Abstract
The “red chilto” (Solanum betaceum Cav) is a native fruit from the Yungas forest of Argentina. Red chilto is a neglected and underutilized native species (NUS). The objective of this work was to evaluate the potentiality of pulp, seed and skin from “red chilto” as a functional food ingredient to add value to a native resource of Argentine Yungas to promote sustainable integral use of it. The powders have low carbohydrate and sodium content and are a source of vitamin C, phenolic acids (rosmarinic acid and caffeoylquinic acid), anthocyanins, condensed tannins, carotenoids, potassium, and fiber. The phenolics of chilto powders showed, before and after simulated gastroduodenal digestion, antioxidant activity (ABTS•+; H2O2; O2•; HO•) and were able to inhibit enzymes related to metabolic syndrome, such as α-glucosidase, α-amylase and lipase. Chilto powder showed hypoglycemic effect by increasing glucose adsorption, decreasing glucose diffusion rate and by promoting glucose transport across the cell membrane. These results suggest the potential of Argentinean “red chilto” fruits as functional food ingredients or dietary supplements to prevent metabolic syndrome principally by its antioxidant, hypoglycemic and hypolipemic effects.
Keywords: Food science, Solanum betaceum, Red chilto fruit, Powder, Antioxidant, Hypoglycemic, Metabolic syndrome
Food science, Solanum betaceum, Red chilto fruit, Powder Antioxidant, Hypoglycemic, Metabolic syndrome.
1. Introduction
The study of native fruits as unconventional foods is an emerging area in Argentina (Cardozo et al., 2011; Costamagna et al., 2013; Orqueda et al., 2017). In Northwestern Argentina, many native fruits make up the food spectrum of rural communities. Solanum betaceum Cav., called “monte tomato” or “chilto” is one of them. S. betaceum is distributed in Northwest Argentina in the provinces of Catamarca, Salta, Jujuy and Tucumán in the “Selva Pedemontana” of the Yungas forest, between 400 and 700 m.a.s.l. It plays an outstanding ecological role in the context of the Yungas since it has a high biological diversity and serves as a refuge for species of other altitudinal levels of the mountain forest. The Selva Pedemontana has been strongly reduced in size by the transformation of its natural cover in areas of intensive agriculture, in particular, sugar cane.
S. betaceum produces edible fruits with an interesting growing market in its native Andean countries, as well as in other parts of the world (Prohens and Nuez, 2000). The main fruit types were conventionally recognized based on skin colour, namely dark purple, red and orange. At present, the sustainable crop of red and orange chilto is being carried out in Argentina Yungas in three provinces. The orange variety is being grown in Tucumán province and the red variety in Jujuy and Salta provinces. The orange variety of chilto was previously investigated for nutritional and phytochemical composition, antioxidant and inhibitory activity on key enzymes involved in metabolic syndrome (Orqueda et al., 2017). However, so far there have been no studies of the red variety grown in Argentina.
Although the native fruits can be incorporated in the diet as fresh ripe fruits, they have a limited shelf life, as physical, chemical and biological changes can occur after a few days of harvest. Under these circumstances, minimum processing is often necessary to achieve a longer shelf life, preserving their nutritional and functional values as well as their organoleptic properties. The objective of this work was to evaluate the potentiality of pulp, seed and skin from “red chilto” as a functional food ingredient to add value to a native resource of Argentine Yungas and promote sustainable integral use of it.
2. Materials and methods
2.1. Plant material
The fruits of Solanum betaceum (red variety) were harvested at the ripening stage in which they are consumed in Finca del Obispo, Villa Jardín de Reyes, Jujuy, Argentina, in December 2016. Skin, pulp and seeds were separated, freeze-dried and powdered. The powders were vacuum packed and stored at -20 °C. Scanning electron microscopy (SEM) of each powder was carried out. SEM of gold-coated (Fine Coat Ion Sputter JEOL JFC-1100) samples was performed by using a ZEISS SUPRA-55 VP field emission scanning electron microscope at Centro Integral de Microscopía Electrónica (CIME), CONICET-UNT. The micrographs were taken for each sample at a magnification of 10,000 and 50,000 (Figure 1).
Figure 1.
I) Red chilto fruit; II) (A)Seed; (B) pulp and (C) skin powder; III) Scanning electron micrographs of (A) seed powder; (B) pulp powder and (C) skin powder.
2.2. Quality parameters of fresh fruits
The titratable acidity was analyzed for each sample (AOAC, 2000). To determine the different color parameters, a Chroma meter CR-400 colorimeter (Konica Minolta, Tokyo, Japan) was used through the CIELab system. Chromaticity coordinates L *, a * and b * were obtained. The parameters were obtained from three independent measurements in the equatorial region of each whole fruit. Ten fruits were measured in total.
2.3. Determination of metabolic profile of fresh fruits by 1H-NMR
To test the metabolic profile, whole fresh fruits were frozen in liquid nitrogen. Then, was homogenized with potassium phosphate buffer (1 M pH 7.4 in D2O) and centrifuged at 4 °C for 30 min at 14000 rpm. The supernatant was recovered and the pH was adjusted to 7.4. The deuterated sodium salt of 3-trimethylsilyl-1-propane sulfonic acid (TSP) (except for the methyl groups bound to the silicium atom) was added as an internal standard. 1HNMR at 600.13 (MHz Bruker Avance II spectrometer) was used.
2.4. Calculation of sweetness index
The sweetness index for each powder was determined according to Keutgen and Pawelzik (2007). The sweetness index of each part of the chilto fruit, an estimate of total sweetness perception, was calculated based on the amount and sweetness properties of individual carbohydrates presents in the samples. The contribution of each sugar in the samples, taking into account that fructose and glucose are 1.7 and 0.75 times sweeter than sucrose, respectively was estimated. As a result, sweetness index = 0.75 [Glucose] + 1.0 [Sucrose] + 1.7 [Fructose].
2.5. Estimation of taste parameter of the fruit powder
The sugars were repeatedly extracted from each part of the fruit using 80% ethanol at 80 °C until their total depletion. Total soluble sugars were quantified by the Fenol-H2SO4 method proposed by Dubois et al. (1956). Reducing sugars were quantified using the Somogyi-Nelson method (Nelson, 1944; Somogyi, 1945). The glucose content was determined using the enzyme glycemia kit (Wiener lab. 1400101) which is based on the glucose oxidase/peroxidase method. Fructose and sucrose were determined using the resorcinol-thiourea method proposed by Cardini et al. (1955) and Roe and Papadopoulus (1954).
2.6. Proximal composition of chilto powder
2.6.1. Protein, fat, fiber and minerals
To determine of crude protein, lipids and minerals content, the protocols previously described by Orqueda et al. (2017) was used. The total protein content was determined by measuring the total nitrogen (N) by the Kjeldahl method with a conversion factor of 6.25 (AOCS, 1989). For the determination of the total lipid content, the samples were extracted by Soxhlet with petroleum ether (40–60 °C) for 4 h and gravimetrically quantified.
The fiber content was determined according to AOAC (2005). Briefly, 1 g of fat-free powder was weighed, treated with 20 mL of 1.25% H2SO4 and incubated at 100 °C for 30 min. Then, 50 mL of 3.52% NaOH was added and incubated again at 100 °C for 30 min. Finally, the sample was dried at 110 °C until constant weight. It was then incinerated in muffle at 500 °C and the crude fiber content was determined by weight difference. The integrated total dietary fiber kit (WI 96461, Megazyme International Ireland, Ireland) was used to determine the dietary fiber content (McCleary, 2014). Mineral analysis was performed using quadrupole induction plasma mass spectrometry (Q-ICPMS) at the Instituto Superior de Investigación, Desarrollo y Servicios en Alimentos, ISIDSA, Córdoba, Argentina. The following ions were analyzed: sodium, magnesium, potassium, calcium, iron, copper and zinc.
2.6.2. Starch determination
The extraction and determination of the digestible and resistant starch content were carried out according to the method of Holm et al. (1986) with some modifications. The powders (0.1 g) were mixed with 10 mL of 0.1 M HCl and 0.1 g of pepsin at pH 1.5 and kept at 40 °C for 1 h. Then, 0.1 g of sodium azide was added and the mixture was hydrolyzed using 0.5 g of α-amylase (10 U/mg) at pH 6.9 for 16 h at 37 °C. Subsequently, the samples were centrifuged at 6,000 x g. The pellets were dispersed in 2 M KOH and maintained at room temperature for 30 min. Then, the digestible starch and the resistant starch were subjected to a digestion process using 50 μL of a solution of amyloglucosidase 10 mg/mL (14U/mg) pH 4.75 in a water bath for 30 min at 60 °C. The glucose content was determined by the glucose oxidase enzymatic assay (Wiener Lab., Argentina).
2.7. Phytochemical analysis
2.7.1. Extraction and determination of phenolic compounds
The phenolic compounds were obtained from chilto powders at 25 °C with 95° ethanol (1:5, W/V) until exhaustion with ultrasound during 30 min (Pothitirat et al., 2009). Then, the samples were filtered and dried under reduced pressure to obtain the phenolic enriched extract (PEE). In each PEE, total phenolic content and flavonoid content was determined according to Costamagna et al. (2013). Extraction and quantification of tannins and anthocyanins were carried out according to Costamagna et al. (2013).
2.7.2. Vitamin extraction and determination
For vitamin C quantification, the powder was extracted with 2% H3PO4 ultrasound assisted during 10 min and the ascorbic acid content was determined according to Costamagna et al. (2013). Vitamin C was expressed as g L-ascorbic acid (g L-AA)/100 g powder.
For carotenoid quantification, the powder was extracted with petroleum ether:acetone (1:1, v/v) and total carotenoid was calculated according to Costamagna et al. (2013). The results were expressed as g of β-carotene equivalents (g β-CE)/100 g of powder.
2.7.3. Phenolic profiling by HPLC-DAD
The extracts of the chilto samples (peel, pulp, and seeds) were analyzed by HPLC-DAD to obtain a phenolic fingerprint. The HPLC system used was a Shimadzu equipment (Shimadzu Corporation, Kyoto, Japan) consisting of an LC-20AT pump, an SPD-M20A diode array detector, CTO-20AC column oven and a LabSolution software. An Inertsil ® ODS-3 RP-C18 (5μ, 250 × 4.6 mm) column (GL-Sciences Inc, Tokio, Japan) maintained at 25 °C. The analyses were performed using a linear gradient solvent system consisting of 1% formic acid in water (A) and acetonitrile (B) as follow: 90%–85% A over 15 min, maintained to 20 min, then 85% A for 20–25 min, 85% to 70% A for 25–50 min, maintained to 70% A for 50–80 min, and returning to 90% A for 80.01–90 min. The flow rate was 0.5 mL/min and the volume injected was 20 μL. The compounds were monitored at 254, 280, 320 and 350 nm and spectra from 200 to 600 nm were recorded.
The main phenolics were quantified. The reference compounds rosmarinic acid (98%, Sigma Aldrich, St. Louis, MO, USA) and 3-caffeoylquinic acid (98%, Phytolab GmbH, Verstenbergsgreuth, Germany) were used in concentrations ranging from 5-500 mg/L. Results were expressed as mg of the corresponding compound/100g extract.
2.8. Simulated gastroduodenal digestion
The chilto powder was submitted to a simulated gastroduodenal digestion process (GD) according to Orqueda et al. (2017) using 0.5 g of each sample (skin, pulp and seed powder). Then, the powder samples were centrifuged at 10000 x g and the digested powders and without digestion were used to determine adsorption capacity and diffusion of sugars.
The polyphenolic enriched extracts (PEE) were also submitted to GD according to Orqueda et al. (2017). After GD, the polyphenols were extracted from the aqueous phase using ethyl acetate, dried and resuspended in dimethyl sulfoxide (DMSO) to enzymatic inhibition assays and in ethanol 95° to antioxidant assays. In all case, a negative control was performed using each of solvent.
2.9. Inhibition of enzyme involved in metabolic syndrome by chilto PEE
2.9.1. α-Glucosidase inhibition
The α-glucosidase inhibition activity of PEE was tested according to Costamagna et al. (2016). The α-glucosidase enzyme and PEE of each part of the fruit (2.5–20 μg/mL) (before and after simulated GD digestion) were pre-incubated at 4 °C for 10 min. To start the reaction, the substrate p-Nitrophenyl-α-D-glucopyranoside was added. The results were expressed as IC50 values (μg GAE/mL of PEE necessary to inhibit the 50% of enzyme activity). Orlistat (tetrahydrolipstatin, ATC code: A08AB01, Elea Laboratory, Ciudad Autónoma de Buenos Aires, Argentina) was used as a positive control.
2.9.2. α-Amylase inhibition
The inhibitory activity of α-amylase was measured using Amilokit (Wiener Lab Group, Rosario, Argentina, Cat. N 1021001) as reported by Costamagna et al. (2016) and following the manufacturer's instructions. Different concentrations of extract were used (5–40 μg GAE/mL) (before and after the simulated GD) and the results were reported as IC50 values. Acarbose was used as a positive control.
2.9.3. Lipase inhibition
The effect on lipase activity was tested by measuring of enzymatic hydrolysis of p-nitrophenyl palmitate according to Costamagna et al. (2016). The assays were carried out in presence (final concentration between 2.5 and 20 μg/mL) and in absence of each PEE, before and after simulated GD digestion. After pre-incubation (enzyme-PEE) at 4 °C for 10 min, the enzyme reaction was carried out for 20 min. IC50 values were calculated. Orlistat was used as a positive control.
2.10. Antihyperglycemic activity
2.10.1. Determination of glucose adsorption capacity of chilto powder
The glucose adsorption capacity of each powder (before and after GD digestion) was determined by Ahmed et al. (2011). The reaction mixture consisted of 10 mL of 20 mM glucose solution and 0.5 g of powder from each part of the fruit. The mixture was stirred well, incubated in a water bath with a stirrer at 37 °C for 4 h, centrifuged at 4,000 x g for 20 min and the glucose content in the supernatant was determined. The glucose bound to the powder was calculated using the following formula:
| Glucose bound = [(G1-G2)/(Weight of the sample)] × volume of solution |
G1: glucose concentration in the original solution (20 mM).
G2: glucose concentration after 4 h.
2.10.2. Effect of chilto powder on glucose diffusion
The assay was performed according to Ahmed et al. (2011). To carry out the analysis, 5 mL of a 20 mM glucose solution and chilto powder samples (before and after GD digestion) (0.25, 0.5 and 1g) were dialyzed in dialysis bags against 40 mL of distilled water at 37 °C. The glucose content in the dialysate was measured at 30, 60, 120, 180 and 240 min using the enzymatic glycemia kit. A control was carried out without powder.
2.10.3. Effect of PEE on glucose intake by Saccharomyces cerevisiae cells
The assay was carried out according to Bhutkar et al. (2017). To prepare the suspension, commercial yeast was washed three times with distilled water and then centrifuged (3000 x g, 5 min) until the supernatant was clear. Then, a 10% (v/v) S. cerevisiae cells suspension was prepared in distilled water. One hundred microliter of this suspension was contacted with increasing concentrations of PEE (10–100 mg/mL) (before and after simulated GD digestion). Then, 1mL of 20 mM glucose solution was added to this mixture, incubated for 1 h at 37 °C and centrifuged at 2500 x g for 5 min. The glucose concentration in the supernatant was determined by enzymatic glycemia kit. The percentage of increase in glucose consumption by S. cerevisiae cells was calculated using the following formula:
| Increase in glucose uptake = (DO control-DO sample) / (DO control) × 100 |
For scanning electron microscopy, yeasts with and without treatment were coated with gold (Fine Coat Ion Sputter JEOL JFC-1100). ZEISS SUPRA-55 VP field emission scanning electron microscope was used. The micrographs were taken for each sample at a magnification of 10,000 and 50,000.
2.11. Antioxidant activity of PEE
Unless otherwise specified, antioxidant activity tests were carried out in 96 multiwell plates.
2.11.1. Total antioxidant capacity assay
The total antioxidant activity of PEE was tested using the ABTS radical-cation method (ABTS •+) (before and after simulated GD digestion) (Orqueda et al., 2017). Results were expressed as SC50 (the concentration of PEE necessary to scavenge 50% of ABTS). BHT and quercetin were used as reference compounds.
2.11.2. Protection of oxidative hemolysis assay
The capacity of PEE to protect the red blood cell (RBC) membrane from oxidative hemolysis was carried out according to Orqueda et al. (2017), before and after the simulated GD digestion (0.1–2.5 mg GAE/mL). Antioxidant activity was measured using 2 mL cuvettes. The IC50 values were determined as the concentration of PEE necessary to protect the RBC from oxidative hemolysis by 50%. Quercetin was used as a reference compound.
2.11.3. H2O2 scavenging assay
The hydrogen peroxide scavenging assay was assayed according to Fernando and Soysa (2015) method with a few modifications. PEE, before and after simulated GD digestion (2.5–40 mg GAE/mL) and H2O2 were pre-incubated for 3 min at 37 °C. Then, a solution of phenol (12 mM) and 4-aminoantipyrine was added to the mixture. The content of hydrogen peroxide was determined catalyzing its conversion by horseradish peroxidase (HRP) and measured spectrophotometrically by the formation of a coloured quinone at 504 nm. The results were expressed as SC50 values (mg GAE/mL), the concentration necessary to scavenge 50% of H2O2. Quercetin and ascorbic acid were used as controls.
2.11.4. Superoxide radical scavenging assay
The capacity of PEE to scavenging superoxide radicals was measured with the phenazine methosulfate (PMS) method (Cardozo et al., 2011). The superoxide radicals were generated by oxidation of NADH (β-nicotinamide adenine dinucleotide) in a system with PMS and were measured spectrophotometrically by the reduction of nitroblue tetrazolium (NBT). The reaction mixture contained extract, before and after simulated GD digestion at 10–100 mg GAE/mL. Then, the reaction was incubated 20 min at 25 °C and the optical density was read at 560 nm. The SC50 values were measured as mg GAE/mL necessary to inhibit the 50% of superoxide radicals. Quercetin was used as an antioxidant reference compound.
2.11.5. Hydroxyl radical scavenging assay
The HO• scavenging capacity was measured as described by Cardozo et al. (2011). Antioxidant activity was measured using 2 mL cuvettes. The PEE (before and after simulated GD digestion) (0.5–10 mg GAE/mL) were added to a reaction mixture contained in KH2PO4/KOH buffer (pH 7.4), 50 mL of 10.4 mM 2-deoxy-D-ribose, 50 mL of 50 mM FeCl3, 50 mL of 52 mM EDTA. To start the Fenton reaction, 50 μL of 10 mM H2O2 and 50 μL of 1.0 mM ascorbic acid were added and the reaction mixture was incubated 1 h at 37 °C. Then, 500 μL of 2-thiobarbituric acid (1%, w/v) dissolved in trichloroacetic acid (3%, w/v) was added to the mixture and then, was incubated 20 min at 100 °C. The reaction absorbance was read at 532 nm. The antioxidant activity was expressed as SC50 values (mg GAE/mL necessary to inhibit by 50% the degradation of 2-deoxy-D-ribose by the HO•).
2.11.6. Nitric oxide scavenging assay
The ability of the chilto extracts to scavenging nitric oxide was carried out according to Govindarajan et al. (2003). Different concentrations of PEE (before and after simulated GD digestion) (10–60 μg GAE/mL), sodium nitroprusside (100 mM) and sodium phosphate buffer (0.2 M; pH 7.4) were incubated during 60 min at 37 °C. Then, the Griess reagent was added and incubated for 15 min in the dark. The antioxidant capacity was measured spectrophotometrically at 550 nm. SC50 was defined as the phenolic concentration (mg GAE/mL) necessary to scavenge 50% of nitric oxide. Ascorbic acid was used as a positive control.
2.12. Toxicity assays
2.12.1. Acute toxicity
The acute toxic effect of the extracts was tested by the Artemia salina assay. After hatching, nauplius larvae with 24 h of incubation at 25 °C were used for the assay. The larvae (10 per well) were transferred into wells of a microplate containing 100 μL of seawater. Then, increasing concentrations of extracts were added (2.5–250 μg GAE/mL). Negative control with DMSO and positive control with potassium dichromate (10–40 μg/mL) were assayed. After 24 h of exposition, the number of dead shrimps in each well was counted.
2.12.2. Salmonella mutagenicity assay
The mutagenic effect of red chilto skin, pulp and seed extracts were assayed on Salmonella Typhimurium strains TA98 and TA100 (Maron and Ames, 1983). Different concentrations of extracts (125–500 μg GAE/plate) were used. His + revertant colonies were counted and compared to the revertant colonies of the controls (100 μL of DMSO). The reagent 4-nitro-o-phenylenediamine (4-NPD, 10 μg/plate) was used as a positive control. Extracts are considered mutagenic when the average number of revertants colonies was double or higher than the revertants colonies of controls. Three plates per experiment were assayed and two separate sets of experiments were performed in all cases.
2.13. Statistical analyses
Analyses were conducted at least three times with three different samples. Each experimental value is expressed as the mean ± standard deviation (SD). The one-way ANOVA with Tukey posttest at a confidence level of 95% was used for the comparisons between groups. For correlation analyses between total phenolic content and bioactivities Pearson's correlation coefficient (r) at p ≤ 0.05 was used. The analyses were developed using the statistic software InfoStat (Student Version, 2011).
3. Results and discussion
Chilto (orange and red) is a neglected and underutilized native species (NUS) in Argentine. The successful build-up as a commercially important crop requires the selection of cultivars with good fruit quality and the study of its potencialities as functional ingredient or food. For this reason, the study of red variety chilto grown in Argentinean Yungas and its comparison with the orange fruit is proposed.
3.1. Characterization of chilto fresh red fruits
3.1.1. Parameters of physical-chemical quality of fresh fruit
The quality of the fruits is the combination of characteristics, attributes and properties that give value to the food. The quality can also be defined as the set of parameters, such as soluble solids content (SSC), color and total acitdity that determine that a certain product is the taste of a consumer or a group that you want to satisfy. The CIELAB parameters of colour were taken into account to ensure the homogeneity and quality of the sample (L*: 36.51 ± 2.54; a*: 25.66 ± 1.33; b*: 11.74 ± 1.82; hue angle: 22.97; Chroma: 27.26 and CIRG index: 2.46) (Table 1). These values were different of the colorimetric parameters of orange chilto fruits of Northwestern Argentina (L*: 62.33 ± 1.89; a*: 15.72 ± 1.81; b*: 39.92 ± 2.32; Chroma: 42.90; hue angle: 68.50 and CIRG index: 1.06) (Orqueda et al., 2017), showing differences in the shades of the epicarp among both varieties. The SSC value obtained for red fruits (9.20% ± 0.2) was lower than those for red tree tomatoes fruits from Ecuador and Spain (11.00–12.00%) (Vasco et al., 2009; Acosta Quezada et al., 2015). The red fruit of S. betaceum from Argentina showed lower total acidity (0.9%) than the orange fruit from the same place (1.9%) (Orqueda et al., 2017). The difference in the acidity of the two varieties is clear in the taste, so the red chilto have a more pleasant and less acid taste than the orange fruit.
Table 1.
Metabolic profile of red Solanum betaceum fresh fruits by 1H-NMR spectrometry and CIELAB parameters.
| Metabolites | g/100 g fresh weight |
|---|---|
| D-fructose | 0.99 ± 0.27 |
| D-glucose | 0.73 ± 0.14 |
| Sucrose | 0.66 ± 0.20 |
| Gamma-aminobutyric acid | 0.05 ± 0.02 |
| L-alanine | 0.01 ± 0.001 |
| L-asparagine | 0.17 ± 0.09 |
| L-aspartic_acid | 0.14 ± 0.09 |
| Citric acid | 2.67 ± 0.54 |
| L-malic_acid | 0.48 ± 0.13 |
| Alpha-ketoglutaric_acid | 0.17 ± 0.12 |
| Ethanol | 0.05 ± 0.002 |
| Methanol | 0.10 ± 0.007 |
|
| |
| CIELAB parameters of colour | |
| L* | 36.51 ± 2.54 |
| a* | 25.66 ± 1.33 |
| b* | 11.74 ± 1.82 |
| hue angle | 22.97 |
| Chroma | 27.26 |
| CIRG index | 2.46 |
| Sweetness index | 28.97 |
| Total acidity | 0.9 |
| SSC | 9.20% ± 0.2 |
3.1.2. Metabolic profile of fresh red chilto
In fruits, fructose, glucose and sucrose are the main soluble sugars and differ in the level of sweetness. The sugars are important in the flavor of the fruit causing a strong impact on the organoleptic quality of the fruits (D'Angelo et al., 2018). In fresh fruits of red chilto the level of fructose was 9.91 mg/g FW, glucose 7.32 mg/g FW and sucrose 6.62 mg/g FW (Table 1) and accordingly, the sweetness index of red fruits was 28.97. The sweetness index of chilto was higher than pear, apple and blueberries juice index (Rizzolo and Cortellino, 2018).
Citric acid was the more abundant organic acid in the ripe red chilto (26.47 mg/g FW) followed by malic acid (4.86 mg/g FW). The level of GABA, glutamic and succinic acids were low, indicating the absence of GABA shunt pathway activity, and consequently, the lack of feeding to the tricarboxylic acid cycle, which diminishes the supply of NADH to the mitochondrial electron transport chain. Few free amino acids were detected, including L-asparagine and L-aspartic acid, allowing nitrogen assimilation and suppling oxaloacetate for the tricarboxylic acid cycle functioning, respectively. The level of ethanol was low indicating the absence of a fermentative process (Table 1).
In red chilto fruit, the methanol content was 1 mg/g fruit, similar to that reported for apple (Possner et al., 2014). Methanol is present in pectin, a component of the plant cell walls and the middle lamella.
Water-soluble pectin and methanol are released during processing of fruits and vegetables by enzymatic activities. To accurately measure the methanol contents of whole or processing fruits, it is important that pectin methyl esterase (PME) activity in the fruits is inactivated before analysis. In this way, when homogenizing the material, a rapid de-esterification of the pectin will occur altering the amounts of methyl esters and free methanol.
3.2. Chemical and functional characterization of chilto red fruit powder
Removal of skin and seeds of fruits is a common practice by the Argentine food industry, and the population consumes only the fruits pulp (crude or cooked).
For this reason, we analyzed the chilto skin, pulp and seed in order to give an added value to the waste material (skin and seed) in the industrial processing to obtain pulp, juice and other products.
3.2.1. Nutritional composition of the skin, pulp and seed powder
3.2.1.1. Macronutrients analysis
The soluble sugars content (8.04–20.5 g/100g DW) in the three fruit parts of the Argentinean red chilto was higher than in the orange chilto variety (2.23–11.91 g/100g DW) from the same ecoregion of Argentina (Orqueda et al., 2017). The carbohydrates content of red chilto fruits was lower than the ones reported for wild S. cajanumense of Ecuador (28.6 g/100 g DW) (Acosta-Quezada et al., 2015). The levels of glucose and fructose were higher in skin powder (1.02–3.23 g/100g) than in pulp or seeds powder (0.8–0.95 g/100g). These results were similar to those obtained for the orange chilto variety (0.90–2.72 g/100g) and much lower than for red tree tomatoes from Ecuador (9.0 g/100g) (Acosta-Quezada et al., 2015). The sucrose levels in ripe red chilto (4.23, 7.23 and 8.52 g/100g DW for skin, pulp and seeds, respectively) were higher than glucose (0.6–0.99 g/100g DW) and fructose (1.23–3.96 g/100g DW) (Table 2). According to these results, the sweetness index of the three fruit parts was 6.77, 10.71 and 15.99 for skin, pulp and seeds, respectively, higher than in the orange chilto from the Argentine northwestern Yungas. Comparing with other flours or powders, the sweetness index of red chilto powder was lower than apple flours (59.85) (Pires et al., 2018). Digestible starch (DS) (0.02%, 0.01% and 0.006% for seeds, pulp and skin, respectively) and resistant starch (RS) (0.003% only in the pulp) were lower than for wheat flour (65% of total starch) (Swieca et al., 2017). In order to maintain normal postprandial glucose and fatty acid levels and avoid oxidative stress, a decrease in DS consumption must be achieved (Agama-Acevedo et al., 2018).
Table 2.
Macronutrient and micronutrient content of seed, pulp and skin powder of Argentinean red Solanum betaceum.
| Nutrients and mineral content in 100 g powder | Skin | Pulp | Seeds |
|---|---|---|---|
| Soluble total sugar (g GE) | 8.9 ± 0.4a | 10.5 ± 1.8a | 20.5 ± 1.2b |
| Glucose (g GE)* | 0.6 ± 0.01a | 0.91 ± 0.03b | 0.99 ± 0.06b |
| Fructose (g GE)* | 1.23 ± 0.5a | 1.65 ± 0.52a | 3.96 ± 0.2b |
| Sucrose (g GE)* | 4.23 ± 0.9a | 7.23 ± 0.5b | 8.52 ± 0.3b |
| Digerible starch (mg) | 6.53 ± 0.5a | 18.49 ± 0.2b | 22.52 ± 1.2c |
| Resistant starch (mg) | ND | 3.47 ± 0.60 | ND |
| Total protein (g) | 10.51 ± 0,6a | 11.2 ± 0.3a | 21.9 ± 0.2b |
| Soluble protein (g AE)** | 0.09 ± 0.01b | 0.05 ± 0.008a | 0.5 ± 0.01c |
| Fat (g) | 0.8 ± 0.1c | 0.2 ± 0.03a | 0.42 ± 0.03b |
| Total fiber (g) | 23.2 ± 2.0a | 20.8 ± 1.8a | 29.5 ± 1.2b |
| Dietary soluble fiber (g) | 9.72 ± 0.2b | 9.03 ± 0.2b | 5.59 ± 0.1a |
| Minerals (mg/100 g) | |||
| Na | 1.2 ± 0.1 | ND | ND |
| Mg | 90.4 ± 2.0b | 76.4 ± 0.5a | 131.8 ± 3.5c |
| K | 2725.6 ± 69.0b | 1234.2 ± 26.7a | 1234.2 ± 26.7a |
| Ca | 212.9 ± 6.0c | 105.7 ± 1.9b | 56.5 ± 3.5a |
| Fe | 3.4 ± 0.05b | ND | 5.8 ± 0.06a |
| Cu | 3.74 ± 0.008b | 0.81 ± 0.02a | 0.90 ± 0.03a |
| Zn | 0.46 ± 0.04a | 1.20 ± 0.06b | 1.61 ± 0.04b |
bdl: below detection limit. Different letters (a, b, c, d) in the same line show significant differences in the macro and micronutrient content among each part of the fruit (skin, pulp and seeds), according to Tukey's multiple comparison (p ≤ 0.05). *GE: Glucose equivalent; **AE: Albumin equivalent; ND: no detected.
In the three fruit parts, the protein level was between 5.2-16.3 g/100 g powder, higher than the content found in New Zealand fruits, whose values were between 1.9-2.0 g/100g (Athar et al., 2003). Although the protein content of the chilto powder was lower compared to soybean flour (43.2 g/100g) (Van Ee, 2009), it is relatively high for an unconventional flour. This data together with the low fat content (0.42–0.9 g/100 g), makes these unconventional flours or powders an ingredient of interest in the food industry.
The total fiber level of skin, seed and pulp was high and similar to previously found in orange chilto (8.84–20.95 g/100g) (Orqueda et al., 2017). The dietary soluble fiber (DSF) content in red chilto skin, pulp and seeds was 9.72; 9.06 and 5.59%, respectively. The DSF content was higher than the reported in the flours of amaranth and quinoa (Kurek et al., 2018) and similar to wheat flour (Zhang et al., 2018). It is proven that dietary fibers produce certain healthy effects for consumers through increased fecal volume, stimulation of the fermentation of the colon, reduction of postprandial blood glucose and pre-prandial cholesterol level. These physiological effects are also well associated with reduced risk of cardiovascular diseases, cancer, diabetes, respiratory disease, infections, and others (Zhang et al., 2018). In conclusion, this study proved that chilto seeds and skin, two sub-products, as well as the pulp, could be exploited for their content in nutritional and functional components beneficial for human health.
3.2.1.2. Mineral analysis
The three chilto powders were high in potassium, with a content similar to banana (395 mg/100 g FW) (Livsmedelsverket, 2009) and low in sodium (below detection limits in pulp and seed powder) (Table 2), even below the sodium content of the orange variety of chilto (Orqueda et al., 2017). This potassium/sodium ratio in red chilto powders is desirable in a healthy diet. The magnesium and calcium contents were higher than in tree tomatoes from Ecuador (Vasco et al., 2009), principally in seed powder. Compared with orange chilto, the magnesium, potassium and calcium content was lower in red chilto, although the iron content was higher in the seeds of red chilto (Orqueda et al., 2017). Since iron is an important mineral for the prevention of anemia due to iron deficiency, and the diverse clinical manifestations associated with this pathology, the content of this microelement converts chilto powder as an interesting functional ingredient (Abbaspour et al., 2014). However, other studies on the bioavailability of this mineral in chilto powder would be necessary.
3.2.2. Phytochemical composition of the skin, pulp and seed powder
3.2.2.1. Polyphenolic compounds content
Plant foods have large amounts of phytochemicals. There is evidence on the benefit that the consumption of foods containing phenolic compounds is associated with the prevention of chronic disorders such as cardiovascular diseases and type 2 diabetes (Costamagna et al., 2016). In recent times, the interest in these bioactive compounds has increased, as well as their impact on health. The content of phenolic compounds in red chilto was 408.9 ± 2.3, 334.0 ± 1.2 and 623.6 ± 1.6 mg GAE/100 g DW in the skin, pulp and seeds, respectively. The phenolic profile of pulp and skin was very similar. Two main compounds, rosmarinic acid and caffeoylquinic acid, were identified (Figure 2). These two hydroxycinnamoyl derivatives had been reported previously in the orange chilto from Argentina Yungas and in yellow and purple varieties from Ecuador (Espin et al., 2016; Orqueda et al., 2017) but in orange chilto the diversity of phenolic compounds was greater (12 caffeic acid derivatives and related phenolics, 10 rosmarinic acid derivatives and 7 flavonoids), (Orqueda et al., 2017). The total content of each phenolic acid (rosmarinic acid and caffeoylquinic acid) in red chilto skin powder was 0.291 ± 0.001 g/100 g powder and 0.354 ± 0.002 g/100 g powder, respectively. Several biological activities are attributed to these phenolic acids, such as antioxidant, anti-inflammatory, immunomodulatory, hypoglycemic, hypolipidemic, antimutagenic, antitumor, antimicrobial, hepatoprotective, neuroprotective, and nephroprotective properties (Spínola and Castilho, 2017; Costa et al., 2014).
Figure 2.
Phenolic compounds profile of red chilto skin polyphenolic extracts. Caffeoylquinic acid RT 27.1 min; Rosmarinic acid RT 60.1 min.
The food preferences of the population are characteristic of each region and can define the intake of polyphenols in each of them. However, although the recommended intake depends on the region, preferences, age, among others, the presence of these bioactive compounds enhances the nutritional value of the fruits. Regarding diet contribution of phenolics, 100 g of fresh red chilto contributes 84.87 mg of phenolic compounds, including 43.96, 29.79 and 11.12 mg of these compounds in the seeds, pulp and skin, respectively.
Hydrolyzable tannins were not detected in any of the three powders. But, all red chilto powders contain condensed tannins (Table 3), mainly in the seed powder (265.0 mg/100 g DW). This is the first report on the content of condensed tannins for chilto. Condensed tannins are biofunctional compounds of interest for their multiple health attributes that include anti-inflammatory, antimicrobial, antiviral, antitumoral, hypocholesterolemic, and digestive enzymes (α-amylase, α-glucosidase and lipase) inhibitory activities and thus, their benefits in cardiovascular diseases, diabetes, and gastrointestinal disorders (da Silva et al., 2014).
Table 3.
Phytochemical content of seed, pulp and skin powder of Solanum betaceum fruits (red variety).
| Phytochemical content | Skin | Pulp | Seeds |
|---|---|---|---|
| Total phenolic (mg GAE) | 408.9 ± 2.3b | 334.0 ± 1.2a | 623.6 ± 1.6c |
| Flavone and flavonol (mg QE) | 195.3 ± 3.6c | 123.3 ± 0.9a | 180.5 ± 2.0b |
| Condensed tannins (mg procyanidin B2) | 75.7 ± 1.3b | 18.9 ± 1.2a | 265.0 ± 3.5c |
| Hydrolyzable tannins (mg GAE) | ND | ND | ND |
| Anthocyanins (mg C-3GE) | 62.5 ± 0.5b | 10.6 ± 0.6a | 65.23 ± 0.9b |
| Carotenoids (g β carotene) | ND | 0.9 ± 0.08 | ND |
| Ascorbic acid (mg) | 43.5 ± 2.5a | 75.9 ± 2.0b | 45.2 ± 0.6a |
| %Yield (g powder/100 g fruit part) | 26.0 ± 0.8b | 20.4 ± 0.5a | 25.9 ± 1.5b |
Different letters (a, b, c, d) in the same line show significant differences in the phytochemical content among each part of the fruit (skin, pulp and seeds), according to Tukey's multiple comparison (p ≤ 0.05). ND: no detected. GAE: Gallic acid equivalent; QE: Quercetin equivalent; C-3GE: Cyanidin-3 glucoside equivalent.
Anthocyanins are a group of flavonoid compounds responsible of the colours of many plant structures (flowers and fruits) and are related with health-related properties such as effect reducing the risk of cardiovascular diseases (De Pascual-Teresa and Sanchez-Ballesta, 2008). In red chilto, the content of anthocyanins was 10.6, 62.5 and 65.23 mg EC/100 g DW for the pulp, skin and seeds, respectively. The total content of pigment (60.38 mg EC/100 g FW) in red chilto was much higher than the reported to orange chilto (1.78 mg/100 g, only in the skin) from Argentinean Yungas (Orqueda et al., 2017) and similar to that reported in blackberry (58.61 mg/100 g FW; Souza et al., 2014). These results provide evidence of difference in profile and quantities of phenolic compounds among the orange and red varieties. This information is essential for the development of selection and breeding programmes.
3.2.2.2. Vitamins
The ascorbic acid (vitamin C) content of the skin, pulp and seed powders is shown in Table 3. The results show that the powders are rich in vitamin C, a potent antioxidant compound with large biological activities. The vitamin C content of the red chilto powder was similar to orange chilto from Argentina and was higher than that of the edible tissues of oranges Citrus sinensis (Abeysinghe et al., 2007).
Carotenoids were only detected in red chilto pulp (0.9 mg/100 g DW) while en orange chilto from Argentina was found in the three parts. These pigments have multiple biological properties such as oxidative stress protection, anticarcinogenic capacity, macular degeneration prevention or bone health support (Hornero-Mendez et al., 2018).
3.2.3. Functional properties of seed, skin and pulp powders and PEE
The powders of pulp, skin and seed of chilto were submitted to simulated GD. Due to the extensive evidence that phenolic compounds are effective antioxidants and inhibitors of digestive enzymes, and the intention to also use the fruit powders as raw material for phenolic extracts preparation, the effect of gastroduodenal digestion was studied also on phenolics enriched extracts.
3.2.3.1. Hypoglycemic effect of chilto powder and PEE
Metabolic syndrome is a combination of at least three of the cardiovascular risk factors: obesity, dyslipidemia, hypertension and hyperglycaemia or Type 2 diabetes. All these risk factors are related to insulin resistance, oxidative stress and an inflammatory state. The level of postprandial hyperglycemia can be controlled by inhibiting the activities of α-amylase and α-glucosidase, slowing the diffusion and adsorption of glucose in the intestinal epithelium, stimulating insulin secretion by pancreatic cells and inhibiting the transporters of glucose at the cellular level (Costamagna et al., 2016). The commercial medicines used for treating diabetes may cause several side effects, including flatulence and diarrhea. Therefore, it is necessary to investigate natural sources of food for the prevention of postprandial hyperglycemia.
3.2.3.1.1. Effect of chilto powder on glucose adsorption, diffusion and transport
Adsorption
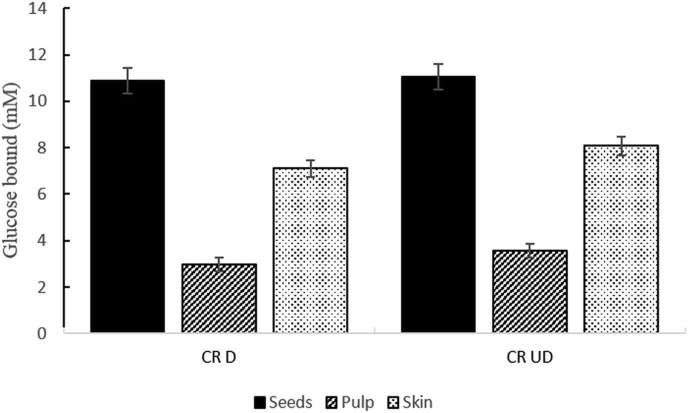
The glucose adsorption capacity of red chilto powders before and after GD was assayed. The seed powder showed higher adsorption capacity than skin and pulp powders (Figure 3). No significant differences were observed between the glucose adsorption capacity of digested and undigested seed and pulp powders. The adsorption capacity of the digested chilto skin powder was greater than undigested. The results revealed that the adsorption capacity of chilto seeds powder was higher than acarbose, wheat bran and oats at a concentration of 10 mM of glucose (Ahmed et al., 2011) and similar to Caesalpinia bonducella and Myristica fragrans seed (Bhutkar et al., 2018). These results indicated that the red chilto powders could decrease the amount of glucose available to be absorbed by the intestinal epithelium resulting in normal blood glucose values. Furthermore, condensed tannins, which are also present in red chilto powders, principally in seed powders could bind to the enzymes at non-specific sites, diminishing their activities and have been proposed as non-competitive inhibitors of these digestive enzymes (Barrett et al., 2018).
Figure 3.
Glucose adsorption capacity of digested and undigested chilto powders. CR D: red chilto digested powders. CR UD: red chilto undigested powders.
Diffusion
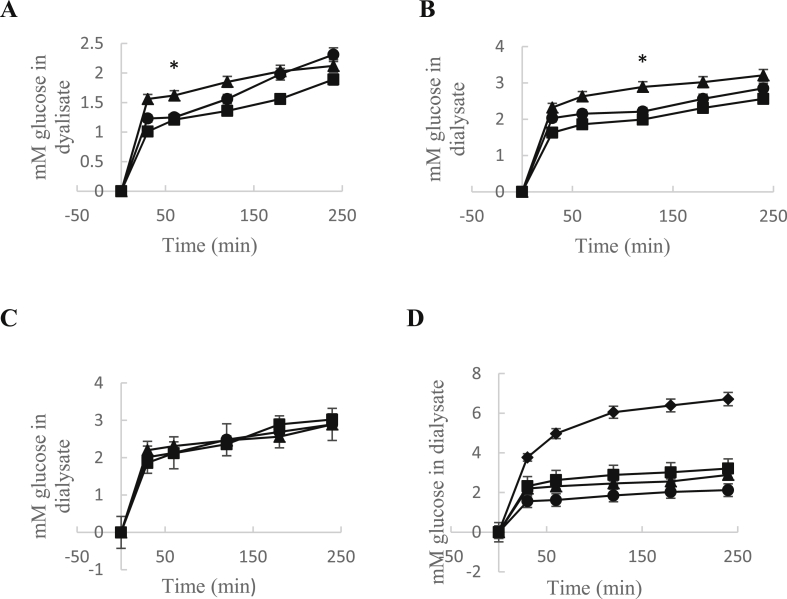
The effect of chilto powder before and after GD on retarding glucose diffusion across the dialysis membrane is represented in Figure 4. During the study, the movement of glucose across the dialysis membrane was monitored every 30 min for 240 min, and it was observed that the powder samples delayed the movement of glucose to the dialysate through the membrane compared to the control. The rate of glucose diffusion across the dialysis membrane was increased over time, but all the samples were able to inhibit glucose diffusion compared to the control (Figure 4). No significant differences were observed between the digested and undigested powders. These effects could be attributed to the network formed by the fibers where glucose is trapped (López et al., 1996). The dietary fiber of the powders forms viscous gels in contact with water, which slows the access of glucose to the small intestine epithelium, decreasing its absorption.
Figure 4.
Effect of different amount of chilto powders on glucose diffusion: (A) Seed; (B) Pulp (B) and (C) Skin powders  0.25 g;
0.25 g;  0.5 g and
0.5 g and  1.0 g. (D) comparative effect on glucose difussion of 0.25 g powders with control
1.0 g. (D) comparative effect on glucose difussion of 0.25 g powders with control  ; seed
; seed  ; pulp
; pulp  ; and skin
; and skin  . The symbol (*) indicates significant differences between seed and pulp extracts compared to skin extracts at a given time.
. The symbol (*) indicates significant differences between seed and pulp extracts compared to skin extracts at a given time.
3.2.3.1.2. Effect of chilto PEE on glucose uptake by S. cerevisiae cells
The yeast model to evaluate the mechanism of glucose transport through the cell membrane is a test for the in vitro evaluation of the possible hypoglycemic effect. The results of the rate of glucose transport across the cell membrane in yeast cell system are represented in Figure 5 I. The presence of phytochemicals of chilto increase the uptake of glucose by the yeast cells, therefore the amount of glucose remaining in the medium is lowest after the incubation time. The area under the curves of glucose uptake obtained with polyphenols from seed powder (5901.52 ± 124.79) was higher than pulp (3504.01 ± 441.78), followed by skin (2789.50 ± 109.58). The chilto seeds polyphenolic extract was more effective to increase glucose transport up to 81% compared to the control (Figure 5I). Enhancement on glucose uptake by low concentration of seeds polyphenols was similar to chia and cinnamon extracts (Woldemariam and Van Winkle, 2015). Furthermore, SEM photographs showed that the cell surface structure of the yeasts was not affected in the presence of chilto extracts (Figure 5II). These results show that the chilto polyphenols could favor the entry of glucose into the cells, resulting in a decrease in blood glucose levels. Further in vivo experiments should be achieved in order to confirm that these natural extracts are possible antidiabetic nutraceuticals.
Figure 5.
I. Effect of S. betaceum extracts on uptake of glucose by S. cerevisiae seed;
seed;  pulp;
pulp;  skin. II. Scanning electron micrographs of (A) control yeast, (B) yeast treated with seed extract, (C) yeast with glucose, (D)yeast treated with polyphenolic enriched extract + glucose.
skin. II. Scanning electron micrographs of (A) control yeast, (B) yeast treated with seed extract, (C) yeast with glucose, (D)yeast treated with polyphenolic enriched extract + glucose.
3.2.3.1.3. Inhibition of carbohydrate metabolism enzymes by PEE
Pancreatic α-amylase and α-glucosidase hydrolyse complex carbohydrates and disaccharides in absorbable monosaccharides in the intestinal epithelium (Costamagna et al., 2016). An objective in the treatment of type 2 diabetes focuses on the inhibition of these enzymes as a way to lower blood glucose levels (Zhang et al., 2015). Anti-diabetic agents that are used in clinical practice, such as acarbose, voglibose, and miglitol are competitive inhibitors of these enzymes which delay the hydrolysis of carbohydrates and thus alleviate postprandial hyperglycemia (Scheen et al., 2015). However, the use of these drugs has some disadvantages including drug resistance, side effects, and even toxicity (Spínola et al., 2020). Due to their strong inhibitory activities, these enzyme inhibitors can produce several side effects, since dangerous ones like the excessive lowering of blood sugar levels when used in combination with other medications used to treat diabetes, to banaler such as abdominal pain, diarrhoea, and flatulence. In this sense, new natural inhibitors with moderate activities and good bioavailability can be useful to manage obesity, insulin resistance or type-2 diabetes. Bioavailability is dependent upon their release from the plant matrix (bioaccessibility), their stability during digestion process, and the efficiency of their transepithelial passage (Manach et al., 2004). So, the inhibitory capacity of amylase and glucosidase of red chilto polyphenolic extracts was determined before and after in vitro gastroduodenal digestion to determine the stability during digestion.
All extracts enriched in polyphenols of red chilto were active to inhibit both enzymes with IC50 values between 25.0-89.3 μg of GAE/mL for α-glucosidase and 50.0–136.5 μg of GAE/mL for α-amylase (Table 4). A strong correlation (r2 ≥ 0.84) between total phenolic contents and the inhibitory capacity of the enzymes (α-glucosidase and α-amylase) was observed with seeds, skin, and pulp extracts (Table 5). However, the correlations were only significant (p < 0.05) with seeds and pulp extracts against α-glucosidase. A very strong significant correlation between total phenolic concentrations and α-glucosidase inhibition was observed with these two extracts (r2 ≥ 0.96, p < 0.05). The seeds powder was the most active extract, probably due to the higher contents of total phenolics and condensed tannins, the last ones recently proved to be effective inhibitors of α-glucosidase and α-amylase (Barrett et al., 2018). The PEE of red chilto were more active towards the α-glucosidase than the acarbose reference compound but had a lower effect on α-amylase. Also, it is noteworthy that the extracts obtained from the orange variety flours proved to be much more active than those of the red variety, probably due to its less phenolic components diversity than orange chilto (Orqueda et al., 2017).
Table 4.
Effect of phenolic-enriched extract (PEE) of Argentinean Solanum betaceum red fruit powder (seed, pulp and skin) before and after simulated gastroduodenal digestion (GD) on enzymes related to carbohydrate and fat metabolism and oxidative processes. Reference compounds are included for comparison.
| S. betaceum | Enzyme IC50 (μg GAE/mL) |
IC50 (μg GAE/mL) |
SC50 (μg GAE/mL) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| α amylase | α glucosidase | Lipase | AAPH | ABTS | O2.- | HO• | H2O2 | NO | |
| Seeds | |||||||||
| Before GD After GD |
50.0 ± 2.3a 35.6 ± 1.2A |
25.0 ± 1.2a 30.5 1.1A |
4.5 ± 0.3a 6.5 ± 0.2A |
1.2 ± 0.05a 3.6 ± 0.02C |
4.5 ± 0.5ab 5.8 ± 1.2AB |
22.1 ± 0.5a 26.6 ± 0.6A |
8.5 ± 0.06c 7.9 ± 0.2B |
5.8 ± 0.5a 7.8 ± 0.3A |
17.1 ± 0.6a 44.0 ± 1.3B |
| Pulp | |||||||||
| Before GD After GD |
115.1 ± 2.1b 120.0 ± 2.3B |
42.3 ± 0.3b 40.3 ± 0.6B |
4.7 ± 0.9a 5.9 ± 0.6A |
1.0 ± 0.03a 0.7 ± 0.06A |
5.7 ± 0.6b 8.2 ± 0.6B |
74.0 ± 1.3b 78.3 ± 2.0C |
5.6 ± 0.1b 10.2 ± 0.03C |
14.6 ± 0.05b 20.6 ± 0.6C |
44.0 ± 3.2b 49.8 ± 2.0B |
| Skin | |||||||||
| Before GD After GD |
118.2 ± 2.6b 136.5 ± 1.2C |
76.0 ± 0.9c 89.3 ± 2.6C |
6.8 ± 0.3b 8.5 ± 0.1B |
2.6 ± 0.04b 2.9 ± 0.01B |
3.4 ± 0.3a 5.6 ± 1.1A |
66.0 ± 0.6b 70.6 ± 0.9B |
4.2 ± 0.09a 6.3 ± 0.1A |
20.6 ± 0.2c 16.5 ± 0.5B |
22.0 ± 2.1a 30.6 ± 1.6A |
| Reference compound | Acarbose | Acarbose | Orlistat | Quercetin | BHT | Quercetin | Quercetin | Quercetin | Ascorbic acid |
| IC50 (μg/mL) |
1.25 ± 0.10 | 25.00 ± 1.00 | 0.08 ± 0.01 | 1.40 ± 0.03 | 0.65 ± 0.01 | 60.50 ± 4.70 | 30.00 ± 2.00 | 17.30 ± 0.50 | 37.19 ± 0.33 |
IC50: Concentration of polyphenolic extract necessary to inhibit 50% of oxidative hemolysis or enzyme activity; SC50: Concentration of PEE necessary to scavenge 50% of ABTS, O2.-, HO•, H2O2, NO; GAE: Gallic acid equivalent; PEE: phenolic enriched extract. Different letters (a, b, c or A, B, C) in the same column in each assay show significant differences among effect of polyphenols on enzyme activity or antioxidant activity according to Tukey's test (p ≤ 0.05).
Table 5.
Pearson's correlation coefficient (r) between total phenolic concentration and functional activities of seeds, pulp, and skin extracts. p-Value less than 0.05 was considered statistically significant.
| Bioactivity | Seeds |
Pulp |
Skin |
|||
|---|---|---|---|---|---|---|
| Pearson's coefficient | p-value | Pearson's coefficient | p-value | Pearson's coefficient | p-value | |
| α-amylase inhibition | 0.84 | 0.1586 | 0.85 | 0.1470 | 0.89 | 0.1078 |
| α-glucosidase inhibition | 0.96 | 0.0380 | 1.00 | 0.0026 | 0.92 | 0.0784 |
| Lipase inhibition | 0.98 | 0.0248 | 0.97 | 0.0273 | 0.90 | 0.0994 |
| AAPH | 0.95 | 0.0040 | 0.64 | 0.1748 | 0.75 | 0.0842 |
| ABTS | 0.99 | 0.0133 | 0.88 | 0.1166 | 1.00 | 0.0015 |
| O2▪ | 0.99 | 0.0096 | 0.96 | 0.0363 | 1.00 | 0.0038 |
| H2O2 | 0.99 | 0.0095 | 0.91 | 0.0863 | 0.98 | 0.0174 |
| HO▪ | 0.97 | 0.0260 | 1.00 | 0.0001 | 0.95 | 0.0455 |
| NO▪ | 0.96 | 0.0412 | 0.96 | 0.0445 | 0.91 | 0.0896 |
Besides, there were no significant differences after subjecting the extracts to the digestive process, contrariwise to the PEE of seeds of orange variety (Table 4).
According to the literature, CQA and RA which are present in red chilto have a great capacity for inhibition of digestive enzymes (Lin et al., 2011; Spínola and Castilho, 2017). Previous reports showed that the concentration of rosmarinic acid is slightly reduced after gastric digestion and that was more stable in the basic pH of the pancreatic environment than in the acidic pH of the gastric juice (Costa et al., 2014; Porfirio et al., 2010). On the other hand, isomerization and hydrolysis of CQA and partial opening of lactones intramolecular ring were observed after incubation of CQA with artificial and human intestinal fluids (pH from 6.3 to 7.7), with slightly losses after 8-h incubation (Farah and Duarte, 2015). The results would indicate that the inhibitory effect could be attributed to both phenolic acids and other minoritary compounds, non-detectable with the used methodologies.
3.2.3.2. Effect of chilto PEE on in vitro fat absorption
Obesity has become a serious health issue around the world due to the connection with a large number of pathological disorders, including metabolic syndrome, diabetes mellitus, hypertension, atherosclerosis and cancer. Inhibition of dietary fat absorption is one of the common approaches to decrease excessive energy intake. Pancreatic lipase hydrolyses lipids in absorbable fatty acids and monoglycerides and, therefore, their inhibition is a key target in the prevention and treatment of obesity. Orlistat is a commonly used antiobesity drug and the reference compound for the inhibition of pancreatic lipase, but it produces several adverse effects. Again, the potency of available commercial inhibitors is mostly responsible for these undesirable effects. Hence the importance of finding moderate lipase inhibitors and natural inhibitors from plant arise as an alternative. In this regard, the inhibitory activity of red chilto PEE was assessed on pancreatic lipase (Table 4) before and after GD digestion. The highest inhibitory activities were observed in the PEE from seeds and pulp before GD digestion (IC50: 4.5 and 4.7 μg GAE/mL, respectively). These both extracts showed a very strong significant correlation between total phenolic contents and the ability to inhibit pancreatic lipase (r2 ≥ 0.97; p < 0.05) (Table 5). The inhibitory capacity of red chilto ethanolic extracts was similar to that of extracts of orange chilto and Geoffroea decorticans (IC50: 4.0 μg GAE/mL) (Costamagna et al., 2016; Orqueda et al., 2017). The ability to inhibit lipase exhibited by the PEE from red chilto could be related to the presence of functional compounds such as caffeoylquinic acids or rosmarinic acid which were previously described as pancreatic lipase inhibitors (Spínola and Castilho, 2017). However, after gastroduodenal digestion, the inhibitory activity of the red chilto PEE decreases by more than 20%, Table 4. The decrease in inhibitory activity after GD digestion may be related to changes in the identity and quantity of bioavailable polyphenols during the passage through the gastrointestinal tract (Costa et al., 2014; Porfirio et al., 2010; Farah and Duarte, 2015).
3.2.3.3. Antioxidant activity of chilto PEE
Several studies indicate that certain complications associated with the metabolic syndrome such as atherosclerosis, hypertension, visceral adiposity and insulin resistance may be associated to an increase in oxidative stress (Costamagna et al., 2016). Some researchers suggest that oxidative stress could be an early event in the pathology of these chronic diseases rather than merely a consequence or an innocent bystander. Considering this, the antioxidant activity of the three PEE was assessed before and after simulated GD (Table 4). Most of the extracts exhibited very strong significant correlation between total phenolic contents and antioxidant activities (r2 ≥ 0.95; p < 0.05) (Table 5). The extracts were able to scavenge ABTS radical with IC50 values between 3.4-8.2 μg GAE/mL. Also, the PEE were very efficient protectors of RBC membrane (0.7–3.6 μg GAE/mL) with major activity of pulps extracts (1 and 0.7 μg GAE/mL, before and after GD digestion). The activity was much higher than extracts of other fruits known for its capacity for the prevention of oxidation and cellular damage, such as quince (Cydonia oblonga) or sweet cherries (Prunus avium) (500 μg GAE/mL and 73 μg GAE/mL, respectively) (Magalhães et al., 2009; Gonçalves et al., 2017); and similar to orange chilto extracts (0.40–1.21 μg GAE/mL, Orqueda et al., 2017). All the extracts were good scavengers of reactive oxygen and nitrogen species (ROS/RNS), with IC50 values between 4.2-78.3 μg GAE/mL. The PEE of seeds showed higher activity than pulp and skin PEE for the depuration of O2.-, H2O2 and NO. However, the HO• radical is considered the most harmful ROS due to its extreme reactivity and oxidative potential, even reacting with compounds such as alkanes (Cardozo et al., 2011). The highest inhibitory potency recorded against this radical was observed in skin powder and was consistent with its higher contents of flavones and flavanols (Table 4). Simulated GD did not significantly affect the protective activity of biomolecules and ROS/RNS scavenging capacity of the PEE. Again, rosmarinic acid and caffeoylquinic acids could be contributing to the functional property of the PEE. Antioxidant capacities of these phenolic compounds were repeatedly demonstrated in both in vitro and in vivo experiments (Zhang et al., 2015; Marković and Tošovic, 2016; Adomako-Bonsu et al., 2017).
3.2.4. Acute toxicity and genotoxicity tests
Toxicity tests are defined as the qualitative and quantitative studies of the deleterious effects caused by chemical or physical agents on the structure and function of living systems. These assays are used for the evaluation of safety and prevention of damage to man. In this sense, the toxicity of S. betaceum extracts was evaluated. None of the extracts of the three parts of the fruit produced significant changes in the number of S. Typhimurium colonies compared to the control at the concentration of 500 μg GAE/plate (data not shown). This result indicates that none of the extracts has genotoxic effects in the cellular systems used, suggesting the absence of compounds that can cause mutations of the frameshift type (TA98) or substitution of bases (TA100). Also, the polyphenols enriched extracts of red chilto had not toxic effect on Artemia salina at the concentrations tested with respect to the negative control (DMSO). With the highest concentration tested (1000 μg GAE/mL) the viability of A. salina was not affected. Besides, no changes in larval movements were observed after the incubation period.
4. Conclusions
The red chilto fruits are a source of appreciated bioactive compounds, such as the rosmarinic acid, caffeoylquinic acids, condensed tannins, anthocyanins, ascorbic acid and soluble dietary fiber. These valuable compounds are also present in the skin, pulp and seed powders, turning them into an excellent source of nutraceuticals and, thus, into dietary supplements or functional foods useful for the management of metabolic syndrome and its related pathologies. Red chilto fruits can alternatively be used as flours or powders to prepare diverse foods or as the raw material for the preparation of functional phenolic extracts. The (before and after GD digestion) seed powder showed high glucose adsorption capacity. The polyphenolic extracts obtained from seed flours were the most active for most of the antioxidant tests as well as to inhibit the enzymes related to the development of the metabolic syndrome and could favor the entry of glucose into the cells, resulting in a decrease in blood glucose levels. The high content of phenolic compounds with high antioxidant activity in seed extracts make them a good functional food candidate and could serve as an ingredient for the preparation of nutraceuticals for human health improvement. Skin powder was also active as an antioxidant supplement. These results provide added value to the different fruit parts that are normally discarded during industrial processes. Pulp powder is attractive because of the presence of bioactive compounds such as phenolic compounds, carotenoids and the high amount of vitamin C, which makes it an excellent dietary supplement with nutritional and phytochemical qualities. The results of the present work showed that both red chilto and the previously studied orange variety are potent inhibitors of digestive enzymes related to metabolic syndrome. The polyphenols of the orange variety displayed higher α-amylase and α-glucosidase inhibitory capacity, while the PEE of the red fruits showed higher inhibition of pancreatic lipase. These results highlight the use of both powder and polyphenolic extracts of red chilto in metabolic syndrome. Besides, natural products often have the advantage of having moderate activity, a wide spectrum of possible multiple/synergistic effects and have fewer side effects with low toxicity, which makes them of special interest for the development of alternative “biologically active products”. However, further research including in vivo studies and clinical trials are necessary to confirm the potential healthy-beneficial effects of red chilto fruits and its powders.
Declarations
Author contribution statement
Maria E. Orqueda: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Sebastian Torres, Iris C. Zampini, Estela M. Valle, Guillermo Schmeda-Hirschman, Maria I. Isla: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Florencia Cattaneo: Performed the experiments; Analyzed and interpreted the data.
Agustina Fernandez Di Pardo, Felipe Jiménez-Aspee: Performed the experiments.
Funding statement
This work was supported by the ANPCyT [PICT 3136 and 4436], ANPCyT-Ministerio de ambiente y desarrollo sustentable [PICTO Bosques 0088], Premio Arcor a la Innovación tecnológica, CONICET PIP 00590 and SCAyT-UNT [PIUNT-G637].
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abbaspour N., Hurrel R., Kelishadi R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014;19:164–174. [PMC free article] [PubMed] [Google Scholar]
- Abeysinghe D.C., Xian L., Sun C., Zhang W., Zhou C., Chen K. Bioactive compounds and antioxidant capacities in different edible tissues of citrus fruit of four species. Food Chem. 2007;104:1338–1344. [Google Scholar]
- Acosta-Quezada P.G., Raigón M.D., Riofrío-Cuenca T., García-Martínez M.D., Plazas M., Burneo M., Figueroa J.G., Vilanova S., Prohens J. Diversity for chemical composition in a collection of different varietal types of tree tomato (Solanum betaceum Cav.), an Andean exotic fruit. Food Chem. 2015;169:327–335. doi: 10.1016/j.foodchem.2014.07.152. [DOI] [PubMed] [Google Scholar]
- Adomako-Bonsu A.G., Chan S.L., Pratten M., Fry J.R. Antioxidant activity of rosmarinic acid and its principal metabolites in chemical and cellular systems: importance of physico-chemical characteristics. Toxicol. Vitro. 2017;40:248–255. doi: 10.1016/j.tiv.2017.01.016. [DOI] [PubMed] [Google Scholar]
- Agama-Acevedo E., Bello-Perez L.A., Lim J., Lee B., Hamaker B.R. Pregelatinized starches enriched in slowly digestible and resistant fractions. LWT - Food Sci. Technol. 2018;97:187–192. [Google Scholar]
- Ahmed F., Sairam S., Urooj A. In vitro hypoglycemic effects of selected dietary fiber sources. J. Food Sci. Technol. 2011;48:285–289. doi: 10.1007/s13197-010-0153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOCS . Association of Official Analytical Chemists; Arlington, VA, USA: 1989. Official Methods of Analysis. [Google Scholar]
- AOAC . Official Methods of Analysis Chemists (17th ed.). Washington, DC. 2000. Association of official analytical chemists. [Google Scholar]
- AOAC . Official Methods of Analysis (18th ed.). Gaithersburg, Maryland. 2005. Association of official analytical chemists. [Google Scholar]
- Athar N., McLaughlin J., Taylor G. 2003. The Concise New Zealand Food Composition Tables. 6th Edition; p. 177. [Google Scholar]
- Barrett A.H., Farhadi N.F., Smith T.J. Slowing starch digestion and inhibiting digestive enzyme activity using plant flavanols/tannins—a review of efficacy and mechanisms. LWT - Food Sci. Technol. 2018;87:394–399. [Google Scholar]
- Bhutkar M.A., Bhinge S.D., Randive D.S., Wadkar G.H. Vol. 55. Bulletin of Faculty of Pharmacy, Cairo University; 2017. Hypoglycemic Effects of Berberis aristata and Tamarindus indica Extracts in Vitro; pp. 91–94. [Google Scholar]
- Bhutkar M.A., Bhinge S.D., Randive D.S., Wadkar G.H., Todkar S.S. In vitro hypoglycemic effects of Caesalpinia bonducella and Myristica fragrans seed extracts. Indian Drugs. 2018;55(1):57–63. [Google Scholar]
- Cardini C.E., Leloir L.F., Chiriboga J. The biosynthesis of sucrose. J. Biol. Chem. 1955;214(1):149–155. [PubMed] [Google Scholar]
- Cardozo M.L., Ordoñez R.M., Alberto M.R., Zampini I.C., Isla M.I. Antioxidant and anti-inflammatory activity characterization and genotoxicity evaluation of Ziziphus mistol ripe berries, exotic Argentinean fruit. Food Res. Int. 2011;44:2063–2071. [Google Scholar]
- Costa P., Grevenstuk T., da Costa A., Gonçalves S., Romano A. Antioxidant and anti-cholinesterase activities of Lavandula viridisL'Hér extracts after in vitro gastrointestinal digestion. Ind. Crop. Prod. 2014;55:83–89. [Google Scholar]
- Costamagna M.S., Ordoñez R.M., Zampini I.C., Sayago J.E., Isla M.I. Nutritional and antioxidant properties and toxicity of Geoffroea decorticans, an Argentinean fruit and products derived from them (flour, arrope, decoction and hydroalcoholic beverage) Food Res. Int. 2013;54:160–168. [Google Scholar]
- Costamagna M.S., Zampini I.C., Alberto M.R., Cuello A.S., Torres S., Pérez J., Quispe C., Schmeda-Hirschmann G., Isla M.I. Polyphenols rich fraction from Geoffroea decorticans fruits flour affects key enzymes involved in metabolic syndrome, oxidative stress and inflammatory process. Food Chem. 2016;190:392–402. doi: 10.1016/j.foodchem.2015.05.068. [DOI] [PubMed] [Google Scholar]
- da Silva S.M., Koehnlein E.A., Bracht A., Castoldi R., de Morais G.R., Baesso M.L., Peralta R.M. Inhibition of salivary and pancreatic α-amylases by a pinhão coat (Araucaria angustifolia) extract rich in condensed tannin. Food Res. Int. 2014;56:1–8. [Google Scholar]
- D'Angelo M., Zanor M.I., Sance M., Cortina P.R., Boggio S.B., Asprelli P., Carrari F., Santiago A.N., Asís R., Peralta I.E., Valle E.M. Contrasting metabolic profiles of tasty tomato fruit of the Andean varieties in comparison with commercial ones. J. Sci. Food Agric. 2018;98:4128–4134. doi: 10.1002/jsfa.8930. [DOI] [PubMed] [Google Scholar]
- De Pascual-Teresa S., Sanchez-Ballesta M.T. Anthocyanins: from plant to health. Phytochemistry Rev. 2008;7:281–299. [Google Scholar]
- Dubois M., Gilles K.A., Hamilton J.K., Rebers P.A., Smith P. Colorimetric methods for determinations of sugar and related substances. Anal. Chem. 1956;28:350–356. [Google Scholar]
- Espin S., Gonzalez-Manzano S., Taco V., Poveda C., Ayuda-Durán B., Gonzalez-Paramas A.M., Santos-Buelga C. Phenolic composition and antioxidant capacity of yellow and purple-red Ecuadorian cultivars of tree tomato (Solanum betaceum Cav.) Food Chem. 2016;194:1073–1080. doi: 10.1016/j.foodchem.2015.07.131. [DOI] [PubMed] [Google Scholar]
- Farah A., Duarte G. Coffee in Health and Disease Prevention. Elsevier; 2015. Bioavailability and metabolism of chlorogenic acids from coffee. Chapter 85. [Google Scholar]
- Fernando C.D., Soysa P. Optimized enzymatic colorimetric assay for determination of hydrogen peroxide (H2O2) scavenging activity of plant extracts. Methods X. 2015;2:283–291. doi: 10.1016/j.mex.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves A.C., Bento C., Silva B.M., Silva L.R. Sweet cherries from Fundão possess antidiabetic potential and protect human erythrocytes against oxidative damage. Food Res. Int. 2017;95:91–100. doi: 10.1016/j.foodres.2017.02.023. [DOI] [PubMed] [Google Scholar]
- Govindarajan R., Rastogi S., Vuayakumae M., Shirwalkar A., Kumar S., Rawat A., Pushpangadan P. Studies on the antioxidant activities of Desmodium gangeticum. Biol. Pharm. Bull. 2003;26:1424–1427. doi: 10.1248/bpb.26.1424. [DOI] [PubMed] [Google Scholar]
- Holm J., Bjorck I., Drews A., Asp N.G. A rapid method for the analysis of starch. Starch Staerke. 1986;38:224–226. [Google Scholar]
- Hornero-Méndez D., Cerrillo I., Ortega Á., Rodríguez-Griñolo M.R., Escudero-López B., Martín F., Fernández-Pachón M.S. β-Cryptoxanthin is more bioavailable in humans from fermented orange juice than from orange juice. Food Chem. 2018;262:215–220. doi: 10.1016/j.foodchem.2018.04.083. [DOI] [PubMed] [Google Scholar]
- Keutgen A., Pawelzik E. Modifications of taste-relevant compounds in strawberry fruit under NaCl salinity. Food Chem. 2007;105:1487–1494. doi: 10.1021/jf071216o. [DOI] [PubMed] [Google Scholar]
- Kurek M., Karp S., Wyrwisz J., Niu Y. Physicochemical properties of dietary fibers extracted from gluten-free sources: quinoa (Chenopodium quinoa), amaranth (Amaranthus caudatus) and millet (Panicum miliaceum) Food Hydrocolloids. 2018;85:321–330. [Google Scholar]
- Lin L., Dong Y., Zhao H., Wen L., Yang B., Zhao M. Comparative evaluation of rosmarinic acid, methyl rosmarinate and pedalitin isolated from Rabdosia serra (Maxim.) Hara as inhibitors of tyrosinase and α-glucosidase. Food Chem. 2011;129:884–889. doi: 10.1016/j.foodchem.2011.05.039. [DOI] [PubMed] [Google Scholar]
- Livsmedelsverket . Livsmedelsverket, National Food Administration; Sweden: 2009. The National Food Administration’s Food Database, Version 19/05/2009. [Google Scholar]
- López G., Ros G., Rincón F., Periago M.J., Martinez M.C., Ortuno J. Relationship between physical and hydration properties of soluble and insoluble fiber of artichoke. J. Agric. Food Chem. 1996;44(9):2773–2778. [Google Scholar]
- Magalhães A.S., Silva B.M., Pereira J.A., Andrade P.B., Valentão P., Carvalho M. Protective effect of quince (Cydonia oblonga Miller) fruits against oxidative hemolysis of human erythrocytes. Food Chem. Toxicol. 2009;47:1372–1377. doi: 10.1016/j.fct.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Manach C., Scalbert A., Morand C., Remesy C., Jimenez L. Polyphenols: foodsources and bioavailability. Am. J. Clin. Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- Marković S., Tošović J. Comparative study of the antioxidative activities of caffeoylquinic and caffeic acids. Food Chem. 2016;210:585–592. doi: 10.1016/j.foodchem.2016.05.019. [DOI] [PubMed] [Google Scholar]
- Maron D.M., Ames B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res. 1983;113:173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- McCleary B.V. Modification to AOAC official methods 2009.01 and 2011.25 to allow for minor overestimation of low molecular weight soluble dietary fiber in samples containing starch. J. AOAC Int. 2014;97:896–901. doi: 10.5740/jaoacint.13-406. [DOI] [PubMed] [Google Scholar]
- Nelson N. A photometric adaptation of the Somogyi method for the determination of glucose. J. Biol. Chem. 1944;153:375–380. [Google Scholar]
- Orqueda M.E., Rivas M., Zampini I.C., Alberto M.R., Torres S., Cuello S., Sayago J., Thomas-Valdes S., Jiménez-Aspee F., Schmeda-Hirschmann G., Isla M.I. Chemical and functional characterization of seed, pulp and skin powder from chilto (Solanum betaceum), an Argentine native fruit. Phenolic fractions affect key enzymes involved in metabolic syndrome and oxidative stress. Food Chem. 2017;216:70–79. doi: 10.1016/j.foodchem.2016.08.015. [DOI] [PubMed] [Google Scholar]
- Pires T.C.S., Dias M.I., Barros L., Alves M.J., Oliveira M.B.P., Santos-Buelga C., Ferreira I.C.F. Antioxidant and antimicrobial properties of dried Portuguese apple variety (Malus domestica Borkh. cv Bravo de Esmolfe) Food Chem. 2018;240:701–706. doi: 10.1016/j.foodchem.2017.08.010. [DOI] [PubMed] [Google Scholar]
- Porfírio S., Falé P.L.V., Madeira P.J.A., Florêncio M.H., Ascensão L., Serralheiro M.L.M. Antiacetylcholinesterase and antioxidant activities of Plectran-thus barbatus tea, after in vitro gastrointestinal metabolism. Food Chem. 2010;122:179–187. [Google Scholar]
- Possner D., Zimmer T., Kurbel P., Dietrich H. Methanol contents of fruit juices and smoothies in comparison to fruits and a simple method for the determination thereof. Dtsch. Lebensm.-Rundsch. 2014:65–69. [Google Scholar]
- Pothitirat W., Chomnawang M.T., Supabphol R., Gritsanapan W. Free radical scavenging and anti-acne activities of mangosteen fruit rind extracts prepared by different extraction methods. Pharmaceut. Biol. 2010;48(2):182–186. doi: 10.3109/13880200903062671. [DOI] [PubMed] [Google Scholar]
- Prohens J., Nuez F. The tamarillo (Cyphomandra betacea): a review of a promising small fruit crop. Small Fruits Rev. 2000;2:43–68. [Google Scholar]
- Rizzolo A., Cortellino G. Beverages based on ricotta cheese whey and fruit juices. Ital. J. Food Sci. 2018;30:289–302. [Google Scholar]
- Roe J.H., Papadopoulos N.M. The determination of fructose-6-phosphate and fructose-1, 6-diphosphate. J. Biol. Chem. 1954;210(2):703–707. [PubMed] [Google Scholar]
- Scheen A.J., Esser N., Paquot N. Antidiabetic agents: potential anti-inflammatory activity beyond glucose control. Diabetes Metabol. 2015;41:183–194. doi: 10.1016/j.diabet.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Somogyi M. A new reagent for the determination of sugars. J. Biol. Chem. 1945;160:61–68. [Google Scholar]
- Souza V.R., Pereira P.A., da Silva T.L., de Oliveira Lima L.C., Pio R., Queiroz F. Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chem. 2014;156:362–368. doi: 10.1016/j.foodchem.2014.01.125. [DOI] [PubMed] [Google Scholar]
- Spínola V., Castilho P.C. Evaluation of Asteraceae herbal extracts in the management of diabetes and obesity. Contribution of caffeoylquinic acids on the inhibition of digestive enzymes activity and formation of advanced glycation end-products (in vitro) Phytochemistry. 2017;143:29–35. doi: 10.1016/j.phytochem.2017.07.006. [DOI] [PubMed] [Google Scholar]
- Spínola V., Llorent-Martínez E., Castilho P.C. Inhibition of α-amylase, α-glucosidase and pancreatic lipase by phenolic compounds of Rumex maderensis (Madeira sorrel) LWT - Food Sci. Technol. 2020;118:108727. [Google Scholar]
- Swieca M., Dziki D., Gawlik-Dziki U. Starch and protein analysis of wheat bread enriched with phenolics-rich sprouted wheat flour. Food Chem. 2017;228:643–648. doi: 10.1016/j.foodchem.2017.02.052. [DOI] [PubMed] [Google Scholar]
- Vasco C., Avila J., Ruales J., Svanberg U., Kamal-Eldin A. Physical and chemical characteristics of golden-yellow and purple-red varieties of tamarillo fruit (Solanum betaceum Cav.) Int. J. Food Sci. Nutr. 2009;60:278–288. doi: 10.1080/09637480903099618. [DOI] [PubMed] [Google Scholar]
- Van Ee J.H. Soy constituents: modes of action in low-density lipoprotein management. Nutr. Rev. 2009;67:222–234. doi: 10.1111/j.1753-4887.2009.00192.x. [DOI] [PubMed] [Google Scholar]
- Woldemariam T., Van Winkle J. In vitro hypoglycemic effect of Salvia hispanica using a yeast glucose uptake model. J. Pharmaceut. Sci. Pharmacol. 2015;2(2):119–122. [Google Scholar]
- Zhang Y., Chen X., Yang L., Zu Y., Lu Q. Effects of rosmarinic acid on liver and kidney antioxidant enzymes, lipid peroxidation and tissue ultrastructure in aging mice. Food Funct. 2015;6:927–931. doi: 10.1039/c4fo01051e. [DOI] [PubMed] [Google Scholar]
- Zhang H., Wang H., Cao X., Wang J. Preparation and modification of high dietary fiber flour: a review. Food Res. Int. 2018;113:24–35. doi: 10.1016/j.foodres.2018.06.068. [DOI] [PubMed] [Google Scholar]