Abstract
Background
The ankle is the second most frequent site, following the knee, that requires cartilage repair. Osteochondral lesion of the talus (OLT) is common among athletes and is a result of talar cartilage detachment with or without subchondral bone fragmentation after a traumatic event. Treatment strategies for OLT can be classified as reparative or replacement interventions, with the former taking precedence. Recent studies show that the growth factors and bioactive components in platelet rich plasma (PRP) could improve cartilage regeneration. The prospect of using autologous blood to obtain a product that could enhance regeneration in damaged cartilage has been regarded as innovative, as it could circumvent the need for a replacement, and potentially join the ranks of first line reparative interventions against cartilage diseases.
Methods
Literature searches were performed across seven search engines for randomized controlled trials using PRP to treat patients with OLT. Outcomes extracted included ankle function and pain measures. Level of evidence and methodological quality were evaluated using relevant guidelines.
Results
Four studies met the eligibility criteria and were systematically appraised. Two studies scored Level 1 and 2 scored Level 2 based on the LOE assessment. MQOE evaluation revealed one study with excellent quality, and three with good quality. Overall results showed that PRP, as an adjunct to microfracture surgery, significantly improved function and reduced pain compared to microfracture surgery alone. Intra-articular PRP injection also demonstrated significantly enhanced recovery of function, and decreased pain scores compared to HA
Conclusion
PRP improves joint function, and reduces pain in patients with OLT regardless of the method of implementation. In addition, inter-study comparison demonstrated that patients that received surgery along with PRP injections improved more than those that received PRP only. The studies that corroborate this conclusion have high levels of evidence with satisfactory methodological quality.
Level of evidence
Level 2, systematic review of Level 1 and 2 studies.
Keywords: Talar, Osteochondral lesion, OLT, OCL, Platelet-rich plasma, PRP, Microfracture
1. Introduction
Found in 70% of ankle sprains and fractures, osteochondral lesions of the talus (OLTs) are common among athletes, affecting about 5.2 per 10,000 athletes.28,57 OLT is a result of talar cartilage detachment with or without subchondral bone fragments after a traumatic event.21 Patients normally present with ankle pain upon weight bearing, reduced range of motion, impaired function, stiffness, functional instability, swelling and locking, impeding their quality of life.4
Treatment strategies for OLT can be classified as conservative and operative, with the former taking precedence.57 Conservative treatment includes restriction of weight bearing activities, non-steroidal anti-inflammatory drugs, rest and immobilization.28 Surgical measures include bone marrow stimulation or autologous osteochondral transplantation depending on the size of the lesion, and aim to induce the formation of regenerative fibrous cartilage.57 However, regeneration is still reportedly unsatisfactory with these interventions. A study by Becher et al., showed that cartilage regeneration after microfracture surgery, a method of bone marrow stimulation, was neither homogenous nor intact suggesting a structure weaker than hyaline.6 Moreover, evidence has shown that regenerated fibrous cartilage is mechanically inferior to hyaline cartilage, and is susceptible to deterioration over time.11,48
Numerous studies have been conducted to develop an intervention that could regenerate hyaline-like articular cartilage, a tissue known to have limited regenerative capacity, in damaged joints.1,6 Platelet rich plasma (PRP) is one candidate that has been gaining popularity, as mounting evidence continues to prove its ability to promote cartilage regeneration.(5) Platelets play a central role in wound healing and as such, contain a number of cytokines whose actions accommodate this purpose, including transforming growth factor-beta (TGF-B), insulin-like growth factors (IGF), fibroblast growth factor, epidermal growth factor, and endothelial cell growth factors to name a few.60 Of these, TGF-B has been discovered to be the greatest inducer of cartilage repair by directing bone-marrow stromal cell towards lesions, inducing chondrocyte proliferation, differentiation and maintaining chondrocyte phenotype to increase overall cartilage formation.68 In vitro studies have shown that PRP was able to induce cell proliferation, and cartilaginous matrix production by mesenchymal stem cells and chondrocytes.66 This is further corroborated by an animal study that demonstrated significantly improved cartilage regeneration, with thicker and better integration of cartilage, using PRP as an adjunct, compared to mosaicplasty only.2 In addition, given its autologous nature, PRP is not only inherently free from transmittable diseases, but have also been reported to have an acceptable safety profile.16,39,64 Preparation of PRP involves drawing and concentrating patients’ blood via centrifugation, with subsequent activation, allowing platelets to release the aforementioned growth factors, before isolating the activated platelet products. After isolation, PRP is injected, with or without radiological guidance, into the lesion site.68 The prospect of using autologous blood to obtain a product that could enhance regeneration in damaged cartilage has been regarded as innovative, as it could circumvent the need for a replacement, and potentially join the ranks of first line reparative interventions against cartilage diseases.
Currently, most studies assess the efficacy of PRP in knee joint pathologies, with only few investigating its potential in the ankle.25 Moreover, the highly heterogenous nature of these studies, as well as the preparations of PRP, make it even more difficult to draw a clear conclusion regarding its efficacy.66 This systematic review aims to establish the efficacy of PRP in improving the clinical outcomes of patients with OLTs, using the highest level of evidence available to this date.
2. Methods
This systematic review was written according to the guidelines for Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA).47
2.1. Search strategy
A literature search was conducted on November 13, 2018, in seven databases (PubMed, EMBASE, CINAHL, Scopus, Proquest, Science Direct, and The Cochrane Library) for clinical studies that used PRP to treat OLT. Search terms input into each search engine were: (Osteochondral lesions OR OCL) AND (Talus OR Talar) AND (Platelet Rich Plasma OR PRP). Study title and abstracts were first screened, followed by full text analysis of selected articles based on previously set eligibility criteria. In addition, the reference lists of review articles and included studies were further analysed to identify other studies that could be included. Screening and analysis were conducted separately by two independent researchers.
2.2. Eligibility criteria
The following inclusion criteria were used to select articles: clinical studies that assessed the effect of PRP on patients with talar osteochondral lesions, including any comparator, using functionality and pain as outcome measures, clinical studies of level of evidence 2 or above, studies that included a control or comparison group, without time or language limitations. Exclusion criteria were: case reports, studies that do not report clinical results, reviews, and in vitro, or animal studies.
2.3. Data extraction
Relevant data pooled from each study article were: patient characteristics, study design and outcome measures which include: American Orthopedic Foot and Ankle Society (AOFAS) Ankle/Hindfoot Scale, and the Visual Analog Scale (VAS) for pain.
2.4. Critical appraisal
Studies were assessed based on their level of evidence (LOE) using criteria published by the Journal of Bone and Joint Surgery, and methodological quality of evidence (MQOE) using the Modified Coleman Methodology Score (MCMS).17,51 The MCMS is comprised of two sections, part A, which assesses the study characteristics, and part B, which evaluates outcome criteria and assessment and subject selection process.17 Studies with a MCMS of 85–100 were considered excellent, 70–84 good, 55–69 fair, and below 55 poor.
2.5. Data analysis and statistical methods
Baseline measurements for AOFAS/Ankle and Hindfoot Scale and VAS pain scores were combined and assessed for comparability between studies. The same was not performed for followup measurements of different time periods.
Statistical analysis was performed using the statistical analysis software, IBM SPSS version 20.0. Normality was tested using Shapiro-Wilk's test. Comparison between data sets were then analysed by parametric or non-parametric tests depending on the distribution. Non-parametric test used to compare baseline values is the Mann-Whitney test.
3. Results
3.1. Search results
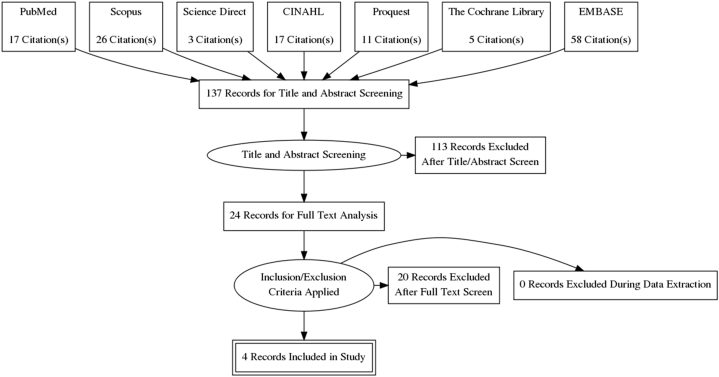
Literature search results are summarized in a PRISMA flow diagram (Fig. 1). A total of four clinical studies were included for systematic review.30, 31, 32,45
Fig. 1.
Prisma flow diagram describing search results.
3.2. Study characteristics
Summary of population demographics across all four studies are summarized in Table 1. A total of 159 ankles with talar osteochondral lesions, diagnosed by radiographs/computed tomography scans and/or magnetic resonance imaging, are included. The study by Mei-Dan et al., used PRP compared to hyaluronic acid (HA) as a form of conservative treatment, with three consecutive intra-articular injections. This study performed three followup measurements at 1, 3 and 7 months.45 Gormeli et al. assessed the efficacy of intra-articular PRP injections, used as an adjunct to arthroscopic microfracture surgery compared to HA and saline for control, on 40 patients followed up to an average of 15 months.30 The 2013 study by Guney et al., compared the difference between arthroscopic microfracture surgery with or without PRP as an adjunct, on 35 patients followed up to an average of 16 months.31 The same authors performed another study to assess the difference between three surgical groups, arthroscopic microfracture surgery with PRP, without PRP, and mosaicplasty. This study was carried out on 54 patients, with a longer followup period of 42 months.32
Table 1.
Population demographics and study characteristics.
| Author, Year | No. of Ankles | Mean Age (years) | Average Followup (months) | Intervention | Comparator | LOE | MQOE |
|---|---|---|---|---|---|---|---|
| Mei-Dan, 201245 | 30 | 36.5 | 1, 3, 7 | Intra-articular PRP injection | Intra-articular HA injection | 1 | 78 |
| Gormeli, 201530 | 40 | PRP: 38.6 | 15.3 | Arthroscopic Microfracture Surgery with subsequent PRP injection | Arthroscopic Microfracture Surgery with subsequent HA injection Arthroscopic Microfracture Surgery with subsequent Saline injection |
1 | 86 |
| HA: 39.7 | |||||||
| C: 40.3 | |||||||
| Guney, 201331 | 35 | I: 42.8 | 16.2 | Arthroscopic Microfracture Surgery with subsequent PRP injection | Arthroscopic Microfracture Surgery only | 2 | 73 |
| C: 38.5 | |||||||
| Guney, 201532 | 54 | 40.1 | 42 | Arthroscopic Microfracture Surgery with subsequent PRP injection | Arthroscopic Microfracture Surgery only Mosaicplasty |
2 | 75 |
PRP: Platelet-Rich Plasma.
HA: Hyaluronic Acid.
3.3. LOE
All studies had a high level of evidence based on the criteria published by the Journal of Bone and Joint Surgery. From the four included studies, two scored level 1 and the other two had level 2 level of evidences (Table 1).
3.4. MQOE
Overall, one study achieved a rating of excellent (85–100), whereas the other three achieved a rating of good (70–84), with an average MCMS of 78 ± 5.7 over 100 points (Table 1).
3.5. AOFAS/ankle and hindfoot scale
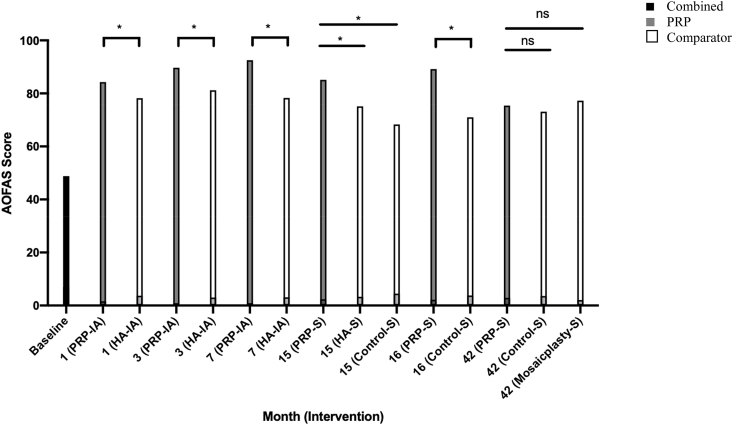
The AOFAS/Ankle and Hindfoot Scale was developed to assess the functionality of a patients’ ankle and hindfoot.40 A higher score meant better functionality, with a maximum score of 100 points. Baseline and followup values for functional assessments using the AOFAS/Ankle and Hindfoot Scale from the four studies are compiled in Table 2 and illustrated in Fig. 2. Baseline values are similar between PRP and comparator groups (P = 0.61) across all studies, with a combined mean of 48.8 ± 14.2. Functional improvements were evident from as early as one month after treatment, and remained consistently better in patients receiving intra-articular PRP injections compared to those that received HA (P < 0.05).45 Fifteen months after arthroscopic microfracture surgery, AOFAS score was significantly higher in the PRP group than in HA or control groups (P < 0.001).30 At 16 months, the followup by Guney et al. yielded a significantly higher score in the PRP group compared to the control group that did not receive PRP injection post arthroscopic microfracture surgery (P < 0.001).31 The 2015 study by Guney et al. showed no significant differences in terms of function, between the group that received PRP post-microfracture surgery and the microfracture surgery or mosaicplasty groups at 42 months followup, despite all groups showing significant recovery of function compared to baseline (P < 0.001).32 All patients experienced a significantly improved function score when compared to baseline.
Table 2.
AOFAS/ankle and hindfoot scale scores from baseline to followup.
| Author, Year | Study Group | Baseline (P = 0.61) | Followup (Months) |
Change from Baseline | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 (P < 0.05) | 3 (P < 0.05) | 7 (P < 0.05) | 15 (P = 0.001) | 16 (P = 0.001) | 42 (n.s.) | ||||
| Mei-Dan, 2012 | PRPIA | 68.0 | 84.3 | 89.7 | 92.5 | P < 0.001 | |||
| HAIA | 66.4 | 78.2 | 81.2 | 78.3 | P < 0.001 | ||||
| Gormeli, 2015 | PRPs | 43.6 | 85.1 | P < 0.001 | |||||
| HAs | 44.9 | 75.1 | P < 0.001 | ||||||
| Salines | 42.7 | 68.3 | P < 0.001 | ||||||
| Guney, 2013 | PRPs | 42.5 | 89.2 | P < 0.05 | |||||
| Non-PRPs | 46.8 | 71.0 | P < 0.05 | ||||||
| Guney, 2015 | PRPs | 44.1 | 75.4 | P < 0.001 | |||||
| Non-PRPs | 46.8 | 73.1 | P < 0.001 | ||||||
| Mosaicplastys | 43.8 | 77.3 | P < 0.001 | ||||||
PRP: Platelet Rich Plasma, HA: Hyaluronic Acid, S: In addition to Arthroscopic Microfracture Surgery, IA: Intra-Articular Injection only, n.s.: statistically not significant.
Fig. 2.
AOFAS/ankle and hindfoot scores through different followup periods across all studies.
Statistical significance is denoted by symbols overlying the graphs: * = P < 0.05, ns = P > 0.05. PRP: Platelet Rich Plasma, HA: Hyaluronic Acid, S: In addition to Arthroscopic Microfracture Surgery, IA: Intra-Articular Injection only.
3.6. VAS score for pain
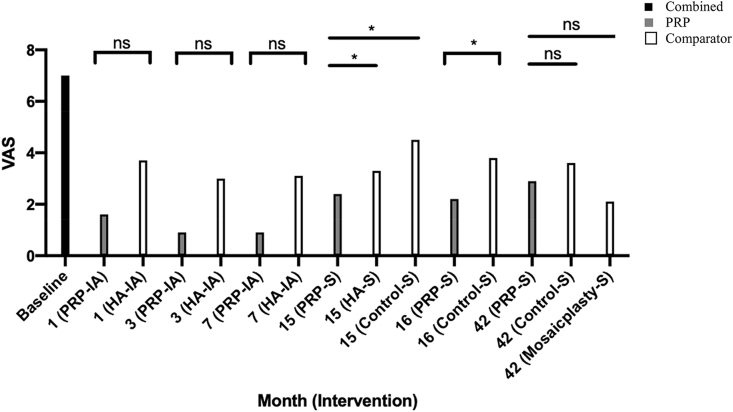
The VAS score for pain allows the researcher to score a patients’ perceived pain by observation. A lower score meant less pain experienced by patient. Baseline and followup values for VAS score for pain are compiled in Table 3 and illustrated in Fig. 3. Baseline values are similar between PRP and comparator groups (P = 0.762) across all studies, with a combined mean of 7.0 ± 1.6. Followup months 1, 3, and 7 in the study by Mei-Dan et al., did not see any significant differences between groups receiving conservative intra-articular PRP or HA injections. Despite this, pain scores were consistently lower in the PRP group.45 The 15 month followup in the study by Gormeli et al. saw a significantly lower VAS score in the PRP group compared to the HA and control groups (P < 0.001).30 At 16 months, the study by Guney et al. reported a significantly lower pain score in PRP treated group compared to the group that did not receive PRP after arthroscopic microfracture surgery (P < 0.001).31 After 42 months, Guney et al. found no significant differences in pain scores, between the group that received PRP post-microfracture surgery and the microfracture surgery or mosaicplasty groups at 42 months followup, despite all groups demonstrating significant reduction in pain scores compared to baseline (P < 0.001).32 All patients experienced significantly less pain from baseline.
Table 3.
VAS scores for pain from baseline to followup.
| Author, Year | Study Group | Baseline (P = 0.762) | Followup (Months) |
Change from Baseline | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 (n.s.) | 3 (n.s.) | 7 (n.s.) | 15 (P = 0.001) | 16 (P = 0.001) | 42 (n.s.) | ||||
| Mei-Dan, 2012 | PRPIA | 4.1 | 1.6 | 0.9 | 0.9 | P < 0.001 | |||
| HAIA | 5.6 | 3.7 | 3.0 | 3.1 | P < 0.001 | ||||
| Gormeli, 2015 | PRPs | 8.0 | 2.4 | P < 0.001 | |||||
| HAs | 7.8 | 3.3 | P < 0.001 | ||||||
| Salines | 7.7 | 4.5 | P < 0.001 | ||||||
| Guney, 2013 | PRPs | 8.0 | 2.2 | P < 0.05 | |||||
| Non-PRPs | 7.3 | 3.8 | P < 0.05 | ||||||
| Guney, 2015 | PRPs | 7.2 | 2.9 | P < 0.001 | |||||
| Non-PRPs | 6.8 | 3.6 | P < 0.001 | ||||||
| Mosaicplastys | 7.8 | 2.1 | P < 0.001 | ||||||
PRP: Platelet Rich Plasma, HA: Hyaluronic Acid, S: In addition to Arthroscopic Microfracture Surgery, IA: Intra-Articular Injection only.
Fig. 3.
VAS pain scores through different followup periods across all studies.
Statistical significance is denoted by symbols overlying the graphs: * = P < 0.05, ns = P > 0.05. PRP: Platelet Rich Plasma, HA: Hyaluronic Acid, S: In addition to Arthroscopic Microfracture Surgery, IA: Intra-Articular Injection on.
4. Discussion
The notion that the regeneration of avascular, non-regenerative cartilage is possible, has spurred researchers to develop an interventional approach that could enhance cartilage regeneration in cartilage diseases. Recently, the bioactive components in PRP has introduced the prospect of using this autologous concoction as one method of approach, and several studies have been conducted to assess its efficacy in treating OLT.
4.1. Summary of findings
Results of the four studies show that PRP promotes recovery of function and reduces pain in OLT patients when administered conservatively or as an adjunct to arthroscopic microfracture surgery.
Ankle function was consistently significantly better in PRP treated groups from 1 to 16 months. The AOFAS/Ankle and Hindfoot scale assesses the pain, activity capacity, range of motion, gait and alignment of the ankle, giving researchers a good idea of the ankle's overall function.40 Consistent with these results, clinical trials have demonstrated that PRP improves joint function in various joint-inhibiting diseases such as rotator cuff tears, Achilles tendon rupture, and knee osteoarthritis.36,67,69
On the contrary, ankle pain was only significantly less in PRP groups at 15 and 16 months, and conservative use showed non-significantly lower VAS scores at 1, 3 and 7 months. This may underscore the inferiority of conservative PRP administration compared to its use post-surgery, in relieving pain in OLT patients.
Both function and pain scores were significantly better than baseline in all patients across all studies. It must not be overlooked that this may be due to the placebo effect, constituting factors that have little or nothing to do with the underlying pathophysiology, such as patient-doctor interaction.46,50 However, seeing as how consistent this finding was across all groups in the four studies, we believe that the influence of the placebo effect may be, if any, low. Another explanation for this may be attributed to the arthrocentesis in surgical procedures, which involves the removal of fluid from the ankles, relieving pressure that may improve both functional and pain scores.34
There was a tendency for a downtrend in terms of function, and an uptrend for pain, as time progresses, this may indicate the short term nature of the benefits derived from PRP in cartilage healing. The lack of difference at 42 months between groups in Guney et al.'s study also support this notion. Other studies, also report that the benefits of PRP use are time-dependent and deteriorate in the long term.5,27 In light of this, Guney et al. still acknowledges the short-term benefits of PRP and recommends future studies confirm the long-term effects of PRP with a larger, controlled study.32
4.2. Side effects
The most frequently reported side effects of PRP injections include post-injective pain and swelling of the affected joint that limits activity and self-limiting adverse events such as dizziness, headaches, nausea, sweating and tachycardia.43,55 With regards to the studies in this review, no adverse effects were reported in both of the studies by Guney et al. Gormeli et al. reported no severe adverse reactions in any groups, and Mei-Dan et al. commented that both PRP and HA injections were associated with minimal side effects.30, 31, 32,45
4.3. Conservative vs surgical
Non-operative methods are the first-line treatment for grade 1–2 lesions, whereas surgical interventions are reserved for when non-operative treatment is unsuccessful or early treatment for grade 3–4 lesions.26,41 Conservative treatment circumvents the risk of possible complications found in surgical methods. Moreover, injections allow surgeons to reach intra-articular lesions wherever they lie within the joint, an area that would be relatively difficult to reach operatively.45 Alpha granules in PRP contain growth factors known to induce overall tissue regeneration including angiogenesis, collagen synthesis, cell proliferation and augmenting healing of tendon, ligament, skeletal muscle, and bone.33,53 With regards to cartilage regeneration, PRP enhances repair of articular cartilage defects by increasing chondrocyte proliferation and inducing cartilaginous matrix formation.12,65
PRP is also known to counteract catabolic tissue environments by inhibiting catabolic cytokines (IL-1β and TNF-α) found in degenerative cartilage diseases and suppressing inflammation by production of factors with known anti-inflammatory properties.7,15,19,49 Moreover, trials that tested conservative methods of cartilage treatment using PRP have shown improved clinical outcomes on patients with various cartilage problems such as knee and hip osteoarthritis.13,54,59 The results from Mei-Dan et al.'s study also confirmed that PRP significantly improves function and pain in OLT patients.45 However, since only one trial has been done on conservative PRP use in OLT, further studies must be done to verify the usefulness of this method.
Current evidence from the studies included in this paper support the surgical use of PRP as an adjunct to prior bone marrow stimulation techniques over conservative use. An explanation for this would be the access to progenitor cells made available by surgical use. Mesenchymal stem cells (MSCs) of the marrow have been implicated in cartilage repair, notably through their gradual differentiation into chondrocytes and migration to damaged areas.56 This formed the basis of bone marrow stimulating techniques for cartilage repair, including the microfracture procedure, which involves the drilling of holes into the subchondral bone to allow undifferentiated MSCs from the marrow to access the damaged articular cartilage.29 PRP has been discovered to selectively promote chondrogenic differentiation of MSCs, without stimulating osteogenic or adipocyte differentiation. In addition, evidence suggests that PRP guides progenitor cell migration towards damaged areas, further enhancing the effects of bone marrow stimulation.42
4.4. Optimisation
It must be acknowledged that no two PRP preparations are the same. The general consensus agrees that PRP simply refers to a blood derivative with at least 200% platelet concentration of peripheral blood count.22,44 This definition in itself is rather vague as many conditions can affect a patient's platelet count. Moreover, PRP preparations have been known to vary over a wide range, with concentrations of up to 800% that of basal levels, resulting in another confounding factor to comparability.44 There are currently two methods to prepare PRP in clinical practice, centrifugation, which is highly technician dependent, and density gradient cell separator, which can be done with many different devices, each with their own features and specifications.62 In addition to platelets, the composition of other cell types also affect the efficacy of PRP. Studies have reported that leukocytes can release reactive oxygen species and matrix metalloproteinases, which are catabolic and may damage articular tissues instead.10 Storage of PRP is also an issue, with freeze-thawing known to impair platelet function and lifespan, fresh administration immediately after preparation is considered the better alternative.63
In the four studies, three of the studies applying surgical interventions achieved comparable PRP concentrations of around 5 fold the original platelet count, whereas Mei-Dan et al. produced a relatively lower concentration of 2–3 times. Several studies have reported a good association between platelet concentration and clinical outcome.22,61 This may explain the lack of significantly better pain score in the PRP group in the study by Mei-Dan et al.45 In addition, Mei-Dan et al. conducted manual centrifugation whereas the other three studies used a high yield (concentration ability) automated micro-centrifuge, the Smart- PReP®️2 system (Harvest Autologous Hemobiologics, Norwell, MA). These methods are known to produce high inter-product variations, hence care must be taken to ensure consistency.14 None of the studies assessed the cell-type composition, or described the storage procedure of their PRP preparations.
4.5. Quality and limitation of studies and review
Overall evidence pooled in this review are of satisfactory LOE, suggesting convincing evidence to better inform clinicians on the efficacy of PRP.51 However, it must be noted that LOE provides only a rough estimate of the overall quality of the study, and sheds little light on the quality of methodology used. A randomized controlled trial with a followup rating of 80% is given an LOE of 1, regardless of other flaws in study design.38 For this reason, an additional assessment was performed using the MCMS to evaluate the MQOE of included studies.3 The MQOE of three of the studies were rated good, and one study was rated excellent.
With regards to part A of the MCMS, all studies achieved the highest rating for sample size at followup (>90%), patient compliance, confirmation of diagnosis by radiograph and/or MRI, and comprehensive descriptions of surgical techniques and postoperative rehabilitation. Followup time period was relatively short in 3 of the studies (<3 years), with only Guney et al.,'s 2015 study performing outcome assessment at longer than 3 years (42 months).32 Sample sizes were also relatively small across all studies, with only 2 studies (Guney 2015 and Gormeli et al.) achieving >40 patient enrolments.30,31 Despite this, Mei-Dan et al. and Gormeli et al. still managed to report sufficiently powered samples, and the 2013 study by Guney et al. reported statistical significance, reducing the likelihood of type 2 error.30,31,45
All studies had similar scores for the criteria in part B. Full scores were achieved for outcome criteria and subject selection subheadings. Outcome measures were well defined, taken upon followup examinations and endorsed by similar studies for their high quality. Multiple studies have reported the high reliability and sensitivity of VAS for pain and AOFAS/ankle and hindfoot scale for functional measurements.8,9,18,20,37 Subject selection processes were also clearly described and reported, with all withdrawals accounted for. With regards to assessing outcome measures, all studies satisfactorily described recruitment and data collection processes, but did not report an independent investigator to collect outcome measures, except for Gormeli et al. whose study was the only one to incorporate observer blinding.30
The high LOE and MQOE of studies in this review further support the reliability of evidence regarding use of PRP for OLT.
4.6. Limitations of studies
In three of the studies, observer blinding was not possible as all study procedures were performed by the operating team. Followup time period was considered short according to the MCMS.17 However, evidence states that the treatment effect of PRP was apparent as early as one week after treatment and maximal effect appeared between 5 and 13 weeks after injection, meaning that followup periods of 4 months or longer should be sufficient.30 In this case, a longer followup period would be beneficial simply to monitor for any potential adverse effects or relapses in function. None of the studies used image guidance to locate the needle during injection. Evidence has shown that the use of imaging guidance for needle-based procedures in soft tissues increases the accuracy of needle-tip placement and helps avoid vascular structures and cutaneous nerves.23,35,58 Postoperative analgesic use was reportedly not recorded by Mei-dan et al. and Gormeli et al., and not mentioned in both studies by Guney et al., presenting a confounding factor for pain measurement. However, Mei-dan et al. and Gormeli et al. expect the effects to be balanced between both groups.30,45
4.7. Recommendations for future trials
Recommended methodological improvements for future studies in this area would be the blinding of observers, longer followup periods, and increased study size. Longer-term followup is particularly important to reaffirm the findings by Guney et al. Since prohibition of analgesic use would be unethical, documentation of analgesic use, and additional subgroup analyses should be done to verify the efficacy of PRP in reducing pain. Image guidance during administration of PRP or other injectates should also be implemented to ensure the location of the needle. Assessment of cell composition and standardization of PRP concentration would also be beneficial to increase inter-study comparability. Additional examinations to assess the quality of repair tissue and regeneration of hyaline cartilage would provide more information regarding the actions of PRP in cartilage regeneration and aid in the development of future treatment modalities aimed at promoting regeneration.
4.8. Limitations of review
Several protocols were used to minimize risk of bias in this review. Review bias was avoided by adherence to the PRISMA guideline, which directs the content of a high quality review paper and is touted by medical journals.47 Literature search and data extraction bias were minimized by compliance to a set of predetermined data extraction criteria, conducted by 2 independent researchers.24 The MCMS serves only as an indicator, not a determinant, of methodological quality as it assesses the quality of reporting found in a study, not the quality of the actual study.52 As such, studies with poor MCMS scores should be interpreted with caution by contacting the authors for additional information. Fortunately, despite the limitations found outside the MCMS criteria, the studies included in this review are all of high methodological quality.
5. Conclusions
PRP improves the clinical outcomes of patients with OLT, enhancing recovery of function and alleviating pain. This is supported by the high quality studies included in this review. Future studies should aim to also establish agreed upon standards for PRP concentration, method of concentration, and discretion in using PRP surgically or conservatively.
Declaration of competing interest
The authors declare no potential conflicts of interest with regards to the study, authorship, and/or publication of this article. No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Contributor Information
Oliver Emmanuel Yausep, Email: oliveremmanuel@hotmail.com.
Imad Madhi, Email: imad.madhi@stft.nhs.uk.
Dionysios Trigkilidas, Email: diotrigkilidas@hotmail.com.
References
- 1.Abrams G.D., Frank R.M., Fortier L.A., Cole B.J. Platelet-rich plasma for articular cartilage repair. Sports Med Arthrosc Rev. 2013;21(4):213–219. doi: 10.1097/JSA.0b013e3182999740. [DOI] [PubMed] [Google Scholar]
- 2.Altan E., Aydin K., Erkocak O., Senaran H., Ugras S. The effect of platelet-rich plasma on osteochondral defects treated with mosaicplasty. Int Orthop. 2014;38(6):1321–1328. doi: 10.1007/s00264-013-2275-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altman D.G., Schulz K.F., Moher D. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med. 2001;134(8):663–694. doi: 10.7326/0003-4819-134-8-200104170-00012. [DOI] [PubMed] [Google Scholar]
- 4.Badekas T., Takvorian M., Souras N. Treatment principles for osteochondral lesions in foot and ankle. Int Orthop. 2013;37(9):1697–1706. doi: 10.1007/s00264-013-2076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Battaglia M., Guaraldi F., Vannini F. Platelet-rich plasma (PRP) intra-articular ultrasound-guided injections as a possible treatment for hip osteoarthritis: a pilot study. Clin Exp Rheumatol. 2011;29(4):754. [PubMed] [Google Scholar]
- 6.Becher C., Driessen A., Hess T., Longo U.G., Maffulli N., Thermann H. Microfracture for chondral defects of the talus: maintenance of early results at midterm follow-up. Knee Surg Sports Traumatol Arthrosc. 2010;18(5):656–663. doi: 10.1007/s00167-009-1036-1. [DOI] [PubMed] [Google Scholar]
- 7.Bendinelli P., Matteucci E., Dogliotti G. Molecular basis of anti-inflammatory action of platelet-rich plasma on human chondrocytes: mechanisms of NF-κB inhibition via HGF. J Cell Physiol. 2010;225(3):757–766. doi: 10.1002/jcp.22274. [DOI] [PubMed] [Google Scholar]
- 8.Bijur P.E., Silver W., Gallagher E.J. Reliability of the visual analog scale for measurement of acute pain. Acad Emerg Med Off J Soc Acad Emerg Med. 2001;8(12):1153–1157. doi: 10.1111/j.1553-2712.2001.tb01132.x. [DOI] [PubMed] [Google Scholar]
- 9.de Boer A.S., Tjioe R.J.C., Van der Sijde F. The American Orthopaedic Foot and Ankle Society Ankle-Hindfoot Scale; translation and validation of the Dutch language version for ankle fractures. BMJ Open. 2017;7(8) doi: 10.1136/bmjopen-2017-017040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boswell S.G., Cole B.J., Sundman E.A., Karas V., Fortier L.A. Platelet-rich plasma: a milieu of bioactive factors. Arthrosc J Arthrosc Relat Surg Off Publ Arthrosc Assoc N Am Int Arthrosc Assoc. 2012;28(3):429–439. doi: 10.1016/j.arthro.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 11.null Buckwalter, null Mow, null Ratcliffe. Restoration of injured or degenerated articular cartilage. J Am Acad Orthop Surg. 1994;2(4):192–201. doi: 10.5435/00124635-199407000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Camargo P.M., Lekovic V., Weinlaender M., Vasilic N., Madzarevic M., Kenney E.B. Platelet-rich plasma and bovine porous bone mineral combined with guided tissue regeneration in the treatment of intrabony defects in humans. J Periodontal Res. 2002;37(4):300–306. doi: 10.1034/j.1600-0765.2002.01001.x. [DOI] [PubMed] [Google Scholar]
- 13.Cerza F., Carnì S., Carcangiu A. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40(12):2822–2827. doi: 10.1177/0363546512461902. [DOI] [PubMed] [Google Scholar]
- 14.Chahla J., Mandelbaum B.R. Biological treatment for osteoarthritis of the knee: moving from bench to bedside—current practical concepts. Arthrosc J Arthrosc Relat Surg. 2018;34(5):1719–1729. doi: 10.1016/j.arthro.2018.01.048. [DOI] [PubMed] [Google Scholar]
- 15.Chen L.X., Lin L., Wang H.J. Suppression of early experimental osteoarthritis by in vivo delivery of the adenoviral vector-mediated NF-kappaBp65-specific siRNA. Osteoarthritis Cartilage. 2008;16(2):174–184. doi: 10.1016/j.joca.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Choi J., Minn K.W., Chang H. The efficacy and safety of platelet-rich plasma and adipose-derived stem cells: an update. Arch Plast Surg. 2012;39(6):585–592. doi: 10.5999/aps.2012.39.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coleman B.D., Khan K.M., Maffulli N., Cook J.L., Wark J.D. Studies of surgical outcome after patellar tendinopathy: clinical significance of methodological deficiencies and guidelines for future studies. Scand J Med Sci Sports. 2000;10(1):2–11. doi: 10.1034/j.1600-0838.2000.010001002.x. [DOI] [PubMed] [Google Scholar]
- 18.Conceição CS da, Gomes Neto M., Costa Neto A., Mendes S.M.D., Baptista A.F., Sá K.N. Análise das propriedades psicométricas do American Orthopaedic Foot and Ankle Society Score (Aofas) em pacientes com artrite reumatoide: aplicação do modelo Rasch. Rev Bras Reumatol. 2016;56(1):8–13. doi: 10.1016/j.rbre.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Daheshia M., Yao J.Q. The interleukin 1beta pathway in the pathogenesis of osteoarthritis. J Rheumatol. 2008;35(12):2306–2312. doi: 10.3899/jrheum.080346. [DOI] [PubMed] [Google Scholar]
- 20.De Boer A.S., Meuffels D.E., Van der Vlies C.H. Validation of the American orthopaedic foot and ankle society ankle-hindfoot scale Dutch language version in patients with hindfoot fractures. BMJ Open. 2017;7(11) doi: 10.1136/bmjopen-2017-018314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Dijk C.N., Reilingh M.L., Zengerink M., van Bergen C.J.A. Osteochondral defects in the ankle: why painful? Knee Surg Sports Traumatol Arthrosc Off J ESSKA. 2010;18(5):570–580. doi: 10.1007/s00167-010-1064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dohan Ehrenfest D.M., Rasmusson L., Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF) Trends Biotechnol. 2009;27(3):158–167. doi: 10.1016/j.tibtech.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Drakonaki E.E., Allen G.M., Watura R. Ultrasound-guided intervention in the ankle and foot. Br J Radiol. 2016;89(1057):20150577. doi: 10.1259/bjr.20150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drucker A.M., Fleming P., Chan A.-W. Research techniques made simple: assessing risk of bias in systematic reviews. J Invest Dermatol. 2016;136(11):e109–e114. doi: 10.1016/j.jid.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 25.Elghawy A.A., Sesin C., Rosselli M. Osteochondral defects of the talus with a focus on platelet-rich plasma as a potential treatment option: a review. BMJ Open Sport Exerc Med. 2018;4(1) doi: 10.1136/bmjsem-2017-000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferkel R.D., Zanotti R.M., Komenda G.A. Arthroscopic treatment of chronic osteochondral lesions of the talus: long-term results. Am J Sports Med. 2008;36(9):1750–1762. doi: 10.1177/0363546508316773. [DOI] [PubMed] [Google Scholar]
- 27.Filardo G., Kon E., Buda R. Platelet-rich plasma intra-articular knee injections for the treatment of degenerative cartilage lesions and osteoarthritis. Knee Surg Sports Traumatol Arthrosc Off J ESSKA. 2011;19(4):528–535. doi: 10.1007/s00167-010-1238-6. [DOI] [PubMed] [Google Scholar]
- 28.Gianakos A.L., Yasui Y., Hannon C.P., Kennedy J.G. Current management of talar osteochondral lesions. World J Orthoped. 2017;8(1):12–20. doi: 10.5312/wjo.v8.i1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gill T.J., Steadman J.R. Articular Cartilage Lesions. Springer New York; New York, NY: 2004. Bone marrow stimulation techniques: microfracture, drilling, and abrasion; pp. 63–72. [Google Scholar]
- 30.Görmeli G., Karakaplan M., Görmeli C.A., Sarıkaya B., Elmalı N., Ersoy Y. Clinical effects of platelet-rich plasma and hyaluronic acid as an additional therapy for talar osteochondral lesions treated with microfracture surgery: a prospective randomized clinical trial. Foot Ankle Int. 2015;36(8):891–900. doi: 10.1177/1071100715578435. [DOI] [PubMed] [Google Scholar]
- 31.Guney A., Akar M., Karaman I., Oner M., Guney B. Clinical outcomes of platelet rich plasma (PRP) as an adjunct to microfracture surgery in osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2015;23(8):2384–2389. doi: 10.1007/s00167-013-2784-5. [DOI] [PubMed] [Google Scholar]
- 32.Guney A., Yurdakul E., Karaman I., Bilal O., Kafadar I.H., Oner M. Medium-term outcomes of mosaicplasty versus arthroscopic microfracture with or without platelet-rich plasma in the treatment of osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1293–1298. doi: 10.1007/s00167-015-3834-y. [DOI] [PubMed] [Google Scholar]
- 33.Hall M.P., Band P.A., Meislin R.J., Jazrawi L.M., Cardone D.A. Platelet-rich plasma: current concepts and application in sports medicine. J Am Acad Orthop Surg. 2009;17(10):602–608. doi: 10.5435/00124635-200910000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Hansford B.G., Stacy G.S. Musculoskeletal aspiration procedures. Semin Intervent Radiol. 2012;29(4):270–285. doi: 10.1055/s-0032-1330061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hari S., Kumari S., Srivastava A., Thulkar S., Mathur S., Veedu P.T. Image guided versus palpation guided core needle biopsy of palpable breast masses: a prospective study. Indian J Med Res. 2016;143(5):597–604. doi: 10.4103/0971-5916.187108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hassan A.S., El-Shafey A.M., Ahmed H.S., Hamed M.S. Effectiveness of the intra-articular injection of platelet rich plasma in the treatment of patients with primary knee osteoarthritis. Egypt Rheumatol. 2015;37(3):119–124. [Google Scholar]
- 37.Hawker G.A., Mian S., Kendzerska T., French M. Measures of adult pain: visual analog scale for pain (VAS pain), numeric rating scale for pain (NRS pain), McGill pain questionnaire (MPQ), short-form McGill pain questionnaire (SF-MPQ), chronic pain grade scale (CPGS), short form-36 bodily pain scale (SF. Arthritis Care Res. 2011;63(S11):S240–S252. doi: 10.1002/acr.20543. [DOI] [PubMed] [Google Scholar]
- 38.Jakobsen R.B., Engebretsen L., Slauterbeck J.R. An analysis of the quality of cartilage repair studies. J Bone Jt Surg Am Vol. 2005;87(10):2232–2239. doi: 10.2106/JBJS.D.02904. [DOI] [PubMed] [Google Scholar]
- 39.Kennedy M.I., Whitney K., Evans T., LaPrade R.F. Platelet-rich plasma and cartilage repair. Curr Rev Muscoskel Med. 2018;11(4):573–582. doi: 10.1007/s12178-018-9516-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kitaoka H.B., Alexander I.J., Adelaar R.S., Nunley J.A., Myerson M.S., Sanders M. Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int. 1994;15(7):349–353. doi: 10.1177/107110079401500701. [DOI] [PubMed] [Google Scholar]
- 41.Kreuz P.C., Steinwachs M., Erggelet C., Lahm A., Henle P., Niemeyer P. Mosaicplasty with autogenous talar autograft for osteochondral lesions of the talus after failed primary arthroscopic management: a prospective study with a 4-year follow-up. Am J Sports Med. 2006;34(1):55–63. doi: 10.1177/0363546505278299. [DOI] [PubMed] [Google Scholar]
- 42.Krüger J.P., Hondke S., Endres M., Pruss A., Siclari A., Kaps C. Human platelet-rich plasma stimulates migration and chondrogenic differentiation of human subchondral progenitor cells. J Orthop Res. 2012;30(6):845–852. doi: 10.1002/jor.22005. [DOI] [PubMed] [Google Scholar]
- 43.Marmotti A., Rossi R., Castoldi F., Roveda E., Michielon G., Peretti G.M. PRP and articular cartilage: a clinical update. BioMed Res Int. 2015;2015:542502. doi: 10.1155/2015/542502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marx R.E. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10(4):225–228. doi: 10.1097/00008505-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 45.Mei-Dan O., Carmont M.R., Laver L., Mann G., Maffulli N., Nyska M. Platelet-rich plasma or hyaluronate in the management of osteochondral lesions of the talus. Am J Sports Med. 2012;40(3):534–541. doi: 10.1177/0363546511431238. [DOI] [PubMed] [Google Scholar]
- 46.Miller F.G., Colloca L., Kaptchuk T.J. The placebo effect: illness and interpersonal healing. Perspect Biol Med. 2009;52(4):518–539. doi: 10.1353/pbm.0.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moher D., Liberati A., Tetzlaff J., Altman D.G., The PRISMA Group Preferred reporting Items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nehrer S., Spector M., Minas T. Histologic analysis of tissue after failed cartilage repair procedures. Clin Orthop. 1999;365:149–162. doi: 10.1097/00003086-199908000-00020. [DOI] [PubMed] [Google Scholar]
- 49.Nurden A.T. Platelets, inflammation and tissue regeneration. Thromb Haemostasis. 2011;105(Suppl 1):S13–S33. doi: 10.1160/THS10-11-0720. [DOI] [PubMed] [Google Scholar]
- 50.Oken B.S. Placebo effects: clinical aspects and neurobiology. Brain. 2008;131(11):2812–2823. doi: 10.1093/brain/awn116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parsa A., Ebrahimzadeh M.H. Level of evidence in the archivs of bone and joint surgery journal. Arch Bone Jt Surg. 2017;5(6):347–350. [PMC free article] [PubMed] [Google Scholar]
- 52.Pinski J.M., Boakye L.A., Murawski C.D., Hannon C.P., Ross K.A., Kennedy J.G. Low level of evidence and methodologic quality of clinical outcome studies on cartilage repair of the ankle. Arthrosc J Arthrosc Relat Surg. 2016;32(1):214–222. doi: 10.1016/j.arthro.2015.06.050. e1. [DOI] [PubMed] [Google Scholar]
- 53.Sánchez M., Anitua E., Orive G., Mujika I., Andia I. Platelet-rich therapies in the treatment of orthopaedic sport injuries. Sports Med. 2009;39(5):345–354. doi: 10.2165/00007256-200939050-00002. [DOI] [PubMed] [Google Scholar]
- 54.Sánchez M., Guadilla J., Fiz N., Andia I. Ultrasound-guided platelet-rich plasma injections for the treatment of osteoarthritis of the hip. Rheumatology (Oxford, England) 2012;51(1):144–150. doi: 10.1093/rheumatology/ker303. [DOI] [PubMed] [Google Scholar]
- 55.Shahid M., Kundra R. Platelet-rich plasma (PRP) for knee disorders. EFORT Open Rev. 2017;2(1):28–34. doi: 10.1302/2058-5241.2.160004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shapiro F., Koide S., Glimcher M.J. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Jt Surg Am Vol. 1993;75(4):532–553. doi: 10.2106/00004623-199304000-00009. [DOI] [PubMed] [Google Scholar]
- 57.Shimozono Y., Yasui Y., Ross A.W., Kennedy J.G. Osteochondral lesions of the talus in the athlete: up to date review. Curr Rev Muscoskel Med. 2017;10(1):131–140. doi: 10.1007/s12178-017-9393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soh E., Li W., Ong K.O., Chen W., Bautista D. Image-guided versus blind corticosteroid injections in adults with shoulder pain: a systematic review. BMC Muscoskel Disord. 2011;12:137. doi: 10.1186/1471-2474-12-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spaková T., Rosocha J., Lacko M., Harvanová D., Gharaibeh A. Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid. Am J Phys Med Rehabil. 2012;91(5):411–417. doi: 10.1097/PHM.0b013e3182aab72. [DOI] [PubMed] [Google Scholar]
- 60.Sun Y., Feng Y., Zhang C.Q., Chen S.B., Cheng X.G. The regenerative effect of platelet-rich plasma on healing in large osteochondral defects. Int Orthop. 2010;34(4):589–597. doi: 10.1007/s00264-009-0793-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Torricelli P., Fini M., Filardo G. Regenerative medicine for the treatment of musculoskeletal overuse injuries in competition horses. Int Orthop. 2011;35(10):1569–1576. doi: 10.1007/s00264-011-1237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tschon M., Fini M., Giardino R. Lights and shadows concerning platelet products for musculoskeletal regeneration. Front Biosci Elite Ed. 2011;3:96–107. doi: 10.2741/e224. [DOI] [PubMed] [Google Scholar]
- 63.Wasterlain A.S., Braun H.J., Dragoo J.L. Contents and formulations of platelet-rich plasma. Operat Tech Orthop. 2012;22(1):33–42. [Google Scholar]
- 64.Wehling P., Evans C., Wehling J., Maixner W. Effectiveness of intra-articular therapies in osteoarthritis: a literature review. Ther Adv Muscoskel Dis. 2017;9(8):183–196. doi: 10.1177/1759720X17712695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu W., Chen F., Liu Y., Ma Q., Mao T. Autologous injectable tissue-engineered cartilage by using platelet-rich plasma: experimental study in a rabbit model. J Oral Maxillofac Surg Off J Am Assoc Oral Maxillofac Surg. 2007;65(10):1951–1957. doi: 10.1016/j.joms.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 66.Xie X., Zhang C., Tuan R.S. Biology of platelet-rich plasma and its clinical application in cartilage repair. Arthritis Res Ther. 2014;16(1):204. doi: 10.1186/ar4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zafarani Z., Mirzaee F., Guity M., Aslani H. Clinical results of platelet-rich plasma for partial thickness rotator cuff tears: a case series. Arch Bone Jt Surg. 2017;5(5):328–331. [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu Y., Yuan M., Meng H.Y. Basic science and clinical application of platelet-rich plasma for cartilage defects and osteoarthritis: a review. Osteoarthritis Cartilage. 2013;21(11):1627–1637. doi: 10.1016/j.joca.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 69.Zou J., Mo X., Shi Z. A prospective study of platelet-rich plasma as biological augmentation for acute Achilles tendon rupture repair. BioMed Res Int. 2016;2016:9364170. doi: 10.1155/2016/9364170. [DOI] [PMC free article] [PubMed] [Google Scholar]