Abstract
This study was designed to investigate the biocompatibility and the degradation behavior of a Zn-3Cu alloy, a Zn-3Cu coating alloy, a Mg-Nd-Zn-Zr (denoted as JDBM) alloy and a JDBM coating alloy to choose the optimal alloy for common bile duct (CBD) surgery. In the in vitro degradation experiments, we observed the surface morphology of the samples and determined the elements of the corrosion products. In the in vitro cytotoxicity experiments, the cell morphology and cytotoxicity were observed and tested. In the in vivo experiments, in addition to analyzing the samples, we also analyzed the variations in serum magnesium, serum creatinine (CREA), blood urea nitrogen (BUN), total bilirubin (TB) and glutamic pyruvic transaminase (GPT). Moreover, important tissue samples from the CBD, liver, kidney and spleen were taken for histological evaluation. The in vitro degradation experiments revealed that the surface corrosion of the JDBM and JDBM coating alloys were more obvious than that of Zn-3Cu and Zn-3Cu coating alloys, and the degradation rate of the JDBM coating alloy was the slowest. The in vitro cytotoxicity assessment showed that the JDBM alloy and JDBM coating alloy extracts were biologically safe for L-929 cells, while the Zn-3Cu alloy and Zn-3Cu coating alloy extracts were harmful to L-929 cells. In the in vivo experiments, neither the JDBM alloy nor the JDBM coating alloy affected the function or morphology of the bile duct, liver, kidney or spleen. Similar to the in vitro degradation behavior, the surface corrosion of the JDBM alloy was more significant than that of the JDBM coating alloy. Our data suggested that the JDBM coating alloy is a safe, biodegradable material for CBD surgery.
Keywords: Magnesium alloys, biocompatibility, degradation behavior, common bile duct surgery
Introduction
Biliary stricture is a thorny issue that commonly and frequently occurs in hepatobiliary disease. Traditionally, T-tube drainage following the surgical exploration of the common bile duct (CBD) is the main method. However, the incidence of T-tube placement-associated adverse events, such as biliary fistula, biliary obstruction and water-electrolyte disturbance [1-3], have been reported to be high. Later, with the development of endoscopic interventional techniques, biliary stent insertion is becoming the most important and preferred method to support CBD. However, biliary stent insertion is still controversial because stent occlusion caused by tumor overgrowth, biliary sludge accumulation and epithelial hyperplasia influences its long-term therapeutic effects. Furthermore, the placed stents are likely to embed into the biliary wall and are difficult to remove after stenosis is relieved. All of these flaws severely limit the widespread application of biliary stents.
Recently, magnesium alloys have been of great interest as promising biodegradable materials. They have been widely used as vascular stent materials, bone fixation materials and porous materials for bone repair in orthopedic fields [4-9]. However, their utility in the biliary duct has been less reported. In existing research works, a Mg-6Zn alloy, which has been indicated to have good biocompatibility and safety, however, this kind of magnesium alloy corroded too fast that couldn’t meet the requirement of clinical work [10-12]. Here, we developed two new biodegradable materials, JDBM, with high elongation ratios, low yield strength and low degradation rates and Zn-3Cu [13]. In this study, we aimed to select the most ideal biodegradable material by comparing the Zn-3Cu and JDBM alloy in experiments in vivo and in vitro.
Materials and methods
Materials
Four different samples, including a Zn-3Cu alloy, a JDBM alloy and their respective coating (MgF2-PDLLA) products, were provided by National Engineering Research Center of Light Alloy Net Forming of Shanghai Jiao Tong University. Each sample was processed into two types: a disk sample and a cylindrical sample. The disk samples were used for the in vitro degradation and cellularity experiments, with a diameter of 12 mm and a thickness of 3.0 mm. Cylindrical samples, with a luminal diameter of 1.5 mm and a length of 8 mm, were put into the CBD of beagles. All samples were polished with SiC paper up to 1200 mesh, followed by ultrasonic cleaning in absolute ethyl alcohol for 10 min. Samples were sterilized with ultraviolet radiation for one hour before the in vitro and in vivo experiments.
Bile was collected from patients who indwelt nasobiliary ducts for biliary decompression. It was orderly filtered with 100 and 200 stainless steel screen mesh, sterilized at 62°C for 30 min and stored in -20°C after packing.
In vitro degradation experiment
In vitro, samples were immersed in bile with a temperature maintained at 37 ± 0.5°C using a water bath. They were taken out every ten days, washed with distilled water and dried at room temperature. The appearance and weight were observed and recorded. After three months of immersion, the surface morphology of the samples was observed using scanning electron microscopy (SEM), and the elements of the corrosion products on the sample surface were determined by energy dispersive spectroscopy (EDS). Then, the samples were rinsed with chromic acid to remove the corrosion products. The surface morphology of the samples was observed once again. The degradation rate (in units of mm/yr) was obtained according to the ASTM-G31-72.
Corrosion rate = (K × W)/(A × T × D). where the coefficient K = 8.76 × 104, W is the weight loss (g), A is the sample area exposed to bile (cm2), T is the immersion time (h) and D is the density of the material (g/cm3).
In vitro cell experiments
Preparation of the alloy extracts
Disk samples were fully immersed in cell culture medium, Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Invitrogen). The ratio of the sample surface area to the cell culture medium was 1.25 cm2/ml. Immersed samples were placed in an atmosphere of 5% CO2 at 37°C for 72 h, and then the extracts were collected, filtered though a 0.22 µm microporous membrane and packaged separately. To observe a dose-response relationship, extracts were diluted with DMEM to three different concentrations (100, 80 and 40% concentration). All extracts were then used in the Cell Counting Kit-8 (CCK-8) assay.
Cytotoxicity assessments
The murine fibroblast cell line L-929 was cultured in DMEM, supplemented with 10% fetal bovine serum (FBS, HyClone) in a humidified incubator with 5% CO2 at 37°C. L-929 cells were first incubated in 96-well culture plates at a density of 1 × 103 cells/well (4 groups, 4 concentrations, each concentration with 3 wells) and placed in a humidified incubator with 95% relative humidity and 5% CO2 at 37°C for 24 h to allow for cell attachment. Then, 10 µl of extraction medium of different concentrations was then added to the culture plates. DMEM medium acted as a negative control in this experiment. At predetermined times (1, 3 and 5 days), the cell morphology was observed first by optical microscopy, 10 µl of CCK-8 was then added to each well (wells without cells acted as a blank group). The plates were incubated for 1-4 h. Optical density (OD) measurements were completed at the test wavelength of 450 nm. The cell relative growth rate (RGR) was obtained according to the following formula: RGR = (ODtest - ODnegative)/ODblank × 100%.
In vivo experiments
Surgery
Beagles with a mean body weight of 10 kg were used in our study. These beagles were randomly assigned to experimental groups and a negative control group. All beagles were treated under general anesthesia by intravenous injection of sodium pentobarbital at 10 mg/kg body weight. In the experimental groups, biodegradable materials were implanted after bile duct incision, and nothing was implanted in the negative control group. The animal experiment was carried out in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals and was approved by the Ethics Committee of Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine.
General condition of all animals
After materials implantation surgery, the surgical incision, the wellbeing and the body weight of the beagles were observed and evaluated every two weeks.
Biochemical tests
Five milliliter blood samples were collected from the femoral vein before surgery and 1, 2 and 3 months after the operation. The blood biochemical tests for serum magnesium, serum creatinine (CREA), blood urea nitrogen (BUN), total bilirubin (TB) and glutamic pyruvic transaminase (GPT) were performed with a Hitachi 7600-020 automatic biochemical analyzer.
Degradation characterization
The implanted alloy materials were retrieved after the beagles were sacrificed. The surface morphology of the samples and the elements of the corrosion products were observed and analyzed using SEM and EDS.
Histological evaluation and immunohistochemistry analysis
At predetermined times, three beagles in each group were sacrificed. The common bile duct of 5 cm2 surrounding the alloy materials, liver, kidney and spleen were removed, fixed in 4% formaldehyde and embedded with paraffin. The paraffin-embedded samples were then cut into slices with a thickness of 4 µm and fixed on the glass slides. All slices were processed and stained with hematoxylin eosin (HE). Immunohistochemical analysis was performed to study the expression of apoptosis-related genes. After deparaffinization and rehydration using xylene and graded concentrations of ethyl alcohol, the slices were microwave treated in citrate buffer (pH 6.0) at 99°C for 10 min. Endogenous peroxidase activity was blocked with 3% H2O2 for 10 min. Slices were then cleaned three times with phosphate-buffered saline (PBS) for 5 min. Nonspecific reactions were blocked with normal goat serum for 20 min at room temperature. The slices were then incubated with antibodies against Bcl-2 (bs-4563R, 1:800, Bioss, China) and caspase-3 (bs-0081R, 1:800, Bioss, China) at 4°C overnight. After washing three times with PBS for 5 min each time, a biotinylated secondary antibody (goat anti-rabbit immunoglobulin) was added to the slices for 30 min at 37°C. After washing three times with PBS, strept avidin-biotin complex (SABC) was added followed by incubation for 20 min at 37°C. Diaminobenzidine (DAB, Sigma) was used as the chromogenic substrate.
Statistical analysis
Statistical analysis was performed with SPSS 22.0 software. The experimental results were analyzed with the two samples t-test and presented in the form of mean values ± standard deviation. The level of statistical significance was set at P < 0.05.
Results
In vitro degradation experiment
Appearance changes and degradation rates of samples
The appearance changes and degradation rates of samples were observed and calculated after 10 days, 30 days, 50 days, 70 days and 90 days of immersion. Figure 1 indicates that all samples corroded after immersion and became more and more pronounced over time. And the surface corrosion on the JDBM alloy and JDBM coating alloy was more obvious than that on the Zn-3Cu alloy and Zn-3Cu coating alloy. Furthermore, the surface corrosion on the JDBM alloy was more obvious and uneven compared with inconspicuous and even surface corrosion on the JDBM coating alloy. As also listed in Table 1, the JDBM alloy and JDBM coating alloy degraded faster than the Zn-3Cu alloy and Zn-3Cu coating alloy. The degradation rates of the Zn-3Cu alloy and Zn-3Cu coating alloy were similar, however, the degradation rate was higher for the JDBM alloy than for the JDBM coating alloy.
Figure 1.
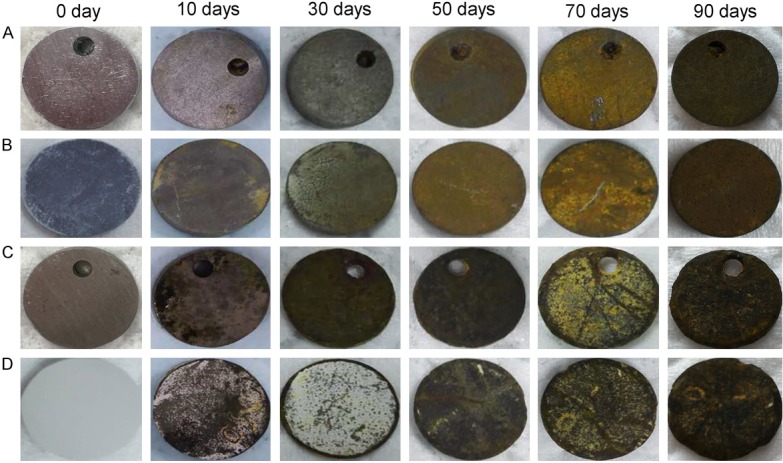
The changes in appearance of the samples after 10 days, 30 days, 50 days, 70 days and 90 days of immersion. The surface corrosion of the JDBM alloy (C) and JDBM coating alloy (D) was more obvious than that of the Zn-3Cu alloy (A) and Zn-3Cu coating alloy (B).
Table 1.
Degradation rates of the samples after 10 days, 30 days, 50 days, 70 days and 90 days of immersion in vitro
| Sample | In vitro (mm. year-1) | ||||
|---|---|---|---|---|---|
|
| |||||
| 10 days | 30 days | 50 days | 70 days | 90 days | |
| Zn-3Cu | 0.173 | 0.115 | 0.102 | 0.074 | 0.077 |
| Zn-3Cu coating | 0.173 | 0.115 | 0.104 | 0.074 | 0.077 |
| JDBM | 2.199 | 1.446 | 1.173 | 1.257 | 1.140 |
| JDBM coating | 0.733 | 0.489 | 0.586 | 0.628 | 0.652 |
Surface morphology and EDS results
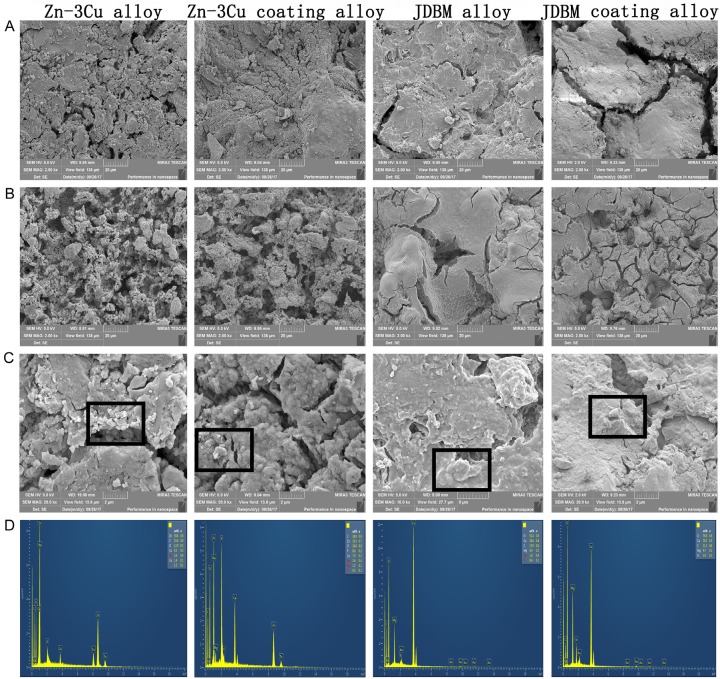
Figure 2 shows the surface morphology of the samples and the EDS results of the surface corrosion products after 3 months of immersion. Figure 2A illustrates the surface morphology of the unwashed samples, and Figure 2B illustrates the surface morphology of the washed samples. As shown in Figure 2A, the corrosion cracks observed on the surface of the JDBM alloy and JDBM coating alloy were larger and deeper than those on the Zn-3Cu alloy and Zn-3Cu coating alloy. Furthermore, there were more corrosion cracks on the surface of the JDBM alloy when compared with those on the JDBM coating alloy. After removing the surface corrosion products with chromic acid, more corrosion pits on the surface of the JDBM alloy and JDBM coating alloy were observed, and the corrosion pits on the JDBM coating alloy were more uniform than those on the JDBM alloy. Figure 2C displays the surface corrosion products of the unwashed samples, and Figure 2D shows the EDS results of the corrosion products. The EDS results demonstrated that the corrosion products from the JDBM alloy and JDBM coating alloy were mainly composed of O, C, Na, Mg, Ca, P, Cl and Si, while the corrosion products from the Zn-3Cu alloy and Zn-3Cu coating alloy were mainly composed of O, C, Cu, Ca, P and Zn.
Figure 2.
The surface morphology of the samples and the EDS results of the surface corrosion products. Cracks on the JDBM alloy and JDBM coating alloy were deeper than those on the Zn-3Cu alloy and Zn-3Cu coating alloy after 3 months of immersion (A). Corrosion pits on the JDBM alloy were the deepest after removing the surface corrosion products (B). The corrosion products on the JDBM alloy and JDBM coating alloy were mainly made of O, C, Na, Mg, Ca, P, Cl and Si, and the corrosion products on the Zn-3Cu alloy and Zn-3Cu coating alloy were mainly composed of O, C, Cu, Ca, P and Zn (C and D, respectively).
In vitro cytotoxicity test
Analysis of cell morphologies and numbers
L-929 cells cultured in different extracts were observed with an inverted microscope after 1, 3 and 5 days of incubation (Figures 3, 4). In cells treated with the Zn-3Cu or Zn-3Cu coating extracts, cell membrane damage and cell nuclei pyknosis occurred, and most cells became round with increasing extract concentration. Furthermore, the number of dead cells after 5 days of incubation increased markedly. However, in the JDBM or JDBM coating extracts, the cell morphologies were fusiform, polygonal or triangular, and the cell numbers increased evidently after 3 and 5 days of incubation, which were similar to that of the negative control group.
Figure 3.
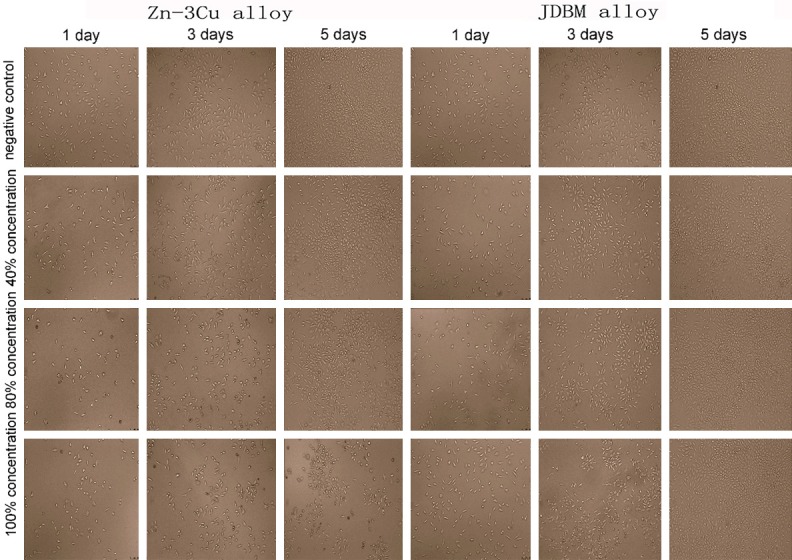
Comparison of the morphologies and numbers of L-929 cells cultured in Zn-3Cu and JDBM extracts. Cells cultured in JDBM extract were fusiform, polygonal or triangular and increased evidently with the prolongation of time, while cells cultured in Zn-3Cu extract became round with increasing extract concentration, and the dead cells increased markedly with the extension of time.
Figure 4.
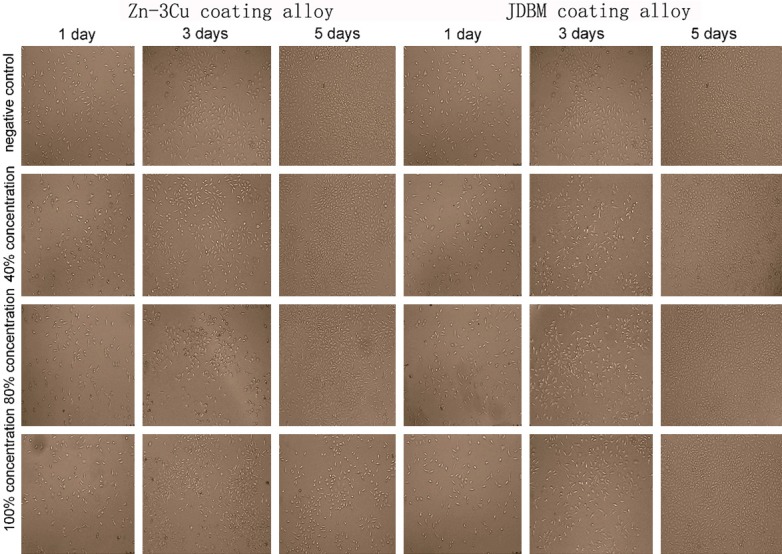
Comparison of the morphologies and numbers of L-929 cells cultured in Zn-3Cu coating and JDBM coating extracts. Cells retained their original morphology and increased in number along with the culture time extension in the JDBM coating extract, while the cells became round and decreased markedly in number with increasing extract concentration in the Zn-3Cu coating extracts.
Analysis of cytotoxicity
Table 2 shows the optical density (OD) values and cell relative growth rates (RGRs). The OD values in different concentrations of JDBM and JDBM coating extracts increased gradually with incubation time, but the OD was lower than the initial value after 5 days of incubation with high concentrations of Zn-3Cu and Zn-3Cu coating extracts. The values of the RGR in the JDBM group and JDBM coating group were all greater than 90%. According to ISO 10993-5: 1999, the cytotoxicity of these extracts was graded 0-1. Conversely, the values of some RGRs in the Zn-3Cu group and Zn-3Cu coating group were less than 75%, and the cytotoxicity of these extracts was graded 2-4. Thus, the JDBM and JDBM coating extracts are safe, while the Zn-3Cu and Zn-3Cu coating extracts are toxic.
Table 2.
The Optical density (OD) and cell relative growth rate (RGR)
| Groups | Extracts (%) | 1 day | 3 days | 5 days | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| OD (Mean ± SD) | RGR (%) | OD (Mean ± SD) | RGR (%) | OD (Mean ± SD) | RGR (%) | ||
| Negative control | 0 | 0.236+0.028 | 100 | 0.843+0.113 | 100 | 1.109+0.172 | 100 |
| Zn-3Cu alloy | 40 | 0.220+0.002 | 76.62 | 0.698+0.067 | 78.54 | 0.939+0.529 | 81.88 |
| 80 | 0.208+0.003 | 57.71 | 0.563+0.086 | 58.41 | 0.599+0.128 | 45.78 | |
| 100 | 0.193+0.009 | 35.33 | 0.462+0.039 | 43.47 | 0.180+0.003 | 1.17 | |
| Zn-3Cu coating | 40 | 0.224+0.002 | 82.58 | 0.739+0.125 | 84.62 | 1.047+0.190 | 93.41 |
| 80 | 0.222+0.002 | 78.61 | 0.704+0.114 | 79.37 | 1.029+0.233 | 91.53 | |
| 100 | 0.219+0.006 | 75.12 | 0.649+0.021 | 70.62 | 0.618+0.149 | 46.74 | |
| JDBM alloy | 40 | 0.245+0.016 | 113.43 | 0.888+0.100 | 106.63 | 1.300+0.146 | 120.29 |
| 80 | 0.240+0.006 | 105.97 | 0.851+0.223 | 101.19 | 1.203+0.118 | 110.00 | |
| 100 | 0.232+0.023 | 94.53 | 0.842+0.042 | 99.85 | 1.042+0.215 | 92.83 | |
| JDBM coating | 40 | 0.293+0.024 | 177.61 | 0.87+0.026 | 103.95 | 1.360+0.211 | 126.74 |
| 80 | 0.279+0.030 | 164.68 | 0.855+0.027 | 101.73 | 1.306+0.149 | 120.99 | |
| 100 | 0.273+0.034 | 155.72 | 0.842+0.045 | 99.9 | 1.048+0.129 | 93.47 | |
In vivo experiment
Analysis of the cytotoxicity indicted that Zn-3Cu and Zn-3Cu coating extracts are toxic for cells, so we only implanted the JDBM and JDBM coating alloys into the bile duct of beagles.
General state of the animals
Animals were evaluated at predetermined times. The beagles in each group grew well and could perform free activities. The diet, drinking and defecation were normal. Postoperative complications such as inflammation, bleeding, pyogenesis and body fluid effusion did not occur in any animals. All animals survived until the end of the trial and maintained a stable body weight.
Biochemical results
After implantation of the samples, no postoperative adverse events occurred in all animals throughout the experimental process. The changes in serum magnesium, ALT, AST, TB, BUN and CREA are presented in Figure 5. The preoperative value of all the biochemical indicators in the different groups showed no meaningful difference (P>0.05). No significant differences were detected in any of the biochemical indicators before and after surgery. Moreover, the postoperative value of biochemical indicators in the same group at different time points and in different groups at same time points did not differ significantly (P>0.05).
Figure 5.
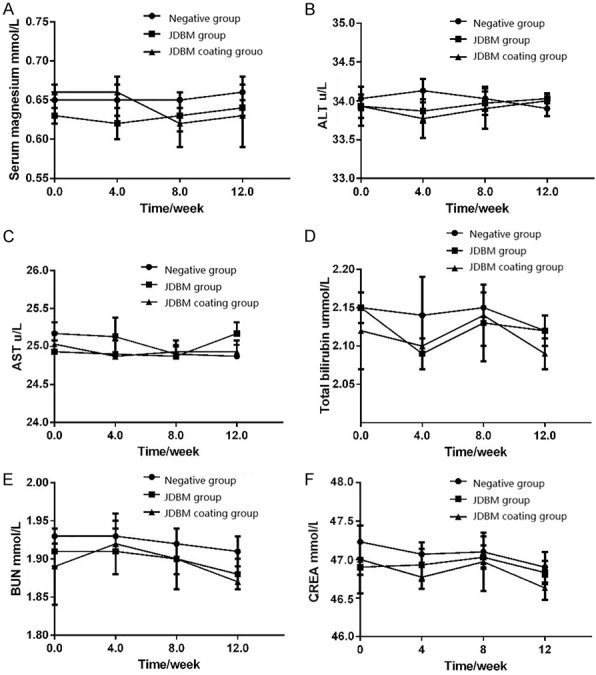
Variations in biochemical indicators. There were no statistically significance differences in serum magnesium (A), ALT (B), AST (C), total bilirubin (D), serum urea nitrogen (E), and creatinine (F) before and after surgery.
Degradation characterization
As listed in Figure 6A, the surface corrosion, which was incomplete, rough and uneven, became increasingly obvious with extension of the implantation time. Moreover, degradation of the JDBM coating alloy was slower than that of the JDBM alloy. Figure 6B, 6C shows the surface morphologies and the EDS results of the residual materials after 3 months of implantation. The surface of the residual materials was covered with corrosion products and was rough and uneven. The surface cracks in the JDBM alloy were greater in number and deeper than those in the JDBM coating alloy, which was consistent with the in vitro degradation experiment. The EDS analysis demonstrated that the corrosion products on the surface of the JDBM alloy consisted of O, C, Ca, Mg and Si, and the corrosion products on the surface of the JDBM coating alloy included O, C, Ca, Mg, Si and P.
Figure 6.
The appearance changes, surface morphologies and EDS results of residual materials after 3 months of implantation. Surface corrosion became increasingly obvious with the extension of implantation time (A). Surface cracks in the JDBM alloy were deeper than those in the JDBM coating alloy (B and C, respectively). The corrosion products on the surface of the JDBM alloy included O, C, Ca, Mg and Si, and those on the surface of the JDBM coating alloy included O, C, Ca, Mg, Si and P (D).
Histological evaluation and immunohistochemistry analysis
Figure 7 provides a picture depicting the results of the histological evaluation. Hepatocytes did not show significant swelling or necrosis, and the hepatic duct did not expand. The spleen and kidney (renal glomerulus and renal tubules) retained their original structure, and no edema or inflammation was observed. No significant cell swelling or necrosis, infiltration of neutrophils and monocytes and apoptotic bodies were detected in the CBD tissue. Figure 8 depicts the results of the immunohistochemistry analysis. The expression levels of capase-3 and Bcl-2 in the JDBM alloy group and JDBM coating alloy group did not differ significantly when compared with the negative control group.
Figure 7.
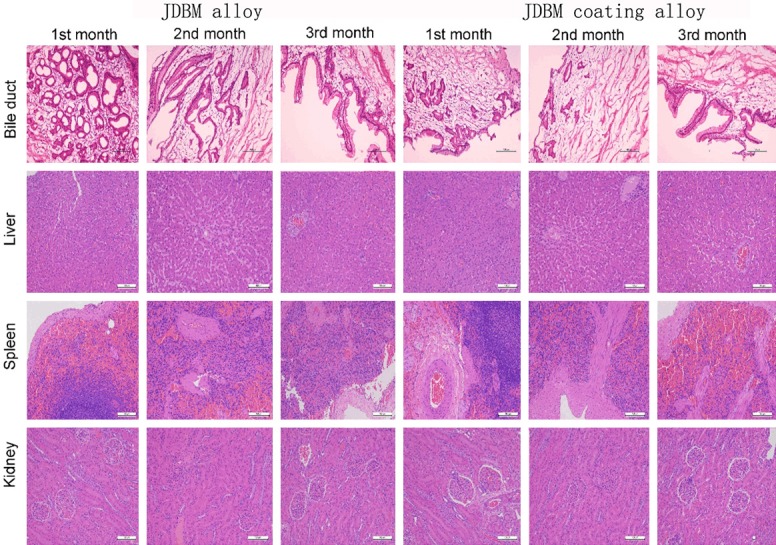
The results of the histological evaluation in the JDBM alloy group and JDBM coating alloy group. The bile duct, liver, spleen and kidney retained their original structure, and no significant cell swelling or necrosis was observed.
Figure 8.
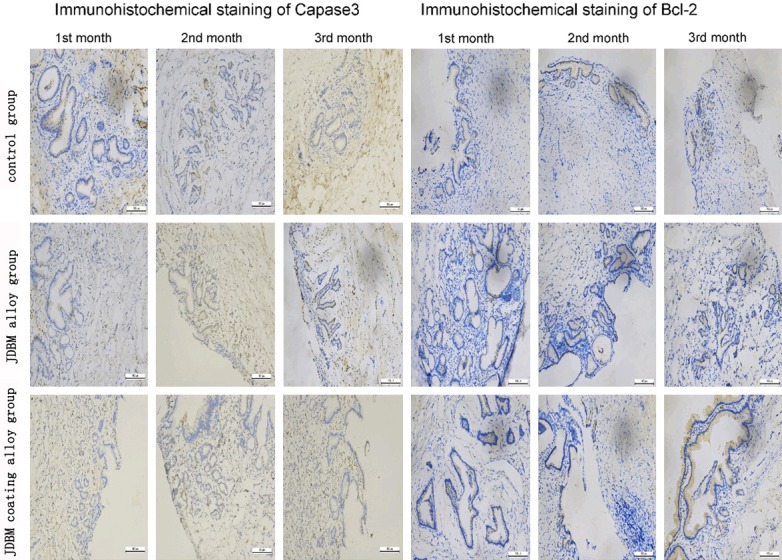
The results of immunohistochemical staining of capase-3 and Bcl-2 (DAB, × 200). The expression levels of capase-3 and Bcl-2 in the JDBM alloy group and JDBM coating alloy group showed no significant difference when compared with the negative control group.
Discussion
Recently, various magnesium alloys have been reported. Among them, Mg-6Zn which with high degradation rate is the most commonly used in the bile duct [10,11,12,14]. In the present study, we developed two new alloys, the Zn-3Cu and the JDBM alloy.
To choose the optimal alloy between the Zn-3Cu alloy, JDBM alloy and their respective coating alloys, we carried out in vivo and in vitro experiments. Our results indicated that JDBM coating alloy is the best for both the biocompatibility and the degradation rate.
Biomaterials must have good biocompatibility to ensure security of clinical application [15]; thus, the evaluation of biocompatibility of biomaterials is an indispensable step before clinical experiments. The cytotoxicity test is a fast, inexpensive and reproducible method to determine biomaterial biocompatibility by detecting the effect of a material or its extract on cell growth. In our study, to ensure the medical safety of the Zn-3Cu alloy, Zn-3Cu coating alloy, JDBM alloy and JDBM coating alloy, we first performed an in vitro cytotoxicity test, and the results indicated that the JDBM alloy extracts and JDBM coating coating alloy extracts showed no damage to cells. Cells cultured in JDBM alloy extracts and JDBM coating alloy extracts were normal and healthy, similar to the negative group cells. The cytotoxicity of the JDBM alloy extracts and JDBM coating alloy extracts were grades 0-1. Furthermore, the Zn-3Cu alloy extracts and Zn-3Cu coating alloy extracts were toxic to cells. Cells cultured in the Zn-3Cu alloy extracts and Zn-3Cu coating alloy extracts were irregular and crumpled, showing a significant difference from that of the negative group. The cytotoxicity of the Zn-3Cu alloy extracts and Zn-3Cu coating alloy extracts were grades 2-4, Similarly, previous study found that the cytotoxicity of JDBM alloy is mild when applied as tracheal stents, vascular stents and bone fixation [16-18]. While, Zn is proved to have a dosedependent effect on some cellular functions like attachment, migration, and spreading [19]. Besides, Li et al. reported that adding Mg, Sr and Ca into Zn could alleviate the cytotoxicity induced by Zn in MG63 cells [20], which may account for the reason why JDBM alloy has little effect on cells though there is small amount of Zn in its composition. For further assessment of the biocompatibility of the JDBM alloy and JDBM coating alloy, we implanted the JDBM alloy and JDBM coating alloy into the common bile duct (CBD) of beagles.
In the present study, the JDBM alloy stents and JDBM coating alloy stents were removed from beagles 1, 2 or 3 months after surgery. The influence of the new material on the local tissue (CBD) and the surrounding organs (such as the liver, kidney and spleen) was assessed. The histological evaluation and immunohistochemistry analysis indicated that the JDBM alloy and JDBM coating alloy (compared with the control group) did not cause organ damage to the CBD, liver, kidney or spleen. In addition, no formation of gallstones or cholestasis was found after the JDBM alloy or JDBM coating alloy stents were inserted into the CBD. The values of the postoperative serum magnesium, ALT, AST, TB, BUN and CREA did not differ between the JDBM alloy group or the JDBM coating alloy group and the negative control group. Taken together, these results showed good biocompatibility of the JDBM alloy and JDBM coating alloy in vivo and in vitro.
Magnesium in aqueous solution dissolves according to the following equations [21-23]: anodic reaction: Mg → Mg2+ + 2e-; cathodic reaction: 2H2O + 2e- → H2 + 2OH-; Mg2+ + 2OH- → Mg(OH)2.
Bile is a complex substance containing organic and inorganic solutes, such as bilirubin, bile acids, cholesterol, phospholipids, Na+, K+, Ca2+, Mg2+, Cl-, HCO3 - and PO4 3- [24]. Thus, the degradation process in bile is more complex than in aqueous solution. The chloride ions (Cl-) from bile can further transform Mg(OH)2 into MgCl2, resulting in an excess of OH- in bile. Ca2+, PO4 3- and OH- are likely to nucleate to form hydroxyapatite and other phosphates and adhere firmly to the magnesium alloy surface. In the present study, after three months of immersion in bile in vitro degradation experiments, all samples were observed using SEM and EDS. The results showed that surface deposits of all samples formed. The surface deposits on the JDBM alloy and JDBM coating alloy were more notable than those in Zn-3Cu alloy and Zn-3Cu coating alloy groups. EDS analysis results indicated that the surface deposits attached to the JDBM alloy and JDBM coating alloy were mainly composed of O, C, Na, Mg, Ca, P, Cl and Si, while the surface deposits attached to the Zn-3Cu alloy and Zn-3Cu coating alloy mainly consisted of O, C, Cu, Ca, P and Zn.
Magnesium in physiological environments exhibits rapid degradation, which destroys the implant’s structural integrity. Through the alloying process, corrosion resistance can be improved effectively. However, magnesium alloys are still unable to meet the clinical needs. Many studies of biodegradable magnesium alloys have reported that they do not perform well because of their rapid degradation [22]. Slower corrosion rates and longer support times are essential for magnesium alloys to be potential biodegradable materials. There are some possible ways to approach this problem. Surface coatings seem to be one of the effective methods to prevent the fast degradation of magnesium implants [25,26]. In this study, we first investigated the in vitro degradation behaviors of four different samples, including a Zn-3Cu alloy, a Zn-3Cu coating alloy, a JDBM alloy and a JDBM coating alloy for the bile duct application. On the basis of the immersion test results, the degradation rates of the Zn-3Cu alloy and Zn-3Cu coating alloy were basically the same, which indicates that the corrosion resistance of the Zn-3Cu alloy is perfect. The degradation rate of the JDBM coating alloy was significantly lower than that of the JDBM alloy, which indicates that coating can effectively lower the degradation rate. Furthermore, the degradation rates of the JDBM alloy and JDBM coating alloy were significantly higher than that of Zn-3Cu alloy and Zn-3Cu coating alloy. After examining the cytotoxicity of the Zn-3Cu alloy and Zn-3Cu coating alloy, we only investigated the in vivo degradation behaviors of the JDBM alloy and JDBM coating alloy. After immersion in bile for 1, 2 or 3 months, the implants were taken out and observed by SEM. The SEM results demonstrated that the surface corrosion of the JDBM alloy was more obvious than that of the JDBM coating alloy. The surface cracks were larger and deeper in the JDBM alloy than those in the JDBM coating alloy. Taken together, these results suggested good corrosion resistance of the JDBM coating alloy.
Conclusion
In the present study, in vitro and in vivo experiments were performed to investigate the biocompatibility and degradation rate of a Zn-3Cu alloy, a Zn-3Cu coating alloy, a JDBM alloy and a JDBM coating alloy. In the in vitro experiments to assess biocompatibility, both the JDBM alloy and JDBM coating alloy extracts were bio-safe for L-929 cells, while the Zn-3Cu alloy and Zn-3Cu coating alloy extracts were toxic to L-929 cells. In the in vivo experiments to evaluate biocompatibility, neither the JDBM alloy nor the JDBM coating alloy affected the function or morphology of the bile duct, liver, kidney or spleen. In the in vitro experiments to explore the degradation rates of the Zn-3Cu alloy, the Zn-3Cu coating alloy, the JDBM alloy and the JDBM coating alloy, the degradation rates of the Zn-3Cu and Zn-3Cu coating alloys were basically the same, while the degradation rate of the JDBM coating alloy was significantly lower than that of JDBM alloy. Taken together, the JDBM coating alloy is better than the Zn-3Cu alloy, Zn-3Cu coating alloy and JDBM alloy. Thus, it can be concluded that the JDBM coating alloy is a safe, biodegradable material for CBD surgery.
Acknowledgements
This study was funded by the National Natural Science Foundation (No. 81572316) from National Natural Science Foundation of China, the Shanghai Municipal Natural Science Foundation (No. 14411966900) from Shanghai Science and Technology Committee and the “Medicine and Engineering Crossover Research Foundation” (YG2014MS24) at Shanghai Jiao Tong University. Special gratitude is expressed to Guangyin Yuan from National Engineering Research Center of Light Alloy Net Forming and State Key Laboratory of Metal Matrix Composite, China for providing the JDBM alloy, Zn-3Cu alloy and their respective coating products for our research.
Disclosure of conflict of interest
None.
References
- 1.El-Geidie AA. Is the use of T-tube necessary after laparoscopic choledochotomy? J Gastrointest Surg. 2010;14:844–848. doi: 10.1007/s11605-009-1133-y. [DOI] [PubMed] [Google Scholar]
- 2.Leida Z, Ping B, Shuguang W, Yu H. A randomized comparison of primary closure and T-tube drainage of the common bile duct after laparoscopic choledochotomy. Surg Endosc. 2008;22:1595–1600. doi: 10.1007/s00464-007-9731-9. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed I, Pradhan C, Beckingham IJ, Brooks AJ, Rowlands BJ, Lobo DN. Is a T-tube necessary after common bile duct exploration? World J Surg. 2008;32:1485–1488. doi: 10.1007/s00268-008-9475-2. [DOI] [PubMed] [Google Scholar]
- 4.Yue Y, Wang L, Yang N, Huang J, Lei L, Ye H, Ren L, Yang S. Effectiveness of biodegradable magnesium alloy stents in coronary artery and femoral artery. J Interv Cardiol. 2015;28:358–364. doi: 10.1111/joic.12217. [DOI] [PubMed] [Google Scholar]
- 5.Waksman R, Pakala R, Kuchulakanti PK, Baffour R, Hellinga D, Seabron R, Tio FO, Wittchow E, Hartwig S, Harder C, Rohde R, Heublein B, Andreae A, Waldmann KH, Haverich A. Safety and efficacy of bioabsorbable magnesium alloy stents in porcine coronary arteries. Catheter Cardiovasc Interv. 2006;68:607–617. doi: 10.1002/ccd.20727. discussion 618-609. [DOI] [PubMed] [Google Scholar]
- 6.Bornapour M, Mahjoubi H, Vali H, Shum-Tim D, Cerruti M, Pekguleryuz M. Surface characterization, in vitro and in vivo biocompatibility of Mg-0.3Sr-0.3Ca for temporary cardiovascular implant. Mater Sci Eng C Mater Biol Appl. 2016;67:72–84. doi: 10.1016/j.msec.2016.04.108. [DOI] [PubMed] [Google Scholar]
- 7.Makkar P, Sarkar SK, Padalhin AR, Moon BG, Lee YS, Lee BT. In vitro and in vivo assessment of biomedical Mg-Ca alloys for bone implant applications. J Appl Biomater Funct Mater. 2018;16:126–136. doi: 10.1177/2280800017750359. [DOI] [PubMed] [Google Scholar]
- 8.Witte F, Ulrich H, Palm C, Willbold E. Biodegradable magnesium scaffolds: part II: peri-implant bone remodeling. J Biomed Mater Res A. 2007;81:757–765. doi: 10.1002/jbm.a.31293. [DOI] [PubMed] [Google Scholar]
- 9.Li Z, Gu X, Lou S, Zheng Y. The development of binary Mg-Ca alloys for use as biodegradable materials within bone. Biomaterials. 2008;29:1329–1344. doi: 10.1016/j.biomaterials.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Yan J, Wang X, Yu S, Wang Z, Zhang X, Zhang S, Zheng Y, Zhao C, Zheng Q. In vivo and in vitro evaluation of effects of Mg-6Zn alloy on apoptosis of common bile duct epithelial cell. Biometals. 2014;27:1217–1230. doi: 10.1007/s10534-014-9784-x. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Yan J, Zhao C, Zhang S, Yu S, Wang Z, Wang X, Zhang X, Zheng Q. In vitro and in vivo assessment of the biocompatibility of an Mg-6Z(n) alloy in the bile. J Mater Sci Mater Med. 2014;25:471–480. doi: 10.1007/s10856-013-5090-3. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Yan J, Wang Z, Yu S, Wang X, Yuan Z, Zhang X, Zhao C, Zheng Q. In vitro and in vivo corrosion measurements of Mg-6Zn alloys in the bile. Mater Sci Eng C Mater Biol Appl. 2014;42:116–123. doi: 10.1016/j.msec.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Seitz JM, Eifler R, Stahl J, Kietzmann M, Bach FW. Characterization of MgNd2 alloy for potential applications in bioresorbable implantable devices. Acta Biomater. 2012;8:3852–3864. doi: 10.1016/j.actbio.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Yan J, Wang Z, Yu S, Yuan Z, Wang X, Zhang X, Zheng Q. Technique for the safe placement of a biodegradable stent into the common bile duct of rabbits. Exp Ther Med. 2013;6:1101–1104. doi: 10.3892/etm.2013.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams DF. On the mechanisms of biocompatibility. Biomaterials. 2008;29:2941–2953. doi: 10.1016/j.biomaterials.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 16.Xue B, Liang B, Yuan G, Zhu L, Wang H, Lu Z, Xu Z. A pilot study of a novel biodegradable magnesium alloy airway stent in a rabbit model. Int J Pediatr Otorhinolaryngol. 2019;117:88–95. doi: 10.1016/j.ijporl.2018.10.047. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Kong N, Niu J, Shi Y, Li H, Zhou Y, Yuan G. Influence of fluoride treatment on surface properties, biodegradation and cytocompatibility of Mg-Nd-Zn-Zr alloy. J Mater Sci Mater Med. 2014;25:791–799. doi: 10.1007/s10856-013-5106-z. [DOI] [PubMed] [Google Scholar]
- 18.Liao Y, Xu Q, Zhang J, Niu J, Yuan G, Jiang Y, He Y, Wang X. Cellular response of chondrocytes to magnesium alloys for orthopedic applications. Int J Mol Med. 2015;36:73–82. doi: 10.3892/ijmm.2015.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niu J, Tang Z, Huang H, Pei J, Zhang H, Yuan G, Ding W. Research on a Zn-Cu alloy as a biodegradable material for potential vascular stents application. Mater Sci Eng C Mater Biol Appl. 2016;69:407–413. doi: 10.1016/j.msec.2016.06.082. [DOI] [PubMed] [Google Scholar]
- 20.Li H, Yang H, Zheng Y, Zhou F, Qiu K, Wang X. Design and characterizations of novel biodegradable ternary Zn-based alloys with IIA nutrient alloying elements Mg, Ca and Sr. Materials & Design. 2015;83:95–102. [Google Scholar]
- 21.Witte F, Fischer J, Nellesen J, Crostack HA, Kaese V, Pisch A, Beckmann F, Windhagen H. In vitro and in vivo corrosion measurements of magnesium alloys. Biomaterials. 2006;27:1013–1018. doi: 10.1016/j.biomaterials.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 22.Heublein B, Rohde R, Kaese V, Niemeyer M, Hartung W, Haverich A. Biocorrosion of magnesium alloys: a new principle in cardiovascular implant technology? Heart. 2003;89:651–656. doi: 10.1136/heart.89.6.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Witte F, Kaese V, Haferkamp H, Switzer E, Meyer-Lindenberg A, Wirth CJ, Windhagen H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials. 2005;26:3557–3563. doi: 10.1016/j.biomaterials.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 24.Reshetnyak VI. Physiological and molecular biochemical mechanisms of bile formation. World J Gastroenterol. 2013;19:7341–7360. doi: 10.3748/wjg.v19.i42.7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hornberger H, Virtanen S, Boccaccini AR. Biomedical coatings on magnesium alloys - a review. Acta Biomater. 2012;8:2442–2455. doi: 10.1016/j.actbio.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 26.Gu X, Zheng Y, Cheng Y, Zhong S, Xi T. In vitro corrosion and biocompatibility of binary magnesium alloys. Biomaterials. 2009;30:484–498. doi: 10.1016/j.biomaterials.2008.10.021. [DOI] [PubMed] [Google Scholar]