prevented τ hyperphosporlylation. However, they found that donepezil, an AChE inhibitor, was unable to modify GSK‐3β phosphorylation. Interestingly, ABT‐239‐stimulated GSK‐3β phosphorylation in the hippocampus was blunted in α7 nicotinic ACh receptor (nAChR)‐KO mice (Bitner et al., 2011). We recently confirmed and expanded these observations: ABT‐239 and donepezil, given as systemic treatments, augment GSK‐3β phosphorylation in cortical and hippocampal homogenates of wild‐type but not of acutely or chronically histamine‐depleted mice. Furthermore, administration of the PI3K inhibitor LY 294002, which blocks GSK‐3β phosphorylation, prevented ABT‐239‐induced procognitive effects as measured in the object recognition test of wild‐type mice (Provensi et al., 2016b). Taken together, these results point to the requirement of an intact histaminergic system for both donepezil and ABT‐239 to exert their procognitive effects, whereas increased ACh release and subsequent α7 nAChR activation seems not to play a major role.
More recently, the efficacy of the H3 antagonist thioperamide was tested in an experimental model of Parkinsonism (Bonito‐Oliva et al., 2014a). This model produces a partial depletion of dopamine without affecting horizontal motor activity, mimicking a relatively early stage of Parkinson's disease (Bonito‐Oliva et al., 2014b). Mice with a partial lesion of the midbrain dopaminergic system fail to recognize a novel object when tested 24 h after the acquisition phase (Bonito‐Oliva et al., 2014b; Masini et al., 2017). This deficit was abolished by systemic treatment with thioperamide 20 min before both the acquisition and test phases (Masini et al., 2017).
Animal social behaviour is often used as a measure to study disorders characterized by alterations in sociability, such as the autism spectrum disorder. Considering this fact, the efficacy of the H3 antagonist ciproxifan was tested in the animal model of autism induced by prenatal exposure to valproic acid (Baronio et al., 2015). This animal model is based on the evidence that treatment with valproic acid during pregnancy is associated with an increased incidence of autism spectrum disorder in children (Bromley et al., 2013). Moreover, in utero exposure of rodents to valproic acid results in neuroanatomical, behavioural and biochemical features replicating some characteristics observed in autistic patients, such as reduced sociability and social novel preference as measured in the three chamber test (Schneider and Przewlocki, 2005). This test is a commonly used method to measure social approach behaviour in mice (Figure 3). The apparatus consists of a three‐chamber arena: one of which contains a stimulus mouse positioned in a wire mesh container, while in the opposite chamber, a similar container holds an inanimate object. After adaptation to the arena, the animal is placed into the middle chamber and left free to explore the apparatus. The tendency to approach or avoid the compartment with the stimulus mouse provides a measure of sociability. In a second session, the object is replaced by a novel unfamiliar mouse, and the time spent in exploring the known and the novel animals is used to calculate the social novelty index (Yang et al., 2011). Using this approach, it was observed that ciproxifan treatment normalized sociability, but not social novelty impairments displayed by animals who had been exposed to valproic acid in utero. Interestingly, the treatment with the H3 antagonist also attenuated the repetitive behaviour detected in mice exposed to valproic acid, as assessed in the marble burying test (Baronio et al., 2015).
Figure 3.
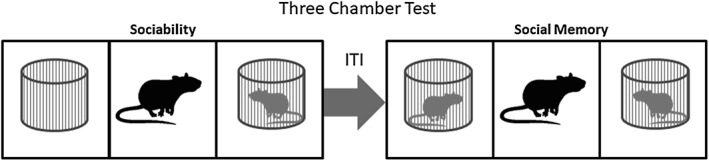
Schematic representation of the three‐chamber test used to evaluate sociability and social memory.
The zebrafish is rapidly becoming a new popular model organism in neuroscience research, due to some similarities with both humans and rodents regarding anatomy, neurotransmitter systems and pharmacology (Kalueff et al., 2014). Zebrafish are highly social and prefer to swim in groups for different reasons, such as mating, foraging or avoiding predators. The behavioural and neurochemical alterations of both larval and adult zebrafish exposed to valproic acid during neural tube formation was recently reported (Baronio et al., 2018). It was found that larvae exposed to valproic acid showed a significant reduction in the number of histaminergic neurons, histamine content as well as HDC, H1, H2 and H3 receptor mRNA expression along with decreased motor activity and an abnormal flash‐dark response. These altered responses to sudden darkness are probably related to reduced histaminergic transmission, since this is a phenotype characteristic of larvae lacking the ability to synthesize histamine (Sundvik and Panula, 2012). No difference in the basal locomotor activity was found between control and valproic acid‐exposed adult zebrafish. However, valproic‐treated zebra fish showed impaired sociability, as they spent less time swimming in the zone closest to the compartment with the stimulus fish. Regarding the histaminergic system, the reduced mRNA expression of HDC and H3 receptors persisted in adult zebrafish exposed to valproic acid, although normal levels of brain histamine were found (Baronio et al., 2018). These findings support the hypothesis that at least some of the main clinical alterations present in autism spectrum disorder could be attenuated by H3 antagonists even in a late developmental stage (Baronio et al., 2015).
Clinical studies
Evidence of the involvement of the central histaminergic system in memory and cognition mainly derive from preclinical animal models. However, over the last decade, evidence has accumulated showing that histamine may play an important role in humans as well, and therefore, the histaminergic system has been considered a possible target for pharmacological interventions to ameliorate cognitive deficits in clinical disorders.
In the late 1980, antihistamines were already widely used for the treatment of allergic diseases, but their sedative properties were a major concern. Car and work accidents as well as a decline in productivity and learning efficiency was observed in patients taking antihistamine drugs. Therefore, the introduction of a second‐generation antihistamines was a major advance in the field. These compounds were argued to have a better safety profile since they were minimally or non‐sedating because of their limited penetration of the blood–brain barrier (Hu et al., 2015). At that time, clinical research was focused on the comparison of first‐ and second‐generation antihistamines regarding their potential side effects with particular attention to psychomotor, sedative and cognitive domains. For instance, Meador et al. (1989) measured the P3‐evoked potential in a placebo‐controlled, double‐blind, randomized trial in healthy adult subjects. The P3 is a cognitively evoked electroencephalographic response that is an objective and sensitive measure of sustained attention and cerebral processing speed. This measure is modified by drugs and disease states, for example, scopolamine slows cognitive processing speed and prolongs P3 latency (Potter et al., 2000). A grater latency was observed in chlorpheniramine‐treated subjects compared to terfenadine or placebo groups, suggesting that second‐generation antihistamines may be particularly advantageous in patients who require alertness and intact cognitive abilities (Meador et al., 1989).
Following this initial study, many others have attempted to investigate the effects of several H1 receptor antihistamines on cognition. The vast majority failed to detect significant memory alterations at therapeutically used doses, with mild effects on memory performance observed only at high doses (Kerr et al., 1994; Vuurman et al., 1994; Patat et al., 1995; Hindmarch and Shamsi, 2001; Tagawa et al., 2002; Theunissen et al., 2004; van Ruitenbeek et al., 2010a; Taubel et al., 2016). One of the possible explanations of these dose‐related effects was that, at higher doses, impaired psychomotor performance and increased sedation may have affected cognitive performance. To address this question, van Ruitenbeek and co‐workers performed a study to clarify the effects of the H1 antagonist dexchlorpheniramine on sedation and memory performance. Memory tasks and cortical activity were measured between 1.5 and 2.5 h after drug administration (i.e. during the peak of psychomotor impairment) and compared to those of placebo and lorazepam, a benzodiazepine known for its sedative and amnesic properties. They found that H1 receptor blockade induced clear sedative effects without affecting memory performance. In contrast, lorazepam affected both working memory and sedation (Van Ruitenbeek et al., 2010b). Therefore, it can be concluded that sedation is not necessarily associated with impaired memory and that the memory impairment observed with potentially sedative drugs could be related to effects on neuronal networks independent of those that affect arousal (Turner et al., 2006).
In the clinical evaluation of the potential cognitive‐enhancing properties of H3 antagonists, Cho et al. (2011) performed a randomized, double‐blind, placebo‐controlled study in healthy subjects. The results obtained show that a single‐dose treatment with the H3 antagonist MK‐3134 ameliorates the decline in cognitive functions induced by scopolamine, particularly attention, psychomotor and executive function to a similar extent as that observed in the group receiving donepezil, a standard AChE inhibitor (Cho et al., 2011).
The results of both preclinical and post‐mortem studies indicate the possible therapeutic use of H3 antagonists in patients with major cognitive impairments such as Alzheimer's disease or dementia with Lewy bodies (Sadek et al., 2016; Lethbridge and Chazot, 2016). However, the clinical trials failed to demonstrate unequivocal cognitive improvements. In a randomized, double‐blind, placebo‐controlled study with a small group of patients (n = 8) with mild‐to‐moderate Alzheimer's disease, it was found that treatment with GSK239512 for 4 weeks, using a titration regimen in order to find the optimal dose for individual patients, resulted in positive effects on attention and memory (Nathan et al., 2013). In a subsequent study using a larger population (99 placebo and 97 treated subjects), it was observed that the treatment with the same drug for 16 weeks improved episodic memory, but not other cognitive domains such as executive functions and working memory (Grove et al., 2014). In both studies, the H3 antagonist had an acceptable safety and tolerability profile. Although promising, these results need to be replicated in larger scale Phase III clinical trials to confirm the efficacy of GSK239512 treatment in Alzheimer's disease patients.
The safety profile of another H3 antagonist, ABT‐288, was assessed in healthy young adults and elderly volunteers. The drug's pharmacokinetics were comparable between the two populations studied. Moreover, single as well as multiple doses up to 3 mg were generally safe and well tolerated, and the most frequently reported adverse events were hot flushes, headaches, abnormal dreams, insomnia, nausea and dizziness (Othman et al., 2013). Therefore, ABT‐288 was advanced to Phase II evaluation in Alzheimer's patients. The proof‐of‐concept, randomized, placebo‐controlled study was designed to evaluate the efficacy and safety of the two doses of ABT‐288 (1 and 3 mg) and compared with that of donepezil (10 mg) in the symptomatic treatment of subjects with mild‐to‐moderate Alzheimer's disease. However, after 12 weeks, the study was prematurely terminated because neither dose of ABT‐288 demonstrated a procognitive effect with respect to placebo, whereas donepezil showed a significant improvement in the primary endpoint. The positive results obtained with the active comparator donepezil suggest that the lack of efficacy of the H3 antagonist treatment was not related to the design and conduct of the trial (Haig et al., 2014). Similar results were obtained in a previous pilot randomized study in which mild‐to‐moderate Alzheimer's disease patients received placebo or the H3 antagonist/inverse agonist MK‐0249 for 4 weeks. Although the treatment was generally well tolerated, no differences in cognitive function were observed between MK‐0249 and placebo‐treated patients (Egan et al., 2012).
Currently, a Phase II clinical study is assessing SAR110894, an H3 receptor antagonist with excellent drug‐like properties, on the cognitive performance of patients with mild‐to‐moderate Alzheimer's disease in comparison to placebo (Griebel et al., 2012). The clinical outcomes have not been disclosed yet (clinicaltrials.gov Identifier: NCT01266525).
Concluding remarks
The actual state of the art indicates that histamine, acting in different brain sites, has an important role as a regulator of memory consolidation/retrieval in various learning paradigms. The role of histamine receptors in recognition memory has been extensively studied using both specific ligands and also transgenic animals. The data collected in our review strongly suggest a role for hippocampal H1, H2 and H3 receptors in object recognition and H2 receptors within the hippocampus and amygdala in social recognition (Figure 4).
Figure 4.
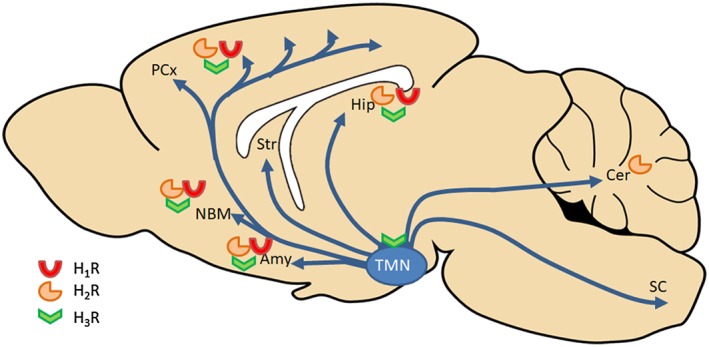
Schematic representation of the brain histaminergic projections involved in cognition. Histamine‐producing cell bodies are restricted to the TMN and histaminergic fibres project to various brain regions including the prefrontal cortex, nucleus basalis magnocellularis (NBM), the striatum (Str), the amygdala (Amy), the hippocampus (Hip), the cerebellum (Cer) and spinal cord (SC). Histamine effects in the brain are mediated by four receptors (H1 to H4 R) with different distribution across the brain. In recognition memory, the role of specific receptor activation in discrete brain regions was just marginally studied as most studies were performed using systemically injected compounds (see text). Only two studies analysed the effects of local drug infusions: H1 and H2 antagonists, as well as an H3 agonist directly infused into the hippocampal CA1 region blocked long‐term consolidation of object recognition memory (da Silveira et al., 2013). An H2 antagonist delivered into hippocampal CA1 or basolateral amygdala impaired social recognition memory (Garrido Zinn et al., 2016).
Due to its actions as an auto/heteroreceptor, regulating not only histamine synthesis but also the release of other important neurotransmitters critically involved in cognition, the H3 receptor has received great attention by the scientific community as a good target for the development of new centrally acting drugs, and many academic groups as well as pharmaceutical companies have synthesized numerous selective and potent H3 receptor ligands. However, despite their excellent ‘drug‐like’ profile, particularly in cognition and attention models (Sadek et al., 2016), which suggests that H3 antagonists could be effective therapeutic compounds, clinical studies have proved that they lack efficacy or have only minor beneficial effects on cognitive performance in Alzheimer's disease patients (Zlomuzica et al., 2016). Although these initial failures have markedly dampened the enthusiasm, it is important to note that recently, pitolisant, the first H3 receptor antagonist tested in humans (Schwartz, 2011), received market approval from the European Medicines Agency for the treatment of narcolepsy, and this could open up the opportunity for also accessing its efficacy for other disorders in off‐label clinical trials.
In summary, more than 30 years have passed since the role of histamine in the regulation of memory consolidation was firstly proposed by De Almeida and Izquierdo in 1986, and since then, many advances have been made, and today, there is compelling evidence that alterations in the central histaminergic system are associated with the cognitive impairments observed in several neurodegenerative disorders. The discrepancies between preclinical data and the results of clinical trials has initiated several questions that need to be addressed in future studies using cutting‐edge technologies.
The data discussed in this review are broadly consistent with the hypothesis that diverse functional roles are served by different subpopulations of histaminergic neurons, implying that histaminergic neurons are presumably organized into distinct circuits responding to selective inputs, and engaged according to their projections to the brain region required for a specific behavioural outcome (Figure 4). In addition to this spatial compartmentalization, that is, number and type of cells participating in a functional pathway, we recently provided experimental evidence of differences in the kinetics of neuronal activation, that is, a temporal regulation of histaminergic activity in different brain regions (Benetti et al., 2015; Fabbri et al., 2016). Therefore, translational histamine research should start afresh given the availability of HDC‐Cre mice that will allow the use of cutting‐edge technologies such as optogenetic or chemogenetic approaches. This will afford a better understanding of the temporal and anatomical modulation by histaminergic neurons. These approaches hopefully will clarify the inconsistent actions of histamine receptors, due presumably to the discrepant actions of histamine receptors in different brain regions and cellular types. Furthermore, histamine receptors are expressed on glial cells (reviewed in Hu and Chen, 2017), and their potential role in learning and memory is as yet unexplored.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a,b).
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
G.P. was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq Brazil, process 201511/2014‐2), Fondazione Umberto Veronesi and Universitá degli Studi di Firenze (DR 175372‐1210) grants; and A.C. was supported by Ministero della Salute, Bando Ricerca Finalizzata e Giovani Ricercatori 2011e2012 (GR‐201102346829).
Provensi G., Costa A., Izquierdo I., Blandina P., and Passani M. B. (2020) Brain histamine modulates recognition memory: possible implications in major cognitive disorders, British Journal of Pharmacology, 177, 539–556, doi: 10.1111/bph.14478.
References
- Acevedo SF, de Esch IJ, Raber J (2007). Sex‐ and histamine‐dependent long‐term cognitive effects of methamphetamine exposure. Neuropsychopharmacology 32: 665–672. [DOI] [PubMed] [Google Scholar]
- Acevedo SF, Ohtsu H, Benice TS, Rizk‐Jackson A, Raber J (2006a). Age‐dependent measures of anxiety and cognition in male histidine decarboxylase knockout (Hdc−/−) mice. Brain Res 1071: 113–123. [DOI] [PubMed] [Google Scholar]
- Acevedo SF, Pfankuch T, Ohtsu H, Raber J (2006b). Anxiety and cognition in female histidine decarboxylase knockout (Hdc(−/−)) mice. Behav Brain Res 168: 92–99. [DOI] [PubMed] [Google Scholar]
- Acevedo SF, Pfankuch T, van Meer P, Raber J (2008). Role of histamine in short‐ and long‐term effects of methamphetamine on the developing mouse brain. J Neurochem 107: 976–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo SF, Raber J (2011). Histamine‐dependent behavioral response to methamphetamine in 12‐month‐old male mice. Brain Res 1393: 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airaksinen MS, Alanen S, Szabat E, Visser TJ, Panula P (1992). Multiple neurotransmitters in the tuberomammillary nucleus: comparison of rat, mouse. and guinea pig. J Comp Neurol 323: 103–116. [DOI] [PubMed] [Google Scholar]
- Alachkar A, Łazewska D, Kieć‐Kononowicz K, Sadek B. (2017). The Histamine H3 Receptor Antagonist E159 Reverses Memory Deficits Induced by Dizocilpine in Passive Avoidance and Novel Object Recognition Paradigm in Rats. Front Pharmacol 8: 709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen TA, Fortin NJ (2013). The evolution of episodic memory. Proc Nat Acad Sci 110 (Suppl. 2): 10379–10386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anaclet C, Parmentier R, Ouk K, Guidon G, Buda C, Sastre JP et al (2009). Orexin/hypocretin and histamine: distinct roles in the control of wakefulness demonstrated using knock‐out mouse models. J Neurosci 29: 14423–14438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonucci R, Porcella A, Pilloni MD (2014). Perinatal asphyxia in the term newborn. J Ped Neonat Ind Med 3: 1–14. [Google Scholar]
- Arrang JM, Garbarg M, Schwartz JC (1983). Auto‐inhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature 302: 832–837. [DOI] [PubMed] [Google Scholar]
- Bardgett ME, Davis NN, Schultheis PJ, Griffith MS (2011). Ciproxifan, an H3 receptor antagonist, alleviates hyperactivity and cognitive deficits in the APP Tg2576 mouse model of Alzheimer's disease. Neurobiol Learn Mem 95: 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baronio D, Castro K, Gonchoroski T, de Melo GM, Nunes GD, Bambini‐Junior V et al (2015). Effects of an H3R antagonist on the animal model of autism induced by prenatal exposure to valproic acid. PloS one 10: e0116363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baronio D, Puttonen HAJ, Sundvik M, Semenova S, Lehtonen E, Panula P (2018). Embryonic exposure to valproic acid affects the histaminergic system and the social behaviour of adult zebrafish (Danio rerio). Br J Pharmacol 175: 797–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE (2010). Histamine in the CNS: multiple functions and potential neurologic implications. Neurology 75: 1472–1479. [DOI] [PubMed] [Google Scholar]
- Benetti F, Furini CR, de Carvalho Myskiw J, Provensi G, Passani MB, Baldi E et al (2015). Histamine in the basolateral amygdala promotes inhibitory avoidance learning independently of hippocampus. Proc Natl Acad Sci 112: E2536–E2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitner RS, Markosyan S, Nikkel AL, Brioni JD (2011). In‐vivo histamine H3 receptor antagonism activates cellular signaling suggestive of symptomatic and disease modifying efficacy in Alzheimer's disease. Neuropharmacology 60: 460–466. [DOI] [PubMed] [Google Scholar]
- Blandina P, Giorgetti M, Bartolini L, Cecchi M, Timmerman H, Leurs R et al (1996). Inhibition of cortical acetylcholine release and cognitive performance by histamine H3 receptor activation in rats. Br J Pharmacol 119: 1656–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandina P, Munari L, Provensi G, Passani MB (2012). Histamine neurons in the tuberomamillary nucleus: a whole center or distinct subpopulations? Front Sys Neurosci 6: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser R, Heyser C (2015). Spontaneous object recognition: a promising approach to the comparative study of memory. Front Behav Neurosci 9: 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boksa P, Krishnamurthy A, Brooks W (1995). Effects of a period of asphyxia during birth on spatial learning in the rat. Pediatric Res 37: 489–496. [DOI] [PubMed] [Google Scholar]
- Bongers G, Leurs R, Robertson J, Raber J (2004). Role of H3‐receptor‐mediated signaling in anxiety and cognition in wild‐type and Apoe−/− mice. Neuropsychopharmacology 29: 441–449. [DOI] [PubMed] [Google Scholar]
- Bonito‐Oliva A, Masini D, Fisone G (2014a). A mouse model of non‐motor symptoms in Parkinson's disease: focus on pharmacological interventions targeting affective dysfunctions. Front Behav Neurosci 8: 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonito‐Oliva A, Pignatelli M, Spigolon G, Yoshitake T, Seiler S, Longo F et al (2014b). Cognitive impairment and dentate gyrus synaptic dysfunction in experimental Parkinsonism. Biol Psychiatry 75: 701–710. [DOI] [PubMed] [Google Scholar]
- Bromley RL, Mawer GE, Briggs M, Cheyne C, Clayton‐Smith J, Garcia‐Finana M et al (2013). The prevalence of neurodevelopmental disorders in children prenatally exposed to antiepileptic drugs. J Neurol Neurosurg Psych 84: 637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, Stevens DR, Haas HL (2001). The physiology of brain histamine. Progr Neurobiol 63: 637–672. [DOI] [PubMed] [Google Scholar]
- Burman OH, Mendl M (2000). Short‐term social memory in the laboratory rat: its susceptibility to disturbance. Appl Anim Behav Sci 67: 241–254. [DOI] [PubMed] [Google Scholar]
- Cavalcante LES, Zinn CG, Schmidt SD, Saenger BF, Ferreira FF, Furini CRG et al (2017). Modulation of the storage of social recognition memory by neurotransmitter systems in the insular cortex. Behav Brain Res 334: 129–134. [DOI] [PubMed] [Google Scholar]
- Chen SY, Wright JW, Barnes CD (1996). The neurochemical and behavioral effects of beta‐amyloid peptide (25–35). Brain Res 720: 54–60. [DOI] [PubMed] [Google Scholar]
- Chen Z, Chen JQ, Kamei C (2001). Effect of H1‐antagonists on spatial memory deficit evaluated by 8‐arm radial maze in rats. Acta Pharmacol Sinica 22: 609–613. [PubMed] [Google Scholar]
- Cho W, Maruff P, Connell J, Gargano C, Calder N, Doran S et al (2011). Additive effects of a cholinesterase inhibitor and a histamine inverse agonist on scopolamine deficits in humans. Psychopharmacology (Berl) 218: 513–524. [DOI] [PubMed] [Google Scholar]
- Chotard C, Ouimet T, Morisset S, Sahm U, Schwartz JC, Trottier S (2002). Effects of histamine H3 receptor agonist and antagonist on histamine co‐transmitter expression in rat brain. J Neural Transm 109: 293–306. [DOI] [PubMed] [Google Scholar]
- Courtney KE, Ray LA (2014). Methamphetamine: an update on epidemiology, pharmacology, clinical phenomenology, and treatment literature. Drug Alcohol Depend 143: 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silveira CK, Furini CR, Benetti F, Monteiro Sda C, Izquierdo I (2013). The role of histamine receptors in the consolidation of object recognition memory. Neurobiol Learn Mem 103: 64–71. [DOI] [PubMed] [Google Scholar]
- Dai H, Kaneko K, Kato H, Fujii S, Jing Y, Xu A et al (2007). Selective cognitive dysfunction in mice lacking histamine H1 and H2 receptors. Neurosci Res 57: 306–313. [DOI] [PubMed] [Google Scholar]
- Dai H, Okuda H, Iwabuchi K, Sakurai E, Chen Z, Kato M et al (2004). Social isolation stress significantly enhanced the disruption of prepulse inhibition in mice repeatedly treated with methamphetamine. Ann N Y Acad Sci 1025: 257–266. [DOI] [PubMed] [Google Scholar]
- Davis RL, Zhong Y (2017). The biology of forgetting – a perspective. Neuron 95: 490–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida MA, Izquierdo I (1986). Memory facilitation by histamine. Arch Int Pharmacodynam Ther 283: 193–198. [PubMed] [Google Scholar]
- Deiana S, Platt B, Riedel G (2011). The cholinergic system and spatial learning. Behav Brain Res 221: 389–411. [DOI] [PubMed] [Google Scholar]
- Delay‐Goyet P, Blanchard V, Schussler N, Lopez‐Grancha M, Menager J, Mary V et al (2016). SAR110894, a potent histamine H3‐receptor antagonist, displays disease‐modifying activity in a transgenic mouse model of tauopathy. Alzheimers Dement 2: 267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dere E, De Souza‐Silva MA, Topic B, Spieler RE, Haas HL, Huston JP (2003). Histidine‐decarboxylase knockout mice show deficient nonreinforced episodic object memory, improved negatively reinforced water‐maze performance, and increased neo‐ and ventro‐striatal dopamine turnover. Learn Mem 10: 510–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dere E, Zlomuzica A, Viggiano D, Ruocco LA, Watanabe T, Sadile AG et al (2008). Episodic‐like and procedural memory impairments in histamine H1 Receptor knockout mice coincide with changes in acetylcholine esterase activity in the hippocampus and dopamine turnover in the cerebellum. Neuroscience 157: 532–541. [DOI] [PubMed] [Google Scholar]
- Di Cara B, Panayi F, Gobert A, Dekeyne A, Sicard D, De Groote L et al (2007). Activation of dopamine D1 receptors enhances cholinergic transmission and social cognition: a parallel dialysis and behavioural study in rats. Int J Neuropsychopharmacol 10: 383–399. [DOI] [PubMed] [Google Scholar]
- Egan M, Yaari R, Liu L, Ryan M, Peng Y, Lines C et al (2012). Pilot randomized controlled study of a histamine receptor inverse agonist in the symptomatic treatment of AD. Current Alzheimer Res 9: 481–490. [DOI] [PubMed] [Google Scholar]
- Eissa N, Khan N, Ojha SK, Lazewska D, Kiec‐Kononowicz K, Sadek B (2018). The histamine H3 receptor antagonist DL77 ameliorates MK801‐induced memory deficits in rats. Front Neurosci 12: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom P, Holmqvist BI, Panula P (1995). Histamine‐immunoreactive neurons in the brain of the teleost Gasterosteus aculeatus L. Correlation with hypothalamic tyrosine hydroxylase‐ and serotonin‐immunoreactive neurons. J Chem Neuroanatomy 8: 75–85. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Wotjak CT, Landgraf R (1995). Social discrimination procedure: an alternative method to investigate juvenile recognition abilities in rats. Physiol & Behav 58: 315–321. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J (1988). A new one‐trial test for neurobiological studies of memory in rats. 1: behavioral data. Behav Brain Res 31: 47–59. [DOI] [PubMed] [Google Scholar]
- Ercan‐Sencicek AG, Stillman AA, Ghosh AK, Bilguvar K, O'Roak BJ, Mason CE et al (2010). L‐histidine decarboxylase and Tourette's syndrome. N Engl J Med 362: 1901–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson KS, Peitsaro N, Karlstedt K, Kaslin J, Panula P (1998). Development of the histaminergic neurons and expression of histidine decarboxylase mRNA in the zebrafish brain in the absence of all peripheral histaminergic systems. Eur J Neurosci 10: 3799–3812. [DOI] [PubMed] [Google Scholar]
- Eriksson TM, Delagrange P, Spedding M, Popoli M, Mathe AA, Ogren SO et al (2012). Emotional memory impairments in a genetic rat model of depression: involvement of 5‐HT/MEK/Arc signaling in restoration. Mol Psych 17: 173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri R, Furini CR, Passani MB, Provensi G, Baldi E, Bucherelli C et al (2016). Memory retrieval of inhibitory avoidance requires histamine H1 receptor activation in the hippocampus. Proc Natl Acad Sci 113: E2714–E2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Femenia T, Magara S, DuPont CM, Lindskog M (2015). Hippocampal‐dependent antidepressant action of the H3 receptor antagonist clobenpropit in a rat model of depression. Int J Neuropsychopharmacol 18 10.1093/ijnp/pyv032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez TV, Sanders SJ, Yurkiewicz IR, Ercan‐Sencicek AG, Kim YS, Fishman DO et al (2012). Rare copy number variants in Tourette syndrome disrupt genes in histaminergic pathways and overlap with autism. Biol Psych 71: 392–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores‐Balter G, Cordova‐Jadue H, Chiti‐Morales A, Lespay C, Espina‐Marchant P, Falcon R et al (2016). Effect of perinatal asphyxia on tuberomammillary nucleus neuronal density and object recognition memory: a possible role for histamine? Behav Brain Res 313: 226–232. [DOI] [PubMed] [Google Scholar]
- Fouquet C, Tobin C, Rondi‐Reig L (2010). A new approach for modeling episodic memory from rodents to humans: the temporal order memory. Behav Brain Res 215: 172–179. [DOI] [PubMed] [Google Scholar]
- Fox GB, Esbenshade TA, Pan JB, Radek RJ, Krueger KM, Yao BB et al (2005). Pharmacological properties of ABT‐239 [4‐(2‐{2‐[(2R)‐2‐methylpyrrolidinyl]ethyl}‐benzofuran‐5‐yl)benzonitrile]: II. Neurophysiological characterization and broad preclinical efficacy in cognition and schizophrenia of a potent and selective histamine H3 receptor antagonist. J Pharmacol Exp Ther 313: 176–190. [DOI] [PubMed] [Google Scholar]
- Fujita A, Bonnavion P, Wilson MH, Mickelsen LE, Bloit J, de Lecea L et al (2017). Hypothalamic tuberomammillary nucleus neurons: electrophysiological diversity and essential role in arousal stability. J Neurosci 37: 9574–9592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuke S, Konosu S (1991). Taste‐active components in some foods: a review of Japanese research. Physiol Behav 49: 863–868. [DOI] [PubMed] [Google Scholar]
- Garrido Zinn C, Clairis N, Silva Cavalcante LE, Furini CR, de Carvalho Myskiw J, Izquierdo I (2016). Major neurotransmitter systems in dorsal hippocampus and basolateral amygdala control social recognition memory. Proc Nat Acad Sci 113: E4914–E4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghi P, Orsetti M, Gamalero SR, Ferretti C (1999). Sex differences in memory performance in the object recognition test. Possible role of histamine receptors. Pharmacol Biochem Behav 64: 761–766. [DOI] [PubMed] [Google Scholar]
- Giannoni P, Passani MB, Nosi D, Chazot PL, Shenton FC, Medhurst AD et al (2009). Heterogeneity of histaminergic neurons in the tuberomammillary nucleus of the rat. Eur J Neurosci 29: 2363–2374. [DOI] [PubMed] [Google Scholar]
- Giovannini MG, Bartolini L, Bacciottini L, Greco L, Blandina P (1999). Effects of histamine H3 receptor agonists and antagonists on cognitive performance and scopolamine‐induced amnesia. Behav Brain Res 104: 147–155. [DOI] [PubMed] [Google Scholar]
- Gomez‐Galan M, De Bundel D, Van Eeckhaut A, Smolders I, Lindskog M (2013). Dysfunctional astrocytic regulation of glutamate transmission in a rat model of depression. Mol Psych 18: 582–594. [DOI] [PubMed] [Google Scholar]
- Griebel G, Pichat P, Pruniaux MP, Beeske S, Lopez‐Grancha M, Genet E et al (2012). SAR110894, a potent histamine H(3)‐receptor antagonist, displays procognitive effects in rodents. Pharmacol Biochem Behav 102: 203–214. [DOI] [PubMed] [Google Scholar]
- Griffin MG, Taylor GT (1995). Norepinephrine modulation of social memory: evidence for a time‐dependent functional recovery of behavior. Behav Neurosci 109: 466–473. [DOI] [PubMed] [Google Scholar]
- Grove RA, Harrington CM, Mahler A, Beresford I, Maruff P, Lowy MT et al (2014). A randomized, double‐blind, placebo‐controlled, 16‐week study of the H3 receptor antagonist, GSK239512 as a monotherapy in subjects with mild‐to‐moderate Alzheimer's disease. Curr Alzheimer Res 11: 47–58. [DOI] [PubMed] [Google Scholar]
- Haas HL, Sergeeva OA, Selbach O (2008). Histamine in the nervous system. Physiol Rev 88: 1183–1241. [DOI] [PubMed] [Google Scholar]
- Haig GM, Pritchett Y, Meier A, Othman AA, Hall C, Gault LM et al (2014). A randomized study of H3 antagonist ABT‐288 in mild‐to‐moderate Alzheimer's dementia. J Alzheimers Dis 42: 959–971. [DOI] [PubMed] [Google Scholar]
- Hakanson R, Ronnberg AL, Sjolund K (1972). Fluorometric determination of histamine with OPT: optimum reaction conditions and tests of identity. Annal Biochem 47: 356–370. [DOI] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindmarch, Shamsi Z (2001). The effects of single and repeated administration of ebastine on cognition and psychomotor performance in comparison to triprolidine and placebo in healthy volunteers. Curr Med Res Op 17: 273–281. [PubMed] [Google Scholar]
- Hu W, Chen Z (2017). The roles of histamine and its receptor ligands in central nervous system disorders: An update. Pharmacol Ther 175: 116–132. [DOI] [PubMed] [Google Scholar]
- Hu Y, Sieck DE, Hsu WH (2015). Why are second‐generation H1‐antihistamines minimally sedating? Eur J Pharmacol 765: 100–106. [DOI] [PubMed] [Google Scholar]
- Huang YW, Chen Z, Hu WW, Zhang LS, Wu W, Ying LY et al (2003). Facilitating effect of histamine on spatial memory deficits induced by dizocilpine as evaluated by 8‐arm radial maze in SD rats. Acta Pharmacol Sinica 24: 1270–1276. [PubMed] [Google Scholar]
- Huang ZL, Mochizuki T, Qu WM, Hong ZY, Watanabe T, Urade Y et al (2006). Altered sleep‐wake characteristics and lack of arousal response to H3 receptor antagonist in histamine H1 receptor knockout mice. Proc Nat Acad Sci 103: 4687–4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudkins RL, Josef KA, Becknell NC, Aimone LD, Lyons JA, Mathiasen JR et al (2014). Discovery of (1R,6S)‐5‐[4‐(1‐cyclobutyl‐piperidin‐4‐yloxy)‐phenyl]‐3,4‐diaza‐bicyclo[4.1.0]hep t‐4‐en‐2‐one (R,S‐4a): histamine H(3) receptor inverse agonist demonstrating potent cognitive enhancing and wake promoting activity. Bioorg Med Chem Lett 24: 1303–1306. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Toda K, Taniuchi I, Panula P, Yamatodani A, Tohyama M et al (1990). An analysis of histaminergic efferents of the tuberomammillary nucleus to the medial preoptic area and inferior colliculus of the rat. Exp Brain Res 80: 374–380. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Yamatodani A, Ando‐Yamamoto M, Tohyama M, Watanabe T, Wada H (1988a). Organization of histaminergic fibers in the rat brain. J Comp Neurol 273: 283–300. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Yamatodani A, Shinoda K, Panula P, Watanabe T, Shiotani Y et al (1988b). Histaminergic nerve fibers in the median eminence and hypophysis of rats demonstrated immunocytochemically with antibodies against histidine decarboxylase and histamine. Brain Res 439: 402–405. [DOI] [PubMed] [Google Scholar]
- Josselyn SA, Kohler S, Frankland PW (2015). Finding the engram. Nature reviews. Neuroscience 16: 521–534. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Stewart AM, Gerlai R (2014). Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol Sci 35: 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannidis I, Dehning S, Sandor P, Tarnok Z, Rizzo R, Wolanczyk T et al (2013). Support of the histaminergic hypothesis in Tourette syndrome: association of the histamine decarboxylase gene in a large sample of families. J Med Gen 50: 760–764. [DOI] [PubMed] [Google Scholar]
- Katoh Y, Niimi M, Yamamoto Y, Kawamura T, Morimoto‐Ishizuka T, Sawada M et al (2001). Histamine production by cultured microglial cells of the mouse. Neurosci Lett 305: 181–184. [DOI] [PubMed] [Google Scholar]
- Kerr JS, Dunmore C, Hindmarch I (1994). The psychomotor and cognitive effects of a new antihistamine, mizolastine, compared to terfenadine, triprolidine and placebo in healthy volunteers. Eur J Clin Pharmacol 47: 331–335. [DOI] [PubMed] [Google Scholar]
- Khateb A, Fort P, Pegna A, Jones BE, Muhlethaler M (1995). Cholinergic nucleus basalis neurons are excited by histamine in vitro. Neuroscience 69: 495–506. [DOI] [PubMed] [Google Scholar]
- Khateb A, Serafin M, Muhlethaler M (1990). Histamine excites pedunculopontine neurones in guinea pig brainstem slices. Neurosci Letters 112: 257–262. [DOI] [PubMed] [Google Scholar]
- Kitanaka J, Kitanaka N, Tatsuta T, Morita Y, Takemura M (2007). Blockade of brain histamine metabolism alters methamphetamine‐induced expression pattern of stereotypy in mice via histamine H1 receptors. Neuroscience 147: 765–777. [DOI] [PubMed] [Google Scholar]
- Köhler C, Swanson LW, Haglund L, Wu JY (1985). The cytoarchitecture, histochemistry and projections of the tuberomammillary nucleus in the rat. Neuroscience 16: 85–110. [DOI] [PubMed] [Google Scholar]
- Kohlhauser C, Kaehler S, Mosgoeller W, Singewald N, Kouvelas D, Prast H et al (1999). Histological changes and neurotransmitter levels three months following perinatal asphyxia in the rat. Life Sci 64: 2109–2124. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Haas HL, Brown RE (2002). Histamine excites GABAergic cells in the rat substantia nigra and ventral tegmental area in vitro. Neurosci Lett 320: 133–136. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Sergeeva OA, Ponomarenko AA, Haas HL (2005). Histamine excites noradrenergic neurons in locus coeruleus in rats. Neuropharmacology 49: 129–134. [DOI] [PubMed] [Google Scholar]
- Kraus MM, Prast H, Philippu A (2013). Facilitation of short‐term memory by histaminergic neurons in the nucleus accumbens is independent of cholinergic and glutamatergic transmission. Br J Pharmacol 170: 214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Ito C, Sakurai E, Watanabe T, Ohtsu H (2002). Increased methamphetamine‐induced locomotor activity and behavioral sensitization in histamine‐deficient mice. J Neurochem 83: 837–845. [DOI] [PubMed] [Google Scholar]
- Kukko‐Lukjanov TK, Panula P (2003). Subcellular distribution of histamine, GABA and galanin in tuberomamillary neurons in vitro . J Chem Neuroanat 25: 279–292. [DOI] [PubMed] [Google Scholar]
- Kuroda M, Ishizaki T, Maruyama T, Takatsuka Y, Kuboki T (2007). Effect of dried‐bonito broth on mental fatigue and mental task performance in subjects with a high fatigue score. Physiol Behav 92: 957–962. [DOI] [PubMed] [Google Scholar]
- Le S, Gruner JA, Mathiasen JR, Marino MJ, Schaffhauser H (2008). Correlation between ex vivo receptor occupancy and wake‐promoting activity of selective H3 receptor antagonists. J Pharmacol Exp Ther 325: 902–909. [DOI] [PubMed] [Google Scholar]
- Leger M, Quiedeville A, Bouet V, Haelewyn B, Boulouard M, Schumann‐Bard P et al (2013). Object recognition test in mice. Nat Protoc 8: 2531–2537. [DOI] [PubMed] [Google Scholar]
- Lemaire M (2003). Social recognition task in the rat. Curr Prot Pharmacol Chapter 5: Unit5 30. [DOI] [PubMed] [Google Scholar]
- Lethbridge NL, Chazot PL (2016). Ligand autoradiographical quantification of histamine H3 receptor in human dementia with Lewy bodies. Pharmacol Res 113: 245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Wisor J, Shiba T, Taheri S, Yanai K, Wurts S et al (2002). Measurement of hypocretin/orexin content in the mouse brain using an enzyme immunoassay: the effect of circadian time, age and genetic background. Peptides 23: 2203–2211. [DOI] [PubMed] [Google Scholar]
- Lin RY, Schwartz LB, Curry A, Pesola GR, Knight RJ, Lee HS et al (2000). Histamine and tryptase levels in patients with acute allergic reactions: an emergency department‐based study. J Allergy Clin Immun 106: 65–71. [DOI] [PubMed] [Google Scholar]
- Lin YT, Hsieh TY, Tsai TC, Chen CC, Huang CC, Hsu KS (2018). Conditional deletion of hippocampal CA2/CA3a oxytocin receptors impairs the persistence of long‐term social recognition memory in mice. J Neurosci 38: 1218–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Juraska JM (2007). Social recognition memory: influence of age, sex, and ovarian hormonal status. Physiol Behav 92: 881–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masini D, Lopes‐Aguiar C, Bonito‐Oliva A, Papadia D, Andersson R, Fisahn A et al (2017). The histamine H3 receptor antagonist thioperamide rescues circadian rhythm and memory function in experimental Parkinsonism. Transl Psychiatry 7: e1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ (2010). Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Annals New York Acad Sci 1186: 190–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador KJ, Loring DW, Thompson EE, Thompson WO (1989). Differential cognitive effects of terfenadine and chlorpheniramine. J Allergy Clin Immunol 84: 322–325. [DOI] [PubMed] [Google Scholar]
- Mohsen A, Yoshikawa T, Miura Y, Nakamura T, Naganuma F, Shibuya K et al (2014). Mechanism of the histamine H(3) receptor‐mediated increase in exploratory locomotor activity and anxiety‐like behaviours in mice. Neuropharmacology 81: 188–194. [DOI] [PubMed] [Google Scholar]
- Moriwaki C, Chiba S, Wei H, Aosa T, Kitamura H, Ina K et al (2015). Distribution of histaminergic neuronal cluster in the rat and mouse hypothalamus. J Chem Neuroanat 68: 1–13. [DOI] [PubMed] [Google Scholar]
- Nathan PJ, Boardley R, Scott N, Berges A, Maruff P, Sivananthan T et al (2013). The safety, tolerability, pharmacokinetics and cognitive effects of GSK239512, a selective histamine H(3) receptor antagonist in patients with mild to moderate Alzheimer's disease: a preliminary investigation. Curr Alzheimer Res 10: 240–251. [DOI] [PubMed] [Google Scholar]
- Nautiyal KM, Ribeiro AC, Pfaff DW, Silver R (2008). Brain mast cells link the immune system to anxiety‐like behavior. Proc Nat Acad Sci 105: 18053–18057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson LE, Guo TZ, Lu J, Saper CB, Franks NP, Maze M (2002). The sedative component of anesthesia is mediated by GABA(A) receptors in an endogenous sleep pathway. Nat Neurosci 5: 979–984. [DOI] [PubMed] [Google Scholar]
- Nelson LE, Lu J, Guo T, Saper CB, Franks NP, Maze M (2003). The alpha2‐adrenoceptor agonist dexmedetomidine converges on an endogenous sleep‐promoting pathway to exert its sedative effects. Anesthesiology 98: 428–436. [DOI] [PubMed] [Google Scholar]
- Nozawa Y, Ishizaki T, Kuroda M, Noguchi T (2008). Effect of dried‐bonito broth intake on peripheral blood flow, mood, and oxidative stress marker in humans. Physiol Behav 93: 267–273. [DOI] [PubMed] [Google Scholar]
- Nozawa Y, Mimura M, Yamada K, Sugita M, Shibakusa T, Koyama N (2014). Dried bonito broth improves cognitive function via the histaminergic system in mice. Biomed Res 35: 311–319. [DOI] [PubMed] [Google Scholar]
- Okuyama T, Kitamura T, Roy DS, Itohara S, Tonegawa S (2016). Ventral CA1 neurons store social memory. Science 353: 1536–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olariu A, Tran MH, Yamada K, Mizuno M, Hefco V, Nabeshima T (2001). Memory deficits and increased emotionality induced by beta‐amyloid (25‐35) are correlated with the reduced acetylcholine release and altered phorbol dibutyrate binding in the hippocampus. J Neural Transm 108: 1065–1079. [DOI] [PubMed] [Google Scholar]
- Othman AA, Haig G, Florian H, Locke C, Zhang J, Dutta S (2013). Safety, tolerability and pharmacokinetics of the histamine H3 receptor antagonist, ABT‐288, in healthy young adults and elderly volunteers. Br J Clin Pharmacol 75: 1299–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Wegener G (2013). The flinders sensitive line rat model of depression – 25 years and still producing. Pharmacol Rev 65: 143–155. [DOI] [PubMed] [Google Scholar]
- Panula P, Airaksinen MS, Pirvola U, Kotilainen E (1990). A histamine‐containing neuronal system in human brain. Neuroscience 34: 127–132. [DOI] [PubMed] [Google Scholar]
- Panula P, Chazot PL, Cowart M, Gutzmer R, Leurs R, Liu WLS et al (2015). International Union of Basic and Clinical Pharmacology. XCVIII. Histamine receptors. Pharmacol Rev 67: 601–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panula P, Nuutinen S (2013). The histaminergic network in the brain: basic organization and role in disease. Nat Rev Neurosci 14: 472–487. [DOI] [PubMed] [Google Scholar]
- Panula P, Pirvola U, Auvinen S, Airaksinen MS (1989). Histamine‐immunoreactive nerve fibers in the rat brain. Neuroscience 28: 585–610. [DOI] [PubMed] [Google Scholar]
- Panula P, Yang HY, Costa E (1984). Histamine‐containing neurons in the rat hypothalamus. Proc Nat Acad Sci 81: 2572–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoli V, Boer‐Saccomani C, Hermant JF (2009). H3 receptor antagonists reverse delay‐dependent deficits in novel object discrimination by enhancing retrieval. Psychopharmacology (Berl) 202: 141–152. [DOI] [PubMed] [Google Scholar]
- Passani MB, Blandina P (2011). Histamine receptors in the CNS as targets for therapeutic intervention. Trends Pharmacol Sci 32: 242–249. [DOI] [PubMed] [Google Scholar]
- Passani MB, Giannoni P, Bucherelli C, Baldi E, Blandina P (2007). Histamine in the brain: beyond sleep and memory. Biochem Pharmacol 73: 1113–1122. [DOI] [PubMed] [Google Scholar]
- Patat A, Perault MC, Vandel B, Ulliac N, Zieleniuk I, Rosenzweig P (1995). Lack of interaction between a new antihistamine, mizolastine, and lorazepam on psychomotor performance and memory in healthy volunteers. Br J Clin Parmacol 39: 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul CM, Magda G, Abel S (2009). Spatial memory: theoretical basis and comparative review on experimental methods in rodents. Behav Brain Res 203: 151–164. [DOI] [PubMed] [Google Scholar]
- Potter DD, Pickles CD, Roberts RC, Rugg MD (2000). The effect of cholinergic receptor blockade by scopolamine on memory performance and the auditory P3. J Psychophysiol 14: 11–23. [Google Scholar]
- Prast H, Argyriou A, Philippu A (1996). Histaminergic neurons facilitate social memory in rats. Brain Res 734: 316–318. [PubMed] [Google Scholar]
- Provensi G, Blandina P, Passani MB (2016a). The histaminergic system as a target for the prevention of obesity and metabolic syndrome. Neuropharmacology 106: 3–12. [DOI] [PubMed] [Google Scholar]
- Provensi G, Costa A, Passani MB, Blandina P (2016b). Donepezil, an acetylcholine esterase inhibitor, and ABT‐239, a histamine H3 receptor antagonist/inverse agonist, require the integrity of brain histamine system to exert biochemical and procognitive effects in the mouse. Neuropharmacology 109: 139–147. [DOI] [PubMed] [Google Scholar]
- Raam T, McAvoy KM, Besnard A, Veenema AH, Sahay A (2017). Hippocampal oxytocin receptors are necessary for discrimination of social stimuli. Nat Comm 8: 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapanelli M, Frick L, Bito H, Pittenger C (2017). Histamine modulation of the basal ganglia circuitry in the development of pathological grooming. Proce Nat Acad Sci 114: 6599–6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisberg D, Heuer F (2005). Memory for Emotional Events. Oxford University Press: New York. [Google Scholar]
- Riveros ME, Forray MI, Torrealba F (2015). Infralimbic cortex activation and motivated arousal induce histamine release. Behav Pharmacol 26: 338–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizk A, Curley J, Robertson J, Raber J (2004). Anxiety and cognition in histamine H3 receptor−/− mice. The Eur J Neurosci 19: 1992–1996. [DOI] [PubMed] [Google Scholar]
- Sadek B, Saad A, Sadeq A, Jalal F, Stark H (2016). Histamine H3 receptor as a potential target for cognitive symptoms in neuropsychiatric diseases. Behav Brain Res 312: 415–430. [DOI] [PubMed] [Google Scholar]
- Sakurai E, Gunji E, Iizuka Y, Hikichi N, Maeyama K, Watanabe T (1994). The disposition of thioperamide, a histamine H3‐receptor antagonist, in rats. J Pharm Pharmacol 46: 209–212. [DOI] [PubMed] [Google Scholar]
- Sandi C, Pinelo‐Nava MT (2007). Stress and memory: behavioral effects and neurobiological mechanisms. Neural Plast 2007: 78970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna MD, Ghelardini C, Thurmond RL, Masini E, Galeotti N (2017). Behavioural phenotype of histamine H4 receptor knockout mice: focus on central neuronal functions. Neuropharmacology 114: 48–57. [DOI] [PubMed] [Google Scholar]
- Scammell TE, Estabrooke IV, McCarthy MT, Chemelli RM, Yanagisawa M, Miller MS et al (2000). Hypothalamic arousal regions are activated during modafinil‐induced wakefulness. J Neurosci 20: 8620–8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicker E, Kathmann M (2017). Role of the histamine H3 receptor in the central nervous system. Handb Exp Pharmacol 241: 277–299. [DOI] [PubMed] [Google Scholar]
- Schneider EH, Neumann D, Seifert R (2014a). Modulation of behavior by the histaminergic system: lessons from H(1)R‐and H(2)R‐deficient mice. Neurosci Biobehav 42: 252–266. [DOI] [PubMed] [Google Scholar]
- Schneider EH, Neumann D, Seifert R (2014b). Modulation of behavior by the histaminergic system: lessons from HDC‐, H3R‐ and H4R‐deficient mice. Neurosci Biobehav Rev 47: 101–121. [DOI] [PubMed] [Google Scholar]
- Schneider EH, Seifert R (2016). The histamine H4‐receptor and the central and peripheral nervous system: a critical analysis of the literature. Neuropharmacology 106: 116–128. [DOI] [PubMed] [Google Scholar]
- Schneider T, Przewlocki R (2005). Behavioral alterations in rats prenatally exposed to valproic acid: animal model of autism. Neuropsychopharmacology 30: 80–89. [DOI] [PubMed] [Google Scholar]
- Schneiderman N, Ironson G, Siegel SD (2005). Stress and health: psychological, behavioral, and biological determinants. Ann Rev Clin Psychol 1: 607–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz JC (2011). The histamine H3 receptor: from discovery to clinical trials with pitolisant. Br J Pharmacol 163: 713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selbach O, Brown RE, Haas HL (1997). Long‐term increase of hippocampal excitability by histamine and cyclic AMP. Neuropharmacology 36: 1539–1548. [DOI] [PubMed] [Google Scholar]
- Shan L, Bao AM, Swaab DF (2017). Changes in histidine decarboxylase, histamine N‐methyltransferase and histamine receptors in neuropsychiatric disorders. Handb Exp Pharmacol 241: 259–276. [DOI] [PubMed] [Google Scholar]
- Sherin JE, Elmquist JK, Torrealba F, Saper CB (1998). Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci 18: 4705–4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman AJ, Millar RP, King JA, Zhuang X, Silver R (1994). Mast cells with gonadotropin‐releasing hormone‐like immunoreactivity in the brain of doves. Proc Nat Acad Sci 91: 3695–3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simola N, Bustamante D, Pinna A, Pontis S, Morales P, Morelli M et al (2008). Acute perinatal asphyxia impairs non‐spatial memory and alters motor coordination in adult male rats. Exp Brain Res 185: 595–601. [DOI] [PubMed] [Google Scholar]
- Sundvik M, Panula P (2012). Organization of the histaminergic system in adult zebrafish (Danio rerio) brain: neuron number, location, and cotransmitters. J Comp Neurol 520: 3827–3845. [DOI] [PubMed] [Google Scholar]
- Tabarean IV (2016). Histamine receptor signaling in energy homeostasis. Neuropharmacology 106: 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagawa M, Kano M, Okamura N, Higuchi M, Matsuda M, Mizuki Y et al (2002). Differential cognitive effects of ebastine and (+)‐chlorpheniramine in healthy subjects: correlation between cognitive impairment and plasma drug concentration. Br J Clin Pharmacol 53: 296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Lin JS, Sakai K (2006). Neuronal activity of histaminergic tuberomammillary neurons during wake‐sleep states in the mouse. J Neurosci 26: 10292–10298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda N, Inagaki S, Taguchi Y, Tohyama M, Watanabe T, Wada H (1984). Origins of histamine‐containing fibers in the cerebral cortex of rats studied by immunohistochemistry with histidine decarboxylase as a marker and transection. Brain Res 323: 55–63. [DOI] [PubMed] [Google Scholar]
- Taubel J, Ferber G, Fernandes S, Lorch U, Santamaria E, Izquierdo I (2016). Pharmacokinetics, safety and cognitive function profile of rupatadine 10, 20 and 40 mg in healthy Japanese subjects: a randomised placebo‐controlled trial. PloS one 11: e0163020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar MM (2011). Histamine in the regulation of wakefulness. Sleep Med Rev 15: 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theunissen EL, Vermeeren A, van Oers AC, van Maris I, Ramaekers JG (2004). A dose‐ranging study of the effects of mequitazine on actual driving, memory and psychomotor performance as compared to dexchlorpheniramine, cetirizine and placebo. Clin Exp Allergy 34: 250–258. [DOI] [PubMed] [Google Scholar]
- Thor DH, Holloway WR (1982). Social memory of the male laboratory rat. J Comp Physiol Psychol 96: 1000–1006. [Google Scholar]
- Toyota H, Dugovic C, Koehl M, Laposky AD, Weber C, Ngo K et al (2002). Behavioral characterization of mice lacking histamine H(3) receptors. Mol Pharmacol 62: 389–397. [DOI] [PubMed] [Google Scholar]
- Trofimiuk E, Braszko JJ (2014). Single dose of H3 receptor antagonist – ciproxifan – abolishes negative effects of chronic stress on cognitive processes in rats. Psychopharmacology (Berl) 231: 209–219. [DOI] [PubMed] [Google Scholar]
- Trottier S, Chotard C, Traiffort E, Unmehopa U, Fisser B, Swaab DF et al (2002). Co‐localization of histamine with GABA but not with galanin in the human tuberomamillary nucleus. Brain Res 939: 52–64. [DOI] [PubMed] [Google Scholar]
- Turner C, Handford AD, Nicholson AN (2006). Sedation and memory: studies with a histamine H‐1 receptor antagonist. J Psychopharmacol 20: 506–517. [DOI] [PubMed] [Google Scholar]
- Valdés JL, Sánchez C, Riveros ME, Blandina P, Contreras M, Farías P et al (2010). The histaminergic tuberomammillary nucleus is critical for motivated arousal. Eur J Neurosci 31: 2073–2085. [DOI] [PubMed] [Google Scholar]
- van der Kooij MA, Sandi C (2012). Social memories in rodents: methods, mechanisms and modulation by stress. Neurosci Biobehav Rev 36: 1763–1772. [DOI] [PubMed] [Google Scholar]
- van Ruitenbeek P, Vermeeren A, Riedel WJ (2010a). Histamine H1 receptor antagonist cetirizine impairs working memory processing speed, but not episodic memory. Br J Pharmacol 161: 456–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ruitenbeek P, Vermeeren A, Riedel WJ (2010b). Memory in humans is unaffected by central H1‐antagonism, while objectively and subjectively measured sedation is increased. Eur Neuropsychopharmacol 20: 226–235. [DOI] [PubMed] [Google Scholar]
- Vogel‐Ciernia A, Wood MA (2014). Examining object location and object recognition memory in mice. Curr Prot Neurosci 69: 31–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuurman EF, Uiterwijk MM, Rosenzweig P, O'Hanlon JF (1994). Effects of mizolastine and clemastine on actual driving and psychomotor performance in healthy volunteers. Eur J Clin Pharmacol 47: 253–259. [DOI] [PubMed] [Google Scholar]
- Wacker DW, Ludwig M (2012). Vasopressin, oxytocin, and social odor recognition. Horm Behav 61: 259–265. [DOI] [PubMed] [Google Scholar]
- Wada H, Inagaki N, Itowi N, Yamatodani A (1991). Histaminergic neuron system in the brain: distribution and possible functions. Brain Res Bull 27: 367–370. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Taguchi Y, Shiosaka S, Tanaka J, Kubota H, Terano Y et al (1984). Distribution of the histaminergic neuron system in the central nervous system of rats; a fluorescent immunohistochemical analysis with histidine decarboxylase as a marker. Brain Res 295: 13–25. [DOI] [PubMed] [Google Scholar]
- Weible AP, Rowland DC, Pang R, Kentros C (2009). Neural correlates of novel object and novel location recognition behavior in the mouse anterior cingulate cortex. J Neurophysiol 102: 2055–2068. [DOI] [PubMed] [Google Scholar]
- Williams MT, Blankenmeyer TL, Schaefer TL, Brown CA, Gudelsky GA, Vorhees CV (2003a). Long‐term effects of neonatal methamphetamine exposure in rats on spatial learning in the Barnes maze and on cliff avoidance, corticosterone release, and neurotoxicity in adulthood. Brain Res Develop Brain Res 147: 163–175. [DOI] [PubMed] [Google Scholar]
- Williams MT, Morford LL, Wood SL, Wallace TL, Fukumura M, Broening HW et al (2003b). Developmental D‐methamphetamine treatment selectively induces spatial navigation impairments in reference memory in the Morris water maze while sparing working memory. Synapse 48: 138–148. [DOI] [PubMed] [Google Scholar]
- Williams RH, Chee MJ, Kroeger D, Ferrari LL, Maratos‐Flier E, Scammell TE et al (2014). Optogenetic‐mediated release of histamine reveals distal and autoregulatory mechanisms for controlling arousal. J Neurosci 34: 6023–6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JT, Insel TR (2004). Neuroendocrine basis of social recognition. Curr Op Neurobiol 14: 248–253. [DOI] [PubMed] [Google Scholar]
- Winters BD, Saksida LM, Bussey TJ (2008). Object recognition memory: neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci Biobehav Rev 32: 1055–1070. [DOI] [PubMed] [Google Scholar]
- Wright C, Shin JH, Rajpurohit A, Deep‐Soboslay A, Collado‐Torres L, Brandon NJ et al (2017). Altered expression of histamine signaling genes in autism spectrum disorder. Transl Psychiatry 7: e1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie JF, Fan K, Wang C, Xie P, Hou M, Xin L et al (2017). Inactivation of the tuberomammillary nucleus by GABAA receptor agonist promotes slow wave sleep in freely moving rats and histamine‐treated rats. Neurochem Res 42: 2314–2325. [DOI] [PubMed] [Google Scholar]
- Xu C, Michelsen KA, Wu M, Morozova E, Panula P, Alreja M (2004). Histamine innervation and activation of septohippocampal GABAergic neurones: involvement of local ACh release. J Physiol 561 (Pt. 3): 657–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakami J, Sakurai E, Kuramasu A, Yanai K, Watanabe T, Tanaka Y (2000). L‐Histidine decarboxylase protein and activity in rat brain microvascular endothelial cells. Inflamm Res 49: 231–235. [DOI] [PubMed] [Google Scholar]
- Yang M, Silverman JL, Crawley JN (2011). Automated three‐chambered social approach task for mice. Curr Protoc Neurosci Chapter 8: Unit 8 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Ye Z, Houston CM, Zecharia AY, Ma Y, Zhang Z et al (2015). Wakefulness is governed by GABA and histamine cotransmission. Neuron 87: 164–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhuang QX, Li B, Wu GY, Yung WH, Zhu JN et al (2016). Selective modulation of histaminergic inputs on projection neurons of cerebellum rapidly promotes motor coordination via HCN channels. Mol Neurobiol 53: 1386–1401. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Silverman AJ, Silver R (1999). Distribution and local differentiation of mast cells in the parenchyma of the forebrain. J Comp Neurol 408: 477–488. [DOI] [PubMed] [Google Scholar]
- Zlomuzica A, Dere D, Binder S, De Souza Silva MA, Huston JP, Dere E (2016). Neuronal histamine and cognitive symptoms in Alzheimer's disease. Neuropharmacology 106: 135–145. [DOI] [PubMed] [Google Scholar]
- Zlomuzica A, Dere D, Dere E (2013). The histamine H1 receptor and recollection‐based discrimination in a temporal order memory task in the mouse. Pharmacol Biochem Behav 111: 58–63. [DOI] [PubMed] [Google Scholar]
- Zlomuzica A, Viggiano D, De Souza Silva MA, Ishizuka T, Gironi Carnevale UA, Ruocco LA et al (2008). The histamine H1‐receptor mediates the motivational effects of novelty. Eur J Neurosci 27: 1461–1474. [DOI] [PubMed] [Google Scholar]


