Abstract
Targeted oncolytic adenoviruses can selectively replicate in cancer cells; combined with traditional chemotherapy drugs, this approach is expected to become an important treatment method for overcoming the current bottleneck of osteosarcoma treatment. Here, we investigate the effect of oncolytic adenovirus Ad11 combined with cisplatin on autophagy in osteosarcoma cells. Immunohistochemistry was used to detect CD46 expression in patients with osteosarcoma. A cytotoxicity assay was employed to detect the killing effect of Ad11, cisplatin and their combination on osteosarcoma cells under different time scenarios. Expression of autophagy proteins Beclin1, ATG3, and LC3A/B under treatment of osteosarcoma cells with Ad11, cisplatin and their combination under different time scenarios was detected by immunofluorescence and western blotting. We found that the oncolytic adenovirus Ad11 synergizes with cisplatin to kill osteosarcoma cells and that the synergistic effect was greatest when cells were first treated with Ad11. This synergy is due to oncolytic adenovirus Ad11-mediated inhibition of autophagy, which enhanced the sensitivity of cells to chemotherapy. In conclusion, this study provides evidence that the oncolytic adenovirus Ad11 can enhance the effect of chemotherapy by inhibiting autophagy. The findings provide a cytological basis for the treatment of osteosarcoma with oncolytic adenovirus combined with cisplatin.
Keywords: Osteosarcoma, virotherapy, oncolytic adenovirus, autophagy, chemotherapy, cisplatin
Introduction
Osteosarcoma (OS), a primary malignant bone tumour derived from mesenchymal tissue, has a worldwide incidence of 3.4 per million people per year [1]. Treatment of this bone cancer, the third most common malignancy in children and adolescents, was once mainly amputation, with very limited efficacy. However, since the mid-1970s, multidrug chemotherapy, including cisplatin, doxorubicin, high-dose methotrexate, and ifosfamide, and surgical strategies have increased the 5-year overall survival rate of OS to 70%-80% [2]. Nonetheless, from the 1970s to the present, the treatment of OS has entered a 50-year bottleneck. Additionally, approximately 20% of OS patients present with metastasis at the time of diagnosis, with 90% occurring in the lungs, with a 5-year survival rate of less than 20% [3,4]. Overall, due to the seriousness of OS, new treatment modalities are urgently needed.
Oncolytic virus therapy, which selectively replicates and kills cancer cells without damaging normal tissues, may constitute the next major breakthrough in cancer treatment [5]. Oncolytic adenovirus is a non-enveloped, dsDNA virus with a linear genome of 36 kb and is capable of selectively replicating in cancer cells. Approved by the China National Food and Drug Administration, adenovirus type 5 enters a cell through host-expressed Coxsackie adenovirus receptor (CAR) (Ad5 receptor) [6,7]. To date, many clinical trials on oncolytic viruses covering four OV agents (Oncorine, OrienX010, KH901, and H103) are ongoing, and additional OV agents are awaiting approval for clinical trials in China. However, there are few studies investigating oncolytic viruses for the treatment of osteosarcoma. Tirino V and others [8] tested in human osteosarcoma cell lines, primary cell cultures, as well as subcutaneous osteosarcoma xenografts, Ad5 demonstrated limited antitumour responses at low-doses of the virus. One limitation in the use of oncolytic adenovirus is that they bind directly to CAR and large number of malignant cells, including osteosarcoma, express low levels of CAR which limits entry of viral particles into osteosarcoma cells [9]. Therefore, many tumour cells do not express or underexpress CAR, which leads to unsatisfactory transfection efficiency of Ad5.
Our previous studies demonstrated that adenovirus serotype 11 (Ad11) [10], a group B adenovirus, utilizes the complement-regulating protein CD46 [11,12], a receptor that is widely expressed in osteosarcoma cell lines [13]. In a previous study, we inserted telomerase reverse transcriptase (TERT) and enhanced green fluorescent protein (GFP) into Ad11, which resulted in a high degree of replication and production of green fluorescence [14,15].
Cisplatin (DDP), a frontline chemotherapeutic drug, is widely used to treat various tumours, including osteosarcoma. The main role of cisplatin in the treatment of OS is to inhibit DNA synthesis at high doses and to inhibit RNA and protein synthesis at low doses [16,17]. This action similar to an alkylating agent is produced by the platinum ion after hydration [18]. In general, cisplatin exerts cytotoxic effects in tumour cells mainly through the generation of DNA-platinum adducts and subsequent DNA damage response. Some patients present with intrinsic or acquired resistant to cisplatin, leading to chemoresistance. However, the underlying mechanisms of cisplatin resistance are still unknown [19].
Autophagy is an intracellular degradation process identified more than 50 years ago. The 2016 Nobel Prize award to Pr Ohsumi for his research on autophagy renewed interest for the involvement of this process in medicine field and autophagy is now recognized as a critical process in osteosarcoma [20]. A large number of studies have confirmed that autophagy dysfunction is inextricably linked to the occurrence and development of malignant tumours [21,22]. Although autophagy’s role in cancer is complex and context-dependent, pharmacological modulation of this process is emerging in clinical trials. However, understanding the relationship between autophagy and osteosarcoma chemotherapy is an essential prerequisite.
Overall, the therapeutic approach of an oncolytic adenovirus combined with chemotherapy is being increasingly researched, and indeed, this has become a hot topic for the treatment of OS [23]. Although chemotherapy is one of the basic treatments for OS, as represented by cisplatin [24], tumour cell resistance and excessive side effects, such as nephrotoxicity, severe nausea and vomiting, myelosuppression, ototoxicity and neurotoxicity, severely limit its efficacy [25]. Numerous studies have focused on finding ways to counteract chemoresistance, and we have reported that autophagy is closely related to chemotherapy resistance [26]. Therefore, our research is dedicated to exploring the role of autophagy in the growth of OS cells [27].
In this study, we applied Ad11-hTERT-EGFP, as described above and previously modified, and cisplatin to treat OS cell lines for different lengths of time and evaluated expression of autophagy-related factors. The findings suggest that oncolytic adenovirus Ad11 combined with cisplatin can be used to treat OS.
Materials and methods
Immunohistochemistry
A total of 20 pairs of osteosarcoma tissue samples and adjacent tissues were obtained from patients with osteosarcoma, who underwent surgical resection at the First Affiliated Hospital of Zhengzhou University between January 2013 and June 2018. Patients receiving chemotherapy or radiotherapy prior to surgical treatment were excluded from the study. The diagnosis and Enneking stage of osteosarcoma was assessed by two experienced pathologists independently from the Department of Pathology of the First Affiliated Hospital of Zhengzhou University, in a blinded manner, and according to the Enneking system [28] and the 2015 World Health Organization Classification of Tumours [29]. Information about the OS patients from whom the samples were obtained, such as age, sex, Enneking stage, tumour location and histological subtype, was collected from medical records (Table 1). All participants signed the informed consent form. The study was approved by the ethics committee of the First Affiliated Hospital of Zhengzhou University.
Table 1.
Clinical characteristics of the 20 osteosarcoma patients
| Clinic pathological parameters | Cases | Percent (%) |
|---|---|---|
| Gender | ||
| Male | 13 | 65 |
| Female | 7 | 35 |
| Age (years) | ||
| < 23.49 | 18 | 90 |
| ≥ 23.49 | 2 | 10 |
| Enneking stage | ||
| I | 1 | 5 |
| II A | 6 | 30 |
| II B | 10 | 50 |
| III | 3 | 15 |
| Tumor location | ||
| Proximal femur | 2 | 10 |
| Distal femur | 7 | 35 |
| Proximal tibia | 6 | 30 |
| Distal tibia | 1 | 5 |
| Proximal humerus | 1 | 5 |
| Distal humerus | 1 | 5 |
| Other | 2 | 10 |
| Histological subtype | ||
| Traditional | 11 | 55 |
| Other | 9 | 45 |
Immunohistochemistry was used to detect CD46 expression in OS pathological sections using a standard immunohistochemistry (IHC) protocol in all cases. Tissue sections (4-µm-thick slice) were cut from paraffin blocks, deparaffinised, dehydrated, and subjected to antigen retrieval. After blocking, each section was incubated with an anti-CD46 antibody (1:100, Sigma-Aldrich, St Louis, MO, USA) overnight at 4°C. A PV-9000 two-step immunohistochemical staining kit (Zhongshan Jinqiao Biotechnology Co. Ltd., Beijing, China) was used, and 3, 3’-diaminobenzidine tetra hydrochloride staining with haematoxylin counterstaining was conducted according to the manufacturer’s protocol. Finally, a neutral resin was applied to fix the sections.
Cell lines and cell cultures
JH293, the human kidney epithelial cell line transformed with Ad11 DNA, was obtained from Cancer Research UK Central Cell Services (London, United Kingdom) and maintained in Dulbecco’s modified essential medium (DMEM) (GIBCO Corporation, USA) with 10% foetal bovine serum (FBS) (GIBCO Corporation, USA). Human OS cell lines MG-63, SaOS2, HOS, and U2OS and the K7M2 mouse cell line were purchased from Shanghai Cell Collection (Chinese Academy of Sciences, China). Confirmation of no Mycoplasma infection and STR (Short Tandem Repeat) proof provided by the institution. MG-63, SaOS2, HOS and K7M2 cells were cultured in (DMEM) containing 10% FBS (GIBCO Corporation, USA), 4 mM glutamine, 100 U/ml penicillin, and 100 U/ml streptomycin. U2OS cells were routinely maintained in RPMI 1640 medium supplemented with 10% FBS, 100 U/ml penicillin and 100 U/ml streptomycin. The culture medium was changed every 3 to 4 days. All cells were maintained at 37°C in a humidified atmosphere containing 5% CO2 and confirmed to be mycoplasma-free before being used experimentally.
Generation and purification of the oncolytic virus
Modified Ad11-hTERT-EGFP was previously constructed by Dr. Zhang, the First Affiliated Hospital of Zhengzhou University, and stored in our laboratory. Our laboratory staff has confirmed no contamination of the wild-type virus. Ad11-hTERT-EGFP was amplified in JH293 cells, and large-scale purification was performed by ultracentrifugation using a caesium chloride gradient, followed by dialysis. Briefly, JH 293 cells were seeded in 96-well plates at 1 × 104 Cells/well in 200 µl of 10% FBS in DMEM. Harvested samples were serially diluted and aliquots of 22 µl added to the JH 293 cells [30]. Virus titres in JH 293 cells were determined by the limiting dilution method (determination of 50% tissue culture infective dose (TCID50)). The calculation used was as follows: TCID50/ml = 10(PD-a)/b, where PD = proportional distance, a = log dilution greater than 50% infected, and b = inoculum volume (ml). PD = % of wells infected over 50%-50%)/(% of wells infected over 50%) - (% of wells infected less than 50%) [31].
Cell viability assay
First, cells were seeded at 2 × 103 per well in 96-well plates in the corresponding medium with 2% FBS and infected with viruses 18 h later at a starting MOI (multiplicity of infection) = 1 × 104 pfu/cell. Cell survival on day 6 after viral infection was determined by the MTS assay, and EC50 values were calculated [30]. Second, we treated each cell line with different concentrations of cisplatin (Selleck, Shanghai, China) to obtain IC25 (25% inhibitory concentration) and IC50 (50% inhibitory concentration) values for each. Lastly, we performed the experiment using two groups: the first was treated with the virus alone, drug alone, and virus for 24 hours before adding the drug; the second was treated with the virus alone, drug alone, and drug for 24 hours before adding the virus. After 48 hours of incubation, MTS and PMS (MTS:PMS 20:1) were added to each well, and after mixing for 4 hours, absorbance at 595 nm was measured using a microplate reader (TECAN, Austria).
Immunofluorescence
Cells (1 × 104 cells/well) were seeded in 24-well culture plates containing slides. After incubation for 24 hours, 4% paraformaldehyde (PFA) was added for 15 minutes to fix the cells, followed by Triton X-100 for 30 minutes to permeabilize the cells. The cells were blocked with PBS containing 10% goat serum and allowed to react with the primary antibody overnight at 4°C. A fluorescein-conjugated goat anti-rabbit secondary antibody (1:100; Cell Signaling Technology, Beverly, MA, USA) was then added for 60 minutes at room temperature. Nuclei were stained with 4, 6-diamidino-2-phenylindole (DAPI) (1 μg/ml; Cell Signaling Technology, Beverly, MA, USA). The cell sheets were fixed with glycerol containing an anti-quenching agent and covered with a coverslip. All samples were examined under an inverted microscope using AxioVision (Axiovert 200 M, Carl Zeiss Jena GmbH, Germany).
Western blot analysis and antibodies
MG63 and U2OS cells were seeded in 6-well plates at 5 × 105 cells per well using the same grouping and processing methods as described above. Total protein was extracted using cell lysis buffer supplemented with IP (Beyotime, Nantong, China) and PMSF (Sigma-Aldrich, St Louis, MO, USA). The Bradford assay was employed to estimate the protein concentration. Equal amounts (50 μg) of protein supplemented with 5 × LSB loading dye were separated by 10% SDS-PAGE and transferred to Turbo Midi PVDF membranes by semi-dry blotting. After blocking for 1 h at room temperature with a blocking solution (10 mmol/l Tris-HCl, pH 7.4, 150 mmol/L NaCl and 0.05% Tween 20 and 5% dry milk), the membrane was incubated overnight with the primary antibody (1:1,000; Cell Signaling Technology, Beverly, MA, USA) diluted in blocking solution at 4°C. The membrane was then probed with horseradish peroxidase-linked sheep anti-rabbit or anti-mouse IgG, and the blots were developed using ECL detection reagents (Amersham Biosciences). To ensure uniform sample loading, the blots were re-probed with an anti-GAPDH (1:1,000; Cell Signaling Technology, Beverly, MA, USA) antibody. The density of the bands on the membrane was scanned and analysed using an image analyser.
Statistical analysis
All results are expressed as the mean ± standard deviation (SD). Statistical analysis of the data was performed using SPSS software, version 21.0. Statistical significance was assessed using a Student’s t test or a Chi-square test, as appropriate, and differences between groups were considered significant at P < 0.05.
Results
Immunohistochemistry (IHC) analysis showed strong expression of CD46 in osteosarcoma patients
To evaluate expression of CD46, we performed pathological analysis of 40 tissue sections, including 20 osteosarcoma tissue sections and 20 adjacent normal tissue sections. Among 20 osteosarcoma pathological sections, 19 exhibited high expression and 1 low expression. In contrast, low expression of CD46 in all the 20 adjacent normal tissue sections (Figure 1).
Figure 1.
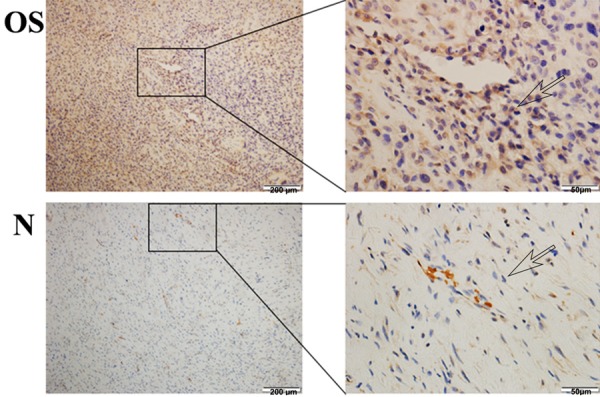
Immunohistochemistry analysis of CD46 expression on osteosarcoma tissues. Illustrations of CD46 expression on osteosarcoma tissues and normal tissue. (Original magnification 200 ×, 400 ×) (OS denotes osteosarcoma; N denotes normal tissue).
Cytotoxicity of Ad11-hTERT-EGFP in four human osteosarcoma cell lines and one murine osteosarcoma cell line
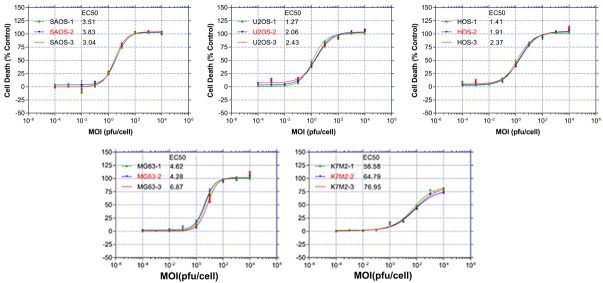
Ad11 infection and killing of four common human osteosarcoma cell lines (MG63, HOS, U2OS and SAOS) and one murine cell line (K7M2) were examined by the MTS assay. According to the results, the growth of the OS cells infected with Ad11-hTERT-EGFP was inhibited to differing degrees, which occurred in a dose-dependent manner (Figure 2). We used EC50 values to indicate the degree of Ad11-hTERT-EGFP-mediated killing in different OS lines, as follows: 5.26 pfu/cell for MG63 cells, 1.90 pfu/cell for HOS cells, 1.92 pfu/cell for U2OS cells, 3.46 pfu/cell for SAOS cells, and 66.11 pfu/cell for K7M2 cells. Obviously, the four human OS cell lines are sensitive to Ad11-hTERT-EGFP, whereas the murine cell line K7M2 was not. We then selected two cell lines, MG63 and U2OS, with moderate EC50 values for the ensuing experiments.
Figure 2.
Mortality of 5 osteosarcoma cell lines following Ad11-hTERT-EGFP infection. After 18 hours of cell culture, tenfold dilutions of virus was added at and a starting dose of MOI = 1 × 104 pfu/cell. Six days later, the EC50 value was calculated by the MTS method. All data are representative of three independent experiments performed in triplicate.
Comparison of the killing effect of Ad11-hTERT-EGFP and cisplatin (DDP) on osteosarcoma cells for different time periods
We first performed a cytotoxicity assay for DDP in MG63 and U2OS cells and calculated their IC25 and IC50 values: for MG63 cells, IC25 and IC50 values of 20 µmol/l and 30 µmol/l were obtained; for U2OS cells, IC25 and IC50 values of 10 µmol/l and 25 µmol/l were obtained. We then examined differences in the cell killing effect of Ad11-hTERT-EGFP and DDP combination therapy at different time points.
The feature of this combination experiment was that after adding Ad11-hTERT-EGFP alone to OS cells for 24 hours, DDP was added, and the culture was continued for 48 hours; alternatively, DDP alone was added for 24 hours, after which Ad11-hTERT-EGFP was added for 48 hours (Figure 3). As shown in Figure 3, the combination of virus and drug had a synergistic killing effect in both cell lines. Moreover, the synergistic effect of the combination with the virus added first was greater than that of DDP added first. After reviewing the literature [32,33], we believe that DDP abolished normal intracellular replication and affects the replication of the virus, rendering it ineffective.
Figure 3.
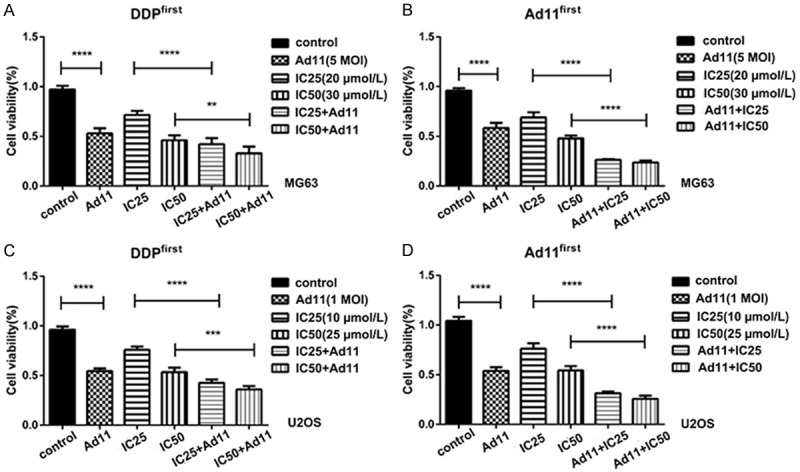
Cell viability after treatment with Ad11 and DDP at different time periods. A. The concentration of DDP was IC25 = 20 µmol/l, and the MOI of Ad11 was 5 pfu/cell. B. The concentration of DDP was IC50 = 30 µmol/l, and the MOI of Ad11 was 5 pfu/cell. C, D. The same treatments using U2OS cells. (** denotes P < 0.05, *** denotes P < 0.01, **** denotes P < 0.001).
Immunofluorescence of autophagy protein LC3A/B in different treatments
To investigate whether autophagy is involved in the synergistic killing effect in combination experiments, we assessed expression of the autophagy marker LC3A/B in OS cells by immunofluorescence. Based on the results shown in Figure 4, the cell morphology of the untreated controls was normal, with good cell growth. DAPI staining revealed very clear nuclei, and no expression of EGFP was found in normal cells. Moreover, the LC3A/B fluorescence distribution was diffuse and weak, with no aggregation. After cisplatin treatment, expression of LC3A/B was significantly enhanced. As presented in the third row of Figure 4, virus-infected cells displayed green fluorescence of EGFP, indicating that the virus replicated normally in these cells. The morphology of the MG63 cells was altered, including rounded, contracted, broken or needle-like shapes, and the number of particles inside the cells increased (Figure 4). Furthermore, expression of LC3A/B in both co-treatment groups was weaker than in the DDP alone group. In addition, expression of LC3A/B was weaker when the virus was added first followed by DDP. Therefore, Ad11 can attenuate the autophagy caused by DDP.
Figure 4.
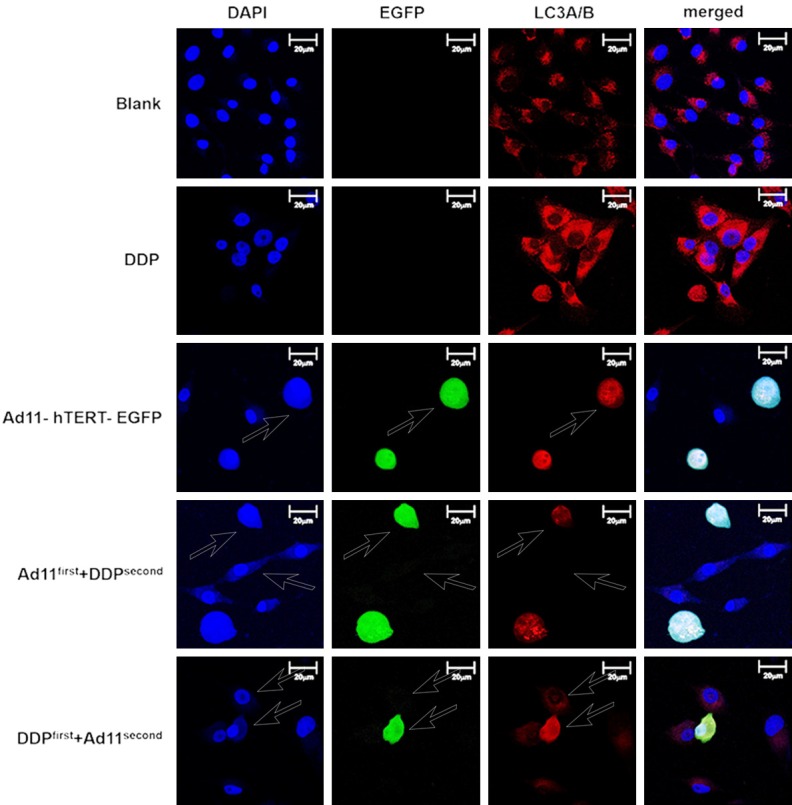
LC3A/B immunofluorescence assay induced by Ad11-hTERT-EGFP and DDP in MG63 cells. Blue fluorescence indicates nuclei stained with DAPI. Green fluorescence indicates Ad11-hTERT-EGFP, and red fluorescence indicates LC3A/B. All images were measured under an inverted microscope using AxioVision at 400 times magnification (Axiovert 200 M, Carl Zeiss Jena GmbH, Germany). Scale bar: 20 µm.
Autophagy is regulated by the combination of Ad11-hTERT-EGFP and chemotherapy
To validate the results of our immunofluorescence experiments and to further explore which autophagy proteins are affected by the combination of virus and chemotherapy, we performed western blot analysis to examine a variety of autophagy-related proteins, focussing on ATG3, Beclin1 and LC3A/B. Due to the damaging effects of chemotherapeutic drugs on cells, autophagy was significantly enhanced after treatment with DDP. However, autophagy proteins ATG3, Beclin1 and LC3A/B were expressed at low levels after treatment with Ad11 in the combination group (Figure 5A). Figure 5B shows a similar phenomenon. Treatment of cells with virus alone resulted in a decrease in autophagy protein expression, and increased autophagy protein expression was found with DDP alone; however, autophagy protein expression was reduced in the combination group. We also performed the same experiment in the U2OS cell line and obtained similar results (Figure 5C, 5D).
Figure 5.
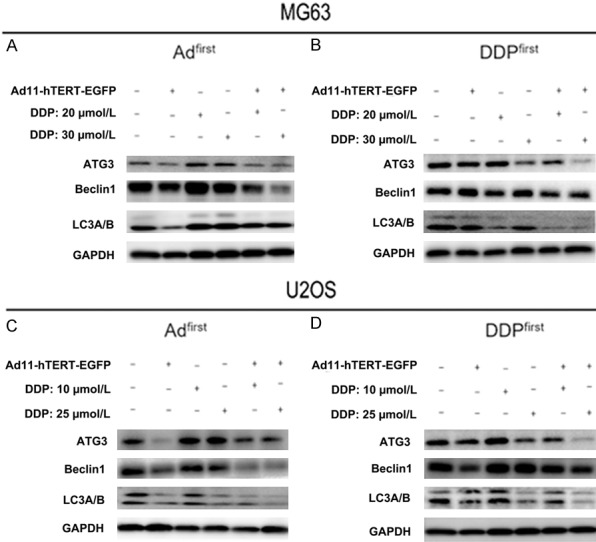
Expression of autophagy-related proteins ATG3, Beclin1 and LC3A/B after different treatments. After treatment, cells were harvested and examined by western blot analysis. GAPDH was used as the internal control (A, B). We repeated the same experiment using the U2OS cell line and obtained the same results (C, D).
Virus replication under different virus and cisplatin treatment times
Ad11-hTERT-EGFP allows for observing the green signal under a fluorescence microscope; the intensity of fluorescence indirectly reflects the degree of virus replication in the cell, and we can thus deduce the relationship between the degree of viral replication and the extent to which autophagy is affected. The fluorescence intensity of the group treated with the virus first was stronger than that of the group treated with DDP first (Figure 6), and this was found in both MG63 and U2OS cells. This finding also indicates that addition of DDP attenuated the replication process of Ad11, further affecting Ad11-hTERT-EGFP-mediated inhibition of autophagy.
Figure 6.
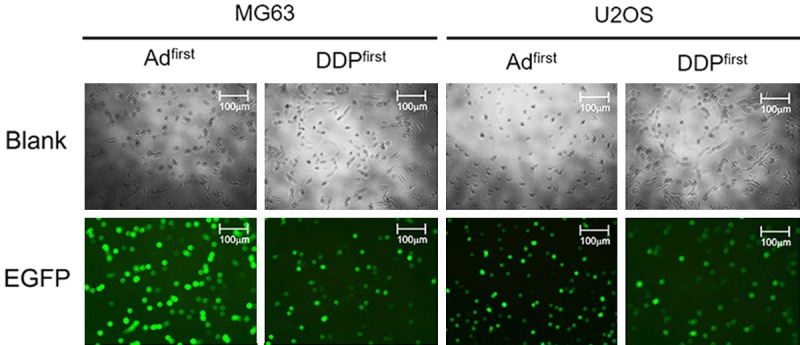
Expression of EGFP after different treatments. The first column shows the results of adding the virus for 24 hours and then adding DPP for 48 hours. The second column shows the results of adding DDP for 24 hours and then adding the virus for 48 hours. The results were observed by fluorescence microscopy at 100 times magnification. The same experiment was repeated using U2OS cells. Scale bar: 100 µm.
Discussion
Since the US Food and Drug Administration (FDA) approved Amgen’s oncolytic virus therapy for the treatment of melanoma in October 2015, an important period of recombinant oncolytic virus research has ensued [34]. Indeed, viral therapy combined with gene therapy, immunotherapy, and chemotherapy is developing rapidly. Based on the results of combination therapy applied for OS in this study, we believe that viral therapy combined with chemotherapy is highly practical.
The Ad11 virus we studied was previously constructed using Ad5 and Ad11 [10]. It contains the entire Ad5 E1A enhancer (including the packaging signal) and the promoter as a replacement of the corresponding region of Ad11, and its infectivity and toxicity are higher than that of Ad11. The receptor for AD11 is CD46, a membrane cofactor protein that is highly expressed on the surface of OS cells [35]. Takagi-Kimura M and others [13] from Hyogo College of Medicine in Japan used flow cytometry to show that CD46 is highly expressed on five OS cell lines commonly used in the laboratory. As shown in Figure 1, we confirmed the high level of CD46 expression. Naiara and others [36] from The Health Research Institute of Navarra in Spain indicate that, in osteosarcoma cell lines, adenovirus replicated in vitro, expressed the PH20 enzyme, and exerted a robust oncolytic effect. The addition of hTERT and EGFP conferred tissue selectivity on Ad11 for OS and facilitated fluorescence microscopy. Therefore, Ad11-hTERT-EGFP has strong potential for the treatment of OS [37,38].
Cisplatin is an effective antitumour agent with a wide spectrum of activity against solid tumours, and the inclusion of cisplatin in osteosarcoma treatment has improved outcome for patients with high grade disease [39]. DDP inhibits replication and transcription, leading to the activation of multiple signaling pathways to induce cell cycle arrest, apoptosis and autophagy. Unfortunately, OS cells can repair damaged DNA by reverse transcription, base excision repair, nucleotide excision repair and mismatch repair, allowing them to continue to proliferate and resulting in drug resistance and weaken effects of chemotherapy drugs [40,41]. Therefore, drug resistance and its main side effects such as nephrotoxicity and myelosuppression by high-dose cisplatin also reduce its benefits in patients with OS and even threaten their lives [42,43]. However, our experiments showed that in OS cells, treatment with Ad11-hTERT-EGFP for 24 h and then treatment with cisplatin for 48 h inhibited the development of drug resistance as well as increased sensitivity to cisplatin and improved its efficacy. In the study of the OS cell lines SaOs-2, MG-63 and U2OS, H C A Graat, VU University Medical Center, The Netherlands, confirmed that simultaneous treatment of OS cell lines with Ad5-Δ24RGD and cisplatin yielded additive to synergistic effects [44].
For patients with poor tolerance to cisplatin, this approach may increase the drug effect of cisplatin, whereby the dose can be reduced until the patient can tolerate it, achieving the same effect. In contrast, when adding the virus after adding cisplatin, the virus exhibited difficulty in entering OS cells, and only a small amount of fluorescence was observed (Figure 6). This may be due to cisplatin destroying the normal DNA replication of these cells or inactivating telomerase; even if the virus enters the cell, it cannot replicate through the hTERT promoter to produce GFP [45,46].
Autophagy is a critical process by which cells self-digest and recycle inessential or ineffectual cellular components to maintain homeostasis, especially under conditions of adverse conditions of survival [47]. Lots of studies have demonstrated that autophagy is used by tumour cells to repress initial steps of carcinogenesis and/or support the survival and growth of established tumours. Autophagy is considered a double-edged sword, with some asserting that it is a cell protection mechanism but others having the opposite view [48]. In osteosarcoma, autophagy appears to be deregulated and could also act both as a pro or anti-tumoural process [49]. Enhancement of autophagy in OS cells may enable cells to avoid apoptosis and adapt to harsh conditions such as starvation, chemotherapy, and radiation therapy [50-52]. Nonetheless, excessive autophagy may kill the cells themselves, as a large number of autophagosomes have been found in the cytoplasm of dead cells, which is why autophagy is called type 2 programmed cell death [53].
In our study, autophagy in OS cells treated with cisplatin was enhanced, which may be a protective mechanism against chemotherapeutic agents [54]. After adding Ad11-hTERT-EGFP, we found reduced levels of autophagy-related proteins, and we suggest that Ad11-hTERT-EGFP disrupts this protection mechanism. In other words, the sensitivity of chemotherapy could be increased by the down-regulating autophagy. In the experiment of Shao et al. [54], osteosarcoma cell lines were exposed to 3-methyladenine (3-MA) (6 mmol/L), autophagy inhibitor, and DDP (1.25, 2.5, 5, 10, 20 and 40 μg/mL) simultaneously, and it was found that the inhibition rates in 3-MA+DDP groups were significantly higher than those in DDP groups. Therefore, we conclude that the Ad11-hTERT-EGFP we studied is likely to play a similar role to the autophagy inhibitor 3-MA.
In the study of autophagy and OS, the autophagy regulatory protein Beclin1 and the PI3K/AKT/mTOR signalling pathways have become the most important research topics [27,55,56]. Beclin1 is a critical regulator of autophagy and is involved in the formation of the autophagosome through the Beclin1-Vps34-Vps15 complex. Beclin1 help the class III phosphoinositide 3 kinase (PI3K) Vps34 to catalyse the formation of PI(3)P, an essential early step during phagophore formation [57], and specifically inhibiting expression of the early autophagy targeting protein Beclin1 can significantly enhance the sensitivity of OS to anticancer drugs [58,59]. Studies in HEK293 cells have revealed that ATG3 promotes formation of the ATG12-ATG5 complex, which is a key step in the conversion of LC3A to LC3B. Specifically, since LC3 has been considered a specific marker of autophagosome formation, it is widely monitored as an autophagy-related protein. LC3A is a precursor protein of LC3B, and LC3A can be converted to LC3B as the autophagy progresses. In general, the ratio of LC3B to LC3A reflects the autophagy process. The higher the ratio, the stronger the autophagy process [60]. Obviously, we believe that ATG3 is one of the key factors involved in the production of the autophagy regulatory protein LC3B [61,62]. Wu et al. used deoxyribozyme to inhibit Beclin1, and by observing LC-II expression, found that inhibition of Beclin1 at the mRNA level significantly suppressed the formation of autophagosomes [52]. After 24 hours of Ad11-hTERT-EGFP treatment, viral replication in MG63 and U2OS cells may have disrupted the Beclin1-related pathway induced by autophagy, resulting in a decrease in both ATG3 and LCA/B expression. Therefore, after adding cisplatin, the cells could not invoke protective autophagy, and cell death eventually increased.
Conclusion
In conclusion, the results of our study indicate that Ad11-hTERT-EGFP combined with cisplatin has a synergistic effect in the treatment of OS cells. The reason may be that the Beclin1-related autophagy pathway is inhibited after treatment with Ad11-hTERT-EGFP, resulting in attenuation of the resistance of cells to cisplatin and increased cell death. Our study provides a cytological basis for future therapies involving viral and chemotherapy combination for the treatment of OS and demonstrates that in such combination therapy, the use of virus before chemotherapy can enhance efficacy.
Acknowledgements
This work was supported by the Science and Technology Program of Henan Province (No. 182102310370, 192102310389). The authors thank the School of medical sciences, Zhengzhou University for the technical guidance.
Disclosure of conflict of interest
None.
References
- 1.Mirabello L, Troisi RJ, Savage SA. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int J Cancer. 2009;125:229–234. doi: 10.1002/ijc.24320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aljubran AH, Griffin A, Pintilie M, Blackstein M. Osteosarcoma in adolescents and adults: survival analysis with and without lung metastases. Ann Oncol. 2009;20:1136–1141. doi: 10.1093/annonc/mdn731. [DOI] [PubMed] [Google Scholar]
- 3.Wesolowski R, Budd GT. Use of chemotherapy for patients with bone and soft-tissue sarcomas. Cleve Clin J Med. 2010;77(Suppl 1):S23–26. doi: 10.3949/ccjm.77.s1.05. [DOI] [PubMed] [Google Scholar]
- 4.Wittig JC, Bickels J, Priebat D, Jelinek J, Kellar-Graney K, Shmookler B, Malawer MM. Osteosarcoma: a multidisciplinary approach to diagnosis and treatment. Am Fam Physician. 2002;65:1123–1132. [PubMed] [Google Scholar]
- 5.Garcia-Moure M, Martinez-Velez N, Patino-Garcia A, Alonso MM. Oncolytic adenoviruses as a therapeutic approach for osteosarcoma: a new hope. J Bone Oncol. 2017;9:41–47. doi: 10.1016/j.jbo.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu TC, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract Oncol. 2007;4:101–117. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- 7.Yu W, Fang H. Clinical trials with oncolytic adenovirus in China. Curr Cancer Drug Targets. 2007;7:141–148. doi: 10.2174/156800907780058817. [DOI] [PubMed] [Google Scholar]
- 8.Tirino V, Desiderio V, Paino F, De Rosa A, Papaccio F, Fazioli F, Pirozzi G, Papaccio G. Human primary bone sarcomas contain CD133+ cancer stem cells displaying high tumorigenicity in vivo. FASEB J. 2011;25:2022–2030. doi: 10.1096/fj.10-179036. [DOI] [PubMed] [Google Scholar]
- 9.Douglas JT, Kim M, Sumerel LA, Carey DE, Curiel DT. Efficient oncolysis by a replicating adenovirus (ad) in vivo is critically dependent on tumor expression of primary ad receptors. Cancer Res. 2001;61:813–817. [PubMed] [Google Scholar]
- 10.Wong HH, Jiang G, Gangeswaran R, Wang P, Wang J, Yuan M, Wang H, Bhakta V, Muller H, Lemoine NR, Wang Y. Modification of the early gene enhancer-promoter improves the oncolytic potency of adenovirus 11. Mol Ther. 2012;20:306–316. doi: 10.1038/mt.2011.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaggar A, Shayakhmetov DM, Lieber A. CD46 is a cellular receptor for group B adenoviruses. Nat Med. 2003;9:1408–1412. doi: 10.1038/nm952. [DOI] [PubMed] [Google Scholar]
- 12.Sirena D, Lilienfeld B, Eisenhut M, Kalin S, Boucke K, Beerli RR, Vogt L, Ruedl C, Bachmann MF, Greber UF, Hemmi S. The human membrane cofactor CD46 is a receptor for species B adenovirus serotype 3. J Virol. 2004;78:4454–4462. doi: 10.1128/JVI.78.9.4454-4462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takagi-Kimura M, Yamano T, Tagawa M, Kubo S. Oncolytic virotherapy for osteosarcoma using midkine promoter-regulated adenoviruses. Cancer Gene Ther. 2014;21:126–132. doi: 10.1038/cgt.2014.7. [DOI] [PubMed] [Google Scholar]
- 14.Li G, Kawashima H, Ogose A, Ariizumi T, Xu Y, Hotta T, Urata Y, Fujiwara T, Endo N. Efficient virotherapy for osteosarcoma by telomerase-specific oncolytic adenovirus. J Cancer Res Clin Oncol. 2011;137:1037–1051. doi: 10.1007/s00432-010-0969-6. [DOI] [PubMed] [Google Scholar]
- 15.Thoma C, Bachy V, Seaton P, Green NK, Greaves DR, Klavinskis L, Seymour LW, Morrison J. Adenovirus serotype 11 causes less long-term intraperitoneal inflammation than serotype 5: implications for ovarian cancer therapy. Virology. 2013;447:74–83. doi: 10.1016/j.virol.2013.08.032. [DOI] [PubMed] [Google Scholar]
- 16.Harder HC, Rosenberg B. Inhibitory effects of anti-tumor platinum compounds on DNA, RNA and protein syntheses in mammalian cells in virtro. Int J Cancer. 1970;6:207–216. doi: 10.1002/ijc.2910060207. [DOI] [PubMed] [Google Scholar]
- 17.Hou Z, Zhou Y, Li J, Zhang X, Shi X, Xue X, Li Z, Ma B, Wang Y, Li M, Luo X. Selective in vivo and in vitro activities of 3,3’-4-nitrobenzylidene-bis-4-hydroxycoumarin against methicillin-resistant Staphylococcus aureus by inhibition of DNA polymerase III. Sci Rep. 2015;5:13637. doi: 10.1038/srep13637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaake-Koning C, van den Bogaert W, Dalesio O, Festen J, Hoogenhout J, van Houtte P, Kirkpatrick A, Koolen M, Maat B, Nijs A, et al. Effects of concomitant cisplatin and radiotherapy on inoperable non-small-cell lung cancer. N Engl J Med. 1992;326:524–530. doi: 10.1056/NEJM199202203260805. [DOI] [PubMed] [Google Scholar]
- 20.Pierrefite-Carle V, Santucci-Darmanin S, Breuil V, Camuzard O, Carle GF. Autophagy in bone: self-eating to stay in balance. Ageing Res Rev. 2015;24:206–217. doi: 10.1016/j.arr.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Choi KS. Autophagy and cancer. Exp Mol Med. 2012;44:109–120. doi: 10.3858/emm.2012.44.2.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su Z, Yang Z, Xu Y, Chen Y, Yu Q. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol Cancer. 2015;14:48. doi: 10.1186/s12943-015-0321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garofalo M, Saari H, Somersalo P, Crescenti D, Kuryk L, Aksela L, Capasso C, Madetoja M, Koskinen K, Oksanen T, Makitie A, Jalasvuori M, Cerullo V, Ciana P, Yliperttula M. Antitumor effect of oncolytic virus and paclitaxel encapsulated in extracellular vesicles for lung cancer treatment. J Control Release. 2018;283:223–234. doi: 10.1016/j.jconrel.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 24.Jaffe N. Historical perspective on the introduction and use of chemotherapy for the treatment of osteosarcoma. Adv Exp Med Biol. 2014;804:1–30. doi: 10.1007/978-3-319-04843-7_1. [DOI] [PubMed] [Google Scholar]
- 25.Ferrari S, Serra M. An update on chemotherapy for osteosarcoma. Expert Opin Pharmacother. 2015;16:2727–2736. doi: 10.1517/14656566.2015.1102226. [DOI] [PubMed] [Google Scholar]
- 26.Zhao D, Yuan H, Yi F, Meng C, Zhu Q. Autophagy prevents doxorubicininduced apoptosis in osteosarcoma. Mol Med Rep. 2014;9:1975–1981. doi: 10.3892/mmr.2014.2055. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Yang Z, Li Y, Xia J, Li D, Li H, Ren M, Liao Y, Yu S, Chen Y, Yang Y, Zhang Y. Cell apoptosis, autophagy and necroptosis in osteosarcoma treatment. Oncotarget. 2016;7:44763–44778. doi: 10.18632/oncotarget.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Present D, Bertoni F, Hudson T, Enneking WF. The correlation between the radiologic staging studies and histopathologic findings in aggressive stage 3 giant cell tumor of bone. Cancer. 1986;57:237–244. doi: 10.1002/1097-0142(19860115)57:2<237::aid-cncr2820570209>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 29.Zambo I, Vesely K. WHO classification of tumours of soft tissue and bone 2013: the main changes compared to the 3rd edition. Cesk Patol. 2014;50:64–70. [PubMed] [Google Scholar]
- 30.Wang Y, Hallden G, Hill R, Anand A, Liu TC, Francis J, Brooks G, Lemoine N, Kirn D. E3 gene manipulations affect oncolytic adenovirus activity in immunocompetent tumor models. Nat Biotechnol. 2003;21:1328–1335. doi: 10.1038/nbt887. [DOI] [PubMed] [Google Scholar]
- 31.Nadgir SV, Hensler HR, Knowlton ER, Rinaldo CR, Rappocciolo G, Jenkins FJ. Fifty percent tissue culture infective dose assay for determining the titer of infectious human herpesvirus 8. J Clin Microbiol. 2013;51:1931–1934. doi: 10.1128/JCM.00761-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma B, Wang Y, Zhou X, Huang P, Zhang R, Liu T, Cui C, Liu X, Wang Y. Synergistic suppression effect on tumor growth of hepatocellular carcinoma by combining oncolytic adenovirus carrying XAF1 with cisplatin. J Cancer Res Clin Oncol. 2015;141:419–429. doi: 10.1007/s00432-014-1835-8. [DOI] [PubMed] [Google Scholar]
- 33.Rein DT, Volkmer A, Bauerschmitz G, Beyer IM, Janni W, Fleisch MC, Welter AK, Bauerschlag D, Schondorf T, Breidenbach M. Combination of a MDR1-targeted replicative adenovirus and chemotherapy for the therapy of pretreated ovarian cancer. J Cancer Res Clin Oncol. 2012;138:603–610. doi: 10.1007/s00432-011-1135-5. [DOI] [PubMed] [Google Scholar]
- 34.Hamid O, Hoffner B, Gasal E, Hong J, Carvajal RD. Oncolytic immunotherapy: unlocking the potential of viruses to help target cancer. Cancer Immunol Immunother. 2017;66:1249–1264. doi: 10.1007/s00262-017-2025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casasnovas JM, Larvie M, Stehle T. Crystal structure of two CD46 domains reveals an extended measles virus-binding surface. EMBO J. 1999;18:2911–2922. doi: 10.1093/emboj/18.11.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinez-Velez N, Xipell E, Vera B, Acanda de la Rocha A, Zalacain M, Marrodan L, Gonzalez-Huarriz M, Toledo G, Cascallo M, Alemany R, Patino A, Alonso MM. The oncolytic adenovirus VCN-01 as therapeutic approach against pediatric osteosarcoma. Clin Cancer Res. 2016;22:2217–2225. doi: 10.1158/1078-0432.CCR-15-1899. [DOI] [PubMed] [Google Scholar]
- 37.Mueller C, Flotte TR. Clinical gene therapy using recombinant adeno-associated virus vectors. Gene Ther. 2008;15:858–863. doi: 10.1038/gt.2008.68. [DOI] [PubMed] [Google Scholar]
- 38.Hastie E, Samulski RJ. Recombinant adeno-associated virus vectors in the treatment of rare diseases. Expert Opin Orphan Drugs. 2015;3:675–689. doi: 10.1517/21678707.2015.1039511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bielack SS, Smeland S, Whelan JS, Marina N, Jovic G, Hook JM, Krailo MD, Gebhardt M, Papai Z, Meyer J, Nadel H, Randall RL, Deffenbaugh C, Nagarajan R, Brennan B, Letson GD, Teot LA, Goorin A, Baumhoer D, Kager L, Werner M, Lau CC, Sundby Hall K, Gelderblom H, Meyers P, Gorlick R, Windhager R, Helmke K, Eriksson M, Hoogerbrugge PM, Schomberg P, Tunn PU, Kuhne T, Jurgens H, van den Berg H, Bohling T, Picton S, Renard M, Reichardt P, Gerss J, Butterfass-Bahloul T, Morris C, Hogendoorn PC, Seddon B, Calaminus G, Michelagnoli M, Dhooge C, Sydes MR, Bernstein M. Methotrexate, doxorubicin, and cisplatin (MAP) plus maintenance pegylated interferon Alfa-2b Versus MAP Alone in patients with resectable high-grade osteosarcoma and good histologic response to preoperative MAP: first results of the EURAMOS-1 good response randomized controlled trial. J. Clin. Oncol. 2015;33:2279–2287. doi: 10.1200/JCO.2014.60.0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Biason P, Hattinger CM, Innocenti F, Talamini R, Alberghini M, Scotlandi K, Zanusso C, Serra M, Toffoli G. Nucleotide excision repair gene variants and association with survival in osteosarcoma patients treated with neoadjuvant chemotherapy. Pharmacogenomics J. 2012;12:476–483. doi: 10.1038/tpj.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hao T, Feng W, Zhang J, Sun YJ, Wang G. Association of four ERCC1 and ERCC2 SNPs with survival of bone tumour patients. Asian Pac J Cancer Prev. 2012;13:3821–3824. doi: 10.7314/apjcp.2012.13.8.3821. [DOI] [PubMed] [Google Scholar]
- 42.Sooriyaarachchi M, White WM, Narendran A, Gailer J. Chemoprotection by D-methionine against cisplatin-induced side-effects: insight from in vitro studies using human plasma. Metallomics. 2014;6:532–541. doi: 10.1039/c3mt00238a. [DOI] [PubMed] [Google Scholar]
- 43.Luetke A, Meyers PA, Lewis I, Juergens H. Osteosarcoma treatment - where do we stand? A state of the art review. Cancer Treat Rev. 2014;40:523–532. doi: 10.1016/j.ctrv.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 44.Graat HC, Witlox MA, Schagen FH, Kaspers GJ, Helder MN, Bras J, Schaap GR, Gerritsen WR, Wuisman PI, van Beusechem VW. Different susceptibility of osteosarcoma cell lines and primary cells to treatment with oncolytic adenovirus and doxorubicin or cisplatin. Br J Cancer. 2006;94:1837–1844. doi: 10.1038/sj.bjc.6603189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Q, Wang J, Liao D, Ai J, Jin L, Gao Q. Degradation of DAXX by adenovirus type 12 E1B-55K circumvents chemoresistance of ovarian cancer to cisplatin. Virology. 2018;521:118–128. doi: 10.1016/j.virol.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 46.Hulin-Curtis SL, Davies JA, Jones R, Hudson E, Hanna L, Chester JD, Parker AL. Histone deacetylase inhibitor trichostatin A sensitises cisplatin-resistant ovarian cancer cells to oncolytic adenovirus. Oncotarget. 2018;9:26328–26341. doi: 10.18632/oncotarget.25242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boya P, Reggiori F, Codogno P. Emerging regulation and functions of autophagy. Nat Cell Biol. 2013;15:713–720. doi: 10.1038/ncb2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Farrill JS, Gordon N. Autophagy in osteosarcoma. Adv Exp Med Biol. 2014;804:147–160. doi: 10.1007/978-3-319-04843-7_8. [DOI] [PubMed] [Google Scholar]
- 49.Camuzard O, Santucci-Darmanin S, Carle GF, Pierrefite-Carle V. Role of autophagy in osteosarcoma. J Bone Oncol. 2019;16:100235. doi: 10.1016/j.jbo.2019.100235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 51.Fesus L, Demeny MA, Petrovski G. Autophagy shapes inflammation. Antioxid Redox Signal. 2011;14:2233–2243. doi: 10.1089/ars.2010.3485. [DOI] [PubMed] [Google Scholar]
- 52.Wu W, Li W, Zhou Y, Zhang C. Inhibition of beclin1 affects the chemotherapeutic sensitivity of osteosarcoma. Int J Clin Exp Pathol. 2014;7:7114–7122. [PMC free article] [PubMed] [Google Scholar]
- 53.Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry WS, Fulda S, Gottlieb E, Green DR, Hengartner MO, Kepp O, Knight RA, Kumar S, Lipton SA, Lu X, Madeo F, Malorni W, Mehlen P, Nunez G, Peter ME, Piacentini M, Rubinsztein DC, Shi Y, Simon HU, Vandenabeele P, White E, Yuan J, Zhivotovsky B, Melino G, Kroemer G. Molecular definitions of cell death subroutines: recommendations of the nomenclature committee on cell death 2012. Cell Death Differ. 2012;19:107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Z, Shao Z, Xiong L, Che B, Deng C, Xu W. Expression of Beclin1 in osteosarcoma and the effects of down-regulation of autophagy on the chemotherapeutic sensitivity. J Huazhong Univ Sci Technolog Med Sci. 2009;29:737–740. doi: 10.1007/s11596-009-0613-3. [DOI] [PubMed] [Google Scholar]
- 55.Ding L, Congwei L, Bei Q, Tao Y, Ruiguo W, Heze Y, Bo D, Zhihong L. mTOR: an attractive therapeutic target for osteosarcoma? Oncotarget. 2016;7:50805–50813. doi: 10.18632/oncotarget.9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Su Z, Yang Z, Xu Y, Chen Y, Yu Q. MicroRNAs in apoptosis, autophagy and necroptosis. Oncotarget. 2015;6:8474–8490. doi: 10.18632/oncotarget.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.von Hoven G, Kloft N, Neukirch C, Ebinger S, Bobkiewicz W, Weis S, Boller K, Janda KD, Husmann M. Modulation of translation and induction of autophagy by bacterial exoproducts. Med Microbiol Immunol. 2012;201:409–418. doi: 10.1007/s00430-012-0271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu D, Yang Y, Liu Q, Wang J. Inhibition of autophagy by 3-MA potentiates cisplatin-induced apoptosis in esophageal squamous cell carcinoma cells. Med Oncol. 2011;28:105–111. doi: 10.1007/s12032-009-9397-3. [DOI] [PubMed] [Google Scholar]
- 59.Song J, Qu Z, Guo X, Zhao Q, Zhao X, Gao L, Sun K, Shen F, Wu M, Wei L. Hypoxia-induced autophagy contributes to the chemoresistance of hepatocellular carcinoma cells. Autophagy. 2009;5:1131–1144. doi: 10.4161/auto.5.8.9996. [DOI] [PubMed] [Google Scholar]
- 60.Maheswari U, Ghosh K, Sadras SR. Licarin A induces cell death by activation of autophagy and apoptosis in non-small cell lung cancer cells. Apoptosis. 2018;23:210–225. doi: 10.1007/s10495-018-1449-8. [DOI] [PubMed] [Google Scholar]
- 61.Tanida I, Tanida-Miyake E, Komatsu M, Ueno T, Kominami E. Human Apg3p/Aut1p homologue is an authentic E2 enzyme for multiple substrates, GATE-16, GABARAP, and MAP-LC3, and facilitates the conjugation of hApg12p to hApg5p. J Biol Chem. 2002;277:13739–13744. doi: 10.1074/jbc.M200385200. [DOI] [PubMed] [Google Scholar]
- 62.Nemoto T, Tanida I, Tanida-Miyake E, Minematsu-Ikeguchi N, Yokota M, Ohsumi M, Ueno T, Kominami E. The mouse APG10 homologue, an E2-like enzyme for Apg12p conjugation, facilitates MAP-LC3 modification. J Biol Chem. 2003;278:39517–39526. doi: 10.1074/jbc.m300550200. [DOI] [PubMed] [Google Scholar]