Highlights
-
•
Patients with anti-NMDAR encephalitis showed decreased ALFF values in the bilateral posterior cingulate gyrus, left precuneus and bilateral cerebellum.
-
•
The functional connectivities between the bilateral posterior cingulate gyrus and the bilateral lingual gyrus, fusiform gyrus, calcarine, cuneus, and right posterior central gyrus were significantly increased in patients with anti-NMDAR encephalitis.
-
•
Functional connectivity strength between the bilateral posterior cingulate gyrus and bilateral cuneus were positively correlated with MoCA memory scores.
-
•
Patients with anti-NMDAR encephalitis exhibited decreased spontaneous neural activities and abnormal functional connectivity, which may participate in the process of cognition and emotion deficits.
Keywords: Anti-N-methyl-D-aspartate receptor encephalitis, Amplitude of low-frequency fluctuation, Functional connectivity, MoCA
Abbreviations: NMDAR, N-methyl-D-aspartate receptor; rs-fMRI, resting-state functional magnetic resonance imaging; ALFF, amplitude of low frequency fluctuations; FC, functional connectivity; MoCA, Montreal Cognitive Assessment; HAMA, Hamilton Anxiety Scale; HAMD24, Hamilton Depression Scale; MNI, Montreal Neurological Institute; PCC, posterior cingulate cortex; ROI, region of interest; L, left; R, right
Abstract
Background
Anti–N-methyl-D-aspartate receptor (NMDAR) encephalitis showing severe neuropsychiatric symptoms is the most common type of autoimmune encephalitis. However, the corresponding standard clinical magnetic resonance imaging (MRI) presents normal or atypical in the majority of patients with anti-NMDAR encephalitis. Here, this study aimed to investigate the alterations in brain functional activity in patients with anti-NMDAR encephalitis and whether these alterations contributed to cognition and mood disorders.
Methods
Seventeen patients with anti-NMDAR encephalitis and eighteen gender, age and education-matched healthy controls were recruited. All participants underwent neuropsychological tests (including Montreal Cognitive Assessment (MoCA), Hamilton Anxiety Scale (HAMA), and Hamilton Depression Scale (HAMD24)) and resting-state functional MRI. MRI data was firstly analyzed by amplitude of low-frequency fluctuation (ALFF), and brain regions with altered ALFF between groups were selected as regions of interest for the further functional connectivity (FC) analysis. Correlation analyses were performed to investigate the associations between brain dysfunction and neuropsychological performance.
Results
Relative to the healthy controls, patients with anti-NMDAR encephalitis performed inferiorly in the MoCA score, and showed anxiety and depression disorders with higher HAMA and HAMD24 scores (all p < 0.05). In the brain functional activity analysis, the patients showed decreased ALFF values in the bilateral posterior cingulate gyrus, left precuneus, and bilateral cerebellum (false- discovery- rate corrected, p < 0.05). Furthermore, relative to the control group, the patients showed significantly increased FC between the left posterior cingulate cortex (PCC) and the bilateral lingual gyrus, right calcarine, right cuneus, also between the right PCC and the right fusiform gyrus, bilateral lingual gyrus, left calcarine, left cuneus, and right posterior central gyrus (false- discovery- rate corrected, p < 0.05). FC strength between the left posterior cingulate gyrus and right cuneus, and between the right posterior cingulate gyrus and left cuneus were both positively correlated with MoCA memory scores (r = 0.485, p = 0.048; r = 0.550, p = 0.022).
Conclusion
The present study highlight that decreased spontaneous neural activities and abnormal FC exhibited in the patients with anti-NMDAR encephalitis, which may participate in the process of cognition and emotion deficits. These results may help to elucidate the clinical radiological contradictions in anti-NMDAR encephalitis and contribute to deeper understanding of the pathophysiological mechanism of the disease.
1. Introduction
Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis is autoimmune disease of the central nervous system mediated by anti-NMDAR antibodies. It is an acute or subacute onset disease, mainly involving the hippocampus, amygdala, insula, and other marginal structures. Patients typically develop memory deficits, behavioral changes, psychosis and epileptic seizures, and the severe stage will be accompanied by dyskinesia, hypoventilation, and decreased levels of consciousness. Despite this severe clinical course, studies have shown that routine brain CT/MRI are normal or atypical in most patients and therefore provide limited diagnostic and prognostic value (Bacchi et al., 2018). The majority of patients have a favorable outcome, although some are left with persistent cognitive deficits, such as impairment of memory, attention, and executive control.
Resting state fMRI (rs-fMRI) does not require specific task demands, which renders it suitable for application in cognitively impaired patients, and can detect functional activity changes in the brain, even when conventional MRI shows no structural changes (Buckner et al., 2008). ALFF and FC are common indicators measured by rs-fMRI, and these measures can reflect the intensity of spontaneous activity of local brain neurons and brain functional activities under physiological and disease states. They have been widely used in neuropsychiatric disorders, such as mild cognitive impairment, Alzheimer's disease and depression.
At present, there are few rs-fMRI studies on anti-NMDAR encephalitis, especially on the resting-state spontaneous brain activity of patients. The discrepancy between the severity of clinical symptoms and routine imaging remains perplexing and has drawn researchers’ attention. Therefore, we used rs-fMRI combined with ALFF and FC approaches to provide a preliminary analysis of the characteristics of the spontaneous activity of brain regions and changes in brain FC in patients with anti-NMDAR encephalitis. Then, we used correlation analyses to investigate whether these abnormal cerebral functional activities were associated with neuropsychological deficits. In summary, this study may help to explore the pathophysiological mechanisms and deepen our understanding of anti-NMDAR encephalitis.
2. Materials and methods
2.1. Subjects
The study was approved by the First Affiliated Hospital of Guangxi Medical University Ethics Committee. All study participants (or family members) provided informed written consent for inclusion in the research and publication. Seventeen patients with anti-NMDAR encephalitis after the acute stage of the disease and 18 sex-matched, age-matched and education-matched healthy control subjects without any neurological or psychiatric diseases were recruited. The patients were recruited in the Department of Neurology of the First Affiliated Hospital of Guangxi Medical University between January 2015 and June 2018. According to the diagnostic criteria of anti-NMDAR encephalitis (Graus et al., 2016), a diagnosis was established in all patients based on characteristic clinical presentation and detection of immunoglobulin G (IgG) NMDAR antibodies. Control subjects were recruited through public advertisements and had similar social backgrounds in comparison with the patients. In all subjects, cognitive function was assessed using the Montreal Cognitive Assessment (MoCA), and the degree of anxiety and depression were assessed using Hamilton Anxiety Scale (HAMA) and Hamilton Depression Scale (HAMD24).
2.2. MRI acquisition
Imaging was performed with an Achieva 3.0 T MR scanner (Philips, Amsterdam, The Netherlands). During the scanning, subjects were instructed to remain in an awake and relaxed state, to avoid thinking anything about a specific topic, and to keep their eyes closed. The resting-state functional imaging was collected using the echo-planar image (EPI) technique (repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, voxel size = 3.44 × 3.44 × 6.00 mm, field of view = 220 × 220 mm², flip angle = 90°, 31 slices, slice thickness = 5 mm, slice gap = 1 mm, scanning time > 360 s).
2.3. Data processing
All rs-fMRI data preprocessing were conducted using DPABI software (http://rfmri.org/dpabi) running on MATLAB R2013b. The first ten time points were discarded to allow magnetization equilibrium. The remaining volumes were processed as follows: slice-timing correction, head motion correction (rotational and translational movements of all subjects were within 2 mm or 2°, respectively, in the x, y, and z planes), spatial normalization to Montreal Neurological Institute (MNI) space with a resampling resolution of 3 × 3 × 3 mm³, spatial smoothing with a 6-mm Gaussian kernel along all three directions, and linear trend removal. Finally, all images were filtered using a typical temporal bandpass filter (0.01–0.08 Hz) to reduce low-frequency drift, physiological high-frequency respiratory and cardiac noise.
2.4. ALFF calculation
ALFF was calculated using DPABI software (http://rfmri.org/dpabi). The ALFF analysis was based on previous preprocessed results. For a given voxel, the time sequences were transformed to the frequency series by fast Fourier transform, and the square root of the power spectrum was calculated and filtered across 0.01–0.08 Hz. The average square root was considered to be the ALFF value. To reduce individual differences among the subjects, the average ALFF value was subtracted from the ALFF value of each voxel and then divided by the standard deviation of the whole-brain ALFF map to obtain the standard ALFF value. The maps of the significant differences in ALFF between the patient and control groups were compared using voxel-wise two-sample t-tests with age, sex and educational level serving as nuisance covariates (false- discovery- rate [FDR] corrected, p < 0.05).
2.5. Functional connectivity analysis
Seed-based analysis was used to analyze FC. Some brain areas with ALFF values that were significantly different from the other brain regions were chosen as regions of interest (ROIs). Then, Pearson's correlation coefficients were calculated between the time courses of each ROI and the signal time series of each voxel across the whole brain to generate an ROI-FC map for each participant. The ROI-FC map was subjected to Fisher's r-to-z transformation to generate a z-score FC map to improve the normality of correlation coefficients. Z-score FC (zFC) maps within the patient group and control group were statistically analyzed using a one-sample t-test (p < 0.05; FDR corrected). After the one-sample t-test, a mask was merged with the statistically significant zFC maps of the patient group and control group for further between-group comparisons. A two-sample t-test (p < 0.05; FDR corrected) was performed to investigate the FC strength of regions with significant differences between the patient and control groups, with age, sex and educational level serving as nuisance covariates.
2.6. Statistical analysis
The analysis of the general demographic data was performed by SPSS22.0 software. The data are reported as the means ± standard deviations. The differences in age, education level, MoCA, HAMA and HAMD24 scores were analyzed using independent two-sample t-tests, while differences in sex were analyzed using a chi-squared test. Individual mean ALFF z-scores and zFC values were extracted for Pearson's correlation analysis with MoCA, HAMA and HAMD24 total scores. Furthermore, a subgroup analysis of the different cognitive domains in MoCA was employed to detect a more specific correlation with the mean ALFF z-scores in the anti-NMDAR encephalitis patients. Significant correlations were determined based on p-values less than 0.05.
3. Results
3.1. Demographic data and scale scores
There was no significant difference between the two groups with regard to age (t = 0.344, p = 0.733), sex distribution (χ2 = 0.030, p = 0.862), or years of education (t = −1.843, p = 0.074). There were significant differences in the MoCA, HAMA and HAMD24 scores between the two groups (p < 0.05). Details of the demographic data and corresponding tests are presented in Table 1.
Table 1.
Demographics and neuropsychological data.
| Variables | Patients | Controls | t/χ2 | p value |
|---|---|---|---|---|
| Age | 28.59 ± 11.07 | 27.50 ± 7.38 | 0.344 | 0.733 |
| Gender (M/F) | 9/8 | 9/9 | 0.030 | 0.862 |
| Education (years) | 12.12 ± 3.95 | 14.61 ± 4.05 | −1.843 | 0.074 |
| Time After Disease Onset (months) | 20.29 ± 14.00 | – | – | – |
| HAMA scores | 4.12 ± 3.44 | 1.17 ± 1.62 | 3.214 | 0.004 |
| HAMD24 scores | 5.59 ± 6.78 | 0.78 ± 1.17 | 2.884 | 0.010 |
| MoCA scores | 24.24 ± 3.51 | 28.44 ± 1.653 | −4.582 | 0.000 |
| Executive | 0.65 ± 0.493 | 0.78 ± 0.428 | 2.746 | 0.107 |
| Language | 1.00 ± 0.707 | 1.78 ± 0.428 | 0.866 | 0.359 |
| Orientation | 5.59 ± 1.278 | 6.00 ± 0.00 | 8.887 | 0.005 |
| Calculate | 2.76 ± 0.562 | 3.00 ± 0.00 | 16.304 | 0.000 |
| Abstraction | 2.18 ± 0.951 | 2.61 ± 0.698 | 1.916 | 0.176 |
| Memory | 2.76 ± 1.522 | 4.50 ± 0.857 | 5.367 | 0.027 |
| Visual perception | 2.53 ± 0.717 | 2.78 ± 0.428 | 6.921 | 0.013 |
| Naming | 3.88 ± 0.332 | 4.00 ± 0.00 | 12.051 | 0.001 |
| Attention | 3.00 ± 0.00 | 3.00 ± 0.00 | – | – |
Values are means ± SD. M = male, F = female, MoCA = the Montreal Cognitive Assessment, HAMA = Hamilton Anxiety Scale, HAMD24 = Hamilton Depression Scale.
3.2. ALFF results
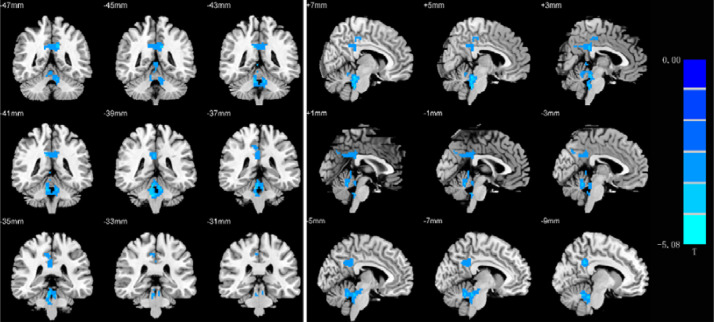
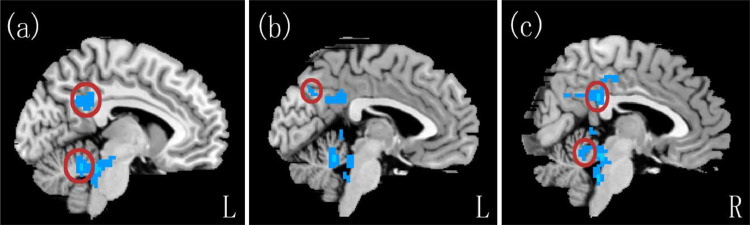
In the ALFF z-score analysis, the patients exhibited decreased ALFF values in the bilateral posterior cingulate gyrus, left precuneus and bilateral cerebellum compared with controls.(p < 0.05, FDR corrected; Table 2, Figs. 1 and 2).
Table 2.
Brain regions with different ALFF values in the anti-NMDAR encephalitis patients compared with controls.
| Brain regions | MNI coordinates of peak point | Voxels | T value | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Cingulum_Post_L | −7 | −44 | 30 | 157 | −3.5549 |
| Cingulum_Post_R | 5 | −45 | 31 | 101 | −3.0245 |
| Precuneus_L | −1 | −66 | 34 | 47 | −1.8732 |
| Cerebelum_3_L | −4 | −51 | −19 | 204 | −4.2805 |
| Cerebelum_3_R | 4 | −48 | −19 | 129 | −3.2367 |
MNI = Montreal Neurological Institute, L = left, R = right.
Fig. 1.
Brain regions showing ALFF differences in the patients compared with the controls (Cool tone represents reduce ALFF).
Fig. 2.
Brain regions showing ALFF differences in the patients compared with the controls: (a) Left posterior cingulate gyrus and left cerebellum. (b) Left precuneus. (c) Right posterior cingulate gyrus and right cerebellum.
3.3. Functional connectivity results
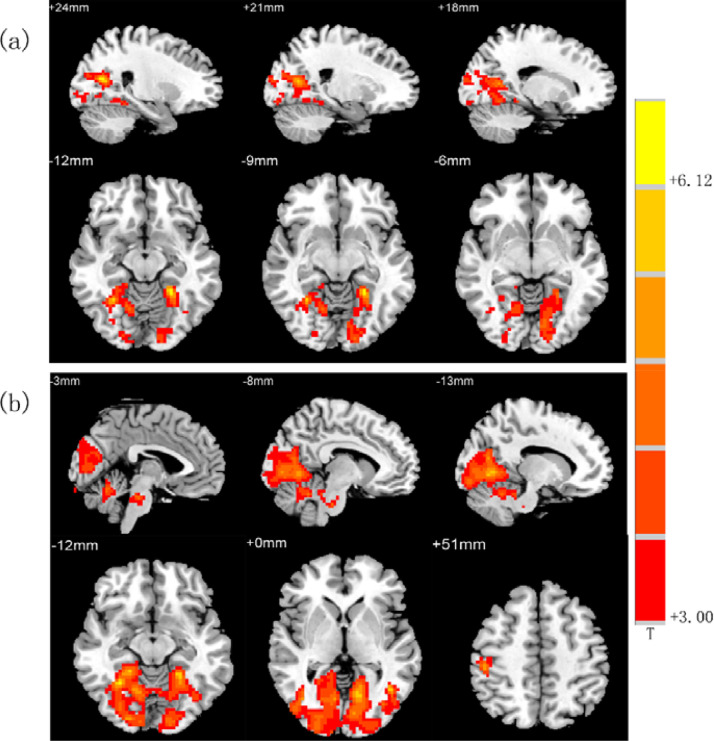
FC analysis was performed using the left posterior cingulate gyrus and the right posterior cingulate gyrus, which showed differences in the ALFF values between patients and controls in the ROI analysis. Compared with controls, patients exhibited increased FC between the left posterior cingulate cortex (PCC) and the bilateral lingual gyrus, right calcarine, right cuneus (p < 0.05, FDR corrected; Table 3 and Fig. 3(a)). Furthermore, we also found increased FC between the right PCC and the right fusiform gyrus, bilateral lingual gyrus, left calcarine, left cuneus, and right posterior central gyrus (p < 0.05, FDR corrected; Table 3 and Fig. 3(b)). No brain regions were found to have reduced FC with the bilateral PCC.
Table 3.
Differences of functional connectivity in patients compared with controls.
| Brain region | MNI coordinates of peak point | Voxels | T value | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Increase FC with PCC_L | |||||
| Lingual_L | −24 | −48 | −9 | 216 | 6.3557 |
| Lingual_R | 18 | −64 | −1 | 179 | 4.0578 |
| Calcarine_R | 24 | −69 | 14 | 169 | 3.8996 |
| Cuneus_R | 18 | −96 | 12 | 185 | 4.1942 |
| Increase FC with PCC_R | |||||
| Fusiform_R | 30 | −54 | −12 | 285 | 6.7398 |
| Lingual_R | 11 | −68 | 0 | 448 | 8.8907 |
| Lingual_L | −13 | −67 | −2 | 355 | 7.6545 |
| Calcarine_L | −10 | −83 | 7 | 380 | 7.9826 |
| Cuneus_L | −3 | −82 | 21 | 177 | 4.0099 |
| Postcentral_R | 48 | −27 | 51 | 126 | 3.1750 |
MNI = Montreal Neurological Institute, FC = functional connectivity, PCC = posterior cingulate cortex, L = left, R = right.
Fig. 3.
Brain regions showing FC differences in the patients compared with the controls: (a) Brain regions which had increased connectivity with left posterior cingulate gyrus. (b) Brain regions which had increased connectivity with right posterior cingulate gyrus. (Warm tones represent increased functional connectivity).
3.4. Correlational analyses
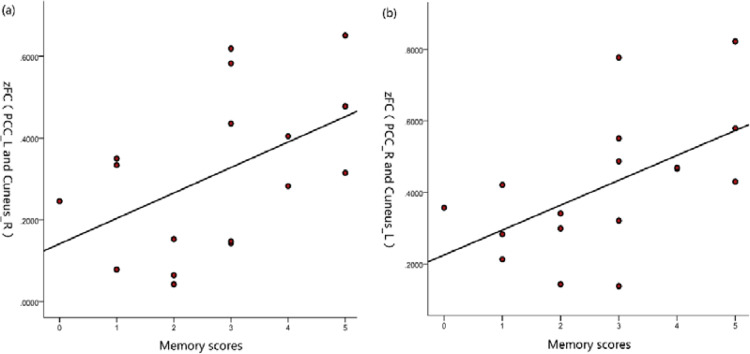
A significant positive correlation was found between zFC values and memory performance in patients (MoCA memory: zFC value between the left posterior cingulate gyrus and right cuneus, r = 0.485, p = 0.048; zFC value between the right posterior cingulate gyrus and left cuneus, r = 0.550, p = 0.022; Table 4 and Fig.4). No correlations were found between ALFF values in the abnormal brain regions and HAMA and HAMD24 scores (p > 0.05).
Table 4.
Functional connectivity of brain regions associated with neuropsychological scores.
| r value | P value | |
|---|---|---|
| zFC values associated with MoCA memory scores | ||
| PCC_L and Cuneus_R | 0.485 | 0.048 |
| PCC_R and Cuneus_L | 0.550 | 0.022 |
PCC = posterior cingulate cortex, L = left, R = right.
Fig. 4.
FCs between the posterior cingulate gyrus and cuneus were positively correlated with MoCA memory scores.
4. Discussion
With the development of imaging analyses, more attention has been focused on the characteristic structural and functional imaging patterns in patients with autoimmune encephalitis (Heine et al., 2015). In patients with anti-NMDAR encephalitis, impaired FC of the hippocampus with the medial prefrontal cortex and atrophy of the hippocampus were observed, and these changes correlated with individuals’ memory deficits (Finke et al., 2016; Finke et al., 2013). Another study found disruptions in large-scale networks such as the sensorimotor, frontoparietal, lateral-temporal, and visual networks. In addition, memory impairment was correlated with hippocampal and medial-temporal-lobe network connectivity, while schizophrenia-like symptoms were associated with FC changes in the frontoparietal networks (Peer et al., 2017).
In this study, our results indicated that compared with controls, patients with anti-NMDAR encephalitis exhibited significantly decreased ALFF in the bilateral posterior cingulate gyrus, left precuneus and bilateral cerebellum. In addition, seed-to-whole-brain voxel analyses showed increased FC between the bilateral PCC and the bilateral lingual gyrus, calcarine, cuneus, fusiform gyrus, and posterior central gyrus.
The decreased ALFF in the bilateral posterior cingulate gyrus, left precuneus and bilateral cerebellum indicated a decrease in spontaneous neural activity and functional impairment in these brain areas. The PCC is a major node within the default mode network (DMN), a set of brain regions that constantly collects information from itself and its surroundings that is related to the episodic memory, the maintenance of a conscious state and normal cognitive function (Buckner et al., 2008; Costigan et al., 2019; Leech et al., 2012). The PCC functionally connects the major cognitive brain regions and centers, and is responsible for the information exchange between different brain regions and the integration of cognitive information to maintain the normal external behavioral performance of the body (such as self-judgment, episodic memory, etc.). Liang et al. (2014) found that mild cognitive impairment and AD patients all showed decreased ALFF in the PCC, which was significantly positively correlated with cognitive impairment as measured by the Mini-Mental State Examination (MMSE). Weiler et al. (2014) and Liu et al. (2014) found that, compared with controls, AD patients showed significantly decreased ALFF values in the PCC, which was a reasonable explanation for the cognitive deficits present in the disease. Our study found that patients exhibited decreased ALFF values in the bilateral posterior cingulate gyrus, which may be related to cognitive impairment, such as impairments in memory and executive control in patients with anti-NMDAR encephalitis. Wang et al. (2016) found that major depressive disorder patients showed altered ALFF in fractional brain regions, including the frontal cortex and precuneus. Ferri et al. (2016) suggested that the precuneus was involved in the processing of emotion-related information, working memory, visual attention and emotional regulation, and that dysfunction of the precuneus would lead to a weakening of the regulation of negative emotions. Therefore, dysfunction of the precuneus may contribute to psychiatric disorders with social and emotional components. The cerebellum is traditionally considered a motor control center and plays an important role in executive motor inhibition, but, there is growing awareness of the role of the cerebellum in emotional and cognitive function (Phillips et al., 2015). Liu et al. (2014) found that major depressive disorder subjects showed decreased ALFF in the cerebellum. Therefore, a growing body of evidence has suggested that cerebellar microstructural abnormalities and abnormal FC contribute to dysfunctional brain circuits in depressive disorders (He et al., 2018; Minichino et al., 2014; Zhao et al., 2016). Cordova-Palomera et al. (2016) suggested that cerebellar alterations in the resting state constituted a key candidate mechanism for depressive psychopathology. We found that patients with anti-NMDAR encephalitis exhibited decreased ALFF values in the left precuneus and bilateral cerebellum, that is, local brain neural activity in the precuneus and cerebellum was restrained. Therefore, we presumed that precuneus and cerebellum dysfunction may be an important contributor to the pathogenesis of depression and anxiety in patients with anti-NMDAR encephalitis.
The lingual gyrus, fusiform gyrus, cuneus and calcarine are all located in the occipital lobe. The occipital lobe acts as the center of the visual cortex, integrates visual, auditory, language-related and other executive functions to process visual information. The fusiform gyrus and lingual gyrus are important components of the visual recognition network, which jointly participate in facial recognition and emotional perception and play an important role in the integration of visual information and introspective stimuli (Cai et al., 2015; Kukolja et al., 2016). The visual cortex is typical sensory granular cortex that located around the calcarine of the occipital lobe; visual input comes from the lateral geniculate body of the thalamus, and then via the primary visual cortex (V1) to output information through the dorsal and ventral streams. Wang et al. (2008) chose the primary visual cortex (PVA) as a ROI to identify the pattern of spontaneous activity during the resting state, and the results showed that activity of the bilateral middle occipital gyrus, bilateral cuneus, bilateral lingual gyrus, precuneus, precentral/postcentral gyrus, middle frontal gyrus, fusiform gyrus, and parahippocampal gyrus were associated with spontaneous activity in the PVA, suggesting that memory-related mental imagery and visual memory consolidation processes may be candidates. Chadick and Gazzaley (2011) found that visual cortical areas that selectively process relevant visual memory tasks were functionally connected with the frontal-parietal network, whereas those that process visual memory tasks with interfering information were simultaneously coupled with the DMN. The authors suggested that the FC of the visual cortex and DMN would resist the negative effect of distraction on working memory performance. The DMN is located far from the visual cortex, and its activity is inhibited by external stimuli whereas it is activated by introspective cognitive processes such as prospective/retrospective memory and internal monitoring. Chen et al. (2018) found that reduced gray matter volume in the lateral occipital cortex was associated with cognitive impairments. Mazzoni et al. (2019) found that posterior visual areas including the precuneus typically become active during later stages of elaboration of personal memories rather than during initial access to those memories. Our study found that FC between the bilateral posterior cingulate gyrus and several brain regions associated with the primary visual cortex, including the lingual gyrus, fusiform gyrus, cuneus and calcarine were enhanced in patients with anti-NMDAR encephalitis, and subsequent analyses showed that FC between the bilateral posterior cingulate gyrus and the bilateral cuneus were positively correlated with MoCA memory scores, which may be a compensatory effect in response to memory dysfunction. Patients with anti-NMDAR encephalitis present with phenomena including decreased memory function, weakened learning ability, and forgetfulness after onset of the illness, and in some patients, these symptoms were persistent and difficult to recover. Therefore, to avoid the impact of these deteriorating conditions, compensatory mechanisms may be activated by enhancing FC with visual brain areas associated with memory, in turn, enhancing the anti-interference ability of the body and then strengthening the efficiency to integrate cognitive information to improve memory function and improve the working efficiency of the memory system.
The posterior central gyrus is part of the somatosensory - motor network and participates in somatosensory processing and regulation. It is mainly responsible for integrating information transmitted by various somatosensory stimuli to achieve correct identification of the object. Previously, abnormal changes in the gray matter of the posterior central gyrus had been found in people with violent tendencies of non-mental disorder (Tiihonen et al., 2008) and people with antisocial behaviors (Aoki et al., 2014). Patients with anti-NMDAR encephalitis often have mental abnormalities and aggressive behaviors, which may be related to abnormal structure and function of the posterior central gyrus. As seen in the convalescence of patients, these symptoms disappear, and enhanced FC is considered a compensation after injury, or these changes may be caused by the proliferation of glial cells at the site of injury during convalescence, which requires further pathological study.
In this study, no brain regions were found to have reduced FC with the bilateral posterior cingulate gyrus, and no brain regions with reduced ALFF values were found to be associated with the MoCA, HAMA and HAMD24 scores. The following reasons may be responsible for these findings: first, the sample size was not large enough to fully reflect the actual situation. Second, anti-NMDAR encephalitis patients have a widespread brain damage, which includes many brain areas. Park et al. (2017) found that, in temporal lobe epilepsy patients with poor seizure control, regional brain correlates of memory impairment did not completely map onto the distribution of abnormalities, but the wider extent of abnormalities appeared to be related to greater vulnerability to memory impairment. The anti-NMDAR encephalitis patients we enrolled had different disease durations and were in the recovery period, and some of the damage had been compensated for. So, the abnormal brain areas identified here may not have an impact on the cognition, anxiety and depression scores. Third, the MoCA, HAMA and HAMD24 scales may not fully reflect the degree of cognitive impairment and emotional abnormalities of anti-NMDAR encephalitis patients due to limitations of the scales and the complexity of the patients' conditions.
Our study had some limitations. First, the sample size was not large enough to ensure the stability of the results. Second, because the patients with anti-NMDAR encephalitis cannot cooperate and undergo the examination during the acute phase, the patients included in this study were all in the convalescence phase, the course of disease varied across patients, and there were differences in brain function. Therefore, the present findings do not fully reflect the actual changes in brain function in patients at the onset of anti-NMDAR encephalitis. Third, longitudinal studies are needed to further understand the impairment and recovery of brain function to help us better understand anti-NMDAR encephalitis. Therefore, future studies should increase the sample size and use multimodal MRI techniques to reveal abnormities in brain structure and function to deepen our understanding of the nature of anti-NMDAR encephalitis.
5. Conclusions
In conclusion, our rs-fMRI study combines ALFF and FC analysis to provide a preliminary exploration of the functional brain characteristics of patients with anti-NMDAR encephalitis. We found decreased ALFF values in the bilateral posterior cingulate gyrus, left precuneus, and bilateral cerebellum and increased FC between the PCC and the visual cortex and central posterior gyrus. Furthermore, FC between the bilateral posterior cingulate gyrus and bilateral cuneus were positively correlated with MoCA memory scores. Those changes may participate in the process of cognition and emotion deficits. In summary, patients with anti-NMDAR encephalitis have both brain functional impairments and compensation. These findings may provide further insight into the underlying neuropathophysiological mechanism of anti-NMDAR encephalitis.
Author contributions
Experimental design: Luhui Cai, Xia Zhou; data collection: Luhui Cai, Yanli Liang, Huanjian Huang; data analysis: Luhui Cai, Xia Zhou. All authors contributed to manuscript preparation and have read and approved the final manuscript.
CRediT authorship contribution statement
Luhui Cai: Writing - original draft. Yanli Liang: Data curation. Huanjian Huang: Investigation. Xia Zhou: Methodology. Jinou Zheng: Funding acquisition.
Declaration of Competing Interest
All other authors declare no competing interests.
Funding and acknowledgments
This study is supported by the National Natural Science Foundation of China (Contract grant numbers: 81560223) and the Innovation Project of Guangxi Graduate Education (grant numbers: YCBZ2019042), which play a role in the collection and analysis of the data.
References
- Aoki Y., Inokuchi R., Nakao T., Yamasue H. Neural bases of antisocial behavior: a voxel-based meta-analysis. Soc. Cognit. Affect. Neurosci. 2014;9(8):1223–1231. doi: 10.1093/scan/nst104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchi S., Franke K., Wewegama D., Needham E., Patel S., Menon D. Magnetic resonance imaging and positron emission tomography in anti-NMDA receptor encephalitis: a systematic review. J. Clin. Neurosci. 2018;52:54–59. doi: 10.1016/j.jocn.2018.03.026. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain’s default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Cai S., Chong T., Zhang Y., Li J., von Deneen K.M., Ren J. Altered functional connectivity of fusiform gyrus in subjects with amnestic mild cognitive impairment: a resting-state fMRI study. Front. Hum. Neurosci. 2015;9:471. doi: 10.3389/fnhum.2015.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadick J.Z., Gazzaley A. Differential coupling of visual cortex with default or frontal-parietal network based on goals. Nat. Neurosci. 2011;14(7):830–832. doi: 10.1038/nn.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.S., Chen H.L., Lu C.H., Chen M.H., Chou K.H., Tsai N.W. Reduced lateral occipital gray matter volume is associated with physical frailty and cognitive impairment in Parkinson’s disease. Eur. Radiol. 2018 doi: 10.1007/s00330-018-5855-7. [DOI] [PubMed] [Google Scholar]
- Cordova-Palomera A., Tornador C., Falcon C., Bargallo N., Brambilla P., Crespo-Facorro B. Environmental factors linked to depression vulnerability are associated with altered cerebellar resting-state synchronization. Sci. Rep. 2016;6:37384. doi: 10.1038/srep37384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costigan A.G., Umla-Runge K., Evans C.J., Hodgetts C.J., Lawrence A.D., Graham K.S. Neurochemical correlates of scene processing in the precuneus/posterior cingulate cortex: a multimodal fMRI and (1) H-MRS study. Hum. Brain Mapp. 2019 doi: 10.1002/hbm.24566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri J., Schmidt J., Hajcak G., Canli T. Emotion regulation and amygdala-precuneus connectivity: focusing on attentional deployment. Cognit. Affect. Behav. Neurosci. 2016;16(6):991–1002. doi: 10.3758/s13415-016-0447-y. [DOI] [PubMed] [Google Scholar]
- Finke C., Kopp U.A., Pajkert A., Behrens J.R., Leypoldt F., Wuerfel J.T. Structural hippocampal damage following anti-N-methyl-D-aspartate receptor encephalitis. Biol. Psychiatry. 2016;79(9):727–734. doi: 10.1016/j.biopsych.2015.02.024. [DOI] [PubMed] [Google Scholar]
- Finke C., Kopp U.A., Scheel M., Pech L.M., Soemmer C., Schlichting J. Functional and structural brain changes in anti-N-methyl-D-aspartate receptor encephalitis. Ann. Neurol. 2013;74(2):284–296. doi: 10.1002/ana.23932. [DOI] [PubMed] [Google Scholar]
- Graus F., Titulaer M.J., Balu R., Benseler S., Bien C.G., Cellucci T. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391–404. doi: 10.1016/S1474-4422(15)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Wang Y., Chang T.T., Jia Y., Wang J., Zhong S. Abnormal intrinsic cerebro-cerebellar functional connectivity in un-medicated patients with bipolar disorder and major depressive disorder. Psychopharmacology. 2018;235(11):3187–3200. doi: 10.1007/s00213-018-5021-6. [DOI] [PubMed] [Google Scholar]
- Heine J., Pruss H., Bartsch T., Ploner C.J., Paul F., Finke C. Imaging of autoimmune encephalitis–Relevance for clinical practice and hippocampal function. Neuroscience. 2015;309:68–83. doi: 10.1016/j.neuroscience.2015.05.037. [DOI] [PubMed] [Google Scholar]
- Kukolja J., Goreci D.Y., Onur O.A., Riedl V., Fink G.R. Resting-state fMRI evidence for early episodic memory consolidation: effects of age. Neurobiol. Aging. 2016;45:197–211. doi: 10.1016/j.neurobiolaging.2016.06.004. [DOI] [PubMed] [Google Scholar]
- Leech R., Braga R., Sharp D.J. Echoes of the brain within the posterior cingulate cortex. J. Neurosci. 2012;32(1):215–222. doi: 10.1523/JNEUROSCI.3689-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P., Xiang J., Liang H., Qi Z., Li K. Alzheimer’s disease neuroimaging I. Altered amplitude of low-frequency fluctuations in early and late mild cognitive impairment and alzheimer’s disease. Curr. Alzheimer Res. 2014;11(4):389–398. doi: 10.2174/1567205011666140331225335. [DOI] [PubMed] [Google Scholar]
- Liu J., Ren L., Womer F.Y., Wang J., Fan G., Jiang W. Alterations in amplitude of low frequency fluctuation in treatment-naive major depressive disorder measured with resting-state fMRI. Hum. Brain Mapp. 2014;35(10):4979–4988. doi: 10.1002/hbm.22526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Wang S., Zhang X., Wang Z., Tian X., He Y. Abnormal amplitude of low-frequency fluctuations of intrinsic brain activity in alzheimer’s disease. J. Alzheimer’s Dis. 2014;40(2):387–397. doi: 10.3233/JAD-131322. [DOI] [PubMed] [Google Scholar]
- Mazzoni G., Clark A., De Bartolo A., Guerrini C., Nahouli Z., Duzzi D. Brain activation in highly superior autobiographical memory: the role of the praecuneus in the autobiographical memory retrieval network. Cortex. 2019 doi: 10.1016/j.cortex.2019.02.020. [DOI] [PubMed] [Google Scholar]
- Minichino A., Bersani F.S., Trabucchi G., Albano G., Primavera M., Delle Chiaie R. The role of cerebellum in unipolar and bipolar depression: a review of the main neurobiological findings. Riv. Psichiatr. 2014;49(3):124–131. doi: 10.1708/1551.16907. [DOI] [PubMed] [Google Scholar]
- Park C.H., Choi Y.S., Jung A.R., Chung H.K., Kim H.J., Yoo J.H. Seizure control and memory impairment are related to disrupted brain functional integration in temporal lobe epilepsy. J. Neuropsychiatry Clin. Neurosci. 2017;29(4):343–350. doi: 10.1176/appi.neuropsych.16100216. [DOI] [PubMed] [Google Scholar]
- Peer M., Pruss H., Ben-Dayan I., Paul F., Arzy S., Finke C. Functional connectivity of large-scale brain networks in patients with anti-NMDA receptor encephalitis: an observational study. Lancet Psychiatry. 2017;4(10):768–774. doi: 10.1016/S2215-0366(17)30330-9. [DOI] [PubMed] [Google Scholar]
- Phillips J.R., Hewedi D.H., Eissa A.M., Moustafa A.A. The cerebellum and psychiatric disorders. Front. Public Health. 2015;3:66. doi: 10.3389/fpubh.2015.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiihonen J., Rossi R., Laakso M.P., Hodgins S., Testa C., Perez J. Brain anatomy of persistent violent offenders: more rather than less. Psychiatry Res. 2008;163(3):201–212. doi: 10.1016/j.pscychresns.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Wang K., Jiang T., Yu C., Tian L., Li J., Liu Y. Spontaneous activity associated with primary visual cortex: a resting-state fMRI study. Cereb. Cortex. 2008;18(3):697–704. doi: 10.1093/cercor/bhm105. [DOI] [PubMed] [Google Scholar]
- Wang L., Kong Q., Li K., Su Y., Zeng Y., Zhang Q. Frequency-dependent changes in amplitude of low-frequency oscillations in depression: a resting-state fMRI study. Neurosci. Lett. 2016;614:105–111. doi: 10.1016/j.neulet.2016.01.012. [DOI] [PubMed] [Google Scholar]
- Weiler M., Teixeira C.V., Nogueira M.H., de Campos B.M., Damasceno B.P., Cendes F. Differences and the relationship in default mode network intrinsic activity and functional connectivity in mild alzheimer’s disease and amnestic mild cognitive impairment. Brain Connect. 2014;4(8):567–574. doi: 10.1089/brain.2014.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Wang Y., Jia Y., Zhong S., Sun Y., Zhou Z. Cerebellar microstructural abnormalities in bipolar depression and unipolar depression: a diffusion kurtosis and perfusion imaging study. J. Affect. Disord. 2016;195:21–31. doi: 10.1016/j.jad.2016.01.042. [DOI] [PubMed] [Google Scholar]