Abstract
Growth factors represent a family of important biological molecules that can also be critical in the pathogenesis of various gastrointestinal cancers. In this study, we conducted a comprehensive analysis of the systemic levels of selected growth factors - hepatocyte, vascular-endothelial, fibroblast, and insulin-like 1 growth factors (HGF, VEGF, FGF, and IGF-1, respectively), as well as granulocyte-colony stimulating factor (G-CSF) in 75 patients with different gastric neoplasms (carcinomas, gastrointestinal stromal tumors - GISTs, neuroendocrine neoplasms - NENs, and lymphomas) and 40 healthy volunteers. Patients with gastric carcinoma or other types of gastric neoplasms had higher HGF and IGF-1 levels than healthy individuals (P < 0.05 in all cases). In comparison to healthy control subjects, systemic VEGF concentrations were elevated in patients with gastric carcinoma (P < 0.05), but not in individuals with other types of gastric malignancies. No statistically significant differences were observed between the analyzed groups in terms of FGF and G-CSF levels. When patients with gastric carcinoma were subdivided according to the Japanese classification system, significantly elevated levels of HGF, VEGF, and IGF-1 concentrations were observed in patients with advanced gastric carcinoma (extending beyond the submucosal layer of the stomach). Only the systemic levels of HGF were associated with tumor node metastasis - TNM staging, the absolute numbers of bone marrow-derived mesenchymal cells, and very small embryonic/epiblast-like stem cells circulating in patients with gastric carcinoma. ROC curves analyses demonstrated that AUC values of systemic levels of examined growth factors ranged from 0.40-0.65 (P > 0.06 in all cases). In conclusion, patients with gastric malignancies showed a systemic biochemical imbalance in multiple growth factors, which appears to be associated with clinical presentation of these neoplasms in humans. However, none of the growth factors examined here seem to be suitable diagnostic biomarkers for detecting or differentiating different types of gastric malignancies in humans.
Keywords: Bone marrow-derived stem cells, gastric cancer, growth factors, neuroendocrine neoplasms
Introduction
The diagnosis and treatment of gastric neoplasms remain a major challenge of modern gastroenterology. These malignancies form a very heterogenic group of tumors that have diverse histological origins and clinical prognoses. Among gastric neoplasms, the most common is gastric cancer. According to the recent reports, over 900,000 new cases of gastric cancer are diagnosed annually worldwide [1,2]. Unfortunately, the vast majority of patients inevitably die from this disease, particularly those diagnosed at later stages. Other types of gastric malignancies, such as gastrointestinal stromal tumors (GISTs) or neuroendocrine neoplasms (NENs), occur less frequently in humans and are usually associated with better clinical outcomes [3-7]. However, despite much effort put into discovering the precise pathogenic mechanisms of gastric tumors in humans, the significance of multiple molecular factors responsible for the development and spread of these malignancies are either unknown or have not been verified in clinical studies.
Growth factors (GFs) are strongly suspected to significantly contribute to the formation, progression, and survival of various (gastrointestinal) neoplasms in humans. This fact is supported by the results of multiple animal and in vitro studies, which revealed that the activities of GFs directly stimulate the proliferation, differentiation, and survival of cells under both physiological and pathological conditions [8-12]. Therefore, within recent years, various researchers have examined the biochemical network of GF interactions in many types of human malignancies including thyroid, prostate, renal, as well as gastrointestinal cancers [13-17]. Among a wide panel of GFs, particularly the hepatocyte, vascular-endothelial, fibroblast, and insulin-like 1 GFs (HGF, VEGF, FGF, and IGF-1, respectively) together with granulocyte-colony stimulating factor (G-CSF) seem to be of significance in the development of gastric cancer [18]. Namely, gastric cancer (stem) cells express these GFs, and their action influences function of these cells on autocrine, paracrine, and juxtacrine levels within the cancer microenvironment [2,19,20]. The activities of HGF and G-CSF seem to especially promote invasion and growth of gastric cancer by inhibiting apoptosis in cancer cells, upregulating heparanase (which modulates the shedding of various cytokines and consequently promotes metastasis), or influencing the homeostasis of (cancer) stem cells [21-26]. In addition, neo-angiogenesis and metastasis in the lymph nodes, which are crucial for the systemic spread of gastric cancer, are thought to be mainly promoted by expression of the GFs, VEGF and FGF [2,19,20,27]. Nevertheless, as for now, no comprehensive evaluation of the eventual clinical associations between the expression levels of these GFs and the development of various gastric tumors has been reported. Moreover, diagnostic value of these substances has not been verified in patients affected by gastric malignancies.
Taking all these facts into consideration we decided to conduct a comprehensive evaluation of the peripheral levels of HGF, VEGF, IGF-1, FGF, and G-CSF in individuals with various types of gastric malignancies. We focused on comparison of values of the systemic levels of GFs examined here between control individuals and patients with gastric malignancies. Moreover, we wanted to verify their, GFs levels, eventual clinical associations with clinical staging of gastric cancer in our patients, and the absolute numbers of different populations of circulating bone marrow-derived stem cells reported previously [26]. Furthermore, we also attempted to estimate (at least preliminarily) if the peripheral levels of examined GFs could be of any diagnostic value for detection of gastric cancer in humans. We hypothesized that in patients with gastric malignancies systemic imbalance in the levels of certain GFs occurs, and this would be associated with the clinical presentation of the disease, as well as, could offer potential diagnostic value for detection and differentiation of gastric cancer in humans.
Material and methods
For these analyses we recruited 115 participants, who were subsequently assigned into three main groups termed “cancer”, “other malignancies”, and “control” groups. The “cancer” group consisted of 50 patients with newly diagnosed gastric carcinoma. The “other malignancies” group included 5 patients with GISTs, 12 individuals with NENs, and 8 patients diagnosed with primary gastric lymphomas. Forty healthy volunteers were recruited to the “control” group.
In every case of suspected malignancy a pathological evaluation of a biopsy specimen was performed, and a definitive diagnosis was made. In order to characterize the staging of the malignancy patients were undergoing imaging tests, that included abdominal USG/CT, endoscopic ultrasonography (EUS), and/or chest x-rays. Patients from the “cancer” group had been evaluated using multiple classifications/scales. TNM staging revealed stage I gastric cancer in 20 individuals, stage II in 4, stage III in 4, while metastatic disease had been observed in 20 cases. According to the Lauren’s classification 32 patients had intestinal, 12 diffuse, and 6 mixed type of gastric cancer. In two gastric carcinoma patients, we were not able to characterize the TNM staging because they died before we were able to perform any further diagnostic assessments. Furthermore, 19 patients presented with early and 29 with advanced gastric carcinoma, classified according to the Japanese criteria.
As mentioned before, to the “others” group we assigned patients with GISTs, NENs and lymphomas. In our study all GISTs cases were localized in the fundus of the stomach; 3 patients had I stage low grade GISTs tumors, and the remaining 2 were diagnosed with II and III stage tumors determined to be of high malignant potential. Among the patients with NEN, there were 9 cases of NEN G1 tumors and 3 cases of NEN G2. All of the NEN patients had non-functional tumors primarily localized in the fundus of the stomach, and they did not present any metastatic lesions within either lymph nodes or other organs. Among the 8 patients with primary gastric lymphomas in 4 cases the diffuse large B-cell lymphoma was diagnosed, 2 patients had Burkitt lymphoma, and the remaining two patients had small lymphocyte lymphoma and mucus-associated lymphoid tissue lymphoma. A comprehensive summary of biochemical and anthropometric evaluation of participants of this study, divided into appropriate groups, is presented in Table 1.
Table 1.
General characteristics of analyzed patients and healthy individuals enrolled in the study (data presented as means ± SD or median [interquartile range])
| Parameter | Control | Cancer | Other |
|---|---|---|---|
| Age (years) | 61 ± 6 | 65 ± 11 | 60 ± 13 |
| Sex (M-male/F-female) | 19-M/21-F | 24-M/26-F | 5-M/20-F |
| BMI (kg/m2) | 26.18 ± 3.16 | 24.57 ± 4.11 | 25.82 ± 5.82 |
| RBC (×1012 cells/L) | 4.82 ± 0.55 | 4.26 ± 0.97 | 4.56 ± 0.40 |
| Hb (g/dL) | 14.19 ± 1.75 | 12.54 ± 2.71 | 13.22 ± 1.59 |
| Platelets count (×109 cells/L) | 220 ± 62 | 262 ± 87 | 250 ± 93 |
| WBC count (×109 cells/L) | 6.05 ± 1.81 | 6.45 ± 2.12 | 6.89 ± 2.35 |
| CRP (mg/L) | 2.10 ± 1.04 | 3.42 [1.10; 12.95] | 1.45 [1.33; 4.96] |
BMI - body mass index; RBC - red blood cells; Hb - hemoglobin; CRP - C-reactive protein; WBC - white blood cells.
From each participant a blood sample was collected (8-10 mL). These were further processed without any unnecessary delay according to the routine laboratory standards. Plasma was separated, and stored at -80°C until further assessment.
The peripheral levels of multiple GFs (HGF, VEGF, FGF, IGF-1, and G-CSF) were analyzed with use of ELISA kits (produced by R&D Systems, Minneapolis, MN, USA). During measurements investigators were following instructions provided by the manufacturer of the kit.
As described previously [28-33] all received results were subjected to comprehensive statistical analysis. Briefly, distribution of the variables was verified using the Shapiro-Wilk test. Continuous variables that were not normally distributed were subjected to log transformation. After verification of the normality of the distribution, mean values of examined parameters between appropriate groups were compared using Student’s t-test (parametric variables) or Mann-Whitney U-test (non-parametric variables). In order to calculate the correlations between parametric and non-parametric variables we used Pearson’s or Spearman’s correlation rank tests (respectively). In addition, we performed a multivariate regression analyses with use of a stepwise selection method. In order to exclude eventual presence of any residual confounding we entered individually the variables that initially were excluded from the constructed model. Finally, we constructed the ROC curves and calculated the AUCs values for all growth factors examined here as eventual diagnostic substances for gastric cancer in humans. These statistical analyses were executed with use of SPSS software and P < 0.05 values were considered as significant.
This study was performed in accordance with appropriate regulations and guidelines highlighted in the “World Medical Association Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects”, as well as its protocol has been approved by the Bioethical Committee of the Pomeranian Medical University in Szczecin. Informed written consents have been obtained from all participants of this study.
Results
Comparison of analyzed groups of patients
A statistical comparison of parameters describing general status of individuals participating in this study is presented in Table 1. This analysis revealed lack of significant differences in the values of presented parameters between analyzed groups. However, comparison of body mass index (BMI) values and hemoglobin levels between cancer patients and healthy controls revealed values close to statistical significance (P = 0.06 and P = 0.07 respectively).
Systemic levels of GFs in examined groups
In Figures 1 and 2 are depicted mean GF values examined in both healthy controls and patients with gastric malignancies. As presented in Figure 1 the systemic levels of HGF, IGF-1, and VEGF were significantly higher in patients with gastric carcinoma than in healthy volunteers (Figure 1). When the levels of examined growth factors were compared between healthy controls and individuals with other than carcinoma types of gastric neoplasms, we found statistically significant differences only in terms of HGF and IGF-1 concentrations (Figures 1 and 2). In our study, no significant differences were observed in systemic FGF and G-CSF concentrations between individuals from the control group and both groups of patients with different types of gastric neoplasms (Figure 2).
Figure 1.
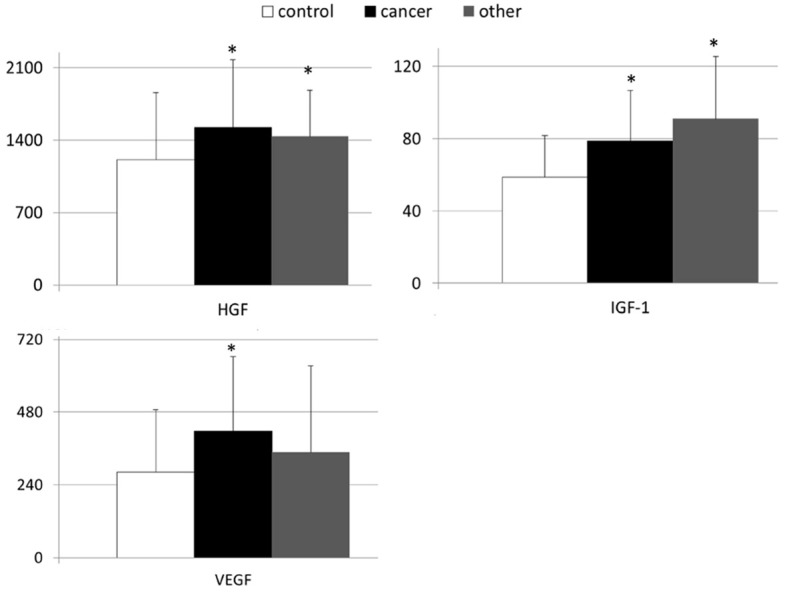
Mean values of peripheral levels of selected growth factors in patients with gastric cancer, other gastric neoplasms and control individuals together with their statistical comparison (mean levels ± standard deviation [pg/mL]). HGF - hepatocyte growth factor; VEGF - vascular-endothelial growth factor; IGF-1 - insulin-like growth factor-1; *P < 0.05 - level of significance (in comparison to “control” group).
Figure 2.
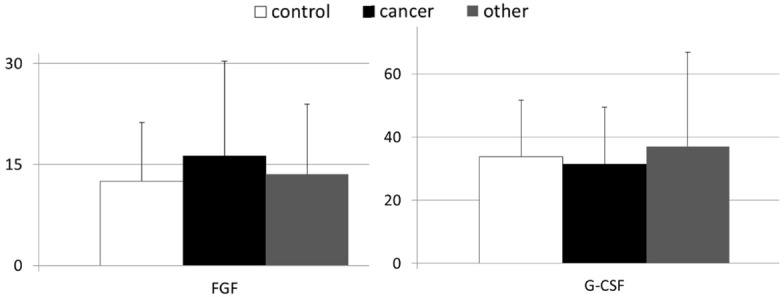
Mean peripheral levels of fibroblast growth factor (FGF) and granulocyte-colony stimulating factor (G-CSF) in patients with gastric cancer, other types of gastric neoplasms and control individuals (means ± standard deviation [pg/mL]).
Evaluation of clinical associations
Next we became interested if the observed values of peripheral concentrations of examined GFs are linked to the clinical staging of gastric carcinoma in our patients. In order to evaluate this aspect we analyzed the concentrations of examined GFs in our cancer patients subdivided into early and advanced gastric cancer subgroups. This analysis demonstrated that in comparison to healthy volunteers, individuals with early gastric cancer had significantly higher systemic levels of IGF-1, but not of HGF and VEGF (Figure 3). Interestingly, IGF-1 levels in patients with advanced gastric were also significantly higher than in control individuals. However, these values were not significantly different from those present in patients with early gastric cancer. The mean peripheral levels of HGF and VEGF were significantly higher in patients with advanced gastric cancer than in other examined (sub)group (Figure 3). We did not observe any statistical differences in values of FGF and G-CSF between healthy controls and both subgroups of patients with gastric carcinoma (Figure 4). In addition, we verified if the peripheral concentrations of GFs examined here were associated with the clinical staging of gastric carcinoma in our patients, that was evaluated according to the established tumor node metastasis (TNM) classification. Statistical analyses with use of multivariate regression revealed that in our patients only the systemic HGF levels were significantly associated with the TNM staging of gastric carcinoma (Table 2).
Figure 3.
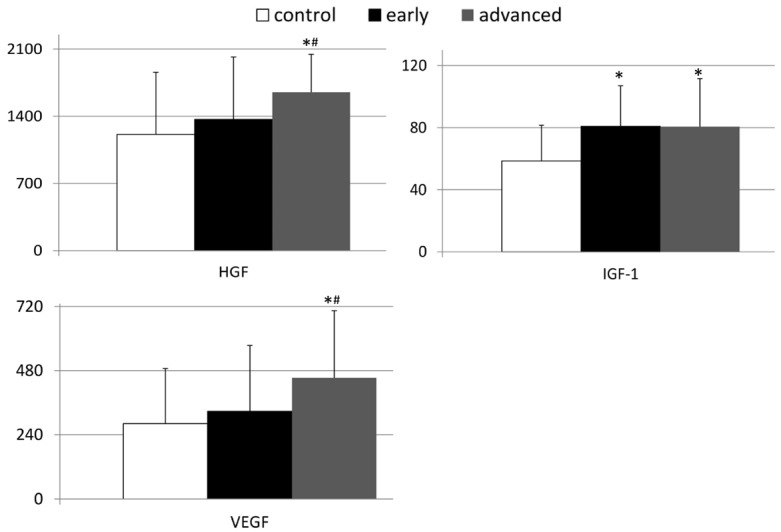
Mean peripheral levels of examined growth factors in patients with early and advanced gastric cancer, as well as in control individuals together with their statistical comparison. (means ± standard deviation [pg/mL]). HGF - hepatocyte growth factor; VEGF - vascular-endothelial growth factor; IGF-1 - insulin-like growth factor-1; *P < 0.05 - level of significance (in comparison to the “control” group); #P < 0.05 - level of significance (in comparison to the “early” group).
Figure 4.
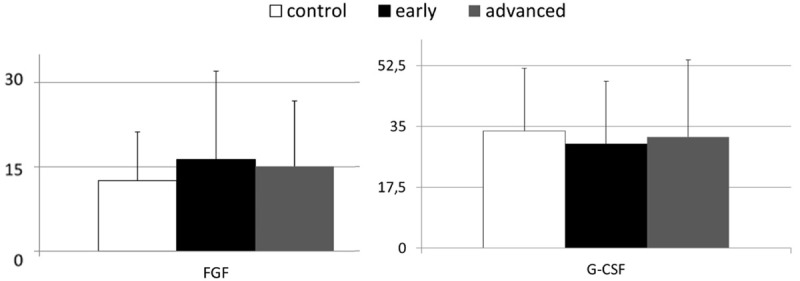
Mean peripheral levels of fibroblast growth factor (FGF) and granulocyte-colony stimulating factor (G-CSF) in healthy controls and patients with gastric cancer, divided into subgroups of early and advanced cancer (means ± standard deviation [pg/mL]).
Table 2.
Analysis of associations between levels of examined growth factors and clinical presentation of gastric cancer in patients (modelling using multivariate regression analysis)
| Dependent variable | Independent variable | β | R2 | p |
|---|---|---|---|---|
| Gastric cancer TNM staging * | HGF | 0.28 | 0.20 | 0.04 |
| VEGF | 0.18 | 0.11 | 0.12 | |
| IGF-1 | 0.21 | 0.13 | 0.07 | |
| FGF | 0.03 | 0.02 | 0.74 | |
| G-CSF | 0.08 | 0.03 | 0.38 |
β - standardized coefficient in the regression equation; p - level of significance; HGF - hepatocyte growth factor; VEGF - vascular-endothelial growth factor; IGF-1 - insulin-like growth factor-1; FGF - fibroblast growth factor; G-CSF - granulocyte-colony stimulating factor;
Variable was created by assigning 1, 2, 3 or 4 value to appropriate TNM stage detected in patients with gastric cancer.
Growth factors and populations of stem cells
We also were interested in verifying whether the concentrations of GFs examined here were correlated with the absolute numbers of circulating bone marrow-derived stem cells (BMSCs), reported previously [26]. We found that in patients with gastric carcinoma the peripheral concentrations of only HGF correlated significantly with the absolute numbers of very small embryonic/epiblast-like stem cells and mesenchymal stem cells, but not hematopoietic stem/progenitor cells or endothelial progenitor cells circulating in the peripheral blood (Table 3).
Table 3.
Coefficients of correlations between levels of examined growth factors and previously reported absolute numbers of circulating populations of bone marrow-derived stem cells in patients with gastric carcinoma (n = 8)
| Population of stem cells | HGF | VEGF | IGF-1 | FGF | G-CSF |
|---|---|---|---|---|---|
| VSEL | 0.31* | NS | NS | NS | NS |
| MSC | 0.32* | NS | NS | NS | NS |
| HSC | NS | NS | NS | NS | NS |
| EPC | NS | NS | NS | NS | NS |
P < 0.05.
P - level of significance; NS - not significant; VSEL - very small embryonic-like stem cells; MSC - mesenchymal stem cells; HSC - hematopoietic stem cells; EPC - endothelial progenitor cells; HGF - hepatocyte growth factor; VEGF - vascular-endothelial growth factor; IGF-1 - insulin-like growth factor-1; FGF - fibroblast growth factor; G-CSF - granulocyte-colony stimulating factor.
Examined GFs as potential novel biomarkers of gastric cancer in humans
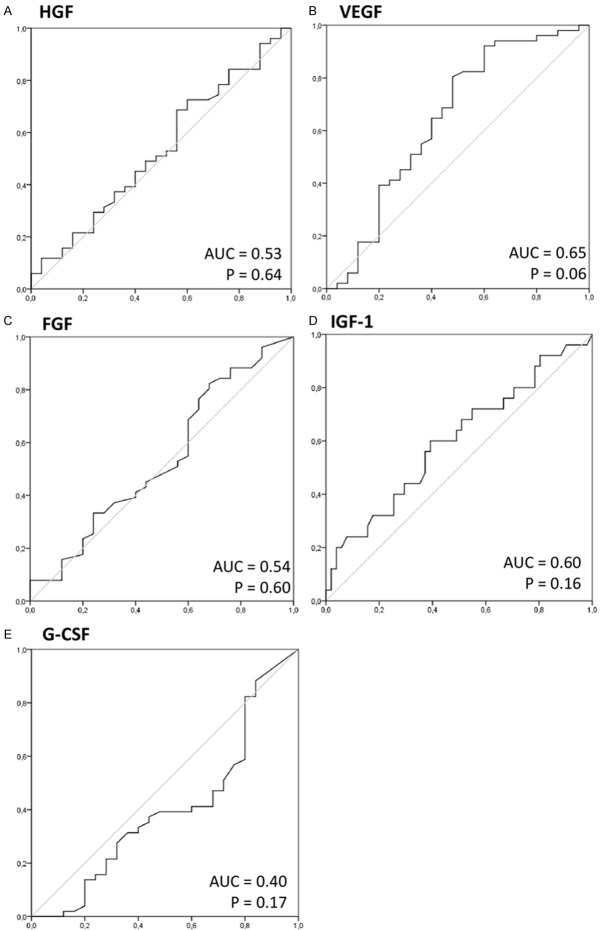
Finally, we wanted to test if the peripheral concentrations of the GFs examined here may possess any value for detection and/or differentiation of gastric carcinoma in humans. In order to realize this goal we performed analyses with use of receiver operator characteristic (ROC) curves and evaluated the respective area under curve (AUC) values to test GFs as potential new substances that could serve as diagnostic markers. Results of our analyses revealed relatively low AUC values for all GFs evaluated here, and these were not statistically significant (presented in Figure 5).
Figure 5.
Receiver operating characteristics (ROC) curves of examined growth factors as clinical indicators of gastric carcinoma. Calculated sensitivity (y-axis) is plotted against 1-specificity formula (x-axis) for examined growth factors, that is (A) HGF, (B) VEGF, (C) FGF, (D) IGF-1 and (E) G-CSF. AUC - area under curve; p - level of significance; HGF - hepatocyte growth factor; VEGF - vascular-endothelial growth factor; IGF-1 - insulin-like growth factor-1; FGF - fibroblast growth factor; G-CSF - granulocyte-colony stimulating factor.
Discussion
Within the last few years, growing evidence has suggested that the activities of a wide array of GFs may be fundamentally involved in the pathogenesis of various human cancers, as well as considered as a promising target for anti-cancer therapies [34-36]. Therefore we decided to perform a comprehensive evaluation of the peripheral concentrations of several GFs that, from a molecular standpoint, could exert the most impact on the development, survival, and systemic spread of gastric malignancies in humans.
We observed that patients with gastric malignancies presented an imbalance in the peripheral concentrations of selected GFs examined here, mainly in the HGF, IGF-1, and VEGF concentrations. Significant elevations in the peripheral levels of HGF and IGF-1 were observed not only in individuals with gastric cancer, but also in patients suffering from other types of gastric malignancies. However, higher VEGF levels were found exclusively in individuals with gastric cancer. Our findings are therefore in agreement with previous results showing that individuals with gastric cancer had significantly elevated peripheral levels of HGF, IGF-1, and VEGF [37-41]. However, our results expanded upon these previous findings, revealing that systemic imbalances in peripheral HGF and IGF-1 levels occurred not only in patients with gastric carcinoma, but also are present in individuals with other types of gastric neoplasms. Furthermore, the results of our analyses demonstrated that significant alterations in peripheral GF concentrations in individuals with gastric cancer are usually present mainly (if not only) in patients with advanced disease, defined according to the Japanese classification.
Currently, insufficient data are available to fully explain why such systemic imbalances occur in patients with gastric carcinoma, or what mechanism(s) might be responsible for this phenomenon. It seems likely that cancer cells present in the tumor microenvironment are not the cause of this phenomenon, as the tissue expression of certain GFs (like HGF and its receptor c-MET) do not correlate with their peripheral levels [37]. Therefore, we speculate that the imbalance in certain GF may result from various exosomes secreted to the periphery from gastric cancer cells, their metabolism, and most likely their interactions with immune-related cytokines (for example IL-8) that have recently been implicated in the pathogenetic scenario and clinical presentation of gastric carcinoma [42-44]. Given that changes in systemic GF levels occur mainly in individuals with gastric cancer that has reached the gastric muscular layer (thereby gaining access to the vasculature), this explanation seems to be the most probable. However, this matter requires verification in further experimental and clinical studies.
It is also very interesting that our data demonstrated significant associations between systemic HGF levels, but not G-CSF concentrations, and the peripheral circulation of certain BMSC populations in patients with gastric carcinoma. Currently, very little is known regarding the biochemical signals that promote communication between the stem cell environment and a developing malignancy. Our data reveal that in case of gastric carcinoma in humans, HGF (but not the widely known stem cell chemoattractant G-CSF) may be involved in such signaling, as higher HGF levels were associated with increased absolute numbers of circulating very small embryonic/epiblast-like stem cells, which represent a BMSC subpopulation with unique and very powerful regenerative and trans-differentiation potential. We observed similar results in previous studies performed in patients with pancreatic cancer [45].
However, independently of the molecular mechanism(s) responsible for the imbalanced peripheral GF levels, our results revealed that this phenomenon was not specific for gastric carcinoma, as certain “components” of it also accompanied presence of other than cancer types of gastric neoplasms. It is difficult to definitively explain the general impact of this phenomenon on the progression of different types of gastric tumors or the clinical prognosis of affected patients. Data from preliminary studies have shown that the presence of elevated peripheral VEGF levels in patients with gastric NENs may be indicative of local and systemic spread, as well as of a worse prognosis [46-49]. However, no studies published to date have addressed this issue in patients with GISTs or gastric lymphomas. It seems probable that this matter will be addressed in greater detail in the upcoming years, given that innovative medications interfering in GF signaling are currently being tested in clinical trials and their effectiveness in the treatment of (gastric) cancers is also being evaluated [50,51]. In this context, it is noteworthy that our results provide important new information that may be helpful for understanding the results of recent clinical trials conducted in patients with gastric cancer, involving medications that interfere with GF activities. For example, very recent results on bevacizumab-containing chemotherapy (anti-VEGF antibodies) demonstrated the lack of additional clinical benefit using this medication on the overall prognosis of patients with early gastric carcinoma [52]. Our results seem to agree with these clinical observations. Namely, we found that although peripheral VEGF levels are elevated in the overall group of patients with gastric cancer, our results suggested that the systemic VEGF level is of greater significance in patients with advanced gastric carcinoma. Moreover, we did not observe any significant differences in the systemic levels of FGF, in agreement with the results of clinical trial on FGF and its receptor inhibitors in patients with gastrointestinal malignancies [53]. In aforementioned clinical trial, the application of such new medications did not improve the overall survival rates of cancer patients, showing a response rate of < 10% in treated individuals [50]. Our data suggest that it would be of interest to test the therapeutic value of novel GF inhibitors in patients with gastric neoplasms other than carcinoma, as these patients also have abnormal systemic levels of various GFs.
Finally, some previous findings have suggested that the peripheral levels of certain GFs may be of some prognostic value in individuals with different gastric malignancies [37,48,54-56]. However, their potential use as biomarkers of gastric tumors has not been examined. Therefore, we investigated several GFs to determine whether they could be of potential diagnostic value for detecting or differentiating different types of human gastric malignancies with lesions located in the stomach tissue. Unfortunately, the GFs examined did not show sufficient sensitivity and specificity to be considered as independent biomarkers of human gastric tumors. Moreover, the results of our study appear to suggest that, due to their low specificity and sensitivity, these GFs most likely will not be helpful in supporting conventional methods/techniques already used for diagnosis of gastric malignancies in humans.
In summary, these results demonstrated that multiple GFs examined here are of significance in both the pathogenetic scenario and clinical presentation of gastric malignancies in humans. Our data indicate that an imbalance in the peripheral levels of the GFs studied here is not specific to patients with gastric cancer, but also occurs in patients affected by other than cancer types of gastric malignancies. Moreover, our study also revealed the clinical significance of HGF in the process of intensified systemic circulation of selected BMSCs in individuals with gastric cancer. Finally, our data demonstrated that the peripheral concentrations of the multiple GFs do not seem to be of sufficient diagnostic potential to be used as independent (bio)markers for discriminating various types of gastric malignancies in patients.
Acknowledgements
This study was financed from grant funds awarded by the Polish Ministry of Science and Higher Education (IP2014003273). The grant founders had no role in the study design, data acquisition and analysis, or in the decision to submit the article for publication.
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Smyth EC, Cunningham D. Gastric cancer in 2012: defining treatment standards and novel insights into disease biology. Nat Rev Clin Oncol. 2013;10:73–74. doi: 10.1038/nrclinonc.2012.228. [DOI] [PubMed] [Google Scholar]
- 3.Soreide K, Sandvik OM, Soreide JA, Giljaca V, Jureckova A, Bulusu VR. Global epidemiology of gastrointestinal stromal tumours (GIST): a systematic review of population-based cohort studies. Cancer Epidemiol. 2016;40:39–46. doi: 10.1016/j.canep.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 4.Isaacson PG, Du MQ. MALT lymphoma: from morphology to molecules. Nat Rev Cancer. 2004;4:644–653. doi: 10.1038/nrc1409. [DOI] [PubMed] [Google Scholar]
- 5.Ilett EE, Langer SW, Olsen IH, Federspiel B, Kjar A, Knigge U. Neuroendocrine carcinomas of the gastroenteropancreatic system: a comprehensive review. Diagnostics. 2015;5:119–176. doi: 10.3390/diagnostics5020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen C, Chen H, Chen H, Yin Y, Han L, Chen J, Tang S, Yin X, Zhou Z, Zhang B, Chen Z. Surgical treatment and prognosis of gastric neuroendocrine neoplasms: a single-center experience. BMC Gastroenterol. 2016;16:111. doi: 10.1186/s12876-016-0505-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szucs Z, Thway K, Fisher C, Bulusu R, Constantinidou A, Benson C, van der Graaf WT, Jones RL. Molecular subtypes of gastrointestinal stromal tumors and their prognostic and therapeutic implications. Future Oncol. 2017;13:93–107. doi: 10.2217/fon-2016-0192. [DOI] [PubMed] [Google Scholar]
- 8.Bikfalvi A. Recent developments in the inhibition of angiogenesis: examples from studies on platelet factor-4 and the VEGF/VEGFR system. Biochem Pharmacol. 2004;68:1017–1021. doi: 10.1016/j.bcp.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 9.Song K, Yuan Y, Lin Y, Wang YX, Zhou J, Gai QJ, Zhang L, Mao M, Yao XX, Qin Y, Lu HM, Zhang X, Cui YH, Bian XW, Zhang X, Wang Y. ERBB3, IGF1R, and TGFBR2 expression correlate with PDGFR expression in glioblastoma and participate in PDGFR inhibitor resistance of glioblastoma cells. Am J Cancer Res. 2018;8:792–809. [PMC free article] [PubMed] [Google Scholar]
- 10.Yakar S, Wu Y, Setser J, Rosen CJ. The role of circulating IGF-I: lessons from human and animal models. Endocrine. 2002;19:239–248. doi: 10.1385/ENDO:19:3:239. [DOI] [PubMed] [Google Scholar]
- 11.Di Domenico M, Giordano A. Signal transduction growth factors: the effective governance of transcription and cellular adhesion in cancer invasion. Oncotarget. 2017;8:36869–36884. doi: 10.18632/oncotarget.16300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallo S, Sala V, Gatti S, Crepaldi T. Cellular and molecular mechanisms of HGF/Met in the cardiovascular system. Clin Sci (Lond) 2015;129:1173–1193. doi: 10.1042/CS20150502. [DOI] [PubMed] [Google Scholar]
- 13.Xiang C, Chen J, Fu P. HGF/Met signaling in cancer invasion: the impact on cytoskeleton remodeling. Cancers (Basel) 2017;9:E44. doi: 10.3390/cancers9050044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trovato M, Campennì A, Giovinazzo S, Siracusa M, Ruggeri RM. Hepatocyte growth Factor/C-Met axis in thyroid cancer: from diagnostic biomarker to therapeutic target. Biomark Insights. 2017;12:1177271917701126. doi: 10.1177/1177271917701126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisermann K, Fraizer G. The androgen receptor and vegf: mechanisms of androgen-regulated angiogenesis in prostate cancer. Cancers (Basel) 2017;9:E32. doi: 10.3390/cancers9040032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roskoski R Jr. Vascular endothelial growth factor (VEGF) and VEGF receptor inhibitors in the treatment of renal cell carcinomas. Pharmacol Res. 2017;120:116–132. doi: 10.1016/j.phrs.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Alvarez-Sola G, Uriarte I, Latasa MU, Urtasun R, Bárcena-Varela M, Elizalde M, Jimenez M, Rodriguez-Ortigosa CM, Corrales FJ, Fernandez-Barrena MG, Berasain C, Avila MA. Fibroblast growth factor 15/19 in hepatocarcinogenesis. Dig Dis. 2017;35:158–165. doi: 10.1159/000450905. [DOI] [PubMed] [Google Scholar]
- 18.Tahara E. Abnormal growth factor/cytokine network in gastric cancer. Cancer Microenviron. 2008;1:85–91. doi: 10.1007/s12307-008-0008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wadhwa R, Song S, Lee JS, Yao Y, Wei Q, Ajani JA. Gastric cancer - molecular and clinical dimensions. Nat Rev Clin Oncol. 2013;10:643–655. doi: 10.1038/nrclinonc.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith MG, Hold LG, Tahara El-Omar EM. Cellular and molecular aspects of gastric cancer. World J Gastroenterol. 2006;12:2979–2990. doi: 10.3748/wjg.v12.i19.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hao NB, Tang B, Wang GZ, Xie R, Hu CJ, Wang SM, Wu YY, Liu E, Xie X, Yang SM. Hepatocyte growth factor (HGF) upregulates heparanase expression via the PI3K/Akt/NF-κB signaling pathway for gastric cancer metastasis. Cancer Lett. 2015;361:57–66. doi: 10.1016/j.canlet.2015.02.043. [DOI] [PubMed] [Google Scholar]
- 22.Lee KH, Kim JR. Regulation of HGF-mediated cell proliferation and invasion through NF-κB, JunB, and MMP-9 cascades in stomach cancer cells. Clin Exp Metastasis. 2012;29:263–272. doi: 10.1007/s10585-011-9449-x. [DOI] [PubMed] [Google Scholar]
- 23.Lee KH, Choi EY, Kim MK, Hyun MS, Eun JR, Jang BI, Kim TN, Kim SW, Song SK, Kim JH, Kim JR. Hepatocyte growth factor promotes cell survival by phosphorylation of BAD in gastric cancer cells. Oncol Res. 2008;17:23–32. doi: 10.3727/096504008784046072. [DOI] [PubMed] [Google Scholar]
- 24.Koh SA, Lee KH. HGF mediated upregulation of lipocalin 2 regulates MMP9 through nuclear factor-κB activation. Oncol Rep. 2015;34:2179–2187. doi: 10.3892/or.2015.4189. [DOI] [PubMed] [Google Scholar]
- 25.Morris KT, Khan H, Ahmad A, Weston LL, Nofchissey RA, Pinchuk IV, Beswick EJ. G-CSF and G-CSFR are highly expressed in human gastric and colon cancers and promote carcinoma cell proliferation and migration. Br J Cancer. 2014;110:1211–1220. doi: 10.1038/bjc.2013.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blogowski W, Zuba-Surma E, Sałata D, Budkowska M, Dołęgowska B, Starzyńska T. Peripheral trafficking of bone-marrow-derived stem cells in patients with different types of gastric neoplasms. Oncoimmunology. 2016;5:e1099798. doi: 10.1080/2162402X.2015.1099798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen S, Zhang X, Peng J, Zhai E, He Y, Wu H, Chen C, Ma J, Wang Z, Cai S. VEGF promotes gastric cancer development by upregulating CRMP4. Oncotarget. 2015;7:17074–17086. doi: 10.18632/oncotarget.7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blogowski W, Madej-Michniewicz A, Marczuk N, Dolegowska B, Starzyńska T. Interleukins 17 and 23 in patients with gastric neoplasms. Sci Rep. 2016;6:37451. doi: 10.1038/srep37451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blogowski W, Serwin K, Budkowska M, Salata D, Dolegowska B, Lokaj M, Prowans P, Starzynska T. Clinical analysis of systemic and adipose tissue levels of selected hormones/adipokines and stromal-derived factor-1. J Biol Regul Homeostat Agents. 2012;26:607–615. [PubMed] [Google Scholar]
- 30.Dolegowska B, Blogowski W, Domanski L. Association between the perioperative antioxidative ability of platelets and early post-transplant function of kidney allografts: a pilot study. PLoS One. 2012;7:e29779. doi: 10.1371/journal.pone.0029779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deskur A, Salata D, Budkowska M, Dolegowska B, Starzynska T, Blogowski W. Selected hemostatic parameters in patients with pancreatic tumors. Am J Transl Res. 2014;6:768–776. [PMC free article] [PubMed] [Google Scholar]
- 32.Bodnarczuk T, Deskur A, Dolegowska K, Dolegowska B, Starzynska T, Blogowski W. Hydroxyeicosatetraenoic acids in patients with pancreatic cancer: a preliminary report. Am J Cancer Res. 2018;8:1865–1872. [PMC free article] [PubMed] [Google Scholar]
- 33.Blogowski W, Dolegowska K, Deskur A, Dolegowska B, Starzynska T. An attempt to evaluate selected aspects of “bone-fat axis” function in healthy individuals and patients with pancreatic cancer. Medicine (Baltimore) 2015;94:e1303. doi: 10.1097/MD.0000000000001303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu TC, Jin X, Wang Y, Wang K. Role of epidermal growth factor receptor in lung cancer and targeted therapies. Am J Cancer Res. 2017;7:187–202. [PMC free article] [PubMed] [Google Scholar]
- 35.Nieto-Estevez V, Defterali C, Vicario-Abejon C. IGF-I: a key growth factor that regulates neurogenesis and synaptogenesis from embryonic to adult stages of the brain. Front Neurosci. 2016;10:52. doi: 10.3389/fnins.2016.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo F, Zhang H, Jia Z, Cui M, Tian J. Chemoresistance and targeting of growth factors/cytokines signalling pathways: towards the development of effective therapeutic strategy for endometrial cancer. Am J Cancer Res. 2018;8:1317–1331. [PMC free article] [PubMed] [Google Scholar]
- 37.Noguchi E, Saito N, Kobayashi M, Kameoka S. Clinical significance of hepatocyte growth factor/c-Met expression in the assessment of gastric cancer progression. Mol Med Rep. 2015;11:3423–3431. doi: 10.3892/mmr.2015.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanaka K, Miki C, Wakuda R, Kobayashi M, Tonouchi H, Kusunoki M. Circulating level of hepatocyte growth factor as a useful tumor marker in patients with early-stage gastric carcinoma. Scand J Gastroenterol. 2004;39:754–760. doi: 10.1080/00365520410005973. [DOI] [PubMed] [Google Scholar]
- 39.Mohri Y, Miki C, Tanaka K, Kawamoto A, Ohi M, Yokoe T, Kusunoki M. Clinical correlations and prognostic relevance of tissue angiogenic factors in patients with gastric cancer. Clin Oncol (R Coll Radiol) 2012;24:610–616. doi: 10.1016/j.clon.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Park DJ, Seo AN, Yoon C, Ku GY, Coit DG, Strong VE, Suh YS, Lee HS, Yang HK, Kim HH, Yoon SS. Serum VEGF-A and tumor vessel VEGFR-2 levels predict survival in caucasian but not asian patients undergoing resection for gastric adenocarcinoma. Ann Surg Oncol. 2015;22:S1508–S1515. doi: 10.1245/s10434-015-4790-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Franciosi CM, Piacentini MG, Conti M, Romano F, Musco F, Caprotti R, Rovelli F, Uggeri F. IGF-1 and IGF-1BP3 in gastric adenocarcinoma. Preliminary study. Hepatogastroenterology. 2003;50:297–300. [PubMed] [Google Scholar]
- 42.Zhang H, Deng T, Liu R, Bai M, Zhou L, Wang X, Li S, Wang X, Yang H, Li J, Ning T, Huang D, Li H, Zhang L, Ying G, Ba Y. Exosome-delivered EGFR regulates liver microenvironment to promote gastric cancer liver metastasis. Nat Commun. 2017;8:15016. doi: 10.1038/ncomms15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee KH, Koh SA, Kim JR. Hepatocyte growth factor-mediated gastrin-releasing peptide induces IL-8 expression through Ets-1 in gastric cancer cells. Oncol Res. 2013;20:393–402. doi: 10.3727/096504013X13657689382770. [DOI] [PubMed] [Google Scholar]
- 44.Madej-Michniewicz A, Budkowska M, Sałata D, Dołęgowska B, Starzyńska T, Błogowski W. Evaluation of selected interleukins in patients with different gastric neoplasms: a preliminary report. Sci Rep. 2015;5:14382. doi: 10.1038/srep14382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Starzynska T, Dabkowski K, Blogowski W, Zuba-Surma E, Budkowska M, Salata D, Dolegowska B, Marlicz W, Lubikowski J, Ratajczak MZ. An intensified systemic trafficking of bone marrow-derived stem/progenitor cells in patients with pancreatic cancer. J Cell Mol Med. 2013;17:792–799. doi: 10.1111/jcmm.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cigrovski Berkovic M, Cacev T, Catela Ivkovic T, Marout J, Ulamec M, Zjacic-Rotkvic V, Kapitanovic S. High VEGF serum values are associated with locoregional spread of gastroenteropancreatic neuroendocrine tumors (GEP-NETs) Mol Cell Endocrinol. 2016;425:61–68. doi: 10.1016/j.mce.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 47.Kuiper P, Hawinkels LJ, de Jonge-Muller ES, Biemond I, Lamers CB, Verspaget HW. Angiogenic markers endoglin and vascular endothelial growth factor in gastroenteropancreatic neuroendocrine tumors. World J Gastroenterol. 2011;17:219–225. doi: 10.3748/wjg.v17.i2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pavel ME, Hassler G, Baum U, Hahn EG, Lohmann T, Schuppan D. Circulating levels of angiogenic cytokines can predict tumour progression and prognosis in neuroendocrine carcinomas. Clin Endocrinol (Oxf) 2005;62:434–443. doi: 10.1111/j.1365-2265.2005.02238.x. [DOI] [PubMed] [Google Scholar]
- 49.Kaltsas GA, Cunningham JL, Falkmer SE, Grimelius L, Tsolakis AV. Expression of connective tissue growth factor and IGF1 in normal and neoplastic gastrointestinal neuroendocrine cells and their clinico-pathological significance. Endocr Relat Cancer. 2010;18:61–71. doi: 10.1677/ERC-10-0026. [DOI] [PubMed] [Google Scholar]
- 50.Rolfo C, Bronte G, Sortino G, Papadimitriou K, Passiglia F, Fiorentino E, Marogy G, Russo A, Peeters M. The role of targeted therapy for gastrointestinal tumors. Expert Rev Gastroenterol Hepatol. 2014;8:875–885. doi: 10.1586/17474124.2014.922870. [DOI] [PubMed] [Google Scholar]
- 51.Bradley CA, Salto-Tellez M, Laurent-Puig P, Bardelli A, Rolfo C, Tabernero J, Khawaja HA, Lawler M, Johnston PG, Van Schaeybroeck S. Targeting c-MET in gastrointestinal tumours: rationale, opportunities and challenges. Nat Rev Clin Oncol. 2017;14:562–576. doi: 10.1038/nrclinonc.2017.40. [DOI] [PubMed] [Google Scholar]
- 52.Cunningham D, Stenning SP, Smyth EC, Okines AF, Allum WH, Rowley S, Stevenson L, Grabsch HI, Alderson D, Crosby T, Griffin SM, Mansoor W, Coxon FY, Falk SJ, Darby S, Sumpter KA, Blazeby JM, Langley RE. Peri-operative chemotherapy with or without bevacizumab in operable oesophagogastric adenocarcinoma (UK Medical Research Council ST03): primary analysis results of a multicentre, open-label, randomised phase 2-3 trial. Lancet Oncol. 2017;18:357–370. doi: 10.1016/S1470-2045(17)30043-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garrett CR, Siu LL, El-Khoueiry A, Buter J, Rocha-Lima CM, Marshall J, LoRusso P, Major P, Chemidlin J, Mokliatchouk O, Velasquez L, Hayes W, Feltquate D, Syed S, Ford S, Kollia G, Galbraith S, Nuyten DS. Phase I dose-escalation study to determine the safety, pharmacokinetics and pharmacodynamics of brivanib alaninate in combination with full-dose cetuximab in patients with advanced gastrointestinal malignancies who have failed prior therapy. Br J Cancer. 2011;105:44–52. doi: 10.1038/bjc.2011.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang L, Chang Y, Xu J, Zhang Q. Predictive significance of serum level of vascular endothelial growth factor in gastric cancer patients. Biomed Res Int. 2016;2016:8103019. doi: 10.1155/2016/8103019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Macedo F, Ladeira K, Longatto-Filho A, Martins SF. Gastric cancer and angiogenesis: is VEGF a useful biomarker to assess progression and remission? J Gastric Cancer. 2017;17:1–10. doi: 10.5230/jgc.2017.17.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takahashi N, Furuta K, Taniguchi H, Sasaki Y, Shoji H, Honma Y, Iwasa S, Okita N, Takashima A, Kato K, Hamaguchi T, Shimada Y, Yamada Y. Serum level of hepatocyte growth factor is a novel marker of predicting the outcome and resistance to the treatment with trastuzumab in HER2-positive patients with metastatic gastric cancer. Oncotarget. 2016;7:4925–4938. doi: 10.18632/oncotarget.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]