Summary
Wnt signaling is involved in the regulation of cancer stem cells (CSCs); however, the molecular mechanism involved is still obscure. SFRP1, a Wnt inhibitor, is downregulated in various human cancers; however, its role in tumor initiation and CSC regulation remains unexplored. Here, we used a skin carcinogenesis model, which showed early tumor initiation in Sfrp1−/− (Sfrp1 knockout) mice and increased tumorigenic potential of Sfrp1−/− CSCs. Expression profiling on Sfrp1−/− CSCs showed upregulation of genes involved in epithelial to mesenchymal transition, stemness, proliferation, and metastasis. Further, SOX-2 and SFRP1 expression was validated in human skin cutaneous squamous cell carcinoma, head and neck squamous cell carcinoma, and breast cancer. The data showed downregulation of SFRP1 and upregulation of SOX-2, establishing their inverse correlation. Importantly, we broadly uncover an inverse correlation of SFRP1 and SOX-2 in epithelial cancers that may be used as a potential prognostic marker in the management of cancer.
Keywords: cancer stem cells, CSCs, epithelial to mesenchymal transition, EMT, epithelial cancer, Sfrp1 (secreted frizzled-related protein), tumorigenic potential, skin squamous cell carcinoma, head and neck squamous cell carcinoma, HNSCC
Highlights
-
•
Loss of Sfrp1 accelerates murine skin tumor initiation and SCC progression
-
•
Sfrp1 loss enhances in vivo tumorigenic potential of murine skin CSCs
-
•
We found enhanced EMT and Sox-2 in Sfrp1−/− murine skin SCC
-
•
Sfrp1 and Sox-2 are inversely correlated in multiple human epithelial cancers
Dr. Waghmare and his colleagues showed the importance of Sfrp1 in mouse skin tumor initiation and CSC regulation. Sfrp1 loss enhanced in vivo tumorigenic potential of CSCs with upregulation of EMT and stemness markers. Sfrp1 and Sox-2 showed an inverse correlation in multiple human epithelial cancers with poor overall survival. Therefore, Sfrp1 can be used as a prognostic marker in human epithelial cancers.
Introduction
Cancer is a heterogeneous disease at both the cellular and the molecular level. The heterogeneity arises from the number of events, including genetic, epigenetic, and transcriptional alterations (Latil et al., 2017). Within the tumor, a subset of cells possesses an unlimited self-renewal activity, higher tumorigenic potential, and resistance to conventional therapies, termed as cancer stem cells (CSCs) (Batlle and Clevers, 2017). CSCs have been isolated from various cancers such as leukemia, breast cancer, head and neck cancers, etc. (Al-Hajj et al., 2003, Bonnet and Dick, 1997, Prince et al., 2007). These CSCs escape chemoradiotherapy thereby leading to recurrence of the tumor followed by metastasis (Nassar and Blanpain, 2016). During the process of epithelial to mesenchymal transition (EMT), epithelial cells lose their properties and acquire the mesenchymal fate, which confers on the cells migratory and invasive properties (Thiery et al., 2009). Although the EMT process is activated during embryonic development for the formation and differentiation of various tissues and organs, its activity in cancer cells was reported to endow stem cell-like properties. Recent findings have shown that the overexpression of EMT markers such as TWIST1, SNAIL, ZEB1, etc., in cancer cells converts them to cancer stem-like cells (Morel et al., 2008, Wellner et al., 2009). Thus, this suggests that there may be a link between the EMT and CSCs. Further, developmental signaling pathways, such as Wnt, Sonic hedgehog, Notch, etc., are involved in the regulation of EMT and CSCs (Karamboulas and Ailles, 2013).
Wnt signaling is involved in self-renewal, cell fate determination, migration, polarity, etc., during both embryonic development and adult tissue homeostasis (Clevers, 2006, Steinhart and Angers, 2018). Wnt signaling is tightly regulated by various secreted antagonists such as secretory frizzled-related proteins (SFRPs), Wnt inhibitory factor-1, and Dickkopf proteins (Kawano and Kypta, 2003). Moreover, intracellular noncanonical Wnt pathways were shown to regulate the canonical Wnt pathway by inhibiting β-catenin (Renstrom et al., 2009). SFRPs are a family of natural Wnt inhibitors that are present in the extracellular compartment, which inhibits Wnt signaling, and are also involved in embryonic development and tissue homeostasis (Matsuyama et al., 2009). Further, Sfrp1 is upregulated in the hair follicle stem cells (HFSCs) (Lien et al., 2011, Tumbar et al., 2004), while it is downregulated in various cancers. In oral squamous cell carcinoma (OSCC), silencing of the SFRP1, SFRP2, and SFRP5 genes was observed, due to methylation, in both oral cancer cell lines and tumor specimens (Sogabe et al., 2008). Further, methylation of the SFRP1 promoter was observed in esophageal squamous cell carcinoma (Meng et al., 2011) and hepatocellular carcinoma (Davaadorj et al., 2016). SFRP1 loss was also observed in invasive breast cancer tissues and cell lines through either gene deletion or promoter hypermethylation (Bernemann et al., 2014, Veeck et al., 2006). In addition, SFRP (1, 2, 4, and 5) gene promoters are hypermethylated in cutaneous squamous cell carcinoma (SCC) in Chinese patient samples (Liang et al., 2015). Moreover, microRNAs such as miR-1301-3p negatively target GSK-3β and SFRP1, and promote the expansion of CSCs in prostate cancer (Song et al., 2018). Although SFRP1 was shown to be lost in multiple epithelial cancers, including skin, OSCC, and breast cancers, its role in tumor initiation and CSC regulation is still obscure. Interestingly, epithelial tissues such as epidermis, oral epithelium, and breast epithelium have been reported to have similarities in tissue architecture and function as well as during tumor progression and metastasis.
Epidermis and oral epithelium are made up of stratified squamous epithelial layers consisting of stratum basale, stratum spinosum, stratum granulosum, and stratum corneum (gingiva and hard palate) (Muroyama and Lechler, 2012, Porcheri et al., 2019). Optimum levels of Wnt signaling are essential for the maintenance and differentiation of both skin and oral epithelia (Lim and Nusse, 2013, Liu and Millar, 2010). Further, Notch signaling drives the differentiation of keratin 5/14-positive basal epithelial cells into keratin 1/10-positive suprabasal cells in skin as well as oral epithelium (Blanpain et al., 2006, Porcheri et al., 2019). Moreover, both tissues express similar kinds of integrins, such as α2β1, α3β1, and α6β4 (in the basal layer) (Larjava et al., 2011, Owens et al., 2003), and terminal differentiation markers such as filaggrin (in the stratum corneum layer of the epidermis and gingiva/hard palate) (De Benedetto et al., 2008, Presland and Dale, 2000). Similarly, breast epithelium also has stratified epithelial organization and consists of basal/myoepithelial cells and luminal cells (Huebner et al., 2014). Importantly, Wnt/β-catenin is involved in the maintenance of basal/myoepithelial cells inhibiting luminal differentiation (Gu et al., 2013). Similar to that of skin, Notch signaling also plays a significant role in the differentiation and stratification of breast epithelium (Regan et al., 2013). The basal/myoepithelial cells also express keratins such as K5 and K14, which are characteristic of the basal layer of stratified epithelia. Further, integrins such as α2β1, α3β1, and α6β4 are also expressed in the basal layer of breast epithelium similar to that of epidermis (Faraldo et al., 2005).
Interestingly, the epithelial tissues also show certain similarities even in tumor progression and metastasis. For instance, head and neck SCC (HNSCC), triple-negative breast cancer (TNBC), and cutaneous SCC overexpress epidermal growth factor receptor, which plays an important role in tumor progression and metastasis (Argiris, 2015, Liao et al., 2019, Uribe and Gonzalez, 2011). Further, Keratin-8, a marker for more invasive and undifferentiated skin SCC (Caulin et al., 1993), is also a known marker for poor prognosis in OSCC (Fillies et al., 2006). In addition, upregulation of α5β6 integrin and matrix metalloprotease-9 promotes invasion and metastasis in basal cell carcinoma of skin, OSCC, and breast cancers (Arihiro et al., 2000, Lu et al., 2008, Ramos et al., 2002). Significantly, SFRP1 loss due to hypermethylation is reported in skin cutaneous SCC (Liang et al., 2015), breast cancer (Veeck et al., 2006), and OSCC (Sogabe et al., 2008). Therefore, owing to the similarity among epithelial tissues at both the tissue and the tumor levels, the information gained from the studies on skin cancer can be extrapolated to other epithelial cancers such as HNSCC and breast cancers.
In the present study, we show that the loss of Sfrp1 in mouse skin leads to early tumor initiation with an early formation of papillomas and SCC. CSCs isolated from Sfrp1−/− tumors showed increased in vivo tumorigenic potential with enhanced tumor propagating cell (TPC) frequency. Importantly, the expression profile on the CSCs of Sfrp1−/− tumors showed an upregulation of genes involved in the regulation of tumor aggressiveness, metastasis (EMT markers), and stemness (Sox-2). Further, we extrapolated our data from mouse to human epithelial cancers and checked for the expression pattern of SFRP1 and SOX-2 in skin cutaneous SCC, HNSCC (OSCC samples), and breast cancer. The results showed SFRP1 was downregulated, whereas SOX-2 was upregulated in all three cancers, establishing an inverse correlation of SFRP1 and SOX-2 in these cancers. Importantly, within the TCGA database, SFRP1 downregulation is associated with increased SOX-2 expression and overall poor survival in multiple epithelial cancers.
Results
Sfrp1 Loss Results in Accelerated Tumor Initiation with Chemical-Induced Carcinogenesis
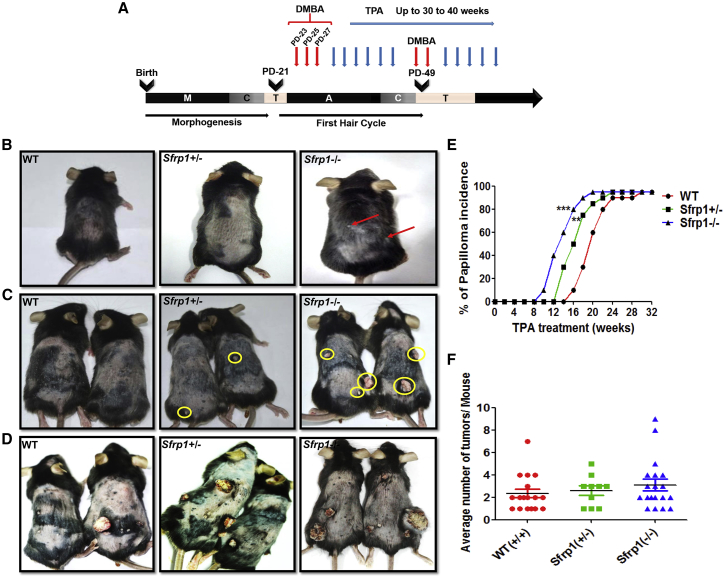
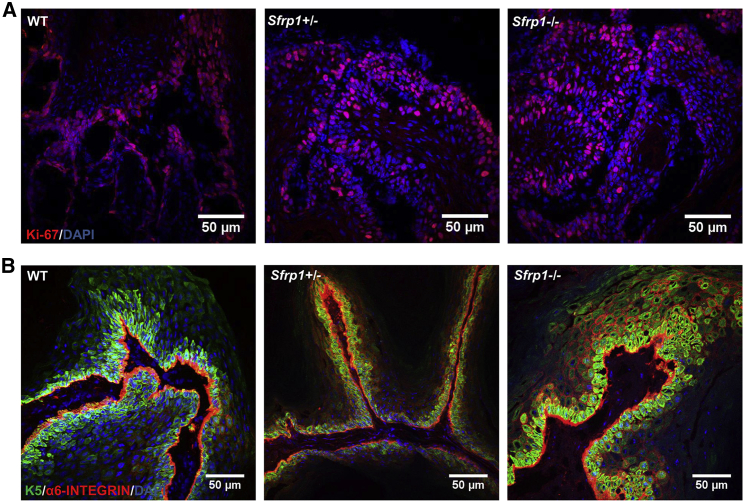
To delineate the role of Sfrp1 in tumor initiation, we used the 7,12-dimethylbenz[a]anthracene (DMBA)/12-O-tetradecanoyl phorbol-13-acetate (TPA)-induced skin carcinogenesis protocol as mentioned in the Experimental Procedures. The wild type (WT), Sfrp1+/− (heterozygous knockout), and Sfrp1−/− (homozygous knockout) mice were treated with DMBA and TPA at various postnatal days as shown in the schematic (Figure 1A). Sfrp1−/− mice showed an early papilloma formation after 10–12 weeks of TPA treatment (Figure 1B), while the Sfrp1+/− mice showed papilloma formation after 12–14 weeks, compared with the WT mice that showed at 16–18 weeks posttreatment. Thus, the study demonstrated that in Sfrp1−/− and Sfrp1+/− mice papilloma formation appears earlier by 3–4 weeks and 2–3 weeks, respectively, compared with WT mice (Figures 1C–1E). Further, we counted the average number of tumors per mouse in the Sfrp1−/− and Sfrp1+/− mice compared with WT mice. Although loss of Sfrp1 showed an early tumor initiation, it does not have any effect on the tumor burden (Figure 1F). Hematoxylin and eosin (H&E) staining showed that the Sfrp1−/− SCCs mostly had the mixed phenotype (tumor containing both epithelial cells and mesenchymal cells), while a few SCCs showed a mesenchymal phenotype. In WT tumors, the SCCs mostly showed a well-differentiated epithelial phenotype and a few SCCs showed a mixed phenotype (Figure S1). Further, we performed an immunofluorescence assay (IFA) using Ki-67 (proliferation marker) and K5 and α6-integrin (basal epithelial markers) in the papillomas of the WT and Sfrp1−/− mice. Our results showed no change in proliferation or in the expression of basal epithelial markers (Figures 2A and 2B). Hence, our results suggest that Sfrp1−/− mice showed accelerated tumor initiation and also the tumors were primarily of mixed phenotype compared with the epithelial phenotype of WT tumors.
Figure 1.
Sfrp1 Loss Accelerates Tumor Initiation and SCC Progression
(A) Schematic representation for two-step chemical-induced carcinogenesis using DMBA/TPA.
(B) WT, Sfrp1+/−, and Sfrp1−/− mice showing difference in tumor formation after 12 weeks of TPA application. Red arrows show papilloma formation in Sfrp1−/− mice.
(C) WT, Sfrp1+/−, and Sfrp1−/− mice showing difference in tumor formation after 18 weeks of TPA application. Yellow circles show papilloma formation in Sfrp1+/− and Sfrp1−/− mice.
(D) WT, Sfrp1+/−, and Sfrp1−/− mice showing difference in tumor formation after 25 weeks of TPA application.
(E) Graph depicting percentage of papilloma incidence versus TPA application in weeks, in WT, Sfrp1+/−, and Sfrp1−/−, mice (n = 11 mice/genotype).
(F) Graph depicting average number of tumors per mouse in WT, Sfrp1+/−, and Sfrp1−/− mice (n = 17 for WT, n = 10 for Sfrp1+/−, and n = 19 for Sfrp1−/−).
WT, wild type; Sfrp1+/−, heterozygous for Sfrp1; Sfrp1−/−, homozygous knockout for Sfrp1; DMBA, 7,12-dimethylbenz[a]anthracene; TPA, 12-O-tetradecanoyl phorbol-13-acetate; A, anagen; C, catagen; T, telogen; M, morphogenesis; SCC, squamous cell carcinoma. Data were analyzed by Student's t test and presented as means ± SEM. ∗∗p < 0.01, ∗∗∗p < 0.001. See also Figure S1.
Figure 2.
Characterization of WT, Sfrp1+/−, and Sfrp1−/− Mouse Papillomas
Immunofluorescence analysis of (A) Ki-67 and (B) keratin 5 (K5) and α6-integrin expression on 7 μm thick cryosections in WT, Sfrp1+/−, and Sfrp1−/− mouse papillomas (n = 4 biological replicates/genotype).
WT, wild type; Sfrp1+/−, heterozygous for Sfrp1; Sfrp1−/−, homozygous knockout for Sfrp1; scale bar: 50 μm.
CSCs of Sfrp1 Knockout Tumors Possess Higher Tumorigenic Potential
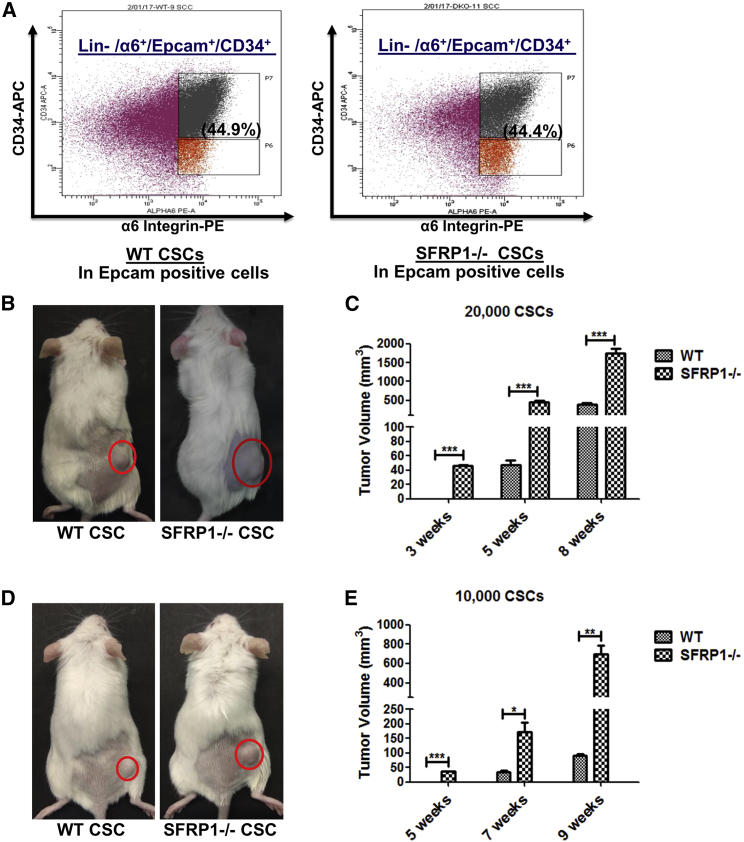
Sfrp1 loss showed accelerated tumor initiation; hence, we further investigated its involvement in CSC regulation. In this regard, we performed flow cytometry to analyze the CSCs from the Sfrp1−/− SCCs and WT SCCs, by using well-defined CSC markers (Lin−/Epcam+/α6-integrin+/CD34+) for skin SCC. The results showed that there was no alteration in the percentage of the CSCs in the WT and Sfrp1−/− mouse skin SCC (Figure 3A). Further, we performed an in vivo tumorigenic potential assay using fluorescence-activated cell sorting (FACS)-sorted CSCs from both the Sfrp1−/− SCCs and WT SCCs by subcutaneously transplanting 20,000 CSCs into NOD/SCID mice. The results showed that the Sfrp1−/− CSCs are able to give rise to tumor after 2–3 weeks of injection, but WT CSCs required 5–6 weeks for the tumor formation (Figures 3B and 3C). In addition, we also performed a serial transplantation assay of CSCs, which is the gold standard assay to determine the presence of CSCs. The results showed that FACS-sorted cells are indeed CSCs, which showed high tumorigenic potential when transplanted into NOD/SCID mice. In addition, 20,000 CSCs isolated from Sfrp1−/− primary tumors, when transplanted into NOD/SCID mice, showed tumor formation (secondary tumors) within 3 weeks. Subsequently, 20,000 CSCs from the Sfrp1−/− secondary tumors, when transplanted into NOD/SCID mice, showed tumors (tertiary tumors) within 10–14 days. This suggests that the CSCs from the Sfrp1−/− secondary tumors are more aggressive compared with Sfrp1−/− primary tumors (Figure S2A). Moreover, we also performed limiting dilution assay where we transplanted 10,000, 5,000, and 1,000 CSCs from both the WT SCCs and Sfrp1−/− SCCs into the NOD/SCID mice. The results showed that mice transplanted with 10,000 Sfrp1−/− CSCs developed tumors within 4–5 weeks of transplantation, whereas mice with 10,000 WT CSCs developed tumors after 7–8 weeks (Figures 3D and 3E). Further, mice with 5,000 Sfrp1−/− CSCs developed tumors after 6–7 weeks and no tumors were observed in mice with 5,000 WT CSCs (Figure S2B). Moreover, no tumors were observed in mice transplanted with 1,000 CSCs from either WT or Sfrp1−/− SCCs (Figure S2C). The TPC frequency was calculated as reported earlier (Hu and Smyth, 2009), and we found that 1/8,442 (estimated value) Sfrp1−/− CSCs and 1/34,761 (estimated value) WT CSCs are able to form tumors when transplanted into NOD/SCID mice (Figure S2D). These results suggest that loss of Sfrp1 results in aggressiveness of the CSCs with increased TPC frequency in Sfrp1−/− CSCs.
Figure 3.
Increased Tumorigenic Potential in CSCs from Sfrp1−/− SCC
(A) FACS analysis of CSCs in both WT SCC and Sfrp1−/− SCC.
(B) In vivo tumorigenesis assay using 20,000 FACS-sorted CSCs from the WT SCC and Sfrp1−/− SCC transplanted into NOD/SCID mice. Tumor growth in NOD/SCID mice after 5 weeks of CSC transplantation is shown (n = 5 mice/genotype).
(C) Graphical representation of tumor volume at 3, 5, and 8 weeks in NOD/SCID mice after transplantation with 20,000 FACS-sorted CSCs from WT SCC and Sfrp1−/− SCC (n = 5 mice/genotype).
(D) In vivo tumorigenesis assay of 10,000 FACS-sorted CSCs from WT SCC and Sfrp1−/− SCC transplanted into NOD/SCID mice. Tumor growth in NOD/SCID mice after 7 weeks of CSC transplantation is shown (n = 5 mice/genotype).
(E) Graphical representation of tumor volume at 5, 7, and 9 weeks in NOD/SCID mice after transplantation with 10,000 FACS-sorted CSCs from WT SCC and Sfrp1−/− SCC (n = 5 mice/genotype).
WT, wild type; Sfrp1−/−, homozygous knockout for Sfrp1; CSC, cancer stem cell; SCC, squamous cell carcinoma; FACS, fluorescence-activated cell sorting; Lin-, lineage negative (CD-31−ve, CD-45−ve, CD-140a−ve), α6+, α6 positive. Data were analyzed by Student's t test and presented as means ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. See also Figure S2.
Expression Profiling on the CSCs of Sfrp1 Knockouts Revealed Altered EMT Regulators and Growth Factor Signaling
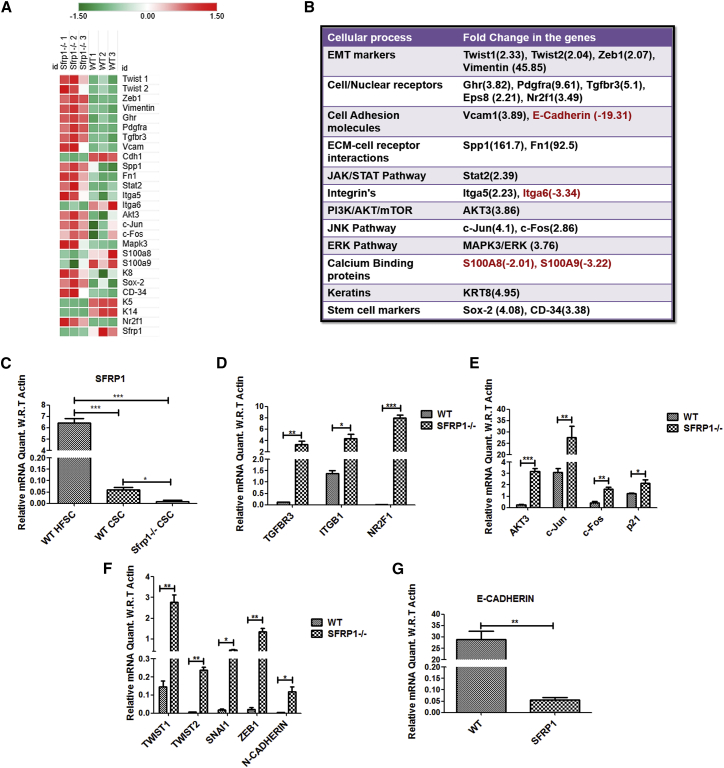
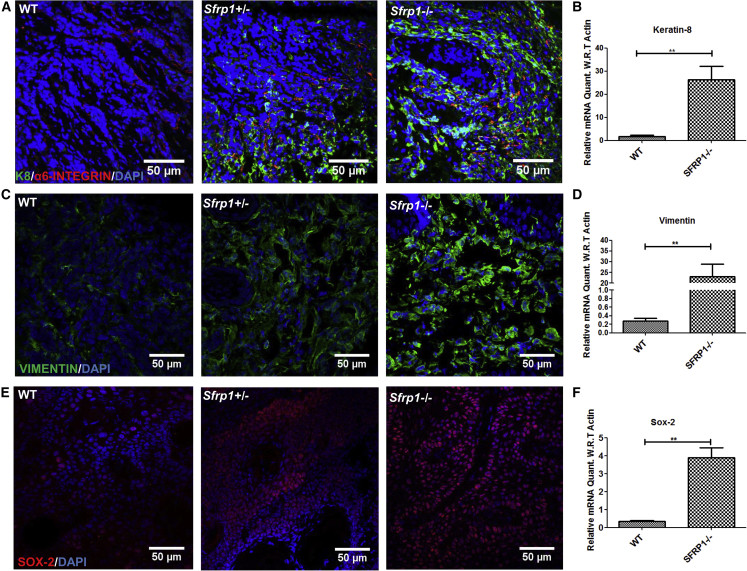
To understand the changes in the expression of Sfrp1 with time, we quantified Sfrp1 mRNA levels in WT mouse epidermis at various time points, such as 5, 10, 15, and 20 weeks, during the DMBA/TPA treatment. The results showed a progressive decrease of Sfrp1 with time in mouse epidermis with TPA treatment (Figure S3A). Further, the mRNA expression levels of Sfrp1 were quantified in both the WT mouse normal epidermis and the chemically induced (DMBA/TPA) WT SCC, which showed a significant decrease in Sfrp1 expression in WT SCC compared with WT normal epidermis (Figure S3B). Moreover, the mRNA quantification of Sfrp1 in the WT CSC population versus the WT non-CSC population showed a significant decrease in Sfrp1 expression in WT CSCs compared with the WT non-CSC population (Figure S3C). Importantly, to investigate the molecular mechanism behind the increased tumorigenic potential of Sfrp1−/− CSCs, we performed expression profiling on WT and Sfrp1−/− CSCs (Figure 4A). The gene expression profile data showed that growth factor receptors (Ghr, Pdgfra, Tgfbr3, and Eps8) and their downstream signaling molecules (Akt3 and Mapk3/Erk1), which are associated with tumor aggressiveness, metastatic potential, and proliferation, were highly upregulated in the Sfrp1−/− CSC population compared with WT CSCs (Figure 4B). Further, the genes involved in the cell to extracellular matrix interaction, such as Spp1, Vcam1, and Fn1, which are known to promote tumor invasion and metastasis, were highly upregulated in Sfrp1−/− CSCs. Moreover, EMT markers such as Twist1, Twist2, Snail, Vimentin, and Zeb1 showed increased expression in the Sfrp1−/− CSCs, while E-cadherin (Cdh1), an epithelial marker, was highly downregulated (Figure 4B). The expression of the stem cell marker Sox-2, involved in regulating tumor initiation and CSC regulation, was upregulated by 4-fold. Further, expression of K8 (K8), a marker for highly invasive and undifferentiated skin tumor, was also higher by 4- to 5-fold. The gene expression profile data were further validated using quantitative real-time PCR, which was in congruence with the microarray data (Figures 4C–4G). This was further confirmed by performing IFA of K8, vimentin (VIM), and SOX-2 in both the Sfrp1−/− and the WT tumors, which showed higher expression of K8, VIM, and SOX-2 in the Sfrp1−/− tumors (Figures 5A–5F).
Figure 4.
Altered Signaling in Sfrp1−/− CSCs Compared with WT CSCs
(A) Heatmap of the significantly deregulated genes between WT CSCs and Sfrp1−/− CSCs (n = 3 biological replicates/genotype).
(B) Expression profile of various pathways in Sfrp1−/− CSCs compared with WT CSCs.
(C) Graph representing the expression levels of the Sfrp1 in WT HFSCs, WT CSCs, and Sfrp1−/− CSCs validated using quantitative real-time PCR (n = 3 biological replicates/genotype).
(D and E) Graphs representing the expression level changes in cell surface receptors and signaling molecules in WT CSCs and Sfrp1−/− CSCs validated using quantitative real-time PCR (n = 3 biological replicates/genotype).
(F and G) Graphs representing the expression level changes in EMT genes in WT CSCs and Sfrp1−/− CSCs validated using quantitative real-time PCR (n = 3 biological replicates/genotype).
FACS, fluorescence-activated cell sorting; HFSC, hair follicle stem cells; CSCs, cancer stem cells; EMT, epithelial to mesenchymal transition; WT, wild type; SFRP1−/−, homozygous knockout for Sfrp1. The mRNA expression levels were normalized to the expression of β-actin. Data were analyzed by Student's t test and presented as means ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. See also Figures S3 and S4.
Figure 5.
Enhanced Epithelial to Mesenchymal Transition and Stemness in Sfrp1−/− SCC
(A) IFA for keratin-8 (K8) and α6-integrin in WT SCC, Sfrp1+/− SCC, and Sfrp1−/− SCC (n = 5 biological replicates/genotype).
(B) Graphical representation of expression level changes in K8 of WT CSCs and Sfrp1−/− CSCs (n = 3 biological replicates/genotype).
(C) IFA for vimentin in WT SCC, Sfrp1+/− SCC, and Sfrp1−/− SCC (n = 5 biological replicates/genotype).
(D) Graphical representation of expression level changes in Vimentin in WT CSCs and Sfrp1−/− CSCs (n = 3 biological replicates/genotype).
(E) IFA for SOX-2 in WT SCC, Sfrp1+/− SCC, and Sfrp1−/− SCC (n = 5 biological replicates/genotype).
(F) Graphical representation of expression level changes in Sox-2 in WT CSCs and Sfrp1−/− CSCs (n = 3biological replicates/genotype).
SCC, squamous cell carcinoma; CSCs, cancer stem cells; IFA, immunofluorescence assay; WT, wild type; Sfrp1+/−, heterozygous for Sfrp1; Sfrp1−/−, homozygous knockout for Sfrp1. Data were analyzed by Student's t test and presented as means ± SEM. Scale bar: 50 μm, ∗∗p < 0.01.
In addition, Sfrp1−/− CSCs showed a decrease in Wnt3A (canonical Wnt ligand) and increase in expression of Wnt7B (noncanonical Wnt ligand) (Figures S4A and S4B). SFRP1 was shown to bind and inhibit WNT7B (Rosso et al., 2005); therefore, loss of SFRP1 could enhance the WNT7B-mediated signaling cascade (WNT7B/JNK/c-JUN/c-FOS pathway) leading to the expression of Sox-2. Hence, we checked the expression of c-Jun and c-Fos, which showed upregulation in Sfrp1−/− CSCs compared with WT CSCs. Overall, the data suggest the loss of Sfrp1 leads to accelerated tumor initiation with aggressiveness in CSCs by enhancing EMT signatures through altered signaling.
Loss of SFRP1 Expression Leads to Upregulation of SOX-2 in Human Skin SCC and HNSCC Tissues
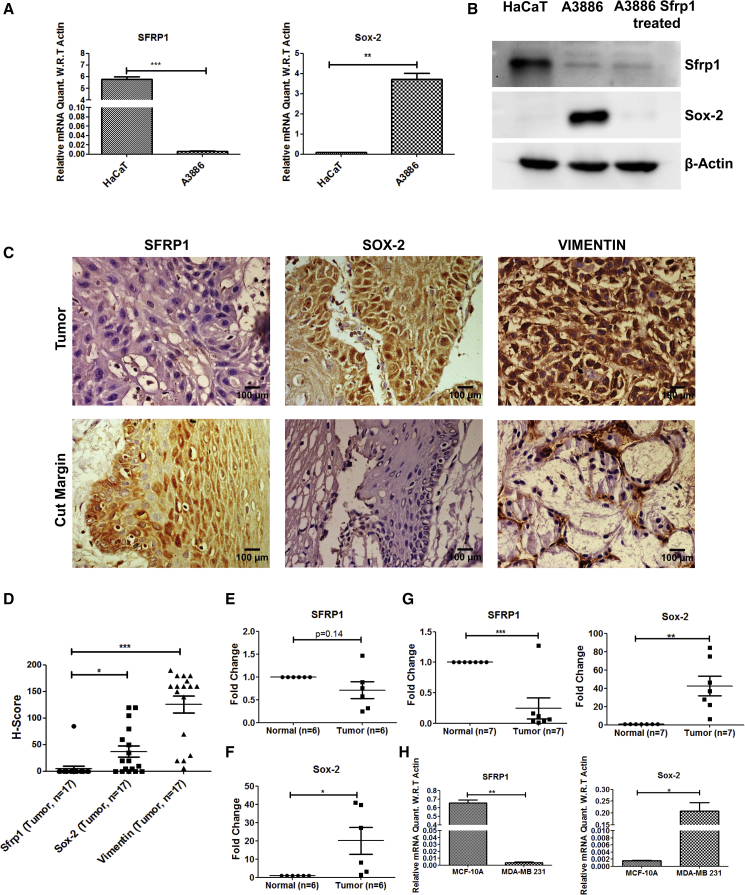
Sfrp1−/− CSCs showed increased tumorigenic potential, and expression profiling revealed higher expression of the stem cell marker Sox-2. In order to determine whether the loss of SFRP1 affects the stemness even in human skin cancers, we checked the expression levels of SFRP1 and SOX-2 in the human cutaneous SCC cell line A3886 and in the human keratinocyte cell line HaCaT. The results showed a decrease in the expression of SFRP1 and increase in the expression of SOX-2, at both RNA and protein levels in A3886 compared with HaCaT. Moreover, treating A3886 cells with SFRP1-containing medium decreased the SOX-2 protein levels within these cells (Figures 6A and 6B). Further, as epidermis and oral epithelium share several structural and functional similarities, we sought to understand if a similar relation exists between SFRP1 and SOX-2 even in the HNSCC tissues. Hence, we performed a validation in human HNSCC (buccal mucosa) samples by doing immunohistochemistry (IHC) using the SOX-2, SFRP1, and VIM markers. We observed that the protein levels of SFRP1 were lower in tumor samples compared with the adjacent cut margins. Further, the expression of SOX-2 and VIM was higher in OSCC tumor sections compared with the adjacent cut margins (Figures 6C, 6D, and S5). These results were also further validated at the expression level in tumor samples of HNSCC (buccal mucosa) compared with their normal counterparts. The results showed a decrease in median expression of SFRP1 and increase in SOX-2 median expression in HNSCC (buccal mucosa) (Figures 6E and 6F). In order to further validate the inverse relation between SFRP1 and SOX-2 in a large cohort of tumor samples, we performed in silico analysis on HNSCC and SKCM (skin cutaneous melanoma) samples from the TCGA database. SFRP1 expression is significantly decreased in HNSCC tumor samples (n = 521), compared with the normal controls (n = 43) (Figure S6A). The observed decrease is also stage dependent (n = 27, 71, 81, and 267 in stage I, stage II, stage III, and stage IV, respectively) (Figure S6B). Moreover, to determine if SFRP1 and SOX-2 are inversely related within HNSCC and SKCM, we first analyzed their expression in TCGA provisional data in HNSCC (n = 521) and in SKCM (n = 480). Further, Z scores were calculated for SFRP1 and SOX-2 tumor samples from normalized log2 transformed counts (Experimental Procedures). SFRP1 Z scores were sorted from low to high expression and the expression of SOX-2 was determined in these samples (Figures S6C and S7A). Log odds ratio of −1.9 suggests a negative correlation between SFRP1 and SOX-2 in HNSCC. However, in SKCM, although we found a log odds ratio of 0.05, the trend of SFRP1 and SOX-2 inverse correlation was observed. Kaplan-Meier analysis of TCGA data of HNSCC and SKCM patients showed poor overall survival in the patients having low expression of SFRP1 in HNSCC (n = 390) and SKCM (n = 345) compared with patients having high expression of SFRP1 in HNSCC (n = 129) and SKCM (n = 114), with p values of 0.023 and 0.001, respectively (Figures S6D and S7D). All these data suggest an inverse correlation of SFRP1 and SOX-2 in HNSCC and SKCM samples.
Figure 6.
SFRP1 Loss Showed Elevated SOX-2 Expression in Skin Cancer, HNSCC, and Breast Cancer
(A) Graphical representation of SFRP1 and SOX-2 expression levels in A3886 (human cutaneous squamous cell carcinoma) cell line compared with HaCaT (human keratinocyte cell line) (n = 3 independent replicates).
(B) Western blot for SFRP1 and SOX-2 in HaCaT, A3886, and A3886 treated with SFRP1-containing medium for 48 h (n = 3 independent replicates).
(C) IHC for SFRP1, SOX-2, and VIM in HNSCC (buccal mucosa) samples and their adjacent cut margins (n = 3 independent replicates).
(D) Graph representing H score for SFRP1, SOX-2, and VIM within the tumor samples.
(E and F) Graphical representations of SFRP1 and SOX-2 expression levels in human HNSCC (buccal mucosa) samples compared with normal buccal mucosa (n = 6 for tumor and control samples).
(G) Graphical representation of SFRP1 and SOX-2 expression levels in breast tumor samples compared with normal breast tissue (n = 7 for tumor and control samples).
(H) Graphical representation of SFRP1 and SOX-2 expression levels in MDA-MB-231 cell line compared with MCF-10A (n = 3 independent replicates).
HNSCC, head and neck squamous cell carcinoma; EMT, epithelial to mesenchymal transition; IHC, immunohistochemistry. Data were analyzed by Student's t test and presented as means ± SEM. Scale bar: 50 μm, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. See also Figures S5–S7.
Inverse Correlation of SFRP1 and SOX-2 Expression in Breast Cancer and Pancreatic Adenocarcinoma
In order to further validate the inverse correlation between SFRP1 and SOX-2 in other epithelial cancers, we checked the expression levels in breast cancer samples (Indian origin) along with their respective controls. The data showed a significant inverse correlation between SFRP1 and SOX-2 even in breast tumor samples (Figure 6G). Further, the levels of SFRP1 and SOX-2 were also assessed in breast cancer cell lines such as MDA-MB-231 (TNBC cell line) and control MCF-10A. The expression levels of SFRP1 were highly reduced, whereas SOX-2 was increased in MDA-MB-231 compared with MCF10A (Figure 6H). Further, we also analyzed SFRP1 and SOX-2 expression in TCGA provisional data in breast invasive carcinoma (n = 1105), and Z scores were calculated. SFRP1 and SOX-2 showed a negative correlation with a log odds ratio of −0.737 (Figure S7B). In addition, we also checked the SFRP1 expression in pancreatic adenocarcinoma (PAAD) (n = 186). Z scores were calculated in PAAD, and we found a negative correlation with a log odds ratio of −3. Altogether, these data demonstrate an inverse correlation between SFRP1 and SOX-2 expression in breast and pancreatic cancer samples (Figures S7B and S7C). Further, Kaplan-Meier analysis of TCGA data of breast invasive carcinoma and PAAD patients showed poor overall survival in the patients with low expression of SFRP1 in breast cancer (n = 808) and PAAD cancer (n = 132) compared with patients with high expression of SFRP1 in breast (n = 273) and PAAD (n = 45), with p values of 0.011 and 0.025, respectively (Figures S7E and S7F). Overall, these data suggest an inverse correlation between SFRP1 and SOX-2 in breast cancer and PAAD.
Discussion
SFRP1 is downregulated in various cancers, including skin, OSCC, breast cancers, etc. (Liang et al., 2015, Sogabe et al., 2008, Veeck et al., 2006). However, its role in tumor initiation and CSC regulation is yet to be discovered. Here, we attempted to unravel the role of Sfrp1 in skin tumor initiation and CSC regulation. We have shown that Sfrp1−/− mice show increased sensitivity to chemical-induced carcinogenesis with an early tumor initiation. The tumor characterization of Sfrp1−/− SCCs predominantly showed SCCs with a mixed phenotype and a few SCCs with a mesenchymal phenotype. However, the WT SCCs mostly showed a well-differentiated epithelial phenotype, with a few SCCs with a mixed phenotype. This result is in congruence with an earlier report, where tumors arising from HFSCs showed mostly a mixed phenotype and a few tumors with a mesenchymal phenotype, while tumors arising from IFE (interfollicular epidermis) stem cells showed a well-differentiated tumor phenotype (Latil et al., 2017). This indicates a possibility that the tumors of Sfrp1−/− mice may arise primarily from HFSCs as most of the Sfrp1−/− tumors are of mixed phenotype and a few Sfrp1−/− tumors have a mesenchymal phenotype. However, WT mouse tumors may mostly arise from IFE stem cells rather than HFSCs, as most of the tumors showed a well-differentiated epithelial phenotype. Moreover, high expression of Sfrp1 in WT HFSCs might also prevent tumor formation from HFSCs within these mice.
Further, K8 expression is important for the tumor progression and EMT of SCC (Caulin et al., 1993). In the present study, Sfrp1−/− CSCs showed higher K8 expression compared with WT CSCs. Hence, Sfrp1 may regulate the expression of K8 in the rapid progression of SCC, thereby leading to higher tumorigenic potential. Further, to explore whether the loss of Sfrp1 has any effect on CSC regulation, we checked the CSC percentage in the WT SCCs and Sfrp1−/− SCCs, which showed no alteration in the percentage of CSCs; however, the in vivo tumorigenesis assay showed increased tumorigenic potential of the Sfrp1−/− CSCs compared with control. In addition, limiting dilution assay using 10,000, 5,000, and 1,000 CSCs from WT SCCs and Sfrp1−/− SCCs showed that Sfrp1−/− CSCs were able to form tumors in NOD/SCID mice at cell numbers as low as 10,000 and 5,000 CSCs with an estimated TPC efficiency of 1/8,442. However, WT CSCs were able to form tumors only from 10,000 CSCs and were unable to form tumors from 5,000 CSCs in NOD/SCID mice, raising the estimated TPC efficiency to 1/34,761. Moreover, no tumors were observed with 1,000 CSCs of both the WT and the Sfrp1−/− SCCs. This suggests that Sfrp1 loss alters the expression of different genes that are required to regulate the tumorigenicity of CSCs. Further, we performed the gene expression profile on the CSCs of Sfrp1−/− SCC compared with control. Our data showed an increased expression of the genes involved in proliferation, such as Akt3. Overexpression of Akt3/Mtor is involved in the proliferation of prostate cancer cells and endows the CSC phenotype (Chang et al., 2013). Further, Akt3 also regulates p21 expression in prostate cancer cells, which is involved in cell proliferation and antiapoptotic activity (Lu et al., 2006). We have also observed an increase in the p21 expression levels in Sfrp1−/− CSCs, which is in congruence with our in vivo tumorigenic data. In addition, Mapk3/Erk1 is overexpressed in Sfrp1−/− CSCs compared with WT CSCs. Activation of ERK1/ERK2 in non-small cell lung cancer (Vicent et al., 2004) and breast cancer (Adeyinka et al., 2002) is associated with tumor advancement and aggressiveness. Therefore, the increased expression of Erk1 may lead to the higher tumorigenic potential of Sfrp1−/− CSCs.
In addition, we have observed an increase in the expression of the Wnt7B (noncanonical Wnt ligand) in the Sfrp1−/− CSCs. It was previously reported that SFRP1 binds and inhibits WNT7B; therefore, knockout of SFRP1 would enhance the WNT7B-mediated noncanonical signaling pathway. Moreover, WNT7B activates JNK, which in turn activates c-JUN (Rosso et al., 2005). Further, c-JUN binds to the promoter region of Sox-2, thereby increasing Sox-2 expression, which is involved in cancer stemness and aggressiveness (Boumahdi et al., 2014, Chang et al., 2013). Therefore, we have checked the expression levels of c-Jun and Sox-2 in Sfrp1−/− CSCs, which showed an increase in c-Jun and Sox-2 expression. Hence, SOX-2 could be involved in the earlier tumor initiation and aggressiveness observed in Sfrp1−/− CSCs compared with WT CSCs.
Taking these results together, we propose a putative model at the molecular level that, in the absence of Sfrp1, WNT7B binds to its receptor and flux through the noncanonical pathway increases, which activates JNK downstream through dishevelled. The activated JNK further activates c-JUN, which then enters into the nucleus, where it binds and increases the expression of Sox-2. Hence, this increased expression of SOX-2 is responsible for increased tumorigenicity and aggressiveness through regulation of EMT markers. However, in-depth understanding of the molecular signaling mechanism should be investigated further.
Importantly, the data obtained from the murine skin model, i.e., the inverse correlation between Sfrp1 and Sox-2, was extrapolated to human skin cancer, as SFRP1 was shown to be lost due to promoter hypermethylation in human cutaneous SCC (Liang et al., 2015). The expression levels of SOX-2 were higher and SFRP1 levels were lower in the A3886 cell line compared with HaCaT. Further, treatment of A3886 cells with SFRP1 externally led to a decrease in the SOX-2 levels, establishing an inverse correlation between SFRP1 and SOX-2. In addition, as epidermis shares certain similarities with oral and breast epithelia in tissue architecture and in tumor progression, we further expanded our studies to these tissues. Moreover, SFRP1 is also lost in OSCC and in breast cancer due to promoter hypermethylation (Sogabe et al., 2008, Veeck et al., 2006). Hence, we sought to understand whether a similar kind of relation of SFRP1 and SOX-2 exists even in OSCC samples of Indian origin. We have observed that SFRP1 levels were lower in OSCC samples compared with the adjacent cut margins. Further, the expression of SOX-2 and VIM was higher in tumor sections compared with the adjacent cut margins, establishing an inverse correlation of SFRP1 and SOX-2 in OSCC samples. In addition we found a similar correlation in both breast tumor tissues and a breast cancer cell line (MDA-MB-231). In order to verify the observed correlation in a large cohort of samples, we used the TCGA database, where we found the inverse correlation in multiple epithelial cancers, such as SKCM, HNSCC, breast cancer, and PAAD.
In summary, we have shown that Sfrp1 loss results in early tumor initiation and CSC regulation (Figure 7). Importantly, the knowledge obtained from the in vivo mouse skin carcinogenesis model was validated in multiple human epithelial cancers that showed that Sfrp1 downregulation is associated with poor survival. This study provides compelling evidence for using murine epithelial models to uncover molecular signaling in human epithelial cancers. Overall, future studies are warranted in understanding the in-depth molecular mechanism of Sfrp1 that is involved in CSC regulation with respect to tumor aggressiveness, proliferation, and EMT regulation, which may pave the way in the development of strategies for cancer.
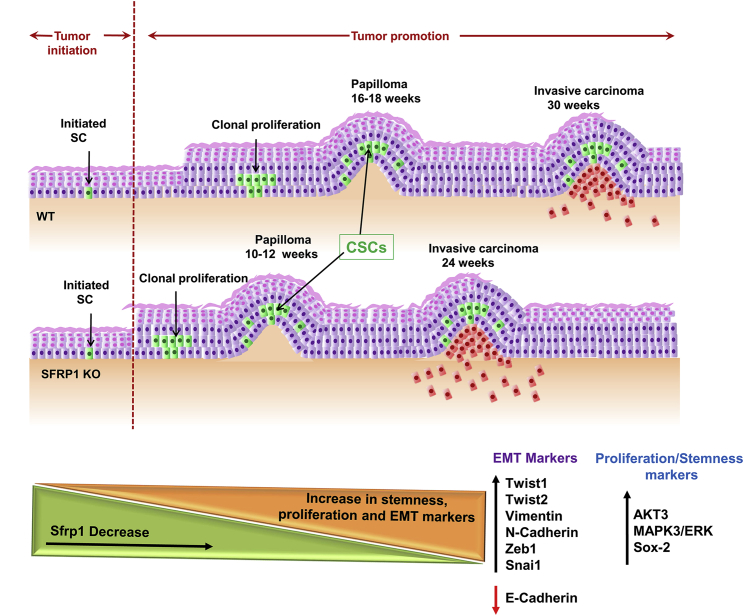
Figure 7.
Schematic Representation of Accelerated Skin Tumor Initiation and CSC Regulation Due to Loss of Sfrp1
Schematic diagram illustrating time points of papilloma and SCC formation in WT and Sfrp1−/− mouse skin upon DMBA and TPA treatment. Induced skin carcinogenesis showed early tumor formation in Sfrp1−/− mice compared with WT mice. As Sfrp1 decreases there is an increase in stemness (Sox-2), proliferation, and EMT markers in Sfrp1−/− CSCs.
WT, wild type; Sfrp1−/−, homozygous knockout for Sfrp1; CSC, cancer stem cells; SCC, squamous cell carcinoma; DMBA, 7,12-dimethylbenz[a]anthracene; TPA, 12-O-tetradecanoyl phorbol-13-acetate; EMT, epithelial to mesenchymal transition.
Experimental Procedures
Mice
Sfrp1 homozygous knockout (Sfrp1−/−) mice were a gift from Dr. Akihiko Shimono, Japan. The animal study was approved by the ACTREC's Institutional Animal Ethics Committee. For the experiments, mice of all three genotypes, WT, Sfrp1+/− (heterozygous knockout), and Sfrp1−/− (homozygous knockout), were obtained by intercrossing Sfrp1+/− mice. The genotyping of these mice was performed as described previously (Satoh et al., 2006).
DMBA/TPA Treatments
We used the two-step chemically induced skin carcinogenesis protocol as described previously (Beck et al., 2015). Mice were topically treated with DMBA (mutagen) and TPA (promoting agent) for skin tumor generation. For complete details, please refer to the Supplemental Information.
Tumor Collection and Digestion for a Single-Cell Suspension
Tumors were dissected from mouse skin, and they were cleaned of any traces of normal skin, blood vessels, and connective tissue attached to them. For complete details please refer to the Supplemental Information.
Isolation of CSCs from SCC
After preparation of a single-cell suspension, the cells were stained using well-defined CSC markers, Lin−/Epcam+/α6 integrin+/CD34+, and CSCs were FACS sorted using FACSAria (BD Biosciences, San Jose, CA). For complete details please refer to the Supplemental Information.
Expression Profiling
FACS-sorted CSCs were utilized for the extraction of RNA. After the RNA quality was assessed, cDNA was prepared and expression profiling was performed using a GeneChip MTA 1.0 array (Affymetrix, USA). For a detailed description of expression profiling and real-time PCR, please refer to the Supplemental Information.
Cell Lines and Tumor Tissue Samples
We have used A3886 (skin cutaneous SCC cell line, a generous gift from Dr. Colin Jamora's lab, Instem, Bangalore), MCF-10A (control), and MDA-MB-231 (TNBC) cell lines. These cell lines were cultured using DMEM containing 10% fetal bovine serum and 1% antibiotics (Invitrogen). The cell lines were passaged using 0.25% trypsin:EDTA solution and maintained at 37°C and 5% CO2. The OSCC (advanced stage treatment naive samples) and breast tumor (invasive ductal carcinoma samples) tissue samples used in the study were approved by the Institutional Ethics Committee under project nos. 188 and 164, respectively.
H&E and Immunostaining on Tumor Sections
Tumor tissues were processed for preparation of paraffin blocks or directly embedded in the OCT compound and stored at −80°C. Subsequently, tumor histology was analyzed by H&E staining on the paraffin tissue sections. IFAs were performed as described previously (Waghmare et al., 2008). For detailed description of IFA and IHC, please refer to the Supplemental Information.
In Silico Analysis
The TCGA PANCAN normalized raw counts were obtained from the UCSC cancer genome browser to determine the expression level changes of SFRP1 and SOX-2 in normal versus tumor samples of HNSCC. The raw data for SKCM, breast, and PAAD cancers were obtained from cBioPortal. The Z scores were calculated and heatmap was constructed using R 3.3.3. For complete details please refer to the Supplemental Information.
In Vivo Tumorigenesis Assay
FACS-sorted CSCs were directly collected in 100 μL of E-Media, mixed with 50 μL of Matrigel, and injected into NOD/SCID mice subcutaneously. Tumor progression was recorded twice a week by taking photographs from the time of transplantation to the experimental endpoint. Tumor size was measured with a Vernier caliper every week from 2 to 15 weeks.
Statistical Analysis
Statistical analysis was performed for tumor incidences, tumor volume, real-time PCR, and FACS analysis by using unpaired two-tailed Student's t test with GraphPad Prism 5. Error bar indicates the mean ± SEM of the mean values: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. The t.test function in R 3.3.3 was used to calculate the p value for TCGA data. The overall survival plots were plotted using Kaplan-Meier analysis. The p values for the survival data were determined using chi-squared analysis.
Author Contributions
S.K.W. conceived and designed the project and analyzed and interpreted the data; R.R.S. performed the experiments and analysis; R.M.S. and R.R.S. performed DMBA/TPA experiments and RNA extraction and analysis; P.S. performed the histology and helped with the animal work; S.S. performed the in silico data analysis and real-time PCR on HNSCC and breast samples; S.K.W. and R.R.S. performed RNA extraction for microarray and analyzed all the data; S.G. designed, analyzed, and wrote the in silico and in vivo validation data; P.C. provided the HNSCC tissue samples; P.G. performed IHC data analysis; S.K.W. analyzed all the data; and S.K.W. and R.R.S. wrote the manuscript.
Acknowledgments
We thank Dr. Akihiko Shimono, Japan, for providing the Sfrp1 knockout mice. We thank Ms. Sayoni Roy for discussion. We thank Colin Jamora, Instem, Bangalore, for providing the A3886 human SCC cell line. We thank the ACTREC animal house, flow cytometry, and microscopy facilities. R.R.S. and S.S. are supported by an ACTREC fellowship. This work was supported by Department of Biotechnology (DBT), India.
Published: January 9, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.stemcr.2019.12.006.
Accession Numbers
The expression profile data have been submitted to the GEO database with accession no. GEO: GSE141176.
Supplemental Information
References
- Adeyinka A., Nui Y., Cherlet T., Snell L., Watson P.H., Murphy L.C. Activated mitogen-activated protein kinase expression during human breast tumorigenesis and breast cancer progression. Clin. Cancer Res. 2002;8:1747–1753. [PubMed] [Google Scholar]
- Al-Hajj M., Wicha M.S., Benito-Hernandez A., Morrison S.J., Clarke M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argiris A. EGFR inhibition for recurrent or metastatic HNSCC. Lancet Oncol. 2015;16:488–489. doi: 10.1016/S1470-2045(15)70178-6. [DOI] [PubMed] [Google Scholar]
- Arihiro K., Kaneko M., Fujii S., Inai K., Yokosaki Y. Significance of alpha 9 beta 1 and alpha v beta 6 integrin expression in breast carcinoma. Breast Cancer. 2000;7:19–26. doi: 10.1007/BF02967183. [DOI] [PubMed] [Google Scholar]
- Batlle E., Clevers H. Cancer stem cells revisited. Nat. Med. 2017;23:1124–1134. doi: 10.1038/nm.4409. [DOI] [PubMed] [Google Scholar]
- Beck B., Lapouge G., Rorive S., Drogat B., Desaedelaere K., Delafaille S., Dubois C., Salmon I., Willekens K., Marine J.C. Cell Stem Cell. Different levels of Twist1 regulate skin tumor initiation, stemness, and progression. 2015;16:67–79. doi: 10.1016/j.stem.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Bernemann C., Hulsewig C., Ruckert C., Schafer S., Blumel L., Hempel G., Gotte M., Greve B., Barth P.J., Kiesel L. Influence of secreted frizzled receptor protein 1 (SFRP1) on neoadjuvant chemotherapy in triple negative breast cancer does not rely on WNT signaling. Mol. Cancer. 2014;13:174. doi: 10.1186/1476-4598-13-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C., Lowry W.E., Pasolli H.A., Fuchs E. Canonical notch signaling functions as a commitment switch in the epidermal lineage. Genes Dev. 2006;20:3022–3035. doi: 10.1101/gad.1477606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet D., Dick J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- Boumahdi S., Driessens G., Lapouge G., Rorive S., Nassar D., Le Mercier M., Delatte B., Caauwe A., Lenglez S., Nkusi E. SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature. 2014;511:246–250. doi: 10.1038/nature13305. [DOI] [PubMed] [Google Scholar]
- Caulin C., Bauluz C., Gandarillas A., Cano A., Quintanilla M. Changes in keratin expression during malignant progression of transformed mouse epidermal keratinocytes. Exp. Cell Res. 1993;204:11–21. doi: 10.1006/excr.1993.1003. [DOI] [PubMed] [Google Scholar]
- Chang L., Graham P.H., Hao J., Ni J., Bucci J., Cozzi P.J., Kearsley J.H., Li Y. Acquisition of epithelial-mesenchymal transition and cancer stem cell phenotypes is associated with activation of the PI3K/Akt/mTOR pathway in prostate cancer radioresistance. Cell Death Dis. 2013;4:e875. doi: 10.1038/cddis.2013.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Davaadorj M., Imura S., Saito Y.U., Morine Y., Ikemoto T., Yamada S., Takasu C., Hiroki T., Yoshikawa M., Shimada M. Loss of SFRP1 expression is associated with poor prognosis in hepatocellular carcinoma. Anticancer Res. 2016;36:659–664. [PubMed] [Google Scholar]
- De Benedetto A., Qualia C.M., Baroody F.M., Beck L.A. Filaggrin expression in oral, nasal, and esophageal mucosa. J. Invest. Dermatol. 2008;128:1594–1597. doi: 10.1038/sj.jid.5701208. [DOI] [PubMed] [Google Scholar]
- Faraldo M.M., Teuliere J., Deugnier M.A., Taddei-De La Hosseraye I., Thiery J.P., Glukhova M.A. Myoepithelial cells in the control of mammary development and tumorigenesis: data from genetically modified mice. J. Mammary Gland Biol. Neoplasia. 2005;10:211–219. doi: 10.1007/s10911-005-9582-8. [DOI] [PubMed] [Google Scholar]
- Fillies T., Werkmeister R., Packeisen J., Brandt B., Morin P., Weingart D., Joos U., Buerger H. Cytokeratin 8/18 expression indicates a poor prognosis in squamous cell carcinomas of the oral cavity. BMC Cancer. 2006;6:10. doi: 10.1186/1471-2407-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu B., Watanabe K., Sun P., Fallahi M., Dai X. Chromatin effector Pygo2 mediates Wnt-notch crosstalk to suppress luminal/alveolar potential of mammary stem and basal cells. Cell Stem Cell. 2013;13:48–61. doi: 10.1016/j.stem.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Smyth G.K. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J. Immunol. Methods. 2009;347:70–78. doi: 10.1016/j.jim.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Huebner R.J., Lechler T., Ewald A.J. Developmental stratification of the mammary epithelium occurs through symmetry-breaking vertical divisions of apically positioned luminal cells. Development. 2014;141:1085–1094. doi: 10.1242/dev.103333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamboulas C., Ailles L. Developmental signaling pathways in cancer stem cells of solid tumors. Biochim. Biophys. Acta. 2013;1830:2481–2495. doi: 10.1016/j.bbagen.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Kawano Y., Kypta R. Secreted antagonists of the Wnt signalling pathway. J. Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- Larjava H., Koivisto L., Hakkinen L., Heino J. Epithelial integrins with special reference to oral epithelia. J. Dental Res. 2011;90:1367–1376. doi: 10.1177/0022034511402207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latil M., Nassar D., Beck B., Boumahdi S., Wang L., Brisebarre A., Dubois C., Nkusi E., Lenglez S., Checinska A. Cell-type-specific chromatin states differentially prime squamous cell carcinoma tumor-initiating cells for epithelial to mesenchymal transition. Cell Stem Cell. 2017;20:191–204.e5. doi: 10.1016/j.stem.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J., Kang X., Halifu Y., Zeng X., Jin T., Zhang M., Luo D., Ding Y., Zhou Y., Yakeya B. Secreted frizzled-related protein promotors are hypermethylated in cutaneous squamous carcinoma compared with normal epidermis. BMC Cancer. 2015;15:641. doi: 10.1186/s12885-015-1650-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W.S., Ho Y., Lin Y.W., Naveen Raj E., Liu K.K., Chen C., Zhou X.Z., Lu K.P., Chao J.I. Targeting EGFR of triple-negative breast cancer enhances the therapeutic efficacy of paclitaxel- and cetuximab-conjugated nanodiamond nanocomposite. Acta Biomater. 2019;86:395–405. doi: 10.1016/j.actbio.2019.01.025. [DOI] [PubMed] [Google Scholar]
- Lien W.H., Guo X., Polak L., Lawton L.N., Young R.A., Zheng D., Fuchs E. Genome-wide maps of histone modifications unwind in vivo chromatin states of the hair follicle lineage. Cell Stem Cell. 2011;9:219–232. doi: 10.1016/j.stem.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim X., Nusse R. Wnt signaling in skin development, homeostasis, and disease. Cold Spring Harb. Perspect. Biol. 2013;5:a008029. doi: 10.1101/cshperspect.a008029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Millar S.E. Wnt/beta-catenin signaling in oral tissue development and disease. J. Dental Res. 2010;89:318–330. doi: 10.1177/0022034510363373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S., Ren C., Liu Y., Epner D.E. PI3K-Akt signaling is involved in the regulation of p21(WAF/CIP) expression and androgen-independent growth in prostate cancer cells. Int. J. Oncol. 2006;28:245–251. [PubMed] [Google Scholar]
- Lu X., Lu D., Scully M., Kakkar V. The role of integrins in cancer and the development of anti-integrin therapeutic agents for cancer therapy. Perspect. Med. Chem. 2008;2:57–73. [PMC free article] [PubMed] [Google Scholar]
- Matsuyama M., Aizawa S., Shimono A. Sfrp controls apicobasal polarity and oriented cell division in developing gut epithelium. PLoS Genet. 2009;5:e1000427. doi: 10.1371/journal.pgen.1000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y., Wang Q.G., Wang J.X., Zhu S.T., Jiao Y., Li P., Zhang S.T. Epigenetic inactivation of the SFRP1 gene in esophageal squamous cell carcinoma. Dig. Dis. Sci. 2011;56:3195–3203. doi: 10.1007/s10620-011-1734-7. [DOI] [PubMed] [Google Scholar]
- Morel A.P., Lievre M., Thomas C., Hinkal G., Ansieau S., Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008;3:e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muroyama A., Lechler T. Polarity and stratification of the epidermis. Semin. Cell Dev. Biol. 2012;23:890–896. doi: 10.1016/j.semcdb.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar D., Blanpain C. Cancer stem cells: basic concepts and therapeutic implications. Annu. Rev. Pathol. 2016;11:47–76. doi: 10.1146/annurev-pathol-012615-044438. [DOI] [PubMed] [Google Scholar]
- Owens D.M., Romero M.R., Gardner C., Watt F.M. Suprabasal alpha6beta4 integrin expression in epidermis results in enhanced tumourigenesis and disruption of TGFbeta signalling. J. Cell Sci. 2003;116:3783–3791. doi: 10.1242/jcs.00725. [DOI] [PubMed] [Google Scholar]
- Porcheri C., Meisel C.T., Mitsiadis T. Multifactorial contribution of notch signaling in head and neck squamous cell carcinoma. Int. J. Mol. Sci. 2019;20:1520. doi: 10.3390/ijms20061520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presland R.B., Dale B.A. Epithelial structural proteins of the skin and oral cavity: function in health and disease. Crit. Rev. Oral Biol. Med. 2000;11:383–408. doi: 10.1177/10454411000110040101. [DOI] [PubMed] [Google Scholar]
- Prince M.E., Sivanandan R., Kaczorowski A., Wolf G.T., Kaplan M.J., Dalerba P., Weissman I.L., Clarke M.F., Ailles L.E. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc. Natl. Acad. Sci. U S A. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos D.M., But M., Regezi J., Schmidt B.L., Atakilit A., Dang D., Ellis D., Jordan R., Li X. Expression of integrin beta 6 enhances invasive behavior in oral squamous cell carcinoma. Matrix Biol. 2002;21:297–307. doi: 10.1016/s0945-053x(02)00002-1. [DOI] [PubMed] [Google Scholar]
- Regan J.L., Sourisseau T., Soady K., Kendrick H., McCarthy A., Tang C., Brennan K., Linardopoulos S., White D.E., Smalley M.J. Aurora A kinase regulates mammary epithelial cell fate by determining mitotic spindle orientation in a Notch-dependent manner. Cell Rep. 2013;4:110–123. doi: 10.1016/j.celrep.2013.05.044. [DOI] [PubMed] [Google Scholar]
- Renstrom J., Istvanffy R., Gauthier K., Shimono A., Mages J., Jardon-Alvarez A., Kroger M., Schiemann M., Busch D.H., Esposito I. Secreted frizzled-related protein 1 extrinsically regulates cycling activity and maintenance of hematopoietic stem cells. Cell Stem Cell. 2009;5:157–167. doi: 10.1016/j.stem.2009.05.020. [DOI] [PubMed] [Google Scholar]
- Rosso S.B., Sussman D., Wynshaw-Boris A., Salinas P.C. Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nat. Neurosci. 2005;8:34–42. doi: 10.1038/nn1374. [DOI] [PubMed] [Google Scholar]
- Satoh W., Gotoh T., Tsunematsu Y., Aizawa S., Shimono A. Sfrp1 and Sfrp2 regulate anteroposterior axis elongation and somite segmentation during mouse embryogenesis. Development. 2006;133:989–999. doi: 10.1242/dev.02274. [DOI] [PubMed] [Google Scholar]
- Sogabe Y., Suzuki H., Toyota M., Ogi K., Imai T., Nojima M., Sasaki Y., Hiratsuka H., Tokino T. Epigenetic inactivation of SFRP genes in oral squamous cell carcinoma. Int. J. Oncol. 2008;32:1253–1261. doi: 10.3892/ijo_32_6_1253. [DOI] [PubMed] [Google Scholar]
- Song X.L., Huang B., Zhou B.W., Wang C., Liao Z.W., Yu Y., Zhao S.C. miR-1301-3p promotes prostate cancer stem cell expansion by targeting SFRP1 and GSK3beta. Biomed. Pharmacother. 2018;99:369–374. doi: 10.1016/j.biopha.2018.01.086. [DOI] [PubMed] [Google Scholar]
- Steinhart Z., Angers S. Wnt signaling in development and tissue homeostasis. Development. 2018;145:dev146589. doi: 10.1242/dev.146589. [DOI] [PubMed] [Google Scholar]
- Thiery J.P., Acloque H., Huang R.Y., Nieto M.A. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Tumbar T., Guasch G., Greco V., Blanpain C., Lowry W.E., Rendl M., Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribe P., Gonzalez S. Epidermal growth factor receptor (EGFR) and squamous cell carcinoma of the skin: molecular bases for EGFR-targeted therapy. Pathol. Res. Pract. 2011;207:337–342. doi: 10.1016/j.prp.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Veeck J., Niederacher D., An H., Klopocki E., Wiesmann F., Betz B., Galm O., Camara O., Durst M., Kristiansen G. Aberrant methylation of the Wnt antagonist SFRP1 in breast cancer is associated with unfavourable prognosis. Oncogene. 2006;25:3479–3488. doi: 10.1038/sj.onc.1209386. [DOI] [PubMed] [Google Scholar]
- Vicent S., Lopez-Picazo J.M., Toledo G., Lozano M.D., Torre W., Garcia-Corchon C., Quero C., Soria J.C., Martin-Algarra S., Manzano R.G. ERK1/2 is activated in non-small-cell lung cancer and associated with advanced tumours. Br. J. Cancer. 2004;90:1047–1052. doi: 10.1038/sj.bjc.6601644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waghmare S.K., Bansal R., Lee J., Zhang Y.V., McDermitt D.J., Tumbar T. Quantitative proliferation dynamics and random chromosome segregation of hair follicle stem cells. EMBO J. 2008;27:1309–1320. doi: 10.1038/emboj.2008.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellner U., Schubert J., Burk U.C., Schmalhofer O., Zhu F., Sonntag A., Waldvogel B., Vannier C., Darling D., zur Hausen A. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat. Cell Biol. 2009;11:1487–1495. doi: 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.