Highlights
-
•
The association between Modic changes and tracer uptake in bone scintigraphy has not previously been studied.
-
•
Our results indicate that Type 1 Modic changes are associated with increased tracer uptake in bone scintigraphy.
-
•
Increased bone tracer uptake reflects increased bone turnover in the area of Type 1 Modic changes.
Abbreviations: MC, Modic changes; MRI, magnetic resonance imaging; 99mTc, 99mTechnetium; 99mTc-HDP, 99m Technetium-labeled hydroxymethylene diphosphonate; M1, Type 1 Modic change; M2, Type 2 Modic change; M3, Type 3 Modic change; M1/2, Type 1/2 mixed Modic change; M1/3, Type 1/3 mixed Modic change; M2/3, Type 2/3 mixed Modic change; LBP, low back pain; κ, Cohen’s kappa; ICC, intraclass correlation coefficients; SPECT, single photon emission computed tomography
Keywords: Modic changes, Magnetic resonance imaging, Bone scintigraphy, Bone turnover
Abstract
Purpose
Our purpose was to evaluate whether Modic changes (MC) revealed in lumbar MRI are associated with increased tracer uptake shown in bone scintigraphy. To our knowledge, this has not previously been studied.
Methods
We included patients with MC shown in lumbar MRI and bone scintigraphy performed within six months before or after MRI. Exclusion criteria included metastasis and other specific lesions in the area of interest such as discitis, tumors or fractures. We compared the level and type of MC to the degree of tracer uptake shown in bone scintigraphy. Tracer uptake was assessed both visually and quantitatively. We calculated the lesion-to-normal-bone ratios between the MC area with increased tracer uptake and the vertebra with normal tracer uptake. We used linear mixed models in statistical analyses.
Results
Our study sample consisted of 93 patients (aged 37–86) with 299 MC (28 Type 1 (M1), 50 mixed Type 1/2 (M1/2), 3 mixed Type 1/3 (M1/3), 211 Type 2 (M2), 6 mixed Type 2/3 (M2/3), and 1 Type 3 (M3)). Of all the MC, 26 (93 %) M1, 34 (64 %) in the combined M1/2 and M1/3 group, and 11 (5 %) in the combined M2, M2/3 and M3 group showed increased tracer uptake. The mean lesion-to-normal-bone ratio was higher for lesions with a Type 1 component (M1, M1/2 and M1/3) than for other types, at 1.55 (SD 0.16) for M1; 1.44 (SD 0.21) for combined M1/2 and M1/3; and 1.28 (SD 0.11) for combined M2, M2/3 and M3; p = 0.001).
Conclusion
In most cases, MC with a Type 1 component showed increased tracer uptake in bone scintigraphy. This indicates that bone turnover is accelerated in the M1 area.
1. Introduction
Modic changes (MC) are vertebral endplate lesions that are visible in magnetic resonance imaging (MRI), and which correlate with intervertebral disc degeneration [1,2] and low back pain (LBP) [[3], [4], [5], [6]]. They are classified on the basis of vertebral end plate signal intensities into Types 1, 2 and 3 [2]. Type 1 changes (M1) show low signal intensity on T1-weighted and high signal intensity on T2-weighted images, indicating bone marrow edema. M1 is connected to increased bone turnover [2]. Type 2 changes (M2) show high signal intensity on T1- and T2-weighted images, representing yellow bone marrow replacement [2], whereas Type 3 changes (M3), showing low signal intensity on T1- and T2-weighted images, are associated with subchondral bone sclerosis [1,7]. The identification of mixed types 1/2 (M1/2) and 2/3 (M2/3) is thought to indicate different stages of the same pathologic process, as Modic types can convert from one to another [[8], [9], [10], [11], [12]]. It has been suggested that injury, inflammation or infection are the etiological factors behind MC. Some researchers believe that these processes are closely interrelated with each other and that each might play a role in the chain of events leading to MC [13,14]. Recently, novel candidate genes have also been identified as a predisposing factor [15].
Bone scintigraphy is a functional imaging method based on the uptake of 99mTechnetium (99mTc) -labelled diphosphonates into the skeleton. The intensity of this uptake is related to bone blood flow, especially to osteoblast activity. Bone scintigraphy shows a focally increased uptake of a bone-seeking radiopharmaceutical in a variety of bone disorders such as metastases, fractures, infections, and inflammations [16,17].
As the relationship between bone scintigraphy and the different types of MC has not been quantified, we aimed to evaluate the relationship between MC and bone scintigraphy findings in terms of detection and quantification. We hypothesized that due to the increased bone turnover, M1 could also be visible as a hot spot with increased tracer uptake in bone scintigraphy.
2. Material and methods
2.1. Study population
The study population consisted of 204 patients who had undergone both lumbar MRI and bone scintigraphy during a six-month period in 2007 or 2008. We retrospectively chose eligible patients from the radiology information system of the University Hospital. The lumbar spine MRI scans of the patients were screened for any type of lumbar MC. We excluded patients with metastasis, tumors, spondylodiscitis, fractures, previous radiation therapy, or prominent image artefacts in the lumbar spine, as well as patients aged under 20. The final study sample consisted of 93 patients (mean age 64, range 37–86; 46 % males). The distribution of the time interval between the lumbar MRI and bone scintigraphy was positively skewed with a median of 28 days and interquartile range of 13–68 days. Fifteen (16 %) patients had a time interval of over 90 days. Table 1 presents the clinical indications of MRI and bone scintigraphy.
Table 1.
Indications of magnetic resonance imaging and scintigraphy among 93 patients.
| Indication | MRI | Scintigraphy |
|---|---|---|
| Malignancy or suspected malignancy | 52 (55.9 %) | 68 (73.1 %) |
| Back pain | 22 (23.7 %) | 4 (4.3 %) |
| Pre- or postoperative study | 7 (7.5 %) | 6 (6.5 %) |
| Neurological symptom or finding | 5 (5.4 %) | 4 (4.3 %) |
| Infection or suspected infection | 3 (3.2 %) | 3 (3.2 %) |
| Other | 4 (4.3 %) | 8 (8.6 %) |
The study was approved by the Ethics Committee of the University Hospital. The research was conducted according to the principles of the Declaration of Helsinki.
2.2. Imaging methods
2.2.1. MR imaging
We obtained MR images using a 1.5Tesla GE Signa with a Phased Array CTL Spine Coil (USA Instruments). The routine lumbar spine imaging protocol consisted of sagittal T1-weighted [e.g. Repetition time (TR) / Echo time (TE) 1810 / 18 ms] fluid-attenuated inversion recovery, sagittal T2-weighted (e.g. TR / TE 3960 / 116 ms) fast spin-echo and axial T2-weighted fast spin-echo (e.g. TR / TE 3000 / 103 ms) imaging of the lumbar spine. The inversion recovery time for the T1-weighted images was 860 ms, and the number of excitations for both the T1- and T2-weighted images was four. The echo train length for the T1-weighted images was 8; for the T2-weighted sagittal images, 29; and for the T2-weighted axial images, 26. The image matrix of the T1-weighted images was 448 × 192; of the T2-weighted sagittal images, 448 × 224; and of the T2-weighted axial images, 256 × 160. The field of view for the sagittal images was 28 × 28 cm and for the axial images 18 × 18. Slice thickness was 4 mm and the interslice gap was 1 mm.
2.2.2. Nuclear imaging
Bone scintigraphy was obtained approximately three hours after an intravenous injection of 550–740 MBq 99mtechnetium-labeled hydroxymethyline diphosphonate (99mTc-HDP, Mallinckrodt Inc.). We performed whole body scans with anterior and posterior views using a dual head gamma camera equipped with a low-energy high-resolution collimator. The camera used was an Adac Vertex (ADAC Laboratories, Milpitas, CA, USA), an Adac Forte, or a Siemens Symbia T2 (Siemens Medical Solutions USA, Inc.). The matrix was 512 × 512, 512 × 1024, or 256 × 1024, respectively. The scanning speed was 8 cm/min. Energy discrimination was provided by a 20 % window centered on the 140-keV peak of 99mTc.
2.3. Image analysis
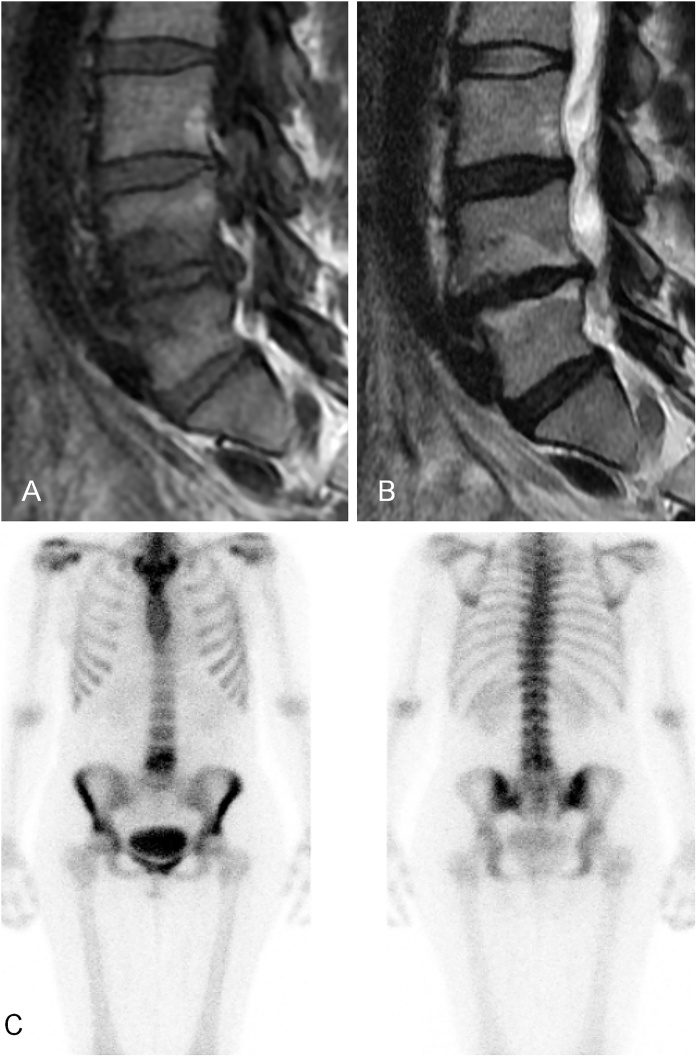
A fellow in musculoskeletal radiology (JJ) analyzed the MR images at a clinical workstation (Neaview Radiology, version 2.23, Neagen corp., Finland). He recorded the MC at the endplates of the lumbar spine (L1 – S1). The type, depth and location of each MC were classified as previously [2,7,8] (Fig. 1). He divided the depth of the MC into three categories: 1–25 %, 26–50 %, and over 50 % of the total height of the lumbar vertebra.
Fig. 1.
Example of Modic 1/2 change at L4/5.
(A) T1-weighted MRI, (B) T2-weighted MRI, and (C) Increased tracer uptake shown in bone scintigraphy.
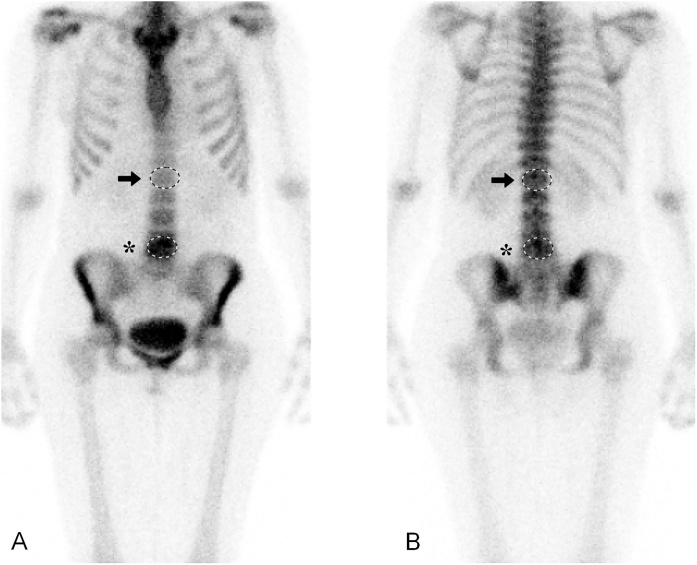
The same researcher (JJ) analyzed the bone scintigraphy images using the same clinical workstation. The scintigraphy images of the patients with MC were screened for spots of increased tracer uptake. If the spot was in the anatomic location of MC, it was considered a hot spot resulting from the increased bone turnover of MC. The intensity of the tracer uptake in the hot spot was measured by the workstation’s region of interest (ROI) tool. This tool indicates the average of the pixel values in the measured area. The greater the average pixel value, the more intense the tracer uptake. To normalize the intensity of the tracer uptake in the hot spot with the normal bone tracer uptake, a lesion-to-normal-bone ratio was determined. For the lesion-to-normal-bone ratio, the ROI was drawn over the hot spot and another ROI of equal size was drawn over the first lumbar vertebra that had normal, symmetrical tracer uptake (Fig. 2). In few cases of MC located at L1/2, the twelfth thoracic vertebra was used as the control. The analyzer measured the tracer uptake intensities from both the anterior and posterior views and used a geometric mean of the pixel values to calculate the lesion-to-normal-bone ratio.
Fig. 2.
Example of measuring pixel values (PV) by placing ROIs on lesion with increased tracer uptake (asterisk) and on control vertebra (arrow).
(A) Anterior image; the average PV on the lesion with increased tracer uptake is 223 (SD 31); and on the control vertebra, 110 (SD 21). Ratio of increased tracer uptake intensity: 223/110 = 2.03. (B) Posterior image; the average PV on the lesion with increased tracer uptake is 190 (SD 33), and on the control vertebra, 160 (SD 35). Ratio of increased tracer uptake intensity: 190/160 = 1.19. Lesion-to-normal-bone ratio is a geometrical mean of anterior and posterior measurements: √2.03 × 1.19 = 1.55.
To estimate the reliability of the image analysis, the first author (JJ) reassessed the MRI scans and bone scintigraphy images of 20 patients, blinded to the original reading. In addition, an experienced musculoskeletal radiologist (JN) evaluated the same patients, also blinded in order to estimate interobserver reliability.
2.4. Statistical analysis
We assessed the prevalence of MC at different lumbar levels using cross-tabulations. We evaluated the number and proportion of MC with increased tracer uptake, and calculated the mean values with standard deviations of the lesion-to-normal-bone ratios separately for each MC type and for the combined group with the M1 component (M1/2 and M1/3) and the combined group without the M1 component (M2, M2/3 and M3). We also made the same calculations for three time-interval groups (< 1 month, 1–3 months, > 3 months) to further specify the effect of the time interval between the imaging studies. We analyzed the relationship between the MC types (M1, combined M1/2 and M1/3 group, and combined M2, M2/3 and M3 group)) and the lesion-to-normal-bone ratio using linear mixed models, because of the correlated measures within the subjects. The lesion-to-normal-bone ratio was treated as a dependent variable, lumbar level as a repeated factor, and the combined MC type groups was treated as a fixed effect. As sensitivity analyses, we conducted the same analyses, excluding those with a time period of over 90 days. We analyzed the intra- and interobserver reliabilities using Cohen’s kappa (κ) for the detection and type of MC, and intraclass correlation coefficients (ICC) for the detection of hot spots and lesion-to-normal-bone ratio measurements [18,19]. We used IBM SPSS Statistics 24 to conduct all the statistical analyses.
3. Results
3.1. Number and distribution of Modic changes
The total number of MCs was 299 (Table 2). They were distributed at the different lumbar levels as follows: 13 (4 %) at L1/2, 38 (13 %) at L2/3, 34 (11 %) at L3/4, 80 (27 %) at L4/5, and 134 (45 %) at L5/S1.
Table 2.
Distribution of Modic types according to lumbar levels.
| Modic type | L1/2 | L2/3 | L3/4 | L4/5 | L5/S1 | Total |
|---|---|---|---|---|---|---|
| M1 | 1 (7.7) | 7 (18.4) | 4 (11.8) | 11 (13.8) | 5 (3.7) | 28 (9.4) |
| M1/2 | 6 (46.2) | 9 (23.7) | 6 (17.6) | 13 (16.3) | 16 (11.9) | 50 (16.7) |
| M1/3 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (2.5) | 1 (0.7) | 3 (1.0) |
| M2 | 6 (46.2) | 22 (57.9) | 22 (64.7) | 54 (67.5) | 107 (79.9) | 211 (70.6) |
| M2/3 | 0 (0.0) | 0 (0.0) | 2 (5.9) | 0 (0.0) | 4 (3.0) | 6 (2.0) |
| M3 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.7) | 1 (0.3) |
| Total | 13 (100) | 38 (100) | 34 (100) | 80 (100) | 134 (100) | 299 (100) |
Numbers (%) indicated.
3.2. Modic types and their association with tracer uptake
The data consisted of 28 M1, 50 M1/2, three M1/3, 211 M2, six M2/3 and one M3. Table 3 presents the numbers and proportions of the Modic types with increased tracer uptake in scintigraphy and the means of the pixel values measured by scintigraphy. In all, 26 (93 %) MC in the M1 group, 34 (64 %) in the combined M1/2 and M1/3 group, and 11 (5 %) in the combined other Modic types group showed increased tracer uptake in bone scintigraphy.
Table 3.
Key figures of Modic changes (MC) with increased tracer uptake shown in scintigraphy.
| Modic type | Number of MC and mean lesion-to-normal-bone ratio |
|||
|---|---|---|---|---|
| n (%) | Mean (SD)a | Mean (SD)b | Pc | |
| M1 | 1.55 (0.16) | |||
| M1 | 26 (93) | 1.55 (0.16) | ||
| M1/2 & M1/3b | 1.44 (0.22) | 0.045 | ||
| M1/2 | 31 (62) | 1.41 (0.21) | ||
| M1/3 | 3 (100) | 1.76 (0.01) | ||
| M2 & M3b | 1.28 (0.11) | < 0.001 | ||
| M2 | 10 (5) | 1.28 (0.12) | ||
| M2/3 | 0 (0) | – | ||
| M3 | 1 (100) | 1.36 (−) | ||
| Total | 71(24) | 1.46 (0.21) | ||
SD = standard deviation.
Each Modic type separately.
Combined Modic types.
Pairwise comparisons to M1 from the linear mixed model.
The mean lesion-to-normal-bone ratio in bone scintigraphy was significantly higher for the MC with an M1 component than for the MC without an M1 component; 1.55 (SD 0.16) for M1, 1.41 (SD 0.21) for M1/2, 1.76 (SD 0.01) for M1/3, 1.28 (SD 0.12) for M2, and 1.36 for M3 (p = 0.001, Table 3). The estimated mean lesion-to-normal-bone ratio for M1 (1.54, standard error (SE) 0.04) was higher than that for the combined M1/2 and M1/3 group (1.44, SE 0.03; p = 0.045) and that for the combined M2, M2/3 and M3 group (1.29, SE 0.06; p < 0.001). The regression coefficients (compared to M1) were −0.10 (95 % C.I. −0.20, −0.002) for the combined M1/2 and M1/3 group, and −0.25 (−0.39, −0.12) for the combined M2, M2/3 and M3 group. When the lesions with a Type 1 component (M1, M1/2, M1/3) were combined as one group, bone scintigraphy showed 60 out of 81 (74 %) MC with increased tracer uptake (mean lesion-to-normal-bone ratio 1.49, SD 0.20). In contrast, scintigraphy showed 10 of the 217 (4.6 %) MC in the combined group with a Type 2 component (M2 and M2/3) as having increased tracer uptake (mean lesion-to-normal-bone ratio 1.28, SD 0.12).
3.3. Effect of time interval between the imaging studies on lesion-to-normal-bone ratios
Only 8 % of the sample had a time interval of over 90 days between the MRI and bone scintigraphy. Table 4 shows the mean lesion-to-normal-bone ratios separately for those with a time interval of 30 days or less, 31–90 days, and over 90 days. When those with a time interval of over 90 days were excluded, the estimated mean lesion-to-normal-bone ratio for M1 (1.53, SE 0.04) was higher than that of the combined M2, M2/3 and M3 group (1.26, SE 0.06; p < 0.001) but did not differ from that of the combined M1/2 and M1/3 group (1.47, SE 0.03; p = 0.212). The regression coefficients (compared to M1) were −0.06 (95 % C.I. −0.16, 0.04) for the combined M1/2 and M1/3 group, and −0.28 (−0.42, −0.14) for the combined M2, M2/3 and M3 group.
Table 4.
Key figures of Modic changes (MC) with increased tracer uptake divided into three time-groups. MRI = magnetic resonance imaging.
| Time interval between MRI and bone scintigraphy |
||||||
|---|---|---|---|---|---|---|
| 30 days or less |
31–90 days |
91–180 days |
||||
| N | Mean (SD)a | N | Mean (SD)a | N | Mean (SD)a | |
| M1 | 20 (76.9) | 1.51 (0.11) | 4 (15.4) | 1.75 (0.27) | 2 (7.7) | 1.59 (0.00) |
| M1/2 & M1/3 | 17 (50.0) | 1.47 (0.16) | 16 (47.0) | 1.42 (0.28) | 1 (2.9) | 1.13 (-) |
| M2 & M3 | 6 (54.5) | 1.25 (0.05) | 2 (18.2) | 1.27 (0.13) | 3 (27.3) | 1.36 (0.20) |
| Total | 43 (60.6) | 22 (31.0) | 6 (8.4) | |||
Mean lesion-to-normal-bone ratio.
3.4. Reliability of image readings
The intraobserver reliability for the type of MC (κ = 0.73) and bone scintigraphy measurements (ICC = 0.72) was good. The interobserver reliability for detecting MCs was very good (κ = 0.96) and for detecting scintigraphy’s hot spots, excellent (ICC = 0.92). The interobserver reliability for the type of MC (κ = 0.69) was good and for bone scintigraphy measurements (ICC = 0.91), excellent.
4. Discussion
We found a significant association between the MC containing M1 and increased bone turnover shown in bone scintigraphy in the same area. In the current study, bone scintigraphy showed that 93 % of M1 and 64 % of the combined M1/2 and M1/3 group had increased tracer uptake, whereas the percentage of MC with an increased tracer uptake was only 5 % among the other types (M2, M2/3, M3). To our knowledge, this is the first publication to report a specific relationship between MC and bone scintigraphy findings. Our results suggest that most M1 may be visible in bone scintigraphy as the lesion of an increased tracer uptake. This association also supports the theory that bone turnover and blood flow are increased in the area of the MC containing an M1 component [2].
Modic et al. [2] investigated histopathological specimens from the areas of degenerative endplate signal changes. The histopathology of M1 showed fibrovascular replacement of normal bone marrow. The amount of reactive woven bone with thickened trabeculae and prominent osteoclasts and osteoblasts was also higher, which indicates rapid bone turnover. However, the histopathology of M1 was originally based on only three specimens. Few studies exist on the bone turnover of MC shown in nuclear imaging. In one reliability study, bone scintigraphy or single photon emission computed tomography (SPECT) showed that M1 and M2 changes had an increased tracer uptake [20]. Lusins et al. [21] proposed that a positive endplate in SPECT in degenerative disc disease is related to marrow changes in the region of the endplate of the disc, and that SPECT may be of value in delineating early end plate changes prior to the MRI showing M1 or M2 in the same area. Their patients’ (n = 48) MRI and SPECT were taken within six weeks of each other. Twenty-six of these displayed M1 and 12 showed M2. Of the 38 individuals with MC, all but one had a positive SPECT scan. In our study, the proportion of M2 with increased tracer uptake in bone scintigraphy was considerably lower. We had only a few MC that contained an M3 component, which was in accordance with other studies [3]. We found one M3, three M1/3 changes and six M2/3 changes. Bone scintigraphy showed increased tracer uptake for all the M3 and M1/3, but none for the M2/3. However, the low number of cases limits any firm conclusions. An Australian study analyzed bone samples from MC using micro-CT to assess bone micro-architectural parameters and remodeling indices. M1 showed the highest bone turnover, which is consistent with our results. The M2 biopsies were indicative of reduced bone formation and remodeling. The M3 biopsies suggested a more stable sclerotic phase, linked to increased bone formation and reduced resorption [22].
The pathophysiology of MC is still not known. M1 may represent an inflammatory reaction of the subchondral bone [[23], [24], [25], [26], [27], [28], [29], [30]]. It has been suggested that repeated trauma to the intervertebral disc results in upregulation of inflammatory mediators in the nucleus pulposus. Diffusion of such toxic chemicals through the vertebral endplates could result in a local inflammatory reaction [28]. The results of Ohtori et al. [26] suggest that endplate abnormalities are related to inflammation, and the number of TNF-immunoreactive cells in the endplates with M1 was significantly higher than that in the endplates with M2. In a French study, the level of high-sensitive C-reactive protein was higher in M1 than in M2 [24]. Furthermore, Dudli et al. [30] concluded that MC require endplate defects that allow bone marrow and nucleus pulposus cells to co-mingle, and an adjacent inflammatory “MC disc” that can amplify the immune response. Recently, a low-grade bacterial infection has also been proposed as an etiological factor of MC. The nuclear tissue of herniated discs demonstrates the presence of low virulent anaerobic microorganisms. A quiet infection in the disc due to these low virulent bacteria may give rise to an inflammation in the adjacent bone around the endplates, which appears as M1 [31]. Our results suggest that blood flow and osteoblast activity are increased in the area of MC that contain an M1 component.
In the diagnostic context, the results of our study may provide an additional tool for physicians who report findings in bone scintigraphy. The clinical relevance of our finding is supported by the observation that MC with an M1 component are more frequently associated with pain than other Modic types [12,27,[32], [33], [34]]. In a Japanese study [26], 73 % of the patients with M1 suffered from low back pain (LBP), in comparison to only 11 % of those with M2. Kääpä et al. [27] showed in their descriptive study that patients with chronic LBP displaying an M1 suffered from significantly more pain and disability than patients with M1/2. Their tentative interpretation was that as an M1 turns into an M2, pain intensity and perceived disability subside. Kjaer et al. [34] found a strong association between LBP and MC, particularly with M1, in a population-based sample. Albert and Manniche [35] observed that pain was more frequent among subjects displaying M1 than among those with M2, although the difference was not statistically significant.
Anatomical imaging such as MRI offers morphological information on tissues such as bone marrow. It may reveal degenerative disc disease, osteophytes and MC in patients with LBP. The anatomical information of bone scintigraphy is inferior to that of MRI, but a potential advantage of such functional imaging is that it can show the physiological activity of an osseous lesion [36]. Although we did not address this in the current study, the amount of tracer uptake may also correlate with MC activity and show a similar pain relationship. The additional information obtained through bone scintigraphy, in combination with MRI findings, may have clinical importance, as MC most likely present different stages of the same pathological process [8], and the temporal evolution of MC may take years [12,37]. Routine MRI may not be enough to differentiate between the active and silent phases of MC. Further studies on the association of the tracer uptake of MC, using a more sensitive and specific method such as SPECT and with concurrent pain and disability reports on the patient, would clarify the clinical relevance of the increased bone turnover.
Our study has some limitations. First, the bone scintigraphy results were analyzed by one reader according to the MRI findings and were hence not blinded. We did not analyze the bone scintigraphy results in a blinded way because we were primarily using scintigraphy not for lesion detection but as an activity indicator of focal bone metabolism. For the lesion-to-normal-bone ratio measurements, we attempted to locate the MC on the scintigraphy images as accurately as possible, according to the MRI findings. For improving the reliability of image reading, two readers assessed the images and were blinded to both the original readings and each other. The reliability of the image analysis varied from good to excellent, which reduces the image reading bias of our study design. The reliability of the interpretation of Modic classification has shown to vary from moderate [39] to excellent [20,[39], [40], [41], [42]]. In a North American study [20], interobserver reliability in the interpretation of bone scintigraphy was moderate, whereas two experienced physicians in nuclear medicine reached good interobserver agreement in the area of the lumbar spine in another study [43]. Second, as bone scintigraphy images are acquired from the planar anterior and posterior orientation, it is not always possible to localize the lesion to a precise skeletal location because of the overlap of the bony structures. Furthermore, the spatial resolution of bone scintigraphy is inferior to SPECT, which allows more accurate anatomic localization of increased tracer uptake [36]. Therefore, SPECT could offer a more sensitive and specific tool for differentiating MCs from other degenerative changes [38]. Third, planar bone scintigraphy is an unspecific imaging method, and the tracer uptake may be similar in many different malignant and benign conditions. We excluded metastasis, tumors and infections such as spondylodiscitis. However, many degenerative changes in the endplates, facet joints or on the edge of the vertebral body, such as spondylosis deformans or paravertebral osteophytes, may also manifest as an increased tracer uptake [36]. Therefore, as it was sometimes impossible to differentiate whether the tracer uptake was due to an MC or, for example, a paravertebral osteophyte, we used the information from the MRI images to place the ROIs in the bone scintigraphy over the area of the MC. The increased tracer uptake may have been due to an osteoarthritic change and not necessarily related to the MC itself. Finally, our patients’ lumbar MRI and bone scintigraphy were taken within six months of each other. Although it has been reported that the conversion of MC from one type to another is a slow process [12,37], we cannot exclude the possibility that the activity of the lesions in the bone scintigraphy may have changed during the six-month time period of the current study. However, most patients’ examinations were taken within three months of each other. We excluded patients with time interval of over three months between their MRI and bone scintigraphy in our sensitivity analysis. As a result, the estimated mean lesion-to-normal-bone ratio for M1 was still higher than that of the combined M2, M2/3 and M3 group, but it did not differ from the combined M1/2 and M1/3 group.
5. Conclusions
This is the first study to report a specific relationship between MC types and bone scintigraphy. MC containing the M1 component were associated with increased tracer uptake shown in bone scintigraphy, whereas changes with the M2 component rarely showed increased tracer uptake. The current findings indicate the increased bone turnover in the M1 area.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
None.
Contributor Information
Jyri Järvinen, Email: jyri.jarvinen@ppshp.fi.
Jaakko Niinimäki, Email: jaakko.niinimaki@ppshp.fi.
Jaro Karppinen, Email: jaro.karppinen@ttl.fi.
Reijo Takalo, Email: takaloreijo@gmail.com.
Marianne Haapea, Email: marianne.haapea@oulu.fi.
Osmo Tervonen, Email: osmo.tervonen@oulu.fi.
References
- 1.de Roos A., Kressel H., Spritzer C., Dalinka M. MR imaging of marrow changes adjacent to endplates in degenerative lumbar disk disease. AJR Am. J. Roentgenol. 1987;149:531–534. doi: 10.2214/ajr.149.3.531. [DOI] [PubMed] [Google Scholar]
- 2.Modic M.T., Steinberg P.M., Ross J.S., Masaryk T.J., Carter J.R. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166:193–199. doi: 10.1148/radiology.166.1.3336678. [DOI] [PubMed] [Google Scholar]
- 3.Jensen T.S., Karppinen J., Sorensen J.S., Niinimäki J., Leboeuf-Yde C. Vertebral endplate signal changes (Modic change): a systematic literature review of prevalence and association with non-specific low back pain. Eur. Spine J. 2008;17:1407–1422. doi: 10.1007/s00586-008-0770-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Järvinen J., Karppinen J., Niinimäki J., Haapea M., Grönblad M., Luoma K., Rinne E. Association between changes in lumbar Modic changes and low back symptoms over a two-year period. BMC Musculoskelet. Disord. 2015;16:98. doi: 10.1186/s12891-015-0540-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Määttä J.H., Wadge S., MacGregor A., Karppinen J., Williams F.M. Vertebral endplate (Modic) change is an independent risk factor for episodes of severe and disabling low back pain. Spine (Phila Pa 1976) 2015;40:1187–1193. doi: 10.1097/BRS.0000000000000937. [DOI] [PubMed] [Google Scholar]
- 6.Mok F.P., Samartzis D., Karppinen J., Fong D.Y., Luk K.D., Cheung K.M. Modic changes of the lumbar spine: prevalence, risk factors, and association with disc degeneration and low back pain in a large-scale population-based cohort. Spine J. 2016;16:32–41. doi: 10.1016/j.spinee.2015.09.060. [DOI] [PubMed] [Google Scholar]
- 7.Modic M.T., Masaryk T.J., Ross J.S., Carter J.R. Imaging of degenerative disk disease. Radiology. 1988;168:177–186. doi: 10.1148/radiology.168.1.3289089. [DOI] [PubMed] [Google Scholar]
- 8.Braithwaite I., White J., Saifuddin A., Renton P., Taylor B.A. Vertebral end-plate (Modic) changes on lumbar spine MRI: correlation with pain reproduction at lumbar discography. Eur. Spine J. 1998;7:363–368. doi: 10.1007/s005860050091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hutton M.J., Bayer J.H., Powell J.M. Modic vertebral body changes: the natural history as assessed by consecutive magnetic resonance imaging. Spine (Phila Pa 1976) 2011;36:2304–2307. doi: 10.1097/BRS.0b013e31821604b6. [DOI] [PubMed] [Google Scholar]
- 10.Kuisma M., Karppinen J., Niinimäki J., Kurunlahti M., Haapea M., Vanharanta H., Tervonen O. A three-year follow-up of lumbar spine endplate (Modic) changes. Spine (Phila Pa 1976) 2006;31:1714–1718. doi: 10.1097/01.brs.0000224167.18483.14. [DOI] [PubMed] [Google Scholar]
- 11.Jensen T.S., Bendix T., Sorensen J.S., Manniche C., Korsholm L., Kjaer P. Characteristics and natural course of vertebral endplate signal (Modic) changes in the Danish general population. BMC Musculoskelet. Disord. 2009;10:81. doi: 10.1186/1471-2474-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitra D., Cassar-Pullicino V.N., McCall I.W. Longitudinal study of vertebral type-1 end-plate changes on MR of the lumbar spine. Eur. Radiol. 2004;14:1574–1581. doi: 10.1007/s00330-004-2314-4. [DOI] [PubMed] [Google Scholar]
- 13.Dudli S., Fields A.J., Samartzis D., Karppinen J., Lotz J.C. Pathobiology of modic changes. Eur. Spine J. 2016;25:3723–3734. doi: 10.1007/s00586-016-4459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crockett M.T., Kelly B.S., van Baarsel S., Kavanagh E.C. Modic type 1 vertebral endplate changes: Injury, inflammation, or infection? AJR Am. J. Roentgenol. 2017;209:167–170. doi: 10.2214/AJR.16.17403. [DOI] [PubMed] [Google Scholar]
- 15.Kraatari M., Skarp S., Niinimäki J., Karppinen J., Männikkö M. A whole exome study identifies novel candidate genes for vertebral bone marrow signal changes (Modic changes) Spine (Phila Pa 1976) 2017;42:1201–1206. doi: 10.1097/BRS.0000000000002049. [DOI] [PubMed] [Google Scholar]
- 16.Leffers D., Collins L. An overview of the use of bone scintigraphy in sports medicine. Sports Med. Arthrosc. 2009;17:21–24. doi: 10.1097/JSA.0b013e3181974314. [DOI] [PubMed] [Google Scholar]
- 17.Kanstrup I.L. Bone scintigraphy in sports medicine: a review. Scand. J. Med. Sci. Sports. 1997;7:322–330. doi: 10.1111/j.1600-0838.1997.tb00161.x. [DOI] [PubMed] [Google Scholar]
- 18.Altman D. 1st ed. Chapman & Hall/CRC; London: 1991. Practical Statistics for Medical Research. [Google Scholar]
- 19.Rosner B. 6th ed. Duxbury Press; Belmont: 2005. Fundamentals of Biostatistics. [Google Scholar]
- 20.Mulconrey D.S., Knight R.Q., Bramble J.D., Paknikar S., Harty P.A. Interobserver reliability in the interpretation of diagnostic lumbar MRI and nuclear imaging. Spine J. 2006;6:177–184. doi: 10.1016/j.spinee.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Lusins J.O., Cicoria A.D., Goldsmith S.J. SPECT and lumbar MRI in back pain with emphasis on changes in end plates in association with disc degeneration. J. Neuroimaging. 1998;8:78–82. doi: 10.1111/jon19988278. [DOI] [PubMed] [Google Scholar]
- 22.Perilli E., Parkinson I.H., Truong L.H., Chong K.C., Fazzalari N.L., Osti O.L. Modic (endplate) changes in the lumbar spine: bone micro-architecture and remodelling. Eur. Spine J. 2015;24:1926–1934. doi: 10.1007/s00586-014-3455-z. [DOI] [PubMed] [Google Scholar]
- 23.Ulrich J.A., Liebenberg E.C., Thuillier D.U., Lotz J.C. ISSLS prize winner: repeated disc injury causes persistent inflammation. Spine (Phila Pa 1976) 2007;32:2812–2819. doi: 10.1097/BRS.0b013e31815b9850. [DOI] [PubMed] [Google Scholar]
- 24.Rannou F., Ouanes W., Boutron I., Lovisi B., Fayad F., Mace Y., Borderie D., Guerini H., Poiraudeau S., Revel M. High-sensitivity C-reactive protein in chronic low back pain with vertebral end-plate Modic signal changes. Arthritis Rheum. 2007;57:1311–1315. doi: 10.1002/art.22985. [DOI] [PubMed] [Google Scholar]
- 25.Modic M.Y., Ross J.S. Lumbar degenerative disk disease. Radiology. 2007;245:43–61. doi: 10.1148/radiol.2451051706. [DOI] [PubMed] [Google Scholar]
- 26.Ohtori S., Inoue G., Ito T., Koshi T., Ozawa T., Doya H., Saito T., Moriya H., Takahashi K. Tumor necrosis factor-immunoreactive cells and PGP 9.5-immunoreactive nerve fibers in vertebral endplates of patients with discogenic low back Pain and Modic Type 1 or Type 2 changes on MRI. Spine. 2006;31:1026–1031. doi: 10.1097/01.brs.0000215027.87102.7c. [DOI] [PubMed] [Google Scholar]
- 27.Kääpä E., Luoma K., Pitkäniemi J., Kerttula L., Grönblad M. Correlation of size and type of Modic type 1 and 2 lesion with clinical symptoms - a descriptive study in a subgroup of chronic low back pain patients based on a university hospital patient sample. Spine (Phila Pa 1976) 2012;37:134–139. doi: 10.1097/BRS.0b013e3182188a90. [DOI] [PubMed] [Google Scholar]
- 28.Crock H.V. Internal disc disruption. A challenge to disc prolapse fifty years on. Spine (Phila Pa 1976) 1986;11:650–653. [PubMed] [Google Scholar]
- 29.Fayad F., Lefevre-Colau M.M., Rannou F., Quintero N., Nys A., Macé Y., Poiraudeau S., Drapé J.L., Revel M. Relation of inflammatory modic changes to intradiscal steroid injection outcome in chronic low back pain. Eur. Spine J. 2007;16:925–931. doi: 10.1007/s00586-006-0301-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dudli S., Liebenberg E., Magnitsky S., Lu B., Lauricella M., Lotz J.C. Modic type 1 change is an autoimmune response that requires a pro-inflammatory milieu provided by the’ modic disc’. Spine J. 2018;18:831–844. doi: 10.1016/j.spinee.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Albert H.B., Lambert P., Rollason J., Sorensen J.S., Worthington T., Pedersen M.B., Nørgaard H.S., Vernallis A., Busch F., Manniche C., Elliott T. Does nuclear tissue infected with bacteria following disc herniations lead to Modic changes in the adjacent vertebrae? Eur. Spine J. 2013;22:690–696. doi: 10.1007/s00586-013-2674-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuisma M., Karppinen J., Niinimäki J., Ojala R., Haapea M., Heliövaara M., Korpelainen R., Taimela S., Natri A., Tervonen O. Modic changes in endplates of lumbar vertebral bodies: prevalence and association with low back and sciatic pain among middle-aged male workers. Spine (Phila Pa 1976) 2007;32:1116–1122. doi: 10.1097/01.brs.0000261561.12944.ff. [DOI] [PubMed] [Google Scholar]
- 33.Toyone T., Takahashi K., Kitahara H., Yamagata M., Murakami M., Moriya H. Vertebral bone-marrow changes in degenerative lumbar disc disease. An MRI study of 74 patients with low back pain. J. Bone Joint Surg. Br. 1994;76:757–764. [PubMed] [Google Scholar]
- 34.Kjaer P., Leboeuf-Yde C., Korsholm L., Sorensen J.S., Bendix T. Magnetic resonance imaging and low back pain in adults: a diagnostic imaging study of 40-year-old men and women. Spine (Phila Pa 1976) 2005;30:1173–1180. doi: 10.1097/01.brs.0000162396.97739.76. [DOI] [PubMed] [Google Scholar]
- 35.Albert H.B., Manniche C. Modic changes following lumbar disc herniation. Eur. Spine J. 2007;16:977–982. doi: 10.1007/s00586-007-0336-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Maeseneer M., Lenchik L., Everaert H., Marcelis S., Bossuyt A., Osteaux M., Beeckman P. Evaluation of lower back pain with bone scintigraphy and SPECT. Radiographics. 1999;19:901–912. doi: 10.1148/radiographics.19.4.g99jl03901. [DOI] [PubMed] [Google Scholar]
- 37.Albert H.B., Kjaer P., Jensen T.S., Sorensen J.S., Bendix T., Manniche C. Modic changes, possible causes and relation to low back pain. Med. Hypotheses. 2008;70:361–368. doi: 10.1016/j.mehy.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 38.Kanmaz B., Collier B.D., Liu Y., Uzum F., Uygur G., Akansel G., Gunes I., Krasnow A.Z., Hellman R.S., Isitman A.T., Carrera G. SPET and three-phase planar bone scintigraphy in adult patients with chronic low back pain. Nucl. Med. Commun. 1998;19:13–21. doi: 10.1097/00006231-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Carrino J.A., Lurie J.D., Tosteson A.N., Tosteson T.D., Carragee E.J., Kaiser J., Grove M.R., Blood E., Pearson L.H., Weinstein J.N., Herzog R. Lumbar spine: reliability of MR imaging findings. Radiology. 2009;250:161–170. doi: 10.1148/radiol.2493071999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arana E., Royuela A., Kovacs F.M., Estremera A., Sarasibar H., Amengual G., Galarraga I., Martinez C., Muriel A., Abraira V., Gil Del Real M.T., Zamora J., Campillo C. Lumbar spine: agreement in the interpretation of 1.5-T MR images by using the Nordic Modic Consensus Group classification form. Radiology. 2010;254:809–817. doi: 10.1148/radiol.09090706. [DOI] [PubMed] [Google Scholar]
- 41.Fayad F., Lefevre-Colau M.M., Drape J.L., Feydy A., Chemla N., Quintero N., Rannou F., Poiraudeau S., Fermanian J., Revel M. Reliability of a modified Modic classification of bone marrow changes in lumbar spine MRI. Joint Bone Spine. 2009;76:286–289. doi: 10.1016/j.jbspin.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 42.Jones A., Clarke A., Freeman B.J., Lam K.S., Grevitt M.P. The Modic classification: inter- and intraobserver error in clinical practice. Spine. 2005;30:1867–1869. doi: 10.1097/01.brs.0000173898.47585.7d. [DOI] [PubMed] [Google Scholar]
- 43.Ore L., Hardoff R., Gips S., Tamir A., Epstein L. Observer variation in the interpretation of bone scintigraphy. J. Clin. Epidemiol. 1996;49:67–71. doi: 10.1016/0895-4356(95)00056-9. [DOI] [PubMed] [Google Scholar]