Abstract
Extra-skeletal Ewing's sarcoma is among the rarest tumors in adults. The primary sites of the tumor dictates symptoms and signs, thus early treatments are compromised when more common tumors are lined up as differentials by the location. We present a case of a 35-year-old pregnant female who developed a renal Ewing sarcoma during pregnancy. A prior simple left kidney cyst in an ultrasound with no tumor signs was spotted. A month after her cesarean section she visited a doctor when she was sent and admitted for surgery with renal cell carcinoma as the primary diagnosis to the Firoozgar hospital. Histology confirmed the final diagnosis. To this end, she completed the standard chemotherapy for the renal Ewing sarcoma with pulmonary metastasis when she was re-evaluated for the general bone pain, diagnosed with multiple bone metastases, and ultimately approached her palliative care. She expired after 2 months. This study demonstrates: a gently progressive mass; palpable in late stages; introduced rise in mean corpuscular volume and lactate dehydrogenase with no drop in the hematocrit. In conclusion, any random parenchymal and/or cortical thickening in primary ultrasound and/or computed tomography demonstrating a cyst—whether displaying internal echo or not— with suggested signs should be furtherly evaluated.
Keywords: Ewing Sarcoma, Imaging diagnosis, Computed tomography, Ultra sound imaging, Tumors
Introduction
Ewing sarcoma families of tumors (ESFT), which are among the rarest of neoplastic diseases, require more regards in the eyes of health care providers. Ewing sarcomas comprise: primary extra-skeletal Ewing tumors as peripheral primitive neuroectodermal tumors, extraosseous Ewing sarcoma (EES), and Askin tumors. Since it is almost atypically presented, easily compromises primary diagnose and management to more popular sarcomas [1], [2], [3], [4]. In this study, for instance, malignancies derived from the kidney—such as renal cell carcinomas or transitional cell carcinomas. Regarding strong predilection of these tumors in children, ESFTs implicate 20% of adults in a total 0.3 per 100,000 incidence rates [5], [6], [7], [8]. The short overall survival, the late offering accompanied by distant metastasis, and high local recurrence are the seamy side revealed by this diagnosis [9]. In is noteworthy that the soft tissue subgroup of extra-axial tumors, arising from neuroectoderm, is the rarest among them all [10]. The tendency of Ewing's tumors invading the bones, mostly in pediatrics, channeled the studies to fundamentally focus on this subgroup. Confusing clinical manifestations, scarce resources of EES, and histologic recognition are the main dilemmas for this disease [11,12]. Usually the first symptom of ESFT is pain [4]—which is divulged by plain radiographs in 2 planes, complemented by computed tomography (CT), and/or magnetic resonance imaging (MRI), which are indicative of a malignant tumor [13], [14], [15]. When most of the articles focused on the treatment outcomes, radiologic findings—particularly EES in adults—is yet a terra incognita [16], [17], [18], [19], [20], [21]. In this case report, scenario of the patient covers: initial symptoms, the obstacles that led to late and unrecognized diagnosis, and compiling past medical signs pertinent to former published studies. The researcher's hope here is to discuss and share congruent imaging data and criteria in hope of never encountering a same scenario in future.
Case presentation
A 35-year-old woman was evaluated for acute rise in blood pressure 25 days after her C-section childbirth. In her drug history, she was administered Sildenafil in the last trimester of her pregnancy and 5 mg folic acid oral route daily because she had been told to have megaloblastic anemia and pulmonary hypertension. On physical examinations, a huge mass was palpated in her left flank as she claimed a dull pain during pregnancy in that area. She was then referred to Firoozgar hospital (Tehran, Iran) for further evaluation. Initial ultrasound showed 117 mm length, 21 mm parenchymal thickening of the left kidney, with a 110 × 117 mm cyst display internal echo plus thick septum (showing vascularity on color Doppler) in the upper pole of the kidney. Suggesting hemorrhagic cyst, further evaluation had then been advised.
In light of past medical records, her ultrasound as an outpatient, approximately 2 years earlier, provided an extra-renal pelvis in the right kidney. The left kidney had been 105 mm in length with 14 mm parenchymal thickness. A 30 × 29 mm cyst on the superior pole containing echogenic component with septa without calcification was identified, in which CT was then recommended for Bosniac staging; however, she did not cooperate.
With this in mind, abdominopelvic CT indicated a huge complex left kidney mass, demonstrating internal cystic structure with 110 × 160 mm estimated size; crossed abdominal midline, compressing intestinal loops. Pelvic veins; particularly left gonadal, showed distention. After contrast injection, heterogeneous enhancement in solid part of the mass was detected. No evidences of adenopathy or vascular invasion shown. A multilocular collection marked the enhancing septum 9 × 33 mm at anterior abdominal wall by the recent incision line. Offering renal cell carcinoma (RCC) and lipid poor angiomyolipoma or sarcoma as differential diagnosis, biopsy under ultrasonography guide was then advised (Figs. 1–3).
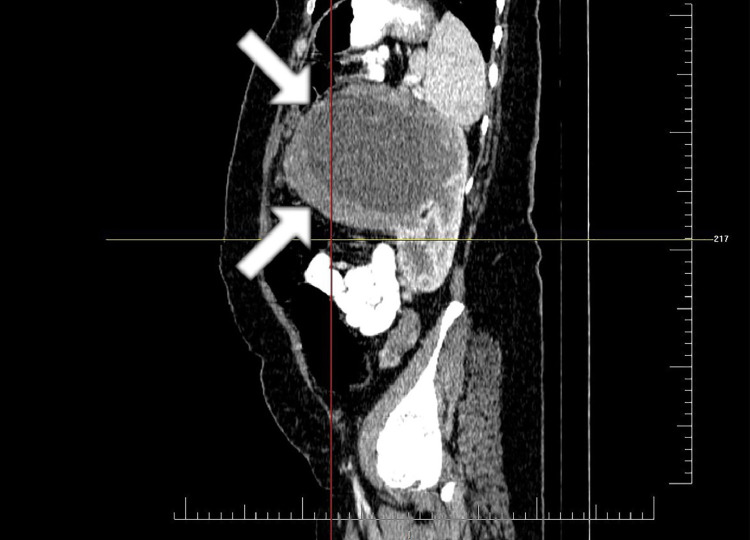
Fig. 2.
The sagittal view of tumor in abdominal CT. The upper limit of tumor is at the level of pancreatic body. The lower limit of tumor is at the level of midpart of left kidney. The tumor mostly grows anteriorly with exophytic pattern. The boundary of the tumor is shown with the white arrow. CT, computed tomography.
Fig.1.
Abdominopelvic CT; there is a huge retroperitoneal, heterogeneously enhancing, solid-cystic mass, originating from left kidney, measuring 164 × 143 × 108 mm. The mass has occupied most of the left upper abdominal cavity, crossing the midline and displacing small bowel loops. There is no accompanying lymphadenopathy or renal vein tumor thrombosis. CT, computed tomography.
Fig. 3.
Coronal view of abdominal CT. The upper limit of tumor is at the level of pancreatic body. The lower limit of tumor is at the level of midpart of left kidney. The tumor mostly grows anteriorly with exophytic pattern. The boundary of the tumor is shown with the white arrow. CT, computed tomography.
Moreover, in pulmonary spiral CT a few noncalcified solid nodules randomly distributed in both lung fields as large as 10 mm diameters at most, in the left upper lobe segments adjoining segmental bronchus. Also a few subpleural nodules in right lung with 9 mm longitude were accounted as the largest (Fig. 4). The whole body bone scan revealed tracer retention in pelvis of right kidney with big halo sign with very faint activity most probability is due to space-occupying lesion in left kidney is present.
Fig. 4.
The pulmonary spiral CT indicating noncalcified solid nodules randomly distributed in both lung fields and in the left upper lobe segments adjoining segmental bronchus. Also a few subpleural nodules in right lung with 9 mm longitude were accounted as the largest. CT, computed tomography.
She underwent radical left nephrectomy for pathologic studies since admitting diagnosis had been renal cell carcinoma. Primary laboratory results are shown in Table 1. The patient was then discharged, and follow-up was made by her pathology report which represented: “a small round cell tumor growing in thick serpentine pattern and with foci of central necrosis. Tumor cells were monotonous, with round nuclei, fine chromatin, and indistinct cytoplasm. No other component seen.” Immunohistochemistry (IHC) staining showed positive reactivity for CD99, but negative for GATA3, Synapophysin, PAN-CK, WT1, CD56, Myogenin, and Desmin. TLE-1 was focally positive and KI67 was 30% positive. After all, the diagnosis was compatible with renal Ewing's sarcoma with lung metastasis. Tumor sized 140 × 130 × 100 mm penetrating renal capsule. Perinephric fat and renal sinus were invaded by tumor; veins, adrenal gland, and ureter margin were free though (Fig. 5).
Table 1.
Initial laboratory findings of the patient.
| Blood sugar | 90 mg/dL | WBC | 6500 × 1000/mm³ |
| Aspartate aminotransferase | 31 U/I | Hb | 13.5 gm/dL |
| Alanine aminotransferase | 47 U/I | HCT | 40 % |
| Alkaline phosphatase | 325 IU/L | MCV | 97.1 fL |
| K | 4 mmol/L | MCH | 32.8 Pgm |
| Na | 142 mmol/L | Platelet | 368 × 1000/mm³ |
| Urea | 17 mg/dL | Cr | 0.9 mg/dL |
Hb, hemoglobin; Cr, creatinine; HCT, hematocrit; MCV, mean corpuscular volume; MCH, mean cell hemoglobin; K, potassium.
Fig. 5.
Low power microscopic view, indicating sheets of monotonous small round cells nuclei with several mitotic; fine chromatin, and indistinct cytoplasm figures.
Coupled with this finding, VAC/IE regimen (Vincristine, Adriamycin, Cyclophosphamide, Ifosfamide, and Etoposide) chemotherapy was initiated for the patients, as her trend of laboratory findings is shown in Table 2 [22]. She took the regime for 12 courses. Due to her generalized pain, she underwent a whole body scan. The latter showed multiple diffuse skeletal metastasis when her therapy inclined toward palliative treatment with opioid analgesics and Zolondronic 4 mg/IV at home with her own consent with no medical monitoring. She was expired 2 months after her palliative care approach.
Table 2.
Remarkable laboratory findings before the chemotherapy cycles.
| Before surgery | 1st | 2nd | 3rd | 4th | 6th | 10th | 11th | 12th | |
|---|---|---|---|---|---|---|---|---|---|
| LDH (U/L) | 801 | 480 | 796 | 381 | 712 | 687 | |||
| Hb (gm/dL) | 13.5 | 11 | 10.7 | 10.5 | 8.7 | 8.6 | 7.7 | 9.3 | 7.8 |
| MCV (fL) | 97.1 | 98.8 | 99.4 | 96.5 | 104.9 | 102.4 | 105 | 107.8 | 107 |
| Cr (mg/dL) | 0.9 | 1.2 | 1.1 | 1 | 1 | 1 | 1 | 1 | |
| Alk.p (IU/L) | 258 | 388 | 295 | 257 | 305 | 238 | |||
LDH, lactate dehydrogenase; Hb, hemoglobin; Cr, creatinine; MCV, mean corpuscular volume; Alk.p, alkaline phosphatase.
Discussion
EES is a far rare disease in adults that can hardly cross one's mind in the primary differentials. Not only diverse symptoms and signs depend on the originating site, but also early treatments are compromised when more common tumors are lined up in differential diagnoses. Surgical excision and histologic findings can differentiate these tumors. Luckily, relevant outcomes in primary imaging can help leading to the accurate diagnosis. A case report in 1993 with renal Ewing sarcoma was diagnosed in pregnancy. Likewise, the scenario is as follows: firstly drop in hematocrit, secondly retroperitoneal mass palpated, and then confirmed on the upper pole of the kidney by ultra sound, which resulted in gastric obstruction, predominantly cystic, thick walled, and septation was then thought to be hemorrhage. T1, T2 weighted axial, and coronal MRI revealed a well-circumscribed mass contiguous with the upper pole of the kidney. Soft tissue excrescences and septations were also seen within the mass on T2-wighted images [15]. Shirkhoda showed the initial contrast-enhanced CT discloses a necrotic soft tissue mass in 4 out of 14 patients and 2 with peripheral enhancement. In 3 patients in whom the mass was homogeneous it did not enhance. Having detected the size of the mass, he used the CT for evaluation of the patient's tumor response to treatments, the integrity of adjacent bone, the medullary cavity, and the presence or absence of metastases. He showed evidence of a dramatic initial response in follow-up CT [23]. To that end, Guimarães et al revealed the importance of PET/CT (Positron Emission Tomography/CT) in restaging and re-evaluation of therapeutic response in patients with Ewing's tumor. The method lies on its capability to provide additional physiological data which generates: clinical implication changes in the therapy scheme, surgical approach, and treatment interruption. Additionally, it is noted that even though PET has a high specificity for pulmonary lesions, the disadvantage is that a negative study does not rule out the presence of metastatic nodules when expression of the glucose transporters and changes in biological behaviors also reduces the FDG (18F-Fluorodeoxyglucose) uptake by the lesions between the primary tumor and its pulmonary metastases [24]. Based on the intensity of FDG uptake by the lesions, PET/CT allows for the detection of tumor regression and progression even before morphologic changes by the anatomic imaging methods such as CT and MRI [25,26]. Barnardt, who compared 2 cases of ESFT by PET/CT and MRI, however, delineated the impossibility of radiologic tumoral differentiation among members of the Ewing's family [27].
Yet in the review of other literatures, extraosseous Ewing tumors unanimously showed similar attenuation to that of muscle in CT [28]. Low attenuation (lower than the muscles) is likely corresponding to areas of hemorrhage or necrosis—with no evident postcontrast enhancement [29]. Hypoechoic and/or hypodensity heterogeneous soft tissue feature was a common unspecific correspondence which did not accord with what we found in CT and ultrasounds [29,30]. In the latter study MRI also showed necrosis and/or cystic enhancement surrounding the tumor.
Although, in older studies [29], it was thought that any noncalcified tumor was a hint to Ewing sarcomas, calcification is seen in 25%-30% of radiological finding in EES according to the radiologic references. Bone surface with cortical erosion or periosteal reaction reflects bone involvement in 40% of cases; however, the retains of medullary cavity (normal fatty marrow attenuation) disrupts the assumption [28,31].
In the most recent retrospective review of 100 cases of pathologically approved ESFT, radiologic features were discussed. Only 2 were retroperitoneal Ewing sarcoma, both were having large heterogeneous enhancement on CT abutting the kidney confined to midline. One of these showed calcification. Then again, this study had no significant imaging gain in the EES [32].
Conclusion
All things considered, attained evidences suggest: high mean corpuscular volume, high lactate dehydrogenase was significant in the laboratory review of this study with no drop in hematocrit. Any parenchymal and/or cortical thickening in ultrasound and/or CT demonstrating a cyst—whether displaying internal echo or not—plus thick septum which sublimes the diagnosis to hemorrhagic cyst, should be furtherly evaluated. It is also noteworthy that the whole body bone scan can reveal tracer retention in pelvis.
Footnotes
Acknowledgment: We would like to warmly appreciate Dr Asaad Moradi, assistant professor of Urology, Iran University of Medical sciences, for his academic support during this study. There was no fund needed during the process. Special thanks to the consent of a member of the patient's immediate family, who is among our colleagues, not to mention the cooperation they kindly bestowed on us.
Declarations of Competing Interest: The authors declare that they have no conflicts of interest.
References
- 1.Ross K.A., Smyth N.A., Murawski C.D., Kennedy J.G. The biology of ewing sarcoma. ISRN Oncol. 2013 doi: 10.1155/2013/759725. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wigger H., Salazar G., Blane W. Extraskeletal Ewing sarcoma. An ultrastructural study. Arch Pathol Lab Med. 1977;101(8):446–449. [PubMed] [Google Scholar]
- 3.Ahmad R., Mayol B.R., Davis M., Rougraff B.T. Extraskeletal Ewing's sarcoma. Cancer: Interdiscip Int J Am Cancer Soc. 1999;85(3):725–731. [PubMed] [Google Scholar]
- 4.Angervall L., Enzinger F. Extraskeletal neoplasm resembling Ewing's sarcoma. Cancer. 1975;36(1):240–251. doi: 10.1002/1097-0142(197507)36:1<240::aid-cncr2820360127>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 5.Maki R.G. Pediatric sarcomas occurring in adults. J Surg Oncol. 2008;97(4):360–368. doi: 10.1002/jso.20969. [DOI] [PubMed] [Google Scholar]
- 6.Teegavarapu P.S., Rao P., Matrana M.R., Cauley D.H., Wood C., Patel S. Outcomes of adults with Ewing's sarcoma family of tumors (ESFT) of the kidney: a single institution experience. Am J Clin Oncol. 2017;40(2):189. doi: 10.1097/COC.0000000000000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valdes M., Nicholas G., Verma S., Asmis T. Systemic therapy outcomes in adult patients with Ewing sarcoma family of tumors. Case Rep Oncol. 2017;10(2):462–472. doi: 10.1159/000475806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Granowetter L., Womer R., Devidas M., Krailo M., Wang C., Bernstein M. Dose-intensified compared with standard chemotherapy for nonmetastatic Ewing sarcoma family of tumors: a Children's Oncology Group Study. J Clin Oncol. 2009;27(15):2536. doi: 10.1200/JCO.2008.19.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang S., Wang G., Chen J., Dong Y. Comparison of clinical features and outcomes in patients with extraskeletal vs skeletal Ewing sarcoma: An SEER database analysis of 3,178 cases. Cancer Manag Res. 2018;10:6227. doi: 10.2147/CMAR.S178979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Applebaum M.A., Worch J., Matthay K.K., Goldsby R., Neuhaus J., West D.C. Clinical features and outcomes in patients with extraskeletal Ewing sarcoma. Cancer. 2011;117(13):3027–3032. doi: 10.1002/cncr.25840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delattre O., Zucman J., Melot T., Garau X.S., Zucker J.-M., Lenoir G.M. The Ewing family of tumors–a subgroup of small-round-cell tumors defined by specific chimeric transcripts. N Engl J Med. 1994;331(5):294–299. doi: 10.1056/NEJM199408043310503. [DOI] [PubMed] [Google Scholar]
- 12.Christie D., Bilous A., Carr P. Diagnostic difficulties in extraosseous Ewing's sarcoma: a proposal for diagnostic criteria. Aust Radiol. 1997;41(1):22–28. doi: 10.1111/j.1440-1673.1997.tb00463.x. [DOI] [PubMed] [Google Scholar]
- 13.Paulussen M., Bielack S., Jürgens H., Casali P., Group E.G.W. Ewing's sarcoma of the bone: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20(suppl_4) doi: 10.1093/annonc/mdp155. iv140-iv2. [DOI] [PubMed] [Google Scholar]
- 14.Galyfos G., Karantzikos G.A., Kavouras N., Sianou A., Palogos K., Filis K. Extraosseous Ewing sarcoma: diagnosis, prognosis and optimal management. Indian J Surg. 2016;78(1):49–53. doi: 10.1007/s12262-015-1399-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francis I.R., Bowerman R.A. Retroperitoneal extraosseous Ewing's sarcoma with renal involvement: US and MRI findings. Clin Imaging. 1993;17(2):149–152. doi: 10.1016/0899-7071(93)90057-t. [DOI] [PubMed] [Google Scholar]
- 16.Gillespie J.J., Roth L.M., Wills E.R., Einhorn L.H., Willman J. Extraskeletal Ewing's sarcoma. Histologic and ultrastructural observations in three cases. Am J Surg Pathol. 1979;3(2):99–108. [PubMed] [Google Scholar]
- 17.Shin J.H., Lee H.K., Rhim S.C., Cho K.-J., Choi C.G., Suh D.C. Spinal epidural extraskeletal Ewing sarcoma: MR findings in two cases. Am J Neuroradiol. 2001;22(4):795–798. [PMC free article] [PubMed] [Google Scholar]
- 18.Khong P., Chan G., Shek T., Tam P., Chan F. Imaging of peripheral PNET: common and uncommon locations. Clin Radiol. 2002;57(4):272–277. doi: 10.1053/crad.2001.0807. [DOI] [PubMed] [Google Scholar]
- 19.Murphey M.D., Senchak L.T., Mambalam P.K., Logie C.I., Klassen-Fischer M.K., Kransdorf M.J. From the radiologic pathology archives: Ewing sarcoma family of tumors: radiologic-pathologic correlation. Radiographics. 2013;33(3):803–831. doi: 10.1148/rg.333135005. [DOI] [PubMed] [Google Scholar]
- 20.Saifuddin A., Robertson R., Smith S. The radiology of Askin tumours. Clin Radiology. 1991;43(1):19–23. doi: 10.1016/s0009-9260(05)80348-4. [DOI] [PubMed] [Google Scholar]
- 21.Castex M.-P., Rubie H., Stevens M.C., Escribano C.C., de Gauzy J.S., Gomez-Brouchet A. Extraosseous localized Ewing tumors: Improved outcome with anthracyclines—the French society of pediatric oncology and international society of pediatric oncology. J Clin Oncol. 2007;25(10):1176–1182. doi: 10.1200/JCO.2005.05.0559. [DOI] [PubMed] [Google Scholar]
- 22.Miser J.S., Kinsella T., Triche T., Tsokos M., Jarosinski P., Forquer R. Ifosfamide with mesna uroprotection and etoposide: an effective regimen in the treatment of recurrent sarcomas and other tumors of children and young adults. J Clin Oncol. 1987;5(8):1191–1198. doi: 10.1200/JCO.1987.5.8.1191. [DOI] [PubMed] [Google Scholar]
- 23.Shirkhoda A., Peuchot M. Extraosseous Ewing's sarcoma computed tomography evaluation before and after chemotherapy. Clin Imaging. 1989;13(2):142–146. doi: 10.1016/0899-7071(89)90097-1. [DOI] [PubMed] [Google Scholar]
- 24.Guimarães J.B., Rigo L., Lewin F., Emerick A. The importance of PET/CT in the evaluation of patients with Ewing tumors. Radiologia Brasileira. 2015;48(3):175–180. doi: 10.1590/0100-3984.2013.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franzius C., Sciuk J., Brinkschmidt C., JÜRGENS H., Schober O. Evaluation of chemotherapy response in primary bone tumors with F-18 FDG positron emission tomography compared with histologically assessed tumor necrosis. Clin Nucl Med. 2000;25(11):874–881. doi: 10.1097/00003072-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Bredella M.A., Caputo G.R., Steinbach L.S. Value of FDG positron emission tomography in conjunction with MR imaging for evaluating therapy response in patients with musculoskeletal sarcomas. Am J Roentgenol. 2002;179(5):1145–1150. doi: 10.2214/ajr.179.5.1791145. [DOI] [PubMed] [Google Scholar]
- 27.Barnardt P., Roux F. The role of imaging in the evaluation of extraskeletal Ewing’s sarcoma. SA J Radiol. 2013;17(1):4. [Google Scholar]
- 28.Robbin M., Murphey M., Jelinek J., Temple H., Flemming D. Radiological Soc North Amer 20th and Northampton STS, Easton, PA 18042 USA. 1998. Imaging of soft tissue Ewing sarcoma and primitive neuroectodermal tumor. [Google Scholar]
- 29.O'Keeffe F., Lorigan J.G., Wallace S. Radiological features of extraskeletal Ewing sarcoma. Br J Radiol. 1990;63(750):456–460. doi: 10.1259/0007-1285-63-750-456. [DOI] [PubMed] [Google Scholar]
- 30.Perouli E., Chrysikopoulos H., Vlachos A., Koskinas A., Batistatou A., Polyzoidis K. Imaging findings in paraspinal extra osseous Ewing sarcoma. JBR-BTR. 2006;89(6):310–312. [PubMed] [Google Scholar]
- 31.SV F.J. Bibliography Current World Literature Vol. 14 No 6 December 2001. Clin Neuropathol. 2000;19 256ą;7. [Google Scholar]
- 32.Patnaik S., Yarlagadda J., Susarla R. Imaging features of Ewing's sarcoma: special reference to uncommon features and rare sites of presentation. J Cancer Res Ther. 2018;14(5):1014. doi: 10.4103/jcrt.JCRT_1350_16. [DOI] [PubMed] [Google Scholar]