Abstract
Objective
Delayed presentation after ST-elevation myocardial infarction (STEMI) and complicated by cardiogenic shock (CS-STEMI) is commonly encountered in developing countries and is a challenging scenario because of a delay in revascularization resulting in infarction of a large amount of the myocardium. We aimed to assess the clinical characteristics, angiographic profile, and predictors of outcome in patients with a delayed presentation after CS-STEMI.
Methods
A total of 147 patients with CS-STEMI with time to appropriate medical care ≥12 h after symptom onset were prospectively recruited at a tertiary referral center.
Results
The median time to appropriate care was 24 h (interquartile range 18–48 h). The mean age was 58.7 ± 11.1 years. Left ventricular pump failure was the leading cause of shock (67.3%), whereas mechanical complications accounted for 14.9% and right ventricular infarction for 13.6% of cases. The overall in-hospital mortality was 42.9%. Acute kidney injury [Odds ratio (OR) 8.04; 95% confidence intervals (CI) 3.08–20.92], ventricular tachycardia (OR 7.04; CI 2.09–23.63), mechanical complications (OR 6.46; CI 1.80–23.13), and anterior infarction (OR 3.18; CI 1.01–9.97) were independently associated with an increased risk of mortality. Coronary angiogram (56.5%) revealed single-vessel disease (45.8%) as the most common finding. Percutaneous coronary intervention was performed in 53 patients (36%), at a median of 36 h (interquartile range 30–72) after symptom onset.
Conclusion
Patients with a delayed presentation after CS-STEMI were younger and more likely to have single-vessel disease. We found a high in-hospital mortality of 42.9%. Appropriate randomized studies are required to evaluate the optimal treatment strategies in these patients.
Keywords: Cardiogenic shock, STEMI, Mortality, PCI, Medical stabilization, Late presentation
1. Introduction
Approximately three-quarters of all global deaths due to cardiovascular diseases occur in low- and middle-income countries.1 Although the coronary artery disease (CAD)–related mortality has declined over the past few decades, the overall incidence of acute coronary syndrome (ACS) has increased significantly, owing to a multitude of factors, especially in less developed countries.2 A significant difference in the presentation of ACS in developing countries of Asia, Africa, and Latin America as compared with the developed countries is the delay in seeking medical care as a result of lack of awareness among patients and inadequate medical facilities.3, 4, 5
In spite of a considerable improvement in the outcome of patients with ACS globally, the mortality of those who present with cardiogenic shock (CS) remains as high as 40–60% even in the developed countries.6, 7, 8 While the randomized Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock (SHOCK) trial established a benefit of early revascularization compared with initial medical stabilization in patients with CS, a large proportion of the cohort presented early after symptom onset, with a median time to revascularization of 11 h from symptom onset and 5 h from the onset of shock.7 A delayed presentation after ST-elevation myocardial infarction (STEMI) is associated with extensive myocardial necrosis and worse outcomes.9 The occurrence of CS with end-organ hypoperfusion further impairs the prognosis. There is lack of data regarding patients presenting late after symptom onset (≥12 h) with ST-elevation myocardial infarction and complicated by cardiogenic shock (CS-STEMI), and the optimal treatment strategies in these patients remain poorly defined. Given the large number of patients in low- and middle-income countries with this profile, it is important to determine the optimum management strategy to improve their outcomes.
2. Methods
2.1. Study design
This was a prospective, nonrandomized, single-centre, observational study of consecutive patients with CS-STEMI presenting to a tertiary care hospital. We aimed to evaluate the etiology of shock, clinical and angiographic profiles, outcome, and predictors of outcomes in patients presenting late with CS-STEMI. The study protocol conforms to the ethical guidelines of the Declaration of Helsinki and was reviewed and cleared by the Ethics Committee of the Post Graduate Institute of Medical Education & Research, Chandigarh. Informed written consent was obtained from all patients or appropriate legally authorized representatives.
2.2. Patient selection
The study enrolled patients over a period of three years (July 2015 to May 2018) with a diagnosis of STEMI presenting ≥12 h after symptom onset and complicated by CS at admission. All patients had time to appropriate medical care (defined as treatment with antiplatelets, heparin, statins, and/or revascularisation) ≥12 h after symptom onset. STEMI was defined as per the European Society of Cardiology (ESC)/American College of Cardiology Foundation (ACCF)/American Heart Association (AHA)/World Heart Federation (WHF) third universal definition.10 CS was defined as a systolic blood pressure <90 mm Hg for at least 30 min or the need of supportive measures to maintain a systolic blood pressure ≥90 mm Hg despite adequate filling pressures and signs of end-organ hypoperfusion.7,11 Two-dimensional echocardiography (Vivid q; GE Healthcare™, New York, USA) was performed to assess the left ventricular (LV) ejection fraction (LVEF) and associated mechanical complications. The severity of mitral regurgitation (MR) was classified in accordance with the established criteria.12 Acute kidney injury was defined as described in the Kidney Disease: Improving Global Outcomes (KDIGO) 2012 practice guideline.13
2.3. Management strategy
Time to revascularization in STEMI is an important determinant of outcome, and a delay is associated with a lesser extent of salvageable myocardium and poor outcomes.14 A strategy of initial stabilization with inotropes, mechanical ventilation, and/or intraaortic balloon pump (IABP) followed by delayed revascularization before hospital discharge was followed. Treatment decisions including the choice of inotropic drugs, use of IABP, and timing of revascularization were left to the discretion of the treating physician. The decision and timing of revascularization were influenced by several factors including the delay from symptom onset, presence of ongoing pain or electrical instability, hemodynamic parameters, end-organ failure, viability of myocardium supplied by infarct-related artery (as assessed by nuclear perfusion imaging), mechanical complications, and patient consenting to undergo revascularization. Patients were treated with aspirin, clopidogrel (or ticagrelor), atorvastatin, and low-molecular-weight heparin (enoxaparin). Femoral artery was the preferred vascular access for patients undergoing a coronary angiogram. Coronary flow was described as per the classification defined by Thrombolysis in Myocardial Infarction (TIMI) study group.15 The default revascularization strategy was culprit vessel percutaneous coronary intervention (PCI), with nonculprit vessels addressed during follow-up. The angiographic profile of each patient was analyzed by two physicians, both of whom were unaware of the patient outcomes. More than 70% stenosis of the left anterior descending artery (LAD), right coronary artery (RCA), left circumflex artery (LCx), and more than 50% stenosis of the left main coronary artery (LMCA) were considered significant. Patients were monitored throughout the duration of hospitalization to assess for outcomes. The primary end-point of the study was in-hospital mortality.
2.4. Statistical analysis
All data were prospectively collected by trained physicians (four authors of the study) and entered into a spreadsheet (Microsoft Excel 2016™; Microsoft Corporation, USA). Statistical analysis was performed using the Statistical package for social sciences (SPSS Inc., version 23.0™; IBM Corporation, Chicago, USA). All continuous variables were summarized as mean ± standard deeviation or median [interquartile range (IQ)]. Categorical variables were described as proportions and frequencies (%). The comparison between two groups for continuous variables was performed by using the Student's t-test or Wilcoxon–Mann–Whitney test. The comparison between two categorical variables was performed by using the chi-square test or Fisher exact test. Subsequently, variables with p ≤ 0.10 on univariate analysis were included in a multivariable regression analysis to identify independent predictors of outcome. All p values are two-tailed and set at a statistical significance of 0.05.
3. Results
3.1. Baseline clinical characteristics
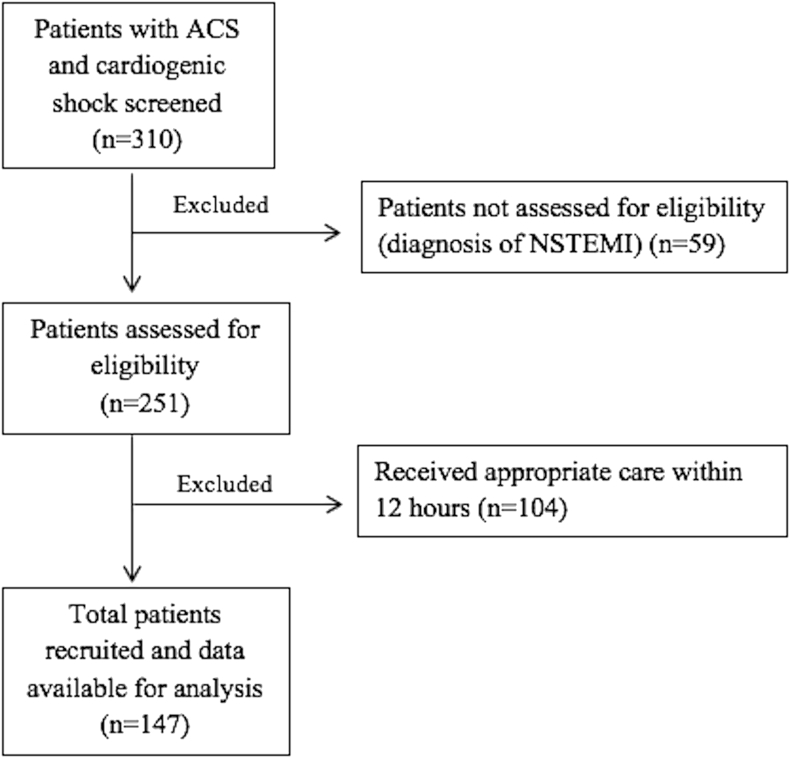
A total of 147 patients were included (Fig. 1). The mean age was 58.7 ± 11.1 years. The location of infarction was anterior in 98 patients (66.6%), followed by inferior in 44 (29.9%), whereas 5 patients (3.4%) had lateral or posterior infarction. The median time to appropriate medical care was 24 h (IQ 18–48), and 101 patients (68.7%) presented ≥24 h after symptom onset. Pump failure due to LV dysfunction was the commonest cause of shock, present in 99 patients (67.3%). Twenty-two patients (14.9%) had mechanical complications as the cause of shock (Table 1). Complete heart block was noted in 31 patients (21.1%), of which 17 (54.8%) had inferior infarction and 14 (45.2%) had anterior infarction.
Fig. 1.
STROBE diagram of patients in the study. ACS = acute coronary syndrome; NSTEMI = Non-ST-elevation myocardial infarction; STROBE = strengthening the reporting of observational studies in epidemiology.
Table 1.
Clinical characteristics of the study population.
| Characteristic | n = 147 | |
|---|---|---|
| Age, years, mean ± SD | 58.7 ± 11.1 | |
| Sex, n (%) | Male, n (%) | 104 (70.7) |
| Female, n (%) | 43 (29.3) | |
| Risk factors, n (%) | ||
| Diabetes mellitus | 62 (42.2) | |
| Hypertension | 63 (42.9) | |
| Smoking | 65 (44.2) | |
| Alcohol consumption | 27 (18.4) | |
| Family history of CAD | 11 (7.5) | |
| History of prior MI | 18 (12.2) | |
| Prior PCI/CABG | 6 (4.1) | |
| Timeline, hours, median (IQ) | ||
| Time to appropriate medical care | 24 (18–48) | |
| Time to admission | 28 (24–48) | |
| Time to fibrinolysisa | 13 (12–21) | |
| Duration of hospital stay | 144 (48–264) | |
| Myocardial territory involved, n (%) | ||
| Anterior | 98 (66.6) | |
| Inferior | 44 (29.9) | |
| Posterior | 4 (2.7) | |
| Lateral | 1 (0.7) | |
| LV ejection fraction, %, mean ± SD | 29.2 ± 9.0 | |
| Acute kidney injury, n (%) | 86 (58.5) | |
| Fibrinolysis, n (%) | 33 (22.4) | |
| Etiology of Shock, n (%) | ||
| Pump Failure | 99 (67.3) | |
| Ventricular septal rupture | 6 (4.1) | |
| Severe mitral regurgitation | 13 (8.8) | |
| LV rupture/tamponade | 3 (2) | |
| Right ventricular infarction | 20 (13.6) | |
| Patients who underwent a coronary angiogram, n (%) | 83 (56.5) | |
| Pattern of vessel involvement, n (%)b | ||
| Left main disease | 5 (6%) | |
| Single-vessel disease | 38 (45.8%) | |
| Two-vessel disease | 26 (31.3%) | |
| Three-vessel disease | 18 (21.7%) | |
| Coronary artery involvement, n (%)b | ||
| LAD | 61 (73.5%) | |
| RCA | 45 (54.2%) | |
| LCX | 38 (45.8%) | |
| Culprit vessel, n (%)b | ||
| LAD | 46 (55.4%) | |
| RCA | 26 (31.3%) | |
| LCX | 10 (12%) | |
| Occlusion of culprit vessel, n (%)b | 39 (46.9%) | |
| Occlusion of nonculprit vessel (CTO), n (%)b | 9 (10.8%) | |
| GP IIb/IIIa inhibitor, n (%)b | 22 (26.5%) | |
| Patients undergoing PCI, n | 53 | |
| Time to PCI, hours, median (IQ)c | 36 (30–72) | |
| Stent placement, n (%)c | 49 (92.4%) | |
| TIMI 3 flow, n (%)c | 46 (86.8%) | |
Occlusion of vessel was defined as TIMI flow 0/1.
CABG = coronary artery bypass grafting; CAD = coronary artery disease; CTO = chronic total occlusion; IQ = interquartile range; LAD = left anterior descending coronary artery; LCX = left circumflex coronary artery; LV = left ventricle; MI = myocardial infarction; PCI = percutaneous coronary intervention; RCA = right coronary artery; SD = standard deviation; TIMI = thrombolysis in myocardial infarction.
The bold values represent a subset of patients who underwent an angiogram. The angiographic findings labelled as ‘b’ in the table are based on these patients.
Values are based on 33 patients who received fibrinolysis.
Values are based on 83 patients who underwent an angiogram.
Values are based on 53 patients who underwent PCI.
3.2. Angiographic profile
Of the 147 patients, 41 (27.9%) died before a coronary angiogram could be perfomed and 23 (15.6%) did not consent for an angiogram. Single-vessel disease was seen in 38 patients (45.8%), two-vessel disease in 26 (31.3%), and three-vessel disease in 18 (21.7%). Of these 83 patients, 30 (36.1%) had nonviable myocardium supplied by the infarct-related artery (as assessed by nuclear perfusion imaging) and did not undergo revascularization, whereas the remaining 53 patients (63.9%) underwent PCI at a median of 36 h (IQ 30–72) after symptom onset (Table 1).
3.3. Outcome and mortality predictors
Sixty-three patients (42.9%) died during initial hospitalization, of whom 20 (13.6%) died in the initial 24 hours. Nonsurvivors were significantly older compared with survivors. The nonsurvivors had a higher occurrence of anterior infarction, ventricular tachycardia (VT), acute kidney injury, mechanical complications, and a lower mean LVEF (Table 2). The nonsurvivors had a higher mean total leukocyte count (15534 ± 5301 vs 12427 ± 3630 cells/mm3; p < 0.001), serum creatinine (2.03 ± 1.26 vs 1.29 ± 0.72 mg/dl; p < 0.001), serum urea (78.9 ± 50.1 vs 58.3 ± 31.1 mg/dl; p = 0.003), alanine aminotransferase [ALT 136 (70–614) vs 85 (48–175) U/L; p = 0.018], aspartate aminotransferase [AST 270 (98–748) vs 89 (56–243) U/L; p = 0.001], and lower mean albumin (3.05 ± 0.6 vs 3.34 ± 0.48 gm/dl; p = 0.007) compared with survivors (Supplementary Table S1).
Table 2.
Comparison of characteristics between survivors and nonsurvivors.
| Characteristic | Survivors (n = 84) | Nonsurvivors (n = 63) | p value | ||
|---|---|---|---|---|---|
| Age, years, mean ± SD | 57.1 ± 9.6 | 61.0 ± 12.5 | 0.034 | ||
| Sex, n (%) | Male | 63 (75) | 41 (65.1) | 0.191 | |
| Female | 21 (25) | 22 (34.9) | |||
| Risk factors, n (%) | |||||
| Diabetes mellitus | 36 (42.9) | 26 (41.3) | 0.847 | ||
| Hypertension | 34 (40.5) | 29 (46.0) | 0.501 | ||
| Smoking | 38 (45.2) | 27 (42.9) | 0.774 | ||
| Alcohol | 21 (25.0) | 6 (9.5) | 0.016 | ||
| Family history of CAD | 4 (4.8) | 7 (11.1) | 0.206 | ||
| Prior MI | 13 (15.5) | 5 (7.9) | 0.168 | ||
| Prior PCI/CABG | 3 (3.6) | 3 (4.8) | 1.00 | ||
| Timeline, hours, median (IQ) | |||||
| Time to AMC | 24 (18–48) | 24 (18–48) | 0.624 | ||
| Time to admission | 29 (24–48) | 24 (24–48) | 0.790 | ||
| Time to fibrinolysisa | 13 (12–16) | 13 (12–23) | 0.258 | ||
| Time to PCIb | 36 (30–72) | 34 (23–38) | 0.242 | ||
| Anterior infarction, n (%) | 48 (57.1) | 50 (79.4) | 0.005 | ||
| LBBB, n (%) | 1 (1.2) | 4 (6.3) | 0.165 | ||
| Arrhythmias, n (%) | CHB | 17 (20.2) | 14 (22.2) | 0.770 | |
| VT/VF | 6 (7.1) | 21 (33.3) | <0.001 | ||
| AF | 1 (1.2) | 4 (6.3) | 0.165 | ||
| LV Ejection fraction, %, mean ± SD | 30.9 ± 8.5 | 26.8 ± 9.2 | 0.006 | ||
| Mechanical complications, n (%) | 7 (8.3) | 15 (23.8) | 0.009 | ||
| VSR | 1 (1.2) | 5 (7.9) | – | ||
| LV rupture/tamponade | 0 (0) | 3 (4.8) | – | ||
| Acute severe MR | 6 (7.1) | 7 (11.1) | – | ||
| Acute kidney injury, n (%) | 33 (39.3) | 53 (84.2) | <0.001 | ||
| Fibrinolysis, n (%) | 19 (22.6) | 14 (22.2) | 0.954 | ||
| GP IIb/IIIa inhibitor, n (%)c | 15 (24.6) | 7 (31.8) | 0.510 | ||
| IABP, n (%)c | 9 (10.7) | 10 (15.9) | 0.356 | ||
| Pattern of vessel involvement, n (%)c | |||||
| Left Main disease | 4 (6.55) | 1 (4.54) | 1.00 | ||
| Single-vessel disease | 29 (47.5) | 9 (40.9) | 0.592 | ||
| Two-vessel disease | 20 (32.8) | 6 (27.3) | 0.633 | ||
| Three-vessel disease | 11 (18.0) | 7 (31.8) | 0.179 | ||
| Coronary artery involvement, n (%)c | |||||
| LAD | 43 (70.5) | 18 (81.8) | 0.302 | ||
| RCA | 33 (54.1) | 12 (54.5) | 0.971 | ||
| LCX | 27 (44.3) | 11 (50) | 0.643 | ||
AF = atrial fibrillation; AMC = appropriate medical contact; CABG = coronary artery bypass grafting; CAD = coronary artery disease; CHB = complete heart block; IABP = intraaortic balloon pump; IQ = interquartile range; LAD = left anterior descending coronary artery; LBBB = left bundle branch block; LCX = left circumflex coronary artery; LV = left ventricle; MI = myocardial infarction; MR = mitral regurgitation; PCI = percutaneous coronary intervention; RCA = right coronary artery; SD = standard deviation; VSR = ventricular septal rupture; VT/VF = ventricular tachycardia/fibrillation.
Values are based on 19 survivors and 14 nonsurvivors who received fibrinolysis.
Values are based on 38 survivors and 15 nonsurvivors who underwent PCI.
Values are based on 61 survivors and 22 nonsurvivors who underwent an angiogram.
Patients with mechanical complications had a median time to appropriate medical care of 34 h (IQ 23–48) after symptom onset. Apart from being older (64.7 ± 8.44 vs 57.75 ± 11.24 years; p = 0.006), patients with mechanical complications did not differ significantly from those without them. The IABP was the only circulatory assist device used because of nonavailability of left ventricular assist devices (LVADs) and limited access to extracorporeal membrane oxygenation (ECMO) along with its attendant costs.
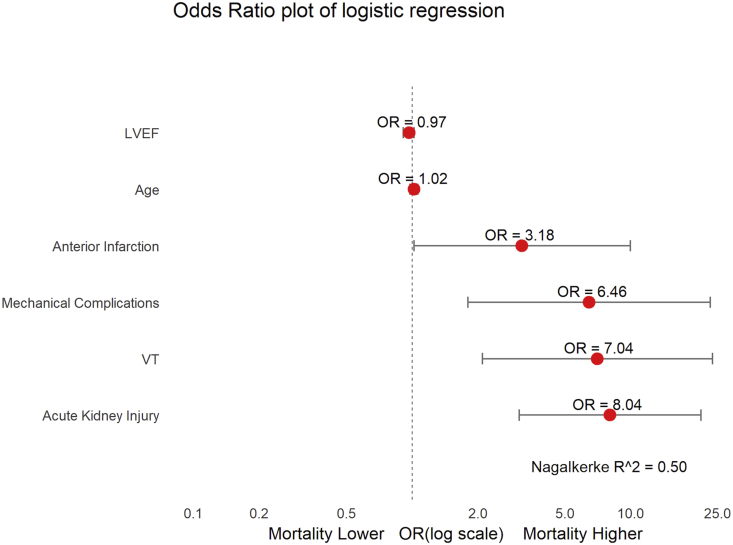
A binary logistic regression was performed to determine the effects of age, ejection fraction, location of myocardial infarction, presence of mechanical complications, VT, and acute kidney injury on in-hospital mortality. Acute kidney injury [Odds ratio (OR) = 8.04; 95% confidence interval (CI): 3.08–20.92], VT (OR = 7.04; CI: 2.09–23.63), presence of mechanical complications (OR = 6.46; CI: 1.80–23.13), and anterior infarction (OR = 3.18; CI: 1.01–9.97) were independently associated with an increased risk of mortality after adjusting for the covariates (Fig. 2 and Table 3).
Fig. 2.
Odds ratio and 95% confidence intervals for in-hospital mortality in patients with STEMI in cardiogenic shock. Mortality was predicted by acute kidney injury, ventricular tachycardia, mechanical complications, and anterior infarction. LVEF = left ventricular ejection fraction; VT = ventricular tachycardia. Mechanical complications include a composite of acute severe mitral regurgitation, ventricular septal rupture, and left ventricular rupture/tamponade.
Table 3.
Logistic regression analysis.
| B | S.E. | Wald | df | Sig. | Exp(B) | 95% CI for EXP(B) |
|||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||
| Step 1 | Age | 0.019 | 0.021 | 0.867 | 1 | 0.352 | 1.020 | 0.979 | 1.062 |
| Anterior infarction | 1.158 | 0.582 | 3.956 | 1 | 0.047 | 3.184 | 1.017 | 9.972 | |
| Mechanical complications | 1.865 | 0.651 | 8.202 | 1 | 0.004 | 6.456 | 1.802 | 23.136 | |
| VT | 1.951 | 0.618 | 9.967 | 1 | 0.002 | 7.038 | 2.096 | 23.636 | |
| Ejection fraction | −0.034 | 0.029 | 1.352 | 1 | 0.245 | 0.966 | 0.912 | 1.024 | |
| AKI | 2.084 | 0.488 | 18.216 | 1 | 0.000 | 8.037 | 3.086 | 20.927 | |
| Constant | −3.086 | 1.751 | 3.106 | 1 | 0.078 | 0.046 | |||
Variable(s) entered on step 1: age, alcohol, anterior infarction, mechanical complications, VT, ejection fraction, AKI.
AKI = acute kidney injury; B = Unstandardized regression weight; D.F = Degree of freedom; Exp (B) = exponentiation of the B coefficient, which is the Odds ratio; S.E. = This is how much the unstandardized regression weight can vary by; Sig = The statistical significance of the test; VT = ventricular tachycardia; Wald = Statistical significance for each of the independent variables.
Linearity of the continuous variables with respect to the logit of the dependent variable was analyzed using the Box-Tidwell procedure. A Bonferroni correction was applied using all 9 elements in the model resulting in statistical significance being accepted when p < 0.006. Based on this assessment, all continuous independent variables were found to be linearly related to the logit of the dependent variable. The logistic regression model was statistically significant [χ2(4) = 67.96, p < 0.0005]. The model explained 50% (Nagelkerke R2) of the variance in in-hospital mortality and correctly classified 80.8% of cases.
4. Discussion
This is one of the largest prospective studies of patients with CS-STEMI presenting late after symptom onset. Late presentation is a common problem in low- and middle-income countries and adversely affects the patient outcomes.3, 4, 5,16,17 Because the extent of salvageable myocardium declines with each passing hour, the benefits of revascularization may be limited while being associated with considerable risks in these patients with end-organ dysfunction. The class IB recommendation for emergency revascularization of the ESC and AHA is based on the SHOCK trial, wherein patients underwent revascularization early after symptom onset.7,18,19 It is thus important to ascertain the optimum management in late-presenting patients to help improve outcomes.
In this study, 147 of 251 (58.6%) patients presenting with CS-STEMI received appropriate care ≥12 h after symptom onset and over two-thirds of these presented ≥24 h after symptom onset. This is in stark contrast to the SHOCK registry, where the median time to presentation was 1.25 h.20,21 The age at presentation was a decade lower than that in the western literature.7,21 Although older patients had worse outcomes, age was not an independent predictor of mortality in multivariable analysis, similar to the some of the published studies.22,23 Several studies24,25 have identified advanced age (>75 years) as an independent predictor of mortality in CS-STEMI. However, the younger age of presentation of our cohort and the small number of patients with age >75 years may have led to the contrasting results in the present study. The low rates of previous myocardial infarction (MI), three-vessel disease (21.7%), and left main disease (6%) are in stark contrast to the published data.6,7 These differences reflect the late presentation of patients in our study. In our cohort, patients with single-vessel disease undergo considerable infarction with severe reduction in LV function leading to shock. In contrast, in developed countries, patients with single-vessel disease undergo revascularization early, have a lower likelihood of developing shock, and are underrepresented, whereas patients with multivessel disease or prior MI develop shock even with a small infarction and are more represented.6,7 Hence, the extent of viable myocardium in late-presenting patients is uncertain, as seen in the present study.
The in-hospital mortality in this study is similar to the published literature6,8,21,26 and may be a consequence of late presentation of these patients and limited use of mechanical circulatory support devices. The delay in seeking medical care leads to infarction of a significant amount of myocardium and attendant end-organ dysfunction, which contributes to the adverse outcomes. The occurrence of mechanical complications further worsens the prognosis of these late-presenting patients. However, a much younger age of presentation of patients and hence, the lower extent of CAD along with a male preponderance may have resulted in a somewhat lower in-hospital mortality compared with the contemporary data of mortality exceeding 50% across many centers, especially in the developing countries.
Table 4 highlights the current and previously published data on late-onset CS-STEMI. As compared with the subgroup of patients with late shock in the SHOCK registry,20 our study cohort was younger (mean age 58.7 vs 69.6 years), had fewer patients who had a prior history of infarction or revascularization, had a much lower prevalence of multivessel and left main disease, and had lower use of mechanical circulatory support devices. Acute kidney injury, the occurrence of VT, presence of mechanical complications (ventricular septal rupture [VSR]/acute severe MR/LV rupture/tamponade), and anterior infarction were significant predictors of mortality after adjusting for covariates in our study. Acute kidney injury has previously been recognized as one of the most important predictors of outcomes in CS-STEMI.6,28 Acute kidney injury was seen in much high proportion of patients compared with the data from developed countries,6,7 reflecting the late presentation and subsequent end-organ hypoperfusion. Anterior MI has also been shown to be associated with pump failure, late-onset CS, and a higher mortality.21,30,34 The presence of mechanical complications, particularly VSR, was associated with a high mortality, similar to the published literature.21 Although the outcomes of VSR and free wall rupture have improved in developed countries, they continue to remain dismal in developing countries.35, 36, 37 In contrast to several studies,27,30,38 LVEF was not an independent predictor of mortality. Because our study included patients with mechanical complications, the wide variation in LVEF may have led to these results, in contrast to most studies7,27,38 which have only included patients with CS secondary to pump failure.
Table 4.
Comparison of current and published literature that addressed late cardiogenic shock.
| Study | No. of patients | Predictors of developing shock | Mortality predictors | Remarks |
|---|---|---|---|---|
| Hands et al27 | 60 | Age >65 years, previous MI, LVEF <35%, CK-MB > 160 IU/L, DM | LVEF and wall motion abnormality | Median time, 76 h. Mortality, 65% |
| Mavric et al28 | 17 | Age, previous MI, lactate, urea, cardiothoracic ratio | – | – |
| Leor et al29 | 89 | Age, female, history of angina, prior stroke, PVD, peak LDH >4 × normal, hyperglycemia | – | Mortality, 97% |
| Webb et al20 | 211 | LAD culprit vessel, multiple Q waves | – | Median time, 51 h. Mortality, 53.6% |
| Obling et al30 | 64 | Age, prior stroke, time to PCI, higher SI, anterior MI, LVEF, LMCA or LAD culprit, multivessel disease | Age, LVEF, PVD, anterior MI, time to intervention | Mortality, 47% |
| Luca G et al31 | 71 | Killip class>1, age, anterior MI, DM, unsuccessful PCI | – | Mortality, 71.8% |
| Lindholm et al32 | 132 | Age, female gender, DM, prior MI, anterior MI, BBB. | – | Mortality, 87% |
| Valente et al33 | 22 | Single-vessel disease, PCI failure | Age, PCI failure, hyperglycemia, mechanical complications. | Mortality, 45.4% |
| Current study | 147 | – | Anterior MI, renal dysfunction, mechanical complications, ventricular tachycardia | Mortality, 42.9% |
BBB = bundle branch block; CK-MB = creatine kinase-MB; CS = cardiogenic shock; LAD = left anterior descending coronary artery; DM = diabetes mellitus; LDH = lactate dehydrogenase; LMCA = left main coronary artery; LVEF = left ventricular ejection fraction; MI = myocardial infarction; PCI = percutaneous coronary intervention; PVD = peripheral vascular disease; SI = Stroke Index.
Improvements in regional STEMI network, including patient and primary physician education, adequate government sponsored insurance coverage and cashless facilities, availability of private and public ambulances, prehospital low-cost high-definition ECGs at first medical contact, and data tele-transportation may help to improve the outcome of patients with STEMI.39 Regional STEMI networks, analogous to those in the developed countries need to be created in low- and middle-income countries. Legislative changes, such as transportation of patients by ambulances to 24 h/7 days a week PCI-equipped centers, prehospital management, emergency department bypass, and single page activation need to be enforced to improve the patient outcomes. Careful monitoring and audit of hospital data and dissemination of STEMI data to the community may lead to earlier presentation and higher rates of primary PCI in STEMI.39,40
The merits of this study include a prospective assessment of the outcome of patients presenting late with CS-STEMI, which is an understudied cohort and a frequent entity in low- and middle-income countries. The study provides real-world data from a developing country, which differ significantly from the developed countries. There are several limitations to the study. This was a single-center, single-arm, nonrandomized, observational study. Hence, all the limitations of a nonrandomized sample apply to our study. In the absence of a comparator group, the use of various treatment approaches remains obscure. In addition, the treatment strategies in our study are quite heterogeneous because of the variabilities in patient presentation and associated end-organ dysfunction. A coronary angiogram was performed in just over half of the cohort, hence, the coronary anatomy is unknown in the other half. Hence, the SYNTAX score, which has been shown to be an independent prognostic marker in CS-STEMI,41,42 was not assessed in the present study. Because just over a third of our patients underwent revascularization, the impact of revascularization strategies remains unknown. The nonavailability of LVADs at our centre (and at most centers in our country) and the limited availability of ECMO because of financial constraints may have contributed to the high in-hospital mortality. In addition, follow-up data are not available to assess the long-term outcomes and cardiac, noncardiac, and all-cause mortality.
To conclude, late presentation after STEMI is a common problem in less developed countries, and more than two-thirds of patients in this study received appropriate care for STEMI only after a delay of more than 24 h after symptom onset with a high in-hospital mortality rate of 42.9%. Given that late presentation is associated with the drawbacks of more myocardial necrosis, lower extent of salvageable myocardium, and higher incidence of end-organ dysfunction, these risks of revascularization may be higher and the optimal management strategy in this cohort of patients is unclear. As this is a considerable and rapidly growing problem in the less developed countries, establishing the best management strategy in this cohort of patients with appropriate randomized studies is important.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
All authors have none to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ihj.2019.11.256.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.WHO . Cardiovascular Diseases (CVDs). Fact sheet – reviewed June. 2016.
- 2.Gupta R., Gupta V.P. Meta-analysis of coronary heart disease prevalence in India. Indian Heart J. 1996;48:241–245. [PubMed] [Google Scholar]
- 3.Kakou-Guikahue M., N'Guetta R., Anzouan-Kacou J.-B. Optimizing the management of acute coronary syndromes in sub-Saharan Africa: a statement from the AFRICARDIO 2015 Consensus Team. Arch Cardiovasc Dis. 2016;109:376–383. doi: 10.1016/j.acvd.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Martínez-Sánchez C., Jerjes-Sánchez C., Nicolau J.C. [Acute coronary syndromes in Latin America: lessons from the ACCESS registry] Rev Medica Inst Mex Seguro Soc. 2016;54:726–737. [PubMed] [Google Scholar]
- 5.Guha S., Sethi R., Ray S. Cardiological Society of India: position statement for the management of ST elevation myocardial infarction in India. Indian Heart J. 2017;69:S63–S97. doi: 10.1016/j.ihj.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Backhaus T., Fach A., Schmucker J. Management and predictors of outcome in unselected patients with cardiogenic shock complicating acute ST-segment elevation myocardial infarction: results from the Bremen STEMI Registry. Clin Res Cardiol Off J Ger Card Soc. 2018;107:371–379. doi: 10.1007/s00392-017-1192-0. [DOI] [PubMed] [Google Scholar]
- 7.Hochman J.S., Sleeper L.A., Webb J.G. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl J Med. 1999;341:625–634. doi: 10.1056/NEJM199908263410901. [DOI] [PubMed] [Google Scholar]
- 8.Kunadian V., Qiu W., Ludman P. Outcomes in patients with cardiogenic shock following percutaneous coronary intervention in the contemporary era: an analysis from the BCIS database (British cardiovascular intervention society) JACC Cardiovasc Interv. 2014;7:1374–1385. doi: 10.1016/j.jcin.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 9.Cerrato E., Forno D., Ferro S. Characteristics, in-hospital management and outcome of late acute ST-elevation myocardial infarction presenters. J Cardiovasc Med Hagerstown Md. 2017;18:567–571. doi: 10.2459/JCM.0000000000000527. [DOI] [PubMed] [Google Scholar]
- 10.Thygesen K., Alpert J.S., Jaffe A.S. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. doi: 10.1161/CIR.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]
- 11.Holmes D.R., Bates E.R., Kleiman N.S. Contemporary reperfusion therapy for cardiogenic shock: the GUSTO-I trial experience. J Am Coll Cardiol. 1995;26:668–674. doi: 10.1016/0735-1097(95)00215-p. [DOI] [PubMed] [Google Scholar]
- 12.Nishimura R.A., Otto C.M., Bonow R.O. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American college of Cardiology/American heart association task force on practice guidelines. Circulation. 2014;129 doi: 10.1161/CIR.0000000000000031. e521–643. [DOI] [PubMed] [Google Scholar]
- 13.Kidney Disease: Improving Global Outcomes (KDIGO) Acute kidney injury work group KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 14.Scholz K.H., Maier S.K.G., Maier L.S. Impact of treatment delay on mortality in ST-segment elevation myocardial infarction (STEMI) patients presenting with and without haemodynamic instability: results from the German prospective, multicentre FITT-STEMI trial. Eur Heart J. 2018;39:1065–1074. doi: 10.1093/eurheartj/ehy004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.TIMI Study Group The Thrombolysis in myocardial infarction (TIMI) trial. Phase I findings. N Engl J Med. 1985;312:932–936. doi: 10.1056/NEJM198504043121437. [DOI] [PubMed] [Google Scholar]
- 16.Mohanan P.P., Mathew R., Harikrishnan S. Presentation, management, and outcomes of 25 748 acute coronary syndrome admissions in Kerala, India: results from the Kerala ACS Registry. Eur Heart J. 2013;34:121–129. doi: 10.1093/eurheartj/ehs219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xavier D., Pais P., Devereaux P.J. Treatment and outcomes of acute coronary syndromes in India (CREATE): a prospective analysis of registry data. Lancet Lond Engl. 2008;371:1435–1442. doi: 10.1016/S0140-6736(08)60623-6. [DOI] [PubMed] [Google Scholar]
- 18.O'Gara P.T., Kushner F.G., Ascheim D.D. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American college of Cardiology foundation/American heart association task force on practice guidelines. Circulation. 2012 doi: 10.1161/CIR.0b013e3182742cf6. CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 19.Ibanez B., James S., Agewall S. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 20.Webb J.G., Sleeper L.A., Buller C.E. Implications of the timing of onset of cardiogenic shock after acute myocardial infarction: a report from the SHOCK Trial Registry. J Am Coll Cardiol. 2000;36:1084–1090. doi: 10.1016/s0735-1097(00)00876-7. [DOI] [PubMed] [Google Scholar]
- 21.Hochman J.S., Buller C.E., Sleeper L.A. Cardiogenic shock complicating acute myocardial infarction–etiologies, management and outcome: a report from the SHOCK Trial Registry. Should we emergently revascularize occluded coronaries for cardiogenic shock? J Am Coll Cardiol. 2000;36:1063–1070. doi: 10.1016/s0735-1097(00)00879-2. [DOI] [PubMed] [Google Scholar]
- 22.Acharya D. Predictors of outcomes in myocardial infarction and cardiogenic shock. Cardiol Rev. 2018;26:255. doi: 10.1097/CRD.0000000000000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayıroğlu M.İ., Keskin M., Uzun A.O. Predictors of in-hospital mortality in patients with ST-segment elevation myocardial infarction complicated with cardiogenic shock. Heart Lung Circ. 2019;28:237–244. doi: 10.1016/j.hlc.2017.10.023. [DOI] [PubMed] [Google Scholar]
- 24.Sutton A.G.C., Finn P., Hall J.A. Predictors of outcome after percutaneous treatment for cardiogenic shock. Heart Br Card Soc. 2005;91:339–344. doi: 10.1136/hrt.2003.021691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bilkova D., Motovska Z., Widimsky P. Shock index: a simple clinical parameter for quick mortality risk assessment in acute myocardial infarction. Can J Cardiol. 2011;27:739–742. doi: 10.1016/j.cjca.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Subban V., Gnanaraj A., Gomathi B. Percutaneous coronary intervention in cardiogenic shock complicating acute ST-elevation myocardial infarction—a single centre experience. Indian Heart J. 2012;64:152–158. doi: 10.1016/S0019-4832(12)60052-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hands M.E., Rutherford J.D., Muller J.E. The in-hospital development of cardiogenic shock after myocardial infarction: incidence, predictors of occurrence, outcome and prognostic factors. The MILIS Study Group. J Am Coll Cardiol. 1989;14:40–46. doi: 10.1016/0735-1097(89)90051-x. discussion 47. [DOI] [PubMed] [Google Scholar]
- 28.Mavrić Z., Zaputović L., Zagar D. Usefulness of blood lactate as a predictor of shock development in acute myocardial infarction. Am J Cardiol. 1991;67:565–568. doi: 10.1016/0002-9149(91)90892-o. [DOI] [PubMed] [Google Scholar]
- 29.Leor J., Goldbourt U., Reicher-Reiss H. Cardiogenic shock complicating acute myocardial infarction in patients without heart failure on admission: incidence, risk factors, and outcome. SPRINT Study Group. Am J Med. 1993;94:265–273. doi: 10.1016/0002-9343(93)90058-w. [DOI] [PubMed] [Google Scholar]
- 30.Obling L., Frydland M., Hansen R. Risk factors of late cardiogenic shock and mortality in ST-segment elevation myocardial infarction patients. Eur Heart J Acute Cardiovasc Care. 2018;7:7–15. doi: 10.1177/2048872617706503. [DOI] [PubMed] [Google Scholar]
- 31.De Luca G., Savonitto S., Greco C. Cardiogenic shock developing in the coronary care unit in patients with ST-elevation myocardial infarction. J Cardiovasc Med. 2008;9:1023–1029. doi: 10.2459/JCM.0b013e328304ae7f. [DOI] [PubMed] [Google Scholar]
- 32.Lindholm M.G., Køber L., Boesgaard S. Cardiogenic shock complicating acute myocardial infarction; prognostic impact of early and late shock development. Eur Heart J. 2003;24:258–265. doi: 10.1016/s0195-668x(02)00429-3. [DOI] [PubMed] [Google Scholar]
- 33.Valente S., Lazzeri C., Chiostri M. Time of onset and outcome of cardiogenic shock in acute coronary syndromes. J Cardiovasc Med. 2008;9:1235–1240. doi: 10.2459/JCM.0b013e3283168a27. [DOI] [PubMed] [Google Scholar]
- 34.Klein L.W., Shaw R.E., Krone R.J. Mortality after emergent percutaneous coronary intervention in cardiogenic shock secondary to acute myocardial infarction and usefulness of a mortality prediction model. Am J Cardiol. 2005;96:35–41. doi: 10.1016/j.amjcard.2005.02.040. [DOI] [PubMed] [Google Scholar]
- 35.Arnaoutakis G.J., Zhao Y., George T.J. Surgical repair of ventricular septal defect after myocardial infarction: outcomes from the society of thoracic surgeons national database. Ann Thorac Surg. 2012;94:436–444. doi: 10.1016/j.athoracsur.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malhotra A., Patel K., Sharma P. Techniques, timing & prognosis of post infarct ventricular septal repair: a Re-look at old dogmas. Braz J Cardiovasc Surg. 2017;32:147–155. doi: 10.21470/1678-9741-2016-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopez-Sendon J., Gonzalez A., Lopez De Sa E. Diagnosis of subacute ventricular wall rupture after acute myocardial infarction: sensitivity and specificity of clinical, hemodynamic and echocardiographic criteria. J Am Coll Cardiol. 1992;19:1145–1149. doi: 10.1016/0735-1097(92)90315-e. [DOI] [PubMed] [Google Scholar]
- 38.Picard M.H., Davidoff R., Sleeper L.A. Echocardiographic predictors of survival and response to early revascularization in cardiogenic shock. Circulation. 2003;107:279–284. doi: 10.1161/01.cir.0000045667.11911.f6. [DOI] [PubMed] [Google Scholar]
- 39.Mehta S., Granger C., Grines C.L. Confronting system barriers for ST- elevation MI in low and middle income countries with a focus on India. Indian Heart J. 2018;70:185–190. doi: 10.1016/j.ihj.2017.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herlitz J., Blohm M., Hartford M. Follow-up of a 1-year media campaign on delay times and ambulance use in suspected acute myocardial infarction. Eur Heart J. 1992;13:171–177. doi: 10.1093/oxfordjournals.eurheartj.a060142. [DOI] [PubMed] [Google Scholar]
- 41.Lin M.-J., Chen C.-Y., Lin H.-D. Prognostic analysis for cardiogenic shock in patients with acute myocardial infarction receiving percutaneous coronary intervention. BioMed Res Int. 2017;2017:8530539. doi: 10.1155/2017/8530539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hayıroğlu M.İ., Keskin M., Uzun A.O. Predictive value of SYNTAX score II for clinical outcomes in cardiogenic shock underwent primary percutaneous coronary intervention; a pilot study. Int J Cardiovasc Imaging. 2018;34:329–336. doi: 10.1007/s10554-017-1241-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.