Abstract
Vaping has emerged as a popular alternative form of inhalation of nicotine and marihuana derivates (including Tetrahydrocannabinol, THC) in part due to the avoidance of combustion byproducts. Unfortunately, THC oil (especially that produced by unregulated individuals) may contain dilutants such as propylene glycol, vitamin E, and flavoring ingredients that can lead to adverse respiratory effects. Acute eosinophilic pneumonia (AEP) has been described in association with e-cigarette and vaping associated lung injury (EVALI) but the majority of bronchoalveolar lavage (BAL) samples reported in the literature do not show eosinophils as the predominant cell lineage. Only two other cases of AEP have been published, and here we present the first case reported in the literature of a patient with EVALI with AEP pattern associated with counterfeit tetrahydrocannabinol (THC) oil vaping and discordant bilateral BAL cell count differential.
1. Introduction
Over 2000 cases of e-cigarette and vaping associated lung injury (EVALI) have been reported to the CDC. To date, no specific causative agent has been identified, although vaping with counterfeit products that may contain dilutants, such as Vitamin E acetate, appears to be associated with this epidemic [1]. There are multiple clinicopathologic syndromes associated with EVALI, including Acute eosinophilic pneumonia(AEP), lipoid pneumonia, hypersensitivity pneumonitis, diffuse alveolar hemorrhage, respiratory bronchiolitis-associated pneumonitis, organizing pneumonia, acute lung injury and acute respiratory distress syndrome [[2], [3], [4], [5], [6], [7]]. Although AEP has been described in association with EVALI, the majority of bronchoalveolar lavage (BAL) samples reported in the literature do not show eosinophils as the predominant cell lineage. Here we present the first case reported in the literature of a patient with EVALI with AEP pattern associated with counterfeit tetrahydrocannabinol (THC) oil vaping and discordant bilateral BAL cell count differential.
2. Case report
A 23-year-old man developed a non-productive cough, dyspnea, nausea, vomiting, and fevers over two weeks that failed to improve with a course of antibiotics requiring hospitalization. He denied prior respiratory infections and reported daily vaping with THC oil that he obtained from a friend for the past 2 months. Denied chest pain, lower extremity swelling, and hemoptysis. He was not taking any medications neither prescribed nor over the counter, denied recent travel and had no exposure to birds. He denied any personal or family history of respiratory problems or any other illness and did not have any past surgical history. Initial physical examination findings revealed temperature 102.8 C, pulse 112 min-1, respiratory rate 22 breaths min-1, SpO2 91% (0.21) and diffuse bilateral crackles. Laboratory examination was significant for WBC 17.21 x 103/μL, 92% granulocytes and 0.1% eosinophils. Chemistry was significant for creatinine 0.62 mg/dL, NT-proBNP 97 pg/ml, procalcitonin 0.65 ng/ml. AST 23 U/L, ALT 19 U/L, ALK PHOS 62 U/L. Urinalysis showed no protein and no red blood cell casts. Chest x-ray showed bilateral lower lobe reticulonodular opacities. Intravenous antibiotics were initiated for community-acquired pneumonia but fevers persisted and progressive hypoxemic respiratory failure requiring high flow oxygen developed; arterial blood gas showed pH 7.45/PCO2 38/PaO2 68 (100% FIO2). Computed tomography of the chest was obtained (Fig. 1). Blood cultures remained sterile, HIV serology was negative, and serum IgE was 279 kU/L.
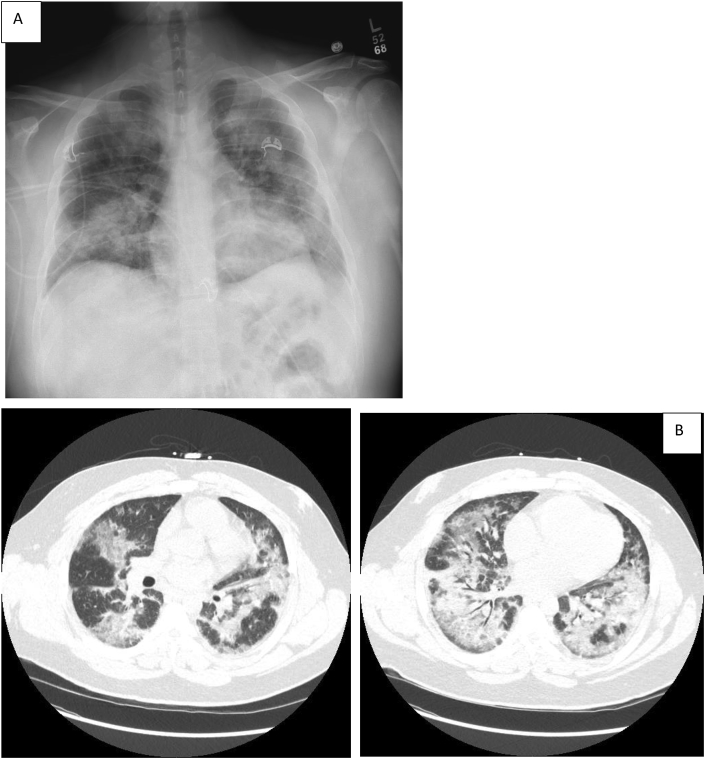
Fig. 1.
At presentation, Chest X-Ray (A) and compute tomography (B) of the chest demonstrated patchy bilateral groundglass opacities, peribronchovascular and centrally located, no bronchiectasis or honeycombing.
Seventy hours after initial presentation, tracheal intubation and invasive mechanical ventilation were required. Shortly thereafter, flexible bronchoscopy with bilateral BAL was performed revealing normal airway mucosa and no airway secretions. No fungal or bacterial organisms were identified. Smears from BAL lingula (Fig. 2A) showed 20 eosinophils/100 inflammatory cells and right middle lobe (Fig. 2B) showed less than 5 eosinophils/100 inflammatory cells. No malignant cells identified. Acute eosinophilic pneumonia was diagnosed and IV methylprednisolone initiated at 1 mg/kg every 8 hours. Within 12 hours, his oxygen requirements improved and fevers abated. Successful extubation occurred 36 hours after the initiation of mechanical ventilation. Systemic steroids were continued and he was discharged on prednisone 40 mg/day with tapering dose over ensuing 10 days. One month after discharge, the patient came back for outpatient follow up. He had no dyspnea, he was able to work out in the gym, he had a normal physical exam and normal pulse oximetry(Fig. 3, follow up chest X-ray and spirometry).
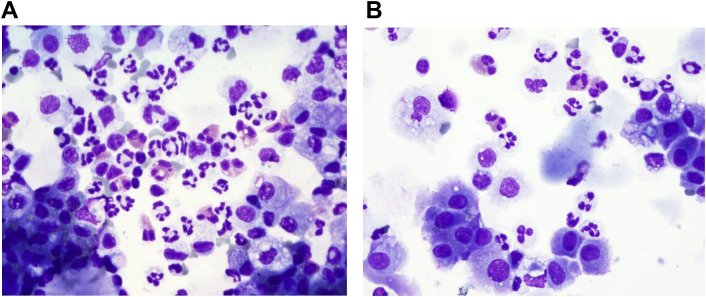
Fig. 2.
Patient's Bronchoalveolar Lavage. Cytological analysis and direct cell count were performed. The direct count was done using a Neubauer hemocytometer followed by manual differential count done on cytospins stained with Wright's stain (not shown).The cytological analysis was done on cytospins stained with Romanowsky and Papanicolaou stains.A:BAL from lingula stained with Romanowsky stain. Concomitant differential count of 20 eosinophils per 100 inflammatory cells.B:BAL from right middle lobe stained with Romanowsky stain.Concomitant differential count of fewer than 5 eosinophils per 100 inflammatory cells.
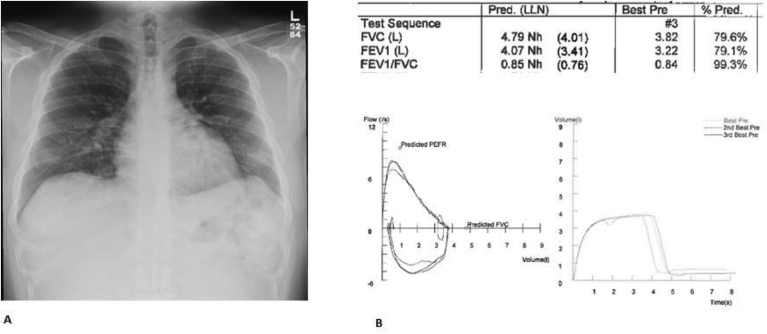
Fig. 3.
One month after discharge from the hospital, Chest X-Ray (A) demonstrating complete resolution of pulmonary infiltrates and spirometry(B)showing non-specific ventilator defect.
3. Discussion
Acute eosinophilic pneumonia (AEP) has been associated with inhalational drugs including marijuana and e-cigarette use. It is typically diagnosed using the modified Philit criteria [8] (Table 1). Treatment involves systemic or oral steroids depending on the severity of illness [9]. Our patient did not meet strict criteria of 25% eosinophils in the BAL and this may be due to probable BAL eosinophils counted as neutrophils due to degranulation and presentation with multiple nuclear lobes.
Table 1.
Modified Philit criteria to diagnose definite acute eosinophilic pneumonia.
| 1) acute respiratory illness of less than or equal to 1 month duration |
| 2) pulmonary infiltrates on chest radiography or computed tomography (CT) |
| 3) pulmonary eosinophilia: more than 25% eosinophils in BAL fluid (can be accompanied by variably increased percentages of lymphocytes and neutrophils) or eosinophilic pneumonia on lung biopsy (bronchoscopic or surgical) |
| 4) Absence of other specific pulmonary eosinophilic diseases. (Eosinophilic granulomatosis with polyangiitis, hypereosinophilic syndrome and allergic bronchopulmonary aspergillosis) |
Acute eosinophilic pneumonia (AEP) has been described as one of the probable presentations of e-cigarette and vaping associated lung injury (EVALI) [[6], [7], [8], [9]]. In the largest case series published to date, BAL samples from patients with EVALI showed eosinophils in a range up to 6% [10], which would not meet the criteria for the diagnosis of AEP. Two other cases of AEP associated with vaping have been published [11,12], but our case is the first to report AEP associated with vaping of counterfeit THC oil with discordant bilateral BAL results. It is unknown whether the BAL results of the cases described in the literature were obtained from both lungs and combined into one sample or analyzed independently from each lung. To date, there is no pathognomonic finding on BAL that can help identify EVALI [[5], [6], [7], [8], [9], [10], [11], [12], [13]].
Vaping has emerged as a popular alternative form of inhalation of nicotine and marihuana derivates (including Tetrahydrocannabinol, THC) in part due to avoidance of combustion byproducts [1,14]. Unfortunately, THC oil (especially that produced by unregulated individuals) may contain dilutants such as propylene glycol, vitamin E, and flavoring ingredients that can lead to adverse respiratory effects [15,16].
Vaping THC oil is a growing trend among adolescents and young adults and is a health hazard. Acute or subacute presentation with systemic, respiratory and gastrointestinal symptoms and radiographic infiltrates in a young adult with recent inhalation of counterfeit THC oil within 90 days of the presentation via an electronic delivery device should alert providers to EVALI as the cause for acute hypoxic respiratory failure. A bronchoscopic examination with bronchoalveolar lavage may be warranted in certain individuals, and providers should be aware of the possibility of discordant bilateral cell count differentials in the BAL results. In the absence of immunosuppression and after infectious etiologies have been ruled out, prompt initiation of oral or intravenous steroids can be considered [7].
CRediT authorship contribution statement
Daniel Puebla Neira: Conceptualization, Investigation, Writing - original draft, Writing - review & editing, Visualization. Sarah Tambra: Investigation. Vibha Bhasin: Resources. Ranjana Nawgiri: Resources. Alexander G. Duarte: Supervision, Writing - review & editing.
Declaration of competing interest
Daniel Puebla Neira: No conflicts of interest to disclose. Sarah Tambra: No conflicts of interest to disclose.Vibha Bhasin: No conflicts of interest to disclose. Ranjana Nawgiri: No conflicts of interest to disclose.Alex Duarte: No conflicts of interest to disclose.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmcr.2020.101015.
Contributor Information
Daniel Puebla Neira, Email: dapuebla@utmb.edu.
Sarah Tambra, Email: satambra@UTMB.EDU.
Vibha Bhasin, Email: vibhasin@UTMB.EDU.
Ranjana Nawgiri, Email: ranawgir@UTMB.EDU.
Alexander G. Duarte, Email: aduarte@UTMB.EDU.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Siegel D.A., Jatlaoui T.C., Koumans E.H., Kiernan E.A., Layer M., Cates J.E., Godfred-Cato S. Update: interim guidance for health care providers evaluating and caring for patients with suspected e-cigarette, or vaping, product use associated lung injury—United States. MMWR (Morb. Mortal. Wkly. Rep.) 2019;68(41):919. doi: 10.15585/mmwr.mm6841e3. October 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carlos W.G., Crotty Alexander L.E., Gross J.E., Dela Cruz C.S., Keller J.M., Pasnick S., Jamil S. Vaping-associated pulmonary illness (VAPI) Am. J. Respir. Crit. Care Med. 2019;200(7):P13–P14. doi: 10.1164/rccm.2007P13. [DOI] [PubMed] [Google Scholar]
- 3.Hswen Y., Brownstein J.S. Real-time digital surveillance of vaping-induced pulmonary disease. N. Engl. J. Med. 2019;381(18):1778–1780. doi: 10.1056/NEJMc1912818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christiani D.C. Vaping-induced lung injury. N. Engl. J. Med. 2019;6(10.1164) doi: 10.1056/NEJMe1912032. [DOI] [PubMed] [Google Scholar]
- 5.Maddock S.D., Cirulis M.M., Callahan S.J., Keenan L.M., Pirozzi C.S., Raman S.M., Aberegg S.K. Pulmonary lipid-laden macrophages and vaping. N. Engl. J. Med. 2019;381(15):1488–1489. doi: 10.1056/NEJMc1912038. [DOI] [PubMed] [Google Scholar]
- 6.Christiani D.C. Vaping-induced lung injury. N. Engl. J. Med. 2019;6(10.1164) doi: 10.1056/NEJMe1912032. [DOI] [PubMed] [Google Scholar]
- 7.Fuentes X.F., Kashyap R., Hays J.T., Chalmers S., von Buchwald C.L., Gajic O., de Moraes A.G. Mayo Clinic Proceedings. Elsevier; 2019, November. VpALI—vaping-related acute lung injury: a new killer around the block. [DOI] [PubMed] [Google Scholar]
- 8.De Giacomi F., Decker P.A., Vassallo R., Ryu J.H. Acute eosinophilic pneumonia: correlation of clinical characteristics with underlying cause. Chest. 2017;152(2):379–385. doi: 10.1016/j.chest.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 9.De Giacomi F., Vassallo R., Yi E.S., Ryu J.H. Acute eosinophilic pneumonia. Causes, diagnosis, and management. Am. J. Respir. Crit. Care Med. 2018;197(6):728–736. doi: 10.1164/rccm.201710-1967CI. [DOI] [PubMed] [Google Scholar]
- 10.Layden J.E., Ghinai I., Pray I., Kimball A., Layer M., Tenforde M., Haupt T. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin—preliminary report. N. Engl. J. Med. 2019 doi: 10.1056/NEJMoa1911614. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Thota D., Latham E. Case report of electronic cigarettes possibly associated with eosinophilic pneumonitis in a previously healthy active-duty sailor. J. Emerg. Med. 2014;47(1):15–17. doi: 10.1016/j.jemermed.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 12.Arter Z.L., Wiggins A., Hudspath C., Kisling A., Hostler D.C., Hostler J.M. Acute eosinophilic pneumonia following electronic cigarette use. Respir. Med. Case Rep. 2019;27:100825. doi: 10.1016/j.rmcr.2019.100825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mukhopadhyay S., Mehrad M., Dammert P., Arrossi A.V., Sarda R., Brenner D.S., Ghobrial M. Lung biopsy findings in severe pulmonary illness associated with E-cigarette use (vaping) A report of eight cases. Am. J. Clin. Pathol. 2020;153(1):30–39. doi: 10.1093/ajcp/aqz182. [DOI] [PubMed] [Google Scholar]
- 14.Abrams D.I., Vizoso H.P., Shade S.B., Jay C., Kelly M.E., Benowitz N.L. Vaporization as a smokeless cannabis delivery system: a pilot study. Clin. Pharmacol. Therapeut. 2007;82(5):572–578. doi: 10.1038/sj.clpt.6100200. [DOI] [PubMed] [Google Scholar]
- 15.Barrington-Trimis J.L., Samet J.M., McConnell R. Flavorings in electronic cigarettes: an unrecognized respiratory health hazard? Jama. 2014;312(23):2493–2494. doi: 10.1001/jama.2014.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen R.P., Luo W., Pankow J.F., Strongin R.M., Peyton D.H. Hidden formaldehyde in e-cigarette aerosols. N. Engl. J. Med. 2015;372(4):392–394. doi: 10.1056/NEJMc1413069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.