Abstract
Intraventricular hemorrhage (IVH) is an independent poor prognostic factor in subarachnoid and intra-parenchymal hemorrhage. The use of intraventricular fibrinolytics (IVF) has long been debated, and its exact effects on outcomes are unknown. A systematic review and meta-analysis were performed in accordance with the PRISMA guidelines to assess the impact of IVF after non-traumatic IVH on mortality, functional outcome, intracranial bleeding, ventriculitis, time until clearance of third and fourth ventricles, obstruction of external ventricular drains (EVD), and shunt dependency. Nineteen studies were included in the meta-analysis, totaling 1020 patients. IVF was associated with lower mortality (relative risk [RR] 0.58; 95% confidence interval [CI] 0.47–0.72), fewer EVD obstructions (RR 0.41; 95% CI 0.22–0.74), and a shorter time until clearance of the ventricles (median difference [MD] − 4.05 days; 95% CI − 5.52 to − 2.57). There was no difference in good functional outcome, RR 1.41 (95% CI 0.98–2.03), or shunt dependency, RR 0.93 (95% CI 0.70–1.22). Correction for publication bias predicted an increased risk of intracranial bleeding, RR 1.67 (95% CI 1.01–2.74) and a lower risk of ventriculitis, RR 0.68 (95% CI 0.45–1.03) in IVH patients treated with IVF. IVF was associated with improved survival, faster clearance of blood from the ventricles and fewer drain obstructions, but further research is warranted to elucidate the effects on ventriculitis, long-term functional outcomes, and re-hemorrhage.
Electronic supplementary material
The online version of this article (10.1007/s12028-019-00786-5) contains supplementary material, which is available to authorized users.
Keywords: Cerebral ventricles, Cerebral hemorrhage, External ventricular drain, Intraventricular, Fibrinolytic agents
Introduction
The presence of an intraventricular hemorrhage (IVH) in subarachnoid hemorrhage (SAH), intra-parenchymal hemorrhage (IPH), and, to a lesser extent, traumatic brain injury is an independent risk factor for poor outcomes [1, 2]. By obstructing the flow of cerebrospinal fluid (CSF), IVH can cause acute hydrocephalus necessitating CSF diversion, typically in the form of an external ventricular drain (EVD) [1]. Management of this EVD is often complicated by obstruction of the shunt with blood clots, which leads to poor intracranial pressure control and an increased risk of infection [3]. Multiple replacements of EVDs and long EVD drainage are regularly needed when managing post-IVH hydrocephalus, which are both linked to an increased risk of ventriculitis [4, 5]. Aside from hydrocephalus, the mass effect of the blood clot can hamper perfusion of local tissue leading to ischemia [6], while the presence of blood and blood-degradation products in the CSF contributes to periventricular edema, neural cell death, and arachnoidal fibrosis [7]. Combined, these factors conduce to the development of communicating hydrocephalus, with many patients showing persistent dependence on CSF diversion long after the dissolvement of the initial blood clot, necessitating placement of permanent shunts [8, 9].
To address the complications related to IVH and IVH-related EVD use, efforts have been made to accelerate the removal of ventricular blood. The intraventricular injection of fibrinolytic agents, referred to as intraventricular fibrinolysis (IVF), in patients suffering from IVH was first described in 1990 [10] and has thus far shown mixed results [11–14]. The American Heart Association/American Stroke Association guidelines conclude that the efficacy and safety of fibrinolytics in IVH are uncertain [15]. Recently, the CLEAR-III study [14] evaluated the effect of IVF for IVH resulting from small IPHs and observed a decreased risk of mortality, which was endorsed by previous meta-analyses [16, 17], while no improvement of good functional outcome (GFO) (modified Rankin Score [mRS] equal to or lower than 3) was observed [14]. Previous systematic reviews have reached different conclusions regarding functional outcome, and discussions regarding the safety of (repeated) injections of fibrinolytic agents into fresh hemorrhages are ongoing [16–19]. Moreover, a thorough meta-analysis including meta-regression and assessment of publication bias in different outcomes has thus far lacked.
In light of the conflicting results in literature and the recent findings of the CLEAR-III trial [14], we sought to provide an updated systematic review and meta-analysis regarding the use of fibrinolytics in the treatment of non-traumatic IVH. We assessed patient mortality, functional outcome, shunt dependency and time until clearance of the ventricles, as well as complications related to EVD treatment including obstruction rate, ventriculitis, and incidence of post-treatment intracerebral hemorrhage.
Methods
Literature Search
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [20]. All data were gathered from publicly available sources. In December of 2017, we searched the PubMed and Embase databases for articles comparing the use of intraventricular fibrinolytics via EVD versus EVD alone in the treatment of non-traumatic IVH. Keywords, MeSH terms, and Emtree terms including IVH, cerebral hemorrhage, clot, thrombus, thrombolysis, fibrinolytic therapy, plasminogen activator, and synonyms were combined with the help of a librarian to form our search strategy (Supplementary Table I). Both the title/abstract screening and the full-text screening were performed independently by two reviewers (T.S. and V.K.), with discrepancies resolved via discussion with a third reviewer (O.A.). The reference lists of full-text articles were screened for additional studies.
Study Selection
Studies were excluded if they were not written in the English language or if the full text was not available. When the outcomes of studies were reported in multiple publications, the publication with the largest patient cohort with relevant outcome data was included in this review. Results from case reports, reviews, registry data, abstracts, and replies/commentaries were excluded. Eligibility of studies was assessed using the following PICOS criteria: Participants (P): patients with a non-traumatic IVH; Intervention (I): EVD with injection of fibrinolytics; Control (C): EVD alone; Outcomes (O): mortality, functional outcome, occurrence of ventriculitis, shunt dependency, intracranial bleeding after start of treatment, obstruction of EVD, and time until clearance of the third and fourth ventricles; and Study design (S): we selected randomized controlled trials (RCTs), prospective cohort studies and, due to the scarcity of studies, also included retrospective cohort studies and matched case–control series. For inclusion criteria and details regarding data extraction, see Supplementary Methods.
Data Extraction
The following data were extracted from each study: name of first author, year of publication, journal of publication, country of origin, trial design, inclusion and exclusion criteria, treatment, number of patients, time of follow-up, end-point mortality, functional outcomes and incidences of ventriculitis, shunt placement, symptomatic bleeding, obstruction rates, and time until clearance of third and fourth ventricles on computed tomography (CT). GFO was defined as a score of 3 or lower on the mRS or 4 and higher on the Glasgow Outcome Scale (GOS), assessed at least 3 months after start of therapy. ‘Intracranial bleeding’ was defined as symptomatic hemorrhage after the start of intraventricular treatment. For patient characteristics, mean age and sex were extracted. The quality of non-randomized studies was assessed using the Newcastle–Ottawa scale (NOS) for non-randomized studies [21] and the Jadad-score [22] for RCTs. Assessment was done by two reviewers (T.S. and V.K.), with discrepancies solved through discussion. A study was considered of higher quality if the score was equal to or higher than the median score of the studies included (4 > = for Jadad, 6 > = for NOS).
Statistical Analysis
For outcome analyses, both random- and fixed-effect models were used to obtain risk ratios (RRs) with 95% confidence intervals (CIs) for dichotomous outcomes. Forest plots were created using the random-effects model. For continuous outcomes, we extracted the reported means and evaluated the mean difference using both the random- and fixed-effect models, with the forest plot created with the random-effects model. Heterogeneity was assessed using Cochran’s Q test (p < 0.10) and the I2 statistic. If I2 > 50%, heterogeneity was deemed considerable [23]. Details considering the meta-regression analysis and assessment of publication bias via the trim-fill method can be found in Supplementary Methods. p values < 0.05 were considered statistically significant unless otherwise specified. All analyses were performed in R v3.4.1 (The R Foundation for Statistical Computing) using the ‘Metafor’ package [24].
Results
Our search strategy resulted in the retrieval of 3518 unique studies. After careful screening and assessment, nineteen articles were included in the meta-analysis (Supplementary Fig. 1) [11–14, 25–39]. Eight studies were classified as RCTs [14, 25, 29, 30, 33, 37–39], six as retrospective cohort studies [11, 12, 28, 31, 34, 35], and five as matched case–control studies [13, 26, 27, 32, 36]. The majority of studies focused on IVH in the setting of IPH (68%) compared to SAH (26%), with the remainder including both IPH and SAH patients (5%). The use of recombinant tissue plasminogen activator ((r)t-PA) increased over the years and was slightly more common than urokinase, 55% versus 45% of the studies, respectively. Additional study details can be found in Table 1.
Table 1.
Study characteristics
| References | Region | Design | Origin of IVH | Patients | Fibrinolytic | Dose | Quality (NOS/Jadad) | Impact factor | |
|---|---|---|---|---|---|---|---|---|---|
| IVF + EVD | EVD | ||||||||
| Akdemir et al. [30] | Middle East | RCT | IPH and SAH | 7 | 9 | UK | 5000 IU/12 h | 2a | 2.06 |
| Coplin et al. [34] | N-America | RCS | IPH | 22 | 18 | UK | 10,000 IU/12 h | 6b | 5.72 |
| Ducruet et al. [35] | N-America | RCS | IPH | 13 | 17 | tPA | 1–3 mg/12 h | 5b | 4.89 |
| Dunatov et al. [12] | Europe | RCS | IPH | 48 | 49 | rt-PA | 1 mg/12 h | 5b | 3.09 |
| Findlay et al. [31] | N-America | RCS | SAH | 21 | 9 | rt-PA | 4 mg/24 h | 5b | 4.89 |
| Gerner et al. [32] | Europe | M-CS | SAH | 14 | 14 | rt-PA | 1 mg/8 h | 7b | 2.75 |
| Hallevi et al. [11] | Middle East | RCS | IPH | 18 | 11 | tPA | 1–2 mg/24 h | 5b | 2.47 |
| Hanley et al. [14] | N-America | RCT | IPH | 249 | 251 | tPA | 1 mg/8 h | 5a | 44 |
| Huttner et al. [36] | Europe | M-CS | IPH | 22 | 22 | rt-PA | 2–4 mg/12 h | 8b | 3.96 |
| King et al. [37] | Asia | RCT | IPH | 7 | 9 | UK | 25,000 IU/12 h | 5a | 1.38 |
| Kramer et al. [33] | N-America | RCT | SAH | 6 | 6 | tPA | 2 mg/12 h | 5a | 2.75 |
| Litrico et al. [38] | Europe | RCT | SAH | 11 | 8 | rt-PA | 3 mg/12 h | 3a | 2.06 |
| Naff et al. [39] | N-America | RCT | IPH | 6 | 5 | UK | 25,000 IU/12 h | 4a | 4.89 |
| Naff et al. [25] | N-America | RCT | IPH | 26 | 22 | rt-PA | 3 mg/12 h | 3a | 5.72 |
| Rainov and Burkert [26] | Europe | M-CS | IPH | 16 | 5 | UK | 10,000 IU/12 h | 6b | 2.06 |
| Todo et al. [27] | Asia | CS | IPH and SAH | 6 | 4 | UK | 10,000 IU/12 h | 4b | 3.74 |
| Torres et al. [28] | Europe | RCS | IPH | 14 | 14 | UK | 10,000 IU/12 h | 6b | 0.96 |
| Tung et al. [29] | Asia | RCT | IPH | 10 | 11 | UK | 50,000/12 h | 1a | 0.96 |
| Varelas et al. [13] | N-America | M-CS | SAH | 10 | 10 | tPA | 2 mg/12 h | 7b | 4.89 |
CS case–control, EVD extraventricular drain, IPH intra-parenchymal hemorrhage, IU international units, IVH intraventricular hemorrhage, IVF intraventricular fibrinolysis, M-CS matched case–control, mg milligram, NA not assessed, NOS Newcastle–Ottawa Outcome Scale, RCS retrospective cohort study, RCT randomized controlled trial, rt-PA recombinant tissue plasminogen activator, SAH subarachnoid hemorrhage, tPA tissue plasminogen activator, UK urokinase
aScored using Jadad scale, 1–5 points
bScored using NOS scale, 1–9 points (high quality defined as a score of 4 > = for Jadad, 6 > = for NOS)
Collectively, 1020 patients were included in this meta-analysis of which 526 received intraventricular fibrinolytics. Male patients constituted 55.9% of the studied population, and the overall mean age was 56 years (median 56 years). Patients who received IVF had a mean age of 56.0 years (median 55.5), while those receiving EVD only had a mean age of 56.1 years (median 56). More study details and the assessment of bias regarding RCTs can be found in Table I, Supplementary Tables 2–5, and in Supplementary Results section.
Mortality
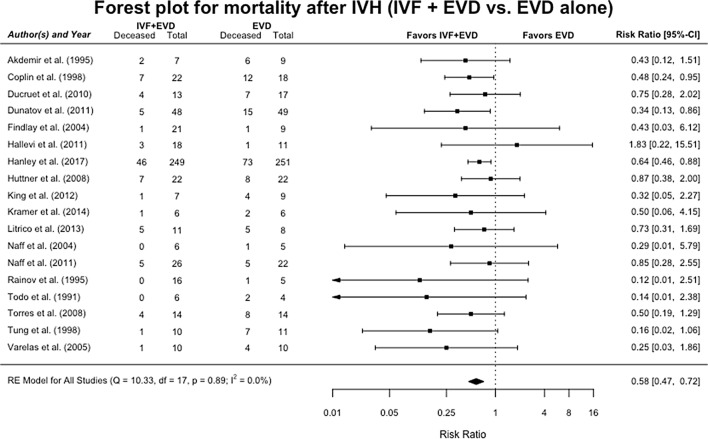
Eighteen studies reported on mortality in patients with IVH [11–14, 25–31, 33–39]. Pooled analysis showed a significant decrease in mortality risk for patients receiving IVF treatment with EVD compared to patients receiving EVD alone in both the fixed- and random-effect models, with RR 0.58 (95% CI 0.47–0.72) for both models (Fig. 1). Heterogeneity was low in the random-effects (RE) model (I2 = 0%, p-heterogeneity = 0.89). Meta-regression found no sources for confounding. Egger’s test and Begg’s test were not significant, with p = 0.14 and p = 0.15, respectively. The funnel plot showed a possible bias but correction via the trim-fill method (Supplementary Fig. 2) did not yield a significantly different model.
Fig. 1.
Forest plot for mortality after intraventricular hemorrhage. Pooled risk ratios for mortality in patients receiving IVF and EVD versus those being treated with EVD alone, in a random-effects model. Solid squares represent the point estimate of each study, with 95% CI being shown in error bars. The diamond represents the pooled estimate of the risk ratios. I2 and p values for heterogeneity are shown. CI confidence interval, EVD external ventricular drain, IVF intraventricular fibrinolysis, IVH intraventricular hemorrhage, RE random effects
Functional Outcome
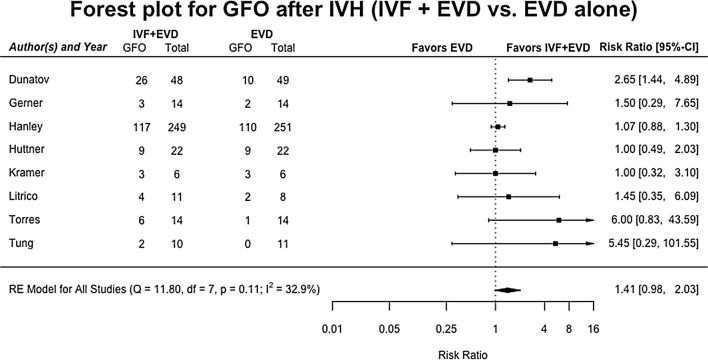
Functional outcome was assessed in eight studies totaling 749 patients [12, 14, 28, 29, 32, 33, 36, 38]. GFO did not differ after IVF treatment compared to patients receiving EVD alone with RR 1.41 (95% CI 0.98–2.03), in both fixed- and random-effect models (Fig. 2). Heterogeneity of the RE model was moderate, at 32.9%, p = 0.11. Meta-regression analysis showed that study quality was an effect modifier with β = 0.83, p = 0.005, as was study design, with retrospective cohort studies having a β = 0.90, p = 0.006, with RCT as reference category. Curiously, the impact factor of the journal in which the study was published was also an effect modifier, with β = 0.02, p = 0.03, as was the country in which the study was done: studies in Europe modified the effect with β = 0.52, p = 0.03, compared to North-American studies (Supplementary Table 6). Egger’s test was significant at p = 0.04, Begg’s test was not, p = 0.11. The funnel plot showed possible bias in reporting, but correction via the trim-and-fill method (Supplementary Fig. 3) did not predict a significant new model, with RR 1.31 (95% CI 0.93–1.85).
Fig. 2.
Forest plot for good functional outcome after intraventricular hemorrhage. Pooled risk ratios for good functional outcome in patients receiving IVF and EVD versus those being treated with EVD alone, in a random-effects model. It should be noted that GFO is a positive outcome, with a higher RR indicating a higher chance of this occurring in the intervention group compared to the control. Solid squares represent the point estimate of each study, with 95% CI being shown in error bars. The diamond represents the pooled estimate of the risk ratios. I2 and p values for heterogeneity are shown. CI confidence interval, EVD external ventricular drain, GFO good functional outcome, IVF intraventricular fibrinolysis, IVH intraventricular hemorrhage, RE random effects
Ventriculitis
Information regarding the incidence of ventriculitis was available from 15 studies [12–14, 25–31, 33–35, 37, 38]. Ventriculitis rates were not significantly lower in patients using IVF with EVD compared to patients receiving EVD alone: 0.68 (95% CI 0.45–1.03) for both the fixed- and random-effect models, p = 0.06 (Supplementary Fig. 4). There was no evidence for heterogeneity in the RE model (I2 = 0%, p = 0.97), and no factor was identified as a source of heterogeneity through meta-regression. Egger’s test (p = 0.44) and Begg’s test (p = 0.86) were not significant. The funnel plot showed a possible indication for bias (Supplementary Fig. 5), and correction via the trim-fill method did yield a significant model, with a decreased risk of ventriculitis (RR 0.61, 95% CI 0.41–0.91, p = 0.02), with no heterogeneity (0%, p = 0.94).
Bleeding
Symptomatic intracranial bleeding after start of therapy was evaluated in 926 patients in 14 studies [12–14, 25–28, 30, 31, 33–36, 38, 39]. The fixed- and random-effect models showed no significant impact of the treatment on outcome (RR 1.50, 95% CI 0.89–2.52), with low heterogeneity (I2: 0%, p = 0.99) in the RE model (Supplementary Fig. 6). Meta-regression showed that none of the factors contributed to heterogeneity. Egger’s (p = 0.44) and Begg’s (p = 0.85) tests were not significant, but the funnel plot showed possible bias (Supplementary Fig. 7). Correction for publication bias via the trim-fill method predicted a significant random-effects model with RR 1.67 (95% CI 1.01–2.74) with low heterogeneity (I2: 0%, p = 0.99).
Obstruction
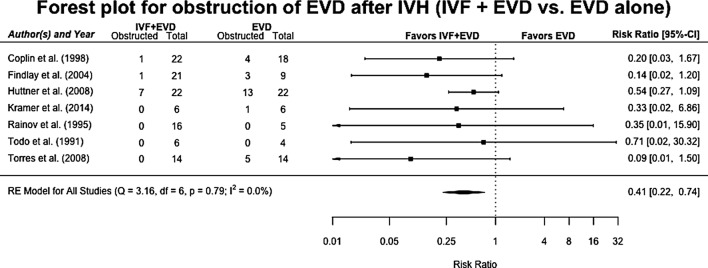
EVD obstruction rate was evaluated in seven studies totaling 185 patients [26–28, 31, 33, 34, 36]. Obstruction rates were significantly lower in patients treated with IVF compared to patients receiving EVD alone with a RR of 0.41 (95% CI 0.22–0.74) in both the fixed- and random-effect models (Fig. 3). In the RE model, heterogeneity was low at I2 = 0% with p = 0.79. No sources of heterogeneity were identified by meta-regression. Funnel plot, Egger’s test (p = 0.26), and Begg’s test (p = 1.0) did not indicate publication bias.
Fig. 3.
Forest plot for obstruction after intraventricular hemorrhage. Pooled risk ratios for obstruction in patients receiving IVF and EVD versus those being treated with EVD alone, in a random-effects model. Solid squares represent the point estimate of each study, with 95% CI being shown in error bars. The diamond represents the pooled estimate of the risk ratios. I2 and p values for heterogeneity are shown. CI confidence interval, EVD external ventricular drain, IVF intraventricular fibrinolysis, IVH intraventricular hemorrhage, RE random effects
Time to IVH Resolution
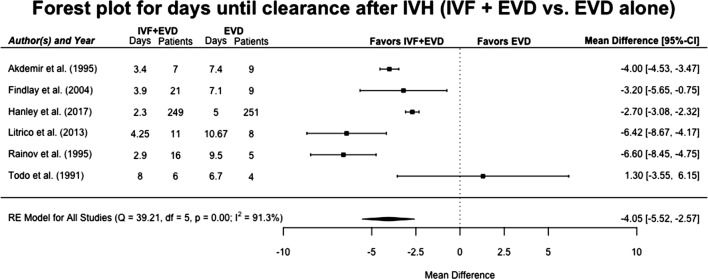
Clearance of the third and fourth ventricles was assessed in six studies totaling 596 patients [14, 26, 27, 30, 31, 38]. The random-effects model showed a significantly faster ventricular clearance in patients receiving IVF with EVD compared to patients receiving EVD alone (mean difference − 4.05 days, 95% CI between − 5.52 and − 2.57) (Fig. 4). The fixed-effect model showed similar, yet slightly weaker, results (mean difference − 3.27 days (95% CI between − 3.57 and − 2.97). Heterogeneity was high in the RE model, however, with I2: 91.3%, p < 0.0001. Study country (Europe: β = −3.82, p < 0.001, Middle East: β = −1.29, p = 0.0001, North America as reference), design (case–control: β = −2.81, p = 0.035, RCT as reference), size (β = 0.003, p < 0.0001), and the impact factor of the journal in which the study was published (β = 0.03, p < 0.0001) all interfered with outcome, as did patient age (β = 0.46, p < 0.0001) (Supplementary Table 6). The funnel plot, Egger’s test (0.52), and Begg’s test (0.72) indicated low possibility of bias.
Fig. 4.
Forest plot for days until clearance of third and fourth ventricles after intraventricular hemorrhage. Mean differences for days until clearance of ventricles in patients receiving IVF and EVD versus those being treated with EVD alone, in a random-effects model. Solid squares represent the point estimate of each study, with 95% CI being shown in error bars. The diamond represents the pooled estimate of the median differences. I2 and p values for heterogeneity are shown. CI confidence interval, EVD external ventricular drain, IVF intraventricular fibrinolysis, IVH intraventricular hemorrhage, RE random effects
Shunt Dependency
Shunt dependency after IVH was assessed in 16 studies [12–14, 26, 27, 29–36, 38, 39]. There was no difference between IVF + EVD and EVD alone in the risk shunt dependency using the random-effect or fixed-effect models (RR 0.93, 95% CI 0.70–1.22, p = 0.59). No heterogeneity was found in the RE model, I2: 0%, p = 0.71. Average age was identified as a possible source of heterogeneity, with β = 0.11 (p = 0.048) (Supplementary Table 6). Egger’s and Begg’s tests were not significant, with p = 0.27 and p = 0.33, respectively. The funnel plot showed possible publication bias (Supplementary Fig. 8), but correction via the trim-fill method did not yield a significantly different model.
IVF in SAH Versus IPH
Since SAH and IPH have very different underlying pathologies, we analyzed differences between these patient groups. The origin of the IVH (IPH or SAH) showed no significant impact on any of the outcomes in our meta-regression analysis (Supplementary Table 6). We performed a subgroup analysis to further evaluate this finding, comparing IPH only studies (12 studies, 885 patients) with SAH only studies (five studies, 109 patients). Risk of mortality, obstruction, and clearance of the ventricles remained significantly improved in IPH patients receiving IVF, while only the risk of faster clearance of the ventricles was significantly improved in SAH patients with IVF (data not shown).
Discussion
Our findings showed that patients receiving IVF for non-traumatic IVH had a lower risk of mortality and EVD obstruction, and a faster clearance of blood from the third and fourth ventricles compared to patients receiving only an EVD. There was no significant difference in GFO, shunt dependency, ventriculitis, and bleeding. However, in a sensitivity analysis, correction for publication bias estimated an increased risk of intracranial bleeding and a lower risk of ventriculitis for patients receiving IVF.
Previous systematic reviews have been cautiously optimistic regarding the use of IVF in IVH [16, 17, 40]. Recently, a systematic review from Wang et al. [16] concluded that IVF was associated with lower risk of mortality (RR 0.63, 95% CI 0.47–0.83) and ventriculitis (RR 0.57, 95% CI 0.35–0.93), but did not change the likelihood of poor functional outcome (RR 0.96, 95% CI 0.83–1.11), shunt dependence (RR 1.06, 95% CI 0.75–1.49), or re-hemorrhage (RR 0.57, 95% CI 0.35–0.93). At the same time, Baker et al. [17] published a systematic review regarding IVF in IPH specifically, and they concluded that IVF-treated patients had an increased likelihood of GFO (RR 0.67, 95% CI 0.55–0.83), with ‘GFO’ defined as having a GOS of 3 or higher. We felt that a GFO should be defined as GOS 4 or higher, as GOS 3 is defined as ‘severe injury with permanent need for help with daily living’ [41]. Our analysis showed no difference in GFO, albeit there was a trend toward improved GFO after IVF. Publication bias was not assessed for GFO by Baker et al. or for any outcome by Wang et al., while our funnel plot with trim-fill analysis indicated the presence of this. However, correction via the trim-fill method did not predict a different model, but did lessen the trend observed in the uncorrected model. We included more patients than previous systematic reviews. Due to our strict inclusion criteria for the purpose of reducing heterogeneity, some studies previously reviewed in other meta-analyses were excluded from our analysis (see Supplementary Table 7). The findings from our meta-analysis aligned with the results of the CLEAR-III trial [14]: Similar outcomes were observed with respect to mortality (hazard ratio 0.60, 95% CI 0.41–0.86) and GFO (RR 1.06, 95% CI 0.88–1.28). Risk of ventriculitis (RR 0.55, 95% CI 0.64–0.90) was lower in their study, which underscores our results from the trim-fill analysis.
An interesting find was the predicted increase in symptomatic hemorrhages in patients receiving IVF, after trim-fill correction for publication bias. The number of these events was small in most studies, with two studies reporting much higher incidences of hemorrhage post-treatment than the average [25, 35]. Both examined IVF specifically in IPH and used rt-PA at a dose of 6 mg/24 h. This is above the median of 4 mg/24 h for all (r)t-PA studies, although Ducruet et al. [35] lowered their dosing during the study. The trim-fill analysis indicated that this serious complication might be underestimated because of publication bias. However, recent studies, such as the CLEAR-III trial, have been developed with stringent safety criteria in place to prevent complications, such as clot stability confirmed on CT scan at least 6 h after EVD placement, low doses spread over 24 h, and daily CT scans to detect complications early [14]. These studies provide better precautions to prevent hemorrhage compared to earlier studies which has increased the safety of IVF use. Future work should stress the importance of these precautions and provide further data on the risk of symptomatic bleeding after IVF.
Despite the obvious differences in underlying pathology, only minor differences in outcomes were observed for IVF in IVH between SAH and IPH. There were fewer studies evaluating the use of IVF in SAH, and thus, not all results were significant, but similar trends were observed. It is important to note that this does not mean outcomes are similar between IPH and SAH, but that IVF does not seem to have a different effect in these different pathologies. Given the small number of studies investigating IVF in SAH, it could be that more nuanced differences were missed in this meta-analysis. Currently, the FIVHeMA study (Intraventricular Fibrinolysis for Aneurysmal SAH, clinicaltrials.gov: NCT03187405) is recruiting patients, which will provide further insights into the use of fibrinolytics for IVH in SAH.
To our knowledge, this is the first systematic review and meta-analysis on this subject that employs meta-regression and assesses publication bias via the trim-fill method. The meta-regression analysis allowed us to analyze the influence of multiple variables on the outcomes after IVH. Not only did we account for different study variables such as quality, region of study, study size, and impact factor of the journal, we were also able to assess the impact of the origin of the IVH and the type of treatment received, although it was not possible to account for dosage. The type of fibrinolytic used did not appear to impact outcomes, contrary to previous publications [16, 40].
Finally, this is the first meta-analysis in which obstruction and time until clearance of ventricles, both important variables for clinicians working with EVD, have been evaluated in relation to IVF.
Previous studies observed a decrease in ventriculitis with the use of IVF [14, 16, 40]. In our meta-analysis, we observed a clear trend, but the difference was not significant. However, possible publication bias was observed and correction via the trim-fill method predicted a significant model with a RR that was in line with results from the CLEAR-III trial, which showed an RR of 0.55, 95% CI 0.31–0.97 [14]. Interestingly, the risk of ventriculitis was shown to decrease, even though repeatedly accessing the ventricles is commonly regarded as increasing the risk of ventriculitis and is commonly avoided in the clinic [18, 42]. This protective association against ventriculitis could probably be attributed to the faster clearance of the ventricles, limiting the time an EVD is in situ, which is also directly related to increased incidence of ventriculitis [4]. Apparently, this outweighs the risk of accessing the EVD repeatedly and injecting medication. The CLEAR-III trial [14] used a saline control, while in other trials the EVDs of control patients were not accessed [26, 27, 30, 38, 43], further highlighting the positive effects of IVF therapy on ventriculitis rates.
Staykov et al. recently showed the benefits of combining IVF with lumbar drainage in patients with IPH-related IVH regarding shunt dependency [43]. None of the 14 included patients treated with IVF and lumbar drainage developed the need for permanent shunting compared to 7 of 16 (44%) patients treated with IVF alone, p = 0.007. Lumbar drainage was outside the scope of this review, but as shunt dependency is an important outcome for patients and clinicians, we evaluated this in our analysis. We did not observe differences in shunt dependency between the two groups. It must be noted, however, that the criteria and thresholds to place permanent shunting varied between studies and were often poorly described, limiting generalizability and pooling for this outcome. Not only did indications for this procedure differ among institutions, they also varied within hospitals and studies, as the timing of permanent shunt placement was generally left to the treating physician. We suggest that future studies provide unified protocols regarding the timing and indication of permanent shunt surgery, and describe this in their papers. This will increase comparability among studies and generalizability of results. The use of lumbar drainage and IVF as described by Staykov et al. also warrants further investigation.
Our study had several limitations. Via the trim-fill method, we estimated the number of ‘missing studies’ due to publication bias in our meta-analysis and were able to provide a prediction of the effect that these studies would have had on the overall outcome [44]. This showed an increased risk of intracranial hemorrhage and a decreased risk of ventriculitis. Nevertheless, it must be noted that the trim-fill method is a statistical model which provides an estimate of the effect of publication bias and cannot provide the true effect it has had on the results. It has been shown to reduce bias in pooled estimates, especially when between-study heterogeneity exists [45], yet it remains a statistical model based on assumptions. Although both of our outcomes had low between-study heterogeneity, results derived from this method should still be considered as estimates and do not replace actual patient data.
The studies included in this review consisted not only of RCTs but also of retrospective and case–control studies. The study quality varied widely between included studies and was moderate on average. Studies like the CLEAR-III trial or by Gerner et al., well designed and performed RCTs, were of much higher quality than most previous trials, and combining their results was not ideal. Most RCTs had a high or unclear risk of bias. Due to the limited number of RCTs available, we opted to include observational studies and employ meta-regression to account for these differences in study quality, size, and design.
Functional outcome was determined at different time points in different studies, with some noting it at discharge, while others after 3 or 6 months. For GFO, we included studies describing functional outcome 3 months or more after initial admission, with time of assessment ranging between 3 and 12 months. Ideally, all studies would assess this outcome at similar time points, for it is possible that longer rehabilitation might benefit one intervention over the other.
To fully evaluate the place of IVF in treating IVH, more data regarding other outcomes are needed; length of intensive care unit stay, length of hospital stay, time until permanent shunt placement, Graeb scores, and Glasgow Coma Scale score on admission are all relevant measures in evaluating the effect of IVF, but have so far been recorded infrequently and in varying details.
In this systematic review and meta-analysis, patients treated with IVF after IVH showed a decreased risk for mortality, fewer obstructions of the external ventricular drains and faster clearance of the ventricles. Functional outcome after 3 months did not differ, as did the risk for shunt dependency. After correcting for publication bias, a possible increase in the risk of symptomatic intracranial hemorrhage and a decrease in the risk of ventriculitis was suggested in patients receiving IVF. In our opinion, the benefits of IVF are not necessarily in improving outcomes, but more in aiding external drain management and possibly preventing ventriculitis. However, more data is needed to fully elucidate these effects and to determine the exact place for IVF in the treatment of IVH.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author Contributions
TvanS performed the study subject and design, data extraction, statistical analysis, interpretation of data and was the author of manuscript. IM contributed to statistical analysis. VK extracted the data. WG was involved in critical revision of manuscript. RM performed statistical analysis, study design, and critical revision of manuscript. MB was involved in critical revision of manuscript and supervision. OA contributed to the study concept and design, critical revision of manuscript, and supervision.
Source of Support
There was no support for this work.
Conflict of interest
The authors declare that they have no conflict of interest.
Informed Consent
For this type of study, formal consent is not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nieuwkamp DJ, de Gans K, Rinkel GJ, Algra A. Treatment and outcome of severe intraventricular extension in patients with subarachnoid or intracerebral hemorrhage: a systematic review of the literature. J Neurol. 2000;247(2):117–121. doi: 10.1007/PL00007792. [DOI] [PubMed] [Google Scholar]
- 2.Atzema C, Mower WR, Hoffman JR, Holmes JF, Killian AJ, Wolfson AB. Prevalence and prognosis of traumatic intraventricular hemorrhage in patients with blunt head trauma. J Trauma. 2006;60(5):1010–1017. doi: 10.1097/01.ta.0000218038.28064.9d. [DOI] [PubMed] [Google Scholar]
- 3.Aucoin PJ, Kotilainen HR, Gantz NM, Davidson R, Kellogg P, Stone B. Intracranial pressure monitors. Epidemiologic study of risk factors and infections. Am J Med. 1986;80(3):369–376. doi: 10.1016/0002-9343(86)90708-4. [DOI] [PubMed] [Google Scholar]
- 4.Kirmani AR, Sarmast AH, Bhat AR. Role of external ventricular drainage in the management of intraventricular hemorrhage; its complications and management. Surg Neurol Int. 2015;6:188. doi: 10.4103/2152-7806.172533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spazzapan P, Bosnjak P. External ventricular drainage infections: a single-centre experience on 100 cases. J Neurol Neurophysiol. 2016;7(4):10–4172. [Google Scholar]
- 6.Mayfrank L, Kissler J, Raoofi R, et al. Ventricular dilatation in experimental intraventricular hemorrhage in pigs. Characterization of cerebrospinal fluid dynamics and the effects of fibrinolytic treatment. Stroke. 1997;28(1):141–148. doi: 10.1161/01.STR.28.1.141. [DOI] [PubMed] [Google Scholar]
- 7.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5(1):53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- 8.Lodhia KR, Shakui P, Keep RF. Hydrocephalus in a rat model of intraventricular hemorrhage. Acta Neurochir Suppl. 2006;96:207–211. doi: 10.1007/3-211-30714-1_45. [DOI] [PubMed] [Google Scholar]
- 9.Ramakrishna R, Sekhar LN, Ramanathan D, et al. Intraventricular tissue plasminogen activator for the prevention of vasospasm and hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2010;67(1):110–117. doi: 10.1227/01.NEU.0000370920.44359.91. [DOI] [PubMed] [Google Scholar]
- 10.Shen PH, Matsuoka Y, Kawajiri K, et al. Treatment of intraventricular hemorrhage using urokinase. Neurol Med Chir (Tokyo) 1990;30(5):329–333. doi: 10.2176/nmc.30.329. [DOI] [PubMed] [Google Scholar]
- 11.Hallevi H, Walker KC, Kasam M, Bornstein N, Grotta JC, Savitz SI. Inflammatory response to intraventricular hemorrhage: time course, magnitude and effect of t-PA. J Neurol Sci. 2011;315(1–2):93–95. doi: 10.1016/j.jns.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 12.Dunatov S, Antoncic I, Bralic M, Jurjevic A. Intraventricular thrombolysis with rt-PA in patients with intraventricular hemorrhage. Acta Neurol Scand. 2011;124(5):343–348. doi: 10.1111/j.1600-0404.2010.01481.x. [DOI] [PubMed] [Google Scholar]
- 13.Varelas PN, Rickert KL, Cusick J, et al. Intraventricular hemorrhage after aneurysmal subarachnoid hemorrhage: pilot study of treatment with intraventricular tissue plasminogen activator. Neurosurgery. 2005;56(2):205–212. doi: 10.1227/01.NEU.0000147973.83688.D8. [DOI] [PubMed] [Google Scholar]
- 14.Hanley DF, Lane K, McBee N, et al. Thrombolytic removal of intraventricular haemorrhage in treatment of severe stroke: results of the randomised, multicentre, multiregion, placebo-controlled CLEAR III trial. Lancet. 2017;389(10069):603–611. doi: 10.1016/S0140-6736(16)32410-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hemphill JC, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(7):2032–2060. doi: 10.1161/STR.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 16.Wang D, Liu J, Norton C, Liu M, Selim M. Local fibrinolytic therapy for intraventricular hemorrhage: a meta-analysis of randomized controlled trials. World Neurosurg. 2017;107:1016–1024. doi: 10.1016/j.wneu.2017.07.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker AD, Rivera Perla KM, Yu Z, et al. Fibrinolytic for treatment of intraventricular hemorrhage: a meta-analysis and systematic review. Int J Stroke. 2017;13(1):11–23. doi: 10.1177/1747493017730745. [DOI] [PubMed] [Google Scholar]
- 18.Fabiano AJ, Gruber TJ, Baxter MS. Increased ventriculostomy infection rate with use of intraventricular tissue plasminogen activator: a single-center observation. Clin Neurol Neurosurg. 2013;115(11):2362–2364. doi: 10.1016/j.clineuro.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 19.Dey M, Stadnik A, Riad F, et al. Bleeding and infection with external ventricular drainage: a systematic review in comparison with adjudicated adverse events in the ongoing Clot Lysis Evaluating Accelerated Resolution of Intraventricular Hemorrhage Phase III (CLEAR-III IHV) trial. Neurosurgery. 2015;76(3):291–300. doi: 10.1227/NEU.0000000000000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339(1):b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet]. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 3 Nov 2018.
- 22.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 25.Naff N, Williams MA, Keyl PM, et al. Low-dose recombinant tissue-type plasminogen activator enhances clot resolution in brain hemorrhage: the intraventricular hemorrhage thrombolysis trial. Stroke. 2011;42(11):3009–3016. doi: 10.1161/STROKEAHA.110.610949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rainov NG, Burkert WL. Urokinase infusion for severe intraventricular haemorrhage. Acta Neurochir (Wien) 1995;134(1–2):55–59. doi: 10.1007/BF01428504. [DOI] [PubMed] [Google Scholar]
- 27.Todo T, Usui M, Takakura K. Treatment of severe intraventricular hemorrhage by intraventricular infusion of urokinase. J Neurosurg. 1991;74(1):81–86. doi: 10.3171/jns.1991.74.1.0081. [DOI] [PubMed] [Google Scholar]
- 28.Torres A, Plans G, Martino J, et al. Fibrinolytic therapy in spontaneous intraventricular haemorrhage: efficacy and safety of the treatment. Br J Neurosurg. 2008;22(2):269–274. doi: 10.1080/02688690701834494. [DOI] [PubMed] [Google Scholar]
- 29.Tung MY, Ong PL, Seow WT, Tan KK. A study on the efficacy of intraventricular urokinase in the treatment of intraventricular haemorrhage. Br J Neurosurg. 1998;12(3):234–239. doi: 10.1080/02688699845050. [DOI] [PubMed] [Google Scholar]
- 30.Akdemir H, Selcuklu A, Pasaoglu A, Oktem IS, Kavuncu I. Treatment of severe intraventricular hemorrhage by intraventricular infusion of urokinase. Neurosurg Rev. 1995;18(2):95–100. doi: 10.1007/BF00417665. [DOI] [PubMed] [Google Scholar]
- 31.Findlay JM, Jacka MJ, et al. Cohort study of intraventricular thrombolysis with recombinant tissue plasminogen activator for aneurysmal intraventricular hemorrhage. Neurosurgery. 2004;55(3):532–537. doi: 10.1227/01.NEU.0000134473.98192.B1. [DOI] [PubMed] [Google Scholar]
- 32.Gerner ST, Kuramatsu JB, Abel H, et al. Intraventricular fibrinolysis has no effects on shunt dependency and functional outcome in endovascular-treated aneurysmal SAH. Neurocrit Care. 2014;21(3):1–9. doi: 10.1007/s12028-014-9961-3. [DOI] [PubMed] [Google Scholar]
- 33.Kramer AH, Roberts DJ, Holodinsky J, et al. Intraventricular tissue plasminogen activator in subarachnoid hemorrhage patients: a prospective, randomized, placebo-controlled pilot trial. Neurocrit Care. 2014;21(2):275–284. doi: 10.1007/s12028-014-9965-z. [DOI] [PubMed] [Google Scholar]
- 34.Coplin WM, Vinas FC, Agris JM, et al. A cohort study of the safety and feasibility of intraventricular urokinase for nonaneurysmal spontaneous intraventricular hemorrhage. Stroke. 1998;29(8):1573–1579. doi: 10.1161/01.STR.29.8.1573. [DOI] [PubMed] [Google Scholar]
- 35.Ducruet AF, Hickman ZL, Zacharia BE, et al. Exacerbation of perihematomal edema and sterile meningitis with intraventricular administration of tissue plasminogen activator in patients with intracerebral hemorrhage. Neurosurgery. 2010;66(4):648–655. doi: 10.1227/01.NEU.0000360374.59435.60. [DOI] [PubMed] [Google Scholar]
- 36.Huttner HB, Tognoni E, Bardutzky J, et al. Influence of intraventricular fibrinolytic therapy with rt-PA on the long-term outcome of treated patients with spontaneous basal ganglia hemorrhage: a case–control study. Eur J Neurol. 2008;15(4):342–349. doi: 10.1111/j.1468-1331.2008.02077.x. [DOI] [PubMed] [Google Scholar]
- 37.King NKK, Lai JL, Tan LB, et al. A randomized, placebo-controlled pilot study of patients with spontaneous intraventricular haemorrhage treated with intraventricular thrombolysis. J Clin Neurosci. 2012;19(7):961–964. doi: 10.1016/j.jocn.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 38.Litrico S, Almairac F, Gaberel T, et al. Intraventricular fibrinolysis for severe aneurysmal intraventricular hemorrhage: a randomized controlled trial and meta-analysis. Neurosurg Rev. 2013;36(4):523–530. doi: 10.1007/s10143-013-0469-7. [DOI] [PubMed] [Google Scholar]
- 39.Naff NJ, Hanley DF, Keyl PM, et al. Intraventricular thrombolysis speeds blood clot resolution: results of a pilot, prospective, randomized, double-blind, controlled trial. Neurosurgery. 2004;54(3):577–584. doi: 10.1227/01.NEU.0000108422.10842.60. [DOI] [PubMed] [Google Scholar]
- 40.Khan NR, Tsivgoulis G, Lee SL, et al. Fibrinolysis for intraventricular hemorrhage: an updated meta-analysis and systematic review of the literature. Stroke. 2014;45:2662–2669. doi: 10.1161/STROKEAHA.114.005990. [DOI] [PubMed] [Google Scholar]
- 41.Jennett B, Bond M. Assessment of outcome after severe brain damage: a practical scale. Lancet. 1975;305(7905):480–484. doi: 10.1016/S0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 42.Korinek AM, Reina M, Boch AL, Rivera AO, De Bels D, Puybasset L. Prevention of external ventricular drain—related ventriculitis. Acta Neurochir (Wien) 2005;147(1):39–46. doi: 10.1007/s00701-004-0416-z. [DOI] [PubMed] [Google Scholar]
- 43.Findlay JM, Kassell NF, Weir BK, et al. A randomized trial of intraoperative, intracisternal tissue plasminogen activator for the prevention of vasospasm. Neurosurgery. 1995;37(1):168–178. doi: 10.1227/00006123-199507000-00041. [DOI] [PubMed] [Google Scholar]
- 44.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341X.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 45.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Performance of the trim and fill method in the presence of publication bias and between-study heterogeneity. Stat Med. 2007;26(25):4544–4562. doi: 10.1002/sim.2889. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.