Abstract
Background
The role of macrophages (Mφs) in tendon injury healing is controversy. The aims of this study were to determine whether there is a shift in Mφs polarisation after an acute and chronic tendon injury and to assess whether the Mφs polarisation between the partial and complete rupture is different.
Methods
This systematic review of the scientific literature was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and Cochrane guidelines. PubMed database and Excerpta Medica Database (EMBASE) were used for specific search criteria. Only studies measuring Mφs using specific cell markers in Achilles tendon tissue and rotator cuff tendon tissue were included, respectively.
Results
Five Achilles tendon injury studies and four rotator cuff injury studies were included. Expression of the pan Mϕs marker Cluster of Differentiation (CD) 68 was significantly upregulated in acute Achilles tendon ruptures compared to intact tendons, while no significant changes were found in Mφs polarisation markers CD80 (M1 Mφs) and CD206 (M2 Mφs). High levels of CD86 (M1 Mφs) and CD206 were observed in acute partial rupture. Expression of CD68 and CD206 were significantly upregulated in chronic rotator cuff tendinopathy and downregulated as structural failure increases. A low level of CD206 was observed in complete tendon rupture regardless of acute or chronic injury.
Discussion and conclusion
In spite of the limited number of articles included, findings from this study suggested that the process of inflammation plays an important role in acute Achilles tendon injuries, indicated by the increased expression of CD68+ Mφs. Low levels of CD206+ Mφs were constantly observed in complete Achilles tendon rupture, while high levels of CD80+ Mφs and CD206+ Mφs were observed in partial Achilles tendon rupture, which suggested the potential correlation between M2 Mφs and tendon structure. For chronic rotator cuff injury, CD68+ Mφs and CD206+ Mφs were higher in tendinopathic tissues in comparison to the intact control tissues. Both CD68+ Mφs and CD206+ Mφs has an inverse relation to the structural failure in the torn rotator cuff tendon. After tendon rupture, the time point of biopsy specimen collection is an important factor, which could occur in the acute phase or chronic phase. Collectively, the understanding of the roles in Mφs after tendon injury is inadequate, and more research efforts should be devoted to this direction.
The translational potential of this article
This article provided a potential implication on how pan Mφs or M2 Mφs might be associated with ruptured or torn tendon structure. Managing Mφs numbers and phenotypes may lead to possible novel therapeutic approaches to the management of early tendinopathy, early acute tendon rupture, hence, promote healing after restoration surgery.
Keywords: Achilles, Macrophages, Rotator cuff, Tendon
Introduction
Tendon tissue connects muscle to bone, allowing coordination of muscle contractions into motion. Tendons contribute to the stable movement of the skeleton with high tear resistance and tensile strength [1]. However, tendon injuries have been considered as the most frequent musculoskeletal complaint for which patients seek medical treatments [2,3]. The musculoskeletal pain, related to tendon injuries, occupied more than 30% of those general practice consultations [4]. Tendon injuries could bring a high burden to patients, which can lead to a significant loss in individual production capacity, as the patients’ quality of life is impaired and induces an enormous economic burden of the health-care system on the whole society [5].
Tendon injuries can divide into acute injuries (e.g., traumatic acute Achilles tendon rupture) and chronic injuries (e.g., degenerative rotator cuff injury). Tendon pathologies range from acute to chronic or chronic to acute injuries with partial or complete rupture [6,7]. A partial rupture refers to tissue that is partly torn without damaging tendon integrity, and a complete rupture is characterised as a gap in the tendon with integrity damage [8]. An acute tendon rupture is a one-time event that can result in immediate pain and decreased the function of the affected joint and may be followed by swelling or bruising. Chronic tendon ruptures may result from: a partial rupture that slowly worsens over a prolonged period, or an acute rupture that goes untreated for several weeks [9].
Although acute or chronic tendon injuries can occur in any parts of our bodies, those with high in vivo loading demands, such as Achilles tendon, rotator cuff, patellar tendon, and forearm extensor tendons, are often affected [[10], [11], [12]]. Sudden exposure to elevated mechanical stresses is one of the risks for acute tendon injuries, while overuse or overloading has been widely considered to be the causative factors contributing to the onset of chronic injury or tendinopathy [[13], [14], [15]]. Nowadays, chronic tendon injuries are common athletic and occupational injuries that account for physician visits. The term tendinopathy is preferred to tendinitis because of the presence of a disordered and degenerative healing process—not inflammation—in the pathologic tendon. However, chronic injury and tendinopathy are usually difficult to separate in elderly patients because researchers may not be able to accurately identify the period between the beginning of chronic or acute injury and tendon specimen collection time point when patients received biopsy or surgery. But the consensus is that there are four major stages to tendon healing after injury, including hemorrhages, inflammation, tissue formation, and remodelling. The self-recovery of tendon injuries can be stimulated by the mechanical load, and is characterised by an early inflammatory phase, then proliferative and differentiation phases [[16], [17], [18], [19], [20], [21]]. First, a scar tissue with an increased cross-sectional area can be produced, and then, it takes several months for tissues to develop from biomechanically inferior scar tissue into new tendon tissue. This period of the healing process is widely considered as a crisis with a high risk of rerupturing [22]. Moreover, the healed tendon tissues only achieve 70% mechanical properties of preinjured tendons [23,24].
The tendon repairing process involves a complex and coordinated series of events. Recent evidence demonstrates that modulation of inflammation in the early stages following tendon repair may help to improve healing [25]. Although numerous cells are involved in the process of tendon healing, macrophages (Mφs) play essential roles in moderate the process of tissue repair through promoting and reducing inflammation [7,[26], [27], [28]]. Mφs can be categorised into two broad phenotypes: classically activated pro-inflammatory Mφs (M1), which can promote extracellular matrix (ECM) breakdown, inflammation, apoptosis, and alternatively activated antiinflammatory Mφs (M2) that coordinate ECM deposition and tissue repair [29,30]. The surface markers of CD68, CD80/CD86, and CD163/CD206 have been wildly accepted as to describe the pan Mφs, M1 Mφs, and M2 Mφs, respectively [[31], [32], [33]]. The previous study on tendon injury has demonstrated the similar concept of inflammation which is consistent with the experimental evidence [34], and the balance between pro-inflammation and antiinflammation, which could be reflected by the ratio of M1 and M2 Mφs, result in a defective repair and impaired tissue function [[35], [36], [37], [38]].
The management of two classical phenotypes of Mφs, M1, and M2 phenotypes, are believed to be the main contributors to the expected beneficial effects of tendon repair [39]. Understanding the transition of Mφs phenotypes during tendon repair may provide insights on how to manage treatment for a tendon injury in the future. This study aims to demonstrate the role of Mφs after acute tendon injury (Achilles tendon partial or complete rupture) and chronic tendon injury (rotator cuff tendinopathy with or without structural failure). The first aim of this article is to determine whether the number of Mφs altered after Achilles tendon injury and rotator cuff injury. The second aim is to access the presence of Mφs, which could be linked with the degree of structural tendon failure after injury.
Method
The PRISMA Statement (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) was used for this systematic review. And the Cochrane handbook was used as guidelines in the development of the study protocol.
Search strategy
Two investigators conducted the literature search. The following electronic databases were utilised: PubMed database and EMBASE (Excerpta Media Database) database. No limit conditions were placed on the year of data entry, and the search was organised in June 2019. The following search term were used: (Achilles [Title/Abstract] OR tendon [Title/Abstract]) AND (rupture [Title/Abstract] OR tear [Title/Abstract] OR partial [Title/Abstract] OR complete [Title/Abstract]) AND (macrophage*[Title/Abstract] OR inflam*[Title/Abstract] OR M1[Title/Abstract] OR M2[Title/Abstract]) for Achilles tendon injury and (rotator cuff [Title/Abstract] OR supraspinatus [Title/Abstract] OR infraspinatus [Title/Abstract] OR subscapularis [Title/Abstract] OR teres minor [Title/Abstract] OR tendon [Title/Abstract]) AND (rupture [Title/Abstract] OR tear [Title/Abstract] OR partial [Title/Abstract] OR complete [Title/Abstract]) AND (macrophage*[Title/Abstract] OR inflam*[Title/Abstract] OR M1[Title/Abstract] OR M2[Title/Abstract]) for rotator cuff injury (Table 1). Additional studies were located by searching papers referenced in listed articles. Those studies identified by the searches were combined, and duplicates excluded. The screening of titles and abstracts were screened before elaboration on the included full-text articles. The titles and abstracts of identified articles were screened first by two authors. In cases of disagreement between the two authors, a consensus was reached by discussion with a third author. Then the full texts of the screened articles were examined. If necessary, corresponding authors of the reviewed articles could be contacted to obtain those missing data.
Table 1.
Operationalization of the search strategy.
| Step | PubMed |
EMBASE |
||
|---|---|---|---|---|
| Achilles tendon injury | Rotator cuff injury | Achilles tendon injury | Rotator cuff injury | |
| #1 | Achilles [Title/Abstract] OR tendon [Title/Abstract] | Rotator cuff [Title/Abstract] OR supraspinatus [Title/Abstract] OR infraspinatus [Title/Abstract] OR subscapularis [Title/Abstract] OR teres minor [Title/Abstract] OR tendon [Title/Abstract] | Achilles:ti,ab,kw OR tendon:ti,ab,kw | ‘rotator cuff”:ti,ab,kw OR supraspinatus:ti,ab,kw OR infraspinatus:ti,ab,kw OR subscapularis:ti,ab,kw OR ‘teres minor”:ti,ab,kw OR tendon:ti,ab,kw |
| #2 | rupture [Title/Abstract] OR tear [Title/Abstract] OR partial [Title/Abstract] OR complete [Title/Abstract] | rupture [Title/Abstract] OR tear [Title/Abstract] OR partial [Title/Abstract] OR complete [Title/Abstract] | Rupture:ti,ab,kw OR tear:ti,ab,kw OR partial:ti,ab,kw OR complete:ti,ab,kw | Rupture:ti,ab,kw OR tear:ti,ab,kw OR partial:ti,ab,kw OR complete:ti,ab,kw |
| #3 | Macrophage*[Title/Abstract] OR inflam*[Title/Abstract] OR M1[Title/Abstract] OR M2[Title/Abstract] | Macrophage*[Title/Abstract] OR inflam*[Title/Abstract] OR M1[Title/Abstract] OR M2[Title/Abstract] | macrophage* OR m1 OR m2 OR inflam* | macrophage* OR m1 OR m2 OR inflam* |
| #4 | #1 and #2 and #3 | #1 and #2 and #3 | #1 and #2 and #3 | #1 and #2 and #3 |
Inclusion criteria and exclusion criteria
The eligibility of each article was determined based on the inclusion and exclusion criteria. The search was limited to articles published in English in the last 20 years, availability of full text, and articles containing original data. Only in vivo articles reporting on Mφs in the tendon tissues of humans or animals were included. Moreover, Mφs related number or proportion was measured in a quantitative or semi-quantitative format, and the quantification/semi-quantification of Mφs cell numbers had to involve the use of specific cell markers, including CD68, CD80, CD86, CD163, and CD206, as defined by the individual studies. Achilles related studies and rotator cuff related studies were searched, respectively. Exclusion criteria consisted of the following: studies without any control groups; patients only with infective or known ‘“inflammatory” tendon disease without subgroup analysis of tendon structural damage; in vitro studies; sampling method described inconsistently; staining methods not using specific markers; case reports, case series, review articles, letters or chapters; not available in English language (Table 2a and Table 2b).
Table 2a.
Inclusion criteria and exclusion criteria for Achilles tendon injury.
| Criteria | |
|---|---|
| Inclusion criteria | 1. Full-text available; |
| 2. Written in English; | |
| 3. Articles published in the last 20 years; | |
| 4. Articles containing original data; | |
| 5. Studies must be related to “macrophages” in the tendon tissue; | |
| 6. Achilles related studies; | |
| 7. Macrophages related number or proportion were measured in a quantitative or semi-quantitative format; | |
| 8. The quantification/semi-quantification of macrophages cell numbers had to involve the use of specific cell markers (CD68, CD80, CD86, CD163, and CD206) as defined by the individual studies. | |
| Exclusion criteria | 1. No control group (control group was defined as either a healthy tendon or a division between different tendinopathy groups); |
| 2. Patients only with infective or known “inflammatory” tendon disease without subgroup analysis of tendon structural damage; | |
| 3. In vitro studies; | |
| 4. Sampling method described inconsistently; | |
| 5. Staining methods not using specific markers; | |
| 6. Case reports, case series, review articles, letters or chapters; | |
| 7. Not available in English language. |
Table 2b.
Inclusion criteria and exclusion criteria for rotator cuff injury.
| Criteria | |
|---|---|
| Inclusion criteria |
|
| |
| |
| |
| |
| |
| |
| |
| Exclusion criteria |
|
| |
| |
| |
| |
| |
|
Studies meeting all criteria were reviewed, and all the included data were chosen based on study heterogeneity and methodological quality. Methodological quality scoring system assessment of human studies was based on the method used by Hegedus et al. [40] and Dean et al. [41]. Animal studies were assessed with another quality system, which was raised by Wells K et al. [42] (Appendix 1a and 1b).
Study selection
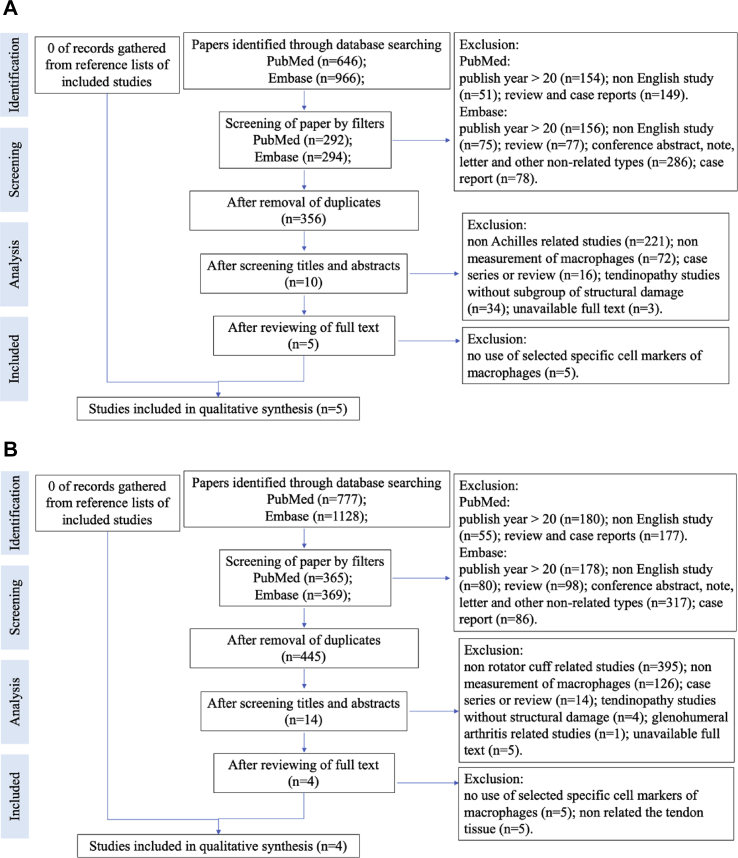
According to these two databases, a total of 1612 results and 1905 results were yield for preliminary screening of Achilles tendon injury and rotator cuff injury, respectively. After screening by database filters and removing duplicates, 356 articles remained for Achilles tendon and 445 articles remained for the rotator cuff. After further assessment by screening titles and abstracts, ten articles on Achilles tendon injury and 14 articles-related rotator cuff injury remained. After reviewing the full text and based on the application of inclusion/exclusion for the above screening procedures, led to the exclusion of non-related articles, five articles, and four articles were included in the final qualitative synthesis, respectively.
Study characteristics
Out of the five included Achilles tendon injury studies, 3 were human studies [[43], [44], [45]], and 2 were animal studies [46,47]. All five studies reported different phenotypes of Mφs with specific cell markers and designed a subgroup of acute Achilles ruptured tendons. All human studies had a tendinopathy subgroup. All studies collected acute and chronic Achilles injury samples from live humans who received surgical or biopsy treatments in a trauma unit or a sports clinic. The animal studies [46,47] used a partial defect model and a surgical transected and repair Achilles model, respectively.
All four included rotator cuff injury studies are on rotator cuff tear or tendinopathy. The major limitation of most rotator cuff injury human studies is that tendon biopsy specimens can be only obtained when patients are symptomatic, so all the biopsy samples use tendinopathy to represent chronic injury rather early phase. All tendon biopsy specimens in the four studies were collected from patients who failed conservative treatment and received shoulder surgery. Ruptured or torn supraspinatus specimens were collected as the experimental group during rotator cuff repair surgery, and subscapularis specimens were collected as the intact or healthy control group during anterior stabilisation surgery of shoulder dislocation. One study [48] established a human model of subscapularis tendinopathy, which can provide biopsy specimens during shoulder surgery of rotator cuff tears. The inclusion criteria of the subscapularis tendinopathy can only be included if there was no clinically detectable evidence MRI scan or macroscopic damage to the subscapularis tendon at the time of arthroscopic surgery. This study analysed differences between the tear group and matched subscapularis tendinopathy group, and an independent control group was obtained, comprising subscapularis tendon collected from patients who were undergoing a stabilisation surgical procedure. Two studies used patients who had a history of recurrent dislocation of the shoulder and without history or evidence of rotator cuff disease as a control group [33,49]. One study [50] used fresh cadaveric supraspinatus tendons without grossly evident tears as controls.
Study methodology and risk of bias
All the included studies stated and described their experiment groups, control groups, and tendon tissue analysis. The summary of the included studies and methodological scores are detailed in Appendix 2.
Out of the five Achilles tendon injury studies, three human studies compared acute ruptures with intact controls, and two animal studies specifically compared tendons with partial defects against the completely transected tendon. All four rotator cuff injury studies analysed the shift in Mφs polarisation across different structural stages of tendinopathy. Three studies were quantitative, and one study was semi-quantitative. The extracted data were categorised into 2 groups: Achilles tendon injury group and rotator cuff injury group. A further categorisation of Achilles tendon injury studies into human studies and animal studies.
During the screening, studies that did not have control groups for comparison were excluded. A control group was defined as either a healthy tendon or a division between different tendinopathy groups. In addition to a formal statistical analysis, studies that used quantitative and/or semi-quantitative methods were included in the results. Meanwhile, studies that used descriptive non-quantitative methods were also included. This methodological assessment means that the results did not include studies with a large degree of bias and that those with higher degrees of potential bias have been highlighted to readers [41]. Due to the natural heterogeneity of measurements across studies, a meta-analysis could not be performed. Therefore, descriptive data, that is, increased, decreased, or unchanged, was also summarised as an outcome measurement.
Outcome measurement
The primary outcome was Mφs cell counts. Secondary outcomes were specific cell markers measurement, including quantitative and descriptive data of CD68, CD80, CD86, CD163, CD206, or other related cell markers.
Results
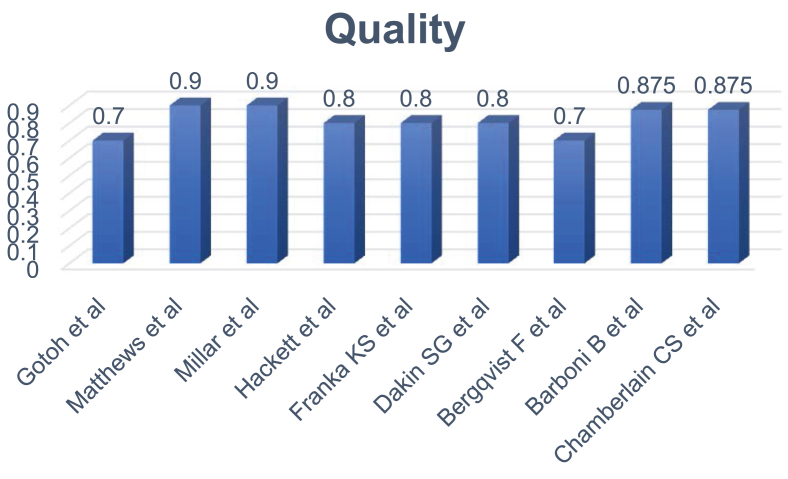
The query resulted in 9 citations, and the flow charts of systematic review protocol are listed in Figure 1A and B. The quality assessment of the included articles was shown in Figure 2. A total of 215 human samples and 93 animal samples were included in this study. The details of the included studies are listed in Table 3.
Figure 1.
(A) Flow chart of systematic review protocol (Achilles tendon injury). (B) Flow chart of systematic review protocol (rotator cuff injury).
Figure 2.
Quality assessment of the included studies.
Table 3.
Details of included studies.
| Author | Year | Journal | Study type | Tendon | Sample demographics |
Subgroup analysis | Inflammatory cell markers | ||
|---|---|---|---|---|---|---|---|---|---|
| Experimental group type and size | Control group type and size | Source | |||||||
| Franka KS et al. [37] | 2018 | Int J Mol Sci | Human | Achilles | Acute (n = 13) vs chronic (n = 6) vs tendinopathy (n = 7) | Intact tendons for RNA analysis (n = 4); for histological evaluation (n = 5) |
|
Acute Achilles rupture vs. chronic Achilles rupture vs. chronic tendinopathy vs. intact | CD3, CD34, CD45, CD68, CD80, CD206 |
| Dakin SG et al. [38] | 2018 | Br J Sports Med | Human | Achilles | Mid-portion Achilles tendinopathy (n = 17) and rupture (n = 19) | Healthy hamstring tendons (n = 15) |
|
Mid-portion tendinopathy vs. rupture | CD14, CD31, CD68, CD106, CD163, CD206, CD248 |
| Bergqvist F et al. [39] | 2019 | Arthritis Res Ther | Human | Achilles | Shoulder tendon cohorts (n = 19) vs Achilles tendon cohort (acute: n = 3; chronic: n = 3) | Hamstring tendon cohort (n = 8) |
|
Tendinopathy vs. rupture | CD31, CD34, CD45, CD68 |
| Barboni B et al. [40] | 2018 | J Tissue Eng Regen Med | Animal (Ovine) | Achilles | Ovine Achilles tendon partial defect model (n = 29) | Health Achilles tendons (n = 10) |
|
Partial defect model vs. health tendon | CD3, CD45, CD68, CD86, CD206 |
| Chamberlain CS et al. [41] | 2019 | Stem Cells | Animal (Mice) | Achilles | Surgical transected Achilles model (n = 27) | Intact contralateral side tendon (n = 27) |
|
Transected tendon vs. intact tendon | CD9, CD14, CD63, CD81, CD206 |
| Gotoh et al. [42] | 1997 | J Orthop Res | Human | Rotator cuff | Supraspinatus insertions: complete tear (n = 8) and incomplete tear (n = 8) | Cadaveric specimens (n = 6) |
|
Partial thickness vs. full thickness tears | CD68 |
| Matthews et al. [43] | 2006 | J Bone Joint Surg Br | Human | Rotator cuff | Torn chronic supraspinatus specimens (n = 40) | Subscapularis specimens from patients with recurrent dislocation (n = 4) |
|
Tear size | CD34, CD45, CD68 |
| Millar et al. [44] | 2010 | Am J Sports Med | Human | Rotator cuff | Ruptured supraspinatus specimens (n = 20) | Tendinopathic subscapularis specimens (n = 10) |
|
Tear vs tendinopathy | CD3, CD34, CD68, CD206 |
| Hackett et al. [45] | 2016 | J Bone Joint Surg Am | Human | Rotator cuff | Torn supraspinatus specimens (n = 10) | Subscapularis specimens (n = 10) |
|
Control vs. tear vs. calcific tendinitis | CD3, CD34, CD68, CD206 |
Achilles tendon injury studies
All results are summarised in Table 4. One human study reported a fourfold upregulation of CD68+ Mφs found in acute Achilles. However, no significant changes were found in Mφs polarisation markers CD80 and CD206 [43]. In immunohistochemistry staining, the ruptured Achilles tendon expressed higher amount of CD68+ Mφs (p = 0.0007) when compare with the normal groups. Immunohistochemistry staining of CD68 was also found in ruptured Achilles tendon [45].
Table 4.
Macrophages cell markers change in acute Achilles ruptured tendon.
| Cell markers |
|||
|---|---|---|---|
| CD68 | CD80/86 | CD163/206 | |
| Increased, unchanged, decreased in acute rupture vs. control or not detected (ND) | a b c |
a d |
a d e |
| Increased, unchanged, decreased in acute rupture vs tendinopathy or not detected (ND) | ND a | / | a b |
Article notes:
a: Franka KS et al., Different Achilles Tendon Pathologies Show Distinct Histological and Molecular Characteristics. Int J Mol Sci 2018.
b: Dakin SG et al., Chronic inflammation is a feature of Achilles tendinopathy and rupture. Br J Sports Med 2018.
c: Bergqvist F et al., Divergent roles of prostacyclin and PGE2 in human tendinopathy. Arthritis Res Ther 2019.
d: Barboni B et al., Therapeutic potential of hAECs for early Achilles tendon defect repair through regeneration. J Tissue Eng Regen Med 2018.
e: Chamberlain CS et al., Extracellular Vesicle-Educated Macrophages Promote Early Achilles Tendon Healing. Stem Cell 2019.
d: an ovine Achilles tendon partial defect model was used.
Target cells/tissue: CD68: Pan macrophages; CD80/CD86: M1 like macrophages; CD163/CD206: M2 like macrophages; CD31/34: Vascular endothelium.
In animal models, a partial defect model of the Achilles tendon [46] and a surgical transected and repaired Achilles model was used to demonstrate the shift in Mφs polarisation [47]. Quantification of M1 or M2 Mφs were expressed as a percentage of CD86+ or CD206+ Mφs per total number of nuclei in the area of healing tendons. The percentage of CD206+ Mφs per total number of nuclei showed a higher expression level than CD86+ Mφs per total number of nuclei in a partial Achilles tendon defect model [46]. Low levels of M2 Mφs (CD206+) were constantly observed in complete ruptured Achilles tendon regardless of the affected portion. Thus, on day 28, after Achilles tendon transection, a high level of M1 Mφs expression was found compared to M2 Mφs [47].
Specific cell markers CD68, CD80, and CD206, were used to demonstrate the Mφs polarisation between ruptured tendon (acute and chronic) and healthy intact tendon [43,44]. Inflammatory change was detected by immunohistochemistry in all tendon pathology groups but was found to be significantly lower in tendons with tendinopathy compared to chronic ruptures. The number of CD68+ Mφs was found to be significantly higher in chronic Achilles tendon when compared to healthy intact controls (p = 0.0015). No significant differences in the number of CD68+ Mφs were found between ruptured Achilles tendon and Achilles tendon with tendinopathy. CD163 messenger RNA (mRNA) was highly expressed in ruptured Achilles (n = 0.03) and in Achilles tendon with tendinopathy (n = 0.002) when compared to healthy controls. CD206 mRNA were also found to be increased in Achilles with tendinopathy compared to ruptured Achilles tendon (p = 0.0002) [44].
In humans, the contribution of Mφs to the pathogenesis was indicated by the increased expression of CD68, while M1 and M2 Mφs showed no significant changes after acute tendon injury. In animals, low levels of CD206+ Mφs were constantly observed in the complete ruptured Achilles tendon, but higher expression levels in partial defect animal models were also shown.
Rotator cuff injury
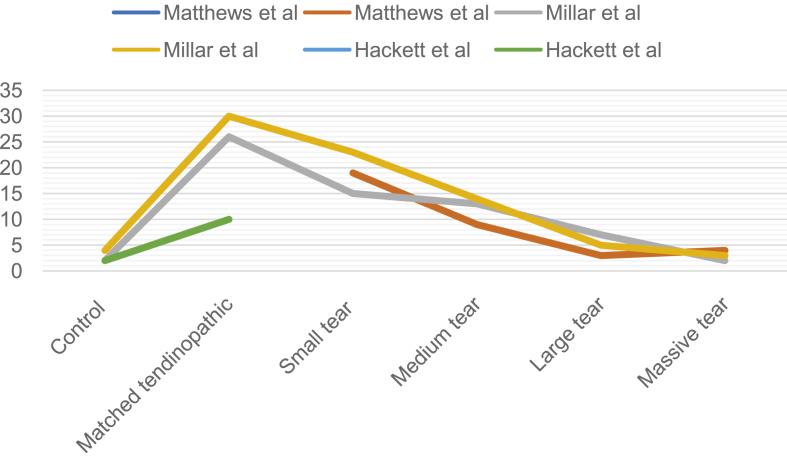
The number of pan Mφs and CD68+ Mφs was significantly higher in matched specimens (subscapularis) with tendinopathy compared to matched healthy control in two studies [48,49]. One study used a descriptive assessment to assess the increase appearance of CD68+ Mφs in a matched specimen with tendinopathy [50]. Compared to healthy control, CD206+ Mφs also showed a coherent increase in matched specimens with tendinopathy [48]. Significant differences of CD206+ Mφs can be found in intact tissue, intact tissue with tendinopathy, and torn tendon by quantitative assessment (p < 0.001) [[45], [46], [47]] (Table 5). Moreover, pan Mφs levels and M2 Mφs levels decrease as the structural tendon failure increases [48,49]. CD68 and CD206 markers also assessed the coherent variety as pan Mφs and M2 Mφs in one study [48]. The other study tested the increased level of cell count and specific cell markers between the intact control and torn tendon specimens [33]. The number of CD68+ Mφs and CD206+ M2 Mφs were higher in tissues with tendinopathy. Moreover, both of CD68+ Mφs and CD206 + M2 Mφs decreased as the structural failure of torn rotator cuff tendon increased (Table 6 & Figure 3).
Table 5.
Macrophages cell and vascular markers change in rotator cuff tendinopathic specimens versus healthy control tendon, and differences as structural tendon failure increases.
| Cell markers |
|||
|---|---|---|---|
| CD68 | CD206 | ||
| Increased, unchanged, decreased in tendinopathic vs. control or not detected (ND) | Quantitative | g h | h |
| Descriptive | f | / | |
| Increased, unchanged, decreased as structural failure increases or not detected (ND) | Quantitative |
g h i |
h |
| Descriptive | f | ND i | |
Article notes:
f: Gotoh M, Hamada K, Yamakawa H et al. Significance of granulation tissue in torn supraspinatus insertions: an immunohistochemical study with antibodies against interleukin-1 beta, cathepsin D, and matrix metalloprotease-1. J Orthop Res 1997.
g: Matthews TJ, Hand GC, Rees JL et al. Pathology of the torn rotator cuff tendon. Reduction in potential for repair as tear size increases. J Bone Joint Surg Br 2006.
h: Millar NL, Hueber AJ, Reilly JH et al. Inflammation is present in early human tendinopathy. Am J Sports Med 2010.
i: Hackett L, AMS, Millar NL et al. Are the Symptoms of Calcific Tendinitis Due to Neoinnervation and/or Neovascularisation? J Bone Joint Surg Am 2016.
i: only show the increase between intact control and torn tendon specimen. No result of sequential variety as structural tendon failure increases.
Target cells/tissue: CD68: Pan macrophages; CD206: M2 like macrophages; CD34: Vascular endothelium.
Table 6.
Macrophages cell count quantitative changes in rotator cuff tendinopathic and torn specimens versus healthy control tendon.
| Authors | Cell counta (Control – matched tendinopathic subscapularis – small tear – medium tear – large tear – massive tear) |
Mean vessel countb (Control – matched tendinopathic subscapularis – small tear – medium tear – large tear – massive tear) | |
|---|---|---|---|
| Pan-macrophages | M2-like macrophages | ||
| Matthews et al. | 2-/-19-9-3-4 | / | 4.5-/-32.2-18.4-6.1-0.5 |
| Millar et al. | 4-30-23-14-5-3 | 2-26-15-13-7-2 | 6-38-28-17-6-1 |
| Hackett et al.c | 2-10 | 2-10 | 5-11 |
Small: <1 cm2, Medium: >1–3 cm2; Large: >3–5 cm2; Massive: >5 cm2.
Control: subscapularis tendon collected from patients undergoing arthroscopic surgery for shoulder stabilisation without rotator cuff tears in the same time period.
Mean number of cells in ten high-power fields of view (magnification ×400).
Mean number of vessels in ten high-power fields of view (magnification ×400).
No more details of the size of torn supraspinatus.
Figure 3.
Macrophages cell counts observed related to the size of rotator cuff tear.
Discussion
The systematic review aims to identify the role of Mφs after a tendon injury, including acute rupture and chronic tendinopathy-tear/rupture stages by analysing Achilles tendon injury and rotator cuff injury. The findings support that Mφs contributes to the pathogenesis of acute rupture and chronic tendinopathy, as indicated by the increased expression of CD68. While pan Mφs level after acute Achilles tendon rupture was higher than intact controls, M1 and M2 Mφs showed no significant changes, as indicated by no upregulated expression of CD80 or CD86, and CD163 or CD206. Pan Mφs and M2 Mφs levels were higher in partial tendon rupture and tissues with tendinopathy compared to intact controls. Both pan Mφs and M2 Mφs decreased as the structural failure of torn rotator cuff tendon increases. Due to the limited amount of studies that investigate Mφs in acute ruptured Achilles tendon tissues and rotator cuff tendinopathy tissues by using specific cell markers and/or quantitative or descriptive analysis, the evidence was based on a small range of studies. However, the findings still provided a potential implication on how to pan Mφs or M2 Mφs might be associated with a ruptured or torn tendon structure. Managing Mφs numbers and phenotypes may lead to possible novel therapeutic approaches to the management of early tendinopathy, early acute tendon rupture, hence, promote healing after restoration surgery.
As an acute injury, Achilles tendon injuries can heal with an acceptable recovery of function, but tendon tissue quality can rarely or unable to return to preinjury levels by using current biological augmentation or surgical interventions [51,52]. Tendon ruptured may occur spontaneously during activities of daily livings, and it has been widely attributed to the accumulation of tissue damage, which is associated with the process of degenerative tissue remodelling [53]. Tendon injury could ultimately result in biological and mechanical propagation of the tendon tear or rupture, and then lead to the catastrophic structural damage. By analogy with a wound or other tissue healing, the tendon injury could be repaired with an initial matrix, which can provide stop-gap mechanical integrity and a tissue template to guide later matrix remodelling [[53], [54], [55]]. Since tissue barriers between intrinsic and extrinsic compartments had been violated after tissue injury, the classic wound healing procedure programmed procedure, including bleeding, clot formation, immune cells, and progenitor cells recruitment-early tissue, early and late tissue remodelling [56,57], which ideally manage the resolution and restoration of the neo-innervation and neo-vasculature [58]. Inflammation plays a crucial role in cross-talk management between the neurovascular immune system and tendon core [59,60]. The term “inflammation” has been primarily considered to describe a serious of clinical symptoms and signals after bony or soft tissue injury. However, the definition of “inflammation” has been subsequently changed and be more complex. In a huge variety of disease processes, including tendon healing process, inflammation may lead to a deeper understanding of the way to manage the repair response, especially during the tendon tissue healing process [61]. Mφs can act as immune sentinels in the front line of tissue defense. When the immune system needs to defect or respond to the initial tissue damage, Mφs would be positioned discretely and programmed transcriptionally [61]. This study has demonstrated that Mφs play a role after a tendon injury, including acute rupture and chronic tendinopathy.
A variety of mechanisms can initiate the inflammatory process of the immune system, during tissue repair, including mechanical stress, chemical or thermal stimulation, and pathogens invasion. Both Achilles tendon and rotator cuff are precisely the connective tissue between tendon and bone, which experiences a high level of intrinsic mechanical stress [62]. The reaction between tendon tissue and abnormal mechanical loading plays a key role after tendon injury. Mechanical forces could act as stimuli, and then detected by tenocytes, and are transduced via intracellular pathways and converted into biochemical signals that elicit cellular responses [63]. As the structural failure of the ruptured or torn tendon structure increases, the intrinsic mechanical stress would fizzle out. The unique abilities of M s and the variable expression of different subtypes are fundamental for maintaining soft tissue homeostasis [64]. Therefore, the shift in Mφs polarisation during the healing process is highly dynamic, and the specific role of M2 Mφs is difficult to discern in the complicated macroenvironment [65]. Chu SY et al. reported that stretch elicits Mφs-predominated inflammation and activated chemokines for Mφs recruitment. The strain-stimulated macroenvironment could recruit Mφs by releasing chemokines and then polarise them into M2 subtypes [66]. However, the precise mechanism of facilitating M2 polarisation by regulating cytokines and metabolic processes still needs further investigation. Previous studies have revealed the important role of the extracellular matrix (ECM) fragments, which can upregulate chemokines, promote Mφs recruitment, enhance phagocytic functions, and induce cytokines [[67], [68], [69], [70]]. Moreover, M2 Mφs can produce a wide array of growth factors. The growth factors and fibroblast growth factors, such as Hgf, Igf1, and Fgf10, are upregulated when stretch is released [66]. Tissue damage, such as rupture of the ECM or cell junction induced by stretch, might play a role in Mφs recruitment or polarisation. Moreover, the links between force distribution, tissue damage, morphogen gradients and cell responses have acquired substantial research interest, which is related to the mechanical stretch—immune response activation—regeneration process axis. Especially M2 Mφs, it might be the key mediators in stretch-induced regeneration. According to our results of this systematic review, Achilles tendon suffered a sudden external disruption, which induces strain-stimulated macroenvironment alteration. Pan Mφs level after acute Achilles tendon rupture increased as indicated by the increased expression of CD68. Alteration of CD206 expression was not found, which could indicate the variety of M2 Mφs. A possible explanation to such a phenomenon would be that M2 Mφs requires a longer time to be induced, and therefore, does not respond immediately with a sudden external disruption after a traumatic injury. However, we found that CD206 expression decreased as structural failure increases in rotator cuff injury, which may indicate that M2 Mφs decreases the tension of the tendon tissue causing the strain-stimulated macroenvironment to weaken. Increased accumulation of Mφs was found in a rodent model with upper extremity overuse injury and another model with calcaneal tendon injury [71,72]. The current systematic review obtained similar results. CD163/CD206 was highly expressed in tendon tissue with tendinopathy, including chronic tendinopathy in Achilles tendon and chronic tendinopathy rotator cuff tendon, which may indicate precisely the mechanical stretch might play a role in facilitating M2 Mφs polarisation through regulation of inflammatory cytokines or metabolic processes. Still, further research on animal models is needed to pinpoint the effect of strain-stimulated macroenvironment, which can recruit Mφs through chemokine release and polarised them into M2 subtypes. Since this conclusion would be difficult to be drawn using human models, where interpersonal differences are not negligible, these two kinds of pathological changes can affect the CD206 expression.
Mφs can be presented in tissues that undergo substantial mechanical stretch. A review intends to highlight Mφs may be mechanically sensitive, and Mφs phenotypes may be partially regulated by the physical factors [73]. Mφs can be mechanically activated when they are probed, leading to discerned alterations in the mechanical properties of the surrounding microenvironment, then transduce those changes into biochemical signals [74,75]. Previous studies have demonstrated that the migration speed of Mφs is inversely correlated to intracellular tensions [76], and M2 primary Mφs has been shown to be much more shifting than M1 Mφs in the absence of chemoattractant in murine [77]. One of the findings from this systematic review demonstrated that CD206 expression decreased as structural failure increased. Demonstrating the correlation between mechanical stretch and M2 Mφs polarisation, as well as how the restoration of mechanical stretch affects Mφs polarisation, may provide a potential mechanism to explain the tendon healing process after tendon repair surgical procedure.
Experimental approaches by means of selectively targeting Mφs with phenotypes or introducing beneficial ones are still far from being successful [78]. However, recent evidence demonstrated that more than one Mφs phenotype is required to regulate the complex tissue healing process. The key point may lie in the sequential activation and timely transition from one phenotype to another. Through biochemical, physical, or combined means of managing the functional plasticity of Mφs, to remodulate them to attain certain phenotypes sequentially may provide a promising therapeutic strategy.
Limitations
The conclusions drawn from this systematic review are limited because of the quantity of the included studies. Meanwhile, in order to avoid the significant study heterogeneity in terms of the included tendon types, the studies of Achilles tendon and rotator cuff tendon were analysed separately. Therefore, the major limitation of this review is the small number of articles included.
The second limitation is that we cannot standardise the time point of collecting tendon specimens in human studies. The time points of biopsy specimen collection relative to an acute tendon rupture or a chronic tendon tear is a significant factor, which could occur in the acute phase or chronic phase. More attention should be paid to note that the time and method of sampling the tendon tissue might contribute to the differences observed, and the amount of loose connective tissue in biopsy may vary between healthy or intact controls and the tissue with tendinopathy. Since researchers may not be able to identify the period between the beginning of chronic or acute injury and tendon specimen collection time point when patients received biopsy or surgery, this serves as a limitation.
The third limitation is that only those studies that use specific cell markers can be included. Although this decision was taken in order to exclude studies, which have still relied on subjective interpretation, some critical studies might be excluded during the screening process.
Conclusions
The existing evidence revealed that inflammation seems to play a role in acute ruptures, as indicated by the increased expression of CD68+ Mφs. Lower levels of CD80/CD86 and CD163/CD206 were constantly observed in acute complete rupture, which show a potential correlation between M2 Mφs and tendon structure. For chronic rotator cuff tendinopathy, pan Mφs and M2 Mφs levels were higher in tissues with tendinopathy than intact controls. Both pan Mφs and M2 Mφs decreases as the structural failure of torn rotator cuff tendon increases. After tendon rupture, the time point of biopsy specimen collection is a significant factor. Collectively, the understanding of the roles of Mφ during tendon injury is still relatively inadequate and more research efforts could be devoted to this direction. The lack of high-quality quantitative studies demonstrated that there is still an explicit need for further researches to better understand the role of Mφs and inflammation in tendon injury, which may lead to a novel intervention.
Conflict of interest
The authors have no conflicts of interest to disclose in relation to this article.
Acknowledgements
This study was supported by the MWLC Associate Member Programme, Ming Wai Lau Centre of Reparative Medicine of Karolinska Institutet to L.O.K., and also by CUHK Research Committee Funding (Direct Grants) to L.C.W. (Reference no. 2018.020).
Contributor Information
Hong-Tao Xu, Email: xhtsmed@gmail.com.
Chien-Wei Lee, Email: icehikki@gmail.com.
Ming-Yan Li, Email: lmyr0807@gmail.com.
Yu-Fan Wang, Email: amanda.yf.wang@gmail.com.
Patrick Shu-Hang Yung, Email: patrickyung@cuhk.edu.hk.
Oscar Kuang-Sheng Lee, Email: oscarlee9203@gmail.com.
Appendix.
Appendix 1a. Methodological quality assessment document for human study (the number of “yes” answers was counted for each study to give a total score out of 10).
| NO. | Criteria | Yes | No | Unclear |
|---|---|---|---|---|
| 1 | Study population clearly described (age/sex) | |||
| 2 | Control group clearly described | |||
| 3 | Sampling method clearly described | |||
| 4 | Sampling method consistent and unbiased | |||
| 5 | Quantitative analysis method or semi-quantitative analysis | |||
| 6 | Blinded image analysis | |||
| 7 | Analysis method representative of whole sample and unbiased | |||
| 8 | Reliability and/or validity of methods described | |||
| 9 | Study limitations mentioned/addressed | |||
| 10 | Statistical level of significance stated |
Dean BJ, Lostis E, Oakley T et al. The risks and benefits of glucocorticoid treatment for tendinopathy: a systematic review of the effects of local glucocorticoid on tendon. Semin Arthritis Rheum 2014; 43:570–6.
Hegedus EJ, Cook C, Brennan M, Wyland D, Garrison JC, Driesner D. Vascularity and tendon pathology in the rotator cuff: a review of literature and implications for rehabilitation and surgery. Br J Sports Med 2010; 44:838–47.
Appendix 1b. Methodological quality assessment document for animal study (the number of “yes” answers was counted for each study to give a total score out of 8).
| Section and topic | No. | Quality criteria | Yes | No |
|---|---|---|---|---|
| Title/Keywords/Introduction | 1 | Were the study hypothesis/aim/objective clearly described | ||
| Method | 2 | Were the animal model for the study well-described | ||
| 3 | Were the method well-described | |||
| 4 | Were the data collected time point clearly defined | |||
| 5 | Were the main outcome measures clearly defined | |||
| 6 | Were the experiment group well compared with the control group | |||
| Discussion | 7 | Were the results well-described | ||
| 8 | Were the articles discussed the limitation |
Kathleen Wells, Julia H. Littell. Study Quality Assessment in Systematic Reviews of Research on Intervention Effects. Research on Social Work Practice. 2009; 19: 52–62.
Appendix 2. Summary of included studies and methodological score.
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Quality assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gotoh et al. | No | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | 7 |
| Matthews et al. | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | 9 |
| Millar et al. | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 9 |
| Hackett et al. | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | 8 |
| Franka KS et al. | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Unclear | Yes | Yes | 8 |
| Dakin SG et al. | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Unclear | Yes | Yes | 8 |
| Bergqvist F et al. | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear | Unclear | Yes | Yes | 7 |
| Barboni B et al. | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 7 | ||
| Chamberlain CS et al. | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 7 |
References
- 1.Nourissat G., Berenbaum F., Duprez D. Tendon injury: from biology to tendon repair. Nat Rev Rheumatol. 2015;11(4):223–233. doi: 10.1038/nrrheum.2015.26. [DOI] [PubMed] [Google Scholar]
- 2.Mccormick A., Charlton J., Fleming D. Assessing health needs in primary care. Morbidity study from general practice provides another source of information. BMJ. 1995;310(6993):1534. doi: 10.1136/bmj.310.6993.1534d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riley G. Tendinopathy–from basic science to treatment. Nat Clin Pract Rheumatol. 2008;4:82–89. doi: 10.1038/ncprheum0700. [DOI] [PubMed] [Google Scholar]
- 4.Kaux J.F., Forthomme B., Goff C.L., Crielaard J.M., Croisier J.L. Current opinions on tendinopathy. J Sport Sci Med. 2011;10:238–253. [PMC free article] [PubMed] [Google Scholar]
- 5.Mcelvany M.D., Mcgoldrick E., Gee A.O., Neradilek M.B., Matsen F.A., 3rd Rotator cuff repair: published evidence on factors associated with repair integrity and clinical outcome. Am J Sports Med. 2015;43(2):491–500. doi: 10.1177/0363546514529644. [DOI] [PubMed] [Google Scholar]
- 6.Docheva D., Muller S.A., Majewski M., Evans C.H. Biologics for tendon repair. Adv Drug Deliv Rev. 2015;84:222–239. doi: 10.1016/j.addr.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voleti P.B., Buckley M.R., Soslowsky L.J. Tendon healing: repair and regeneration. Annu Rev Biomed Eng. 2012;14(1):47–71. doi: 10.1146/annurev-bioeng-071811-150122. [DOI] [PubMed] [Google Scholar]
- 8.Verhagen E., van Mechelen W. Oxford University Press; 2010. Sports injury research. [Google Scholar]
- 9.Flint J.H., Wade A.M., Giuliani J., Rue J.P. Defining the terms acute and chronic in orthopaedic sports injuries: a systematic review. Am J Sports Med. 2014 Jan;42(1):235–241. doi: 10.1177/0363546513490656. [DOI] [PubMed] [Google Scholar]
- 10.Thornton G.M., Hart D.A. The interface of mechanical loading and biological variables as they pertain to the development of tendinosis. J Musculoskelet Neuronal Interact. 2011;11(2):94–105. [PubMed] [Google Scholar]
- 11.Magnusson S.P., Langberg H., Kjaer M. The pathogenesis of tendinopathy: balancing the response to loading. Nat Rev Rheumatol. 2010;6(5):262–268. doi: 10.1038/nrrheum.2010.43. [DOI] [PubMed] [Google Scholar]
- 12.Sharma Pankaj. Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am. 2005;87(1):187–202. doi: 10.2106/JBJS.D.01850. [DOI] [PubMed] [Google Scholar]
- 13.Järvinen T.A., Kannus P., Maffulli N., Khan K.M. Achilles tendon disorders: etiology and epidemiology. Foot Ankle Clin. 2005;10(2):255–266. doi: 10.1016/j.fcl.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Willett T.L., Labow R.S., Avery N.C., Lee J.M. Increased proteolysis of collagen in an in vitro tensile overload tendon model. Ann Biomed Eng. 2007;35(11):1961–1972. doi: 10.1007/s10439-007-9375-x. [DOI] [PubMed] [Google Scholar]
- 15.Soslowsky L.J., Thomopoulos S., Tun S., Flanagan C.L., Keefer C.C., Mastaw J. Neer Award 1999. Overuse activity injures the supraspinatus tendon in an animal model: a histologic and biomechanical study. J Shoulder Elb Surg. 2000;9(2):79–84. [PubMed] [Google Scholar]
- 16.Bring K.I., Reno C., Renstrom P., Salo P., Hart D.A., Ackermann P.W. Joint immobilization reduces the expression of sensory neuropeptide receptors and impairs healing after tendon rupture in a rat model. J Ortho Res. 2009;27(2):274–280. doi: 10.1002/jor.20657. [DOI] [PubMed] [Google Scholar]
- 17.Palmes D., Spiegel H.U., Schneider T.O., Langer M., Stratmann U., Budny T. Achilles tendon healing: long-term biomechanical effects of postoperative mobilization and immobilization in a new mouse model. J Ortho Res. 2002;20(5):939–946. doi: 10.1016/S0736-0266(02)00032-3. [DOI] [PubMed] [Google Scholar]
- 18.Virchenko O., Aspenberg P. How can one platelet injection after tendon injury lead to a stronger tendon after 4 weeks? Interplay between early regeneration and mechanical stimulation. Acta Orthop. 2006;77(5):806–812. doi: 10.1080/17453670610013033. [DOI] [PubMed] [Google Scholar]
- 19.Eliasson P., Fahlgren A., Aspenberg P. Mechanical load and BMP signaling during tendon repair: a role for follistatin? Clin Orthop Relat Res. 2008;466(7):1592–1597. doi: 10.1007/s11999-008-0253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eliasson P., Andersson T., Aspenberg P. Rat Achilles tendon healing: mechanical loading and gene expression. J Appl Physiol. 2009;107(2):399–407. doi: 10.1152/japplphysiol.91563.2008. [DOI] [PubMed] [Google Scholar]
- 21.Aspenberg P. Stimulation of tendon repair: mechanical loading, GDFs and platelets. A mini-review. Int Orthop. 2007;31:783–789. doi: 10.1007/s00264-007-0398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Müller S.A., Todorov A., Heisterbach P.E., Martin I., Majewski M. Tendon healing: an overview of physiology, biology, and pathology of tendon healing and systematic review of state of the art in tendon bioengineering. Knee Surg Sport Traumatol Arthrosc. 2013;7:2097–2105. doi: 10.1007/s00167-013-2680-z. [DOI] [PubMed] [Google Scholar]
- 23.Hogan M.V., Bagayoko N., James R., Starnes T., Katz A., Chhabra A.B. Tissue engineering solutions for tendon repair. J Am Acad Orthop Surg. 2011;19:134–142. doi: 10.5435/00124635-201103000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Sarrafian T.L., Wang H., Hackett E.S., Yao J.Q., Shih M.S., Ramsay H.L. Comparison of Achilles tendon repair techniques in a sheep model using a cross-linked acellular porcine dermal patch and platelet-rich plasma fibrin matrix for augmentation. J Foot Ankle Surg. 2010;49:128–134. doi: 10.1053/j.jfas.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Hays P.L., Kawamura S., Deng X.H., Dagher E., Mithoefer K., Ying L. The role of macrophages in early healing of a tendon graft in a bone tunnel. J Bone Joint Surg Am. 2008;90:565–579. doi: 10.2106/JBJS.F.00531. [DOI] [PubMed] [Google Scholar]
- 26.Hope M., Saxby T.S. Tendon healing. Foot Ankle Clin. 2007;12(4):553–567. doi: 10.1016/j.fcl.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Fukasawa M., Bryant S.M., Nakamura R.M., Nakamura R.M., diZerega G.S. Modulation of fibroblast proliferation by postsurgical macrophages. J Surg Res. 1987;43(6):513–520. doi: 10.1016/0022-4804(87)90124-7. [DOI] [PubMed] [Google Scholar]
- 28.Leadbetter W.B. Cell-matrix response in tendon injury. Clin Sports Med. 1992;11:533–578. [PubMed] [Google Scholar]
- 29.Laskin D.L. Macrophages and inflammatory mediators in chemical toxicity: a battle of forces. Chem Res Toxicol. 2009;22:1376–1385. doi: 10.1021/tx900086v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sica A., Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Investig. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park H.C., Quan H., Zhu T., Kim Y., Kim B., Yang H.C. The effects of M1 and M2 macrophages on odontogenic differentiation of human dental pulp cells. J Endod. 2017;43(4):596–601. doi: 10.1016/j.joen.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Dakin S.G., Werling D., Hibbert A., Abayasekara D.R., Young N.J., Smith R.K. Macrophage sub-populations and the lipoxin A4 receptor implicate active inflammation during equine tendon repair. PLoS One. 2012;7(2) doi: 10.1371/journal.pone.0032333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hackett L., Millar N.L., Lam P., Murrell G.A. Are the symptoms of calcific tendinitis due to neoinnervation and/or neovascularization? J Bone Joint Surg Am. 2016;98(3):186–192. doi: 10.2106/JBJS.O.00417. [DOI] [PubMed] [Google Scholar]
- 34.Sugg K.B., Lubardic J., Gumucio J.P., Mendias C.L. Changes in macrophage phenotype and induction of epithelial-to-mesenchymal transition genes following acute Achilles tenotomy and repair. J Orthop Res. 2014;32:944–951. doi: 10.1002/jor.22624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krzyszczyk P., Schloss R., Palmer A., Berthiaume F. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front Physiol. 2018;9:419. doi: 10.3389/fphys.2018.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stahl M., Schupp J., Jager B., Schmid M., Zissel G., Muller-Quernheim J. Lung collagens perpetuate pulmonary fibrosis via CD204 and M2 macrophage activation. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0081382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bility M.T., Cheng L., Zhang Z., Luan Y., Li F., Chi L. Hepatitis B virus infection and immunopathogenesis in a humanized mouse model: induction of human-specific liver fibrosis and M2-like macrophages. PLoS Pathog. 2014;10(3) doi: 10.1371/journal.ppat.1004032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore J.P., Vinh A., Tuck K.L., Sakkal S., Krishnan S.M., Chan C.T. Drummond, M2 macrophage accumulation in the aortic wall during angiotensin II infusion in mice is associated with fibrosis, elastin loss, and elevated blood pressure. Am J Physiol Heart Circ Physiol. 2015;309:H906–H917. doi: 10.1152/ajpheart.00821.2014. [DOI] [PubMed] [Google Scholar]
- 39.Thomopoulos S., Parks W.C., Rifkin D.B., Derwin K.A. Mechanisms of tendon injury and repair. J Ortho Res. 2015;33(6):832–839. doi: 10.1002/jor.22806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hegedus E.J., Cook C., Brennan M., Wyland D., Garrison J.C., Driesner D. Vascularity and tendon pathology in the rotator cuff: a review of literature and implications for rehabilitation and surgery. Br J Sports Med. 2010;44:838–847. doi: 10.1136/bjsm.2008.053769. [DOI] [PubMed] [Google Scholar]
- 41.Dean B.J., Lostis E., Oakley T., Rombach I., Morrey M.E., Carr A.J. The risks and benefits of glucocorticoid treatment for tendinopathy: a systematic review of the effects of local glucocorticoid on tendon. Semin Arthritis Rheum. 2014;43:570–576. doi: 10.1016/j.semarthrit.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 42.Wells K., Littell J.H. Study quality assessment in systematic reviews of research on intervention effects. Res Soc Work Pract. 2009;19(1):53–62. [Google Scholar]
- 43.Klatte-Schulz F., Minkwitz S., Schmock A., Bormann N., Kurtoglu A., Tsitsilonis S. Different Achilles tendon pathologies show distinct histological and molecular characteristics. Int J Mol Sci. 2018;19(2):404. doi: 10.3390/ijms19020404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dakin S.G., Newton J., Martinez F.O., Hedley R., Gwilym S., Jones N. Chronic inflammation is a feature of Achilles tendinopathy and rupture. Br J Sports Med. 2018;52(6):359–367. doi: 10.1136/bjsports-2017-098161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bergqvist F., Carr A.J., Wheway K., Watkins B., Oppermann U., Jakobsson P.J. Divergent roles of prostacyclin and PGE2 in human tendinopathy. Arthritis Res Ther. 2019;21(1):74. doi: 10.1186/s13075-019-1855-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barboni B., Russo V., Gatta V., Bernabo N., Berardinelli P., Mauro A. Therapeutic potential of hAECs for early Achilles tendon defect repair through regeneration. J Tissue Eng Regenerat Med. 2017;12(3):e1594–e1608. doi: 10.1002/term.2584. [DOI] [PubMed] [Google Scholar]
- 47.Chamberlain C.S., Clements A.B., Kink J.A., Choi U., Baer G.S., Halanski M.A. Extracellular vesicle-educated macrophages promote early Achilles tendon healing. Stem Cells. 2019;37(5):652–662. doi: 10.1002/stem.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Millar N.L., Hueber A.J., Reilly J.H., Xu Y., Fazzi U.G., Murrell G.A. Inflammation is present in early human tendinopathy. Am J Sports Med. 2010;38(10):2085–2091. doi: 10.1177/0363546510372613. [DOI] [PubMed] [Google Scholar]
- 49.Matthews T.J., Hand G.C., Rees J.L., Athanasou N.A., Carr A.J. Pathology of the torn rotator cuff tendon. Reduction in potential for repair as tear size increases. J Bone Joint Surg Br. 2006;88(4):489–495. doi: 10.1302/0301-620X.88B4.16845. [DOI] [PubMed] [Google Scholar]
- 50.Gotoh M., Hamada K., Yamakawa H., Tomonage A., Inoue A., Fukuda H. Significance of granulation tissue in torn supraspinatus insertions: an immunohistochemical study with antibodies against interleukin-1 beta, cathepsin D, and matrix metalloprotease-1. J Orthop Res. 1997;15(1):33–39. doi: 10.1002/jor.1100150106. [DOI] [PubMed] [Google Scholar]
- 51.Gelberman R.H., Amifl D., Gonsalves M., Woo S., Akeson W.H. The influence of protected passive mobilization on the healin of flexor tendons: a biochemical and microangiographic study. Hand. 1981;13(2):120–128. doi: 10.1016/s0072-968x(81)80051-4. [DOI] [PubMed] [Google Scholar]
- 52.Gelberman R.H., Vande Berg J.S., Lundborg G.N., Akeson W.H. Flexor tendon healing and restoration of the gliding surface. An ultrastructural study in dogs. J Bone Joint Surg Am. 1983;65(1):70–80. [PubMed] [Google Scholar]
- 53.Kannus P., Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73(10):1507–1525. [PubMed] [Google Scholar]
- 54.Galatz L.M., Gerstenfeld L., Heber-Katz E., Rodeo S.A. Tendon regeneration and scar formation: the concept of scarless healing. J Orthop Res. 2015;33(6):823–831. doi: 10.1002/jor.22853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Herdrich B.L., Danzer E., DaveyMG, Bermudez D.M., Radu A., Zhang L. Liechty, fetal tendon wound size modulates wound gene expression and subsequent wound phenotype. Wound Repair Regen. 2010;18(5):543–549. doi: 10.1111/j.1524-475X.2010.00615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharma P., Maffulli N. Biology of tendon injury: healing, modeling and remodeling. J Musculoskelet Neuronal Interact. 2006;6(2):181–190. [PubMed] [Google Scholar]
- 57.Liu C.F., Aschbacher-Smith L., Barthelery N.J., Dyment N., Butler D., Wylie C. What we should know before using tissue engineering techniques to repair injured tendons: a developmental biology perspective. Tissue Eng B Rev. 2011;17(3):165–176. doi: 10.1089/ten.teb.2010.0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lomas A.J., Ryan C.N., Sorushanova A., Shologu N., Sideri A.I., Tsioli V. The past, present and future in scaffold-based tendon treatments. Adv Drug Deliv Rev. 2015;84:257–277. doi: 10.1016/j.addr.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 59.Millar N.L., Murrell G.A., McInnes I.B. Inflammatory mechanisms in tendinopathy – towards translation. Nat Rev Rheumatol. 2017;13(2):110–122. doi: 10.1038/nrrheum.2016.213. [DOI] [PubMed] [Google Scholar]
- 60.Ackermann P.W. Neuronal regulation of tendon homoeostasis. Int J Exp Pathol. 2013;94(4):271–286. doi: 10.1111/iep.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weiss U. Inflammation. Nature. 2008;454(7203):427. doi: 10.1038/454427a. [DOI] [PubMed] [Google Scholar]
- 62.Benjamin M., Mcgonagle D. The enthesis organ concept and its relevance to the spondyloarthropathies. Adv Exp Med Biol. 2009;649:57–70. doi: 10.1007/978-1-4419-0298-6_4. [DOI] [PubMed] [Google Scholar]
- 63.Humphrey J.D., Dufresne E.R., Schwartz M.A. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol. 2014;15(12):802–812. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mills C.D. Anatomy of a discovery: M1 and M2 macrophages. Front Immunol. 2015;6:212. doi: 10.3389/fimmu.2015.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Das A., Sinha M., Datta S., Abas M., Chaffee S., Sen C.K. Monocyte and macrophage plasticity in tissue repair and regeneration. Am J Pathol. 2015;185(10):2596–2606. doi: 10.1016/j.ajpath.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chu S.Y., Chou C.H., Huang H.D., Yen M.H., Hong H.C., Chao P.H. Mechanical stretch induces hair regeneration through the alternative activation of macrophages. Nat Commun. 2019;10(1):1524. doi: 10.1038/s41467-019-09402-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Adair-Kirk T.L., Atkinson J.J., Kelley D.G., Arch D.G., Miner J.H., Senior R.M. A chemotactic peptide from laminin alpha 5 functions as a regulator of inflammatory immune responses via TNF alpha-mediated signaling. J Immunol. 2005;174(3):1621–1629. doi: 10.4049/jimmunol.174.3.1621. [DOI] [PubMed] [Google Scholar]
- 68.Hance K.A., Tataria M., Ziporin S.J., Lee J.K., Thompson R.W. Monocyte chemotactic activity in human abdominal aortic aneurysms: role of elastin degradation peptides and the 67-kD cell surface elastin receptor. J Vasc Surg. 2002;35(2):254–261. doi: 10.1067/mva.2002.120382. [DOI] [PubMed] [Google Scholar]
- 69.Adair-Kirk T.L., Senior R.M. Fragments of extracellular matrix as mediators of inflammation. Int J Biochem Cell Biol. 2008;40(6–7):1101–1110. doi: 10.1016/j.biocel.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McKee C.M., Penno M.B., Cowman M., Burdick M.D., Strieter R.M., Bao C. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J Clin Investig. 1996;98(10):2403–2413. doi: 10.1172/JCI119054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kietrys D.M., Barr-Gillespie A.E., Amin M., Wade C.K., Popoff S.N. Aging contributes to inflammation in upper extremity tendons and declines in forelimb agility in a rat model of upper extremity overuse. PLoS One. 2012;7(10) doi: 10.1371/journal.pone.0046954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pingel J., Wienecke J., Kongsgaard M., Behzad H., Abraham T., Langberg H. Increased mast cell numbers in a calcaneal tendon overuse model. Scand J Med Sci Sport. 2013;23(6):e353–e360. doi: 10.1111/sms.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mcwhorter F.Y., Davis C.T., Liu W.F. Physical and mechanical regulation of macrophage phenotype and function. Cell Mol Life Sci. 2015;72(7):1303–1316. doi: 10.1007/s00018-014-1796-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Friedl P., Weigelin B. Interstitial leukocyte migration and immune function. Nat Immunol. 2008;9(9):960–969. doi: 10.1038/ni.f.212. [DOI] [PubMed] [Google Scholar]
- 75.Santiago-Garcıa J., Kodama T., Pitas R.E. The class A scavenger receptor binds to proteoglycans and mediates adhesion of macrophages to the extracellular matrix. J Biol Chem. 2003;278(9):6942–6946. doi: 10.1074/jbc.M208358200. [DOI] [PubMed] [Google Scholar]
- 76.Block M.R., Badowski C., Million-Fremillon A., Bouvard D., Bouin A.P., Faurobert E. Podosome-type adhesions and focal adhesions, so alike yet so different. Eur J Cell Biol. 2008;87(8–9):491–506. doi: 10.1016/j.ejcb.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 77.Vereyken E.J., Heijnen P.D., Baron W., de Vries E.H., Dijkstra C.D., Teunissen C.E. Classically and alternatively activated bone marrow derived macrophages differ in cytoskeletal functions and migration towards specific CNS cell types. J Neuroinflammation. 2011;8:58. doi: 10.1186/1742-2094-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jetten N., Roumans N., Gijbels M.J., Romano A., Post M.J., de Winther M.P. Wound administration of m2-polarized macrophages does not improve murine cutaneous healing responses. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0102994. [DOI] [PMC free article] [PubMed] [Google Scholar]