Abstract
Osteoarthritis (OA) is the most common cause of disability in worldwide population, which is characterized by cartilage breakdown, synovial fibrosis, osteophyte formation and pain. Synovial inflammation is usually found in both early and late stages in most of the OA patients. Macrophages, the major component of the mononuclear phagocyte system, play a critical role in OA pathogenesis through the induction of inflammatory mediators, growth factors and proteinases. So, drugs that can target macrophages and macrophage-associated inflammatory pathways at an appropriate stage may help to inhibit or slow down the progression of OA. However, despite an emerging role of synovial macrophages in OA pathogenesis, little is known about the biology of synovial tissue macrophages, and attempts to target macrophages therapeutically have had limited success. But the use of selective targets of macrophages may minimize the side effects and support the promising therapeutic strategy in the treatment of OA. More pre-clinical animal models and clinical trials are necessary to evaluate the role of selective targets of macrophages in the prevention and treatment of OA. This review article discusses the association of macrophages in OA development and possible OA therapeutics by targeting macrophages.
Keywords: Osteoarthritis, inflammation, macrophages, cytokines, synovial tissue
Introduction
Macrophages are bone marrow-derived cells that consist of blood monocytes, and tissue macrophages. Macrophages are widely distributed throughout the body, contributing to physiologic homeostasis by responding to internal and external changes within the body throughout the life [1,2]. As essential effectors of the innate immune system, macrophages play a central role in inflammation and host defense. A major characteristic of macrophages is their ability to recognize, internalize, and destroy harmful endogenous and foreign substances in response to inflammatory signals in a process called phagocytosis [3,4]. They are highly heterogeneous cells that can rapidly change their function in response to different tissue environments. Activated macrophages may secrete proinflammatory cytokines, which play important role in bone loss in inflammatory bone disease [5,6].
Osteoarthritis (OA) is the most prevalent form of joint disease that causes disability in adults due to pain and impaired joint function. According to the World Health Organization (WHO), the prevalence of OA is more than 150 million in people worldwide [7,8]. OA is characterized by pain, stiffness, locomotor restriction, bony enlargement, and sometimes swelling of the specific joints such as knees, hips, hands and spine, all of which can result in impaired function [9-11]. The exact mechanisms involved in the pathogenesis of OA are not well understood yet. The pathogenesis of OA seemed to be the result of the complex interaction between mechanical, cellular and inflammatory factors. With the understanding of pathogenesis of OA, it is considered to be predominantly mechanical in a so-called “wear and tear” process whose immune system was unlikely to be affected. OA cannot be cured totally. Only conventional therapy for OA is directed toward pain management as pain is an important factor in strategies to manage OA. The available conventional therapeutic strategies include physiotherapy, pharmacological agents and surgery [12-14]. However, these treatments are not always satisfactory as they are not powerful enough to modify the course of the underlying disease and are not able to prevent cartilage degenerative processes. Therefore, there is a large demand for disease-modifying therapies for the treatment of OA [15,16].
Targeting activated macrophages at an appropriate stage may help to inhibit or to slow the progression of bone loss in patients with OA. In this review, we discuss the pathogenic and protective functions of macrophage in OA.
Functional roles of macrophages
Macrophages are the major component of the mononuclear phagocyte system that arises from yolk sac and fetal liver progenitors during embryonic development. They are present in mammals from midgestation period, and play a central role in host defense as well as in normal physiological processes such as the maintenance of tissues throughout the life [17,18]. Macrophages can be found in almost all organs in the body, including the liver, spleen, brain, bones, lymph nodes and lungs. They have specific functions in each organ and the surrounding environments influence their properties during differentiation [2,19]. Macrophages may function as scavengers by their ability to recognize, internalize, and destroy harmful endogenous and foreign substances. They sometimes play a role in host antimicrobial defense, antitumor immune responses and anti-inflammatory responses, tissue repair, and homeostasis while they sometimes promote inflammation and tumor growth [20-22]. Generally, it is considered that embryonic-derived macrophages play a strong role in the maintenance of tissue homeostasis while bone marrow derived macrophages are related to host defense reactions and inflammatory diseases [2].
In response to microenvironmental stimuli, macrophages (both resident and inflammatory macrophages) can be classified on basis of the activation: classically activated macrophages (proinflammatory M1) and alternatively activated macrophages (antiinflammatory M2). M1 macrophages have proinflammatory functions and are responsible for the release of molecules crucial for joint inflammation. M1 macrophages are stimulated by interferon (IFN)-γ or lipopolysaccharide (LPS), granulocyte macrophage colony-stimulating factor or other toll-like receptor (TLR) ligands to produce high levels of proinflammatory cytokines, such as IL-1β, IL-12, tumor necrosis factor-α (TNF-α), and superoxide anions; induce Th1 immune response, and mediate defense of the host from various bacteria, protozoa and viruses. M1 macrophages also mediate antitumour immune responses [23-25]. Conversely, M2 macrophages have an anti-inflammatory function and contribute to tissue repair and resolution of inflammation. M2 macrophages can also regulate wound healing. M2 macrophages are stimulated by interleukin (IL)-4 or IL-13 to produce anti-inflammatory cytokines such as IL-10, IL-1α, transforming growth factor (TGF)-β and arginase-1 (Arg1); induce the activation of the Th2 immune response and antiinflammatory functions (Figure 1) [26,27]. There are some other less-well-defined macrophages, including tumor-associated macrophages (TAM), which suppress antitumor immunity; “immature” monocyte-like (GR1/Ly6C+) or “mature” neutrophil-like (GR1/Ly6G+) myeloid-derived suppressor cells (MDSCs) [28,29].
Figure 1.
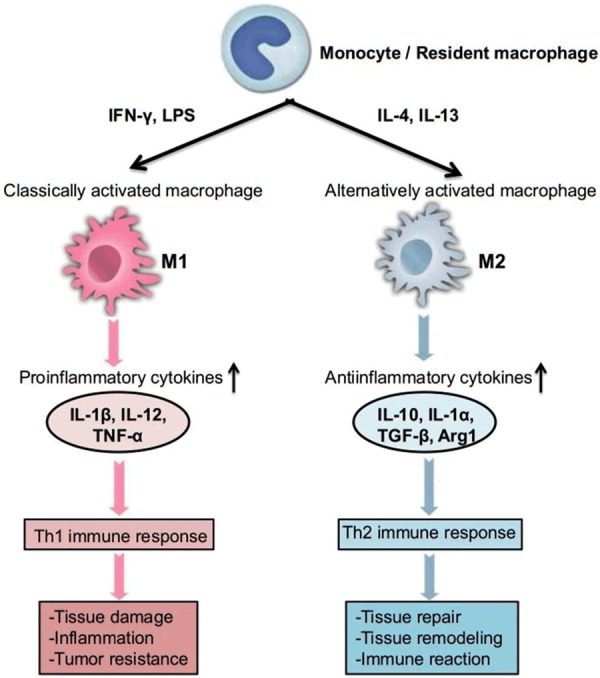
Functional role of macrophages. In response to microenvironmental stimuli, M1 macrophages are stimulated by IFN-γ or LPS to produce high levels of proinflammatory cytokines, induce Th1 immune response and mediate defense of the host. Conversely, M2 macrophages are stimulated by interleukin IL-4 or IL-13 to produce anti-inflammatory cytokines, induce the activation of the Th2 immune response and mediate antiinflammatory functions.
Under normal conditions, most macrophages display an M2 phenotype to maintain tissue homeostasis [30]. In inflammation, macrophages are activated and polarized to an M1 phenotype. These M1 macrophages have three major function; antigen presentation, phagocytosis, and immunomodulation through production of nitric oxide and proinflammatory cytokines, which can lead to tissue damage [4,17]. During the resolution of inflammation, macrophages are predominantly polarized to an M2 phenotype. They can repair damages tissues, clear debris, and restore tissue homeostasis through production of anti-inflammatory cytokines and cytokine antagonists (Figure 2). Macrophages use pattern recognition receptors (PRRs), including TLRs, C-type lectin receptors, scavenger receptors, retinoic acid-inducible gene 1 (RIG1)-like helicase receptors (RLRs) and NOD-like receptors, to recognize, internalize, and destroy harmful foreign substances and dead or dying cells [31-33].
Figure 2.
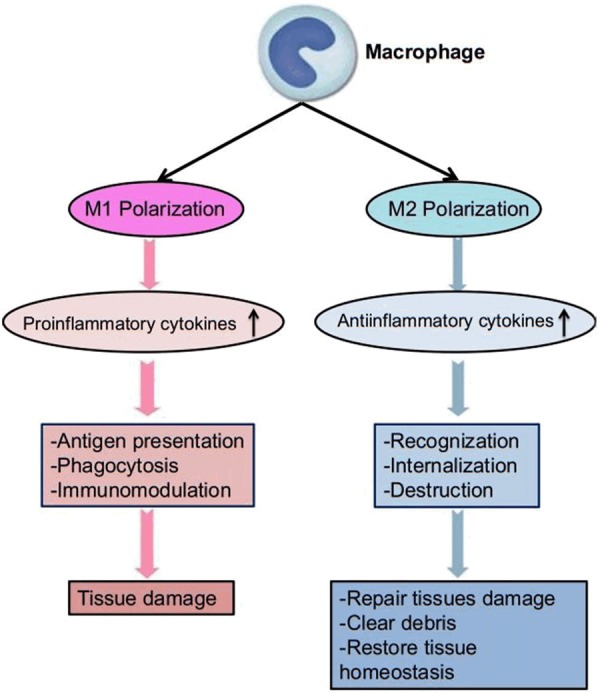
Basic mechanism of macrophages in inflammation. During inflammation, macrophages are activated and polarized to M1 phenotype. These M1 macrophages can lead to tissue damage through production of proinflammatory cytokines. During the resolution of inflammation, macrophages are predominantly polarized to M2 phenotype. They can repair damaged tissues, clear debris, and restore tissue homeostasis through production of antiinflammatory cytokines.
Association of macrophages in osteoarthritis
OA is the most common cause of disability of people worldwide characterized by cartilage breakdown, synovial fibrosis, osteophyte (bony outgrowths at the joint margin) formation and pain. It has been demonstrated that synovial inflammation can be found in both early and late stages in most OA patients [34-36]. Abundant proinflammatory cytokines that are responsible for inflammation and cartilage degradation have been found in the synovium of patients with OA. The accumulation of macrophages in the synovial lining can be recognized is a hallmark of synovitis [37,38]. It has been implicated that both inflammatory and destructive responses are dependent largely on macrophages which play a critical role in OA pathogenesis through the induction of inflammatory mediators, growth factors and proteinases [39,40]. As M1 macrophage is believed to be pro-inflammatory whereas M2 macrophage anti-inflammatory, the balance between M1 and M2 macrophages might be distorted in OA and the degree of imbalance was associated with severe level of OA. The failure of synovial macrophages to transform from M1 to M2 subtypes may contribute to the initiation and progression of OA (Figure 3) [41-43]. In the synovium of OA patients, M1 cytokines, including IL-12, IL-1β and TNF-α are increased while M2 cytokine such as IL-1α is reduced. The overproduction of cytokines and growth factors from the inflamed synovium can influence the cartilage degradation through the production of other pro- and anti-inflammatory cytokines, production of matrix metalloproteinases (MMPs), and expression of aggrecanases in the OA synovium. The polarization of macrophages also has an effect on the progression of OA. The increased macrophages found in the synovium and subchondral bone of OA patients can be identified by cell surface markers, including CD163, CD68, CD14, MHC class II genes and F4/80. When patients with OA are examined, an increase in CD14 and CD163 indicates inflammatory phenotypes and OA severity [27,39,44]. In OA, macrophage activation can occur due to cartilage damage through the secretion of MMPs (MMP-1, -3 and -9), cytokines and growth factors. Potential mediators including damage-associated molecular patterns (DAMPs) leak into the synovial fluid by damaged cartilage and activate synovial macrophages. Activation of synovial macrophages leads to release of pro-inflammatory cytokines as well as catabolic and anabolic factors, which can induce osteophyte formation [45,46].
Figure 3.
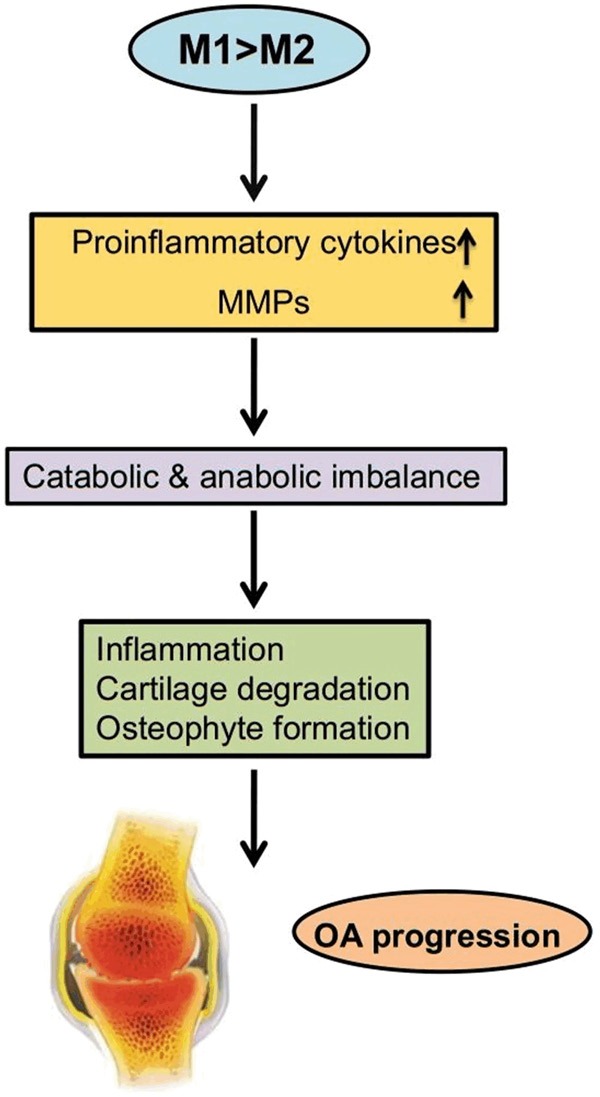
Pathogenic mechanism of macrophages in OA progression. In OA, macrophage activation leads to release of proinflammatory cytokines as well as MMPs which induce cartilage damage and osteophyte formation through catabolic and anabolic imbalance.
Therapeutic aspects of macrophages in osteoarthritis treatment
OA is one of the most common causes of chronic disability in adults due to the result of deep pathologic changes in the articular tissues. There is no effective treatment strategy of OA [47,48]. Therapeutic approaches involved in macrophage phenotype modulation are promising. Drugs targeting macrophages and macrophage-associated inflammatory pathways might be a promising therapeutic strategy in the treatment of OA. Direct inhibition of M1 or promotion of M2 polarization may be useful therapeutic approaches in the treatment of OA [49,50].
Bondeson et al. [38] examined OA cultures of synovial cells that have the advantage of spontaneously producing a variety of both pro- and anti-inflammatory cytokines as well as the major MMPs and tissue inhibitors of MMPs (TIMPs). They demonstrated that CD14 deficiency is correlated to delayed cartilage degradation in OA. In this study, they depleted synovial macrophages by anti-CD14 conjugated magnetic beads resulting in reduced production of IL-1β, TNF-α and MMPs by synovial fibroblasts and reduced cartilage damage. Moreover, they injected clodronateladen liposomes in destabilized medial meniscus (DMM) OA models in mice, leading to the inhibition of osteophyte formation through the depletion of macrophages. In another mouse model of papain-induced OA treated with triamcinolone acetonide (TA), Siebelt et al. [51] reported that enhanced macrophage infiltration reduced osteophyte formation by increased proportions of CD163/FRβ-positive M2 anti-inflammatory macrophages. Re-balance of the ratio of M1/M2 might be used as a novel therapeutic alternative for OA. Glucocorticoids increase synovial macrophage expression of CD163 and slightly reduce CD68+ macrophages in the lining layer in patients with OA [42]. Utomo et al. [52] added dexamethasone to synovium explants of OA patients and showed that dexamethasone suppressed the pro-inflammatory M1 macrophages and enhanced the anti-inflammatory M2 macrophages. Choi [53] used Tissuegene-C (a cell-mediated gene therapy) for localized delivery of TGF-β1 in OA patients. Their findings suggested that Tissuegene-C induces an anti-inflammatory environment in the joint of OA patients. In a rat MIA model, they found elevated production of IL-10 and other M2 macrophage markers in joints of the Tissuegene-C group as compared to the control group. Tissuegene-C is currently being tested in phase II clinical trials in OA. Targeting cytokines has yielded disappointing results. Chevalier et al. [16] reported that anti-NGF-β therapy in OA patients resulted in substantial pain reduction but was accompanied by serious side effects. However, broadly-acting anti-inflammatory drugs have yielded positive effects. Akasaki et al. [54] found that statins inhibited CCL2 and MMPs, reduced infiltration of CD68+ macrophages and decreased articular cartilage degradation in the OA subintima of ACLT rabbits. Similarly, CCL9 neutralization reduced macrophage and CD4+ T cell infiltration and pro-inflammatory IL-1β expression whereas decreased osteoclast formation and MMP-13 expression in ACLT mice [55]. Furthermore, a study in patients with advanced OA revealed that celecoxib treatment decreased macrophage infiltration and cytokine expression in the synovial membrane [56]. Methotrexate (MTX) may exert a positive effect on OA as the folate receptor β (FRβ) on synovial macrophages may serve as an entry route and assessment of FRβ expression by non-invasive macrophage imaging could prove a useful diagnostic tool to identify OA patients eligible for MTX therapy. A pragmatic phase III trial of MTX in OA patients is currently running [44,57,58].
Future direction
OA is a multifactorial pathology characterized by inflammation and immune response in the joint. Macrophages are the most common immune cell type present in this inflamed synovial tissue. These are thought to be an important player in promoting the production of inflammatory and degenerative mediators in OA. As macrophages play critical role in the pathogenesis of OA, modulating synovial macrophages might be sufficient to alleviate OA symptoms and prevent progression [59-61]. Despite an emerging role for synovial macrophages in OA pathogenesis, little is known about the biology of synovial tissue macrophages and attempts to target macrophages therapeutically have had limited success. The etiopathogenesis of OA is still object of intense pre-clinical and clinical research [61-63]. Development of targeted therapies for OA is critical for gaining clinical benefit without adverse effects. A better understanding of the role of human synovial macrophages in the regulation of joint homeostasis offers the prospect of new regenerative therapeutic strategies for OA [64,65].
Conclusion
OA is a slowly progressive disease which includes the involvement of macrophage, leading to macrophage-related inflammation and degradation of the local cartilage. A better understanding regarding the pathogenic mechanisms of OA is necessary for the development of novel and effective therapeutic strategies. Development of OA therapeutic drug will require the use of biologic approaches that may alter the pathologic responses and activities of macrophages. The use of selective targets of macrophages may minimize the side effects and support the promising therapeutic strategy in the treatment of OA. More pre-clinical animal models and clinical trials are necessary to evaluate the role of selective targets of macrophages in the prevention and treatment of OA.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81902303, 81902233, 81874030, 81402224), the Provincial Science Foundation of Hunan (No. 2015JJ3139, 2018JJ2636), the Key Research and Development Program of Hunan Province (No. 2018SK2076), the Shenzhen Science and Technology Project (201606018), the Clinical and Rehabilitation Research Foundation of Xiangya Hospital and Weiming of Peking University (xywm2015II04).
Disclosure of conflict of interest
None.
References
- 1.Gordon S, Pluddemann A, Martinez Estrada F. Macrophage heterogeneity in tissues: phenotypic diversity and functions. Immunol Rev. 2014;262:36–55. doi: 10.1111/imr.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirayama D, Iida T, Nakase H. The phagocytic function of macrophage-enforcing innate immunity and tissue homeostasis. Int J Mol Sci. 2017;19 doi: 10.3390/ijms19010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smythies LE, Sellers M, Clements RH, Mosteller-Barnum M, Meng G, Benjamin WH, Orenstein JM, Smith PD. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005;115:66–75. doi: 10.1172/JCI19229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma WT, Gao F, Gu K, Chen DK. The role of monocytes and macrophages in autoimmune diseases: a comprehensive review. Front Immunol. 2019;10:1140. doi: 10.3389/fimmu.2019.01140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang DH, Yang MY. The role of macrophage in the pathogenesis of osteoporosis. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20092093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Musumeci G, Aiello FC, Szychlinska MA, Di Rosa M, Castrogiovanni P, Mobasheri A. Osteoarthritis in the XXIst century: risk factors and behaviours that influence disease onset and progression. Int J Mol Sci. 2015;16:6093–6112. doi: 10.3390/ijms16036093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrell CR, Markovic BS, Fellabaum C, Arsenijevic A, Volarevic V. Mesenchymal stem cell-based therapy of osteoarthritis: current knowledge and future perspectives. Biomed Pharmacother. 2019;109:2318–2326. doi: 10.1016/j.biopha.2018.11.099. [DOI] [PubMed] [Google Scholar]
- 9.Abhishek A, Doherty M. Diagnosis and clinical presentation of osteoarthritis. Rheum Dis Clin North Am. 2013;39:45–66. doi: 10.1016/j.rdc.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Cucchiarini M, de Girolamo L, Filardo G, Oliveira JM, Orth P, Pape D, Reboul P. Basic science of osteoarthritis. J Exp Orthop. 2016;3:22. doi: 10.1186/s40634-016-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hugle T, Geurts J. What drives osteoarthritis?-synovial versus subchondral bone pathology. Rheumatology (Oxford) 2017;56:1461–1471. doi: 10.1093/rheumatology/kew389. [DOI] [PubMed] [Google Scholar]
- 12.Lane NE, Brandt K, Hawker G, Peeva E, Schreyer E, Tsuji W, Hochberg MC. OARSI-FDA initiative: defining the disease state of osteoarthritis. Osteoarthritis Cartilage. 2011;19:478–482. doi: 10.1016/j.joca.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 13.de Rezende MU, de Campos GC, Pailo AF. Current concepts in osteoarthritis. Acta Ortop Bras. 2013;21:120–122. doi: 10.1590/S1413-78522013000200010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conaghan PG, Cook AD, Hamilton JA, Tak PP. Therapeutic options for targeting inflammatory osteoarthritis pain. Nat Rev Rheumatol. 2019;15:355–363. doi: 10.1038/s41584-019-0221-y. [DOI] [PubMed] [Google Scholar]
- 15.Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, Towheed T, Welch V, Wells G, Tugwell P American College of Rheumatology. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012;64:465–674. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 16.Chevalier X, Eymard F, Richette P. Biologic agents in osteoarthritis: hopes and disappointments. Nat Rev Rheumatol. 2013;9:400–410. doi: 10.1038/nrrheum.2013.44. [DOI] [PubMed] [Google Scholar]
- 17.Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:281–286. doi: 10.2174/1568010054022024. [DOI] [PubMed] [Google Scholar]
- 18.Gordon S, Martinez-Pomares L. Physiological roles of macrophages. Pflugers Arch. 2017;469:365–374. doi: 10.1007/s00424-017-1945-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ginhoux F, Guilliams M. Tissue-resident macrophage ontogeny and homeostasis. Immunity. 2016;44:439–449. doi: 10.1016/j.immuni.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 20.Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005;23:901–944. doi: 10.1146/annurev.immunol.23.021704.115816. [DOI] [PubMed] [Google Scholar]
- 21.Underhill DM, Goodridge HS. Information processing during phagocytosis. Nat Rev Immunol. 2012;12:492–502. doi: 10.1038/nri3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franklin RA, Liao W, Sarkar A, Kim MV, Bivona MR, Liu K, Pamer EG, Li MO. The cellular and molecular origin of tumor-associated macrophages. Science. 2014;344:921–925. doi: 10.1126/science.1252510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon S, Pluddemann A, Mukhopadhyay S. Sinusoidal immunity: macrophages at the lymphohematopoietic interface. Cold Spring Harb Perspect Biol. 2014;7:a016378. doi: 10.1101/cshperspect.a016378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu XQ, Dai Y, Yang Y, Huang C, Meng XM, Wu BM, Li J. Emerging role of microRNAs in regulating macrophage activation and polarization in immune response and inflammation. Immunology. 2016;148:237–248. doi: 10.1111/imm.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan HY, Wang N, Li S, Hong M, Wang X, Feng Y. The reactive oxygen species in macrophage polarization: reflecting its dual role in progression and treatment of human diseases. Oxid Med Cell Longev. 2016;2016:2795090. doi: 10.1155/2016/2795090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 27.Gu Q, Yang H, Shi Q. Macrophages and bone inflammation. J Orthop Translat. 2017;10:86–93. doi: 10.1016/j.jot.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meazza C, Travaglino P, Pignatti P, Magni-Manzoni S, Ravelli A, Martini A, De Benedetti F. Macrophage migration inhibitory factor in patients with juvenile idiopathic arthritis. Arthritis Rheum. 2002;46:232–237. doi: 10.1002/1529-0131(200201)46:1<232::AID-ART10059>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 29.Swidrowska-Jaros J, Orczyk K, Smolewska E. Macrophages-silent enemies in juvenile idiopathic arthritis. Postepy Hig Med Dosw (Online) 2016;70:743–750. doi: 10.5604/17322693.1208887. [DOI] [PubMed] [Google Scholar]
- 30.Dey A, Allen J, Hankey-Giblin PA. Ontogeny and polarization of macrophages in inflammation: blood monocytes versus tissue macrophages. Front Immunol. 2014;5:683. doi: 10.3389/fimmu.2014.00683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schenten D, Medzhitov R. The control of adaptive immune responses by the innate immune system. Adv Immunol. 2011;109:87–124. doi: 10.1016/B978-0-12-387664-5.00003-0. [DOI] [PubMed] [Google Scholar]
- 32.Mills CD, Thomas AC, Lenz LL, Munder M. Macrophage: SHIP of immunity. Front Immunol. 2014;5:620. doi: 10.3389/fimmu.2014.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watanabe S, Alexander M, Misharin AV, Budinger GRS. The role of macrophages in the resolution of inflammation. J Clin Invest. 2019;130:2619–2628. doi: 10.1172/JCI124615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iagnocco A, Filippucci E, Ossandon A, Ciapetti A, Salaffi F, Basili S, Grassi W, Valesini G. High resolution ultrasonography in detection of bone erosions in patients with hand osteoarthritis. J Rheumatol. 2005;32:2381–2383. [PubMed] [Google Scholar]
- 35.Loeuille D, Chary-Valckenaere I, Champigneulle J, Rat AC, Toussaint F, Pinzano-Watrin A, Goebel JC, Mainard D, Blum A, Pourel J, Netter P, Gillet P. Macroscopic and microscopic features of synovial membrane inflammation in the osteoarthritic knee: correlating magnetic resonance imaging findings with disease severity. Arthritis Rheum. 2005;52:3492–3501. doi: 10.1002/art.21373. [DOI] [PubMed] [Google Scholar]
- 36.Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6:625–635. doi: 10.1038/nrrheum.2010.159. [DOI] [PubMed] [Google Scholar]
- 37.Takano S, Uchida K, Miyagi M, Inoue G, Aikawa J, Fujimaki H, Minatani A, Sato M, Iwabuchi K, Takaso M. Synovial macrophage-derived IL-1beta regulates the calcitonin receptor in osteoarthritic mice. Clin Exp Immunol. 2016;183:143–149. doi: 10.1111/cei.12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun AR, Friis T, Sekar S, Crawford R, Xiao Y, Prasadam I. Is synovial macrophage activation the inflammatory link between obesity and osteoarthritis? Curr Rheumatol Rep. 2016;18:57. doi: 10.1007/s11926-016-0605-9. [DOI] [PubMed] [Google Scholar]
- 39.Bondeson J, Wainwright SD, Lauder S, Amos N, Hughes CE. The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res Ther. 2006;8:R187. doi: 10.1186/ar2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bondeson J, Blom AB, Wainwright S, Hughes C, Caterson B, van den Berg WB. The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Arthritis Rheum. 2010;62:647–657. doi: 10.1002/art.27290. [DOI] [PubMed] [Google Scholar]
- 41.Barboza E, Hudson J, Chang WP, Kovats S, Towner RA, Silasi-Mansat R, Lupu F, Kent C, Griffin TM. Profibrotic infrapatellar fat pad remodeling without M1 macrophage polarization precedes knee osteoarthritis in mice with diet-induced obesity. Arthritis Rheumatol. 2017;69:1221–1232. doi: 10.1002/art.40056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu B, Zhang M, Zhao J, Zheng M, Yang H. Imbalance of M1/M2 macrophages is linked to severity level of knee osteoarthritis. Exp Ther Med. 2018;16:5009–5014. doi: 10.3892/etm.2018.6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xue YZB, Niu YM, Tang B, Wang CM. PCL/EUG scaffolds with tunable stiffness can regulate macrophage secretion behavior. Prog Biophys Mol Biol. 2019;148:4–11. doi: 10.1016/j.pbiomolbio.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 44.Daghestani HN, Pieper CF, Kraus VB. Soluble macrophage biomarkers indicate inflammatory phenotypes in patients with knee osteoarthritis. Arthritis Rheumatol. 2015;67:956–965. doi: 10.1002/art.39006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blom AB, van Lent PL, Libregts S, Holthuysen AE, van der Kraan PM, van Rooijen N, van den Berg WB. Crucial role of macrophages in matrix metalloproteinase-mediated cartilage destruction during experimental osteoarthritis: involvement of matrix metalloproteinase 3. Arthritis Rheum. 2007;56:147–157. doi: 10.1002/art.22337. [DOI] [PubMed] [Google Scholar]
- 46.Liu-Bryan R. Synovium and the innate inflammatory network in osteoarthritis progression. Curr Rheumatol Rep. 2013;15:323. doi: 10.1007/s11926-013-0323-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malemud CJ. Biologic basis of osteoarthritis: state of the evidence. Curr Opin Rheumatol. 2015;27:289–294. doi: 10.1097/BOR.0000000000000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lopa S, Leijs MJ, Moretti M, Lubberts E, van Osch GJ, Bastiaansen-Jenniskens YM. Arthritic and non-arthritic synovial fluids modulate IL10 and IL1RA gene expression in differentially activated primary human monocytes. Osteoarthritis Cartilage. 2015;23:1853–1857. doi: 10.1016/j.joca.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 49.Kraus VB, McDaniel G, Huebner JL, Stabler TV, Pieper CF, Shipes SW, Petry NA, Low PS, Shen J, McNearney TA, Mitchell P. Direct in vivo evidence of activated macrophages in human osteoarthritis. Osteoarthritis Cartilage. 2016;24:1613–1621. doi: 10.1016/j.joca.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xie J, Huang Z, Yu X, Zhou L, Pei F. Clinical implications of macrophage dysfunction in the development of osteoarthritis of the knee. Cytokine Growth Factor Rev. 2019;46:36–44. doi: 10.1016/j.cytogfr.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 51.Siebelt M, Korthagen N, Wei W, Groen H, Bastiaansen-Jenniskens Y, Muller C, Waarsing JH, de Jong M, Weinans H. Triamcinolone acetonide activates an anti-inflammatory and folate receptor-positive macrophage that prevents osteophytosis in vivo. Arthritis Res Ther. 2015;17:352. doi: 10.1186/s13075-015-0865-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Utomo L, van Osch GJ, Bayon Y, Verhaar JA, Bastiaansen-Jenniskens YM. Guiding synovial inflammation by macrophage phenotype modulation: an in vitro study towards a therapy for osteoarthritis. Osteoarthritis Cartilage. 2016;24:1629–1638. doi: 10.1016/j.joca.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 53.Choi H. Tissuegene-c (INVOSSATM) induces an anti-inflammatory environment in the arthritic knee joint via macrophage polarization. Osteoarthritis Cartilage. 2016;24(Suppl 1):S335. [Google Scholar]
- 54.Akasaki Y, Matsuda S, Nakayama K, Fukagawa S, Miura H, Iwamoto Y. Mevastatin reduces cartilage degradation in rabbit experimental osteoarthritis through inhibition of synovial inflammation. Osteoarthritis Cartilage. 2009;17:235–243. doi: 10.1016/j.joca.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 55.Shen PC, Wu CL, Jou IM, Lee CH, Juan HY, Lee PJ, Chen SH, Hsieh JL. T helper cells promote disease progression of osteoarthritis by inducing macrophage inflammatory protein-1gamma. Osteoarthritis Cartilage. 2011;19:728–736. doi: 10.1016/j.joca.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 56.Alvarez-Soria MA, Largo R, Santillana J, Sanchez-Pernaute O, Calvo E, Hernandez M, Egido J, Herrero-Beaumont G. Long term NSAID treatment inhibits COX-2 synthesis in the knee synovial membrane of patients with osteoarthritis: differential proinflammatory cytokine profile between celecoxib and aceclofenac. Ann Rheum Dis. 2006;65:998–1005. doi: 10.1136/ard.2005.046920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xia W, Hilgenbrink AR, Matteson EL, Lockwood MB, Cheng JX, Low PS. A functional folate receptor is induced during macrophage activation and can be used to target drugs to activated macrophages. Blood. 2009;113:438–446. doi: 10.1182/blood-2008-04-150789. [DOI] [PubMed] [Google Scholar]
- 58.Kingsbury SR, Tharmanathan P, Arden NK, Batley M, Birrell F, Cocks K, Doherty M, Edwards CJ, Garrood T, Grainger AJ, Green M, Hewitt C, Hughes R, Moots R, O’Neill TW, Roddy E, Scott DL, Watt FE, Torgerson DJ, Conaghan PG. Pain reduction with oral methotrexate in knee osteoarthritis, a pragmatic phase iii trial of treatment effectiveness (PROMOTE): study protocol for a randomized controlled trial. Trials. 2015;16:77. doi: 10.1186/s13063-015-0602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karsdal MA, Michaelis M, Ladel C, Siebuhr AS, Bihlet AR, Andersen JR, Guehring H, Christiansen C, Bay-Jensen AC, Kraus VB. Disease-modifying treatments for osteoarthritis (DMOADs) of the knee and hip: lessons learned from failures and opportunities for the future. Osteoarthritis Cartilage. 2016;24:2013–2021. doi: 10.1016/j.joca.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 60.Li YS, Luo W, Zhu SA, Lei GH. T cells in osteoarthritis: alterations and beyond. Front Immunol. 2017;8:356. doi: 10.3389/fimmu.2017.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vitale ND, Vandenbulcke F, Chisari E, Iacono F, Lovato L, Di Matteo B, Kon E. Innovative regenerative medicine in the management of knee OA: the role of autologous protein solution. J Clin Orthop Trauma. 2019;10:49–52. doi: 10.1016/j.jcot.2018.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Izawa T, Hutami IR, Tanaka E. Potential role of rebamipide in osteoclast differentiation and mandibular condylar cartilage homeostasis. Curr Rheumatol Rev. 2018;14:62–69. doi: 10.2174/1573397113666171017113441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wood MJ, Leckenby A, Reynolds G, Spiering R, Pratt AG, Rankin KS, Isaacs JD, Haniffa MA, Milling S, Hilkens CM. Macrophage proliferation distinguishes 2 subgroups of knee osteoarthritis patients. JCI Insight. 2019;4 doi: 10.1172/jci.insight.125325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Orlowsky EW, Kraus VB. The role of innate immunity in osteoarthritis: when our first line of defense goes on the offensive. J Rheumatol. 2015;42:363–371. doi: 10.3899/jrheum.140382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gaffney L, Warren P, Wrona EA, Fisher MB, Freytes DO. Macrophages’ role in tissue disease and regeneration. Results Probl Cell Differ. 2017;62:245–271. doi: 10.1007/978-3-319-54090-0_10. [DOI] [PubMed] [Google Scholar]


