Abstract
Background
The distant metastasis (DM) mode and treatment efficacies in the advanced biliary tract cancer (BTC) were obscure, and a credible evaluation is urgently needed.
Method
A total of 6348 advanced BTC patients (ICC, intrahepatic cholangiocarcinoma, n = 1762; PHCC, perihilar cholangiocarcinoma, n = 1103; GBC, gallbladder cancer, n = 2580; DCC, distal cholangiocarcinoma, n = 538; AVC, carcinoma of Vater ampulla, n = 365) were enrolled from the Surveillance, Epidemiology, and End Results (SEER) database. Propensity score matching (PSM) process was carried out for less bias.
Result
The proportion of M1 patients in each subtype at first diagnosis was 26.4% (ICC), 37.2% (PHCC), 41. 0% (GBC), 24.5% (DCC), and 12.7% (AVC), and the constitution of DM sites in different subtypes varied apparently. Moreover, the survival of metastasis sites was different (P < .05 in all the subtypes) where the multi‐metastasis and distant lymph node (dLN) only always indicated the worst and best prognosis, respectively. Chemotherapy presented the most significant survival impact with the lowest hazard ratio by multivariate cox model and still provided a survival improvement after PSM (all P < .001) in all subtypes. However, the median months manifested different between patients with and without chemotherapy among the subtypes (ICC, from 5 to 9; PHCC, from 6 to 10; AVC, from 4 to 9; GBC, from 6 to 7; DCC from 6 to 8).
Conclusion
We provided a landscape about the detailed DM mode of the advanced BTC in a large population, found the survival differences among DM sites, and revealed the different chemotherapy efficacies in the BTC subtypes.
Keywords: biliary tract cancer, chemotherapy, distant metastasis, metastasis mode, PSM
Using a large population, we provided a detailed landscape about the DM mode of the advanced biliary tract cancer (BTC), found the survival differences among DM sites, and revealed the different chemotherapy efficacies in the BTC subtypes. The constitution and the survival of the DM sites varied significantly in different subtypes, and the chemotherapy efficacies manifested different among the subtypes.

1. INTRODUCTION
Biliary system is a tree‐like network of tubular structures, and could be classified into five segments according to the anatomic position, which are intrahepatic bile duct, hilar bile duct, gallbladder, distal bile duct, and ampulla of Vater from distal to proximal, respectively. Correspondingly, biliary tract cancer (BTC), originated from biliary system, have five subtypes, including intrahepatic cholangiocarcinoma (ICC), perihilar cholangiocarcinoma (PHCC), gallbladder cancer (GBC), distal cholangiocarcinoma (DCC), and carcinoma of Vater ampulla (AVC).1 BTC is a kind of uncommon but relatively aggressive malignancy and made up about 4% of malignant digestive system tumors, with a gradually increasing incidence in the past years.2, 3
Complete surgical resection is the only curative treatment for BTC, but quite a few patients have no chance for radical surgery due to distant metastasis (DM) at initial diagnosis.4, 5 Although tremendous efforts have been made to explore the mechanisms of DM, the clinical features of DM in advanced BTC were poorly understood. Due to the low morbidity of BTC, the knowledge about the detailed DM mode mainly came from anatomy and clinical experience, and few clinical researches with large populations worked on it.6, 7
Proper treatments are vital for advanced BTC patients. According to the NCCN clinical practice guideline of hepatobiliary cancers, palliative surgery was not encouraged owing to the high risk and the limited benefit, and chemotherapy and radiation were recommended. Even though great progress on the treatments has been made, the survival time and life quality were still frustrating.8 To understand the DM mode and therapy efficacy of the advanced BTC, we analyzed the metastasis sites of each cancer subtype and assessed the efficacy of different treatments in a large population.
2. METHOD
2.1. Patients and data collection
Eligible patients were enrolled from the Surveillance, Epidemiology, and End Results (SEER) database of the US National Cancer Institute. The TNM information of the SEER cohort was obtained based on the following codes: Derived AJCC Stage Group, 7th ed (7th edition; 2010‐2015), Derived AJCC T (7th edition; 2010‐2015), Derived AJCC N (7th edition; 2010‐2015), Derived AJCC M (7th edition; 2010‐2015), Derived SEER Cmb Stg Grp (2016+), Derived SEER Combined T (2016+), Derived SEER Combined N (2016+), Derived SEER Combined M (2016+). The DM site was classified into liver only, distant lymph node (dLN) only, lung only, brain only, bone only, multi‐metastasis, and others, based on SEER combined mets at DX‐bone (2010+), SEER combined mets at DX‐brain (2010+), SEER combined mets at DX‐liver (2010+), SEER combined mets at DX‐lung (2010+), CS mets at DX (2004‐2015), and mets at DX‐distant LN (2016+). In the 7th AJCC staging system, “intrahepatic metastasis” was defined as T2b disease in ICC; however, these cases were recorded as liver only metastasis in the SEER database inconsequently, and we had to conduct analysis according to the record of SEER database.
Inclusion criteria included: (a) patients diagnosed with BTC between 2010 and 2016; (b) accurate tumor stage classification according to the 7th AJCC staging system; and (c) complete available follow‐up information. Patients whose BTC were not the first and only malignant primary tumor or who were lost to follow‐up were excluded in the cohort 1. Note that patients who died within 2 months after initial diagnosis confirmed or received radiation or palliative surgery were excluded in the propensity score matching (PSM) process for less bias in the cohort 2.
The following clinicopathological variables were collected: clinic‐pathological factors (age at initial diagnosis, year at initial diagnosis, gender, race, tumor size, tumor differentiation, TNM stage, and metastasis site), economic factors (marital status, income, insurance, region, and residence city), and treatments (radiation, palliative surgery, and chemotherapy). Data regarding the detailed regimes of radiation, palliative surgery, and chemotherapy were unavailable. Survival duration was calculated from the date of initial diagnosis until the date of death or last follow‐up. The flow chart of inclusion and exclusion is shown in Figure 1.
Figure 1.
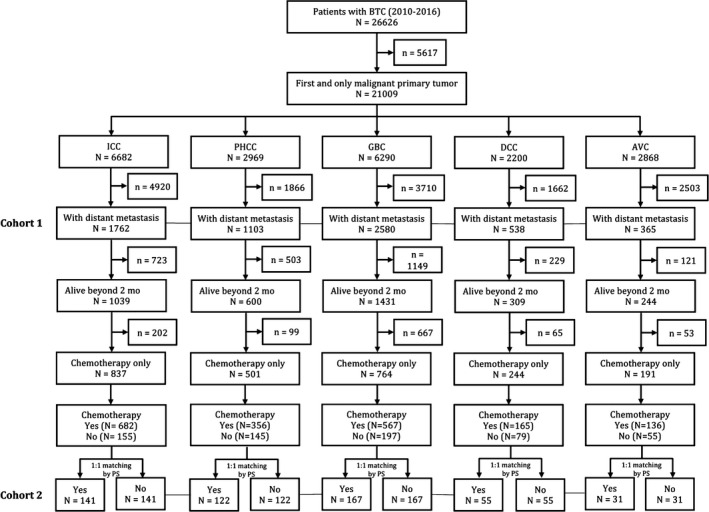
Flow chart of inclusion and exclusion in the study. The cohort 1 was for the analysis of distant metastasis mode and cox regression. The cohort 2 was for the survival analysis of chemotherapy efficacy and was built up by integrating the five subgroups. AVC, Carcinoma of Vater ampulla; BTC, biliary tract cancer DCC, Distal cholangiocarcinoma; GBC, Gallbladder cancer; ICC, Intrahepatic cholangiocarcinoma; PHCC, Perihilar cholangiocarcinoma; PS, Propensity score
2.2. Statistical analysis
Continuous variables were analyzed by Student's t test, and categorical variables were calculated by Chi‐squared test. Survival duration was calculated by the median overall survival (OS), and survival curves were constructed using the Kaplan‐Meier method. Analyses were performed using MedCalc 15.2.2 (MedCalc Software bvba, Ostend, Belgium) and GraphPad Prism 8.0.0 (GraphPad Software, San Diego, California USA, http://www.graphpad.com). PSM process was performed by MatchIt package in R version 3.4.0 (Bell Laboratories, Murray Hill, NJ). A two‐sided P‐value less than 0.05 was considered statistically significant.
3. RESULTS
3.1. Baseline characteristics
Our study included 6348 BTC patients with DM (ICC: 1762, PHCC: 1103, GBC: 2580, DCC: 538, AVC: 365) who were diagnosed between 2010 and 2016. As depicted in Table 1, most of the factors appeared to differ significantly among the five subtypes except residence, which suggested enormous diversity inside the BTC.
Table 1.
Baseline characteristic of metastatic biliary tract cancer patients at initial diagnosis
| Factors | ICC, n (%) | PHCC, n (%) | GBC, n (%) | DCC, n (%) | AVC, n (%) | P | Total, n (%) |
|---|---|---|---|---|---|---|---|
| Number of patients | 1762 | 1103 | 2580 | 538 | 365 | 6348 | |
| Age, years | |||||||
| ≤59 | 573 (32.5) | 280 (25.4) | 625 (24.2) | 127 (23.6) | 89 (24.4) | <.001 | 1694 (26.7) |
| 60‐69 | 577 (32.7) | 364 (33.0) | 751 (29.1) | 184 (34.2) | 99 (27.1) | 1975 (31.1) | |
| 70‐79 | 417 (23.7) | 287 (26.0) | 688 (26.7) | 133 (24.7) | 103 (28.2) | 1628 (25.6) | |
| ≥80 | 195 (11.1) | 172 (15.6) | 516 (20.0) | 94 (17.5) | 74 (20.3) | 1051 (16.6) | |
| Sex | |||||||
| Male | 924 (52.4) | 537 (48.7) | 791 (30.7) | 276 (51.3) | 200 (54.8) | <.001 | 2728 (43.0) |
| Female | 838 (47.6) | 566 (51.3) | 1789 (69.3) | 262 (48.7) | 165 (45.2) | 3620 (57.0) | |
| Race | |||||||
| White | 1359 (77.1) | 842 (76.3) | 1900 (73.6) | 415 (77.1) | 283 (77.5) | <.001 | 4799 (75.6) |
| Black | 144 (8.2) | 104 (9.4) | 381 (14.8) | 58 (10.8) | 46 (12.6) | 733 (11.5) | |
| Others | 259 (14.7) | 157 (14.2) | 299 (11.6) | 65 (12.1) | 36 (9.9) | 816 (12.9) | |
| Year at diagnosis | |||||||
| 2010‐1013 | 787 (44.7) | 577 (52.3) | 1417 (54.9) | 293 (54.5) | 203 (55.6) | <.001 | 3277 (51.6) |
| 2014‐2016 | 975 (55.3) | 526 (47.7) | 1163 (45.1) | 245 (45.5) | 162 (44.4) | 3071 (48.4) | |
| Marriage | |||||||
| Yes | 1031 (58.5) | 588 (53.1) | 1290 (50.0) | 290 (53.9) | 190 (52.1) | <.001 | 3389 (53.4) |
| No | 731 (41.5) | 515 (46.7) | 1290 (50.0) | 248 (46.1) | 175 (47.9) | 2959 (46.6) | |
| Income | |||||||
| Low | 816 (46.3) | 582 (52.8) | 1327 (51.4) | 289 (53.7) | 187 (51.2) | .001 | 3201 (50.4) |
| High | 946 (53.7) | 521 (47.2) | 1253 (48.6) | 249 (46.3) | 178 (48.8) | 3147 (49.6) | |
| Insurance | |||||||
| Yes | 1699 (96.4) | 1043 (94.6) | 2428 (94.1) | 512 (95.2) | 347 (95.1) | .016 | 6029 (95.0) |
| No | 63 (3.6) | 60 (5.4) | 152 (5.9) | 26 (4.8) | 18 (4.9) | 319 (5.0) | |
| Region | |||||||
| East | 632 (35.9) | 350 (31.7) | 966 (37.4) | 222 (41.3) | 122 (33.4) | <.001 | 2292 (36.1) |
| Pacific Coast | 922 (52.3) | 646 (58.6) | 1265 (49.0) | 241 (44.8) | 200 (54.8) | 3274 (51.6) | |
| Northern Plain | 131 (7.4) | 72 (6.5) | 222 (8.6) | 54 (10.0) | 28 (7.7) | 507 (7.99) | |
| Alaska/Southwest | 77 (4.4) | 35 (3.2) | 127 (4.9) | 21 (3.9) | 15 (4.1) | 275 (4.33) | |
| Residence city | |||||||
| Small | 262 (14.9) | 158 (14.3) | 404 (15.7) | 107 (19.9) | 57 (15.6) | .087 | 988 (15.6) |
| Middle | 391 (22.2) | 257 (23.3) | 527 (20.4) | 113 (21.0) | 73 (20.0) | 1361 (21.4) | |
| Large | 1109 (62.9) | 688 (62.4) | 1649 (63.9) | 318 (59.1) | 235 (64.4) | 3999 (63.0) | |
| Tumor size, mm | |||||||
| ≤30 | 115 (6.5) | 165 (15.0) | 394 (15.3) | 118 (21.9) | 123 (33.7) | <.001 | 915 (14.4) |
| 30‐59 | 213 (12.1) | 129 (11.7) | 439 (17.0) | 59 (11.00 | 61 (16.7) | 901 (14.2) | |
| >60 | 576 (32.7) | 97 (8.8) | 354 (13.7) | 25 (4.6) | 13 (3.6) | 1065 (16.8) | |
| Unknown | 858 (48.7) | 712 (64.5) | 1393 (54.0) | 336 (62.5) | 168 (46.0) | 3467 (54.6) | |
| Differentiation | |||||||
| I‐II | 263 (14.9) | 105 (9.5) | 490 (19.0) | 52 (9.7) | 123 (33.7) | <.001 | 1033 (16.3) |
| III‐IV | 277 (15.7) | 135 (12.2) | 686 (26.6) | 75 (13.9) | 103 (28.2) | 1276 (20.1) | |
| Unknown | 1222 (69.4) | 863 (78.3) | 1404 (54.4) | 411 (76.4) | 139 (38.1) | 4039 (63.6) | |
| T stage | |||||||
| T1‐T2 | 946 (53.7) | 339 (30.7) | 500 (19.4) | 140 (26.0) | 127 (34.8) | <.001 | 2052 (32.3) |
| T3‐T4 | 335 (19.0) | 161 (14.6) | 1340 (51.9) | 183 (34.0) | 140 (38.4) | 2159 (34) | |
| TX | 481 (27.3) | 603 (54.7) | 740 (28.7) | 215 (40.0) | 98 (26.9) | 2137 (33.7) | |
| N stage | |||||||
| N0 | 739 (41.9) | 468 (42.4) | 1073 (41.6) | 272 (50.6) | 176 (48.2) | .003 | 2728 (43.0) |
| N1 | 693 (39.3) | 412 (37.4) | 1034 (40.1) | 175 (32.5) | 134 (36.7) | 2448 (38.6) | |
| Nx | 330 (18.7) | 223 (20.2) | 473 (18.3) | 91 (16.9) | 55 (15.1) | 1172 (18.5) | |
| Metastasis site | |||||||
| Bone only | 109 (6.2) | 24 (2.2) | 39 (1.5) | 12 (2.2) | 4 (1.1) | <.001 | 188 (3.0) |
| Brain only | 2 (0.1) | 1 (0.1) | 3 (0.1) | 1 (0.2) | 3 (0.8) | 10 (0.2) | |
| Liver only | 344 (19.5) | 481 (43.6) | 1281 (49.7) | 248 (46.1) | 200 (54.8) | 2554 (40.2) | |
| Lung only | 159 (9.0) | 51 (4.6) | 79 (3.1) | 31 (5.8) | 33 (9.0) | 353 (5.6) | |
| dLN only | 254 (14.4) | 115 (10.4) | 255 (9.9) | 46 (8.6) | 16 (4.4) | 686 (10.8) | |
| Other | 288 (16.4) | 158 (14.3) | 463 (18.0) | 96 (17.8) | 54 (14.8) | 1059 (16.7) | |
| Multi‐metastasis | 606 (34.4) | 273 (24.8) | 460 (17.8) | 104 (19.3) | 55 (15.1) | 1498 (23.6) | |
| Radiation | |||||||
| Yes | 193 (11.0) | 89 (8.1) | 193 (7.5) | 46 (8.6) | 29 (7.9) | .002 | 550 (8.7) |
| No | 1569 (89.0) | 1014 (91.9) | 2387 (92.5) | 492 (91.4) | 336 (92.1) | 5798 (91.3) | |
| Palliative surgery | |||||||
| Yes | 71 (4.0) | 50 (4.5) | 858 (33.3) | 43 (8.0) | 50 (13.7) | <.001 | 1072 (16.9) |
| No | 1691 (96.0) | 1053 (95.5) | 1722 (66.7) | 495 (92.1) | 315 (86.3) | 5276 (83.1) | |
| Chemotherapy | |||||||
| Yes | 1049 (59.5) | 527 (47.8) | 1283 (49.7) | 261 (48.5) | 212 (58.1) | <.001 | 3332 (52.5) |
| No | 713 (40.5) | 576 (52.2) | 1297 (50.3) | 277 (51.5) | 153 (41.9) | 3016 (47.5) | |
P < .05 is considered statistically significant (bold).
Abbreviation: AVC, Carcinoma of Vater ampulla; dLN, Distant lymph node; DCC, Distal cholangiocarcinoma; GBC, Gallbladder cancer; ICC, Intrahepatic cholangiocarcinoma; PHCC, Perihilar cholangiocarcinoma.
3.2. Distant metastasis mode
DM mode was an important form of the diversity. As shown in Figure 2A, the proportion of M1 patients in each subtype at first diagnosis was 26.4% in the ICC, 37.2% in the PHCC, 41. 0% in the GBC, 24.5% in the DCC, and 12.7% in the AVC, respectively. The GBC had the highest DM rate and the highest liver only rate (22.1%), which meant that more than 2/5 of the GBC patients had the DM, and over half of them were the liver only DM. The DM rate and the liver only rate of the PHCC were slightly less than that in the GBC, while its multi‐metastasis rate was the highest. Although the ICC and the DCC had similar DM rate (more than 1/3), the most common DM site of the ICC and the DCC were the multi‐metastasis (9.1%) and the liver only (11.3%), respectively. Conversely, only less than 1/5 of the M1 ICC patients had the liver only DM, and nearly half of the M1 DCC patients had the liver only DM. More than 1/3 of the M1 DCC patients had the multi‐metastasis, while only 1/6 of the M1 ICC patients had the multi‐metastasis. The AVC had the lowest DM rate (12.7%), and nearly half of them were liver only DM.
Figure 2.
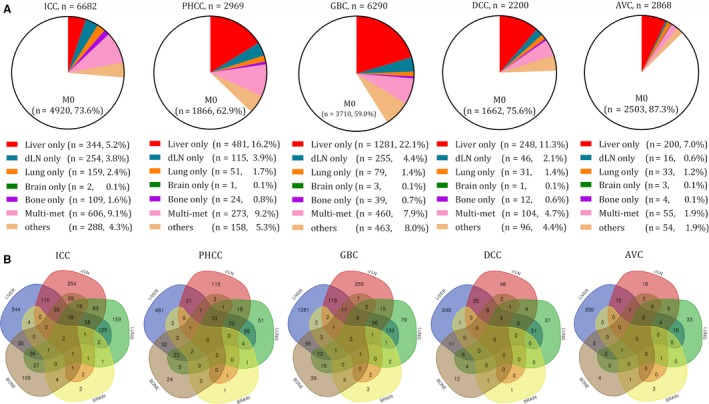
Distant metastasis mode of each subtype of the BTC. A, proportion of the M1 patients at first diagnosis in each subtype. B, Venn diagrams of the distribution of distant metastatic sites in each subtype. BTC, biliary tract cancer; ICC, Intrahepatic cholangiocarcinoma; PHCC, Perihilar cholangiocarcinoma; GBC, Gallbladder cancer; DCC, Distal cholangiocarcinoma; AVC, Carcinoma of Vater ampulla; dLN, Distant lymph node
As other metastasis sites, the dLN only was commonly seen in the ICC (3.8%), the PHCC (3.9%), and the GBC (4.4%); the bone only was mostly found in the ICC (1.6%); the lung only rate was similar in all the subtypes (1.2%‐2.4%), and the brain only was extremely rare in all the subtypes (all ≤0.1%). In addition, there were still part of patients without accurate record of the metastasis sites in all the subtypes.
The multi‐metastasis could be found in some patients at initial diagnosis. The most common combination was liver plus lung, and the second one was liver plus dLN. This was in accord with the fact that liver, dLN, and lung were the top three DM sites in most of the BTC (Figure2B and Figure S2‐S7), indicating that the combinations of the multi‐metastasis were closely related to the DM rate of a single site. However, although the rate of liver plus lung was higher than that of liver plus dLN (liver plus lung rate vs liver plus dLN rate: ICC, 7.1% vs 6.2%; PHCC, 7.8% vs 4.6%; GBC, 5.0% vs 4.6%; DCC, 5.8% vs 4.7%; AVC, 4.9% vs 3.3%), the dLN DM rate was higher than the lung DM rate in most of the BTC (dLN vs lung: ICC, 14.4% vs 9.0%; PHCC, 10.4% vs 4.6%; GBC, 9.9% vs 3.1%; DCC, 8.6% vs 5.8%; AVC, 4.4% vs 9.0%) (Figure 2B, Figure S2‐S7), suggesting that the multi‐site metastasis might be progressed step by step but not randomly combined. All the specific combinations of different sites are shown in Figure 2B, and more detailed information about metastasis mode is shown in Figure S2‐S7.
Although all the M1 BTC patients shared a compromised prognosis, survival of different metastasis sites displayed significantly different (P < .001 in the ICC, P = .005 in the PHCC, P < .001 in the GBC, P = .011 in the DCC, and P < .001 in the AVC, respectively) (Figure 3). In our survival analysis, multi‐metastasis always indicated a shortest survival, while dLN only always indicated a better prognosis. Moreover, the bone only in the ICC, GBC, and DCC, the lung only in the PHCC, and the liver only in the GBC were also poor predictors for OS. The brain only was excluded in this analysis for the insufficient patient numbers.
Figure 3.
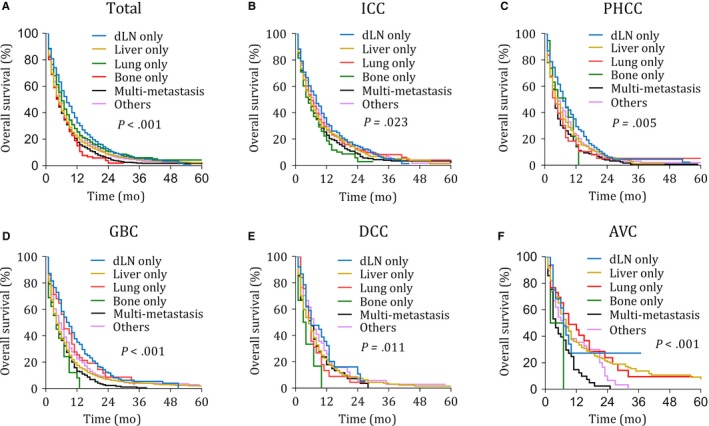
Kaplan–Meier curves of overall survival according to metastasis sites in the total cohort and each subtype. AVC, Carcinoma of Vater ampulla; BTC, biliary tract cancer; DCC, Distal cholangiocarcinoma; dLN, Distant lymph node; GBC, Gallbladder cancer; ICC, Intrahepatic cholangiocarcinoma; PHCC, Perihilar cholangiocarcinoma
3.3. Univariate and multivariate cox analysis
Univariate and multivariate cox analysis were conducted to further identify the potential factors that might influence survival besides metastasis sites. In the univariate cox analysis, age, race, marital status, insurance, tumor size, differentiation, T stage, N stage, metastasis sites, and treatments (radiation, palliative surgery, and chemotherapy) were identified to be significantly associated with OS in at least two subtypes (Table S1). Besides, sex in the ICC, and year at diagnosis and residence in the GBC were also significant predictors.
These factors proved to be significant predictors above were then entered into the multivariate cox proportional hazards model of different subtypes separately. As shown in Table 2, the independent prognostic indicators were demonstrated in the ICC (age, sex, differentiation, metastasis site, radiation, palliative surgery, and chemotherapy), the PHCC (age, race, region, tumor size, metastasis site, palliative surgery, and chemotherapy), the GBC (age, income, tumor size, differentiation, N stage, metastasis site, palliative surgery, and chemotherapy), the DCC (palliative surgery and chemotherapy), and the AVC(differentiation, metastasis site, palliative surgery, and chemotherapy). Based on the results, the clinic‐pathological factors (age, race, sex, differentiation, tumor size, and metastasis site) and the treatments (palliative surgery and chemotherapy) play important roles in patient survival, while the economic factors' (region and income) influence was rather limited. Interestingly, T stage and N stage also only had limited influence. Furthermore, factors identified above in the ICC, PHCC, and GBC were more abundant compared with that in the DCC and the AVC, where the treatments had the most significant influence in patient prognosis.
Table 2.
Multivariate cox regression models of prognostic factors for overall survival
| Factors | ICC (n = 1762) | PHCC (n = 1103) | GBC (n = 2580) | DBDC (n = 538) | AVC (n = 365) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Age, years | ||||||||||
| 60‐69 vs ≤ 59 | 1.143 (1.004‐1.302) | .045 | 1.268 (1.071‐1.502) | .006 | 1.091 (0.971‐1.226) | 0.147 | 0.906 (0.704‐ 1.165) | .443 | 0.868 (0.618‐1.218) | .415 |
| 70‐79 vs ≤ 59 | 1.270 (1.099‐1.466) | .001 | 1.374 (1.146‐1.647) | <.001 | 1.177 (1.044‐1.326) | .008 | 1.258 (0.963‐1.645) | .095 | 1.152 (0.826‐1.607) | .406 |
| ≥80 vs ≤ 59 | 1.410 (1.168‐1.703) | <.001 | 1.226 (0.991‐1.517) | .063 | 1.422 (1.246‐1.622) | <.001 | 1.299 (0.961‐1.755) | .091 | 0.974 (0.662‐1.432) | .892 |
| Sex | ||||||||||
| Male vs Female | 0.772 (0.694‐0.858) | <.001 | ||||||||
| Race | ||||||||||
| Black vs White | 0.919 (0.738‐1.145) | .456 | 1.105 (0.818‐1.492) | .517 | ||||||
| Others vs White | 0.661 (0.546‐0.802) | <.001 | 0.846 (0.630‐1.136) | .268 | ||||||
| Year at diagnosis | ||||||||||
| 14‐16 vs 10‐13 | 0.958 (0.878‐1.045) | .334 | ||||||||
| Marriage | ||||||||||
| Yes vs No | 1.106 (0.993‐1.233) | .069 | 1.054 (0.967‐1.149) | .237 | 1.179 (0.977‐1.423) | .088 | ||||
| Income | ||||||||||
| Low vs High | 1.155 (1.062‐1.256) | <.001 | ||||||||
| Insurance | ||||||||||
| No vs Yes | 1.001 (0.759‐1.322) | .993 | 0.949 (0.793‐1.135) | .566 | ||||||
| Region | ||||||||||
| Pacific Coast vs East | 1.142 (0.934‐1.396) | .197 | 0.724 (0.555‐0.943) | .016 | ||||||
| Northern Plain vs East | 0.972 (0.868‐1.089) | .629 | 0.908 (0.786‐1.049) | .193 | ||||||
| Alaska/Southwest vs East | 0.910 (0.701‐1.183) | .484 | 0.926 (0.632‐1.338) | .663 | ||||||
| Residence city | ||||||||||
| Middle vs Large | 1.018 (0.915‐1.132) | .745 | ||||||||
| Small vs Large | 1.136 (0.947‐1.209) | .281 | ||||||||
| Tumor size, mm | ||||||||||
| 30‐59 vs ≤ 29 | 0.871 (0.672‐1.128) | .298 | 1.104 (0.863‐1.412) | .435 | 1.115 (0.967‐1.149) | .168 | 1.155 (0.822‐1.622) | .410 | ||
| >60 vs ≤ 29 | 0.985 (0.783‐1.240) | .899 | 1.363 (1.037‐1.791) | .017 | 1.243 (1.056‐1.462) | .009 | 1.010 (0.621‐1.642) | .969 | ||
| Unknown vs ≤ 29 | 1.023 (0.815‐1.285) | .844 | 1.252 (1.042‐1.504) | .017 | 1.276 (1.119‐1.456) | <.001 | 1.099 (0.862‐1.401) | .449 | ||
| Differentiation | ||||||||||
| III/ IV vs I‐II | 1.312 (1.085‐1.588) | .005 | 1.460 (1.282‐1.663) | <.001 | 1.167 (0.778‐1.749) | .458 | 1.816 (1.333‐2.474) | <.001 | ||
| Unknown vs I‐II | 1.373 (1.178‐1.601) | <.001 | 1.242 (1.081‐1.426) | .002 | 1.137 (0.803‐1.609) | .471 | 1.122 (0.849‐1.484) | .421 | ||
| T stage | ||||||||||
| T3‐T4 vs T1‐T2 | 0.948 (0.825‐1.089) | .448 | 0.955 (0.849‐1.075) | .447 | ||||||
| TX vs T1‐T2 | 0.905 (0.790‐1.037) | .154 | 0.940 (0.812‐1.087) | .406 | ||||||
| N stage | ||||||||||
| N1 vs N0 | 1.001 (0.860‐1.165) | .990 | 1.024 (0.928‐1.130) | .635 | ||||||
| Nx vs N0 | 1.169 (0.984‐1.389) | .107 | 1.129 (1.001‐1.273) | .049 | ||||||
| Metastasis site | ||||||||||
| Liver vs DLN | 0.970 (0.803‐1.171) | .751 | 1.243 (0.986‐ 1.569) | .068 | 1.348 (1.153‐1.575) | <.001 | 0.968 (0.680‐1.378) | .856 | 0.840 (0.452‐1.559) | .582 |
| Lung vs DLN | 1.113 (0.895‐1.383) | .338 | 1.346 (0.950‐1.908) | .097 | 1.022 (0.771‐1.355) | .882 | 1.047 (0.635‐1.725) | .859 | 0.789 (0.388‐1.606) | .515 |
| Brain vs DLN | 3.075 (0.761‐12.434) | .117 | 2.027 (0.274‐14.979) | .491 | 1.419 (0.453‐4.447) | .551 | 5.244 (0.710‐38.728) | .106 | 2.127 (0.578‐7.833) | .259 |
| Bone vs DLN | 1.323 (1.030‐1.700) | .029 | 1.089 (0.673‐1.762) | .731 | 1.901 (1.306‐2.766) | <.001 | 1.594 (0.811‐3.133) | .178 | 3.499 (1.096‐11.167) | .035 |
| Others vs DLN | 1.079 (0.894‐1.303) | .432 | 1.121 (0.859‐1.463) | .403 | 1.307 (1.097‐1.558) | <.001 | 0.801 (0.539‐1.190) | .274 | 0.934 (0.473‐1.844) | .844 |
| Multi vs DLN | 1.332 (1.126‐1.576) | <.001 | 1.612 (1.266‐2.053) | <.001 | 1.470 (1.236‐1.748) | <.001 | 1.204 (0.820‐1.768) | .346 | 1.683 (0.869‐3.260) | .125 |
| Radiation | ||||||||||
| Yes vs No | 0.750 (0.631‐0.891) | .001 | 0.895 (0.704‐1.138) | .368 | 0.950 (0.804‐1.123) | .553 | ||||
| Palliative surgery | ||||||||||
| Yes vs No | 0.528 (0.398‐0.699) | <.001 | 0.402 (0.290‐0.558) | <.001 | 0.636 (0.561‐0.720) | <.001 | 0.452 (0.286‐0.714) | <.001 | 0.356 (0.237‐0.534) | <.001 |
| Chemotherapy | ||||||||||
| Yes vs No | 0.274 (0.243‐0.310) | <.001 | 0.381 (0.330‐0.438) | <.001 | 0.403 (0.367‐0.443) | <.001 | 0.350 (0.285‐0.429) | <.001 | 0.309 (0.234‐0.409) | <.001 |
P<0.05 is considered statistically significant (bold).
Abbreviations:AVC, Carcinoma of Vater ampulla; CI, Confidence interval; dLN, Distant lymph node; GBC, Gallbladder cancer; DCC, Distal cholangiocarcinoma; HR, Hazard ratio; ICC, Intrahepatic cholangiocarcinoma; PHCC, Perihilar cholangiocarcinoma.
3.4. Chemotherapy efficacy in different BTC subtypes
Treatments for the M1 BTC patients included radiation, palliative surgery, and chemotherapy. Radiation only worked in the ICC with a higher hazard ratio (HR) than chemotherapy (Table 2), and palliative surgery was always considered harmful in the NCCN clinical practice guideline of hepatobiliary cancers, and only very few patients received it (Table 1). Chemotherapy was the main choice at present, and it proved to be a significant method to prolong patient survival in multivariate cox model (yes vs no: ICC, HR (95%CI): 0.274 (0.243‐0.310), P < .001; PHCC, HR (95%CI): 0.381 (0.330‐0.438), P < .001; GBC, HR (95%CI): 0.403 (0.367‐0.443), P < .001; DCC, (95%CI): 0.350 (0.285‐0.429), P < .001; AVC, HR (95%CI), 0.309 (0.234‐0.409), P < .001).
To further assess the therapeutic value of chemotherapy, Kaplan‐Meier survival analysis was adopted and median survival was calculated simultaneously. Patients lived less than 2 months or received radiation or palliative surgery were excluded in this analysis to reduce possible bias between chemotherapy group and no chemotherapy group. However, significant difference of baseline characteristics existed between two groups in the total cohort (Table 3) and all the subtypes (Table S2‐S6). PSM was conducted to correct bias in each subtype separately with an appropriate caliper of 0.04, and then all the baseline characteristics between two groups presented no difference in the five subtypes (Table S2‐S6). The total cohort was integrated from the five subtypes (Table 3).
Table 3.
Baseline characteristics of the without (No) and with chemotherapy (Yes) groups before and after PSM
| Factors | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|
| No (n = 631) | Yes (n = 1906) | P | No (n = 516) | Yes (n = 516) | P | |
| Location | ||||||
| ICC | 155 | 682 | <.001 | 141 | 141 | 1.000 |
| PHCC | 145 | 356 | 122 | 122 | ||
| GBC | 197 | 567 | 167 | 167 | ||
| DCC | 79 | 165 | 55 | 55 | ||
| AVC | 55 | 136 | 31 | 31 | ||
| Age, years | ||||||
| ≤59 | 132 | 675 | <.001 | 125 | 112 | .499 |
| 60‐69 | 163 | 655 | 154 | 169 | ||
| 70‐79 | 159 | 451 | 136 | 167 | ||
| ≥80 | 177 | 125 | 101 | 68 | ||
| Sex | ||||||
| Male | 262 | 856 | .149 | 226 | 240 | .416 |
| Female | 369 | 1050 | 290 | 276 | ||
| Race | ||||||
| White | 455 | 1453 | .011 | 379 | 379 | .669 |
| Black | 72 | 219 | 58 | 68 | ||
| Others | 104 | 234 | 79 | 69 | ||
| Year at diagnosis | ||||||
| 2010‐1013 | 361 | 994 | .031 | 293 | 286 | .707 |
| 2014‐2016 | 270 | 912 | 223 | 230 | ||
| Marriage | ||||||
| Yes | 266 | 1172 | <.001 | 246 | 259 | .455 |
| No | 365 | 734 | 270 | 257 | ||
| Income | ||||||
| Low income | 308 | 990 | 0.187 | 255 | 256 | 1.000 |
| High income | 323 | 916 | 261 | 260 | ||
| Insurance | ||||||
| Yes | 594 | 1851 | <.001 | 17 | 25 | .271 |
| No | 37 | 55 | 499 | 491 | ||
| Region | ||||||
| East | 190 | 738 | <.001 | 165 | 195 | .209 |
| Pacific Coast | 48 | 152 | 35 | 36 | ||
| Northern Plain | 365 | 946 | 293 | 268 | ||
| Alaska/Southwest | 28 | 70 | 23 | 17 | ||
| Residence city | ||||||
| Small | 420 | 1214 | .327 | 348 | 320 | .168 |
| Middle | 129 | 403 | 102 | 114 | ||
| Large | 82 | 289 | 66 | 82 | ||
| Tumor size, mm | ||||||
| ≤30 | 90 | 247 | .602 | 72 | 77 | .912 |
| 30‐59 | 93 | 272 | 76 | 67 | ||
| >60 | 75 | 369 | 69 | 76 | ||
| Unknown | 373 | 1018 | 299 | 296 | ||
| Differentiation | ||||||
| I‐II | 92 | 271 | .474 | 74 | 65 | .965 |
| III‐IV | 76 | 289 | 59 | 78 | ||
| Unknown | 463 | 1346 | 383 | 373 | ||
| T stage | ||||||
| T1‐T2 | 186 | 661 | .001 | 157 | 158 | .981 |
| T3‐T4 | 183 | 595 | 154 | 156 | ||
| TX | 262 | 650 | 205 | 202 | ||
| N stage | ||||||
| N0 | 284 | 786 | .336 | 238 | 229 | .839 |
| N1 | 199 | 807 | 168 | 191 | ||
| Nx | 148 | 313 | 110 | 96 | ||
| Metastasis site | ||||||
| dLN only | 68 | 243 | <.001 | 56 | 68 | .515 |
| Liver only | 294 | 677 | 227 | 217 | ||
| Lung only | 44 | 132 | 33 | 26 | ||
| Brain only | 2 | 0 | 2 | 0 | ||
| Bone only | 15 | 37 | 13 | 5 | ||
| Other | 115 | 305 | 100 | 80 | ||
| Multi‐metastasis | 93 | 512 | 85 | 120 | ||
P < .05 is considered statistically (bold).
Abbreviations: AVC, Carcinoma of Vater ampulla; dLN, Distant lymph node; DBDC, Distal cholangiocarcinoma; GBC, Gallbladder cancer; ICC, Intrahepatic cholangiocarcinoma; PHCC, Perihilar cholangiocarcinoma.
Survival analysis was carried out subsequently, and the results are presented in Figure 4. In the total cohort, chemotherapy was significantly associated with improved survival months (chemotherapy vs no‐chemotherapy: 8, 95%CI: 8‐9 vs 5, 95%CI: 5‐6 for median OS) (P < .001). The improvement on OS after receiving chemotherapy also existed in each subtype (all P < .001); however, the level of survival improvement manifested different among the subtypes. The median OS for the ICC, PHCC, and AVC were elevated from 5 months to 9 months, from 6 months to 10 months, and from 4 months to 9 months after receiving chemotherapy, respectively. While, the elevation of survival time for the GBC and the DCC was only from 6 months to 7 months and from 6 months to 8 months, respectively, demonstrating insufficient efficacy of current chemotherapy regimens for the GBC and the DCC.
Figure 4.
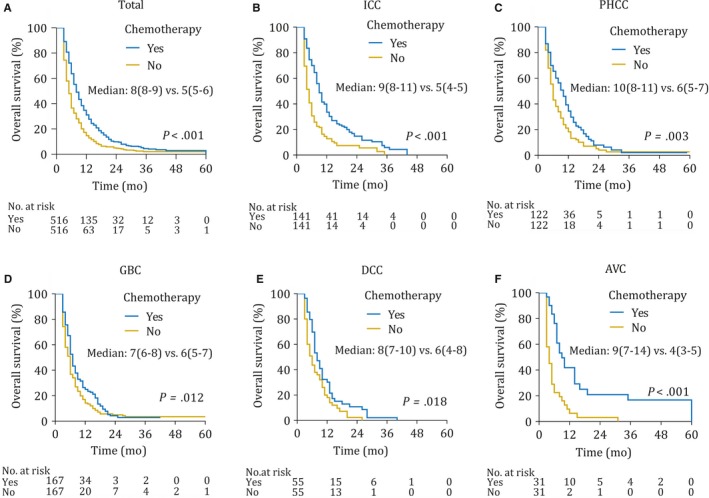
Kaplan‐Meier curves of overall survival according to whether or not receiving chemotherapy in each subtype of the BTC. AVC, Carcinoma of Vater ampulla; DCC, Distal cholangiocarcinoma; GBC, Gallbladder cancer; ICC, Intrahepatic cholangiocarcinoma; PHCC, Perihilar cholangiocarcinoma
4. DISCUSSION
Correct understanding for the DM mode of the advanced BTC is helpful for accurate diagnosis and optimal treatment selection. Meanwhile, in consideration of the fact that clinical researches about treatment strategies in the BTC always included multiple subtypes, a valid and credible evaluation to the efficacy of current therapy in each subtype is urgently needed. In this study, we described the DM modes of the BTC in a large population, revealed the survival differences among DM sites, and found the different efficacy of current treatments in each subtype.
The DM mode of each subtype was apparently unique. Although liver only and dLN only were the top two metastasis sites in all the subtypes, DM rates of these subtypes extremely varied, ranging from 12.7% to 41.0%, and the proportions of the metastasis sites differed distinctively. About the differences of DM modes, there were some theoretical bases in clinical features and anatomy. Obstructive jaundice might develop at an early stage in the DCC and the AVC, while the GBC and the PHCC are always symptom‐free or just manifested by some nonspecific symptoms, such as sour regurgitation, nausea, and abdominal pain.9 Pathological difference might be an important factor, because the GBC and the PHCC are always more malignant and have a higher recurrence rate and a higher mortality rate after resection. Adjacency to visceral organ and complex vascular lymphatic system might also play an important role. The underlying mechanism is complicated and requires further investigations.
Despite the poor prognosis of the advanced BTC in whole, these metastasis sites still manifested different survival. Multi‐metastasis always indicated the shortest survival in our study, and dLN only always indicated a better prognosis, which might suggest the different disease progression, although they were both in M1 stage. Also, some metastasis sites showed different survival among the subtypes, which indicated the peculiarity of the DM mode of each subtype. Besides metastasis sites, there were some other clinic‐pathological factors, economic factors, and treatments relating to the patient survival. Most of the factors have been proved to influence the prognosis of the resectable BTC patients before, such as marital status, differentiation, and treatment, but the influence of T stage and N stage in the advanced BTC was fairly limited and not clearly as that in resectable cases.10, 11, 12 Interestingly, there is no factor having a significant survival effect in all the five subtypes except treatments. Choosing a proper treatment seemed the only thing that we could do for the advanced BTC patients, and a valid and credible evaluation to the efficacy of different therapies is of great value.
Radiation and palliative surgery did not work in the BTC according to our study, the previous researches, and the NCCN guideline.13 The remaining choice for M1 BTC patients was chemotherapy. As the gold standard, GemCis regimen (gemcitabine‐cisplatin) could elevate the median survival of the unresectable BTC patients from 8.1 to 11.7 months compared with gemcitabine monotherapy and did not reduce the quality of life.14, 15 A similar magnitude of benefit from the GemCis regimen was seen in the BT‐22 study.16 Besides, the GEMOX (gemcitabine‐oxaliplatin) also proved to be active and well tolerated in the unresectable BTC.17 However, these clinical researches included all the subtypes regardless of the different pathological basis, which severely limited the clinical application. In spite of the unavailability of the detailed regimes of chemotherapy in our study, improvement on survival after receiving chemotherapy also existed in each subtype. However, the level of survival improvement manifested different among the subtypes. In the PSM analysis, the median OS of patients receiving chemotherapy was nearly two times longer than those not receiving chemotherapy in the ICC, PHCC, and AVC, but the elevation of survival time for the GBC and the DCC was no more than 2 months. In view of the insufficient efficacy of chemotherapy, the role of targeted therapy and the immunotherapy needs further study. Targeted therapy and the immunotherapy showed effective results in some cancers, but the effect of them on the advanced BTC is still obscure. The effect of the EGFR inhibitors, the HER‐2 inhibitors, and the VEGF inhibitors did not demonstrate a better prognosis compared with GEMOX regimen, but part of patients could get a complete or partial remission.18, 19, 20, 21, 22 Immunotherapy might be a promising treatment for the advanced BTC because the expression of PD‐1/PD‐L1 elevated in the BTC tissue. Further researches are being carried out all over the world.23, 24
This study has some inevitable limitations primarily caused by the nature of the SEER database. First, the study was limited by its retrospective nature. It is necessary to validate our results in a prospective study or another large‐volume database. Second, there were still potential bias caused by the lack of some clinical characteristics, including palliative surgical procedures and the data regarding the detailed regimes of chemotherapy and radiation. Third, subgroup analyses for the chemotherapy efficacy of different metastasis sites in each subtype were not conducted for the insufficient patient numbers. At last, the record of peritoneal carcinomatosis was unavailable in SEER database, which is one of the common metastatic sites by BTC. Given these limitations, more efforts should be undertaken to validate our conclusion prior to clinical application.
5. CONCLUSION
In conclusion, we provided a detailed landscape of the DM mode of the advanced BTC in a large population, found the survival differences among DM sites, and revealed the different chemotherapy efficacy of the BTC subtypes.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
AUTHOR CONTRIBUTIONS
J. Wang, X. Bo and L. Nan for acquisition of data, analysis and interpretation of data, statistical analysis and drafting of the manuscript; C. Wang, Z. Gao, T. Suo, X. Ni, and H. Liu for technical and material support; H. Liu, Y. Wang, and P. Lu for study concept and design, analysis and interpretation of data, drafting of the manuscript, obtained funding and study supervision. All authors read and approved the final manuscript.
Supporting information
ACKNOWLEDGMENTS
This study was funded by grants from the National Natural Science Foundation of China (81872352), the Foundation of Shanghai Science and Technology Committee (16411952000), JianFeng project of XuHui Provincial Commission of Health and Family Planning (SHXH201703) and the Shanghai Medical Discipline of Key Programs for General Surgery (2017ZZ02007) and Clinical Study of Zhongshan Hospital(2018ZSLC24). All these study sponsors have no roles in the study design, in the collection, analysis, and interpretation of data. All authors have contributed significantly to the content of the manuscript.
Wang J, Bo X, Nan L, et al. Landscape of distant metastasis mode and current chemotherapy efficacy of the advanced biliary tract cancer in the United States, 2010‐2016. Cancer Med. 2020;9:1335–1348. 10.1002/cam4.2794
Jie Wang, Xiaobo Bo, and Lingxi Nan contributed equally to this work.
Contributor Information
Pinxiang Lu, Email: pinxianglu@163.com.
Yueqi Wang, Email: yueqiwang@fudan.edu.cn.
Houbao Liu, Email: houbaoliu@aliyun.com.
DATA AVAILABILITY STATEMENT
The raw data that generated cohort 1 and cohort 2 in this study are available upon request in the SEER (https://seer.cancer.gov/). The detailed information about metastasis mode and baseline characteristics of each subtype before and after PSM is available as Supporting Information.
REFERENCES
- 1. Valle JW, Borbath I, Khan SA, et al. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2016;27(suppl 5):v28‐v37. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA: Cancer J Clin. 2017;67(1):7‐30. [DOI] [PubMed] [Google Scholar]
- 3. Chen W. Cancer statistics: updated cancer burden in China. Chin J Cancer Res. 2015;27(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145(6):1215‐1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kurahara H, Maemura K, Mataki Y, et al. Indication of extrahepatic bile duct resection for gallbladder cancer. Langenbecks Arch Surg. 2018;403(1):45‐51. [DOI] [PubMed] [Google Scholar]
- 6. Nath MC, Torbenson MS, Erickson LA. Perihilar cholangiocarcinoma. Mayo Clin Proc. 2018;93(3):397‐398. [DOI] [PubMed] [Google Scholar]
- 7. Katayose YU, Nakagawa K, Yamamoto K, et al. Lymph nodes metastasis is a risk factor for bone metastasis from extrahepatic cholangiocarcinoma. Hepatogastroenterology. 2012;59(118):1758‐1760. [DOI] [PubMed] [Google Scholar]
- 8. Valle JW. Advances in the treatment of metastatic or unresectable biliary tract cancer. Annals Oncol. 2010;21(suppl_7):vii345‐vii348. [DOI] [PubMed] [Google Scholar]
- 9. Roberts RH, Krige JE, Bornman PC, Terblanche J. Pancreaticoduodenectomy of ampullary carcinoma. Am Surg. 1999;65(11):1043‐1048. [PubMed] [Google Scholar]
- 10. Yifan T, Zheyong L, Miaoqin C, Liang S, Xiujun C. A predictive model for survival of gallbladder adenocarcinoma. Surg Oncol. 2018;27(3):365‐372. [DOI] [PubMed] [Google Scholar]
- 11. Groot Koerkamp B, Fong Y. Outcomes in biliary malignancy. J Surg Oncol. 2014;110(5):585‐591. [DOI] [PubMed] [Google Scholar]
- 12. Li X, Liu YE, Wang YI, et al. The influence of marital status on survival of gallbladder cancer patients: a population‐based study. Sci Rep. 2017;7(1):5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pollom EL, Alagappan M, Park LS, Whittemore AS, Koong AC, Chang DT. Does radiotherapy still have a role in unresected biliary tract cancer? Cancer Med. 2017;6(1):129‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273‐1281. [DOI] [PubMed] [Google Scholar]
- 15. Bridgewater J, Lopes A, Palmer D, et al. Quality of life, long‐term survivors and long‐term outcome from the ABC‐02 study. Br J Cancer. 2016;114(9):965‐971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Okusaka T, Nakachi K, Fukutomi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. 2010;103(4):469‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. André T, Tournigand C, Rosmorduc O, et al. Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol. 2004;15(9):1339‐1343. [DOI] [PubMed] [Google Scholar]
- 18. Chen JS, Hsu C, Chiang NJ, et al. A KRAS mutation status‐stratified randomized phase II trial of gemcitabine and oxaliplatin alone or in combination with cetuximab in advanced biliary tract cancer. Ann Oncol. 2015;26(5):943‐949. [DOI] [PubMed] [Google Scholar]
- 19. Leone F, Marino D, Cereda S, et al. Panitumumab in combination with gemcitabine and oxaliplatin does not prolong survival in wild‐type KRAS advanced biliary tract cancer: A randomized phase 2 trial (Vecti‐BIL study). Cancer. 2016;122(4):574‐581. [DOI] [PubMed] [Google Scholar]
- 20. Javle M, Churi C, Kang HC, et al. HER2/neu‐directed therapy for biliary tract cancer. J Hematol Oncol. 2015;8:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhu AX, Meyerhardt JA, Blaszkowsky LS, et al. Efficacy and safety of gemcitabine, oxaliplatin, and bevacizumab in advanced biliary‐tract cancers and correlation of changes in 18‐fluorodeoxyglucose PET with clinical outcome: a phase 2 study. Lancet Oncol. 2010;11(1):48‐54. [DOI] [PubMed] [Google Scholar]
- 22. Valle JW, Wasan H, Lopes A, et al. Cediranib or placebo in combination with cisplatin and gemcitabine chemotherapy for patients with advanced biliary tract cancer (ABC‐03): a randomised phase 2 trial. Lancet Oncol. 2015;16(8):967‐978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sabbatino F, Villani V, Yearley JH, et al. PD‐L1 and HLA class I antigen expression and clinical course of the disease in intrahepatic cholangiocarcinoma. Clin Cancer Res. 2016;22(2):470‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gani F, Nagarajan N, Kim Y, et al. Program death 1 immune checkpoint and tumor microenvironment: implications for patients with intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2016;23(8):2610‐2617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data that generated cohort 1 and cohort 2 in this study are available upon request in the SEER (https://seer.cancer.gov/). The detailed information about metastasis mode and baseline characteristics of each subtype before and after PSM is available as Supporting Information.


