Abstract
Objectives
To estimate the prevalence and to identify potential risk factors of silent myocardial ischemia in a cohort of patients with asymptomatic type 2 Diabetes (diabetes) for early detection of coronary risk by employing objective noninvasive clinical screening tools for Subclinical Atherosclerosis.
Methods
The study is a clinic-based observational study on 338 consecutive diabetes patients attending an urban health center from Eastern India. The response rate was 96.57% out of 350 eligible subjects, comprising 176 (52.1%) males and 162 (47.9%) females. Clinical, anthropometric, biochemical parameters were collected in all participants. Both tools, i.e., treadmill test (TMT) to identify subjects with silent myocardial ischemia, and carotid imaging to detect subclinical atherosclerosis by evaluating carotid intima-media thickness (CIMT), were assessed. Significant determinants were predicted by multivariable logistic regression.
Results
The study group was divided into a TMT negative (n = 260), and a TMT positive group (n = 78). These 78 TMT positive subjects (23.1%) were identified to have silent myocardial ischemia. The prevalence of silent myocardial ischemia was more common in males (28.4%) than in females (17.3%). The mean CIMT in our study group was 0.6741 ± 0.034 mm (males – 0.684 ± 0.034 mm and females – 0.663 ± 0.032 mm). Age ≥50 years, CIMT ≥0.70 mm, hypercholesterolemia, and hypertriglyceridemia were significant determinants for identifying asymptomatic diabetics at risk for silent myocardial ischemia.
Conclusion
Silent myocardial ischemia is highly prevalent at about one in four asymptomatic diabetic patients. An increased CIMT can be a surrogate marker of higher coronary risk amongst these asymptomatic diabetics.
Keywords: Silent myocardial ischemia, Subclinical atherosclerosis, CHD, Type 2 diabetes, CIMT, Coronary risk
1. Introduction
The Indian subcontinent, along with South Asian neighbors, has the dubious distinction of very high prevalence of diabetes totaling 84 million, i.e., one-fifth of all the global diabetes burden.1 We previously reported a high prevalence of diabetes at about 11% in an urban population from the state of Odisha in Eastern India,2 and similar results have been reported from recent ICMR-INDIAB population-based cross-sectional study across India.3 Related cardiometabolic risk factors, such as hypertension, central obesity, smoking, and physical inactivity, are also showing a rapid increase across all socioeconomic groups in India and elsewhere.4,5 Previous well-established evidence from the Framingham Study,6 the MRFIT,7 and the UKPDS8 have demonstrated the potential areas of cardiovascular prevention and control. However, a community-based preventive cardiovascular intervention targeting both co-morbidity and associated risk factors, including diabetes,9 in an underprivileged population setting, with a heterogeneous mix of ethnicity and religious background, is limited. Such evidence is crucial for establishing consistency in study findings, and reproducibility, as well as providing supporting indirect evidence of the cost-effectiveness of these interventions.
Diabetes, as an independent risk factor for cardiovascular disease, and also being a co-morbid condition for CVD,10 needs a population-level approach to be identified earlier in a potentially high-risk population. One such approach can be to detect Silent Myocardial Ischemia in asymptomatic diabetic patients.11,12 Subclinical atherosclerosis by quantitative measurements of carotid intima-media thickness (CIMT)13,14 and Silent Myocardial Ischemia (Silent MI) by treadmill exercise stress testing11,12 are two recommended clinical strategies to address this population-level approach, which can be both cost-effective and noninvasive. Taken together, we set out with the following two specific study objectives.
-
1.
To estimate the prevalence of silent MI objectively, and to identify the associated risk factors of silent MI in a cohort of asymptomatic diabetes patients attending an urban health center in Eastern India.
-
2.
To explore if CIMT can be a surrogate marker of coronary risk in asymptomatic diabetes patients.
2. Methods
The detailed study methodology has already been published.15 We have provided a brief overview of the methods below. Our study is a cross-sectional clinico-observational study in an urban population setting of individuals without any known cardiovascular ailments, i.e., a cohort of asymptomatic type 2 diabetes patients. The study setting is Berhampur City, a major city of Odisha state, and one of the underdeveloped regions of Eastern India bordering an affluent Andhra Pradesh state of Southern India. Therefore, the residents here are a diverse mix of socioeconomic class, language, faith, and customs. The study is a clinic-based observational study on 350 consecutive patients of diabetes at an urban health center, Berhampur, India, over a period of 6 months from September 2017 to February 2018 with a predefined inclusion and exclusion criteria.
Inclusion criteria: Patients of diabetes on insulins or oral hypoglycemic agents with a normal resting ECG.
Exclusion criteria: Diabetes patients with cerebrovascular disease, cardiovascular diseases, and chronic kidney disease at baseline.
Silent Myocardial ischemia (based on exercise tolerance testing)11,12 and subclinical atherosclerosis (identified through carotid-intima media measurements of the common carotid artery)14,15 are the primary outcome measures. The methods are described in the prepublished protocol.15 Details of other variables of interest are explained below.15 Given the nature of the research question, no potential confounders or effect modifiers were identified apriori. There is no primary exposure of interest in this research question. However, potential risk factors for the outcome of interest were identified and were factored into the analysis. We have taken adequate care to ensure a good response rate by explaining the recruited subjects about the importance of the study to self and also the public health implications of the study. A previously validated questionnaire tool that was applied to our earlier studies has been adopted.4 In short, we have followed the WHO stepwise approach for data collection.16 Demographic, socioeconomic, and self-reported behavioral information (smoking, alcohol, physical activity, diet), objective measures of anthropometry (height, weight, waist and hip circumferences), biochemical parameters (blood glucose, glycosylated hemoglobin, lipids, uric acid, creatinine levels, microalbuminuria),electrocardiographic and CIMT measurements were also obtained. Health conditions have been documented based on self-reported history of diabetes, hypertension, and cardiovascular disease (chest pain, heart attack, or stroke). Family histories for all of the above conditions were collected. Current estimates of silent MI in diabetic patients amongst South Asians are less well documented. Based on the literature review,17 we assumed a prevalence of silent MI amongst diabetic patients to be 25%. Based on this prevalence (+/− 5%), we computed the required sample size using Open-Epi online software, assuming an alpha level of 0.05 (95% CI) to be 289. The estimated sample size is 350 on factoring 20% nonresponse.
2.1. Statistical analysis
The entire statistical analyses were carried out using SPSS software version 22. All continuous variables (including CIMT) were reported as Means ± Standard deviation. The prevalence of various cardiovascular risk factors in study participants was described in percentages. The mean values and frequencies of cardio-metabolic risk factors were analyzed across two subgroups, namely those with silent MI positive (TMT Positive), and those with silent MI negative group (TMT Negative) using Students’ t-test (for continuous variables) and Chi-square tests for categorical variables. Multivariate logistic regression using a backward elimination method was used to predict the significant risk factors of silent MI (only one variable with the highest ‘’ p-value, which does not show significance was excluded in the next modeling step). A priori subgroup analyses within silent MI patients were undertaken. For instance, within the silent MI patients, two risk groups (namely high and low) were compared and contrasted based on known cardiometabolic risk factors employing multivariable logistic regression analyses. Finally, the mean difference and the 1-SD difference in CIMT readings were estimated to predict subclinical atherosclerosis in our study subjects employing multivariable logistic regression techniques. Statistically, the significance is assumed at p < 0.05.
2.2. Ethics
The study is in agreement with the Indian Council of Medical Research guidelines on bioethics. The institutional ethical committee of Kalinga Institute of Medical Sciences, Bhubaneswar, India has accepted the study proposal. All the study participants have given their written informed consent before the study.
3. Results
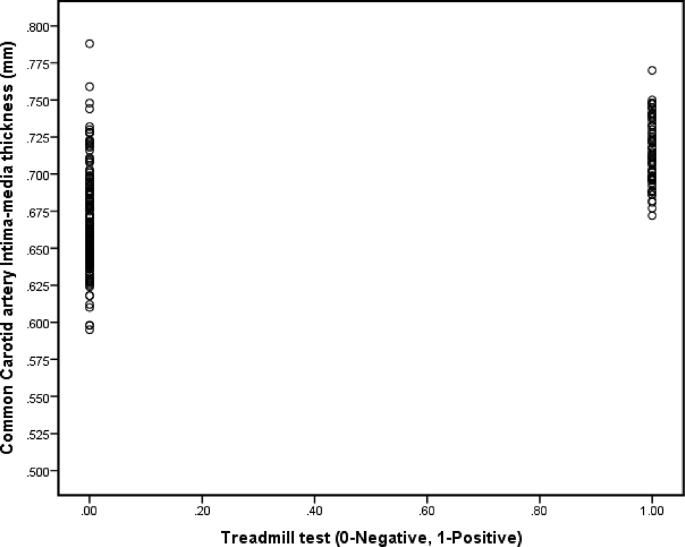
A total of 338 subjects out of 350 eligible subjects have completed the study. The response rate is 96.57% of which males are 176 (52.1%), and females are 162 (47.9%). Of the 12 dropouts, five patients did not report to TMT lab because of knee problems, two patients had hypersensitivity to the carotid bulb, one patient had to be shifted to acute care, and four patients have not turned up for followup at all. There is an increased prevalence of coronary risk factors, physical inactivity, hypertension, hypercholesterolemia, and obesity, as well as factors peculiar to South Asians, such as central obesity, low fruit intake, low HDL-cholesterol, and hypertriglyceridemia in this cohort of diabetic subjects. Moreover, risk factors like physical inactivity, central obesity, overweight, and low HDL cholesterol are more prevalent in females in our study subjects. The estimated prevalence (crude) of silent MI amongst subjects of asymptomatic type 2 Diabetes was 23.1%, objectively measured through TMT tests. Males had a relatively higher prevalence (28.4%) compared to females (17.3%). The mean IMT in our study group was 0.6741 ± 0.034 mm (males – 0.684 ± 0.034 mm and females – 0.663 ± 0.032 mm). Table 1, Table 2 show the respective baseline characteristics of anthropometric, behavioral, clinical, biochemical, and imaging parameters by TMT status across males and females. Of note in Table 2, the mean CIMT was higher in subjects within the TMT positive group, i.e., 0.7133 ± 0.0215 versus 0.6624 ± 0.0286 mm in TMT negative group. Furthermore, by regression analysis, the sensitivity and specificity of CIMT are best observed at 0.70 mm to predict Silent MI (sensitivity of 63% and specificity of 90%), as evident from Table 3. The correlation of CIMT with silent MI (TMT positive: coded as 1 and TMT negative: coded as 0) is depicted in Fig. 1. Pearson’s correlation coefficient shows a statistically significant positive correlation (r = 0.621) with CIMT. The figure shows that higher CIMT values are observed in silent MI group compared to TMT negative group.
Table 1.
Anthropometric/Behavioral/Clinical risk factors in TMT positive versus TMT negative subjects.
| Risk Parameters | Tread Mill Test |
||
|---|---|---|---|
| Positive [+ve] (n = 78) | Negative [-ve] (260) | Sig | |
| Age (Years), (mean ± SD) | 59.5 ± 8.1 | 51.5 ± 11.5 | 0.001* |
| Body mass Index (kg/m2), (mean ± SD) | 26.7 ± 4.7 | 25.6 ± 4.1 | 0.027* |
| Waist Circumference, (mean ± SD) | 93.1 ± 11.6 | 89.9 ± 10.8 | 0.023* |
| Central Obesity, n (%) | 59 (75.6) | 161 (61.9) | 0.026* |
| Obesity, n (%) | 46 (59.0) | 131 (50.4) | 0.183 |
| Overweight, n (%) | 61 (78.2) | 187 (71.9) | 0.271 |
| Physical inactivity, n (%) | 52 (66.7) | 146 (56.2) | 0.098 |
| Smokers, n (%) | 28 (35.9) | 52 (20.0) | 0.004* |
| Alcohol consumers, n (%) | 20 (25.6) | 61 (23.5) | 0.692 |
| Low/No Fruit intake, n (%) | 36 (46.2) | 144 (55.4) | 0.152 |
| Systolic BP(mmHg), (mean ± SD) | 145.31 ± 13.6 | 138.79 ± 14.3 | 0.001* |
| Diastolic BP(mmHg), (mean ± SD) | 87.36 ± 6.8 | 85.31 ± 8.2 | 0.044* |
| Hypertension, n (%) | 61 (78.2) | 151 (58.1) | 0.001* |
| Duration of Diabetes (years), (mean ± SD) | 7.8 ± 3.8 | 5.57 ± 3.3 | 0.001* |
| Family History-CHD, n (%) | 8 (10.3) | 20 (7.7) | 0.471 |
| Family history-Diabetes, n (%) | 36 (46.2) | 113 (43.5) | 0.674 |
| Family history –HTN, n (%) | 30 (38.5) | 80 (30.8) | 0.203 |
* Significant with p < 0.01, p < 0.05 using student t-test, chi-square test, SD. Standard Deviation
BP:Blood Pressure,CHD:Cornary heart disease, HTN,Hypertension.
Table 2.
Comparison of Biochemical/Imaging risk parameters in TMT Positive versus TMT Negative subjects.
| Risk Parameters | Tread Mill Test |
||
|---|---|---|---|
| Positive [+ve] (n = 78) | Negative [-ve] (260) | Sig | |
| Fasting blood sugar [mg/dl], (mean ± SD) | 141.9 ± 37.6 | 130.8 ± 33.7 | 0.014* |
| Post prandial sugar [mg/dl], (mean ± SD) | 241.6 ± 50.8 | 226.9 ± 52.9 | 0.030* |
| Glycosylated hemoglobin [%], (mean ± SD) | 7.52 ± 0.70 | 7.37 ± 0.78 | 0.136 |
| Microalbuminuria. n (%) | 32 (41.0) | 30 (11.5) | 0.001* |
| Total cholesterol [mg/dl], (mean ± SD) | 206.9 ± 45.1 | 183.3 ± 36.3 | 0.001* |
| Hypercholesterolemia n (%) | 38 (48.7) | 68 (26.2) | 0.001* |
| Triglycerides [mg/dl], (mean ± SD) | 216.7 ± 57.6 | 175.7 ± 45.7 | 0.001* |
| Hypertriglyceridemia, n (%) | 70 (89.7) | 180 (69.2) | 0.001* |
| HDL cholesterol [mg/dl], (mean ± SD) | 41.3 ± 6.2 | 47.3 ± 7.4 | 0.001* |
| Low HDL, n (%) | 40 (51.3) | 96 (36.9) | 0.023* |
| LDL cholesterol [mg/dl], (mean ± SD) | 121.4 ± 45.8 | 101.1 ± 35.8 | 0.001* |
| Increased LDL, n (%) | 29 (37.2) | 59 (22.7) | 0.011* |
| CIMT [mm], (mean ± SD) | 0.7133 ±0.022 | 0.6624 ±0.029 | 0.001* |
* Significant with p < 0.01, p < 0.05 using student t-test, chi-square test, SD. Standard Deviation
CHD:Cornary heart disease, HTN,Hypertension,HDL:High Density Lipoprotein,LDL:Low Density Lipoprotein.
TMT: Treadmill test, CIMT: Common Carotid artery Intima-media thickness,mm: millimetre, mg: milligram.
Table 3.
Correlation of CIMT with silent myocardial ischemia.
| CIMT | Sensitivity | Specificity | PPV | NPV | Accuracy | OR | RR | p-value |
|---|---|---|---|---|---|---|---|---|
| 0.60 mm | 0.100 | 0.41 | 0.34 | 0.100 | 0.55 | – | – | a |
| 0.65 mm | 0.100 | 0.41 | 0.34 | 0.100 | 0.55 | – | – | a |
| 0.70 mm | 0.63 | 0.90 | 0.65 | 0.89 | 0.84 | 15.2 | 5.9 | <0.000* |
| 0.75 mm | 0.26 | 0.99 | 0.50 | 0.77 | 0.77 | 3.40 | 2.20 | 0.199 |
| 0.80 mm | 0.0 | 0.100 | – | 0.77 | 0.77 | – | – | a |
| 0.85 mm | 0.0 | 0.100 | – | 0.77 | 0.77 | – | – | a |
| 0.90 mm | 0.0 | 0.100 | – | 0.77 | 0.77 | – | – | a |
*Significant with p < 0.05; p < 0.01.
CIMT: Common Carotid artery Intima-media thickness; PPV: Positive predictive value.
NPV: Negative predictive value; OR: Odds ratio; RR: Relative risk; mm: millimetre.
Nil significance due to insufficient data.
Fig. 1.
Correlation between CIMT and Silent Myocardial ischemia.
Various potential risk factors for silent MI like LDL, HDL, Total Cholesterol, Triglycerides, Smoking status, Duration of Diabetes, Microalbuminuria, Hypertension, Age, Central obesity, and CIMT were identified by univariate analyses, which were later factored in multivariable logistic regression analyses. Table 4 Identifies the significant risk factors of silent MI through multivariable logistic regression analyses. Only variables like Hypercholesterolemia, Hypertriglyceridemia, Age, and CIMT remained statistically significant in the final multivariable model. For instance, those who had increased CIMT (≥0.70 mms) were ten times as likely to develop silent MI compared to those who had lower CIMT (adjusted OR: 9.78; 95% CI: 5.0–19.1).
Table 4.
Multivariate logistic regression analysis to predict significant risk factors for silent myocardial ischemia amongst study population.
| Risk factors | Odds ratio (95% Confidence Interval) | p-value | Risk factors Eliminated in each step |
|---|---|---|---|
| Initial [Step 1] | |||
| Increased LDL | 1.19 (0.46–3.11) | 0.718 | 2 |
| Diabetes duration ≥10 years | 1.23 (0.52–2.89) | 0.635 | 3 |
| Low HDL | 1.17 (0.56–2.43) | 0.682 | 4 |
| Hypertension | 1.39 (0.65–2.96) | 0.398 | 5 |
| Microalbuminuria | 1.38 (0.58–3.25) | 0.468 | 6 |
| Central Obesity | 1.67 (0.80–3.47) | 0.173 | 7 |
| Smoking status | 1.97 (0.93–4.16) | 0.075 | 8 |
| Hypertriglyceridemia | 2.38 (0.94–5.99) | 0.066 | |
| Hypercholesterolemia | 2.51 (0.99–6.32) | 0.050 | |
| Age [≥50 years] | 3.22 (1.25–8.28) | 0.015* | |
| CIMT [≥0.7000 mm] | 8.03 (4.00–16.11) | <0.001* | |
| Final [Step 8] | |||
| Hypertriglyceridemia | 2.62 (1.09–6.29) | 0.031* | |
| Hypercholesterolemia | 2.91 (1.47–5.74) | 0.002* | |
| Age [≥50 years] | 4.09 (1.69–9.88) | 0.002* | |
| CIMT [≥0.7000 mm] | 9.78 (5.0–19.1) | <0.000* | |
HDL:High Density Lipoprotein,LDL:Low Density Lipoprotein.
CIMT: Common Carotid artery Intima-media thickness,mm: millimetre.
4. Discussion
4.1. Key results
The main findings of this cross-sectional study based on the two specific study objectives are:
-
a)
The estimated prevalence (crude) of silent MI in this study population was 23.1% objectively measured through TMT tests; males had a relatively high prevalence (28.4%) compared to females (17.3%).
-
(b)
Age, CIMT, Hypercholesterolemia, and Hypertriglyceridemia are the only significant risk factors for silent MI in our cohort of diabetic patients.
-
(c)
Those who had higher CIMT ≥0.70 mms were ten times as likely to develop silent MI compared to those who had lower CIMT.
-
(d)
CIMT can be used as a potential surrogate marker of coronary risk, amongst asymptomatic Diabetic patients.
Our study findings are broadly consistent with evidence documented earlier.18, 19, 20 Several studies based in India have reported a wide variation in the prevalence of silent MI,18,21, 22, 23 which may be due to the methodological differences in these studies. Globally, our study findings are also in agreement with the published literature related to the prevalence of silent MI.12,24, 25, 26, 27 Second, we have reported in this study that Age, CIMT, Hypercholesterolemia, and Hypertriglyceridemia were identified as potential significant risk factors of silent MI drawn on the variables that were measured or available to this study, which are consistent with evidence published elsewhere.21, 22, 23,26,28 However, other cardiometabolic risk factors, such as duration of diabetes, lipid parameters, smoking status, central obesity, hypertension, and microalbuminuria, were significantly associated in the univariate analyses but did not reach statistical significance in multivariable logistic regression analyses. Comparable to earlier studies,12,21,24 our study has also not shown any significance of glycaemic control in predicting silent MI, although it is of significance to prevent further complications of diabetes. Therefore, there is this potential of residual confounding due to unmeasured and unknown risk factors.
Finally, we also explored the possibility of CIMT being considered a surrogate measure of coronary risk among asymptomatic diabetic patients. Our study findings are in favor of this direction. A number of large-scale studies have established the relationship between increased CIMT and cardiovascular events independent of usual cardiometabolic risk factors both locally29 and internationally.13 The Chennai Urban Population study evaluated that the mean IMT in South Indian subjects with diabetes (0.95 ± 0.31 mm) was significantly higher than those of the nondiabetic subjects (0.74 ± 0.14 mm).29 But the mean CIMT in our study group was 0.6741 ± 0.034 mm (males – 0.684 ± 0.034 mm and females – 0.663 ± 0.032 mm). This may be because the majority of our patients were already diagnosed cases of diabetes and on regular treatment. But a recent SCORE-India study highlights that the CIMT values were also higher in diabetic subjects at (0.635 ± 0.10 mm) as compared to nondiabetics (0.589 ± 0.12 mm).30 Hence our observations are consistent with the above nationwide SCORE-India study. Our study shows that the mean CIMT in the silent MI positive group was 0.713 ± 0.021 mm as compared to 0.662 ± 0.028 mm in the negative group. This difference was statistically significant, and there was a direct correlation between CIMT and the occurrence of silent MI in our study. Both males and females had higher CIMT in the silent MI group than in a negative group.
4.2. Strengths and limitations of the study
Our study had certain limitations that are worth considering. Although this is one of the large studies to determine coronary risk profile in asymptomatic diabetes subjects, it is still small given the overall size and the diversity of the Indian population. The interpretation of the prevalence of coronary risk in the present study is affected by possible recall and measurement biases. However, they are minimized through the administration of the previously validated questionnaire, trained interviewers, and the utility of calibrated diagnostic and anthropometric tools based on well-defined measurement criteria. Selection bias may be a possibility in this study design, but this may be minimal, given that the study participants were recruited from the source population (study base) consecutively.
Moreover, this is only a cross-sectional study done at one point in time, and therefore, no causal inferences can be drawn. Furthermore, we could not evaluate our patients, whether tested positive or not for silent myocardial ischemia either by coronary interventional or radionuclide studies solely due to logistical and resource constraints. The study has been undertaken in a low-resource setting, with minimal resources wherein a universal health coverage is a distant dream.31,32 The same is further corroborated by the fact that about 60% of ST-elevation myocardial infarction (STEMI) patients deserving primary Percutaneous coronary intervention (PCI) and pharmaco-invasive approach to coronary reperfusion are untreated in India.33 However, evidence suggests that exercise tolerance tests report a sensitivity of 68% and a specificity of 77%, which can be acceptable in a research setting, such as the current one.34 Therefore, additional investments in coronary interventional and radionuclide imaging may not be justifiable.
One of the main strengths of this study is that this study is from a population setting wherein the resources and logistics are limited but has heterogeneous socio-cultural characteristics, and no such similar studies have been undertaken in such an environment, which adds to the novelty of our study rationale.
5. Conclusion
The observations of this study apply to both clinical and preventive cardiovascular practice and are more pertinent to low and middle-income countries with limited health care infrastructure. An estimated prevalence of 23% for silent MI among asymptomatic diabetic patients is relatively high. However, the four potential significant factors thus identified to predict silent myocardial ischemia can be further developed into a risk assessment clinical tool. Although the study is a cross-sectional design, the findings suggest that patients of diabetes with a mean duration of ten years and above need to undergo carotid imaging, and those with a CIMT greater than 0.70 mm may need further exercise tolerance testing to detect silent MI. However, prospective longitudinal studies with a larger sample size will provide additional insights into this.
5.1. Generalizability
Although our study is a regional study, but is of adequate statistical power and representative of the target population of asymptomatic diabetic patients bordering two neighboring states from Eastern and Southern India. Further, our study has pursued the Strengthening the Reporting of observational studies in Epidemiology (STROBE) criteria for global comparisons.35
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
All authors have none to declare.
Acknowledgements
We acknowledge our thanks to all participants in the PRISM study and all the staff involved in the data collection at Heart Centre.
Contributor Information
D.S. Prasad, Email: drdsprasad@gmail.com.
Zubair Kabir, Email: z.kabir@ucc.ie.
K. Revathi Devi, Email: drrevathidevi@gmail.com.
Pearline Suganthy Peter, Email: pearlinepeter@gmail.com.
B.C. Das, Email: bhagabaticharan.das677@gmail.com.
References
- 1.IDF . 8th ed. International Diabetes Federation; Brussels,Belgium: 2017. Diabetes Atlas. [Google Scholar]
- 2.Prasad D.S., Kabir Z., Dash A.K., Das B.C. Prevalence and risk factors for diabetes and impaired glucose tolerance in Asian Indians: a community survey from urban eastern India. Diabetes Metab Syndrome. 2012;6:96–101. doi: 10.1016/j.dsx.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 3.Anjana R.M., Deepa M., Pradeepa R. Prevalence of diabetes and prediabetes in 15 states of India: results from the ICMR-INDIAB population-based cross-sectional study. Lancet Diabetes Endocrinol. 2017;5:585–596. doi: 10.1016/S2213-8587(17)30174-2. [DOI] [PubMed] [Google Scholar]
- 4.Prasad D.S., Kabir Z., Dash A.K., Das B.C. Coronary risk factors in South Asians: a prevalence study in an urban populace of Eastern India. CVD Prev Control. 2010:125–132. [Google Scholar]
- 5.Shah B., Mathur P. Surveillance of cardiovascular disease risk factors in India: the need & scope. Indian J Med Res. 2010;132:634–642. doi: 10.4103/0971-5916.73420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kannel W.B., McGee D.L. Diabetes and cardiovascular disease: the framingham study. J Am Med Assoc. 1979;241:2035–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 7.Stamler J., Vaccaro O., Neaton J.D., Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the multiple risk factor intervention trial. Diabetes Care. 1993;16:434. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- 8.Implications of the United Kingdom prospective diabetes study. Diabetes Care. 2003;26(suppl 1):s28–s32. doi: 10.2337/diacare.26.2007.s28. [DOI] [PubMed] [Google Scholar]
- 9.Yki-Jarvinen H. Management of type 2 diabetes mellitus and cardiovascular risk: lessons from intervention trials. Drugs. 2000;60:975–983. doi: 10.2165/00003495-200060050-00001. [DOI] [PubMed] [Google Scholar]
- 10.Viberti G. The need for tighter control of cardiovascular risk factors in diabetic patients. J Hypertens Suppl. 2003;21:S3–S6. [PubMed] [Google Scholar]
- 11.Di Carli M.F., Hachamovitch R. Should we screen for occult coronary artery disease among asymptomatic patients with diabetes? J Am Coll Cardiol. 2005;45:50–53. doi: 10.1016/j.jacc.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 12.Wackers F.J.T., Young L.H., Inzucchi S.E. Detection of silent myocardial ischemia in asymptomatic diabetic subjects: the DIAD study. Diabetes Care. 2004;27:1954–1961. doi: 10.2337/diacare.27.8.1954. [DOI] [PubMed] [Google Scholar]
- 13.Lorenz M.W., Markus H.S., Bots M.L., Rosvall M., Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness. Circulation. 2007;115(4):459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 14.Stein J.H., Korcarz C.E., Hurst R.T. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American society of echocardiography carotid intima-media thickness task force endorsed by the society for vascular medicine. J Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Prasad D.S., Kabir Z., Devi K.R., Dash A.K., Das B.C. Subclinical atherosclerosis and silent myocardial ischaemia in patients with type 2 diabetes: a protocol of a clinico-observational study. Open Heart. 2014;1(1) doi: 10.1136/openhrt-2014-000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonita R., De Courten M., Dwyer T., Jamrozik K., Winkelmann R. World Health Organisation; Geneva: 2001. Surveillance of Risk Factors for Non Communicable Disease: The WHO STEP Wise approach.Summary. [Google Scholar]
- 17.Valensi P., Lorgis L., Cottin Y. Prevalence, incidence, predictive factors and prognosis of silent myocardial infarction: a review of the literature. Arch Cardiovasc Dis. 2011;104(3):178–188. doi: 10.1016/j.acvd.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Dodani S., Sharma G.K. Presence of coronary artery disease in diabetic and non diabetic South Asian immigrants. Indian Heart J. 2018;70:50–55. doi: 10.1016/j.ihj.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohan V., Venkatraman J.V., Pradeepa R. Epidemiology of cardiovascular disease in type 2 diabetes: the Indian scenario. J Diabetes Sci Technol. 2010 Jan 1;4(1):158–170. doi: 10.1177/193229681000400121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prabhakaran D., Yusuf S., Mehata S. Two-year outcomes in patients admitted with non-ST elevation acute coronary syndrome: results of the OASIS registry 1 and 2. Indian Heart J. 2005;57:217–225. [PubMed] [Google Scholar]
- 21.Agarwal A.K., Singla S., Singla S. Prevalence of coronary risk factors in type 2 diabetics without manifestations of overt coronary heart disease. J Assoc Phys India. 2009;57:135–142. [PubMed] [Google Scholar]
- 22.Bhatia L.C., Singal R., Jain P., Mishra N., Mehra V. Detection of silent myocardial ischaemia in asymptomatic diabetic patients during treadmill exercise testing. High Blood Press Cardiovasc Prev. 2012;19:137–142. doi: 10.1007/BF03262463. [DOI] [PubMed] [Google Scholar]
- 23.Sharada M., Soni A.K., Meena S., Nigam H., Singh A. A prospective study on utility of exercise treadmill test in type 2 diabetes mellitus patients. J Assoc Phys India. 2016;64:32–37. [PubMed] [Google Scholar]
- 24.Bacci S., Villella M., Villella A. Screening for silent myocardial ischaemia in type 2 diabetic patients with additional atherogenic risk factors: applicability and accuracy of the exercise stress test. Eur J Endocrinol. 2002;147:649–654. doi: 10.1530/eje.0.1470649. [DOI] [PubMed] [Google Scholar]
- 25.Blanchet Deverly A., Amara M., Larifla L. Silent myocardial ischaemia and risk factors in a diabetic Afro-Caribbean population. Diabetes Metab. 2011;37(6):533–539. doi: 10.1016/j.diabet.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Milan Study on Atherosclerosis and Diabetes (MiSAD) Group Prevalence of unrecognized silent myocardial ischemia and its association with atherosclerotic risk factors in noninsulin-dependent diabetes mellitus. Am J Cardiol. 1999;79:134–139. doi: 10.1016/s0002-9149(96)00699-6. [DOI] [PubMed] [Google Scholar]
- 27.Naka M., Hiramatsu K., Aizawa T. Silent myocardial ischemia in patients with non-insulin-dependent diabetes mellitus as judged by treadmill exercise testing and coronary angiography. Am Heart J. 1992;123:46–53. doi: 10.1016/0002-8703(92)90745-h. [DOI] [PubMed] [Google Scholar]
- 28.Premalatha G., Anirudhan M.K., Mohan V., Sastry N.G. Treadmill(Cardiac Stress) test in the diagnosis of ischaemic heart disease in NIDDM patients: usefulness and safety. Int J Diabetes Dev Ctries. 1995;15:3–6. [Google Scholar]
- 29.Ravikumar R., Deepa R., Shanthirani C.S., Mohan V. Comparison of carotid intima-media thickness, arterial stiffness, and brachial artery flow mediated dilatation in diabetic and nondiabetic subjects (The Chennai Urban Population Study [CUPS-9]) Am J Cardiol. 2002;90:702–707. doi: 10.1016/s0002-9149(02)02593-6. [DOI] [PubMed] [Google Scholar]
- 30.Kasliwal R.R., Bansal M., Desai N. A Study to derive distribution of carotid intima media thickness and to determine its COrrelation with cardiovascular Risk factors in asymptomatic nationwidE Indian population (SCORE-India) Indian Heart J. 2016;68(6):821–827. doi: 10.1016/j.ihj.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaul U., Bhatia V. Perspective on coronary interventions & cardiac surgeries in India. Indian J Med Res. 2010;132:543–548. [PMC free article] [PubMed] [Google Scholar]
- 32.Patel V., Parikh R., Nandraj S. Assuring health coverage for all in India. Lancet. 2015 Dec 12;386(10011):2422–2435. doi: 10.1016/S0140-6736(15)00955-1. [DOI] [PubMed] [Google Scholar]
- 33.Khanna N.N., Rao S. Cardiological Society of India-Cardiology Update; 2017. Growth of Interventional Cardiology in India: The Relevance of National Interventional Council (CSI-NIC) pp. 212–219. [Google Scholar]
- 34.Ahmed A.H., Shankar K., Eftekhari H. Silent myocardial ischemia: current perspectives and future directions. Exp Clin Cardiol. 2007;12(4):189–196. [PMC free article] [PubMed] [Google Scholar]
- 35.Vandenbroucke J.P., Elm Ev, Altman D.G. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Ann Intern Med. 2007;147:163–194. doi: 10.7326/0003-4819-147-8-200710160-00010-w1. [DOI] [PubMed] [Google Scholar]