Short abstract
Background
We developed a new approach for the treatment of enterovirus infections, the consecutive alternating administration (CAA) of a combination of enterovirus inhibitors. On the model of coxsackievirus B1 (CVB1) in mice, two phenomena were observed: absence of drug resistance and increased susceptibility to the antivirals. This study aims to clarify the genetic basis of these phenomena.
Methods
Brain samples from CVB1-infected mice subjected to a CAA course with the combination pleconaril/MDL-860/oxoglaucine were used for viral RNA extraction and next generation sequencing. In parallel, samples from monotherapeutic courses of the three substances included in the combination were studied. Whole genome sequence analysis was carried out on all samples.
Results
Samples of pleconaril monotherapy showed mutations in 5′untranslated region, VP3, 2C, 3C and 2A regions of viral RNA, translated in amino acid substitution of the 2A protein. The MDL-860 course induced changes in CVB1 RNA in the VP3 and 2C regions. The oxoglaucine monotherapy samples showed RNA mutation and amino acid substitution in the VP1 region and nucleotide substitution in the 3D region. In the specimens taken from mice subjected to the CAA course with pleconaril/MDL-860/oxoglaucine, the following RNA mutations were established: 5′ untranslated region, 2A, and 2B, and amino acids substitutions in VP3 and 2A, which differ from those mentioned above. These changes could be the reason for the prevention of drug resistance development and also to be considered as the basis for the phenomenon of increased drug susceptibility.
Conclusions
The results reveal that the high anti-enteroviral efficacy of the CAA course is substantiated by the appearance of specific changes in the viral genome.
Keywords: Drug combination, drug resistance, enteroviruses, genomic analysis
Introduction
Enterovirus (EV) infections are a significant cause of morbidity and mortality throughout the world. EVs have been associated with many human diseases, including myocarditis; pericarditis; dilated cardiomyopathy; Bornholm disease; aseptic meningitis; poliomyelitis; juvenile insulin-dependent diabetes; hand, foot, and mouth disease; common cold; uveitis; and so on. Currently, clinically effective antivirals for use in the treatment of EV infections do not exist due to the development of drug resistance during the routinely applied or trialed monotherapy. This phenomenon is based on the unusually high degree of mutation in Picornaviridae resulting in viral progeny consisting of countless quasi species.
The genome of coxsackieviruses as EVs consists of a positive single-stranded RNA molecule of approximately 7400 nucleotides long flanked by 5′ and 3′ untranslated regions (UTRs). This RNA is translated in a polyprotein which is then proteolytically processed to yield the capsid proteins VP1, VP2, VP3, and VP4, non-structural proteins 2A, 2B, 2C, 3A, 3B, 3C, and viral RNA polymerase 3D.1–4
Viral and host cell proteins involved in viral RNA replication induce a change in the host membrane permeability and the production of membranous structures playing a significant role in viral replication, especially in the formation and functioning of the viral replication complexes. The viral replication complex, consisting of viral RNA replicative form, viral RNA polymerase molecules, and (+)RNA strands (from initiation to complete elongation), has been found to be strongly associated with virus-induced membranous vesicles and various replication-associated viral proteins, such as 2B, 2BC, 3A, and 3D, as well as with host proteins.5
In previous studies, we proposed a new, effective treatment approach for EV (coxsackievirus B) infections in mice, which involved consecutive alternating administration (CAA) of triple combinations of enteroviral replication inhibitors.6–10 (Vassileva-Pencheva and Galabov, 2016).
A study on the effect of the CAA course in newborn mice infected with coxsackievirus B1 (CVB1) neuroinfection and treated with the triple combination disoxaril + guanidine.HCl + oxoglaucine observed a suppression of the development of drug resistance followed by the appearance of drug-increased sensitivity.6 This phenomenon was strongly established and proved during subsequent studies of CAA with the combinations pleconaril + guanidine.HCl + oxoglaucine (PGO) and pleconaril + MDL-860 + oxoglaucine (PMO).9,10 To clarify the mechanism of this phenomenon, we analyzed the genomes of coxsackievirus B1 brain isolates from mice subjected to either a CAA course with the PMO triple combination mentioned above or to monotherapies of the compounds. This provided a unique opportunity to investigate any potential virus adaptations to the tested compounds, such as specific genomic features that would sustain dissemination.
After sequence analysis of the collected specimens, nucleotide and amino acid changes were observed in the viral proteins VP1, VP3, 2A, 2B, and 2C.
Materials and methods
Virus
Coxsackievirus B1 (Connecticut-5 strain) for in vivo experiments was obtained through intracerebral passages (0.02 mL/mouse) in newborn albino mice and prepared as a 10% wt/volume brain suspension in phosphate-buffered saline (PBS). The virus underwent at least three intracerebral passages in newborn mice (without intermediary passages in cell cultures).
Mice
ICR random-bred newborn albino mice (obtained from the Experimental and Breeding Base for Laboratory Animals of the Bulgarian Academy of Sciences, Slivnitza, Bulgaria) were used. Each dam was housed in specially designed, well-ventilated acrylic cage containers, with free access to water and food, and maintained in the Animal House Facility of the Stephan Angeloff Institute of Microbiology, BAS. Animal husbandry and experiments were conducted in accordance with the guidelines of Bulgaria’s Directorate of Health Prevention and Humane Behaviour toward Animals.
Compounds
Pleconaril, 3-{3,5-dimethyl-4-[3–(3-methyl-1,2-isoxazol-5-yl)propoxy]phenyl}-5-(trifluoromethyl)-1,2,4-oxadiazole (VP 63843, WIN 63843, Picovir®), was synthesized by Dr Vadim Makarov (State Research Center for Antibiotics, Moscow, Russia). It was dissolved in polyethylene glycol 400 (PEG400).
MDL-860 was obtained from Professor Gerhard Pürstinger (Institute of Pharmacy, University of Innsbruck, Innsbruck, Austria), and an additional amount was synthesized by Dr Vladimir Dimitrov’s team (Institute of Organic Chemistry with Centre of Phytochemistry, Bulgarian Academy of Sciences, Sofia, Bulgaria). The compound was dissolved in PEG400.
Oxoglaucine, {1,2,9,10-tetramethoxy-7h-dibenzo[de,g]quinolin-7-one}, an aporphinoid alkaloid from Glaucium flavum Cranz (yellow horned poppy), was obtained by Dr Stefan Philipov from the Institute of Organic Chemistry with the Centre of Phytochemistry, Bulgarian Academy of Sciences. The compound was dissolved in 1:9 v/v dimethyl sulfoxide/saline.
Coxsackievirus B1 infection in newborn mice: Testing the anti-enteroviral triple combination
Prior to treatment, newborn mice received subcutaneous inoculations of CVB1 at 20 MLD50 (mouse lethal dose). CAA treatment groups received PMO compounds, administered consecutively, starting 1 h post virus inoculation (pvi) (Day 1) and continuing through Day 12. Pleconaril was administered orally, while MDL-860 and oxoglaucine were injected subcutaneously. Each compound was administered every third day, one compound per day, beginning with pleconaril, followed by MDL-860, and ending with oxoglaucine. In addition to the placebo group (received 1/1 v:v PEG400/saline every day), control groups received monotherapies of pleconaril, MDL-860, or oxoglaucine.
Virus samples
In accordance with Stoyanova et al.,10 the following CVB1 mouse brain isolates were selected and prepared as 10% brain suspensions in PBS for a study on in vivo experimental neuroinfection for whole genome (RNA) sequencing analysis: placebo—taken at Day 4 pvi; pleconaril (25 mg/kg) monotherapy—Day 13 pvi; MDL-860 (75 mg/kg) monotherapy—Day 7 pvi; oxoglaucine (25 mg/kg) monotherapy—Day 4 pvi; treatment with CAA course of the triple combination pleconaril (25 mg/kg) + MDL-860 (75 mg/kg) + oxoglaucine (25 mg/kg)—Day 13 pvi. Each sample was prepared by mixing all individual brain samples of animals of the respective test group.
Sequencing
Viral RNA was extracted using High Pure Viral RNA kit (Roche Diagnostics, Meylan, France) according to the manufacturer’s instructions. For entire length genome amplification, two sets of primers were used. The ∼4.1 kb region encoding the viral structural proteins was amplified using primer HT-C004 (5′-GGG AAGCTTT AATA CGACT CACTATAGGGTTAAA ACAGCYYKDGGGTTG) and primer CB1-7075R (5′-AATCCACTCCATCCCTTTGC). The ≈4.3 kb region encoding the viral nonstructural proteins was amplified using primer CB1-3040F (5′-GGAGTCT ACGGGATCAACAC) and primer EVB-polyT (5′-T TTTTTTTTTTTTTTTCCGCACCG). The two overlapping amplicons were synthesized by using One-Step RT-PCR Kit (ref G174, Applied Biological Materials Inc.) as already described.11 The two PCR products were pooled and purified and then sent to the sequencing platform PIBNet (Pasteur International Bioresources Network, Institut Pasteur Paris) where the products were sequenced using Illumina NextSeq HiSeq. Data analysis was performed using CLC Genomics Workbench 8.5 (CLCbio). Then de novo assembly was performed using CLC Main Workbench (CLCbio). Variant frequencies were obtained using ViVan pipeline v 0.43.12
Results
Viral RNA of CVB1 was subjected to next generation sequencing in order to determine and identify possible mutations arising during treatment with antivirals.
The degree of identity of the established whole genome sequences between the placebo sample and antiviral-treated samples was around 99.88%. We found no deletions or insertions but only nucleotide substitutions in the studied samples.
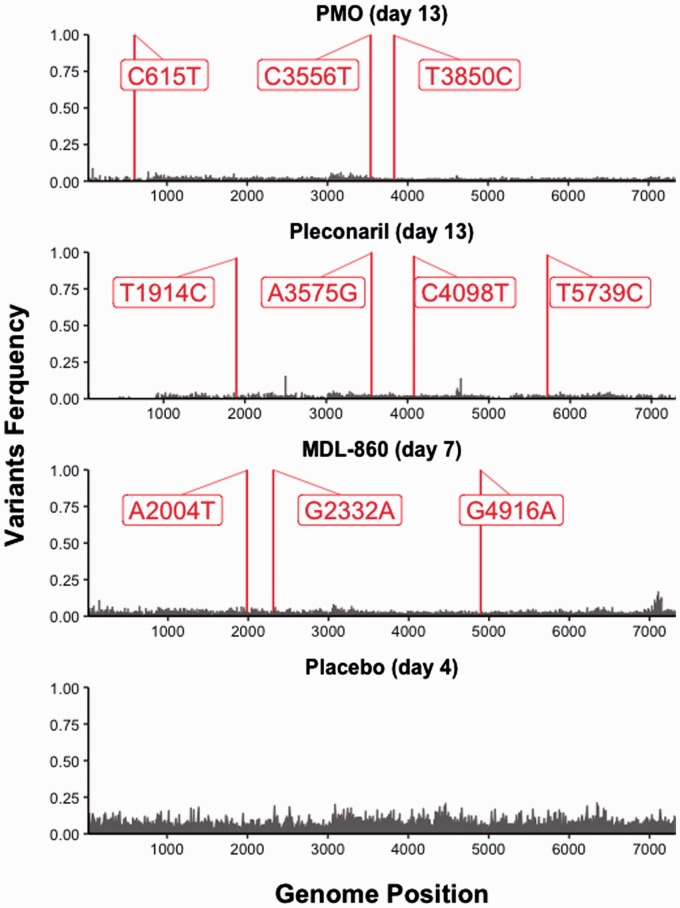
Alignment analyses (nucleotide (NA) and amino acid (AA)) (Table 1) of all virus-containing brain samples were made in comparison to the placebo sample from Day 4 pvi. This sample was used because there were no surviving animals in the placebo group after that day. Major variants (frequency > 0.5) are represented in Figure 1. The figure was generated using R package ggplot2 (R version 3.6.1).
Table 1.
Nucleotide mutations and amino acid substitutions in the studied samples.
|
Region |
5UTR |
VP3 |
VP1 |
2A |
2B |
2C |
3C |
3D |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Position in genome | 109 | 615 | 1914 | 2004 | 2332 | 3040 | 3556 | 3575 | 3850 | 4098 | 4916 | 5739 | 6876 |
| Placebo (Day 4) | T | C | T | A | G | A | C | A | T | C | G | T | C |
| PMO (Day 13) | T | T | T | A | G | A | T | A | C | C | G | T | C |
| P→Sa | |||||||||||||
| Pleconaril (Day 13) | C | C | C | A | G | A | C | G | T | T | G | C | C |
| H→Ra | |||||||||||||
| MDL-860 (Day 7) | T | C | T | T | A | A | C | A | T | C | A | T | C |
| V→Ia | R→Ka | ||||||||||||
| Oxoglaucine (Day 4) | T | C | T | A | G | T | C | A | T | C | G | T | T |
| T→Sa | |||||||||||||
aAmino acid substitution.
PMO: pleconaril/MDL-860/oxoglaucine.
Figure 1.
Major sequence variants obtained via NGS.
NGS: next generation sequencing.
Alignment of the full genome sequences of viruses resistant to pleconaril (25 mg/kg) taken at Day 13 pvi revealed five nucleotide mutations—T109C (5′UTR), T1914C (VP3 region), A3575G (2A region), C4098T (2C region), and T5739C (3C region).
The AA sequences of viruses with placebo (Day 4) and those showing resistance to pleconaril on Day 13 showed one amino acid substitution—H945R (in 2A region). This histidine to arginine substitution located within the 2A protein is a conservative change that replaces an aromatic hydrophilic polar residue with one non-aromatic positively charged amino acid.
Three nucleotide substitutions were found in the MDL-860 Day 7 pvi sample—A2004T (VP3 region), G2332A (VP3 region), and G4916A (2C region)—which led to two amino acid substitutions—V531I (in VP3 region) and R1392K (in 2C). The amino acid substitutions—valine to isoleucine substitution (V531I) located in VP3 protein and arginine to lysine substitution (R1392K) located in 2C protein—were both conservative substitutions. Sample from Day 7 of this monotherapy course was chosen due to the mortality of animals in this group after that day.
The genome analysis of the sample taken at Day 4 pvi of the oxoglaucine monotherapy showed two nucleotide mutations—A3040T (VP1 region) and T6876C (3D region)—and AA registered one substitution—T767S (VP1 region). Day 4 of this sample was chosen due to the mortality of animals in this group after that day.
The NA analysis of the sample treated with the CAA course of PMO and taken on Day 13 sample manifested in three nucleotide substitutions—C615T (5′UTR), C3556T (2A region), and T3850C (2B region); however, AA showed only one amino acid change—P939S (2A region). The proline to serine substitution located within the 2A protein is a non-conservative change that replaces an aromatic hydrophilic polar residue with one non-aromatic positively charged amino acid.
Discussion
Mutation and recombination are well-known phenomena in EV evolution. The infidelity of EV 3D polymerase leads to mutation rates of around one per genome per replication.4,13,14 Mutations in various regions such as 5′UTR, VP1, VP2, 2A, 2C, and 3D of EV have been shown to be associated with alterations in virulence in experimental animal models and in humans.15,16
In this work, we clarify the mechanism of the suppression of drug resistance and the appearance of the drug-increased sensitivity phenomenon during the CAA course of the triple combination of EV replication inhibitors.
Experimental data from the pleconaril monotherapy in the CVB1-infected mice show the development of marked drug resistance after Day 5 pvi, attaining a maximum level at Days 12–13 pvi. The PGO combination using the CAA course prevents this phenomenon.10 Sequence analysis of mouse brain isolates from the monotherapy samples established the appearance of nucleotide substitution in the first loop of the internal ribosomal entry site (IRES) in 5′UTR and amino acid substitution in the 2A region. The 5′UTR of coxsackievirus B1 contains putative stem-loop structures. These structures are essential for viral RNA synthesis and comprise the IRES necessary for translation initiation. The boundaries of the IRES, and the importance of specific sequences within the element, have been mapped for poliovirus; the results indicate that the motif encompasses nt ∼140–631. The maintenance of these structural elements is critical for viral viability.17,18 The viral protein 2A is a cysteine protease that cleaves viral polyprotein and specific host proteins. It is responsible for the cleavage between the P1 and P2 regions, with the first cleavage occurring in the polyprotein. Moreover, it also cleaves the host translation initiation factor EIF4G1 to shut down the capped cellular mRNA translation. Finally, it inhibits the host nucleus–cytoplasm protein and RNA trafficking by cleaving host members of the nuclear pores.
In addition, analyses of Day 13 pleconaril monotherapy samples found the appearance of mutation in the 5′-UTR IRES within the treatment course. These changes could be related to the development of drug resistance. The well-known changes in VP1 protein in the WIN compounds-resistant mutants of CVB1 (Nikolova et al., 2011) were not observed. One of the aims of this study was to determine the prevalent mutations related to the viral phenotype of interest by sequencing directly brain homogenates. However, this approach conceals the low-rate mutations, and we believe that this is the reason for the lack of the characteristic mutations in the pleconaril resistant progeny.
The well-manifested development of drug resistance in the course of MDL-860 monotherapy could be linked to the registered mutations in VP3 and 2C regions. Protein 2C displays RNA-binding, nucleotide-binding, and NTPase activities. Although the mutation found in the 2C region is silent, we think it may play a role in virion morphogenesis and viral RNA encapsidation by interacting with the capsid protein VP3.5,19–21
When following the influence of the PMO CAA course, we found (1) nucleotide mutation in the sixth loop of 5′-UTR IRES; (2) mutation in the 2A region with one amino acid substitution; (3) mutations with a lack of the amino acid substitution in VP3, 2A, and 2C, which were present in the samples of the monotherapy courses. These mutations led to hampering of the development of resistance to each of the antivirals included in the combination. In addition, they could explain the phenomenon of the increased drug susceptibility observed in the CAA course with triple combination PMO against experimental CVB1 infection in vivo. A significant role in this phenomenon could be played by either the mutation localized in the sixth loop of 5′-UTR IRES, which may disturb the functioning of EV replicative complexes, or the change in the properties of the 2A protein due to the amino acid substitutions of aromatic hydrophilic polar residue with one non-aromatic positively charged amino acid.
The observed phenotypic and genotypic mutations led to CVB1 drug resistance in the cases of monotherapy (pleconaril, MDL-860, and oxoglaucine) and to the virus’s higher sensitivity to these substances when included in the CAA treatment.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Marie-Line Joffret https://orcid.org/0000-0002-5244-5429
Angel S Galabov https://orcid.org/0000-0001-8554-1655
References
- 1.Lin J-Y, Chen T-C, Weng K-Fet al. Viral and host proteins involved in picornavirus life cycle. J Biomed Sci 2009; 16: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yip CCY, Lau SKP, Woo PCYet al. Human enterovirus 71 epidemics: What’s next? Emerg Health Threats J 2013; 6. DOI: 10.3402/ehtj.v6i0.19780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ao D, Sun SQ, Guo HC. Topology and biological function of enterovirus non-structural protein 2B as a member of the viroporin family. Vet Res 2014; 45: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nidaira M, Kuba Y, Saitoh Met al. Molecular evolution of VP3, VP1, 3C(pro) and 3D(pol) coding regions in coxsackievirus group A type 24 variant isolates from acute hemorrhagic conjunctivitis in 2011 in Okinawa, Japan. Microbiol Immunol 2014; 58: 227–238. [DOI] [PubMed] [Google Scholar]
- 5.de Jong AS, Schrama IW, Willems PHet al. Multimerization reactions of coxsackievirus proteins 2B, 2C and 2BC: A mammalian two-hybrid analysis. J Gen Virol 2002; 83: 783–793. [DOI] [PubMed] [Google Scholar]
- 6.Vassileva-Pencheva R, Galabov AS. Avoiding drug-resistance development by novel approach of combining anti-enteroviral substances against coxsackievirus B1 infection in mice. Antiviral Res 2010; 85: 366–372. [DOI] [PubMed] [Google Scholar]
- 7.Galabov AS, Nikolaeva-Glomb L, Nikolova Iet al. Perspectives for effective chemotherapy of enterovirus infections In: Najdenski H, Angelova M, Stoitsova S. (eds) New trends of microbiology (65th Anniversary of the Stephan Angeloff Institute of Microbiology). Sofia: The Stephan Angeloff Institute of Microbiology, 2012, pp.47–81. [Google Scholar]
- 8.Galabov AS, Nikolova I, Vassileva-Pencheva Ret al. Antiviral combination approach: A perspective to combat enterovirus infections. Prilozi/Contributions 2015; 26: 91–99. [DOI] [PubMed] [Google Scholar]
- 9.Stoyanova A, Nikolova I, Galabov AS. Effect of consecutive alternative administration (CAA) of a triple anti-enteroviral combination on Coxsackievirus B1 neuroinfection in mice. Antiviral Res 2015; 121: 138–144. [DOI] [PubMed] [Google Scholar]
- 10.Stoyanova A, Nikolova I, Pürstinger Get al. Anti-enteroviral triple combination of viral replication inhibitors: Activity on Coxsackievirus B1 induced neuroinfection in mice. Antivir Chem Chemother 2016; DOI: 10.1177/2040206616671571. [DOI] [PMC free article] [PubMed]
- 11.Joffret ML, Polston PM, Razafindratsimandresy Ret al. Whole genome sequencing of enteroviruses species A to D by high-throughput sequencing: Application for viral mixture. Front Microbiol 2018; 9: 2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isakov O, Bordería A, Golan Det al. Deep sequencing analysis of viral infection and evolution allows rapid and detailed characterization of viral mutant spectrum. Bioinformatics 2015; 31: 2141–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agol VI, Pilipenko EV, Slobodskaya OR. Modification of translation control elements as a new approach to design of attenuated picornavirus strains. J Biotechnol 1996; 44: 119–128. [DOI] [PubMed] [Google Scholar]
- 14.Wang H-Y, Tsao K-C, Hsieh C-Het al. Inferring nonneutral evolution from contrasting patterns of polymorphisms and divergences in different protein coding regions of enterovirus 71 circulating in Taiwan during 1998-2003. BMC Evol Biol 2010; 10: 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cordey S, Petty TJ, Schibler Met al. Identification of site-specific adaptations conferring increased neural cell tropism during human enterovirus 71 infection. PLoS Pathog 2012; 8: e1002826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang T, Yu B, Lin Let al. A functional nuclear localization sequence in the VP1 capsid protein of coxsackievirus B3. Virology 2012; 433: 513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao C, Bator-Kelly CM, Rieder Eet al. The crystal structure of coxsackievirus A21 and its interaction with ICAM-1. Structure 2005; 13: 1019–1033. [DOI] [PubMed] [Google Scholar]
- 18.Hunziker IP, Cornell CT, Whitton JL. Deletions within the 5’UTR of coxsackievirus B3: Consequences for virus translation and replication. Virology 2007; 360: 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burns C, Shaw J, Campagnoli Ret al. Modulation of poliovirus replicative fitness in HeLa cells by deoptimization of synonymous codon usage in the capsid region. J Virol 2006; 80: 3259–3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burns C, Campagnoli R, Shaw Jet al. Genetic inactivation of poliovirus infectivity by increasing the frequencies of CpG and UpA dinucleotides within and across synonymous capsid region codons. J Virol 2009; 83: 9957–9969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asare E, Mugavero J, Jiang Pet al. A single amino acid substitution in poliovirus nonstructural protein 2CATPase causes conditional defects in encapsidation and uncoating. J Virol 2016; 90: 6174–6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kato S, Tsutsumi R, Sato S. Nucleotide sequence of the 5’nontranslated and virion polypeptides regions of coxsackievirus B6. Microbiol Immunol 1999; 43: 871–883. [DOI] [PubMed] [Google Scholar]
- 23.Yang CH, Li HC, Jiang JGet al. Enterovirus type 71 2A protease functions as a transcriptional activator in yeast. J Biomed Sci 2010; 17: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nikolova I, Petkova R, Galabov ASet al. Disoxaril mutants of Coxsackievirus B1: Phenotypic characteristics and analysis of the target VP1 gene.- Z. Z Naturforsch, C, J Biosci 2011; 66c: 627–636. [DOI] [PubMed] [Google Scholar]