Abstract
Elemental impurities in drug samples can generate unwanted pharmacological–toxicological effects, therefore they must be carefully monitored. In order to update the elemental analysis of pharmaceutical products, new regulations for elemental impurities were published by the United States Pharmacopoeia (USP). This work presents elemental analysis of 23 analytes in omeprazole drug samples from seven different commercial brands considering reference, similar and generic medicines using inductively coupled plasma mass spectrometry (ICP-MS). Microwave-assisted digestion using 2.0 mol L−1 HNO3 (partial digestion) was applied successfully for omeprazole drugs. Most analytes were below the respective limits of quantification, except for As, Ba, Cd, Co, Cu, Cr, Li, Mo, Ni, Pb, Sb and V. However, the determined concentrations for these analytes were lower than the limits proposed by the USP Chapter 232 and similar for all products, inferring that for the seven analyzed samples there is no difference among reference, similar and generic drugs considering contaminants contents. Discussions considering potential risks of elemental contamination taking into account diverse brands were presented.
Keywords: Analytical chemistry, Pharmaceutical chemistry, Pharmacology, Elemental contamination, Generic drugs, USP, ICP-MS
Analytical chemistry; Pharmaceutical chemistry, Pharmacology, Elemental contamination; Generic drugs; USP; ICP-MS
1. Introduction
According to data published by the Brazilian Health Regulatory Agency in 2017 [1], the Brazilian pharmaceutical market generated approximately US$ 17.4 billion, which represents a growth of 9.4% compared to the previous year. Originally approved by the U.S. Food and Drug Administration (FDA) in 1989, omeprazole is an important drug, considered a "blockbuster", used to treat certain stomach diseases and esophagus problems, such as gastroesophageal reflux, peptic ulcer, and other diseases characterized by the over secretion of gastric acid [2]. In the ranking of the most commercially drugs in 2017, omeprazole is the 17th best seller [1].
In addition to reference medicines marketed in the Brazilian pharmaceutical sector, there are also similar and generic drugs [1, 3, 4]. The use of the similar and generic drugs is growing due to their comparatively lower cost, being the generic the lowest one. Similar drugs differ from reference drugs only by shelf-life, size, format, packaging, labeling, excipients and they must be identified by commercial name or brand [3]. The generic drugs must contain the same dosage, active principle and administration route than the reference drugs, but not necessarily preclinical and clinical data [4]. In 2010 and 2011, omeprazole was in the ranking of the ten generic drugs most sold in Brazil [5]. The efficiency, according to their bioequivalence and bioavailability, of generic and similar drugs compared to reference medicines were discussed [6, 7]. However, studies about elemental impurities in generic and similar drugs are less frequent and we did not find any recent paper comparing amounts of elemental impurities in generic and similar omeprazole drugs.
The Elemental Impurities Guidelines of the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH Q3D) [8] is applied for new drugs, products containing medicinal substances, medicines consisting of purified proteins and polypeptides and their derivatives. For the assessment of toxicity for potential elemental impurities, the guideline establishes a permissible daily exposure value (PDE) for each element of toxicological concern, separating these into three classes based on their toxicities and probability of occurrence [8, 9].
In order to update the elemental analysis of pharmaceutical products, new regulations for elemental impurities were published by the United States Pharmacopoeia (USP) [10, 11]. The Chapters 232 [10] and 233 [11] proposed analytical procedures for elemental impurities determination in drug substances, excipients and drug products. A new version of Chapter 232 [12], harmonized with the final version of the Elemental Impurities Guidelines (ICH Q3D) [8], became official in December 2017 and was implemented in January 2018. Chapter 232 [12] specifies 24 elemental impurities and their toxicity limits and Chapter 233 [11] describes procedures for elemental determinations by using either inductively coupled plasma optical emission spectrometry (ICP OES) or inductively coupled plasma mass spectrometry (ICP-MS).
Elemental impurities include catalysts and environmental contaminants that may be present in active pharmaceutical ingredients (APIs), excipients and final medications at different stages of their formulation and production, which may occur naturally, be added intentionally, or be introduced inadvertently (e.g. through the interaction with equipment, containers and surfaces during processing or by contamination of the constituent components of the drug, such as raw materials, reagents and excipients (stabilizers, fillers, binders, flavorings, colorants, and coatings) which can generate unwanted and unknown pharmacological–toxicological effects [9, 13, 14, 15, 16].
During the synthesis of APIs, the use of catalysts (e.g. Ir, Os, Pd, Pt, Rh and Ru) or incorporated metals and metalloids (e.g. antimicrobial agents containing Ag and Au, imaging agents containing Ba; psychotic disease drugs which contain Li and Pt-based agents) may also lead to elemental contamination [14, 15]. Trace elements, such as As, Cd, Hg and Pb, are considered toxic and concentrations higher than those allowed may be critical to health. On the other hand, Co, Cu, Mo and Se are classified as essential elements for human body, but in high concentrations they are also harmful to health [8, 15, 16, 17].
Elemental impurities are also associated with the manufacturing process, hence, we showed here an analysis of 23 elemental impurities in omeprazole drug samples of seven different commercial brands, including reference, similar and generic drugs, using ICP-MS. Microwave-assisted digestion was performed using dilute nitric acid solution (partial digestion) as recently recommended by Pinheiro et al. [18] for digestion of nine different products commonly available in Brazilian pharmaceutical market. In addition, according to twelve elemental impurities concentrations determined in omeprazole samples, a discussion considering the potential risk of elemental contamination taking into account responsible brands by sale, registration, manufacture and packaging and also by the constituent excipients was presented.
2. Experimental
2.1. Instrumentation
A Quadrupole ICP-MS model 7800 (Agilent Technologies, Tokyo, Japan) was used for all performed experiments. Argon (99.999%, White Martins-Praxair, (Sertãozinho, SP, Brazil) was used in all measurements and nitrogen (99.9%, White Martins-Praxair) was used for pressurization of the Ultrawave (Milestone, Sorisole, Italy) microwave oven. This equipment is based on single reaction chamber technology. Plasma operating conditions used in ICP-MS were previously established by Pinheiro et al. [18].
2.2. Omeprazole drug samples and sample preparation
Seven omeprazole drug samples from seven different commercial brands (Om1 - Om7) in tablets form (oral administration route) were analyzed. All samples were purchased in local pharmacies in São Carlos, SP, Brazil. The drug samples are classified as generic drug (Om1, Om4, Om5 and Om6); similar drug (Om2 and Om3); and “name-brand” or reference drug (Om7) [1,3,4].
Sample preparation was performed as previously established by Pinheiro et al. [18]. All omeprazole drug samples were ground using pestle and mortar. Masses of approximately 100 and 500 mg were accurately weighed directly in the perfluoroalkoxy alkanes (PFA) digestion vessels and microwave-assisted digested (in triplicate) in 7 mL of inverse aqua regia 3HNO3:1HCl v v−1 and 7 mL of 2.0 mol L−1 HNO3, respectively. Microwave heating program was applied according to conditions previously recommended [18].
Subsequently, digests were diluted to 50.0 mL with distilled-deionized water. After, an aliquot of each digest obtained using inverse aqua regia was appropriately 3-fold diluted (final dilution of 300-fold, which implies in a residual acidity of 4.7% v v−1) followed by quantification using ICP-MS. Samples digested with 2.0 mol L−1 HNO3 were directly analyzed without any further dilution (final dilution of 100-fold, which implies in solids content of 1.0% m v−1 and a residual acidity of 2.0% v v−1).
2.3. Reagents and standard solutions
All working solutions were prepared using HNO3 (Synth, Diadema, SP, Brazil) purified in a sub-boiling distillation apparatus Distillacid™ BSB-939-IR (Berghof, Eningen, Germany), HCl (Qhemis, São Paulo, SP, Brazil) purified in a DuoPur sub-boiling distillation system (Milestone, Sorisole, Italy) and ultrapure water, resistivity higher than 18.2 MΩ cm, (Milli-Q®, Millipore, Bedford, MA, USA). Standard solutions for the determined elements were prepared from a mixture of all elements by appropriate dilution of stock standard solutions containing 1000 mg L−1 (Qhemis, São Paulo, SP, Brazil) in 0.14 mol L−1 HNO3 medium. The concentrations of the analytical solutions used for calibration ranged from 0.050 to 50 μg L−1. Accuracies were evaluated by addition and recovery experiments in concentrations of 1.0 μg L−1 for all analyzed samples. Memory effects for Hg were avoided by cleaning the sample introduction system with 0.06 mol L−1 HCl [18, 19].
3. Results and discussion
3.1. Determination of elemental impurities in omeprazole drug samples
For analysis of omeprazole drug samples, two microwave-assisted sample preparation procedures were performed: 1) microwave-assisted sample preparation adopted as reference procedure using inverse aqua regia [20, 21, 22, 23, 24] and 2) microwave-assisted sample digestion using 2.0 mol L−1 HNO3, as previously proposed by Pinheiro et al. [18].
When applying the reference procedure using inverse aqua regia, complete digestion was obtained for most samples, except to samples Om2 and Om5. In fact, all analyzed drug samples contained the same active principle, so incomplete digestions for these two samples can be related to the use of different excipients for preparing omeprazole drug samples (Table 1) or even some pharmaceutical substance which was not specified in the drug label, which imply in distinctive different matrix complexities. On the other hand, partial digestions were obtained for all samples digested using 2.0 mol L−1 HNO3.
Table 1.
Possible excipients of omeprazole drug samples (Om1-Om7) of seven commercial brands.
| Sample | Excipient |
|---|---|
| Om1 | hypromellose, acetone, cetyl alcohol, hypromellose phthalate, isopropyl alcohol, lactose, mannitol and sucrose |
| Om2 | hypromellose, sodium laurylsulfate, titanium dioxide, calcium carbonate, cetyl alcohol, dibasic sodium phosphate, lactose, mannitol and sucrose |
| Om3 | ethyl phthalate, hypromellose, polissorbato 80, sodium laurylsulfate, titanium dioxide, calcium carbonate, manitol, sucrose and talc |
| Om4 | hypromellose, polysorbate 80, titanium dioxide, cetyl alcohol, dibasic sodium phosphate, hypromellose phthalate, lactose, mannitol, sucrose and talc |
| Om5 | ethyl polymethacrylate polyacrylate, hypromellose, hyplosis, macrogol, magnesium carbonate, silicon dioxide, sodium hydroxide, starch and sucrose |
| Om6 | ethyl polymethacrylate polyacrylate, hyplosis, macrogol, magnesium carbonate, silicon dioxide, sodium hydroxid e and starch |
| Om7 | ethyl polymethacrylate polyacrylate, hypromellose, polysorbate 80, sodium laurylsulfate, titanium dioxide, calcium carbonate, dibasic sodium phosphate, ethyl phthalate, manitol, sodium hydroxide, sucrose and talc |
The concentrations of As, Ba, Cd, Co, Cu, Cr, Li, Mo, Ni, Pb, Sb and V determined in omeprazole drug samples for both sample preparation procedures are shown in Table 2. The other 11 elemental impurities established by the USP Chapter 232 were below respective LOQs for both procedures. As previously observed for determination of 15 elemental impurities in nine drug samples [18], microwave-assisted digestion using 2.0 mol L−1 HNO3 was also applied successfully for omeprazole drug samples, since it did not present significant differences (t-paired test with 95% of confidence level) when compared with those concentrations determined using inverse aqua regia.
Table 2.
Determination of As, Ba, Cd, Co, Cu, Cr, Li, Mo, Ni, Pb, Sb and V (μg g−1, mean ± standard deviation, n = 3) by ICP-MS in omeprazole drug samples of seven commercial drugs (Om1-Om7) digested using two microwave-assisted digestion procedures.
| Analyte | Sample | Sample preparation |
Analyte | Sample | Sample preparation |
||
|---|---|---|---|---|---|---|---|
| 3HNO3:1HCl v v−1a | HNO3 2 mol L−1b | 3HNO3:1HCl v v−1a | HNO3 2 mol L−1b | ||||
| As | Om1 | <0.62 | 0.07 ± 0.01 | Ba | Om1 | 0.3 ± 0.2 | 0.46 ± 0.08 |
| Om2 | <0.62 | 0.107 ± 0.007 | Om2 | 1.8 ± 0.4 | 1.9 ± 0.2 | ||
| Om3 | <0.62 | 0.073 ± 0.006 | Om3 | 0.38 ± 0.04 | 0.41 ± 0.04 | ||
| Om4 | <0.62 | 0.05 ± 0.02 | Om4 | 0.50 ± 0.04 | 0.46 ± 0.09 | ||
| Om5 | <0.62 | <0.020 | Om5 | <0.22 | 0.19 ± 0.01 | ||
| Om6 | <0.62 | 0.08 ± 0.01 | Om6 | 0.54 ± 0.04 | 0.6 ± 0.1 | ||
| Om7 | <0.62 | 0.051 ± 0.009 | Om7 | <0.22 | 0.20 ± 0.02 | ||
| Cd | Om1 | <0.017 | 0.005 ± 0.001 | Co | Om1 | 0.027 ± 0.004 | 0.024 ± 0.004 |
| Om2 | <0.017 | 0.012 ± 0.001 | Om2 | 0.031 ± 0.002 | 0.024 ± 0.001 | ||
| Om3 | <0.017 | 0.003 ± 0.001 | Om3 | <0.021 | 0.015 ± 0.009 | ||
| Om4 | <0.017 | 0.004 ± 0.001 | Om4 | 0.025 ± 0.001 | 0.024 ± 0.002 | ||
| Om5 | <0.017 | <0.0010 | Om5 | <0.021 | <0.0010 | ||
| Om6 | <0.017 | <0.0010 | Om6 | <0.021 | 0.016 ± 0.003 | ||
| Om7 | <0.017 | <0.0010 | Om7 | <0.021 | 0.005 ± 0.002 | ||
| Cu | Om1 | <0.72 | 0.12 ± 0.03 | Cr | Om1 | <0.23 | 0.075 ± 0.003 |
| Om2 | <0.72 | 0.035 ± 0.008 | Om2 | <0.23 | 0.16 ± 0.02 | ||
| Om3 | <0.72 | 0.07 ± 0.01 | Om3 | <0.23 | 0.06 ± 0.01 | ||
| Om4 | <0.72 | 0.06 ± 0.01 | Om4 | <0.23 | 0.09 ± 0.01 | ||
| Om5 | <0.72 | <0.0030 | Om5 | <0.23 | <0.010 | ||
| Om6 | <0.72 | 0.025 ± 0.008 | Om6 | <0.23 | 0.117 ± 0.008 | ||
| Om7 | <0.72 | 0.03 ± 0.02 | Om7 | <0.23 | 0.070 ± 0.009 | ||
| Li | Om1 | <0.42 | 0.020 ± 0.004 | Mo | Om1 | <0.47 | 0.088 ± 0.003 |
| Om2 | <0.42 | 0.050 ± 0.001 | Om2 | 7.4 ± 0.4 | 7.5 ± 0.2 | ||
| Om3 | <0.42 | 0.011 ± 0.001 | Om3 | <0.47 | 0.11 ± 0.03 | ||
| Om4 | <0.42 | 0.016 ± 0.002 | Om4 | <0.47 | 0.122 ± 0.002 | ||
| Om5 | <0.42 | 0.038 ± 0.002 | Om5 | <0.47 | 0.14 ± 0.03 | ||
| Om6 | <0.42 | 0.019 ± 0.002 | Om6 | <0.47 | 0.072 ± 0.005 | ||
| Om7 | <0.42 | 0.010 ± 0.001 | Om7 | 0.56 ± 0.01 | 0.55 ± 0.02 | ||
| Ni | Om1 | 0.33 ± 0.03 | 0.31 ± 0.06 | Pb | Om1 | <0.083 | <0.010 |
| Om2 | 0.42 ± 0.01 | 0.41 ± 0.02 | Om2 | <0.083 | <0.010 | ||
| Om3 | <0.32 | 0.09 ± 0.01 | Om3 | <0.083 | <0.010 | ||
| Om4 | <0.32 | 0.29 ± 0.02 | Om4 | <0.083 | <0.010 | ||
| Om5 | 3.43 ± 0.05 | 3.39 ± 0.09 | Om5 | <0.083 | <0.010 | ||
| Om6 | <0.32 | 0.09 ± 0.02 | Om6 | <0.083 | <0.010 | ||
| Om7 | <0.32 | 0.09 ± 0.03 | Om7 | <0.083 | 0.014 ± 0.007 | ||
| Sb | Om1 | <0.033 | <0.0010 | V | Om1 | <0.28 | 0.143 ± 0.006 |
| Om2 | <0.033 | <0.0010 | Om2 | <0.28 | 0.202 ± 0.002 | ||
| Om3 | <0.033 | 0.003 ± 0.001 | Om3 | <0.28 | 0.13 ± 0.06 | ||
| Om4 | <0.033 | <0.0010 | Om4 | <0.28 | 0.140 ± 0.005 | ||
| Om5 | <0.033 | <0.0010 | Om5 | <0.28 | <0.0030 | ||
| Om6 | <0.033 | <0.0010 | Om6 | <0.28 | <0.0030 | ||
| Om7 | <0.033 | 0.008 ± 0.002 | Om7 | <0.28 | 0.060 ± 0.003 | ||
Sample masses digested of 100 mg.
Sample masses digested of 500 mg.
According to the elemental impurities classification based on their toxicities and probability of occurrence from ICH [8] (Table 3), among the twelve elemental impurities determined in omeprazole drug samples, three elements are Class 1 (As, Cd and Pb) and six elements are Class 3 (Ba, Cr, Cu, Li, Mo and Sb). So, considering the elements from Class 1 and Class 3, only Hg and Sn, respectively, were below the respective LOQs for all samples. These four elements are toxic to humans and have limited or no use in the manufacture of pharmaceuticals. Their presence in drug products typically comes from commonly used materials (e.g. excipients). On the other hand, the elements from Class 3 have relatively low toxicities when administered via oral route [8, 9].
Table 3.
Limits of quantification reached by ICP-MS for elemental impurities determined in omeprazole drug samples digested using two microwave-assisted sample preparation procedures [18].
| Element | Class a | PDE (μg g−1) |
Isotope | Sample preparation |
|
|---|---|---|---|---|---|
| 3HNO3:1HCl v v−1 LOQ (μg g−1) b |
HNO3 2.0 mol L−1 LOQ (μg g−1) c |
||||
| Ag | 2B | 15 | 107Ag | 0.033 | 0.0030 |
| As | 1 | 1.5 | 75As | 0.62 | 0.020 |
| Au | 2B | 10 | 197Au | 0.17 | 0.0030 |
| Ba | 3 | 140 | 138Ba | 0.22 | 0.0030 |
| Cd | 1 | 0.5 | 112Cd | 0.017 | 0.0010 |
| Co | 2A | 5.0 | 59Co | 0.021 | 0.0010 |
| Cr | 3 | 1100 | 52Cr | 0.23 | 0.010 |
| Cu | 3 | 300 | 63Cu | 0.72 | 0.0030 |
| Hg | 1 | 3.0 | 202Hg | 0.23 | 0.013 |
| Ir | 2B | 10 | 193Ir | 0.13 | 0.0010 |
| Li | 3 | 55 | 7Li | 0.42 | 0.0030 |
| Mo | 3 | 300 | 98Mo | 0.47 | 0.0030 |
| Ni | 2A | 20 | 58Ni | NA | 0.013 |
| 58Ni | 0.32 | NA | |||
| Os | 2B | 10 | 190Os | 0.50 | 0.10 |
| Pb | 1 | 0.5 | 208Pb | 0.083 | 0.010 |
| Pd | 2B | 10 | 108Pd | 0.65 | 0.0030 |
| Pt | 2B | 10 | 195Pt | 0.58 | 0.013 |
| Rh | 2B | 10 | 103Rh | 0.033 | 0.0010 |
| Ru | 2B | 10 | 102Ru | 0.033 | 0.0010 |
| Sb | 3 | 120 | 123Sb | 0.033 | 0.0010 |
| Se | 2B | 15 | 78Se | 6.2 | 0.60 |
| Sn | 3 | 600 | 120Sn | 0.083 | 0.0030 |
| Tl | 2B | 0.8 | 205Tl | 0.0080 | 0.0010 |
| V | 2A | 10 | 51V | 0.28 | 0.0030 |
Class according ICH [8].
Sample masses digested of 100 mg.
Sample masses digested of 500 mg; NA: not applicable.
In addition, all elements of Class 2A (Co, Ni and V) were determined in omeprazole drug samples and none of Class 2B (Ag, Au, Ir, Os, Pd, Pt, Rh, Ru, Se and Tl). Elements of Class 2 are further divided in sub-classes 2A and 2B based on their possibilities of occurrence in drugs. Class 2A has relatively high probability of occurrence in drug products and thus must be evaluated in all potential sources of contamination. Class 2B has a reduced probability of occurrence in drug products and they may be excluded from the risk assessment unless they are intentionally added during the manufacture of excipients or other components of the drug product [8, 9].
The highest values for As, Ba, Cd, Co, Li, Mo and V were observed for sample Om2. Nevertheless, determined concentrations of these analytes in all drug samples were lower than the limits established by the Chapter 232 (PDE values showed in Table 3) considering a maximum daily dose of 10 g day−1. In reality the daily dose for omeprazole drug is 20 mg and thus the risk is very low, consequently, the limits established considering this maximum daily dose are even higher.
3.2. Accuracy and analytical performance
Analytical performance of ICP-MS was evaluated as previously proposed by Pinheiro et al. [18]. Therefore, Octopole Reaction System (ORS) was used with He in collision mode for correcting for spectral interferences for determination of 51V+, 52Cr+, 63Cu+, 75As+ and 78Se+ in both sample preparation procedures and HMI system allows the use of conditions with minimum dilution of digests, that is, introduction of samples with total dissolved solids around 1% m v−1 (for digests using dilute nitric acid solution), and residual acidity around 5% v v−1 (for digests using inverse aqua regia). Isotopes determined and LOQs [25] obtained for all analytes are shown for each sample preparation procedure in Table 3 [18].
For some omeprazole samples analyte concentrations determined applying the procedure using 2.0 mol L−1 HNO3 could not be compared with the concentrations determined using inverse aqua regia due to the higher LOQs (Table 3) reached for this later procedure, as observed for the concentrations of As, Cd, Cr, Cu, Li, Pb, Sb and V determined only for samples digested using dilute nitric acid solution. Higher LOQs obtained when using inverse aqua regia can be explained considering that the sample mass reduction from 500 to 100 mg caused LOQs 5 times higher and, due to the higher residual acidity, digests obtained using inverse aqua regia were 3-fold diluted with deionized water before quantification by ICP-MS and digests obtained using dilute nitric acid solution were directly analyzed without any further dilution. As expected, addition of higher volume of reagents implies in higher analytical blanks.
Sample preparation using 2.0 mol L−1 HNO3 were validated by comparison with the concentration determined in digests obtained using inverse aqua regia. In addition, the accuracy was evaluated by spike experiments at level of 1.0 μg L−1 and recoveries ranging from 70 to 127% were obtained for all analytes (Table 4). It is important to highlight that osmium has not been evaluated since in nitric acid medium, the specie OsO4, which is volatile and toxic, is formed [9, 18, 24].
Table 4.
Recovery percentages and relative standard deviation (%) for spiked in digested omeprazole drug samples (Om1 – Om7) by ICP-MS (n = 3).
| Isotope | Omeprazole drug samples |
||||||
|---|---|---|---|---|---|---|---|
| Om1 | Om2 | Om3 | Om4 | Om5 | Om6 | Om7 | |
| 7Li | 94 (1) | 93.1 (0.5) | 98.2 (0.5) | 101 (1) | 107.2 (0.1) | 115 (1) | 119 (4) |
| 51V | 70 (6) | 82 (1) | 84.5 (0.7) | 90 (2) | 93.9 (0.9) | 112 (3) | 115 (3) |
| 52Cr | 95.5 (0.4) | 107 (3) | 102.5 (0.4) | 102 (8) | 105 (1) | 102 (4) | 115 (4) |
| 58Ni | 92 (1) | 96.5 (0.3) | 100.5 (0.9) | 103 (5) | 110 (2) | 111 (4) | 119 (5) |
| 59Co | 92 (1) | 93.5 (0.2) | 97 (3) | 101 (1) | 106.0 (0.1) | 108 (6) | 112 (5) |
| 63Cu | 89 (1) | 100 (1) | 99.8 (0.4) | 106 (6) | 108 (6) | 110 (1) | 112 (3) |
| 75As | 95 (9) | 92 (1) | 95 (2) | 102 (7) | 113.5 (0.2) | 111 (5) | 118 (5) |
| 78Se | 119 (3) | 86 (4) | 92 (2) | 100 (2) | 122.2 (0.1) | 122 (9) | 93 (3) |
| 98Mo | 99 (1) | 100.1 (0.3) | 102.5 (0.3) | 106 (2) | 110 (4) | 119.0 (0.8) | 127 (4) |
| 102Ru | 91.5 (0.5) | 90.0 (0.2) | 92.5 (0.8) | 97.0 (0.1) | 103.1 (0.5) | 109 (3) | 114 (3) |
| 103Rh | 91.6 (0.5) | 91 (4) | 93.1 (0.8) | 97.5 (0.8) | 102 (1) | 112 (1) | 116 (4) |
| 107Ag | 94.5 (0.1) | 84 (4) | 72 (6) | 108 (2) | 118.0 (0.1) | 112.0 (0.4) | 116 (4) |
| 108Pd | 94 (1) | 84 (4) | 71 (7) | 107 (3) | 118.1 (0.9) | 124 (7) | 110 (4) |
| 111Cd | 97.5 (0.1) | 95.1 (0.5) | 100 (2) | 105 (4) | 111 (2) | 115 (3) | 118 (2) |
| 120Sn | 91 (3) | 90 (4) | 89.1 (0.5) | 99 (2) | 94 (2) | 111 (2) | 116 (4) |
| 123Sb | 99 (1) | 97.3 (0.3) | 99.8 (0.8) | 106 (2) | 110 (2) | 100 (9) | 117 (4) |
| 138Ba | 91 (1) | 91.7 (0.6) | 94.5 (0.5) | 98 (3) | 102 (2) | 107 (5) | 117 (3) |
| 193Ir | 96 (5) | 88 (1) | 92 (5) | 97 (7) | 101.5 (0.9) | 114 (7) | 115 (1) |
| 195Pt | 88.5 (0.6) | 93 (2) | 97 (1) | 102 (1) | 105 (1) | 108 (8) | 114 (3) |
| 197Au | 99 (1) | 86.4 (0.5) | 94.5 (0.6) | 114 (2) | 111 (2) | 103 (7) | 113 (2) |
| 202Hg | 98.0 (0.4) | 92 (2) | 100 (1) | 114.0 (0.2) | 117.5 (0.5) | 108 (2) | 97 (3) |
| 205Tl | 99.2 (0.4) | 77 (7) | 81 (1) | 86 (1) | 90 (1) | 96 (1) | 112 (4) |
| 208Pb | 87 (2) | 92 (2) | 92 (0.5) | 93 (5) | 102 (3) | 113 (4) | 109 (3) |
3.3. Omeprazole drug samples and possible contamination sources of elemental impurities
Determined concentrations of Ba, Mo and Ni differed for some samples, for example, concentration of Ba in sample Om2 (similar drug); concentrations of Mo in samples Om2 and Om7 (reference drug); and concentration of Ni in sample Om5 (generic drug) are significantly higher than for other samples. Nevertheless, for other elements, the determined concentrations were similar, inferring that in general there are no differences among reference, similar and generic drugs considering contaminants contents.
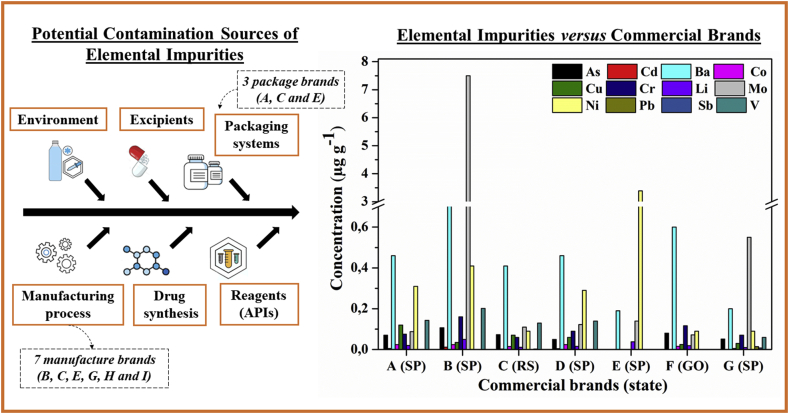
Aiming to evaluate possible contamination sources of elemental impurities in the samples analyzed, omeprazole drug samples were organized according to the commercial brands, classification, registration, manufacture and packaging (possibility of contamination during sample processing) and according to the constituent excipients (possibility of contamination by constituent components of each drug). Figure 1 shows a graphical representation of elemental impurities determined in omeprazole drug samples of each commercial brand analyzed. Table 5 show the samples classified according to the commercial brand (A-G), register brand (A-E, G and H), manufacture brand (B, C, E, G, H and I) and package brand (A, C and E). These differences between the brands are explained because some drug samples are marketed by a particular pharmaceutical company, but registered, manufactured and/or packaged either by the same company or by a different one.
Figure 1.
Commercial brands of omeprazole drug samples versus determined elemental impurities and possible contamination sources.
Table 5.
Omeprazole drug samples (Om1-Om7) of seven commercial brands (A-G) sold in Brazil.
| Sample | Commercial brand (state) | Classification | Register brand (state) | Manufacture brand (state) | Package brand (state) |
|---|---|---|---|---|---|
| Om1 | A (SP) | generic | A (SP) | I∗ (AM) | A (SP) |
| Om2 | B (SP) | similar | J∗ (SP) | B (MG) | NI |
| Om3 | C (RS) | similar | C (RS) | C (RS) | C (RS) |
| Om4 | D (SP) | generic | D (SP) | I∗ (AM) | A (SP) |
| Om5 | E (SP) | generic | E (SP) | E (SP) | E (SP) |
| Om6 | F (GO) | generic | H∗ (GO) | H∗ (GO) | NI |
| Om7 | G (SP) | reference | G (SP) | G (DF) | NI |
Registered and manufactured brands different from commercial brands analyzed; NI: not informed; SP: São Paulo State; GO: Goiás State; RS: Rio Grande do Sul State; AM: Amazonas State; MG: Minas Gerais State; DF: Federal District.
Only the samples Om3 and Om5 are marketed, registered, manufactured and packaged by the same company, C and E, respectively. The samples Om2, Om6 and Om7 are marketed, registered and manufactured by brands B, F and G, respectively, but they did not specify the responsible for package. The sample with the highest contents of elemental impurities (Om2) is the only marketed, registered and manufactured for the commercial brand B and the brand B is not responsible for any stage of production for the other samples analyzed. Samples Om1 and Om4 are marketed by brands A and D, respectively, but both are manufactured and packaged by the brands I and A, respectively. It can be seen, when comparing determined concentrations of analytes for samples Om1 and Om4, both generic products, that similar concentration were observed for Ba, Cd, Co, Li, Ni and V.
Although there is no other interconnection among the evaluated brands, as above mentioned, the brand I it is not responsible for any stage of production for other samples. It can be inferred that analytes found for samples Om1 and Om4 may be introduced during the manufacture and/or package stage. However, it should be highlighted that none significant contamination was observed.
During the formulation and drug production, manufacturing equipments commonly contain hastelloy, stainless steel and glass materials, due to their superior chemical resistance. The elements Ni, Co, V, Mo, Cr and Cu are usually found in those equipments and under extreme/corrosive reaction conditions (as high temperature and low/high pH) may be leached [13, 17, 26, 27].
However, considering the constituent excipients of omeprazole drug samples (Table 1), similar excipients are used in most commercial brands analyzed, consequently, there is no difference considering contaminants contents in the analyzed drugs. Therefore, it is possible to infer that none excipient eventually used in drug preparation show high elemental impurities concentrations. In addition, the elemental impurity risk associated with excipients is low [16]. A database with elemental impurities excipient [16] confirms that elemental impurity concentrations in excipients are generally low and when used in typical proportions are unlikely to pose a significant contamination source risk.
4. Conclusions
The new regulations for elemental impurities provide more meaningful results that can be used to identify the presence of specific elemental impurities and inform potential impact of the drug on patient safety. The sample preparation procedure using dilute nitric acid solution proposed by Pinheiro et al. [18] was applied successfully for omeprazole drugs. Different commercial brands (reference, similar and generic drugs) of omeprazole were analyzed and, in general, contaminants were not present or they were present in lower concentrations than those allowed by maximum daily doses established by the USP Chapter 232. It can be concluded that there were no critical differences among several commercial products considering contaminants contents.
Declarations
Author contribution statement
Joaquim Araujo Nobrega: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Fernanda Costa Pinheiro, Ariane Isis Barros: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, 141634/2017-0, 305201/2018-2 and 428558/2018-6) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/PNPD – Graduate Program in Chemistry, Federal University of São Carlos). This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001. This work was supported by the Agilent Technologies (São Paulo, SP, Brazil) and Milestone (Sorisole, BG, Italy). This work was supported by Instituto Nacional de Ciências e Tecnologias Analíticas Avançadas – CNPq, Grant No. 573894/2008-6 and FAPESP, Grant No. 2014/50951-4.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Agência Nacional de Vigilância Sanitária . 2018. Third Statistical Yearbook of the Pharmaceutical Market.http://portal.anvisa.gov.br/documents/374947/3413536/Anu%C3%A1rioEstat%C3%ADsticodoMercadoFarmac%C3%AAutico-2017/3179a522-1af4-4b4c-8014-cc25a90fb5a7 [Google Scholar]
- 2.Strand D.S., Kim D., Peura D.A. 25 Years of proton pump inhibitors: a comprehensive review. Gut Liver. 2017;11:27–37. doi: 10.5009/gnl15502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monteiro W.M., Melo G.C., Massunari G.K., Hübner D.V., Tasca R.S. Avaliação da disponibilidade de medicamentos genéricos em farmácias e drogarias de Maringá (PR) e comparação de seus preços com os de referência e similares. Rev. Bras. Cienc. Farm. 2005;41:333–343. [Google Scholar]
- 4.Jalali R.K., Rasaily D. Generic drug and bioequivalence studies, pharma. Medtrans. Clin. Res. 2018:327–339. [Google Scholar]
- 5.Pfarma . 2011. Profile of Consumption of Generic Drugs Changes in Brazil.http://pfarma.com.br/noticia-setor-farmaceutico/mercado/876-10-medicamentos-genericos-mais-consumidos-2011.html [Google Scholar]
- 6.Shimatani T., Inou M., Kuroiwa T., Xub J., Mienoc H., Tazuma S. Acid-suppressive effects of generic omeprazole: comparison of three brands of generic omeprazole with original omeprazole. Digest. Liver Dis. 2006;38:554–559. doi: 10.1016/j.dld.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 7.Liu J., Shentu J.Z., Wu L.H., Dou J., Xu Q.Y., Zhou H.L., Wu G.L., Huang M.Z., Hu X.J., Chen J.C. Relative bioavailability and pharmacokinetic comparison of two different enteric formulations of omeprazole. Univ. Sci. B. 2012;13:348–355. doi: 10.1631/jzus.B1100272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.International conference on harmonization of technical requirements for registration of pharmaceuticals for human use, guideline for elemental impurities. Q3D Step. December 16, 2014;4 [Google Scholar]
- 9.Barin J.S., Mello P.A., Mesko M.F., Duarte F.A., Flores E.M.M. Determination of elemental impurities in pharmaceutical products and related matrices by ICP-based methods: a review. Anal. Bioanal. Chem. 2016;408:4547–4566. doi: 10.1007/s00216-016-9471-6. [DOI] [PubMed] [Google Scholar]
- 10.The United States Pharmacopeia Convention . 2013. The United States Zharmacopeia, Chapter 232: Elemental Impurities - Limits, Revision Bulletin, Official February 1. [Google Scholar]
- 11.The United States Pharmacopeia Convention . 2013. The United States Pharmacopeia, Chapter 233: Elemental Impurities - Procedures, Revision Bulletin, Official February 1. [Google Scholar]
- 12.The United States Pharmacopeia Convention . 2017. The United States Pharmacopeia, Chapter 232: Elemental Impurities - Limits, USP 40 – NF 35, First Supplement, Official December 1. [Google Scholar]
- 13.Lewen N. Preparation of pharmaceutical samples for elemental impurities analysis: some potential approaches. Spectroscopy. 2016;31:36–43. [Google Scholar]
- 14.Holm R., Elder D.P. Analytical advances in pharmaceutical impurity profiling. Eur. J. Pharmaceut. Sci. 2016;87:118–135. doi: 10.1016/j.ejps.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Balaram V. Recent advances in the determination of elemental impurities in pharmaceuticals – Status, challenges and moving frontiers. TrAC Trend Anal. Chem. 2016;80:83–95. [Google Scholar]
- 16.Boetzel R., Ceszlak A., Day C., Drumm P., Bejar J.G., Glennon J., Harris L., Heghes C.I., Horga R., Jacobs P.L., Keurentjes W.J.T.M., King F., Lee C.W., Lewen N., Marchant C.A., Maris F.A., Nye W., Powell S., Rockstroh H., Rutter L., Schweitzer M., Shannon E., Smallshaw L., Teasdale A., Thompson S., Wilkinson D. An elemental impurities excipient database: a viable tool for ICH Q3D drug product risk assessment. J. Pharm. Sci. 2018;107:2335–2340. doi: 10.1016/j.xphs.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Fllszar K.A., Walker D., Allain L. Profiling of metal ions leached from pharmaceutical packaging materials. PDA J. Pharm. Sci. Technol. 2016;60:337–342. [PubMed] [Google Scholar]
- 18.Pinheiro F.C., Barros A.I., Nóbrega J.A. Microwave-assisted sample preparation of medicines for determination of elemental impurities in compliance with United States Pharmacopeia: how simple can it be? Anal. Chim. Acta. 2019;1065:1–11. doi: 10.1016/j.aca.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 19.Amaral C.D.B., Amais R.S., Fialho L.L., Schiavo D., Amorim T., Nogueira A.R.A., Rocha F.R.P., Nóbrega J.A. A novel strategy to determine As, Cr, Hg and V in drinking water by ICP-MS/MS. Anal. Methods. 2015;7:1215–1220. [Google Scholar]
- 20.Støving C., Jensen H., Gammelgaard B., Stürup S. Development and validation of an ICP-OES method for quantitation of elemental impurities in tablets according to coming US pharmacopeia chapters. J. Pharm. Biomed. Anal. 2013;84:209–214. doi: 10.1016/j.jpba.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Muller A.L.H., Oliveira J.S.S., Mello P.A., Muller E.I., Flores E.M.M. Study and determination of elemental impurities by ICP-MS in active pharmaceutical ingredients using single reaction chamber digestion in compliance with USP requirements. Talanta. 2013;136:161–169. doi: 10.1016/j.talanta.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 22.Lewen N., Mathew S., Schenkenberger M., Raglione T. A rapid ICP-MS screen for heavy metals in pharmaceutical compounds. J. Pharm. Biomed. Anal. 2004;35:739–752. doi: 10.1016/j.jpba.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 23.Silva C.S., Pinheiro F.C., Amaral C.D.B., Nóbrega J.A. Determination of As, Cd, Hg and Pb in continuous use drugs and excipients by plasma-based techniques in compliance with the United States Pharmacopeia requirements. Spectrochim. Acta Part B. 2017;138:14–17. [Google Scholar]
- 24.Van Hoecke K., Catry C., Vanhaecke F. Optimization of sample preparation and a quadrupole ICP-MS measurement protocol for the determination of elemental impurities in pharmaceutical substances in compliance with USP guidelines. J. Anal. At. Spectrom. 2012;27:1909–1919. [Google Scholar]
- 25.Thomsen V., Schatzlein D., Mercuro D. Limits of detection in spectroscopy. Spectroscopy. 2003;18:112–114. [Google Scholar]
- 26.Pohl P., Bielawska-Pohl A., Dzimitrowicz A., Jamroz P., Welna M. Impact and practicability of recently introduced requirements on elemental impurities. TrAC Trend Anal. Chem. 2018;101:43–55. [Google Scholar]
- 27.Thomas R.J. first ed. Boca Raton: CRC Press; New York: 2018. Measuring Elemental Impurities in Pharmaceuticals: A Practical Guide. [Google Scholar]