Abstract
Histamine, acting via distinct histamine H1, H2, H3, and H4 receptors, regulates various physiological and pathological processes, including pain. In the last two decades, there has been a particular increase in evidence to support the involvement of H3 receptor and H4 receptor in the modulation of neuropathic pain, which remains challenging in terms of management. However, recent data show contrasting effects on neuropathic pain due to multiple factors that determine the pharmacological responses of histamine receptors and their underlying signal transduction properties (e.g., localization on either the presynaptic or postsynaptic neuronal membranes). This review summarizes the most recent findings on the role of histamine and the effects mediated by the four histamine receptors in response to the various stimuli associated with and promoting neuropathic pain. We particularly focus on mechanisms underlying histamine‐mediated analgesia, as we aim to clarify the analgesic potential of histamine receptor ligands in neuropathic pain.
Linked Articles
This article is part of a themed section on New Uses for 21st Century. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v177.3/issuetoc
Abbreviations
- CaM
calmodulin
- DRG
dorsal root ganglion
- GSK3β
glycogen synthase kinase 3β
- IP3
inositol triphosphate
- KO
knockout
- LC
locus coeruleus
- PIP2
phosphatidylinositol 4,5‐bisphosphate
- PNS
peripheral nervous system
- SP
substance P
1. NEUROPATHIC PAIN AND ITS TREATMENT
Neuropathic pain was first defined by the International Association for the Study of Pain as “pain initiated or caused by a primary lesion or dysfunction in the nervous system” (Merskey & Bogduk, 1994). Fourteen years later, Treede et al. (2008) revised this definition and redefined it as “pain arising as a direct consequence of a lesion or disease affecting the somatosensory system.” In the revised definition of neuropathic pain, two terms have received particular attention. First, the term “disease” which refers to all types of abnormal conditions including inflammation, autoimmune syndromes, and ion channel disorders (channelopathies) replaced the term “dysfunction.” Second, to avoid misdiagnosis of neuropathic pain as another type of pain originating from the nervous system, such as spasticity and rigidity of the muscles and bone (e.g., musculoskeletal pain), the term “nervous system” was replaced by the term “somatosensory system” (Finnerup et al., 2016). This revised definition of neuropathic pain describes the nature of this condition more precisely and is, therefore, now widely accepted and approved by the Neuropathic Pain Special Interest Group of the International Association for the Study of Pain. Neuropathic pain can be divided into two subtypes, peripheral or central, based on the anatomical location of the lesion or the disease, within the peripheral nervous system (PNS) or central nervous system (CNS), respectively.
It is estimated that the worldwide prevalence rate of neuropathic pain in the general population lies between 7% and 10%; however, this figure differs for different countries (van Hecke, Austin, Khan, Smith, & Torrance, 2014). The highest prevalence rates for neuropathic pain were recorded in Canada (17.9%) and in the United States (9.8–12.4%; VanDenKerkhof et al., 2016; Yawn et al., 2009), while a relatively low prevalence rate was noted in Austria and Netherlands (3.3% and 1%, respectively; Bouhassira, Lanteri‐Minet, Attal, Laurent, & Touboul, 2008; Gustorff et al., 2008; Harifi et al., 2013). In the United Kingdom, France, and Brazil, it is reported that 7–10% of chronic pain sufferers have been affected by neuropathic pain (Fayaz, Croft, Langford, Donaldson, & Jones, 2016). As a consequence of extended life expectancy, it is predicted that the worldwide prevalence rate of neuropathic pain is likely to increase further, because this type of chronic pain occurs with many common age‐related diseases. Neuropathic pain is triggered by a lesion within the somatosensory system, trauma, or to toxic effects of certain medications (Colloca et al., 2017; Yu et al., 2013). Pathological conditions that are responsible for the development of neuropathic pain through injury include metabolic diseases (e.g., diabetic neuropathy), infection (e.g., postherpetic neuralgia), vascular disease (e.g., stroke), trauma (e.g., orofacial neuropathy), and cancer (Campbell & Meyer, 2006). Neuropathic pain is a complex condition that can either be constant or periodic and presents with a range of different symptoms. These symptoms can increase throughout the day with clinically relevant morning–evening differences and can be affected by gender and underlying aetiology (Gilron, Bailey, & Vandenkerkhof, 2013). Sufferers of neuropathic pain have ongoing, spontaneous pain that has a significant negative impact on quality of life and daily functioning, including physical, emotional, and social well‐being (Jensen, Chodroff, & Dworkin, 2007).
The mechanisms underlying neuropathic pain are complex and multidimensional. Numerous pathophysiological and biochemical changes cause morphological and functional adaptations in the nervous system, including an increase in excitatory neurotransmitters and neuropeptides, for example, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1204, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=649, 5‐HT, and glutamate, which leads to hyperexcitability (Baron, Binder, & Wasner, 2010). Likewise, injured peripheral nerve fibres give rise to an intense and prolonged input of ectopic activity to the CNS and may induce secondary changes to the excitability of the spinal cord dorsal horn neurons (Colloca et al., 2017; Ossipov & Porreca, 2005). These morphological changes and functional adaptations lead to abnormal sensory signs in patients with neuropathic pain presented as, for example, allodynia (pain due to a stimulus that does not normally activate the nociceptive system), hyperalgesia (an increased response to a stimulus that is normally painful), or sensory loss (hypoesthesia). Clinically, neuropathic pain manifests as evoked pain and presents in many ways such as burning, tingling, prickling, shooting, electric shock‐like, jabbing, squeezing, spasm, or cold (Rice, Finnerup, Kemp, Currie, & Baron, 2018).
Animal models of neuropathic pain are essential in understanding the plethora of mechanisms that may drive neuropathic pain, allowing the field to identify potential therapeutic targets for the effective management of this condition. Extensive research in the pain field has developed and characterized a wide variety of animal models of neuropathic pain. The four most commonly used models, also adopted in some studies discussed in this review, are chronic constriction injury (Bennett & Xie, 1988), partial sciatic ligation (Seltzer, Dubner, & Shir, 1990), spinal nerve ligation (Kim & Chung, 1992), and spared nerve injury (Decosterd & Woolf, 2000) models. These models aim to simulate some of the clinical features of neuropathic pain, in the preclinical setting (e.g., allodynia and hyperalgesia), because a large proportion of peripheral neuropathic pain models, which are currently used in research, share alterations in hind‐limb cutaneous sensory thresholds following partial injury of a peripheral (usually sciatic) nerve. This is associated with the development of neuropathic pain symptoms, such as hyperalgesia and allodynia (Ma et al., 2003; Wall et al., 1979). There are also limitations associated with the use of animal models of neuropathic pain. These limitations are linked to challenges associated with (a) reliable and objective measures of behavioural responses to noxious stimuli, since animals cannot self‐report and the experimenter can be biased, (b) appropriateness of the outcome measures, for example, sleep disturbance to reflect spontaneous pain, and (c) complexity of mechanisms underlying the development of neuropathic pain and their relevance to humans (Colleoni & Sacerdote, 2010).
Despite advances in the understanding of the underlying causes and mechanisms leading to the development and maintenance of neuropathic pain, 40% of Europeans who suffer from chronic pain did not achieve satisfactory pain control (Breivik, Collett, Ventafridda, Cohen, & Gallacher, 2006), and to date, no medication has shown long‐term efficacy and tolerability for neuropathic pain conditions. A significant contributing factor to these limited therapeutic strategies is that neuropathic pain has different aetiology and pathophysiology to any other type of chronic pain, making the management of this type of chronic pain particularly difficult and challenging (Finnerup et al., 2015). Consequently, there is a pressing need for the identification of new therapeutic strategies to improve management of neuropathic pain that will directly improve the outcome for pain sufferers.
The histamine system has been a target for multiple therapeutic interventions. Recently, growing evidence has supported the use of selective ligands of histamine http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=264 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=265 for the treatment of neuropathic pain (Bhowmik, Khanam, & Vohora, 2012; Chaumette et al., 2018; Popiolek‐Barczyk et al., 2018; Sanna, Mello, Masini, & Galeotti, 2018). Approval in the European Union for the use of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=8924 (Wakix™), an antagonist/inverse agonist of H3 receptors for the treatment of narcolepsy (Kollb‐Sielecka et al., 2017), presents an opportunity to explore its clinical use for other conditions. Consequently, it seems an appropriate time to reconsider the histamine system as a therapeutic target for the management of neuropathic pain. This review aims to summarize the most recent findings on the role of histamine and its effects, mediated by different subtypes of histamine receptors, on neuropathic pain, with particular regard to the mechanisms underlying histamine‐mediated analgesia.
2. HISTAMINE, HISTAMINE RECEPTORS, AND PAIN
2.1. Histamine and pain
Histamine (2‐(4‐imidazolyl)‐ethylamine), one of the most extensively studied amino acid‐derived neurotransmitters in the CNS and PNS, is involved in various physiological and pathological processes, including sleep‐waking cycle, homeostasis, synaptic plasticity, and learning (Panula et al., 2015; Parsons & Ganellin, 2006; Pini, Obara, Battell, Chazot, & Rosa, 2016). Histamine is synthesized from the amino acid http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3310 through oxidative decarboxylation via catalysis with the histidine decarboxylase enzyme (Bodmer, Imark, & Kneubuhl, 1999; Haas, Sergeeva, & Selbach, 2008) and is arguably the most pleiotropic molecule in the human and animal body, being present in many cell types (Lindskog, 2017). Histamine is released by neuronal and non‐neuronal sources and is responsible for many physiological processes, including the contraction of smooth muscles of the lungs, uterus, and intestine, secretion of gastric acid in the stomach, and vasodilation, and modulation of heart rate and contractility (Parsons & Ganellin, 2006). Histamine also functions as a neurotransmitter, within the nervous system, regulating a variety of body functions, such as temperature control, memory, wakefulness, and pain sensation (Panula & Nuutinen, 2013).
Histamine is a key mediator in the processing of nociceptive information, acting in an antinociceptive manner in the CNS while, conversely, in a nociceptive manner in the PNS (Khalilzadeh, Azarpey, Hazrati, & Vafaei Saiah, 2018). In the PNS, histamine is released in response to tissue injury/damage, and, through the sensitization of polymodal nociceptors resulting in increased firing rates, it contributes to the generation of pain hypersensitivity. In neuropathic pain, histamine released in the periphery by mast cells has been shown to play an important role in the development of hypersensitivity following nerve injury. This pathological process is associated with recruitment of macrophages and neutrophils, and as histamine is a powerful chemoattractant of mast cells, it regulates this recruitment (Smith, Haskelberg, Tracey, & Moalem‐Taylor, 2007; Zuo, Perkins, Tracey, & Geczy, 2003). Interestingly, it was also observed that peripherally acting histamine could interact with mechanisms underlying pruritus (itch) and pain. Findings suggest that low concentrations of histamine, acting on sensory neurons, produce pruritus with a high concentration leading to pain (Baron, Schwarz, Kleinert, Schattschneider, & Wasner, 2001; Hough & Rice, 2011; LaMotte, Simone, Baumann, Shain, & Alreja, 1987; Parsons & Ganellin, 2006; Pini et al., 2016; Simone, Alreja, & LaMotte, 1991). There is also evidence to show that histamine‐induced itch can convert into pain associated with neuropathic hyperalgesia (Baron et al., 2001). Indeed, multiple itch pathways were identified indicating the presence of distinct itch‐generating types of neuron, one responsible for transmitting itch sensation and the other, ultimately, for transmitting pain (Usoskin et al., 2015). In contrast to histamine activity in PNS, multiple behavioural studies have shown that histamine injected directly into the various brain areas (e.g., somatosensory cortex or hippocampus) attenuated pain (Erfanparast, Tamaddonfard, Farshid, & Khalilzadeh, 2010; Tamaddonfard & Hamzeh‐Gooshchi, 2014).
Histamine exerts its effects via four distinct GPCR subtypes: http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=262, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=263, H3, and H4 receptors. These receptors differ in their pharmacology and signal transduction properties (Panula et al., 2015; Parsons & Ganellin, 2006; Simons & Simons, 2011). Thus, histamine has differential effects on neuropathic pain dependent upon the histamine receptor subtype it is bound to. As mentioned previously, this review aims to summarize histamine‐mediated effects on neuropathic pain. Therefore, the next sections of this review focus on mechanisms underlying histamine‐mediated analgesia.
2.2. Histamine receptors and pain
Excitatory histamine receptor signalling in nociceptive pathways is associated with increased pain symptoms (Gangadharan & Kuner, 2013; Mobarakeh et al., 2000), whereas inhibition of histamine receptor signalling predominantly causes neuroprotective and antinociceptive effects (Bhowmik et al., 2012; Chazot & Care, 2005; Popiolek‐Barczyk et al., 2018). Different subtypes of histamine receptors are expressed in both presynaptic and postsynaptic neuronal membranes (Brown, Stevens, & Haas, 2001; Parsons & Ganellin, 2006; Zhang et al., 2013). Presynaptic histamine receptors function as autoreceptors or heteroreceptors providing either positive or negative feedback regulation of neurotransmitter release from the axon terminals into the synaptic cleft (Nieto‐Alamilla, Marquez‐Gomez, Garcia‐Galvez, Morales‐Figueroa, & Arias‐Montano, 2016). It could be concluded that the resultant excitatory or inhibitory physiological effect of histamine receptors depends on the action of the neurotransmitter and the subsequent downstream cascade.
Specificity of localization of histamine receptors in different parts of the nervous system, on either presynaptic or postsynaptic membranes, is determined by their physiological relevance (Parsons & Ganellin, 2006). Among the four subtypes of histamine receptors, H1 and H2 receptors are predominantly identified postsynaptically (Brown et al., 2001; Connelly et al., 2009; Zhang et al., 2013), with the location of the H4 receptor requiring further investigation (Connelly et al., 2009). Cross‐desensitization and agonist‐induced heterodimerization of H1 and H2 receptors (Alonso et al., 2013) may suggest a possible partnership between histamine receptors. Expression of H3 receptors was initially reported as exclusively presynaptic in the rat cerebral cortex (Arrang, Garbarg, & Schwartz, 1983; Clark & Hill, 1996), while postsynaptic expression of H3 receptors could not be completely excluded (Nieto‐Alamilla et al., 2016). H3 receptors are predominantly expressed in neurons and, together with H4 receptors, have higher affinity (nM range) for histamine than H1 and H2 receptors (μM range; Parsons & Ganellin, 2006). Expression of H3 and H4 receptors on the opposite sides of the synaptic cleft may contribute to their effects in neuropathic pain, although the neuronal topology of the H4 receptor still remains controversial. The use of selective ligands for histamine receptors has led to a better understanding of the physiological and pathophysiological roles of these receptors. The next section summarizes the effects produced by histamine receptor ligands on neuropathic pain.
Besides their presynaptic or postsynaptic localization, the physiological effects of histamine receptors are, to a great extent, determined by the type of guanine nucleotide‐binding proteins (G‐proteins) to which they are coupled (Leung & Wong, 2017). The difference in underlying signalling pathways may directly determine the effect on pain perception produced by selective ligands, even when they act at the same histamine receptor, as described below.
2.2.1. H1 receptor
H1 receptors are excitatory receptors, which couple with Gq‐type proteins, leading to downstream activation of http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=274 and hydrolysis of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2387 (PIP2) to produce DAG and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4222 (IP3). DAG subsequently activates PKC at the membrane, while IP3 diffuses and binds to IP3 receptors on the endoplasmic reticulum to mobilize stored calcium (Ca2+). These changes cause PIP2 depletion and increased intracellular concentration of Ca2+. This increased concentration of Ca2+ activates PKC‐dependent phosphorylation and forms a complex with http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2351 (Ca2+CaM), both of which suppress http://www.guidetopharmacology.org/GRAC/FamilyIntroductionForward?familyId=81 (Figure 1), leading to depolarization and increased nociception (Brown & Passmore, 2009; Chen, Li, Hiett, & Obukhov, 2016).
Figure 1.
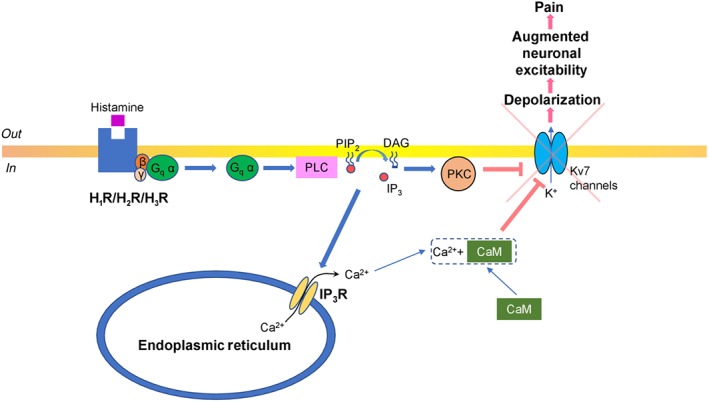
Diagram illustrating histamine receptor signalling—Gq pathway. Histamine binds to histamine receptors (H1, H2, or H3 receptor subtypes) that are coupled with the Gq‐type protein. Gq α subunit activates PLC which hydrolyses phosphatidylinositol 4,5‐bisphosphate (PIP2), subsequently producing DAG, that remains in the inner leaflet of the plasma membrane activating PKC, and water soluble inositol triphosphate (IP3), which binds to http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=123 (IP3R) and stimulates Ca2+ release from endoplasmic reticulum. This intracellular Ca2+ forms a complex with calmodulin (CaM) Ca2+CaM and induces PKC‐dependent phosphorylation. This suppresses the activity of potassium voltage‐gated channels type 7 (Kv7 channels), which depolarizes the neurons, and leads to the augmentation of neuronal excitability, which manifests as increased pain symptoms
2.2.2. H2 receptor
H2 receptors are postsynaptic, predominantly associated with http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=257 via coupling to Gs and, in a similar fashion to H1 and H3 receptors, Gq proteins, both pathways initiating excitatory downstream signalling. Thus, H2 receptor inhibition yielded efficient antinociceptive effects (Mojtahedin, 2016). Gs α subunit stimulates AC with consequent augmented production of cAMP and consequent activation of http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=284 (Brown et al., 2001). Elevated cAMP concentration up‐regulates PKA, which as reported for hippocampal neurons, could activate ligand gated http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=75 with resultant depolarization and increase of neuronal firing (Park et al., 2016). Also, as it was reported for after‐hyperpolarization in enteric neurons, PKA inhibits small conductance http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=69 with resultant augmented neuronal excitability (Figure 2; Vogalis, Harvey, & Furness, 2003).
Figure 2.
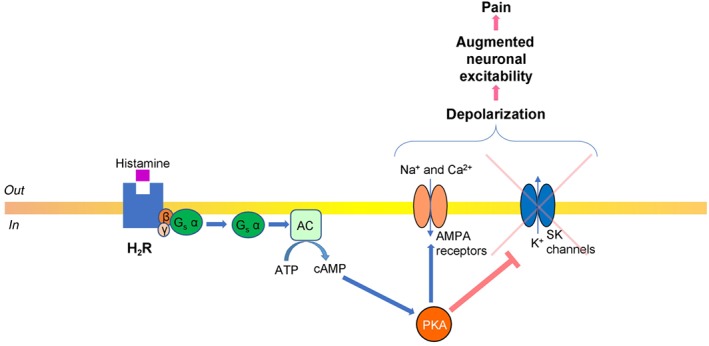
Diagram illustrating histamine receptor signalling—Gs pathway. Histamine binds to the H2 receptor subtype that is coupled with the Gs‐type protein. Gs α subunit activates AC, with subsequent production of cAMP, which then stimulates PKA. PKA‐dependent phosphorylation activates ligand gated α‐AMPA receptors, which open and facilitate influx of Na+ and, less commonly, Ca2+. PKA also suppresses K+ efflux through small conductance Ca2+‐activated potassium channels (SK channels). Both the activation of AMPA receptors and inhibition of SK channels depolarize the neurons, with consequent augmentation of their excitability and increased pain signalling
2.2.3. H3 receptor
Presynaptic H3 receptors are coupled with Gi (AC inhibitory) proteins (Nieto‐Alamilla et al., 2016; Schlicker & Kathmann, 2017). Gi α subunit‐mediated AC inhibition results in a decreased intracellular concentration of cAMP and subsequent down‐regulation of PKA (Nieto‐Alamilla et al., 2016). In sympathetic and sensory neurons, it was reported that H3 receptor activation stimulated dissociation of Gi β and γ subunits from Gi α subunit, which then inhibited voltage‐gated Ca2+ influx through N‐, P‐, and Q‐type Ca2+ channels (Zamponi & Currie, 2013), and stimulated http://www.guidetopharmacology.org/GRAC/FamilyIntroductionForward?familyId=74 (Luscher & Slesinger, 2010). Both mechanisms could hyperpolarize presynaptic neurons, reduce neuronal excitability, and produce pain relief (Figure 3).
Figure 3.
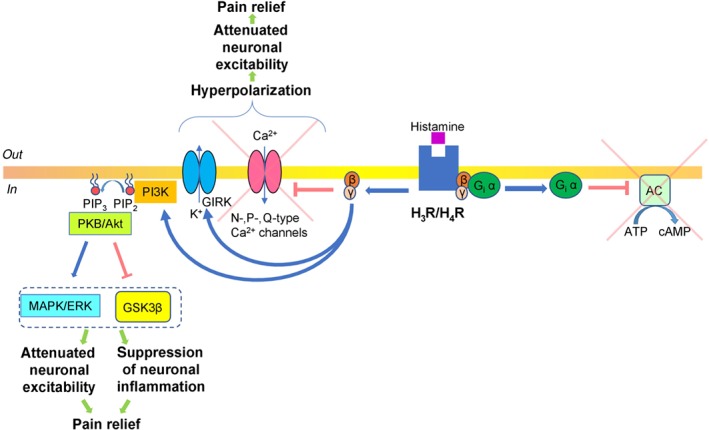
Diagram illustrating histamine receptor signalling—Gi pathway. Histamine binds to the histamine receptors (presynaptic and postsynaptic H3 or H4 receptor subtypes) that are coupled with Gi‐type protein. The Gi α subunit inhibits AC with subsequent suppression of cAMP production and inhibition of PKA activity. Also, Gi β and γ subunits can inhibit Ca2+ influx through voltage‐gated N‐, P‐, and Q‐type Ca2+ channels and stimulate G protein‐coupled inwardly rectifying potassium (GIRK) channels, with resultant K+ efflux. Both effects on N‐type Ca2+ and GIRK channels result in the development of hyperpolarization, attenuation of neuronal excitability, and resultant pain relief. Besides Gi α subunit effects, H3 receptor activation could produce analgesic effects through Gi β and γ subunits, which up‐regulate the PI3K pathway, with the subsequent production of phosphatidylinositol 2,4,5‐trisphosphate (PIP3) from phosphatidylinositol 4,5‐bisphosphate (PIP2). PIP3 recruits PKB (Akt), which phosphorylates and inactivates glycogen synthase kinase 3β (GSK3β). In parallel, PKB (Akt)‐dependent phosphorylation additionally activates the MAPK/ERK cascade. The action on both GSK3β and MAPK/ERK decreases neuronal excitability, inhibits mechanisms of neuronal inflammation, and, therefore, produces pain relief
Besides the Gi pathway, postsynaptic activation of H3 receptors was reported to stimulate PLC in a subpopulation of striatal neurons, with subsequent activation of the IP3 pathway followed by increased intracellular concentrations of Ca2+ (Rivera‐Ramírez et al., 2016). Thus, it is analogous to the mechanisms described for H1 /H2 receptors coupled to Gq proteins (Figure 1). Similarly, the H1 and H2 receptor Gq cascade PLC signalling pathways modulate neuronal excitability with resultant potential facilitation of pain sensitivity. Furthermore, H3 receptor activation was established to inhibit http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2030 (GSK3β) and MAPK/http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=514 pathways (Bongers et al., 2007; Morita, Aida, & Miyamoto, 1983). These effects are translated via Gi β and γ subunits (Lai et al., 2016), which up‐regulate the http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=781 pathway with subsequent production of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2353 (PIP3) from PIP2, which results in the recruitment of http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=285 (Akt). PKB is initially activated by http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1519 (PDK1) and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2109 complex 2‐dependent phosphorylation (Dzamko, Zhou, Huang, & Halliday, 2014). PKB, via phosphorylation, inactivates GSK3β, which decreases neuronal excitability (Paul et al., 2016), inhibits neuronal inflammation (Maixner & Weng, 2013), and, subsequently, relieves pain. PKB‐dependent phosphorylation that activates the MAPK/ERK cascade was also reported to be a highly efficient neuroprotective mechanism for chronic inflammatory and neuropathic pain (Cruz & Cruz, 2007; Figure 3).
2.2.4. H4 receptor
The role of H4 receptors in the nervous system is poorly understood (Schneider & Seifert, 2016). H4 receptors are known to be coupled to Gi proteins, and their downstream pathways are postulated to be similar to those described for H3 receptors (Figure 3). Compared to the other three types of histamine receptors, the H4 receptor is not expressed abundantly in the CNS and PNS. By quantitative single‐cell Ca2+ imaging, it was demonstrated that histamine induces a Ca2+ increase in a subset of sensory neurons (3–10%) via activation of the H1 and H4 receptors as well as inhibition of the H3 receptor. It is assumed that the decreased threshold in response to H3 receptor antagonism, which accounts for the analgesic effect of H3 receptor antagonists, activates H1 and H4 receptors on sensory neurons, which in turn results in the excitation of histamine‐sensitive afferents and, therefore, may result in a modulation of pain sensitivity (Rossbach et al., 2011).
3. HISTAMINE RECEPTOR LIGANDS AND NEUROPATHIC PAIN
3.1. H1 and H2 receptor ligands and neuropathic pain
The best‐known roles for the H1 receptor are regulation of vasodilation and bronchoconstriction on multiple cell types, including endothelial and smooth muscle cells, while the H2 receptor is primarily involved in the modulation of gastric secretion on parietal cells (Barocelli & Ballabeni, 2003; Simons, 2003). There is also evidence for their expression in the nervous system, where they regulate some neuronal functions (Haas et al., 2008; Kashiba, Fukui, & Senba, 2001; Murakami et al., 1999).
Both H1 and H2 receptors have been implicated in the role of histamine in nociception and chronic pain (Table 1). Interestingly, with the discovery of H1 and H2 receptor ligands in the 1950s, controlled clinical studies using these H1 and H2 receptor antagonists reported mild analgesic activity and their potential as analgesic adjuvants, particularly in conditions where pain was induced by histamine. Most of the clinical studies focused on http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1224 (first generation H1 receptor antagonist) and showed its analgesic potential in the treatment of dysmenorrhea, atypical head and face pain, trigeminal neuralgia, and thalamic syndrome (Rumore & Schlichting, 1986). In addition, diphenhydramine, when combined with opioids, showed its potential as an analgesic adjuvant in refractory cancer pain (Santiago‐Palma, Fischberg, Kornick, Khjainova, & Gonzales, 2001). In addition to clinical evidence for the analgesic potential of H1 and H2 receptor antagonists, preclinical studies identified the expression of H1 and H2 receptors in nociceptive pathways and, therefore, further supported the roles of H1 and H2 receptors in the regulation of pain. There are limited anatomical data available for H2 receptors, despite the report of H2 receptor mRNA expression in human spinal cord (Murakami et al., 1999). The potential involvement of H1 receptors in the modulation of neuropathic pain has been investigated more extensively. In studies using in situ hybridization techniques in the guinea pig, the H1 receptor mRNA was shown to be expressed in about 15–20% of the central trigeminal and lumbar dorsal root ganglion (DRG) neurons. These sensory neurons are fundamental to nociceptive processes, potentially responding to histamine by acting on H1 receptors. These neurons are exclusively small in size and coexpress isolectin B4, but not http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2098 (SP) or http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3586, suggesting characteristics of unmyelinated C‐fibres involved in acute nociception. Interestingly, the potential role of H1 receptors in the regulation of neuropathic pain sensitivity can be explained by the marked (up to fourfold) increase in H1 receptor expression in the mainly small‐sized DRG neurons, 1–5 days after a crush injury of the sciatic nerve. Moreover, this study showed new characteristics of peptidergic (SP/CGRP) sensory neurons not detected prior to nerve injury, suggesting that the sensory modalities evoked by histamine acting via H1 receptors in normal and neuropathic pain states may result in different effects. This demonstration of the potential up‐regulation of H1 receptor number in injured afferent nerves further supports the involvement of H1 receptors in the regulation of neuropathic pain hypersensitivity, presumably expressed on unmyelinated C‐fibres (Kashiba et al., 2001). The earliest electrophysiological study to probe the histaminergic system in neuropathic pain transmission reported that daily injections of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2603, a CNS‐sparing H1 receptor antagonist, acting via peripheral histamine‐sensitive C‐fibres, blocked ectopic spontaneous discharges from the neuroma and suppressed autotomy following sciatic and saphenous neurectomy (Seltzer et al., 1991; Yu et al., 2013). More recently, i.c.v. injection of histamine blocked mechanical and thermal hypersensitivity associated with neuropathic pain (Sanna et al., 2015; Wei et al., 2016). However, these pain‐modulatory effects of histamine can vary depending on the dose of histamine administered i.c.v., due to an action on H2 receptors and the involvement of adrenoceptors (Wei et al., 2014; Wei et al., 2016).
Table 1.
A summary of the effects produced by histamine receptor ligands in animal models of neuropathic pain
| Drug | Model | Strain | Test | Effect | Reference |
|---|---|---|---|---|---|
| H1 antagonists | |||||
| Astemizole | Neurectomy | Sabra rats | Score | i.p. suppressed autotomy | Seltzer, Paran, Eisen, & Ginzburg, 1991 |
| Chlorpheniramine | TNT | Wistar rats | Acetone | i.p. reduced allodynia and prevented cold plate avoidance behaviour | Khalilzadeh et al., 2018 |
| von Frey | |||||
| Double plate | |||||
| Open field | |||||
| PLSN | Wistar rats | Hargreaves | i.p. suppressed and alleviated hyperalgesia | Zuo et al., 2003 | |
| Randall‐Selito | |||||
| Fexofenadine | TNT | Wistar rats | Acetone | p.o. reduced allodynia and prevented cold plate avoidance behaviour | Khalilzadeh et al., 2018 |
| von Frey | |||||
| Double plate | |||||
| Open field | |||||
| http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7216 | Peripheral axotomy | Sprague–Dawley rats | Score of autotomy | i.p. did not block analgesic effects of histidine on pain behaviour but alone suppressed autotomy | Yu et al., 2013 |
| Mepyramine (pyrilamine) | PLSN | Sprague–Dawley rats | von Frey | Intrathecally, i.c.v. blocked analgesic effects of histidine on pain behaviour | Yu et al., 2016 |
| IR laser | |||||
| SNL | Hannover‐Wistar rats | von Frey | Intrathecally did not attenuate the antihypersensitivity effect of histamine | Wei, Viisanen, You, & Pertovaara, 2016 | |
| Radiant heat | |||||
| SNL | Hannover‐Wistar rats | von Frey test | Into LC did not attenuate the antihypersensitivity effect of histamine and alone failed to influence pain | Wei, Jin, Viisanen, You, & Pertovaara, 2014 | |
| Radiant heat | |||||
| PLSN | Rats | von Frey | i.c.v was ineffective | Huang, Adachi, Nagaro, Liu, & Arai, 2007 | |
| http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7282 | Vincristine induced | Wistar albino rats | Pinprick | i.p. reduced hyperalgesia and allodynia | Jaggi, Kaur, Bali, & Singh, 2017 |
| Acetone | |||||
| Hot plate | |||||
| H2R antagonists | |||||
| Cimetidine | PLSN | Sprague–Dawley rats | von Frey | Intrathecally, i.c.v. did not block histidine's analgesic effects on pain behaviour | Yu et al., 2016 |
| IR laser | |||||
| PLSN | Wistar rats | Hargreaves | i.p. suppressed and alleviated hyperalgesia | Zuo et al., 2003 | |
| Randall‐Selito | |||||
| Famotidine | TNT | Wistar rats | Acetone | p.o. was ineffective in all tests | Khalilzadeh et al., 2018 |
| von Frey | |||||
| Double plate | |||||
| Open field | |||||
| PLSN | Sprague–Dawley rats | von Frey | i.p. reduced allodynia and hyperalgesia | Yue et al., 2014 | |
| Diode laser | |||||
| Ranitidine | TNT | Wistar rats | Acetone | i.p. reduced allodynia and prevented cold plate avoidance behaviour | Khalilzadeh et al., 2018 |
| von Frey | |||||
| Double plate | |||||
| Open field | |||||
| Vincristine induced | Wistar rats | Pinprick | i.p. reduced hyperalgesia and allodynia | Jaggi et al., 2017 | |
| Acetone | |||||
| Hot plate | |||||
| PLSN | Rats | von Frey | i.c.v. increased hypersensitivity | Huang et al., 2007 | |
| Zolantidine | SNL | Hannover‐Wistar rats | von Frey | i.t. attenuated the antihypersensitivity effect of histamine | Wei et al., 2016 |
| Radiant heat | |||||
| SNL | Hannover‐Wistar rats | von Frey | Into LC attenuated histamine's analgesic effect but alone failed to influence pain | Wei et al., 2014 | |
| Radiant heat | |||||
| H3R antagonists and H3R antagonists/inverse agonist | |||||
| A‐960656 | SNL | Hannover‐Wistar rats | von Frey | Into LC reduced hypersensitivity | Wei et al., 2014 |
| Radiant heat | |||||
| SNL | Hannover‐Wistar rats | von Frey | i.t. reduced hypersensitivity | Wei et al., 2016 | |
| Radiant heat | |||||
| SNL | Sprague–Dawley rats | von Frey | p.o. reduced hypersensitivity | Cowart et al., 2012 | |
| http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1265 | SNI | BL6 mice | Hargreaves | i.p. was ineffective | Zhang et al., 2012 |
| Dynamic plantar aesthesiometer | |||||
| E‐162 | CCI | Swiss CD1 mice | von Frey | i.p. reduced hypersensitivity | Popiolek‐Barczyk et al., 2018 |
| Cold plate | |||||
| Tail‐flick | |||||
| GSK189254 | CCI | Random‐hooded rats | von Frey | p.o. reduced allodynia and hyperalgesia | Medhurst et al., 2008 |
| Randall‐Selito | |||||
| SNL | Sprague–Dawley rats | von Frey | i.p. reduced allodynia | Hsieh et al., 2010 | |
| GSK334429 | CCI | Random‐hooded rats | von Frey | p.o. reduced allodynia and hyperalgesia | Medhurst et al., 2008 |
| Randall‐Selito | |||||
| Pitolisant | SNI | BL6 mice | Hargreaves | i.p. was ineffective, high doses increased thermal but not mechanical hypersensitivity | Zhang et al., 2012 |
| Dynamic plantar aesthesiometer | |||||
| S38093 | CCI and oxaliplatin induced | Sprague–Dawley rats | Randall‐Selito | p.o. reduced hypersensitivity | Chaumette et al., 2018 |
| Tail‐immersion (10°C) | |||||
| ST‐889 | SNI | BL6 mice | Hargreaves | i.p. was ineffective | Zhang et al., 2012 |
| Dynamic plantar aesthesiometer | |||||
| Thioperamide | PLSN | Rats | von Frey | i.c.v. increased but i.p. reduced hypersensitivity | Huang et al., 2007 |
| PLSN | Sprague–Dawley rats | Randall‐Selito | i.p. increased hypersensitivity | Smith et al., 2007 | |
| H4R agonists | |||||
| ST‐1006 | SNI | CD1 mice | Hargreaves | i.c.v. reduced allodynia and hyperalgesia | Sanna et al., 2015 |
| Dynamic plantar aesthesiometer | |||||
| VUF‐8430 | SNI | CD1 mice | Hargreaves | i.c.v. reduced allodynia and hyperalgesia | Sanna et al., 2015 |
| Dynamic plantar aesthesiometer | |||||
| SNI | CD1 mice | Hargreaves | i.t.reduced allodynia and hyperalgesia | Sanna, Lucarini, et al., 2017 | |
| Dynamic plantar aesthesiometer | |||||
| PLSN | Sprague–Dawley rats | Randall‐Selito | i.p. reduced hypersensitivity | Smith et al., 2007 | |
| H4R antagonists | |||||
| JNJ7777120 | PLSN | Sprague–Dawley rats | Randall‐Selito | i.p. increased hypersensitivity | Smith et al., 2007 |
| SNL | Sprague–Dawley rats | von Frey | i.p. reduced allodynia | Hsieh et al., 2010 | |
| JNJ10191584 | SNI | CD1 mice | Hargreaves | p.o. blocked the analgesic effect of i.c.v. ST‐1006 and VUF‐8430 and was ineffective alone | Sanna et al., 2015 |
| Dynamic plantar aesthesiometer | |||||
| SNI | CD1 mice | Hargreaves | p.o. blocked the analgesic effect of i.t. VUF‐8430 and was ineffective alone | Sanna et al., 2015 | |
| Dynamic plantar aesthesiometer | |||||
| TR‐7 | CCI | Swiss CD1 mice | von Frey | i.p. reduced hypersensitivity | Popiolek‐Barczyk et al., 2018 |
| Cold plate | |||||
| Tail‐flick | |||||
Abbreviations: CCI, chronic constriction nerve injury; LC, locus coeruleus; PLSN, partial ligation of sciatic nerve; SNI, spared nerve injury; SNL, spinal nerve ligation; TNT, tibial nerve transection.
To further support the role of H1 and H2 receptors in the regulation of pain, separate studies using knockout (KO) mice lacking H1 and H2 receptors demonstrated that these mice displayed significantly lower responses to nociceptive stimuli when compared to their wild‐type controls (Mobarakeh et al., 2002; Mobarakeh, Takahashi, Sakurada, Kuramasu, & Yanai, 2006). Interestingly, the antinociceptive phenotype of H2 receptor KO mice was relatively less prominent when compared to H1 receptor KO mice, suggesting a potentially distinct role for these receptors in the modulation of pain. Indeed, behavioural studies using a model of neuropathic pain, induced by the partial ligation of the sciatic nerve, showed that the CNS‐permeable H1 receptor antagonist http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1227, but not the H2 receptor CNS‐sparing antagonist http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1231, blocked the effects of histidine on neuropathic pain hypersensitivity and spinal microglia activity (Yu et al., 2016). In addition, Jaggi et al. (2017) suggested that the H1 receptor plays a more important role in a vincristine‐induced model of neuropathic pain, when compared to H2 receptors. However, Khalilzadeh et al. (2018) observed different behavioural effects upon tibial nerve transection‐induced neuropathic pain with respect to the extent of brain penetration of the ligands, in a study focused on centrally active and centrally sparing H1 and H2 receptor antagonists. Specifically, both http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6976, a centrally and peripherally active H1 receptor antagonist, and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4819, an H1 receptor, centrally sparing antagonist, were found to profoundly decrease the mechanical hypersensitivity associated with the development of neuropathic pain. In contrast, while http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1234, a widely used centrally permeable H2 receptor antagonist, also improved mechanical hypersensitivity, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7074, a centrally sparing H2 receptor antagonist, was ineffective. These results indicate that both blood brain barrier penetrating and poorly penetrating histamine H1 receptor antagonists can block neuropathic pain hypersensitivity, but only the blood brain barrier penetrating histamine H2 receptor antagonist can generate an analgesic effect in neuropathic pain. In line with this observation, histamine‐induced mechanical hypersensitivity was prevented by spinal pretreatment with zolantidine, a brain penetrating H2 receptor antagonist, as well as localized peripheral administration of cimetidine (H2 receptor antagonist) and chlorpheniramine (H1 receptor antagonist) into the plantar side of the hindpaw (Zuo et al., 2003).
Taken together, these results indicate that the brain histamine, acting particularly via central H1 and H2 receptors, may be involved in the modulation of neuropathic pain. These studies consistently support the idea that CNS‐permeable H1 and H2 receptor antagonists may potentially be used as analgesics for patients with neuropathic pain. The involvement of central H2 receptors in the regulation of neuropathic pain hypersensitivity was also demonstrated in studies where histamine (presumably postsynaptically‐ induced) facilitated mechanical hypersensitivity mediated by http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=75 receptors as well as, in a dose‐dependent manner, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=585 channel expression in primary afferent neurons in the sciatic nerve and L4/L5 DRG (Wei et al., 2016; Yue et al., 2014). While sodium channels are responsible for the development and maintenance of neuropathic pain (Ossipov & Porreca, 2005; Yue et al., 2014), the above studies highlight the importance of histamine acting via H2 receptors in the regulation of mechanisms associated with neuropathic pain states. The influence of the H2 receptor on non‐neuronal cells (mast cells) is discussed later in this review.
3.2. H3 receptor ligands and neuropathic pain
H3 receptors are mostly presynaptic, expressed as autoreceptors on histaminergic neurons involved in the negative feedback control of histamine levels (Arrang et al., 1983; Hough & Rice, 2011), while H3 heteroreceptors on postsynaptic nonhistaminergic neurons also regulate negatively the release of neurotransmitters, such as http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=294, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=940, 5‐HT, and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=484 (Blandina, Munari, Giannoni, Mariottini, & Passani, 2010; Gemkow et al., 2009; Giannoni et al., 2010). Since the cloning of H3 receptors (Lovenberg et al., 1999), there has been an increased interest within the pharmaceutical industry in developing ligands for this receptor to target several diseases, including neuropathic pain. This interest was strongly fuelled by the report of H3 receptor expression in nociceptive pathways, suggesting its functional involvement in the regulation of nociceptive transmission (Cannon et al., 2007). Indeed, the histamine H3 receptor consists of several functional isoforms expressed in both the CNS and PNS, particularly along the ascending nociceptive pathway and descending pain‐control pathway, that are critical for the processing of nociceptive information. Within the CNS, this receptor has been found in various brain areas, such as thalamus, hypothalamus, prefrontal cortex, and periaqueductal grey area (Drutel et al., 2001), and in the spinal cord (Cannon et al., 2007; Heron, Rouleau, Cochois, Pillot, & Schwartz, 2001; Medhurst et al., 2008). In the periphery, the expression of H3 receptors has been identified in DRG, superior cervical ganglia, and dermal tissues (Cannon et al., 2007; Medhurst et al., 2008).
However, while the localization of H3 receptors strongly suggests its functional involvement in the regulation of nociceptive transmission, pharmacological studies using agonists and antagonists of H3 receptors are confusing as these drugs have different effects on the nociceptive threshold depending on the pain model used, the nociceptive stimulus selected, together with the affinity and selectivity for the histamine receptors, and the dose and routes of administration (Huang et al., 2007; Smith et al., 2007). Several studies have reported inhibitory effects on pain following activation of H3 receptors using agonists (Cannon et al., 2003; Hasanein, 2011). The involvement of H3 receptors in neuropathic pain has been implicated using a range of H3 receptor antagonists/inverse agonists (Table 1). The antagonism at H3 receptors results in reduced mechanical and cold hypersensitivity associated with neuropathic pain (chronic constriction injury model or spinal nerve ligation model) as shown in studies using E‐162 or GSK189254, selective H3 receptor antagonists, where its strong analgesic effect was observed after a single systemic (i.p.) dose (Hsieh et al., 2010; Popiolek‐Barczyk et al., 2018). In addition, repeated, orally delivered doses of GSK189254, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=9103, S38093, and A‐960656, selective H3 receptor antagonists/inverse agonists, significantly reduced paw withdrawal threshold to mechanical stimuli or elicited an analgesic effect in the vocalization test of neuropathic pain (chronic constriction injury model or spinal nerve ligation model), showing comparable efficacy to http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5484 or http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5483, which are both used clinically as first‐line treatments (Chaumette et al., 2018; Cowart et al., 2012; Medhurst et al., 2008). The analgesic efficacy of S38093 was also confirmed in other models of neuropathic pain with different aetiologies, such as diabetic and chemotherapeutic agent‐induced neuropathy, where the drug again showed analgesic potency similar to pregabalin and gabapentin (Chaumette et al., 2018). Analgesia induced by the blockade of the H3 receptor is possibly the result of the regulation of histamine levels in the CNS, as depolarization activates histamine synthesis in nerve endings, a process that is controlled by H3 autoreceptors (Arrang et al., 1983; Hough & Rice, 2011). Indeed, Wei et al. (2016) proposed that blocking the autoinhibitory H3 receptor on histaminergic terminals in the pontine locus coeruleus (LC), which receives efferent projections from the histaminergic tuberomammillary nucleus, facilitated endogenous release of histamine leading to neuropathic hypersensitivity inhibition through the regulation of descending noradrenergic pathways. In addition, there is accumulating evidence to support the idea that the analgesic effects of H3 receptor antagonists/inverse agonists in neuropathic pain can be partially mediated by http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=4 desensitization (induced by H3 receptor antagonists/inverse agonists) in the LC and spinal cord. This suggests an inhibitory role for the central heteroreceptor noradrenergic transmission in the efficacy of H3 receptor antagonists/inverse agonists. In agreement with this idea, systemic administration of α2 adrenoceptor agonists or nerve injury‐induced activation of these α2 adrenoceptors decreases the firing activity of LC noradrenergic cells, resulting in the dampening of noradrenaline release in the terminal area (e.g., prefrontal cortex or spinal cord) and promoting neuropathic hypersensitivity by attenuating descending inhibition (Chaumette et al., 2018; Wei et al., 2010; Wei et al., 2014). In contrast, treatment with H3 receptor antagonists/inverse agonists restores LC and decreases α2 adrenoceptor activity, respectively, potentially leading to relief in neuropathic pain hypersensitivity (Chaumette et al., 2018; Wei et al., 2010). To further support this proposed mechanism, it was shown that bilateral lesion of the LC, transection of the spinal cord, or direct injection of a α2 agonist (fadolmidine) into the LC reversed the antihyperalgesic effect produced by H3 receptor antagonists, A‐960656 or GSK189254 (Chaumette et al., 2018; McGaraughty, Chu, Cowart, & Brioni, 2012; Wei et al., 2014). In line with this, electrophysiological studies performed in anaesthetized animals indicated that, after systemic administration, GSK189254 dose‐dependently decreased both evoked and spontaneous firing of wide dynamic range neurons in neuropathic, but not sham‐operated rats (McGaraughty et al., 2012). However, analgesia induced by the blockade of the H3 receptor can also be mediated via H3 heteroreceptors that regulate other neurotransmitters' release; the blockade of the H3 receptor is known to increase the release of ACh, dopamine, 5‐HT, noradrenaline, and SP in the CNS (Blandina et al., 2010; Gemkow et al., 2009; Giannoni et al., 2010).
Interestingly, the majority of the behavioural observations published indicate that H3 receptor antagonists/inverse agonists do not produce any antinociceptive effects in naïve rodents, suggesting a possibility that H3 receptors are not involved or tonically activated in nociception (at least in relation to acute mechanical nociception), but are critical for pathological pain states, particularly for mechanical hypersensitivity (Chaumette et al., 2018; McGaraughty et al., 2012). Also, H3 receptor KO mice showed unaltered response to mechanical pinch (Cannon et al., 2003), and multiple studies suggest modality (mechanical vs. heat) and intensity (preferential responses to low‐intensity tail pinch stimulation; Cannon et al., 2003) with specific antinociceptive effects mediated by H3 receptors. H3 receptor antagonists/inverse agonists at a dose that produced a significant reduction of mechanical hypersensitivity in neuropathic pain did not attenuate heat hypersensitivity indicating that the antihyperalgesic effect was due to selective depression of spinal sensory rather than motor neurons (Wei et al., 2014; Wei et al., 2016). To support this, in situ hybridization studies revealed H3 receptor mRNA transcripts in the sensory neurons of the dorsal horn and DRG (Heron et al., 2001). Moreover, receptor autoradiography studies, using [3H] GSK189254, showed specific H3 receptor binding sites in the dorsal horn of the spinal cord and DRG, confirming these as sites of action of H3 receptor antagonists within structures receiving histaminergic innervation and are critical for processing of pain information (Medhurst et al., 2008). The modality‐ and intensity‐specific antinociceptive effects of H3 receptor activation/inhibition may also suggest involvement of a specific population of sensory fibres that regulate mechanical hypersensitivity. In line with this, immunohistochemical studies identified localization of H3 receptors (confirmed by H3 receptor KO mice) on medium‐size cell bodies in DRG and on small‐calibre periarterial, peptidergic Aδ fibres that ramified in dorsal horn laminae I, II, and V and coexpress immunoreactivity for acid‐sensing ion channel 3 and 200‐kD neurofilament protein. This strongly supports the involvement of H3 receptors in the regulation of mechanical sensitivity (Cannon et al., 2007). In addition, Medhurst et al. (2007) showed that GSK207040 and GSK334429, selective H3 receptor antagonists, blocked the secondary mechanical allodynia in the capsaicin‐induced model of pain. Secondary mechanical hypersensitivity is known to be exclusively signalled by A‐fibres and amplified by sensitized dorsal horn neurons (Magerl, Fuchs, Meyer, & Treede, 2001; Treede & Magerl, 2000). Thus, presumably reducing the sensitivity of H3 receptor‐positive A‐fibres with selective H3 receptor antagonists resulted in a diminished input to the dorsal horn and the subsequent amplification of the A‐fibres response, confirming the potential role for H3 receptors in the modulation of central sensitization processes. In contrast, thermal (heat) hypersensitivity is generally regarded as a sign of the peripheral sensitization of C‐fibres, which do not express H3 receptors (Cannon et al., 2007; Gold & Gebhart, 2010). The only study that reported a significant increase in thermal (radiant heat in Hargreaves test), but not mechanical, threshold in the spared nerve injury model of neuropathic pain used the selective H3 receptor inverse agonist pitolisant (Wakix™). The drug produced this unexpected effect at a dose 5× higher than its clinically relevant dose, and pharmacological analysis of this effect suggested at least partial involvement of transient receptor potential cation channel subfamily V member 1 (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=507), without any contribution of H3 receptors (Zhang et al., 2012). Interestingly, the H3 receptor antagonist/inverse agonist E‐162, at a dose that produced a significant reduction in mechanical hypersensitivity, also attenuated the response to cold in neuropathic pain (Popiolek‐Barczyk et al., 2018). The signalling of cool temperatures that become aversive in neuropathic pain is known to be mediated via the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=500 receptor, a member of the TRP channel family (Knowlton et al., 2013). It was reported that the number of TRPM8‐positive Aδ‐fibres (but not C‐fibres) increases after nerve injury (Ji, Zhou, Kochukov, Westlund, & Carlton, 2007); thus, it is possible that H3 receptor‐positive A‐fibres are probably sensitive to cooling and may contribute to cold hypersensitivity in neuropathic pain.
The most significant inconsistencies in behavioural outcomes in neuropathic pain can be found in studies on the role of a first‐generation imidazole‐based molecule, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1267 (H3 receptor antagonist, H3 /H4 receptor inverse agonist), in the regulation of mechanical hypersensitivity in neuropathic pain. On the one hand, blocking H3 receptors (and H4 receptors) by thioperamide resulted in a significant enhancement of mechanical hyperalgesia in a rat model of neuropathic pain induced by partial ligation of the sciatic nerve. Specifically, i.c.v. (Huang et al., 2007) or s.c. injection (Smith et al., 2007) of thioperamide directly into the operated hindpaw resulted in a significantly reduced mechanical withdrawal threshold as compared to controls. On the other hand, systemic (i.p.) injection of thioperamide significantly increased mechanical withdrawal threshold indicating an analgesic effect (Huang et al., 2007). The reason for this discrepancy may lie in the drug's dual affinity for both H3 and H4 receptors (e.g., the effect of thioperamide on neurotransmitter release in the anterior hypothalamic area of rats is nonreversible by an H3 receptor agonist, suggesting the involvement of H4 receptors; Yamamoto, Mochizuki, Okakura‐Mochizuki, Uno, & Yamatodani, 1997) and on the behavioural effects resulting from the route (localized vs. systemic) and dose of thioperamide administration. In addition, the involvement of other histaminergic mechanisms of action in the behavioural effects produced by thioperamide is suggested by the observation that thioperamide increases the density of intact mast cells in the injured nerve (Smith et al., 2007). While nerve injury causes a decrease in mast cell numbers as a consequence of degranulation (Zuo et al., 2003), thioperamide's action leads to an opposite effect that may represent a prevention of mast cell degranulation and stabilization or redistribution of mast cells in the injured nerve that theoretically would result in the inhibition of hyperalgesia, rather than its enhancement. This effect could be linked to the observation that histamine (acting through H1 and H3 receptors) inhibits the release of the pro‐inflammatory cytokine http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5074 from alveolar macrophages (Sirois, Ménard, Moses, & Bissonnette, 2000), and antagonism of H3 receptors on macrophages resulted in an increase in TNF‐α and, subsequent, enhancement of mechanical hyperalgesia (Smith et al., 2007).
Taken together, the interpretation of the thioperamide data is complicated further since the drug has high affinity, not only for H3 and H4 receptors but also for http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=68 (Leurs et al., 1995). Studies with more selective H3 receptor antagonists/inverse agonists suggest that these ligands may be beneficial for the improvement of mechanical and cold hypersensitivity associated with neuropathic pain, particularly given their ability to modulate histamine levels, as well as several neurotransmitters, including ACh, histamine, noradrenaline, dopamine, and SP. However, due to the wide presynaptic and postsynaptic distribution of H3 receptors throughout the CNS and PNS, more research is certainly needed to clarify the involvement of peripheral, spinal, and brain H3 receptors in various neuropathic pain states, thus determining their full potential in neuropathic pain.
3.3. H4 receptor ligands and neuropathic pain
The H4 receptor, which has low homology with other histamine receptors, can be primarily found in bone marrow, intestinal tissue, spleen, thymus, and also in various immune cells, such as T cells, mast cells, neutrophils, and eosinophils, showing modulatory effects on these cells, including activation, migration, and production of cytokines and chemokines, suggesting its principal role in the regulation of immune/inflammatory mechanisms (Takeshita, Sakai, Bacon, & Gantner, 2003; Zhu et al., 2001). Interestingly, recent reports also indicate the presence of H4 receptors on peripheral sensory nerves, in the DRG, with more intense staining of small‐ and medium‐diameter cells, and in the spinal cord, especially laminae I and II (Sanna, Lucarini, et al., 2017; Strakhova et al., 2009). This neuronal localization supports H4 receptors involvement in the regulation of neuronal function related to the modulation of nociceptive transmission (Sanna, Ghelardini, Thurmond, Masini, & Galeotti, 2017; Sanna, Lucarini, et al., 2017).
The involvement of H4 receptors in both acute (Galeotti, Sanna, & Ghelardini, 2013) and persistent inflammatory pain (Hsieh et al., 2010) is relatively well documented, and recently, the role of H4 receptors in the modulation of neuropathic pain was identified in H4 receptor‐KO mice through the observation that these animals, when subjected to neuropathic pain, induced by spared nerve injury of sciatic nerve, showed enhanced hypersensitivity to mechanical and thermal stimuli compared to wild‐type controls (Sanna, Ghelardini, et al., 2017). Interestingly, H4 receptor deficiency does not support a role for H4 receptors in the physiological maintenance of pain threshold, as H4 receptor‐KO mice did not show any change in thermal or mechanical nociceptive thresholds, suggesting that the H4 receptor is specifically involved in the regulation of hypersensitivity associated with pathological chronic pain induced by nerve injury (Sanna, Ghelardini, et al., 2017). This observation in H4 receptor‐KO neuropathic mice is particularly important as H4 receptor mRNA expression in humans and rodents supports their involvement in the regulation of neuronal function, including regulation of neuropathic pain. The controversy around the generation of consistently specific H4 receptor antibodies highlights the need for cautious interpretation of some of the immunohistochemical outcomes (Beermann, Seifert, & Neumann, 2012; Gutzmer et al., 2012; Schneider & Seifert, 2016). In line with the observation from H4 receptor KO mice, blockade of H4 receptors by the specific H4 receptor antagonist JNJ7777120, injected s.c. directly into the operated hindpaw, resulted in a significant increase in mechanical hyperalgesia compared to controls (Smith et al., 2007). Subsequently, activation of H4 receptors by localized administration of potent and selective agonists, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=8983 (i.c.v.) and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1274 (i.c.v., intrathecally, and s.c. directly into the operated hindpaw), resulted in a significantly reduced mechanical and thermal withdrawal threshold in mice subjected to neuropathic pain induced by spared nerve injury or partial nerve ligation of the sciatic nerve (Sanna et al., 2015; Sanna, Lucarini, et al., 2017; Smith et al., 2007). The analgesia produced by VUF8430 has been shown to be associated with a reduction in neuroinflammation and oxidative stress mediated by neuronal H4 receptors in the spinal cord and sciatic nerve (Sanna, Lucarini, et al., 2017), and the involvement of H4 receptors in the behavioural effects produced by ST‐1006 and VUF8430 was confirmed with http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1277, H4 receptor antagonist also known as VUF6002, which fully prevented the analgesic effects produced by these H4 receptor agonists (Sanna et al., 2015).
Interestingly, similar to the H3 receptor, pharmacological studies using agonists and antagonists of H4 receptors demonstrate that these drugs can have different effects on the nociceptive threshold depending on the routes of administration and target cells (Popiolek‐Barczyk et al., 2018; Sanna et al., 2015). In contrast to the studies above that used H3 receptor agonists/antagonists after localized application, the antagonism of H4 receptors produced by systemic administration resulted in the alleviation of mechanical and cold hypersensitivity associated with neuropathic pain (chronic constriction injury model). Studies using TR‐7, a selective H4 receptor antagonist, elicited a strong analgesic effect after a single systemic (i.p.) dose, which was as effective as http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1627, a gold standard in pain treatment (Popiolek‐Barczyk et al., 2018). In addition, JNJ7777120 reduced mechanical hypersensitivity after a systemic (i.p.) administration in neuropathic pain (chronic constriction injury model and spinal nerve ligation model; Hsieh et al., 2010). Given that H4 receptors are expressed on the immune cells, in addition to the well‐documented involvement of H4 receptors in the regulation of immune/inflammatory mechanisms (Takeshita et al., 2003; Zhu et al., 2001), it is possible that the antinociceptive action of H4 receptor antagonists, particularly after systemic administration, may result from a reduction in ongoing inflammatory processes at the site of nerve injury, since the analgesic effect produced by JNJ7777120 was weaker (secondary) than its anti‐inflammatory effect (Hsieh et al., 2010). An underlying mechanism may be associated with stabilization of mast cells that are known to regulate the recruitment of neutrophils and macrophages and, subsequently, to modulate the development of hyperalgesia in neuropathic pain (Smith et al., 2007; Zuo et al., 2003). It should be further noted that similar observations have been described for the closely related H3 receptor.
The H4 receptor is known to activate the MAPK signalling pathway in mast cells (Desai & Thurmond, 2011). Interestingly, Sanna et al. (2015, 2018) also identified the effect of H4 receptor stimulation on the activity of the MAPK signalling pathway in neurons. They demonstrated that modulation of this signalling pathway within the neurons of the DRG, spinal cord, and sciatic nerve underpinned H4 receptor agonist‐induced antiallodynic activity. They also revealed that neuropathic pain hypersensitivity observed in H4 receptor‐KO mice is associated with an overactivation of the spinal ERK–http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2734 pathway in DβH immunoreactive neurons, supporting a potential association between the noradrenergic system and H4 receptor‐mediated analgesia. In summary, increasing evidence arising from H4 receptor KO mice and the use of selective ligands support H4 receptor as an interesting neuronal target for the treatment of chronic, particularly neuropathic, pain.
4. HISTAMINE AND NON‐NEURONAL CELLS IN NEUROPATHIC PAIN
Following peripheral nerve injury, the immune system seems to play a vital role in the development of persistent inflammation and chronic neuropathic pain (Marchand, Perretti, & McMahon, 2005). Non‐neuronal astrocytes, satellite glia cells, microglia, and mast cells play key roles in communication between the immune system and the CNS via the production of neuroinflammatory mediators, including histamine, 5‐HT, chemokines, and growth factors (Zhuang, Gerner, Woolf, & Ji, 2005). The neuroimmune interactions between these two systems may reflect distinct roles in the development of chronic neuropathic pain (Zhao et al., 2017). Stimulation of H1 receptors via a PKC/MAPK/http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2062 signalling pathway has recently been shown to elicit release of the key pro‐inflammatory cytokines http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4974 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4998 with, subsequent, regulation of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5026 release from astrocytes (reviewed recently in Jurič, Kržan, & Lipnik‐Stangelj, 2016). Satellite glial cells, prominent in the PNS, including the DRGs, with active roles in persistent neuropathic pain are also known to secrete the cytokine IL‐6 in the chronic constriction injury neuropathic pain model, but the identity profile of the histamine receptor in these cells has yet to be established (Dubový, Klusáková, Svíženská, & Brázda, 2010). Activated microglia also release a myriad of pro‐inflammatory cytokines, including notably, IL‐6, IL‐1β, and TNF‐α (Kempuraj et al., 2016; Mika, Zychowska, Popiolek‐Barczyk, Rojewska, & Przewlocka, 2013). H3 and H4 receptor activation of primary and clonal microglia has been shown to inhibit these cytokines (Ferreira et al., 2012).
Mast cells are professional cellular suppliers of histamine and contribute to the histamine‐based effects in neuropathic pain. For example, mast cell depletion prevented mechanical allodynia in a mouse model of postoperative pain (Kaur, Singh, & Jaggi, 2017). Recently, it was shown that the administration of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7121, a second‐generation H1 receptor antagonist and mast cell stabilizer, blocked the development of mechanical allodynia and inhibited mast cell degranulation in mice with http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7433‐induced mechanical allodynia pain. The H1 and H4 receptors are likely molecular players in this process (Sakamoto, Andoh, & Kuraishi, 2016). For example, genetically silencing the H4 receptor inhibited the production of IL‐1β for human mast cells (Ebenezer, Prasad, Rajan, Thangam, & Transduction, 2018), and H4 receptor activation was shown to stimulate a number of cytokines, including IL‐6 (Jemima, Prema, & Thangam, 2014). In addition, while H1 and H2 receptor antagonism reduced hypersensitivity following nerve injury, it is possible that histamine released by mast cells contributes to the recruitment of neutrophils and macrophages in neuropathic pain and, acting via these histamine receptors, contributes to the regulation of hypersensitivity in this type of chronic pain (Jaggi et al., 2017; Smith et al., 2007; Zuo et al., 2003). There is an important aspect associated with H2 receptor antagonism, which should be considered for its therapeutic potential in neuropathic pain control. In vitro studies using CHO and HEK‐293 cells identified time‐ and dose‐dependent up‐regulation of H2 receptors upon long‐term exposure to H2 receptor antagonists (e.g., ranitidine), which may underlie the development of tolerance after prolonged clinical use of these ligands and result in the rebound hypersecretion of gastric acid and anaphylaxis that can occur after withdrawal of treatment (Allen, Chazot, & Dixon, 2018; Smit et al., 1996). Thus, side effects linked to pharmacological tolerance may potentially compromise long‐term efficacy and tolerability of H2 receptor antagonists in neuropathic pain. Little is known about the role of the H3 receptors in non‐neuronal cells in neuropathic pain states.
Overall, non‐neuronal cells play a key, but poorly, defined role in the mechanisms underlying histamine‐mediated neuropathic pain. We propose that neuronal H1 and H4 receptors (Ferreira et al., 2012) may orchestrate these mechanisms, with IL‐6 and IL‐1β cytokines as common denominator mediators. Further complications arise from the recent observation that activated mast cells trigger microglial activation (Zhang, Wang, Dong, Xu, & Zhang, 2016). These cell types and their interactions may potentially go some way to explain the paradoxical effects of histamine ligands, particularly for the H4 receptor, seen in animal pain models.
5. HISTAMINE AND ITS INTERACTION WITH OPIOID SYSTEM IN NEUROPATHIC PAIN
Interestingly, in neuropathic pain, high doses of opioids are required to achieve pain relief, and pharmacological tolerance to analgesic effect of opioids develops rapidly (Osikowicz, Mika, Makuch, & Przewlocka, 2008). This phenomenon significantly restricts the clinical usefulness of opioids. In addition, the misuse of and addiction to opioids, including prescription pain relievers, morphine and heroin, as well as synthetic opioids, such as fentanyl, is a serious international crisis that affects public health as well as social and economic welfare (Lipman & Webster, 2015).
An interaction between histaminergic and opioidergic systems within the CNS was suggested nearly 30 years ago, through an observation that morphine administration resulted in the release of histamine and its increased turnover in the periaqueductal grey (Nishibori, Oishi, Itoh, & Saeki, 1985), suggesting that analgesia produced by opioids may be associated with the stimulation of histamine receptors at the supraspinal level. There are also data suggesting that ligands of histamine receptors may modulate the analgesic action of opioids; however, the site and mode of this interaction differ between the spinal or supraspinal level, and depend on the subtype of histamine receptor involved (Mobarakeh et al., 2002; Mobarakeh et al., 2006; Mobarakeh, Takahashi, & Yanai, 2009). Specifically, a series of studies over the last two decades has shown that in H1, H2, or H3 receptor‐KO mice, morphine‐induced antinociception was significantly augmented when compared to the wild‐type controls in models of acute pain. H1 receptor‐KO mice showed a reduced spontaneous nociceptive threshold as they responded to significantly lower pain stimuli when compared to their controls (Mobarakeh et al., 2002), while thresholds for pain perception in H2 receptor‐KO mice were higher when compared to their corresponding controls (Mobarakeh et al., 2006). Intrathecal administration of morphine in H1 and H3 receptor‐KO mice and i.c.v. morphine injection in H2 receptor‐KO mice produce enhanced analgesic effects (Mobarakeh et al., 2002; Mobarakeh et al., 2006). Interestingly, pharmacological blockade of H1 and H3 receptors by either intrathecal administration of the first‐generation antihistamine chlorpheniramine (H1 receptor antagonist) or thioperamide (H3 receptor antagonist, H3 /H4 receptor inverse agonist), or H2 receptor antagonism, produced by zolantidine (i.c.v. route), resulted in the potentiation of the morphine analgesic effect (Mobarakeh et al., 2002; Mobarakeh et al., 2006). These behavioural studies, in both KO mice and involving pharmacological interventions, clearly demonstrated that blocking H1, H2 and H3 receptors in combination with morphine may have beneficial effects on analgesia and suggested that endogenous histamine may exert an inhibitory effect on morphine‐induced analgesia acting via H1 and H3 receptors at the spinal cord level and via H2 receptors at the supraspinal level.
Importantly, the observations observed with H3 receptor‐KO mice are consistent with a pharmacological study using a preclinical model of neuropathic pain induced by chronic constriction injury of the sciatic nerve. Here, Popiolek‐Barczyk et al. (2018) showed that blockade of H3 receptors by a selective antagonist (E‐162) significantly enhanced morphine antinociception assessed with both mechanical and cold stimuli. Pharmacological analysis of these effects revealed an additive effect. Interestingly, Popiolek‐Barczyk et al. (2018) also showed that TR‐7, a selective H4 receptor antagonist, significantly enhanced morphine antinociception in neuropathic pain. This latter study is the first demonstration of the involvement of H4 receptors in the regulation of morphine efficacy in chronic pain.
To the best of our knowledge, the literature does not provide evidence for the mechanisms underlying histamine and opioid system interactions, in relation to the modulation of morphine analgesic effects. Given that the analgesic effects produced by modulation of the activity of both the histamine and opioid systems could be associated with blocking SP release from peripheral nerve terminals (Barnes et al., 1986; Przewłocki & Przewłocka, 2001), it is possible that an interaction that would result in potentiation of analgesic efficacy of morphine may involve, together with other possible mechanisms, the inhibition of peripheral SP accumulation. Such an outcome may be useful for the management of neuropathic pain, particularly when peripheral administration of drugs is possible, thus affording reduction of the undesired secondary effects associated with opioid administration and peripheral mechanisms of action (e.g., constipation). However, centrally acting drugs administered by peripheral routes should be taken into consideration due to the potential serious interactions related to their pharmacodynamics and central mechanisms of action. For example, chlorpheniramine (a first‐generation H1 receptor antagonist) was reported to potentiate fentanyl‐induced sedation and respiratory depression after surgery (Anwari & Iqbal, 2003).
6. CONCLUSIONS AND FUTURE DIRECTIONS
Findings from the last two decades indicate that selective pharmacological antagonism of neurons expressing H3 receptors could provide important and promising therapeutic approaches for the control of mechanical and cold hypersensitivity in peripheral neuropathies (Table 1). The analgesic effectiveness of H3 receptor antagonists/inverse agonists was comparable to gabapentin and pregabalin, first‐line treatments for neuropathic pain. Importantly, multiple examples of behavioural, electrophysiological, and molecular evidence strongly support the rationale for this neuropathic pain strategy, particularly given their ability to modulate histamine levels as well as several neurotransmitters critical for chronic pain processing. Moreover, the recent registered approval of pitolisant (Wakix™), an antagonist/inverse agonist of H3 receptors, for the treatment of narcolepsy in patients, has opened the door for the potential use of H3 receptor ligands for other conditions, including chronic neuropathic pain. However, due to the wide presynaptic and postsynaptic distribution of H3 receptors throughout the CNS and PNS, more research is certainly needed to clarify the involvement of peripheral, spinal, and brain H3 receptors in various pain states, before determining their full potential in neuropathic pain.
Recent findings also suggest the use of centrally permeable H2 receptor antagonists as promising new drug candidates for the treatment of neuropathic pain, in view of their analgesic effects and metabolic stability. Interestingly, however, despite the discovery of the most recently discovered histamine receptor, the role of the H4 receptor in neuropathic pain transmission is still controversial after nearly 20 years, with apparent confounding effects of both agonists and antagonists on hypersensitivity associated with neuropathic pain. This may be due to biased signalling of histamine and H4 receptor agonist ligands and differential effects on multiple signalling pathways in central and peripheral parts of the sensory nervous system. Furthermore, the paucity of detailed mechanistic definitions of histamine‐mediated analgesia, and the additive effects with the opioid system, requires attention to provide a rationale to the field of histamine and development of neuropathic pain control therapeutics.
A better understanding of the interaction between histaminergic signalling pathway molecules (Figures 1, 2, 3) and histamine receptors may result in the identification of further novel pharmacological targets to improve neuropathic pain control. The literature available provides some evidence for potential pharmacological target molecules. One potential strategy exploits the role of Ca2+ channels in the regulation of cellular excitability associated with nociception (e.g., N‐type Ca2+ channels). Evidence has shown that Ca2+ channel blockers (e.g., http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2536) offer interesting analgesic potential in treating neuropathic pain (Vanegas & Schaible, 2000). However, there is no evidence for the effect produced by a combination of Ca2+ channel blockers and histamine receptor ligands, and we propose that their interaction should be taken into consideration. Another potential target involves the contribution of the MAPK/ERK signalling pathway to the regulation of pain hypersensitivity. Recently, Sanna et al. (2015) showed that H4 receptor stimulation, which led to analgesic activity in neuropathic pain, was modulated by MAPK/ERK signalling in the neurons of the DRG, spinal cord, and sciatic nerve. While the MAPK/ERK signalling pathway regulates pain sensitivity and, for a while, has been considered as a target for the treatment of neuropathic pain (Ma & Quirion, 2005), further studies on the interaction between this pathway and H4 receptors may lead to the identification of more efficient therapeutic strategies to control neuropathic pain.
6.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org/, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Christopoulos et al., 2017; Alexander, Fabbro et al., 2017; Alexander, Peters et al., 2017; Alexander, Striessnig et al., 2017).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGEMENT
Supported by the funding from the Medical Research Council Confidence in Concept scheme to Drs P. L. Chazot and I. Obara (MC/PC/17157). We acknowledge Drs Janet Chazot and Natalie Young for their editorial assistance on the manuscript.
Obara I, Telezhkin V, Alrashdi I, Chazot PL. Histamine, histamine receptors, and neuropathic pain relief. Br J Pharmacol. 2020;177:580–599. 10.1111/bph.14696
REFERENCES
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Marrion, N. V. , Peters, J. A. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. British Journal of Pharmacology, 174, S17–S129. 10.1111/bph.13878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174(S1), S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Peters, J. A. , Kelly, E. , Marrion, N. V. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators (2017). THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: Ligand‐gated ion channels. British Journal of Pharmacology, 174(S1), S130–S159. 10.1111/bph.13879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Striessnig, J. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: Voltage‐gated ion channels. British Journal of Pharmacology, 174(S1), S160–S194. 10.1111/bph.13884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, S. J. , Chazot, P. L. , & Dixon, C. (2018). Can H2‐receptor upregulation and raised histamine explain an anaphylactoid reaction on cessation of ranitidine in a 19‐year‐old female? A case report. British Journal of Clinical Pharmacology, 84, 1611–1616. 10.1111/bcp.13578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso, N. , Fernandez, N. , Notcovich, C. , Monczor, F. , Simaan, M. , Baldi, A. , … Shayo, C. (2013). Cross‐desensitization and cointernalization of H1 and H2 histamine receptors reveal new insights into histamine signal integration. Molecular Pharmacology, 83, 1087–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwari, J. S. , & Iqbal, S. (2003). Antihistamines and potentiation of opioid induced sedation and respiratory depression. Anaesthesia, 58, 494–495. 10.1046/j.1365-2044.2003.03154_18.x [DOI] [PubMed] [Google Scholar]
- Arrang, J. M. , Garbarg, M. , & Schwartz, J. C. (1983). Auto‐inhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature, 302, 832–837. [DOI] [PubMed] [Google Scholar]
- Barnes, P. , Brown, M. , Dollery, C. , Fuller, R. , Heavey, D. , & Ind, P. (1986). Histamine is released from skin by substance P but does not act as the final vasodilator in the axon reflex. British Journal of Pharmacology, 88, 741–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barocelli, E. , & Ballabeni, V. (2003). Histamine in the control of gastric acid secretion: A topic review. Pharmacological Research, 47, 299–304. 10.1016/S1043-6618(03)00009-4 [DOI] [PubMed] [Google Scholar]
- Baron, R. , Binder, A. , & Wasner, G. (2010). Neuropathic pain: Diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurology, 9, 807–819. 10.1016/S1474-4422(10)70143-5 [DOI] [PubMed] [Google Scholar]
- Baron, R. , Schwarz, K. , Kleinert, A. , Schattschneider, J. , & Wasner, G. (2001). Histamine‐induced itch converts into pain in neuropathic hyperalgesia. Neuroreport, 12, 3475–3478. [DOI] [PubMed] [Google Scholar]
- Beermann, S. , Seifert, R. , & Neumann, D. (2012). Commercially available antibodies against human and murine histamine H4‐receptor lack specificity. Naunyn‐Schmiedeberg's Archives of Pharmacology, 385, 125–135. 10.1007/s00210-011-0700-4 [DOI] [PubMed] [Google Scholar]
- Bennett, G. J. , & Xie, Y.‐K. (1988). A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain, 33, 87–107. 10.1016/0304-3959(88)90209-6 [DOI] [PubMed] [Google Scholar]
- Bhowmik, M. , Khanam, R. , & Vohora, D. (2012). Histamine H3 receptor antagonists in relation to epilepsy and neurodegeneration: A systemic consideration of recent progress and perspectives. British Journal of Pharmacology, 167, 1398–1414. 10.1111/j.1476-5381.2012.02093.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandina, P. , Munari, L. , Giannoni, P. , Mariottini, C. , & Passani, M. B. (2010). Histamine neuronal system as a therapeutic target for the treatment of cognitive disorders. Future Neurology, 5, 543–555. 10.2217/fnl.10.30 [DOI] [Google Scholar]
- Bodmer, S. , Imark, C. , & Kneubuhl, M. (1999). Biogenic amines in foods: Histamine and food processing. Inflammation Research, 48, 296–300. 10.1007/s000110050463 [DOI] [PubMed] [Google Scholar]
- Bongers, G. , Sallmen, T. , Passani, M. B. , Mariottini, C. , Wendelin, D. , Lozada, A. , … Leurs, R. (2007). The Akt/GSK‐3β axis as a new signaling pathway of the histamine H3 receptor. Journal of Neurochemistry, 103, 248–258. 10.1111/j.1471-4159.2007.04752.x [DOI] [PubMed] [Google Scholar]
- Bouhassira, D. , Lanteri‐Minet, M. , Attal, N. , Laurent, B. , & Touboul, C. (2008). Prevalence of chronic pain with neuropathic characteristics in the general population. Pain, 136, 380–387. 10.1016/j.pain.2007.08.013 [DOI] [PubMed] [Google Scholar]
- Breivik, H. , Collett, B. , Ventafridda, V. , Cohen, R. , & Gallacher, D. (2006). Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. European Journal of Pain, 10, 287–333. [DOI] [PubMed] [Google Scholar]
- Brown, D. A. , & Passmore, G. M. (2009). Neural KCNQ (Kv7) channels. British Journal of Pharmacology, 156, 1185–1195. 10.1111/j.1476-5381.2009.00111.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, R. E. , Stevens, D. R. , & Haas, H. L. (2001). The physiology of brain histamine. Progress in Neurobiology, 63, 637–672. 10.1016/S0301-0082(00)00039-3 [DOI] [PubMed] [Google Scholar]
- Campbell, J. N. , & Meyer, R. A. (2006). Mechanisms of neuropathic pain. Neuron, 52, 77–92. 10.1016/j.neuron.2006.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon, K. E. , Chazot, P. L. , Hann, V. , Shenton, F. , Hough, L. B. , & Rice, F. L. (2007). Immunohistochemical localization of histamine H3 receptors in rodent skin, dorsal root ganglia, superior cervical ganglia, and spinal cord: Potential antinociceptive targets. Pain, 129, 76–92. 10.1016/j.pain.2006.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon, K. E. , Nalwalk, J. W. , Stadel, R. , Ge, P. , Lawson, D. , Silos‐Santiago, I. , & Hough, L. B. (2003). Activation of spinal histamine H3 receptors inhibits mechanical nociception. European Journal of Pharmacology, 470, 139–147. [DOI] [PubMed] [Google Scholar]
- Chaumette, T. , Chapuy, E. , Berrocoso, E. , Llorca‐Torralba, M. , Bravo, L. , Mico, J. A. , … Sors, A. (2018). Effects of S 38093, an antagonist/inverse agonist of histamine H3 receptors, in models of neuropathic pain in rats. European Journal of Pain, 22, 127–141. 10.1002/ejp.1097 [DOI] [PubMed] [Google Scholar]
- Chazot, P. L. , & Care, C. (2005). Histamine H3 receptors: Potential novel target for analgesia. Current Anaesthesia and Critical Care, 16, 94–98. 10.1016/j.cacc.2005.03.003 [DOI] [Google Scholar]
- Chen, X. , Li, W. , Hiett, S. C. , & Obukhov, A. G. (2016). Novel roles for Kv7 channels in shaping histamine‐induced contractions and bradykinin‐dependent relaxations in pig coronary arteries. PLoS ONE, 11, e0148569 10.1371/journal.pone.0148569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, E. A. , & Hill, S. J. (1996). Sensitivity of histamine H3 receptor agonist‐stimulated [35S]GTPγ [S] binding to pertussis toxin. European Journal of Pharmacology, 296, 223–225. 10.1016/0014-2999(95)00800-4 [DOI] [PubMed] [Google Scholar]
- Colleoni, M. , & Sacerdote, P. (2010). Murine models of human neuropathic pain. Biochimica et Biophysica Acta, 1802, 924–933. 10.1016/j.bbadis.2009.10.012 [DOI] [PubMed] [Google Scholar]
- Colloca, L. , Ludman, T. , Bouhassira, D. , Baron, R. , Dickenson, A. H. , Yarnitsky, D. , … Raja, S. N. (2017). Neuropathic pain. Nature Reviews. Disease Primers, 3, 17002 10.1038/nrdp.2017.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly, W. , Shenton, F. , Lethbridge, N. , Leurs, R. , Waldvogel, H. , Faull, R. , … Chazot, P. L. (2009). The histamine H4 receptor is functionally expressed on neurons in the mammalian CNS. B J Pharmacol, 157, 55–63. 10.1111/j.1476-5381.2009.00227.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowart, M. , Hsieh, G. , Black, L. A. , Zhan, C. , Gomez, E. J. , Pai, M. , … Brioni, J. D. (2012). Pharmacological characterization of A‐960656, a histamine H3 receptor antagonist with efficacy in animal models of osteoarthritis and neuropathic pain. European Journal of Pharmacology, 684, 87–94. 10.1016/j.ejphar.2012.03.048 [DOI] [PubMed] [Google Scholar]
- Cruz, C. D. , & Cruz, F. (2007). The ERK 1 and 2 pathway in the nervous system: From basic aspects to possible clinical applications in pain and visceral dysfunction. Current Neuropharmacology, 5, 244–252. 10.2174/157015907782793630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decosterd, I. , & Woolf, C. J. (2000). Spared nerve injury: An animal model of persistent peripheral neuropathic pain. Pain, 87, 149–158. 10.1016/S0304-3959(00)00276-1 [DOI] [PubMed] [Google Scholar]
- Desai, P. , & Thurmond, R. L. (2011). Histamine H4 receptor activation enhances LPS‐induced IL‐6 production in mast cells via ERK and PI3K activation. European Journal of Immunology, 41, 1764–1773. 10.1002/eji.201040932 [DOI] [PubMed] [Google Scholar]
- Drutel, G. , Peitsaro, N. , Karlstedt, K. , Wieland, K. , Smit, M. J. , Timmerman, H. , … Leurs, R. (2001). Identification of rat H3 receptor isoforms with different brain expression and signaling properties. Molecular Pharmacology, 59, 1–8. [PubMed] [Google Scholar]
- Dubový, P. , Klusáková, I. , Svíženská, I. , & Brázda, V. (2010). Satellite glial cells express IL‐6 and corresponding signal‐transducing receptors in the dorsal root ganglia of rat neuropathic pain model. Neuron Glia Biology, 6, 73–83. [DOI] [PubMed] [Google Scholar]
- Dzamko, N. , Zhou, J. , Huang, Y. , & Halliday, G. M. (2014). Parkinson's disease‐implicated kinases in the brain; insights into disease pathogenesis. Frontiers in Molecular Neuroscience, 7, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebenezer, A. J. , Prasad, K. , Rajan, S. , Thangam, E. B. , & Transduction, S. (2018). Silencing of H4R inhibits the production of IL‐1β through SAPK/JNK signaling in human mast cells. Journal of Receptor Research, 38, 204–212. [DOI] [PubMed] [Google Scholar]
- Erfanparast, A. , Tamaddonfard, E. , Farshid, A. A. , & Khalilzadeh, E. (2010). Effect of microinjection of histamine into the dorsal hippocampus on the orofacial formalin‐induced pain in rats. European Journal of Pharmacology, 627, 119–123. 10.1016/j.ejphar.2009.10.063 [DOI] [PubMed] [Google Scholar]
- Fayaz, A. , Croft, P. , Langford, R. M. , Donaldson, L. J. , & Jones, G. T. (2016). Prevalence of chronic pain in the UK: A systematic review and meta‐analysis of population studies. BMJ Open, 6, e010364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira, R. , Santos, T. , Gonçalves, J. , Baltazar, G. , Ferreira, L. , Agasse, F. , & Bernardino, L. (2012). Histamine modulates microglia function. J Neuroinflammation, 9, 90 10.1186/1742-2094-9-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnerup, N. B. , Attal, N. , Haroutounian, S. , McNicol, E. , Baron, R. , Dworkin, R. H. , … Wallace, M. (2015). Pharmacotherapy for neuropathic pain in adults: A systematic review and meta‐analysis. Lancet Neurology, 14, 162–173. 10.1016/S1474-4422(14)70251-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnerup, N. B. , Haroutounian, S. , Kamerman, P. , Baron, R. , Bennett, D. L. , Bouhassira, D. , … Jensen, T. S. (2016). Neuropathic pain: An updated grading system for research and clinical practice. Pain, 157, 1599–1606. 10.1097/j.pain.0000000000000492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeotti, N. , Sanna, M. D. , & Ghelardini, C. (2013). Pleiotropic effect of histamine H4 receptor modulation in the central nervous system. Neuropharmacology, 71, 141–147. [DOI] [PubMed] [Google Scholar]
- Gangadharan, V. , & Kuner, R. (2013). Pain hypersensitivity mechanisms at a glance. Disease Models & Mechanisms, 6, 889–895. 10.1242/dmm.011502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemkow, M. J. , Davenport, A. J. , Harich, S. , Ellenbroek, B. A. , Cesura, A. , & Hallett, D. (2009). The histamine H3 receptor as a therapeutic drug target for CNS disorders. Drug Discovery Today, 14, 509–515. 10.1016/j.drudis.2009.02.011 [DOI] [PubMed] [Google Scholar]
- Giannoni, P. , Medhurst, A. D. , Passani, M. B. , Giovannini, M. G. , Ballini, C. , Corte, L. D. , & Blandina, P. (2010). Regional differential effects of the novel histamine H3 receptor antagonist 6‐[(3‐cyclobutyl‐2,3,4,5‐tetrahydro‐1H‐3‐benzazepin‐7‐yl)oxy]‐N‐methyl‐3‐pyridine carboxamide hydrochloride (GSK189254) on histamine release in the central nervous system of freely moving rats. The Journal of Pharmacology and Experimental Therapeutics, 332, 164–172. 10.1124/jpet.109.158444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilron, I. , Bailey, J. M. , & Vandenkerkhof, E. G. (2013). Chronobiological characteristics of neuropathic pain: Clinical predictors of diurnal pain rhythmicity. The Clinical Journal of Pain, 29, 755–759. 10.1097/AJP.0b013e318275f287 [DOI] [PubMed] [Google Scholar]
- Gold, M. S. , & Gebhart, G. F. (2010). Nociceptor sensitization in pain pathogenesis. Nature Medicine, 16, 1248–1257. 10.1038/nm.2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustorff, B. , Dorner, T. , Likar, R. , Grisold, W. , Lawrence, K. , Schwarz, F. , & Rieder, A. (2008). Prevalence of self‐reported neuropathic pain and impact on quality of life: A prospective representative survey. Acta Anaesthesiologica Scandinavica, 52, 132–136. [DOI] [PubMed] [Google Scholar]
- Gutzmer, R. , Werfel, T. , Baumer, W. , Kietzmann, M. , Chazot, P. L. , & Leurs, R. (2012). Well characterized antihistamine 4 receptor antibodies contribute to current knowledge of the expression and biology of the human and murine histamine 4 receptor. Naunyn‐Schmiedeberg's Archives of Pharmacology, 385, 853–860. 10.1007/s00210-012-0744-0 [DOI] [PubMed] [Google Scholar]
- Haas, H. L. , Sergeeva, O. A. , & Selbach, O. (2008). Histamine in the nervous system. Physiological Reviews, 88, 1183–1241. 10.1152/physrev.00043.2007 [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR . (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res, 46, D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harifi, G. , Amine, M. , Ait Ouazar, M. , Boujemaoui, A. , Ouilki, I. , Rekkab, I. , … el Hassani, S. (2013). Prevalence of chronic pain with neuropathic characteristics in the Moroccan general population: A national survey. Pain Medicine, 14, 287–292. 10.1111/pme.12009 [DOI] [PubMed] [Google Scholar]
- Hasanein, P. (2011). Effects of histamine H3 receptors on chemical hyperalgesia in diabetic rats. Neuropharmacology, 60, 886–891. [DOI] [PubMed] [Google Scholar]
- Heron, A. , Rouleau, A. , Cochois, V. , Pillot, C. , & Schwartz, J. (2001). Expression analysis of the histamine H3 receptor in developing rat tissues. Mechanisms of Development, 105, 167–173. 10.1016/S0925-4773(01)00389-6 [DOI] [PubMed] [Google Scholar]
- Hough, L. B. , & Rice, F. L. (2011). H3 receptors and pain modulation: Peripheral, spinal, and brain interactions. The Journal of Pharmacology and Experimental Therapeutics, 336, 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh, G. C. , Honore, P. , Pai, M. , Wensink, E. J. , Chandran, P. , Salyers, A. K. , … Brioni, J. D. (2010). Antinociceptive effects of histamine H3 receptor antagonist in the preclinical models of pain in rats and the involvement of central noradrenergic systems. Brain Research, 1354, 74–84. [DOI] [PubMed] [Google Scholar]
- Huang, L. , Adachi, N. , Nagaro, T. , Liu, K. , & Arai, T. (2007). Histaminergic involvement in neuropathic pain produced by partial ligation of the sciatic nerve in rats. Regional Anesthesia and Pain Medicine, 32, 124–129. 10.1097/00115550-200703000-00006 [DOI] [PubMed] [Google Scholar]
- Jaggi, A. S. , Kaur, G. , Bali, A. , & Singh, N. (2017). Pharmacological investigations on mast cell stabilizer and histamine receptor antagonists in vincristine‐induced neuropathic pain. Naunyn‐Schmiedeberg's Archives of Pharmacology, 390, 1087–1096. 10.1007/s00210-017-1426-8 [DOI] [PubMed] [Google Scholar]
- Jemima, E. A. , Prema, A. , & Thangam, E. B. (2014). Functional characterization of histamine H4 receptor on human mast cells. Molecular Immunology, 62, 19–28. [DOI] [PubMed] [Google Scholar]
- Jensen, M. P. , Chodroff, M. J. , & Dworkin, R. H. (2007). The impact of neuropathic pain on health‐related quality of life: review and implications. Neurology, 68, 1178–1182. 10.1212/01.wnl.0000259085.61898.9e [DOI] [PubMed] [Google Scholar]
- Ji, G. , Zhou, S. , Kochukov, M. , Westlund, K. , & Carlton, S. (2007). Plasticity in intact Aδ‐and C‐fibers contributes to cold hypersensitivity in neuropathic rats. Neuroscience, 150, 182–193. 10.1016/j.neuroscience.2007.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurič, D. M. , Kržan, M. , & Lipnik‐Stangelj, M. (2016). Histamine and astrocyte function. Pharmacological Research, 111, 774–783. [DOI] [PubMed] [Google Scholar]
- Kashiba, H. , Fukui, H. , & Senba, E. (2001). Histamine H1 receptor mRNA is expressed in capsaicin‐insensitive sensory neurons with neuropeptide Y‐immunoreactivity in guinea pigs. Brain Research Reviews, 901, 85–93. 10.1016/S0006-8993(01)02287-9 [DOI] [PubMed] [Google Scholar]
- Kaur, G. , Singh, N. , & Jaggi, A. S. (2017). Mast cells in neuropathic pain: an increasing spectrum of their involvement in pathophysiology. Reviews in the Neurosciences, 28, 759–766. 10.1515/revneuro-2017-0007 [DOI] [PubMed] [Google Scholar]
- Kempuraj, D. , Thangavel, R. , Natteru, P. A. , Selvakumar, G. P. , Saeed, D. , Zahoor, H. , … Zaheer, A. (2016). Neuroinflammation induces neurodegeneration. J Neurol Neurosurg Spine, 1, 1003. [PMC free article] [PubMed] [Google Scholar]
- Khalilzadeh, E. , Azarpey, F. , Hazrati, R. , & Vafaei Saiah, G. (2018). Evaluation of different classes of histamine H1 and H2 receptor antagonist effects on neuropathic nociceptive behavior following tibial nerve transection in rats. European Journal of Pharmacology, 834, 221–229. 10.1016/j.ejphar.2018.07.011 [DOI] [PubMed] [Google Scholar]
- Kim, S. H. , & Chung, J. M. (1992). An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain, 50, 355–363. [DOI] [PubMed] [Google Scholar]
- Knowlton, W. M. , Palkar, R. , Lippoldt, E. K. , McCoy, D. D. , Baluch, F. , Chen, J. , & McKemy, D. D. (2013). A sensory‐labeled line for cold: TRPM8‐expressing sensory neurons define the cellular basis for cold, cold pain, and cooling‐mediated analgesia. The Journal of Neuroscience, 33, 2837–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollb‐Sielecka, M. , Demolis, P. , Emmerich, J. , Markey, G. , Salmonson, T. , & Haas, M. (2017). The European Medicines Agency review of pitolisant for treatment of narcolepsy: Summary of the scientific assessment by the Committee for Medicinal Products for Human Use. Sleep Medicine, 33, 125–129. 10.1016/j.sleep.2017.01.002 [DOI] [PubMed] [Google Scholar]
- Lai, X. , Ye, L. , Liao, Y. , Jin, L. , Ma, Q. , Lu, B. , … Zhou, N. (2016). Agonist‐induced activation of histamine H3 receptor signals to extracellular signal‐regulated kinases 1 and 2 through PKC‐, PLD‐, and EGFR‐dependent mechanisms. Journal of Neurochemistry, 137, 200–215. 10.1111/jnc.13559 [DOI] [PubMed] [Google Scholar]
- LaMotte, R. , Simone, D. , Baumann, T. , Shain, C. , & Alreja, M. (1987). Hypothesis for novel classes of chemoreceptors mediating chemogenic pain and itch. Pain, 30, S15. [Google Scholar]
- Leung, C. C. Y. , & Wong, Y. H. (2017). Role of G protein‐coupled receptors in the regulation of structural plasticity and cognitive function. Molecules, 22, 1239 10.3390/molecules22071239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leurs, R. , Tulp, M. T. , Menge, W. M. , Adolfs, M. J. , Zuiderveld, O. P. , & Timmerman, H. (1995). Evaluation of the receptor selectivity of the H3 receptor antagonists, iodophenpropit and thioperamide: an interaction with the 5‐HT3 receptor revealed. British Journal of Pharmacology, 116, 2315–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindskog, M. (2017). Histamine receptors in the cross‐talk between periphery and brain. The International Journal of Neuropsychopharmacology, 20, 400–402. 10.1093/ijnp/pyx018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman, A. , & Webster, L. (2015). The economic impact of opioid use in the management of chronic nonmalignant pain. J Manag Care Spec Pharm, 21, 891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovenberg, T. W. , Roland, B. L. , Wilson, S. J. , Jiang, X. , Pyati, J. , Huvar, A. , … Erlander, M. G. (1999). Cloning and functional expression of the human histamine H3 receptor. Molecular Pharmacology, 55, 1101–1107. 10.1124/mol.55.6.1101 [DOI] [PubMed] [Google Scholar]
- Luscher, C. , & Slesinger, P. A. (2010). Emerging roles for G protein‐gated inwardly rectifying potassium (GIRK) channels in health and disease. Nature Reviews. Neuroscience, 11, 301–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, C. , Shu, Y. , Zheng, Z. , Chen, Y. , Yao, H. , Greenquist, K. W. , … LaMotte, R. H. (2003). Similar electrophysiological changes in axotomized and neighboring intact dorsal root ganglion neurons. Journal of Neurophysiology, 89, 1588–1602. 10.1152/jn.00855.2002 [DOI] [PubMed] [Google Scholar]
- Ma, W. , & Quirion, R. (2005). The ERK/MAPK pathway, as a target for the treatment of neuropathic pain. Expert Opinion on Therapeutic Targets, 9, 699–713. 10.1517/14728222.9.4.699 [DOI] [PubMed] [Google Scholar]
- Magerl, W. , Fuchs, P. N. , Meyer, R. A. , & Treede, R. D. (2001). Roles of capsaicin‐insensitive nociceptors in cutaneous pain and secondary hyperalgesia. Brain, 124, 1754–1764. 10.1093/brain/124.9.1754 [DOI] [PubMed] [Google Scholar]
- Maixner, D. W. , & Weng, H. R. (2013). The role of glycogen synthase kinase 3 beta in neuroinflammation and pain. The Journal of Pharmacy and Pharmacology, 1, 001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand, F. , Perretti, M. , & McMahon, S. B. (2005). Role of the immune system in chronic pain. Nature Reviews. Neuroscience, 6, 521–532. [DOI] [PubMed] [Google Scholar]
- McGaraughty, S. , Chu, K. L. , Cowart, M. D. , & Brioni, J. D. (2012). Antagonism of supraspinal histamine H3 receptors modulates spinal neuronal activity in neuropathic rats. The Journal of Pharmacology and Experimental Therapeutics, 343, 13–20. 10.1124/jpet.112.194761 [DOI] [PubMed] [Google Scholar]
- Medhurst, A. D. , Briggs, M. A. , Bruton, G. , Calver, A. R. , Chessell, I. , Crook, B. , … Wilson, D. M. (2007). Structurally novel histamine H3 receptor antagonists GSK207040 and GSK334429 improve scopolamine‐induced memory impairment and capsaicin‐induced secondary allodynia in rats. Biochemical Pharmacology, 73, 1182–1194. [DOI] [PubMed] [Google Scholar]
- Medhurst, S. J. , Collins, S. D. , Billinton, A. , Bingham, S. , Dalziel, R. G. , Brass, A. , … Chessell, I. P. (2008). Novel histamine H3 receptor antagonists GSK189254 and GSK334429 are efficacious in surgically‐induced and virally‐induced rat models of neuropathic pain. Pain, 138, 61–69. 10.1016/j.pain.2007.11.006 [DOI] [PubMed] [Google Scholar]
- Merskey, H. , & Bogduk, N. (1994). Classification of chronic pain (2nd ed.) (p. 212). Seattle: IASP Press. [Google Scholar]
- Mika, J. , Zychowska, M. , Popiolek‐Barczyk, K. , Rojewska, E. , & Przewlocka, B. (2013). Importance of glial activation in neuropathic pain. European Journal of Pharmacology, 716, 106–119. [DOI] [PubMed] [Google Scholar]
- Mobarakeh, J. I. , Sakurada, S. , Hayashi, T. , Orito, T. , Okuyama, K. , Sakurada, T. , … Yanai, K. (2002). Enhanced antinociception by intrathecally‐administered morphine in histamine H1 receptor gene knockout mice. Neuropharmacology, 42, 1079–1088. [DOI] [PubMed] [Google Scholar]
- Mobarakeh, J. I. , Sakurada, S. , Katsuyama, S. , Kutsuwa, M. , Kuramasu, A. , Lin, Z. Y. , … Yanai, K. (2000). Role of histamine H1 receptor in pain perception: A study of the receptor gene knockout mice. European Journal of Pharmacology, 391, 81–89. 10.1016/S0014-2999(00)00060-1 [DOI] [PubMed] [Google Scholar]
- Mobarakeh, J. I. , Takahashi, K. , Sakurada, S. , Kuramasu, A. , & Yanai, K. (2006). Enhanced antinociceptive effects of morphine in histamine H2 receptor gene knockout mice. Neuropharmacology, 51, 612–622. 10.1016/j.neuropharm.2006.05.003 [DOI] [PubMed] [Google Scholar]
- Mobarakeh, J. I. , Takahashi, K. , & Yanai, K. (2009). Enhanced morphine‐induced antinociception in histamine H3 receptor gene knockout mice. Neuropharmacology, 57, 409–414. 10.1016/j.neuropharm.2009.06.036 [DOI] [PubMed] [Google Scholar]
- Mojtahedin, A. (2016). Effects of cholinergic system in antinociception induced by H‐1 and H‐2 receptor antagonists on somatic pain in rats. Int J Med Res Health Sci, 5, 378–383. [Google Scholar]
- Morita, Y. , Aida, N. , & Miyamoto, T. (1983). Role of phospholipase A2 activation in histamine release from human basophils. Allergy, 38, 413–418. 10.1111/j.1398-9995.1983.tb05084.x [DOI] [PubMed] [Google Scholar]
- Murakami, H. , Sun‐Wada, G. H. , Matsumoto, M. , Nishi, T. , Wada, Y. , & Futai, M. (1999). Human histamine H2 receptor gene: Multiple transcription initiation and tissue‐specific expression. FEBS Letters, 451, 327–331. 10.1016/S0014-5793(99)00618-3 [DOI] [PubMed] [Google Scholar]
- Nieto‐Alamilla, G. , Marquez‐Gomez, R. , Garcia‐Galvez, A. M. , Morales‐Figueroa, G. E. , & Arias‐Montano, J. A. (2016). The histamine H3 receptor: Structure, pharmacology, and function. Molecular Pharmacology, 90, 649–673. 10.1124/mol.116.104752 [DOI] [PubMed] [Google Scholar]
- Nishibori, M. , Oishi, R. , Itoh, Y. , & Saeki, K. (1985). Morphine‐induced changes in histamine dynamics in mouse brain. Journal of Neurochemistry, 45, 719–724. 10.1111/j.1471-4159.1985.tb04051.x [DOI] [PubMed] [Google Scholar]
- Osikowicz, M. , Mika, J. , Makuch, W. , & Przewlocka, B. (2008). Glutamate receptor ligands attenuate allodynia and hyperalgesia and potentiate morphine effects in a mouse model of neuropathic pain. Pain, 139, 117–126. 10.1016/j.pain.2008.03.017 [DOI] [PubMed] [Google Scholar]
- Ossipov, M. H. , & Porreca, F. (2005). Challenges in the development of novel treatment strategies for neuropathic pain. NeuroRx, 2, 650–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panula, P. , Chazot, P. L. , Cowart, M. , Gutzmer, R. , Leurs, R. , Liu, W. L. , … Haas, H. L. (2015). International union of basic and clinical pharmacology. XCVIII. Histamine receptors. Pharmacological Reviews, 67, 601–655. 10.1124/pr.114.010249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panula, P. , & Nuutinen, S. (2013). The histaminergic network in the brain: Basic organization and role in disease. Nature Reviews. Neuroscience, 14, 472–487. [DOI] [PubMed] [Google Scholar]
- Park, P. , Sanderson, T. M. , Amici, M. , Choi, S. L. , Bortolotto, Z. A. , Zhuo, M. , … Collingridge, G. L. (2016). Calcium‐permeable AMPA receptors mediate the induction of the protein kinase A‐dependent component of long‐term potentiation in the hippocampus. The Journal of Neuroscience, 36, 622–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons, M. E. , & Ganellin, C. R. (2006). Histamine and its receptors. British Journal of Pharmacology, 147(Suppl 1), S127–S135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul, J. R. , DeWoskin, D. , McMeekin, L. J. , Cowell, R. M. , Forger, D. B. , & Gamble, K. L. (2016). Regulation of persistent sodium currents by glycogen synthase kinase 3 encodes daily rhythms of neuronal excitability. Nature Communications, 7, 13470 10.1038/ncomms13470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pini, A. , Obara, I. , Battell, E. , Chazot, P. L. , & Rosa, A. C. (2016). Histamine in diabetes: Is it time to reconsider? Pharmacological Research, 111, 316–324. 10.1016/j.phrs.2016.06.021 [DOI] [PubMed] [Google Scholar]
- Popiolek‐Barczyk, K. , Łażewska, D. , Latacz, G. , Olejarz, A. , Makuch, W. , Stark, H. , … Mika, J. (2018). Antinociceptive effects of novel histamine H3 and H4 receptor antagonists and their influence on morphine analgesia of neuropathic pain in the mouse. British Journal of Pharmacology, 175, 2897–2910. 10.1111/bph.14185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przewłocki, R. , & Przewłocka, B. (2001). Opioids in chronic pain. European Journal of Pharmacology, 429, 79–91. 10.1016/S0014-2999(01)01308-5 [DOI] [PubMed] [Google Scholar]
- Rice, A. S. C. , Finnerup, N. B. , Kemp, H. I. , Currie, G. L. , & Baron, R. (2018). Sensory profiling in animal models of neuropathic pain: A call for back‐translation. Pain, 159, 819–824. 10.1097/j.pain.0000000000001138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera‐Ramírez, N. , Montejo‐López, W. , López‐Méndez, M.‐C. , Guerrero‐Hernández, A. , Molina‐Hernández, A. , García‐Hernández, U. , & Arias‐Montaño, J. A. (2016). Histamine H3 receptor activation stimulates calcium mobilization in a subpopulation of rat striatal neurons in primary culture, but not in synaptosomes. Neurochemistry International, 101, 38–47. [DOI] [PubMed] [Google Scholar]
- Rossbach, K. , Nassenstein, C. , Gschwandtner, M. , Schnell, D. , Sander, K. , Seifert, R. , … Bäumer, W. (2011). Histamine H1, H3 and H4 receptors are involved in pruritus. Neuroscience, 190, 89–102. 10.1016/j.neuroscience.2011.06.002 [DOI] [PubMed] [Google Scholar]
- Rumore, M. M. , & Schlichting, D. A. (1986). Clinical efficacy of antihistaminics as analgesics. Pain, 25, 7–22. 10.1016/0304-3959(86)90004-7 [DOI] [PubMed] [Google Scholar]
- Sakamoto, A. , Andoh, T. , & Kuraishi, Y. (2016). Involvement of mast cells and proteinase‐activated receptor 2 in oxaliplatin‐induced mechanical allodynia in mice. Pharmacological Research, 105, 84–92. [DOI] [PubMed] [Google Scholar]
- Sanna, M. D. , Ghelardini, C. , Thurmond, R. L. , Masini, E. , & Galeotti, N. (2017). Behavioural phenotype of histamine H4 receptor knockout mice: Focus on central neuronal functions. Neuropharmacology, 114, 48–57. [DOI] [PubMed] [Google Scholar]
- Sanna, M. D. , Lucarini, L. , Durante, M. , Ghelardini, C. , Masini, E. , & Galeotti, N. (2017). Histamine H4 receptor agonist‐induced relief from painful peripheral neuropathy is mediated by inhibition of spinal neuroinflammation and oxidative stress. B J Pharmacol, 174, 28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna, M. D. , Mello, T. , Masini, E. , & Galeotti, N. (2018). Activation of ERK/CREB pathway in noradrenergic neurons contributes to hypernociceptive phenotype in H4 receptor knockout mice after nerve injury. Neuropharmacology, 128, 340–350. 10.1016/j.neuropharm.2017.10.025 [DOI] [PubMed] [Google Scholar]
- Sanna, M. D. , Stark, H. , Lucarini, L. , Ghelardini, C. , Masini, E. , & Galeotti, N. (2015). Histamine H4 receptor activation alleviates neuropathic pain through differential regulation of ERK, JNK, and P38 MAPK phosphorylation. Pain, 156, 2492–2504. 10.1097/j.pain.0000000000000319 [DOI] [PubMed] [Google Scholar]
- Santiago‐Palma, J. , Fischberg, D. , Kornick, C. , Khjainova, N. , & Gonzales, G. (2001). Diphenhydramine as an analgesic adjuvant in refractory cancer pain. Journal of Pain and Symptom Management, 22, 699–703. 10.1016/S0885-3924(01)00311-6 [DOI] [PubMed] [Google Scholar]
- Schlicker, E. , & Kathmann, M. (2017). Role of the Histamine H3 Receptor in the Central Nervous System. Handbook of Experimental Pharmacology, 241, 277–299. 10.1007/164_2016_12 [DOI] [PubMed] [Google Scholar]
- Schneider, E. H. , & Seifert, R. (2016). The histamine H4‐receptor and the central and peripheral nervous system: A critical analysis of the literature. Neuropharmacology, 106, 116–128. 10.1016/j.neuropharm.2015.05.004 [DOI] [PubMed] [Google Scholar]
- Seltzer, Z. , Dubner, R. , & Shir, Y. (1990). A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain, 43, 205–218. [DOI] [PubMed] [Google Scholar]
- Seltzer, Z. , Paran, Y. , Eisen, A. , & Ginzburg, R. (1991). Neuropathic pain behavior in rats depends on the afferent input from nerve‐end neuroma including histamine‐sensitive C‐fibers. Neuroscience Letters, 128, 203–206. 10.1016/0304-3940(91)90261-Q [DOI] [PubMed] [Google Scholar]
- Simone, D. A. , Alreja, M. , & LaMotte, R. H. (1991). Psychophysical studies of the itch sensation and itchy skin (“alloknesis”) produced by intracutaneous injection of histamine. Somatosensory & Motor Research, 8, 271–279. 10.3109/08990229109144750 [DOI] [PubMed] [Google Scholar]
- Simons, F. E. , & Simons, K. J. (2011). Histamine and H1‐antihistamines: Celebrating a century of progress. The Journal of Allergy and Clinical Immunology, 128, 1139–1150. 10.1016/j.jaci.2011.09.005 [DOI] [PubMed] [Google Scholar]
- Simons, F. E. R. (2003). H1‐antihistamines: More relevant than ever in the treatment of allergic disorders. The Journal of Allergy and Clinical Immunology, 112, S42–S52. [DOI] [PubMed] [Google Scholar]
- Sirois, J. , Ménard, G. , Moses, A. S. , & Bissonnette, E. Y. (2000). Importance of histamine in the cytokine network in the lung through H2 and H3 receptors: Stimulation of IL‐10 production. Journal of Immunology Research, 164, 2964–2970. [DOI] [PubMed] [Google Scholar]
- Smit, M. J. , Leurs, R. , Alewijnse, A. E. , Blauw, J. , Van Nieuw Amerongen, G. P. , Van De Vrede, Y. , … Timmerman, H. (1996). Inverse agonism of histamine H2 antagonist accounts for upregulation of spontaneously active histamine H2 receptors. Proceedings of the National Academy of Sciences of the United States of America, 93, 6802–6807. 10.1073/pnas.93.13.6802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, F. M. , Haskelberg, H. , Tracey, D. J. , & Moalem‐Taylor, G. (2007). Role of histamine H3 and H4 receptors in mechanical hyperalgesia following peripheral nerve injury. Neuroimmunomodulation, 14, 317–325. 10.1159/000125048 [DOI] [PubMed] [Google Scholar]
- Strakhova, M. I. , Nikkel, A. L. , Manelli, A. M. , Hsieh, G. C. , Esbenshade, T. A. , Brioni, J. D. , & Bitner, R. S. (2009). Localization of histamine H4 receptors in the central nervous system of human and rat. Brain Research, 1250, 41–48. 10.1016/j.brainres.2008.11.018 [DOI] [PubMed] [Google Scholar]
- Takeshita, K. , Sakai, K. , Bacon, K. B. , & Gantner, F. (2003). Critical role of histamine H4 receptor in leukotriene B4 production and mast cell‐dependent neutrophil recruitment induced by zymosan in vivo. The Journal of Pharmacology and Experimental Therapeutics, 307, 1072–1078. 10.1124/jpet.103.057489 [DOI] [PubMed] [Google Scholar]
- Tamaddonfard, E. , & Hamzeh‐Gooshchi, N. (2014). Effects of administration of histamine and its H1, H2, and H3 receptor antagonists into the primary somatosensory cortex on inflammatory pain in rats. Iran J Basic Med Sci, 17, 55. [PMC free article] [PubMed] [Google Scholar]
- Treede, R. D. , Jensen, T. S. , Campbell, J. N. , Cruccu, G. , Dostrovsky, J. O. , Griffin, J. W. , … Serra, J. (2008). Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology, 70, 1630–1635. [DOI] [PubMed] [Google Scholar]
- Treede, R‐D. , & Magerl, W . (2000). Multiple mechanisms of secondary hyperalgesia In Progress in Brain Research. (pp 331–341). Elsevier; 10.1016/S0079-6123(00)29025-0 [DOI] [PubMed] [Google Scholar]
- Usoskin, D. , Furlan, A. , Islam, S. , Abdo, H. , Lonnerberg, P. , Lou, D. , … Ernfors, P. (2015). Unbiased classification of sensory neuron types by large‐scale single‐cell RNA sequencing. Nature Neuroscience, 18, 145–153. 10.1038/nn.3881 [DOI] [PubMed] [Google Scholar]
- van Hecke, O. , Austin, S. K. , Khan, R. A. , Smith, B. H. , & Torrance, N. (2014). Neuropathic pain in the general population: A systematic review of epidemiological studies. Pain, 155, 654–662. 10.1016/j.pain.2013.11.013 [DOI] [PubMed] [Google Scholar]
- VanDenKerkhof, E. G. , Mann, E. G. , Torrance, N. , Smith, B. H. , Johnson, A. , & Gilron, I. (2016). An epidemiological study of neuropathic pain symptoms in Canadian adults. Pain Research & Management, 2016, 9815750 10.1155/2016/9815750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanegas, H. , & Schaible, H.‐G. (2000). Effects of antagonists to high‐threshold calcium channels upon spinal mechanisms of pain, hyperalgesia and allodynia. Pain, 85, 9–18. 10.1016/S0304-3959(99)00241-9 [DOI] [PubMed] [Google Scholar]
- Vogalis, F. , Harvey, J. R. , & Furness, J. B. (2003). PKA‐mediated inhibition of a novel K+ channel underlies the slow after‐hyperpolarization in enteric AH neurons. The Journal of Physiology, 548, 801–814. 10.1113/jphysiol.2002.037325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall, P. , Devor, M. , Inbal, R. , Scadding, J. , Schonfeld, D. , Seltzer, Z. , & Tomkiewicz, M. M. (1979). Autotomy following peripheral nerve lesions: Experimental anesthesia dolorosa. Pain, 7, 103–113. 10.1016/0304-3959(79)90002-2 [DOI] [PubMed] [Google Scholar]
- Wei, F. , Dubner, R. , Zou, S. , Ren, K. , Bai, G. , Wei, D. , & Guo, W. (2010). Molecular depletion of descending serotonin unmasks its novel facilitatory role in the development of persistent pain. The Journal of Neuroscience, 30, 8624–8636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, H. , Jin, C. Y. , Viisanen, H. , You, H. J. , & Pertovaara, A. (2014). Histamine in the locus coeruleus promotes descending noradrenergic inhibition of neuropathic hypersensitivity. Pharmacological Research, 90, 58–66. 10.1016/j.phrs.2014.09.007 [DOI] [PubMed] [Google Scholar]
- Wei, H. , Viisanen, H. , You, H. J. , & Pertovaara, A. (2016). Spinal histamine in attenuation of mechanical hypersensitivity in the spinal nerve ligation‐induced model of experimental neuropathy. European Journal of Pharmacology, 772, 1–10. [DOI] [PubMed] [Google Scholar]
- Yamamoto, Y. , Mochizuki, T. , Okakura‐Mochizuki, K. , Uno, A. , & Yamatodani, A. (1997). Thioperamide, a histamine H3 receptor antagonist, increases GABA release from the rat hypothalamus. Methods and Findings in Experimental and Clinical Pharmacology, 19, 289–298. [PubMed] [Google Scholar]
- Yawn, B. P. , Wollan, P. C. , Weingarten, T. N. , Watson, J. C. , Hooten, W. M. , & Melton, L. J. 3rd (2009). The prevalence of neuropathic pain: Clinical evaluation compared with screening tools in a community population. Pain Medicine, 10, 586–593. 10.1111/j.1526-4637.2009.00588.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, J. , Lou, G. D. , Yue, J. X. , Tang, Y. Y. , Hou, W. W. , Shou, W. T. , … Chen, Z. (2013). Effects of histamine on spontaneous neuropathic pain induced by peripheral axotomy. Neuroscience Bulletin, 29, 261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, J. , Tang, Y. Y. , Wang, R. R. , Lou, G. D. , Hu, T. T. , Hou, W. W. , … Chen, Z. (2016). A critical time window for the analgesic effect of central histamine in the partial sciatic ligation model of neuropathic pain. Journal of Neuroinflammation, 13, 163 10.1186/s12974-016-0637-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue, J. X. , Wang, R. R. , Yu, J. , Tang, Y. Y. , Hou, W. W. , Lou, G. D. , … Chen, Z. (2014). Histamine upregulates Nav1.8 expression in primary afferent neurons via H2 receptors: Involvement in neuropathic pain. CNS Neuroscience & Therapeutics, 20, 883–892. 10.1111/cns.12305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamponi, G. W. , & Currie, K. P. (2013). Regulation of Ca(V)2 calcium channels by G protein coupled receptors. Biochimica et Biophysica Acta, 1828, 1629–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, D. D. , Sisignano, M. , Schuh, C. D. , Sander, K. , Stark, H. , & Scholich, K. (2012). Overdose of the histamine H3 inverse agonist pitolisant increases thermal pain thresholds. Inflammation Research, 61, 1283–1291. 10.1007/s00011-012-0528-5 [DOI] [PubMed] [Google Scholar]
- Zhang, X. , Wang, Y. , Dong, H. , Xu, Y. , & Zhang, S. (2016). Induction of microglial activation by mediators released from mast cells. Cellular Physiology and Biochemistry, 38, 1520–1531. 10.1159/000443093 [DOI] [PubMed] [Google Scholar]
- Zhang, X. Y. , Yu, L. , Zhuang, Q. X. , Peng, S. Y. , Zhu, J. N. , & Wang, J. J. (2013). Postsynaptic mechanisms underlying the excitatory action of histamine on medial vestibular nucleus neurons in rats. British Journal of Pharmacology, 170, 156–169. 10.1111/bph.12256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, H. , Alam, A. , Chen, Q. , Eusman M, A. , Pal, A. , Eguchi, S. , … Ma, D. (2017). The role of microglia in the pathobiology of neuropathic pain development: What do we know? British Journal of Anaesthesia, 118, 504–516. 10.1093/bja/aex006 [DOI] [PubMed] [Google Scholar]
- Zhu, Y. , Michalovich, D. , Wu, H. , Tan, K. B. , Dytko, G. M. , Mannan, I. J. , … Fitzgerald, L. R. (2001). Cloning, expression, and pharmacological characterization of a novel human histamine receptor. Molecular Pharmacology, 59, 434–441. 10.1124/mol.59.3.434 [DOI] [PubMed] [Google Scholar]
- Zhuang, Z. Y. , Gerner, P. , Woolf, C. J. , & Ji, R. R. (2005). ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain, 114, 149–159. 10.1016/j.pain.2004.12.022 [DOI] [PubMed] [Google Scholar]
- Zuo, Y. , Perkins, N. M. , Tracey, D. J. , & Geczy, C. L. (2003). Inflammation and hyperalgesia induced by nerve injury in the rat: A key role of mast cells. Pain, 105, 467–479. 10.1016/S0304-3959(03)00261-6 [DOI] [PubMed] [Google Scholar]


