Abstract
Osteoarthritis (OA) is considered to be a joint-associated disorder and one of leading reasons for disability, however, potential mechanism has never been clarified. The purpose of this research was to evaluate protective-effects of Achyranthes Bidentata extracts (ABE) on chondrocytes function in osteoarthritis. We performed a systematic investigation of transcriptional and proteomic landscapes to identify the underlying mechanisms behind effects of ABE on chondrocytic functions. OA animal models were generated in the present research. Chondrocytes were isolated and cultured, and then prepared for GeneChip analysis. Two-dimensional gel electrophoresis and LC-MS/MS analysis were conducted to analyze samples. Quantitative real-time PCR (qRT-PCR) and western blotting were used to evaluate expression of protein kinase B (AKT), β-tubulin and β-action. Apoptosis and glycolysis pathway were significantly compromised in chondrocytes with ABE stimulation as revealed by both transcriptional and proteomic data. Consistently, ABE suppressed chondrocytes apoptosis and glycolytic activity in vitro through modulating multiple genes, such as Plk2, Casp1/12 and Cers1 as well as Pkm2, Eno1/3 and Pgk2. Mechanically, ABE activated MAPK signaling pathway and suppressed AKT signaling pathway, therefore, reducing the glycolysis to provide survival benefits. We extended our analysis by verifying insulin-like growth factor 1 (IGF-1) and MAP kinase 1 (MEK1) in chondrocytes function. Depletion of either IGF-1 or MEK1 impaired AKT expression and phosphorylation, leading to the enhanced chondrocyte apoptosis and reduced cell proliferation. In conclusion, our study provided systematic view and molecular basis for ABE to serve as potential intervention of OA via suppressing AKT signaling.
Keywords: Achyranthes bidentata, osteoarthritis, AKT, GLUT1, glycolysis
Introduction
Osteoarthritis (OA) is considered to be a joint-associated disorder and one of leading reasons for disability, influencing appropriate 250 million patients in the whole world [1]. A cohort study [2] shows the prevalence of symptomatic knee osteoarthritis in adults ranges from 7% to 17%, increasing with age, especially post age of 65 years. Multiple risk factors, including age, prior injury, overuse, obesity, and genetic predisposition are particularly relevant with the higher risk of OA [3].
OA is also a degenerative and poor progressive disease, without restoration for OA caused damage. For decades, despites continued efforts have been performed to fully understand OA pathogenesis and treatment, there is currently no cure for OA. Therefore, the present strategies mainly eliminate the stiffness, painful signals and improve the life-quality. Clinically, strategies treating OA mainly includes surgical operation, administrating with drugs or non-drug treatments. Pharmacological therapies in clinical guidelines include capsaicin, topical nephrogenic syndrome of inappropriate anti-diuresis (NSIADs), and salicylates [4-7]. Due to the side-effects of drugs and the lower efficacy, it isn’t recommended to administrate medications for long periods. In recent years, several new strategies have also been applied, including mesenchymal stem cells (MSCs) transplantation, injecting intra-articularly with platelet rich plasma (PRP), both of which have been proven to be safe and well tolerated for relieving pain and improving knee functions [8-10]. These are still under investigation and more studies and standardization of these therapies would be required.
Therefore, sustained attempts are indispensable for discoveries of novel cellular targets and effective intervention of OA. Nowadays, OA has been proven to be correlated with the metabolic diseases, such as diabetes mellitus type 2 (T2DM), especially for T2DM occurring in older patients. The above description suggests that the metabolic alterations might be a potential link with OA [11-14]. While it is well-characterized that tumor cells prefer to a shift toward increased glycolysis even in presence of oxygen. Meanwhile, an adaptation to stressful and dynamic microenvironment of solid tumor would facilitate the tumor proliferation and invasiveness [15-17]. Strikingly, the microenvironment in swollen joints is considered to be of hypoxia and insufficient nutrients supply [18,19]. Increased glucose consumption has been observed in swollen joints by fluoro-deoxy-glucose positron emission tomography (FDG-PET) [20-25]. These observations suggested increase of glycolysis might be a critical factor driving the disease progression of OA, therefore, implying targeting glycolysis might provide therapeutic intervention for OA.
Radix achyranthes bidentata (AB), a Traditional Chinese Medicinal Herb, has been extensively used in Chinese Medicinal formulations for treating a variety of diseases, such as OA [26-31]. The underlying molecular basis is poorly clarified, which has limited its potential use of OA therapy. In present study, we have performed Affymetrix GeneChip and proteomic approach for identifying potential effectors or specific signaling events that favor the protecting effects of AB extract (ABE). Interestingly, GeneChip analysis reveals that glycolysis and hypoxia-inducible factor 1 (Hif-1) signaling pathways in chondrocytes were significantly compromised in response to ABE treatment. Of note, mRNA expressions of protein kianse B (AKT2) and glucose transporter 1 (GLUT1) are identified and verified to be remarkably down-regulated upon ABE treatment. Meanwhile, the increased mitogen-activated protein kinase 1 (MAP2K1) and enhanced insulin-like growth factor 1 (IGF-1) are also found. Moreover, proteomic-dissection of altered protein profiles using two-dimensional gel electrophoresis and liquid chromatography-mass spectrometry (LC-MS) also showed that ABE predominately targets the glycolysis. We previously observed that the reduced protein levels of AKT2 are considered as a critical regulator of glucose metabolism. The functional investigations also revealed that depletion of IGF-1 or MAP2K1 dramatically suppresses chondrocytes proliferation and facilitates apoptosis induced by ABE. On the contrary, over-expression of AKT or GLUT1 significantly results in the opposite effects. Furthermore, ablation of IGF-1 or MAP2K1 showed reduced AKT levels and impaired AKT signaling. Collectively, our study for the first time provided experimental evidences that ABE treatment impaired glycolysis in chondrocytes. Therefore, the ABE treatment could facilitate the chondrocytes viability through suppressing AKT signaling, and provide the basis for treating OA by combining ABE treatment with the glycolysis inhibition.
Materials and methods
Animals and OA model establishment
Total of 6 New Zealand white rabbits (male) were employed to generate OA models. Left knee joints were mobilized (1.5 cm below groin to 3 cm above rear-ankle) for 6 weeks with the plaster bandages, keeping knee-flexed at angle of 30°-40°. On each-side, the dorsalis pedis pulses were examined and observed. The plaster tightness was detected for 3 days post the establishment of models. Rabbits were than fed in single cages and were moved or exercise. The other untreated 6 rabbits were employed as the normal control. The successful modeling was evaluated using Pelletier grading and staging system and further confirmed by hematoxylin-eosin (HE) staining and immunohistochemistry (IHC) staining of Collagen II. All of the experiments or tests have been approved by Institutional Animal Care and Use Committee of Shenzhen Traditional Chinese Medicine Hospital.
Isolation and primary culture of chondrocytes
Knee articular cartilages were isolated from OA rabbits models. The isolated chondrocytes were cut into thin-slices. The cartilage was digested using 0.25% Trypsin (Beyotime Beotech. Shanghai, China) for 30 min and then cells were obtained. Subsequently, the cells were cultured in F12 medium containing 0.1% collagenase II and 10% fetal bovine serum (FBS) and supplementing with penicillin (100 U/mL) and streptomycin (100 mg/mL) in 5% CO2 for 4 h at 37°C. Chondrocytes were passaged with a ratio of 1:3. Finally, the third passaged chondrocytes were utilized for the following experiments or tests.
Sample preparation and GeneChip analysis
Chondrocytes were plated into 6-cm dish. When the cell confluence reaching 80%, chondrocytes were treated with 50 μg/ml and 100 μg/ml ABE contained DMEM:F12 (1:1) solution for low dose and high dose, respectively. Meanwhile, the 10 ng/ml bFGF was also added to the DMEM:F12 (1:1) for 24 h. Cells were then washed with phosphate buffered saline (PBS) for 3 times, followed by RNA extraction. The gene expression profiles were monitored using Agilent SurePrint G3 Rat GE (8*60K, Design ID: 028279) according to protocol provided by manufacture. In order to obtain the raw data, the array-images were analyzed using Feature Extraction software (version 10.7.1.1, Agilent Technologies, Santa Clare, CA, USA). Then, the basic analysis was conducted using Genespring (version 13.1, Agilent Technologies), with raw data as template. Raw data in this study was normalized using quantile algorithm, while probes illustrating more than 100% of values in anyone out of conditions have flags in “Detected” were selected for the following data analysis. The differentially-expressed genes were subsequently identified based on the fold-changes. When a fold change more than 2.0, the up-regulation and down-regulation of genes were assigned. Finally, kyoto encyclopedia of genes and genomes (KEGG) analysis and gene ontology (GO) analysis were conducted to evaluate roles of the above differentially-expressed genes.
Two-dimensional gel electrophoresis and LC-MS/MS analysis
Cells and samples were prepared as described above. Briefly, cells with indicated treatment were lysed with radio-immuno-precipitation assay (RIPA). The total protein was extracted and quantified using bicinchoninic acid (BCA) assay. Equal amount of total proteins were loaded by separating with 2D gel electrophoresis. The separation between one or more protein “spots” on the scanned image of a 2-DE gel was analyzed using the software packages, including Delta2D, ImageMaster, Melanie, PDQuest, Progenesis and REDFIN software, as previously described [32].
The excised spots were further analyzed using in-gel digestion and LC-MS/MS as previously described [33]. Briefly, liquid chromatography (LC) was conducted on a Nano-Acquity UPLC system (Waters Corporation, Milford, USA) combining with a mass spectrometer (mode: LTQ Orbitrap XL, Thermo Scientific, Bremen, Germany). Peptides were re-suspended with 20 µl solvent A (5% acetonitrile, 0.1% formic acid in water). Total of 18 µl peptide solution was then loaded onto trap-column (100 μm×2.0 mm Acclaim PepMap C18, Thermo Fisher Scientific, San Jose, CA, USA) at a 20 μl/min flow rate of solvent. Database search and protein identification were performed according to protocol in previous study. Raw files were processed by MaxQuant 1.5.3.8 for peptide/protein identification and quantification with the human International Protein Index database (IPI human 3.45, 71983 entries). The searching parameters were set up as the follows: full trypsin (KR)-cleavage with two-missed cleavage was considered, the oxidation of methionine was specified as the variable modification. Fragment ion tolerance was 0.5 Da and the peptide mass tolerance was 10 ppm. At least two peptides were required for the match of each protein identified.
Quantitative real-time PCR (RT-PCR) and western blotting
Total RNAs were extracted and purified using Trizol and the reverse transcription reaction was performed with the First-strand complementary DNA (cDNA) Synthesis Kit. The resulting cDNAs were used as templates for amplifying the indicated genes. qRT-PCR assay was performed using SYBR® Premix Ex Taq™ according to the manual protocol provided by TaKaRa. PCR reactions were done in triplicates with ABI 7500 device (Applied Biosystems Incorporation). Fold enrichment was calculated with the 2-ΔΔCt method relative to β-actin.
For western blot assay, the cells were lysed using RIPA lysis buffer (Beyotime Beotech.) supplementing with protease inhibitor (Sigma-Aldrich, St. Louis, Missouri, USA) for 30 min. The supernatants were separated with sodium dodecyl sulphate-polyAcrylamide gel electrophoresis (SDS-PAGE) and subjected for western blotting analysis against indicated antibodies.
Statistical analysis
Data were recorded as mean ± standard deviation (SD) and analyzed using SPSS software (version: 20.0, SPSS Inc., Chicago, Ull, USA). The Tukey’s post-hoc test validated analysis of variance (ANOVA) was used to analyze differences of measurement data among groups. A statistical significance was defined when P<0.05.
Results
Isolation and characterization of chondrocytes in rabbit model of OA
OA modeling was established using knee immobilization, which is reported to achieve a reproducible model to investigate the pathogenesis and therapy of OA [34-36]. The successful modeling was evaluated using Pelletier grading and staging system. The cartilage lesions were obviously discovered in femoral condyles isolating from the knee joints undergoing knee immobilization (Figure 1A). Pelletier grading showed significantly higher scores in modeling group (Figure 1B). HE staining suggested severe fibrillation and erosion of articular cartilage following modeling (Figure 1C). IHC staining of college II indicated extensive loss of college II, which suggests the cartilage destruction (Figure 1D). These evidences implied the successful modeling of rabbit OA. Chondrocyte was isolated according to the previously described. Chondrocytes displayed fibroblast-like morphology, which is similar to previously described chondrocytes. The identity of isolated chondrocytes was further confirmed using Toluidine Blue O (TBO) staining as well as IHC, which demonstrates the chondrocytes characteristics.
Figure 1.
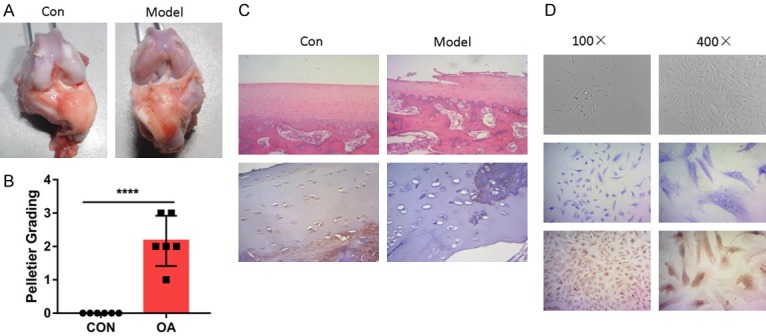
Rabbit OA model was successfully established for chondrocytes isolation. A. Macroscopic observations of rabbit articular cartilage in indicated group. Typical changes of the cartilage lesions were illustrated. B. Modeling was evaluated using Pelletier grading. C. Hematoxylin-eosin (HE) staining and immunohistochemistry (IHC) staining verified the proper modeling of OA in rabbit. The IHC-staining was conducted with the anti-Collagen II antibody. D. Isolated chondrocytes was evaluated using Microscopic observation, HE and IHC staining.
Gene expression profiling of chondrocytes identified the differentially expressed mRNAs upon ABE treatment
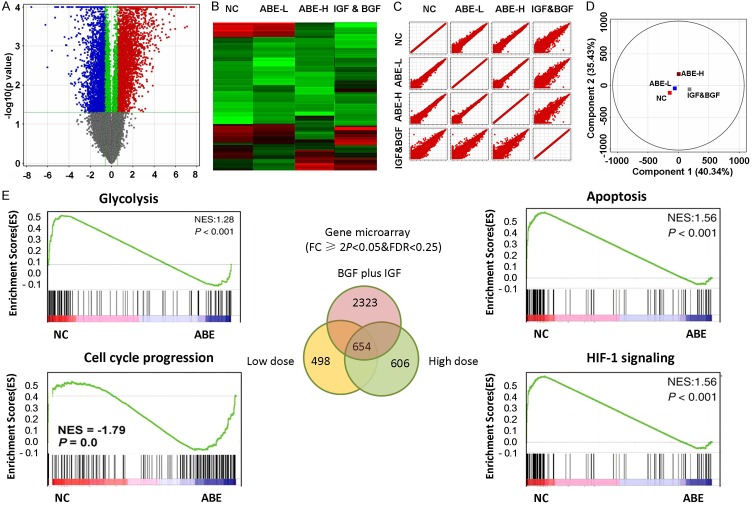
To gain insight into the underlying mechanism by which ABE mediated the protecting effect of chondrocytes, we performed GeneChip analysis of RNA expressions in chondrocytes treated with low dose or high dose of ABE. Treatment of chondrocytes with fibroblast growth factor (bFGF) together with insulin-like growth factor-1 (IGF-1) was used in parallel to evaluate the effect of ABE. Differentially expressed mRNAs were filtered through fold changes. Volcano plot filtering analysis (Figure 2A) and the heat map analysis (Figure 2B) demonstrated the differentially-expressed genes, illustrating significant differences among the four groups. Scatter Plot (Figure 2C) and principal component analysis (PCA) (Figure 2D) further supported the good separation and expression signatures among these groups. By the threshold set of fold change ≥2.0, P<0.05 and FDR <0.05, a total of 498 genes versus 606 genes was found to be differentially regulated. Among which 153 genes and 232 genes were up-regulated, while 374 and 345 down-regulated mRNAs were observed in the low dose and high dose groups respectively, compared with the control group (Figure 2E). Notably, in contrast to low dose treatment, 287 genes were discovered to be changed in chondrocytes with high dose treatment, suggesting a dose-dependent effect of ABE. Strikingly, 2323 genes were significantly altered in bFGF plus IGF-1 including 1232 up-regulated and 1091 down-regulated genes. Venn diagram revealed 654 genes were simultaneously regulated among these groups. These data suggest that ABE exerts transcriptome-wide changes of genes expression in chondrocytes.
Figure 2.
Gene expression profiles revealed ABE predominately targeting glycolysis and apoptosis pathway. A. Volcano plots were used to visualize the differentially-expressed genes among different conditions. Vertical lines correspond to 2.0 folds (log2 scaled) were assigned as up-regulation and down-regulation, respectively. The horizontal line represented a P-value of 0.05 (-log10 scaled). The red and blue points in plot represent the differentially expressed genes with statistical significance. B. Heatmap and hierarchical cluster analysis of the most up and down regulated genes. C. Scatter plots were used to evaluate the difference in the expression of genes between experiment group and control group. Values plotted on the X and the Y axes were the mean normalized signal values for each group (log2 scaled). D. PCA plot revealed the expression profiles in the indicated groups showed good separation. E. GSEA was used to identify the most enriched signaling pathways. Top enriched pathways were showed.
ABE treatment connected specific signaling pathways and transcriptional programs for anti-apoptotic response
The gene expression data sets among these groups were integrated and analyzed using multiple bioinformatics methods, including Gene Set Enrichment Analysis (GSEA), GO analysis and KEGG analysis. To this end, the differentially expressed genes were functionally classified into several pathways, including glycolysis, Hif-1 signaling, cell cycle progression, apoptosis and PI3K/AKT signaling pathway and the others. Given that Hif-1 signaling and PI3K/AKT signaling pathway are critical regulators of glycolysis, we assumed that ABE treatment might predominately suppress glucose metabolism. Moreover, cell cycle progression as well as apoptosis downstream of PI3K/AKT signaling is also remarkably enriched. We extended our analysis through integrating key pathway modules and network reconstruction. As showed in Figure 3A, we hypnotized that ABE treatment might mediate the protecting effect through modulating the glycolysis pathway and cell cycle progression through PI3K/AKT signaling pathway.
Figure 3.
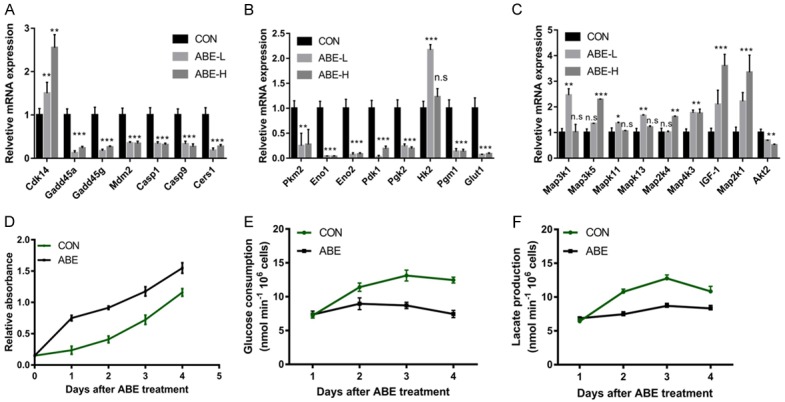
ABE treatment suppressed glycolysis and apoptosis. A. Cell cycle and apoptosis-related genes were examined using qRT-PCR. B. Glycolysis-related genes were examined using qRT-PCR. C. MAPK pathway-related genes were examined using qRT-PCR. D. ABE treatment inhibited chondrocytes proliferation. E. ABE treatment repressed glucose uptake. F. ABE treatment inhibited lactate production. *, **, ***means P<0.05, P<0.01, and P<0.001 by comparing with CON.
ABE treatment facilitated chondrocytes cell proliferation and suppressed glycolysis
Next, we performed qRT-PCR analysis to verify the GeneChip data. Cdk14, which positively controlling cell proliferation, was up-regulated in response to ABE stimulation. Plk2, and the canonical targeting genes of p53, including Gadd45a, Gadd45g and Mdm2, were found to be repressed by ABE treatment, suggesting the suppression of negative cell cycle regulators (Figure 3A). Casp1 and Casp12, two potent components of apoptotic cascades, showed reduced levels in chondrocytes following ABE treatment (Figure 3A). Cers1 could encode the CerS1 enzyme implicated in de novo ceramide biosynthesis [37]. Meanwhile, CerS1 mainly generates the C18-Ceramide, which is a critical intracellular inducer of apoptosis [37]. Interestingly, Cers1 was observed to be significantly decreased by ABE stimulation. This evidence indicated that ABE stimulation could promote chondrocytes cell proliferation through suppression of key modulators that negatively govern cell cycle progression. Furthermore, the ABE stimulation could also inhibit the apoptotic process via distinct mechanisms. In agreement with these observations, examination of the proliferation activity and apoptosis showed that the ABE treatment indeed facilitates chondrocytes cell proliferation and represses cell death.
We found the mRNA expressions of certain important metabolic enzymes encoded by Pkm2, Eno1, Eno2, Pdk1, Pgk2, Hk2, were reduced in chondrocytes treated with ABE (Figure 3B, 3C). SLC2A1, as a glucose transporter encoding Glut1, was also decreased. This result suggests the glycolysis pathway is compromised following ABE treatment, possible providing survival benefits for chondrocytes. Since many genes involved glycolysis were reduced upon ABE treatment in chondrocytes, therefore, ABE treatment indeed affected the glucose metabolism (Figure 3D). Evaluation of the glucose uptake (Figure 3E) and lactate production (Figure 3F) suggested that glycolytic rate decreased by 40% following ABE treatment.
Proteomic-dissection revealed ABE suppresses chondrocytes cell apoptosis and glycolysis
To verify and interpret the transcriptional data, we determined to examine the protein profile of chondrocytes cell following ABE treatment. Two-dimensional gel electrophoresis (2-DE) coupled with LC-MS/MS was a powerful strategy for comparative proteomics research (Figure 4A). Therefore, 2-DE was used to identify the differentiated expressed proteins. A total of 61 proteins were discovered, and were functionally categorized (Figure 4B). The top enriched pathways mainly include cytoskeletal regulation, apoptosis, integrin pathway, glycolysis, FGF signaling, ECM regulation, transcription factors and EGF signaling. In lines with the transcriptional investigation, these findings here strongly supported that ABE might predominately modulate apoptosis and glycolysis signaling events. Interestingly, we found the protein level of AKT2 was significantly down-regulated by ABE treatment (Figure 4C). This line of evidence might imply that the functions of ABE towards chondrocytes cell apoptosis and glycolysis are probably through activating AKT signaling cascade.
Figure 4.
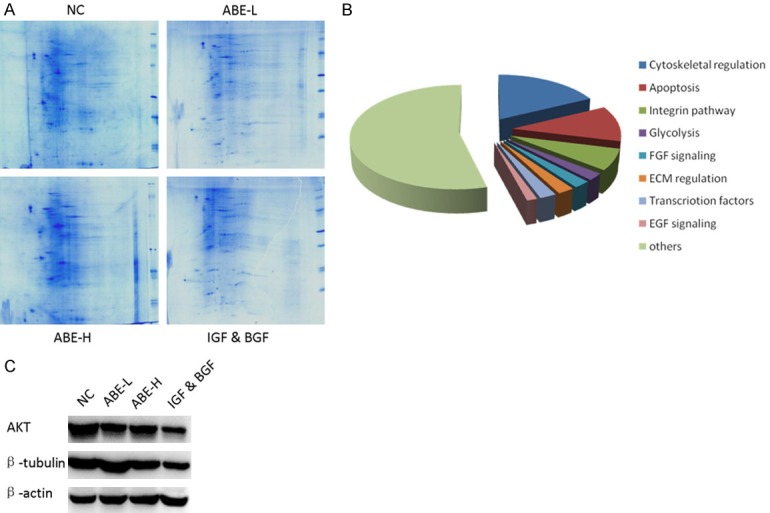
2-D gel separation and LC-MS/MS of protein profiles in chondrocytes upon ABE treatment. A. 2-D gel separation and commassie blue staining in indicated groups. B. Functional classification of identified proteins. Top enriched pathways were showed. C. The protein levels of AKT2 and β-tubulin were down-regulated in chondrocytes upon ABE treatment.
Depletion of IGF-1 and MEK1 in chondrocytes led to increased apoptosis through modulation of AKT
Next, we performed in vitro investigation of selected genes for the effects on chondrocytes phenotypes. IGF-1 and MEK1, two newly discovered genes induced by ABE, were selected to verify the following experiments. Ablation of IGF-1 and MEK1 using short hairpin RNA (shRNA) in chondrocytes resulted in 3-5 folds enhanced cell apoptosis, suggesting IGF-1 (Figure 5A) or MEK1 (Figure 5B) were required for chondrocytes survival. Moreover, knockdown of either IGF-1 (Figure 5C) or MEK1 (Figure 5D) led to decreased cell proliferation activity. Since IGF-1 acts as an upstream molecule of the MAPK signaling pathway and AKT signaling pathway [38-40], we therefore determined whether IGF-1 and/or MEK1 would play roles through AKT signaling pathway. The protein levels of AKT2, as well as phos-AKT were remarkably increased in chondrocytes undergoing the IGF-1 or MEK1 silencing (Figure 5E). These findings suggest that the pro-survival functions of IGF-1 and MEK1 were played through modulating AKT signaling pathway.
Figure 5.
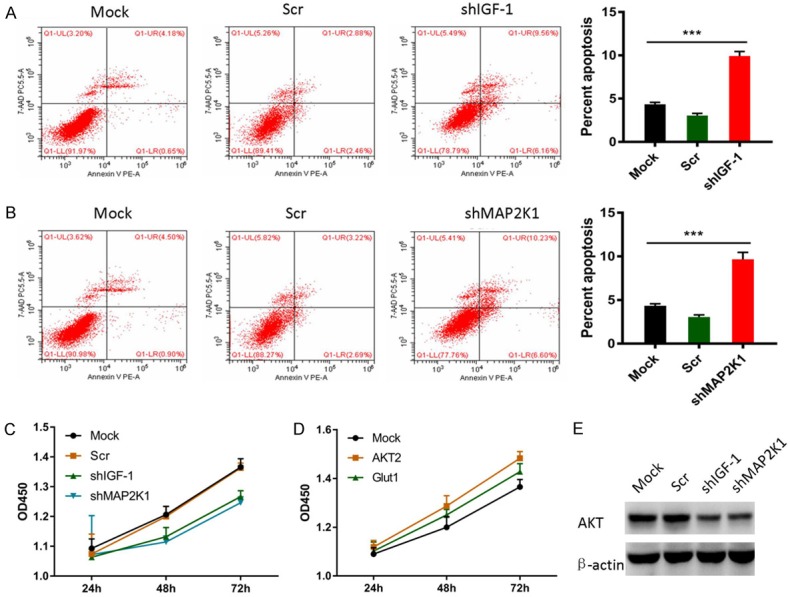
IGF-1 and MEK1 suppressed chondrocyte apoptosis through activating AKT signaling pathway. A. Knockdown of IGF-1 promoted chondrocyte apoptosis as revealed by Annexin V-FITC/PI. B. Knockdown of MEK1 facilitated chondrocyte apoptosis as revealed by Annexin V-FITC/PI. C. Knockdown of IGF-1 suppressed chondrocyte proliferation as evidenced by CCK8 assays. D. Ablation of MEK1 suppressed chondrocyte proliferation as evidenced by CCK8 assays. E. Depletion of IGF-1 and MEK1 up-regulated AKT protein levels and activated AKT signaling.
Discussion
OA, caused by the irreversible cartilage damage, is considered as a widespread disabling disorder [1,2]. Although continued efforts have been made to fully understand the OA pathogenesis and therapy, the current management of pharmacotherapies and non-pharmacological therapies has been reported to have limited effectiveness. Considering the side effects, it is not recommended for long-term use. A few new therapeutic approaches and administrations are also undergone the identification, therefore, the effects of these approaches are also needed to be explored [8-10]. Traditional Chinese Medicine might provide alterative intervention for OA. Radix Achyranthes bidentata (AB), a Traditional Chinese Medicinal Herb, has been extensively used in Chinese medicinal formulations for treating a variety of diseases, such as OA [26-31]. Till now, the protecting effects of ABE on OA seem also ambiguous and the underlying molecular basis of ABE remains poorly understood, therefore, it’s needed to investigate the potential use of ABE on treating OA.
Chondrocytes constitute of the essential component of articular cartilage and are crucial for maintaining the physical structures of cartilage [41,42]. Here, we performed a systematic study to explore the potential mechanisms hinting in ABE’s effects on the chondrocytic functions. Interestingly, glycolysis pathway was found to be significantly impaired in chondrocytes with ABE stimulation. Moreover, Hif-1 and AKT signaling cascades upstream of glycolysis were spontaneously attenuated. Many key genes, such as Pkm2, Eno1, Eno2, Pdk1, Pgk2 and Hk2, were reduced in chondrocytes treated with ABE. 2-DE coupled with LC-MS/MS also strongly supported that ABE might predominately modulate apoptosis and glycolysis signaling events. Consistently, OA has been proven to be correlated with the metabolic disorders or disturbances [11,12]. Furthermore, the growing cases of OA and T2DM provide sufficient clinical evidence for correlation between OA and the abnormal metabolism [13,14]. Enhanced glucose uptake has been seen in swollen joints by FDG-PET [20-25]. These observations increased glycolysis might be a critical factor that drives the disease progression of OA. Our data indicated here that ABE could target glycolysis to protect the functions of chondrocytes. Furthermore, dual treatment of ABE and glycolysis inhibition might provide therapeutic intervention for OA, which requires further investigation in the future work.
In this study, ABE was found to regulate cell proliferation and apoptosis through multiple mechanisms. On one hand, ABE inhibited the expressions of negative cell cycle regulators, such as Plk2, and the canonical target genes of p53, including Gadd45a, Gadd45g and Mdm2. On the other hand, ABE suppressed the mRNA levels of Casp1 and Casp12, components of apoptotic pathways. Interestingly, Cers1 was observed to be significantly decreased by ABE stimulation. Cers1 was involved in de novo biosynthesis of mainly C18-Ceramide, which potently induces cell apoptosis and autophagy [37]. These findings suggest that ABE could facilitate the cell proliferation via distinct manners.
Eventually, two differentiated genes (IGF-1 and MEK1) were used to verify effects of ABE on chondrocytic functions. Knockdown of IGF-1 or MEK1 significantly promoted cell apoptosis and impaired cell proliferation, suggesting IGF-1 and MEK1 involve in preventing chondrocytes viability. Since ABE primarily modulates glycolysis and apoptosis, the AKT serves as master regulator of glycolysis and apoptosis. Of note, certain genes of upstream of AKT signaling pathway, including Rictor, Pik3ap1 and Inpp5d functions upstream of AKT signaling were found to be simultaneously regulated. Also, many MAPK signaling pathway associated molecules were identified to be regulated by ABE treatment. The previous literatures have demonstrated functions of IGF-1 in MAPK signaling pathway and AKT signaling pathway [38-40]. Therefore, we reasoned whether IGF-1 and/or MEK1 would play functions through activating the AKT signaling pathway. IGF-1 or MEK1 ablation remarkably increased AKT2 protein levels and activated the AKT molecule, suggesting IGF-1 or MEK1 indeed plays roles through activating the AKT signaling pathways to reduce glycolysis.
In conclusion, our study provided systematic view and molecular basis for ABE to serve as potential intervention of OA. Meanwhile, ABE also highlighted that ABE predominately targeting glycolysis via suppressing the AKT signaling pathway.
Acknowledgements
This work was funded by National Natural Science Foundation of China (Grant No. 81804124) and Project supported by the Natural Science Foundation of Guangdong Province, China (Grant No. 2018A0303130138).
Disclosure of conflict of interest
None.
References
- 1.Hunter DJ, Schofield D, Callander E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol. 2014;10:437–441. doi: 10.1038/nrrheum.2014.44. [DOI] [PubMed] [Google Scholar]
- 2.Vina ER, Kwoh CK. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol. 2018;30:160–167. doi: 10.1097/BOR.0000000000000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bortoluzzi A, Furini F, Scirè CA. Osteoarthritis and its management - Epidemiology, nutritional aspects and environmental factors. Autoimmun Rev. 2018;17:1097–1104. doi: 10.1016/j.autrev.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Racine J, Aaron RK. Pathogenesis and epidemiology of osteoarthritis. R I Med J (2013) 2013;96:19–22. [PubMed] [Google Scholar]
- 5.Wu X, Cao L, Li F, Ma C, Liu G, Wang Q. Interleukin-6 from subchondral bone mesenchymal stem cells contributes to the pathological phenotypes of experimental osteoarthritis. Am J Transl Res. 2018;10:1143–1154. [PMC free article] [PubMed] [Google Scholar]
- 6.Valdes AM, Spector TD. Genetic epidemiology of hip and knee osteoarthritis. Nat Rev Rheumatol. 2011;7:23–32. doi: 10.1038/nrrheum.2010.191. [DOI] [PubMed] [Google Scholar]
- 7.Valdes AM, Spector TD. The genetic epidemiology of osteoarthritis. Curr Opin Rheumatol. 2010;22:139–143. doi: 10.1097/BOR.0b013e3283367a6e. [DOI] [PubMed] [Google Scholar]
- 8.Cook CS, Smith PA. Clinical update: why PRP should be your first choice for injection therapy in treating osteoarthritis of the knee. Curr Rev Musculoskelet Med. 2018;11:583–592. doi: 10.1007/s12178-018-9524-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meng M, Liu Y, Wang W, Wei C, Liu F, Du Z, Xie Y, Tang W, Hou Z, Li Q. Umbilical cord mesenchymal stem cell transplantaion in the treatment of multiple sclerosis. Am J Transl Res. 2018;10:212–223. [PMC free article] [PubMed] [Google Scholar]
- 10.Im GI. Tissue engineering in osteoarthritis: current status and prospect of mesenchymal stem cell therapy. BioDrugs. 2018;32:183–192. doi: 10.1007/s40259-018-0276-3. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Wei J, Zeng C, Yang T, Li H, Cui Y, Xie D, Xu B, Liu Z, Li J, Jiang S, Lei G. Association between serum magnesium concentration and metabolic syndrome, diabetes, hypertension and hyperuricaemia in knee osteoarthritis: a cross-sectional study in Hunan Province, China. BMJ Open. 2018;8:e019159. doi: 10.1136/bmjopen-2017-019159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adeyemi WJ, Olayaki LA. Diabetes escalates knee osteoarthritis in rats: evidence of adaptive mechanism. Environ Toxicol Pharmacol. 2018;61:1–7. doi: 10.1016/j.etap.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Courties A, Sellam J. Osteoarthritis and type 2 diabetes mellitus: what are the links? Diabetes Res Clin Pract. 2016;122:198–206. doi: 10.1016/j.diabres.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 14.Aiello FC, Trovato FM, Szychlinska MA, Imbesi R, Castrogiovanni P, Loreto C, Musumeci G. Molecular links between diabetes and osteoarthritis: the role of physical activity. Curr Diabetes Rev. 2017;13:50–58. doi: 10.2174/1573399812666151123104352. [DOI] [PubMed] [Google Scholar]
- 15.Liao W, Liu J, Zhang D, Huang W, Chen R. Butein inhibited in vitro hexokinase-2 mediated tumor glycolysis in hepatocellular carcinoma by blocking epidermal growth factor receptor (EGFR) Med Sci Monit. 2018;24:3283–3292. doi: 10.12659/MSM.906528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajeshkumar NV, Dutta P, Yabuuchi S, de Wilde RF, Martinez GV, Le A, Kamphorst JJ, Rabinowitz JD, Jain SK, Hidalgo M, Dang CV, Gillies RJ, Maitra A. Therapeutic targeting of the Warburg effect in pancreatic cancer relies on an absence of p53 function. Cancer Res. 2015;75:3355–3364. doi: 10.1158/0008-5472.CAN-15-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nie H, Li J, Yang XM, Cao QZ, Feng MX, Xue F, Wei L, Qin W, Gu J, Xia Q, Zhang ZG. Mineralocorticoid receptor suppresses cancer progression and the Warburg effect by modulating the miR-338-3p-PKLR axis in hepatocellular carcinoma. Hepatology. 2015;62:1145–1159. doi: 10.1002/hep.27940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gelse K, Pfander D, Obier S, Knaup KX, Wiesener M, Hennig FF, Swoboda B. Role of hypoxia-inducible factor 1 alpha in the integrity of articular cartilage in murine knee joints. Arthritis Res Ther. 2008;10:R111. doi: 10.1186/ar2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters CL, Morris CJ, Mapp PI, Blake DR, Lewis CE, Winrow VR. The transcription factors hypoxia-inducible factor 1alpha and Ets-1 colocalize in the hypoxic synovium of inflamed joints in adjuvant-induced arthritis. Arthritis Rheum. 2004;50:291–296. doi: 10.1002/art.11473. [DOI] [PubMed] [Google Scholar]
- 20.Shao F, Zhang M, Wang Y, Zou Y, Chen Y. Widespread amyloidosis around major joints on 18F-FDG PET/CT. Clin Nucl Med. 2017;42:76–78. doi: 10.1097/RLU.0000000000001442. [DOI] [PubMed] [Google Scholar]
- 21.Mabray MC, Brus-Ramer M, Behr SC, Pampaloni MH, Majumdar S, Dillon WP, Talbott JF. (18)F-sodium Fluoride PET-CT hybrid imaging of the lumbar facet joints: tracer uptake and degree of correlation to CT-graded arthropathy. World J Nucl Med. 2016;15:85–90. doi: 10.4103/1450-1147.174698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yonemoto Y, Okamura K, Kaneko T, Okura C, Kobayashi T, Suto T, Tsushima Y, Takagishi K. Effect of total knee arthroplasty on other joints in patients with rheumatoid arthritis evaluated by 18-FDG-PET. Int J Rheum Dis. 2017;20:702–707. doi: 10.1111/1756-185X.12855. [DOI] [PubMed] [Google Scholar]
- 23.Kamasaki T, Hayashida N, Miyamoto I, Usui T, Chiba K, Kudo T, Takamura N. PET/CT shows subjective pain in shoulder joints to be associated with uptake of (18)F-FDG. Nucl Med Commun. 2014;35:44–50. doi: 10.1097/MNM.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 24.Basu S, Shejul Y. Regional lymph node hypermetabolism corresponding to the involved joints on FDG-PET in newly diagnosed patients of rheumatoid arthritis: observation and illustration in symmetrical and asymmetric joint involvement. Rheumatol Int. 2014;34:413–415. doi: 10.1007/s00296-012-2604-0. [DOI] [PubMed] [Google Scholar]
- 25.Fischer DR, Pfirrmann CW, Zubler V, Stumpe KD, Seifert B, Strobel K, Tamborrini G, von Schulthess GK, Michel BA, Ciurea A. High bone turnover assessed by 18F-fluoride PET/CT in the spine and sacroiliac joints of patients with ankylosing spondylitis: comparison with inflammatory lesions detected by whole body MRI. EJNMMI Res. 2012;2:38. doi: 10.1186/2191-219X-2-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma C, Zhang C, Li X. Intervention and effect analysis of Achyranthes bidentata blume combined with aerobic exercise to interfere with type 2 diabetes. Pak J Pharm Sci. 2018;31:1151–1156. [PubMed] [Google Scholar]
- 27.Zhang M, Wang Y, Zhang Q, Wang C, Zhang D, Wan JB, Yan C. UPLC/Q-TOF-MS-based metabolomics study of the anti-osteoporosis effects of achyranthes bidentata polysaccharides in ovariectomized rats. Int J Biol Macromol. 2018;112:433–441. doi: 10.1016/j.ijbiomac.2018.01.204. [DOI] [PubMed] [Google Scholar]
- 28.Peng S, Wang C, Ma J, Jiang K, Jiang Y, Gu X, Sun C. Achyranthes bidentata polypeptide protects dopaminergic neurons from apoptosis in Parkinson’s disease models both in vitro and in vivo. Br J Pharmacol. 2018;175:631–643. doi: 10.1111/bph.14110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang Z, Qian J, Dong H, Yang J, Yu X, Chen J, Chen H, Shi Q, Jia L. The traditional Chinese medicine achyranthes bidentata and our de novo conception of its metastatic chemoprevention: from phytochemistry to pharmacology. Sci Rep. 2017;7:3888. doi: 10.1038/s41598-017-02054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang L, Jiang H, Yan ML, Xing XD, Zhang YY, Wei N, Yang BY, Wang QH, Kuang HX. A new phytoecdysteroid from the roots of achyranthes bidentata Bl. Nat Prod Res. 2017;31:1073–1079. doi: 10.1080/14786419.2016.1272114. [DOI] [PubMed] [Google Scholar]
- 31.He G, Guo W, Lou Z, Zhang H. Achyranthes bidentata saponins promote osteogenic differentiation of bone marrow stromal cells through the ERK MAPK signaling pathway. Cell Biochem Biophys. 2014;70:467–473. doi: 10.1007/s12013-014-9942-3. [DOI] [PubMed] [Google Scholar]
- 32.Murphy S, Dowling P, Ohlendieck K. Comparative skeletal muscle proteomics using two-dimensional gel electrophoresis. Proteomes. 2016;4 doi: 10.3390/proteomes4030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Liu Y, Duan J, Yan H, Zhang J, Zhang H, Fan Q, Luo F, Yan G, Qiao K, Liu J. Hippocalcin-like 1 suppresses hepatocellular carcinoma progression by promoting p21 (Waf/Cip1) stabilization by activating the ERK1/2-MAPK pathway. Hepatology. 2016;63:880–897. doi: 10.1002/hep.28395. [DOI] [PubMed] [Google Scholar]
- 34.Jiang S, He R, Zhu L, Liang T, Wang Z, Lu Y, Ren J, Yi X, Xiao D, Wang K. Endoplasmic reticulum stress-dependent ROS production mediates synovial myofibroblastic differentiation in the immobilization-induced rat knee joint contracture model. Exp Cell Res. 2018;369:325–334. doi: 10.1016/j.yexcr.2018.05.036. [DOI] [PubMed] [Google Scholar]
- 35.Campbell TM, Reilly K, Laneuville O, Uhthoff H, Trudel G. Bone replaces articular cartilage in the rat knee joint after prolonged immobilization. Bone. 2018;106:42–51. doi: 10.1016/j.bone.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 36.Jeon HS, Hwang S, Woo YK. The effect of ankle and knee immobilization on postural control during standing. Knee. 2013;20:600–604. doi: 10.1016/j.knee.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 37.Jazwinski SM, Kim S, Dai J, Li L, Bi X, Jiang JC, Arnold J, Batzer MA, Walker JA, Welsh DA, Lefante CM, Volaufova J, Myers L, Su LJ, Hausman DB, Miceli MV, Ravussin E, Poon LW, Cherry KE, Welsch MA Georgia Centenarian Study and the Louisiana Healthy Aging Study. HRAS1 and LASS1 with APOE are associated with human longevity and healthy aging. Aging Cell. 2010;9:698–708. doi: 10.1111/j.1474-9726.2010.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Padmanabhan S, Mukhopadhyay A, Narasimhan SD, Tesz G, Czech MP, Tissenbaum HA. A PP2A regulatory subunit regulates C. elegans insulin/IGF-1 signaling by modulating AKT-1 phosphorylation. Cell. 2009;136:939–951. doi: 10.1016/j.cell.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levine AJ, Feng Z, Mak TW, You H, Jin S. Coordination and communication between the p53 and IGF-1-AKT-TOR signal transduction pathways. Genes Dev. 2006;20:267–275. doi: 10.1101/gad.1363206. [DOI] [PubMed] [Google Scholar]
- 40.Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. 2004;14:395–403. doi: 10.1016/s1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- 41.Intekhab-Alam NY, White OB, Getting SJ, Petsa A, Knight RA, Chowdrey HS, Townsend PA, Lawrence KM, Locke IC. Urocortin protects chondrocytes from NO-induced apoptosis: a future therapy for osteoarthritis? Cell Death Dis. 2013;4:e717. doi: 10.1038/cddis.2013.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prasadam I, Akuien A, Friis TE, Fang W, Mao X, Crawford RW, Xiao Y. Mixed cell therapy of bone marrow-derived mesenchymal stem cells and articular cartilage chondrocytes ameliorates osteoarthritis development. Lab Invest. 2018;98:106–116. doi: 10.1038/labinvest.2017.117. [DOI] [PubMed] [Google Scholar]