Abstract
Background:
Angiopoietin-2 (Ang-2), as one of the ligands of endothelial receptor Tie2, is known to be significant for vessel maturation and stabilization after birth. Previous studies showed the relationship between Ang-2 level and the risk of mortality in patients with acute respiratory distress syndrome (ARDS). However, the link between circulating Ang-2 and the risk of mortality in patients with ARDS varied in different investigations.
Results:
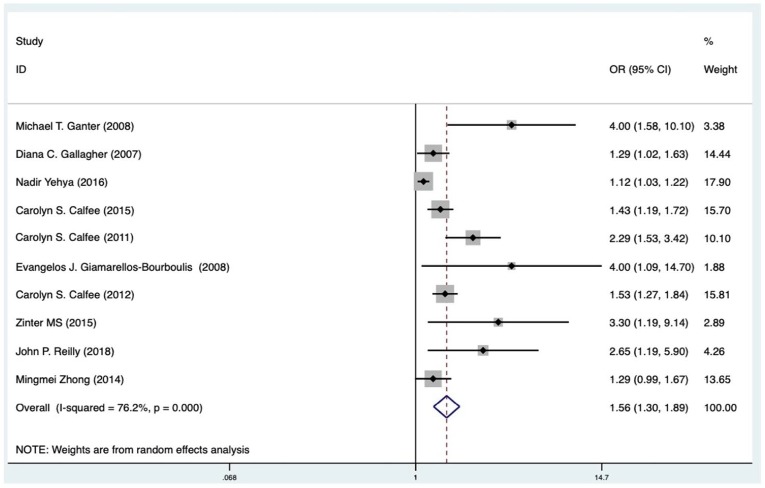
We performed a systematic review and meta-analysis of all available cohort studies regarding the association between baseline circulating Ang-2 and mortality in patients with ARDS. Among the 10 eligible studies, pooled odds ratio (OR) showed that high Ang-2 level contributed to ARDS mortality [OR = 1.56, 95% confidence interval (CI): 1.30–1.89, I2 = 76.2%]. Stratified analysis revealed that higher circulating Ang-2 was related to a 30% higher risk in the high-quality scores group (OR = 1.68, 95% CI: 1.33–2.68, I2 = 62.4%). The I2 of the bad compliance group decreased from 76.2% to 8.5%, which suggested that compliance is a significant source of heterogeneity. This association may be blunted by potential bias, although the results was not meaningfully changed by omitting only one study at a time. Further subgroup analysis and meta-regression support that compliance of patients also affects the results significantly, compared with the publication year, follow-up duration, the samples, or population characteristics.
Conclusion:
Participants with higher baseline Ang-2 were at a higher risk for future risk of mortality in patients with ARDS. Higher circulating Ang-2 levels could independently predict the risk of mortality in patients with ARDS. However, further large scale prospective cohorts or even interventional studies are warranted to evaluate the diagnostic power of Ang-2 and its causative role on ARDS outcome.
The reviews of this paper are available via the supplemental material section.
Keywords: acute respiratory distress syndrome, angiopoietin-2, meta-analysis, mortality
Introduction
The endothelial receptors are known to be critical in vessel maturation and stabilization after birth, which are risk factors for many kinds of respiratory diseases.1 Angiopoietin is one of the members of the vascular growth factor family, which contributes to embryonic and postnatal angiogenesis. Angiopoietin signaling directly interacts with angiogenesis, the process involved in the formation of new arteries and veins.2 It is responsible for disassembling and assembling the endothelial lining of blood vessels. Angiopoietin-2 (Ang-2), a ligand of the endothelial receptor Tie2, blocks Tie2 phosphorylation3 and contributes to vessel destabilization and proinflammatory states.4,5 Acute respiratory distress syndrome (ARDS) is induced by pathological permeability of the epithelium and pulmonary endothelium, which leads to intra-alveolar edema rich in inflammatory mediators and leukocytes.5–7 Patients manifest severe oxygenation failure and display a high mortality clinically. They progress to death in approximately 20–40% of cases.8–10 Notably, effects of endothelial receptors and changes in Ang-2 levels have been involved in the underlying mechanisms contributing to the link between circulating Ang-2 and the risk of mortality in patients with ARDS.11,12 Indeed, an early prospective cohort study showed that increased plasma Ang-2 levels independently predicts mortality in over 900 patients with acute lung injury (ALI) or ARDS.13 Subsequent studies showed that selective temporal blockade of Ang-2 function greatly improved PaO2/FiO2, decreased lung protein leak and indices of inflammation, and finally improved overall survival in murine models of ARDS.12 Although a large number of cohort studies in patients with ARDS or ALI further confirmed the prognostic role of circulating Ang-2 levels for ARDS outcome,14,15 some studies failed to display a specific association between Ang-2 level and ARDS prognosis.15,16 Therefore, in this study, we performed a systematic review and meta-analysis of all available studies to quantitatively evaluate the association between baseline circulating Ang-2 and the risk of mortality in patients with ARDS.
Methods
Search strategy
PubMed, Embase, as well as Cochrane library databases were searched from the inception up to 31 March 2019 for relevant studies without language restriction. Methods used are consistent with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement for reporting systematic reviews and the meta-analysis of observational studies in epidemiology (MOOSE) guidelines.17 Supplementary materials display the PRISMA checklist. The search terms used were as follows: PubMed: (((((((“Acute Lung Injury”[Mesh]) OR Acute Lung Injuries[Title/Abstract]) OR Lung Injuries, Acute[Title/Abstract]) OR Lung Injury, Acute[Title/Abstract])) OR (((((((((“Respiratory Distress Syndrome, Adult”[Mesh]) OR Shock Lung[Title/Abstract]) OR Lung, Shock[Title/Abstract]) OR ARDS, Human[Title/Abstract]) OR ARDSs, Human[Title/Abstract]) OR Human ARDS[Title/Abstract]) OR Respiratory Distress Syndrome, Acute[Title/Abstract]) OR Acute Respiratory Distress Syndrome[Title/Abstract]) OR Adult Respiratory Distress Syndrome[Title/Abstract]))) AND ((“Angiopoietin-2”[Mesh]) OR Angiopoietin 2[Title/Abstract]). Embase: (‘adult respiratory distress syndrome’/exp OR ‘acute lung injury’/exp OR ‘acute lung injuries’: ab,ti OR ‘lung injuries, acute’:ab,ti OR ‘lung injury, acute’:ab,ti) AND (‘angiopoietin 2’/exp OR ‘angiopoietin 2’:ab,ti). To identify additional eligible studies, we also screened the references of included papers and published meta-analyses.
Selection criteria
Criteria of the current systematic review and meta-analysis were as follows: (a) Investigations conducted in human; (b) serum or plasma Ang-2 level was measured at baseline; (c) mortality in patients with ARDS were displayed as the end-points; (d) Odds ratios (OR) with confidence intervals (CI) were shown or estimated for the association between baseline Ang-2 level and mortality in patients with ARDS. Patients were diagnosed according to the American–European Consensus Conference diagnostic criteria for ARDS or the Berlin Definition of ARDS. Acute lung injury was defined with the same criteria except for patients with PaO2/FiO2 less than 300. We eliminated studies that are animal experiments, case reports, narrative reviews, or present insufficient data for pooling. Two independent reviewers performed the database searching and subsequent review of the literature. Consultation was also launched with a senior investigator to resolve any remaining discrepancies.
Data extraction
Country of origin, surname of the first author, sample size, year of publication, research design, patients’ diseases, gender, age, follow-up period, serum or plasma Ang-2, time-points of Ang-2 measurement, disease outcome, adjustment variables, and ORs with their corresponding 95% CIs were extracted for each potentially included study. The outcome assessed in the meta-analysis was the risk of mortality in patients with ARDS. The data extraction was conducted by two independent investigators via a predesigned data extraction form. Divergences were resolved by consensus or by consulting a senior investigator.
Quality evaluation
The risk of bias for included studies based on study group was evaluated via the Newcastle–Ottawa Scale (NOS),18 including group comparability and ascertainment of exposure or outcome. Quality of evidence in GRADE system was assessed via Grade Pro 4.04 (designed by Grade Working Group) and the results are displayed in Supplementary Table S2. Two reviewers independently evaluated the quality of each study.
Statistical analysis
ORs and their corresponding CIs were used to evaluate the risk of mortality in patients with ARDS with different levels of Ang-2. The definitions of quartiles of baseline Ang-2 were in line with the original definition in the literature. Heterogeneity among studies was assessed via the I2 statistic, Cochran’s Q test and p value. When subgroup analysis was performed, although some groups had less than five studies, a random-effects model was applied to all meta-analyses for which both fixed-effect and random-effects models were used to calculate pooled ORs. A fixed-effect model was also used when heterogeneity was absent (I2 = 0%). Potential sources of heterogeneity were explored via stratified analysis. A meta-regression model fitted with covariables including country, publishing year, population, follow-up days, samples, design, adjusted covariate, grade scores, and clinical compliance were analyzed to explore potential sources of heterogeneity. In sensitivity analysis, we assessed the influence of each single study on overall estimates. To assess the influence of new studies on overall effects, cumulative meta-analysis according to the year of publication for individual studies was performed. Selective reporting as well as publication bias were examined by visual inspection of the asymmetry of the funnel plot, in which the standard error of log OR was plotted against OR. Begg’s and Egger’s linear regression tests were performed to determine statistical significance.19 The trim-and-fill approach was utilized to determine the number of additional studies required to overcome potential bias and provide adjusted effects. All statistical analyses were conducted with STATA software, version 15.1 (StataCorp LP, College Station, TX, USA). A two-sided p value < 0.05 was considered statistically significant.
Results
Literature search
In total, 278 records from Embase, PubMed, as well as Cochrane Library databases were retrieved. After removing 93 duplicates, the remaining 185 records had their titles and abstracts examined further. Among these papers, 144 were not associated with the theme and 19 were not clinical studies. Full-text review further excluded 12 articles, yielding a sum of 10 studies for the meta-analysis.
Of these studies, one study drew a conclusion about the relationship between Ang-2 and mortality in patients with ARDS but did not provide OR.15 Six studies did not provide ORs with 95% CI.20–25 Three studies were from the same database.26–28 In addition, another three investigations did not follow the clinical rules.29–31 A flow chart describing the process of study selection is displayed in Figure 1.
Figure 1.
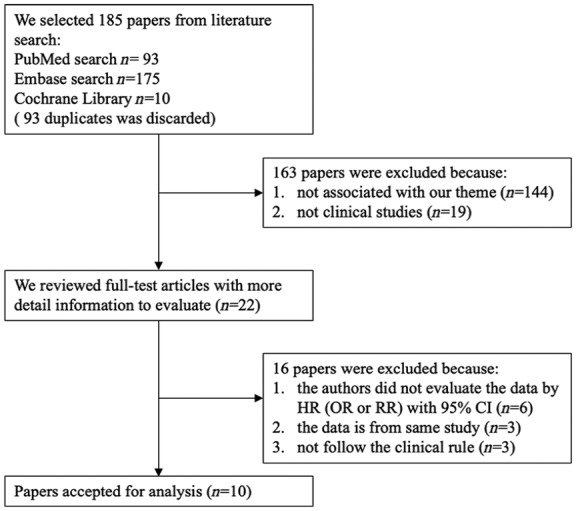
Flowchart of database search and study identification.
CI, confidence interval; HR, hazard ratio; OR, odds ratio; RR, risk ratio.
Study characteristics
The detailed characteristics of the 10 prospective studies published from 2007 to 2018 are shown in Table 1. Among these studies, seven were conducted in the United States,13,16,32–36 while others were performed in United Kingdom,37 Greece,38 and China.39 Follow-up durations of these studies ranged from 15 to 100 days. Children16,34 and adults13,32,33,35–39 were both included in this study. Generally, the size of study cohorts varied from 53 to 931 participants, with a total of 3723 subjects included in the final meta-analysis. The outcome reported from these studies was mortality in patients with ARDS. All included studies were displayed as low to high quality, as indicated by individual NOS scores ranging from 4–8.
Table 1.
Characteristics of included studies.
| Author | Country | Population | Follow-up days |
Male (%) | Age | Assay | Sample | Design | Patients status |
Quality score |
|---|---|---|---|---|---|---|---|---|---|---|
| Calfee and colleagues13 | USA | Adult | 90 | 53.0 | 50 ± 16 | ELISA | Plasma | PCS | Non-severity | 4 |
| Yehya and colleagues16 | USA | Children | 28 | 50.0 | 4.1 (0.6–13) | ELISA | Plasma | RCT | Non-severity | 4 |
| Gallagher and colleagues32 | USA | Adult | 60 | 48.0 | 67 ± 17 | ELISA | Plasma | PCS | Severity | 6 |
| Calfee and colleagues33 | USA | Adult | 90 | 52.0 | 51 ± 16 | ELISA | Plasma | RCT | Severity | 5 |
| Zinter and colleagues34 | USA | Children | 100 | 55.4 | 5.2 (1.1–13.2) | ELISA | Plasma | RCT | Severity | 7 |
| Reilly and colleagues35 | USA | Adult | 30 | 58.8 | 60 (51–69) | ELISA | Plasma | RCT | Severity | 7 |
| Calfee and colleagues36 | USA | Adult | 90 | N/A | N/A | N/A | Plasma | RCT | Severity | 5 |
| Ganter and colleagues37 | UK | Adult | 28 | 75.0 | 41 (27–63) | ELISA | Plasma | RCT | Severity | 8 |
| Giamarellos-Bourboulis and colleagues38 | Greece | Adult | 15 | N/A | N/A | ELISA | Serum | RCT | Non-severity | 4 |
| Zhong and colleagues39 | China | Adult | 28 | 66.0 | 59.2 ± 17.4 | ELISA | Plasma | PCS | Severity | 5 |
ELISA, enzyme-linked immunosorbent assay; PCS, prospective cohort study; RCT, randomized controlled trial; UK, United Kingdom; USA, United States.
Ang-2 and mortality in patients with ARDS
All ten included studies, which included 3723 participants, reported the potential association between circulating blood Ang-2 and risk of mortality in patients with ARDS.13,21,32–35,37–39 Meta-analysis showed that elevated blood levels of Ang-2 were independently associated with increased mortality in patients with ARDS (OR 1.56, 95% CI: 1.30–1.89). However, a considerable heterogeneity was noticed among different studies (I2 = 76.2%, p < 0.001; Figure 2). Subsequently, we performed a subgroup analysis to evaluate the potential effects of country, publishing year, population, follow-up days, samples, design, adjusted covariate, quality scores, and clinical compliance on the association between baseline Ang-2 and mortality in patients with ARDS. We found that higher Ang-2 levels were associated with an increase of overall mortality in high-quality papers (OR = 1.68, 95% CI: 1.33–2.13, I2 = 62.4%) (Supplementary Figure S4A). Studies of patients with high or low compliance both showed that the I2 of the bad compliance group decreased from 76.2% to 8.5%, which suggested that compliance is a significant source of heterogeneity (Supplementary Figure S4B). For sample subgroups, fixed-effect model was used to estimate pooled ORs (serum: OR = 4.00, 95% CI: 1.09–14.70; plasma: OR = 1.49, 95% CI: 1.24– 1.79). Furthermore, pooled hazard ratios from studies performed in the other countries (OR = 1.51, 95% CI: 1.24–1.85) were not statistically significant, while those in the United States showed a 50% higher risk of mortality in patients with increased Ang-2 levels (OR = 2.41, 95% CI: 0.97– 5.99). Additional subgroup analyses showed similar increases of mortality risk in studies with different follow-up days (follow-up ⩽30 days: OR = 1.65, 95% CI: 1.14–2.39, I2 = 74.5%; follow-up >30 days: OR = 1.55, 95% CI: 1.30–1.86, I2 = 52.9%). As all included studies have been published within 10 years in this meta-analysis, we checked if the year of publication affected the pooled OR by cumulative analysis (Supplementary Figure S5). The association of baseline Ang-2 and mortality risk remained after the addition of more recent studies, which was further confirmed by the results of meta-regression for publication year (p = 0.187). Fitting other variables, including country, publishing year, population, follow-up days, samples, design, adjusted covariate, quality scores, and clinical compliance into the meta-regression model did not indicate additional sources of heterogeneity (Table 2). Results of sensitivity analysis did not support a certain study impacting the overall outcome significantly (Supplementary Figure S6). According to the results, no individual study was found responsible for the observed heterogeneity.
Figure 2.
Forest plot (random-effects model) for the association between primary Angiopoietin-2 (Ang-2) level or increased Ang-2 level and acute respiratory distress syndrome associated mortality: overall meta-analysis results.
CI, confidence interval; OR, odds ratio.
Table 2.
Stratified analysis of pooled odds ratio risks of ARDS associated mortality.
| Stratified analysis | Pooled HR (95% CI) | Heterogeneity | Meta regression (p-value) |
|---|---|---|---|
| Country | 0.501 | ||
| Non-USA | 2.41 (0.97–5.99) | Q = 7.74, p = 0.021, I2 = 74.2% | |
| USA | 1.51 (1.24–1.85) | Q = 28.87, p < 0.001, I2 = 76.2% | |
| Follow-up days | 0.782 | ||
| ⩽30 | 1.65 (1.14–2.39) | Q = 15.69, p = 0.003, I2 = 74.5% | |
| >30 | 1.55 (1.30–1.86) | Q = 8.48, p = 0.075, I2 = 52.9% | |
| Publishing year | 0.187 | ||
| Before 2011 | 2.20 (1.28–3.79) | Q = 12.00, p = 0.007, I2 = 75.0% | |
| After 2011 | 1.41 (1.16–1.71) | Q = 19.93, p < 0.001, I2 = 74.9% | |
| Population | 0.044 | ||
| ARDS/ALI | 1.60 (1.33–1.90) | Q = 15.91, p = 0.026, I2 = 56.0% | |
| ARDS/ALI (children) | 1.70 (0.61–4.76) | Q = 4.30, p = 0.038, I2 = 76.7% | |
| Samples | 0.193 | ||
| Plasma | 1.49 (1.24–1.79) | Q = 31.56, p < 0.001, I2 = 77.8% | |
| Serum | 4.00 (1.09–14.70) | — | |
| Adjustment | 0.495 | ||
| Adjusted | 1.89 (1.20–3.20) | Q = 7.65, p = 0.022, I2 = 73.9% | |
| Non-Adjusted | 1.48 (1.20–1.81) | Q = 25.69, p < 0.001, I2 = 76.6% | |
| Quality score | 0.360 | ||
| >4 | 1.68 (1.33–2.13) | Q = 15.95, p = 0.014, I2 = 62.4% | |
| ⩽4 | 1.38 (1.00–1.92) | Q = 12.58, p = 0.002, I2 = 84.1% | |
| Compliance of patients | 0.323 | ||
| Good | 1.89 (1.33–2.68) | Q = 32.43, p < 0.001, I2 = 84.6% | |
| Bad | 1.38 (1.20–1.58) | Q = 3.28, p = 0.351, I2 = 8.5% | |
| Study design | 0.198 | ||
| RCT | 1.92 (1.38–2.66) | Q = 32.96, p < 0.001, I2 = 81.8% | |
| PCS | 1.40 (1.23–1.59) | Q = 1.77, p = 0.412, I2 = 0 | |
ALI, acute lung injury; ARDS, acute respiratory distress syndrome; HR, hazard ratio; PCS, prospective cohort study; RCT, randomized controlled trial; USA, United States.
The funnel plot showed obvious asymmetry, indicating the presence of publication bias (Supplementary Figure S1). Application of a trim-and-fill model suggested that three more studies may be required to eliminate the publication bias, although the pooled effect was markedly different (Supplementary Figure S2A and S2B). Nonetheless, statistics using the Egger’s (p = 0.599) and Begg’s (p = 0.371) regression tests did not show a significant bias in publication (Supplementary Figure S3A and S3B). We also used the standard ‘risk of bias’ tool and NOS to evaluate bias (Supplementary Figure S7A andS7B; Table 1 and Supplementary Table S3).
Discussion
In this study, by pooling the data from 10 available prospective cohort studies incorporating 3723 participants, we found that higher circulating Ang-2 at baseline was independently associated with a 56% increase in the risk of mortality in patients with ARDS. Although significant heterogeneity was detected, subsequent subgroup and meta-analyses found that the quality scores of papers may be responsible for the heterogeneity. Characteristics of country, publishing year, population, follow-up days, samples, design, adjusted covariate, and clinical compliance had few significant effects on the association between Ang-2 and the risk of mortality in patients with ARDS. These results suggest that participants with higher baseline Ang-2 were at a higher risk of future risk of mortality in patients with ARDS. Future studies are needed to determine if circulating Ang-2 confers prognostic efficacy for ARDS.
The function of endothelial cells (ECs) is mediated by the competitive binding of EC growth factors during angiogenesis. Ang-2, a ligand of endothelial receptor Tie2, is expressed on ECs primarily.40,41 Ang-1, produced by pericytes, smooth muscle cells, as well as fibroblasts, is found on the extracellular matrix. Ang-1/Tie2 binding is thought to be anti-inflammatory and promote vessel stabilization, prosurvival and antipermeability signaling through phosphorylation of Tie2.42 In contrast, activated ECs rapidly release stored preformed Ang-2 in an autocrine response. Ang-2/Tie2 binding is believed to induce vessel destabilization, pulmonary leakage and inflammation.43,44 Previous clinical investigations have reported elevated EC growth factor, Ang-2, in the peripheral blood from ARDS patients.26,37,45 The shock/sepsis model for the development of ARDS demonstrated effective reduction in indices of inflammation and lung tissue injury following small interfering RNA inhibition of Ang-2 protein synthesis.45 While this method is useful in identifying potential target proteins for therapeutic intervention, similar usage is not currently feasible for treating ARDS in the human patient population.
Nevertheless, the results on the link between Ang-2 level and risk of mortality of patients with ARDS remain limited and inconsistent. For instance, Ang-2 exhibited no significant predictive value on mortality in an observational cohort including 931 patients with ARDS.13 A similar result was obtained from a more recent US study in patients with ARDS.16 More intriguingly, inhibition of Ang-2 confers significant protection against ARDS rather than predisposing to deleterious outcome.45 To the best of our knowledge, ours is the first systematic review and meta-analysis exploring the prognostic value of Ang-2 in ARDS that has been published so far. Results of our study further confirmed that higher Ang-2 at baseline was associated with an increased risk of mortality in patients with ARDS. Moreover, our subsequent subgroup analyses found that the vast disagreement among studies may attribute to various aspects, including quality scores (>4 and ⩽4) and compliance of patients (good and bad). Good management of patients may be found in studies with a rigorous design. Also, patients are prone to obey the management in large medical centers. Compliance in our meta-analysis is a comprehensive assessment based on the previous factors. What’s more, only studies conducted in the US exhibited a significant overall OR for the high Ang-2 effect on mortality. In spite of this, studies focused on children did not suggest a correlation between increased Ang-2 and a higher risk of mortality in patients with ARDS with statistical significance. Therefore, the result remains uncertain given the limited number of studies, and further studies with larger sample sizes are needed to confirm our results.
Strength and limitations
Our study demonstrated the potential prognostic power of Ang-2 in a variety of populations with ARDS. To our knowledge, this is the first meta-analysis that shows a positive correlation between a high Ang-2 level and an adverse ARDS outcome. In this study, we included only prospective cohorts and followed the PRISMA and MOOSE guidelines. Potential sources of heterogeneity were explored using different methods, and trim-and-fill strategy was applied to solve the possibility of publication bias. All of the previous aspects added to the power of the study.
Our study also has potential limitations, which should be considered when interpreting the results. First, studies that did not provide sufficient data for pooling were excluded from the meta-analysis, which may raise the risk of bias in the overall effects by Ang-2. A considerable risk of publication bias was identified for the association between high Ang-2 and mortality in patients with ARDS, leading to a dubitative conclusion on the relationship. Second, considerable heterogeneities existed in baseline clinical characteristics including age, gender, and race across individual studies. In addition, uncontrolled confounding factors including basic diseases and genetic variation may significantly affect the concentration of Ang-2. Inclusion of those studies without comprehensive adjustment may yield inaccurate effects. Third, the lack of continuous data made it difficult to draw a quantitative result on the differences of Ang-2 between subjects with and without events. Finally, results of our meta-analysis of observational studies could only provide the potential temporal association between increased circulating Ang-2 and the subsequent risk of mortality in patients with ARDS. Whether increased Ang-2 was causative to a poor ARDS outcome deserves further investigation.
Conclusion
Participants with higher baseline Ang-2 were at a higher risk for future risk of mortality in patients with ARDS. Further large scale prospective cohorts or even interventional studies are warranted to evaluate the diagnostic power of Ang-2 and its causative role on ARDS outcome.
Supplemental Material
Supplemental material, Author_response_1 for Circulating angiopoietin-2 and the risk of mortality in patients with acute respiratory distress syndrome: a systematic review and meta-analysis of 10 prospective cohort studies by Fengyuan Li, Rulan Yin and Qiang Guo in Therapeutic Advances in Respiratory Disease
Supplemental material, PRISMA-2009-Checklist-MS-Word for Circulating angiopoietin-2 and the risk of mortality in patients with acute respiratory distress syndrome: a systematic review and meta-analysis of 10 prospective cohort studies by Fengyuan Li, Rulan Yin and Qiang Guo in Therapeutic Advances in Respiratory Disease
Supplemental material, PRISMA-2009-Flow-Diagram-MS-Word for Circulating angiopoietin-2 and the risk of mortality in patients with acute respiratory distress syndrome: a systematic review and meta-analysis of 10 prospective cohort studies by Fengyuan Li, Rulan Yin and Qiang Guo in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Circulating angiopoietin-2 and the risk of mortality in patients with acute respiratory distress syndrome: a systematic review and meta-analysis of 10 prospective cohort studies by Fengyuan Li, Rulan Yin and Qiang Guo in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Circulating angiopoietin-2 and the risk of mortality in patients with acute respiratory distress syndrome: a systematic review and meta-analysis of 10 prospective cohort studies by Fengyuan Li, Rulan Yin and Qiang Guo in Therapeutic Advances in Respiratory Disease
Supplemental material, Supplement_Materials_1 for Circulating angiopoietin-2 and the risk of mortality in patients with acute respiratory distress syndrome: a systematic review and meta-analysis of 10 prospective cohort studies by Fengyuan Li, Rulan Yin and Qiang Guo in Therapeutic Advances in Respiratory Disease
Acknowledgments
FY Li and Q Guo conceived and designed the study. FY Li analyzed the data and wrote the paper; RL Yin analyzed the data; Q Guo reviewed the article. All authors read and approved the manuscript.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from Six talent peaks project in Jiangsu Province (WSN021). The 13th Five-Year Jiangsu Province Health, Science and Technology Key Project (ZDRCA2016046). Jiangsu Health Planning Committee of Jiangsu project (H201618). Key Health Talents in Gusu (GSWS2019009).
Conflict of interest statement: The authors declare that there is no conflict of interest.
Ethics approval and consent to participate: Not applicable.
Patient consent for publication: Not applicable.
ORCID iD: Qiang Guo  https://orcid.org/0000-0002-8853-9822
https://orcid.org/0000-0002-8853-9822
Availability of data and materials: All data generated or analyzed during this study are included in this published article.
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Fengyuan Li, Department of Critical Care Medicine, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, China.
Rulan Yin, Department of Nursing, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, China.
Qiang Guo, Department of Critical Care Medicine, The First Affiliated Hospital of Soochow University, Suzhou, 899# Pinghai Road, Suzhou, Jiangsu, 215006, China.
References
- 1. Suri C, Jones PF, Patan S, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 1996; 87: 1171–1180. [DOI] [PubMed] [Google Scholar]
- 2. Alves BE, Montalvao SA, Aranha FJ, et al. Imbalances in serum angiopoietin concentrations are early predictors of septic shock development in patients with post chemotherapy febrile neutropenia. BMC Infect Dis 2010; 10: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Daly C, Pasnikowski E, Burova E, et al. Angiopoietin-2 functions as an autocrine protective factor in stressed endothelial cells. Proc Natl Acad Sci U S A 2006; 103: 15491–15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kumpers P, Lukasz A, David S, et al. Excess circulating angiopoietin-2 is a strong predictor of mortality in critically ill medical patients. Crit Care 2008; 12: R147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roviezzo F, Tsigkos S, Kotanidou A, et al. Angiopoietin-2 causes inflammation in vivo by promoting vascular leakage. J Pharmacol Exp Ther 2005; 314: 738–744. [DOI] [PubMed] [Google Scholar]
- 6. Maniatis NA, Kotanidou A, Catravas JD, et al. Endothelial pathomechanisms in acute lung injury. Vascul Pharmacol 2008; 49: 119–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest 2012; 122: 2731–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Matthay MA. Resolution of pulmonary edema. Thirty years of progress. Am J Respir Crit Care Med 2014; 189: 1301–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matthay MA. Alveolar fluid clearance in patients with ARDS: does it make a difference? Chest 2002; 122: 340S–343S. [DOI] [PubMed] [Google Scholar]
- 10. Pediatric Acute Lung Injury Consensus Conference Group. Pediatric acute respiratory distress syndrome: consensus recommendations from the pediatric acute lung injury consensus conference. Pediatr Crit Care Med 2015; 16: 428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Araujo CB, de Oliveira Neves FM, de Freitas DF, et al. Angiopoietin-2 as a predictor of acute kidney injury in critically ill patients and association with ARDS. Respirology 2019; 24: 345–351. [DOI] [PubMed] [Google Scholar]
- 12. Lomas-Neira JL, Heffernan DS, Ayala A, et al. Blockade of endothelial growth factor, angiopoietin-2, reduces indices of ARDS and mortality in mice resulting from the dual-insults of hemorrhagic shock and sepsis. Shock 2016; 45: 157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Calfee CS, Gallagher D, Abbott J, et al. Plasma angiopoietin-2 in clinical acute lung injury: prognostic and pathogenetic significance. Crit Care Med 2012; 40: 1731–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Choi JS, Kwak KA, Park MJ, et al. Ratio of angiopoietin-2 to angiopoietin-1 predicts mortality in acute lung injury induced by paraquat. Med Sci Monit 2013; 19: 28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ando M, Miyazaki E, Abe T, et al. Angiopoietin-2 expression in patients with an acute exacerbation of idiopathic interstitial pneumonias. Respir Med 2016; 117: 27–32. [DOI] [PubMed] [Google Scholar]
- 16. Yehya N, Thomas NJ, Meyer NJ, et al. Circulating markers of endothelial and alveolar epithelial dysfunction are associated with mortality in pediatric acute respiratory distress syndrome. Intensive Care Med 2016; 42: 1137–1145. [DOI] [PubMed] [Google Scholar]
- 17. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 2000; 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 18. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25: 603–605. [DOI] [PubMed] [Google Scholar]
- 19. Chaimani A, Mavridis D, Salanti G. A hands-on practical tutorial on performing meta-analysis with Stata. Evid Based Ment Health 2014; 17: 111–116. [DOI] [PubMed] [Google Scholar]
- 20. Xu Z, Wu GM, Li Q, et al. Predictive value of combined LIPS and ANG-2 level in critically ill patients with ARDS risk factors. Mediators Inflamm 2018; 2018: 1739615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hendrickson CM, Matthay MA. Endothelial biomarkers in human sepsis: pathogenesis and prognosis for ARDS. Pulm Circ 2018; 8: 2045894018769876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kumpers P, Lukasz A. The curse of angiopoietin-2 in ARDS: on stranger TI(E)des. Crit Care 2018; 22: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tsangaris I, Tsantes A, Vrigkou E, et al. Angiopoietin-2 levels as predictors of outcome in mechanically ventilated patients with acute respiratory distress syndrome. Dis Markers 2017; 2017: 6758721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang H, Cade BE, Chen H, et al. Variants in angiopoietin-2 (ANGPT2) contribute to variation in nocturnal oxyhaemoglobin saturation level. Hum Mol Genet 2016; 25: 5244–5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fisher J, Douglas JJ, Linder A, et al. Elevated plasma angiopoietin-2 levels are associated with fluid overload, organ dysfunction, and mortality in human septic shock. Crit Care Med 2016; 44: 2018–2027. [DOI] [PubMed] [Google Scholar]
- 26. van der Heijden M, van Nieuw Amerongen GP, Koolwijk P, et al. Angiopoietin-2, permeability oedema, occurrence and severity of ALI/ARDS in septic and non-septic critically ill patients. Thorax 2008; 63: 903–909. [DOI] [PubMed] [Google Scholar]
- 27. van der Heijden M, van Nieuw Amerongen GP, van Hinsbergh VW, et al. The interaction of soluble Tie2 with angiopoietins and pulmonary vascular permeability in septic and nonseptic critically ill patients. Shock 2010; 33: 263–268. [DOI] [PubMed] [Google Scholar]
- 28. van der Heijden M, Pickkers P, van Nieuw Amerongen GP, et al. Circulating angiopoietin-2 levels in the course of septic shock: relation with fluid balance, pulmonary dysfunction and mortality. Intensive Care Med 2009; 35: 1567–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Parke R, Bihari S, Dixon DL, et al. Fluid resuscitation associated with elevated angiopoietin-2 and length of mechanical ventilation after cardiac surgery. Crit Care Resusc 2018; 20: 198–208. [PubMed] [Google Scholar]
- 30. Hoeboer SH, Groeneveld AB, van der Heijden M, et al. Serial inflammatory biomarkers of the severity, course and outcome of late onset acute respiratory distress syndrome in critically ill patients with or at risk for the syndrome after new-onset fever. Biomark Med 2015; 9: 605–616. [DOI] [PubMed] [Google Scholar]
- 31. Gores KM, Delsing AS, Kraus SJ, et al. Plasma angiopoietin 2 concentrations are related to impaired lung function and organ failure in a clinical cohort receiving high-dose interleukin 2 therapy. Shock 2014; 42: 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gallagher DC, Parikh SM, Balonov K, et al. Circulating angiopoietin 2 correlates with mortality in a surgical population with acute lung injury/adult respiratory distress syndrome. Shock 2008; 29: 656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Calfee CS, Janz DR, Bernard GR, et al. Distinct molecular phenotypes of direct vs indirect ARDS in single-center and multicenter studies. Chest 2015; 147: 1539–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zinter MS, Spicer A, Orwoll BO, et al. Plasma angiopoietin-2 outperforms other markers of endothelial injury in prognosticating pediatric ARDS mortality. Am J Physiol Lung Cell Mol Physiol 2016; 310: L224–L231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Reilly JP, Wang F, Jones TK, et al. Plasma angiopoietin-2 as a potential causal marker in sepsis-associated ARDS development: evidence from Mendelian randomization and mediation analysis. Intensive Care Med 2018; 44: 1849–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Calfee CS, Gallagher D, Abbott J, et al. Effect of conservative fluid management on endothelial inflammation (angiopoietin-2) in patients with acute lung injury. Am J Respir Crit Care Med 2011; 183: abstract 3986. [Google Scholar]
- 37. Ganter MT, Cohen MJ, Brohi K, et al. Angiopoietin-2, marker and mediator of endothelial activation with prognostic significance early after trauma? Ann Surg 2008; 247: 320–326. [DOI] [PubMed] [Google Scholar]
- 38. Giamarellos-Bourboulis EJ, Kanellakopoulou K, Pelekanou A, et al. Kinetics of angiopoietin-2 in serum of multi-trauma patients: correlation with patient severity. Cytokine 2008; 44: 310–313. [DOI] [PubMed] [Google Scholar]
- 39. Zhong M, Zhang L, Wang F, et al. The levels of angiopoietin-2 in patients with acute respiratory distress syndrome and its value on prognosis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2014; 26: 804–809. [DOI] [PubMed] [Google Scholar]
- 40. Gale NW, Thurston G, Hackett SF, et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev Cell 2002; 3: 411–423. [DOI] [PubMed] [Google Scholar]
- 41. Teichert-Kuliszewska K, Maisonpierre PC, Jones N, et al. Biological action of angiopoietin-2 in a fibrin matrix model of angiogenesis is associated with activation of Tie2. Cardiovasc Res 2001; 49: 659–670. [DOI] [PubMed] [Google Scholar]
- 42. Childs EW, Tharakan B, Byrge N, et al. Angiopoietin-1 inhibits intrinsic apoptotic signaling and vascular hyperpermeability following hemorrhagic shock. Am J Physiol Heart Circ Physiol 2008; 294: H2285–H2295. [DOI] [PubMed] [Google Scholar]
- 43. Lemieux C, Maliba R, Favier J, et al. Angiopoietins can directly activate endothelial cells and neutrophils to promote proinflammatory responses. Blood 2005; 105: 1523–1530. [DOI] [PubMed] [Google Scholar]
- 44. Fiedler U, Reiss Y, Scharpfenecker M, et al. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med 2006; 12: 235–239. [DOI] [PubMed] [Google Scholar]
- 45. Lomas-Neira J, Venet F, Chung CS, et al. Neutrophil-endothelial interactions mediate angiopoietin-2-associated pulmonary endothelial cell dysfunction in indirect acute lung injury in mice. Am J Respir Cell Mol Biol 2014; 50: 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Author_response_1 for Circulating angiopoietin-2 and the risk of mortality in patients with acute respiratory distress syndrome: a systematic review and meta-analysis of 10 prospective cohort studies by Fengyuan Li, Rulan Yin and Qiang Guo in Therapeutic Advances in Respiratory Disease
Supplemental material, PRISMA-2009-Checklist-MS-Word for Circulating angiopoietin-2 and the risk of mortality in patients with acute respiratory distress syndrome: a systematic review and meta-analysis of 10 prospective cohort studies by Fengyuan Li, Rulan Yin and Qiang Guo in Therapeutic Advances in Respiratory Disease
Supplemental material, PRISMA-2009-Flow-Diagram-MS-Word for Circulating angiopoietin-2 and the risk of mortality in patients with acute respiratory distress syndrome: a systematic review and meta-analysis of 10 prospective cohort studies by Fengyuan Li, Rulan Yin and Qiang Guo in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Circulating angiopoietin-2 and the risk of mortality in patients with acute respiratory distress syndrome: a systematic review and meta-analysis of 10 prospective cohort studies by Fengyuan Li, Rulan Yin and Qiang Guo in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Circulating angiopoietin-2 and the risk of mortality in patients with acute respiratory distress syndrome: a systematic review and meta-analysis of 10 prospective cohort studies by Fengyuan Li, Rulan Yin and Qiang Guo in Therapeutic Advances in Respiratory Disease
Supplemental material, Supplement_Materials_1 for Circulating angiopoietin-2 and the risk of mortality in patients with acute respiratory distress syndrome: a systematic review and meta-analysis of 10 prospective cohort studies by Fengyuan Li, Rulan Yin and Qiang Guo in Therapeutic Advances in Respiratory Disease