Abstract
Testing for vector-borne pathogens in livestock is largely reliant upon blood and tissue. The role of biopsy samples remains poorly explored for detecting tick-borne bacteria in animals.
In a 2-year survey, animals of veterinary importance from farms throughout the northern part of Greece were routinely checked for the presence of biopsy samples. Where detected, either a portion or a biopsy was collected together with whole blood samples and any ticks at the site of the biopsy sample. Molecular testing was carried out by real-time PCR targeting the internal transcribed spacer gene of Bartonella species. A total of 68 samples (28 blood samples, 28 biopsy samples and 12 ticks (nine Rhipicephalus bursa and three Rhipicephalus turanicus)) were collected from goats (64 samples) and cattle (four samples). Eight (11.8%) of the 68 samples were positive for Bartonella species. Of the biopsy and whole blood samples, four (14.3%) of each type were positive for Bartonella species. None of the ticks tested positive for Bartonella species. All pairs of positive biopsy samples/whole blood samples originated from the same animals. Positive samples were identified as Bartonella vinsonii subsp. arupensis. Although many more samples from a much wider spectrum of animal species is required before concluding upon the merit of biopsy samples in the study of tick-borne diseases, the significance of our finding warrants further study, both for clinical consequences in small ruminants and for those humans who are farming infected animals.
Keywords: Animals of veterinary importance, Bartonella, biopsy sample, tick
Introduction
Bartonella are considered as emerging pathogens, being increasingly associated with a number of diseases both in humans (trench fever, Carrion's disease, bacillary angiomatosis, endocarditis, cat scratch disease and neuroretinitis) [1], as well as, in animals (including ruminants, cattle, cats, rodents, dogs and a wide range of wild animals) [2]. In vertebrates, Bartonella parasitize erythrocytes and endothelial cells [3], typically for protracted periods [4].
Established and proposed new members of Bartonella species have increased exponentially over recent years. Over 30 species have been recognized with some having global distribution and infecting a wide variety of vertebrates [5]. A wide variety of vectors are involved in the transmission of Bartonella species, including body lice, fleas, ticks, mites and sandflies [6]. Examples of bacteria of the genus of Bartonella associated with vector transmission are Bartonella bacilliformis, which is transmitted by sand flies, Bartonella henselae (transmitted by cat fleas) and Bartonella quintana (transmitted by the human body louse). The role of ticks in the ecology of Bartonella is hypothesized [[7], [8], [9]], despite their notable ability to serve as arthropod vectors/reservoirs of various agents of medical and veterinary health significance [10], and an upsurge in the incidence of tick-borne diseases in many regions of the world [11].
The association between Bartonella and their mammalian hosts is varied, with some strictly limited whereas others are less restricted [12]. Cats play the role of the main reservoir for B. henselae causing cat-scratch disease. Furthermore, several strains have been isolated from various rodent [13,14] and ruminant [15,16] species throughout the world. Ruminants can also become infected with Bartonella schoenbuchensis, Bartonella chomelii and Bartonella bovis, which have been isolated from blood in Europe, Africa and North America [15,17,18]. Among cattle, B. bovis has been implicated in causing bovine endocarditis [19], and B. chomelii has also been isolated from the same animal species [20], although no clinical consequence has been demonstrated for the latter species. Moreover, Bartonella rochalimae causes infection in domestic animals, wild carnivores and humans [21].
In cases where vertebrate hosts, vectors and wild animal species interact with each other, deciphering the transmission cycles of zoonotic agents seems quite challenging [22]. Proper sampling plays a crucial role in the accurate approach to the study of a zoonotic disease. Serological analysis has been used extensively, especially in epidemiological studies, but is limited in its ability to discriminate closely related pathogen genotypes. Moreover, detection of antibodies does not necessarily conclude bacteraemia or even infection of the host; whereas detection of the pathogen in the host’s blood or from a direct sample (biopsy sample for example) would seem a more secure approach.
The purpose of the current study was to compare biopsy samples (removed scab) with whole blood or tick vectors for detection of tick-borne bacteria in livestock to assess the diagnostic merits of various sample types for the detection of Bartonella species.
Materials and methods
Sampling
In a 2-year survey carried out in the laboratory of Clinical Bacteriology, Parasitology, Zoonoses and Geographical Medicine of Crete (Greece) in conjunction with the Veterinary department of the Aristotle University of Thessalonica (Greece), animals of veterinary importance (sheep, goats, cattle) from farms throughout the northern part of Greece were routinely checked for the presence of biopsy samples. Where detected, either a portion or a biopsy was collected together with whole blood samples and any ticks at the site of the biopsy sample. Data on animal species, farm location, time of collection, etc. were recorded.
Ticks removed from animals were placed in separate 1.5-mL tubes with 70% ethanol and were uniquely coded according to individual animal, livestock and region; then transported to the Aristotle University of Thessalonica where they were kept at –80°C before testing. Each tick was identified to species using existing taxonomic keys [23] at the laboratory of Clinical Bacteriology of the University of Crete, in Greece.
Blood samples and biopsy samples were similarly removed, transferred into individual 1.5-mL tubes, labelled and stored frozen until assessed.
Molecular analysis
DNA extraction from whole blood samples (QIAamp DNA blood mini kit; Qiagen, Hilden, Germany) or biopsy samples and ticks (QIAamp Tissue extraction kit; Qiagen) was undertaken according to the manufacturer’s instructions at the laboratory of Clinical Bacteriology, Parasitology, Zoonoses and Geographical Medicine of Crete. Each tick and biopsy sample was washed in 70% alcohol, rinsed in sterile water and dried on sterile filter paper. Consequently, samples were triturated individually into sterile tubes and a portion of them was used for further DNA extraction. Once extracted, DNA samples were kept at –20°C until further analysis.
Molecular testing was undertaken at the University of East London using an initial real-time PCR targeting the internal transcribed spacer (ITS) gene of Bartonella species to screen as previously described [24]. Master mix was prepared containing PCR buffer, dNTPs (0.2 mM each), MgCl2 (5 mM), Taq DNA polymerase (0.06 mM; Invitrogen, Carlsbad, CA, USA), as well as primers (1 μM each) and probe (0.1 μM; (Sigma Genosys, St Louis, MO, USA) at a final volume of 25 μL. Agilent 96-well plates and cap strips were used. Nucleotide-free sterile H2O was used as negative control. At least four randomly selected wells in each plate were used as negative controls. A single well was used as the positive control in each assay, the positive control being a verified positive B. quintana DNA isolated from human blood. The master mix preparation room, the DNA addition room and the amplification room were all separated from each other to avoid any chance of contamination. All positive and/or ambiguous samples were re-tested at least once in order to demonstrate reproducibility using conditions similar to those described above. Only samples producing cT values of <35 were considered to be positive. All amplifications were performed using an Agilent Aria Mx cycler.
Positive samples were further tested by conventional PCR (targeting ITS) to obtain amplicons that were further used for sequencing as previously described [25]. All primers and probes used both for real-time PCR and for the conventional PCR are summarized in Table 1. Amplicons were purified using the PCR product purification kit (QIAquick Qiagen) and sequenced in both directions by Sanger sequencing (Durham) using the same primers used for PCR. All sequences obtained were aligned using ClustalW. Sequences were compared for similarity with those at GenBank using the nucleotide BLAST program (National Centre for Biotechnology Information; http://www.ncbi.nlm.nih.gov/BLAST) the ClustalW online software (http://www.ebi.ac.uk/Tools/msa/clustalw2/) and the MEGA v. X software.
Table 1.
Primers and probes used to target the internal transcribed spacer gene either by real-time PCR or by conventional PCR
| Sequence | Gene targeted | |
|---|---|---|
| Real-time PCR | ITS | |
| Primer forward | GGGGCCGTAGCTCAGCTG | |
| Primer reverse | TGAATATATCTTCTCTTCACAATTTC | |
| Probe | 6-carboxyfluorescein-CGATCCCGTCCGGCTCCACCA-6-carboxytetramethylrhodamine | |
| PCR | ITS | |
| Primer forward (438s) | GGTTTTCCGGTTTATCCCGGAGGGC | |
| Primer reverse (1100as) | GAACCGACGACCCCCTGCTTGCAAAGC | |
Results
A total of 68 samples (n = 28 blood samples; n = 28 biopsy samples; and n = 12 ticks) were collected and tested for Bartonella species. Livestock included goats (12 ticks, 26 eschars, 26 blood samples) and bovine animals (two eschars and two blood samples).
Of the 12 ticks collected, nine were characterized as Rhipicephalus bursa and three as Rhipicephalus turanicus. Ticks were collected from goats only.
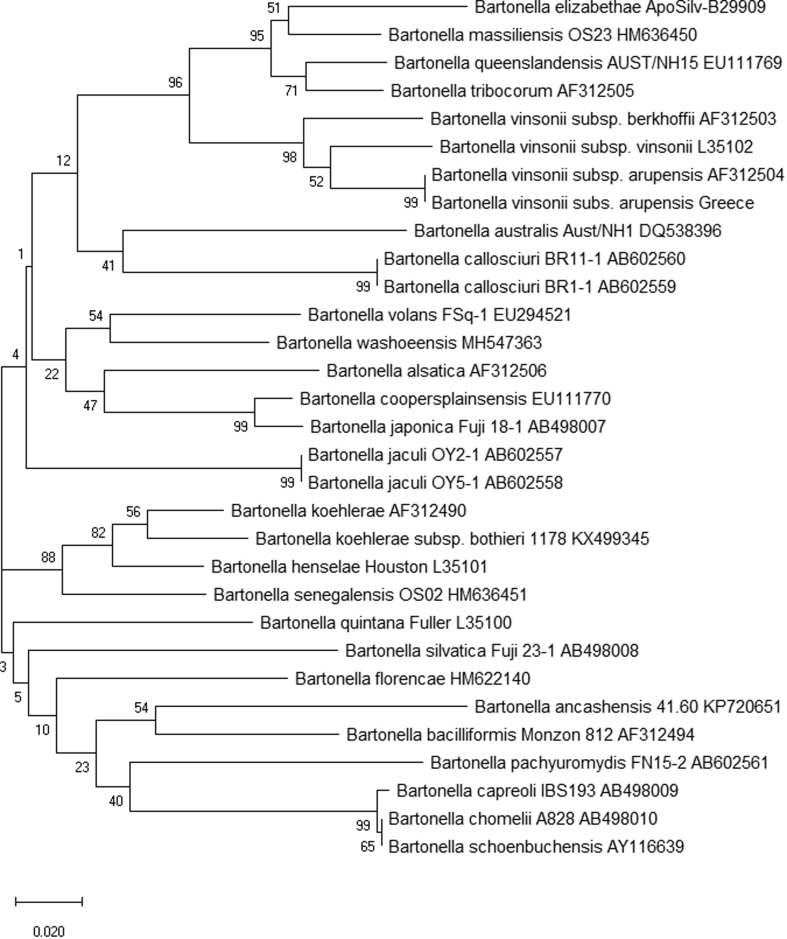
Eight (11.8%) of the 68 samples revealed the presence of Bartonella species with Ct values ranging from 29.07 to 34.44 (see Table 2). All positive samples were verified by a second amplification. Of the biopsy sample and whole blood samples, eight (four from each sample type; 14.3%) were positive for Bartonella species. All pairs of positive biopsy samples/whole blood samples originated from the same animals. All remaining samples were negative. Of the eight positive samples, we amplified and sequenced a 408-bp portion of ITS from six samples (sample numbers 11–16) that revealed identical sequence in both directions. All positive samples despite their origin were identified as Bartonella vinsonii subsp. arupensis showing 100% (408/408 bp) similarity to the already published sequence AF312504 and 99% (404/408) similarity to the already published sequence AF442952. To further explore the extent of the relatedness of our sequences with published ones, partial ITS sequences for another 32 Bartonella species were aligned to construct a phylogenetic tree (Fig. 1) in which the position of our sequences against the sequences of other Bartonella species was demonstrated.
Table 2.
Sample types and origins tested for Bartonella species
| Animals | Ticks | Biopsy samples | Blood samples | Blood sample and eschar (pairs)a | Blood sample, tick and eschar (triad)b | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | n | n | Pos. (%) | n | Pos. (%) | n | Pos. (%) | n | Pos. (%) | n | Pos. (%) |
| Bovine | 2 | 0 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 0 |
| Goat | 26 | 12 | 0 | 26 | 4 (15.4) | 26 | 4 (15.4) | 26 | 4 (15.4) | 12 | 0 |
| Total | 28 | 12 | 0 | 28 | 4 (14.3) | 28 | 4 (14.3) | 28 | 4 (14.3) | 12 | 0 |
Corresponds to cases where both eschar and whole blood samples were collected from the same animal.
Corresponds to cases where biopsy sample, whole blood sample and a tick were collected from the same animal.
Fig. 1.
Internal transcribed spacer (ITS) phylogeny for a 408-bp fragment of the 16S–23S intergenic linker region of 33 Bartonella species. The evolutionary history was inferred using the neighbour-joining method. The optimal tree with the sum of branch length 1.67495836 is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) is shown next to the branches. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the maximum composite likelihood method and are in the units of the number of base substitutions per site. Codon positions included were 1st+2nd+3rd+Noncoding. All positions containing gaps and missing data were eliminated (complete deletion option) [61].
All bovine samples and all ticks tested were negative for Bartonella species. The results are summarized in Table 2.
Discussion
Interest in zoonotic tick-borne diseases has increased in the last few decades, because these are considered as important zoonoses in Europe [26]; among them are Bartonellaceae.
Bartonella vinsonii was described as the Canadian vole agent back in 1946 [27], and almost four decades later (1982) Weiss and Dasch further characterized the agent and named it after Rochalimaea vinsonii [28]. Fifteen years later (1999), its first isolation was recorded, from a 62-year-old man with bacteraemia [29].
A number of genes are used as targets for the identification of Bartonella species, including the 16S rRNA and citrate synthase (gltA) [30], the 16S/23S rRNA intergenic spacer region (ITS) [31], which shows a high degree of interspecies variability among Bartonella species, the ftsZ [32] and the GroEL [33] genes. In our case, we did not have enough DNA to go through the amplification of further genes, nevertheless, the successful detection of Bartonella in four animals, in both biopsy and blood samples, demonstrates the robustness of our findings. Control samples were included in all assays and verified correct performance of the tests reported. Sanger sequencing revealed that in all cases we had detected B. vinsonii subsp. arupensis, close to B. vinsonii subsp. vinsonii, which is rodent-associated, and to B. vinsonii subsp. berkhoffii, which has been described in dogs.
Rodent infections caused by Bartonella tend to be asymptomatic; however, whether they could serve as a pathogen in other vertebrates is a cause for concern. As far as ruminants (including water buffalo, several deer species, cattle, camels and moose) and animals of veterinary importance are concerned, a number of Bartonella species have been associated with these animal species, such as B. bovis, B. capreoli, B. chomelii, B. dromedarii and B. schoenbuchensis [15,16,34]. In contrast to the large ruminants above, the isolation of Bartonella species from small ruminants (including sheep and goats, which we studied herein) has been more puzzling. Indeed, several studies have failed to detect any Bartonella species from sheep or goats [35,36], whereas others have detected B. melophagi from domestic sheep samples [37] despite the great difficulties in the isolation of this group of bacteria.
The natural reservoirs of B. vinsonii subsp. arupensis are small rodents with mice believed to show persistent infection [34]. Further reports have detected this agent in deer mice in North America [38], in rodents in Mexico [39,40], in Brazil [2] and in the USA (California) [41]. Its zoonotic potential was revealed by its isolation from a human with endocarditis [42], in pre-enriched blood of four individuals in Thailand [43] and in a child where it caused hepatic granulomatous lesions [44]. Bartonella vinsonii subsp. berkhoffii is now established as a canine pathogen with the ability to cause endocarditis [45]. Interestingly, B. vinsonii subsp. arupensis has also been detected in the blood of stray dogs in Thailand [46]. The role of this organism as a pathogen in other vertebrate species remains to be clarified. Our detection of B. vinsonii subsp. arupensis in goats is intriguing. Whether it has pathogenic potential in this small ruminant is worthy of further exploration.
Importantly, this study reports the validity of biopsy samples for detection of Bartonella infection in livestock. Infection was confirmed by the demonstration of Bartonella in the blood of all biopsy-sample-positive animals. To the best of our knowledge, this is the first time that the presence of Bartonella DNA in veterinary biopsy samples has been recorded; on the other hand, simultaneous detection of the same Bartonella species in ruminants and in the vectors they carry (deer keds and cattle tail louse), has been described [37]. A biopsy sample or cutaneous necrosis is caused by vasculitis at the tick-bite site of inoculation, known as tache noire (‘black spot’) and usually it is pathognomonic for infection by Rickettsia. The presence of an eschar plays a significant role in both human clinical and laboratory diagnosis [[47], [48], [49], [50]]. In contrast to humans, the role of biopsy samples in animals of veterinary importance has not been studied. Epidemiological surveys for tick-borne diseases infecting animals are generally restricted to use of serum and whole blood alone. The limitation presented with serum antibodies is that, if present, they might correspond to past infection; furthermore, only IgG antibodies can be used as a screening method. Furthermore, whole blood often fails to yield a positive PCR because bacteraemia is rare in this case in animals and is not always a feature of vector-borne pathogens.
It seems that ticks may have the potential to act as vectors of Bartonella species [51]. Bartonella has been detected in questing ticks (Ixodes pacificus, Dermacentor and Rhipicephalus sanguineus) in the USA [16], while other European studies (the Netherlands, France, Poland and Austria) have demonstrated the presence of Bartonella in Ixodes ricinus ticks obtained from vegetation, either by molecular means [52] or following isolation of the pathogen (B. henselae in Ixodes ricinus) [53].
Although R. turanicus is considered as a species frequently associated with sheep [54], it is R. bursa that is considered a major ectoparasite of sheep in the Mediterranean basin [54]. In our study, although we collected ticks belonging to both these species, we failed to detect any Bartonella DNA in any of them. Nevertheless, although the total number of ticks collected in the current survey was low (12 samples), our finding agrees with previous studies [[54], [55], [56], [57], [58], [59]] that failed to detect pathogenic species in R. turanicus. In an earlier study carried out in Palestine, the DNA of Bartonella species was detected in R. sanguineus collected from dogs and from camels; however, all ticks collected from sheep or goats were negative [60]. A study of R. bursa ticks removed from goats reported limited detection of Bartonella species from Sardinia [54].
The limitations of our study are that our numbers and range of livestock and ticks tested was small. Furthermore, insufficient material was available to enable exhaustive molecular typing to confirm the identity of the Bartonella vinsonii subsp. arupensis present in small ruminants.
Conclusion
We report the presence of B. vinsonii subsp. arupensis species in goats from Greece, with four animals showing positive blood and biopsy samples. The significance of this finding warrants further study, both for clinical consequences in small ruminants and for those humans farming infected animals. Certainly, many more samples from a much wider spectrum of animal species are required before concluding upon the merit of biopsy samples in the study of tick-borne diseases; however, we provide valuable proof-of-concept data that should promote future research.
Conflicts of interest
None.
Acknowledgements
This work was performed under the frame of EurNegVec Cost Action TD1303.
References
- 1.Prutsky G., Domecq J.P., Mori L., Bebko S., Matzumura M., Sabouni A. Treatment outcomes of human bartonellosis: a systematic review and meta-analysis. Int J Infect Dis. 2013;17:e811–e819. doi: 10.1016/j.ijid.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 2.Favacho A.R., Andrade M.N., de Oliveira R.C., Bonvicino C.R., D'Andrea P.S., de Lemos E.R. Zoonotic Bartonella species in wild rodents in the state of Mato Grosso do Sul, Brazil. Microbe. Infect. 2015;17:889–892. doi: 10.1016/j.micinf.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 3.Harms A., Dehio C. Intruders below the radar: molecular pathogenesis of Bartonella spp. Clin Microbiol Rev. 2012;25:42–78. doi: 10.1128/CMR.05009-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birtles R.J. Bartonellae as elegant hemotropic parasites. Ann N Y Acad Sci. 2005;1063:270–279. doi: 10.1196/annals.1355.044. [DOI] [PubMed] [Google Scholar]
- 5.Regier Y., OR F., Kempf V.A. Bartonella spp. – a chance to establish One Health concepts in veterinary and human medicine. Parasites Vectors. 2016;9:261. doi: 10.1186/s13071-016-1546-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai Y.L., Chang C.C., Chuang S.T., Chomel B.B. Bartonella species and their ectoparasites: selective host adaptation or strain selection between the vector and the mammalian host? Comp Immunol Microbiol Infect Dis. 2011;34:299–314. doi: 10.1016/j.cimid.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Bown K.J., Bennet M., Begon M. Flea-borne Bartonella grahamii and Bartonella taylorii in bank voles. Emerg Infect Dis. 2004;10:684–687. doi: 10.3201/eid1004.030455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang C.C., Chomel B.B., Kasten R.W., Romano V., Tietze N. Molecular evidence of Bartonella spp. in questing adult Ixodes pacificus ticks in California. J Clin Microbiol. 2001;39:1221–1226. doi: 10.1128/JCM.39.4.1221-1226.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stevenson H.L., Bai Y., Kosoy M.Y., Montenieri J.A., Lowell J.L., Chu M.C. Detection of novel Bartonella strains and Yersinia pestis in prairie dogs and their fleas (Siphonaptera: Ceratophyllidae and Pulicidae) using multiplex polymerase chain reaction. J Med Entomol. 2003;40:329–337. doi: 10.1603/0022-2585-40.3.329. [DOI] [PubMed] [Google Scholar]
- 10.Colwell D.D., Dantas-Torres F., Otranto D. Vector-borne parasitic zoonoses: emerging scenarios and new perspectives. Vet Parasitol. 2011;182:14–21. doi: 10.1016/j.vetpar.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Brites-Neto J., Duarte K.M., Martins T.F. Tick-borne infections in human and animal population worldwide. Vet World. 2015;8:301–315. doi: 10.14202/vetworld.2015.301-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vayssier-Taussat M., Le Rhun D., Deng H.K., Biville F., Cescau S., Danchin A. The Trw type IV secretion system of Bartonella mediates host-specific adhesion to erythrocytes. PLoS Pathog. 2010;6(6) doi: 10.1371/journal.ppat.1000946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bermond D., Heller R., Barrat F., Delacour G., Dehio C., Alliot A. Bartonella birtlesii sp. nov., isolated from small mammals (Apodemus spp.) Int J Syst Evol Microbiol. 2000;50:1973–1979. doi: 10.1099/00207713-50-6-1973. [DOI] [PubMed] [Google Scholar]
- 14.Kosoy M.Y., Regnery R.L., Tzianabos T., Marston E.L., Jones D.C., Green D. Distribution, diversity, and host specificity of Bartonella in rodents from the Southeastern United States. Am J Trop Med Hyg. 1997;57:578–588. doi: 10.4269/ajtmh.1997.57.578. [DOI] [PubMed] [Google Scholar]
- 15.Bermond D., Boulouis H.J., Heller R., Van Laere G., Monteil H., Chomel B.B. Bartonella bovis Bermond et al. sp. nov. and Bartonella capreoli sp. nov., isolated from European ruminants. Int J Syst Evol Microbiol. 2002;52:383–390. doi: 10.1099/00207713-52-2-383. [DOI] [PubMed] [Google Scholar]
- 16.Chang C.C., Chomel B.B., Kasten R.W., Heller R.M., Kocan K.M., Ueno H. Bartonella spp. isolated from wild and domestic ruminants in North America. Emerg Infect Dis. 2000;6:306–311. doi: 10.3201/eid0603.000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breitschwerdt E.B., Sontakke S., Cannedy A., Hancock S.I., Bradley J.M. Infection with Bartonella weissii and detection of Nanobacterium antigens in a North Carolina beef herd. J Clin Microbiol. 2001;39:879–882. doi: 10.1128/JCM.39.3.879-882.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raoult D., La Scola B., Kelly P.J., Davoust B., Gomez J. Bartonella bovis in cattle in Africa. Vet Microbiol. 2005;105:155–156. doi: 10.1016/j.vetmic.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Erol E., Jackson C., Bai Y., Sells S., Locke S., Kosoy M. Bartonella bovis isolated from a cow with endocarditis. J Vet Diagn Invest. 2013;25:288–290. doi: 10.1177/1040638713477408. [DOI] [PubMed] [Google Scholar]
- 20.Antequera-Gomez M.L., Lozano-Almendral L., Barandika J.F., Gonzalez-Martin-Nino R.M., Rodriguez-Moreno I., Garcia-Perez A.L. Bartonella chomelii is the most frequent species infecting cattle grazing in communal mountain pastures in Spain. Appl Environ Microbiol. 2015;81:623–629. doi: 10.1128/AEM.03159-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaefer J.D., Moore G.M., Namekata M.S., Kasten R.W., Chomel B.B. Seroepidemiology of Bartonella infection in gray foxes from Texas. Vector Borne Zoonotic Dis. 2012;12:428–430. doi: 10.1089/vbz.2011.0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salkeld D.J., Lane R.S. Community ecology and disease risk: lizards, squirrels, and the Lyme disease spirochete in California, USA. Ecology. 2010;91:293–298. doi: 10.1890/08-2106.1. [DOI] [PubMed] [Google Scholar]
- 23.Tsatsaris A., Chochlakis D., Papadopoulos B., Petsa A., Georgalis L., Angelakis E. Species composition, distribution, ecological preference and host association of ticks in Cyprus. Exp Appl Acarol. 2016;70:523–542. doi: 10.1007/s10493-016-0091-9. [DOI] [PubMed] [Google Scholar]
- 24.Raoult D., Roblot F., Rolain J.M., Besnier J.M., Loulergue J., Bastides F. First isolation of Bartonella alsatica from a valve of a patient with endocarditis. J Clin Microbiol. 2006;44:278–279. doi: 10.1128/JCM.44.1.278-279.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beard A.W., Maggi R.G., Kennedy-Stoskopf S., Cherry N.A., Sandfoss M.R., DePerno C.S. Bartonella spp. in feral pigs, southeastern United States. Emerg Infect Dis. 2011;17:893–895. doi: 10.3201/eid1705.100141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parola P., Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32:897–928. doi: 10.1086/319347. [DOI] [PubMed] [Google Scholar]
- 27.Baker J.A. A rickettsial infection in Canadian voles. J Exp Med. 1946;84:37–50. [PubMed] [Google Scholar]
- 28.Weiss E., Dasch G.A. Differential characteristics of strains of Rochalimaea: Rochalimaea vinsonii sp. nov., the Canadian vole agent. Int J Syst Bacteriol. 1982;32:305–314. [Google Scholar]
- 29.Welch D.F., Carroll K.C., Hofmeister E.K., Persing D.H., Robison D.A., Steigerwalt A.G. Isolation of a new subspecies, Bartonella vinsonii subsp. arupensis, from a cattle rancher: identity with isolates found in conjunction with Borrelia burgdorferi and Babesia microti among naturally infected mice. J Clin Microbiol. 1999;37:2598–2601. doi: 10.1128/jcm.37.8.2598-2601.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Houpikian P., Fournier P.E., Raoult D. Phylogenetic position of Bartonella vinsonii subsp. arupensis based on 16S rDNA and gltA gene sequences. Int J Syst Evol Microbiol. 2001;51:179–182. doi: 10.1099/00207713-51-1-179. [DOI] [PubMed] [Google Scholar]
- 31.Houpikian P., Raoult D. 16S/23S rRNA intergenic spacer regions for phylogenetic analysis, identification, and subtyping of Bartonella species. J Clin Microbiol. 2001;39:2768–2778. doi: 10.1128/JCM.39.8.2768-2778.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeaiter Z., Liang Z., Raoult D. Genetic classification and differentiation of Bartonella species based on comparison of partial ftsZ gene sequences. J Clin Microbiol. 2002;40:3641–3647. doi: 10.1128/JCM.40.10.3641-3647.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeaiter Z., Fournier P.E., Ogata H., Raoult D. Phylogenetic classification of Bartonella species by comparing groEL sequences. Int J Syst Evol Microbiol. 2002;52:165–171. doi: 10.1099/00207713-52-1-165. [DOI] [PubMed] [Google Scholar]
- 34.Bai Y., Calisher C.H., Kosoy M.Y., Root J.J., Doty J.B. Persistent infection or successive reinfection of deer mice with Bartonella vinsonii subsp. arupensis. Appl Environ Microbiol. 2011;77:1728–1731. doi: 10.1128/AEM.02203-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dahmani M., Sambou M., Scandola P., Raoult D., Fenollar F., Mediannikov O. Bartonella bovis and Candidatus Bartonella davousti in cattle from Senegal. Comp Immunol Microbiol Infect Dis. 2017;50:63–69. doi: 10.1016/j.cimid.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 36.Kho K.L., Koh F.X., Jaafar T., Nizam Q.N., Tay S.T. Prevalence and molecular heterogeneity of Bartonella bovis in cattle and Haemaphysalis bispinosa ticks in Peninsular Malaysia. BMC Vet Res. 2015;11:153. doi: 10.1186/s12917-015-0470-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kosoy M., Bai Y., Enscore R., Rizzo M.R., Bender S., Popov V. Bartonella melophagi in blood of domestic sheep (Ovis aries) and sheep keds (Melophagus ovinus) from the southwestern US: cultures, genetic characterization, and ecological connections. Vet Microbiol. 2016;190:43–49. doi: 10.1016/j.vetmic.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 38.Andre A., Mouton A., Millien V., Michaux J. Liver microbiome of Peromyscus leucopus, a key reservoir host species for emerging infectious diseases in North America. Infect Genet Evol. 2017;52:10–18. doi: 10.1016/j.meegid.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 39.Schulte Fischedick F.B., Stuckey M.J., Aguilar-Setien A., Moreno-Sandoval H., Galvez-Romero G., Salas-Rojas M. Identification of Bartonella species isolated from rodents from Yucatan, Mexico, and isolation of Bartonella vinsonii subsp. yucatanensis subsp. nov. Vector Borne Zoonotic Dis. 2016;16:636–642. doi: 10.1089/vbz.2016.1981. [DOI] [PubMed] [Google Scholar]
- 40.Rubio A.V., Avila-Flores R., Osikowicz L.M., Bai Y., Suzan G., Kosoy M.Y. Prevalence and genetic diversity of Bartonella strains in rodents from northwestern Mexico. Vector Borne Zoonotic Dis. 2014;14:838–845. doi: 10.1089/vbz.2014.1673. [DOI] [PubMed] [Google Scholar]
- 41.Ziedins A.C., Chomel B.B., Kasten R.W., Kjemtrup A.M., Chang C.C. Molecular epidemiology of Bartonella species isolated from ground squirrels and other rodents in northern California. Epidemiol Infect. 2016;144:1837–1844. doi: 10.1017/S0950268816000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fenollar F., Sire S., Raoult D. Bartonella vinsonii subsp. arupensis as an agent of blood culture-negative endocarditis in a human. J Clin Microbiol. 2005;43:945–947. doi: 10.1128/JCM.43.2.945-947.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bai Y., Kosoy M.Y., Diaz M.H., Winchell J., Baggett H., Maloney S.A. Bartonella vinsonii subsp. arupensis in humans, Thailand. Emerg Infect Dis. 2012;18:989–991. doi: 10.3201/eid1806.111750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Melzi M.L., Ferrari G.M., D'Adda A., Bovo G., Foresti S., Cavallero A. Hepatic granulomatous lesions caused by systemic Bartonella vinsonii subsp. arupensis infection in a child. Pediatr Infect Dis J. 2015;34:1416–1417. doi: 10.1097/INF.0000000000000904. [DOI] [PubMed] [Google Scholar]
- 45.Cadenas M.B., Bradley J., Maggi R.G., Takara M., Hegarty B.C., Breitschwerdt E.B. Molecular characterization of Bartonella vinsonii subsp. berkhoffii genotype III. J Clin Microbiol. 2008;46:1858–1860. doi: 10.1128/JCM.02456-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bai Y., Kosoy M.Y., Boonmar S., Sawatwong P., Sangmaneedet S., Peruski L.F. Enrichment culture and molecular identification of diverse Bartonella species in stray dogs. Vet Microbiol. 2010;146:314–319. doi: 10.1016/j.vetmic.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 47.Harrison N., Burgmann H., Forstner C., Ramharter M., Szell M., Schotta A.M. Molecular diagnosis of African tick bite fever using eschar swabs in a traveller returning from Tanzania. Wien Klin Wochenschr. 2016;128:602–605. doi: 10.1007/s00508-016-1047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dubourg G., Socolovschi C., Del Giudice P., Fournier P.E., Raoult D. Scalp eschar and neck lymphadenopathy after tick bite: an emerging syndrome with multiple causes. Eur J Clin Microbiol Infect Dis. 2014;33:1449–1456. doi: 10.1007/s10096-014-2090-2. [DOI] [PubMed] [Google Scholar]
- 49.Germanakis A., Chochlakis D., Angelakis E., Tselentis Y., Psaroulaki A. Skin lesions and inoculation eschars at the tick bite site in spotted fever group rickettsioses: experience from a patient series in eastern crete, Greece. Dermatology. 2014;228:332–337. doi: 10.1159/000360525. [DOI] [PubMed] [Google Scholar]
- 50.Faccini-Martinez A.A., Garcia-Alvarez L., Hidalgo M., Oteo J.A. Syndromic classification of rickettsioses: an approach for clinical practice. Int J Infect Dis. 2014;28:126–139. doi: 10.1016/j.ijid.2014.05.025. [DOI] [PubMed] [Google Scholar]
- 51.Angelakis E., Billeter S.A., Breitschwerdt E.B., Chomel B.B., Raoult D. Potential for tick-borne bartonelloses. Emerg Infect Dis. 2010;16:385–391. doi: 10.3201/eid1603.091685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Billeter S.A., Miller M.K., Breitschwerdt E.B., Levy M.G. Detection of two Bartonella tamiae-like sequences in Amblyomma americanum (Acari: Ixodidae) using 16S-23S intergenic spacer region-specific primers. J Med Entomol. 2008;45:176–179. doi: 10.1603/0022-2585(2008)45[176:dotbts]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 53.Vayssier-Taussat M., Moutailler S., Michelet L., Devillers E., Bonnet S., Cheval J. Next generation sequencing uncovers unexpected bacterial pathogens in ticks in western Europe. PLoS One. 2013;8 doi: 10.1371/journal.pone.0081439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Satta G., Chisu V., Cabras P., Fois F., Masala G. Pathogens and symbionts in ticks: a survey on tick species distribution and presence of tick-transmitted micro-organisms in Sardinia, Italy. J Med Microbiol. 2011;60:63–68. doi: 10.1099/jmm.0.021543-0. [DOI] [PubMed] [Google Scholar]
- 55.Harrus S., Perlman-Avrahami A., Mumcuoglu K.Y., Morick D., Eyal O., Baneth G. Molecular detection of Ehrlichia canis, Anaplasma bovis, Anaplasma platys, Candidatus Midichloria mitochondrii and Babesia canis vogeli in ticks from Israel. Clin Microbiol Infect. 2011;17:459–463. doi: 10.1111/j.1469-0691.2010.03316.x. [DOI] [PubMed] [Google Scholar]
- 56.Millan J., Proboste T., Fernandez de Mera I.G., Chirife A.D., de la Fuente J., Altet L. Molecular detection of vector-borne pathogens in wild and domestic carnivores and their ticks at the human–wildlife interface. Ticks Tick Borne Dis. 2016;7:284–290. doi: 10.1016/j.ttbdis.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 57.Mancini F., Di Luca M., Toma L., Vescio F., Bianchi R., Khoury C. Prevalence of tick-borne pathogens in an urban park in Rome, Italy. Ann Agric Environ Med. 2014;21:723–727. doi: 10.5604/12321966.1129922. [DOI] [PubMed] [Google Scholar]
- 58.Marie J.L., Davoust B., Socolovschi C., Mediannikov O., Roqueplo C., Beaucournu J.C. Rickettsiae in arthropods collected from red foxes (Vulpes vulpes) in France. Comp Immunol Microbiol Infect Dis. 2012;35:59–62. doi: 10.1016/j.cimid.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 59.Loftis A.D., Reeves W.K., Szumlas D.E., Abbassy M.M., Helmy I.M., Moriarity J.R. Rickettsial agents in Egyptian ticks collected from domestic animals. Exp Appl Acarol. 2006;40:67–81. doi: 10.1007/s10493-006-9025-2. [DOI] [PubMed] [Google Scholar]
- 60.Ereqat S., Nasereddin A., Vayssier-Taussat M., Abdelkader A., Al-Jawabreh A., Zaid T. Molecular evidence of Bartonella species in Ixodid ticks and domestic animals in Palestine. Front Microbiol. 2016;7:1217. doi: 10.3389/fmicb.2016.01217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]