Abstract
Background
Polycystic ovary syndrome (PCOS) is a common condition affecting 8% to 13% of reproductive‐aged women. In the past clomiphene citrate (CC) used to be the first‐line treatment in women with PCOS. Ovulation induction with letrozole should be the first‐line treatment according to new guidelines, but the use of letrozole is off‐label. Consequently, CC is still commonly used. Approximately 20% of women on CC do not ovulate. Women who are CC‐resistant can be treated with gonadotrophins or other medical ovulation‐induction agents. These medications are not always successful, can be time‐consuming and can cause adverse events like multiple pregnancies and cycle cancellation due to an excessive response. Laparoscopic ovarian drilling (LOD) is a surgical alternative to medical treatment. There are risks associated with surgery, such as complications from anaesthesia, infection, and adhesions.
Objectives
To evaluate the effectiveness and safety of LOD with or without medical ovulation induction compared with medical ovulation induction alone for women with anovulatory polycystic PCOS and CC‐resistance.
Search methods
We searched the Cochrane Gynaecology and Fertility Group (CGFG) trials register, CENTRAL, MEDLINE, Embase, PsycINFO, CINAHL and two trials registers up to 8 October 2019, together with reference checking and contact with study authors and experts in the field to identify additional studies.
Selection criteria
We included randomised controlled trials (RCTs) of women with anovulatory PCOS and CC resistance who underwent LOD with or without medical ovulation induction versus medical ovulation induction alone, LOD with assisted reproductive technologies (ART) versus ART, LOD with second‐look laparoscopy versus expectant management, or different techniques of LOD.
Data collection and analysis
Two review authors independently selected studies, assessed risks of bias, extracted data and evaluated the quality of the evidence using the GRADE method. The primary effectiveness outcome was live birth and the primary safety outcome was multiple pregnancy. Pregnancy, miscarriage, ovarian hyperstimulation syndrome (OHSS), ovulation, costs, and quality of life were secondary outcomes.
Main results
This updated review includes 38 trials (3326 women). The evidence was very low‐ to moderate‐quality; the main limitations were due to poor reporting of study methods, with downgrading for risks of bias (randomisation and allocation concealment) and lack of blinding.
Laparoscopic ovarian drilling with or without medical ovulation induction versus medical ovulation induction alone
Pooled results suggest LOD may decrease live birth slightly when compared with medical ovulation induction alone (odds ratio (OR) 0.71, 95% confidence interval (CI) 0.54 to 0.92; 9 studies, 1015 women; I2 = 0%; low‐quality evidence). The evidence suggest that if the chance of live birth following medical ovulation induction alone is 42%, the chance following LOD would be between 28% and 40%. The sensitivity analysis restricted to only RCTs with low risk of selection bias suggested there is uncertainty whether there is a difference between the treatments (OR 0.90, 95% CI 0.59 to 1.36; 4 studies, 415 women; I2 = 0%, low‐quality evidence). LOD probably reduces multiple pregnancy rates (Peto OR 0.34, 95% CI 0.18 to 0.66; 14 studies, 1161 women; I2 = 2%; moderate‐quality evidence). This suggests that if we assume the risk of multiple pregnancy following medical ovulation induction is 5.0%, the risk following LOD would be between 0.9% and 3.4%.
Restricting to RCTs that followed women for six months after LOD and six cycles of ovulation induction only, the results for live birth were consistent with the main analysis.
There may be little or no difference between the treatments for the likelihood of a clinical pregnancy (OR 0.86, 95% CI 0.72 to 1.03; 21 studies, 2016 women; I2 = 19%; low‐quality evidence). There is uncertainty about the effect of LOD compared with ovulation induction alone on miscarriage (OR 1.11, 95% CI 0.78 to 1.59; 19 studies, 1909 women; I2 = 0%; low‐quality evidence). OHSS was a very rare event. LOD may reduce OHSS (Peto OR 0.25, 95% CI 0.07 to 0.91; 8 studies, 722 women; I2 = 0%; low‐quality evidence).
Unilateral LOD versus bilateral LOD
Due to the small sample size, the quality of evidence is insufficient to justify a conclusion on live birth (OR 0.83, 95% CI 0.24 to 2.78; 1 study, 44 women; very low‐quality evidence).
There were no data available on multiple pregnancy.
The likelihood of a clinical pregnancy is uncertain between the treatments, due to the quality of the evidence and the large heterogeneity between the studies (OR 0.57, 95% CI 0.39 to 0.84; 7 studies, 470 women; I2 = 60%, very low‐quality evidence). Due to the small sample size, the quality of evidence is not sufficient to justify a conclusion on miscarriage (OR 1.02, 95% CI 0.31 to 3.33; 2 studies, 131 women; I2 = 0%; very low‐quality evidence).
Other comparisons
Due to lack of evidence and very low‐quality data there is uncertainty whether there is a difference for any of the following comparisons: LOD with IVF versus IVF, LOD with second‐look laparoscopy versus expectant management, monopolar versus bipolar LOD, and adjusted thermal dose versus fixed thermal dose.
Authors' conclusions
Laparoscopic ovarian drilling with and without medical ovulation induction may decrease the live birth rate in women with anovulatory PCOS and CC resistance compared with medical ovulation induction alone. But the sensitivity analysis restricted to only RCTs at low risk of selection bias suggests there is uncertainty whether there is a difference between the treatments, due to uncertainty around the estimate. Moderate‐quality evidence shows that LOD probably reduces the number of multiple pregnancy. Low‐quality evidence suggests that there may be little or no difference between the treatments for the likelihood of a clinical pregnancy, and there is uncertainty about the effect of LOD compared with ovulation induction alone on miscarriage. LOD may result in less OHSS.
The quality of evidence is insufficient to justify a conclusion on live birth, clinical pregnancy or miscarriage rate for the analysis of unilateral LOD versus bilateral LOD. There were no data available on multiple pregnancy.
Plain language summary
Laparoscopic application of heat or laser to the ovaries to cause ovulation in women with polycystic ovary syndrome who do not ovulate
Review question
Cochrane authors reviewed the evidence about the effect of a surgical procedure called laparoscopic ovarian drilling (LOD) compared with medical treatment to cause ovulation in women with polycystic ovary syndrome (PCOS) who do not ovulate. We also reviewed the effect of different LOD techniques.
Background
Women with PCOS have problems with ovulating and therefore may have difficulty becoming pregnant. In the past clomiphene citrate (CC) used to be the first‐line treatment in women with PCOS. Ovulation induction with letrozole should be the first‐line treatment according to new guidelines, but the use of letrozole is not officially approved. Consequently, clomiphene citrate is still commonly used. Approximately 20% of women on CC do not ovulate. When this occurs, we call it CC‐resistant PCOS. For women with CC‐resistant PCOS there are different medications available to induce ovulation, such as gonadotrophins, metformin or aromatase inhibitors, but these medications are not always successful and can cause adverse events like multiple pregnancies and cycle cancellation due to an excessive response. Another option for treatment is a surgical procedure called laparoscopic ovarian drilling (LOD). This involves applying heat or laser to the ovaries with a laparoscope (a camera) passed through a small cut, usually just below the belly button. This procedure is thought to improve the way the ovaries produce and respond to hormones, increasing the chance of ovulation. However, there are risks associated with surgery, such as complications from anaesthesia, infection, and adhesions. LOD is a surgical alternative to medical treatment, and this review aimed to determine its benefits and risks.
Study characteristics
In this updated review we included 38 controlled trials comparing LOD with medical ovulation induction or comparing different techniques of LOD. The evidence is current to October 2019
Key results
Our main analysis with low‐quality evidence shows that LOD with and without medical ovulation induction may decrease the live birth rate slightly in women with anovulatory PCOS and CC‐resistance compared with medical ovulation induction alone. Analysis including only the higher‐quality RCTs shows uncertainty about any difference between the treatments. The evidence suggests that if the chance of live birth following medical ovulation induction alone is 44%, the chance following LOD would be between 32% and 52%. Moderate‐quality evidence shows that LOD probably reduces the number of multiple pregnancies. The evidence suggests that if we assume the chance of a multiple pregnancy following medical ovulation induction alone to be 5.0%, the chance following LOD would be between 0.9% and 3.4%.
There may be little or no difference between the treatments for clinical pregnancy, and there is uncertainty about the effect of LOD compared with ovulation induction alone on miscarriage. Ovarian hyperstimulation syndrome (OHSS) may occur less often following LOD.
The quality of the evidence is not sufficient to justify a conclusion on live birth, clinical pregnancy or miscarriage for the analysis of unilateral LOD versus bilateral LOD.
The results of the primary outcomes for the other interventions were insufficient to enable us to draw any conclusions.
Quality of the evidence
The evidence was of very low to moderate quality. The main limitations in the evidence were poor reporting of study methods, the presence of bias introduced by the selection of individuals and variability in the results.
Summary of findings
Background
Description of the condition
Polycystic ovary syndrome (PCOS) is a common condition, affecting 8% to 13% of reproductive‐aged women. PCOS is commonly diagnosed with the Rotterdam PCOS diagnostic criteria (two of clinical or biochemical hyperandrogenism, ovulatory dysfunction, or polycystic ovaries on ultrasound (ESHRE 2018)). Problems in inducing ovulation are well recognised in women with PCOS. Surgical ovarian wedge resection by laparotomy was the first established treatment for women with anovulatory PCOS (Stein 1939), but was largely abandoned because of the risk of post‐surgical adhesion formation, which converted endocrinological subfertility to mechanical subfertility as a result of scarring (Adashi 1981; Buttram 1975). Wedge resection was replaced by medical ovulation induction (Franks 1985). In the past clomiphene citrate (CC) used to be the first‐line treatment in women with PCOS. According to new guidelines, ovulation induction with letrozole should be the first‐line treatment, but the use of letrozole is off‐label (ESHRE 2018). Ovulation induction with CC is not always successful, with approximately 20% of women described as 'clomiphene citrate‐resistant' (Imani 1998). CC resistance is defined as lack of ovulation with the use of CC. Women who are CC‐resistant can be treated with gonadotrophins, other medical ovulation induction agents or a surgical therapy using laparoscopic techniques known as laparoscopic ovarian drilling (LOD).
Description of the intervention
LOD was first described by Gjönnaess 1984. Both laparoscopic ovarian cautery and laser vaporisation using carbon dioxide (CO2), argon or neodymium‐doped yttrium aluminium garnet (Nd:YAG; Nd:Y3Al5O12) crystal lasers have been used to create multiple perforations (approximately 10 holes per ovary) in the ovarian surface and stroma (inner area of the ovary). The procedure can be done on an outpatient basis with less trauma and fewer postoperative adhesions than with ovarian wedge resection. Uncontrolled observational studies claim that it is followed, at least temporarily, by a high rate of spontaneous postoperative ovulation and conception (Armar 1990; Armar 1993; Greenblatt 1987; Kovacs 1991), or that subsequent medical ovulation induction becomes easier (Farhi 1995).
How the intervention might work
The mechanism of action of LOD is thought to be similar to that of ovarian wedge resection. Both procedures may destroy ovarian androgen‐producing tissue and reduce the peripheral conversion of androgens to oestrogens (one of the many disturbances of endocrine physiology that occur in women with PCOS). A fall in the serum levels of androgens and luteinising hormone (LH) and an increase in follicle‐stimulating hormone (FSH) levels have been demonstrated after ovarian drilling (Armar 1990; Greenblatt 1987). The endocrine changes following the surgery are thought to convert the adverse androgen‐dominant intrafollicular environment to an oestrogenic one (Aakvaag 1985), and to restore the hormonal environment to normal by correcting disturbances of the ovarian‐pituitary feedback mechanism (Balen 1993). Thus, both local and systemic effects are thought to promote follicular recruitment, maturation and subsequent ovulation.
Why it is important to do this review
Women who are CC‐resistant can be treated with gonadotrophins or other medical ovulation‐induction agents. These medications are not always successful and can cause adverse events like multiple pregnancies and cycle cancellation due to an excessive response. Gonadotrophin therapy requires daily injections and the need for intensive monitoring with ultrasound which makes them expensive, inconvenient and time‐consuming (ESHRE 2018). LOD is a surgical alternative to medical treatment. There are risks associated with surgery, such as complications from anaesthesia, infection, and adhesions.There might be a small risk of reduced ovarian reserve or loss of ovarian function. Clarification of the role of LOD is needed, in comparison to other treatments, in infertile women with PCOS. This review aimed to determine its benefits, safety, and costs.
Objectives
To evaluate the effectiveness and safety of laparoscopic ovarian drilling (LOD) with or without medical ovulation induction compared with medical ovulation induction alone for women with anovulatory polycystic ovary syndrome (PCOS) and clomiphene citrate resistance.
Methods
Criteria for considering studies for this review
Types of studies
We include randomised controlled trials (RCTs), but exclude quasi‐randomised trials.
Types of participants
Women with anovulatory polycystic ovary syndrome (PCOS), diagnosed by the Rotterdam criteria for PCOS, who had been shown to be resistant to clomiphene (100 mg/day or more). Clomiphene resistance was defined as lack of proven ovulation with the use of clomiphene citrate (CC).
Types of interventions
Laparoscopic ovarian drilling (LOD) with or without medical ovulation induction versus medical ovulation induction alone, including all different types of medical ovulation induction and different time periods of follow‐up
LOD in women undergoing artificial reproductive technologies (ART) such as LOD plus in vitro fertilisation (IVF) versus IVF
LOD with second‐look laparoscopy versus LOD with expectant management
Techniques for LOD, including:
LOD of one ovary (unilateral) versus LOD of both ovaries (bilateral)
monopolar versus bipolar
adjusted thermal dose versus fixed thermal dose
laser versus diathermy
We excluded trials that only compared the number of punctures to each ovary, and echoscopic transvaginal hydrolaparoscopic ovarian surgery, since the Cochrane Review Zhang 2019 includes these studies.
Types of outcome measures
Primary outcomes
Live birth (defined as delivery of a live fetus after 20 completed weeks of gestation)
Multiple pregnancy
Secondary outcomes
Clinical pregnancy (defined as evidence of a gestational sac, confirmed by ultrasound)
Miscarriage
Ovarian hyperstimulation syndrome (OHSS)
Ovulation
Costs
Quality of life
Search methods for identification of studies
For the 2020 update we searched for all published and unpublished RCTs of LOD, without language restriction and in consultation with the Cochrane Gynaecology and Fertility Group (CGFG) Information Specialist.
Electronic searches
We searched the following databases for relevant trials:
The CGFG Specialised Register of Controlled Trials, searched 8 October 2019 (Procite platform) (Appendix 1);
Cochrane Central Register of Controlled Trials (CENTRAL); searched 8 October 2019 via the Cochrane Register of Studies Online (CSRO Web platform) (Appendix 2);
MEDLINE, searched from 1946 to 8 October 2019 (Ovid platform) (Appendix 3);
Embase, searched from 1980 to 8 October 2019 (Ovid platform) (Appendix 4);
PsycINFO, searched from 1806 to 8 October 2019 (Ovid platform) (Appendix 5);
Cumulative Index to Nursing and Allied Health Literature (CINAHL), searched from 1961 to 8 October 2019 (Ebsco platform) (Appendix 6).
We combined the MEDLINE search with the Cochrane highly sensitive search strategy for identifying randomised trials, which appears in the Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1.0, Chapter 6, 6.4.11; Higgins 2011). We combined the Embase, PsycINFO, and CINAHL searches with trial filters developed by the Scottish Intercollegiate Guidelines Network (www.sign.ac.uk/search‐filters.html).
Other electronic sources of trials include the following:
Trial registers for ongoing and registered trials: www.clinicaltrials.gov (a service of the US National Institutes of Health) and www.who.int/trialsearch/Default.aspx (the World Health Organization International Trials Registry Platform search portal);
LILACS and other Spanish and Portuguese language databases (Latin American and Caribbean Health Science Information database (from 1982 ongoing)), found in the Virtual Health Library Regional Portal (VHL) pesquise.bvsalud.org/portal/.
PubMed and Google Scholar, for recent trials not yet indexed in the major databases.
Searching other resources
We handsearched the reference lists of relevant trials and systematic reviews retrieved by the search, and contacted experts in the field to obtain additional data. We also handsearched for relevant journals and conference abstracts that were not covered in the CGFG register, in liaison with the Information Specialist.
Data collection and analysis
Selection of studies
For the 2020 update, after an initial screen of titles and abstracts retrieved by the search, conducted by EB and LR, we retrieved the full texts of all the potentially eligible studies. Two review authors (EB and LR or JM and JB) then independently examined the full‐text articles for compliance with the inclusion criteria and to select eligible studies. We intended to contact study investigators if required, to clarify study eligibility. We resolved disagreements by discussion with a third review author (MW). We documented the 2020 update selection process with a PRISMA flow chart.
Data extraction and management
Two review authors (EB and LR or JB and BN) independently extracted data from eligible studies using a data extraction form, and resolving any disagreements by discussion with a third review author (MW). Data extraction included study characteristics and outcome data (see Characteristics of included studies tables). We reported studies with multiple publications under a single study ID with multiple references. We contacted study investigators for further data on methods and results, if required.
Assessment of risk of bias in included studies
Two review authors (EB and LR or BN and JB) independently assessed the included studies for risks of bias, using the Cochrane 'Risk of bias' assessment tool (Higgins 2017) to assess: selection (random sequence generation and allocation concealment); performance (blinding of participants and personnel); detection (blinding of outcome assessors); attrition (incomplete outcome data); reporting (selective reporting); and other potential bias. We resolved disagreements by discussion with a third review author (MW). We described all judgements fully and presented the conclusions in the 'Risk of bias' table, which we incorporated into the interpretation of review findings by means of sensitivity analyses.
Measures of treatment effect
For dichotomous data, we used the numbers of events in the control and intervention groups of each study and calculated Mantel‐Haenszel odds ratios (ORs) or Peto ORs. For continuous data (e.g. costs), if all studies reported exactly the same outcomes, we calculated mean differences (MDs) between treatment groups. If similar outcomes were reported on different scales we calculated the standardised mean difference (SMD). We treated ordinal data (e.g. quality‐of‐life scores) as continuous data. We present 95% confidence intervals (CIs) for all outcomes.
Unit of analysis issues
The primary analysis was by woman randomised. We counted multiple births as one live birth event. If data did not allow valid analysis (e.g. 'by cycle' data) we contacted the primary authors for data by woman randomised, and did not include the 'by cycle' data in the meta‐analyses. For cross‐over trials, we included only first‐phase data.
Dealing with missing data
We analysed the data on an intention‐to‐treat basis as far as possible (i.e. including all randomised participants in analysis, in the groups to which they were randomised). We tried to obtain missing data from the original trialists, by contacting the primary authors. We analysed only the available data, without imputation.
Assessment of heterogeneity
We considered whether clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity by the I2 statistic. We took an I2 measurement greater than 50% as an indication of substantial heterogeneity (Deeks 2017).
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, we aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by being alert for duplication of data. We used a funnel plot to explore the possibility of small‐study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies), where 10 studies or more contributed to the analysis.
Data synthesis
If studies were sufficiently similar, we combined the data using a fixed‐effect model in the following comparisons:
LOD with or without medical ovulation induction versus medical ovulation induction alone
LOD in women undergoing IVF versus IVF
LOD with second‐look laparoscopy versus LOD with expectant management
Techniques for LOD, including:
LOD of one ovary (unilateral) versus LOD of both ovaries (bilateral)
monopolar versus bipolar
fixed thermal dose versus adjusted thermal dose
laser versus diathermy
We performed statistical analysis using Review Manager 5.3 (Review Manager 2014).
Subgroup analysis and investigation of heterogeneity
We conducted subgroup analyses for the different medical ovulation‐induction agents, to determine the separate evidence for:
clomiphene citrate (CC)
CC + metformin
CC + tamoxifen
CC + rosiglitazone
gonadotrophins
gonadotrophins (rFSH) + metformin
letrozole
letrozole + metformin
metformin
Sensitivity analysis
We conducted sensitivity analyses for the primary outcomes of live birth and multiple pregnancy, to determine whether the conclusions were robust to arbitrary decisions made about eligibility and analysis. These analyses included consideration of whether the review conclusions would have differed if:
Eligibility had been restricted to studies at low risk of bias (defined as studies at low risk of selection bias);
High levels of heterogeneity were present;
Follow‐up in the individual trials had lasted for at least six months or six cycles.
Overall quality of the body of evidence
We updated the 'Summary of findings' tables using GRADEpro and Cochrane methods (Gradepro GDT 2015; Schünemann 2017). Table 1 presents the overall quality of the body of evidence for the main review outcomes (live birth, multiple pregnancy, clinical pregnancy, miscarriage and OHSS) for the main review comparison (LOD with or without medical ovulation induction compared with medical ovulation induction alone). We produced an additional 'Summary of findings' table (Table 2) for the main review outcomes for one other important comparison: Unilateral LOD versus bilateral LOD. We evaluated the quality of the evidence using GRADE criteria: risk of bias, consistency of effect, imprecision, indirectness and publication bias. Two review authors (EB and LR), working independently, made judgements about the evidence quality (high, moderate, low or very low), resolving disagreements by discussion with a third review author (MW). The judgements were justified, documented, and incorporated into the reporting of results for each outcome.
Summary of findings for the main comparison. LOD with and without medical ovulation compared to medical ovulation induction alone.
| Laparoscopic ovarian drilling with and without medical ovulation compared to medical ovulation induction alone | ||||||
| Patient or population: women with anovulatory PCOS and CC resistance Setting: fertility clinics Intervention: laparoscopic ovarian drilling with and without medical ovulation Comparison: medical ovulation induction alone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with medical ovulation induction alone | Risk with LOD ±medical ovulation | |||||
| Live birth | 418 per 1000 | 338 per 1000 (279 to 398) | OR 0.71 (0.54 to 0.92) | 1015 (9 RCTs) | ⊕⊕⊝⊝ Lowa | |
| Live birth (sensitivity analysis) | 439 per 1000 | 413 per 1000 (316 to 516) |
OR 0.90 (0.59 to 1.36) |
415 (4 RCTs) |
⊕⊕⊝⊝ Lowb,c | |
| Multiple pregnancy | 50 per 1000 | 18 per 1000 (9 to 34) | Peto OR 0.34 (0.18 to 0.66) | 1161 (14 RCTs) | ⊕⊕⊕⊝ Moderateb | |
| Clincial pregnancy | 460 per 1000 | 423 per 1000 (380 to 467) | OR 0.86 (0.72 to 1.03) | 2016 (21 RCTs) | ⊕⊕⊝⊝ Lowa | |
| Miscarriage | 64 per 1000 | 71 per 1000 (51 to 99) | Peto OR 1.11 (0.78 to 1.59) | 1909 (19 RCTs) | ⊕⊕⊝⊝ Lowa | |
| OHSS | 23 per 1000 | 6 per 1000 (2 to 21) | Peto OR 0.25 (0.07 to 0.91) | 722 (8 RCTs) | ⊕⊕⊝⊝ Lowb,c | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded by two levels for very serious risk of bias; inadequate randomisation or allocation concealment and no evidence of blinding.
bDowngraded by one level for serious risk of bias; no evidence of blinding.
cDowngraded by one level for serious imprecision.
Summary of findings 2. LOD of one ovary (unilateral) versus LOD of both ovaries (bilateral).
| LOD of one ovary (unilateral) versus LOD of both ovaries (bilateral) | ||||||
| Patient or population: women with anovulatory PCOS and CC resistance Setting: fertility clinics Intervention: bilateral LOD Comparison: unilateal LOD | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with Bilateral | Risk with Unilateral | |||||
| Live birth | 409 per 1000 | 365 per 1000 (142 to 658) | OR 0.83 (0.24 to 2.78) | 44 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | ‐ |
| Multiple pregnancy | ‐ | ‐ | ‐ | ‐ | ‐ | No data were reported for this outcome. |
| Clinical pregnancy | 464 per 1000 | 331 per 1000 (253 to 421) | OR 0.57 (0.39 to 0.84) | 470 (7 RCTs) | ⊕⊕⊝⊝ Lowa | ‐ |
| Miscarriage | 91 per 1000 | 93 per 1000 (30 to 250) | Peto OR 1.02 (0.31 to 3.33) | 131 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b | ‐ |
| OHSS | ‐ | ‐ | ‐ | ‐ | ‐ | No data were reported for this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded by two levels for very serious risk of bias; inadequate randomisation or allocation concealment and no evidence of blinding.
bDowngraded by one level for serious imprecision.
Results
Description of studies
Results of the search
The original review retrieved 19 full‐text articles and included nine RCTs. For the 2012 update we identified 86 potential articles, from which 16 trials met the inclusion criteria. The 2020 update includes a further 15 trials (Darwish 2016; Elgafor 2013; El‐Sayed 2017; Fernandez 2015; Giampaolino 2016; Ibrahim 2017; Jamal 2000; Liu 2015; Malkawi 2003; Mamonov 2000; Mehrabian 2012; Rezk 2016; Sorouri 2015; Yadav 2018; Zakherah 2011). We placed two studies which were included in the previous update to studies awaiting classification (Abu Hashim 2010; Abu Hashim 2011). This gives a total of 38 trials (3326 women) now included. See Figure 1 for the PRISMA flow chart.
1.
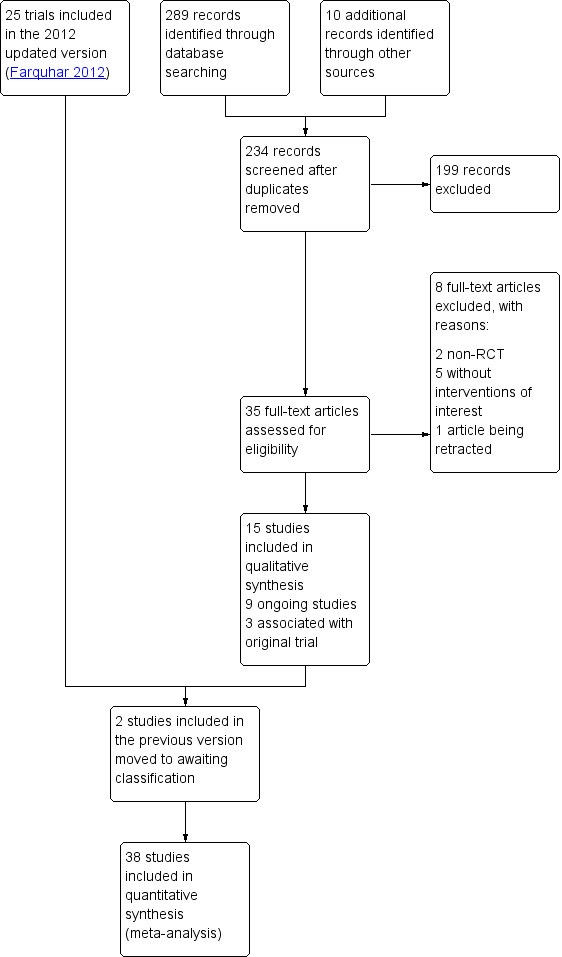
Study flow diagram.
We added three papers associated with the Bayram 2004 trial, one paper associated with the Farquhar 2002 trial and one paper associated with the Zakherah 2011 trial.
There are currently nine ongoing studies (IRCT138903291306N2; NCT02239107; NCT02305693; NCT02381184; NCT02775734; NCT03009838; NCT03206892; NCT03664050; PACTR201411000886127). In future updates we will check whether data from these trials have been published.
We exclude a total of 29 studies, with eight trials excluded from the 2020 update (Franz 2016; Kandil 2018; Roy 2018; Salah 2013; Seyam 2018; Sunj 2013; Wang 2015; Zeng 2012).
See study tables: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; and Characteristics of ongoing studies.
Included studies
Study design and setting
We include 38 trials in this systematic review. All studies are parallel‐design randomised controlled trials (RCTs). All of the trials recruited women with fertility problems who were attending fertility clinics. Twelve were from Egypt (Abdellah 2011; Darwish 2016; Elgafor 2013; El‐Sayed 2017; Hamed 2010; Ibrahim 2017; Nasr 2013; Nasr 2015; Rezk 2016; Youssef 2007; Zakherah 2010; Zakherah 2011), four from India (Roy 2009; Roy 2010; Sharma 2006; Yadav 2018), four from Iran (Ashrafinia 2009; Ghafarnegad 2010; Mehrabian 2012; Sorouri 2015), four from Italy (Fernandez 2015; Palomba 2004; Palomba 2010; Vegetti 1998), four from the UK (Al‐Mizyen 2000; Amer 2009; Balen 1994; Rimington 1997), two from Turkey (Gürgan 1992; Kaya 2005), one from China (Liu 2015), one from France (Fernandez 2015), one from Jordan (Malkawi 2003), one from the Netherlands (Bayram 2004), one from New Zealand (Farquhar 2002), one from Saudi Arabia (Jamal 2000), one from Ukraine (Mamonov 2000), and one from Yugoslavia (Lazoviz 1998).
Participants
1. LOD with or without medical ovulation induction versus medical ovulation induction alone
Twenty‐one trials including 1031 women in the LOD groups and 985 women in the medical ovulation induction‐alone groups (Abdellah 2011; Amer 2009; Bayram 2004; Elgafor 2013; Farquhar 2002; Fernandez 2015; Ghafarnegad 2010; Hamed 2010; Ibrahim 2017; Kaya 2005; Lazoviz 1998; Liu 2015; Malkawi 2003; Mamonov 2000; Mehrabian 2012; Palomba 2004; Palomba 2010; Roy 2010; Vegetti 1998; Yadav 2018; Zakherah 2010). All of the women had subfertility and PCOS.
2. LOD plus IVF versus IVF
One trial (Rimington 1997) included 25 women who had undergone LOD plus IVF and 25 women who had undergone IVF. The mean age of the women in the LOD+ IVF group was 31.8 years and in the IVF group 31 years.
3. LOD with second‐look laparoscopy versus LOD with expectant management
One trial (Gürgan 1992) included 20 women who had undergone second‐look laparoscopy and 20 women who had received expectant management. The mean age of the women was 25.2 years.
Techniques of ovarian drilling
4. Unilateral LOD versus bilateral LOD
Nine trials included 233 women in the unilateral LOD groups and 237 women in the bilateral LOD group (Al‐Mizyen 2000; Balen 1994; El‐Sayed 2017; Jamal 2000; Nasr 2013; Rezk 2016; Roy 2009; Sorouri 2015; Youssef 2007). The mean age of women in the unilateral group was 28.8 years and in the bilateral group 28 years. Jamal 2000 did not give details of the number of women in each group (total n = 35).
5. Monopolar versus bipolar
Three trials included 175 women in the monopolar groups and 176 women in the bipolar groups (Darwish 2016; Giampaolino 2016; Sharma 2006).
6. Adjusted thermal dose versus fixed thermal dose
Two trials including 100 women in the adjusted thermal dose groups and 100 women in the fixed thermal dose groups (Nasr 2015; Zakherah 2011).
Interventions
1. LOD with or without medical ovulation induction versus medical ovulation induction alone
1/22 trials compared LOD with clomiphene citrate (Amer 2009);
2/22 trials compared LOD with CC + metformin ( Palomba 2004; Palomba 2010);
1/22 trials compared LOD with CC + tamoxifen (Zakherah 2010);
1/22 trials compared LOD with CC + rosiglatazone (Roy 2010);
9/22 trials compared LOD with gonadotrophins (Bayram 2004; Farquhar 2002; Ghafarnegad 2010; Kaya 2005; Lazoviz 1998; Mamonov 2000; Mehrabian 2012; Vegetti 1998; Yadav 2018);
1/22 trials compared LOD with gonadotrophins (rFSH) + metformin (Fernandez 2015);
3/22 trials compared LOD with letrozole (Abdellah 2011; Ibrahim 2017; Liu 2015);
1/22 trials compared LOD with letrozole + metformin (Elgafor 2013);
3/22 trials compared LOD with metformin (Ashrafinia 2009; Hamed 2010; Malkawi 2003).
Fifteen of the trials followed women for six months after LOD and six cycles of ovulation induction (Abdellah 2011; Amer 2009; Ashrafinia 2009; Elgafor 2013; Fernandez 2015; Hamed 2010; Ibrahim 2017; Lazoviz 1998; Liu 2015; Palomba 2004; Palomba 2010; Roy 2010; Vegetti 1998; Yadav 2018; Zakherah 2010). Two trials had no details on the timing of follow‐up (Mehrabian 2012; Malkawi 2003), two trials followed women for six months after LOD and three cycles of gonadotrophins within six months (Farquhar 2002; Kaya 2005), one trial followed women for 12 months after LOD and six cycles of gonadotrophins within 12 months (Bayram 2004), one trial followed women for four months after LOD and four cycles of gonadotrophins (Ghafarnegad 2010), and one trial followed women for 18 months after LOD and six cycles of gonadotrophins (Mamonov 2000).
2. LOD plus IVF versus IVF
1/1 trial compared LOD plus IVF versus IVF (Rimington 1997). Follow‐up was for one cycle in each group.
3. LOD with second‐look laparoscopy versus LOD with expectant management
1/1 trial compared second‐look laparoscopy versus expectant management (Gürgan 1992). Follow‐up was for six months in each group.
Techniques of LOD
Nine trials compared unilateral and bilateral drilling. Four trials followed women for six months (Rezk 2016; El‐Sayed 2017; Nasr 2013; Sorouri 2015), three trials for 12 months (Al‐Mizyen 2000; Roy 2009; Youssef 2007), and two trials for three months (Balen 1994; Jamal 2000)
Three trials compared monopolar versus bipolar technique, of which two trials followed women for six months (Darwish 2016; Giampaolino 2016), and one trial for three months (Sharma 2006)
Two trials compared adjusted thermal dose versus fixed thermal dose (Nasr 2015; Zakherah 2011). Follow‐up was for six months in each group.
Outcomes
1. Outcomes for LOD with or without medical ovulation induction versus medical ovulation induction alone
9/22 reported live birth (Abdellah 2011; Bayram 2004; Farquhar 2002; Ghafarnegad 2010; Liu 2015; Palomba 2004; Palomba 2010; Yadav 2018; Zakherah 2010);
14/22 reported multiple pregnancy (Abdellah 2011; Amer 2009; Bayram 2004; Farquhar 2002; Fernandez 2015; Kaya 2005; Lazoviz 1998; Malkawi 2003; Mehrabian 2012; Palomba 2004; Palomba 2010; Roy 2010; Vegetti 1998; Yadav 2018);
21/22 reported clinical pregnancy (Abdellah 2011; Amer 2009; Bayram 2004; Elgafor 2013; Farquhar 2002; Fernandez 2015; Ghafarnegad 2010; Hamed 2010; Ibrahim 2017; Kaya 2005; Lazoviz 1998; Liu 2015; Malkawi 2003; Mamonov 2000; Mehrabian 2012; Palomba 2004; Palomba 2010; Roy 2010; Vegetti 1998; Yadav 2018; Zakherah 2010);
19/22 reported miscarriage (Abdellah 2011; Bayram 2004; Elgafor 2013; Farquhar 2002; Fernandez 2015; Ghafarnegad 2010; Hamed 2010; Ibrahim 2017; Lazoviz 1998; Liu 2015; Malkawi 2003; Mamonov 2000; Mehrabian 2012; Palomba 2004; Palomba 2010; Roy 2010; Vegetti 1998; Yadav 2018; Zakherah 2010);
8/22 reported OHSS (Amer 2009; Bayram 2004; Farquhar 2002; Kaya 2005; Malkawi 2003; Mehrabian 2012Roy 2010; Yadav 2018);
10/22 reported ovulation (Amer 2009; Elgafor 2013; Farquhar 2002; Hamed 2010; Ibrahim 2017; Malkawi 2003; Palomba 2010; Roy 2010; Yadav 2018; Zakherah 2010);
4/22 reported costs (Bayram 2004; Farquhar 2002; Kaya 2005; Palomba 2010);
1/22 reported quality of life (Bayram 2004).
One trial was identified that met all of the inclusion criteria associated with the population and interventions but did not report on any obstetric outcomes (Ashrafinia 2009). We have contacted the authors for information but there has been no response to date.
2. Outcomes for LOD plus IVF versus IVF
1/1 reported live birth (Rimington 1997)
1/1 reported multiple pregnancy (Rimington 1997)
1/1 reported clinical pregnancy (Rimington 1997)
1/1 reported miscarriage (Rimington 1997)
1/1 reported OHSS (Rimington 1997)
3. Outcomes for LOD with second‐look laparoscopy versus LOD with expectant management
1/1 reported clinical pregnancy (Gürgan 1992)
1/1 reported miscarriage (Gürgan 1992)
1/1 reported ovulation (Gürgan 1992)
4. Outcomes for techniques of LOD: unilateral versus bilateral
1/9 studies reported live birth (Roy 2009);
7/9 studies reported clinical pregnancy (Al‐Mizyen 2000; Balen 1994; El‐Sayed 2017; Rezk 2016; Roy 2009; Sorouri 2015; Youssef 2007);
2/9 studies reported miscarriage (Roy 2009; Youssef 2007);
6/9 studies reported ovulation (Balen 1994; El‐Sayed 2017; Rezk 2016; Roy 2009; Sorouri 2015; Youssef 2007).
5. Outcomes for techniques of LOD: monopolar versus bilateral
3/3 studies reported clinical pregnancy (Darwish 2016; Giampaolino 2016; Sharma 2006);
2/3 studies reported ovulation (Darwish 2016; Sharma 2006)
6. Outcomes for techniques of LOD: adjusted thermal dose versus fixed thermal dose
2/2 studies reported clinical pregnancy (Nasr 2015; Zakherah 2011);
1/2 studies reported miscarriage (Zakherah 2011);
2/2 studies reported ovulation (Nasr 2015; Zakherah 2011).
Jamal 2000 did not report any data in their conference abstract. Nasr 2013 reported only on anti‐Mullerian hormone as their outcome, which was not a prespecified outcome for this review.
Excluded studies
We excluded 29 studies from the review, for the following reasons (refer to Characteristics of excluded studies for further details):
11/29 were not RCTs (Abdel Gadir 1990; Gadir 1992; Al‐Mizyen 2000; Sunj 2013; Gürgan 1991; Heylen 1994; Keckstein 1990; Malkawi 2005; Muenstermann 2000; Rath 2006; Seyam 2018);
13/29 had comparisons that were not of interest (Badawy 2009; Franz 2016; Foroozanfard 2010; Kamel 2004; Kandil 2018; Kocak 2006; Nasr 2010; Roy 2018; Salah 2013; Saravelos 1996; Tabrizi 2005; Zeng 2012; Zhu 2010);
1/29 had participants not of interest (Abu Hashim 2011b);
1/29 had interventions not of interest (Vrbikova 1998);
1/29 had ovaries as the unit of randomisation (Greenblatt 1993);
1/29 was retracted by the journal (Wang 2015);
1/29 is a conference abstract; we tried to obtain the details, but had no response from the authors, so excluded it for lack of usable data (Lockwood 1995).
Risk of bias in included studies
The risks of bias of included studies are illustrated in Figure 2; Figure 3.
2.
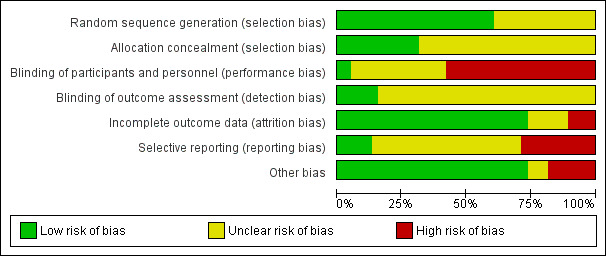
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
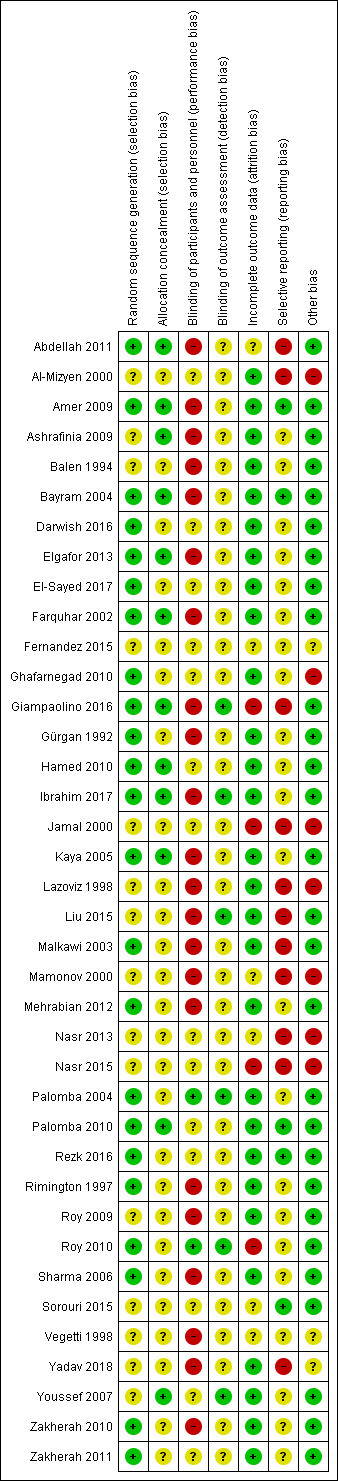
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Twenty‐three trials were at low risk of bias due to random sequence generation, as they clearly explained the methods used (Abdellah 2011; Amer 2009; Bayram 2004; Darwish 2016; Elgafor 2013; El‐Sayed 2017; Farquhar 2002; Ghafarnegad 2010; Giampaolino 2016; Gürgan 1992; Hamed 2010; Ibrahim 2017; Kaya 2005; Malkawi 2003; Mehrabian 2012; Palomba 2004; Palomba 2010; Rezk 2016; Rimington 1997; Roy 2010; Sharma 2006; Zakherah 2010; Zakherah 2011).
Fifteen trials did not provide an adequate explanation of the randomisation process and were judged to be at unclear risk of bias (Al‐Mizyen 2000; Ashrafinia 2009; Balen 1994; Fernandez 2015; Jamal 2000; Lazoviz 1998; Liu 2015; Mamonov 2000; Nasr 2013; Nasr 2015; Roy 2009; Sorouri 2015; Vegetti 1998; Yadav 2018; Youssef 2007).
Allocation concealment
Twelve trials were at low risk of selection bias related to allocation concealment, as they used central allocation concealment or sealed opaque sequentially‐numbered envelopes (Abdellah 2011; Amer 2009; Ashrafinia 2009; Bayram 2004; Elgafor 2013; Farquhar 2002; Giampaolino 2016; Hamed 2010; Ibrahim 2017; Kaya 2005; Palomba 2010; Youssef 2007).
Twenty‐six trials did not provide adequate details to establish whether an appropriate method of allocation concealment had been used, and were judged to be of unclear risk of selection bias (Al‐Mizyen 2000; Balen 1994; Darwish 2016; El‐Sayed 2017; Fernandez 2015; Ghafarnegad 2010; Gürgan 1992; Jamal 2000; Lazoviz 1998; Liu 2015; Malkawi 2003; Mamonov 2000; Mehrabian 2012; Nasr 2013; Nasr 2015; Palomba 2004; Rezk 2016; Rimington 1997; Roy 2009; Roy 2010; Sharma 2006; Sorouri 2015; Vegetti 1998; Yadav 2018; Zakherah 2010; Zakherah 2011).
Blinding
Performance bias
We rated two trials at low risk of performance bias (Palomba 2004; Roy 2010). There was insufficient detail to tell if researchers or participants had been blinded in 14 trials that we judged to be at unclear risk of performance bias (Al‐Mizyen 2000; Darwish 2016; El‐Sayed 2017; Fernandez 2015; Ghafarnegad 2010; Hamed 2010; Jamal 2000; Nasr 2013; Nasr 2015; Palomba 2010; Rezk 2016; Sorouri 2015; Youssef 2007; Zakherah 2011). For the remaining 22 trials there was no blinding of participants or researchers and we judged these trials to be at high risk of bias (Abdellah 2011; Amer 2009; Ashrafinia 2009; Balen 1994; Bayram 2004; Elgafor 2013; Farquhar 2002; Giampaolino 2016; Gürgan 1992; Ibrahim 2017; Kaya 2005; Lazoviz 1998; Liu 2015; Malkawi 2003; Mamonov 2000; Mehrabian 2012; Rimington 1997; Roy 2009; Sharma 2006; Vegetti 1998; Yadav 2018; Zakherah 2010).
Detection bias
We judged six trials to be at low risk of detection bias, as the outcome assessors were blinded to treatment allocation ( Giampaolino 2016; Ibrahim 2017; Liu 2015; Palomba 2004; Roy 2010; Youssef 2007).
There was insufficient detail to tell if researchers or participants had been blinded in the remaining 32 trials that we judged to be at unclear risk of detection bias (Abdellah 2011; Al‐Mizyen 2000; Amer 2009; Ashrafinia 2009; Balen 1994; Bayram 2004; Darwish 2016; Elgafor 2013; El‐Sayed 2017; Farquhar 2002; Fernandez 2015; Ghafarnegad 2010; Gürgan 1992; Hamed 2010; Jamal 2000; Kaya 2005; Lazoviz 1998; Malkawi 2003; Mamonov 2000; Mehrabian 2012; Nasr 2013; Nasr 2015; Palomba 2010; Rezk 2016; Rimington 1997; Roy 2009; Sharma 2006; Sorouri 2015; Vegetti 1998; Yadav 2018; Zakherah 2010; Zakherah 2011).
Incomplete outcome data
We judged 28 trials to be at low risk of attrition bias ( Al‐Mizyen 2000; Amer 2009; Ashrafinia 2009; Balen 1994; Bayram 2004; Darwish 2016; Elgafor 2013; El‐Sayed 2017; Farquhar 2002;Ghafarnegad 2010; Gürgan 1992; Hamed 2010; Ibrahim 2017; Kaya 2005; Lazoviz 1998; Liu 2015; Malkawi 2003; Mehrabian 2012; Palomba 2004; Palomba 2010; Rezk 2016; Rimington 1997; Roy 2009; Sharma 2006; Yadav 2018; Youssef 2007; Zakherah 2010; Zakherah 2011).
We rated six trials at unclear risk of attrition bias, due to insufficient details (Abdellah 2011; Fernandez 2015; Mamonov 2000; Nasr 2013; Sorouri 2015; Vegetti 1998).
We considered four trials to be at high risk of attrition bias (Giampaolino 2016; Jamal 2000; Nasr 2015; Roy 2010). Roy 2010 was rated at high risk of bias because the attrition of women in the trials was not adequately explained and intention‐to‐treat analysis was not conducted.
Selective reporting
We checked four of the original trial protocols, and considered four to be at low risk of bias (Amer 2009; Bayram 2004; Palomba 2010; Sorouri 2015). In these studies all the outcomes mentioned in the protocol were presented in the published report.
We could not retrieve protocols for the other trials. Most of them did report on all of the outcomes listed in the methods section of the papers. We rated 11 trials at high risk of bias (Abdellah 2011; Al‐Mizyen 2000; Giampaolino 2016; Jamal 2000; Lazoviz 1998; Liu 2015; Malkawi 2003; Mamonov 2000; Nasr 2013; Nasr 2015; Yadav 2018), with most reporting on outcomes that had not been listed in the Methods section.
Lazoviz 1998 and Nasr 2013 were published in conference abstract form only, and we could find no full study report, while Mamonov 2000 did not list any outcomes in the Methods section of their conference abstract.
Other potential sources of bias
We judged three trials to be at unclear risk of bias. Fernandez 2015 reported that the trial stopped early due to difficulties in the inclusion criteria, and Vegetti 1998 only reported interim results for which we could find no full publication. Women with LOD received CC or gonadotrophins in Yadav 2018. We rated seven trials at high risk of other bias, as they were only published as abstracts (Al‐Mizyen 2000; Ghafarnegad 2010; Jamal 2000; Lazoviz 1998; Mamonov 2000; Nasr 2013; Nasr 2015). We rated the remaining trials at low risk of bias.
Effects of interventions
1. LOD with or without medical ovulation induction versus medical ovulation induction alone
1.1 Live birth
Nine trials including 1015 women reported live birth rate by woman (Abdellah 2011; Bayram 2004; Farquhar 2002; Ghafarnegad 2010; Liu 2015; Palomba 2004; Palomba 2010; Yadav 2018; Zakherah 2010). The meta‐analysis shows that LOD may decrease live birth slightly when compared with medical ovulation induction alone (odds ratio (OR) 0.71, 95% confidence interval (CI) 0.54 to 0.92; 9 studies, 1015 women; I2 = 0%; low‐quality evidence; Analysis 1.1; Figure 4).
1.1. Analysis.
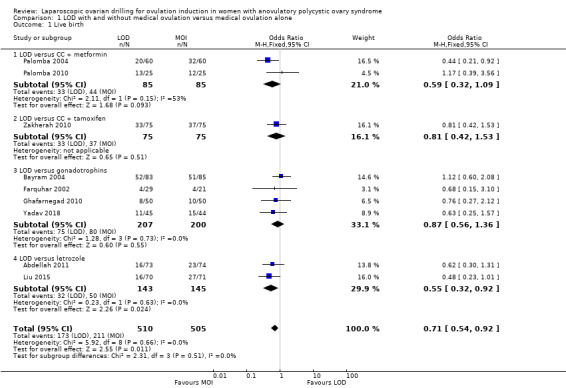
Comparison 1 LOD with and without medical ovulation versus medical ovulation alone, Outcome 1 Live birth.
4.
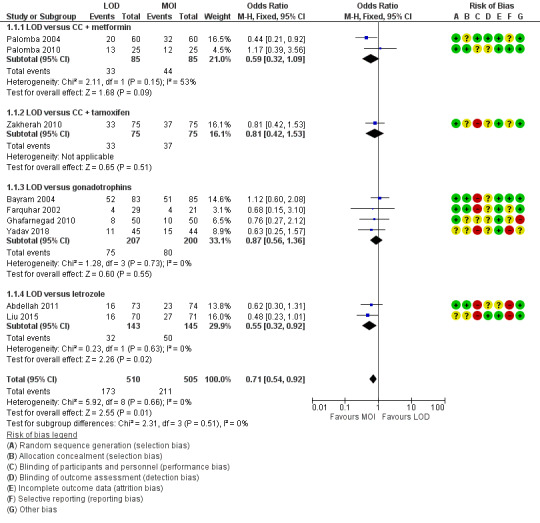
Forest plot of comparison: 1 LOD with and without medical ovulation versus medical ovulation alone, outcome: 1.1 Live birth.
MOI: Medical ovulation induction alone
LOD: laparoscopic ovarian drilling with or without medical ovulation induction
The evidence suggest that if the chance of live birth following medical ovulation induction alone is 42%, the chance following LOD would be between 28% and 40%. The funnel plot did not indicate publication bias (Figure 5). Our sensitivity analysis restricting to RCTs with low risk of selection bias (Abdellah 2011; Bayram 2004; Farquhar 2002; Palomba 2010) suggests there is uncertainty whether there is a difference between the treatments (OR 0.90, 95% CI 0.59 to 1.36; 4 studies, 415 women; I2 = 0%, low‐quality evidence; Analysis 7.1; Figure 6). This result suggests that if the chance of live birth following medical ovulation induction alone is 44%, the chance following LOD would be between 32% and 52%.
5.
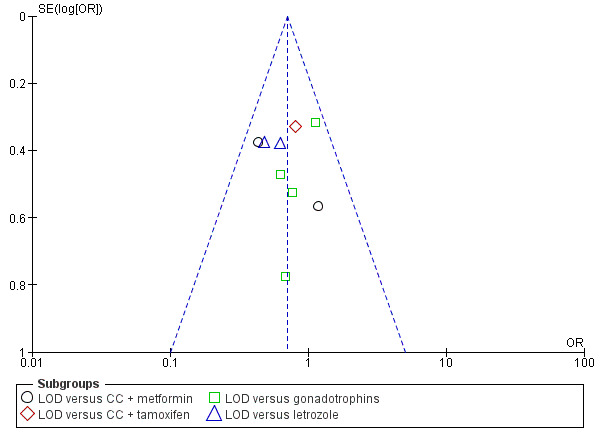
Funnel plot of comparison: 1 LOD with and without medical ovulation versus medical ovulation alone, outcome: 1.1 Live birth.
LOD: laparoscopic ovarian drilling with or without medical ovulation induction
7.1. Analysis.
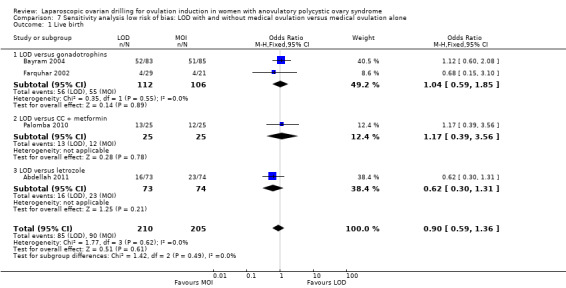
Comparison 7 Sensitivity analysis low risk of bias: LOD with and without medical ovulation versus medical ovulation alone, Outcome 1 Live birth.
6.
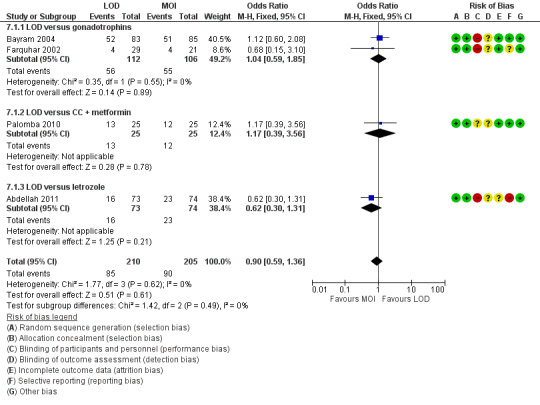
Forest plot of comparison: 5 Sensitivity analysis low risk of bias: LOD with and without medical ovulation versus medical ovulation alone, outcome: 5.1 Live birth.
MOI: Medical ovulation induction alone
LOD: laparoscopic ovarian drilling with or without medical ovulation induction
Only one small trial had no treatment time/follow‐up of at least six months (Ghafarnegad 2010). Restricting to studies with at least six months of follow‐up resulted in a similar estimate for live birth. There were four different comparisons with LOD: clomiphene citrate (CC) and metformin (Palomba 2004; Palomba 2010), CC and tamoxifen (Zakherah 2010), gonadotrophins (Bayram 2004; Farquhar 2002; Ghafarnegad 2010; Yadav 2018) and letrozole (Abdellah 2011; Liu 2015). Subgroup analysis did not identify any between‐group differences.
One of the trials (Bayram 2004) continued longitudinal follow‐up for a mean of 133.5 months for 95% of the original sample. At this extended follow‐up point 86% of couples having LOD and 81% of couples having recombinant FSH (rFSH) had conceived and reported a live birth (P = 0.63). However, LOD resulted in significantly reduced requirements for stimulated cycles to reach a live birth outcome (44/71 live births in the LOD group versus 65/69 live births in the rFSH group; RR 0.69, 95% CI 0.55 to 0.88). Significantly more women in the LOD group had a second live birth compared with the rFSH group (61% versus 46%; RR 1.30, 95% CI 1.01 to 1.80; P = 0.03). Of those women achieving a second live birth in the LOD group 24% required additional treatment, as did 19% of those in the rFSH group who had a second live birth. At the end of follow‐up there had been 134 live births in the LOD group and 124 in the rFSH group (P = 0.09). Of the 175 pregnancies in the LOD group, five were ectopic and 31 miscarriages occurred, compared with three ectopic pregnancies in a total of 159 pregnancies in the rFSH group (risk ratio (RR) 1.50, 95% CI 0.37 to 6.20) and 23 miscarriages (RR 1.20, 95% CI 0.75 to 2.0).
1.2 Multiple pregnancy
Fourteen trials including 1161 women reported on multiple pregnancies (Abdellah 2011; Amer 2009; Bayram 2004; Farquhar 2002; Fernandez 2015; Kaya 2005; Lazoviz 1998; Malkawi 2003; Mehrabian 2012; Palomba 2004; Palomba 2010; Roy 2010; Vegetti 1998; Yadav 2018). The meta‐analysis shows that LOD probably reduces multiple pregnancy rates compared with medical ovulation induction alone (Peto OR 0.34, 95% CI 0.18 to 0.66; 14 studies, 1161 women; I2 = 2%; moderate‐quality evidence; Analysis 1.2; Figure 7). This suggests that if we assume the risk of multiple pregnancy following medical ovulation induction alone is 5.0%, the risk following LOD would be between 0.9% and 3.4%. Caution is advised in interpreting the analysis, as event rates are very low, with 10/602 in the LOD group and 28/559 in the other treatment group. Sensitivity analysis: after restricting to only RCTs with low risk of selection bias; the result for multiple pregnancy was consistent with the main analysis (Analysis 7.2). Analysis per pregnancy showed similar results (Peto OR 0.34, 95% CI 0.17 to 0.66; 14 studies, 577 women, I2 = 22%; Analysis 1.11). Subgroup analysis did not identify any between‐group differences. There were no cases of multiple pregnancies in either group for CC (Amer 2009), CC and metformin (Palomba 2004; Palomba 2010), gonadotrophins (Farquhar 2002 only), gonadotrophins (rFSH) + metformin (Fernandez 2015) or letrozole (Abdellah 2011 only) compared with LOD. Only one small trial had no treatment time/follow‐up of at least six months (Ghafarnegad 2010), and consequently restricting to studies with at least six months of follow‐up resulted in a similar estimate for multiple pregnancy.
1.2. Analysis.
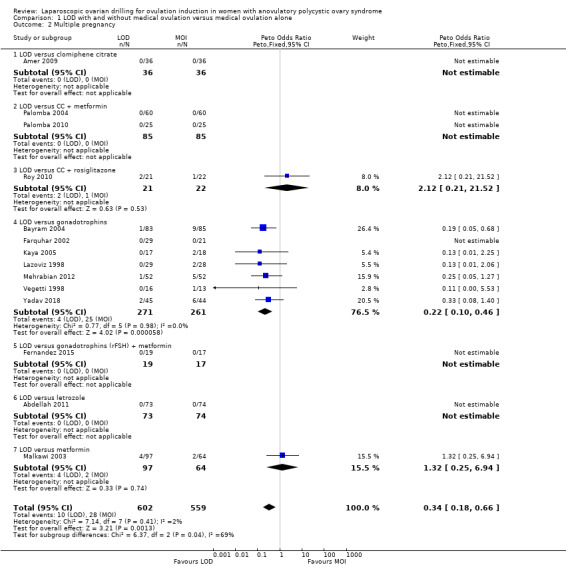
Comparison 1 LOD with and without medical ovulation versus medical ovulation alone, Outcome 2 Multiple pregnancy.
7.
Forest plot of comparison: 1 LOD with and without medical ovulation versus medical ovulation alone, outcome: 1.4 Multiple pregnancy rate (per ongoing pregnancy).
MOI: Medical ovulation induction alone
LOD: laparoscopic ovarian drilling with or without medical ovulation induction
7.2. Analysis.
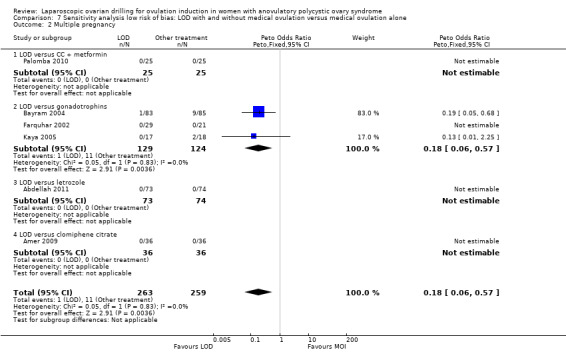
Comparison 7 Sensitivity analysis low risk of bias: LOD with and without medical ovulation versus medical ovulation alone, Outcome 2 Multiple pregnancy.
1.11. Analysis.
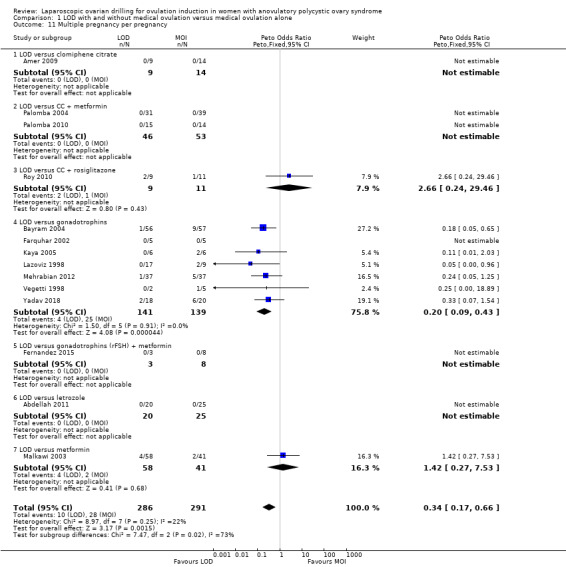
Comparison 1 LOD with and without medical ovulation versus medical ovulation alone, Outcome 11 Multiple pregnancy per pregnancy.
1.3 Clinical pregnancy
Twenty‐one trials including 2016 women reported on the clinical pregnancy rate (Abdellah 2011; Amer 2009; Bayram 2004; Elgafor 2013; Farquhar 2002; Fernandez 2015; Ghafarnegad 2010; Hamed 2010; Ibrahim 2017; Kaya 2005; Lazoviz 1998; Liu 2015; Malkawi 2003; Mamonov 2000; Mehrabian 2012; Palomba 2004; Palomba 2010; Roy 2010; Vegetti 1998; Yadav 2018; Zakherah 2010). The analysis suggests there may be little or no difference between LOD and medical ovulation induction alone, but the quality of the evidence was low (OR 0.86, 95% CI 0.72 to 1.03; 21 studies, 2016 women; I2 = 19%; low‐quality evidence; Analysis 1.3).
1.3. Analysis.
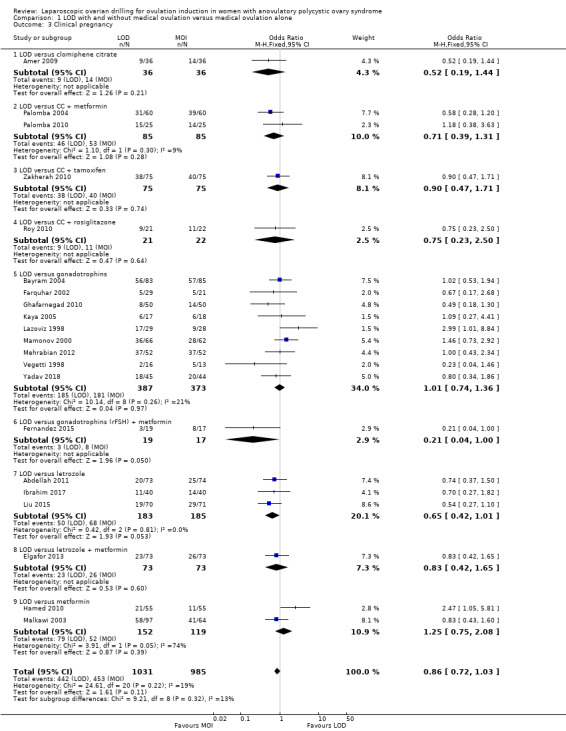
Comparison 1 LOD with and without medical ovulation versus medical ovulation alone, Outcome 3 Clinical pregnancy.
Subgroup analysis did not identify any differences between the groups with different ovulation induction therapies.
1.4 Miscarriage
Nineteen trials including 1909 women reported on miscarriage (Abdellah 2011; Bayram 2004; Elgafor 2013; Farquhar 2002; Fernandez 2015; Ghafarnegad 2010; Hamed 2010; Ibrahim 2017; Lazoviz 1998; Liu 2015; Malkawi 2003; Mamonov 2000; Mehrabian 2012; Palomba 2004; Palomba 2010; Roy 2010; Vegetti 1998; Yadav 2018; Zakherah 2010). There is uncertainty about the effect of LOD compared with ovulation induction alone, due to large uncertainty around the estimate and the low quality of the evidence (OR 1.11, 95% CI 0.78 to 1.59; 19 studies, 1909 women; I2 = 0%; low‐quality evidence; Analysis 1.4). Analysis per pregnancy showed similar results (OR 1.28, 95% CI 0.88 to 1.88; 19 studies, 900 women; I2 = 0%; Analysis 1.12).
1.4. Analysis.
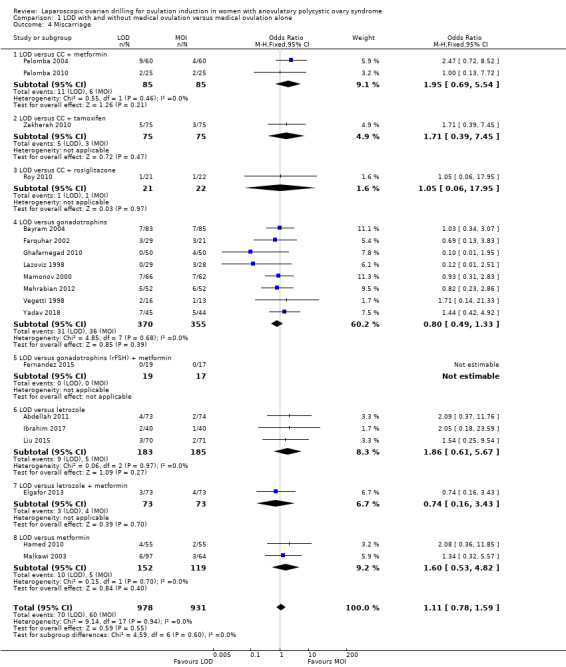
Comparison 1 LOD with and without medical ovulation versus medical ovulation alone, Outcome 4 Miscarriage.
1.12. Analysis.
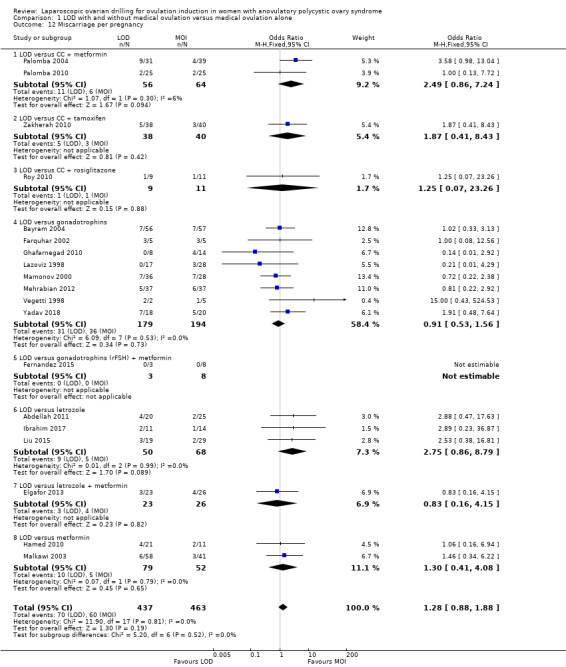
Comparison 1 LOD with and without medical ovulation versus medical ovulation alone, Outcome 12 Miscarriage per pregnancy.
Subgroup analysis did not identify any differences between the groups with different ovulation induction therapies.
In Farquhar 2002 one pregnancy ended with a termination and was reported in the text as such. Fernandez 2015 reported no events of miscarriage in either group.
1.5 Ovarian hyperstimulation syndrome (OHSS)
Eight trials including 722 women reported on rates of OHSS (Amer 2009; Bayram 2004; Farquhar 2002; Kaya 2005; Malkawi 2003; Mehrabian 2012; Roy 2010; Yadav 2018). The analysis suggests that LOD may reduce OHSS (Peto OR 0.25, 95% CI 0.07 to 0.91; 8 studies, 722 women; I2 = 0%; low‐quality evidence; Analysis 1.5). Caution is advised when interpreting the data, due to the low event rates in both groups. There were two cases of OHSS associated with LOD among the 8 trials (2/380), and eight cases (8/342) for the medical ovulation induction‐alone group. Subgroup analysis did not identify any between‐group differences.
1.5. Analysis.
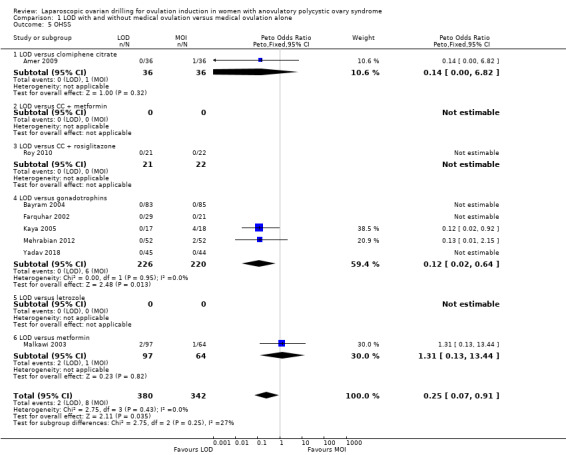
Comparison 1 LOD with and without medical ovulation versus medical ovulation alone, Outcome 5 OHSS.
1.6 Ovulation
Ten trials including 951 women reported on ovulation (Amer 2009; Elgafor 2013; Farquhar 2002; Hamed 2010; Ibrahim 2017; Malkawi 2003; Palomba 2010; Roy 2010; Yadav 2018; Zakherah 2010). There is uncertainty about the effect of LOD compared with ovulation induction alone, due to large uncertainty around the estimate, and the low quality of the evidence (OR 0.96, 95% CI 0.73 to 1.28; 10 studies, 951 women; I2 = 0%; low‐quality evidence; Analysis 1.6). Subgroup analysis did not identify any between‐group differences.
1.6. Analysis.
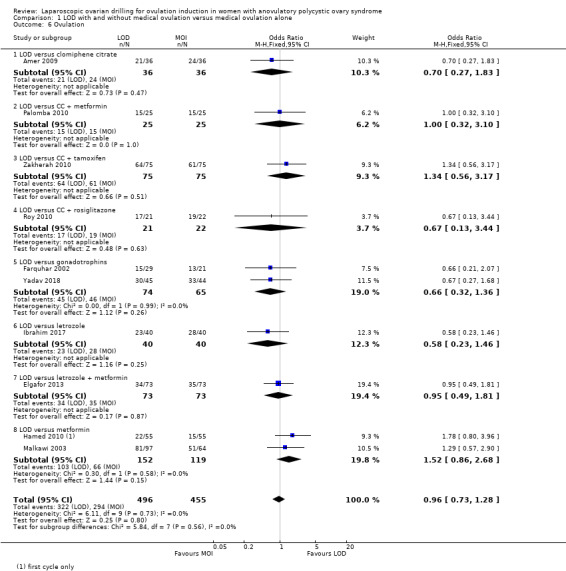
Comparison 1 LOD with and without medical ovulation versus medical ovulation alone, Outcome 6 Ovulation.
For ovulation rate, we included only first‐cycle data in the meta‐analyses of the trial reported in Palomba 2010. Abdellah 2011 and Liu 2015 reported ovulation rates by cycle data and not by woman randomised, and we could not include these data in the meta‐analysis.
1.7 Costs
Both direct and indirect cost data were collected in five papers from four studies (Bayram 2004; Farquhar 2002; Kaya 2005; Palomba 2004). Heterogeneity was high, with I2 = 99%, which is probably due to the currencies used and the different factors taken into account when calculating costs. We have reported only as subgroups. In Bayram 2004 the addition of LOD to the diagnostic laparoscopy added 20 minutes to the procedure, but total costs following LOD were lower due to lower requirement of medical ovulation induction, with a difference of EUR 754 (95% CI 1666.1 to 155.1). In the Discussion section of this paper the cost per term pregnancy was estimated at EUR 14,489 for gonadotrophins and EUR 11,301 for LOD followed by medical induction therapy. The long‐term costs at 10‐year follow‐up were reported in a 2011 economic analysis of Bayram 2004. The costs were significantly lower for the treatment strategy starting with LOD when compared to the gonadotrophin strategy (mean difference EUR 2235; 95% CI 80 to 3790).
The costs associated with Farquhar 2002 were reported in a 2004 publication. The authors reported that the costs of a live birth were one‐third lower in the group that underwent LOD compared to the women who received gonadotrophins (NZD 19,640 and NZD 29,836, respectively). The costs were based on hospital and clinic direct and indirect costs. No estimates of a standard deviation were reported, so we have not included these data in the analysis. Refer to Table 10.
1. Costs.
| Study | LOD ± CC | Other treatment | P value |
| Palomba 2004 | EUR 1050 | Metformin ± CC EUR 50 |
< 0.05 |
| Farquhar 2002 | Total cost per patient NZD 2953 Chance of pregnancy 28% Cost per pregnancy NZD 10,938 Chance of live birth 14% Cost per live birth NZD 21,095 |
Gonadotrophin Total cost per woman NZD 5461 Chance of pregnancy 33% Cost per pregnancy NZD 16,549 Chance of live birth 19% Cost per live birth NZD 28,744 |
NS NS |
Kaya 2005 reported that the costs of LOD were almost half that of treatment with gonadotrophins (USD 1081 ± 234 versus USD 2214 ± 356).
Palomba 2004 reported that LOD was significantly more expensive (P < 0.05) than metformin treatment in a six‐month treatment programme (EUR 1050 versus EUR 50 respectively). Refer to Table 10.
1.8 Quality of life
Only Bayram 2004 reported on health‐related quality of life, using the SF‐36, Rotterdam Symptom Checklist and depression scales (CES‐D). The intention‐to‐treat analysis comparing LOD and rFSH showed no clear evidence of a treatment effect on any of the SF‐36 subscales (Analysis 1.8). The intention‐to‐treat analysis comparing LOD and rFSH showed no clear evidence of treatment or time effects for physical symptoms, psychological measures or overall quality of life on the Rotterdam Symptom Checklist (Analysis 1.9). The intention‐to‐treat analysis comparing LOD and rFSH showed no statistically significant treatment or time effects on the depression scales (CES‐D) (Analysis 1.10).
1.8. Analysis.
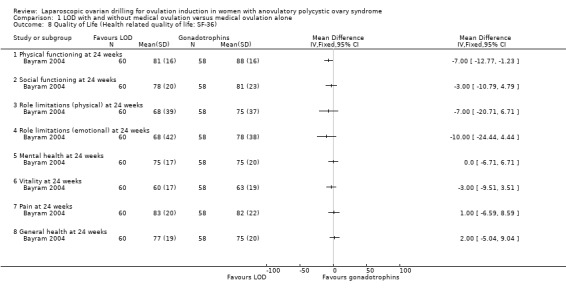
Comparison 1 LOD with and without medical ovulation versus medical ovulation alone, Outcome 8 Quality of Life (Health related quality of life: SF‐36).
1.9. Analysis.
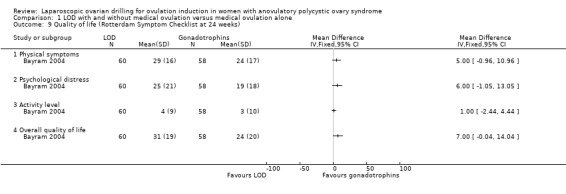
Comparison 1 LOD with and without medical ovulation versus medical ovulation alone, Outcome 9 Quality of life (Rotterdam Symptom Checklist at 24 weeks).
1.10. Analysis.
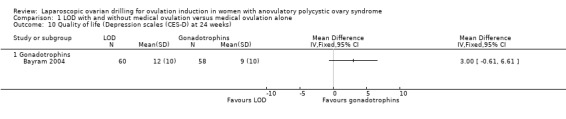
Comparison 1 LOD with and without medical ovulation versus medical ovulation alone, Outcome 10 Quality of life (Depression scales (CES‐D) at 24 weeks).
2. LOD plus IVF versus IVF
We found one trial including 50 women that compared LOD plus IVF with IVF (Rimington 1997). Due to the small sample size, the quality of evidence is not sufficient to justify a conclusion for any of the outcomes.
2.1 Live birth
We are uncertain if LOD plus IVF improves live birth rate compared to IVF alone (OR 1.26, 95% CI 0.33 to 4.84; 1 study, 50 women; very low‐quality evidence; Analysis 2.1).
2.1. Analysis.
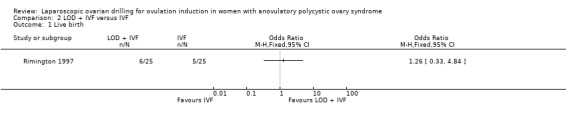
Comparison 2 LOD + IVF versus IVF, Outcome 1 Live birth.
2.2 Multiple pregnancy
We are uncertain if LOD plus IVF reduces multiple pregnancy rate compared to IVF alone (Peto OR 1.0, 95% CI 0.06 to 16.45; 1 study, 50 women; very low‐quality evidence; Analysis 2.2).
2.2. Analysis.
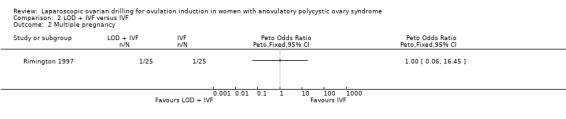
Comparison 2 LOD + IVF versus IVF, Outcome 2 Multiple pregnancy.
2.3 Clinical pregnancy
We are uncertain if LOD plus IVF improves clinical pregnancy rate compared to IVF alone (OR 1.20, 95% CI 0.37 to 3.86; 1 study, 50 women; very low‐quality evidence; Analysis 2.3).
2.3. Analysis.
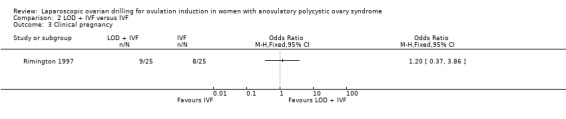
Comparison 2 LOD + IVF versus IVF, Outcome 3 Clinical pregnancy.
2.4 Miscarriage
We are uncertain if LOD plus IVF reduces miscarriage rate compared to IVF alone miscarriage (OR 1.00, 95% CI 0.18 to 5.51; 1 study, 50 women; very low‐quality evidence; Analysis 2.4).
2.4. Analysis.
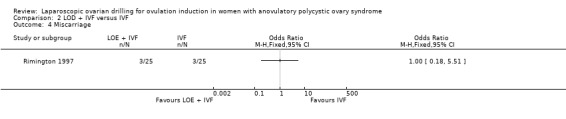
Comparison 2 LOD + IVF versus IVF, Outcome 4 Miscarriage.
2.5 OHSS
We are uncertain if LOD plus IVF improves OHSS rate compared to IVF alone (Peto OR 0.27, 95% CI 0.04 to 1.69; 1 study, 50 women; very low‐quality evidence; Analysis 2.5).
2.5. Analysis.
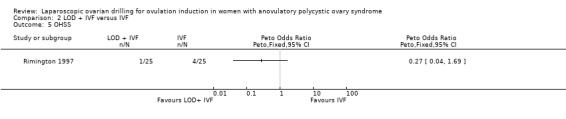
Comparison 2 LOD + IVF versus IVF, Outcome 5 OHSS.
Ovulation
No data were reported for ovulation.
Costs
No data were reported for costs.
Quality of life
No data were reported for quality of life.
3. LOD with second‐look laparoscopy versus LOD with expectant management
We found one trial including 40 women that compared LOD by laser or diathermy and second‐look laparoscopy adhesiolysis three to four weeks later, compared with expectant management (no second‐look laparoscopy) (Gürgan 1992). Due to the small sample size, the quality of the evidence is not sufficient to justify a conclusion for any of the outcomes.
Live birth
No data were reported for live birth.
Multiple pregnancy
No data were reported for multiple pregnancy.
3.1 Clinical pregnancy
We are uncertain if LOD with second‐look laparoscopy improves clinical pregnancy rate (OR 0.67, 95% CI 0.19 to 2.33; 1 study, 40 women; Analysis 3.1).
3.1. Analysis.
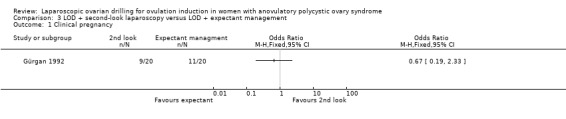
Comparison 3 LOD + second‐look laparoscopy versus LOD + expectant management, Outcome 1 Clinical pregnancy.
3.2 Miscarriage
We are uncertain if LOD with second‐look laparoscopy reduces miscarriage rate (OR 1.00, 95% CI 0.13 to 7.89; 1 study, 40 women; Analysis 3.2).
3.2. Analysis.
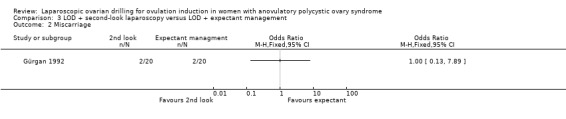
Comparison 3 LOD + second‐look laparoscopy versus LOD + expectant management, Outcome 2 Miscarriage.
OHSS
No data were reported for OHSS.
3.3 Ovulation
We are uncertain if LOD with second‐look laparoscopy improves ovulation rate (OR 6.33, 95% CI 0.67 to 60.16; 1 study, 40 women; Analysis 3.3).
3.3. Analysis.
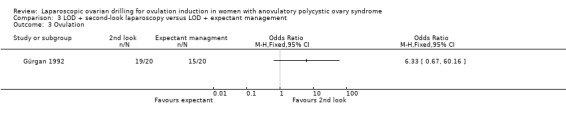
Comparison 3 LOD + second‐look laparoscopy versus LOD + expectant management, Outcome 3 Ovulation.
Costs
No data were reported for costs.
Quality of life
No data were reported for quality of life.
4. Techniques for LOD: unilateral versus bilateral
4.1 Live birth
Live birth was reported in one trial (Roy 2009). Due to the small sample size, the quality of evidence is not sufficient to justify a conclusion for live birth (OR 0.83, 95% CI 0.24 to 2.78; 1 study, 44 women; very low‐quality evidence; Analysis 4.1).
4.1. Analysis.
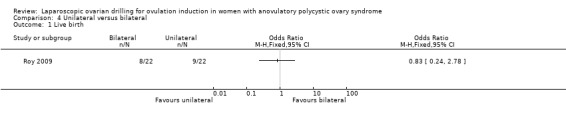
Comparison 4 Unilateral versus bilateral, Outcome 1 Live birth.
Multiple pregnancy
No data were reported for multiple pregnancy.
4.2 Clinical pregnancy
Clinical pregnancy rate was reported in seven trials (Al‐Mizyen 2000; Balen 1994; El‐Sayed 2017; Rezk 2016; Roy 2009; Sorouri 2015; Youssef 2007). For the likelihood of a clinical pregnancy there is uncertainty whether there is a difference between unilateral and bilateral LOD, due to the quality of the evidence and the large heterogeneity between the studies (OR 0.57, 95% CI 0.39 to 0.84; 7 studies, 470 women; I2 = 60%, very low‐quality evidence; Analysis 4.2). Rezk 2016 reports data at six months, unlike the other trials reporting this outcome. The removal of this trial from the analysis makes I2 = 0% and also changes the overall treatment effect. In this subgroup there is uncertainty whether there is a difference between the treatments, due to great uncertainty around the estimate (OR 0.79, 95% CI 0.51 to 1.21; 6 studies, 362 women; analysis not shown).
4.2. Analysis.
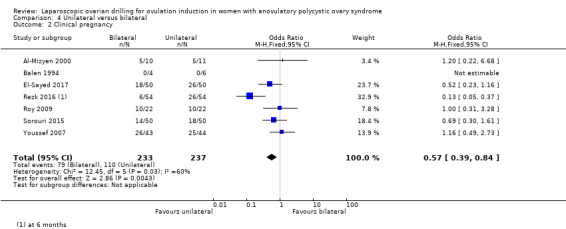
Comparison 4 Unilateral versus bilateral, Outcome 2 Clinical pregnancy.
4.3 Miscarriage Miscarriage was reported in two trials (Roy 2009; Youssef 2007). Due to the small sample size, the quality of evidence is not sufficient to justify a conclusion for miscarriage (OR 1.02, 95% CI 0.31 to 3.33; 2 studies, 131 women; I2 = 0%; very low‐quality evidence; Analysis 4.3). Analysis per pregnancy showed similar results (OR 0.97, 95% CI 0.28 to 3.36; 2 studies, 71 women; I2 = 0%; Analysis 4.5).
4.3. Analysis.
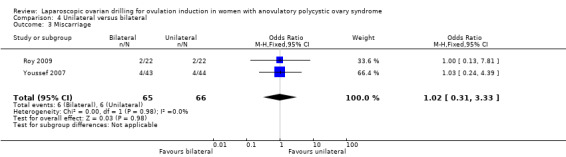
Comparison 4 Unilateral versus bilateral, Outcome 3 Miscarriage.
4.5. Analysis.
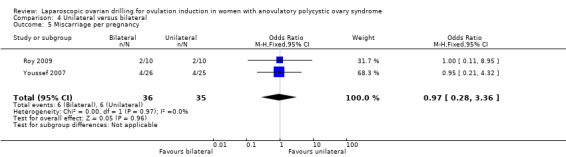
Comparison 4 Unilateral versus bilateral, Outcome 5 Miscarriage per pregnancy.
OHSS
No data were reported for OHSS.
4.4 Ovulation
Ovulation rate was reported in six trials (Balen 1994; El‐Sayed 2017; Rezk 2016; Roy 2009; Sorouri 2015; Youssef 2007). Unilateral LOD might decrease the ovulation rate slightly compared with bilateral LOD (OR 0.60, 95% CI 0.40 to 0.90; 6 studies, 449 women; I2 = 38%; very low‐quality evidence; Analysis 4.4).
4.4. Analysis.
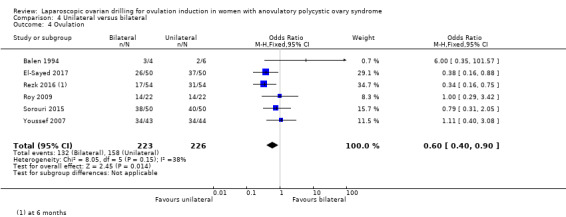
Comparison 4 Unilateral versus bilateral, Outcome 4 Ovulation.
Costs
No data were reported for costs.
Quality of life
No data were reported for quality of life.
5. Techniques for LOD: monopolar verus bipolar
Due to the small sample size, the quality of evidence is not sufficient to justify a conclusion for any of the outcomes.
Live birth
No data were reported for live birth.
Multiple pregnancy
No data were reported for multiple pregnancy.
5.1 Clinical pregnancy
Clinical pregnancy rate was reported in three trials (Darwish 2016; Giampaolino 2016; Sharma 2006) (OR 0.94, 95% CI 0.62 to 1.44; 3 studies, 3541 women; I2 = 710%; very low‐quality evidence; Analysis 5.1).
5.1. Analysis.
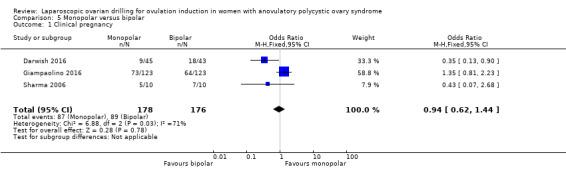
Comparison 5 Monopolar versus bipolar, Outcome 1 Clinical pregnancy.
Miscarriage
No data were reported for miscarriage.
OHSS
No data were reported for OHSS.
5.2 Ovulation
Ovulation was reported in two trials (Darwish 2016; Sharma 2006) (OR 0.33, 95% CI 0.14 to 0.76; 2 studies, 108 women; I2 = 0%; very low‐quality evidence; Analysis 5.2).
5.2. Analysis.
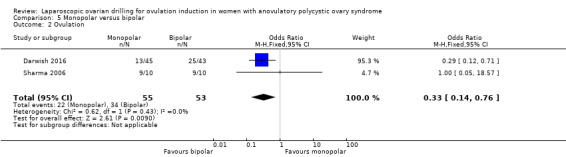
Comparison 5 Monopolar versus bipolar, Outcome 2 Ovulation.
Costs
No data were reported for costs.
Quality of life
No data were reported for quality of life.
6. Techniques for LOD: adjusted thermal dose versus fixed thermal dose
Due to the small sample size, the quality of evidence is not sufficient to justify a conclusion for any of the outcomes.
Live birth
No data were reported for live birth.
Multiple pregnancy
No data were reported for multiple pregnancy.
6.1 Clinical pregnancy
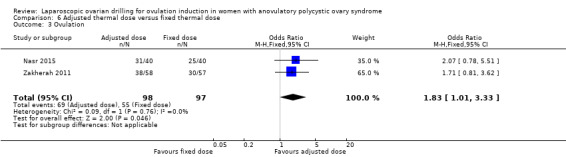
Clinical pregnancy was reported in two trials (Nasr 2015; Zakherah 2011) (OR 1.84, 95% CI 1.04 to 3.26; 2 studies, 195 women; I2 = 0%; very low‐quality evidence; Analysis 6.1).
6.1. Analysis.
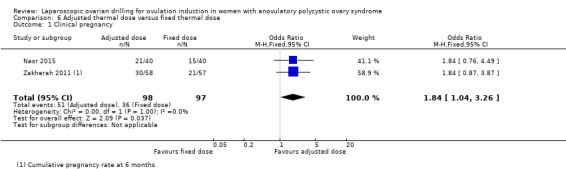
Comparison 6 Adjusted thermal dose versus fixed thermal dose, Outcome 1 Clinical pregnancy.
6.2 Miscarriage
Miscarriage was reported in one trial (Zakherah 2011) (OR 1.33, 95% CI 0.28 to 6.24; 1 study, 115 women; very low‐quality evidence; Analysis 6.2).
6.2. Analysis.
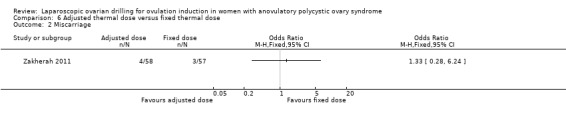
Comparison 6 Adjusted thermal dose versus fixed thermal dose, Outcome 2 Miscarriage.
OHSS
No data were reported for OHSS.
6.3 Ovulation
Ovulation was reported in two trials (Nasr 2015; Zakherah 2011) (OR 1.83, 95% CI 1.01 to 3.33; 2 studies, 195 women; I2 = 0%; very low‐quality evidence; Analysis 6.3).
6.3. Analysis.
Comparison 6 Adjusted thermal dose versus fixed thermal dose, Outcome 3 Ovulation.
Costs
No data were reported for costs.
Quality of life
No data were reported for quality of life.
Discussion
Summary of main results
In women with anovulatory polycystic ovary syndrome (PCOS) and clomiphene citrate (CC) resistance, the main analysis including all studies suggests that LOD with and without medical ovulation induction may decrease live birth compared with medical ovulation induction alone. The evidence suggests that if the chance of live birth following medical ovulation induction alone is 42%, the chance following laparoscopic ovarian drilling (LOD) would be between 28% and 40%; the quality of the evidence was low. The sensitivity analysis restricting to RCTs with low risk of selection bias suggests there might be little or no difference, although there is uncertainty around the estimate.
We found that LOD with and without medical ovulation induction probably reduces the number of multiple pregnancies compared with medical ovulation induction alone. This suggests that if we assume that the risk of multiple pregnancy following medical ovulation induction alone is 5.0%, the risk following LOD would be between 0.9% and 3.4%. The quality of the evidence was moderate, and sensitivity analyses were consistent with the main analysis.
We performed subgroup analysis for the different ovulation‐induction agents, which did not identify any between‐group differences. Virtually all studies had a follow‐up time of at least six months following LOD.
Low‐quality evidence suggests there may be little or no difference in clinical pregnancy between the treatments and that there is uncertainty about the effect of LOD compared with ovulation induction alone for miscarriage. LOD may reduce OHSS , but there was a very low occurrence rate of OHSS. LOD will not by itself induce OHSS, but ovulation induction may induce OHSS (ESHRE 2018).
The quality of the evidence is not sufficient to justify a conclusion from the comparison of unilateral LOD versus bilateral LOD about live birth, clinical pregnancy or miscarriage. There were no data available on multiple pregnancy.
Due to lack of evidence and very low‐quality data there is uncertainty whether there is a difference for any of the following comparisons: LOD with IVF versus IVF alone, LOD with second‐look laparoscopy versus expectant management, monopolar versus bipolar LOD, or adjusted thermal dose versus fixed thermal dose.
Overall completeness and applicability of evidence
Although the number of studies for each drug comparison was limited, the evidence does appear to encompass all available treatments for anovulatory women with PCOS seeking a fertility outcome. As all women included were CC‐resistant, results are probably generalisable for this population, irrespective of the specific diagnostic criteria used. There may have been studies that our searches did not find. We could not find specific data on intra‐operative and post‐operative risks or for long‐term ovarian function. Although there is no superiority of LOD over medical ovulation induction agents, LOD may provide an effective alternative. Specifically, when a laparoscopy is indicated for another reason in women with anovulatory PCOS and there are no other infertility factors, LOD could be considered.
Quality of the evidence
Overall certainty of the evidence was very low to moderate (Table 1; Table 2). This was mainly due to inadequate explanations of randomisation, allocation concealment, and lack of detail or no blinding. All comparisons had relatively few included studies. Randomisation was adequately explained in 23 of the 38 included trials and allocation concealment was adequately explained in 12 of the 38 trials. None of the included trials blinded participants. Outcome assessors were blinded in only seven of the trials, with the remaining trials either unclear about blinding or not conducting blinding at all.
The strengths of this systematic review include the extensive search strategy, and the performance of subgroup and sensitivity analyses. One limitation is that more than half of the included trials did not report the effectiveness outcome of live birth. A second limitation is that due to small sample sizes in many of the interventions the quality of the evidence was very low and we therefore could not justify drawing conclusions about the effects of these interventions.
Potential biases in the review process
The authors of this systematic review believe we have conducted a rigorous search of the evidence. The evidence includes published and unpublished data and there were no restrictions by language.
Agreements and disagreements with other studies or reviews
We agree with the current guideline of ESHRE 2018 that LOD is an intervention that can lead to a singleton birth in women with PCOS. There is no convincing evidence of the superiority of medical ovulation‐induction agents over LOD, there is no need for monitoring (because of mono‐ovulation), and only a small risk of multiple pregnancy. However, it is important to note that LOD is an invasive surgical intervention; long‐term ovarian function and intra‐operative and post‐operative risks should be considered.
Our sensitivity analysis shows uncertainty about whether there is a difference in live birth between LOD with and without medical ovulation induction compared with medical ovulation induction alone. Similarly, a recent meta‐analysis comparing letrozole with LOD also suggests there might be no differences in the live birth rate (Yu 2019).
Although surgically‐related complications associated with LOD seem rare, a case of pelvic infection following LOD highlights the need for caution when offering this treatment over gonadotrophin therapy (Deans 1997). There are also the associated risks and morbidity of laparoscopy under general anaesthetic, postoperative adhesion formation (Greenblatt 1993), and the as yet theoretical long‐term risk of premature ovarian failure. However, a 10‐year follow‐up study did not find any indication for adhesion formation, nor for premature ovarian failure (Nahuis 2014).
Authors' conclusions
Implications for practice.
Our main analysis with low‐quality evidence shows that laparoscopic ovarian drilling (LOD) with and without medical ovulation induction may slightly decrease the live birth rate in women with anovulatory polycystic ovary syndrome and clomiphene citrate resistance, compared with medical ovulation induction alone. But in the sensitivity analysis restricted to only RCTs with low risk of selection bias there is uncertainty whether there is a difference between the treatments, due to large uncertainty around the estimate. Moderate‐quality evidence shows that LOD probably reduces the number of multiple pregnancies. Low‐quality evidence suggests that there may be little or no difference between the treatments for the likelihood of a clinical pregnancy. There is uncertainty about the effect of LOD compared with ovulation induction alone on miscarriage. LOD may result in less ovarian hyperstimulation syndrome (OHSS).The quality of evidence is not sufficient to justify conclusions about live birth, clinical pregnancy or miscarriage rate for the comparison of unilateral LOD versus bilateral LOD. There were no data available on multiple pregnancy.
Implications for research.
Further RCTs should primarily be focused on the role of LOD in association with medical ovulation induction. These trials will require sample sizes of at least 600 women to enable the determination of realistic differences, and will require a follow‐up period of at least six months. Studies should not just evaluate the outcomes of live birth and clinical pregnancy rates, but should also include outcomes such as the ease of medical ovulation induction, adverse effects (multiple pregnancy, miscarriage, OHSS and surgical complications), cost benefit analyses and consumer satisfaction.The long‐term benefits (spontaneous resumption of ovulation and menstruation) and potential risks of LOD (such as premature ovarian failure) will also need to be addressed. Further trials of optimising techniques need to address how to perform LOD in the least invasive way.
Feedback
Query about study inclusion
Summary
The protocol states that eligible participants were subfertile women with clomiphene‐resistant PCOS. Although the term 'clomiphene‐resistant' is not defined in the review, it is generally accepted to mean that women have not responded with proven ovulation to the use of clomiphene. Clomiphene failure, on the other hand, means that women have ovulated on clomiphene but have failed to achieve a successful outcome. In my opinion, the meta‐analysis has therefore incorrectly included the study of Abu Hashim et al (Abu Hashim et al, 2011b), as participants in this study were infertile women with clomiphene citrate failure rather than clomiphene‐resistance. (Summary of comments received from Associate Professor Luk Rombauts)
Reply
The authors agree that Abu Hashim 2011b should not have been included in this review and we have now excluded this study. We have also added a definition of clomiphene resistance in the Methods section. We would like to thank Associate Professor Rombauts for his comments.
Contributors
Associate Professor Luk Rombauts, Obstetrics and Gynaecology, Monash University
Cindy Farquhar, Julie Brown and Jane Marjoribanks, Obstetrics and Gynaecology, University of Auckland
What's new
| Date | Event | Description |
|---|---|---|
| 14 October 2019 | New citation required but conclusions have not changed | The addition of new studies has not led to changes in our conclusion. |
| 14 October 2019 | New search has been performed | Added new studies: Darwish 2016; Elgafor 2013; El‐Sayed 2017; Giampaolino 2016; Ibrahim 2017; Liu 2015; Nasr 2013; Nasr 2015; Rezk 2016; Sorouri 2015; Yadav 2018; Zakherah 2011, and amendments to review text. Placed Abu Hashim 2010a and Abu Hashim 2011a to awaiting classification. |
History
Protocol first published: Issue 2, 1998 Review first published: Issue 2, 1998
| Date | Event | Description |
|---|---|---|
| 20 March 2014 | Amended | Correction of effect estimate (from RR to OR) for one outcome in comparison 1, and consequential amendments to review text. |
| 6 August 2012 | Feedback has been incorporated | Abu Hashim 2011a excluded in response to feedback |
| 15 May 2012 | New citation required but conclusions have not changed | There is insufficient evidence for the conclusions to this review to be changed. |
| 15 May 2012 | New search has been performed | This review was first published in 1998. Updates were published in 2001 and 2007. Nine trials were included in the 2007 version. In the current update an additional 16 studies have been added to the meta‐analysis: Abdellah 2011; Abu Hashim 2010; Abu Hashim 2011; Abu Hashim 2011b; Ashrafinia 2009; Amer 2009; Ghafarnegad 2010; Hamed 2010; Palomba 2004; Palomba 2010; Rimington 1997; Roy 2009; Roy 2010; Sharma 2006; Youssef 2007; Zakherah 2009; Zakherah 2010. |
| 11 November 2008 | Amended | Converted to new review format. |
| 1 May 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The review authors would like to acknowledge the contribution of M Arnot to the original review. The review authors also wish to acknowledge the contribution of Richard Lilford, Patrick Vandekerckhove, Jane Marjoribanks, and Cindy Farquhar as authors in previous versions of the review. The review authors would like to acknowledge the contribution of Elena Kostova (Managing Editor of the CGFG) to the updated version. We would like to thank Roger Hart, Katie Stocking, and Edgardo Somigliana for the valuable peer review comments.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility (CGFG) specialised register search
Searched 8 October 2019
Procite platform
Keywords CONTAINS "polycystic ovary morphology" or "polycystic ovary syndrome" or"PCOS" or Title CONTAINS "polycystic ovary morphology" or "polycystic ovary syndrome" or "PCOS"
AND
Keywords CONTAINS "laparoscopic coagulation techniques" or "laparoscopic electrocautery" or "laparoscopic ovarian cautery" or "laparoscopic ovarian cystectomy" or "laparoscopic ovarian diathermy" or "laparoscopic ovarian drilling" or "laparoscopic ovarian electrocauterization" or "laparoscopic ovarian electrodrilling" or "laser" or "Diathermy" or "electrocautery" or "Electrocoagulation" or "electrosurgical" or "cystectomy" or "thermocoagulation" or "ovarian cystectomy" or "ovarian diathermy" or "ovarian drilling" or "ovarian adhesions" or "ovarian electrocautery" or "ovarian surgery" or Title CONTAINS "laparoscopic coagulation techniques" or "laparoscopic electrocautery" or "laparoscopic ovarian cautery" or "laparoscopic ovarian cystectomy" or "laparoscopic ovarian diathermy" or "laparoscopic ovarian drilling" or "laparoscopic ovarian electrocauterization" or "laparoscopic ovarian electrodrilling"
(113 records)
Appendix 2. CENTRAL search strategy
Searched 8 October 2019
via the Central Register of Studies Online (CRSO) web platform
#1 MESH DESCRIPTOR Polycystic Ovary Syndrome EXPLODE ALL TREES 1336 #2 (PCOS or PCOD):TI,AB,KY 2619 #3 (stein leventhal syndrome):TI,AB,KY 30 #4 (polycystic ovar*):TI,AB,KY 3203 #5 #1 OR #2 OR #3 OR #4 3511 #6 MESH DESCRIPTOR Diathermy EXPLODE ALL TREES 992 #7 MESH DESCRIPTOR Laparoscopy EXPLODE ALL TREES 5275 #8 MESH DESCRIPTOR Cautery EXPLODE ALL TREES 754 #9 MESH DESCRIPTOR Electrocoagulation EXPLODE ALL TREES 691 #10 cauter*:TI,AB,KY 718 #11 electrocauter*:TI,AB,KY 613 #12 cystectomy:TI,AB,KY 1313 #13 diathermy:TI,AB,KY 711 #14 drilling:TI,AB,KY 414 #15 electrocoagulation:TI,AB,KY 867 #16 thermocoagulation:TI,AB,KY 127 #17 MESH DESCRIPTOR Laser Coagulation EXPLODE ALL TREES 513 #18 (laparoscop* adj5 ovar*):TI,AB,KY 475 #19 laser*:TI,AB,KY 16999 #20 photocoagulation:TI,AB,KY 1420 #21 surg*:TI,AB,KY 217850 #22 electrosurg*:TI,AB,KY 534 #23 #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 230901 #24 #5 AND #23 283
Appendix 3. MEDLINE search strategy
Searched from 1946 to 8 October 2019
Ovid platform
1 Polycystic Ovary Syndrome/ (13783) 2 (polycystic adj5 ovar$).tw. (15849) 3 PCOS.tw. (10482) 4 PCOD.tw. (288) 5 (stein‐leventhal or leventhal).tw. (722) 6 (ovar$ adj (scelerocystic or polycystic or degeneration)).tw. (93) 7 or/1‐6 (18862) 8 exp Diathermy/ (14948) 9 Laparoscopy/ (82328) 10 exp cautery/ or exp electrocoagulation/ or argon plasma coagulation/ (13238) 11 cauter*.tw. (4468) 12 cystectomy.tw. (13677) 13 diathermy.tw. (2918) 14 drilling.tw. (7339) 15 electrocauter*.tw. (3491) 16 electrocoagulation.tw. (3026) 17 thermocoagulation.tw. (934) 18 Laser Coagulation/ (7395) 19 (laparoscop$ adj5 ovar$).tw. (2568) 20 laser.tw. (249887) 21 photocoagulation.tw. (9142) 22 surg$.tw. (1821592) 23 electrosurg*.tw. (3478) 24 or/8‐23 (2112511) 25 randomized controlled trial.pt. (490860) 26 controlled clinical trial.pt. (93307) 27 randomized.ab. (456000) 28 placebo.tw. (206702) 29 clinical trials as topic.sh. (188610) 30 randomly.ab. (319021) 31 trial.ti. (205456) 32 (crossover or cross‐over or cross over).tw. (81833) 33 or/25‐32 (1270919) 34 exp animals/ not humans.sh. (4625030) 35 33 not 34 (1167599) 36 7 and 24 and 35 (145)
Appendix 4. Embase search strategy
Searched from 1980 to 8 October 2019
Ovid platform
1 exp ovary polycystic disease/ or exp stein leventhal syndrome/ (25731) 2 (polycystic adj5 ovar$).tw. (22274) 3 PCOS.tw. (16338) 4 PCOD.tw. (401) 5 (stein‐leventhal or leventhal).tw. (309) 6 (ovar$ adj (scelerocystic or polycystic or degeneration)).tw. (94) 7 or/1‐6 (29877) 8 exp Diathermy/ (4500) 9 Laparoscopy/ (71638) 10 cystectom$.tw. (22067) 11 diathermy.tw. (3104) 12 drilling.tw. (8571) 13 electrocauter$.tw. (5039) 14 electrocoagulat$.tw. (3159) 15 thermocoagulat$.tw. (1220) 16 Laser Coagulation/ (19715) 17 laser$.tw. (257937) 18 (laparoscop$ adj5 ovar$).tw. (4111) 19 photocoagulation.tw. (10939) 20 surg$.tw. (2368961) 21 cauter$.tw. (6403) 22 electrosurg$.tw. (4400) 23 exp cauterization/ or exp electrosurgery/ or exp electrocoagulation/ or exp laser surgery/ (81651) 24 or/8‐23 (2661781) 25 Clinical Trial/ (954205) 26 Randomized Controlled Trial/ (571370) 27 exp randomization/ (84709) 28 Single Blind Procedure/ (36882) 29 Double Blind Procedure/ (164012) 30 Crossover Procedure/ (61044) 31 Placebo/ (329928) 32 Randomi?ed controlled trial$.tw. (213457) 33 Rct.tw. (34258) 34 random allocation.tw. (1918) 35 randomly allocated.tw. (33472) 36 allocated randomly.tw. (2484) 37 (allocated adj2 random).tw. (810) 38 Single blind$.tw. (23527) 39 Double blind$.tw. (196701) 40 ((treble or triple) adj blind$).tw. (1018) 41 placebo$.tw. (292739) 42 prospective study/ (556114) 43 or/25‐42 (2094903) 44 case study/ (64768) 45 case report.tw. (384379) 46 abstract report/ or letter/ (1075862) 47 or/44‐46 (1515022) 48 43 not 47 (2043020) 49 7 and 24 and 48 (535)
Appendix 5. PsycINFO search strategy
Searched from 1806 to 8 October 2019
Ovid platform
1 exp Endocrine Sexual Disorders/ (1726) 2 (polycystic adj5 ovar$).tw. (404) 3 PCOS.tw. (265) 4 PCOD.tw. (7) 5 (stein‐leventhal or leventhal).tw. (296) 6 (ovar$ adj (scelerocystic or polycystic or degeneration)).tw. (0) 7 or/1‐6 (2287) 8 Diathermy.tw. (30) 9 cystectomy.tw. (35) 10 drilling.tw. (291) 11 electrocautery.tw. (11) 12 electrocoagulation.tw. (72) 13 thermocoagulation.tw. (58) 14 laser.tw. (3258) 15 (laparoscop$ adj5 ovar$).tw. (8) 16 laser.tw. (3258) 17 photocoagulation.tw. (33) 18 surg$.tw. (47688) 19 electrosurgery.tw. (4) 20 or/8‐19 (51212) 21 7 and 20 (164) 22 random.tw. (56335) 23 control.tw. (431633) 24 double‐blind.tw. (22388) 25 clinical trials/ (11453) 26 placebo/ (5373) 27 exp Treatment/ (1015784) 28 or/22‐27 (1401802) 29 21 and 28 (104)
Appendix 6. CINAHL search strategy
Cumulative Index to Nursing & Allied Health Literature
Searched from 1961 to 8 October 2019
Ebsco platform
| # | Query | Results |
| S38 | S25 AND S37 | 126 |
| S37 | S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 | 1,350,648 |
| S36 | TX allocat* random* | 10,967 |
| S35 | (MH "Quantitative Studies") | 23,381 |
| S34 | (MH "Placebos") | 11,451 |
| S33 | TX placebo* | 59,249 |
| S32 | TX random* allocat* | 10,967 |
| S31 | (MH "Random Assignment") | 56,159 |
| S30 | TX randomi* control* trial* | 176,191 |
| S29 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 1,030,772 |
| S28 | TX clinic* n1 trial* | 251,547 |
| S27 | PT Clinical trial | 86,654 |
| S26 | (MH "Clinical Trials+") | 267,630 |
| S25 | S5 AND S24 | 556 |
| S24 | S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 | 814,589 |
| S23 | TX electrosurg* | 1,359 |
| S22 | TX surg* | 794,505 |
| S21 | TX photocoagulation | 886 |
| S20 | TX laser | 29,283 |
| S19 | TX laparoscop* N5 ovar* | 544 |
| S18 | (MM "Laser Therapy+") | 7,115 |
| S17 | TX thermocoagulation | 141 |
| S16 | TX electrocoagulation | 985 |
| S15 | TX electrocauter* | 612 |
| S14 | TX drilling | 1,560 |
| S13 | TX diathermy | 676 |
| S12 | TX cystectomy | 2,323 |
| S11 | (MM "Cystectomy") | 869 |
| S10 | TX electrocautery | 570 |
| S9 | TX cauter* | 1,152 |
| S8 | (MM "Cautery+") | 11,718 |
| S7 | (MM "Surgery, Laparoscopic+") | 4,587 |
| S6 | (MM "Diathermy+") OR (MM "Electrocoagulation+") | 13,284 |
| S5 | S1 OR S2 OR S3 OR S4 | 4,752 |
| S4 | TX polycystic ovar* | 4,141 |
| S3 | TX stein leventhal syndrome | 10 |
| S2 | TX PCOS or TX PCOD | 2,578 |
| S1 | (MM "Polycystic Ovary Syndrome") | 2,596 |
Data and analyses
Comparison 1. LOD with and without medical ovulation versus medical ovulation alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth | 9 | 1015 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.54, 0.92] |
| 1.1 LOD versus CC + metformin | 2 | 170 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.32, 1.09] |
| 1.2 LOD versus CC + tamoxifen | 1 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.42, 1.53] |
| 1.3 LOD versus gonadotrophins | 4 | 407 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.56, 1.36] |
| 1.4 LOD versus letrozole | 2 | 288 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.32, 0.92] |
| 2 Multiple pregnancy | 14 | 1161 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.34 [0.18, 0.66] |
| 2.1 LOD versus clomiphene citrate | 1 | 72 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 LOD versus CC + metformin | 2 | 170 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 LOD versus CC + rosiglitazone | 1 | 43 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.12 [0.21, 21.52] |
| 2.4 LOD versus gonadotrophins | 7 | 532 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.22 [0.10, 0.46] |
| 2.5 LOD versus gonadotrophins (rFSH) + metformin | 1 | 36 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 LOD versus letrozole | 1 | 147 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.7 LOD versus metformin | 1 | 161 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.32 [0.25, 6.94] |
| 3 Clinical pregnancy | 21 | 2016 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.72, 1.03] |
| 3.1 LOD versus clomiphene citrate | 1 | 72 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.19, 1.44] |
| 3.2 LOD versus CC + metformin | 2 | 170 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.39, 1.31] |
| 3.3 LOD versus CC + tamoxifen | 1 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.47, 1.71] |
| 3.4 LOD versus CC + rosiglitazone | 1 | 43 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.23, 2.50] |
| 3.5 LOD versus gonadotrophins | 9 | 760 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.74, 1.36] |
| 3.6 LOD versus gonadotrophins (rFSH) + metformin | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.04, 1.00] |
| 3.7 LOD versus letrozole | 3 | 368 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.42, 1.01] |
| 3.8 LOD versus letrozole + metformin | 1 | 146 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.42, 1.65] |
| 3.9 LOD versus metformin | 2 | 271 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.75, 2.08] |
| 4 Miscarriage | 19 | 1909 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.78, 1.59] |
| 4.1 LOD versus CC + metformin | 2 | 170 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.69, 5.54] |
| 4.2 LOD versus CC + tamoxifen | 1 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.39, 7.45] |
| 4.3 LOD versus CC + rosiglitazone | 1 | 43 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.06, 17.95] |
| 4.4 LOD versus gonadotrophins | 8 | 725 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.49, 1.33] |
| 4.5 LOD versus gonadotrophins (rFSH) + metformin | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.6 LOD versus letrozole | 3 | 368 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.86 [0.61, 5.67] |
| 4.7 LOD versus letrozole + metformin | 1 | 146 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.16, 3.43] |
| 4.8 LOD versus metformin | 2 | 271 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.53, 4.82] |
| 5 OHSS | 8 | 722 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.25 [0.07, 0.91] |
| 5.1 LOD versus clomiphene citrate | 1 | 72 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 6.82] |
| 5.2 LOD versus CC + metformin | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 LOD versus CC + rosiglitazone | 1 | 43 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 LOD versus gonadotrophins | 5 | 446 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.12 [0.02, 0.64] |
| 5.5 LOD versus letrozole | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.6 LOD versus metformin | 1 | 161 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.31 [0.13, 13.44] |
| 6 Ovulation | 10 | 951 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.73, 1.28] |
| 6.1 LOD versus clomiphene citrate | 1 | 72 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.7 [0.27, 1.83] |
| 6.2 LOD versus CC + metformin | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.32, 3.10] |
| 6.3 LOD versus CC + tamoxifen | 1 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.56, 3.17] |
| 6.4 LOD versus CC + rosiglitazone | 1 | 43 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.13, 3.44] |
| 6.5 LOD versus gonadotrophins | 2 | 139 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.32, 1.36] |
| 6.6 LOD versus letrozole | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.23, 1.46] |
| 6.7 LOD versus letrozole + metformin | 1 | 146 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.49, 1.81] |
| 6.8 LOD versus metformin | 2 | 271 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.86, 2.68] |
| 7 Costs | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 LOD versus CC + metformin | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 3711.3 [3585.17, 3837.43] |
| 7.2 LOD versus gonadotrophins only (short‐term) | 2 | 203 | Mean Difference (IV, Fixed, 95% CI) | ‐1115.75 [‐1309.72, ‐921.77] |
| 7.3 LOD versus gonadotrophins only (long‐term) | 1 | 168 | Mean Difference (IV, Fixed, 95% CI) | ‐2235.0 [‐4433.16, ‐36.84] |
| 8 Quality of Life (Health related quality of life: SF‐36) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Physical functioning at 24 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Social functioning at 24 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Role limitations (physical) at 24 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.4 Role limitations (emotional) at 24 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.5 Mental health at 24 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.6 Vitality at 24 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.7 Pain at 24 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.8 General health at 24 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Quality of life (Rotterdam Symptom Checklist at 24 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Physical symptoms | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Psychological distress | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Activity level | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.4 Overall quality of life | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Quality of life (Depression scales (CES‐D) at 24 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 Gonadotrophins | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Multiple pregnancy per pregnancy | 14 | 577 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.34 [0.17, 0.66] |
| 11.1 LOD versus clomiphene citrate | 1 | 23 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 LOD versus CC + metformin | 2 | 99 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 LOD versus CC + rosiglitazone | 1 | 20 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.66 [0.24, 29.46] |
| 11.4 LOD versus gonadotrophins | 7 | 280 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.20 [0.09, 0.43] |
| 11.5 LOD versus gonadotrophins (rFSH) + metformin | 1 | 11 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.6 LOD versus letrozole | 1 | 45 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.7 LOD versus metformin | 1 | 99 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.42 [0.27, 7.53] |
| 12 Miscarriage per pregnancy | 19 | 900 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.88, 1.88] |
| 12.1 LOD versus CC + metformin | 2 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.49 [0.86, 7.24] |
| 12.2 LOD versus CC + tamoxifen | 1 | 78 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.87 [0.41, 8.43] |
| 12.3 LOD versus CC + rosiglitazone | 1 | 20 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.07, 23.26] |
| 12.4 LOD versus gonadotrophins | 8 | 373 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.53, 1.56] |
| 12.5 LOD versus gonadotrophins (rFSH) + metformin | 1 | 11 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.6 LOD versus letrozole | 3 | 118 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.75 [0.86, 8.79] |
| 12.7 LOD versus letrozole + metformin | 1 | 49 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.16, 4.15] |
| 12.8 LOD versus metformin | 2 | 131 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.41, 4.08] |
1.7. Analysis.
Comparison 1 LOD with and without medical ovulation versus medical ovulation alone, Outcome 7 Costs.
Comparison 2. LOD + IVF versus IVF.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Multiple pregnancy | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 3 Clinical pregnancy | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Miscarriage | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 OHSS | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 6 Multiple pregnancy per pregnancy | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 7 Miscarriage per pregnancy | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
2.6. Analysis.
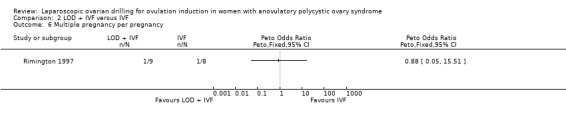
Comparison 2 LOD + IVF versus IVF, Outcome 6 Multiple pregnancy per pregnancy.
2.7. Analysis.
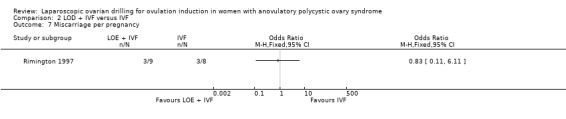
Comparison 2 LOD + IVF versus IVF, Outcome 7 Miscarriage per pregnancy.
Comparison 3. LOD + second‐look laparoscopy versus LOD + expectant management.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical pregnancy | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Miscarriage | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Ovulation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Miscarriage per pregnancy | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
3.4. Analysis.
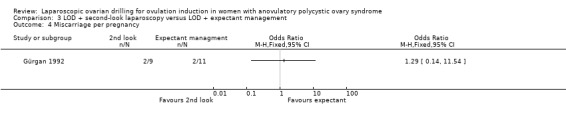
Comparison 3 LOD + second‐look laparoscopy versus LOD + expectant management, Outcome 4 Miscarriage per pregnancy.
Comparison 4. Unilateral versus bilateral.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Clinical pregnancy | 7 | 470 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.39, 0.84] |
| 3 Miscarriage | 2 | 131 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.31, 3.33] |
| 4 Ovulation | 6 | 449 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.40, 0.90] |
| 5 Miscarriage per pregnancy | 2 | 71 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.28, 3.36] |
Comparison 5. Monopolar versus bipolar.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical pregnancy | 3 | 354 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.62, 1.44] |
| 2 Ovulation | 2 | 108 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.14, 0.76] |
Comparison 6. Adjusted thermal dose versus fixed thermal dose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical pregnancy | 2 | 195 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.84 [1.04, 3.26] |
| 2 Miscarriage | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Ovulation | 2 | 195 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.01, 3.33] |
Comparison 7. Sensitivity analysis low risk of bias: LOD with and without medical ovulation versus medical ovulation alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth | 4 | 415 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.59, 1.36] |
| 1.1 LOD versus gonadotrophins | 2 | 218 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.59, 1.85] |
| 1.2 LOD versus CC + metformin | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.39, 3.56] |
| 1.3 LOD versus letrozole | 1 | 147 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.30, 1.31] |
| 2 Multiple pregnancy | 6 | 522 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.18 [0.06, 0.57] |
| 2.1 LOD versus CC + metformin | 1 | 50 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 LOD versus gonadotrophins | 3 | 253 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.18 [0.06, 0.57] |
| 2.3 LOD versus letrozole | 1 | 147 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 LOD versus clomiphene citrate | 1 | 72 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdellah 2011.
| Methods | Randomised trial conducted in Eygpt Timing: July 2007 to February 2010 |
|
| Participants | 156 women assessed for eligibility in fertility clinics and 147 randomised Mean age of women in the letrozole group was 23.9 ± 3.2 years and in the LOD group was 23.6 ± 3.2 years Inclusion: Women with clomiphene‐resistant PCOS, primary or secondary infertility because of anovulation and clomiphene resistance for at least 1 year, normal sperm analysis from partner, patent tubes as seen by hysterosalpingography or diagnostic laparoscopy Exclusion: Age < 20 or > 35 years, hormonal treatment within 3 months prior to study, hyperprolactinaemia, any other endocrine, hepatic or renal disorder, presence of an organic pelvic mass, history of abdominal surgery that might have caused pelvic factor infertility |
|
| Interventions | Letrozole 5 mg/day for 5 days starting on day 3 of menses for a maximum of 6 cycles (n = 74), versus LOD ‐ each ovary was punctured 4 to 6 times depending on the size of the ovary (n = 73) Follow‐up for 6 months |
|
| Outcomes | Endometrial thickness, biochemical pregnancy, clinical pregnancy, spontaneous abortion, ovulation rate | |
| Notes | No conflict of interest Clinical trial registration number: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated random numbers table" |
| Allocation concealment (selection bias) | Low risk | Quote: "achieved using serially numbered opaque envelopes that were only opened once the interventions were assigned" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There are no details of blinding in the paper. Blinding was unlikely to have occurred as the interventions were oral medication versus surgery. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There are no details of outcome assessors being blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 147 randomised; 4 in the letrozole group and 3 in the LOD dropped out of the trial, all for non‐compliance. Intention‐to‐treat analysis was not conducted |
| Selective reporting (reporting bias) | High risk | We could not retrieve the original protocol. Live birth rate was reported in the Results section and was not listed as an outcome in the Methods section of the paper. Adverse effects on the mother and congenital malformations were also addressed in the Discussion section of the paper but had not been reported in the results section |
| Other bias | Low risk | No evidence of other risk of bias |
Al‐Mizyen 2000.
| Methods | Randomised controlled trial conducted in UK Timing: not stated. |
|
| Participants | 21 women randomised (this may be a typographical error in the abstract). Mean age 27 and 28 years; mean duration of infertility was 5.0 versus 4.8 years and the mean BMI was 19 versus 17 kg/m2 Included: women with clomiphene‐resistant PCOS (150 mg clomiphene) with chronic anovulation, and 5 were resistant to FSH ovulation induction |
|
| Interventions | Bilateral ovarian surgery by diathermy (n = 10), versus
Unilateral ovarian surgery (n = 11).
LOS was performed with a diathermy needle creating 4 punctures/ovary 12 months follow‐up |
|
| Outcomes | Pregnancy rate (by participant) | |
| Notes | Conflict of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "allocated randomly"; no other details in conference abstract |
| Allocation concealment (selection bias) | Unclear risk | No details in conference abstract. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No evidence of blinding of researchers, participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No evidence of blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants appear to have been followed through the study and all those randomised were analysed |
| Selective reporting (reporting bias) | High risk | No live birth data |
| Other bias | High risk | Conference abstract only |
Amer 2009.
| Methods | Randomised trial conducted in UK fertility clinic Timing: March 2002 to March 2006 |
|
| Participants | 72 anovulatory women with PCOS. Mean age of women in LOD group 28.1 ± 4.3 years and in CC group 29.1 ± 4.8 years Inclusion: Women with anovulatory infertility with PCOS. Aged 18 to 39 years, BMI ≤ 32 kg/m2, duration of infertility ≥ 1 year. At least 1 patent fallopian tube on hysterosalpingogram and normal semen analysis Exclusion: Inability to give informed consent, contra‐indication to clomiphene citrate or general anaesthetic. Any ovarian induction therapy in previous 6 months |
|
| Interventions | Laparoscopic ovarian diathermy: 4 punctures per ovary in both ovaries. CC was also given if there was no ovulation 6 ‐ 8 weeks after surgery (n = 36), versus CC daily dose increasing from 50 mg to 150 mg on days 2 to 6 of a menstrual period or after a progestogen withdrawal bleed using medroxyprogesterone acetate. Treatment for 6 cycles and then offered LOD (n = 36) |
|
| Outcomes | Ovulation, pregnancy (biochemical, cumulative), multiple pregnancies, live birth rate | |
| Notes | Conflict of interest: not stated Supported by a grant from the University of Sheffield Clinical trial registration number: NCT00220545 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...block randomisation method using a random number table .." |
| Allocation concealment (selection bias) | Low risk | Quote: "held centrally by a trial administrator" Comment: Appears to be central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no blinding; once randomised the allocation was revealed to the investigator and the participant |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | LOD: 3 conceived before LOD, 1 discontinued and 1 postponed. 33 /36 were analysed CC: 3 conceived before CC and 1 postponed treatment. 32 were analysed |
| Selective reporting (reporting bias) | Low risk | We found the registered protocol on ClinicalTrials.gov (NCT00220545). All the outcomes mentioned in the protocol were presented in the published report |
| Other bias | Low risk | No evidence of other risk of bias |
Ashrafinia 2009.
| Methods | Prospective randomised trial conducted in Iran from March 2006 to February 2008 | |
| Participants | 126 women attending a fertility clinic aged 15 to 45 years with a history of infertility for at least 1 year and 3 treatment cycles of clomiphene citrate with no response. Mean age of women in LOD group was 26.54 ± 4.72 years and in the metformin group was 25.13 ± 3.47 years Inclusion: Irregular menstruation, clinical and biochemical signs of hyperandrogenism, polycystic ovaries Exclusion: Diseases that would disturb clinical and hormonal responses, pregnancy during follow‐up, BMI > 30 or < 17 |
|
| Interventions | LOD performed 4 times in each ovary (n = 63), versus Metformin 1500 g daily (n = 63) Follow‐up for 6 months |
|
| Outcomes | Menstrual regularity, hormonal levels, Ferriman‐Gallwey score | |
| Notes | No conflict of interest We have contacted authors for obstetric outcomes Clinical trial registration number: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details in paper |
| Allocation concealment (selection bias) | Low risk | Quote: "serially numbered opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no evidence that participants or researchers were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants appear to have been followed through the study and all those randomised were analysed |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol. All outcomes mentioned in the Method section are presented in the Results. There are no reproductive outcomes. Authors have been contacted. |
| Other bias | Low risk | No evidence of other risk of bias |
Balen 1994.
| Methods | Prospective randomised controlled trial conducted in UK (Middlesex Hospital, London) Timing: not stated |
|
| Participants | 10 women randomised. Refractory PCO. Mean age (range) of the women was 29.5 (27 to 33) years and mean duration (range) of infertility was 5.6 years (4 to 8). Infertility work‐up consisted of tubal patency testing by laparoscopy, semen analysis, endocrinology. In one case the tubes were blocked, 2 had pelvic adhesions, 3 had severe oligospermia or azoospermia and underwent donor insemination. Mean BMI 23 kg/m2 Study duration and timing not stated. | |
| Interventions | Bilateral ovarian surgery by diathermy (N=6), versus
Unilateral ovarian surgery (N=4)
LOD was performed with a diathermy needle creating 4 punctures/ovary, cooled with normal saline Follow‐up for 3 months |
|
| Outcomes | Pregnancy rate (by participant) Ovulation rate (by participant) | |
| Notes | Conflict of interest: not stated Definitions: PCO: not defined. Refractory PCO: failure to ovulate on 100 mg/day (duration not specified); some had also been treated previously with tamoxifen or gonadotrophins Pregnancy: not defined Ovulation: not defined |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details in paper |
| Allocation concealment (selection bias) | Unclear risk | No details in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No evidence of blinding of researchers or participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data reported from all 10 women |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol. The outcomes mentioned in the Method section are presented in the Results section of the abstract. No live birth |
| Other bias | Low risk | No evidence of other risk of bias |
Bayram 2004.
| Methods | Parallel randomised controlled trial. Multicentre (n = 25 centres) in The Netherlands Timing: February 1998 to October 2001 |
|
| Participants | 168 women randomised Time of randomisation: during diagnostic laparoscopy, after determining eligibility. Invited to participate: 213 consecutive women. 45 excluded (27 refused, 3 too obese for surgery, 1 had language barrier, 5 became pregnant while awaiting laparoscopy, 9 excluded during diagnostic laparoscopy due to endometriosis (1), adhesions (5), tubal occlusion (2) or infeasibility of electrocautery (1)). Mean age 29 years, mean duration of infertility was 2.8 years and the mean BMI was 27 kg/m2. Infertility was primary in 76% of women Inclusion criteria: women with clomiphene‐resistant PCOS (150 mg clomiphene) with chronic anovulation Exclusion criteria: women with tubal obstruction, other causes of infertility including severe male‐factor infertility, aged > 40 years |
|
| Interventions | Laparoscopic electrocautery of the ovaries strategy: each ovary was punctured 5 to 10 times depending on its size. If the woman ovulated in 6 subsequent cycles, no further treatment was given. If ovulatory cycles were not established 8 weeks after surgery or the woman became anovulatory again then clomiphene citrate was given in increasing doses. If the woman still remained anovulatory, rFSH was given in increasing, doses starting at 75 IU daily (n = 83) versus 6 cycles of rFSH. Women were treated until 6 subsequent cycles were achieved within 6 months (n = 85) | |
| Outcomes | Primary: ongoing pregnancy rate within 12 months, defined as a viable pregnancy of at least 12 weeks
Secondary: live birth, miscarriage, multiple pregnancy, cost‐related quality of life Followed up to 1 year |
|
| Notes | Analyses on an intention‐to‐treat basis
Powered to detect a 10% difference in ongoing pregnancy rate No conflict of interest Funding: Serono Benelux provided financial support for rFSH during the first eight months of the study when this drug was not funded by the health services. FvdV was supported by a grant from the Health Insurance Funds Council (OG 97/007), Amstelveen, Netherlands. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomisation, stratified by centre |
| Allocation concealment (selection bias) | Low risk | Telephone call to central office |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no evidence of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women randomised were analysed in the primary study |
| Selective reporting (reporting bias) | Low risk | The original protocol was supplied by the authors. All the outcomes mentioned in the protocol were presented in the published report |
| Other bias | Low risk | No evidence of other risk of bias |
Darwish 2016.
| Methods | Parallel randomised controlled trial conducted in Womens Health University Hospital, Eygpt Timing: June 2013 to November 2014 |
|
| Participants | 88 women randomised. 80 women analysed. Mean age of women in monopolar group was 25 ± 4.7 years and for the bipolar group was 24.8 ± 4.4 years Inclusion criteria: Clomiphene‐resistant PCOS (Rotterdam 2003) Exclusion criteria: Male‐factor infertility, tubal or peritoneal factor infertility and endometriosis. One or both tubes blocked. Pelvic adhesions |
|
| Interventions | Monopolar LOD: monopolar needle. 4 seconds with 40 W, 4 punctures to each ovary. Energy for each ovary 640 J (n = 45), versus Bipolar LOD: bipolar needle. 4 seconds with 40 W, 4 punctures to each ovary. Energy for each ovary 640 J (n = 43) Follow‐up for 6 months |
|
| Outcomes | Regularity of menstrual cycle, ovulation rate, pregnancy rate | |
| Notes | No conflict of interest No funding Clinical trial registration number: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned" "computerized random table" |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 45 women allocated to monopolar group; 5 cases lost to follow‐up due to difficulty in travelling and follow‐up by own doctor 43 women allocated to bipolar group; 3 cases lost to follow‐up due to difficulty in travelling and follow‐up by own doctor |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol. The outcomes mentioned in the Methods section are presented in the Results section |
| Other bias | Low risk | No evidence of other risk of bias |
El‐Sayed 2017.
| Methods | Parallel arm "randomized clinical study" conducted in Zagazig University Hospital, Egypt Timing: November 2015 to January 2017 |
|
| Participants | 100 women randomised (50 per group), 95 women analysed (48 in group 1 and 47 in group 2). Mean age: Group 1: 27.5 ± 4.25; Group 2: 28.03 ± 4.32 Inclusion criteria: Infertile women with clomiphene citrate‐resistant PCOS (150 mg/day for 5 days), aged between 25 and 35 years, infertility duration of ≤ 3 years, BMI < 30 kg/m2 luteinising hormone ≥ 10 IU/ml or LH/FSH ratio ≥ 2, Free androgen index ≥ 4, normal semen analysis in the husband, normal oral glucose tolerance test Exclusion criteria: Hyper‐androgenic disorders such as late onset congenital adrenal hyperplasia, hyperprolactinaemia, thyroid diseases, Cushing's syndrome, androgen‐secreting tumours |
|
| Interventions | Unilateral laparoscopic ovarian surgery on the right side, using thermal dose adjusted according to ovarian volume (n = 50), versus Bilateral laparoscopic ovarian surgery using thermal dose adjusted to ovarian volume on both sides (n = 50) Follow‐up for 6 months |
|
| Outcomes | Menstrual cycle resumption, ovulation rate, cumulative pregnancy rate | |
| Notes | Further information confirming methods requested from authors 2 August 2017 No conflict of interest Clinical trial registration number: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done using a computer |
| Allocation concealment (selection bias) | Unclear risk | No details in the paper |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details in the paper but unlikely to have occurred |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details in the paper |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Unilateral LOD group: 2 participants excluded; 1 had a tubal disease which was identified during laparoscopy and 1 missed the follow‐up Bilateral LOD group: 3 participants excluded; 1 was excluded due to endometriosis which was diagnosed during laparoscopy, and 2 participants missed follow‐up |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol. The outcomes mentioned in the Methods section are presented in the Results section |
| Other bias | Low risk | No evidence of other risk of bias |
Elgafor 2013.
| Methods | Parallel randomised controlled trial conducted in Zagazig University Hospital infertility clinic, Egypt Timing: not stated |
|
| Participants | 146 women randomised. Mean age of women in LOD group 25.1 ± 2.1 years; mean age for metformin + letrozole group 24.7 ± 1.8 years Inclusion criteria: Women with PCOS (Rotterdam 2003 criteria) and clomiphene resistance (failure to achieve adequate follicular maturation after 3 consecutive induction cycles with clomiphene citrate 150 mg/day for 5 days) Exclusion criteria: Women with other causes of infertility, endocrine disorders, women who had received hormonal treatment or ovulation induction drugs in the previous 3 months |
|
| Interventions | Bilateral LOD: 4 punctures to ovary then the ovary cooled by irrigating with normal saline and 500 ml of this solution was left in the pelvis at the end of the procedure (n = 73), versus Metformin + letrozole: Metformin started from the first day with a dose of 850 mg/day and increased after 1 week up to 1700 mg/day. Letrozole 5 mg was added for 5 days from day 3 of spontaneous or induced bleeding. Metformin was stopped only when pregnancy was documented (n = 73) Follow‐up for 6 months |
|
| Outcomes | Serum LH and FSH, fasting glucose concentration, testosterone concentration, menstrual calender, ovulation, biochemical pregnancy, clinical pregnancy, spontaneous abortion | |
| Notes | No evidence of sample size calculation No conflict of interest Clinical trial registration number: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated random numeric table" |
| Allocation concealment (selection bias) | Low risk | Quote: "The random allocation sequence was concealed in sealed dark envelopes..." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No evidence of blinding. Blinding unlikely as 1 intervention is a surgical procedure, versus oral medication. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details of blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women randomised appear to be analysed |
| Selective reporting (reporting bias) | Unclear risk | We found the registered protocol on ClinicalTrials.gov (NCT01693289), but it was first posted retrospective. All the outcomes mentioned in the protocol were presented in the published report |
| Other bias | Low risk | Baseline data of groups appeared balanced |
Farquhar 2002.
| Methods | Randomised trial conducted in Fertility Plus, National Women's Hospital, New Zealand Timing: mid 1996 to late 1999 |
|
| Participants | 50 women randomised,3 cycles/participant, mean age 30 years, mean BMI 28 kg/m2, mean length of infertility: 36 months in the LOD group and 29 months in the gonadotrophin group Included: women aged 20 to 38 years with clomiphene‐resistant PCOS (150 mg clomiphene for 5 days), BMI < 32 (for European women) and < 34 (for Polynesian women) Excluded: Other known causes of infertility, including male‐factor infertility |
|
| Interventions | Bilateral ovarian drilling by diathermy, versus
3 cycles of gonadotrophins (HMG or rFSH)
Laparoscopic ovarian drilling was performed with a diathermy needle creating 10 punctures/ovary, cooled with normal saline Follow‐up for 6 months |
|
| Outcomes | Pregnancy rate 6 months after drilling or after 3 cycles of gonadotrophins (per participant), live birth, ovulation rate (per participant), costs | |
| Notes | Analyses on an intention‐to‐treat basis.
Powered to detect a 10% difference in ongoing pregnancy rate.
Definitions
PCO: clinical (oligo‐ or amenorrhoea) + ovarian appearance on ultrasound (criteria by Adams 1986)
Refractory PCO: failure to conceive after 3 cycles of ovulation induction with clomiphene citrate (150 mg/day)
Pregnancy: positive HCG and fetal heart on ultrasound
Ovulation: disappearance of a leading follicle or appearance of a corpus luteum on ultrasound OR mid‐luteal phase serum progesterone > 20 mmol/l Conflict of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated sequences.." |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed numbered opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no evidence that researchers or participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details of blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol. All outcomes listed in Methods were reported in the Results |
| Other bias | Low risk | No evidence of other risk of bias |
Fernandez 2015.
| Methods | Parallel randomised controlled trial conducted in France Timing: June 2009 to June 2012 |
|
| Participants | 40 of 252 women randomised, as trial stopped early. Mean age in LOD group was 28 ± 3 years and in the metformin + FSH group was 27 ± 3 years Inclusion criteria: Clomiphene‐resistant, polycystic ovaries Exclusion criteria: Other causes of infertility including tubal factors, male factor, > 36 years of age, thyroid dysfunction |
|
| Interventions | LOD: Bipolar needle, 10 punctures at 100 to 130 W 8 mm depth and 2 mm diameter. (n = 19), versus Recombinant FSH plus metformin: 3 months treatment by metformin (start dose 500 g up to a max 1500 g a day) followed by 3 hyperstimulation by FSH + insemination Follow‐up for 6 months |
|
| Outcomes | Pregnancy, BMI, hormone levels, follicle count, changing strategy during the study follow‐up | |
| Notes | No conflict of interest Clinical trial registration number: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Central randomisation through a website |
| Allocation concealment (selection bias) | Unclear risk | Centralised randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Planned to recruit 126 women but only recruited 40 before trial stopped. 4 women failed to consent and 2 women were lost to follow‐up. Not stated which group they were allocated to. States that all women were analysed regardless of the group they were randomised to |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol. All outcomes prespecified in the paper appear to have been reported |
| Other bias | Unclear risk | Trial stopped early due to "difficulty in the inclusion criteria with absence of final agreement by team included". |
Ghafarnegad 2010.
| Methods | Randomised trial conducted in Iran Timing: not stated |
|
| Participants | 100 infertile, clomiphene‐resistant women with PCOS | |
| Interventions | Gonadotrophin (n = 50), versus Laparoscopic ovarian electrocautery (n = 50) Follow‐up for 4 months |
|
| Outcomes | Pregnancy, live birth | |
| Notes | Conflict of interest: not stated Clinical trial registration number: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomised". Awaiting further details in translation but numbers are equal in both groups so probably satisfactory |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women accounted for at trial end and intention‐to‐treat data reported |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol |
| Other bias | High risk | Only abstract available |
Giampaolino 2016.
| Methods | Parallel randomised controlled trial, conducted in Department of Obstetrics and Gynaecology, University of Naples, Italy Timing: December 2009 to July 2015 |
|
| Participants | 246 women randomised, 201 analysed. Mean age of women in LOD group was 30.1 ± 7.5 years and in the THL group was 27.5 ± 6.8 years Inclusion criteria: Age 18 to 40 years, PCOS (Rotterdam 2003 criteria), clomiphene resistant Exclusion criteria: endocrine anomalies other than PCOS, any disease potentially responsible for ovarian adhesions, previous abdominal or pelvic surgery, presence of adhesions, fixed retroverted uterus, lateral displacement of the cervix, suspected pelvic tumour, vaginal infection, abnormalities at vaginal examination and transvaginal ultrasound, psychiatric disorder preventing ability to participate, obliteration of the Pouch of Douglas or inability to perform vaginal examination or any other contraindication to THL or laparoscopy |
|
| Interventions | Laparoscopic ovarian drilling: Unipolar needle electrode with a power setting of 40 W for 4 to 5 seconds set at 30 W per ovary. 3 ‐ 6 punctures per ovary (n = 123), versus Transvaginal hydrolaparoscopy ovarian drilling: Bipolar electrosurgical probe and 3 ‐ 6 points per ovary drilled at a power setting of 110 ‐ 130 W (n = 123) . At 6 months, all women offered follow‐up with THL and asked to monitor menstrual cycles for next 12 months for spontaneous pregnancy |
|
| Outcomes | Presence and type of adhesions, peri‐ and post‐operative complications, cumulative pregnancy rate, multiple pregnancy rate | |
| Notes | Only overall cumulative pregnancy rate reported in the paper. We contacted the authors 25 October 2016 for additional data on pregnancy rate by group No conflict of interest Clinical trial registration number: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated" |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation sequence was concealed from the researchers' 'sequentially numbered opaque, sealed and stapled envelope" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of surgeons or participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors of participants were blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 246 women were randomised. 19 women in the LOD group refused follow‐up with THL and therefore follow‐up was completed on 104 women. 26 women in the THL group refused follow‐up with THL and therefore follow‐up was completed on 97 women. Unclear if cumulative pregnancy rate is for all 246 women or only for those who had follow‐up with THL |
| Selective reporting (reporting bias) | High risk | We could not retrieve the original protocol. Data for cumulative pregnancy are given as an overall value and not by group. Pregnancy rate and multiple pregnancy rate are not prespecified as outcomes in the Methods |
| Other bias | Low risk | Groups were balanced at baseline |
Gürgan 1992.
| Methods | Randomised trial conducted in Turkey at the University of Hecettepi, Ankara, Turkey. Time of randomisation: after initial laparoscopic ovarian drilling. Timing: not stated | |
| Participants | 40 women randomised, clomiphene‐resistant PCOS patients (see definitions). Mean age (range) of the participants was 25.2 years (21 to 31) and mean duration of infertility was 4.4 years. 33 participants had primary and 7 had secondary infertility. Infertility work‐up consisted of semen analysis (normal in 36 participants and mildly oligo/asthenospermia in 4) and normal HSG. All women were anovulatory There were no clear inclusion or exclusion criteria specified |
|
| Interventions | 2nd look laparoscopic adhesiolysis following ovarian laser drilling, versus Ovarian laser drilling only Ovarian laser drilling consisted of creating 20 to 25 holes/ovary using beam power of 50 W with the Nd:YAG laser followed by pelvic irrigation with Ringer lactate. Laparoscopic adhesiolysis with sharp or blunt dissection was done 3 to 4 weeks later | |
| Outcomes | Pregnancy rate (by participant), ovulation rate (by participant), miscarriage rate (by pregnancy), multiple pregnancy rate (by pregnancy) Follow‐up for 6 months |
|
| Notes | Conflict of interest: not stated Definitions: PCO: clinical (oligomenorrhoea, hirsutism, obesity) + LH/FSH ratio > 2 + elevated testosterone and/or androstenedione (not specified) Clomiphene resistant: failure to ovulate on 200 mg/day for 5 days (duration not stated) Pregnancy: ultrasound (not specified) Ovulation: biphasic BBT + luteal serum progesterone > 3 ng/ml |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | No details in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details in paper but blinding unlikely to have occurred |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 40 women randomised, 1 refused second‐look laparoscopy |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol. A priori outcomes in Methods section of paper were reported in Results section |
| Other bias | Low risk | No evidence of other risk of bias |
Hamed 2010.
| Methods | Randomised trial conducted in Egypt Timing: May 2007 to September 2008 |
|
| Participants | 110 participants. The mean age of the women in the metformin group was 23.6 ± 2.6 years and in the LOD group was 24.3 ± 4.5 years Inclusion: Women with diagnosis of PCOS attending infertility clinic. Clomiphene resistance. Age 20 to 35 years. Patent fallopian tubes shown by hysterosalpingography, insulin resistance, normal semen analysis Exclusion: women < 20 years and > 35 years, received gonadotrophins or hormonal contraception in previous 3 months, having hyperprolactinaemia, or other endocrine, hepatic, or renal disorders, having organic pelvic mass, or previous abdominal surgery suggesting pelvic factor infertility |
|
| Interventions | 850 mg metformin orally twice daily (n = 55), versus LOD using 4 to 8 punctures (n = 55) Follow‐up for 6 cycles/30 weeks |
|
| Outcomes | BMI, ovulation, pregnancy (biochemical, clinical), miscarriage, resuming regular cycles, glucose/insulin ratio | |
| Notes | No conflict of interest Clinical trial registration number: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "..computer generated random numbers tables" Comment: Satisfactory method. |
| Allocation concealment (selection bias) | Low risk | Quote: '..using serially numbered opaque envelopes" Comment: Satisfactory method |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | There were no details in the paper on blinding, but blinding unlikely due to differences in interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were 55 women allocated to each group and there were no losses to follow‐up or discontinuation of medication. All women were analysed |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol. Report on adverse effects of treatment that were not prespecified as outcomes in the Methods section of the paper |
| Other bias | Low risk | No evidence of other risk of bias |
Ibrahim 2017.
| Methods | Randomised controlled trial conducted in Minia University Hospital, El‐Minia, Egypt Timing: August 2015 to March 2016 |
|
| Participants | 80 women randomised and analysed (40 per group); Mean age: Group A: 28.8 ± 3.13; Group B: 29.7 ± 3.65 Inclusion criteria: Between 20 and 35 years of age, diagnosis of PCOS based on the Revised 2003 Consensus Diagnostic Criteria for PCOS (must meet 2 of the 3 following criteria: ultrasound diagnosis of polycystic ovaries, oligo‐ or anovulation clinically diagnosed as oligo‐ or amenorrhoea, and clinical and biochemical hyperandrogenism), normal hysterosalpingogram, partner has normal semen analysis Exclusion criteria: Age < 20 or > 35 years, non‐PCOS, hyperprolactinaemia, hypo‐ and hyperthyroidism, diabetes, Cushing's syndrome, current or previous (within last 6 months) non‐classical congenital adrenal hyperplasia, use of oral contraceptives, glucocorticoids, antiandrogens, antidiabetic or anti‐obesity drugs or any other hormonal drugs, any neoplastic, metabolic, hepatic or cardiovascular disorder or other concurrent medical illness, pelvic diseases, previous pelvic surgery, suspected peritoneal factor infertility, tubal infertility, male‐factor infertility |
|
| Interventions | Laparoscopic ovarian drilling (n = 40), versus Letrozole 2.5 mg orally twice daily for 5 days from the 3rd day of menses, repeated for up to 6 cycles if ovulation failed (n = 40) Follow‐up for 6 months |
|
| Outcomes | Ovulation rate, pregnancy rate | |
| Notes | No conflict of interest No funding Clinical trial registration number: not stated We requested further information on methods from the authors on 03 August 2017 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved by the use of a randomisation number allocated prior to dosing |
| Allocation concealment (selection bias) | Low risk | Randomisation schedule was produced by an interactive voice response system vendor |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Once the participants had been allocated to 1 of the 2 groups, the treatment was revealed to the investigator |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The doctor responsible for performing the transvaginal ultrasound follow‐up assessment was blinded to the treatment groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the Results there was no loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol. Outcomes in the Method section were reported in the Results section |
| Other bias | Low risk | No evidence of other risk of bias |
Jamal 2000.
| Methods | Parallel randomised controlled trial conducted in University Hospital, Jeddah, Saudi Arabia Timing: 1995 to 1998 |
|
| Participants | 35 women randomised Inclusion criteria: not clearly specified but included women with refractory anovulatory infertility with polycystic ovaries with unsuccessful medical treatment Exclusion criteria: no details |
|
| Interventions | Unilateral laparoscopic ovarian drilling of 5 points in each ovary for 5 seconds, versus Bilateral laparoscopic ovarian drilling of 5 points in each ovary for 5 seconds Follow‐up for 3 months |
|
| Outcomes | Ovulaton rate and endocrine changes (no details) | |
| Notes | Conference abstract only Conflict of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | States that 35 women randomised but no details on the numbers in each group, no details for any losses. Conference abstract only |
| Selective reporting (reporting bias) | High risk | Conference abstract only including no data that could be included in an analysis |
| Other bias | High risk | Conference abstract only. Unable to judge if groups were balanced at baseline |
Kaya 2005.
| Methods | Randomised prospective trial conducted in Turkey Timing: January 2000 to January 2004 |
|
| Participants | Clomiphene‐resistant PCOS participants (see definitions). Mean age of LOMNT group was 26.3 ± 4.3 years and for gonadotrophin group 25.6 ± 4.08 years. All women had anovulatory infertility for > 1 year Exclusions: History of abdominopelvic surgery, systemic disease, proven or suspected pelvic inflammatory disease or ectopic pregnancy |
|
| Interventions | Bilateral ovarian drilling by diathermy (n = 17), versus
3 cycles of gonadotrophins (step up protocol) plus IUI (n = 18) Laparoscopic ovarian drilling was performed with a specially‐designed instrument which was then applied across the ovary and then squeezed Follow‐up for 6 months |
|
| Outcomes | Pregnancy rate by participant, multiple pregnancy rate and ovarian hyperstimulation rate, costs by treatment 8/17 who underwent ovarian drilling had second‐look laparoscopy for adhesion formation All women followed up for 6 months |
|
| Notes | Conflict of interest: not stated Definitions: PCO: clinical (oligomenorrhoea, hirsutism, obesity) + LH/FSH ratio > 2 + elevated testosterone or androstenedione or both (not specified) Clomiphene‐resistant: failure to ovulate on 200 mg/day for 5 days (duration not stated) Pregnancy: ultrasound (not specified) Ovulation: biphasic BBT + luteal serum progesterone > 3 ng/ml |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated random sequence" |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details of blinding, which is unlikely to have occurred |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 woman in the LOD group and 2 women in the gonadotrophin group were lost to follow‐up, but their data were included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol. A priori outcomes stated in the Methods section of the paper were reported in the Results section |
| Other bias | Low risk | No evidence of other risk of bias |
Lazoviz 1998.
| Methods | Randomised trial, cross‐over design, data available prior to cross‐over. Study conducted in Institute for Obstetrics and Gynaecology, University of Belgrade, Belgrade, Yugoslavia Timing: not stated. |
|
| Participants | 56 participants randomised, 6 cycles/patient. Clomiphene‐resistant PCOS participants (high LH). Mean age, duration of infertility, infertility work‐up, mean BMI not stated | |
| Interventions | Ovarian drilling with diathermy or laser vaporisation with CO2 (n = 28), versus Gonadotrophins (FSH or hMG) for ovulation induction for 6 cycles. Number of drill holes per ovary is not stated. (n = 28) Follow‐up for 6 months |
|
| Outcomes | Pregnancy rate (by participant), miscarriage rate (by pregnancy), multiple pregnancy rate (by pregnancy) | |
| Notes | Conflict of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details in paper |
| Allocation concealment (selection bias) | Unclear risk | No details in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details of blinding, but unlikely to have occurred. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants appear to be included in the analysis |
| Selective reporting (reporting bias) | High risk | This is a conference abstract only. No full paper was identified |
| Other bias | High risk | Conference abstract |
Liu 2015.
| Methods | Parallel randomised controlled trial conducted in Centre for Reproductive Medicine, Tongji University, China Timing: Not stated |
|
| Participants | 141 women randomised. Mean age of women in the LOD group 28.1 ± 3.6 years and in the letrozole group was 29.5 ± 3.3 years Inclusion criteria: Diagnosed with PCOS (Revised 2003 Consensus Diagnostic Criteria for PCOS); clomiphene resistance, patent fallopian tubes, normal semen analysis for partner, normal serum prolactin, TSH and 17‐OH progesterone; no systemic disease; no gonadotropin or other hormonal drug treatment during preceding 3 months, normal blood count and blood chemistry; normal glucose and urinalysis Exclusion criteria: Infertility for other reasons than PCOS; uterine cavity lesions or ovarian cyst; > 40 years of age; BMI > 26 kg/m2; contraindications to general anaesthesia; history of pelvic surgery; other endocrine diseases; or a history of liver or renal disease |
|
| Interventions | LOD: Both ovaries cauterised at 4 to 6 points, each for 4 seconds at 40 W at a depth of 7 to 8 mm and a diameter of 3 to 5 mm using a monopolar electrosurgical needle (n = 70), versus Letrozole 2.5 mg orally administered on the 5th day of menses and then every day for 5 days. Treatment was repeated for up to 6 cycles (n = 71). Follow‐up for 6 months. Natural intercourse advised |
|
| Outcomes | Ovulation, biochemical pregnancy, clinical pregnancy | |
| Notes | Conflict of interest: not stated Clinical trial registration number: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allocated" Comment: no other details. |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Once the allocation had been made the intervention was revealed to the investigator |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The doctor responsible for performing the transvaginal ultrasound follow‐up assessment was blinded to the treatment groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 141 women randomised and 141 women analysed |
| Selective reporting (reporting bias) | High risk | Live birth and spontaneous abortion were reported as outcomes, but not prespecified in the Methods |
| Other bias | Low risk | Groups balanced at baseline. No other bias identified |
Malkawi 2003.
| Methods | Randomised controlled trial conducted in King Hussein Medical Centre, Amman, Jordan Timing: January 2000 to December 2001 |
|
| Participants | 161 women were randomised, 64 assigned to receive metformin and 97 to undergo LOD. Mean age: Metformin group = 27.4 ± 3.0; LOD group = 27.1 ± 4.4 Inclusion criteria: Clomiphene citrate‐resistant PCOS, normal uterine cavity and tubal patency on hysterosalpingography, normal semen parameters in male partner Exclusion criteria: Congenital adrenal hyperplasia, Cushing's syndrome, hyperprolactinaemia and thyroid disease |
|
| Interventions | Metformin 850 mg twice daily throughout the cycle (n = 64), versus LOD (n = 97) Follow‐up: not stated |
|
| Outcomes | Ovulation rate, pregnancy rate, multiple pregnancies, miscarriage rate, ectopic pregnancy rate, OHSS, hormonal profile | |
| Notes | Conflict of interest: not stated Clinical trial registration number: not stated We contacted the authors in August 2017 to provide confirmation of randomisation and allocation concealment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was by random‐number table |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details of blinding, but unlikely due to nature of intervention and comparison |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details of blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | High risk | We could not retrieve the original protocol. Ovulation rate and pregnancy rate were prespecified in the study report, but ovarian hyperstimulation, menstrual cycle regularity, and hormone profile were not prespecified outcomes |
| Other bias | Low risk | Appears free of other bias |
Mamonov 2000.
| Methods | Prospective randomised trial conducted in the Ukraine Timing: not stated |
|
| Participants | 128 women with clomiphene‐resistant PCOS. 84% were obese | |
| Interventions | Metrodin High Purity for up to 6 cycles (n = 62), versus Laparoscopic electrocoagulation of the ovarian surface (n = 66). Follow‐up for 1½ years |
|
| Outcomes | Pregnancy, miscarriage | |
| Notes | Conflict of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "..were randomized.." Comment: no other details in abstract |
| Allocation concealment (selection bias) | Unclear risk | No details in abstract |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No evidence of blinding of researchers or participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear details |
| Selective reporting (reporting bias) | High risk | No outcomes were listed in the Methods section. Study only available in abstract form |
| Other bias | High risk | Conference abstract only |
Mehrabian 2012.
| Methods | Parallel randomised controlled trial conducted in Obstetrics and Gynaecology clinic, Isfahan, Iran Timing: not stated |
|
| Participants | 104 women randomised. Mean age of women in LOD group was 29.2 ± 5.5 years and in gonadotropin group was 28.5 ± 5.5 years Inclusion criteria: Nuliparous, aged < 40 years, clomiphene‐resistant, PCOS Exclusion criteria: Male‐factor or tubal‐factor infertility |
|
| Interventions | LOD: 10 to 15 punctures per ovary depending on size (n = 52), versus Gonadotropin: HMG given after the bleeding withdrawal and from day 3 of the cycle with 10 mg medroxyprogesterone (n = 52) Follow‐up: not stated |
|
| Outcomes | Pregnancy, miscarriage, ectopic pregnancy, OHSS, multiple pregnancy | |
| Notes | Conflict of interest: not stated Clinical trial registration number: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer ‐generated random numbers" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details, but unlikely due to different interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women randomised were analysed |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol. Prespecified outcomes appear to be reported |
| Other bias | Low risk | Groups balanced at baseline |
Nasr 2013.
| Methods | Parallel randomised controlled trial in university‐affiliated tertiary centre, Egypt Timing: Not stated |
|
| Participants | 80 women randomised Mean age of unilateral drilling group was 28.4 ± 2.2 years and in bilateral group was 29.2 ± 1.9 years Inclusion criteria: Clomiphene‐resistant PCOS Exclusion criteria: No details |
|
| Interventions | Unilateral drilling (n = 40), versus Bilateral drilling (n = 40) 40 normally‐ovulating women were included as controls but not included in this review and were not randomised Follow‐up for 6 months |
|
| Outcomes | Serum anti‐Mullerian hormone at 6 months follow‐up | |
| Notes | Conflict of interest: not stated Clinical trial registration number: not stated Conference abstract only |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized" Comment: no other details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 80 women randomised but not clear if all women were analysed at 6 months |
| Selective reporting (reporting bias) | High risk | Conference abstract that only reported on anti‐Mullerian hormone, which was not a prespecified outcome for this review |
| Other bias | High risk | States that groups were balanced at baseline but conference abstract only. No tables or P values identified |
Nasr 2015.
| Methods | Parallel randomised controlled trial conducted in Women's Health Centre, Assiut University. Egypt Timing: Not stated |
|
| Participants | 80 women randomised. Mean age of women in adjusted group was 27.7 ± 2.1 years and in the fixed group was 28.5 ± 1.9 years Inclusion criteria: Clomiphene‐resistant PCOS Exclusion criteria: No details |
|
| Interventions | Adjusted thermal dose based on ovarian volume (n = 40), versus Fixed thermal dose 600 J per ovary through 4 punctures regardless of size (n = 40) A third group of normally‐ovulating women acted as controls but are not included in these analyses Follow‐up for 6 months |
|
| Outcomes | AMH levels, ovulation, conception (no details), early abortion rates | |
| Notes | Conflict of interest: not stated Clinical trial registration number: not stated Conference abstract |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details are provided of method used to generate the random sequence |
| Allocation concealment (selection bias) | Unclear risk | No details are provided of the method used to conceal allocation to treatment groups |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided of blinding of participants or trial personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided of blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No details provided of levels of attrition |
| Selective reporting (reporting bias) | High risk | Only available as a conference abstract, no full publication available |
| Other bias | High risk | Conference abstract only |
Palomba 2004.
| Methods | Randomised double‐blind study conducted in Italy Timing: October 2001 to December 2002 |
|
| Participants | 120 women; mean age of metformin group was 26.8 ± 2.2 and in LOD group 27.5 ± 2.4 years Inclusion: Overweight (BMI 25 ‐ 30 kg/m2) women with PCOS, clomiphene‐resistant Exclusion: Age < 22 or > 34 years; hypothyroidism, hyperprolactinaemia, Cushings syndrome, nonclassical congenital adrenal hyperplasia, and current or previous (within 6 months) use of oral contraceptives, glucocorticoids, antiandrogens, ovulation induction agents, antidiabetic or anti‐obesity drugs, or other hormonal drugs; neoplasms, metabolic, hepatic, or cardiovascular disorder or other concurrent medical illness; women who were intending to start a diet or a specific programme of physical activity; having organic pelvic disease, previous pelvic surgery, suspected peritoneal factor infertility , and tubal or male infertility |
|
| Interventions | Diagnostic laparoscopy followed by metformin cloridrate 850 mg twice daily. If anovulatory at 6 months clomiphene citrate 150 mg daily from Day 3 ‐ 7 (n = 60), versus LOD (3 to 6 punctures in each ovary depending on size of ovary) followed by multivitamins twice daily. If anovulatory at 6 months clomiphene citrate 150 mg daily from day 3 ‐7 (n = 60) Follow‐up for 6 months |
|
| Outcomes | Live birth, adverse events, menstrual cycle characteristics, ovulation rate, pregnancy, miscarriage, costs | |
| Notes | Conflict of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation was carried out using online software to generate a random allocation sequence in double block as method of restriction" |
| Allocation concealment (selection bias) | Unclear risk | Quote: 'The random allocation sequence was concealed until the interventions were assigned" Comment: there were no further details in the paper |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 women in metformin group and 5 in the LOD group. Reasons given were evidence of minimal endometriosis by laparoscopy (4 in Group A and 2 from Group B) and non‐compliance (1 from each group). 1 woman from Group A and 2 from group B were excluded for weight loss observed in the first 3 months of the study |
| Selective reporting (reporting bias) | Unclear risk | The original protocol could not be retrieved. All outcomes cited in the Methods section were reported |
| Other bias | Low risk | No evidence of other risk of bias |
Palomba 2010.
| Methods | Randomised trial conducted in Italy Timing: February 2003 to May 2004 |
|
| Participants | 50 participants Inclusion: Anovulatory, clomiphene‐resistant, with PCOS, seeking pregnancy Exclusion: < 18 or > 35 years, BMI > 35 kg/m2, neoplastic, metabolic, endocrine, hepatic, renal , and cardiovascular disorders, or other concurrent medical illnesses; and current or previous use of any drug that affected hormone levels, metabolism or appetite. Organic or pelvic diseases, previous pelvic surgery, suspected peritoneal factor infertility/ subfertility, and tubal or male‐factor infertility or subfertility that was excluded by hysterosalpingogram and semen analysis. Wanting to start a diet or a specific programme of physical activity, cigarette smokers or alcoholic beverage abusers |
|
| Interventions | LOD followed by 6 cycles of observation (n = 25), versus Clomiphene citrate (incremental dose) plus metformin (850 mg increasing to 1700g daily) for 6 cycles (n = 25) Follow‐up for 6 months |
|
| Outcomes | Live birth, pregnancy rates, multiple pregnancy, miscarriage, ovulation rate, adverse events, compliance, cost | |
| Notes | Conflict of interest: not stated Clinical trial registration number: NCT00558077 No funding |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "achieved using online software (www.randomization.it)" |
| Allocation concealment (selection bias) | Low risk | Concealed in sealed dark envelopes until the interventions were assigned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details but blinding unlikely due to differences in the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 women (1 in the LOD group and 2 in the CC + metformin group) were lost to follow‐up because they missed a follow‐up visit |
| Selective reporting (reporting bias) | Low risk | We found the registered protocol on ClinicalTrials.gov (NCT00558077). All the outcomes mentioned in the protocol were presented in the published report |
| Other bias | Low risk | No evidence of other risk of bias |
Rezk 2016.
| Methods | Parallel randomised controlled trial. Single centre, Department of Obstetrics and Gynaecology, Menoufia University Hospital, Egypt Timing: October 2014 to July 2015 |
|
| Participants | 108 women randomised. Mean age of women in unilateral ovarian drilling group was 29.7 ± 1.5 years and in bilateral ovarian drilling group was 29.8 ± 1.4 years Inclusion criteria: Clomiphene‐resistant PCOS (revised Rotterdam criteria); normal semen analysis for partner, normal uterine cavity, bilateral tubal patency Exclusion criteria: FSH > 15 IU/ml, medical disorders such as diabetes and hypertension, contraindications for laparoscopy, endocrine disorders, hyperprolactinaemia, thyroid disorder, Cushing syndrome, acromegaly, pelvic organ disease, abnormal semen analysis from partner |
|
| Interventions | Unilateral ovarian drilling of the larger ovary. Number of punctures was calculated as Np = 60 J/cm3/ 30 W x 4 seconds (n = 52), versus Bilateral ovarian drilling: 5 punctures per ovary at 30 W for 4 seconds. Each ovary received 600 J (n = 53) Follow‐up for 6 months |
|
| Outcomes | Ovulation rate, clinical pregnancy, ovarian reserve measures. | |
| Notes | Clinical trial registration number: PACTR201405000757313 No conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned into two groups" "computer generated simple random tables" |
| Allocation concealment (selection bias) | Unclear risk | No details provided . |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided . |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided . |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 108 women randomised.105 women analysed (2 lost in Unilateral group and 1 lost in Bilateral group ‐ reasons were loss to follow‐up) |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | Groups balanced at baseline. No other bias identified |
Rimington 1997.
| Methods | Randomised prospective study conducted in a Fertility clinic in Wales, UK Timing: not stated |
|
| Participants | 50 women, mean age in IVF group was 31 (95% CI 29.8 to 32.2) and for LOE + IVF the mean age was 31.8 (95% CI 30.3 to 33.2) Inclusion: Diagnosis of PCOS, requiring IVF for reasons other than anovulation, at least 1 previous unsuccessful ovarian stimulation cycle with gonadotrophins Exclusion: Aged > 40 years, history of > 2 miscarriages, severe male‐factor infertility |
|
| Interventions | IVF (n = 25), versus Ovarian electrocautery and IVF (grid of holes 10 mm apart) ovarian stimulation started 1 week after LOE (n = 25). Follow‐up for 1 cycle |
|
| Outcomes | Number of abandoned cycles, OHSS, pregnancy, miscarriage | |
| Notes | Conflict of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Blocked method of randomisation.." |
| Allocation concealment (selection bias) | Unclear risk | No details in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no evidence of blinding of researchers or participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women randomised appear to be analysed |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol. All outcomes listed in the Methods section were reported in the Results |
| Other bias | Low risk | No evidence of other risk of bias |
Roy 2009.
| Methods | Prospective randomised trial conducted in India Timing: June 2005 to June 2007 |
|
| Participants | 44 women with PCOS, normal hysterosalpingography, normal semen parameters in partners; women were also clomiphene‐resistant. Mean age of women in unilateral group was 28.2 ± 12.7 and in the bilateral group was 28.8 ± 2.9 years Exclusion: Other causes of infertility like hypothalamic amenorrhoea, Cushing syndrome, premature ovarian failure, congenital adrenal hyperplasia, androgenic ovarian tumours, endometrial tuberculosis, abnormal TSH and prolactin; had already received other regimens of ovulation induction; tubal obstruction, extensive adhesions of the ovaries or fallopian tubes and endometriosis |
|
| Interventions | Unilateral laparoscopic drilling (n = 22), versus Bilateral laparoscopic drilling (n = 22) 5 drills performed per ovary. If there was no ovulation evident within 3 months, the women were started on clomiphene citrate 50 mg daily for 5 days increasing up to a maximum of 150 mg daily for 5 days for a maximum of 6 cycles Follow‐up for 1 year |
|
| Outcomes | Clinical and biochemical response, ovulation rate and pregnancy rate | |
| Notes | No conflict of interest Clinical trial registration number: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "..randomly allocated.." Comment: No other details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No evidence of blinding of researchers or participant |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women randomised appear to have been analysed |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol. The outcomes listed in the Methods section were reported in the Results |
| Other bias | Low risk | No evidence of other risk of bias |
Roy 2010.
| Methods | Prospective randomised trial conducted in India Timing: January 2006 to January 2009 |
|
| Participants | Women from a gynaecological clinic. Mean age of rosiglitazone group was 27.32 ± 4.25 and for LOD group was 28.42 ± 3.65 years Inclusion: Age between 20 and 40 years, having primary infertility with clomiphene‐resistant PCOS, documented patent tubes on hysterosalpingography and no other infertility factor, normal semen parameters in partner Exclusion: Other PCOS‐like syndromes such as Cushings syndrome, congenital adrenal hyperplasia, androgen producing tumours, hyperprolactinaemia and hypothyroidism |
|
| Interventions | All participants had laparoscopy Unilateral LOD using 5 punctures + multivitamins twice daily + CC (n = 25), versus Rosiglitazone 4 mg twice daily + CC (n = 25). Treatment continued for 6 months after laparoscopy |
|
| Outcomes | Ovulation, pregnancy, number of follicles, serum E2, endocrine parameters | |
| Notes | No conflict of interest Clinical trial registration number: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using online software to generate a random number table" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "opening sealed envelopes containing numbers from the computer generated random table" Comment: Method looks okay but unclear if envelopes were opaque and if they were opened sequentially |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor was blinded to allocation group |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5 women were lost to follow‐up, an additional 2 women refused to participate before randomisation and therefore 43 were analysed. The reasons for loss to follow‐up are not described |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol. The outcomes listed in the Methods section were reported in the Results |
| Other bias | Low risk | No evidence of other risk of bias |
Sharma 2006.
| Methods | Randomised prospective pilot study, conducted in India Timing: not stated |
|
| Participants | 20 women with clomiphene‐resistant PCOS, patent tubes on hysterosalpingography and normal partner semen. Average age of unipolar group was 27.3 (range 21 to 32), and for the bipolar group was 25.5 (range 23 to 30) years No exclusion criteria detailed. |
|
| Interventions | Unipolar (n = 10), versus Bipolar ovarian drilling (n = 10) The average number of punctures across both groups was 14.85 per ovary Follow‐up for 3 months and if no evidence of ovulation then started on clomiphene citrate |
|
| Outcomes | Ovulation and pregnancy rate, androgen and biochemical measurements | |
| Notes | Conflict of interest: not stated Clinical trial registration number: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned by using computerized random table" |
| Allocation concealment (selection bias) | Unclear risk | No details in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No evidence of blinding of researchers or participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Although not stated it appears as though all women randomised were analysed |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol. The outcomes listed in the Methods section were reported in the Results |
| Other bias | Low risk | No evidence of other risk of bias |
Sorouri 2015.
| Methods | Parallel randomised controlled trial. Single centre in Fertility clinic, Al‐Zahara Hospital, Iran Timing: June 2011 to July 2012. |
|
| Participants | 100 women randomised. Mean age of women in the unilateral group was 27.6 ± 4.3 years and in bilateral group was 28.0 ± 4.3 years Inclusion criteria: Women with PCOS (Rotterdam 2003 criteria) and clomiphene resistance Exclusion criteria: Tubal disease, peritoneal adhesions to tubes or ovaries, endometriosis, endocrine abnormality, concomitant male infertility. |
|
| Interventions | Unilateral ovarian drilling (right ovary) (n = 50), versus Bilateral ovarian drilling (n = 50) Unipolar diathermy needle, 8 mm, 60 W and 5 points per ovary Follow‐up for 6 months |
|
| Outcomes | Menstrual calender, serum LH and FSH, ovulation, clinical pregnancy | |
| Notes | No conflict of interest Funding from Guilan University of Medical Sciences Clinical trial registration number: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Blocked sample randomisation, no other details |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Surgeons were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 45 women in each group were analysed. In the unilateral group 2 women were excluded with tubal disease found during laparoscopy and 3 missed follow‐up visit. In the bilateral group, 1 woman was excluded because of endometriosis found during laparoscopy and 4 were excluded as they missed follow‐up visits |
| Selective reporting (reporting bias) | Low risk | We found the registered protocol on irct.ir (IRCT138903291306N2). All the outcomes mentioned in the protocol were presented in the published report |
| Other bias | Low risk | Groups balanced at baseline |
Vegetti 1998.
| Methods | Randomised trial, no method stated. Conducted at First Department of Obstetrics and Gynaecology, University of Milan and Gynaecology Unit, University of Pavia, Varese, Italy Timing: May 1996 to April 1997 | |
| Participants | 29 participants randomised, 6 cycles/participant. Clomiphene‐resistant PCO women (high LH). Mean age not stated Duration of infertility 2 to 6.5 years, Infertility work‐up not stated, mean BMI not stated | |
| Interventions | Ovarian drilling with diathermy (at least 20 drill holes per ovary), (N = 16) versus
Gonadotrophins (pure FSH with low‐dose step‐up protocol) (N = 13) for ovulation induction for 6 cycles Follow‐up for 6 months |
|
| Outcomes | Pregnancy rate (per participant), miscarriage rate (per pregnancy), multiple pregnancy rate (per pregnancy) | |
| Notes | Interim results only ‐ further patients will be randomised and a later publication is expected Conflict of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or study personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol |
| Other bias | Unclear risk | Interim details only |
Yadav 2018.
| Methods | Randomized controlled prospective trial conducted in India Timing: January 2012 to May 2015 |
|
| Participants | 109 women randomised. The mean age of women was 26.23 ± 2.9 years in gonadotropin group and 26.11 ± 2.7 years in ovarian drilling group Inclusion criteria: chronic anovulation, polycystic ovaries diagnosed by transvaginal ultrasonography, clomiphene citrate‐resistant, shown by anovulation after taking 150 mg clomiphene citrate daily for 5 days for at least 3 cycles. Aged between 21 and 35 years. Exclusion criteria: severe male‐factor subfertility, other causes of infertility like tubal obstruction and extensive adhesion (endometriosis) stages III and IV according to the classification of the American Fertility Society Secondary exclusion criteria identified during diagnostic laparoscopy: tubal obstruction, extensive adhesion of the ovaries or fallopian tubes, and endometriosis stage III or IV |
|
| Interventions | Gonadotrophins (N = 44), versus LOD with CC or gonadotrophins (N = 45) (4 to 5 puncture sites, 40 W, monopolar needle) Follow‐up for 6 months |
|
| Outcomes | Ovulation rate, pregnancy rate, live birth, abortion, ectopic, multiple pregnancies | |
| Notes | No conflict of interest No funding Clinical trial registration number: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly allocated2 Comment: no other details in the paper |
| Allocation concealment (selection bias) | Unclear risk | No details in the paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no evidence that participants or researchers were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details in the paper |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Out of 109 women, 8 were excluded after diagnostic laparoscopy because of the presence of endometriosis and adhesions. 12 women did not complete the study protocol |
| Selective reporting (reporting bias) | High risk | We could not retrieve the original protocol. The primary outcome in the Method section was ongoing pregnancy within 12 months. The primary outcome in the Result section was a positive urine pregnancy test after 3 and 6 cycles |
| Other bias | Unclear risk | Women with LOD received CC or gonadotrophins |
Youssef 2007.
| Methods | Randomised trial conducted in Egypt Timing: January 2003 to December 20 |
|
| Participants | 87 women with PCOS. Mean age of unilateral group was 31.1 ± 4.2, and for the bilateral group was 29.8 ± 3.7 years Inclusion: infertility secondary to anovulation, unsuccessful treatment with clomiphene citrate and gonadotrophins |
|
| Interventions | Weight reduction and insulin sensitising drugs were tried first for 3 months Clomiphene citrate 50 mg daily for 5 days from day 3 to 7. If no response then increased up to 150 mg daily for 5 days. If still no response HMG used to stimulate ovulation Unilateral LOD: If both ovaries equal size the right one was drilled, if of unequal size then the larger one was treated (n = 43), versus Bilateral LOD (n = 44). Ovaries were cauterised at 4 points Follow‐up for 1 year |
|
| Outcomes | Postoperative pain, postoperative nausea, ovulation, pregnancy, miscarriage | |
| Notes | Conflict of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided in paper |
| Allocation concealment (selection bias) | Low risk | Quote: "randomly allocated by an independent investigator blinded to the treatment group...using the closed envelope method" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women appear to have been followed up and analysed |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol. All outcomes listed in the Methods section were reported in the Results |
| Other bias | Low risk | No evidence of other risk of bias |
Zakherah 2010.
| Methods | Randomised trial conducted in Egypt Timing: January 2007 to February 2009 |
|
| Participants | 150 women with clomiphene‐resistant PCOS attending an infertility clinic. Mean age for CC + tamoxifen group 25.6 ± 3.5 years, LOD group 25.6 ± 4.1 years Inclusion: Age between 18 and 38 years, at least 2 years of primary or secondary infertility due to anovulation, patent fallopian tubes on hysterosalpingography or diagnostic laparoscopy, no hormonal treatment in previous 3 months and normal semen values |
|
| Interventions | CC (150 mg) + tamoxifen (40 mg) from day 3 to day 7 for a maximum of 6 consecutive cycles (n = 75), versus LOD performed through triple‐puncture laparoscopy (4 to 6 puncture points were made through the ovarian capsule of each ovary) (n = 75) Follow‐up for 6 months |
|
| Outcomes | Pregnancy (biochemical, clinical, live birth), miscarriage, endometrial thickness, ovulation rate (follicles ≥ 18 mm) | |
| Notes | No conflicts of interest Clinical trial registration number: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a computer generated random number table.." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "sealed envelopes" Comment: Not clear if opaque and serially numbered |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No details provided but unlikely that there was blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss to follow‐up and all 150 women were analysed |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol. All a priori outcomes in paper were reported |
| Other bias | Low risk | No evidence of other risk of bias |
Zakherah 2011.
| Methods | Parallel randomised controlled trial conducted in Women's Health Centre and Physiology Department, Assiut University, Egypt Timing: January 2007 to December 2009 |
|
| Participants | 120 women randomised. Mean age of women in adjusted thermal dose group was 25.7 ± 5.9 years and in the fixed‐dose group was 25.4 ± 5.7 years Inclusion criteria: PCOS (Rotterdam 2003); aged 18 to 38 years, clomiphene‐resistant, anovulatory infertility for 2 years or more, confirmed patent tubes, normal semen analysis from male partner Exclusion criteria: Endocrine abnormalities or pelvic pathology |
|
| Interventions | Adjusted thermal dose thermal dose based on ovarian volume (4 to 9 holes delivering a thermal dose of 480 to 1080 J per ovary) (n = 60), versus Fixed 4‐puncture thermal dose 600 J per ovary regardless of size (n = 60) Monopolar diathermy set at 30 W x 5 secs x 4 punctures Follow‐up for 6 months. If no pregnancy after 6 months then evaluated using second‐look laparoscopy for presence of adhesions |
|
| Outcomes | Ovulation rate, menstrual cycle regularity, pregnancy. Serum AMH, FSH, AFC and ovarian volume | |
| Notes | Conflict of interest: not stated Clinical trial registration number: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "assigned randomly" "computer generated random number table" |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 60 women allocated to each group. Adjusted thermal dose group lost 2 women to follow‐up (no reasons provided) analysed 58 women. The fixed‐dose group lost 3 women to follow‐up (no reasons provided), analysed 57 women |
| Selective reporting (reporting bias) | Unclear risk | We could not retrieve the original protocol. Miscarriage rate reported but not prespecified in Methods |
| Other bias | Low risk | Groups balanced at baseline |
AMH: anti‐Müllerian hormone; AFC: antral follicle count; BBT: basal body temperature; BMI: body mass index; CC: clomiphene citrate; FSH: follicle stimulating hormone; hMG: human menopausal (urinary) gonadotrophins; IUI: intra uterine insemination; J: joules; LH: luteinizing hormone; LOD: laparoscopic ovarian drilling; LOE: laparoscopic ovarian electrocautery; OCP: oral contraceptive pill; OHSS: ovarian hyperstimulation syndrome; PCOS: polycystic ovary syndrome; rFSH: recombinant follicle stimulating hormone; THL: transvaginal hydrolaparoscopy; TSH: thyroid stimulating hormone
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdel Gadir 1990 | Serial randomisation |
| Abu Hashim 2011b | Participants had CC failure (defined as failure to achieve pregnancy despite successful CC‐induced ovulation for 6 cycles) as opposed to CC resistance |
| Al‐Mizyen 2007 | Randomisation was by cards numbered 1 to 20; even numbers allocated to one group and odd numbers to another group |
| Badawy 2009 | Trial compared methods of drilling only |
| Foroozanfard 2010 | Compared 5 to 10 punctures in each ovary |
| Franz 2016 | Ineligible intervention: transabdominal versus transvaginal laparoscopic ovarian drilling |
| Gadir 1992 | Serial method of randomisation |
| Greenblatt 1993 | RCT comparing drilling by diathermy + Interceed to 1 ovary versus drilling only to the other ovary 1. Unit of randomisation: ovaries, not participants 2. Only outcome is adhesion formation at second‐look laparoscopy |
| Gürgan 1991 | Use of concurrent controls |
| Heylen 1994 | Use of concurrent controls |
| Kamel 2004 | Compared re‐electrocautery with FSH |
| Kandil 2018 | Compares transvaginal ovarian needle drilling with LOD |
| Keckstein 1990 | Non‐randomised controlled trial comparing Nd:YAG laser drilling versus CO2 laser drilling Different duration of follow‐up between the 2 groups (8 versus 18 to 30 months) |
| Kocak 2006 | Ineligible comparisons. LOD was compared with LOD + metformin |
| Lockwood 1995 | Conference abstract only; lack of usable data; we were not able to obtain data after multiple attempts to contact the authors. |
| Malkawi 2005 | Not an RCT |
| Muenstermann 2000 | Randomisation used an 'alternate' allocation method |
| Nasr 2010 | Both groups underwent LOD |
| Rath 2006 | Quasi‐RCT |
| Roy 2018 | Ineligible intervention: LOD by harmonic scalpel versus monopolar drilling needle |
| Salah 2013 | Ineligible intervention: RCT comparing LOD under local anaesthetic versus general anaesthetic |
| Saravelos 1996 | RCT comparing LOD + interceed to 1 ovary versus drilling only to the other ovary Outcome is adhesion formation at second‐look laparoscopy |
| Seyam 2018 | Not an RCT; prospective controlled study |
| Sunj 2013 | Not an RCT; quasi‐random allocation |
| Tabrizi 2005 | RCT comparing 5 versus 10 versus 15 points electrocautery of the ovary |
| Vrbikova 1998 | No interventions of interest |
| Wang 2015 | Excluded due to article being retracted |
| Zeng 2012 | Ineligible intervention: trial comparing needle puncture drainage with unipolar electrocoagulation drilling |
| Zhu 2010 | This trial compared different numbers of coagulation points |
CC: clomiphene citrate; FSH: follicle stimulating hormone; LOD: laparoscopic ovarian drilling; RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Abu Hashim 2010a.
| Methods | Prospective randomised trial conducted in Egypt |
| Participants | 260 women attending fertility clinics. Mean age of women in letrozole group was 27.3 ± 2.6 years and in the LOD group was 26.4 ± 2.4 years Inclusion: Clomiphene‐resistant PCOS, patent fallopian tubes assessed by hysterosalpingography, normal semen analysis from partner, normal serum prolactin, thyroid stimulating hormone and 17‐hydroyprogesterone Exclusion: Other causes of infertility, age > 40 years, BMI > 35, contraindications to anaesthesia, previous history of LOD, and having received metformin, gonadotrophin, other hormonal drugs or OCP in preceding 6 months. Women intending to start a diet or a specific programme of physical activity were also excluded |
| Interventions | Letrozole 2.5 mg orally daily from day 3 of the menses for 5 days for 6 cycles (n = 128), versus LOD ‐ each ovary was cauterised at 4 points and women were followed up for 6 months (n = 132) |
| Outcomes | Biochemical pregnancy, clinical pregnancy, ovulation, miscarriage, live birth rates, endometrial thickness |
| Notes |
Abu Hashim 2011a.
| Methods | Randomised prospective trial conducted in Egypt |
| Participants | 282 women attending fertility clinics in Egypt. Mean age of women in the metformin group was 27.2 ± 2.5 years and in the LOD group was 26.5 ± 2.3 years Inclusion: Clomiphene‐resistant PCOS, patent fallopian tubes assessed by hysterosalpingography, normal semen analysis from partner, normal serum prolactin, thyroid stimulating hormone and 17‐hydroyprogesterone Exclusion: Other causes of infertility, age > 40 years, contraindications to anaesthesia and having received metformin, gonadotrophin or OCP in preceding 6 months |
| Interventions | Metformin 500 mg 3 times a day for 6 to 8 weeks, followed by 100 mg of clomiphene citrate for 5 days starting on day 3 of spontaneous or induced menstruation. Dosage increased by 50 mg at next cycle if still anovulatory; treated for 6 cycles (n = 138), versus LOD: each ovary was cauterised at 4 points and women were followed up for 6 months (n = 144). |
| Outcomes | Pregnancy, miscarriage, ovulation rate, endometrial thickness |
| Notes | Author contacted in September 2011 for details on pregnancy rates by woman rather than by cycle |
Characteristics of ongoing studies [ordered by study ID]
IRCT138903291306N2.
| Trial name or title | Comparison of ovulation rate after laparoscopic electrocautery in infertile women with clomiphene‐resistant PCOS |
| Methods | Randomised, double‐blinded, parallel‐assignment |
| Participants | Inclusion criteria: Age: 20 ‐ 38, BMI < 32, infertile women with polycystic ovarian syndrome and infertility duration > 1 year caused by disorder of ovulation, normal semen analysis in partner, normal hysterosalpingography, resistance to CC, absence of any major disease Exclusion criteria: Other cause of infertility, use of other indicated ovulation diets like metformin, any abnormalities of fallopian tubes and endometriosis during laparoscopy Age minimum 20 years, maximum 38 years |
| Interventions | Laparoscopic bilateral ovarian cauterisation, versus Laparoscopic unilateral ovarian cauterisation |
| Outcomes | Primary outcome: ovulation rate Secondary outcomes: CC dose for induction ovulation (If ovulation spontaneously does not return), pregnancy rate, serum hormonal level (FSH, LH, testosterone) |
| Starting date | 23 July 2010 |
| Contact information | Dr Ziba Zahiri, Iran |
| Notes |
NCT02239107.
| Trial name or title | N‐acetyl cysteine for ovulation induction in clomiphene citrate‐resistant polycystic ovary syndrome |
| Methods | Randomised controlled trial, parallel‐assignment |
| Participants | Inclusion criteria: 18 to 39 years; PCOS women according to Rotterdam criteria who failed to respond to 6 months ovulation induction therapy with clomiphene citrate; normal semen analysis of partner; normal tubo‐peritoneal anatomy as assessed by laparoscopy Exclusion Criteria: Other causes of infertility; receiving gonadotrophin ovulation induction |
| Interventions | LOD versus LOD plus N‐Acetyl cysteine 1200 mg daily in 2 divided doses starting on cycle day 2 for 6 months |
| Outcomes | Primary outcome measures: ovulation rate Secondary outcome measures: pregnancy rate |
| Starting date | January 2012 |
| Contact information | Esraa Yousef Badran, Egypt |
| Notes |
NCT02305693.
| Trial name or title | Comparison between letrozole and LOD in women with clomiphene‐resistant PCOS |
| Methods | Randomised controlled trial, parallel‐assignment |
| Participants | Inclusion criteria: 20 to 40 years, clomiphene‐resistant PCOS women Exclusion Criteria: Other causes of infertility, hyperprolactinaemia, BMI > 35, previous letrozole or LOD |
| Interventions | LOD versus Letrozole 2.5 mg |
| Outcomes | Primary outcome measures: ovulation Secondary outcome measures: pregnancy |
| Starting date | November 2014 |
| Contact information | AbdelGany MA Hassan, Cairo University, Egypt |
| Notes |
NCT02381184.
| Trial name or title | Extended CC regimen versus LOD for ovulation Induction in clomiphene‐resistant women With PCOS |
| Methods | Randomised controlled trial, parallel‐assignment. |
| Participants | Inclusion criteria: Aged 18 ‐ 35 years,> 2 years infertility, serum level of FSH < 10 U/L in the early follicular phase, CC‐resistant PCOS, as they failed to ovulate with a dose of CC of 150 mg/day for 5 days per cycle for at least 3 consecutive cycles. All women had patent fallopian tubes proved by hysterosalpingography or laparoscopy and their partners satisfied the normal parameters of semen analysis according to the modified WHO criteria Exclusion Criteria: Infertility due to causes other than CC‐ resistant PCOS or due to combined factors, BMI ≥ 35 Kg/m2, use of metformin, gonadotropins, hormonal contraception or diet regimen within the last 6 months; women with congenital adrenal hyperplasia, hyperprolactinaemia or abnormal thyroid function, hypersensitivity or contraindications to letrozole or clomiphene treatment; previous LOD |
| Interventions | CC 100 mg daily for 10 days starting on day 3 of cycle, versus LOD |
| Outcomes | Primary outcome measures: ovulation rate Secondary outcome measures: endometrial thickness, rates of clinical pregnancy |
| Starting date | June 2014 |
| Contact information | Khalid Abd Aziz Mohamed, Benha University, Egypt |
| Notes |
NCT02775734.
| Trial name or title | N‐acetyl‐cysteine in clomiphene‐resistant PCOS after LOD: a randomised controlled trial |
| Methods | Randomised controlled trial, parallel‐assignment |
| Participants | Inclusion criteria: Aged 18 to 35 years; BMI between 25 and 30 Kg/m2; CC‐resistant PCOS Exclusion criteria: BMI < 25 or > 30 Kg/m2, hyper‐ or hypothyroidism, or hyperprolactinaemia; current or previous (within the last 6 months) use of oral contraceptives, glucocorticoids, antiandrogens, antidiabetic and anti‐obesity drugs or other hormonal drugs; intention to start a diet or a specific programme of physical activity; organic pelvic diseases; tubal or male‐factor infertility; interval of earlier treatment with any of the fertility drugs of < 6 months; contraindication to clomiphene citrate; liver disease, undiagnosed abnormal uterine bleeding, uterine fibroids, endometrial cancer, ovarian enlargement or OHSS or HCG injection: ovarian enlargement or hyper stimulation |
| Interventions | LOD + N‐acetyl‐cysteine + CC, versus LOD + CC |
| Outcomes | Primary outcome measures: Biochemical pregnancy rate Secondary outcome measures: Clinical pregnancy rate, live birth rate, ovulation rate, follicles ≥ 18 mm, pre‐ovulatory endometrial thickness, mid‐luteal sub‐endometrial doppler blood flow indices, incidence of side effects |
| Starting date | May 2016 |
| Contact information | Mohamed S Sweed, Ain Shams University, Cairo, Egypt |
| Notes |
NCT03009838.
| Trial name or title | Letrozole versus LOD in PCOS |
| Methods | Randomised controlled trial, parallel‐assignment |
| Participants | Inclusion criteria: Aged 20 to 35 years; history of at least 1 year of infertility either primary or secondary; BMI 25 ‐ 35; normal fallopian tubes; normal semen analysis of the husband; women who will agree to participate in the study Exclusion criteria: BMI > 35; contraindication to general anaesthesia; previous laparoscopic drilling; presence of other causes of infertility; had received metformin, gonadotrophin, oral contraceptives or other hormonal drugs during the preceding 6 months; intended to start a diet programme; refuse to participate in the study |
| Interventions | LOD versus Letrozole 2.5 mg oral tablets |
| Outcomes | Primary outcome: ovulation rate Secondary outcome: mid‐cyclic endometrial thickness |
| Starting date | January 2017 |
| Contact information | Ahmed Mohamed Abbas, Assiut Univeristy, Egypt |
| Notes |
NCT03206892.
| Trial name or title | LESS surgery versus conventional multiport laparoscopy in ovarian drilling |
| Methods | Randomised controlled trial, parallel‐assignment |
| Participants | Inclusion criteria: Aged 16 to 50 years; PCOS according to Rotterdam Criteria (2 out of 3): polycystic ovaries (12 or more follicles in each ovary and/or increased ovarian volume > 10 cm3), oligo‐ or an‐ovulation, clinical and/or biochemical hyperandrogenism after exclusion of other aetiologies for irregular cycles. Indications of laparoscopic ovarian drilling: clomiphene citrate‐resistance or failure: failure to conceive after 6 to 9 cycles, other indications for laparoscopy; before gonadotropin administration to decrease risk of OHSS and multiple pregnancy; before ART to decrease risk of severe OHSS in women who previously had cancelled IVF cycles due to OHSS risk or who suffered from OHSS in a previous treatment. Exclusion criteria: Previous 2 or more laparotomies; chronic pelvic pain, endometriosis or pelvic inflammatory diseases to avoid pelvic adhesions and bias in the quantification of postoperative pain; high BMI (> 35kg/m2); do not possess a native umbilicus; advanced gynaecological surgeries or malignant disorders (total laparoscopic hysterectomy, laparoscopically assisted vaginal hysterectomy , laparoscopic myomectomy); contraindication to any laparoscopy‐like medical condition worsened by pneumoperitoneum or Trendelnburg position |
| Interventions | Laparoscopic ovarian drilling for PCOS in infertile women using laparo‐endoscopic single‐site surgery (LESS surgery: single incision through the umbilicus using modified Hasson technique), versus Conventional multi‐port laparoscopy LOD for PCOS |
| Outcomes | Primary outcome: successful surgical procedure Secondary outcome: operative time, intraoperative blood loss, intraoperative complications, postoperative hospital stay, postoperative pain, postoperative complications, cosmetic outcome |
| Starting date | August 2017 |
| Contact information | Ahmed Mohamed Bahaa Eldin Ahmed, Ain Shams Maternity Hospital, Egypt |
| Notes |
NCT03664050.
| Trial name or title | LOD versus letrozole In clomiphene‐resistant polycystic ovary |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria: diagnosed as PCOS according to Roterdam (2003) criteria; clomiphene‐resistance, i.e. failure to ovulate following 100 mg CC for 5 days for at least 3 cycles; patent fallopian tubes, confirmed by hysterosalpingography or hysteroscopic diagnosis; normal semen analysis parameters of the spouse according to the modified criteria of the World Health Organization; normal serum prolactin, thyroid stimulating hormone and 17‐OH progesterone; no systemic disease; no gonadotropin or other hormonal drug treatment during the preceding 3 months Exclusion criteria: Infertility induced by reasons other than PCOS; uterine cavity lesions or ovarian cyst; > 40 years old; BMI > 26 kg/m2; contraindications to general anaesthesia; history of pelvic surgery; other endocrine diseases; a history of liver or kidney disease |
| Interventions | 2.5 mg letrozole oral tablets on the 2nd ‐ 3rd day of menses and then every day for 5 days. Treatment to be repeated for up to 3 cycles if the participant failed to conceive, versus Bilateral LOD: each ovary will be cauterised at 4 points, each for 4 sec at 40 W, at a depth of 7 ‐ 8 mm and a diameter of 3 ‐ 5 mm, using a monopolar electrosurgical needle according to the size of each ovary |
| Outcomes | Primary outcome: ovulation rate Secondary outcomes: biochemical pregnancy rate, clinical pregnancy rate |
| Starting date | September 2018 |
| Contact information | Ahmed Abdelshafy, Ain shams university maternity hospital, Cairo, Egypt |
| Notes |
PACTR201411000886127.
| Trial name or title | Impact of unilateral versus bilateral LOD on ovarian reserve and pregnancy rate: A randomised clinical trial |
| Methods | Parallel randomised |
| Participants | Inclusion criteria: women with PCOS who were resistant to CC; Age minimum 20 years, maximum 32 years Exclusion criteria: women with adrenal hyperplasia, thyroid disease, Cushings syndrome, hyperprolactinaemia and a tumour‐related excess of androgen |
| Interventions | Bilateral LOD versus Unilateral LOD |
| Outcomes | Clinical pregnancy rate, ovarian reserve measures, ovulation rate |
| Starting date | 10 January 2014 |
| Contact information | Hytham Hamza, Egypt |
| Notes |
ART: assisted reproductive technology; BMI: body mass index; CC: clomiphene citrate; FSH: follicle stimulating hormone; HCG: human chorionic gonadotropin; LOD: laparoscopic ovarian drilling; OHSS: ovarian hyperstimulation syndrome; PCOS: polycystic ovary syndrome; WHO: World Health Organization
Differences between protocol and review
In the original review the only comparison was with gonadotrophins alone. In the 2012 update the comparison was expanded to include other medical treatments. It also included women undergoing ART. In the current (2020) update we changed the title from Laparoscopic 'drilling' by diathermy or laser for ovulation induction in anovulatory polycystic ovary syndrome to Laparoscopic ovarian drilling for ovulation induction in women with anovulatory polycystic ovary syndrome. For dichotomous data, we calculated Peto odds ratios for rare events.
Contributions of authors
In this update Esmée Bordewijk, Lidija Rakic, Julie Brown, and Tineke Crawford selected trials for inclusion, extracted and entered data. Bonnie Ng contributed to data extraction. Disagreements were resolved by discussion with a third review author (Madelon van Wely). Esmée Bordewijk conducted the analyses and prepared the initial draft. All the other authors commented on drafts and approved the final version.
Sources of support
Internal sources
University of Auckland, New Zealand.
Yorkshire Regional Health Authority, UK.
External sources
No sources of support supplied
Declarations of interest
Esmée Bordewijk: none known Ka Ying Bonnie Ng: none known Lidija Rakic: none known Ben Willem Mol reports grants from NHMRC, personal fees from ObsEva, personal fees from Merck Merck KGaA, personal fees from Guerbet, personal fees from iGenomix, outside the submitted work. Julie Brown: none known Tineke Crawford: none known Madelon van Wely: none known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Abdellah 2011 {published data only}
- Abdellah M. Reproductive outcome after letrozole versus laparoscopic ovarian drilling for clomiphene resistant polycystic ovary syndrome. International Journal of Gynaecology and Obstetrics 2011;113(3):218‐21. [DOI] [PubMed] [Google Scholar]
Al‐Mizyen 2000 {published data only}
- Al‐Mizyen E, Grudzinskas JG. Unilateral versus bilateral laparoscopic ovarian diathermy in the management of infertility women with polycystic ovarian syndrome. Abstracts of the 16th Annual Meeting of the ESHRE. 2000:P262.
Amer 2009 {published data only}
Ashrafinia 2009 {published data only}
Balen 1994 {published data only}
- Balen A, Jacobs H. A prospective study comparing unilateral and bilateral laparoscopic ovarian diathermy in women with the polycystic ovary syndrome (PCOS). Abstracts of the 2nd International Meeting of the British Fertility Society. Glasgow, 1994. [DOI] [PubMed]
- Balen A, Jacobs H. A prospective study comparing unilateral and bilateral laparoscopic ovarian diathermy in women with the polycystic ovary syndrome (PCOS). Fertility and Sterility 1994;62(5):921‐5. [DOI] [PubMed] [Google Scholar]
Bayram 2004 {published data only}
- Bayram N, Wely M, Bossuyt P, Veen F. Randomised clinical trial of laparoscopic electrocoagulation of the ovaries versus recombinant FSH for ovulation induction in subfertility associated with polycystic ovary syndrome. 17th Annual Meeting of the ESHRE, Lausanne, Switzerland. July 2001:Abstract 0‐148.
- Bayram N, Wely M, Kaaijk EM, Bossunyt PMM, Veen F. Using an electrocautery strategy or recombinant follicle stimulating hormone to induce ovulation in polycystic ovarian syndrome. BMJ 2004;328(192):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahuis M, Oude Loohuis EJ, Kose N, Bayram N, Hompes P, Oosterhuis G, et al. Long term follow‐up of clomiphene citrate resistant women with PCOS treated with laparoscopic electrocautery of the ovaries or gonadotrophins ‐ an economic evaluation. Human Reproduction 2011;Suppl 1:i297‐8. [DOI] [PubMed] [Google Scholar]
- Nahuis MJ, Kose N, Dessel H, Braat D, Hamilton C, Hompes P, et al. Long term outcomes in women with polycystic ovary syndrome initially randomized to receive laparoscopic electrocautery of the ovaries or ovulation induction with gonadotrophins. Human Reproduction 2011;26(7):1899‐904. [DOI] [PubMed] [Google Scholar]
- Nahuis MJ, Oude Lohuis E, Kose N, Bayram N, Hompes P, Oosterhuis GJ, et al. Long‐term follow‐up of laparoscopic electrocautery of the ovaries versus ovulation induction with recombinant FSH in clomiphene citrate‐resistant women with polycystic ovary syndrome: An economic evaluation. Human Reproduction 2012;27(12):3577‐82. [DOI] [PubMed] [Google Scholar]
- Nahuis MJ, Oude Lohuis EJ, Bayram N, Hompes PG, Oosterhuis GJ, Veen F, et al. Pregnancy complications and metabolic disease in women with clomiphene citrate‐resistant anovulation randomized to receive laparoscopic electrocautery of the ovaries or ovulation induction with gonadotropins: A 10‐year follow‐up. Fertility and Sterility 2014;101(1):270‐4. [DOI] [PubMed] [Google Scholar]
- Oude Loohuis EJ, Nahuis MJ, Bayram N, Hompes PG, Oosterhuis GJ, Bossuyt PM, et al. Long term follow up of CC resistant women with PCOS treated with laparoscopic electrocautery of the ovaries or gonadotrophins ‐ Ovarian function and metabolic syndrome. Human Reproduction 2011;26(Suppl 1):i297‐i8 Abstract no: P‐452. [Google Scholar]
- Wely M, Bayram N, Bossunyt PM, Veen F. Laparoscopic electrocautery of the ovaries or recombinant FSH in clomiphene citrate‐resistant polycystic ovary syndrome: Impact on women's health‐related quality of life. Human Reproduction 2004;19(10):2244‐50. [DOI] [PubMed] [Google Scholar]
- Wely M, Bayram N, Bossunyt PMM, Veen F. An economic comparison of a laparoscopic electrocautery strategy and ovulation induction with recombinant FSH in women with clomiphene citrate‐resistant polycystic ovary syndrome. Human Reproduction 2004;19(8):1741‐5. [DOI] [PubMed] [Google Scholar]
Darwish 2016 {published data only}
- Darwish AM, Metwally AB, Shaaban MM, Mohamed S. Monopolar versus bipolar laparoscopic ovarian drilling in clomiphene‐resistant polycystic ovaries (PCO): a preliminary study. Gynecological Surgery 2016;13(3):179‐85. [Google Scholar]
Elgafor 2013 {published data only}
- Elgafor I. Efficacy of combined metformin‐letrozole in comparison with bilateral ovarian drilling in clomiphene‐resistant infertile women with polycystic ovarian syndrome. Archives of Gynecology and Obstetrics 2013;288(1):119‐23. [DOI] [PubMed] [Google Scholar]
El‐Sayed 2017 {published data only}
- El‐Sayed ML, Ahmed MA, Mansour MA, Mansour SA. Unilateral versus bilateral laparoscopic ovarian drilling using thermal dose adjusted according to ovarian volume in CC‐resistant PCOS, a randomized study. Journal of Obstetrics and Gynaecology of India 2017;67(5):356‐62. [DOI: 10.1007/s13224-017-1010-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Farquhar 2002 {published data only}
- Farquhar CM, Williamson K, Garland J, Brown P. An economic evaluation of laparoscopic ovarian diathermy versus gonadotrophin therapy for women with clomiphene citrate resistant polycystic ovary syndrome. Human Reproduction 2004;19(5):1110‐5. [DOI] [PubMed] [Google Scholar]
- Farquhar CM, Williamson K, Gudex G, Johnson NP, Garland J, Sadler L. A randomized controlled trial of laparoscopic ovarian diathermy versus gonadotrophin therapy for women with clomiphene‐resistant polycystic ovarian syndrome. Fertility and Sterility 2002;78(2):404‐11. [DOI] [PubMed] [Google Scholar]
- Mohiuddin S, Bessellink D, Farquhar C. Long‐term follow up of women with laparoscopic ovarian diathermy for women with clomiphene‐resistant polycystic ovarian syndrome. Australian & New Zealand Journal of Obstetrics & Gynaecology 2007;47(6):508‐511. [DOI] [PubMed] [Google Scholar]
Fernandez 2015 {published data only}
- Fernandez H, Cedrin‐Durnerin I, Gallot V, Rongieres C, Watrelot A, Mayenga‐Mankezi JM, et al. Using an ovarian drilling by hydrolaparoscopy or recombinant follicle stimulating hormone plus metformin to treat polycystic ovary syndrome: Why did a randomized controlled trial fail? [Drilling ovarien par fertilioscopie ou stimulation par FSH plus metformine dasn le traitement du syndrome des ovaries polykystiques: porquoi un essai thérapeutique peut ětre un échec?]. Journal de Gynecologie Obstetrique et Biologie de la Reproduction 2015;44(8):692‐8. [DOI] [PubMed] [Google Scholar]
Ghafarnegad 2010 {published data only}
- Ghafarnegad M, Arjmand N, Khazaeipour Z. Pregnancy rate of gonadotrophin therapy and laparoscopic ovarian electrocautery in polycystic ovary syndrome resistant to clomiphene citrate: A comparative study. Tehran University Medical Journal 2010; Vol. 67, issue 10:712‐7.
Giampaolino 2016 {published data only}
- Bifulco G, Sparice S, Della Corte L, Giampaolino P, Morra I, Nappi C. Post‐operative serum AMH levels after ovarian drilling in patients with PCOS: A randomized study comparing laparoscopy and transvaginal hydrolaparoscopy. Gynecological Surgery 2016;13(Suppl 1):S145. [DOI: 10.1007/s10397-016-0977-x] [DOI] [PubMed] [Google Scholar]
- Giampaolino P, Morra I, Della Corte L, Sparice S, Carlo C, Nappi C, et al. Serum anti‐Mullerian hormone levels after ovarian drilling for the second‐line treatment of polycystic overy syndrome: a pilot randomized study comparing laparoscopy and transvaginal hydrolaparoscopy. Gynecological Endocrinology 2017;33(1):26‐9. [DOI: 10.1080/09513590.2016.1188280] [DOI] [PubMed] [Google Scholar]
- Giampaolino P, Morra I, Russo G, Nappi C, Bifulco G. A randomized study comparing conventional laparoscopy and transvaginal hydrolaparoscopy to reduce ovarian adhesion formation after ovarian drilling. Gynecological Surgery 2016;13(Suppl 1):S42. [DOI: 10.1007/s10397-016-0977-x] [DOI] [PubMed] [Google Scholar]
- Giampaolino P, Morra I, Tommaselli GA, Carlo C, Nappi C, Bifulco G. Post‐operative ovarian adhesion formation after ovarian drilling: a randomized study comparing conventional laparoscopy and transvaginal hydrolaparoscopy. Archives of Gynecology and Obstetrics 2016;294(4):791‐6. [DOI] [PubMed] [Google Scholar]
Gürgan 1992 {published data only}
- Gürgan T, Urman B, Aksu T, Yarali H, Develioglu O, Kisnisci H. The effect of short‐interval laparoscopic lysis of adhesions on pregnancy rates following Nd‐YAG laser photocoagulation of polycystic ovaries. Obstetrics and Gynecology 1992;80(1):45‐7. [PubMed] [Google Scholar]
Hamed 2010 {published data only}
Ibrahim 2017 {published data only}
- Ibrahim MH, Tawfic M, Hassan MM, Sedky OH. Letrozole versus laparoscopic ovarian drilling in infertile women with PCOS resistant to clomiphene citrate. Middle East Fertility Society Journal 2017;22:251‐4. [DOI: 10.1016/j.mefs.2017.02.003] [DOI] [Google Scholar]
Jamal 2000 {published data only}
- Jamal HS. Bilateral or unilateral KTP laser ovarian drilling in polycystic ovarian disease. Annals of Saudi Medicine 2000;20(2):22. [DOI] [PubMed] [Google Scholar]
Kaya 2005 {published data only}
- Kaya H, Sezik M, Ozkaya O. Evaluation of a new surgical approach for the treatment of clomiphene citrate‐resistant infertilty in polycystic ovary syndrome: Laparoscopic ovarian multi‐needle intervention. Journal of Minimally Invasive Gynecology 2005;12(4):355‐8. [DOI] [PubMed] [Google Scholar]
Lazoviz 1998 {published data only}
- Lazovic G, Milacic D, Terzic M, Spremovic S, Mitijasevic S. Medicaments or surgical therapy of PCOS (Abstract only). Fertility and Sterility 1998;70(3):472. [Google Scholar]
Liu 2015 {published data only}
- Liu W, Dong S, Li Y, Shi L, Zhou W, Liu Y, et al. Randomized controlled trial comparing letrozole with laparoscopic ovarian drilling in women with clomiphene citrate‐resistant polycystic ovary syndrome. Experimental and Therapeutic Medicine 2015;10(4):1297‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Malkawi 2003 {published data only}
- Malkawi H, Qublan H, Hamaideh A. Medical vs. surgical treatment for clomiphene citrate resistant women with polycystic ovary syndrome. Journal of Obstetrics and Gynaecology 2003;23(3):289‐93. [DOI] [PubMed] [Google Scholar]
Mamonov 2000 {published data only}
- Mamonov A, Chaika V. Management of clomiphene‐resistant patients with PCO syndrome: Metrodin HP vs. laparoscopic electrocoagulation of the ovarian surface (LEOS). XVI FIGO World Congress of Obstetrics and Gynaecology. 2000; Vol. FC2:12.05.
Mehrabian 2012 {published data only}
- Mehrabian F, Eessaei F. The laparoscopic ovarian electrocautery versus gonadotropin therapy in infertile women with clomiphene citrate‐resistant polycystic ovary syndrome; a randomized controlled trial. Journal of the Pakistan Medical Association JPMA 2012;62(Suppl 2(3)):S42‐4. [PubMed] [Google Scholar]
Nasr 2013 {published data only}
- Nasr A. Impact of unilateral versus bilateral laparoscopic ovarian drilling on ovarian reserve in clomiphene citrate‐resistant PCOS women. Fertility and Sterility 2013;100 Suppl(3):S114. [Google Scholar]
Nasr 2015 {published data only}
- Nasr A. Impact of ovarian volume‐based adjusted thermal dose versus fixed‐puncture dosage in laparoscopic ovarian drilling on ovarian reserve in clomiphene citrate‐resistant PCOS women. Fertility and Sterility 2015;104(Suppl 3):e124. [Google Scholar]
Palomba 2004 {published data only}
- Palomba S, Orio F, Falbo A, Russo T, Tolino A, Zullo F. Plasminogen activator inhibitor 1 and miscarriage after metformin treatment and laparoscopic drilling in patients with polycystic ovary syndrome. Fertility and Sterility 2005;84(3):761‐5. [DOI] [PubMed] [Google Scholar]
- Palomba S, Orio F, Nardo L, Falbo A, Russo T, Corea D, et al. Metformin administration versus laparoscopic ovarian diathermy in clomiphene citrate‐resistant women with polycystic ovary syndrome: A prospective parallel randomized double blind placebo controlled trial. Journal of Clinical Endocrinology and Metabolism 2004;89(10):4801‐9. [DOI] [PubMed] [Google Scholar]
Palomba 2010 {published data only}
- Palomba S, Falbo A, Battista L, Russo T, Venturella R, Tolino A, et al. Laparoscopic ovarian diathermy vs clomiphene citrate plus metformin as second‐line strategy for infertile anovulatory patients with polycystic ovary syndrome: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2010; Vol. 202, issue 6:577.e1‐8. [DOI] [PubMed]
Rezk 2016 {published data only}
- Rezk M, Sayyed T, Saleh S. Impact of unilateral versus bilateral laparoscopic ovarian drilling on ovarian reserve and pregnancy rate: A randomized clinical trial. Gynecological Endocrinology 2016;32(5):399‐402. [DOI] [PubMed] [Google Scholar]
Rimington 1997 {published data only}
- Rimington M, Walker S, Shaw R. The use of laparoscopic ovarian electrocautery in preventing cancellation of in vitro fertilization treatment cycles due to risk of ovarian hyperstimulation syndrome in women with polycystic ovaries. Human Reproduction 1997;12(7):1443‐7. [DOI] [PubMed] [Google Scholar]
Roy 2009 {published data only}
Roy 2010 {published data only}
- Roy K, Baruah J, Sharma A, Sharma J, Kumar S, Kachava G, et al. A prospective randomized trial comparing the clinical and endocrinological outcome with rosiglitazone versus laparoscopic ovarian drilling in patients with polycystic ovarian disease resistant to ovulation induction with clomiphene citrate. Archives of Gynecology and Obstetrics 2010;281(5):939‐44. [DOI] [PubMed] [Google Scholar]
Sharma 2006 {published data only}
- Sharma M, Kriplani A, Agarwal N. Laparoscopic bipolar versus unipolar ovarian drilling in infertile women with resistant polycystic ovarian syndrome. Journal of Gynaecologic Surgery 2006;22:105‐11. [Google Scholar]
Sorouri 2015 {published data only}
- Sorouri ZZ, Sharami SH, Tahersima Z, Salamat F. Comparison between unilateral and bilateral ovarian drilling in clomiphene citrate resistance polycystic ovary syndrome patients: A randomized clinical trial of efficacy. International Journal of Fertility and Sterility 2015;9(1):9‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vegetti 1998 {published data only}
- Vegetti W, Ragni G, Baroni E, Testa G, Marsico, S, Riccaboni A, et al. Laparoscopic ovarian drilling versus low‐dose pure FSH in anovulatory clomiphene‐resistant patients with polycystic ovarian syndrome: randomized prospective study (Abstract only). Human Reproduction 1998;13(1):120. [Google Scholar]
Yadav 2018 {published data only}
- Yadav P, Singh S, Singh R, Jain M, Awasthi S, Raj P. To study the effect on fertility outcome by gonadotropins vs laparoscopic ovarian drilling in clomiphene‐resistant cases of polycystic ovarian syndrome. Journal of the South Asian Federation of Obstetrics and Gynaecology 2017;9(4):336‐40. [DOI: 10.5005/jp-journals-10006-1525] [DOI] [Google Scholar]
Youssef 2007 {published data only}
- Youssef H, Atallah M. Unilateral ovarian drilling in polycystic ovarian syndrome: a prospective randomized trial. Reproductive Biomedicine Online 2007;15(4):457‐62. [DOI] [PubMed] [Google Scholar]
Zakherah 2010 {published data only}
- Zakherah M. Combined clomiphene citrate (CC) and tamoxifen versus laparoscopic ovarian drilling (LOD) in women with clomiphene resistant polycystic syndrome (PCOS): A randomized clinical trial (abstract). 2009; Vol. 107, issue Suppl 2:389.
- Zakherah MS, Nasr A, Saman AM, Shaaban OM, Shahin AY. Clomiphene citrate plus tamoxifen versus laparoscopic ovarian drilling in women with clomiphene‐resistant polycystic ovary syndrome. International Journal of Gynaecology and Obstetrics 2010; Vol. 108, issue 3:240‐3. [DOI] [PubMed]
Zakherah 2011 {published data only}
- Zakherah MS. Ovarian reserve after fixed versus adjusted laparoscopic ovarian drilling in clomiphene resistant PCOS patients. Fertility and Sterility 2014;102(Suppl 3):e145. [Google Scholar]
- Zakherah MS, Kamal MM, Hamed HO. Laparoscopic ovarian drilling in polycystic ovary syndrome: Efficacy of adjusted thermal dose based on ovarian volume. Fertility and Sterility 2011;95(3):1115‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abdel Gadir 1990 {published data only}
- Abdel Gadir A, Mowafi R, Alnaser H, Alrashid A, Alonezi O, Shaw R. Ovarian electrocautery versus human menopausal gonadotrophins and pure follicle stimulating hormone therapy in the treatment of patients with polycystic ovarian disease. Clinical Endocrinology 1990;33(5):585‐92. [DOI] [PubMed] [Google Scholar]
Abu Hashim 2011b {published data only}
- Abu Hashim H, Foda O, Ghayaty E, Elawa A. Laparoscopic ovarian diathermy after clomiphene failure in polycystic ovary syndrome: is it worthwhile? A randomized controlled trial. Archives of Gynecology and Obstetrics 2011;284(5):1303‐9. [DOI] [PubMed] [Google Scholar]
Al‐Mizyen 2007 {published data only}
- Al‐Mizyen E, Gedis Grudzinskas J. Unilateral laparoscopic ovarian diathermy in infertile women with clomiphene citrate resistant polycystic ovary syndrome. Fertility and Sterility 2007;88(6):1678‐9. [DOI] [PubMed] [Google Scholar]
Badawy 2009 {published data only}
- Badawy A, Khiary M, Ragab A, Sherif L. Ultrasound‐guided transvaginal ovarian needle drilling (UTND) for treatment of polycystic ovary syndrome: A randomized controlled trial. Human Reproduction 2009;24 Suppl 1:i179 P‐448 Poster. [DOI] [PubMed] [Google Scholar]
Foroozanfard 2010 {published data only}
- Foroozanfard F, Abedzadeh M, Moosavi Gh S. The effect of the number of laparascopic ovarian drilling in improving reproductive outcome in patients having polycystic ovarian syndrome resistant to clomiphen. Iranian Journal of Reproductive Medicine 2010;8(Suppl 1):69 Abstract no: P‐9. [Google Scholar]
Franz 2016 {published data only}
- Franz M, Marschalek J, Ott J, Pavlik R, Watrelot A, Thaler CJ. A comparison of transabdominal versus transvaginal laparoscopic ovarian drilling for polycystic ovary syndrome. Geburtshilfe Frauenheilkd 2016;76:216. [DOI: 10.1055/s-0036-1592765] [DOI] [Google Scholar]
Gadir 1992 {published data only}
- Gadir AA, Alnaser H, Mowafi R, Shaw R. The response of patients with polycystic ovarian disease to human menopausal gonadotropin therapy after ovarian electrocautery or a luteinizing hormone‐releasing hormone agonist. Fertility and Sterility 1992;57(2):309‐13. [DOI] [PubMed] [Google Scholar]
Greenblatt 1993 {published data only}
- Greenblatt E, Casper R. Adhesion formation after laparoscopic ovarian cautery for polycystic ovarian syndrome: lack of correlation with pregnancy rate. Fertility and Sterility 1993;60(5):766‐70. [PubMed] [Google Scholar]
Gürgan 1991 {published data only}
- Gürgan T, Kişnişçi H, Yarali H, Develioğlu O, Zeyneloğlu H, Aksu T. Evaluation of adhesion formation after laparoscopic treatment of polycystic ovarian disease. Fertility and Sterility 1991;56(6):1176‐8. [DOI] [PubMed] [Google Scholar]
Heylen 1994 {published data only}
- Heylen S, Puttemans P, Brosens I. Polycystic ovarian disease treated by laparoscopic argon laser capsule drilling: comparison of vaporization versus perforation technique. Human Reproduction 1994;9(6):1038‐42. [DOI] [PubMed] [Google Scholar]
Kamel 2004 {published data only}
- Kamel MA, Abdel Hamid A. Laparoscopic ovarian re‐electro cautery versus ovulation induction with FSH for persistent anovulation after laparoscopic PCOS treatment. Middle East Fertility Society Journal 2004;9:70‐8. [Google Scholar]
Kandil 2018 {published data only}
- Kandil M, Rezk M, Al‐Halaby A, Emarh M, El‐Nasr IS. Impact of ultrasound‐guided transvaginal ovarian needle drilling versus laparoscopic ovarian drilling on ovarian reserve and pregnancy rate in polycystic ovary syndrome: a randomized clinical trial. Journal of Minimally Invasive Gynecology 2018;25(6):1075‐9. [DOI: 10.1016/j.mig.2018.01.036] [DOI] [PubMed] [Google Scholar]
Keckstein 1990 {published data only}
- Keckstein G, Rossmanith W, Spatzier K, Schneider V, Borschers K, Steiner R. The effect of laparoscopic treatment of polycystic ovarian disease by CO2‐laser or Nd:YAG laser. Surgical Endoscopy 1990;4(2):103‐7. [DOI] [PubMed] [Google Scholar]
Kocak 2006 {published data only}
Lockwood 1995 {published data only}
- Lockwood G, Ledger W, Barlow D. Randomised cross over trial to assess the efficacy of 3 alternative treatments for ovulation induction in infertile women with clomiphene resistant polycystic ovarian syndrome (PCOS). Abstracts of 15th World Congress on Fertility and Sterility. Montpellier (France), 1995.
Malkawi 2005 {published data only}
- Malkawi HY, Qublan HS. Laparoscopic ovarian drilling in the treatment of polycystic ovary syndrome: how many punctures per ovary are needed to improve the reproductive outcomes. Journal of Obstetrics and Gynaecology Research 2005;31:115‐9. [DOI] [PubMed] [Google Scholar]
Muenstermann 2000 {published data only}
- Muenstermann U, Kleinstein J. Long term GnRH analogue treatment is equivalent to laparoscopic laser diathermy in polycystic ovarian syndrome patients with severe ovarian dysfunction. Human Reproduction 2000;15(12):2526‐30. [DOI] [PubMed] [Google Scholar]
Nasr 2010 {published data only}
- Nasr A. Effect of N‐acetyl‐cysteine after ovarian drilling in clomiphene citrate resistant PCOS women: a pilot study. Reproductive BioMedicine Online 2010;20(3):403‐9. [DOI] [PubMed] [Google Scholar]
Rath 2006 {published data only}
- Rath SK, Jalandhar MH, Duggal BS, Sharma RK. Surgical approach for polycystic ovarian syndrome in management of infertility. Medical Journal, Armed Forces India 2006;62(2):119‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roy 2018 {published data only}
- Roy KK, Maddirala H, Kumar S, Singhal S, Meena J. Evaluation of laparoscopic ovarian drilling by harmonic scalpel versus monopolar drilling needle in cases of clomiphene citrate resistant polycystic ovarian response. Journal of Minimally Invasive Gynecology 2018;25(7):S1–S256. [DOI: 10.1016/j.jmig.2018.09.383] [DOI] [Google Scholar]
Salah 2013 {published data only}
- Salah IM. Office microlaparoscopic ovarian drilling (OMLOD) versus conventional laparoscopic ovarian drilling (LOD) for women with polycystic ovary syndrome. Archives of Gynecology and Obstetrics 2013;287(2):361‐7. [DOI] [PubMed] [Google Scholar]
Saravelos 1996 {published data only}
- Saravelos H, Li T. Postoperative adhesions after laparoscopic electrosurgical treatment to the polycystic ovarian syndrome with the application of Interceed to one ovary: a prospective randomized controlled study. Human Reproduction 1996;11:992‐7. [DOI] [PubMed] [Google Scholar]
Seyam 2018 {published data only}
- Seyam E, Hefzy E. Laparoscopic ovarian drilling versus GnRH antagonist combined with cabergoline as a prophylaxis against the re‐development of ovarian hyperstimulation syndrome. Gynecological Endocrinology 2018;34(7):616‐22. [DOI: 10.1080/09513590.2018] [DOI] [PubMed] [Google Scholar]
Sunj 2013 {published data only}
- Sunj M, Canic T, Baldani DP, Tandara M, Jeroncic A, Palada I. Does unilateral laparoscopic diathermy adjusted to ovarian volume increase the chances of ovulation in women with polycystic ovary syndrome?. Human Reproduction 2013;28(9):2417‐24. [DOI] [PubMed] [Google Scholar]
- Sunj M, Canic T, Jeroncic A, Karelovic D, Tandara M, Juric S, et al. Anti‐Mullerian hormone, testosterone and free androgen index following the dose‐adjusted unilateral diathermy in women with polycystic ovary syndrome. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2014;179:163‐9. [DOI] [PubMed] [Google Scholar]
Tabrizi 2005 {published data only}
- Tabrizi NM, Mohammad K, Dabirashrafi H, Nia FI, Salehi P, Dabirashrafi B, et al. Comparison of 5‐, 10‐, and 15‐point laparoscopic ovarian electrocauterization in patients with polycystic ovarian disease: a prospective, randomized study. JSLS: Journal of the Society of Laparoendoscopic Surgeons 2005;9(4):439‐41. [PMC free article] [PubMed] [Google Scholar]
Vrbikova 1998 {published data only}
- Vrbikova J, Kuzel D, Rezabek K, Zivny J, Starka L, Vondra K, et al. Endocrine‐metabolic changes after different extents of laparoscopic ovarian drilling in clomiphene citrate resistant PCOS women (Abstract only). Fertility and Sterility 1998;70(Suppl 1):5497‐8. [Google Scholar]
Wang 2015 {published data only}
- Wang XH, Wang JQ, Xu Y, Huang LP. Therapeutic effects of metformin and laparoscopic ovarian drilling in treatment of clomiphene and insulin‐resistant polycystic ovary syndrome. Archives of Gynecology and Obstetrics 2015;291(5):1089‐94. [DOI] [PubMed] [Google Scholar]
Zeng 2012 {published data only}
- Zeng L, Zeng C, Tao LL. Comparative study on Chinese medical syndrome typing and treatment combined different surgical methods for treating clomiphene‐resistant polycystic ovary syndrome. Chinese Journal of Integrated Traditional and Western Medicine 2012;32(11):1492‐5. [PubMed] [Google Scholar]
Zhu 2010 {published data only}
- Zhu W, Fu Z, Chen X, Li X, Tang Z, Zhou Y, et al. Transvaginal ultrasound‐guided ovarian interstitial laser treatment in anovulatory women with polycystic ovary syndrome: a randomized clinical trial on the effect of laser dose used on the outcome. Fertility and Sterility 2010; Vol. 94, issue 1:268‐75. [DOI] [PubMed]
References to studies awaiting assessment
Abu Hashim 2010a {published data only}
- Abu Hashim H, Mashaly AM, Badawy A. Letrozole versus laparoscopic ovarian diathermy for ovulation induction in clomiphene resistant women with polycystic ovary syndrome: A randomized controlled trial. Archives of Gynecology and Obstetrics 2010;282(5):567‐71. [DOI] [PubMed] [Google Scholar]
Abu Hashim 2011a {published data only}
- Abu Hashim H, Lakany N, Sherief L. Combined metformin and clomiphene citrate versus laparoscopic ovarian diathermy for ovulation induction in clomiphene‐resistant women with polycystic ovary syndrome: A randomized controlled trial. The Journal of Obstetrics and Gynaecology Research 2011;37(3):169‐77. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
IRCT138903291306N2 {unpublished data only}
- IRCT138903291306N2. Comparison of ovulation rate after laparoscopic electrocautery in infertile women with Clomiphene citrate resistant polycystic ovarian syndrome. en.irct.ir/trial/538 (first received 3 October 2010).
NCT02239107 {unpublished data only}
- NCT02239107. N‐acetyl cysteine for ovulation induction in clomiphene citrate resistant polycystic ovary syndrome. clinicaltrials.gov/ct2/show/NCT02239107 (first received 12 September 2014).
NCT02305693 {unpublished data only}
- NCT02305693. Comparison between letrozole and laparoscopic ovarian drilling in women with clomiphene resistant polycystic ovarian syndrome. clinicaltrials.gov/ct2/show/NCT02305693 (first received 3 December 2014).
NCT02381184 {unpublished data only}
- NCT02381184. Extended clomiphene citrate regimen versus laparoscopic ovarian drilling for ovulation induction in clomiphene citrate‐resistant women with polycystic ovary syndrome. clinicaltrials.gov/ct2/show/NCT02381184 (first received 6 March 2015).
NCT02775734 {unpublished data only}
- NCT02775734. N‐acetyl‐cysteine in clomiphene citrate resistant polycystic ovary syndrome after laparoscopic ovarian drilling: a randomized controlled trial. clinicaltrials.gov/ct2/show/NCT02775734 (first received 18 May 2016).
NCT03009838 {unpublished data only}
- NCT03009838. Letrozole versus laparoscopic ovarian drilling in polycystic ovary syndrome. clinicaltrials.gov/ct2/show/NCT03009838 (first received 4 January 2017).
NCT03206892 {unpublished data only}
- NCT03206892. LESS surgery versus conventional multiport laparoscopy in ovarian drilling. clinicaltrials.gov/ct2/show/NCT03206892 (first received 2 July 2017).
NCT03664050 {unpublished data only}
- NCT03664050. Laparoscopic ovarian drilling versus letrozole in clomiphene citrate resistant polycystic ovary. clinicaltrials.gov/ct2/show/NCT03664050 (first received 10 September 2018).
PACTR201411000886127 {unpublished data only}
Additional references
Aakvaag 1985
- Aakvaag A, Gjønnaess H. Hormonal response to electrocautery of the ovary in patients with polycystic ovarian disease. British Journal of Obstetrics and Gynaecology 1985;92(12):1258‐64. [DOI] [PubMed] [Google Scholar]
Adams 1986
- Adams J, Polson DW, Franks S. Prevalence of polycystic ovaries in women with anovulation and idiopathic hirsutism. Br Med J (Clin Res Ed) 1986;293(6543):355–359. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Adashi 1981
- Adashi EY, Rock JA, Guzick D, Wentz AC, Jones GS, Jones HW Jr. Fertility following bilateral ovarian wedge resection: a critical analysis of 90 consecutive cases of the polycystic ovary syndrome. Fertility and Sterility 1981;36(3):30‐5. [DOI] [PubMed] [Google Scholar]
Armar 1990
- Armar N, McGarrigle H, Honour J, Holownia P, Jacobs H, Lachelin G. Laparoscopic ovarian diathermy in the management of anovulatory infertility in women with polycystic ovaries: endocrine changes and clinical outcomes. Fertility and Sterility 1990;53:45‐9. [DOI] [PubMed] [Google Scholar]
Armar 1993
- Armar N, Lachelin G. Laparoscopic ovarian diathermy: an effective treatment for anti‐oestrogen resistant anovulatory infertility in women with polycystic ovaries. British Journal of Obstetrics and Gynaecology 1993;100(2):161‐4. [DOI] [PubMed] [Google Scholar]
Balen 1993
- Balen A, Tan SL, Jacobs H. Hypersecretion of luteinising hormone. A significant cause of infertility and miscarriage. British Journal of Obstetrics and Gynaecology 1993;100(12):1082‐9. [DOI] [PubMed] [Google Scholar]
Buttram 1975
- Buttram VC, Vaquero C. Post‐ovarian wedge resection adhesive disease. Fertility and Sterility 1975;26(9):874‐6. [DOI] [PubMed] [Google Scholar]
Deans 1997
- Deans A, Wayne C, Toplis P. Pelvic infection: a complication of laparoscopic ovarian drilling. Gynaecological Endocrinology 1997;6:301‐3. [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG, editor(s) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking metaanalyses. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
ESHRE 2018
- ESHRE. International evidence based guideline for the assessment and management of polycystic ovary syndrome. www.monash.edu/medicine/sphpm/mchri/pcos/guideline 2018 (accessed 17 January 2020).
Farhi 1995
- Farhi J, Soule S, Jacobs HS. Effect of laparoscopic ovarian electrocautery on ovarian response and outcome of treatment with gonadotropins in clomiphene citrate‐resistant patients with polycystic syndrome. Fertility and Sterility 1995;64(5):930‐3. [DOI] [PubMed] [Google Scholar]
Franik 2018
- Franik S, Eltrop SM, Kremer JA, Kiesel L, Farquhar C. Aromatase inhibitors (letrozole) for subfertile women with polycystic ovary syndrome. Cochrane Database of Systematic Reviews 2018, Issue 5. [DOI: 10.1002/14651858.CD010287.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Franks 1985
- Franks S, Adams J, Mason H, Polson D. Ovulation disorders in women with polycystic ovary syndrome. Clinical Obstetrics and Gynecology 1985;12(3):605‐33. [PubMed] [Google Scholar]
Gjönnaess 1984
- Gjönnaess H. Polycystic ovarian syndrome treated by ovarian electrocautery through the laparoscope. Fertility and Sterility 1984;41(1):20‐25. [DOI: 10.1016/S0015-0282(16)47534-5] [DOI] [PubMed] [Google Scholar]
Gradepro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Hmilton (ON): McMaster University (developed by Evidence Prime), Accessed 17 January 2020.
Greenblatt 1987
- Greenblatt E, Casper RF. Endocrine changes after laparoscopic ovarian cautery in polycystic ovarian syndrome. American Journal of Obstetrics and Gynecology 1987;156(2):279‐85. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s),.Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Imani 1998
- Imani B, Eijkemans MJ, Velde ET, Habbema JD, Fauser BC. Predictors of patients remaining anovulatory during clomiphene citrate induction of ovulation in normogonadotropic oligoamenorrheic infertility. Journal of Clinical Endocrinology and Metabolism 1998;83(7):2361‐5. [DOI] [PubMed] [Google Scholar]
Kovacs 1991
- Kovacs G, Buckler H, Bangah M, Outch K, Burger H, Healy D, et al. Treatment of anovulation due to polycystic ovarian syndrome by laparoscopic ovarian electrocautery. British Journal of Obstetrics and Gynaecology 1991;98(1):30‐5. [DOI] [PubMed] [Google Scholar]
Nahuis 2014
- Nahuis MJ, Oude Lohuis EJ, Bayram N, Hompes PG, Oosterhuis GJ, Veen F, et al. Pregnancy complications and metabolic disease in women with clomiphene citrate‐resistant anovulation randomized to receive laparoscopic electrocautery of the ovaries or ovulation induction with gonadotropins: A 10‐year follow‐up. Fertility and Sterility 2014;101(1):270‐4. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2017
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Akl E, et al. on behalf of the Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Stein 1939
- Stein IF, Cohen MR. Surgical treatment of bilateral polycystic ovaries. American Journal of Obstetrics and Gynecology 1939;38:465‐73. [Google Scholar]
Yu 2019
- Yu Q, Hu S, Wang Y, Cheng G, Xia W, Zhu C. Letrozole versus laparoscopic ovarian drilling in clomiphene citrate‐resistant women with polycystic ovary syndrome: a systematic review and meta‐analysis of randomized controlled trials. Reproductive Biology and Endocrinology : RB&E 2019;17(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2019
- Zhang J, Tang L, Kong L, Wu T, Xu L, Pan X, et al. Ultrasound‐guided transvaginal ovarian needle drilling for clomiphene‐resistant polycystic ovarian syndrome in subfertile women. Cochrane Database of Systematic Reviews 2019, Issue 7. [DOI: 10.1002/14651858.CD008583.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Farquhar 2001
- Farquhar C, Vandekerckhove P, Lilford R. Laparoscopic "drilling" by diathermy or laser for ovulation induction in anovulatory polycystic ovary syndrome. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD001122] [DOI] [PubMed] [Google Scholar]
Farquhar 2005
- Farquhar C, Lilford RJ, Marjoribanks J, Vandekerckhove P. Laparoscopic "drilling" by diathermy or laser for ovulation induction in anovulatory polycystic ovary syndrome. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD001122.pub2] [DOI] [PubMed] [Google Scholar]
Farquhar 2007
- Farquhar C, Lilford R, Marjoribanks J, Vandekerckhove P. Laparoscopic drilling by diathermy or laser for ovulation induction in anovulatory polycystic ovary syndrome. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD001122.pub2] [DOI] [PubMed] [Google Scholar]
Farquhar 2012
- Farquhar C, Brown J, Marjoribanks J. Laparoscopic drilling by diathermy or laser for ovulation induction in anovulatory polycystic ovary syndrome. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD001122.pub4] [DOI] [PubMed] [Google Scholar]


